Abstract
Elevated levels of circulating high-sensitivity cardiac troponin T (hs-cTnT) are associated with cardiovascular disease. This study aimed to examine whether hs-cTnT levels are associated with incident stroke in the elderly population.
The Iwate Tohoku Medical Megabank Organization pooled participant data for a community-based cohort study (n = 15,063, 69.6 ± 3.4 years), with a mean follow-up period of 5.23 years for all-cause death and incident stroke. The follow-up revealed 316 incident strokes, including atherothrombotic (n = 98), cardioembolic (n = 54), lacunar (n = 63), hemorrhagic (n = 101), and 178 all-cause deaths. Participants were classified into quartiles according to hs-cTnT levels (Q1 ≦ 4 ng/L, Q2: 5–6 ng/L, Q3: 7–9 ng/L, and Q4 > 9 ng/L). After adjusting for sex, age, smoking, drinking, systolic blood pressure, estimated glomerular filtration rate, N-terminal pro-brain natriuretic peptide, hemoglobin A1c, and lipid profile, a Cox proportional hazard model showed that higher hs-cTnT levels were associated with ischemic stroke (Q1 vs. Q4, hazard ratio [HR] = 2.24, 95 % confidence interval [CI] = 1.12–4.51, p = 0.023). The incident of total stroke was not associated with hs-cTnT levels (Q1 vs. Q4, HR 1.39, 95 % CI = 0.89–1.74, p = 0.145). Numerical differences were highest regarding incident lacunar stroke subtypes; however, this association was not statistically significant.
Higher hs-cTnT concentrations were associated with ischemic stroke in the elderly Japanese population.
Keywords: Biomarker, Cerebrovascular disease, Follow-up study, Ischemic stroke
Highlights
-
•
High-sensitivity cardiac troponin T (hs-cTnT) are associated with cardiovascular diseases.
-
•
Higher hs-cTnT concentrations are associated with incident stroke in the elderly Japanese.
-
•
Hs-cTnT level may be helpful in predicting of stroke in the elderly Japanese.
1. Introduction
Stroke remains a common and life-threatening disease in the general elderly population, despite recent advances in stroke management, such as intravenous thrombolysis with recombinant tissue plasminogen activator [1], [2]. The Japan Public Health Center Study developed a scoring system to predict the 10-year stroke risk in the general population using variables such as smoking, body mass index, blood pressure, antihypertensive medication, diabetes mellitus, age, and sex [3]. However, blood prognostic biomarkers predicting the occurrence of stroke in the general Japanese population have not been confirmed.
High-sensitivity cardiac troponin T (hs-cTnT) is a highly sensitive assay of cTnT and is used as a diagnostic marker for acute coronary syndrome [4], [5]. Previous studies have shown that an increase in hs-cTnT levels is associated with a risk of future cardiovascular disease (CVD) [6], [7], [8]. Additionally, several studies have reported hs-cTnT as a useful predictive marker of all-cause death and CVD, even in the general population without a history of CVD [9], [10]. Recently, an association between hs-cTnT levels and stroke has been reported [11], [12]. A large European cohort study reported an association between troponin I levels and ischemic and hemorrhagic stroke [13]. In addition, a meta-analysis showed an association between serum troponin levels and ischemic or hemorrhagic stroke incidents [14]. Moreover, elevated cTnT levels have been shown to be associated with a risk of cardioembolic or other non-lacunar ischemic strokes in the general population aged <65 years [15], and hs-cTnT was independently associated with magnetic resonance imaging-defined asymptomatic stroke [16]. However, the association between stroke and hs-cTnT levels in the general elderly population (≥65 years) remains uncertain. This study aimed to examine whether hs-cTnT levels were associated with incident stroke and all-cause death in the elderly Japanese general population and whether hs-cTnT could be used as a prognostic biomarker of stroke.
2. Methods
We used the cohort data from the Tohoku Medical Megabank Community-based Cohort Study, a prospective cohort study [17], [18]. Participants were recruited from the Iwate prefecture between May 2013 and March 2016 [17], [18]. The study population comprised individuals aged ≥65 years and living in Iwate (n = 15,727). Participants were excluded from the study if they had undergone pacemaker implantation, continuous dialysis, or had self-reported CVD (n = 483), such as myocardial infarction, angina pectoris, aortic aneurysm, aortic dissection, heart failure, atrial fibrillation, ventricular fibrillation, or stroke. This study excluded participants lacking baseline characteristics and laboratory (n = 99) or follow-up data (n = 82). Ultimately, 15,063 participants were included in the analysis. This study was approved by the Ethics Committee of Iwate Medical University (HGH25-2). Informed consent was obtained from all participants.
