Highlights
-
•
A DLLME/GC–MS method for analyzing α-dicarbonyl compounds in sesame oil was developed.
-
•
Chloroform and methanol was used as optimal solvents for the efficient extraction.
-
•
Derivatization with o-phenylenediamine achieved the maximum extraction efficiency.
-
•
Roasting for 30 min at 210 °C increased the methylglyoxal levels in sesame oil.
-
•
This DLLME/GC–MS approach was both sensitive and eco-friendly.
Keywords: Diacetyl, Dispersive liquid–liquid microextraction, Glyoxal, Methylglyoxal, Sesame oil
Abstract
Glyoxal, methylglyoxal, and diacetyl are toxic α-dicarbonyl compounds found in heat-processed foods, including edible oils. Dispersive liquid–liquid microextraction was combined with gas chromatography mass spectrometry to determine the glyoxal, methylglyoxal, and diacetyl contents in sesame oil. Chloroform and methanol were selected as the optimal extraction and dispersive solvents, respectively. The maximum derivatization efficiency was obtained using 500 µg of the derivatization agent, o-phenylenediamine. The derivatization of glyoxal was completed in 1 h, whereas those of methylglyoxal and diacetyl were completed immediately. The optimized method was validated, and was found to exhibit a good linearity, recovery, intraday repeatability, and interday reproducibility. The α-dicarbonyl compound concentrations in the oils were dependent on the roasting temperature. The sesame oil concentrates contained 0–175.4, 0–990.5, and 0–220.9 ng g−1 of glyoxal, methylglyoxal, and diacetyl, respectively. For the perilla oils, the respective concentrations were 0–96.4, 0–410.8, and 0–197.5 ng g−1.
1. Introduction
Glyoxal, methylglyoxal, and diacetyl (or 2,3-butanedione) are potentially hazardous α-dicarbonyl compounds that are formed by endogenous (in the body) or exogenous (in foods) reactions, such as lipid peroxidation and the Maillard reaction under high-temperature conditions (Jang et al., 2013, Jiang et al., 2013). The generation of endogenous glyoxal and methylglyoxal under diabetic conditions or their consumption from heated foods facilitates the formation of advanced glycation end products (AGEs) in blood vessels, which readily accumulate in tissues and are associated with metabolic diseases such as cardiovascular and renal diseases (Wattanapitayakul et al., 2023, Burns et al., 2006, Zieman and Kass, 2004). Diacetyl is a highly volatile compound, and the inhalation of large quantities of this compound can lead to various lung diseases (Harber et al., 2006, Kovacic and Cooksy, 2010).
Sesame (Sesamum indicum) oil is an unrefined and desirable edible oil because of its high nutrient content (Woo et al., 2019, Burns et al., 2006, Zieman and Kass, 2004). It is also commonly consumed as a flavor enhancer in many Asian cuisines. However, it has been reported that α-dicarbonyl compounds can accumulate in edible oils, such as sesame oil, during production and/or cooking at high temperatures (Jiang et al., 2013). In addition, sesame seeds are roasted at high temperatures (160–250 °C) prior to oil extraction to enhance the flavor, color, and palatability of the oil. However, this roasting process can result in the formation of α-dicarbonyl compounds (Jang et al., 2013). Teruhisa et al. first detected the formation of glyoxal, methylglyoxal, and diacetyl during lipid peroxidation using the headspace extraction method (Hirayama et al., 1984); however, since then, no additional extraction methods for the determination of α-dicarbonyl compounds in sesame oil have been established. Moreover, only a few studies have investigated the generation of α-dicarbonyl compounds during sesame oil production.
The dispersive liquid–liquid microextraction (DLLME) technique uses a combination of dispersive and extraction solvents to extract compounds from various media (Rezaee et al., 2006; Yoo et al., 2022). Use of both dispersive and extraction solvents allows the rapid dispersion of the extraction solvent in an aqueous solution even, with when only a small amount of the extraction solvent is present. As a result, the extraction time and degree of solvent usage can be reduced, while the enrichment factor can be increased (Viñas et al., 2014). To date, the DLLME approach has been applied to extract both volatiles and semi-volatiles, such as pesticides, polycyclic aromatic hydrocarbons, mycotoxins, and bioactive compounds, from various food matrices (Chormey et al., 2017, Makoś et al., 2018, Mu et al., 2017, Rodríguez-Cáceres et al., 2017). Recent studies have reported that glyoxal and/or methylglyoxal can be successfully extracted from alcoholic beverages (Rodríguez-Carrasco et al., 2017) and biological samples (Campillo et al., 2017, Pastor-Belda et al., 2017) using the DLLME approach. In a previous study, our group developed a DLLME method coupled with gas chromatography–mass spectrometry (DLLME/GC–MS) to determine the contents of toxic α-dicarbonyl compounds in red ginseng products (Lee et al., 2019).
Thus, to build on our previous study, we herein report the development of a DLLME/GC–MS method for the determination of toxic α-dicarbonyl compounds (glyoxal, methylglyoxal, and diacetyl) in sesame oil. The types and volumes of both the extraction and dispersive solvents are optimized, along with the amount of o-phenylenediamine dihydrochloride (oPD) as a derivatizing agent, and the corresponding derivatization time. Subsequently, the optimized DLLME method is validated in terms of its accuracy, linearity, intra- and interday precisions, limit of detection (LOD), and limit of quantification (LOQ).
