Abstract
A polyomavirus mutant (315YF) blocked in binding phosphatidylinositol 3-kinase (PI 3-kinase) has previously been shown to be partially deficient in transformation and to induce fewer tumors and with a significant delay compared to wild-type virus. The role of polyomavirus middle T antigen-activated PI 3-kinase in apoptosis was investigated as a possible cause of this behavior. When grown in medium containing 1d-3-deoxy-3-fluoro-myo-inositol to block formation of 3′-phosphorylated phosphatidylinositols, F111 rat fibroblasts transformed by wild-type polyomavirus (PyF), but not normal F111 cells, showed a marked loss of viability with evidence of apoptosis. Similarly, treatment with wortmannin, an inhibitor of PI 3-kinase, stimulated apoptosis in PyF cells but not in normal cells. Activation of Akt, a serine/threonine kinase whose activity has been correlated with regulation of apoptosis, was roughly twofold higher in F111 cells transformed by either wild-type virus or mutant 250YS blocked in binding Shc compared to cells transformed by mutant 315YF. In the same cells, levels of apoptosis were inversely correlated with Akt activity. Apoptosis induced by serum withdrawal in Rat-1 cells expressing a temperature-sensitive p53 was shown to be at least partially p53 independent. Expression of either wild-type or 250YS middle T antigen inhibited apoptosis in serum-starved Rat-1 cells at both permissive and restrictive temperatures for p53. Mutant 315YF middle T antigen was partially defective for inhibition of apoptosis in these cells. The results indicate that unlike other DNA tumor viruses which block apoptosis by inactivation of p53, polyomavirus achieves protection from apoptotic death through a middle T antigen–PI 3-kinase–Akt pathway that is at least partially p53 independent.
Programmed cell death occurs during normal development and under certain pathological conditions. In mammalian cells, apoptosis can be induced by a variety of stimuli, including DNA damage (45), virus infection (54, 57), oncogene activation (25), and serum withdrawal (34, 37). Apoptosis can also be blocked by a number of factors, including adenovirus E1B 55- or 19-kDa proteins (9, 16), baculovirus p35 and iap genes (10), Bcl-2 (36, 61), and survival factors (12, 21). DNA tumor viruses have evolved mechanisms that both trigger and inhibit apoptosis. These frequently involve binding and inactivation of tumor suppressor proteins. E7 in some papillomaviruses (22), E1A in adenovirus (31, 43, 64), and large T antigen in simian virus 40 (SV40) (17) bind Rb and/or p300 and lead to upregulation of p53, which is thought to trigger apoptosis in virus-infected cells. The same viruses also inhibit apoptosis by inactivating p53 by various mechanisms (44, 63, 67). In contrast, the mechanism by which polyomavirus interacts with apoptotic pathways in the cell is not known; no direct interaction with p53 by any of the proteins encoded by this virus has been demonstrated (19, 62).
The principal oncoprotein of polyomavirus is the middle T antigen. Neoplastic transformation by polyomavirus middle T antigen has as a central feature its association with and activation of members of the Src family of tyrosine kinases p60c-src (13) and p62c-yes (42). The major known consequence of these interactions is phosphorylation of middle T antigen on specific tyrosine residues creating binding sites for other signaling proteins. Phosphorylation at tyrosines 250, 315, and 322 promotes binding to Shc (18), the p85 regulatory subunit of phosphatidylinositol 3-kinase (PI 3-kinase) (59), and phospholipase Cγ-1 (58), respectively. Recognition of multiple signaling pathways emanating from middle T antigen has led to a keen interest in identifying their downstream biochemical effects, which collectively lead to the emergence of neoplastic transformation and presumably underlie the dramatic ability of the virus to induce many kinds of tumors in the mouse.
Previous work has shown that the binding of PI 3-kinase to middle T antigen is essential for full transformation of rat fibroblasts in culture (8) and for rapid development of a broad spectrum of tumors in mice (30), for translocation of the GLUT1 transporter (68), and activation of p70 S6 kinase (14). While the mutant 315YF (blocked in PI 3-kinase activation) was able to induce some tumors, it did so at reduced frequencies and with an average latency three times longer than that of either the wild-type virus or a mutant, 250YS, blocked in binding Shc (4, 30). Recent studies have indicated a role of PI 3-kinase in blocking apoptosis in nonviral systems. Growth factor receptors acting through protein tyrosine kinases may prevent apoptosis by activating PI 3-kinase in PC12 cells, T lymphocytes, hematopoietic progenitors, and rat fibroblasts (7, 48, 56, 65, 66). The failure of mutant 315YF to induce full transformation of cells in culture and to induce the rapid development of tumors in mice could therefore be related, at least in part, to a failure to block apoptosis. In this study, we focus on the question of whether middle T antigen–PI 3-kinase interaction is involved in blocking apoptosis in cells transformed by polyomavirus.
MATERIALS AND METHODS
Cells.
