Abstract
Lymphocryptoviruses (LCVs) naturally infecting Old World nonhuman primates are closely related to the human LCV, Epstein-Barr virus (EBV), and share similar genome organization and sequences, biologic properties, epidemiology, and pathogenesis. LCVs can efficiently immortalize B lymphocytes from the autologous species, but the ability of a given LCV to immortalize B cells from other Old World primate species is variable. We found that LCV from rhesus monkeys did not immortalize human B cells, and EBV did not immortalize rhesus monkey B cells. In this study, baboon LCV could not immortalize human peripheral blood B cells but could readily immortalize rhesus monkey B cells. Thus, efficient LCV-induced B-cell immortalization across distant Old World primate species appears to be restricted by a species-specific block. To further characterize this species restriction, we first cloned the rhesus monkey LCV major membrane glycoprotein and discovered that the binding epitope for the EBV receptor, CD21, was highly conserved. Stable infections of human B cells with recombinant amplicons packaged in rhesus monkey or baboon LCV envelopes were also consistent with a species-restricted block occurring after virus binding and penetration. Transient infections of human B cells with simian LCV resulted in latent LCV EBNA-2 gene expression and activation of cell CD23 gene expression. EBV-immortalized human B cells could be coinfected with baboon LCV, and the simian virus persisted and replicated in human B cells. Thus, several lines of evidence indicate that the species restriction for efficient LCV-induced B-cell immortalization occurs beyond virus binding and penetration. This has important implications for the study of LCV infection in Old World primate models and for human xenotransplantation where simian LCVs may be inadvertently introduced into humans.
Old World nonhuman primates are naturally infected with a herpesvirus in the same lymphocryptovirus (LCV) subgroup as Epstein-Barr virus (EBV). These nonhuman herpesviruses (referred to here as simian LCVs) share considerable molecular and biologic properties with EBV. Simian LCVs can immortalize B cells from the host species and express a similar repertoire of viral latent nuclear and membrane genes in EBV-immortalized B cells (8, 11, 14–16, 19, 21, 22, 25, 27, 41). Like EBV infection of humans, simian LCVs infect nearly all Old World primates, both those raised in captivity and those in the wild, and persist asymptomatically for life in infected animals (14, 19). Simian LCV can also cause malignant B-cell tumors in animals immunosuppressed by infection with simian immunodeficiency virus similar to the development of EBV-induced B-cell tumors in AIDS patients (3, 12, 13).
These similarities prompted us to use rhesus monkey LCV infection of naive rhesus monkeys as an animal model for acute and persistent EBV infection (31). Previous attempts at infecting Old World primates with EBV were unsuccessful (1, 14, 24). In some cases, failure can be retrospectively attributed to the use of nontransforming deletion mutants of EBV, namely P3HR1 (24), and potential cross-reactive immunity to EBV infection from endemic simian LCV infection (14, 19). However, in other studies it remains unclear why EBV was unable to infect Old World primates. A species restriction to LCV-induced B-cell immortalization may be one reason why infection of Old World primates with EBV was unsuccessful.
Previous reports vary regarding the ability of a given LCV to infect and efficiently immortalize B cells across Old World primate species. There are several reports that EBV can immortalize chimpanzee B cells, and chimpanzee LCV (referred to elsewhere as herpesvirus pan) can immortalize human B cells (19, 21). Similarly, LCV from a given cercopithicine species can often immortalize B cells from a different cercopithicine species, further suggesting that LCV can readily immortalize B cells from closely related Old World primates (17, 19, 34). The ability of LCV to immortalize B cells from more distantly related Old World species is less clear. Rhesus monkey and baboon LCVs have been reported to be incapable of immortalizing human B cells (10). In contrast, others have reported that baboon LCV can immortalize human cord blood B cells, but the process is much less efficient compared to EBV, since immortalized cell lines were successfully produced in only 60% of the attempts (19, 34). In the present study, we readdress the abilities of rhesus monkey, baboon, and human LCVs to immortalize adult peripheral blood B cells from humans and Old World species and begin to characterize the level at which this species restriction to efficient B-cell immortalization may occur.
MATERIALS AND METHODS
Cell lines.
B95-8 is a marmoset B-cell line infected with EBV (human herpesvirus 4) from a patient with infectious mononucleosis (30). S594 is a baboon LCV (referred to elsewhere as cercopithicine herpesvirus 12 or herpesvirus papio)-infected B-cell line derived by spontaneous growth from baboon peripheral blood lymphocytes (34). LCL8664 is a rhesus monkey LCV (cercopithicine herpesvirus 15)-infected B-cell line derived from a retro-orbital B-cell lymphoma in a rhesus monkey (35). BJAB-ZE1 is an EBV-negative human B-lymphoma-cell line, BJAB, that has been stably transfected with an EBV EBNA-1 expression vector to enhance recovery of amplicon containing the EBV latent origin of replication (ori-P) (38). BL41 is an EBV-negative human B-lymphoma-cell line. All cells were propagated in RPMI medium supplemented with 10% fetal bovine serum (FBS). BJAB-ZE1 cells were propagated in the presence of 3 mg of G418/ml.
EBV, simian LCV, and recombinant amplicon preparations.
Cell-free virus supernatants were harvested by filtration (pore size, 0.45 μm) from 3-day-old cultures of 107 virus-producing cells electroporated with 30 μg of pSVNaeI-Z to induce viral replication (7). EBV and rhesus monkey LCV titers were typically 106 and 5 × 104 transforming units/ml, respectively, when assayed on isogeneic B cells. Baboon LCV titers were typically 5 × 105 transforming units/ml with rhesus monkey peripheral blood mononuclear cells (PBMCs). Rhesus monkey PBMCs were obtained from the New England Primate Research Center in accordance with institutional guidelines for animal care. The amplicon plasmid, BSAII, contains the EBV lytic origin of replication (ori-lyt), the EBV packaging and cleavage signals within the terminal repeats, the EBV ori-P for episomal maintenance, and the hygromycin phosphotransferase gene (39). LCV-enveloped amplicons were produced by cotransfecting BSAII and pSVNaeI-Z into B95-8, S594, LCL8664, and BJAB (negative control), and harvesting cell-free supernatants through 0.45-μm-pore-size filters after 3 days of culture. BJAB-ZE1 and BL41 cells were infected with virus-amplicon supernatants for 2 h at 37°C. Cells were then washed and cultured in RPMI 1640 and 10% FBS. Cells stably infected with amplicons were selected in medium supplemented with 400 μg of hygromycin/ml, 24 h after infection.
Infection of primary lymphocytes.
