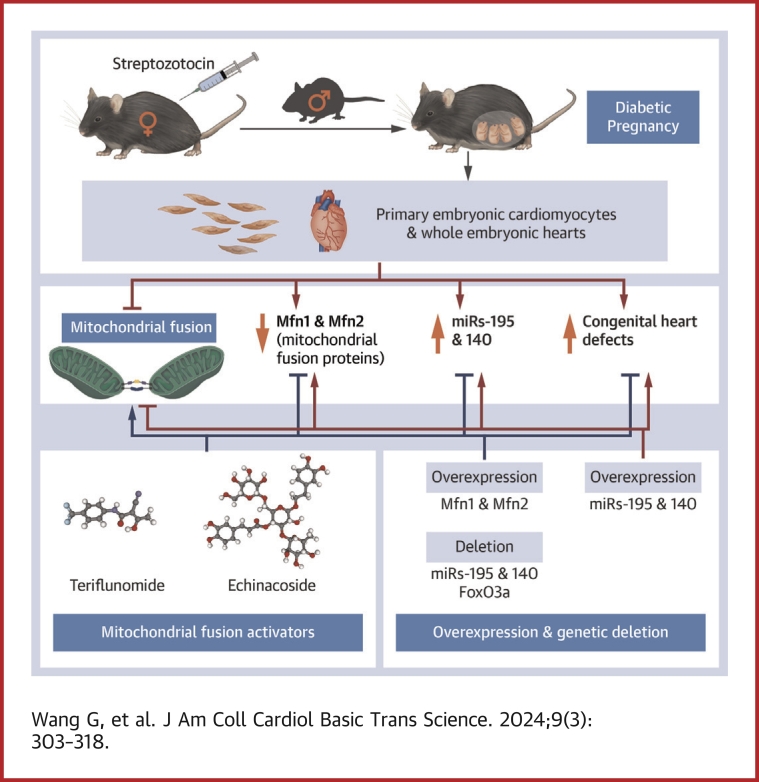
Visual Abstract
Key Words: congenital heart defect, maternal diabetes, microRNA, mitochondrial fusion, mitofusin 1, mitofusin 2
Highlights
-
•
Most CHD cases are attributed to nongenetic factors, whereas the mechanisms underlying nongenetic factor–induced CHDs are elusive. Maternal diabetes is one of the nongenetic factors inducing CHDs.
-
•
The study reveals an innovative epigenetic mechanism underlying maternal diabetes–induced CHDs.
-
•
Maternal diabetes-activated transcription factor FoxO3a increases miR-140 and miR-195, which in turn represses Mfn1 and Mfn2, leading to mitochondrial fusion defects and CHDs.
-
•
Two mitochondrial fusion activators, teriflunomide (an FDA-approved drug) and echinacoside (a naturally occurring compound), increase the expression level of Mfn1 and Mfn2, restore mitochondrial fusion, and prevent CHD formation. These 2 activators show potential value in preventing CHDs in diabetic pregnancy.
Summary
Most congenital heart defect (CHD) cases are attributed to nongenetic factors; however, the mechanisms underlying nongenetic factor–induced CHDs are elusive. Maternal diabetes is one of the nongenetic factors, and this study aimed to determine whether impaired mitochondrial fusion contributes to maternal diabetes–induced CHDs and if mitochondrial fusion activators, teriflunomide and echinacoside, could reduce CHD incidence in diabetic pregnancy. We demonstrated maternal diabetes-activated FoxO3a increases miR-140 and miR-195, which in turn represses Mfn1 and Mfn2, leading to mitochondrial fusion defects and CHDs. Two mitochondrial fusion activators are effective in preventing CHDs in diabetic pregnancy.
The United States has the highest infant mortality rate, which is the basic measure of public health, among developed countries.1 Congenital heart defects (CHDs) are the most common cause of infant death.2 Furthermore, CHDs are the most prevalent birth defects, occurring in approximately 4 to 10 per 1,000 live births.2 Epidemiological studies in CHD prevention suggest a controversial effect of maternal folic acid supplementation,3,4 which is the only effective intervention to prevent neural tube defects, another type of potentially fatal birth defect. However, a mechanism-based means of preventing CHDs is still lacking.
Human epidemiological studies have demonstrated that the major contributing factors to the occurrence of CHDs are nongenetic factors.2,5 Among all nongenetic factors that cause CHDs, maternal diabetes is the major factor.2,6 The rate of CHDs in infants born to mothers with diabetes is approximately 4 to 6 times higher than mothers without diabetes.6, 7, 8 More than 60 million women of reproductive age worldwide have diabetes, and this number will likely double by 2030 due to the current global epidemic of obesity.9 Even under the best clinical care, women with diabetes are still 3 to 4 times more likely to have a child with CHDs than women without diabetes.10 Thus, given these incidences and the lack of means to prevent CHDs, its occurrence is an unmet clinical need, and therefore uncovering the cellular and molecular events underlying its development will aid in the identification of effective preventions.
Here, using a maternal diabetes mouse model of CHDs, we show that this pathology occurs due to activation of the transcription factor FoxO3a, which stimulates expression of miR-140 and miR-195 that, in turn, represses mitofusin 1 (Mfn1) and mitofusin 2 (Mfn2) expression, respectively. Treating this model with 2 activators of mitochondrial fusion, teriflunomide, a U.S. Food and Drug Administration (FDA)-approved drug, and echinacoside, a natural compound, we found prevention of CHDs. This amelioration correlated with re-expression of Mfn1 and Mfn2, improved mitochondrial dynamics and cell proliferation, and reduced apoptosis. Thus, pharmacological restoration of mitochondrial fusion may be an effective approach to reduce the risk of CHDs resulting from diabetic pregnancy.
Methods
The model of maternal diabetes-induced CHDs
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine. The mouse diabetic embryopathy model has been described previously.11, 12, 13 Detailed information is included in the Supplemental Appendix.
Statistical analysis
All experiments were repeated in triplicate. Specifically, for immunostaining, 3 embryonic samples from 3 L were stained for each group and average signal intensity was calculated. For immunoblotting, 1 embryonic heart from 1 litter in each group was used for 1 run. Each experiment was repeated 3 times with 3 embryonic hearts from 3 different litters in each group. For reverse-transcriptase quantitative polymerase chain reaction, 3 embryonic hearts from different litters in each group were analyzed. Data are presented as the mean ± SD. Student’s t-test was used for 2-group comparison. One-way analysis of variance with Tukey's post hoc test for multiple pairwise comparisons was used for comparisons of more than 2 groups. CHD incidence is presented as count with percentage and compared using chi-square test. Differences were considered statistically significant when P < 0.05.