Rat F111 cells, as well as PyF, PyF-315YF, and PyF-250YS cell lines derived from F111 cells, were described previously (14). Cells were routinely cultured in Dulbecco’s modified Eagle medium (DMEM) containing 10% calf serum, 0.375% sodium bicarbonate, 100 U of penicillin, and 100 μg of streptomycin per ml in a 5% CO2 atmosphere at 37°C. Rat-1 clones stably expressing vector alone (Neo), wild-type, 315YF, and 250YS middle T antigens were prepared by electroporation of Rat-1 cells with defective murine retroviral vectors containing the cloned middle T antigen genes (15, 68). Stably transfected cells were selected with Geneticin (G418) at 400 μg/ml, and clones were isolated by limiting dilution. To establish cell lines expressing temperature-sensitive p53 (p53val135), pBabepuro was cotransfected with a 10-fold excess of pLTRcGp53val135 (47) by electroporation into Rat-1 clones stably expressing vector alone (Neo), wild-type, 315YF, and 250YS middle T antigens. Cells were selected with 2 μg of puromycin per ml and cloned by limiting dilution. Cell lines expressing similar levels of middle T antigen or p53val135 were maintained in DMEM supplemented with 10% calf serum, sodium bicarbonate, penicillin-streptomycin, 100 μg of G418 per ml, and 0.5 to 1 μg of puromycin per ml.
Cell growth assays.
Cells plated at a density of 4 × 103 cells per well in 96-well plates were grown for 4 days in myo-inositol-free DMEM (GIBCO) supplemented with 10% calf serum, 5 μM myo-inositol, and increasing levels of 1d-3-deoxy-3-fluoro-myo-inositol (38). Viable cells were assayed with the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay (49). [3H]1d-3-deoxy-3-fluoro-myo-inositol was obtained from Moravek Biochemicals, Inc.
Ca2+ uptake assays.
To measure Ca2+ uptake, cells grown in myo-inositol-free DMEM supplemented with 10% dialyzed calf serum, 5 μM myo-inositol and 2 mM analog for 72 h were changed to 20 mM HEPES (pH 7.4), 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 50 μM K2HPO4, and 0.1% glucose (buffer A) containing 0.1 mM CaCl2. The reaction was started by adding 2 μCi of 45Ca2+ (NEN) per ml and 100 μM lysophosphatidic acid. After 1 min, the cells were washed five times with buffer A containing 10 mM CaCl2 and solubilized in 1% sodium dodecyl sulfate for scintillation counting and protein determination (bicinchoninic acid assay; Pierce).
Apoptosis assays.
To measure the effect of the myo-inositol analog on apoptosis, F111 and PyF cells were plated on coverslips at a density of 5 × 104 cells per well in 9.6-cm2 wells. After 2 days, the medium was changed to myo-inositol-free DMEM supplemented with 10% dialyzed calf serum, 5 μM myo-inositol, and 2 mM analog, and cells were then grown for 2 days. To measure the effect of serum starvation on apoptosis, 1 × 105 to 4 × 105 cells were plated on coverslips in 8-cm2 plates and grown to 60 to 80% confluence. Cells were washed twice with serum-free medium and incubated at the appropriate temperature. Rat-1 clones expressing p53val135 were cultured at 38.5°C before temperature shift. 4′,6-Diamidino-2-phenylindole (DAPI) staining and the terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assay (Oncor) with attached cells were used to detect apoptotic cells.
Kinase assays.
PI 3-kinase activity in immune complexes prepared with anti-T serum was assayed as described previously (14). Akt kinase was assayed as described previously (20) with histone H2B as the substrate. Cell lysates used for Akt kinase assays were prepared with 20 mM Tris (pH 7.4), 140 mM NaCl, 1% Nonidet P-40, 10 mM NaF, 1 mM Na3VO4, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 25 μg of leupeptin per ml, and 10 μg of aprotinin per ml. Polyclonal antibody 31 to Akt (M. Birnbaum, HHMI, University of Pennsylvania School of Medicine) was used to prepare Akt immune complexes. Akt kinase activity was quantitated with a Molecular Imager (Bio-Rad).
RESULTS
1d-3-Deoxy-3-fluoro-myo-inositol inhibits net growth and stimulates apoptotic death in polyomavirus transformed but not in nontransformed F111 rat fibroblasts.
Analogs of myo-inositol with substitution at the d-3 position are potential antagonists for cells exhibiting a constitutively activated PI 3-kinase and are known to inhibit growth of v-sis-transformed NIH 3T3 cells (52). Several studies have shown that 1d-3-deoxy-3-fluoro-myo-inositol acts as a substrate for mammalian phosphatidylinositol synthase and is incorporated into phosphatidylinositols (38, 51, 52). Signaling via phosphatidylinositol 4,5-bisphosphate and phospholipase C appears to be unaffected by the analog, as indicated by the response of intracellular calcium levels to growth factors (52).