PBMCs were prepared by centrifugation of peripheral blood on an equal volume of Ficoll or with Vacutainer CPT tubes (Becton Dickinson) and washed. Mononuclear cells were resuspended in viral supernatants (106 mononuclear cells/ml), incubated for 2 h at 37°C, washed, and cultured in 96-well microtiter plates at a density of 2 × 105 cells per well in RPMI 1640 containing 20% FBS, 20 mM HEPES, 10−5 M β-mercaptoethanol and 0.5 μg of cyclosporin A/ml. Mononuclear cells from several human donors were T cell depleted prior to infection by incubation with sheep erythrocytes treated with 2-amino ethylisothiouronium bromide, followed by removal of rosetted T cells on a second Ficoll gradient. T-cell-depleted mononuclear cells were cultured in 96-well plates at a density of 105 per well in the presence of 104 gamma-irradiated rat-1 fibroblast feeders per well. Propagation of virus from LCL15 was carried out by incubation of 105 gamma-irradiated LCL15 cells (5,000 rads) with 107 PBMCs in a 96-well plate. Proliferation of PBMCs in response to cell-free viral supernatants was determined by a colorimetric tetrazolium-formazan assay as previously described (36).
DNA cloning and PCR.
Genomic DNA from LCL8664 cells was digested with BamHI and cloned into the BamHI site of plasmid Bluescript. Clones were identified by hybridization with a DNA fragment containing the B95-8 EBV gp350 open reading frame. Filters were washed at 50°C with 0.5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 1% sodium dodecyl sulfate (SDS). Nucleotide sequencing of the rhesus monkey EBV DNA fragment RE3 indicated that it was homologous to EBV gp350 beginning at amino acid 288. The 5′ coding region of the rhesus monkey LCV membrane glycoprotein was PCR amplified from LCL8664 virus supernatants with primers derived from the EBV B95-8 gp350 nucleotide sequence upstream of the ATG initiation codon (5′AGTGTGAGACTCACCAACACCG3′) and from the rhesus monkey LCV RE3 sequence (5′GGCATGTCCTGAATAGTGGG3′). Amplification was carried out at an annealing temperature of 45°C for 30 cycles with Taq DNA polymerase (Gibco BRL). Sequence analysis and homology were carried out using Lasergene and PCgene softwares. Amplification of EBV EBNA-2 sequences was carried out with the primer pair 5′GCGCGGATCCCAGCGCAGGGATGCCTGGAC3′ and 5′GCGCGAATTCTGGCACCGTTAGTGTTGCAG3′ at an annealing temperature of 65°C. Amplification of baboon LCV EBNA-1 sequences was carried out with the primer pair 5′GCAGGAGTCTGCACTCCCTG3′ and 5′CTGGGACTACGTGGCCTCTT3′ at an annealing temperature of 64°C.
Southern blot analyses.
DNA was extracted from hygromycin-resistant cells with DNAzol (Gibco BRL), digested with BamHI, and analyzed by electrophoresis through a 0.7% agarose gel. The DNA was transferred to a nylon membrane and probed with a 32P-labeled hygromycin phosphotransferase DNA fragment. For detection of viral DNA, the blot was stripped with 0.4 M NaOH at 65°C and hybridized with an EBV BamHI W DNA fragment. All hybridizations were carried out at 68°C. Filters hybridized with the hygromycin phosphotransferase probe were washed at 68°C in 0.25× SSC and 1% SDS, and filters hybridized with the BamHI W probe were washed at 50°C in 0.5× SSC and 1% SDS.
Immunodetection of EBNA-2, CD23, and LMP1.
A total of 107 B-lymphoma cells were exposed to LCV preparations for 2 h at 37°C, washed, and incubated for 48 h. For detection of EBNA-2, cells were lysed in 50 mM Tris-HCl (pH 7.7)–150 mM NaCl–2 mM EDTA–1% Nonidet P-40–1% deoxycholate–0.1% SDS and immunoprecipitated with 5 μl of PE2 anti-EBNA-2 hybridoma supernatant and protein G-Sepharose. Proteins were resolved on 7% denaturing polyacrylamide gels, transferred to a nitrocellulose membrane, and detected with PE2 monoclonal antibody. Cell surface CD23 expression in BL41 cells was detected by using a fluorescence-activated cell sorter (FACS) with phycoerythrin-conjugated anti-CD23 monoclonal antibody (Bio-Source International). EBNA-2 and LMP1 expression in lymphoblastoid cell lines (LCLs) was determined by Western blot analysis of lysates from 400,000 cells using the PE2 and S12 monoclonal antibodies, respectively.
RESULTS
Species-specific B-cell immortalization with EBV, rhesus monkey LCV, and baboon LCV.
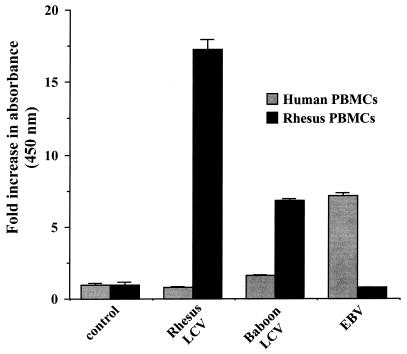
We tested the abilities of baboon LCV, rhesus monkey LCV, and EBV to immortalize PBMCs from rhesus monkeys and humans. Cell-free preparations of rhesus monkey LCV efficiently immortalized PBMCs from five different rhesus monkey donors. The typical titer of transforming virus obtained from the rhesus monkey LCV-producing cell line LCL8664 was approximately 5 × 104 transforming units per ml. However, the same rhesus monkey LCV supernatants failed to immortalize PBMCs from five different human donors. EBV containing supernatants, typically at 106 transforming units per ml, efficiently immortalized the same human PBMC preparations but failed to generate any immortalized cell lines from the same rhesus monkey PBMC preparations. Baboon LCV readily immortalized rhesus monkey PBMCs, but no immortalized B-cell lines were obtained from the same PBMC preparations of the five human donors exposed to baboon LCV. Development of immortalized B-cell lines correlated in all instances with cell proliferation of human and rhesus monkey peripheral blood lymphocytes in response to the different viral preparations (Fig. 1). Thus, rhesus monkey and human LCVs efficiently immortalize B cells from the autologous species but not from the heterologous species. The species restriction for efficient B-cell immortalization is not absolute, since baboon LCV can readily immortalize B cells from rhesus monkeys, a closely related species in the same cercopithicine family as baboons.
FIG. 1.
Proliferation of human and rhesus monkey peripheral blood lymphocytes after infection with EBV, rhesus monkey LCV, or baboon LCV. Cell proliferation was measured by metabolic reduction of soluble tetrazolium salts at 14 days of culture and is expressed as the ratio of infected to noninfected mononuclear cells. Standard deviations are indicated by error bars (n = 6).