Results
Pharmacological activation of mitochondrial fusion ameliorates CHDs
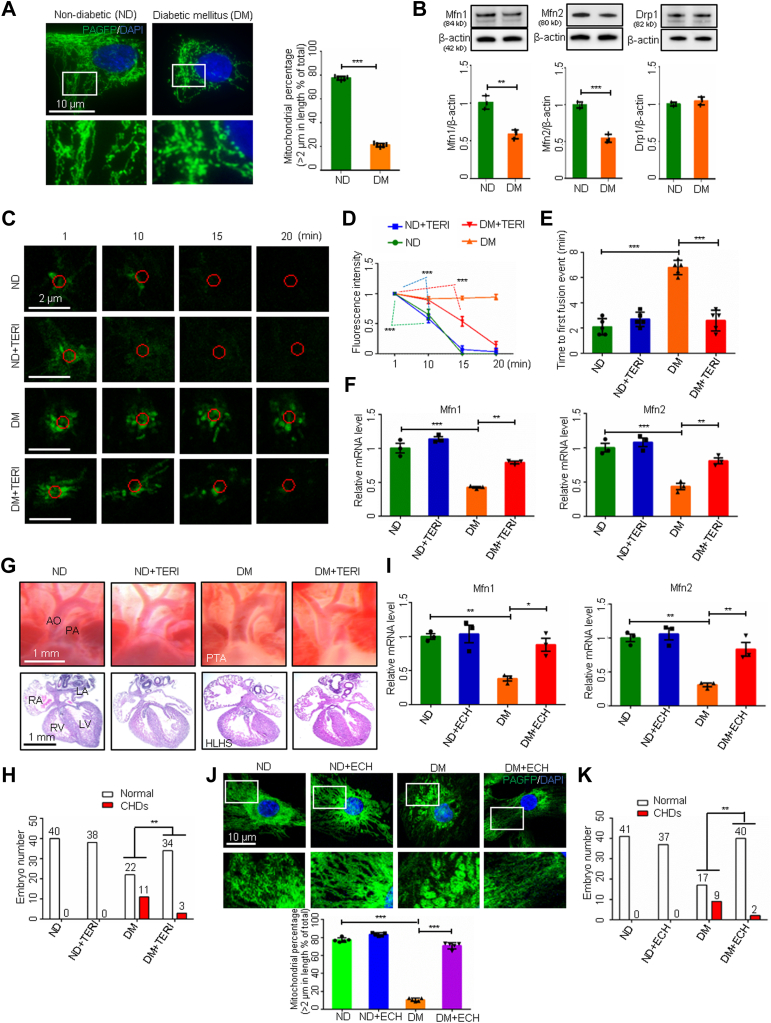
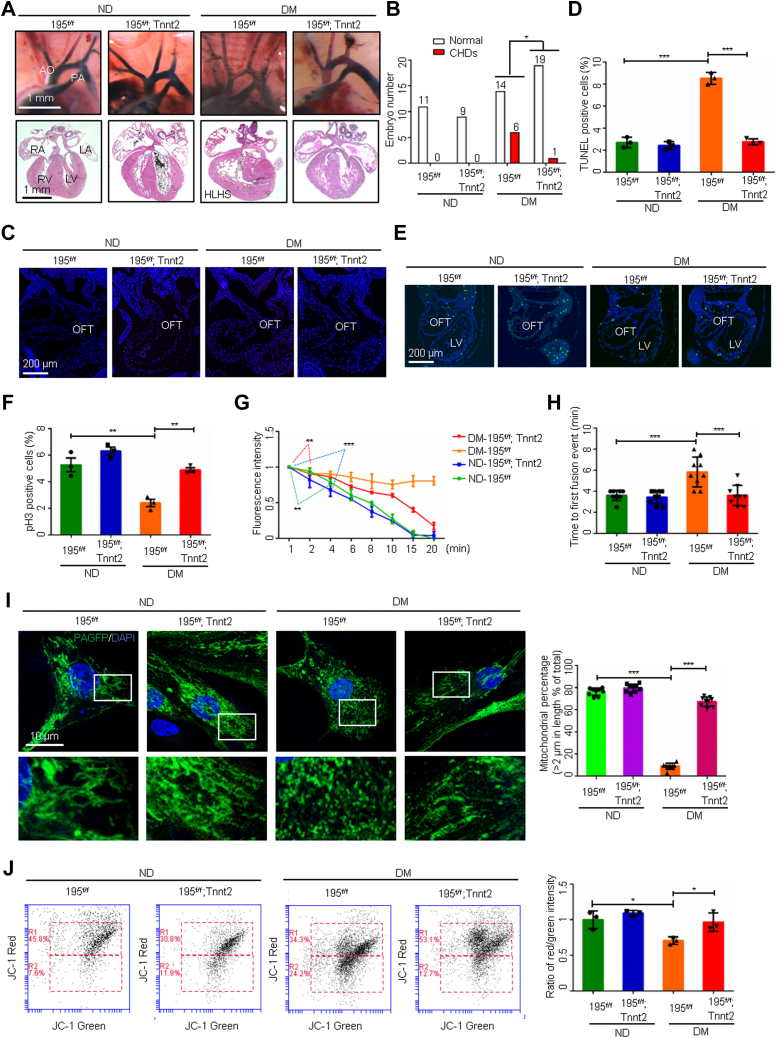
Heart cells, especially cardiomyocytes, are enriched in mitochondria.14 Mitochondrial fusion and fission dynamics play an important role in the regulation of cardiomyocyte viability.15 Because abrogating mitochondrial fusion leads to CHDs16 similar to those observed in diabetic pregnancy,17 mitochondrial morphology and fusion were examined in cardiomyocytes of embryonic mouse hearts from normal healthy dams and those with maternal diabetes induced before mating by treatment with streptozotocin (STZ). Under nondiabetic conditions, mitochondria in embryonic cardiomyocytes exhibited tubular morphology, whereas maternal diabetes triggered fragmented and small spherical mitochondria (Figure 1A). The sizes of mitochondria in cardiomyocytes from embryos exposed to maternal diabetes were significantly smaller than those from nondiabetic dams (Figure 1A). Mitochondrial fusion and fission modulate mitochondrial morphology dynamics.18 The proteins Mfn1 and Mfn2 are essential for mitochondrial fusion, and dynamin-related protein 1 (Drp1) induces mitochondrial fission.18 Maternal diabetes was associated with lower Mfn1 and Mfn2 expression in the developing heart compared with nondiabetic controls but did not affect Drp1 expression (Figure 1B). These findings suggest that maternal diabetes represses mitochondrial fusion leading to reduced mitochondrial sizes. Because the double ablation of Mfn1 and Mfn2 in the developing heart results in complex CHDs and lethality at middle gestation,16 we hypothesized that reduced levels of Mfn1 and Mfn2, and the consequent repression of mitochondrial fusion, are responsible for maternal diabetes-induced CHDs.
Figure 1.
Mitochondrial Fusion Activators Ameliorate Maternal Diabetes–Induced CHDs
(A and J) Primary cardiomyocytes transfected with mitochondrial matrix-targeted photoactive green fluorescent protein (mito-PAGFP) and percentage of mitochondria (>2 μm) per area in cells (n = 5). (B) Protein levels in embryonic day 12.5 (E12.5) hearts (n = 3). (C) mito-PAGFP in primary cardiomyocytes. Red circles: photoactivated regions. (D) Mito-PAGFP intensity (n = 3). (E) Time to first fusion event (n = 5). (F and I) Messenger RNA levels in E12.5 hearts (n = 3). (G) Heart vessels (upper) and sections (lower). (H and K) Numbers of embryos. Data are presented as mean ± SD in A, B, D, E, F, I, and J. Student’s t-test in A and B. One-way analysis of variance with Tukey’s post hoc test in D, E, F, I, and J. Chi-square test in H and K. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. AO = aorta; CHD = congenital heart defect; ECH = echinacoside; HLHS = hypoplastic left heart syndrome; LA/RA = left/right atrium; LV/RV = left/right ventricle; PA = pulmonary artery; PTA = persistent truncus arteriosus; TERI = teriflunomide.
We assessed mitochondrial fusion in primary cardiomyocytes from embryonic day 12.5 (E12.5) hearts using our validated assay that monitors the redistribution of mitochondrial matrix-target photoactivable green fluorescent protein (mito-PAGFP).19 Time-lapse imaging showed the mito-PAGFP intensity in cardiomyocytes from nondiabetic dams began to be significantly reduced at an average of 10 minutes and diminished at 15 minutes after green fluorescent protein (GFP) photoactivation (Figures 1C and 1D), indicating active mitochondrial fusion because of the diffusion of mito-PAGFP into non-GFP mitochondria.19 The first mitochondrial fusion event, defined at the time of mito-PAGFP flow into a non-GFP mitochondrion (Figure 1E), occurred at 2 minutes post-photoactivation in the nondiabetic group. In contrast, the mito-PAGFP intensity in cardiomyocytes from diabetic dams remained unchanged during the entire 20-minute imaging period and mitochondrial fusion did not occur in these cells (Figures 1C and 1D). Treatment with the mitochondrial fusion activator teriflunomide,20 an FDA-approved drug for treating relapsing multiple sclerosis, restored Mfn1 and Mfn2 expression and mitochondrial fusion suppressed by maternal diabetes (Figures 1C to 1F, Supplemental Figure 1A). Teriflunomide did not further accelerate mitochondrial fusion under nondiabetic conditions, and mitochondrial fusion appeared to reach the maximum level (Figures 1C and 1D). Consequently, teriflunomide significantly ameliorated CHD formation under diabetic conditions (Figures 1G and 1H, Supplemental Table 1). Another mitochondrial fusion activator echinacoside,21 a naturally occurring small molecule compound, also restored Mfn1 and Mfn2 expression (Figure 1I, Supplemental Figure 1B) and mitochondrial sizes (Figure 1J), and alleviated maternal diabetes–induced CHDs (Figure 1K, Supplemental Table 2).