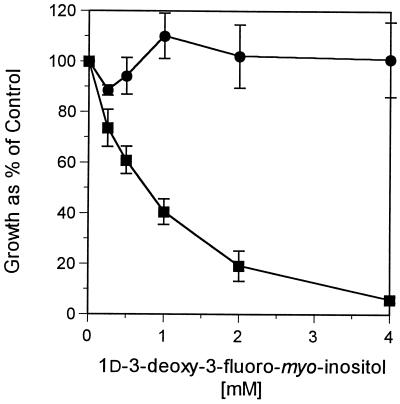
To determine the effect of 1d-3-deoxy-3-fluoro-myo-inositol on the growth of nonvirus-transformed (F111) and polyomavirus-transformed F111 fibroblasts (PyF) (14, 26), cells were grown with increasing levels of 1d-3-deoxy-3-fluoro-myo-inositol (38). The data in Fig. 1 show that PyF cells exhibited marked growth inhibition compared to F111 cells when grown in the presence of 1d-3-deoxy-3-fluoro-myo-inositol (up to 4 mM). Differences in viability were not due to differences in uptake, since studies showed that the analog was incorporated into phospholipid equally well by both cell types (data not shown).
FIG. 1.
Growth of cells in the presence of 1d-3-deoxy-3-fluoro-myo-inositol. Normal F111 (•) and polyomavirus-transformed PyF (▪) cells were plated at subconfluency in myo-inositol-free DMEM containing 10% dialyzed calf serum, 5 μM myo-inositol, and 1d-3-deoxy-3-fluoro-myo-inositol at the concentrations shown and grown for 4 days. Growth was measured by the MTT viability assay as described in Methods and Materials and plotted as a percentage of that of the control without 1d-3-deoxy-3-fluoro-myo-inositol.
The effect of the analog on phospholipase C-dependent pathways was studied by measuring Ca2+ uptake induced by lysophosphatidic acid (40) and was found to be similar in cells grown in the absence or presence of 1d-3-deoxy-3-fluoro-myo-inositol (Fig. 2). Taken together, the data show that while normal and transformed cells take up the 3-deoxy-3-fluoro analog of myo-inositol equally, the growth inhibitory effect was more evident in transformed cells. The effect of the inositol analog on cell viability was apparently unrelated to signal transduction via phospholipase C. Previous work has shown that an increased level of inositol triphosphate in cells expressing wild-type middle T antigen is dependent on p60c-arc but independent of PI 3-kinase activation (33).
FIG. 2.
Effect of 1d-3-deoxy-3-fluoro-myo-inositol on 45Ca2+ uptake induced by lysophosphatidic acid in F111 and PyF cells. Cells were grown on medium containing 5 μM myo-inositol with or without 2 mM 1d-3-deoxy-3-fluoro-myo-inositol for 3 days. 45Ca2+ uptake was measured in the absence (open bars) or presence (hatched bars) of 100 μM lysophosphatidic acid.
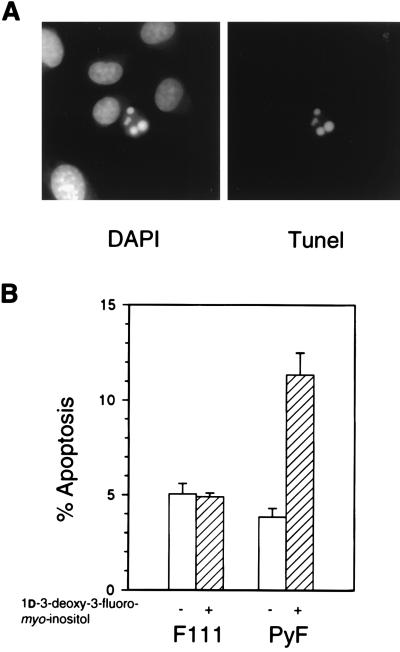
To determine whether the myo-inositol analog induced apoptosis preferentially in PyF cells, F111 and PyF cells were subjected to DAPI staining and the TUNEL assay after growing on 2 mM analog (Fig. 3A). In the presence of 1d-3-deoxy-3-fluoro-myo-inositol, the percentage of apoptosis of PyF cells rose nearly threefold over the level seen in the absence of analog, whereas that of F111 cells remained unchanged (Fig. 3B). These results show that blocking the PI 3-kinase pathway in cells transformed by wild-type polyomavirus drives cells toward apoptosis.
FIG. 3.
Effect of 3-deoxy-3-fluoro-myo-inositol on apoptosis. (A) DAPI and TUNEL staining of PyF cells showing DNA condensation and fragmentation. (B) Percentage of apoptotic cells in cultures grown for 2 days on medium containing 5 μM myo-inositol (open bars) or 5 μM myo-inositol and 2 mM 1d-3-deoxy-3-fluoro-myo-inositol (hatched bars) and quantitated by counting >1,000 cells in duplicate experiments.
Wortmannin stimulates apoptosis in polyomavirus-transformed but not nontransformed F111 cells.
Further evidence that PI 3-kinase helps to prevent apoptosis in polyomavirus-transformed cells was sought by using wortmannin, a potent inhibitor of PI 3-kinase both in vivo and in vitro (53, 60). F111 and PyF cells growing in 10% calf serum were treated with increasing concentrations of wortmannin for 2 h before fixation. TUNEL-positive PyF cells were observed with >10 nM wortmannin, whereas F111 cells failed to undergo apoptosis after treatment with wortmannin at concentrations up to 1,000 nM (Fig. 4A). Wortmannin was shown to inhibit in vitro PI 3-kinase of PyF cells with a similar sensitivity (Fig. 4B). The results support the involvement of PI 3-kinase in preventing apoptosis in cells transformed by polyomavirus but not in nonvirus-transformed F111 cells.