We collected data regarding the lifestyle and medical histories of the participants using self-administered questionnaires and height, weight, and blood pressure measurements based on physiological examinations. Participants who had an alcohol consumption of >34 g of ethanol per day were defined as drinkers [19].
Peripheral venous blood samples for blood tests were collected from the upper arm in resting and seated positions. The samples were centrifuged, and the supernatant was collected and frozen at −80 °C before measurement. Serum hs-cTnT levels were measured using EcLusys high-sensitivity troponin T assay (Roche Diagnostics K.K., Tokyo, Japan). The limit of detection for the assay was 3 ng/L. The 99th percentile upper reference limit for the hs-cTnT level was 13.5 ng/L [5]. Estimated glomerular filtration rate (eGFR) was determined using serum creatinine level according to the 2002 KDOQI Clinical Practice Guidelines for Chronic Kidney Disease [20], and calculated using the following equation: eGFR = 194 × serum cystatin C−1.094 × age−0.287 [×0.739 for women] mL/min/1.73 m [2], [21].
A follow-up survey assessing all-cause death, migration, and stroke incidence was conducted after the baseline study until December 31, 2019. Death and migration were confirmed using official resident registration data issued by the local government offices. Stroke incidence was identified using the Iwate Stroke Registry data at all regional hospitals [22]. The stroke registration program has been coordinated by the Iwate prefecture government and Iwate Medical Association; the medical records of all medical facilities within the survey area are verified to ensure complete recording of all data. Registration forms are submitted to the registration office of the Iwate Medical Association by mail when a patient with stroke leaves the medical facility. The research group, including physicians or trained research nurses, visited the hospitals and reviewed medical charts.
The diagnostic criteria for stroke in this study were based on the criteria of the International Statistical Classification of Diseases and Related Health Problems by the World Health Organization [23], [24]. Patients with transient ischemic attack and traumatic hemorrhagic stroke were excluded from registration. Stroke was defined as the sudden onset of a focal neurological deficit lasting ≥24 h and confirmed using brain computed tomography or magnetic resonance imaging [3], [25]. In cases that involved surgically implanted medical devices, claustrophobia, tattoos, or magnetic dentures, brain computed tomography was used for diagnosis. The different types of stroke included ischemic (atherothrombotic and cardioembolic), lacunar, and hemorrhagic, based on the patients' neuroimaging and medical records.
Participants were classified into quartiles according to hs-cTnT levels. Baseline characteristics of participants are presented as means ± standard deviations for continuous variables and as numbers and percentages for categorical variables. Continuous variables were compared among quartiles using an analysis of variance for normally distributed variables, and the Kruskal-Wallis test was used for skewed variables. Categorical variables were analyzed among quartiles using the chi-squared test. Event-free curves were estimated using the Kaplan–Meier method and compared using the log-rank test. Cox proportional hazard models were used to examine the association between hs-cTnT levels and incident stroke in two models: the unadjusted model was a crude model, and the adjusted model was a multivariate adjustment model, adjusted for sex, age, smoking, drinking, systolic blood pressure, low-density lipoprotein cholesterol, eGFR, N-terminal pro-brain natriuretic peptide (NT-proBNP), and hemoglobin A1c. We defined the reference group for the Cox hazard model as the quartile with the lowest hs-cTnT levels. We performed a logistical analysis on stroke incidents. All data were analyzed using IBM SPSS Statistics, version 25, for Windows (IBM Corp., Armonk, NY, USA); p-values < 0.05 were considered statistically significant.
3. Results
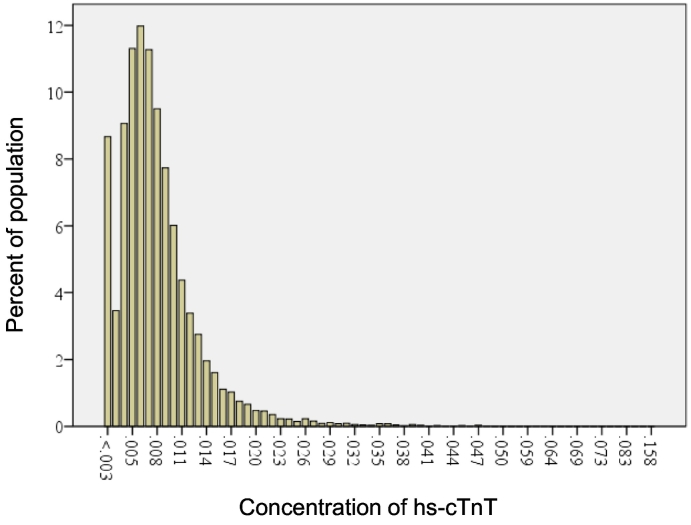
Baseline characteristics of the participants are shown in Table 1. The range of hs-cTnT level was 3–35 ng/L, with a median value of 5 ng/L (interquartile, 5–9 ng/L) (Fig. 1). Baseline characteristics of the study population in quartiles according to hs-cTnT levels (Q1 ≦ 4 ng/L, Q2: 5–6 ng/L, Q3: 7–9 ng/L, and Q4 > 9 ng/L) are shown in Table 2. Significant differences were observed in the prevalence of smoking, diabetes mellitus, and hypertension; levels of NT-proBNP, uric acid, and hemoglobin A1c; and systolic blood pressure (Table 2).