Major viral membrane glycoprotein is conserved in nonhuman LCV and EBV.
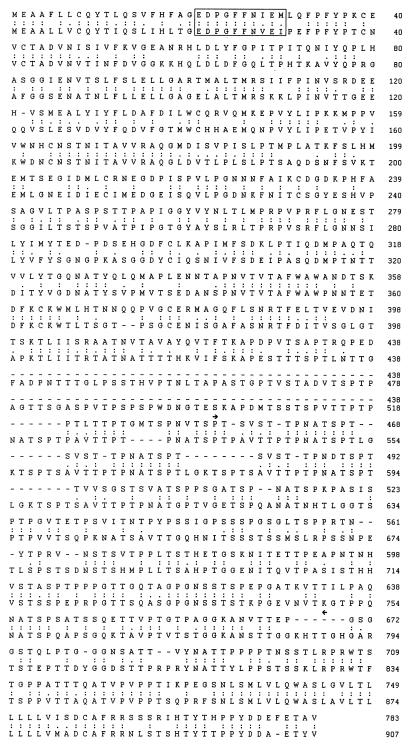
The EBV gp350/220 membrane glycoprotein binds to the EBV receptor on B cells, CD21, and contributes to the cellular host range of EBV (23). We cloned the EBV gp350 membrane glycoprotein homolog from rhesus monkey LCV to determine whether this receptor-binding viral protein had diverged significantly between the human and rhesus monkey LCVs. The amino acid sequence of the rhesus monkey LCV membrane glycoprotein encoded by the predicted open reading frame is 783 amino acids in length and is 56% identical and 68% similar to EBV gp350 (Fig. 2). The rhesus monkey LCV membrane glycoprotein is 124 residues shorter than the human homolog, and the region of greatest divergence between these proteins is within the spliced domain of gp220. The spliced domain present in EBV gp350 encompasses a 13-amino-acid sequence (AVTTPTPNATSPT) that is repeated five times. The rhesus monkey LCV membrane glycoprotein contains a related 12-amino-acid sequence that is repeated three times (SVSTTPNA/DTSPT). The rhesus monkey LCV membrane glycoprotein contains 27 N-X-T/S potential asparagine-linked glycosylation sites, 22 of which are positionally conserved in EBV gp350. The peptide sequence (EDPGFFNVEI) in EBV gp350 which mediates binding to the cell receptor for EBV, CD21, is identical to the peptide sequence in rhesus monkey LCV membrane glycoprotein (EDPGFFNIEM) except for conserved substitutions at positions 8 and 10, suggesting that rhesus monkey LCV is likely to bind to CD21 on human B cells (4, 32, 37).
FIG. 2.
Amino acid comparison of rhesus monkey LCV membrane glycoprotein (top sequence) and EBV (B95-8) major membrane glycoprotein gp350 (lower sequence). The gp350 peptide epitope sufficient for binding to the EBV receptor, CD21, is boxed. Identical (:) and similar (.) amino acids are shown. Amino acid sequence coded by the RNA region spliced in EBV gp220 is shown (▸◂).
A recombinant amplicon packaged with simian LCV envelopes can infect human B cells.
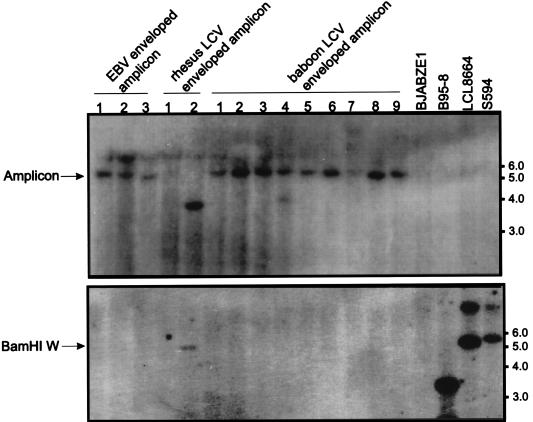
We tested whether simian LCV envelopes are capable of infecting human B cells by using a system that packages a marker plasmid with different LCV envelopes. A recombinant amplicon plasmid carrying a hygromycin phosphotransferase gene as a marker and the signals required for DNA replication and packaging (39) was transfected into EBV (B95-8)-, rhesus monkey LCV (LCL8664)-, and baboon LCV (S594)-infected cell lines. The transfected cells were induced for viral replication by cotransfection of an expression vector for the EBV lytic transactivator BZLF1, whereupon wild-type viruses and amplicons carrying the marker plasmid packaged by the respective LCV envelope were produced. These EBV- or simian LCV-enveloped particles in the supernatant of transfected cells were used in turn to infect an EBV-negative human B-lymphoma-cell line constitutively expressing EBV EBNA-1, BJAB-ZE1. Hygromycin-resistant clones were derived from BJAB-ZE1 cells exposed to EBV-, rhesus monkey LCV-, and baboon LCV-enveloped amplicons (4, 2, and 23 clones, respectively). Amplicon infection could be confirmed by detection of hygromycin phosphotransferase DNA on Southern blots of cell DNA from hygromycin-resistant clones infected with EBV- or simian LCV-enveloped amplicons (Fig. 3, top). Hygromycin phosphotransferase DNA was not detected on blots of cell DNA from uninfected BJAB-ZE1 cells or nontransfected virus-producing cell lines (B95-8, LCL8664, and S594). Low-stringency hybridization with an EBV DNA BamHI W probe was negative in all clones except for hygromycin-resistant clone 2, derived after exposure to rhesus monkey LCV, suggesting the presence of the rhesus monkey LCV genome in this clone by superinfection with wild-type rhesus monkey LCV (Fig. 3, bottom). The different-sized restriction fragments hybridizing with hygromycin phosphotransferase DNA in this clone may be due to integration of the plasmid DNA into the viral or cell genome. This result indicates that simian LCVs are capable of infecting human B cells and suggests that a species restriction for efficient B-cell immortalization is likely to occur at a step beyond virus infection and penetration.
FIG. 3.
Transfer of hygromycin phosphotransferase DNA (top) and viral DNA (bottom) by infection of EBV-negative B-lymphoma cells (BJAB-ZE1) with EBV-, rhesus monkey LCV-, or baboon LCV-enveloped amplicons. Genomic DNAs from hygromycin-resistant BAJB-ZE1 clones exposed to EBV-, rhesus monkey LCV-, and baboon LCV-enveloped amplicons were hybridized with a radiolabelled hygromycin phosphotransferase DNA at high stringency (top) or an EBV BamHI W DNA probe at lower stringency (bottom). Uninfected BJAB-ZE1 cells and EBV-, rhesus monkey LCV-, and baboon LCV-infected cells (B95-8, LCL8664, and S594, respectively) were used as controls. Molecular size markers (kilobases) are shown at right.