FIG. 4.
Effect of wortmannin on apoptosis of F111 (•) and PyF (▪) cells (A) and in vitro PI 3-kinase activity in anti-T-antigen immunoprecipitates of PyF (▪) cell extracts (B). Cells growing on DMEM containing 10% calf serum were treated with wortmannin for 2 h before fixation for DAPI and the TUNEL assay. The percentage of apoptotic cells was quantitated by counting >1,000 cells in duplicate experiments.
Activation of Akt requires binding of PI 3-kinase to middle T antigen in F111 cells transformed by polyomavirus.
Phosphatidylinositol 3,4-bisphosphate, a product of PI 3-kinase, directly regulates Akt (27), the serine/threonine kinase also designated PKB or Rac (2, 11, 39). Akt has been implicated in inhibition of apoptosis by serum and certain growth factors (5, 20, 41). Receptors blocked in PI 3-kinase binding fail to activate Akt (28), and dominant negative Akt expression induces apoptosis (20). To determine whether transformation by polyomavirus leads to activation of Akt, in vitro kinase assays of Akt immunoprecipitates were performed with extracts from serum-starved cells. Akt activity, shown in Fig. 5, was roughly twofold higher in cells transformed by wild-type and 250YS polyomavirus than in parental F111 cells or cells transformed by mutant 315YF. The level of constitutive activation in PyF approached maximal levels achieved by adding back 15% serum. The levels of phosphatidylinositol 3,4-bisphosphate are known to be elevated in 250YS and wild-type middle T antigen-expressing F111 cells compared to 315YF middle T antigen-expressing cells (14). When F111 cells expressing wild-type or mutant middle T antigens were serum starved and examined for apoptosis, cells expressing 315YF showed a twofold elevation in the percentage of apoptotic cells over the percentage seen in either wild-type or 250YS middle T antigen-expressing cells. These data suggest that activation of Akt depends on binding of PI 3-kinase to middle T antigen and correlates with inhibition of apoptosis.
FIG. 5.
In vitro activation of Akt by polyomavirus middle T antigen. Akt was immunoprecipitated from lysates prepared from cells that had been starved for serum for 24 h. F111, normal rat fibroblasts; PyF, F111 cells transformed by wild-type virus; PyF-315YF, F111 cells transformed by 315YF virus; PyF-250YS, F111 cells transformed by 250YS virus. Data are averaged from two to seven experiments. Error bars show standard deviations.
Wild-type middle T antigen blocks apoptosis induced by serum withdrawal in Rat-1 cells by a p53-independent process.
Rat-1 cells are dependent upon serum for survival and undergo apoptosis when treated with wortmannin (65). To extend the finding of a role for PI 3-kinase in the prevention of apoptosis by middle T antigen, Rat-1 cells were transformed with retroviral vectors encoding wild-type or mutant middle T antigens and selected with G418. In an attempt to determine if the apoptotic response of these cells is p53 dependent, clones stably expressing the middle T antigens were also transfected with a temperature-sensitive p53 gene, p53val135 (47), and selected for puromycin resistance. p53val135 behaves like a dominant inhibitory mutant at the restrictive temperature (38.5°C) and exhibits wild-type function at 32°C. At 38.5°C, p53val135 cooperates with E1A or ras to transform primary cells which also express wild-type endogenous p53 (16, 35, 47). p53val135 is impaired in transcription activation (23), repression (1), and nuclear translocation (6, 32, 46) at the nonpermissive temperature.
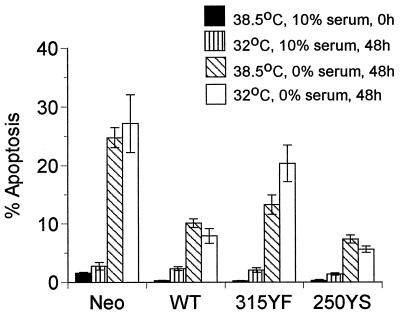
To determine the effect of p53 on apoptosis, all clones were grown with 10% calf serum at 38.5°C and either fixed for apoptosis assays at 0 h or grown for 2 days more either at 38.5°C in serum-free medium or at 32°C in medium with or without calf serum and then fixed (Fig. 6). On shifting to 32°C in the presence of serum, there was only a slight increase in the percentage of apoptotic cells in all clones. However, when normal cells or cells expressing 315YF mutant middle T antigen were shifted down and the serum was removed, there was roughly a 10-fold increase in the percent of apoptotic cells after 2 days. Cells expressing either wild-type middle T antigen or mutant 250YS middle T antigen showed a roughly threefold increase in apoptotic cells under the same conditions, indicating an effect of middle T antigen-activated PI 3-kinase in partially blocking the apoptotic response brought on by serum withdrawal. Protection against apoptotic death upon serum withdrawal by wild-type and 250YS middle T antigens was not significantly temperature sensitive in any of the clones. The protection afforded by middle T antigen–PI 3-kinase interaction thus appeared to be p53 independent, at least to a large extent.
