Abstract
Objective:
This study aims to analyze adverse drug events (ADEs) associated with cenobamate from the FAERS database, covering the third quarter of 2020 to the second quarter of 2023.
Methods:
Data related to cenobamate-associated ADEs from the third quarter of 2020 to the second quarter of 2023 were collected. After standardizing the data, various signal quantification techniques, including ROR, MHRA, BCPNN, and MGPS, were employed for analysis.
Results:
Among 2535 ADE reports where cenobamate was the primary suspected drug, 94 adverse reactions involving 11 different System Organ Class (SOC) categories were identified through the application of four signal quantification techniques. More specifically, neurological disorders and injuries resultant from complications are frequent adverse reactions associated with cenobamate.
Conclusion:
Our research findings align with established results, affirming the favorable safety profile of cenobamate. Effective prevention of adverse reactions induced by cenobamate can be achieved through the establishment of efficient blood concentration monitoring and dose adjustments.
Keywords: FAERS database, cenobamate, adverse drug events, epilepsy, real-world study
1 Introduction
Epilepsy, as a prevalent neurological disorder, is characterized by sudden abnormal discharges of brain neurons, leading to transient cerebral dysfunction and significantly impacting the physical and mental wellbeing as well as daily life of affected individuals. According to the 2019 Global Burden of Disease (GBD) study, epilepsy affects over 50 million people worldwide (Shu et al., 2023). Despite the fact that the majority of epilepsy patients can achieve seizure control through pharmacological intervention, a subset of patients exhibits poor responsiveness to existing antiepileptic drugs (Panebianco et al., 2023). Hence, the urgent need to identify more effective and less adverse-reactive antiepileptic drugs persists.
The third-generation antiepileptic drug, cenobamate, received approval from the U.S. FDA in November 2019. Its primary mechanisms involve blocking sodium ion channels and positively modulating GABA receptor activity, exhibiting antiepileptic effects. Currently, both the FDA and EMA have sanctioned its use for the treatment of focal epilepsy. Clinical studies demonstrate that, compared to other antiepileptic drugs, cenobamate significantly excels in reducing focal epilepsy seizures (Makridis and Kaindl, 2023). Furthermore, several real-world studies substantiate its significant benefits in treating many drug-resistant epilepsy patients (Beltran-Corbellini et al., 2023; Schmitz et al., 2023). Despite the broad therapeutic potential of cenobamate in managing epilepsy, attention should be directed towards its safety.
The FDA Adverse Event Reporting System (FAERS) serves as a platform for collecting and analyzing drug adverse events (ADEs) related to drug utilization (Iyer et al., 2014). These data represent a crucial resource for evaluating drug safety and effectiveness. The purpose of this article is to analyze adverse event signals related to cenobamate in the real-world using data mining techniques, providing insights for the clinical use of the drug.
2 Methods
Using the trade name “XCOPRI” as the search term in the U.S. FAERS database, we retrieved ADEs reports related to cenobamate from the third quarter of 2020 to the second quarter of 2023. Descriptions and classifications of ADE reports were based on the Preferred Term (PT) and System Organ Class (SOC) concentrated in the Medical Dictionary for Regulatory Activities (MedDRA) terminology set (version 24.0) released by the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use.