Simian LCV EBNA-2 is expressed after simian LCV infection of human B cells.
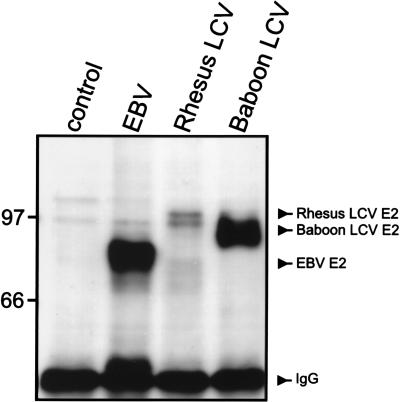
We tested whether transformation-associated latent viral genes are expressed after acute simian LCV infection in human B cells. Antibodies specific for baboon and rhesus monkey LCV latent gene products have not yet been developed, and the human and nonhuman LCV latent genes are generally more divergent than the lytic genes (15, 16, 27, 41). The PE2 monoclonal antibody specific for the EBV EBNA-2 does detect the EBNA-2 gene product from rhesus monkey and baboon LCVs (31, 42), despite the marked sequence divergence of the baboon LCV EBNA-2 (27). Expression of LCV-specific EBNA-2 was detected after acute infection of human B-lymphoma cells with human, rhesus monkey, and baboon LCVs (Fig. 4), and the relative levels of EBNA-2 expression correlated with the relative virus titers (106, 5 × 104, and 5 × 105 transforming units/ml, respectively). Since the EBV promoters responsible for EBNA-2 expression also drive expression of all other EBNAs (23), baboon LCV EBNA-2 expression is probably coincident with expression of all simian LCV EBNAs.
FIG. 4.
EBNA-2 expression in EBV-negative B-lymphoma cells, BL41, after acute infection with control medium, EBV, rhesus monkey LCV, and baboon LCV. The EBV (88 kDa), rhesus monkey LCV (100 kda), and baboon LCV (94 kda) EBNA-2 proteins were immunoprecipitated from cells 2 days after infection and detected by Western blotting with the monoclonal antibody PE2, which recognizes a conserved EBNA-2 epitope. E2, EBNA-2; IgG, immunoglobulin G. Molecular mass markers (kilodaltons) are at left.
Simian LCV infection induces CD23 expression in human B cells.
Latent EBV nuclear and membrane proteins act in synergy to induce cell gene expression, such as the B-cell activation marker CD23 (38). We assayed for cell surface CD23 expression to determine whether simian LCV infection could affect human B-cell gene expression. FACS analysis demonstrated that CD23 expression was reproducibly induced by acute baboon LCV infection of human B cells (Table 1). Only a subset of B-lymphoma cells typically express viral proteins after acute EBV infection in vitro, and the relative number of CD23-positive cells correlated with the percentage of virus-infected, EBNA-2-positive cells. Dual immunostaining with anti-EBNA-2 and anti-CD23 antibodies confirmed expression of CD23 in the EBNA-2-expressing population of EBV- and baboon LCV-infected BL41 cells (data not shown). Rhesus monkey LCV-induced gene expression could not be adequately assessed in this assay due to the relatively low virus titer. Thus, simian LCV infection of human B cells activates at least one cell gene previously identified as a target for EBV latent gene function.
TABLE 1.
Induction of cell surface CD23 in human B-lymphoma cells, BL41, by acute infection with baboon LCV
| Virus | % CD23-positive cells
|
||
|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | |
| Control | 0.7 | 0.8 | 0.2 |
| EBV | 4.5 | 5.4 | NDa |
| Baboon LCV | 4.7 | 6.4 | 7.5 |
ND, not done.
Baboon LCV can superinfect, persist, and replicate in EBV-immortalized human B cells.
An experiment using PBMCs from a sixth human donor provided additional data showing that baboon LCV can superinfect, persist, and replicate in EBV-immortalized human B cells. A high rate of spontaneous LCLs arose from in vitro culture of 107 T-cell-depleted uninfected PBMCs from this human donor, reflecting a relatively high viral load of EBV-infected B cells (28 of 96 microtiter wells positive). When PBMCs from the same individual were exposed to baboon LCV, several immortalized cell lines were retrieved (51 of 96 wells positive). To determine whether these cell lines were infected with EBV, baboon LCV, or both, 12 spontaneous cell lines and 43 cell lines exposed to baboon LCV were expanded for detection of viral DNA (eight cell lines exposed to baboon LCV were lost due to technical problems, e.g., bacterial contamination).
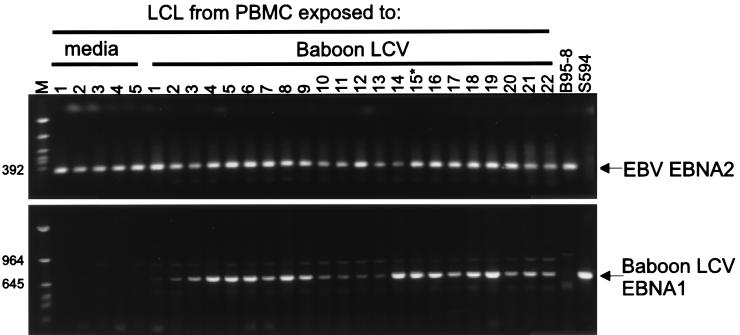
Oligonucleotide primers specific for the EBV EBNA-2 sequence amplified a 341-bp PCR product from a control EBV-infected cell line, B95-8 (Fig. 5, top), but not from a control baboon LCV-infected cell line, S594. EBV EBNA-2 could be amplified from all 12 spontaneous cell lines, indicating that these spontaneous LCLs derived from human peripheral blood were infected with EBV. Figure 5 (top) shows results from representative cell lines, and data are also summarized in Table 2. Similarly, EBV EBNA-2 could be amplified from all 43 cell lines arising after exposure to baboon LCV (Fig. 5 [top]; Table 2), indicating that EBV infection is contributing to immortalization of all these cell lines.
FIG. 5.
Coinfection of EBV-immortalized B cells with baboon LCV. Spontaneous, immortalized B-cell lines were recovered from PBMCs exposed to medium or baboon LCV, and evidence of EBV or baboon LCV infection was evaluated by PCR amplification of genomic DNA. PCR amplifications for EBV EBNA-2 sequences (top) and for baboon LCV EBNA-1 DNA (bottom) are shown. LCL15 (indicated by asterisk) was expanded and used for recovery of coinfecting EBV and baboon LCV shown in Fig. 6. Molecular mass markers (in base pairs) are at left.
