Abstract
The human cytomegalovirus (CMV) US28 gene encodes a functional CC chemokine receptor. However, this activity was observed in cells transfected to express US28 and might not correspond to the actual role of the protein in the CMV life cycle. Expression of US28 allows human immunodeficiency virus type 1 (HIV-1) entry into certain CD4+ cells and their fusion with cells expressing HIV-1 envelope (Env) proteins. Such properties were initially reported for the cellular chemokine receptors CCR5 and CXCR4, which behave as CD4-associated HIV-1 coreceptors. We found that coexpression of US28 and either CXCR4 or CCR5 in CD4+ cells resulted in enhanced synctium formation with HIV-1 Env+ cells. This positive effect of US28 on cell fusion seems to be distinct from its HIV-1 coreceptor activity. Indeed, enhancement of cell fusion was also observed when US28 was expressed on the HIV-1 Env+ cells instead of an CD4+ target cells. Furthermore, US28 could enhance cell fusion mediated by other viral proteins, in particular, the G protein of vesicular stomatitis virus (VSV-G). The HIV-1 coreceptor and fusion-enhancing activities could be affected by mutations in different domains of US28. The fusion-enhancing activity of US28 seems to be cell type dependent. Indeed, cells coexpressing VSV-G and US28 fused more efficiently with human, simian, or feline target cells, while US28 had no apparent effect on fusion with the three mouse or rat cell lines tested. The positive effect of US28 on cell fusion might therefore require its interaction with a cell-specific factor. We discuss a possible role for US28 in the fusion of the CMV envelope with target cells and CMV entry.
G protein-coupled receptors (GCRs) form an extremely large family of signal-transducing proteins involved in numerous biological functions (50). Viral proteins with seven predicted membrane-spanning domains and other features indicating their homology with cellular GCRs were identified initially in human cytomegalovirus (CMV), as the products of the US27, US28, and UL33 genes (9), and later in herpesvirus saimiri (37), equine herpesvirus (51), mouse CMV (MCMV) (44), and human herpesviruses 6 (23), 7 (36), and 8 (known as Kaposi’s sarcoma-associated herpesvirus [KSHV]) (8). Some of these viral proteins are only putative GCRs, as their ligands are unknown (orphan receptors). Functionality in terms of ligand binding and signal transduction has been shown for the US28 protein of CMV (22, 35), the ECRF3 protein of herpesvirus saimiri (2), and the product of KSHV open reading frame 74 (3). Their ligands belong to the family of cellular chemokines, which are small soluble proteins (60 to 80 amino acids) involved in leukocyte chemotaxis (4). Chemokines from the CC subgroup activate US28, while ECRF3 is activated by interleukin-8 and other CXC chemokines. The KSHV-encoded GCR can also bind interleukin-8, but it is constitutively activated, which may play a role in its oncogenic and angiogenic properties (5).
The role played by these GCRs or putative GCRs in the viral life cycle is unknown. Expression of the M33 protein of MCMV was shown to be necessary for virus dissemination in vivo but not in tissue culture (14). Virally encoded GCRs might confer on infected cells responsiveness to cellular chemokines, or to other ligands in the case of orphan receptors. However, it cannot be ascertained that the GCR activity observed in cells transfected to express these viral proteins is relevant to their role in vivo. The US27, US28, and UL33 genes are transcribed late after CMV infection (53), which suggests that they encode structural proteins rather than regulating proteins. The presence of the UL33 protein in CMV particles was indeed demonstrated (29).
Expression of the CMV-encoded chemokine receptor US28 in CD4+ cells could allow their infection by human immunodeficiency virus type 1 (HIV-1) or 2 (HIV-2) or their fusion with cells expressing HIV-1 or HIV-2 envelope (Env) proteins (42). Therefore, US28 apparently shares properties with the cellular chemokine receptors CCR5 and CXCR4, which behave as CD4-associated coreceptors for HIV-1 or HIV-2 (17, 33). Here we show that US28 can enhance cell-cell fusion by a mechanism apparently distinct from HIV coreceptor activity, which leads us to discuss a possible role in CMV entry.
MATERIALS AND METHODS
Cell lines.