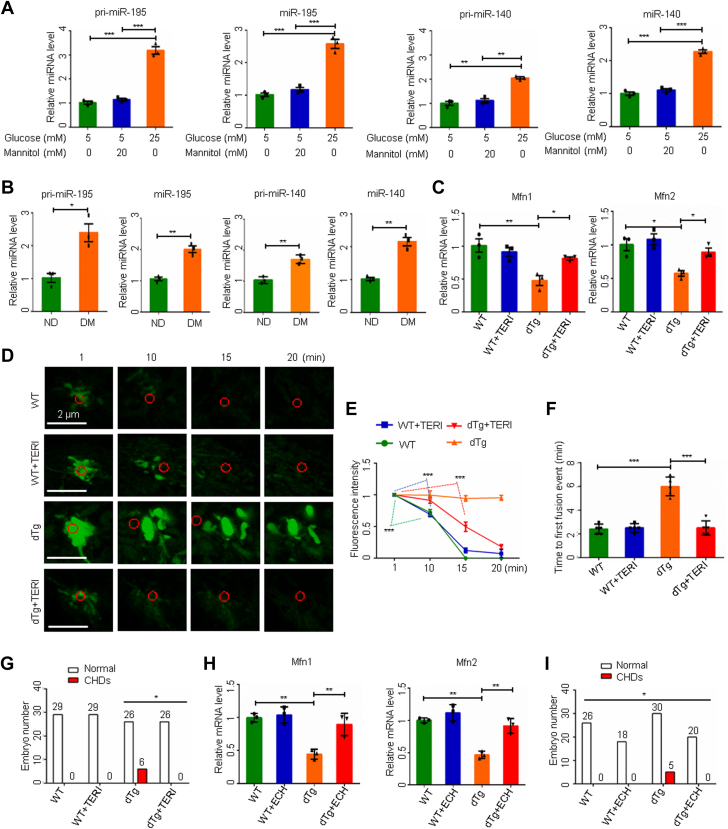
Next, we searched for molecular intermediates that mediate the teratogenicity of maternal diabetes in the developing heart. High-glucose conditions in vitro and maternal diabetes in vivo significantly increased the expression of miR-195 and miR-140 (Figures 2A and 2B). Several microRNAs (miRNAs) negatively regulate mitochondrial fusion.22 Transgenic overexpression of both miR-195 and miR-140 in the developing heart mimicked the effects of maternal diabetes on the repression of Mfn1 and Mfn2, the blockage of mitochondrial fusion, and the induction of CHDs (Figures 2C to 2G). Teriflunomide blocked miR-195/140-suppressed mitochondrial fusion and thus reduced CHD formation (Figures 2D to 2G, Supplemental Table 3). Likewise, echinacoside sustained Mfn1 and Mfn2 expression and ameliorated CHDs induced by miR-195/140 upregulation (Figures 2H and 2I, Supplemental Table 4). Thus, impaired mitochondrial fusion leads to CHD formation and fusion activators can reduce the incidence of CHDs induced by maternal diabetes and upregulation of specific miRNAs.
Figure 2.
Mitochondrial Fusion Activators Reduce CHDs Induced by miR-195/140 Transgenic Overexpression
MicroRNA (miRNA) levels in primary cardiomyocytes (n = 3) (A) and in embryonic day 12.5 (E12.5) hearts (B) (n = 3). (C and H) mRNA levels in E12.5 hearts (n = 3). (D) Time-lapse images of mitochondrial matrix-targeted photoactive green fluorescent protein (mito-PAGFP) in primary cardiomyocytes. (E) mito-PAGFP fluorescence intensity (n = 3). (F) Time to first mitochondrial fusion event (n = 5). (G and I) Numbers of E17.5 embryos. Data are presented as mean ± SD in A, B, C, E, F, and H. One-way analysis of variance with Tukey’s post hoc test in A, C, E, F, and H. Student’s t-test in (B). Chi-square test in G and I. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. dTg = miR-195 and miR-140 double transgenic; WT = wild-type; other abbreviations as in Figure 1.
FoxO3a upregulates miR-195/140 and represses mitochondrial fusion
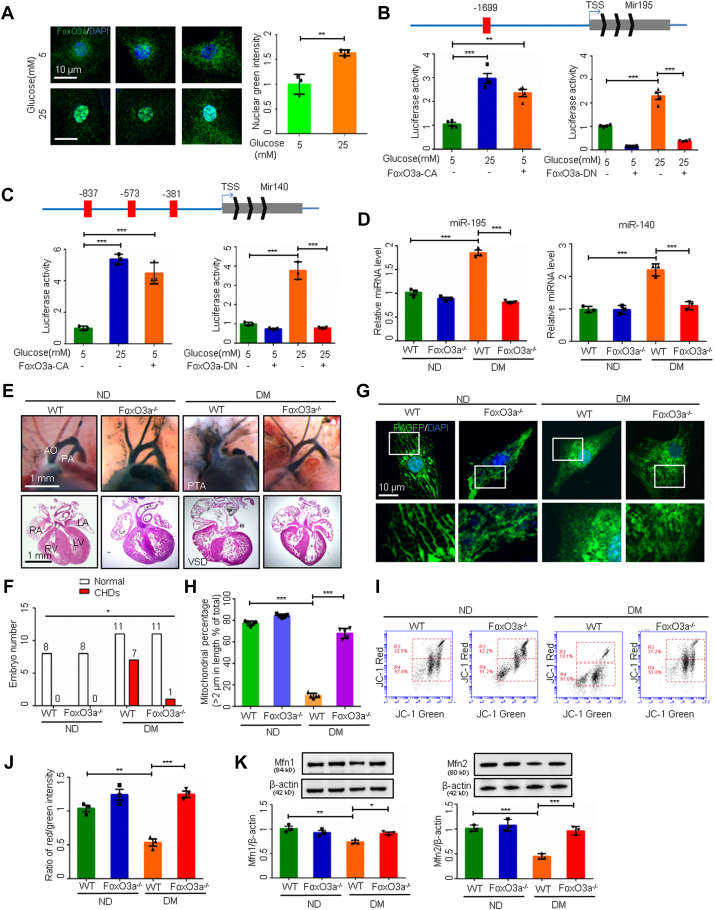
To determine how maternal diabetes induces teratogenic miR195/miR-140 expression in the heart, we focused on the transcription factor FoxO3a. FoxO3a is critically involved in diabetes-induced embryonic noncardiac anomalies.23 High-glucose conditions activated FoxO3a by triggering its nuclear translocation in embryonic cardiomyocytes (Figure 3A). Constitutively active–FoxO3a mimicked the high-glucose–increased Mir195 and Mir140 promoter activity, and the dominant negative–FoxO3a inhibited the promoter activity (Figures 3B and 3C). Foxo3a germline deletion in mice abolished the maternal diabetes–increased expression of miR-195 and miR-140 (Figure 3D), reduced CHD formation (Figures 3E and 3F, Supplemental Table 5), restored mitochondrial lengths (Figures 3G and 3H) and function (Figures 3I and 3J), and reversed the downregulation of Mfn1 and Mfn2 (Figure 3K). These findings indicate that hyperglycemia-induced activation of the transcriptional factor FoxO3a leads to the upregulation of specific miRNAs and the development of CHDs in diabetic pregnancy by repressing mitofusion gene expression.
Figure 3.
