Abstract
Purpose
This study investigated whether Ki-67 labeling index (LI) correlated with clinical outcomes after SRS for atypical meningiomas.
Methods
This retrospective study examined 39 patients with atypical meningiomas who underwent SRS over a 10-year study period. Ki-67 LI was categorized into 3 groups: low (< 5%), intermediate (5%–10%), and high (> 10%). Local tumor control rates (LCRs), progression-free rates (PFRs), disease-specific survival (DSS) rates, and adverse radiation-induced events (AREs) were evaluated.
Results
The median follow-up periods were 26 months. SRS was performed at a median prescription dose of 18 Gy for tumors with a median Ki-67 LI of 9.6%. The 3-year LCRs were 100%, 74%, and 25% in the low, intermediate, and high LI groups, respectively (p = 0.011). The 3-year PFRs were 100%, 40%, and 0% in the low, intermediate, and high LI groups (p = 0.003). The 5-year DSS rates were 100%, 89%, and 50% in the low, intermediate, and high LI groups (p = 0.019). Multivariable Cox proportional hazard analysis showed a significant correlation of high LI with lower LCR (hazard ratio [HR], 3.92; 95% confidence interval [CI] 1.18–13.04, p = 0.026), lower PFR (HR 3.80; 95% CI 1.46–9.88, p = 0.006), and shorter DSS (HR 6.55; 95% CI 1.19–35.95, p = 0.031) compared with intermediate LI. The ARE rates were minimal (8%) in the entire group.
Conclusion
Patients with high Ki-67 LI showed significantly more tumor progression and tumor-related death. Ki-67 LI might offer valuable predictive insights for the post-SRS management of atypical meningiomas.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11060-023-04537-7.
Keywords: Atypical meningioma, Ki-67 labeling index, Recurrence pattern, Stereotactic radiosurgery
Introduction
Atypical meningiomas account for approximately 15% of all meningiomas and are diagnosed with a pathological confirmation of 4–19 high mitotic cells per 10 high power fields or brain invasion, categorized in the 2021 World Health Organization (WHO) Classification of Tumor of Central Nervous System as grade 2 [1, 2]. Diagnosis is confirmed based on the number of mitoses observed as well as brain invasion on histological examination. Overall, the prognosis is worse than that of WHO grade 1 meningiomas with higher recurrence rates seen, varying between 38 to 66% in previous studies [3–6]. Fractionated radiotherapy for postoperative remnants is recommended based on guidelines from the European Association of Neuro-Oncology [1, 7]. However, postoperative management of atypical meningiomas remains controversial, and there is debate whether to observe patients after gross total resection or to perform upfront adjuvant radiotherapy to the resection site. Additionally, there is a lack of consensus regarding the optimal management of tumor recurrences [8–11]. In light of this, a more detailed classification based on post-treatment outcomes is required.
Stereotactic radiosurgery (SRS) is an effective treatment option for meningiomas because of its ability to deliver highly conformal and focused doses to the tumor margin while sparing surrounding neurovascular structures [12]. Several studies have already demonstrated the effectiveness of upfront adjuvant SRS for atypical meningiomas [13–25]. Those results indicated that tumor recurrence could be classified as intrafield recurrence (tumor progression within the irradiated area) and marginal or remote recurrence (tumor progression outside the irradiation area) [25, 26]. Moreover, multiple radiotherapy treatments for frequent recurrences can increase the risks of adverse radiation effects (AREs) [13, 15]. Hence, it may be beneficial to predict not just the response to SRS but also the pattern of recurrence in atypical meningiomas to determine if SRS treatment paradigms need to be modified.
The Ki-67 labeling index (LI) is a valuable tool that reflects tumor proliferation capability and has been shown to correlate with progression after surgery for benign and high-grade meningiomas [27–31]. Recent studies have reported that it can correlate with progression of benign and high-grade meningiomas after SRS [13, 32]. Increased Ki-67 LI found in atypical meningiomas might serve as a predictor of recurrence [33]. However, the use of Ki-67 LI to assess the outcomes following SRS in atypical meningiomas is unclear. This study aimed to evaluate outcomes after SRS for atypical meningiomas and stratify the risk of tumor progression, recurrence patterns, and disease-specific survival (DSS) based on Ki-67 LI.
Methods
Patients and tumor characteristics
The clinical data of 451 consecutive patients with meningiomas treated with single-session SRS between June 1992 and February 2022 at our institution were collected from an institutional Gamma Knife database. Among these patients, 47 patients with atypical meningiomas were identified, and patients with a follow-up period of < 3 months (n = 8) were excluded. In total, 39 atypical meningiomas were analyzed for this study. Patients who underwent external-beam radiotherapy before SRS were included. Tumor tissue from all surgically obtained specimens were reviewed, and all diagnoses were confirmed based on the 2016 WHO Classification of Tumors of the Central Nervous System criteria. Tissue from seven tumors that were resected and diagnosed as atypical meningioma before 2007 were re-evaluated and reconfirmed to meet the 2016 criteria [2, 34]. Formalin-fixed, paraffin-embedded specimens were subjected to immunohistochemistry for Ki-67, and the LI within the hotspot was calculated under a light microscope by board-certified pathologists. All participants in the present study provided written informed consent, and all components of this study were authorized by the appropriate Institutional Review Board (The Research Ethics Committee of our institution, #2231).
SRS procedures and techniques
SRS was performed using a Leksell GammaKnife (Elekta AB, Stockholm, Sweden). After head fixation using a Leksell frame (Elekta Instruments, Stockholm, Sweden), stereotactic imaging (computed tomography [CT] before July 1996, magnetic resonance imaging [MRI] between August 1996 and January 2018, and cone-beam CT thereafter) was performed to obtain the precise three-dimensional coordinates. Thin-slice MR images (gadolinium-contrasted T1-weighted images and fast imaging employing steady-state acquisition) obtained the day before SRS were co-registered to define the tumor, surrounding cranial nerves, and vasculature. Radiosurgical treatment was planned and approved by the dedicated neurosurgeons and radiation oncologists involved with the procedure. All treatment planning was performed using commercially available software programs (KULA treatment planning system until 1998 and Leksell GammaPlan® [Elekta Instruments]).