TABLE 2.
Superinfection and persistence of baboon LCV in EBV-transformed PBMCsa
| Treatment | No. of LCLs generated | No. of LCLs positive for EBV EBNA-2/no. of LCLs analyzed | No. of LCLs positive for baboon LCV EBNA-1/no. of LCLs analyzed |
|---|---|---|---|
| Control medium | 28 | 12/12 | 0/12 |
| Baboon LCV | 51 | 43/43 | 33/43 |
A total of 107 T-cell-depleted human PBMCs were exposed to control medium or baboon LCV and cultured in a 96-well microtiter plate. Subsequent LCLs were analyzed for the presence of EBV DNA and baboon LCV DNA by amplification for EBV EBNA-2 and LCV EBNA-1 sequences, respectively (Fig. 5).
Primers specific for baboon LCV EBNA-1 amplified a 700-bp PCR product from the control baboon LCV-infected cell line S594 but not from a control EBV-infected cell line, B95-8 (Fig. 5, bottom). Baboon LCV EBNA-1 DNA could be amplified from 33 of 43 cell lines that arose after exposure to baboon LCV, suggesting that a significant percentage of cell lines were coinfected with baboon LCV (Fig. 5 [bottom]; Table 2). No cell lines contained baboon LCV alone without EBV, consistent with the interpretation that baboon LCV does not efficiently immortalize human B cells.
To confirm the presence of EBV and baboon LCV coinfection detected by PCR analysis, immortalizing viruses were recovered from a coinfected cell line, LCL15, by lethal gamma irradiation and cocultivation with either human or rhesus monkey PBMCs. Control wells containing only lethally irradiated LCL15 cells or PBMCs showed no growth. However, immortalized cell lines were recovered from cultures of primary human or rhesus monkey lymphocytes cocultivated with irradiated LCL15 cells (two and three LCLs, respectively).
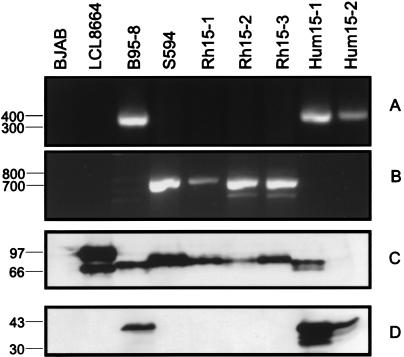
The recovery of both EBV and baboon LCV from LCL15 was confirmed by PCR and Western blotting for EBNA-2. Two cell lines derived from human lymphocytes cocultivated with LCL 15 were PCR positive for EBV EBNA-2 (Fig. 6A) but not for baboon LCV EBNA-1 (Fig. 6B). All three cell lines derived from rhesus monkey lymphocytes cocultivated with LCL15 were PCR positive for baboon LCV EBNA-1 (Fig. 6B) but not for EBV EBNA-2 (Fig. 6A). The identity of the EBV-infected human cells and baboon LCV-infected rhesus monkey cells could be further confirmed by Western blotting for EBNA-2 and LMP1 expression. As shown in Fig. 6C, the relative molecular mass can be used to identify the origin of the EBNA-2 gene product from baboon LCV (S594), EBV (B95-8), or rhesus monkey LCV (LCL8664). The rhesus monkey LCV EBNA-2 migrates with the largest molecular mass and is sometimes associated with a second band, most likely a degradation product. By Western blotting, the cell lines derived from rhesus monkey lymphocytes cocultivated with LCL15 were shown to express a protein similar in size to baboon LCV EBNA-2, consistent with recovery of baboon LCV from the coinfected cell line LCL15. The S12 monoclonal antibody detects EBV LMP1 but not rhesus monkey or baboon LCV LMP1 (Fig. 6D), and all cell lines derived from rhesus monkey lymphocytes cocultivated with LCL15 are negative when blotted with S12, also consistent with a baboon LCV origin. All cell lines derived from human lymphocytes cocultivated with LCL15 express EBV EBNA-2 and EBV LMP1 proteins, indicating recovery of the EBV coinfecting LCL15. EBV EBNA-2 expression was confirmed on repeated Western blot analysis when equal levels of cell lysates were loaded (not shown). Thus, baboon LCV can persist and replicate in human B cells by superinfection of EBV-immortalized B cells.
FIG. 6.
Propagation of EBV and baboon LCV from gamma-irradiated LCL15 cells by incubation with human or rhesus monkey PBMCs. The LCLs generated from human lymphocytes (Hum15-1 and Hum15-2) contained EBV DNA but not baboon LCV DNA, and LCLs generated from the rhesus monkey lymphocytes (Rh15-1, Rh15-2, and Rh15-3) contained baboon LCV but not EBV. (A) PCR amplification for EBV EBNA-2; (B) PCR amplification for baboon LCV EBNA-1; (C) Western blot for EBNA-2; (D) Western blot for EBV LMP1. Molecular mass markers (in base pairs for panels A and B and in kilodaltons for panels C and D) are at left.
DISCUSSION
This study addresses the basis for a species restriction for efficient B-cell immortalization by human and nonhuman LCVs. In previous surveys, EBV immortalized chimpanzee B cells but not baboon, gibbon, cynomolgus macaque, rhesus monkey, and stump-tailed monkey B cells (9, 14, 21). Rhesus LCV immortalized rhesus monkey and cynomolgus macaque B cells but not those of baboon, African green monkey, stump-tailed monkey, or human origin (reference 35 and this report). A negative finding for B-cell immortalization can be difficult to interpret. Virus titer, immune status of the B-cell donor, and use of purified B-cell preparations and cyclosporin A may all be relevant for the efficiency of LCV-induced B-cell immortalization (5). In this study, we used rhesus monkey LCV and EBV preparations with titers of 5 × 104 and 1 × 106 transforming units/ml, respectively, when assayed on lymphocytes from the autologous species. Serologic testing revealed that LCV-immune human and nonhuman donors were used, and use of purified B-cell populations or cyclosporin A had no effect on the findings with the heterologous viruses. Thus, our initial findings are consistent with previous reports of EBV- and rhesus monkey LCV-induced B-cell immortalization in various species. The inability for EBV to immortalize rhesus monkey B cells suggests one possible explanation for why experimental infection of rhesus monkeys with EBV has been unsuccessful (14, 18, 24).