FoxO3a Upregulates miR-140/195 and Represses Mitochondrial Fusion
(A) FoxO3a immunostaining in primary cardiomyocytes and quantification of fluorescence intensity in nuclei (n = 3). FoxO3a binding sites on the Mir195 (B) and Mir140 (C) promoters and promoter-driven luciferase reporter activity in H9C2 cells (n = 3). (D) MicroRNA (miRNA) levels in embryonic day 12.5 (E12.5) hearts (n = 3). (E) E17.5 heart vessels (upper) and sections (lower). (F) Numbers of E17.5 embryos. (G) Representative images of primary cardiomyocytes. (H) Percentage of mitochondria (>2 μm) per area in cells (n = 5). (I and J) Flow cytometry analysis and ratios of fluorescence intensity (red/green) in primary cardiomyocytes (n = 3). (K) Protein levels (n = 3). Data are presented as mean ± SD in A, B, C, D, H, J, and K. Student’s t-test in A. One-way analysis of variance with Tukey’s post hoc test in B, C, D, H, J, and K. Chi-square test in F. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. DM = diabetes mellitus; ND = nondiabetic; TSS = transcription start site; VSD = ventricular septum defect; other abbreviations as in Figures 1 and 2.
miR-195 deletion restores mitochondrial fusion by de-repressing Mfn2
The gain-of-function of miR-195 and miR-140 in transgenic (Tg) mice resembles diabetes-induced CHDs (Figures 2G and 2I). We next aimed to determine the individual roles of miR-195 and miR-140 in the induction of CHDs in diabetic pregnancy using loss-of-function approaches. Cardiomyocyte-specific Mir195 deletion alleviated diabetes-induced CHDs (Figures 4A and 4B, Supplemental Table 6) and cardiac cell apoptosis (Figures 4C and 4D), and restored cell proliferation in the embryonic hearts (Figures 4E and 4F). Because double Tg expression of miR-195 and miR-140 inhibited mitochondrial fusion (Figures 2D to 2F), it is plausible that miR-195 mediates the inhibitory effect of maternal diabetes on mitochondrial fusion. Indeed, Mir195 deletion prevented maternal diabetes–inhibited mitochondrial fusion, which was manifested by the fast diffusion and eventual disappearance of mito-PAGFP and the restoration of the first mitochondrial fusion event in cardiomyocytes (Figures 4G and 4H). Consequently, miR-195 deficiency restored mitochondrial lengths and function in cardiomyocytes (Figures 4I and 4J).
Figure 4.
miR-195 Deletion Restores Mitochondrial Fusion
(A) Images of embryonic day 17.5 (E17.5) heart vessels (upper) and sections (lower). (B) Numbers of E17.5 embryos. TUNEL-positive cells in E9.5 hearts (C) and the quantification (D) (n = 3). p-H3-positive cells in E9.5 hearts (E) and the quantification (F) (n = 3). (G) Mitochondrial matrix-targeted photoactive green fluorescent protein (mito-PAGFP) intensity in primary cardiomyocytes (n = 3). (H) Time to first mitochondrial fusion event (n = 9). (I) Representative images of primary cardiomyocytes and percentage of mitochondria (>2 μm) per area in cells (n = 9). (J) Flow cytometry analysis and ratios of fluorescence intensity (red/green) in primary cardiomyocytes (n = 3). Data are presented as mean ± SD in D, F, G, H, I, and J. Chi-square test in (B). One-way analysis of variance with Tukey’s post hoc test in D, F, G, H, I, and J. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. OFT = outflow tract; other abbreviations as in Figure 1, Figure 2, Figure 3.
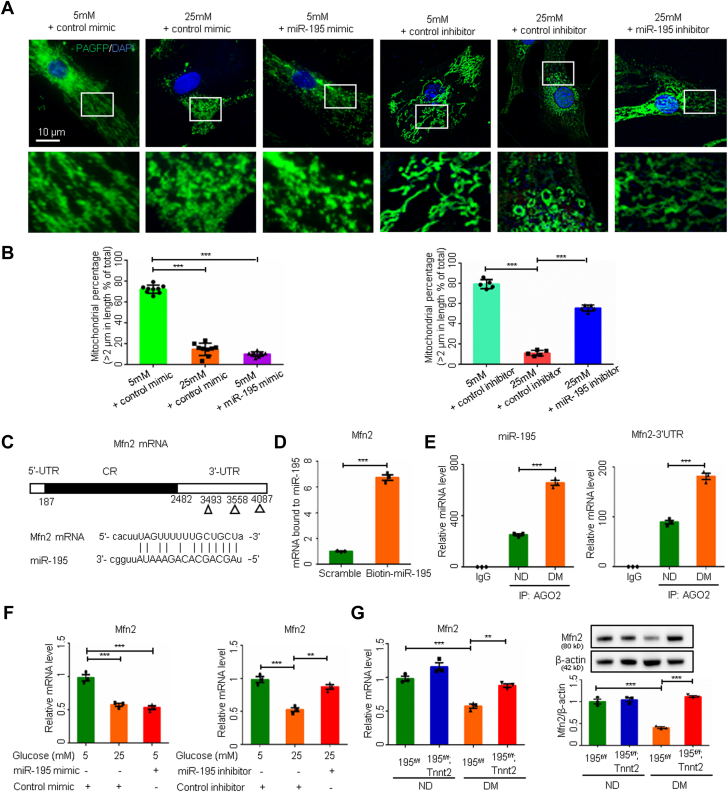
We further showed that miR-195 could directly reduce mitochondrial lengths by decreasing Mfn2 expression. Although an miR-195 mimic replicated the high-glucose–reduced mitochondrial lengths in cardiomyocytes, an miR-195 inhibitor blocked the shortening of mitochondrial lengths in high-glucose conditions (Figures 5A and 5B). A pull-down assay using biotin-labeled miR-195 demonstrated the direct binding of miR-195 to Mfn2 messenger RNA (mRNA) (Figures 5C and 5D), and miR-195 and Mfn2 mRNA were coenriched in the AGO2 RNA-induced silencing complex in embryonic hearts exposed to maternal diabetes (Figure 5E). However, we did not identify any miR-195 binding sites in Mfn1 mRNA. The miR-195 mimic resembled high-glucose conditions in repressing Mfn2 mRNA expression, and the miR-195 inhibitor blocked the high-glucose–induced inhibition of Mfn2 expression (Figure 5F). Deletion of miR-195 in the heart reversed the decrease in Mfn2 protein and mRNA levels in embryos exposed to maternal diabetes (Figure 5G). These findings establish that the inhibition of Mfn2 by miR-195 and miR-195 deficiency in cardiomyocytes is sufficient to block the teratogenicity of diabetes and the mitochondrial fusion inhibition elicited by maternal diabetes.
Figure 5.
Inhibition of miR-195 Improves Mitochondrial Fusion by Upregulating Mfn2
(A) Representative images of primary cardiomyocytes. (B) Percentage of mitochondria (>2 μm) per area in cells (n = 9 or 5). (C) miR-195 binding site on Mfn2 mRNA. (D) Mfn2 mRNA level bound to biotin-labeled miR-195 (n = 3). (E) miR-195 and Mfn2 mRNA in RNA co-immunoprecipitation (IP) in embryonic day 12.5 (E12.5) hearts (n = 3). (F) Mfn2 mRNA in primary cardiomyocytes (n = 3). (G) Mfn2 mRNA and protein levels (n = 3). Data are presented as mean ± SD in B, D, E, F, and G. One-way analysis of variance with Tukey’s post hoc test in B, F, and G. Student’s t-test in D and E. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. IgG = immunoglobulin G; IP = immunoprecipitation; UTR = untranslated region; other abbreviations as in Figure 1, Figure 2, Figure 3.