ADE reports primarily implicating cenobamate were selected, and duplicates were excluded to minimize bias in ADE risk signal identification. This study employed four methods for ADEs signal mining, including the Reporting Odds Ratio (ROR) method, the Medicines Healthcare Products Regulatory Agency (MHRA) method, Bayesian Confidence Propagation Neural Network (BCPNN) method, and Multi-Item Gamma Poisson Shrinker (MGPS) method (Sakaeda et al., 2013). The ROR method originated from the Lareb laboratory of the Dutch Pharmacovigilance Centre, characterized by less bias and higher sensitivity, hence it is widely applied (Moore et al., 2005). The MHRA method is an extension of the PRR method, combining the PRR value, absolute report numbers, and chi-square values on the premise of ensuring a minimum combination of cases. It is known for its high sensitivity and stability of results and is currently extensively used by the Medicines and MHRA of the United Kingdom (Rothman et al., 2004; Hou et al., 2014). However, studies have shown that the sensitivity of this method decreases as the number of reports increases (Zhang et al., 2017). At present, the BCPNN method is a mature signal detection technique applied both domestically and internationally. It is capable of early signal detection even with fewer data or in case of missing data, and its detection results become more stable as the number of reports increases (van Puijenbroek et al., 2000), but the method is computationally complex and lacks transparency. Additionally, the MGPS method has the advantage of detecting signals for rare events (Jiang et al., 2024). Although there is no gold standard for signal detection methods, each method has its characteristics, with respective advantages and disadvantages in terms of applicability and feasibility in the database. Consequently, this study employed a combination of four methods to obtain signals with strong associations. These four methods compare the ratio of target AEs for the target drug to the ratio of target AEs for all other drugs. If this ratio exceeds a set threshold, it is deemed imbalanced, indicating the generation of potential AEs signals. In this study, a positive signal for drug-related AEs is considered when at least one of the four algorithms meets the criteria; when all four algorithms meet the criteria, it suggests a strong association of AEs, thereby avoiding potential false-positive signals. The parameters required for the ROR and other formulas are calculated based on a 2 × 2 contingency table, which is specifically available in Table 1. Specific formulas and signal detection criteria for the four algorithms can be found in Table 2 (Bate et al., 1998; Evans et al., 2001; van Puijenbroek et al., 2002; Sakaeda et al., 2013).
TABLE 1.
Fourfold table for calculation, used for comparing the association between a specific drug and the occurrence of a specific adverse event.
| Cenobamate-related ADEs | Non-cenobamate-related ADEs | Total | |
|---|---|---|---|
| Cenobamate | a | b | a + b |
| Non-cenobamate | c | d | c + d |
| Total | a + c | b + d | N = a + b + c + d |
ADE, adverse drug events. a is the number of cases where a specific adverse event occurred after using cenobamate, b is the number of cases where cenobamate was used but the specific adverse event did not occur, c is the number of cases where the specific adverse event occurred without the use of cenobamate, d is the number of cases where neither cenobamate was used nor the specific adverse event occurred.
TABLE 2.
Four main algorithms are used to evaluate the correlation between cenobamate and AEDs. This includes ROR, MHRA, BCPNN, and EBGM methods, formulas, and thresholds.
| Method | Formula | Threshold |
|---|---|---|
| ROR | a>3 and 95% CI (lower limit) > 1 | |
| MHRA | a>3, PRR>2 and χ2 > 4 | |
| BCPNN | IC025 > 0 | |
| MGPS | EBGM05 > 2 | |
N, the number of reports; a is the number of cases where a specific adverse event occurred after using cenobamate, b is the number of cases where cenobamate was used but the specific adverse event did not occur, c is the number of cases where the specific adverse event occurred without the use of cenobamate, d is the number of cases where neither cenobamate was used nor the specific adverse event occurred; ROR, reporting odds ratio; γ, γij represent the parameters of the Dirichlet distribution; α, αi, β, βj represent the parameters of the Beta distribution; SD, standard deviation; MHRA, healthcare products regulatory agency; BCPNN, bayesian confidence propagation neural network; MGPS, Multi-Item Gamma Poisson Shrinker; PRR, proportional reporting ratio; EBGM, empirical bayes geometric mean; χ2, chi-squared; IC, information component; IC025, the lower limit of 95% CI, for the IC; E(IC), the IC, expectations; V(IC), the variance of IC; EEBGM05, the lower limit of the 95% CI, for EBGM.
We used SPSS software version 26.0 (IBM, United States), Microsoft Excel 2019, and R software version 4.3.1 for statistical analysis. The creation of figures relied on the “ggplot2” package in the R language.
3 Results
3.1 Descriptive analysis
Following the exclusion of duplicates, data from reports logged between the third quarter of 2020 and the second quarter of 2023 were extracted from the FAERS database. Among 2,535 reports, cenobamate was identified as the primary drug used. The specific relevant information and calculated figures are provided in Supplementary Material S1. The majority of these reports originated from the United States (n = 2,378), with the United Kingdom contributing the second-highest number (n = 29). Within the pool of reports, a cumulative total of 770 serious ADEs were recorded, encompassing instances of fatalities, life-threatening outcomes, disability, and permanent damage. Of these, 315 reports indicated ADEs necessitating hospital admission, 375 reports noted other significant medical events of severity, and there were 36 reports marked with fatalities.