Table 1.
Baseline characteristics of participants.
| Parameter | All |
|---|---|
| Number | 15,063 |
| Age | 69.6 ± 3.4 |
| Sex, % men | 6264 (41.6) |
| Body mass index, kg/m2 | 23.8 ± 3.3 |
| Current or former smoker, % | 5456 (36.2) |
| Drinker, % | 6297(41.8) |
| Diabetes, % | 980 (6.5) |
| Hypertension, % | 5597 (37.2) |
| Dyslipidemia, % | 1942 (12.9) |
| Median NT-proBNP, pg/mL | 66 (40–109) |
| Median hs-cTnT, ng/L | 5 (5–9) |
| Uric acid, mg/dL | 5.0 ± 1.3 |
| eGFR, mL/min/1.73 m2 | 76.1 ± 16.0 |
| Hemoglobin A1c, % | 5.8 ± 0.6 |
| HDL-cholesterol, U/L | 62.6 ± 16.4 |
| LDL-cholesterol, U/L | 117.8 ± 28.5 |
| Systolic blood pressure, mm Hg | 131.0 ± 17.8 |
Values are mean ± SD, or number of subjects (percentage). eGFR = estimated glomerular filtration rate, HDL = high-density lipoprotein, hs-cTnT = high-sensitivity cardiac troponin T, LDL = low-density lipoprotein, NT-proBNP = N-terminal pro-brain natriuretic peptide.
Fig. 1.
Distribution map of hs-cTnT levels. Hs-cTnT, high-sensitivity cardiac troponin T.
Table 2.
Baseline characteristics of participants by hs-cTnT levels.
| Parameter | Q1 hs-cTnT ≤ 4 ng/L |
Q2 4 ng/L < hs-cTnT ≤ 6 ng/L |
Q3 6 ng/L < hs-cTnT ≤ 9 ng/L |
Q4 hs-cTnT > 9 ng/L |
p value |
|---|---|---|---|---|---|
| Number | 3482 | 4224 | 3843 | 3514 | |
| Age | 68.5 ± 2.9 | 69.1 ± 3.1 | 69.7 ± 3.3 | 70.5 ± 3.6 | p < 0.001 |
| Sex, % men | 1212 (34.8) | 1601 (37.9) | 1687 (43.9) | 1764 (50.2) | p < 0.001 |
| Body mass index, kg/m2 | 23.1 ± 3.1 | 23.4 ± 3.2 | 23.8 ± 3.3 | 24.4 ± 3.5 | p < 0.001 |
| Current or former smoker, % | 1123 (32.2) | 1649 (39.0) | 1271 (33.0) | 1413 (40.2) | p < 0.001 |
| Drinker, % | 1421 (40.8) | 1688 (40.0) | 1637 (42.5) | 1551(44.1) | p = 0.01 |
| Diabetes, % | 209 (6.0) | 234 (5.5) | 267 (6.9) | 270 (7.7) | p < 0.001 |
| Hypertension, % | 1231 (35.3) | 1575 (37.2) | 1468 (38.1) | 1323 (37.6) | p < 0.001 |
| Dyslipidemia, % | 452 (12.9) | 533 (12.6) | 495 (12.8) | 463 (13.1) | p < 0.001 |
| Median NT-proBNP, pg/mL | 54 (35–86) | 59 (37–95) | 67 (41–108) | 84 (50–153) | p < 0.001 |
| Uric acid, mg/dL | 4.7 ± 1.1 | 4.9 ± 1.2 | 5.0 ± 1.3 | 5.3 ± 1.4 | p < 0.001 |
| eGFR, mL/min/1.73 m2 | 100.8 ± 15.5 | 96.1 ± 16.2 | 92.9 ± 16.7 | 86.5 ± 18.9 | p < 0.001 |
| Hemoglobin A1c, % | 5.8 ± 0.5 | 5.7 ± 0.5 | 5.8 ± 0.4 | 5.8 ± 0.6 | p = 0.03 |
| HDL-cholesterol, U/L | 63.1 ± 16.0 | 63.0 ± 16.3 | 62.9 ± 16.7 | 61.6 ± 16.6 | p < 0.001 |
| LDL-cholesterol, U/L | 121.3 ± 28.6 | 119.1 ± 28.0 | 117.2 ± 27.9 | 119.1 ± 29.2 | p < 0.001 |
| Systolic blood pressure, mm Hg | 127.3 ± 17.3 | 130.7 ± 17.5 | 131.5 ± 17.6 | 133.5 ± 18.1 | p < 0.001 |
Values are mean ± SD, or number of subjects (percentage). eGFR = estimated glomerular filtration rate; HDL = high-density lipoprotein; hs-cTnT = high-sensitivity cardiac troponin T; LDL = low-density lipoprotein; NT-proBNP = N-terminal pro-brain natriuretic peptide.