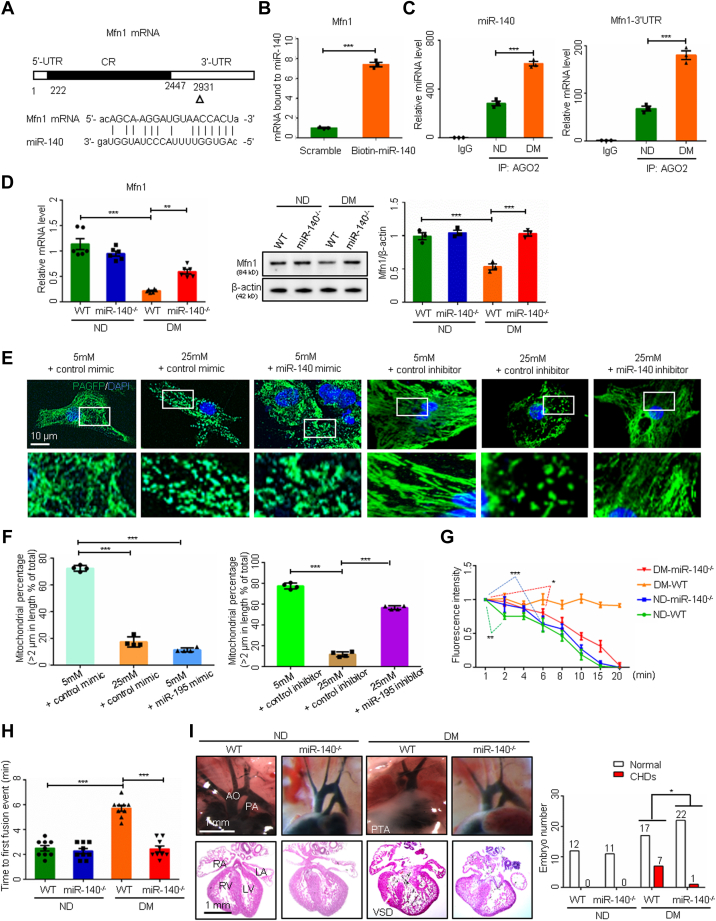
miR-140 deficiency removes the blockage of Mfn1 and mitochondrial fusion
miR-140 binds to Mfn1 mRNA in its 3′ untranslated region (Figures 6A and 6B). miR-140 and Mfn1 mRNA were coenriched in the mRNA degradation AGO2 complex under maternal diabetic conditions (Figure 6C), suggesting that Mfn1 is an miR-140 target. miR-140 deletion blocked the downregulation of Mfn1 at both the mRNA and protein levels in embryonic hearts exposed to diabetes (Figure 6D). Because miR-140 degrades Mfn1, it should inhibit mitochondrial fusion, leading to mitochondrial fragmentation. Indeed, the effect of an miR-140 mimic resembled high-glucose conditions, as the mitochondrial lengths were reduced. In addition, an miR-140 inhibitor ablated mitochondrial length shortening in embryonic cardiomyocytes under high-glucose conditions (Figures 6E and 6F). The inhibition of mitochondrial fusion was abrogated by miR-140 deletion, which was manifested by faster dispersal of mito-PAGFP and a shorter time to reach the first mitochondrial fusion event in cardiomyocytes from diabetic dams compared with nondiabetic dams (Figures 6G and 6H). Consequently, miR-140 deletion reduced the incidence of CHDs in diabetic pregnancy (Figure 6I, Supplemental Table 7), and recovered cell proliferation and survival in the developing heart (Supplemental Figures 2A to 2C). Thus, miR-140 mediates the teratogenic effect of maternal diabetes by suppressing mitochondrial fusion leading to formation of CHDs.
Figure 6.
miR-140 Deficiency Restores Mfn1 Expression and Mitochondrial Fusion
(A) The miR-140 binding site on Mfn1 mRNA. (B) Mfn1 mRNA bond to biotin-labeled miR-140 (n = 3). (C) miR-140 and Mfn1 mRNA levels in RNA immunoprecipitation (n = 3). (D) Mfn1 mRNA and protein levels (n = 3). (E) Representative images of primary cardiomyocytes. (F) Percentage of mitochondria (>2 μm) per area in cells (n = 4). (G) Mitochondrial matrix-targeted photoactivable green fluorescent protein intensity in primary cardiomyocytes (n = 3). (H) Time to first mitochondrial fusion event (n = 9). (I) Images of embryonic day 17.5 heart vessels (upper) and sections (lower). Data are presented as mean ± SD in B, C, D, F, G, and H. Student’s t-test in B and C. One-way analysis of variance with Tukey’s post hoc test in D, F, G, and H. Chi-square test in I. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. Abbreviations as in Figure 1, Figure 2, Figure 3, Figure 4, Figure 5.
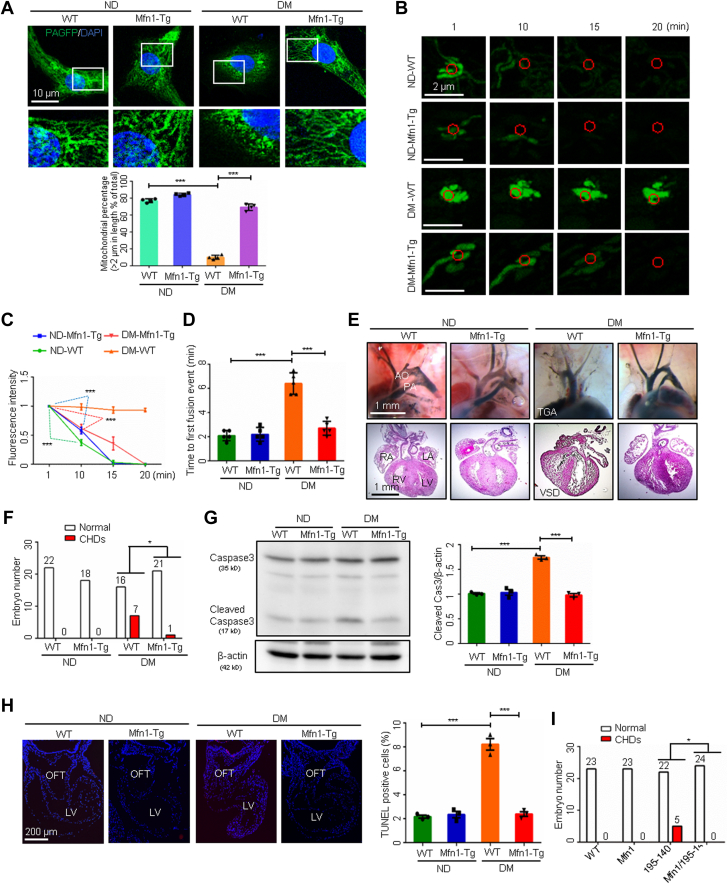
Restoring Mfn1 or Mfn2 expression abrogates the teratogenicity of maternal diabetes
The Tg overexpression of Mfn1 in the developing heart prevented the shortening of mitochondrial lengths (Figure 7A) and reversed the inhibition of the dispersal of mito-PAGFP and the first mitochondrial fusion event in cardiomyocytes (Figures 7B to 7D). There was a significant reduction in CHDs in embryos exposed to diabetes under the condition of Mfn1 Tg overexpression (Figures 7E and 7F, Supplemental Table 8). Furthermore, Mfn1 overexpression blunted maternal diabetes–induced caspase 3 cleavage and cell apoptosis (Figures 7G and 7H). This was particularly true in the outflow tract, which is critical for cardiac septation. Mfn1 overexpression also blocked the formation of CHDs under the conditions of miR-140 and miR-195 overexpression (Figure 7I, Supplemental Table 9), suggesting that maintaining the proper level of Mfn1 is essential for normal cardiac morphogenesis.
Figure 7.