3.2 Signal detection
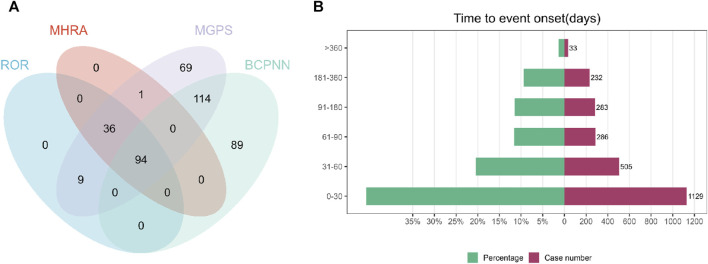
Using four distinct algorithms, including the ROR method and BCPNN method, 139 PTs were found using the ROR method, 131 PTs were separated using the MHRA method, 323 PTs were separated using the EBGM method, and 295 PTs were separated using the BCPNN method. Ultimately, a total of 94 effective PTs were identified, as detailed in Figure 1A. The most prevalent PTs included Seizure (n = 648), Product Dose Omission Issue (n = 446), and Fatigue (n = 340). The top 30 PTs with the strongest associations is displayed in Table 3, according to the frequency of occurrence, while the detailed information for all positive signals is available in Supplementary Table S2. Furthermore, we probed the onset times of each PTs, as depicted in Figure 1B. It was observed that the PTs predominantly clustered within the first month post-medication (n = 1,129), thereafter exhibiting a decremental pattern over time. This insight could hasten the recognition and governance of safety issues related to cenobamate, thereby enabling prompt modifications in therapy to mitigate adverse reactions and augment the effectiveness of the treatment.
FIGURE 1.
(A) The meticulous application of four distinct methodologies culminated in the identification of 94 efficacious PTs. Out of an assemblage of 882 signals, the ROR method surfaced 139 relevant signals, the MHRA method segregated 131, the EBGM method segregated 323, and the BCPNN method segregated 295 effective signals. (B) Onset Time of Adverse Reactions Related to Cenobamate.
TABLE 3.
The top 30 signal strength of adverse events of cenobamate ranked by number of incidence cases at the PTs level in FAERS database.
| PTs | SOC | Case reports | ROR (95% CI) | PRR | EBGM | EBGM05 | IC025 |
|---|---|---|---|---|---|---|---|
| Seizure | Nervous system disorders | 648 | 52.52 (48.45–56.93) | 48.27 | 29,817.45 | 27,871.31 | 3.92 |
| Product dose omission issue | Injury, poisoning and procedural complications | 446 | 20.89 (18.99–22.99) | 19.76 | 7,943.02 | 7,331.75 | 2.63 |
| Fatigue | General disorders and administration site conditions | 340 | 3.58 (3.22–4.00) | 3.47 | 605.79 | 553.13 | 0.13 |
| Somnolence | Nervous system disorders | 334 | 13.39 (12.00–14.94) | 12.86 | 3,659.10 | 3,338.16 | 2.02 |
| Dizziness | Nervous system disorders | 268 | 4.27 (3.78–4.82) | 4.16 | 647.31 | 584.56 | 0.39 |
| Fall | Injury, poisoning and procedural complications | 155 | 3.66 (18.99–22.99) | 3.61 | 293.89 | 257.27 | 0.19 |
| Feeling abnormal | General disorders and administration site conditions | 130 | 4.03 (3.39–4.79) | 3.98 | 291.21 | 251.88 | 0.33 |
| Gait disturbance | General disorders and administration site conditions | 121 | 4.86 (4.06–5.82) | 4.80 | 364.89 | 313.97 | 0.60 |
| Wrong technique in product usage process | Injury, poisoning and procedural complications | 110 | 4.70 (3.89–5.67) | 4.65 | 315.39 | 269.42 | 0.55 |
| Balance disorder | Nervous system disorders | 109 | 9.65 (7.98–11.66) | 9.53 | 831.92 | 710.12 | 1.58 |
| Memory impairment | Nervous system disorders | 85 | 4.78 (3.86–5.92) | 4.74 | 251.26 | 210.10 | 0.58 |