Follow-up and treatment outcomes
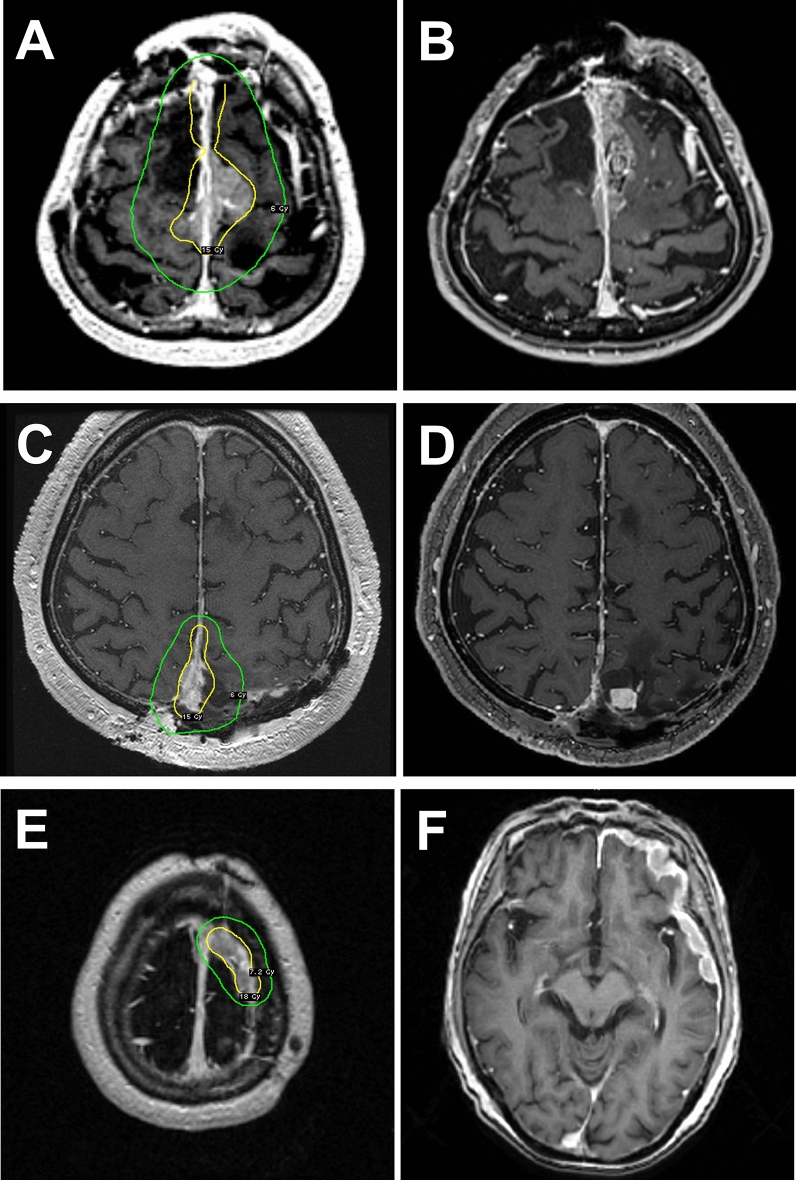
Clinical evaluations and MRI were performed every 3–6 months after the date of SRS. Data on tumor status and SRS-related complications were prospectively collected for the institutional Gamma Knife database. Two neurosurgeons and two neuroradiologists independently assessed radiologic evidence of recurrence. Tumor progression was defined as one of three types of events: (1) “intrafield recurrence,” defined as a > 10% increase in volume inside the 50% isodose line on two or more consecutive imaging studies (Fig. 1A, B), (2) “marginal recurrence,” defined as new tumor progression between the lines of 50% and 20% isodose (Fig. 1C, D), and (3) “remote recurrence,” defined as new tumor progression outside the 20% isodose line (Fig. 1E, F) [25, 26, 32]. AREs were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Peritumoral T2 signal change expanding beyond a 2-mm margin from the tumor after SRS was also considered to be an ARE.
Fig. 1.
Demonstrative cases representing intrafield recurrence, marginal recurrence, and remote recurrence. A, B “Intrafield recurrence” was defined as a > 10% increase in volume inside the 50% isodose line. C, D “Marginal recurrence” was defined as new tumor progression between 50 and 20% isodose lines. E, F “Remote recurrence,” defined as new tumor progression outside the 20% isodose line, was also collected. Yellow lines indicate 50% isodose, and green lines indicate 20% isodose
Statistical analyses
Baseline patient, tumor, and SRS dosimetry characteristics were summarized. Continuous variables were presented as medians and interquartile ranges (IQRs), while categorical variables were presented as numbers and percentages. The Kaplan–Meier method was used to evaluate therapeutic effects, with outcomes comprising local tumor control rates (LCRs), progression-free rates (PFRs), and DSS rates. Local tumor control was defined as the absence of intrafield recurrence, and progression-free status was defined as the absence of any recurrence pattern including a lack of intrafield, margin, or remote recurrence. DSS was defined as the absence of any mortality associated with the treated tumors. To further analyze the association between Ki-67 LI and SRS outcomes, the patients were classified into three groups based on Ki-67 LI: low LI (< 5%), intermediate LI (5%–10%), and high LI (> 10%) groups, based on the previous studies on Ki-67 LI in meningiomas [33, 35]. The LCRs, PFRs, and DSS rates between groups were compared using the log-rank test, and multiple curves were compared using the log-rank test with the Bonferroni correction. In addition, receiver operating curve (ROC) analyses on Ki-67 LI were performed to calculate each area under the curve (AUC), sensitivity, and specificity on intrafield recurrence, any progression, and disease-specific mortality, and to indicate a cut-off Ki-67 LI increasing each risks using Youden index. Continuous variables (age, maximum diameter, target volume, and radiosurgical dose) were entered into the models after dichotomization using the median values. Factors associated with local tumor recurrence and AREs were examined using bivariate and multivariable Cox proportional hazards analyses. Statistical significance was set at p < 0.05. All statistical analyses were performed using the JMP® Pro 17.0.0 software (SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics
Baseline patient characteristics and dosimetry data are summarized in Table 1. The median (IQR) age and follow-up period were 73 (63–77) years and 26 (18–59) months, respectively. The minimum follow-up period among alive patients at the last visit was 9 months. The median (IQR) Ki-67 LI was 9.6% (5.0–10.0%), with 5, 25, and 9 tumors in the low (< 5%), intermediate (5%–10%), and high (> 10%) LI groups, respectively. Postoperative adjuvant SRS was performed for seven (18%) patients after tumor resection before any noted progression. External-beam radiotherapy was performed for 13 (33%) patients before SRS. The median (IQR) central and prescription doses were 36 Gy (32–40 Gy) and 18 Gy (16–18 Gy), respectively.
Table 1.