Restoring Mfn1 Expression Abrogates the Teratogenicity of Maternal Diabetes
(A) Representative images of primary cardiomyocytes and percentage of mitochondria (>2 μm) per area in cells (n = 4). (B) Time-lapse images of mitochondrial matrix-targeted photoactivable green fluorescent protein (mito-PAGFP) in primary cardiomyocytes. (C) mito-PAGFP intensity in primary cardiomyocytes (n = 3). (D) Time to first mitochondrial fusion event (n = 5). (E) Embryonic day 17.5 (E17.5) heart vessels (upper) and sections (lower). (F and I) Numbers of E17.5 embryos. (G) Cleaved caspase-3 levels (n = 3). (H) TUNEL-positive cells in E9.5 hearts and the quantification (n = 3). Data are presented as mean ± SD in A, C, D, G, and H. One-way analysis of variance with Tukey’s post hoc test in A, C, D, G, and H. Chi-square test in F and I. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. TGA = transposition of the great arteries; other abbreviations as in Figure 1, Figure 2, Figure 3, Figure 4, Figure 5.
Similarly, Tg overexpression of Mfn2 also significantly reduced the incidence of CHDs in diabetic pregnancy (Supplemental Figure 3A, Supplemental Table 10), suggesting that Mfn1 and Mfn2 are redundant for mitochondrial fusion during cardiac development.
Discussion
It is conventionally accepted that organ development is orchestrated from the cell nucleus and that the mitochondria simply follow along; however, a recent study demonstrated that mitochondria orchestrate developmental events of the mouse heart, and the disturbance of mitochondrial function contributes to CHD formation.16 Mitochondrial dynamics are governed by fusion and fission events essential for proper heart development.16 Mitochondria fuse via the function of Mfn1 and Mfn2. Deleting the Mfn1 and Mfn2 genes in early heart muscle cells results in severely underdeveloped hearts.16 Furthermore, mouse embryonic stem cells missing Mfn2 and Opa1 (optic atrophy protein 1), a mitochondrial fusion facilitator, do not develop into beating cardiomyocytes.16 Reduced mitochondrial fusion resulting from Mfn1 and Mfn2 deletion disrupts several signaling pathways implicated in CHDs.16 This evidence suggests that altered mitochondrial dynamics drive cardiac dysmorphogenesis.
Impaired mitochondrial fusion leads to mitochondrial dysfunction and subsequently alters cardiac morphogenesis. Mitochondrial dysfunction is an evident cellular defect in cardiomyocytes exposed to maternal diabetes.17,24 Intrinsic abnormalities are present in cardiomyocytes derived from inducible pluripotent stem cells of patients with CHDs that lack an underlying genetic cause,25 suggesting that cell development is a key factor in cardiac morphogenesis. These cellular organelle defects continue to persist after the establishment of CHDs and may contribute to sustainable cardiomyocyte dysfunction in patients with CHD. The present study illustrates for the first time that maternal diabetes increases 2 key miRNAs that impair mitochondrial fusion and enhance mitochondrial fragmentation in mouse embryonic cardiomyocytes in vitro and in vivo.
miRNAs are critically involved in virtually all aspects of cardiac development and disease.26 miR-1 overexpression disrupts mouse embryonic heart development,27 and miR-133a overexpression in cardiomyocytes leads to decreased cell proliferation and the formation of cardiac septation defects.28 The present study demonstrates that the upregulation of miR-140 and miR-195 mediates the teratogenicity of maternal diabetes leading to CHDs. During development, miR-140 is predominantly expressed in embryonic chondrocytes.29 miR-140 induces cardiomyocyte apoptosis via the intrinsic mitochondrial pathway.14 In contrast to miR-140, miR-195 is expressed early in the developing human heart.30 miR-195 inhibits cell proliferation and induces apoptosis by repressing multiple prosurvival proteins.31,32 Multiple lines of evidence suggest that miR-140 and miR-195 always work together. They both trigger mitochondrial dysfunction,14,31 participate in stem cell aging,31 and are elevated in adult heart diseases.33 In agreement with the coherence between these 2 miRNAs, we found that deleting the mir140 gene or the mir195 gene significantly ameliorated maternal diabetes–induced CHDs, and that overexpressing these 2 miRNAs in the heart mimicked maternal diabetes in inducing CHDs.
The transcription factor FoxO3a is activated by maternal diabetes.23 FoxO3a upregulates miRNAs in cancer cells.34 In the present study, FoxO3a transcriptionally induced miR-140 and miR-195 expression and thus inhibited mitochondrial fusion in embryonic cardiomyocytes. FoxO3a reduces the size of cardiomyocytes in rats.35 FoxO3a is a cell death trigger that acts through the mitochondrial apoptosis pathway in conditions of heart failure and hypertrophy.36,37 Our previous study indicated that the deletion of FoxO3a could inhibit maternal diabetes–induced apoptosis in cardiac progenitor cells in vivo.38 Here, we showed that Foxo3a gene deletion ameliorates maternal diabetes–induced CHDs by suppressing mitochondrial fragmentation and dysfunction. Thus, we reveal the downstream effectors of FoxO3a, miR-140 and miR-195, in defective heart development.
Mitochondrial fusion, a prosurvival event, maintains mitochondrial homeostasis by removing dysfunctional mitochondria.39,40 Cells lacking both Mfn1 and Mfn2 have completely fragmented mitochondria with no detectable mitochondrial fusion.40 Mitochondrial fusion is important for the maintenance of mitochondrial morphology, cell growth, membrane potential, and respiration.39 Reduced fusion could be a key factor contributing to diabetes- or miRNA-induced mitochondrial dysfunction. Maternal diabetes induces cellular dysfunction in cells required for cardiac septation leading to CHDs.17,24 Enhanced mitochondrial fusion stimulates cell proliferation by promoting cell cycle progression.41 Cells with double knockout of Mfn1 and Mfn2 proliferate much slower than their corresponding wild-type counterparts.42 Here, we showed that reduced Mfn1 and Mfn2 expression cause cellular dysfunction and alterations in cardiac septation leading to CHDs under conditions of maternal diabetes exposure and miRNA overexpression.
Small molecule drugs are the pillars of traditional medicine. Teriflunomide, a small molecule compound, is approved by the FDA for use in the treatment of multiple sclerosis; however, studies also showed that teriflunomide could activate mitochondrial fusion. One study indicated that teriflunomide upregulates mitofusins and also induces mitochondrial elongation by depletion of the cellular pyrimidine pool secondary to the inhibition of dihydroorotate dehydrogenase.20 Another study indicated that teriflunomide increases Mfn2 transcriptional activity and mitofusin mRNA levels in Hela cells.43 Echinacoside, another small molecule compound, is currently being investigated for anti-apoptotic and neuroprotective effects.44,45 Similarly, this compound also can function as a mitochondrial fusion activator. A study found that echinacoside selectively binds to the previously uncharacterized casein kinase 2 (CK2) α′ subunit (CK2α′) as a direct cellular target and allosterically regulates CK2α′ conformation to recruit basic transcription factor 3 (BTF3) to form a binary protein complex, and then the CK2α′/BTF3 complex facilitates β-catenin nuclear translocation to activate T-cell factor/lymphoid enhancer factor transcription factors and stimulates transcription of the mitochondrial fusion gene Mfn2.21 These findings are consistent with our current study. We demonstrated that teriflunomide and echinacoside, acting as mitochondrial fusion activators, increase the expression levels of Mfn1 and Mfn2 and improve mitochondrial fusion in cardiomyocytes under diabetic conditions, and in turn, prevent CHD formation in diabetic pregnancy. We did not observe any side effect of the 2 compounds in pregnant mice or embryos at the adopted dosage (15 mg/kg); however, we found teriflunomide often caused abortion at a higher dosage (30 mg/kg) in our preliminary study.
