ABSTRACT
Background
Benign migratory glossitis or geographic tongue is a chronic recurring inflammatory condition of the oral cavity. With its ephemeral characteristics, there has been reported literature showing its association with the administration of certain drugs including angiogenesis inhibitors. The antiangiogenic drugs act by selectively inhibiting the vascular endothelial growth factor (VEGF) signaling. It has been widely used as an adjunct and a maintenance agent for the treatment of various cancers.
Aims
This study aims to report probable characteristic oral mucosal changes in a patient with juvenile-onset recurrent respiratory papillomatosis (JORRP) under maintenance therapy with an antiangiogenesis drug.
Case description
The patient was presented with a burning sensation on having spicy food. This occurred after the completion of three cycles of bevacizumab infusion. It was associated with the appearance of migratory lesions over the tongue and evolved periods of remission and exacerbation. Clinical examination revealed lesions characteristic of the geographic tongue on the anterior two-thirds of the dorsal surface as well as the lateral surface of the tongue classified as type 2, according to Hume criteria. Oral examination revealed dental caries in relation to 52, 54, 62, 63, 74, and 85 teeth and grossly decayed 64. Topical lignocaine gel was instituted for symptomatic relief of the lesion. Full mouth rehabilitation with preventive and restorative therapeutic interventions was carried out.
Clinical significance and conclusion
The documented literature along with this report put forth a probable association of geographic tongue with the use of bevacizumab drugs which requires further detailed studies. These lesions generally require symptomatic treatment with assurance only. The etiology is poorly understood.
How to cite this article
Kalra N, Tyagi R, Khatri A, et al. Angiogenesis Inhibitor Drug-induced Benign Migratory Glossitis in a Patient of Juvenile-onset Recurrent Respiratory Papillomatosis under Maintenance Therapy. Int J Clin Pediatr Dent 2024;17(1):92–96.
Keywords: Angiogenesis inhibitor, Benign migratory glossitis, Case report, Juvenile-onset recurrent respiratory papillomatosis, Oral mucosal
Introduction
Geographic tongue is a benign ephemeral recurring oral condition affecting the tongue. It holds various synonyms like benign migratory glossitis, erythema migrans, annulus migrans, and the wandering rash of the tongue. The prevalence of geographic tongue ranges from 1 to 2.5% of the population.1 The etiology of the lesions remains largely unknown though they have an association with multiple systemic diseases, hereditary influences, stress, and occasionally with certain drugs.1,2
Angiogenesis inhibitors are recombinant monoclonal immunoglobulin G1 (IgG1) antibody that affects the cancer cell protein called vascular endothelial growth factor (VEGF). It has been validated for the treatment of multiple solid tumors in advanced and metastatic stages.3,4 The use of these targeted therapies controls the growth and multiplication of malignant cells by limiting the microvascular growth of blood vessels that leads to desirable apoptosis of neoplastic cells.5,6 While its beneficial impact on these tumor cells, these VEGF receptors have been expressed at normal sites that may account for their adverse events. The most common side effects include hypertension, proteinuria, thromboembolic events, cardiomyopathy, hemorrhage, wound complications, gastrointestinal perforation, and reversible posterior leukoencephalopathy syndrome.6 Mucocutaneous toxicities, however, are significantly less commonly reported. However, oral stomatitis with aphthae, gingival inflammation, and pain have been observed in patients receiving bevacizumab in combination with certain antineoplastic drugs like 5-fluorouracil plus leucovorin, and capecitabine.7,8 Martinez-Garcia et al.9 and Sundar and Burge10 have recently described the association of geographic tongue with angiogenesis inhibitor drugs with evidence. Antiangiogenesis drugs have been employed as an adjuvant agent for the treatment of juvenile-onset recurrent respiratory papillomatosis (JORRP).11
Juvenile-onset recurrent respiratory papillomatosis (JORRP) is a rare potentially life-threatening benign neoplasm characterized by multiple papillomatous excrescences in the respiratory tract with the most common laryngeal presentation described as laryngeal papillomatosis. Although the aggressive form of the condition is often caused by the human papillomavirus (HPV) 11 followed by HPV 6.12 The management of JORRP includes a combination therapy with surgical treatment augmented by pharmacological therapy.