The U373MG-CD4 (24), U87MG-CD4 (10), and HeLa P4 (CD4+) (12) cell lines and the HeLa P4 CCR5+ derivative HeLa P5 cell line (42) are stably transfected with Escherichia coli lacZ under transcriptional control of the HIV-1 long terminal repeat (LTRlacZ). The HeLa-Env/LAI (46) and HeLa-Env/ADA (42) cell lines stably express Env from HIV-1LAI and HIV-1ADA, respectively. Cell lines expressing HIV-1 Tat derived from HeLa (46), NIH 3T3 (18), and Dunni and XC (15) cells have been described previously. The Tat+ derivative of the B5 rhesus macaque cell line (ATCC CL-160) was obtained from M. Sitbon (Centre National de la Recherche Scientifique, Montpellier, France). The cat cell line CrFK (38) was obtained from J. Richardson (Institut Cochin de Génétique Moléculaire). All cell lines were propagated in Dulbecco’s modified Eagle’s medium (Gibco) supplemented with antibiotics (60 μg of penicillin per ml and 100 μg of streptomycin per ml) and 10% fetal calf serum (or 10% newborn calf serum in the case of NIH 3T3 cells).
Expression vectors.
The HIV-1LAI Env expression vector was pMA243, a Δgag-pol HIV-1LAI provirus also expressing Tat and Rev (46). The pCEL (15) and pCMV-G (58) vectors allow expression of human T-cell leukemia virus type 1 strain CR (HTLV-1CR) Env and vesicular stomatitis virus G protein (VSV-G), respectively, from the CMV immediate-early promoter. Chemokine receptors and GCR cDNAs were subcloned in Rc/CMV (InVitrogen, La Jolla, Calif.) or pCDNA-3 (Clontech, Palo Alto, Calif.) downstream from the CMV immediate-early promoter. The expression vectors for CXCR4 (41), CCR3 (48), and CCR5, US28, and MYC-tagged US28 (42) are described elsewhere. Expression vectors for CCR1, CCR4, CXCR1, and CXCR2 were obtained from N. Sol (Institut Cochin de Génétique Moléculaire). The corresponding open reading frames were PCR amplified from HeLa cells DNA and subcloned in Rc/CMV. The deduced amino acid sequences of these GCRs were identical to those reported previously (34, 35, 43). The US27 and UL33 open reading frames were PCR amplified from fibroblasts infected with the CMV AD169 strain and subcloned in Rc/CMV. The US27 and UL33 sequences were identical to those reported previously (9). The pCDNA/M33 expression vector (14) was obtained from N. Davis-Poynter (University of Western Australia, Nedlands, Australia). A pCDNA.3 vector expressing KSHV GCR (3) was obtained from M. N. Gershengorn (Cornell University, New York, N.Y.). The US28 mutants listed in Table 1 were obtained by oligonucleotide-directed mutagenesis (sequences of oligonucleotides are available upon request). Mutants were screened for the creation of restriction enzyme sites and checked by nucleotide sequencing.
TABLE 1.
HIV-1 coreceptor activities and fusion-enhancing activities of WT and mutant US28
| US28 expression vector | HIV-1 core- ceptor activity (no. of syncytia)a | Effect on VSV-G fusion (no. of syncytia)b | Fusion enhancement (fold) |
|---|---|---|---|
| None (Rc/CMV) | 2 ± 0.8 | 564 ± 147 | 1 |
| WT US28 | 643 ± 176 | 1,033 ± 127 | 1.8 |
| MYC-tagged US28 (NT insertion) | 163 ± 102 | 1,533 ± 125 | 2.7 |
| Mutant US28 | |||
| Δ2-22 (NT deletion) | 1.3 ± 0.5 | 1,313 ± 123 | 2.3 |
| Δ317 (CT deletion) | 713 ± 75 | 1,850 ± 234 | 3.3 |
| K158V K159V (ECL2) | 770 ± 64 | 653 ± 148 | 1.2 |
| K257V (ECL3) | 115 ± 26 | 1,243 ± 95 | 2.2 |
| E266V R267V (ECL3) | 1 ± 0.8 | 1,569 ± 81 | 2.8 |
US28 mutants were expressed in HeLa P4 cells (CD4+ LTRlacZ) and cocultured with HeLa-Env/ADA cells. The numbers of blue-stained foci per well (six-well trays; means and standard deviations for triplicate wells) after staining with X-Gal are shown.
US28 mutants and VSV-G were coexpressed in HeLa-Tat cells and cocultured with U87MG-CD4-LTRlacZ cells. The numbers of blue-stained foci per well (six-well trays; means and standard deviations) after staining with X-Gal are shown. No fusion was detected if VSV-G was omitted.
Transfection of cells and syncytium formation assays.