2. Materials and methods
2.1. Reagents and samples
Glyoxal (40 % in H2O, w/v), methylglyoxal (40 % in H2O, w/v), diacetyl (97 %), 1-methylpyrazole (1-MP, 99 %), quinoxaline (Q, 99 %), 2-methylquinoxaline (2-MQ, 97 %), 2,3-dimethylquinoxaline (2,3-DMQ 97 %), sodium dihydrogen phosphate, and disodium hydrogen phosphate were purchased from Sigma-Aldrich (St. Louis, MO, USA). oPD (99 %) was purchased from Acros Organics (Geel, Belgium). HPLC-grade methanol, chloroform, and water were purchased from J.T. Baker (Phillipsburg, NJ, USA). Stock solutions (1000 mg/L) of glyoxal, methylglyoxal, diacetyl, and 1-MP (internal standard) were dissolved in HPLC-grade methanol and stored at − 20 °C prior to use. The working standard solutions were freshly prepared in sodium phosphate buffer solution (0.25 M, pH 7.6) and stored at 4 °C. The sesame and perilla oil samples were purchased from local supermarkets (Gyeonggi-do, Republic of Korea).
2.2. Sample preparation
The oil samples (0.2 g) were placed in a 15 mL glass conical centrifuge tube. Subsequently, n-hexane (5 mL), sodium phosphate buffer (5 mL, 0.25 M, pH 7.6), and 1-MP (100 μL, 10 mg/L) were added. The solution was then mixed by vigorously shaking by hand for 2 min, after which time the mixture was subjected to centrifugation for 5 min at 3000 rpm. The majority of the supernatant (organic phase) was decanted, and an aliquot (3 mL) of the sedimented phase (aqueous phase) was transferred to another glass conical tube. For derivatization of the target analytes, an aliquot (100 μL) of the oPD solution (10 g/L) was added, and the reaction was allowed to proceed for 1 h at room temperature.
2.3. DLLME procedure
The derivatized solution was used for DLLME. Initially, a mixture of methanol (1 mL) and chloroform (100 μL) was rapidly injected into the sample solution using a 1.5 mL syringe. The mixture was gently shaken by hand for a few seconds, and a cloudy solution was obtained. This mixture was then subjected to centrifugation for 3 min at 3000 rpm. The sedimented phase was withdrawn using a 50 μL micro-syringe, transferred to a 0.15 mL glass insert vial, and placed in the autosampler vial for GC–MS analysis. All experiments were performed in triplicate.
2.4. Instrumentation
GC–MS analysis was performed using a 7890A gas chromatograph/5975C mass-selective detector (Agilent Technologies, Palo Alto, CA, USA). The gas chromatograph was equipped with a 30 m DB-WAX column (0.25 mm i.d., 0.25 μm thickness; Agilent Technologies, Palo Alto, CA, USA). The temperature of the injection port was maintained at 250 °C and the sample (1 μL) was introduced in splitless mode at a flow rate of 1.0 mL min−1. Helium was used as the carrier gas. The purge flow was 50 mL min−1, and the purge time was 0.75 min. The oven temperature was held at 50 °C for 2 min, increased to 220 °C at a rate of 10 °C min−1, and then held for 15 min at 220 °C. The temperatures of the transfer line, ion source, and quadrupole were maintained at 250, 230, and 150 °C, respectively. The mass spectra were obtained by electron ionization at 70 eV. The selected ion monitoring (SIM) mode was used for confirmation and quantification. For SIM, three ions were monitored for each compound, i.e., m/z 76, 103, and 130 for Q; m/z 76, 117, and 144 for 2-MQ; m/z 76, 117, and 158 for 2,3-DMQ; and m/z 54, 81, and 82 for 1-MP.
2.5. Extraction of the sesame and perilla oils
To extract the sesame and perilla oils, the raw seeds (200 g) were either unheated or heated in 250 mL swing-top bottles at 100, 150, or 210 °C for 15 or 30 min, and pressed using an oil presser (Cat. No. HD-333, GGAEBAKSA, Gyeongsangnam-do, Republic of Korea). The obtained oils were stored at 4 °C until required for analysis.
2.6. Statistical processing
SigmaPlot v12.5 (Systat Software, Rockville, USA) was used for statistical data processing. The data were analyzed using analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. Values with P values < 0.05 were considered significant.