This case report would provide a probable characteristic oral mucosal effect of angiogenesis inhibitor for the maintenance phase in a patient with JORRP. The probability of the drug causing oral mucosal adverse effects has been calculated by the Naranjo adverse drug reaction probability scale13 and reported as 7 on a scale of 10.
Case Description
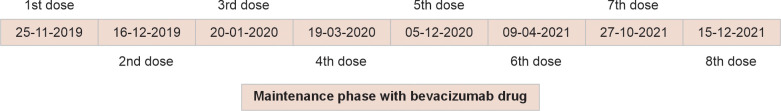
A 5-year-old girl child was diagnosed with JORRP (histological was referred to our Department of Pedodontics and Preventive Dentistry due to throbbing pain and swelling over the lower left back tooth region with the recently developed burning sensation of the tongue on spicy food). The history of the patient revealed that JORRP was diagnosed in March 2018 and has been receiving treatment from the otorhinolaryngology department since she was 2 years old. The patient had undergone laser ablation with carbon dioxide laser (4 watts in continuous mode) (Fig. 1) several times and bevacizumab infusion (100 mg/mL) which was commenced after 1 year of the treatment, resulting in complete remission. The patient was tracheostomized with an uncuffed tube (size 4) and put on permanent tracheostomy. Subsequently, the patient was under maintenance therapy with bevacizumab (100 mg/mL) every 21 days and had eight cycles completed. The graphic timeline showing the treatment cycle evolution has been depicted (Fig. 2).
Fig. 1.
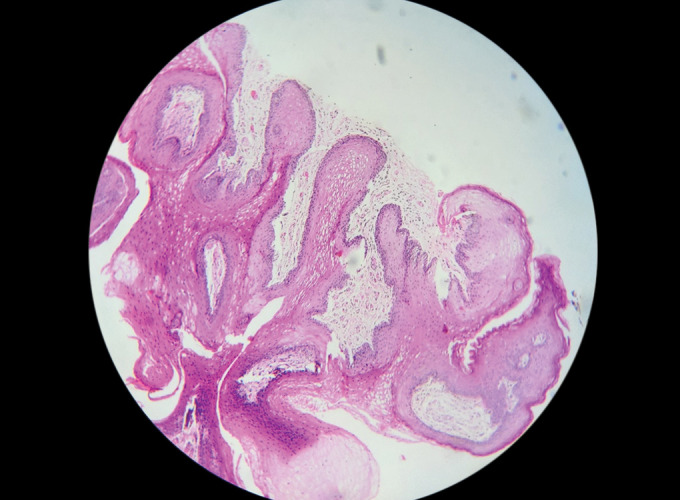
Cross-sectional view of the pathological tissue excised from the larynx observes the multiple branching mucosal projections made up of hyperplastic squamous epithelium overlying thin fibrovascular cores (with hematoxylin and eosin, 10×)
Fig. 2.
Graphical timeline representation showing treatment cycle evolution
The patient was presented with a burning sensation with spicy food after the completion of three cycles of bevacizumab infusion. She had also complained of white lesions on the tongue. These lesions appeared after the third dose but became mild within 3 weeks at the end of the 3rd week when the drug was readministered the lesions reappeared in full form (as at the end of 3rd week). There was also a repeat history of these lesions settling down after 3 weeks when it was time for the next dose (time for the sixth dose). For the lesions, the pattern of waxing and waning continued. The burning sensation was a continued feature that the parents managed with a bland diet.