Baboon LCV has been reported to immortalize B cells from gibbons, cynomolgus macaques, rhesus monkeys, and stump-tailed monkeys (11, 19). Although some reports have cited no immortalization of human B cells by baboon LCV (10), two studies have reported that baboon LCV can transform human cord blood lymphocytes. Given the uncertainties associated with negative results, one might conclude that baboon LCV can readily immortalize human B cells. However, on closer examination of these studies, growth transformation occurred only in 60% of the attempts, significantly less efficient than with EBV (19, 34). Thus, our present findings are not inconsistent with these previous reports and reinforce the interpretation that baboon LCV is much less efficient than EBV in inducing human B-cell growth transformation. Some of the differences may be from our use of adult peripheral blood lymphocytes versus cord blood lymphocytes used in previous studies. Cord blood B cells are generally regarded as being more susceptible to EBV-induced B-cell immortalization and less permissive for EBV replication than adult peripheral blood B cells.
Taken together, these data suggest that LCVs may be able to immortalize B cells from more closely related species but would do so significantly less efficiently for B cells from more distantly related species. One hypothetical mechanism is that the envelope membrane glycoproteins have diverged significantly through evolution so that binding and penetration of human B cells by simian LCV is less efficient. However, the EBV gp350 peptide epitope sufficient for binding to the EBV receptor, CD21, is conserved with 80% identity and 100% similarity in the rhesus monkey LCV membrane glycoprotein, and similar degrees of homology have been cited for the baboon LCV membrane glycoprotein (29). In addition, our results in this study place the species-specific block for efficient LCV-induced B-cell immortalization beyond virus binding and penetration. We demonstrate that simian LCV-enveloped amplicons can infect human B cells, EBNA-2 and CD23 are expressed in human B cells after acute simian LCV infection, and baboon LCV can coinfect and persist in spontaneous EBV-infected human PBMCs. The fact that baboon LCV coinfection was frequently recovered in the spontaneous LCL from the sixth human donor indicates that baboon LCV must be reasonably efficient at binding and penetrating human B cells. Only a minute fraction of B cells in the peripheral blood of seropositive humans is EBV infected, so that many more EBV-negative B cells must be infected by baboon LCV alone without subsequent growth transformation. This study also rules out the possibility of a dominant negative effect, since baboon LCV is able to stably coinfect and persist in EBV-immortalized B cells.
A more likely mechanism for the species-specific block to efficient B-cell immortalization may be at the level of latent gene function and interaction with species-specific host cell proteins or signaling pathways. However, all the LCV latent genes identified to date are capable of interacting with their respective pathways in human B cells. For example, baboon and rhesus monkey LCV EBNA-1 can maintain the EBV ori-P in human cells (31a, 41) and the EBV EBNA-1 can maintain the baboon LCV ori-P in human cells (33). The baboon LCV EBNA-2 can bind human CBF1/RBP Jk, act as a transcriptional transactivator in human cells, and contribute to CD23 induction after baboon LCV infection of human B cells (reference 26 and this report). Baboon LCV LMP2A is tyrosine phosphorylated in human B cells and induces phosphorylation of syc in human B cells (15). Baboon and rhesus monkey LCV LMP1 can bind human TRAF3 and activate NF-κB activity and ICAM-1 expression in human cells (16). Thus, a potential species-specific latent gene effector function that is essential for growth transformation is not yet obvious. A species-specific block at the level of transcriptional regulation also cannot be excluded since certain latent gene effector functions, e.g., LMP1 effects, may be closely linked to expression level. Genetic experiments to produce chimeric viruses may provide further clues to the underlying mechanism.
The cloning of the rhesus monkey LCV gp350 homolog provides further insight into the evolution of the LCV lytic and latent gene sequences. The most divergent evolution between human and nonhuman LCV has been found among the coding sequences for the latent genes, e.g., EBNA-2, LMP1, and the first exon of LMP2A (37, 32, and 31% amino acid homologies, respectively) (15, 16, 26). The EBNA-1 genes in baboon and rhesus monkey LCVs are also modestly divergent from those of EBV (51 and 48% amino acid identities, respectively) (31a, 41). The homology of the lytic gene, gp350, between EBV and rhesus monkey LCV (56% amino acid identity) is closer to that of the EBNA-1 genes and less than that typically seen with the lytic genes and noncoding sequences which are usually highly conserved (often 70 to 90% homology [28, 41]). One hypothesis is that the LCV latent genes are relatively new genes which have evolved for B-cell immortalization. In contrast, the lytic genes are relatively older genes which share properties of virus replication common to all herpesviruses. In this view, it is not surprising that the LCV major membrane glycoprotein that determines B-cell tropism might be closer to latent than lytic gene evolution. Conservation of the LCV membrane glycoprotein provides additional evidence that the rhesus monkey animal model, which can recapitulate the natural oral route of LCV transmission, will provide a valid and useful model for EBV vaccine development.
The ability of nonhuman primate viruses to infect human cells has become highly relevant for the field of xenotransplantation. EBV-induced lymphomas are a major cause of transplant morbidity. Like humans, most Old World primates, either those raised in captivity or those in the wild, are LCV infected, and viruses persist asymptomatically for life in infected animals (14, 19). EBV can be transmitted to other humans by infected B cells present in blood transfusions or bone marrow transplants (2, 20). Thus, there is a high likelihood for transferring simian LCV to humans by transplanting primate organs such as baboon bone marrow.
The present study characterizes the interaction of simian LCV with human cells in vitro and leads to several interpretations regarding the potential biohazards of simian LCV infection introduced by xenotransplantation. First, this study indicates that simian LCV infection in humans is unlikely to cause immediate malignant proliferation of human B cells. Similarly, simian B cells introduced into humans are unlikely to be immortalized by EBV. Second, simian LCV may still cause lymphoproliferative disease of simian B-cell origin in humans after xenotransplantation, and the increased immunosuppression required for xenotransplantation may increase the risk of simian LCV-induced lymphoproliferation. Third, the ability of simian LCV to coinfect, persist, and replicate in EBV-immortalized B cells sustains the theoretical possibility for recombination with EBV to produce novel chimeras. Two recent reports of novel EBV strains derived by potential recombination of type 1 and type 2 EBVs in vivo suggest that this remains a clinically significant possibility (6, 40).
The use of LCV-negative donors would significantly reduce the risk of simian LCV transmission. Most animals, even from typical closed domestic breeding colonies, are usually infected due in part to the persistent and asymptomatic nature of LCV infection. Our preliminary analysis of a specific-pathogen-free breeding colony suggests that breeding LCV-negative animals is possible (31a). Furthermore, we have been able to experimentally infect seronegative rhesus monkeys with rhesus LCV, thereby confirming that LCV-naive animals can be raised domestically (31).
ACKNOWLEDGMENTS
These studies were supported by grants from the U.S. Public Health Service (CA68051 and CA65319).
Peripheral blood from rhesus monkeys was kindly provided by the New England Regional Primate Research Center (USPHS P51RR00168-35).