3. Results and discussion
3.1. Selection of the extraction and dispersive solvents
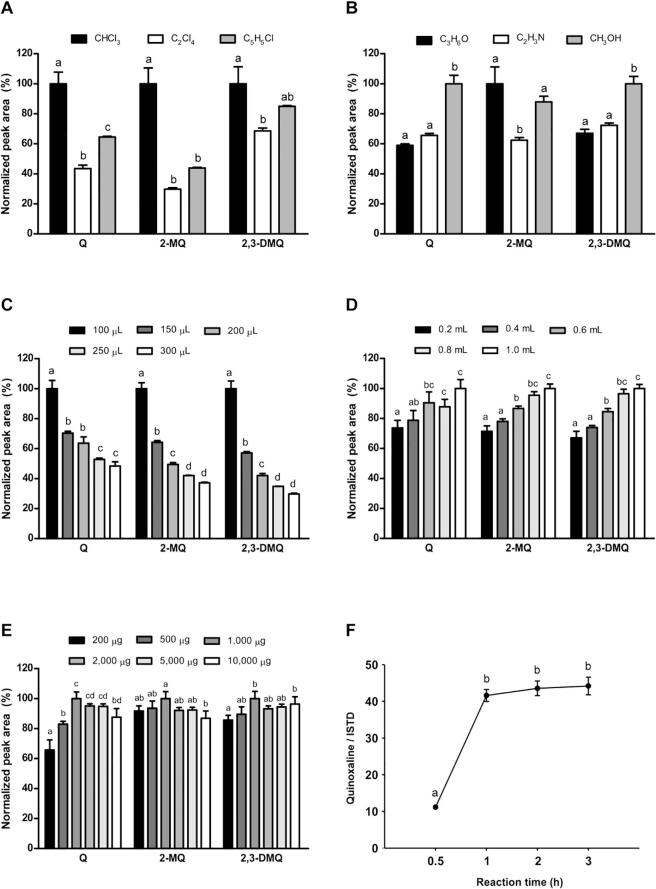
The types of extraction and dispersive solvents employed are known to have a significant impact on the analyte extraction efficiency in the DLLME method (Yan & Wang, 2013). To maximize the extraction efficiency, three extraction solvents (i.e., chloroform (CHCl3), tetrachloroethylene (C2Cl4), and chlorobenzene (C6H5Cl)) and three dispersive solvents (i.e., acetone (C3H6O), acetonitrile (C2H3N), and methanol (CH3OH)) were evaluated. When chloroform was used as the extraction solvent, the highest extraction efficiencies were obtained for glyoxal, methylglyoxal, and diacetyl (P < 0.05) (Fig. 1A), and when methanol was used as the dispersive solvent, significantly higher extraction efficiencies were obtained for these three compounds (P < 0.05) (Fig. 1B). These results suggest that chloroform and methanol are the optimal extraction and dispersive solvents, respectively, for the efficient extraction of glyoxal, methylglyoxal, and diacetyl from sesame oil.
Fig. 1.
Optimization of the extraction and derivatization conditions. (A, B) Optimization of the extraction and dispersive solvents. (A) Selection of the extraction solvent (aqueous phase vol. = 3 mL; compound concentration = 250 ng mL−1; o-phenylenediamine dihydrochloride (oPD) = 0.5 mg; derivatization time = 2 h; dispersive solvent = methanol; dispersive solvent vol. = 0.2 mL; buffer = 0.5 M sodium phosphate buffer at pH 7.6). (B) Selection of the dispersive solvent (aqueous phase vol. = 3 mL; compound concentration = 250 ng mL−1; oPD = 0.5 mg; derivatization time = 2 h; extraction solvent = chloroform; extraction solvent volume = 100 µL; buffer = 0.5 M sodium phosphate buffer at pH 7.6). Data are shown as the mean ± standard deviation (n = 3). (C, D) Optimization of the extraction and dispersive solvent volumes. (C) Optimization of the extraction solvent volume (extraction conditions: aqueous phase vol. = 3 mL; compound concentration = 250 ng mL−1; oPD = 0.5 mg; derivatization time = 2 h; dispersive solvent = methanol; dispersive solvent vol. = 0.2 mL; extraction solvent = chloroform; buffer = 0.5 M sodium phosphate buffer at pH 7.6). (D) Optimization of the dispersive solvent volume (extraction conditions: aqueous phase vol. = 3 mL; compound concentration = 250 ng mL−1; oPD = 0.5 mg; derivatization time = 2 h; dispersive solvent = methanol; extraction solvent = chloroform; extraction solvent vol. = 100 µL; buffer = 0.5 M sodium phosphate buffer at pH 7.6). Data are shown as the mean ± standard deviation (n = 3). (E, F) Optimization of the derivatization parameters. (E) Optimization of the amount of derivatizing agent (aqueous phase = 3 mL; compound concentration = 250 ng mL−1; oPD = 0.5 mg; derivatization time = 2 h; extraction solvent = chloroform; extraction solvent volume = 100 µL; buffer = 0.5 M sodium phosphate buffer at pH 7.6). (F) Optimization of the derivatization time. Data are shown as the mean ± standard deviation (n = 3). Q, quinoxaline; 2-MQ, 2-methylquinoxaline; 2,3-DMQ, 2,3-dimethylquinoxaline.
3.2. Optimization of the extraction and dispersive solvent volumes
The volumes of the extraction and dispersive solvents were optimized to maximize the extraction efficiencies of glyoxal, methylglyoxal, and diacetyl. Five volumes of chloroform were tested (100, 150, 200, 250, and 300 µL), and as shown in Fig. 1C, the peak areas corresponding to the α-dicarbonyl compounds were significantly higher when 100 µL of chloroform was used. Subsequently, six volumes of methanol were evaluated (0.2, 0.4, 0.6, 0.8, and 1.0 mL). The peak areas for glyoxal, methylglyoxal, and diacetyl increased in a volume-dependent manner and reached their highest values when 1.0 mL of methanol was used (Fig. 1D). Hence, 100 µL of chloroform and 1.0 mL of methanol were considered to be the optimal volumes for α-dicarbonyl extraction from sesame oil.