About 105 cells per well were seeded in six-well trays and incubated overnight at 37°C in complete medium. The medium was replaced 2 to 4 h before addition of the DNA-calcium phosphate precipitate (4 μg of DNA per well) and after overnight incubation with the precipitate. Cocultures were initiated 24 h after transfection by adding fusion partner cells or by detaching transfected cells with trypsin, mixing with fusion partner cells, and seeding a 12-well tray. In all cases, the fusion partner cells were in a 1:1 ratio. Cocultures were ended after 24 h by fixing cells with 0.5% glutaraldehyde and staining with the β-galactosidase substrate X-Gal (5-bromo-4-chloro-3-indoyl-β-d-galactopyranoside) as described previously (19). Blue foci were scored at a magnification of ×20. Numbers of >200 were extrapolated from randomly selected fields.
Flow cytometry.
HeLa P4 cells were cotransfected with EGFP-N1 (Clontech), a green fluorescent protein (GFP) expression vector, and with one or two chemokine receptor expression vectors or Rc/CMV (controls). The weight ratio of EGFP-N1 to the other plasmids was 1:6 or 1:4. Cells were detached 36 h after transfection with phosphate-buffered saline containing 1 mM EDTA, stained with antibodies in phosphate-buffered saline containing 1% fetal calf serum, fixed, and analyzed by flow cytometry as described previously (42). The following monoclonal antibodies were used: Leu3A (Becton Dickinson, San Jose, Calif.), anti-CD4, 0.5 μg/ml; 12G5 (20) anti-CXCR4, 6 μg/ml; 2D7 (6) anti-CCR5, 0.8 μg/ml; 9E10 (Boerhinger, Mannheim, Germany), anti-MYC, 0.4 μg/ml; and W6/32 (Dako, Glostrub, Denmark), anti-major histocompatibility complex class I (MHC-I), 3.5 μg/ml. 12G5 and 2D7 were obtained from the National Institutes of Health AIDS Reagent Program. Only Leu3A was directly coupled to phycoerythrin (PE). In the other cases, cells were stained with a secondary PE-coupled goat antimouse antiserum (Dako) used at 16 μg/ml.
RESULTS
US28 enhances HIV-1 fusion.
We have developed simple and sensitive assays allowing detection and quantification of the fusion of cells to form syncytia. These assays are based on the presence of the HIV-1 transactivating protein (Tat) in one cell type and of a Tat-inducible reporter gene, such as the E. coli β-galactosidase gene lacZ linked to the HIV-1 long terminal repeat (LTRlacZ), in the other. Upon fusion of these cell types and cytoplasm mixing, Tat gains access to the nucleus and activates lacZ transcription. The high level of β-galactosidase activity in syncytia allows their detection by histochemical staining (19). This technique can be used to test the activity of candidate HIV-1 coreceptors. For example, HeLa P4 cells (LTRlacZ CD4+) naturally express CXCR4 and can form syncytia with cells expressing Env from a cell line-adapted HIV-1 strain (HeLa-Env/LAI cells) but not from a macrophage (M)-tropic HIV-1 strain (HeLa-Env/ADA cells). Fusion with HeLa-Env/ADA cells can be observed when HeLa P4 cells are transfected with vectors allowing expression of CCR5 or US28 (42).
Transfection of HeLa P4 cells with a US28 expression vector increased the number of syncytia detected in cocultures with HeLa-Env/LAI cells (Fig. 1A). This effect was not observed when HeLa P4 cells were transfected with a CCR5 expression vector, as expected, or with a CXCR4 expression vector. The coreceptor activity of US28 toward cell line-adapted HIV-1 is relatively low (42) and was unlikely to explain the markedly increased number of syncytia observed in this experiment. By measuring the β-galactosidase activity in HeLa P4 cells after transfection with the US28 expression vector or with US28 and Tat expression vectors, we ruled out a direct effect of US28 on LTRlacZ expression or Tat-mediated transactivation (data not shown).
FIG. 1.
Enhancement of HIV-1 Env-mediated cell-cell fusion by US28. HeLa- CD4-LTRlacZ cells (A and B) or U373MG-CD4-LTRlacZ cells (C and D) were transfected with Rc/CMV (Mock) or with CXCR4, CCR5, and US28 expression vectors, as indicated. Cocultures were performed with HeLa cells stably expressing Tat and Env from cell line-adapted HIV-1LAI (A and C) or from M-tropic HIV-1ADA (B and D). Transfections were performed in six-well trays with 4 μg of vector, or with 2 μg of each vector when chemokine receptors were coexpressed. Cells from a subconfluent well were detached with trypsin 24 h posttransfection. Half of them were seeded with an equivalent number of Env+ cells in one well from a 12-well tray. Cells were fixed and stained with X-Gal after a 24-h coculture. Bars represent mean numbers (with standard deviations) of blue-stained foci, indicating cell fusion events, in duplicate wells.