Baseline characteristics and dosimetry data for the entire cohort and stratified by the Ki-67 labeling index (LI) groups
| Variables | All (n = 39) | Low LI group (n = 5) | Intermediate LI group (n = 25) | High LI group (n = 9) | p-value |
|---|---|---|---|---|---|
| Median [interquartile range], N (%) | |||||
| Ki-67 LI, % | 9.6 [5.0–10.0] | 4.0 [3.0–4.0] | 8.0 [5.0–10.0] | 20.0 [15.0–27.5] | 0.001* |
| Age at SRS, years | 73 [63–77] | 75 [62–81] | 74 [67–78] | 67 [61–72] | 0.244 |
| Female sex | 19 (49%) | 2 (40%) | 13 (52%) | 4 (44%) | 0.850 |
| Follow-up period, months | 26 [18–59] | 33 [20–72] | 27 [20–66] | 25 [15–50] | 0.574 |
| History of radiation therapy | 13 (33%) | 2 (40%) | 7 (28%) | 4 (44%) | 0.631 |
| Upfront adjuvant | 11 (24%) | 0 (0%) | 4 (16%) | 3 (33%) | 0.195 |
| Maximum tumor diameter, mm | 33 [22–45] | 45 [23–53] | 31 [21–41] | 33 [26–51] | 0.477 |
| Target volume, mL | 7.5 [3.8–15.4] | 10.3 [5.6–16.8] | 7.2 [3.7–12.4] | 7.5 [4.1–22.1] | 0.463 |
| Central dose, Gy | 36 [32–40] | 36 [33–38] | 36 [32–40] | 36 [34–43] | 0.443 |
| Prescription dose, Gy | 18 [16–18] | 18 [14–19] | 16 [16–18] | 18 [17, 18] | 0.563 |
| Locations | 0.062 | ||||
| Convexity | 7 (18%) | 0 (0%) | 3 (12%) | 4 (44%) | |
| Parasagittal/falx | 11 (28%) | 0 (0%) | 9 (36%) | 2 (22%) | |
| Anterior skull base | 4 (12%) | 2 (40%) | 2 (8%) | 0 (0%) | |
| Middle skull base | 12 (31%) | 3 (60%) | 7 (28%) | 2 (22%) | |
| Posterior skull base | 5 (13%) | 0 (0%) | 4 (16%) | 1 (11%) | |
LI labeling index, N number, SRS stereotactic radiosurgery
*p-values of < 0.05 are considered significant
Tumor control
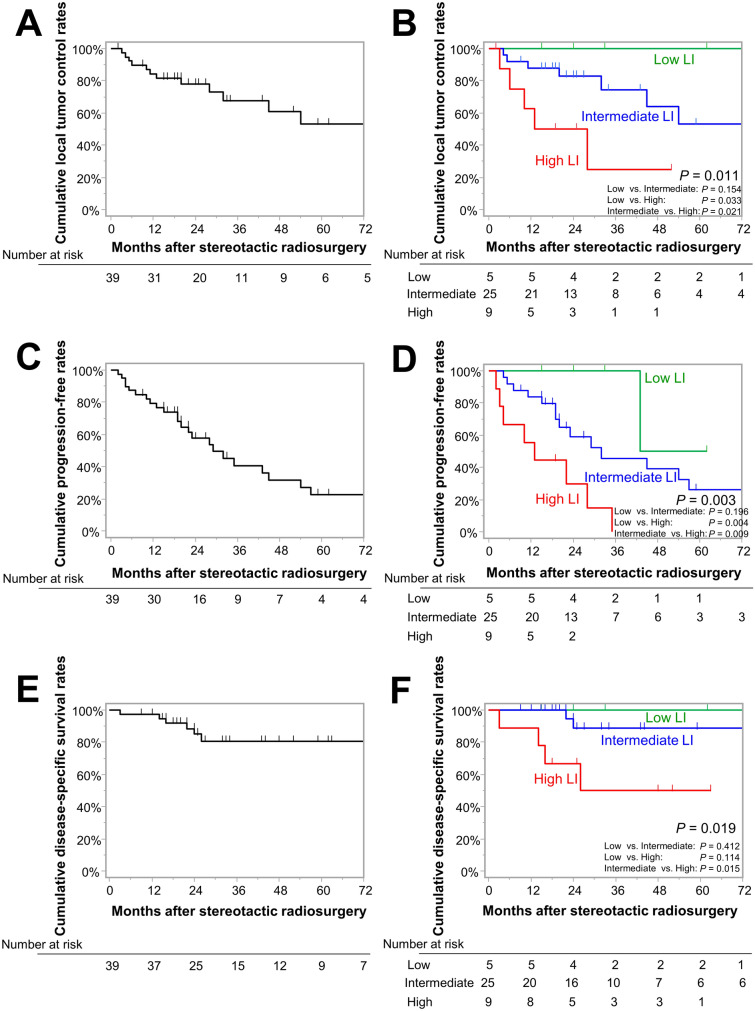
In the entire cohort, 14 (36%) patients experienced intrafield recurrence, and 12 (31%) experienced marginal recurrence (Supplementary Table 1). One patient experienced intrafield and marginal recurrence simultaneously. Remote recurrence was observed in three (8%) patients, and all lesions were detected simultaneously with marginal recurrence. In the entire cohort, the cumulative 1-, 3-, and 5-year LCRs were 84%, 68%, and 53%, respectively (Fig. 2A); and the PFRs were 79%, 41%, and 23%, respectively (Fig. 2C).
Fig. 2.
Kaplan–Meier curves for: A, B local tumor control rates for the entire cohort and compared between three groups stratified by a Ki-67 labeling index of < 5%, 5%–10%, and > 10%, C, D progression-free rates for the entire cohort and compared between three groups stratified by a Ki-67 labeling index of < 5%, 5%–10%, and > 10%, E, F disease-specific survival rates for the entire cohort and compared between three groups stratified with a Ki-67 labeling index of < 5%, 5%–10%, and > 10%
Using the three groups stratified by Ki-67 LI, the 1-, 3-, and 5-year LCRs with low LI were all 100%, and LCRs with intermediate LI were 88%, 74%, and 53%, respectively; the 1- and 3-year LCRs with high LI were 63% and 25%, respectively (Fig. 2B; p = 0.011). Differences between low and high LI (p = 0.033) and between intermediate and high LI (p = 0.021) were marginally significant. The ROC analysis for intrafield recurrence and Ki-67 LI showed an AUC of 0.797 (Supplementary Fig. 1). The Youden index identified a cutoff at Ki-67 LI = 8%, with a sensitivity of 1.000 and specificity of 0.600. Bivariate and multivariable regression analyses were performed to determine if Ki-67 LI was associated with recurrence. In addition to Ki-67 LI, sex and tumor volume were used for multivariable analysis. Tumors with high Ki-67 LI were associated with greater intrafield recurrence risk using bivariate (hazard ratio [HR] 3.75, 95% confidence interval [CI] 1.13–12.49, p = 0.031; Table 2) and multivariable (HR 6.10, 95% CI 1.56–23.95, p = 0.010; Table 2) analyses. Larger tumor volume was associated with greater intrafield recurrence risk only in multivariable analysis (HR 1.08, 95% CI 1.01–1.16, p = 0.023; Table 2). Furthermore, 1-, 3-, and 5-year PFRs with low, and intermediate LI were 100%, 100%, and 50%; and 84%, 46%, and 26%, respectively; the 1- and 3-year PFRs with high LI were 56% and 0%, respectively (Fig. 2D p = 0.003). Differences between low and high LI (p = 0.009) and between intermediate and high LI (p = 0.004) were significant as well. The ROC analysis for any progression and Ki-67 LI showed an AUC of 0.793 (Supplementary Fig. 1). The Youden index indicated a cutoff at Ki-67 LI = 7%, with sensitivity of 0.880 and specificity of 0.714. Tumors with high Ki-67 LI were identified as risk factors of lower PFRs using bivariate (high vs. low, HR 11.97, 95% CI 1.46–98.04, p = 0.021: high vs. intermediate, HR 3.43, 95% CI 1.35–8.70, p = 0.010; Table 3) and multivariable (high vs. low, HR 12.71, 95% CI 1.51–107.23, p = 0.019: high vs. intermediate, HR 4.12, 95% CI 1.54–11.04, p = 0.005; Table 3) analyses in which tumor volume and indication of SRS in addition to Ki-67 LI were included. Tumor volume was not associated with lower PFRs.