3.3. Optimization of the derivatization parameters
The α-dicarbonyl compounds examined herein are highly reactive and can adsorb on the GC column and/or be lost in the injector, thereby leading to a poor analytical performance (Pizarro et al., 2010). Thus, to address these issues, a derivatization step is required prior to analysis by GC. The derivatization conditions (i.e., the amount of the oPD derivatizing agent and the derivatization time) were optimized for the current system to maximize the derivatization efficiency. Five amounts of oPD (200, 500, 1000, 2000, 5000, and 10000 µg) were evaluated, and it was found that when 500 µg of oPD was used, the extraction efficiencies for quinoxaline, 2-methylquinoxaline, and 2,3-dimethylquinoxaline (the derivatives of glyoxal, methylglyoxal, and diacetyl, respectively) reached their highest values (Fig. 1E). In terms of the derivatization time, the glyoxal peak area increased until a time of 1 h, indicating that derivatization was complete at this point (Fig. 1F). In contrast, methylglyoxal and diacetyl were immediately derivatized to 2-methylquinoxaline and 2,3-dimethylquinoxaline (data not shown), and so no further investigation into the derivatization time was required. Hence, a 1 h derivatization time combined with 500 µg of the derivatizing agent were considered to be the optimal derivatization conditions for the extraction of α-dicarbonyl compounds from sesame oil. It should be noted here that in our previous study, the optimal DLLME conditions for determining the α-dicarbonyl compound compounds in red ginseng products were based on the use of chloroform (100 μL) as the extraction solvent, methanol (200 μL) as the dispersive solvent, 0.5 g of the derivatizing agent, and a 1 h derivatization time (Lee et al., 2019). These two optimized methods differed in the volume of the dispersion solvent and the amount of derivatization reagent employed, thereby indicating that the optimal conditions for analyzing α-dicarbonyl compounds using the DLLME method depend on the sample matrix.
3.4. Method validation
The optimized DLLME method was validated in terms of accuracy, linearity, intra- and interday precisions, LOD, and LOQ (Jang et al., 2022). Recovery tests were performed using pooled sesame oil samples (n = 5) at spiked concentrations of 250, 500, and 1000 ng g−1. The relative recoveries were 115–116.1, 98–116.9, and 95.6–124.5 % for glyoxal, methylglyoxal, and diacetyl, respectively (Table 1). The method linearities for the three compounds were tested at concentrations of 0.2–100 ng mL−1, and a high coefficient of determination (R2 > 0.998) was obtained. The intra- and interday precisions were evaluated by measuring the relative standard deviations for three replications carried out over a single day or over 3 d, respectively, and were found to be < 9.6 % and < 9.8 %, respectively. The LOD and LOQ were calculated as the concentrations corresponding to signal-to-noise ratios (S/N) of 3 and 10, respectively. Thus, using the pooled sesame oil sample solution, the LOD and LOQ were determined to be 0.22–0.86 and 0.66–2.60 ng mL−1, respectively (Table 2). In our previous study, determination of the glyoxal, methylglyoxal, and diacetyl contents in red ginseng products gave LOD and LOQ values of 1.30 and 4.33 μg/L for glyoxal, 1.86 and 6.20 μg/L for methylglyoxal, and 1.45 and 4.82 μg/L for diacetyl. Moreover, the relative recoveries ranged from 92.4 to 110.7 % and the relative standard deviations were < 4.95 % and 5.80 % for the intra- and interday analyses, respectively. To minimize the matrix effect during our previous analysis, the red ginseng concentrate sample was diluted 20 times with water, resulting in good performance (Y. Y. Lee et al., 2019). In the current study, the sesame oil specimen (0.2 g) was diluted in a mixture of n-hexane and 0.25 M sodium phosphate buffer (pH 7.6) in a 1:1 (v/v) ratio (10 mL total volume), which effectively reduced the matrix effect.
Table 1.
Relative recoveries of the optimized DLLME method.
| Compound | Content (ng g−1) | 250 ng g−1 spiked |
500 ng g−1 spiked |
1000 ng g−1 spiked |
|||
|---|---|---|---|---|---|---|---|
| Found (ng g−1) | Relative recovery (%) | Found (ng g−1) | Relative recovery (%) | Found (ng g−1) | Relative recovery (%) | ||
| Glyoxal | 143.7 ± 5.7 | 431.9 ± 17.3 | 115 ± 6.9 | 724.2 ± 29.0 | 116.1 ± 5.8 | 1154.1 ± 51.9 | 115.4 ± 5.2 |
| Methylglyoxal | 544.4 ± 21.8 | 789.4 ± 31.6 | 98.0 ± 12.6 | 1129.2 ± 45.2 | 116.9 ± 9.0 | 1159.3 ± 68.1 | 115.9 ± 6.8 |
| Diacetyl | 155.1 ± 6.2 | 394.0 ± 15.8 | 95.6 ± 6,3 | 777.6 ± 31.1 | 124.5 ± 6.2 | 1196.3 ± 54.1 | 119.6 ± 5.4 |
*Recovery tests were performed in triplicate at each concentration.