| Hypersomnia | Nervous system disorders | 76 | 21.08 (16.81–26.43) | 20.88 | 1,434.68 | 1,187.19 | 2.71 |
| Product use issue | Injury, poisoning and procedural complications | 74 | 3.28 (2.61–4.12) | 3.26 | 115.96 | 95.74 | 0.04 |
| Vision blurred | Eye disorders | 70 | 4.07 (3.22–5.15) | 4.04 | 160.44 | 131.76 | 0.35 |
| Diplopia | Eye disorders | 69 | 21.24 (16.75–26.93) | 21.06 | 1,314.43 | 1,077.64 | 2.73 |
| Lethargy | Nervous system disorders | 57 | 7.54 (5.81–9.78) | 7.49 | 320.51 | 257.69 | 1.24 |
| Generalised tonic-clonic seizure | Nervous system disorders | 55 | 28.86 (22.13–37.65) | 28.67 | 1,462.33 | 1,170.72 | 3.17 |
| Product availability issue | Product issues | 51 | 28.41 (21.56–37.44) | 28.23 | 1,334.12 | 1,059.04 | 3.15 |
| Dysarthria | Nervous system disorders | 46 | 9.32 (6.97–12.46) | 9.27 | 339.16 | 266.07 | 1.54 |
| Adverse event | General disorders and administration site conditions | 46 | 3.89 (2.91–5.20) | 3.88 | 98.26 | 77.09 | 0.29 |
| Therapy interrupted | Surgical and medical procedures | 43 | 8.47 (6.27–11.43) | 8.43 | 281.20 | 218.79 | 1.41 |
| Aura | Nervous system disorders | 33 | 141.87 (100.41–200.46) | 141.28 | 4,496.20 | 3,366.85 | 5.44 |
| Disturbance in attention | Nervous system disorders | 33 | 4.57 (3.25–6.44) | 4.56 | 91.73 | 68.90 | 0.52 |
| Feeling drunk | General disorders and administration site conditions | 32 | 32.01 (22.60–45.33) | 31.88 | 952.52 | 711.86 | 3.32 |
| Irritability | Psychiatric disorders | 31 | 3.82 (2.68–5.43) | 3.81 | 64.18 | 47.77 | 0.26 |
| Amnesia | Nervous system disorders | 30 | 3.42 (2.39–4.89) | 3.41 | 51.11 | 37.86 | 0.10 |
| Speech disorder | Nervous system disorders | 29 | 4.20 (2.92–6.05) | 4.19 | 70.37 | 51.86 | 0.40 |
| Anger | Psychiatric disorders | 28 | 6.06 (4.18–8.79) | 6.04 | 117.79 | 86.34 | 0.93 |
| Abnormal behaviour | Psychiatric disorders | 27 | 4.81 (3.29–7.01) | 4.79 | 81.04 | 59.07 | 0.59 |
| Partial seizures | Nervous system disorders | 26 | 40.56 (27.56–59.68) | 40.43 | 993.43 | 45.59 | 3.66 |
PT, preferred term; SOC, system organ class; ROR, reporting odds ratio; PRR, proportional reporting ratio; CI, confidence interval; IC, information component; IC025, the lower limit of the 95% CI, for IC; EBGM, empirical bayes geometric mean; EBGM05, the lower limit of the 95% CI, for EBGM.
3.3 Signals of system organ class
The 94 positive signals of PTs were classified according to the MedDRA 24.0 version SOC, revealing that 11 organ systems are impacted by AEs associated with cenobamate. Table 4 elucidates the signal intensities of the cenobamate-linked AEs stratified by SOCs. The positive signals predominantly clustered within three SOCs, namely: Nervous System Disorders (n = 2069), Injury, Poisoning and Procedural Complications (n = 865), and General Disorders and Administration Site Conditions (n = 705), with the comprehensive details of the remaining SOCs available in Table 4. Specifically, neurological disorders along with injuries due to complications such as falls or cranial impacts are noted as common adverse reactions to cenobamate.
TABLE 4.