Baseline characteristics were compared among the four groups using an analysis of variance (ANOVA) for normally distributed variables, and the Kruskal-Wallis test was used for skewed variables. The chi-squared test was used to analyze between-group differences in categorical variables.
The mean follow-up period was 5.23 ± 2.01 years (range, 0.6–6.6 years). During the follow-up period, all-cause death, ischemic stroke, and hemorrhagic stroke were identified in 178, 215, and 101 participants, respectively. Among the 215 patients with ischemic stroke, atherothrombotic, cardioembolic, and lacunar strokes were reported in 98, 54, and 63 participants, respectively. The subgroup of ischemic stroke is described as a supplement because of the small number of cases.
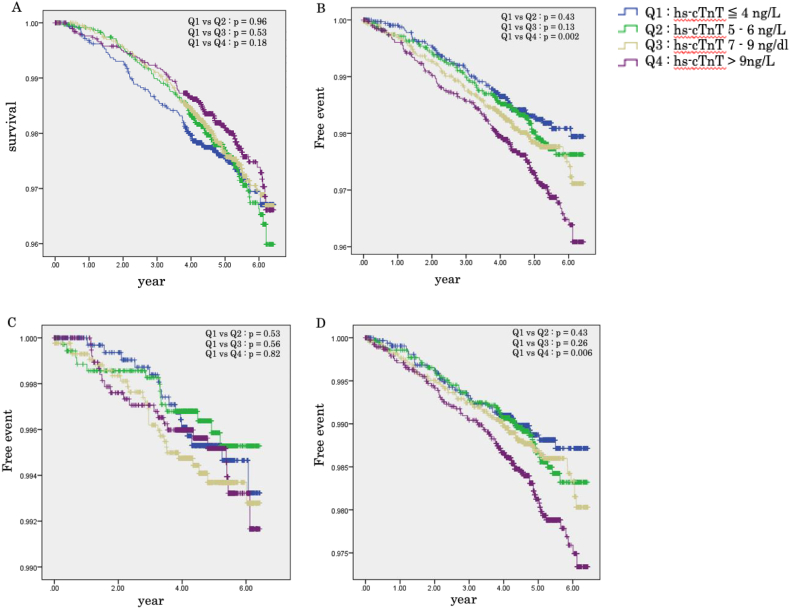
Kaplan–Meier curves for all-cause death and stroke-free rates that were based on quartiles according to hs-cTnT levels in all participants are shown in Fig. 2. Total stroke- and ischemic stroke-free rates were lower in the Q4 quartile than in the Q1 quartile (all p < 0.05, log-rank test). There were no significant differences between the incidences of hemorrhagic stroke among the four groups (Fig. 2).
Fig. 2.
Kaplan–Meier survival and event-time curve according to hs-cTnT levels. (A) All-cause death, (B) total stroke, (C) intracerebral stroke, and (D) ischemic stroke-free probability based on quartiles according to hs-cTnT levels (Q1 ≦ 4 ng/L, Q2: 5–6 ng/L, Q3: 7–9 ng/L, and Q4 > 9 ng/L). Hs-cTnT, high-sensitivity cardiac troponin.