To look for a possible effect of US28 on cell fusion mediated by Env from HIV-1ADA, HeLa P4 cells were transfected with 4 μg of the CCR5 or the US28 vector or with 2 μg of each vector, and cocultures were performed with HeLa-Env/ADA cells. Higher numbers of syncytia were detected when HeLa P4 cells were cotransfected with the CCR5 and US28 vectors (Fig. 1B). As expected, transfection of a CXCR4 expression vector did not allow fusion with HeLa-Env/ADA cells. Therefore, cell fusion mediated by an M-tropic Env seemed to be more efficient when target cells coexpressed the CCR5 coreceptor and US28.
Positive effects of US28 on syncytium formation were also observed with the LTRlacZ CD4+ derivative of the U373MG astroglioma cell line, which is naturally resistant to infection by both M-tropic and cell line-adapted HIV-1 (24, 42). Higher numbers of syncytia were formed with Env+ cells when U373MG-CD4 cells were cotransfected with the US28 and CXCR4 vectors or with the US28 and CCR5 vectors than when parallel transfections with the same quantity of DNA from each of these vectors were performed (Fig. 1C and D). These experiments showed that cell fusion mediated by the HIV-1 envelope proteins was enhanced when CD4+ cells target cells coexpressed the US28 chemokine receptor and a HIV-1 coreceptor, either CXCR4 or CCR5.
The surface expression of CD4, MHC-I, and CXCR4 was assessed by flow cytometry after transfection of HeLa P4 cells with US28, CCR5, or CCR1 vectors, or with Rc/CMV (control cells), and a GFP expression vector. Surface expression of CD4, MHC-1, and CXCR4 among GFP-positive cells, considered to represent the fraction of HeLa P4 cells expressing transfected DNA, was measured. The presence of US28, CCR5, or CCR1 had no apparent effect on the surface expression of CD4 and MHC-I (Fig. 2A). The surface expression of CXCR4 was downregulated in cells transfected with the US28 vector, in comparison with control cells, or with cells transfected with the CCR5 or CCR1 vector. In a similar experiment, we found that CCR5 surface expression was slightly lower in cells cotransfected with CCR5 and US28 vectors than in cells cotransfected with CCR5 and CXCR4 vectors (Fig. 2B). The mechanism by which US28 influenced the surface expression of CXCR4 or CCR5 was not further explored in this study. These experiments ruled out the possibility that the positive effects of US28 on cell fusion were due to an increase in the surface expression of CD4 or the HIV-1 coreceptors.
FIG. 2.
Flow cytometry analysis of cell surface markers in HeLa P4 cells transfected with different chemokine receptor expression vectors. (A) Surface expression of CD4, MHC-1, or CXCR4 after transfection with two vectors, i.e., EGFP-N1 (GFP expression vector) and either Rc/CMV-US28, Rc/CMV-CCR5, Rc/CMV-CCR1, or Rc/CMV (control), in a 1:6 ratio. (B) Surface expression of CCR5 after transfection with three vectors, i.e., EGFP-N1, Rc/CMV-CCR5, and either Rc/CMV-US28, Rc/CMV-CXCR4, or Rc/CMV (control), in a 1:2:2 ratio. Cells were stained with PE-coupled antibodies (red fluorescence) 36 h after transfection and analyzed as indicated in Materials and Methods. The graphs show red fluorescence intensity (x axis, arbitrary units, log scale) and numbers of cells (y axis) among GFP-positive cells (transfected cells). Thick lines, transfections with chemokine receptor expression vectors; thin lines and gray areas, control transfections with Rc/CMV.
Coexpression of US28 and viral fusiogenic proteins.