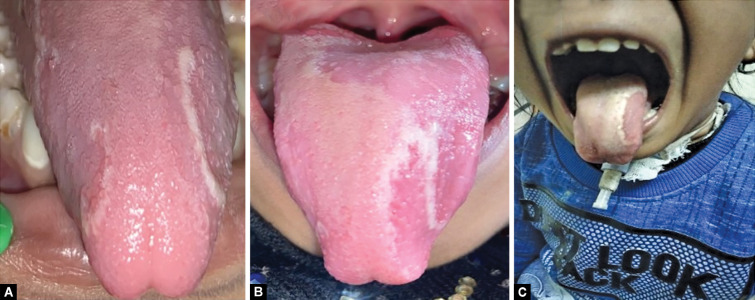
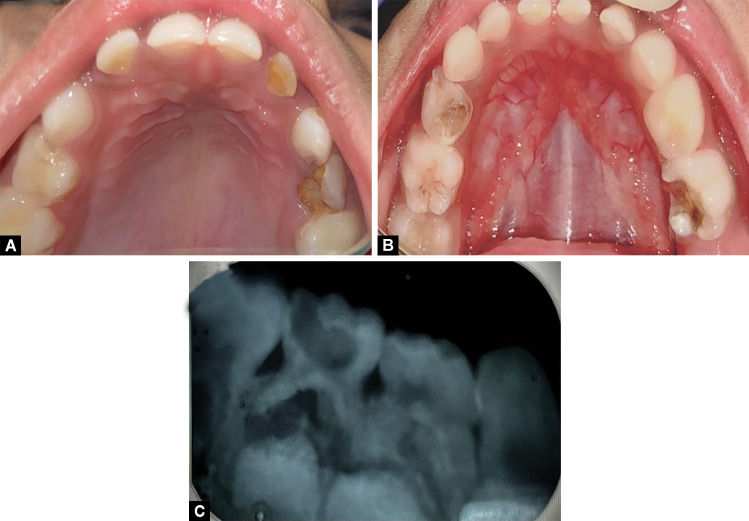
On clinical examination of the oral cavity mucosal lesions characteristic of the geographic tongue were observed on the anterior two-thirds of the dorsal surface as well as the lateral surface of the tongue (Fig. 3). Oblate pattern occurrence of white lesions with erythematous atrophic patches and loss of filiform papillae with white borders. The lesion was type III according to Hume's (1975) classification.14 A scraping was done and tested and candidiasis was ruled out. Also, oral examination revealed dental caries in relation to 52, 54, 62, 63, 74, and 85 teeth and with a carious breakdown of 64 (Fig. 4).
Figs 3 A to C.
(A and B) The wandering lesion over the dorsal surface of the tongue suggestive of geographic tongue; (C) Shows the permanent tracheostomy tube in the child
Figs 4 A to C.
(A) Preoperative intraoral occlusal view; (B) Preoperative intraoral mandibular view; (C) Preoperative intraoral periapical radiograph showing deep caries of 75
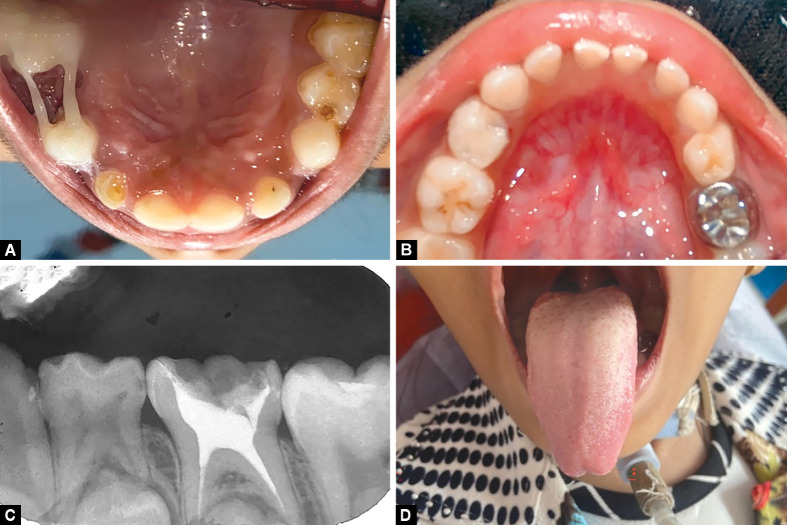
A provisional diagnosis of benign migratory glossitis was concluded. Benign migratory glossitis is known to have a transient character and self-limiting nature and was treated symptomatically with topical local anesthesia (LA) gel—benzocaine −7.5% W/W (Mucopain gel, ICPA Health Products Ltd., Ankaleshwar, India), with reassurance, increase in fluid intakes and meticulous oral hygiene maintenance. Casein phosphopeptide—amorphous calcium phosphate-based varnish (GC Tooth Mousse, GC Dental, India) was applied using the paint-on method to all teeth for the child. This was done to prevent new caries and also helped to de-stress the patient. With subsequent follow-up after 2 weeks, the patient reported improvement with symptomatic relief on the application of the topical LA gel over the lesion. Preventive restorative treatment for 64, 65, 84, and 85 with pit and fissure sealant (GC, Fuji® VII, Sydney, Australia) and definitive restoration of carious teeth—52, 43, 62, and 63 with glass ionomer cement (GIC) (GC, Tokyo, Japan) were performed (Figs 5A and B). A total of 54 were extracted under 2% LA with 1:100000 adrenaline with a peristome followed by placement of polyethylene fiber-reinforced composite (Ribbond, Inc., Seattle, Washington, United States of America) loop space maintainer (as a prevention for space loss due to premature extraction). After plaque removal with a prophylactic paste at the beginning of the procedure, a rubber dam was placed. Pulp therapy was performed for the deep carious 75 followed by permanent restoration with a stainless steel crown (3M™ ESPE™ Stainless steel crown, 3M, St Paul, Minnesota, United States of America) (Fig. 5C).