The signal strength of ADEs of cenobamate at the SOC level in FAERS database.
| System organ class | SOC code | Case reports |
|---|---|---|
| Nervous system disorders | 10,029,205 | 2069 |
| Injury, poisoning and procedural complications | 10,022,117 | 865 |
| General disorders and administration site conditions | 10,018,065 | 705 |
| Psychiatric disorders | 10,037,175 | 199 |
| Eye disorders | 10,015,919 | 155 |
| Surgical and medical procedures | 10,042,613 | 78 |
| Product issues | 10,077,536 | 62 |
| Musculoskeletal and connective tissue disorders | 10,028,395 | 16 |
| Investigations | 10,022,891 | 15 |
| Respiratory, thoracic and mediastinal disorders | 10,038,738 | 11 |
| Social circumstances | 10,041,244 | 6 |
SOC, system organ class; ADE, adverse drug events.
4 Discussion
Cenobamate, as one of the latest antiepileptic drugs, is commonly employed for the treatment of focal seizures in adult patients, offering advantages such as lower cost and improved tolerability (Specchio et al., 2021; Laskier et al., 2023). Functioning not only as a blocker of voltage-gated sodium channels and a positive modulator of GABA receptors, cenobamate also activates the PI3K/Akt-CREB-BDNF pathway, leading to elevated anti-apoptotic factor levels and reduced pro-apoptotic factor levels. This induction inhibits apoptosis, thereby enhancing neuronal survival (Wicinski et al., 2021).
In terms of pharmacokinetic studies on cenobamate, research by Roberti et al. indicates its nonlinear pharmacokinetics. The recommended initial dose of cenobamate is 12.5 mg/day, titrated gradually to the target daily dose of 200 mg, with the possibility of increasing to a maximum of 400 mg/day based on clinical response (Roberti et al., 2021). Some central nervous system-related side effects are more prevalent, including drowsiness, dizziness, diplopia, and gait and coordination disturbances, particularly when the daily dose exceeds 300 mg (Roberti et al., 2021).
Concurrently, studies support the significant improvement in seizure control among adults with uncontrolled focal seizures when cenobamate is used as adjunctive therapy at a dose of 200 mg/day, with good tolerability (Chung et al., 2020; Smith et al., 2022).
Based on clinical trial experience, cenobamate exhibits minor side effects, primarily consisting of dizziness and drowsiness (Catalan-Aguilar et al., 2023; Villanueva et al., 2023). Considering that various neurological and psychiatric conditions are common ADEs) associated with antiepileptic drugs, our study results corroborate this conclusion. Additionally, in patients treated with cenobamate, our study identified high-frequency and strong-signal ADEs such as Seizure (n = 648, ROR = 52.52, IC025 = 3.92) and generalized tonic-clonic seizure (n = 55, ROR = 28.86, IC025 = 3.17), which may be linked to treatment failure with cenobamate. Past research has established a close correlation between antiepileptic drug efficacy and blood drug concentration: elevated concentrations increase toxicity and the likelihood of ADEs, while insufficient concentrations fail to control seizures (Alldredge, 1999).
Furthermore, we observed adverse signals such as Fall (n = 155, ROR = 3.66, IC025 = 0.19) and Head banging (n = 4, ROR = 53.16, IC025 = 4.05). Although some falls may be attributed to poorly controlled seizure symptoms (Jung et al., 2023), numerous studies indicate that less than half of falls and fractures are directly associated with seizures. Falls are also frequent among patients taking antiepileptic drugs (Leppik et al., 2017), posing greater risks and severe consequences, particularly in elderly individuals. However, in another literature on falls in the elderly from the FAERS database, we found that the ROR value for cenobamate is lower than that for common antiepileptic drugs (Zhou et al., 2022), suggesting a favorable effect of cenobamate. Additionally, antiepileptic medications may impinge upon the functionality of the nervous system, encompassing balance and coordination capabilities, thereby elevating the risk of cranial impacts. Although the incidence of head collisions under cenobamate therapy appears to be infrequent, we must nevertheless maintain vigilance regarding this adverse reaction. In summary, monitoring blood drug concentrations during clinical use of antiepileptic drugs is necessary and holds significance for dose adjustments in epilepsy patients. Additionally, our observations revealed that cenobamate may trigger certain skin conditions, such as pruritic rash, possibly due to drug-induced allergic reactions. While generally mild, these skin reactions may serve as precursors to severe allergic reactions (Zgolli et al., 2023). Thus, seeking timely help and advice from healthcare professionals for appropriate diagnosis and treatment is crucial.