FIG. 6.
Serum starvation-induced apoptosis in Rat-1 clones expressing p53val135 along with the neomycin resistance vector alone (Neo [control]) or T antigens (wild type [WT], 315YF, or 250YS). Four plates of each cell type were grown in DMEM containing 10% calf serum at 38.5°C to 70% confluence. Two plates remained in 10% serum, and two plates were changed to serum-free medium. Cells in 10% serum were either fixed at 0 h (solid bars) or shifted to 32°C (vertically striped bars) and fixed at 48 h. Cells in 0% serum either remained at 38.5°C (hatched bars) or were shifted to 32°C (open bars), and then both were fixed at 48 h. Data are averaged from four to seven separate experiments. Error bars show the standard errors of the means.
DISCUSSION
Apoptosis in F111 rat fibroblasts transformed by polyomavirus is shown to be enhanced when PI 3-kinase activity is inhibited by either growth of cells on 1d-3-deoxy-3-fluoro-myo-inositol or treatment with wortmannin, indicating involvement of PI 3-kinase in blocking apoptosis. In contrast, normal F111 cells are much less dependent on PI 3-kinase for survival, as indicated by their resistance to death induced by the myo-inositol analog and by wortmannin. Transformation of F111 by polyomavirus mutant 315YF blocked in activation of PI 3-kinase significantly enhances the susceptibility of these cells to apoptosis induced by serum withdrawal compared to that in cells transformed by the wild-type or 250YS polyomavirus that encodes middle T antigens that activate PI 3-kinase. Cells transformed by mutant 315YF also have a lower constitutive activity of Akt, a serine/threonine kinase regulated by phosphatidylinositol 3,4-bisphosphate (27) and known to be associated with regulation of apoptosis (20, 41). This suggests that signal transduction via middle T antigen through PI 3-kinase and Akt is important for survival and protection from apoptosis.
In contrast to F111 rat fibroblasts, nontransformed Rat-1 fibroblasts show a marked serum dependence for survival and are susceptible to wortmannin-induced apoptosis (65). Apoptosis induced by serum withdrawal in Rat-1 cells expressing p53val135 appears to be principally p53 independent, since the level of apoptosis at 32°C is not significantly higher than that at 38.5°C. In Rat-1 cells expressing wild-type or 250YS middle T antigen, there is significant protection against apoptosis upon serum withdrawal and the degree of protection is largely temperature independent. Rat-1 cells expressing 315YF mutant middle T antigen show less protection against apoptotic death, indicating a role of PI 3-kinase in blocking apoptosis under these conditions. While Rat-1 cells stably transfected with pLTRcGp53val135 possibly retain a low level of functional p53 at the nonpermissive temperature, which might arise either from unquenched endogenous wild-type p53 or p53val135 that retains partial wild-type activity, the absolute amount of functional p53 should be markedly lower at 38.5°C than at 32°C. Consistent with our findings, p53 independence of apoptosis induced by serum withdrawal has also been observed in other cell types (3, 24, 37, 55).
The ability of a virus to delay host cell death is thought to be essential for virus growth (54). Whether polyomavirus can block apoptosis has been unclear. Other DNA tumor viruses, such as adenovirus and SV40, inhibit programmed cell death by inactivation of p53. Since polyomavirus has no known direct interaction with p53 (19), a separate antiapoptotic mechanism is indicated. The results presented here indicate that in polyomavirus-infected cells, middle T antigen, acting through PI 3-kinase and Akt, blocks apoptosis. Interestingly, viruses that handle p53 directly by inactivation or degradation (i.e., adenovirus, SV40, and papillomavirus) lack direct mechanisms for activating PI 3-kinase. Recent evidence suggests that PI 3-kinase and Akt block apoptosis by inhibiting the Ced3/ICE-like activity (41), and Ced3/ICE-like proteases are thought to lie on apoptotic pathways downstream of both p53 (43) and the FAS pathway (50).
Mutant 315YF polyomavirus, whose middle T antigen fails to bind PI 3-kinase, is associated with delayed appearance of tumors in neonatally infected mice (8, 14, 29). In contrast, the 250YS mutant, whose middle T antigen binds PI 3-kinase but fails to bind Shc, induces tumors broadly and with little or no delay compared to wild-type virus (4). The delay in tumor induction by the mutant 315YF may be due to failure to protect against apoptosis in the lytic phase of viral growth or in tumor development.
ACKNOWLEDGMENTS
We thank Geoffrey Cooper for providing Rat-1 cells, Phil Hinds for providing the temperature-sensitive p53 expression plasmid pLTRcGp53val135, and Morris Birnbaum for providing anti-Akt.
This work was supported by grants from the National Cancer Institute to T.L.B. (R35-CA44343) and D.C.B. (R01-CA45795).