Table 2.
Results of bivariate and multivariate analyses for local tumor progression after stereotactic radiosurgery
| Bivariate | Multivariate | |||
|---|---|---|---|---|
| HR [95% CI] | p-value | HR [95% CI] | p-value | |
| Age, years (continuous) | 1.02 [0.97–1.08] | 0.422 | – | – |
| Age > 70 years (vs. ≤ 70 years) | 1.00 [0.33–3.02] | 0.994 | – | – |
| Male (vs. female) | 0.49 [0.15–1.64] | 0.249 | 0.37 [0.11–1.31] | 0.123 |
| Convexity, midline (vs. skull base) | 0.77 [0.26–2.29] | 0.634 | – | – |
| Maximum diameter, mm (continuous) | 1.02 [0.98–1.08] | 0.270 | – | – |
| Maximum diameter > 35 mm (vs. ≤ 35 mm) | 1.44 [0.48–4.33] | 0.521 | – | – |
| Volume, mL (continuous) | 1.06 [0.99–1.13] | 0.095 | 1.08 [1.01–1.16] | 0.023* |
| Volume > 8 mL (vs. ≤ 8 mL) | 0.95 [0.32–2.86] | 0.934 | – | – |
| Ki-67 LI, % (continuous) | 1.04 [0.97–1.10] | 0.196 | – | – |
| High LI (vs. low LI) | NA | NA | NA | NA |
| High LI (vs. intermediate LI) | 3.75 [1.13–12.49] | 0.031* | 6.10 [1.56–23.95] | 0.010* |
| History of radiation therapy | 1.34 [0.43–4.13] | 0.611 | – | – |
| Salvage SRS (vs. adjuvant SRS) | 0.61 [0.17–2.24] | 0.460 | – | – |
| Central dose, Gy (continuous) | 1.0 [0.90–1.13] | 0.932 | – | – |
| Central dose > 36 Gy (vs. ≤ 36 Gy) | 0.99[0.32–3.05] | 0.984 | – | – |
| Marginal dose, Gy (continuous) | 0.95 [0.72–1.25] | 0.736 | – | – |
| Marginal dose > 18 Gy (vs. ≤ 18 Gy) | 1.00 [0.33–3.03] | 0.998 | – | – |
CI confidence interval, HR hazard ratio, LI labeling index, NA not adequately calculated, SRS stereotactic radiosurgery
*p-values of < 0.05 are considered significant;
Table 3.
Results of bivariate and multivariate analyses for tumor progression after stereotactic radiosurgery
| Bivariate | Multivariate | |||
|---|---|---|---|---|
| HR [95% CI] | p-value | HR [95% CI] | p-value | |
| Age, years (continuous) | 1.02 [0.98–1.06] | 0.385 | – | – |
| Age > 70 years (vs. ≤ 70 years) | 1.03 [0.46–2.32] | 0.942 | – | – |
| Male (vs. female) | 1.23 [0.53–2.83] | 0.628 | – | – |
| Convexity, midline (vs. skull base) | 0.88 [0.39–1.96] | 0.748 | – | – |
| Maximum diameter, mm (continuous) | 1.00 [0.97–1.03] | 0.858 | – | – |
| Maximum diameter > 35 mm (vs. ≤ 35 mm) | 0.78 [0.33–1.73] | 0.511 | – | – |
| Volume, mL (continuous) | 1.02 [0.97–1.08] | 0.378 | 1.03 [0.97–1.09] | 0.315 |
| Volume > 8 mL (vs. ≤ 8 mL) | 0.85 [0.38–1.92] | 0.694 | – | – |
| Ki-67 LI, % (continuous) | 1.04 [1.00–1.09] | 0.047* | – | – |
| High LI (vs. low LI) | 11.97 [1.46–98.04] | 0.021* | 12.71 [1.51–107.23] | 0.019* |
| High LI (vs. intermediate LI) | 3.43 [1.35–8.70] | 0.010* | 4.12 [1.54–11.04] | 0.005* |
| History of radiation therapy | 0.69 [0.27–1.75] | 0.438 | – | – |
| Salvage SRS (vs. adjuvant SRS) | 0.50 [0.20–1.29] | 0.152 | 0.59 [0.21–1.64] | 0.309 |
| Central dose, Gy (continuous) | 1.04 [0.96–1.13] | 0.395 | – | – |
| Central dose > 36 Gy (vs. ≤ 36 Gy) | 1.42 [0.63–3.19] | 0.401 | – | – |
| Marginal dose, Gy (continuous) | 1.15 [0.94–1.40] | 0.160 | – | – |
| Marginal dose > 18 Gy (vs. ≤ 18 Gy) | 0.54 [0.12–2.33] | 0.408 | – | – |
CI confidence interval, HR hazard ratio, LI labeling index, SRS stereotactic radiosurgery
*p-values of < 0.05 are considered significant
DSS
Among the 39 patients, 29 (74%) were alive by last follow-up, 7 (18%) had died of uncontrolled tumor progression, and 3 (8%) had died from unrelated causes. The cumulative 1-, 3-, and 5-year DSS rates were 97%, 81%, and 81%, respectively (Fig. 2E). Stratified by Ki-67 LI, the 1-, 3-, 5-year DSS rates with low LI were all 100%, those with intermediate LI were 100%, 100%, and 89%, respectively; and those with high LI were 89%, 50%, and 50%, respectively (p = 0.019; Fig. 2F). The ROC analysis for disease-specific mortality and Ki-67 LI showed an AUC of 0.766 (Supplementary Fig. 1). The Youden index identified a cutoff at Ki-67 LI = 8%, with sensitivity of 1.000 and specificity of 0.469. A significant difference in DSS rates between intermediate and high LI groups was identified (p = 0.015), and high LI compared with intermediate LI was identified as a risk factor related to shorter DSS using bivariate (HR 6.27, 95% CI 1.14–34.26, p = 0.034; Supplementary Table 2) and multivariable (HR 6.55, 95% CI 1.19–35.95, p = 0.035; Supplementary Table 2) analyses in which tumor volume and Ki-67 LI were included. Larger tumor volume was also associated with shorter DSS in both bivariate (HR 1.09, 95% CI 1.00–1.19, p = 0.032; Supplementary Table 2) and multivariable (HR 1.09, 95% CI 1.00–1.20, p = 0.043; Supplementary Table 2) analyses.