Table 3 shows the results of the analysis of the association between hs-cTnT levels and all-cause death and incident stroke. The unadjusted model showed that the Q4 quartile had significantly higher risk of total stroke (hazard ratio [HR] = 1.68, 95 % confidence interval [CI] = 1.21–2.32, p = 0.002) and ischemic stroke (HR = 1.75, 95 % CI = 1.17–2.62, p = 0.006) than the Q1 quartile. For all-cause death and hemorrhagic stroke, no significant association was observed. Multivariate Cox analysis showed that the Q4 quartile was significantly associated with a higher risk for ischemic stroke (HR = 2.24, 95 % CI = 1.12–4.51, p = 0.023) than the Q1 quartile. However, hs-cTnT levels were not associated with all-cause death (Q1 vs. Q4, HR = 0.93, 95 % CI = 0.60–1.39, p = 0.735) and incidents of total stroke (Q1 vs. Q4, HR = 1.39, 95 % CI = 0.89–1.74, p = 0.145) and hemorrhagic stroke (Q1 vs. Q4, HR = 1.12, 95 % CI = 0.77–1.92, p = 0.452).
Table 3.
Hazard ratios (95 % confidence intervals) for incident stroke according to hs-cTnT levels.
| Model | Q1, hs-cTnT ≤ 4 ng/L | Q2, 4 ng/L < hs-cTnT ≤ 6 ng/L | Q3, 6 ng/L < hs-cTnT ≤ 9 ng/L | Q4, hs-cTnT > 9 ng/L |
|---|---|---|---|---|
| Number of participants | 3482 | 4224 | 3843 | 3514 |
| All-cause death | ||||
| Person-years | 16,007.7 | 21,737.1 | 19,339.8 | 17,683.9 |
| Number of deaths | 48 | 70 | 99 | 115 |
| Unadjusted model HR (95 % CI), p value |
1 (reference) | 0.99 (0.74–1.34), p = 0.962 | 0.91 (0.68–1.22), p = 0.530 | 0.81 (0.60–1.12), p = 0.187 |
| Adjusted model HR (95 % CI), p value |
1 (reference) | 0.94 (0.69–1.39), p = 0.700 | 1.09 (0.64–1.57), p = 0.813 | 0.93 (0.60–1.39), p = 0.735 |
| Total stroke | ||||
| Person-years | 15,898.2 | 21,526.2 | 19,132.3 | 17,548.8 |
| Number of cases | 30 | 50 | 59 | 76 |
| Unadjusted model HR (95 % CI), p value |
1 (reference) | 1.14 (0.80–1.63), p = 0.472 | 1.29 (0.92–1.80), p = 0.142 | 1.68 (1.21–2.32), p = 0.002 |
| Adjusted model HR (95 % CI), p value |
1 (reference) | 1.02 (0.64–1.45), p = 0.587 | 1.14 (0.83–1.41), p = 0.249 | 1.39 (0.89–1.74), p = 0.145 |
| Ischemic stroke | ||||
| Number of cases | 14 | 26 | 24 | 37 |
| Unadjusted model HR (95 % CI), p value |
1 (reference) | 1.19 (0.77–1.85), p = 0.438 | 1.27 (0.83–1.92), p = 0.291 | 1.75 (1.17–2.62), p = 0.006 |
| Adjusted model HR (95 % CI), p value |
1 (reference) | 1.37 (0.68–2.78), p = 0.372 | 1.61 (0.90–2.90), p = 0.108 | 2.24 (1.12–4.51), p = 0.023 |
| Hemorrhagic stroke | ||||
| Number of cases | 21 | 27 | 25 | 28 |
| Unadjusted model HR (95 % CI), p value |
1 (reference) | 0.79 (0.39–1.62), p = 0.525 | 1.20 (0.64–2.24), p = 0.567 | 1.09 (0.57–2.09), p = 0.798 |
| Adjusted model HR (95 % CI), p value |
1 (reference) | 0.86 (0.62–1.12), p = 0.371 | 0.97 (0.67–1.49), p = 0.641 | 1.12 (0.77–1.92), p = 0.452 |
CI = confidence interval; hs-cTnT = high-sensitivity cardiac troponin T; HR = hazard ratio.
Unadjusted model was univariate cox regression analysis using hs-cTnT.
Adjusted model was multivariate cox regression analysis using hs-cTnT adjusted for sex, age, smoking, drinker, systolic blood pressure, low-density lipoprotein cholesterol, estimated glomerular filtration rate, N-terminal pro-brain natriuretic peptide, and hemoglobin A1c.