REFERENCES
- 1.Avantaggiati M L, Ogryzko V, Gardner K, Giordano A, Levine A S, Kelly K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell. 1997;89:1175–1184. doi: 10.1016/s0092-8674(00)80304-9. [DOI] [PubMed] [Google Scholar]
- 2.Bellacosa A, Testa J R, Staal S P, Tsichlis P N. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 3.Blandino G, Scardigli R, Rizzo M G, Crescenzi M, Soddu S, Sacchi A. Wild-type p53 modulates apoptosis of normal, IL-3 deprived hematopoietic cells. Oncogene. 1995;10:731–737. [PubMed] [Google Scholar]
- 4.Bronson R, Dawe C, Carroll J, Benjamin T. Tumor induction by a transformation-defective polyoma virus mutant blocked in signaling through Shc. Proc Natl Acad Sci USA. 1997;94:7954–7958. doi: 10.1073/pnas.94.15.7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgering B M T, Coffer P J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 6.Caelles C, Helmberg A, Karin M. p53-dependent apoptosis in the absence of transcriptional activation of p53-target genes. Nature. 1994;370:220–223. doi: 10.1038/370220a0. [DOI] [PubMed] [Google Scholar]
- 7.Cai Y C, Cefai D, Schneider H, Raab M, Nabavi N, Rudd C E. Selective CD28pYMNM mutations implicate phosphatidylinositol 3-kinase in CD86-CD28-mediated costimulation. Immunity. 1995;3:417–426. doi: 10.1016/1074-7613(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 8.Carmichael G, Schaffhausen B S, Mandel G, Liang T J, Benjamin T L. Transformation by polyoma virus is drastically reduced by substitution of phenylalanine for tyrosine at residue 315 of middle sized tumor antigen. Proc Natl Acad Sci USA. 1984;81:679–683. doi: 10.1073/pnas.81.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiou S-K, Tseng C-C, Rao L, White E. Functional complementation of the adenovirus E1B 19-kilodalton protein with Bcl-2 in the inhibition of apoptosis in infected cells. J Virol. 1994;68:6553–6566. doi: 10.1128/jvi.68.10.6553-6566.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clem R J, Fechheimer M, Miller L K. Prevention of apoptosis by a baculovirus gene during infection of insect cells. Science. 1991;254:1388–1390. doi: 10.1126/science.1962198. [DOI] [PubMed] [Google Scholar]
- 11.Coffer P J, Woodgett J R. Molecular cloning and characterization of a novel putative protein-serine kinase related to the cAMP-dependent and protein kinase C families. Eur J Biochem. 1992;201:475–481. doi: 10.1111/j.1432-1033.1991.tb16305.x. [DOI] [PubMed] [Google Scholar]
- 12.Collins M K L, Perkins G R, Rodriguez-Tarduchy G, Nieto M A, López-Rivas A. Growth factors as survival factors: regulation of apoptosis. Bioessays. 1994;16:133–138. doi: 10.1002/bies.950160210. [DOI] [PubMed] [Google Scholar]
- 13.Courtneidge S A, Smith A E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature (London) 1983;303:435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- 14.Dahl J, Freund R, Blenis J, Benjamin T L. Studies of partially transforming polyomavirus mutants establish a role for phosphatidylinositol 3-kinase in activation of pp70 S6 kinase. Mol Cell Biol. 1996;16:2728–2735. doi: 10.1128/mcb.16.6.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dahl J, Thathamangalam U, Freund R, Benjamin T L. Functional asymmetry of the regions juxtaposed to the membrane-binding sequence of polyomavirus middle T antigen. Mol Cell Biol. 1992;12:5050–5058. doi: 10.1128/mcb.12.11.5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Debbas M, White E. Wild-type p53 mediates apoptosis by E1A, which is inhibited by E1B. Genes Dev. 1993;7:546–554. doi: 10.1101/gad.7.4.546. [DOI] [PubMed] [Google Scholar]
- 17.DeCaprio J A, Ludlow J W, Figge J, Shew J Y, Huang C M, Lee W H, Marsillio E, Paucha E, Livingston D M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988;54:275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- 18.Dilworth S M, Brewster C E, Jones M D, Lanfrancone L, Pelicci G, Pelicci P G. Transformation by polyoma virus middle T-antigen involves the binding and tyrosine phosphorylation of Shc. Nature (London) 1994;367:87–90. doi: 10.1038/367087a0. [DOI] [PubMed] [Google Scholar]
- 19.Doherty J, Freund R. Polyomavirus large T antigen overcomes p53 dependent growth arrest. Oncogene. 1997;14:1923–1931. doi: 10.1038/sj.onc.1201025. [DOI] [PubMed] [Google Scholar]
- 20.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 21.Dulbecco R. Topoinhibition and serum requirement of transformed and untransformed cells. Nature. 1970;227:802–806. doi: 10.1038/227802a0. [DOI] [PubMed] [Google Scholar]
- 22.Dyson N, Howley P M, Munger K, Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989;243:934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- 23.El-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 24.El-Deiry W S, Harper J W, O’Conner P M, Velculescu V E, Canman C E, Jackman J, Peitenpol J A, Burrell M, Hill D E, Wang Y, Wiman K G, Mercer W E, Kastan M B, Kohn K W, Elledge S J, Kinzler K W, Vogelstein B. WAF/CIP1 is induced in p53 mediated G1 arrest and apoptosis. Cancer Res. 1994;54:1169–1174. [PubMed] [Google Scholar]