Adverse radiation-induced events
AREs were observed in three (8%) patients, and all were peritumoral T2 signal changes on MRI 3–9 months after SRS. Two patients were asymptomatic (CTCAE grade 1); although one patient experienced increased convulsions likely related to expanding T2 signal changes and required escalation of antiepileptic therapy, his symptoms and AREs were transient and well-controlled after medical therapy alone (CTCAE grade 2).
Discussion
Previous studies have reported on the recurrence rate of atypical meningioma following SRS, with a PFS of 33%–83% after 3 years and 20%–59% after 5 years [13–25]. However, limited studies have focused on the recurrence patterns according to intrinsic tumor characteristics, which is clinically significant as atypical meningiomas often exhibit marginal recurrences outside the radiation field [25]. Ki-67 LI provides insight into the proliferative nature of tumors, and while it has been used as a prognostic factor for postoperative recurrence in atypical meningiomas [13, 27, 28, 30, 31], few studies have evaluated Ki-67 LI as a prognosticator for atypical meningioma SRS outcomes. Shepard et al. reported that a higher LI was associated with shorter PFS after SRS in a cohort including atypical and anaplastic meningiomas classified as a Ki-67 LI of > 15% and ≤ 15% [13]. However, Kowalchuk et al. did not find an association between Ki-67 and PFS in grade 2 meningiomas [14]. Prognostic models with Ki-67 LI stratification for patterns of recurrence or DSS after SRS have also not been well-studied. Our results indicate that Ki-67 stratification was prognostic across different types of recurrence patterns (intrafield only and any form of intracranial progression) and for DSS.
For tumors with low Ki-67 LI (< 5%), no intrafield recurrences were observed, and marginal recurrences were observed only after 3 years, suggesting a more benign course similar to that of grade 1 meningiomas [12, 36]. However, tumors with intermediate and high Ki-67 LI (> 10%) were associated with intrafield recurrences with 3-year LCRs of 53% and 25%, respectively. Especially in the high LI group, patients experienced intrafield recurrence more frequently, similar to anaplastic meningioma which has 5-year PFRs of 17%–50% after SRS [5, 13, 16, 17, 20]. The prescription dose in prior studies (some including anaplastic meningiomas) has ranged from 13–15 Gy [5, 13, 16, 17, 20]; the prescription dose in the present study was 16–18 Gy. Considering that the Ki-67 LI increase was associated with a gradual increase in the risk of intrafield recurrence, it may be reasonable to gradually increase the prescription dose accordingly.
In addition to considering escalation of the prescription dose for higher Ki-67 LI, the present study’s findings suggest that treatment volumes may also need to be larger. Tumor progression tended to occur more frequently outside the 50% isodose line as demonstrated by more rapid decrease in PFR with lower rates of LCR. Marginal recurrences were frequently observed, particularly in atypical meningiomas with intermediate and high Ki-67 LI, indicating the presence of tumor cells in the dural tail or the adjacent brain parenchyma. Previous reports on SRS for meningiomas could not conclude whether the dural tail and the adjacent brain parenchyma should be included in the irradiation field [37–39]. The present study indicates that consideration for more extensive coverage including dural tail should be based on the Ki-67 LI because of the high frequency of marginal recurrence in a relatively short period after SRS for tumors with intermediate to high Ki-67 LI. Nonetheless, expansion of the radiation field should be modest.
Regarding patient survival in atypical meningioma treated with SRS, Shepard et al. found that a Ki-67 LI > 15% was a risk factor for worse survival based on univariate analysis, but there was no significant difference in multivariable analysis [13]. This was attributed to the heterogeneity of their cohort, which included anaplastic and atypical meningiomas. The present study suggests that atypical meningioma is a diverse population in terms of recurrence patterns and DSS, and that Ki-67 may be useful in predicting these outcomes. High Ki-67 LI and poor survival following treatment of atypical meningiomas is supported by the current study and prior reports as well [29, 40, 41]. In addition, the incidence of AREs after SRS was low at 8% in our cohort. However, previous reports have shown a higher occurrence of AREs with some indicating a significant risk increase after external-beam radiotherapy [13, 15]. If further escalation of radiation dose or volume coverage is considered for patients with high Ki-67 LI, this must be balanced with the potential increased risk of adverse effects in normal tissues. Recent research has shown the efficacy of multisession radiosurgery and additional hypofractionated SRS following external-beam radiotherapy [42, 43], and further research on fractionated irradiation is warranted. To this point, several prospective studies on fractionated radiotherapy for atypical meningioma and ongoing randomized trials are being conducted [44–46]. Considering that multimodal treatments should be considered in the management of atypical meningiomas, systemic therapeutic drugs have the potential to improve the prognosis of patients with atypical meningiomas [47, 48]. Additionally, several new studies on genomic alterations observed in meningiomas are providing a paradigm shift for identifying high-risk tumors [49, 50]. Prospective studies on drug therapy targeting some of these genetic mutations are also being conducted, creating hope for future genetic-based personalized medicine for atypical meningiomas [51].
Limitations
This study had some limitations. First, this was a single-center retrospective study, and the number of patients analyzed was limited. As all patients were surgically pretreated, the heterogeneity potentially leads to selection bias. Second, the relatively short follow-up period could undermine the reliability of the results. Third, we did not perform tumor genetic mutation analysis in all cases. As a result, we cannot guarantee the exclusion of the group classified as having grade 3 anaplastic meningioma in 2021 based on TERT mutation or CDKN2A/B homozygous deletion. Therefore, future prospective studies in larger cohorts of patients are warranted to address these limitations.
Conclusion
Ki-67 LI stratification of atypical meningioma into low (< 5%), intermediate (5%–10%), and high (> 10%) groups was correlated with outcomes after SRS in terms of LCRs, PFRs, and DSS rates. Further studies with larger sample sizes are warranted to verify these findings. Additionally, more work is needed to determine if higher doses or expanded SRS fields are needed to further decrease the risk of recurrence in patients with high Ki-67 LI.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MU, YS, HH, AK, AS and NS. The first draft of the manuscript was written by MU, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding provided by The University of Tokyo. This work was supported by JSPS KAKENHI (Grant Number 22K20815 to Motoyuki Umekawa).
Data availability
The authors confirm that data collected for the study and analysis methods will be shared upon reasonable request from any qualified investigator.
Declarations
Competing interest
The authors declare no conflict of interest.
Consent to participate
All participants in the study provided written informed consent.