Table 2.
Linear range, precision, LOD, and LOQ of the optimized DLLME method.
| Compound | Linear range (ng mL−1) |
R2 | Slope | Y-intercept | Precision (20 ng mL−1, RSD %) |
LOD (ng mL−1) |
LOQ (ng mL−1) |
|
|---|---|---|---|---|---|---|---|---|
| Intraday (n = 3) | Interday (n = 3) | |||||||
| Glyoxal | 0.2–100 | 0.9988 | 0.0705 ± 0.0013 | 0.0722 ± 0.0091 | 0.5 | 9.8 | 0.43 | 1.30 |
| Methylglyoxal | 0.2–100 | 0.9993 | 0.0738 ± 0.0006 | 0.0763 ± 0.0049 | 0.9 | 6.7 | 0.22 | 0.66 |
| Diacetyl | 0.2–100 | 0.9995 | 0.0776 ± 0.0034 | 0.1086 ± 0.0202 | 9.6 | 1.3 | 0.86 | 2.60 |
3.5. Application of the optimized DLLME method to sesame and perilla oil samples
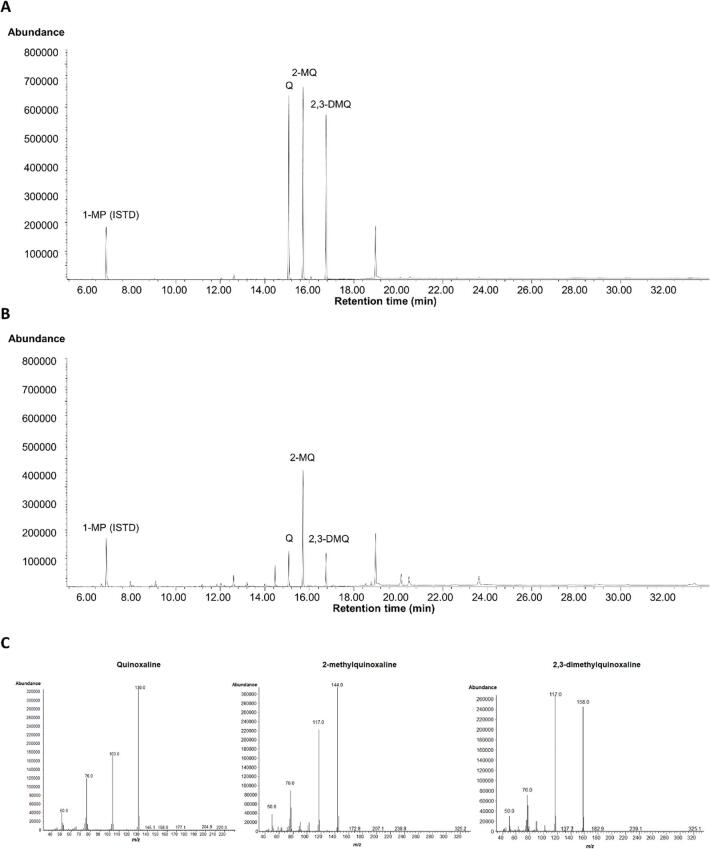
The validated DLLME/GC–MS method was used to determine the contents of toxic α-dicarbonyl compounds in sesame and perilla oil samples. As a result, the glyoxal, methylglyoxal, and diacetyl contents were determined to be 0–175.4, 0–990.5, and 0–220.9 ng g−1, respectively, in the sesame oil samples, and 0–96.4, 0–410.8, and 0–197.5 ng g−1, respectively, in the perilla oil samples. Notably, no α-dicarbonyl compounds were detected in unroasted raw sesame oil (sample #8) or raw perilla oil (sample #3) specimens (Table 3). Representative chromatograms and mass spectra for the α-dicarbonyl compounds detected in the sesame oil sample and in the standard solution are shown in Fig. 2.
Table 3.
Concentrations of the glyoxal, methylglyoxal, and diacetyl compounds in sesame and perilla oils.