- 25.Evan G I, Wyllie A H, Gilbert C S, Littlewood T D, Land H, Brooks M, Waters C M, Penn L Z, Hancock D C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 26.Fluck M M, Benjamin T L. Comparison of two early gene functions essential for transformation in polyoma virus and SV-40. Virology. 1979;96:205–228. doi: 10.1016/0042-6822(79)90185-5. [DOI] [PubMed] [Google Scholar]
- 27.Franke T F, Kaplan D R, Cantley L C, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 28.Franke T F, Yang S, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 29.Freund R, Dawe C J, Carroll J P, Benjamin T L. Changes in frequency, morphology, and behavior of tumors induced in mice by a polyoma virus mutant with a specifically altered oncogene. Am J Pathol. 1992;141:1409–1425. [PMC free article] [PubMed] [Google Scholar]
- 30.Freund R, Sotnikov A, Bronson R T, Benjamin T L. Polyoma virus middle T is essential for virus replication and persistence as well as for tumor induction in mice. Virology. 1992;191:716–723. doi: 10.1016/0042-6822(92)90247-m. [DOI] [PubMed] [Google Scholar]
- 31.Furlong E E M, Rein T, Martin F. YY1 and NF1 both activate the human p53 promoter by alternatively binding to a composite element, and YY1 and E1A cooperate to amplify p53 promoter activity. Mol Cell Biol. 1996;16:5933–5945. doi: 10.1128/mcb.16.10.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ginsberg D, Michael-Michalovitz D, Ginsberg D, Oren M. Induction of growth arrest by a temperature-sensitive p53 mutant is correlated with increased nuclear localization and decreased stability of the protein. Mol Cell Biol. 1991;11:582–585. doi: 10.1128/mcb.11.1.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorga F R, Riney C E, Benjamin T L. Inositol trisphosphate levels in cells expressing wild-type and mutant polyomavirus middle T antigens: evidence for activation of phospholipase C via activation of pp60c-src. J Virol. 1990;64:105–112. doi: 10.1128/jvi.64.1.105-112.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrington E A, Bennett A F, Evan G I. c-Myc-induced apoptosis in fibroblasts is inhibited by specific cytokines. EMBO J. 1994;13:3286–3295. doi: 10.1002/j.1460-2075.1994.tb06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinds P, Finlay C, Levine A J. Mutation is required to activate the p53 gene for cooperation with the ras oncogene and transformation. J Virol. 1989;63:739–746. doi: 10.1128/jvi.63.2.739-746.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hockenbery D, Nuñez G, Milliman C, Schreiber R D, Korsmeyer S J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 37.Ishizaki Y, Cheng L, Mudge A W, Raff M C. Programmed cell death by default in embryonic cells, fibroblasts, and cancer cells. Mol Biol Cell. 1995;6:1443–1458. doi: 10.1091/mbc.6.11.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson S C, Dahl J, Shih T-L, Schedler D J A, Anderson L, Benjamin T L, Baker D C. Synthesis and evaluation of 3-modified 1d-myo-inositols as inhibitors and substrates of phosphatidylinositol synthase and inhibitors of myo-inositol uptake by cells. J Med Chem. 1993;36:3628–3635. doi: 10.1021/jm00075a018. [DOI] [PubMed] [Google Scholar]
- 39.Jones P F, Jakubowicz T, Pitossi F J, Maurer F, Hemmings B A. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc Natl Acad Sci USA. 1991;88:4171–4175. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaplan P L, Ozanne B. Polyoma virus-transformed cells produce transforming growth factor(s) and grow in serum-free medium. Virology. 1982;123:372–380. doi: 10.1016/0042-6822(82)90270-7. [DOI] [PubMed] [Google Scholar]
- 41.Kennedy S G, Wagner A J, Conzen S D, Jordan J, Bellacosa A, Tsichlis P N, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 42.Kornbluth S, Sudol M, Hanafusa H. Association of the polyomavirus middle-T antigen with c-yes protein. Nature (London) 1987;325:171–173. doi: 10.1038/325171a0. [DOI] [PubMed] [Google Scholar]
- 43.Levine A J. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 44.Levine A J. The tumor suppressor genes. Annu Rev Biochem. 1993;62:523–651. doi: 10.1146/annurev.bi.62.070193.003203. [DOI] [PubMed] [Google Scholar]
- 45.Lowe S W, Ruley H E. Stabilization of the p53 tumor suppressor is induced by adenovirus 5 E1A and accompanies apoptosis. Genes Dev. 1993;7:535–545. doi: 10.1101/gad.7.4.535. [DOI] [PubMed] [Google Scholar]
- 46.Martinez J, Georgoff I, Martinez J, Levine A J. Cellular localization and cell cycle regulation by a temperature-sensitive p53 protein. Genes Dev. 1991;5:151–159. doi: 10.1101/gad.5.2.151. [DOI] [PubMed] [Google Scholar]