Ethical approval
All components of this study were authorized by the appropriate Institutional Review Board (The Research Ethics Committee of our institution, #2231).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Goldbrunner R, Stavrinou P, Jenkinson MD, Sahm F, Mawrin C, Weber DC, Preusser M, Minniti G, Lund-Johansen M, Lefranc F, Houdart E, Sallabanda K, Le Rhun E, Nieuwenhuizen D, Tabatabai G, Soffietti R, Weller M. EANO guideline on the diagnosis and management of meningiomas. Neuro Oncol. 2021;23:1821–1834. doi: 10.1093/neuonc/noab150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Streckert EMS, Hess K, Sporns PB, Adeli A, Brokinkel C, Kriz J, Holling M, Eich HT, Paulus W, Spille DC, van Eck A, Raleigh DR, McDermott MW, Stummer W, Brokinkel B. Clinical, radiological, and histopathological predictors for long-term prognosis after surgery for atypical meningiomas. Acta Neurochir (Wien) 2019;161:1647–1656. doi: 10.1007/s00701-019-03956-8. [DOI] [PubMed] [Google Scholar]
- 4.Champeaux C, Dunn L. World Health Organization grade II meningiomas. Acta Neurochir (Wien) 2016;158:921–929. doi: 10.1007/s00701-016-2771-y. [DOI] [PubMed] [Google Scholar]
- 5.Piscevic I, Villa A, Milicevic M, Ilic R, Nikitovic M, Cavallo LM, Grujicic D. The influence of adjuvant radiotherapy in atypical and anaplastic meningiomas: a series of 88 patients in a single institution. World Neurosurg. 2015;83:987–995. doi: 10.1016/j.wneu.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Cao X, Hao S, Wu Z, Wang L, Jia G, Zhang L, Zhang J. Treatment response and prognosis after recurrence of atypical meningiomas. World Neurosurg. 2015;84:1014–1019. doi: 10.1016/j.wneu.2015.05.032. [DOI] [PubMed] [Google Scholar]
- 7.Goldbrunner R, Minniti G, Preusser M, Jenkinson MD, Sallabanda K, Houdart E, von Deimling A, Stavrinou P, Lefranc F, Lund-Johansen M, Moyal EC, Brandsma D, Henriksson R, Soffietti R, Weller M. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17:e383–391. doi: 10.1016/S1470-2045(16)30321-7. [DOI] [PubMed] [Google Scholar]
- 8.Pan PC, Pisapia DJ, Ramakrishna R, Schwartz TH, Pannullo SC, Knisely JPS, Chiang GC, Ivanidze J, Stieg PE, Liechty B, Brandmaier A, Fine HA, Magge RS. Outcomes following upfront radiation versus monitoring in atypical meningiomas: 16-year experience at a tertiary medical center. Neurooncol Adv. 2021;3:vda094. doi: 10.1093/noajnl/vdab094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Momin AA, Shao J, Soni P, Almeida JP, Suh JH, Murphy ES, Chao ST, Angelov L, Mohammadi AM, Barnett GH, Recinos PF, Kshettry VR. Outcomes of salvage radiation for recurrent world health organization grade II meningiomas: a retrospective cohort study. J Neurooncol. 2021;152:373–382. doi: 10.1007/s11060-021-03711-z. [DOI] [PubMed] [Google Scholar]
- 10.Sun SQ, Cai C, Murphy RK, DeWees T, Dacey RG, Grubb RL, Rich KM, Zipfel GJ, Dowling JL, Leuthardt EC, Simpson JR, Robinson CG, Chicoine MR, Perrin RJ, Huang J, Kim AH. Radiation therapy for residual or recurrent atypical meningioma: the effects of modality, timing, and tumor pathology on long-term outcomes. Neurosurgery. 2016;79:23–32. doi: 10.1227/NEU.0000000000001160. [DOI] [PubMed] [Google Scholar]
- 11.Unterberger A, Nguyen T, Duong C, Kondajji A, Kulinich D, Yang I. Meta-analysis of adjuvant radiotherapy for intracranial atypical and malignant meningiomas. J Neurooncol. 2021;152:205–216. doi: 10.1007/s11060-020-03674-7. [DOI] [PubMed] [Google Scholar]
- 12.Kondziolka D, Patel AD, Kano H, Flickinger JC, Lunsford LD. Long-term outcomes after gamma knife radiosurgery for meningiomas. Am J Clin Oncol. 2016;39:453–457. doi: 10.1097/COC.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 13.Shepard MJ, Xu Z, Kearns K, Li C, Chatrath A, Sheehan K, Sheehan D, Faramand A, Niranjan A, Kano H, Gurewitz J, Bernstein K, Liscak R, Guseynova K, Grills IS, Parzen JS, Cifarelli CP, Rehman AA, Atik A, Bakhsheshian J, Zada G, Chang E, Giannotta S, Speckter H, Wu HM, Kondziolka D, Golfinos JG, Mathieu D, Lee CC, Warnick RE, Lunsford LD, Sheehan JP. Stereotactic radiosurgery for atypical (World Health Organization II) and anaplastic (World Health Organization III) Meningiomas: results from a multicenter, international cohort study. Neurosurgery. 2021;88:980–988. doi: 10.1093/neuros/nyaa553. [DOI] [PubMed] [Google Scholar]
- 14.Kowalchuk RO, Shepard MJ, Sheehan K, Sheehan D, Faramand A, Niranjan A, Kano H, Gurewitz J, Bernstein K, Liscak R, Guseynova K, Grills IS, Parzen JS, Cifarelli CP, Rehman AA, Atik A, Bakhsheshian J, Zada G, Chang E, Giannotta S, Speckter H, Wu HM, Kondziolka D, Mathieu D, Lee CC, Warnick RE, Lunsford LD, Trifiletti DM, Sheehan JP. Treatment of WHO grade 2 meningiomas with stereotactic radiosurgery: identification of an optimal group for SRS using RPA. Int J Radiat Oncol Biol Phys. 2021;110:804–814. doi: 10.1016/j.ijrobp.2021.01.048. [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa H, Vakharia K, Link MJ, Stafford SL, Brown PD, Parney IF, Burns TC, Yan ES, Mahajan A, Laack NN, Pollock BE. The role of single-fraction stereotactic radiosurgery for atypical meningiomas (WHO grade II): treatment results based on a 25-year experience. J Neurooncol. 2021;155:335–342. doi: 10.1007/s11060-021-03882-9. [DOI] [PubMed] [Google Scholar]
- 16.Kim M, Cho YH, Kim JH, Kim CJ, Roh SW, Kwon DH. Role of gamma knife radiosurgery for recurrent or residual World Health Organization grade II and III intracranial meningiomas. Br J Neurosurg. 2020;34:239–245. doi: 10.1080/02688697.2020.1726285. [DOI] [PubMed] [Google Scholar]
- 17.Helis CA, Hughes RT, Cramer CK, Tatter SB, Laxton AW, Bourland JD, Munley MT, Chan MD. Stereotactic radiosurgery for atypical and anaplastic meningiomas. World Neurosurg. 2020;144:e53–e61. doi: 10.1016/j.wneu.2020.07.211. [DOI] [PubMed] [Google Scholar]