| Sample No. | Concentration (ng g−1) |
|||||
|---|---|---|---|---|---|---|
| Sesame oil |
Perilla oil |
|||||
| Glyoxal | Methylglyoxal | Diacetyl | Glyoxal | Methylglyoxal | Diacetyl | |
| 1 | 26.2 ± 0.8a | 113.6 ± 6.4 | 35.5 ± 1.5 | 96.4 ± 4.5 | 153.8 ± 5.6 | 46.8 ± 1.4 |
| 2 | 175.4 ± 2.0 | 774.1 ± 6.1 | 196.4 ± 9.1 | 50.1 ± 2.1 | 251.9 ± 16.3 | 197.5 ± 15.0 |
| 3 | 141.4 ± 6.2 | 412.5 ± 18.1 | n.q.c | n.d. | n.d. | n.d. |
| 4 | 96.4 ± 4.2 | 598.5 ± 36.2 | 204.1 ± 0.6 | 73.6 ± 3.2 | 410.8 ± 8.5 | 172.6 ± 9.1 |
| 5 | 128.7 ± 3.3 | 843.2 ± 19.4 | 220.9 ± 2.4 | 14.7 ± 0.4 | 130.6 ± 2.4 | 154.8 ± 2.2 |
| 6 | 89.0 ± 6.8 | 432.2 ± 24.0 | 165.6 ± 8.3 | n.d. | 57.2 ± 2.8 | n.q. |
| 7 | 41.1 ± 2.0 | 263.2 ± 6.0 | 77.8 ± 1.7 | n.d. | 37.3 ± 1.7 | 38.9 ± 1.6 |
| 8 | n.d.b | n.d. | n.d. | n.d. | 92.2 ± 1.8 | 41.9 ± 0.9 |
| 9 | 109.4 ± 3.4 | 990.5 ± 32.1 | 215.0 ± 5.0 | n.d. | 58.4 ± 4.4 | 74.8 ± 2.0 |
| 10 | 92.5 ± 5.1 | 968.4 ± 35.9 | 182.0 ± 6.7 | 25.0 ± 1.5 | 248.3 ± 11.1 | 112.2 ± 7.0 |
a Data represent mean ± standard deviation (n = 3); b n.d. = Not detected; c n.q. = Not quantified. Sesame oil #8 and perilla oil #3 were raw oils.
Fig. 2.
Representative chromatograms obtained in the selected ion monitoring (SIM) mode. (A) SIM chromatogram of the standard glyoxal, methylglyoxal, and diacetyl solutions (20 ng mL−1 in each case). (B) SIM chromatogram of the sample (sesame oil No. 1). Q, quinoxaline; 2-MQ, 2-methylquinoxaline; 2,3-DMQ, 2,3-dimethylquinoxaline; 1-MP, 1-methylpyrazine (internal standard, ISTD). (C) Mass spectra of Q, 2-MQ, and 2,3-DMQ in the sample (sesame oil No.1) obtained in the SIM mode.
3.6. Effects of the roasting temperature and time on the generation of α-dicarbonyl compounds in sesame and perilla seed oils
To evaluate the effects of the roasting conditions on the formation of glyoxal, methylglyoxal, and diacetyl, in the sesame and perilla oils, the corresponding seeds were roasted at different temperatures (100, 150, or 210 °C) for 15 or 30 min. In the case of the sesame oil, the roasting conditions did not significantly affect the glyoxal and diacetyl contents, while that of methylglyoxal was significantly increased following roasting at 210 °C for 30 min (c.f., the non-roasted sample; P < 0.05). For the perilla oil specimen, the glyoxal content was unaffected by the roasting conditions, while the contents of methylglyoxal and diacetyl significantly increased when the seeds were roasted at 210 °C for 30 min (P < 0.05) (Fig. 3). These results suggest that relatively high roasting temperatures (210 °C) and long roasting times (30 min) promote the formation of methylglyoxal and diacetyl in sesame and perilla oils.
Fig. 3.

Effects of the roasting temperature and time on the generation of α-dicarbonyl compounds in the oils of sesame and perilla seeds. (A) Effect of the roasting temperature and time on the α-dicarbonyl compound contents in the sesame oil. (B) Effect of the roasting temperature and time on the α-dicarbonyl compound contents in the perilla oils. Data are shown as the mean ± standard deviation (n = 3).
Recent studies have investigated the formation and presence of α-dicarbonyl compounds in various foods, particularly focusing on coffee beans and soy sauce. For example, one study investigated the effects of organic acid-soaking and sonication on Robusta coffee beans, highlighting a significant decrease in the α-dicarbonyl compound contents after organic acid pretreatment (Lee et al., 2023, Lee et al., 2023). Additionally, optimal roasting conditions have been identified to maximize flavor compound production while minimizing α-dicarbonyl generation (Lee et al., 2023, Lee et al., 2023). Furthermore, another study focused on analyzing the α-dicarbonyl compounds present in soy sauce, and provided an analytical method for their determination and quantification (Kim et al., 2022). It was proposed that this method could potentially aid in the reduction of α-dicarbonyl compound levels in soy sauce, thereby improving its quality. Moreover, several other studies have explored the correlations between roasting and brewing methods and the levels of α-dicarbonyl compounds in coffee, emphasizing the impact of various parameters, such as the temperature, time, and coffee bean particle size (Hyong et al., 2021, Kwon et al., 2021). These findings underscore the importance of understanding and controlling α-dicarbonyl formation during food processing to enhance the flavor of the final product and to minimize potential health risks.
4. Conclusion
A dispersive liquid–liquid microextraction (DLLME) approach combined with gas chromatography mass spectrometry (GC–MS) was developed for the quantification of toxic α-dicarbonyl compounds (i.e., glyoxal, methylglyoxal, and diacetyl) in sesame oil. The extraction and dispersive solvent volumes for the DLLME process were optimized to maximize the extraction efficiency, and the conditions for derivatization were also optimized. Subsequently, the optimized DLLME method was validated in terms of its linearity, accuracy, precision, and sensitivity using GC–MS. Real samples of sesame and perilla oils were successfully quantified. Overall, the obtained results suggest that the developed DLLME/GC–MS method is a sensitive and ecofriendly approach for the determination of glyoxal, methylglyoxal, and diacetyl in sesame oil.