We next asked if US28 could enhance syncytium formation when it was expressed in the Env+ cells instead of the target cells. A Tat+ HeLa cell line was cotransfected to express HIV-1LAI Env and either US28 or CCR5, and cocultures were performed with HeLa P4 cells. The number of syncytia was markedly higher when cells expressed US28 (Fig. 3A). Similar numbers of syncytia were detected when cells were transfected with the CCR5 vector or with Rc/CMV. As expected, there was no detectable fusion when Tat+ HeLa cells expressed US28 (or CCR5) in the absence of Env (data not shown). A positive effect on cell fusion was also observed when US28 was expressed in HeLa-Env/ADA cells and coculture was performed with HeLa P5, a CCR5+ cell line derived from HeLa P4 (Fig. 3B). In these experiments, the positive effects on US28 cell fusion could not be explained by its HIV-1 coreceptor activity or by the modulation of CD4, CXCR4, or CCR5 surface expression.
FIG. 3.
Coexpression of US28 and viral fusiogenic proteins. HeLa-Tat cells were cotransfected with expression vector for HIV-1LAI Env (A), HTLV-1 Env (C), or VSV-G (D) and with either Rc/CMV (mock), Rc/CMV-CCR5, or Rc/CMV-US28, as indicated. Each of these Rc/CMV vectors was also transfected in cells stably expressing Env from HIV-1ADA (B). Cocultures (six-well trays) were initiated 24 h later by adding an equivalent number of HeLa P4 cells (A, C, and D) or their CCR5+ derivatives, HeLa P5 cells (B). Cells were fixed and stained with X-Gal after a 24-h coculture. Bars represent mean numbers (with standard deviations) of blue-stained foci in triplicate wells.
We next looked for an effect of US28 on cell-cell fusion mediated by the gp46 and gp21 envelope proteins of another retrovirus, HTLV-1, and by the rhabdovirus protein VSV-G. The ability of gp46 and gp21 to mediate syncytium formation among cells from different mammalian species and tissue origins is well documented (15, 49). As expected, expression of gp46 and gp21 in Tat+ HeLa cells allowed their fusion with HeLa P4 cells (Fig. 3C). VSV-G was shown to induce syncytium formation only after exposure of cells to a mildly acidic pH (55), in agreement with the pH-dependent entry of VSV (30). However, we found that transfection of a VSV-G expression vector into Tat+ HeLa cells allowed their fusion with HeLa P4 cells under normal tissue culture pH conditions (Fig. 3D). The reason for this apparent discrepancy is unknown. The sensitivity of our cell fusion assay might be higher, allowing detection of activity of VSV-G under suboptimal conditions. Alternatively, the activity of VSV-G might be different in certain cell types, in particular when expressed by transfection. Similar discrepancies are known for some murine leukemia virus strains, which also apparently infect cells by a pH-dependent pathway yet induce syncytium formation in certain cell types or under certain experimental conditions (25, 56).
Cocultures were performed with HeLa P4 cells and Tat+ HeLa cells cotransfected to express HTLV-1 gp46-gp21 or VSV-G and either CCR5 or US28. Higher numbers of syncytia were detected when cells were transfected with the US28 vector (Fig. 3C and D). Flow cytometry experiments showed no effect of US28 on the surface expression of gp46 (data not shown). The surface expression of HIV-1 Env or VSV-G has not been analyzed.
Effects of other chemokine receptors on cell-cell fusion.
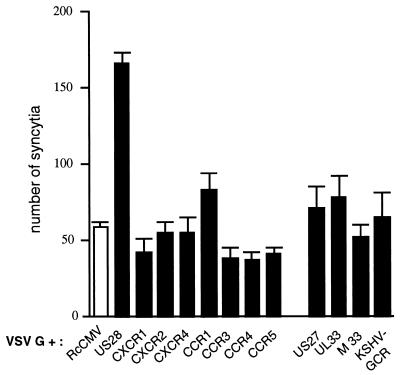
Although CCR5 or CXCR4 had no apparent effect on cell-cell fusion, besides their HIV-1 coreceptor activity, a panel of GCRs was tested by coexpression with VSV-G in Tat+ HeLa cells and coculture with HeLa P4 cells. Among cellular chemokine receptors, only CCR1 had a modest positive effect on cell fusion (Fig. 4), which was not seen in other experiments (see Fig. 5; other data not shown). Notably, flow cytometry analysis of cells transfected with epitope-tagged chemokine receptors suggests that the surface expression is high in the case of CCR1 (11) and relatively low in the case of US28 (42). The KSHV-encoded chemokine receptor and the putative GCRs encoded by the US27 and UL33 genes of human CMV or by the M33 gene of MCMV did not seem to enhance cell fusion in a way comparable to that for US28 (Fig. 4).
FIG. 4.