The STZ-induced type 1 diabetic embryopathy mouse model, which can mimic hyperglycemia in human maternal diabetes, is widely accepted in the field of maternal diabetes–induced birth defects.11,46,47 STZ used to induce diabetes is not a complicating factor because STZ is cleared from the bloodstream rapidly (serum half-life is 5 minutes with no drug measurable by 2 hours),48 and pregnancy is not established until 1 to 2 weeks after STZ injection.49 Insulin treatment of STZ-induced diabetic embryopathy effectively reduces hyperglycemia and embryonic malformations,49 indicating that hyperglycemia is the primary cause of teratogenicity and that pregestational STZ injections do not cause any toxicity to the developing embryo. In the current study, this mouse model produced 20% to 40% CHDs, including ventricular septum defect, persistent truncus arteriosus, transposition of the great arteries, and hypoplastic left heart syndrome, in embryos exposed to diabetes, whereas embryos from nondiabetic controls had zero CHDs. Among all the CHD cases, ventricular septum defect cases were the most common, which is almost identical to that in humans.6,50 In addition, we observed very few neural tube defect cases at E17.5 when embryos were harvested for CHD rate determination, as well as the infrequent cases of kidney defects and eye defects. We used 2 miRNA modified mouse models in this study, miR-140 global knockout and miR-195 conditional knockout mice, and did not observe any cardiac or noncardiac defects because of the deletion of each miRNA.
Unlike neural tube defects, which can be reduced by folate supplementation, prevention methods for CHDs are lacking. The present study reveals a mechanism-based method for the prevention of CHDs induced by nongenetic factors that are primarily causal factors in humans. Treatment of the diabetic pregnant dams with small molecule activators of mitochondrial fusion restored Mfn1 and Mfn2 expression at the transcriptional level and subsequently rescued mitochondrial fusion in cardiomyocytes. We also uncovered the molecular pathway that leads to the inhibition of mitochondrial fusion in the CHD models, thus providing new targets for the design of prevention approaches.
Study limitations
The 2 mitochondrial fusion activators were not tested in human pregnancy; however, mechanism-based prevention needs be developed in preclinical animal models such as those described in the present study. Because maternal diabetes–induced mitochondrial fusion deficit and other functional defects are persistent in cardiomyocytes,51 they allow us to effectively analyze mitochondrial fusion in isolated cardiomyocytes by transfecting a light-activated mitochondrial probe, a reliable in vitro mitochondrial fusion detection method. We are not aware of any in vivo method in detecting mitochondrial fusion. Additional justification for our mitochondrial fusion assay is the fact that high glucose mimics maternal diabetes in inducing mitochondrial fusion impairment in cultured primary cardiomyocytes. Mitochondrial biogenesis and mitophagy, which both play pivotal roles in the regulation of mitochondrial quality and function, were not evaluated in the current study; however, they might be involved in maternal diabetes-affected embryonic hearts and it is worthwhile to be further investigated in the future.
Conclusions
We conclude that reduced mitochondrial fusion is a key event in the formation of CHDs induced by either miR-195/miR-140 Tg expression or exposure to a maternal diabetic milieu. Maternal diabetes–activated FoxO3a increases miR-140 and miR-195, which in turn represses Mfn1 and Mfn2, leading to mitochondrial fusion defects and CHDs. Maternal treatment with either of teriflunomide and echinacoside restores Mfn1 and Mfn2 expression and mitochondrial fusion in cardiomyocytes of embryonic hearts exposed to diabetes, implicating that activating mitochondrial fusion could be a potent means to prevent CHDs induced by maternal nongenetic factors.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: There is no preventive measure for the most common structural birth defect, CHDs. Most CHDs are induced by nongenetic factors. Maternal diabetes is a major nongenetic factor that induces a 4- to 5-fold CHD rate in the offspring. Identifying mitochondrial fusion impairment as the cause of CHDs in diabetic pregnancy reveals a major mechanistic insight for aiding the design of prevention for CHDs. Testing 2 mitochondrial fusion activators in CHD animal models can be translated to the clinic.
TRANSLATIONAL OUTLOOK 1: Repurposing FDA-approved drugs or dietary supplementations of naturally occurring compounds, which re-activates mitochondrial fusion, may ameliorate maternal diabetes–induced CHDs.
TRANSLATIONAL OUTLOOK 2: Mitochondrial fusion impairment may contribute to CHD formation induced by other environmental factors. Mitochondrial fusion activators may be applicable in prevention of other environmental factors–induced CHDs.
Funding Support and Author Disclosures
This work was financially supported by National Institutes of Health grants R01DK083243, R01DK101972, R01DK103024, R01HL131737, R01HL134368, R01HL139060, R01HL153141, and R01HL151108. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgment
The authors thank Ms Hua Li at the University of Maryland School of Medicine for her technical support.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental methods, tables, and figures, please see the online version of this paper.
Contributor Information
Guanglei Wang, Email: gwang@som.umaryland.edu.
Peixin Yang, Email: pyang@som.umaryland.edu.
Appendix
References
- 1.Mathews T.J., Driscoll A.K. Trends in infant mortality in the United States, 2005-2014. NCHS Data Brief. 2017;(279):1–8. [PubMed] [Google Scholar]
- 2.Virani S.S., Alonso A., Benjamin E.J., et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 3.Botto L.D., Mulinare J., Erickson J.D. Occurrence of congenital heart defects in relation to maternal mulitivitamin use. Am J Epidemiol. 2000;151:878–884. doi: 10.1093/oxfordjournals.aje.a010291. [DOI] [PubMed] [Google Scholar]
- 4.Oyen N., Olsen S.F., Basit S., et al. Association between maternal folic acid supplementation and congenital heart defects in offspring in birth cohorts from Denmark and Norway. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.011615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenkins K.J., Correa A., Feinstein J.A., et al. Noninherited risk factors and congenital cardiovascular defects: current knowledge: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115:2995–3014. doi: 10.1161/CIRCULATIONAHA.106.183216. [DOI] [PubMed] [Google Scholar]
- 6.Oyen N., Diaz L.J., Leirgul E., et al. Prepregnancy diabetes and offspring risk of congenital heart disease: a nationwide cohort study. Circulation. 2016;133:2243–2253. doi: 10.1161/CIRCULATIONAHA.115.017465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J., Cummings E.A., O'Connell C., Jangaard K. Fetal and neonatal outcomes of diabetic pregnancies. Obstet Gynecol. 2006;108:644–650. doi: 10.1097/01.AOG.0000231688.08263.47. [DOI] [PubMed] [Google Scholar]
- 8.Loffredo C.A., Wilson P.D., Ferencz C. Maternal diabetes: an independent risk factor for major cardiovascular malformations with increased mortality of affected infants. Teratology. 2001;64:98–106. doi: 10.1002/tera.1051. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence J.M., Contreras R., Chen W., Sacks D.A. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999-2005. Diabetes Care. 2008;31:899–904. doi: 10.2337/dc07-2345. [DOI] [PubMed] [Google Scholar]
- 10.Correa A., Gilboa S.M., Besser L.M., et al. Diabetes mellitus and birth defects. Am J Obstet Gynecol. 2008;199:237:e1–e9. doi: 10.1016/j.ajog.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu C., Shen W.B., Reece E.A., et al. Maternal diabetes induces senescence and neural tube defects sensitive to the senomorphic rapamycin. Sci Adv. 2021;7(27) doi: 10.1126/sciadv.abf5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang P., Xu C., Reece E.A., et al. Tip60- and sirtuin 2-regulated MARCKS acetylation and phosphorylation are required for diabetic embryopathy. Nat Commun. 2019;10:282. doi: 10.1038/s41467-018-08268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang F., Xu C., Reece E.A., et al. Protein kinase C-alpha suppresses autophagy and induces neural tube defects via miR-129-2 in diabetic pregnancy. Nat Commun. 2017;8 doi: 10.1038/ncomms15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J., Li Y., Jiao J., et al. Mitofusin 1 is negatively regulated by microRNA 140 in cardiomyocyte apoptosis. Mol Cell Biol. 2014;34:1788–1799. doi: 10.1128/MCB.00774-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ong S.B., Hall A.R., Hausenloy D.J. Mitochondrial dynamics in cardiovascular health and disease. Antioxid Redox Signal. 2013;19:400–414. doi: 10.1089/ars.2012.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasahara A., Cipolat S., Chen Y., Dorn G.W., 2nd, Scorrano L. Mitochondrial fusion directs cardiomyocyte differentiation via calcineurin and Notch signaling. Science. 2013;342:734–737. doi: 10.1126/science.1241359. [DOI] [PubMed] [Google Scholar]