Figs 5 A to D.
Full mouth rehabilitation. (A) Postoperative maxillary occlusal view; (B) Postoperative mandibular view; (C) Intraoral periapical radiograph showing pulpectomy on 75; (D) Resolved geographic tongue with the cessation of the drug
An analysis was made on the Naranjo adverse drug reaction scale (Table 1). This was done to see the probability of the antiangiogenesis drug causing oral adverse effects. It was found to be four which indicates a possible association of drug reaction. Apart from this the detailed history given by the mother of the pattern followed by geographic lesion and administration of the VEGF further points to a stronger possible association. There was a resolution of the lesion observed on the cessation of the drug (Fig. 5D).
Table 1.
Naranjo adverse drug reaction probability scale for the concerned drug
| Question | Yes | No | Do not know | Score |
|---|---|---|---|---|
| 1. Are there previous conclusive reports on this reaction? | +1 | 0 | 0 | +1 |
| 2. Did the adverse event appear after the suspected drug was administered? | +2 | −1 | 0 | +2 |
| 3. Did the adverse event improve when the drug was discontinued or a specific antagonist was administered? | +1 | 0 | 0 | +1 |
| 4. Did the adverse event reappear when the drug was readministered? | +2 | −1 | 0 | 0 |
| 5. Are there alternative causes that could on their own have caused the reaction? | −1 | +2 | 0 | −1 |
| 6. Did the reaction reappear when a placebo was given? | −1 | +1 | 0 | 0 |
| 7. Was the drug detected in blood or other fluids in concentrations known to be toxic? | +1 | 0 | 0 | 0 |
| 8. Was the reaction more severe when the dose was increased or less severe when the dose was decreased? | +1 | 0 | 0 | 0 |
| 9. Did the patient have a similar reaction to the same or similar drugs in any previous exposure? | +1 | 0 | 0 | +1 |
| 10. Was the adverse event confirmed by any objective evidence? | +1 | 0 | 0 | 0 |
| Total score = 4 | ||||
Discussion
This case report presents a patient with an association of geographic tongue induction of VEGF inhibitors as a therapeutic drug for the treatment of JORRP.