CRediT authorship contribution statement
Jangho Lee: Writing – original draft, Methodology, Investigation, Data curation. Tae Gyu Nam: Methodology, Investigation. Hyo-Kyoung Choi: Investigation, Data curation. Hae Won Jang: Writing – review & editing, Supervision, Resources, Project administration, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. NRF Project RS-2023-00213840). This research was by the Main Research Program (Project No.: E0210400) of the Korea Food Research Institute funded by the Ministry of Science and ICT and by the National Research Foundation.
Contributor Information
Jangho Lee, Email: jhlee@kfri.re.kr.
Tae Gyu Nam, Email: tgzoo0706@kyonggi.ac.kr.
Hyo-Kyoung Choi, Email: chkyoung@kfri.re.kr.
Hae Won Jang, Email: hwjang@sungshin.ac.kr.
Data availability
No data was used for the research described in the article.
References
- Burns W.C., Twigg S.M., Forbes J.M., Pete J., Tikellis C., Thallas-Bonke V., Thomas M.C., Cooper M.E., Kantharidis P. Connective tissue growth factor plays an important role in advanced glycation end product–induced tubular epithelial-to-mesenchymal transition: Implications for diabetic renal disease. Journal of the American Society of Nephrology. 2006;17(9):2484–2494. doi: 10.1681/ASN.2006050525. [DOI] [PubMed] [Google Scholar]
- Campillo N., Viñas P., Marín J., Hernández-Córdoba M. Glyoxal and methylglyoxal determination in urine by surfactant-assisted dispersive liquid–liquid microextraction and LC. Bioanalysis. 2017;9(4):369–379. doi: 10.4155/bio-2016-0217. [DOI] [PubMed] [Google Scholar]
- Chormey D.S., Karakuş Y., Karayaka S., Özsöyler Ç., Bozdoğan A.E., Bakırdere S. Multivariate optimization of dispersive liquid–liquid microextraction for the determination of paclobutrazol and triflumizole in water by GC–MS. Journal of Separation Science. 2017;40(23):4541–4548. doi: 10.1002/jssc.201700853. [DOI] [PubMed] [Google Scholar]
- Harber P., Saechao K., Boomus C. Diacetyl-induced lung disease. Toxicological Reviews. 2006;25(4):261–272. doi: 10.2165/00139709-200625040-00006. [DOI] [PubMed] [Google Scholar]
- Hirayama T., Yamada N., Nohara M., Fukui S. The existence of the 1, 2-dicarbonyl compounds glyoxal, methyl glyoxal and diacetyl in autoxidised edible oils. Journal of the Science of Food and Agriculture. 1984;35(12):1357–1362. doi: 10.1002/jsfa.2740351213. [DOI] [Google Scholar]
- Hyong S., Chu M., Park H., Park J., Lee K.G. Analysis of α-dicarbonyl compounds and 4-methylimidazole in coffee made with various roasting and brewing conditions. LWT - Food Science and Technology. 2021;151(7) doi: 10.1016/j.lwt.2021.112231. [DOI] [Google Scholar]
- Jang H.W., Jiang Y., Hengel M., Shibamoto T. Formation of 4 (5)-methylimidazole and its precursors, α-dicarbonyl compounds, in Maillard model systems. Journal of Agricultural and Food Chemistry. 2013;61(28):6865–6872. doi: 10.1021/jf401958w. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Hengel M., Pan C., Seiber J.N., Shibamoto T. Determination of toxic α-dicarbonyl compounds, glyoxal, methylglyoxal, and diacetyl, released to the headspace of lipid commodities upon heat treatment. Journal of Agricultural and Food Chemistry. 2013;61(5):1067–1071. doi: 10.1021/jf3047303. [DOI] [PubMed] [Google Scholar]
- Kim T.E., Yoo G.J., Lee M.H., Kim B.G., Jang H.W. Novel QuEChERS-ultra-performance liquid chromatography–atmospheric-pressure chemical ionization tandem mass spectrometry method for the simultaneous determination of vitamin D and vitamin K in vitamin-fortified nanoemulsions. Food Chemistry. 2022;389 doi: 10.1016/j.foodchem.2022.133009. [DOI] [PubMed] [Google Scholar]
- Kovacic P., Cooksy A.L. Electron transfer as a potential cause of diacetyl toxicity in popcorn lung disease. Reviews of Environmental Contamination and Toxicology. 2010;204:133–148. doi: 10.1007/978-1-4419-1440-8_2. [DOI] [PubMed] [Google Scholar]
- Kwon J., Ahn H., Lee K.G. Analysis of alpha-dicarbonyl compounds in coffee (Coffea arabica) prepared under various roasting and brewing methods. Food Chemistry. 2021;343 doi: 10.1016/j.foodchem.2020.128525. [DOI] [PubMed] [Google Scholar]
- Lee G., Lee M., Ahn J., Kim Y., Lee K.G. Correlation analysis between volatile compounds and α-dicarbonyl compounds in various beans in response to different roasting conditions. LWT - Food Science and Technology. 2023;177 doi: 10.1016/j.lwt.2023.114544. [DOI] [Google Scholar]