Analysis results for the ischemic stroke subtypes are shown in Supplemental Table 1. The unadjusted model showed that the Q4 quartile had a significantly higher risk of lacunar stroke than the Q1 quartile (HR = 2.59, 95 % CI = 1.17–5.75, p = 0.019). There was no significant association between hs-cTnT levels and atherothrombotic and cardioembolic stroke incidents. Multivariate Cox analysis showed that hs-cTnT levels were not associated with incident of atherothrombotic (Q1 vs. Q4, HR = 1.16, 95 % CI = 0.58–2.35, p = 0.674), cardioembolic (HR = 1.02, 95 % CI = 0.42–2.47; p = 0.971), and lacunar stroke (HR = 1.79, 95 % CI = 0.89–3.99, p = 0.186).
We performed a logistic regression analysis using mean eGFR and NT-proBNP levels. The cut-off for detectable hs-cTnT levels was 9 ng/L. Logistical analysis showed that hs-cTnT levels >9 ng/L were strongly associated with stroke incidence (hs-cTnT > 9 ng/dL odds ratio [OR] 1.52, 95 % CI = 1.35–1.82; hypertension OR 1.35, 95 % CI = 1.28–1.50; dyslipidemia OR 1.29, 95 % CI = 1.25–1.32) (Table 4).
Table 4.
Association between the stroke incident and stroke risk factors including levels of high sense cardiac troponin T using binomial logistic regression analysis.
| Variable | OR 95 % CI | p value |
|---|---|---|
| Age | 1.28 (1.25–1.32) | <0.001 |
| Men | 1.32 (1.04–1.65) | 0.013 |
| Diabetes | 1.31 (1.22–1.41) | 0.002 |
| Hypertension | 1.35 (1.28–1.50) | <0.001 |
| Dyslipidemia | 1.29 (1.25–1.32) | <0.001 |
| NT-proBNP > 66 pg/mL | 1.03 (1.01–1.07) | 0.02 |
| hs-cTnT > 9 ng/L | 1.52 (1.35–1.82) | <0.001 |
| eGFR > 60 mL/min/1.73 m2 | 0.95 (0.90–0.98) | <0.001 |
OR = odds ratio; CI = confidence interval.
NT-proBNP = N-terminal pro-brain natriuretic peptide; hs-cTnT = high-sensitivity cardiac troponin T; LDL = low-density lipoprotein.
4. Discussion
The prevalence of CVD risk factors, such as smoking, diabetes mellitus, and hypertension, was higher in higher hs-cTnT concentrations. The Kaplan–Meier curves showed that the total stroke- and ischemic stroke-free rates were lower in the group with highest hs-cTnT levels than in that with lowest hs-cTnT levels (all p < 0.05, log-rank test). Furthermore, this study showed that higher hs-cTnT concentrations were significantly associated with incident ischemic stroke. Multivariate Cox analysis showed an association between ischemic stroke and higher hs-cTnT concentrations.
Compared with a standard cTnT assay, the high-sensitivity assay for cTnT can detect much lower levels of myocardial injury and may be useful in the detection of subclinical CVD and assessment of future CVD risk in the general population [6]. Our previous study reported a novel distribution of hs-cTnT levels and their association with CVD parameters in the general Japanese population [6]. Additionally, this study demonstrated that circulating hs-cTnT levels were significantly higher in participants with traditional CVD risk factors, such as hypertension, diabetes mellitus, and smoking, than in participants without them. These observations suggest that elevated hs-cTnT levels may reflect the progression of atherosclerosis. Furthermore, hs-cTnT has been reported to be associated with cell apoptosis, which may have an additional impact [26].
The Atherosclerosis Risk in Communities Study reported that cTnT levels are positively associated with the incidence of total and non-lacunar ischemic stroke but not with incidence of lacunar or hemorrhagic stroke [15], which corroborates the findings of this study. Here, higher hs-cTnT concentrations were strongly associated with the occurrence of ischemic stroke but not with that of cardioembolic stroke, because participants with atrial fibrillation or CVD were excluded from the study. There was an age difference between the participants of this study and those of the Atherosclerosis Risk in Communities Study (>65 years vs. 45–65 years) [15].
Age is a very significant factor of mortality. In contrast to previous studies, our study included a large proportion of older people and excluded cases of CVD. Although hs-cTnT was associated with total mortality in previous studies [14], our study did not find a significant result. An elevated hs-cTnT level is known to be associated with poor mortality in the presence of a previous history of CVD [27], which may account for differences between our study's findings and those of previous studies.