The adverse effects of antiepileptic drugs can significantly encroach upon a patient’s quality of life, precipitating physical discomforts such as fatigue, dizziness, and visual disturbances; psychological health issues, including mood fluctuations and depression; as well as cognitive impairments characterized by diminished memory and attention. These detriments may lead to reduced medication adherence, a decline in quality of life, increased economic strain, limited vocational choices, and an intensified sensation of social isolation, as reported in the literature (Kowski et al., 2016; Lin et al., 2016). Furthermore, patients who reduce or discontinue medication due to adverse reactions may experience escalated risks of epilepsy symptom recurrence (Shinnar and Berg, 1995; Ramos-Lizana et al., 2010). This scenario can result in a pernicious cycle that severely compromises the quality of life for many individuals living with epilepsy. To break this cycle, it is imperative to identify antiepileptic medications with fewer adverse reactions and minimal impact on quality of life. Our research observed that severe outcomes comprised 30.4% of the total reports, which signifies that cenobamate has achieved commendable results in clinical therapy, suggesting it might be a preferable treatment option.
Overall, this study, based on the FAERS database and utilizing the ROR method and PRR, among other algorithms, comprehensively presents the safety signal spectrum of cenobamate. It further substantiates cenobamate as a well-tolerated antiepileptic drug.
There are still some limitations in this study. Firstly, while the FAERS database boasts substantial volume and broad coverage, it is marred by incomplete data, with some reports lacking critical information such as age and gender. Additionally, as reporting is voluntary, there is an inherent risk of underreporting, delayed reporting, and misreporting of incomplete information, which introduces potential bias. Secondly, the utilization of analytical methods such as the ROR and PRR can only elucidate the association strength between the medication and ADEs, and cannot directly confirm causality. The actual relationship requires corroboration with existing literature and clinical application. Furthermore, our current study investigated only one limited safety dataset, with all reports predominantly originating from European and American countries. Given regional and ethnic variabilities, these findings may not be extrapolated to other populations, such as those in Asia. Lastly, given cenobamate’s relatively recent introduction to the market, larger-scale clinical trials in the future may unearth additional potential adverse signals. Hence, clinicians should remain vigilant regarding drug safety and promote the judicious use of cenobamate.
5 Conclusion
Our study, predicated upon the data derived from the FAERS database, indicates that cenobamate exhibits a commendable safety profile. We have deliberated on the preventive potential of adverse reactions associated with cenobamate, which can be effectively actualized through the establishment of vigilant therapeutic drug monitoring and meticulous dosage titration. These insights proffer substantive guidance for the clinical utilization of cenobamate in the treatment of epilepsy, further buttressing the assurance of patient safety and therapeutic efficacy during the administration of cenobamate.
Acknowledgments
We would like to thank everyone who participated in this study.
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
SC: Conceptualization, Formal Analysis, Writing–original draft, Writing–review and editing. WF: Formal Analysis, Visualization, Writing–original draft. LZ: Formal Analysis, Writing–original draft. HX: Funding acquisition, Writing–original draft.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1369384/full#supplementary-material
References
- Alldredge B. K. (1999). Seizure risk associated with psychotropic drugs: clinical and pharmacokinetic considerations. Neurology 53, S68–S75. [PubMed] [Google Scholar]