- 18.Zhang G, Zhang Y, Zhang G, Li D, Wu Z, Wang Y, Zhang J. Outcome and prognostic factors for atypical meningiomas after first recurrence. J Clin Neurosci. 2019;63:100–105. doi: 10.1016/j.jocn.2019.01.032. [DOI] [PubMed] [Google Scholar]
- 19.Acker G, Meinert F, Conti A, Kufeld M, Jelgersma C, Nguyen P, Kluge A, Lukas M, Loebel F, Pasemann D, Kaul D, Budach V, Vajkoczy P, Senger C. Image-Guided robotic radiosurgery for treatment of recurrent grade II and III meningiomas. A Single-Center Study. World Neurosurg. 2019;131:e96–e107. doi: 10.1016/j.wneu.2019.07.058. [DOI] [PubMed] [Google Scholar]
- 20.Wang WH, Lee CC, Yang HC, Liu KD, Wu HM, Shiau CY, Guo WY, Pan DH, Chung WY, Chen MT. Gamma knife radiosurgery for atypical and anaplastic meningiomas. World Neurosurg. 2016;87:557–564. doi: 10.1016/j.wneu.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 21.Aboukais R, Zairi F, Lejeune JP, Le Rhun E, Vermandel M, Blond S, Devos P, Reyns N. Grade 2 meningioma and radiosurgery. J Neurosurg. 2015;122:1157–1162. doi: 10.3171/2014.9.JNS14233. [DOI] [PubMed] [Google Scholar]
- 22.Ferraro DJ, Funk RK, Blackett JW, Ju MR, DeWees TA, Chicoine MR, Dowling JL, Rich KM, Drzymala RE, Zoberi I, Simpson JR, Jaboin JJ. A retrospective analysis of survival and prognostic factors after stereotactic radiosurgery for aggressive meningiomas. Radiat Oncol. 2014;9:38. doi: 10.1186/1748-717X-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardesty DA, Wolf AB, Brachman DG, McBride HL, Youssef E, Nakaji P, Porter RW, Smith KA, Spetzler RF, Sanai N. The impact of adjuvant stereotactic radiosurgery on atypical meningioma recurrence following aggressive microsurgical resection. J Neurosurg. 2013;119:475–481. doi: 10.3171/2012.12.JNS12414. [DOI] [PubMed] [Google Scholar]
- 24.Pollock BE, Stafford SL, Link MJ, Garces YI, Foote RL. Stereotactic radiosurgery of World Health Organization grade II and III intracranial meningiomas: treatment results on the basis of a 22-year experience. Cancer. 2012;118:1048–1054. doi: 10.1002/cncr.26362. [DOI] [PubMed] [Google Scholar]
- 25.Attia A, Chan MD, Mott RT, Russell GB, Seif D, Daniel Bourland J, Deguzman AF, Ellis TL, McMullen KP, Munley MT, Tatter SB, Shaw EG. Patterns of failure after treatment of atypical meningioma with gamma knife radiosurgery. J Neurooncol. 2012;108:179–185. doi: 10.1007/s11060-012-0828-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanakita S, Koga T, Igaki H, Murakami N, Oya S, Shin M, Saito N. Role of gamma knife surgery for intracranial atypical (WHO grade II) meningiomas. J Neurosurg. 2013;119:1410–1414. doi: 10.3171/2013.8.JNS13343. [DOI] [PubMed] [Google Scholar]
- 27.Ren L, Cheng H, Chen J, Deng J, Wang D, Xie Q, Wakimoto H, Hua L, Gong Y. Progesterone receptor expression and prediction of benefits of adjuvant radiotherapy in de novo atypical meningiomas after gross-total resection. J Neurosurg. 2023;139:49–58. doi: 10.3171/2022.9.JNS221530. [DOI] [PubMed] [Google Scholar]
- 28.Przybylowski CJ, Suki D, Raza SM, DeMonte F. Volumetric extent of resection and survival for recurrent atypical meningioma. J Neurosurg. 2023 doi: 10.3171/2022.12.JNS221815. [DOI] [PubMed] [Google Scholar]
- 29.Chang WI, Kim IH, Choi SH, Kim TM, Lee ST, Won JK, Park SH, Kim MS, Kim JW, Kim YH, Park CK, Lee JH. Risk stratification to define the role of radiotherapy for benign and atypical meningioma: a recursive partitioning analysis. Neurosurgery. 2022;90:619–626. doi: 10.1227/neu.0000000000001904. [DOI] [PubMed] [Google Scholar]
- 30.Champeaux C, Dunn L. World health organization grade II meningioma: a 10-year retrospective study for recurrence and prognostic factor assessment. World Neurosurg. 2016;89:180–186. doi: 10.1016/j.wneu.2016.01.055. [DOI] [PubMed] [Google Scholar]
- 31.Ho DM, Hsu CY, Ting LT, Chiang H. Histopathology and MIB-1 labeling index predicted recurrence of meningiomas: a proposal of diagnostic criteria for patients with atypical meningioma. Cancer. 2002;94:1538–1547. doi: 10.1002/cncr.10351. [DOI] [PubMed] [Google Scholar]
- 32.Shinya Y, Hasegawa H, Shin M, Kawashima M, Umekawa M, Katano A, Ikemura M, Ushiku T, Ohara K, Okano A, Teranishi Y, Miyawaki S, Saito N. Long-term outcomes of stereotactic radiosurgery for postoperative world health organization grade I Skull base meningioma: utility of Ki-67 labeling index as a prognostic indicator. Neurosurgery. 2023 doi: 10.1227/neu.0000000000002546. [DOI] [PubMed] [Google Scholar]
- 33.Lee SH, Lee EH, Sung KS, Kim DC, Kim YZ, Song YJ. Ki67 index is the most powerful factor for predicting the recurrence in atypical meningioma : retrospective analysis of 99 patients in two institutes. J Korean Neurosurg Soc. 2022;65:558–571. doi: 10.3340/jkns.2021.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 35.Mirian C, Skyrman S, Bartek J, Jr, Jensen LR, Kihlstrom L, Forander P, Orrego A, Mathiesen T. The Ki-67 proliferation index as a marker of time to recurrence in intracranial meningioma. Neurosurgery. 2020;87:1289–1298. doi: 10.1093/neuros/nyaa226. [DOI] [PubMed] [Google Scholar]
- 36.Sheehan JP, Starke RM, Kano H, Barnett GH, Mathieu D, Chiang V, Yu JB, Hess J, McBride HL, Honea N, Nakaji P, Lee JY, Rahmathulla G, Evanoff WA, Alonso-Basanta M, Lunsford LD. Gamma knife radiosurgery for posterior fossa meningiomas: a multicenter study. J Neurosurg. 2015;122:1479–1489. doi: 10.3171/2014.10.JNS14139. [DOI] [PubMed] [Google Scholar]