Previous reports and meta-analyses have reported an association between hemorrhagic stroke and serum troponin levels [13], [14]. However, there was no statistical association between higher hs-cTnT concentrations and incident hemorrhagic stroke in this study. Similar to our findings, it has been previously reported that cTnT levels were not associated with the occurrence of hemorrhagic stroke [15]. Cerebral aneurysms are the most common cause of subarachnoid hemorrhage [28]. Hemorrhagic stroke is also caused by cerebral amyloid angiopathy and hypertension [29]. In addition, the deposition of β-amyloid protein in the cerebral cortex and leptomeningeal vessels is a risk factor for intracerebral hemorrhage, especially in the elderly [28]. These observations suggest differences between the pathogenesis of stroke and cerebral hemorrhage.
This study had several limitations. The number of cases with hemorrhagic and ischemic strokes classified by type in this study was small and insufficient for analysis. Therefore, we plan to perform a follow-up study. This study excluded patients with CVD and heart failure, including atrial fibrillation, according to self-reported CVD or electrocardiogram; therefore, we could not perform any closed examinations for CVD diagnosis. This study also did not include participants taking antiplatelet or anticoagulant medication because we excluded participants with self-reported CVD and investigated their medical records. In addition, we could not account for competing mortality risks in models that predicted stroke.
In conclusion, higher hs-cTnT concentrations were associated with the incidence of ischemic stroke in the elderly Japanese general population.
The following is the supplementary data related to this article.
Hazard ratios (95 % confidence intervals) for ischemic stroke subtype according to hs-cTnT levels.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
I would like to thank the following individuals who were involved in this study and disclose the CRediT author statement. Takahito Nasu: Conceptualization, Methodology, Formal analysis, Writing - Review & Editing, Supervision. Mamoru Satoh: Writing - Review & Editing. Yuka Kotozaki: Data Curation. Kozo Tannno: Data Curation. Koichi Asahi: Data Curation. Hideki Ohmomo: Data Curation. Atsushi Shimizu: Data Curation. Shinichi Omama: Data Curation. Hiroto Kikuchi: Writing - Review & Editing. Satoru Taguchi: Writing - Review & Editing. Yoshihiro Morino: Writing - Review & Editing, Project administration. Kenji Sobue: Data Curation, Project administration. Makoto Sasaki: Data Curation, Project administration.
The authors would like to thank Editage (www.editage.com) for English language editing.
Funding support
This study was supported by the Reconstruction Agency, Ministry of Education, Culture, Sports, Science and Technology (MEXT), and Japan Agency for Medical Research and Development (AMED) (grant no. JP21tm0124006). Funders played no role in the study design, data collection, analysis, decision to publish, or manuscript preparation.
References
- 1.Matsui H., Fushimi K., Yasunaga H. Variation in risk-standardized mortality of stroke among hospitals in Japan. PLoS One. 2015;10 doi: 10.1371/journal.pone.0139216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caprio F.Z., Sorond F.A. Cerebrovascular disease: primary and secondary stroke prevention. Med. Clin. N. Am. 2019;103:295–308. doi: 10.1016/j.mcna.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Yatsuya H., Iso H., Yamagishi K., et al. Development of a point-based prediction model for the incidence of total stroke: Japan public health center study. Stroke. 2013;44:1295–1302. doi: 10.1161/STROKEAHA.111.677534. [DOI] [PubMed] [Google Scholar]
- 4.Lindahl B., Venge P., Wallentin L. Relation between troponin T and the risk of subsequent cardiac events in unstable coronary artery disease. The FRISC study group. Circulation. 1996;93:1651–1657. doi: 10.1161/01.cir.93.9.1651. [DOI] [PubMed] [Google Scholar]
- 5.Giannitsis E., Kurz K., Hallermayer K., et al. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin. Chem. 2010;56:254–261. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi Y., Satoh M., Ohmomo H., et al. Association between high-sensitivity cardiac troponin T and future cardiovascular incidence in a general japanese population: results from the Tohoku medical megabank project. Biomarkers. 2019;24:566–573. doi: 10.1080/1354750X.2019.1606278. [DOI] [PubMed] [Google Scholar]