- Lee H., Yim J., Lee Y., Lee K.G. Effect of organic acid-soaking and sonication on the formation of volatile compounds and alpha-dicarbonyl compounds in robusta coffee. Ultrasonic Sonochemistry. 2023;99 doi: 10.1016/j.ultsonch.2023.106580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.Y., Shibamoto T., Ha S.D., Ha J., Lee J., Jang H.W. Determination of glyoxal, methylglyoxal, and diacetyl in red ginseng products using dispersive liquid–liquid microextraction coupled with GC–MS. Journal of Separation Science. 2019;42(6):1230–1239. doi: 10.1002/jssc.201800841. [DOI] [PubMed] [Google Scholar]
- Makoś P., Fernandes A., Boczkaj G. Method for the simultaneous determination of monoaromatic and polycyclic aromatic hydrocarbons in industrial effluents using dispersive liquid–liquid microextraction with gas chromatography–mass spectrometry. Journal of Separation Science. 2018;41(11):2360–2367. doi: 10.1002/jssc.201701464. [DOI] [PubMed] [Google Scholar]
- Mu J., Gao X., Li Q., Yang X., Yang W., Sun X., Bi K., Zhang H. Vortex-ultrasound-assisted dispersive liquid–liquid microextraction coupled with gas chromatography–mass spectrometry for the analysis of volatile bioactive components and comparative pharmacokinetic study of the herb–herb interactions in guanxin shutong capsule. Journal of Separation Science. 2017;40(16):3267–3278. doi: 10.1002/jssc.201700500. [DOI] [PubMed] [Google Scholar]
- Pastor-Belda M., Fernández-García A.J., Campillo N., Pérez-Cárceles M.D., Motas M., Hernández-Córdoba M., Viñas P. Glyoxal and methylglyoxal as urinary markers of diabetes. determination using a dispersive liquid–liquid microextraction procedure combined with gas chromatography–mass spectrometry. Journal of Chromatography. A. 2017;1509:43–49. doi: 10.1016/j.chroma.2017.06.041. [DOI] [PubMed] [Google Scholar]
- Pizarro C., Sáenz-González C., Perez-Del-Notario N., González-Sáiz J.M. Optimisation of a dispersive liquid–liquid microextraction method for the simultaneous determination of halophenols and haloanisoles in wines. Journal of Chromatography. A. 2010;1217(49):7630–7637. doi: 10.1016/j.chroma.2010.10.032. [DOI] [PubMed] [Google Scholar]
- Rezaee M., Assadi Y., Milani Hosseini M.R., Aghaee E., Ahmadi F., Berijani S. Determination of organic compounds in water using dispersive liquid–liquid microextraction. Journal of Chromatography. A. 2006;1116(1–2):1–9. doi: 10.1016/j.chroma.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Cáceres M.I., Palomino-Vasco M., Mora-Diez N., Acedo-Valenzuela M.I. Dispersive liquid–liquid microextraction for a rapid determination of glyoxal in alcoholic beverages. Talanta. 2017;168:100–104. doi: 10.1016/j.talanta.2017.03.031. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Carrasco Y., Moltó J.C., Mañes J., Berrada H. Development of microextraction techniques in combination with GC–MS/MS for the determination of mycotoxins and metabolites in human urine. Journal of Separation Science. 2017;40(7):1572–1582. doi: 10.1002/jssc.201601131. [DOI] [PubMed] [Google Scholar]
- Viñas P., Campillo N., López-García I., Hernández-Córdoba M. Dispersive liquid–liquid microextraction in food analysis. a critical review. Analytical and Bioanalytical Chemistry. 2014;406(8):2067–2099. doi: 10.1007/s00216-013-7344-9. [DOI] [PubMed] [Google Scholar]
- Wattanapitayakul S.K., Jarisarapurin W., Kunchana K., Setthawong V., Chularojmontri L. Unripe Carica papaya fresh fruit Extract protects against methylglyoxal-mediated aging in human dermal skin fibroblasts. Preventive Nutrition and Food Science. 2023;28(3):235. doi: 10.3746/pnf.2023.28.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo M.J., Han S.K., Song Y.O. Sesame oil attenuates renal oxidative stress induced by a high fat diet. Preventive nutrition and food science. 2019;24(2):114. doi: 10.3746/pnf.2019.24.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H., Wang H. Recent development and applications of dispersive liquid–liquid microextraction. Journal of Chromatography. A. 2013;1295:1–15. doi: 10.1016/j.chroma.2013.04.053. [DOI] [PubMed] [Google Scholar]
- Yoo G.J., Kim T.E., Lee M.H., Kim B.G., Jang H.W. Determination of vitamin K encapsulated into lipid nanocarriers by dispersive liquid–liquid microextraction combined with liquid chromatography–atmospheric pressure chemical ionization–tandem mass spectrometry. Food Science and Nutrition. 2022;11:688–695. doi: 10.1002/fsn3.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieman S.J., Kass D.A. Advanced glycation end product cross-linking: Pathophysiologic role and therapeutic target in cardiovascular disease. Congestive Heart Failure. 2004;10(3):144–149. doi: 10.1111/j.1527-5299.2004.03223.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.