The VEGF is a heparin-binding homodimer glycoprotein structurally comprising eight cysteines related to the platelet-derived growth factor supergene family as evidenced by its ability to function as a potent angiogenic factor as well as mitogenic for endothelial cells. VEGF-A functions as a proangiogenic factor increases vascular permeability and stimulates the migration of macrophage lineage and endothelial cells. It induces dilation of the blood vessels favoring their permeability initiating angiogenesis and potentiating the process of wound healing and inflammation.15,16 VEGFR-1 (Flt-1) and VEGFR-2 (KDR/Flk1 in mice) receptor regulates the vascular proliferation and angiogenesis through VEGF-A, whereas VEGFR-C/VEGF-D with its receptor VEGFR-3 (Flt-4) regulates the process of lymph angiogenesis.15
The VEGF is expressed naturally in proliferating endometrium, corpus luteum formation, embryogenesis, and pathological conditions, such as wound healing, tissue inflammation, and tumor-associated neovascularization.17 Pammer et al. in their study emphasized that VEGF is vital for the normal equilibrium and homeostasis of oral tissue in both physiological and pathological circumstances complementary to other tissues. In addition, VEGF messenger ribonucleic acid (mRNA) is expressed in salivary glands and provides a protective and healing role. In normal salivary glands, VEGF mRNA and protein are expressed in acinar cells, whereas little or no VEGF was found in ductal cells. In chronically inflamed glands, VEGF protein was present in ductal elements and in infiltrating mononuclear cells.18 A three-dimensional organotypic oral mucosa model, correlated with cell survival, exhibited increased expression of VEGF in the basal and suprabasal layers. VEGF inhibitors reduce the human vascular endothelial cell invasion of the oral mucosa which substantiates the hampering of mucosal healing and repairing of the epithelium that may be a plausible mechanism accountable for these alterations observed over the tongue. Moreover, being developed from the stratum germinativum, tongue epithelium turnovers continuously with replacement observed for 3–5 days in animals which may explain the remission and recurrent nature of the lesion over the tongue in the present case.19
Bevacizumab acts by selectively binding circulating VEGF, reducing tissue interstitial pressure, raising vascular permeability, and thus favoring apoptosis of neoplastic endothelial cells. An in vivo study on vascular regrowth, upon interruption of anti-VEGF therapy in mice observed resumed mitosis which reached the baseline growth in 7 days.19 Bevacizumab is taken up by tumor cell VEGF which is a derivative of platelets, releasing them at the sites of endothelial damage, thereby delivering at higher concentration to these procoagulatory angiogenic tumor sites.4
The inhibition of platelet VEGF resulted in the appearance and exhibition of certain side effects that include hypertension, proteinuria, arterial thromboembolic events, cardiomyopathy, hemorrhage, impaired wound healing, gastrointestinal perforation, etc.7 Bevacizumab [anatomical therapeutic classification (ATC) code of L01XC07] is a medication that is known and reported to induce xerostomia, salivary gland hypofunction, or sialorrhea with higher and moderate levels of evidence according to the ATC system of World Health Organization.20 Study by Martinez-Garcia et al. documented the association of geographic tongue with bevacizumab use and hypothesized that the impediment of normal reparative capacity of lingual mucosa with the blockade of VEGF-A may be responsible for the occurrence of the lesion.9 Hubiche et al. and Gavrilovic et al. favored the argument that therapeutic inhibition of specific targets by angiogenesis inhibitors could induce geographic tongue.7,21 Compared with the mammalian target of rapamycin (mTOR) inhibitors, the clinical presentation of oral adverse events induced by angiogenesis inhibitors is much less characterized. Fang et al. in their systematic review on the effect of bevacizumab treatment on hepatocellular carcinoma observed xerostomia in patients on bevacizumab therapy for hepatocellular carcinoma, which might account for the increased observance of the carious teeth in this child.22 Maluf et al. have observed an association of bevacizumab-related osteoradionecrosis of the jaw in a patient with metastatic cancer who underwent dental implant treatment.23 Maluf et al. and Santos-Silva et al. substantiated the observation in their study too.23,24 Takahashi in his study proposed a possible role of bevacizumab in inducing serious oral mucositis in a patient undergoing colorectal chemotherapy with bevacizumab.25