- 37.Piper K, Yu S, Taghvaei M, Fernandez C, Mouchtouris N, Smit RD, Yudkoff C, Collopy S, Reyes M, Lavergne P, Karsy M, Prashant GN, Shi W, Evans J. Radiation of meningioma dural tail may not improve tumor control rates. Front Surg. 2022;9:908745. doi: 10.3389/fsurg.2022.908745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bulthuis VJ, Hanssens PE, Lie ST, van Overbeeke JJ. Gamma Knife radiosurgery for intracranial meningiomas: do we need to treat the dural tail? A single-center retrospective analysis and an overview of the literature. Surg Neurol Int. 2014;5:S391–395. doi: 10.4103/2152-7806.140192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DiBiase SJ, Kwok Y, Yovino S, Arena C, Naqvi S, Temple R, Regine WF, Amin P, Guo C, Chin LS. Factors predicting local tumor control after gamma knife stereotactic radiosurgery for benign intracranial meningiomas. Int J Radiat Oncol Biol Phys. 2004;60:1515–1519. doi: 10.1016/j.ijrobp.2004.05.073. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen MP, Morshed RA, Dalle Ore CL, Cummins DD, Saggi S, Chen WC, Choudhury A, Ravi A, Raleigh DR, Magill ST, McDermott MW, Theodosopoulos PV. Supervised machine learning algorithms demonstrate proliferation index correlates with long-term recurrence after complete resection of WHO grade I meningioma. J Neurosurg. 2023;138:86–94. doi: 10.3171/2022.4.JNS212516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kent CL, Mowery YM, Babatunde O, Wright AO, Barak I, McSherry F, Herndon JE, 2nd, Friedman AH, Zomorodi A, Peters K, Desjardins A, Friedman H, Sperduto W, Kirkpatrick JP. Long-term outcomes for patients with atypical or malignant meningiomas treated with or without radiation therapy: a 25-year retrospective analysis of a single-institution experience. Adv Radiat Oncol. 2022;7:100878. doi: 10.1016/j.adro.2021.100878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pontoriero A, Critelli P, Conti A, Cardali S, Angileri FF, Germano A, Lillo S, Carretta A, Brogna A, Santacaterina A, Parisi S, Pergolizzi S. The "Combo" radiotherapy treatment for high-risk grade 2 meningiomas: dose escalation and initial safety and efficacy analysis. J Neurooncol. 2022 doi: 10.1007/s11060-022-04107-3. [DOI] [PubMed] [Google Scholar]
- 43.Marchetti M, Pinzi V, Iezzoni C, Morlino S, Tramacere I, De Martin E, Cane I, Fariselli L. Multisession radiosurgery for grade 2 (WHO), high risk meningiomas. A phase II clinical trial. J Neurooncol. 2022;157:397–403. doi: 10.1007/s11060-022-03978-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rogers CL (2023) Phase III Trial of Observation Versus Irradiation for a Gross Totally Resected Grade II Meningioma (NCT03180268). https://clinicaltrials.gov/ct2/show/NCT03180268. Accessed 19th Sept 2023
- 45.Rogers CL, Won M, Vogelbaum MA, Perry A, Ashby LS, Modi JM, Alleman AM, Galvin J, Fogh SE, Youssef E, Deb N, Kwok Y, Robinson CG, Shu HK, Fisher BJ, Panet-Raymond V, McMillan WG, de Groot JF, Zhang P, Mehta MP. High-risk meningioma: initial outcomes from NRG oncology/RTOG 0539. Int J Radiat Oncol Biol Phys. 2020;106:790–799. doi: 10.1016/j.ijrobp.2019.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jenkinson MD, Javadpour M, Haylock BJ, Young B, Gillard H, Vinten J, Bulbeck H, Das K, Farrell M, Looby S, Hickey H, Preusser M, Mallucci CL, Hughes D, Gamble C, Weber DC. The ROAM/EORTC-1308 trial: radiation versus observation following surgical resection of atypical meningioma: study protocol for a randomised controlled trial. Trials. 2015;16:519. doi: 10.1186/s13063-015-1040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Preusser M, Silvani A, Le Rhun E, Soffietti R, Lombardi G, Sepulveda JM, Brandal P, Brazil L, Bonneville-Levard A, Lorgis V, Vauleon E, Bromberg J, Erridge S, Cameron A, Lefranc F, Clement PM, Dumont S, Sanson M, Bronnimann C, Balana C, Thon N, Lewis J, Mair MJ, Sievers P, Furtner J, Pichler J, Bruna J, Ducray F, Reijneveld JC, Mawrin C, Bendszus M, Marosi C, Golfinopoulos V, Coens C, Gorlia T, Weller M, Sahm F, Wick W. Trabectedin for recurrent WHO grade 2 or 3 meningioma: a randomized phase II study of the EORTC Brain Tumor Group (EORTC-1320-BTG) Neuro Oncol. 2022;24:755–767. doi: 10.1093/neuonc/noab243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graillon T, Sanson M, Campello C, Idbaih A, Peyre M, Peyriere H, Basset N, Autran D, Roche C, Kalamarides M, Roche PH, Fuentes S, Tabouret E, Barrie M, Cohen A, Honore S, Boucekine M, Baumstarck K, Figarella-Branger D, Barlier A, Dufour H, Chinot OL. Everolimus and octreotide for patients with recurrent meningioma: results from the phase II CEVOREM trial. Clin Cancer Res. 2020;26:552–557. doi: 10.1158/1078-0432.CCR-19-2109. [DOI] [PubMed] [Google Scholar]
- 49.Youngblood MW, Miyagishima DF, Jin L, Gupte T, Li C, Duran D, Montejo JD, Zhao A, Sheth A, Tyrtova E, Ozduman K, Iacoangeli F, Peyre M, Boetto J, Pease M, Avsar T, Huttner A, Bilguvar K, Kilic T, Pamir MN, Amankulor N, Kalamarides M, Erson-Omay EZ, Gunel M, Moliterno J. Associations of meningioma molecular subgroup and tumor recurrence. Neuro Oncol. 2021;23:783–794. doi: 10.1093/neuonc/noaa226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nassiri F, Liu J, Patil V, Mamatjan Y, Wang JZ, Hugh-White R, Macklin AM, Khan S, Singh O, Karimi S, Corona RI, Liu LY, Chen CY, Chakravarthy A, Wei Q, Mehani B, Suppiah S, Gao A, Workewych AM, Tabatabai G, Boutros PC, Bader GD, de Carvalho DD, Kislinger T, Aldape K, Zadeh G. A clinically applicable integrative molecular classification of meningiomas. Nature. 2021;597:119–125. doi: 10.1038/s41586-021-03850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mair MJ, Berghoff AS, Brastianos PK, Preusser M. Emerging systemic treatment options in meningioma. J Neurooncol. 2023;161:245–258. doi: 10.1007/s11060-022-04148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that data collected for the study and analysis methods will be shared upon reasonable request from any qualified investigator.