- 17.Wang F., Fisher S.A., Zhong J., Wu Y., Yang P. Superoxide dismutase 1 in vivo ameliorates maternal diabetes mellitus-induced apoptosis and heart defects through restoration of impaired Wnt signaling. Circ Cardiovasc Genet. 2015;8:665–676. doi: 10.1161/CIRCGENETICS.115.001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H., Detmer S.A., Ewald A.J., Griffin E.E., Fraser S.E., Chan D.C. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karbowski M., Arnoult D., Chen H., Chan D.C., Smith C.L., Youle R.J. Quantitation of mitochondrial dynamics by photolabeling of individual organelles shows that mitochondrial fusion is blocked during the Bax activation phase of apoptosis. J Cell Biol. 2004;164:493–499. doi: 10.1083/jcb.200309082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miret-Casals L., Sebastián D., Brea J., et al. Identification of new activators of mitochondrial fusion reveals a link between mitochondrial morphology and pyrimidine metabolism. Cell Chem Biol. 2018;25:268–278.e4. doi: 10.1016/j.chembiol.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Zeng K.W., Wang J.K., Wang L.C., et al. Small molecule induces mitochondrial fusion for neuroprotection via targeting CK2 without affecting its conventional kinase activity. Signal Transduct Target Ther. 2021;6:71. doi: 10.1038/s41392-020-00447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang G.Q., Wang S.Q., Chen Y., et al. MicroRNAs regulating mitochondrial function in cardiac diseases. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.663322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang P., Li X., Xu C., et al. Maternal hyperglycemia activates an ASK1-FoxO3a-caspase 8 pathway that leads to embryonic neural tube defects. Sci Signal. 2013;6:ra74. doi: 10.1126/scisignal.2004020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang F., Wu Y., Quon M.J., Li X., Yang P. ASK1 mediates the teratogenicity of diabetes in the developing heart by inducing ER stress and inhibiting critical factors essential for cardiac development. Am J Physiol Endocrinol Metab. 2015;309:E487–E499. doi: 10.1152/ajpendo.00121.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitani T., Tian L., Zhang T., et al. RNA sequencing analysis of induced pluripotent stem cell-derived cardiomyocytes from congenital heart disease patients. Circ Res. 2020;126:923–925. doi: 10.1161/CIRCRESAHA.119.315653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouyang Z., Wei K. miRNA in cardiac development and regeneration. Cell Regen. 2021;10:14. doi: 10.1186/s13619-021-00077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y., Samal E., Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005;436:214–220. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 28.Liu N., Bezprozvannaya S., Williams A.H., et al. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev. 2008;22:3242–3254. doi: 10.1101/gad.1738708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuddenham L., Wheeler G., Ntounia-Fousara S., et al. The cartilage specific microRNA-140 targets histone deacetylase 4 in mouse cells. FEBS Lett. 2006;580:4214–4217. doi: 10.1016/j.febslet.2006.06.080. [DOI] [PubMed] [Google Scholar]
- 30.Zhou J., Dong X., Zhou Q., et al. microRNA expression profiling of heart tissue during fetal development. Int J Mol Med. 2014;33:1250–1260. doi: 10.3892/ijmm.2014.1691. [DOI] [PubMed] [Google Scholar]
- 31.Okada M., Kim H.W., Matsu-ura K., Wang Y.G., Xu M., Ashraf M. Abrogation of age-induced microRNA-195 rejuvenates the senescent mesenchymal stem cells by reactivating telomerase. Stem Cells. 2016;34:148–159. doi: 10.1002/stem.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Rooij E., Sutherland L.B., Liu N., et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A. 2006;103:18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Divakaran V., Mann D.L. The emerging role of microRNAs in cardiac remodeling and heart failure. Circ Res. 2008;103:1072–1083. doi: 10.1161/CIRCRESAHA.108.183087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song Y., Zeng S., Zheng G., et al. FOXO3a-driven miRNA signatures suppresses VEGF-A/NRP1 signaling and breast cancer metastasis. Oncogene. 2021;40:777–790. doi: 10.1038/s41388-020-01562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skurk C., Izumiya Y., Maatz H., et al. The FOXO3a transcription factor regulates cardiac myocyte size downstream of AKT signaling. J Biol Chem. 2005;280:20814–20823. doi: 10.1074/jbc.M500528200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaanine A.H., Jeong D., Liang L., et al. JNK modulates FOXO3a for the expression of the mitochondrial death and mitophagy marker BNIP3 in pathological hypertrophy and in heart failure. Cell Death Dis. 2012;3:265. doi: 10.1038/cddis.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X., Du N., Zhang Q., et al. MicroRNA-30d regulates cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic cardiomyopathy. Cell Death Dis. 2014;5:e1479. doi: 10.1038/cddis.2014.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang P., Yang W.W., Chen X., Kaushal S., Dong D., Shen W.B. Maternal diabetes and high glucose in vitro trigger Sca1(+) cardiac progenitor cell apoptosis through FoxO3a. Biochem Biophys Res Commun. 2017;482:575–581. doi: 10.1016/j.bbrc.2016.11.076. [DOI] [PubMed] [Google Scholar]
- 39.Detmer S.A., Chan D.C. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 40.Chen H., McCaffery J.M., Chan D.C. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130:548–562. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 41.Mitra K., Wunder C., Roysam B., Lin G., Lippincott-Schwartz J. A hyperfused mitochondrial state achieved at G1-S regulates cyclin E buildup and entry into S phase. Proc Natl Acad Sci U S A. 2009;106:11960–11965. doi: 10.1073/pnas.0904875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen H., Chomyn A., Chan D.C. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- 43.Duvezin-Caubet S., Jagasia R., Wagener J., et al. Proteolytic processing of OPA1 links mitochondrial dysfunction to alterations in mitochondrial morphology. J Biol Chem. 2006;281:37972–37979. doi: 10.1074/jbc.M606059200. [DOI] [PubMed] [Google Scholar]
- 44.Liu J., Yang L., Dong Y., Zhang B., Ma X. Echinacoside, an inestimable natural product in treatment of neurological and other disorders. molecules. 2018;23:1213. doi: 10.3390/molecules23051213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deng M., Zhao J.Y., Tu P.F., Jiang Y., Li Z.B., Wang Y.H. Echinacoside rescues the SHSY5Y neuronal cells from TNFalpha-induced apoptosis. Eur J Pharmacol. 2004;505:11–18. doi: 10.1016/j.ejphar.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 46.Li R., Thorens B., Loeken M.R. Expression of the gene encoding the high-Km glucose transporter 2 by the early postimplantation mouse embryo is essential for neural tube defects associated with diabetic embryopathy. Diabetologia. 2007;50:682–689. doi: 10.1007/s00125-006-0579-7. [DOI] [PubMed] [Google Scholar]
- 47.Salbaum J.M., Kruger C., Zhang X., et al. Altered gene expression and spongiotrophoblast differentiation in placenta from a mouse model of diabetes in pregnancy. Diabetologia. 2011;54:1909–1920. doi: 10.1007/s00125-011-2132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schein P.S., Loftus S. Streptozotocin: depression of mouse liver pyridine nucleotides. Cancer Res. 1968;28:1501–1506. [PubMed] [Google Scholar]
- 49.Yang P., Zhao Z., Reece E.A. Activation of oxidative stress signaling that is implicated in apoptosis with a mouse model of diabetic embryopathy. Am J Obstet Gynecol. 2008;198:130:e1–e7. doi: 10.1016/j.ajog.2007.06.070. [DOI] [PubMed] [Google Scholar]
- 50.Wren C., Birrell G., Hawthorne G. Cardiovascular malformations in infants of diabetic mothers. Heart. 2003;89:1217–1220. doi: 10.1136/heart.89.10.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin X., Yang P., Reece E.A., Yang P. Pregestational type 2 diabetes mellitus induces cardiac hypertrophy in the murine embryo through cardiac remodeling and fibrosis. Am J Obstet Gynecol. 2017;217:216:e1–e13. doi: 10.1016/j.ajog.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.