Genetic variations in VEGF and its receptors conceivably act as markers for the evaluation of individual sensitivity and response to the drug. The clearance of bevacizumab depends on the neonatal Fc receptor which is an IgG receptor that takes up the antibody to bind to the neonatal Fc receptor (FcRn) delaying the degradation of the antibody; thus, prolonging the systemic elimination.3 Biomarker identifications like circulating endothelial cells and endothelial progenitor cells should assist in patient selection for the drug that would individually serve to measure the response to therapy.15
Conclusion
The possible adverse effects of bevacizumab were observed in our patient. The timeline for the same has been brought out and explained. Our patient presented with benign migratory glossitis. This condition requires only symptomatic treatment and reassurance to the patient. In our report also, the condition resolved with the cessation of the drug but reappeared when the drug was restarted. Since benign migratory glossitis is a self-limiting lesion in response to this drug it is of importance to the treating doctors. On weighing the benefits of the drug, it may be valuable for the patient to continue with the drug with the possible risk of the oral condition. Repeated dental checkup before and during the bevacizumab therapy is recommended by the authors.
Ethics Statement
Investigations were undertaken with ethical approval abiding by the Declaration of Helsinki as updated in the version promulgated in June 2013. Informed consent to publish clinical information and pictures was obtained from the affected persons’ parent, with a signature from a health professional as a witness.
Orcid
Khadeeja Kulood https://orcid.org/0000-0003-3838-740X
Footnotes
Source of support: Nil
Conflict of interest: Dr Rishi Tyagi is associated as the National Editorial Board member of this journal and this manuscript was subjected to this journal's standard review procedures, with this peer review handled independently of this editorial board member and his research group.
Patient consent statement: The author(s) have obtained written informed consent from the patient's parents/legal guardians for publication of the case report details and related images.
REFERENCES
- 1.Campana F, Vigarios E, Fricain JC, et al. Geographic stomatitis with palate involvement. An Bras Dermatol. 2019;94(4):449–451. doi: 10.1590/abd1806-4841.20197774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nandini DB, Bhavana SB, Deepak BS, et al. Paediatric geographic tongue: a case report, review and recent updates. J Clin Diagn Res. 2016;10(2):ZE05–ZE09. doi: 10.7860/JCDR/2016/16452.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kazazi-Hyseni F, Beijnen JH, Schellens JH. Bevacizumab. Oncologist. 2010;15(8):819–825. doi: 10.1634/theoncologist.2009-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia J, Hurwitz HI, Sandler AB, et al. Bevacizumab (Avastin®) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treat Rev. 2020;86:102017. doi: 10.1016/j.ctrv.2020.102017. [DOI] [PubMed] [Google Scholar]
- 5.Pérez-Herrero E, Fernández-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm. 2015;93:52–79. doi: 10.1016/j.ejpb.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Lam SY, Lee CS, Sharma S, et al. Bevacizumab-induced dysphonia: a case report with brief review of literature. J Oncol Pharm Pract. 2020;26(4):1032–1036. doi: 10.1177/1078155219889388. [DOI] [PubMed] [Google Scholar]