REFERENCES
- 1.Ablashi D V, Gerber P, Easton J. Oncogenic herpesviruses of nonhuman primates. Comp Immunol Microbiol Infect Dis. 1979;2:229–241. doi: 10.1016/0147-9571(79)90011-0. [DOI] [PubMed] [Google Scholar]
- 2.Alfieri C, Tanner J, Carpentier L, Perpete C, Savoie A, Paradis K, Delage G, Joncas J. Epstein-Barr virus transmission from a blood donor to an organ transplant recipient with recovery of the same virus strain from the recipient’s blood and oropharynx. Blood. 1996;87:812–817. [PubMed] [Google Scholar]
- 3.Baskin G B, Roberts E D, Kuebler D, Martin L N, Blauw B, Heeney J, Zurcher C. Squamous epithelial proliferative lesions associated with rhesus Epstein-Barr virus in simian immunodeficiency virus-infected rhesus monkeys. J Infect Dis. 1995;172:535–539. doi: 10.1093/infdis/172.2.535. [DOI] [PubMed] [Google Scholar]
- 4.Beisel C, Tanner J, Matsuo T, Thorley-Lawson D, Kezdy F, Kieff E. Two major outer envelope glycoproteins of Epstein-Barr virus are encoded by the same gene. J Virol. 1985;54:665–674. doi: 10.1128/jvi.54.3.665-674.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bird A G, McLachlan S M, Britton S. Cyclosporin A promotes spontaneous outgrowth in vitro of Epstein-Barr virus induced B-cell lines. Nature. 1981;1:300–330. doi: 10.1038/289300a0. [DOI] [PubMed] [Google Scholar]
- 6.Burrows J M, Khanna R, Sculley T B, Alpers M P, Moss D J, Burrows S R. Identification of a naturally occurring recombinant Epstein-Barr virus isolate from New Guinea that encodes both type 1 and type 2 nuclear antigen sequences. J Virol. 1996;70:4829–4833. doi: 10.1128/jvi.70.7.4829-4833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Countryman J, Jenson H, Seibl R, Wolf H, Miller G. Polymorphic proteins encoded within BZLF1 of defective and standard Epstein-Barr viruses disrupt latency. J Virol. 1987;61:3672–3679. doi: 10.1128/jvi.61.12.3672-3679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dilner J, Rabin H, Letvin N, Henle W, Henle G, Klein G. Nuclear DNA-binding proteins determined by the Epstein-Barr virus related simian lymphotropic herpesviruses H. gorilla, H. pan, H. pongo and H. papio. J Gen Virol. 1987;68:1587–1596. doi: 10.1099/0022-1317-68-6-1587. [DOI] [PubMed] [Google Scholar]
- 9.Falk L, Wolfe L, Deinhardt F, Paciga J, Dombos L, Klein G, Henle W, Henle G. Epstein-Barr virus: transformation of non-human primate lymphocytes in vitro. Int J Cancer. 1974;13:363–376. doi: 10.1002/ijc.2910130312. [DOI] [PubMed] [Google Scholar]
- 10.Falk L A, Henle G, Henle W, Deinhardt F, Schudel A. Transformation of lymphocytes by Herpesvirus papio. Int J Cancer. 1977;20:219–266. doi: 10.1002/ijc.2910200209. [DOI] [PubMed] [Google Scholar]
- 11.Falk L A J. A review of Herpesvirus papio, a B-lymphotropic virus of baboons related to EBV. Comp Immunol Microbiol Infect Dis. 1979;2:257–264. doi: 10.1016/0147-9571(79)90013-4. [DOI] [PubMed] [Google Scholar]
- 12.Feichtinger H, Li S L, Kaaya E, Putkonen P, Grunewald K, Weyrer K, Bottiger D, Ernberg I, Linde A, Biberfeld G, et al. A monkey model for Epstein Barr virus-associated lymphomagenesis in human acquired immunodeficiency syndrome. J Exp Med. 1992;176:281–6. doi: 10.1084/jem.176.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feichtinger H, Putkonen P, Parravicini C, Li S T, Kaya E E, Bottiger D, Biberfeld P. Malignant lymphomas in cynomolgus monkeys infected with simian immunodeficiency virus. Am J Pathol. 1990;137:1311–1315. [PMC free article] [PubMed] [Google Scholar]
- 14.Frank A, Andiman W A, Miller G. Epstein-Barr virus and nonhuman primates: natural and experimental infection. Adv Cancer Res. 1976;23:171–201. doi: 10.1016/s0065-230x(08)60546-1. [DOI] [PubMed] [Google Scholar]
- 15.Franken M, Annis B, Ali A, Wang F. 5′ coding and regulatory region sequence divergence with conserved function of the Epstein-Barr virus LMP2A homolog in herpesvirus papio. J Virol. 1995;69:8011–8019. doi: 10.1128/jvi.69.12.8011-8019.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franken M, Devergne O, Rosenzweig M, Annis B, Kieff E, Wang F. Comparative analysis identifies conserved tumor necrosis factor receptor-associated factor 3 binding sites in the human and simian Epstein-Barr virus oncogene LMP1. J Virol. 1996;70:7819–7826. doi: 10.1128/jvi.70.11.7819-7826.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujimoto K, Terato K, Miyamoto J, Ishiko H, Fujisaki M, Cho F, Honjo S. Establishment of a B-lymphoblastoid cell line infected with Epstein-Barr-related virus from a cynomolgus monkey (Macaca fascicularis) J Med Primatol. 1990;19:21–30. [PubMed] [Google Scholar]
- 18.Gerber P, Branch J W, Rosenblum E N. Attempts to transmit infectious mononucleosis to rhesus monkeys and marmosets and to isolate herpeslike virus. Proc Soc Exp Biol Med. 1969;130:14–19. doi: 10.3181/00379727-130-33478. [DOI] [PubMed] [Google Scholar]
- 19.Gerber P, Kalter S S, Schidlovsky G, Peterson W J, Daniel M D. Biologic and antigenic characteristics of Epstein-Barr virus-related herpesviruses of chimpanzees and baboons. Int J Cancer. 1977;20:448–459. doi: 10.1002/ijc.2910200318. [DOI] [PubMed] [Google Scholar]
- 20.Gratama J W, Oosterveer M A, Zwaan F E, Lepoutre J, Klein G, Ernberg I. Eradication of Epstein-Barr virus by allogeneic bone marrow transplantation: implications for sites of viral latency. Proc Natl Acad Sci USA. 1988;85:8693–8696. doi: 10.1073/pnas.85.22.8693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishida T, Yamamoto K. Survey of nonhuman primates for antibodies reactive with Epstein-Barr virus (EBV) antigens and susceptibility of their lymphocytes for immortalization with EBV. J Med Primatol. 1987;16:359–371. [PubMed] [Google Scholar]