- Bate A., Lindquist M., Edwards I. R., Olsson S., Orre R., Lansner A., et al. (1998). A Bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 54, 315–321. 10.1007/s002280050466 [DOI] [PubMed] [Google Scholar]
- Beltran-Corbellini A., Romeral-Jimenez M., Mayo P., Sanchez-Miranda Roman I., Iruzubieta P., Chico-Garcia J. L., et al. (2023). Cenobamate in patients with highly refractory focal epilepsy: a retrospective real-world study. Seizure 111, 71–77. 10.1016/j.seizure.2023.07.026 [DOI] [PubMed] [Google Scholar]
- Catalan-Aguilar J., Hampel K. G., Cano-Lopez I., Garces M., Lozano-Garcia A., Tormos-Pons P., et al. (2023). Prospective study of cenobamate on cognition, affectivity, and quality of life in focal epilepsy. Epilepsia Open 9, 223–235. 10.1002/epi4.12857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. S., French J. A., Kowalski J., Krauss G. L., Lee S. K., Maciejowski M., et al. (2020). Randomized phase 2 study of adjunctive cenobamate in patients with uncontrolled focal seizures. Neurology 94, e2311–e2322. 10.1212/WNL.0000000000009530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S. J., Waller P. C., Davis S. (2001). Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 10, 483–486. 10.1002/pds.677 [DOI] [PubMed] [Google Scholar]
- Hou Y., Ye X., Wu G., Cheng G., Du X., He J. (2014). A comparison of disproportionality analysis methods in national adverse drug reaction databases of China. Expert Opin. Drug Saf. 13, 853–857. 10.1517/14740338.2014.915938 [DOI] [PubMed] [Google Scholar]
- Iyer S. V., Harpaz R., LePendu P., Bauer-Mehren A., Shah N. H. (2014). Mining clinical text for signals of adverse drug-drug interactions. J. Am. Med. Inf. Assoc. 21, 353–362. 10.1136/amiajnl-2013-001612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Zhou L., Shen Y., Zhou Q., Ji Y., Zhu H. (2024). Safety assessment of Brexpiprazole: real-world adverse event analysis from the FAERS database. J. Affect Disord. 346, 223–229. 10.1016/j.jad.2023.11.025 [DOI] [PubMed] [Google Scholar]
- Jung Y. S., Suh D., Kim E., Park H. D., Suh D. C., Jung S. Y. (2023). Medications influencing the risk of fall-related injuries in older adults: case-control and case-crossover design studies. BMC Geriatr. 23, 452. 10.1186/s12877-023-04138-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowski A. B., Weissinger F., Gaus V., Fidzinski P., Losch F., Holtkamp M. (2016). Specific adverse effects of antiepileptic drugs--A true-to-life monotherapy study. Epilepsy Behav. 54, 150–157. 10.1016/j.yebeh.2015.11.009 [DOI] [PubMed] [Google Scholar]
- Laskier V., Agyei-Kyeremateng K. K., Eddy A. E., Patel D., Mulheron S., James S., et al. (2023). Cost-effectiveness of cenobamate for focal seizures in people with drug-resistant epilepsy. Epilepsia 64, 843–856. 10.1111/epi.17506 [DOI] [PubMed] [Google Scholar]
- Leppik I. E., Yang H., Williams B., Zhou S., Fain R., Patten A., et al. (2017). Analysis of falls in patients with epilepsy enrolled in the perampanel phase III randomized double-blind studies. Epilepsia 58, 51–59. 10.1111/epi.13600 [DOI] [PubMed] [Google Scholar]
- Lin C. Y., Chen H., Pakpour A. H. (2016). Correlation between adherence to antiepileptic drugs and quality of life in patients with epilepsy: a longitudinal study. Epilepsy Behav. 63, 103–108. 10.1016/j.yebeh.2016.07.042 [DOI] [PubMed] [Google Scholar]
- Makridis K. L., Kaindl A. M. (2023). Real-world experience with cenobamate: a systematic review and meta-analysis. Seizure 112, 1–10. 10.1016/j.seizure.2023.09.006 [DOI] [PubMed] [Google Scholar]
- Moore N., Thiessard F., Begaud B. (2005). The history of disproportionality measures (reporting odds ratio, proportional reporting rates) in spontaneous reporting of adverse drug reactions. Pharmacoepidemiol Drug Saf. 14, 285–286. 10.1002/pds.1058 [DOI] [PubMed] [Google Scholar]