Coexpression of VSV-G and chemokine receptors or virally encoded GCRs. Transfections of HeLa-Tat cells with expression vectors for VSV-G and for the indicated GCRs and cocultures with HeLa P4 cells were performed as described for Fig. 3. US27 and UL33 are putative GCRs encoded by human CMV; M33 is a putative GCR from MCMV. Bars represent mean numbers (with standard deviations) of blue-stained foci in triplicate wells.
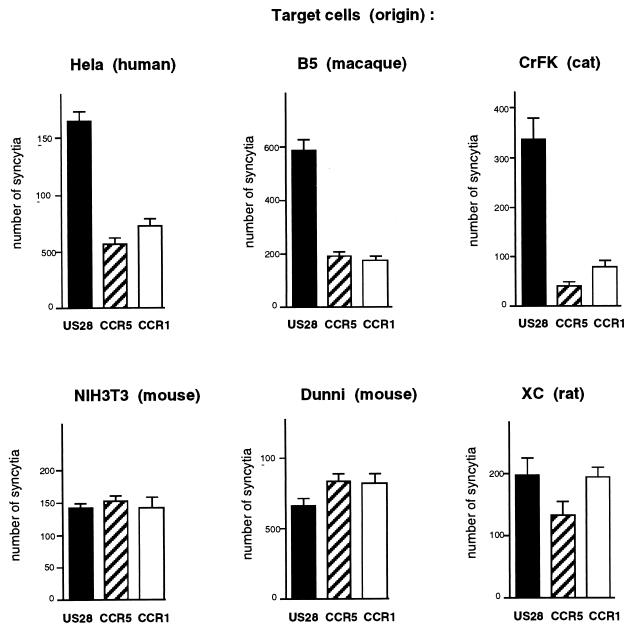
FIG. 5.
Cell type restriction of the fusion-enhancing activity of US28. HeLa P4 cells were cotransfected in six-well trays with expression vectors for VSV-G and for either US28, CCR5, or CCR1. An equivalent number of the indicated target cells, stably expressing HIV-1 Tat or transiently transfected with Rc/CMV-Tat (CrFK), was added 24 h later. Cells were fixed and stained with X-Gal after a 24-h coculture. Bars represent mean numbers (with standard deviations) of blue-stained foci in triplicate wells.
Properties of mutant US28.
A series of US28 mutants was tested for their HIV-1 coreceptor activity by transfection of HeLa P4 cells and coculture with HeLa-Env/ADA cells and for their fusion-enhancing activity by coexpression with VSV-G in Tat+ HeLa cells and coculture with the human astroglioma cell line U87MG-CD4-LTRlacZ. The results of these experiments are summarized in Table 1. The insertion of a 15-amino-acid (aa) sequence from the human c-MYC oncoprotein at the amino terminus of US28 allows its detection at the cell surface by staining with the 9E10 antibody (42). When this mutant (MYC-tagged US28) was expressed in HeLa P4 cells, the number of syncytia formed with HeLa-Env/ADA cells was about fourfold lower than that with wild-type (WT) US28. In contrast, the positive effect of MYC-tagged US28 on cell-cell fusion mediated by the VSV-G was higher. The coreceptor and fusion-enhancing activities could require different amounts of US28 at the cell surface or could be mediated by distinct domains. The importance of the amino-terminal (NT) extracellular domain for the HIV-1 coreceptor activity is shown by the phenotype of the Δ2-22 US28 mutant, in which most of the 33-aa-long NT domain is deleted. This mutant US28 had no detectable HIV-1 coreceptor activity, while it could enhance cell-cell fusion in a way comparable to that for WT US28.
Truncation of the carboxy-terminal (CT) cytoplasmic domain of US28 (aa 296 to 355) beyond aa 317 had minor effects on HIV-1 coreceptor activity and apparently increased fusion-enhancing effects. Flow cytometry analysis of cells transfected with MYC-tagged forms of WT and mutant US28 suggests that the truncation of the CT domain results in a higher level of cell surface expression (Table 2).
TABLE 2.
Surface expression of WT and mutant US28a
| MYC-tagged receptor | GFP-positive cells (%) | MYC-positive cellsb (%) |
|---|---|---|
| None (Rc/CMV vector) | 24.1 | 0.3 |
| WT US28 | 19.2 | 15.5 |
| US28 Δ317 | 21.7 | 25 |
| US28 K158V K159V | 19.9 | 11.4 |
| US28 K257V | 24.8 | 9.9 |
| US28 E266V RS267V | 20.2 | 9.6 |
HeLa P4 cells were cotransfected in six-well trays with pCDNA3 expression vectors for different MYC-tagged forms of US28 and with EGFP-N1, a GFP expression vector (6:1 ratio). Cells were harvested 36 h later, stained with the anti-MYC monoclonal antibody 9E10 and with a PE-conjugated secondary antibody, and analyzed for GFP expression (green fluorescence) and MYC expression (red fluorescence). Results are from the same series of transfections.