- 7.Gavrilovic IT, Balagula Y, Rosen AC, et al. Characteristics of oral mucosal events related to bevacizumab treatment. Oncologist. 2012;17(2):274–278. doi: 10.1634/theoncologist.2011-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Lorenzo G, Porta C, Bellmunt J, et al. Toxicities of targeted therapy and their management in kidney cancer. Eur Urol. 2011;59(4):526–540. doi: 10.1016/j.eururo.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Garcia M, Roman-Sainz J, Silvestre-Torner N, et al. Bevacizumab-induced geographic tongue. Dermatol Pract Concept. 2021;11(3):e2021043. doi: 10.5826/dpc.1103a43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sundar S, Burge F. Geographical tongue induced by axitinib. BMJ Case Rep. 2015;2015:bcr2015211318. doi: 10.1136/bcr-2015-211318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan MA, Leu GR, Upchurch PA, et al. Systemic Bevacizumab (Avastin) for juvenile-onset recurrent respiratory papillomatosis: a systematic review. Laryngoscope. 2021;131(5):1138–1146. doi: 10.1002/lary.29084. [DOI] [PubMed] [Google Scholar]
- 12.Strong MS, Vaughan CW, Cooperband SR, et al. Recurrent respiratory papillomatosis: management with the CO2 laser. Ann Otol Rhinol Laryngol. 1976;85(4 Pt 1):508–516. doi: 10.1177/000348947608500412. [DOI] [PubMed] [Google Scholar]
- 13.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 14.Hume WJ. Geographic stomatitis: a critical review. J Dent. 1975;3(1):25–43. doi: 10.1016/0300-5712(75)90020-2. [DOI] [PubMed] [Google Scholar]
- 15.Shibuya M. Vascular Endothelial Growth Factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer. 2011;2(12):1097–1105. doi: 10.1177/1947601911423031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niu G, Chen X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr Drug Targets. 2010;11(8):1000–1017. doi: 10.2174/138945010791591395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson KE, Wilgus TA. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care (New Rochelle) 2014;3(10):647–661. doi: 10.1089/wound.2013.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pammer J, Weninger W, Mildner M, et al. Vascular endothelial growth factor is constitutively expressed in normal human salivary glands and is secreted in the saliva of healthy individuals. J Pathol. 1998;186(2):186–191. doi: 10.1002/(SICI)1096-9896(1998100)186:2<186::AID-PATH148>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 19.Cutright DE, Bauer H. Cell renewal in the oral mucosa and skin of the rat. I. Turnover time. Oral Surg Oral Med Oral Pathol. 1967;23(2):249–259. doi: 10.1016/0030-4220(67)90104-1. [DOI] [PubMed] [Google Scholar]
- 20.Wolff A, Joshi RK, Ekström J, et al. A guide to medications inducing salivary gland dysfunction, xerostomia, and subjective sialorrhea: a systematic review sponsored by the world workshop on oral medicine VI. Drugs R D. 2017;17(1):1–28. doi: 10.1007/s40268-016-0153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubiche T, Valenza B, Chevreau C, et al. Geographic tongue induced by angiogenesis inhibitors. Oncologist. 2013;18(4):e16–e17. doi: 10.1634/theoncologist.2012-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang P, Hu JH, Cheng ZG, et al. Efficacy and safety of bevacizumab for the treatment of advanced hepatocellular carcinoma: a systematic review of phase II trials. PLoS One. 2012;7(12):e49717. doi: 10.1371/journal.pone.0049717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maluf G, Caldas RJ, Fregnani ER, et al. A rare case of bevacizumab-related osteonecrosis of the jaw associated with dental implants. Int J Implant Dent. 2019;5(1):34. doi: 10.1186/s40729-019-0188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos-Silva AR, Belizário Rosa GA, Castro Júnior Gd, et al. Osteonecrosis of the mandible associated with bevacizumab therapy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115(6):e32–e36. doi: 10.1016/j.oooo.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi H, Sato M, Tsukada K, et al. A retrospective study of oral adverse events with colorectal cancer chemotherapy using bevacizumab. Gan To Kagaku Ryoho. 2011;38(6):959–962. [PubMed] [Google Scholar]