- 22.Kalter S S, Heberling R L, Ratner J J. EBV antibody in sera of non-human primates. Nature. 1972;238:353–354. doi: 10.1038/238353a0. [DOI] [PubMed] [Google Scholar]
- 23.Kieff E. Epstein-Barr virus and its replication. In: Fields B N, Knipe D M, Howley P M, editors. Virology. Philadelphia, Pa: Raven Press; 1996. pp. 2343–2396. [Google Scholar]
- 24.Levine P H, Leiseca S A, Hewetson J F, Traul K A, Andrese A P, Granlund D J, Fabrizio P, Stevens D A. Infection of rhesus monkeys and chimpanzees with Epstein-Barr virus. Arch Virol. 1980;66:341–351. doi: 10.1007/BF01320630. [DOI] [PubMed] [Google Scholar]
- 25.Li S L, Feichtinger H, Kaaya E, Migliorini P, Putkonen P, Biberfeld G, Meddeldorp J M, Biberfeld P, Ernberg I. Expression of Epstein-Barr-virus-related nuclear antigens and B-cell markers in lymphomas of SIV-immunosuppressed monkeys. Int J Cancer. 1993;55:609–615. doi: 10.1002/ijc.2910550416. [DOI] [PubMed] [Google Scholar]
- 26.Ling P D, Hayward S D. Contribution of conserved amino acids in mediating the interaction between EBNA2 and CBF1/RBPJk. J Virol. 1995;69:1944–1950. doi: 10.1128/jvi.69.3.1944-1950.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ling P D, Ryon J J, Hayward S D. EBNA-2 of herpesvirus papio diverges significantly from the type A and type B EBNA-2 proteins of Epstein-Barr virus but retains an efficient transactivation domain with a conserved hydrophobic motif. J Virol. 1993;67:2990–3003. doi: 10.1128/jvi.67.6.2990-3003.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loeb D D, Sung N S, Pesano R L, Sexton C J, Hutchison C D, Pagano J S, Hutchison C. Plasmid origin of replication of herpesvirus papio: DNA sequence and enhancer function. J Virol. 1990;64:2876–2883. doi: 10.1128/jvi.64.6.2876-2883.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mackett M, Pepper S de V, Hampson I, Jenson H, Janjua N, Arrand J. Conservation of gp340 between Epstein-Barr virus (EBV) strains and Herpesvirus papio (HVP), abstr. 312. Presented at the 22nd International Herpesvirus Workshop. 1997. [Google Scholar]
- 30.Miller G, Niederman J C, Stitt D A. Infectious mononucleosis: appearance of neutralizing antibody to Epstein-Barr virus measured by inhibition of formation of lymphoblastoid cell lines. J Infect Dis. 1972;125:403–406. doi: 10.1093/infdis/125.4.403. [DOI] [PubMed] [Google Scholar]
- 31.Moghaddam A, Rosenzweig M, Lee-Parritz D, Annis B, Johnson R P, Wang F. An animal model for acute and persistent Epstein-Barr virus infection. Science. 1997;276:2030–2033. doi: 10.1126/science.276.5321.2030. [DOI] [PubMed] [Google Scholar]
- 31a.Moghaddam, A., and F. Wang. Unpublished data.
- 32.Nemerow G R, Houghten R A, Moore M D, Cooper N R. Identification of an epitope in the major envelope protein of Epstein-Barr virus that mediates viral binding to the B-lymphocyte EBV receptor (CR2) Cell. 1989;56:369–377. doi: 10.1016/0092-8674(89)90240-7. [DOI] [PubMed] [Google Scholar]
- 33.Pesano R L, Pagano J S. Herpesvirus papio contains a plasmid origin of replication that acts in cis interspecies with an Epstein-Barr virus trans-acting function. J Virol. 1986;60:1159–1162. doi: 10.1128/jvi.60.3.1159-1162.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rabin H, Neubauer R H, Hopkins R F, Dzhikidze E K, Shevtsova Z V, Lapin B A. Transforming activity and antigenicity of an Epstein-Barr-like virus from lymphoblastoid cell lines of baboons with lymphoid disease. Intervirology. 1977;8:240–249. doi: 10.1159/000148899. [DOI] [PubMed] [Google Scholar]
- 35.Rangan S R, Martin L N, Bozelka B E, Wang N, Gormus B J. Epstein-Barr virus-related herpesvirus from a rhesus monkey (Macaca mulatta) with malignant lymphoma. Int J Cancer. 1986;38:425–432. doi: 10.1002/ijc.2910380319. [DOI] [PubMed] [Google Scholar]
- 36.Scudiero D A, Shoemaker R H, Paull K D, Monks A, Tierney S, Nofziger T H, Currens M J, Seniff D, Boyd M R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- 37.Tanner J, Whang Y, Sample J, Sears A, Kieff E. Soluble gp350/220 and deletion mutant glycoproteins block Epstein-Barr virus adsorption to lymphocytes. J Virol. 1988;62:4452–4464. doi: 10.1128/jvi.62.12.4452-4464.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang F, Gregory C, Sample C, Rowe M, Liebowitz D, Murray R, Rickinson A, Kieff E. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J Virol. 1990;64:2309–2318. doi: 10.1128/jvi.64.5.2309-2318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang F, Li X, Annis B, Faustman D L. Tap-1 and Tap-2 gene therapy selectively restores conformationally dependent HLA class I expression in type I diabetic cells. Hum Gene Ther. 1995a;6:1005–1017. doi: 10.1089/hum.1995.6.8-1005. [DOI] [PubMed] [Google Scholar]
- 40.Yao Q Y, Tierney R J, Croom-Carter D, Cooper G M, Ellis C J, Rowe M, Rickinson A B. Isolation of intertypic recombinants of Epstein-Barr virus from T-cell immunocompromised individuals. J Virol. 1996;70:4895–4903. doi: 10.1128/jvi.70.8.4895-4903.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yates J L, Camiolo S M, Ali S, Ying A. Comparison of the EBNA-1 proteins of Epstein-Barr virus and Herpesvirus papio in sequence and function. Virology. 1996;222:1–13. doi: 10.1006/viro.1996.0392. [DOI] [PubMed] [Google Scholar]
- 42.Young L, Alfieri C, Hennessy K, Evans H, O’Hara C, Anderson K C, Ritz J, Shapiro R S, Rickinson A, Kieff E, et al. Expression of Epstein-Barr virus transformation-associated genes in tissues of patients with EBV lymphoproliferative disease. N Engl J Med. 1989;321:1080–1085. doi: 10.1056/NEJM198910193211604. [DOI] [PubMed] [Google Scholar]