Among GFP-positive cells.
Point mutations were created in the third and fourth extracellular domains of US28, corresponding to the second and third extracellular loops (ECL), respectively. The replacement of two basic residues (lysine) by neutral residues (valine) at positions 158 and 159 in ECL2 had no apparent effect on the HIV-1 coreceptor activity of US28 but abolished its fusion-enhancing activity. Opposite results were observed with two mutants corresponding to amino acid substitutions in ECL3. Both had fusion-enhancing activities comparable to that of WT US28, while their coreceptor activities were markedly reduced (in the case of the K257V mutant) or null (in the case of the E266V R267V mutant). The surface expression of MYC-tagged forms of these mutants was reduced by comparison with that of WT US28 (Table 2). The amount of US28 available at the cell surface might be more important for the fusion-enhancing activity than for the HIV-1 coreceptor activity, or these properties might require interactions with different domains of US28.
Effect of US28 on different cell types.
In all previous experiments, US28 enhanced syncytium formation between fusion effector and target cells that were both of human origin (HeLa, U373MG, or U87MG cell lines). Since the VSV-G protein does not require a cell-specific receptor to mediate cell fusion, it was possible to test the activity of US28 with target cells from different species in the same experimental setting. HeLa P4 cells were cotransfected with expression vectors for VSV-G and for either US28, CCR1, or CCR5, and cocultures were performed with cell lines from human, simian, feline, or murine origin, stably or transiently expressing Tat. For all target cells tested, similar numbers of syncytia were detected when HeLa P4 cells were transfected with the CCR5 or CCR1 vector (Fig. 5) or with Rc/CMV (data not shown), confirming that CCR5 or CCR1 did not enhance cell fusion mediated by VSV-G. The numbers of fusion events that were detected varied according to the target cell type, which might reflect differences in fusion efficiency but also in expression of Tat and/or efficiency of LTRlacZ transactivation. The lower numbers of syncytia detected with CrFK cells are probably due to their transient transfection to express Tat. Transfection of the US28 vector resulted in markedly increased numbers of syncytia formed with human HeLa cells, as expected, or with macaque B5 cells or feline CrFK cells (Fig. 5), indicating that the fusion-enhancing activity of US28 was not restricted to cells of human origin. In contrast, US28 had no apparent effect on VSV-G-mediated fusion with mouse NIH 3T3 and Dunni cells or with rat XC cells, which suggests that its fusion-enhancing activity is dependent on target cells.
DISCUSSION
Expression of the CMV-encoded chemokine receptor US28 in CD4+ cell lines allows their infection by HIV-1 and their fusion with cells expressing HIV-1 envelope proteins (Env+ cells) (42). Such properties were initially reported for the cellular chemokine receptors CCR5 and CXCR4 (17, 33), which were later shown to interact with the gp120 envelope protein and to behave as CD4-associated HIV-1 coreceptors (27, 52, 57). The interaction of gp120 with two cellular components, CD4 and a coreceptor, is thought to trigger conformational changes that eventually activate the fusiogenic properties of the transmembrane Env subunit gp41.
Coreceptor activity of US28.
Besides CCR5 and CXCR4, several chemokine receptors and related orphan GCRs have been found to be able to mediate CD4-dependent HIV-1 entry, with various efficacies (11, 16, 21, 45). Although their interaction with gp120 was not formally established, these GCRs were inferred to behave as HIV-1 coreceptors, like CXCR4 or CCR5. In the case of US28, it can be wondered if the promiscuous fusion-enhancing activity might not account for its HIV-1 coreceptor activity. In other terms, could US28 allow HIV-1 entry by a mechanism different from that of CXCR4 or CCR5? It might be envisioned that the CD4+ cells used to test candidate HIV-1 coreceptors (e.g., HeLa or U373MG cells) have an intrinsic ability to fuse with Env+ cells, too low to be detected by current assays but revealed by the promiscuous activity of US28 on cell-cell fusion. We found that a mutation in the third ECL of US28 or a deletion in the NT domain abolished its HIV-1 coreceptor activity, while these changes had no apparent effect on the fusion-enhancing activity. Moreover, opposite effects resulted from mutations in the second ECL. These results indicate that the promiscuous effect of US28 on cell-cell fusion is not required for its HIV-1 coreceptor activity. The mechanism by which US28 and other GCRs allow infection of CD4+ cells by HIV-1 or their fusion with Env+ cells is probably similar to the coreceptor activity of CCR5 or CXCR4.