- Panebianco M., Bresnahan R., Marson A. G. (2023). Lamotrigine add-on therapy for drug-resistant focal epilepsy. Cochrane Database Syst. Rev. 12, CD001909. 10.1002/14651858.CD001909.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Lizana J., Aguirre-Rodriguez J., Aguilera-Lopez P., Cassinello-Garcia E. (2010). Recurrence risk after withdrawal of antiepileptic drugs in children with epilepsy: a prospective study. Eur. J. Paediatr. Neurol. 14, 116–124. 10.1016/j.ejpn.2009.05.006 [DOI] [PubMed] [Google Scholar]
- Roberti R., De Caro C., Iannone L. F., Zaccara G., Lattanzi S., Russo E. (2021). Pharmacology of cenobamate: mechanism of action, pharmacokinetics, drug-drug interactions and tolerability. CNS Drugs 35, 609–618. 10.1007/s40263-021-00819-8 [DOI] [PubMed] [Google Scholar]
- Rothman K. J., Lanes S., Sacks S. T. (2004). The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 13, 519–523. 10.1002/pds.1001 [DOI] [PubMed] [Google Scholar]
- Sakaeda T., Tamon A., Kadoyama K., Okuno Y. (2013). Data mining of the public version of the FDA adverse event reporting system. Int. J. Med. Sci. 10, 796–803. 10.7150/ijms.6048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz B., Lattanzi S., Vonck K., Kalviainen R., Nashef L., Ben-Menachem E. (2023). Cenobamate in refractory epilepsy: overview of treatment options and practical considerations. Epilepsia Open 8, 1241–1255. 10.1002/epi4.12830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnar S., Berg A. T. (1995). Withdrawal of antiepileptic drugs. Curr. Opin. Neurol. 8, 103–106. 10.1097/00019052-199504000-00002 [DOI] [PubMed] [Google Scholar]
- Shu Y., Wu Z., Yang X., Song M., Ye Y., Zhang C., et al. (2023). The burden of epilepsy in the People's Republic of China from 1990 to 2019: epidemiological trends and comparison with the global burden of epilepsy. Front. Neurol. 14, 1303531. 10.3389/fneur.2023.1303531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. C., Klein P., Krauss G. L., Rashid S., Seiden L. G., Stern J. M., et al. (2022). Dose adjustment of concomitant antiseizure medications during cenobamate treatment: expert opinion consensus recommendations. Neurol. Ther. 11, 1705–1720. 10.1007/s40120-022-00400-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specchio N., Pietrafusa N., Vigevano F. (2021). Is cenobamate the breakthrough we have been wishing for? Int. J. Mol. Sci. 22, 9339. 10.3390/ijms22179339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Puijenbroek E. P., Bate A., Leufkens H. G., Lindquist M., Orre R., Egberts A. C. (2002). A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 11, 3–10. 10.1002/pds.668 [DOI] [PubMed] [Google Scholar]
- van Puijenbroek E. P., Egberts A. C., Heerdink E. R., Leufkens H. G. (2000). Detecting drug-drug interactions using a database for spontaneous adverse drug reactions: an example with diuretics and non-steroidal anti-inflammatory drugs. Eur. J. Clin. Pharmacol. 56, 733–738. 10.1007/s002280000215 [DOI] [PubMed] [Google Scholar]
- Villanueva V., Santos-Carrasco D., Cabezudo-Garcia P., Gomez-Ibanez A., Garces M., Serrano-Castro P., et al. (2023). Real-world safety and effectiveness of cenobamate in patients with focal onset seizures: outcomes from an Expanded Access Program. Epilepsia Open 8, 918–929. 10.1002/epi4.12757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicinski M., Puk O., Malinowski B. (2021). Cenobamate: neuroprotective potential of a new antiepileptic drug. Neurochem. Res. 46, 439–446. 10.1007/s11064-020-03188-8 [DOI] [PubMed] [Google Scholar]
- Zgolli F., Aouinti I., Charfi O., Kaabi W., Hamza I., Daghfous R., et al. (2023). Cutaneous adverse effects of antiepileptic drugs. Therapie 2023. 10.1016/j.therap.2023.09.005 [DOI] [PubMed] [Google Scholar]
- Zhang T., Ye X., Guo X., Wu G., Hou Y., Xu J., et al. (2017). Signal detection based on time to onset algorithm in spontaneous reporting system of China. Drug Saf. 40, 343–350. 10.1007/s40264-016-0503-0 [DOI] [PubMed] [Google Scholar]
- Zhou S., Jia B., Kong J., Zhang X., Lei L., Tao Z., et al. (2022). Drug-induced fall risk in older patients: a pharmacovigilance study of FDA adverse event reporting system database. Front. Pharmacol. 13, 1044744. 10.3389/fphar.2022.1044744 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.