- 7.Rubin J., Matsushita K., Lazo M., et al. Determinants of minimal elevation in high-sensitivity cardiac troponin T in the general population. Clin. Biochem. 2016;49:657–662. doi: 10.1016/j.clinbiochem.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willeit P., Welsh P., Evans J.D.W., et al. High-sensitivity cardiac troponin concentration and risk of first-ever cardiovascular outcomes in 154,052 participants. J. Am. Coll. Cardiol. 2017;70:558–568. doi: 10.1016/j.jacc.2017.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao W.K., Cao R.H., Liu Y., et al. Association of high-sensitivity cardiac troponin T with mortality and cardiovascular events in a community-based prospective study in Beijing. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-013431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saunders J.T., Nambi V., de Lemos J.A., et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the atherosclerosis risk in communities study. Circulation. 2011;123:1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He L., Wang J., Dong W. The clinical prognostic significance of hs-cTnT elevation in patients with acute ischemic stroke. BMC Neurol. 2018;18:118. doi: 10.1186/s12883-018-1121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terceno M., Silva Y., Bashir S., et al. Troponin T predicts cardioembolic aetiology and clinical outcome in undetermined ischaemic stroke in hyperacute phase. J. Stroke Cerebrovasc. Dis. 2020;29 doi: 10.1016/j.jstrokecerebrovasdis.2019.104528. [DOI] [PubMed] [Google Scholar]
- 13.Camen S., Palosaari T., Reinikainen J., et al. Cardiac troponin I and incident stroke in european cohorts: insights from the BiomarCaRE project. Stroke. 2020;51:2770–2777. doi: 10.1161/STROKEAHA.120.029452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broersen L.H.A., Stengl H., Nolte C., et al. Association between high-sensitivity cardiac troponin and risk of stroke in 96 702 individuals: a meta-analysis. Stroke. 2020;51:1085–1093. doi: 10.1161/STROKEAHA.119.028323. [DOI] [PubMed] [Google Scholar]
- 15.Folsom A.R., Nambi V., Bell E.J., et al. Troponin T, N-terminal pro-B-type natriuretic peptide, and incidence of stroke: the atherosclerosis risk in communities study. Stroke. 2013;44:961–967. doi: 10.1161/STROKEAHA.111.000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dadu R.T., Fornage M., Virani S.S., et al. Cardiovascular biomarkers and subclinical brain disease in the atherosclerosis risk in communities study. Stroke. 2013;44:1803–1808. doi: 10.1161/STROKEAHA.113.001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuriyama S., Yaegashi N., Nagami F., et al. The Tohoku medical megabank project: design and mission. J. Epidemiol. 2016;26:493–511. doi: 10.2188/jea.JE20150268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hozawa A., Tanno K., Nakaya N., et al. Study profile of the Tohoku medical megabank community-based cohort study. J. Epidemiol. 2021;31:65–76. doi: 10.2188/jea.JE20190271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiyohara Y., Kato I., Iwamoto H. The impact of alcohol and hypertension on stroke incidence in a general Japanese population. The Hisayama Study. Stroke. 1995;26:368–372. doi: 10.1161/01.str.26.3.368. [DOI] [PubMed] [Google Scholar]
- 20.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am. J. Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 21.Matsuo S., Imai E., Horio M., et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 22.Omama S., Yoshida Y., Ogawa A., et al. Differences in circadian variation of cerebral infarction, intracerebral haemorrhage and subarachnoid haemorrhage by situation at onset. J. Neurol. Neurosurg. Psychiatry. 2006;77:1345–1349. doi: 10.1136/jnnp.2006.090373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization MONICA Project. Section 2: Stroke event registration data component. MONICA manual, Part IV, Event registration. November 1990. http://www.thl.fi/publications/monica/manual/part4/iv-2.htm
- 24.World Health Organization International statistical classification of diseases and related health problems, 10th rev. Geneva: WHO
- 25.Cui R., Iso H., Yamagishi K., et al. Trends in the proportions of stroke subtypes and coronary heart disease in the japanese men and women from 1995 to 2009. Atherosclerosis. 2016;248:219–223. doi: 10.1016/j.atherosclerosis.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J., Zhang J., Yu P., et al. Remote ischaemic preconditioning and sevoflurane postconditioning synergistically protect rats from myocardial injury induced by ischemia and reperfusion partly via inhibition TLR4/MyD88/NF-κB signaling pathway. Cell. Physiol. Biochem. 2017;41:22–32. doi: 10.1159/000455815. [DOI] [PubMed] [Google Scholar]
- 27.Grodin J.L., Neale S., Wu Y., et al. Prognostic comparison of different sensitivity cardiac troponin assays in stable heart failure. Am. J. Med. 2015;128:276–282. doi: 10.1016/j.amjmed.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macdonald R.L., Schweizer T.A. Spontaneous subarachnoid haemorrhage. Lancet. 2017;389:655–666. doi: 10.1016/S0140-6736(16)30668-7. [DOI] [PubMed] [Google Scholar]
- 29.O'Donnell H.C., Rosand J., Knudsen K.A., et al. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. N. Engl. J. Med. 2000;342:240–245. doi: 10.1056/NEJM200001273420403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hazard ratios (95 % confidence intervals) for ischemic stroke subtype according to hs-cTnT levels.