- 47.Michalovitz D, Halevy O, Oren M. Conditional inhibition of transformation and of cell proliferation by a temperature-sensitive mutant of p53. Cell. 1990;62:671–680. doi: 10.1016/0092-8674(90)90113-s. [DOI] [PubMed] [Google Scholar]
- 48.Minshall C, Arkins S, Freund G G, Kelley K W. Requirement for phosphatidylinositol 3′-kinase to protect hemopoietic progenitors against apoptosis depends upon the extracellular survival factor. J Immunol. 1996;156:939–947. [PubMed] [Google Scholar]
- 49.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 50.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 51.Offer J, Metcalfe J C, Smith G A. The uptake of 3H-labelled monodeoxyfluoro-myo-inositols into thymocytes and their incorporation into phospholipid in permeabilized cells. Biochem J. 1993;291:553–560. doi: 10.1042/bj2910553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Powis G, Aksoy I A, Melder D C, Aksoy S, Eichinger H, Fauq A H, Kozikowski A P. d-3-Deoxy-3-substituted myo-inositol analogues as inhibitors of cell growth. Cancer Chemother Pharmacol. 1991;29:95–104. doi: 10.1007/BF00687317. [DOI] [PubMed] [Google Scholar]
- 53.Powis G, Bonjouklian R, Berggren M M, Gallegos A, Abraham R, Ashendel C, Zalkow L, Matter W F, Dodge J, Grindey G, Vlahos C J. Wortmannin, a potent and selective inhibitor of phosphatidylinositol 3-kinase. Cancer Res. 1994;54:2419–2423. [PubMed] [Google Scholar]
- 54.Razvi E S, Welsh R M. Apoptosis in viral infections. Adv Virus Res. 1995;45:1–60. doi: 10.1016/s0065-3527(08)60057-3. [DOI] [PubMed] [Google Scholar]
- 55.Sakamuro D, Eviner V, Elliott K J, Showe L, White E, Predergast G C. c-Myc induces apoptosis in epothelial cells by both p53-dependent and p53-independent mechanisms. Oncogene. 1995;11:2411–2418. [PubMed] [Google Scholar]
- 56.Scheid M P, Lauener R W, Duronio V. Role of phosphatidylinositol 3-OH-kinase activity in the inhibition of apoptosis in haemopoietic cells: phosphatidylinositol 3-OH-kinase inhibitors reveal a difference in signalling between interleukin-3 and granulocyte-macrophage colony stimulating factor. Biochem J. 1995;312:159–162. doi: 10.1042/bj3120159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen Y, Shenk T E. Viruses and apoptosis. Curr Opin Gen Dev. 1995;5:105–111. doi: 10.1016/s0959-437x(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 58.Su W, Liu W, Schaffhausen B S, Roberts T M. Association of polyomavirus middle tumor antigen with phospholipase C-gamma1. J Biol Chem. 1995;270:12331–12334. doi: 10.1074/jbc.270.21.12331. [DOI] [PubMed] [Google Scholar]
- 59.Talmage D A, Freund R, Young A T, Dahl J, Dawe C J, Benjamin T L. Phosphorylation of middle T by pp60c-src: a switch for binding of phosphatidylinositol 3-kinase and optimal tumorigenesis. Cell. 1989;59:55–65. doi: 10.1016/0092-8674(89)90869-6. [DOI] [PubMed] [Google Scholar]
- 60.Thelen M, Wymann M P, Langen H. Wortmannin binds specifically to 1-phosphatidylinositol 3-kinase while inhibiting guanine nucleotide-binding protein-coupled receptor signaling in neutrophil leukocytes. Proc Natl Acad Sci USA. 1994;91:4960–4964. doi: 10.1073/pnas.91.11.4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vaux D L, Cory S, Adams J M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 62.Wang E H, Friedman P N, Prives C. The murine p53 protein blocks replication of SV40 DNA in vitro by inhibiting the initiation function of SV40 large T antigen. Cell. 1989;57:379–392. doi: 10.1016/0092-8674(89)90913-6. [DOI] [PubMed] [Google Scholar]
- 63.Werness B A, Levine A J, Howley P M. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 64.Whyte P, Buchkovich K, Horowitz J M, Friend S H, Raybuck M, Weinberg R A, Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988;334:124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- 65.Yao R, Cooper G M. Growth factor-dependent survival of rodent fibroblasts requires phosphatidylinositol 3-kinase but is independent of pp70S6K activity. Oncogene. 1996;13:343–351. [PubMed] [Google Scholar]
- 66.Yao R, Cooper G M. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science. 1995;267:2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]
- 67.Yew P R, Berk A J. Inhibition of p53 transactivation required for transformation by adenovirus early 1B protein. Nature. 1992;357:82–85. doi: 10.1038/357082a0. [DOI] [PubMed] [Google Scholar]
- 68.Young A T, Dahl J, Hausdorff S F, Bauer P H, Birnbaum M J, Benjamin T L. Phosphatidylinositol 3-kinase binding to polyoma virus middle tumor antigen mediates elevation of glucose transport by increasing translocation of the GLUT1 transporter. Proc Natl Acad Sci USA. 1995;92:11613–11617. doi: 10.1073/pnas.92.25.11613. [DOI] [PMC free article] [PubMed] [Google Scholar]