Mechanism of fusion enhancement.
The expression of US28 enhanced the efficiency of cell-cell fusion mediated by envelope proteins from three different viruses, HIV-1, HTLV-1, and VSV. This effect was observed when US28 was expressed in target cells or in cells bearing the viral fusiogenic proteins. These elements strongly suggest that the effect of US28 on syncytium formation is not due to a direct interaction with the fusiogenic proteins or their cellular receptors.
The mechanism by which viral proteins mediate virus entry, or syncytium formation, is not known in its molecular details. The energy stored in their conformation is used to overcome repulsive hydration forces between membranes in order to allow their close apposition. The next steps seem to be the formation of a fusion pore, generally viewed as a proteinaceous structure forming a bridge between membranes, and its dilatation (28, 32, 54). Physicochemical features of the membranes, such as charge and lipid composition, influence their ability to engage in fusion. For example, compounds decreasing the surface potential of membranes, such as polyethylene glycol, are well known to induce cell-cell fusion. High cholesterol concentrations in target membranes can enhance the efficiency of fusion mediated by viral proteins (26). The positive effect of amphotericin B and related polyene macrolides on cell-cell fusion induced by HIV-1 Env or by other viral proteins (39, 40) could be related to their ability to interact with membrane cholesterol (7).
High local concentrations of a protein with multiple membrane-spanning domains, such as US28, might affect the fluidity or other physical properties of membranes in a way favorable to fusion. However, positive effects on cell-cell fusion were not observed, or were considerably less efficient, for proteins sharing the membrane topology of US28 and apparently expressed at a higher level, for example, CCR1. A major argument against a direct effect of US28 on membranes was its lack of fusion-enhancing activity in cocultures with murine target cells. Indeed, US28 could enhance VSV-G-mediated fusion with three human cell lines (HeLa, U87MG, and U373MG), the simian cell line B5, and the cat cell line CrFK but not that with NIH 3T3 or Dunni mouse cells or rat XC cells. In this experiment, the fusion-enhancing activity of US28 depended upon the target cell and probably upon the presence or absence of a component of its plasma membrane. The most likely hypothesis seems to be that the fusion-enhancing activity requires the interaction of US28 with a membrane component. Such a putative US28 ligand would be present in human cell lines of different tissue origin but also in simian and feline cells, suggesting a certain degree of conservation among species. On the other hand, it should either be absent in the rat or mouse cells that we have tested or be too different in these species to be functional. Analysis of other cell types from different species is necessary to confirm these views and evaluate the biochemical nature of this factor. Its interaction with US28 might either promote cell-cell contact or result in an indirect effect on the US28-expressing cell promoting membrane fusion. It would be of interest to test the effect on fusion of a mutant US28 devoid of cell signalling activity.
Possible role of US28 in CMV entry.
It is often envisioned that US28 confers on CMV-infected cells responsiveness to CC chemokines, thereby modulating viral gene expression or contributing to establishment of latent infection (22). Changes in the intracellular concentrations of calcium, diacylglycerol, and other second messengers were indeed reported for CMV-infected cells (1), but it cannot be ascertained that these phenomena are mediated by US28. The US28, US27, and and UL33 genes were found to be transcribed with late CMV genes (53), which generally encode structural proteins. According to that study, US28, US27, or UL33 would be expressed soon before virus release and cell death, which seems to disfavor a possible role in the regulation of CMV gene expression. It remains possible that smaller amounts of US28 are expressed earlier in infected cells (31) or that the pattern of expression is different in other cell types or in vivo. Alternatively, it can be envisioned that the main function of US28 is not to regulate CMV expression in response to CC chemokines through its GCR activity.
The pattern of expression of US28 and its membrane topology are compatible with its association with the lipidic envelope of virions. The putative GCR encoded by the UL33 gene was indeed shown to be associated with the CMV envelope (29). If US28 is also expressed in the CMV envelope, its role could be to direct virions to sites of inflammation, where high concentrations of CC chemokines are found. However, the nature of the viral and cellular proteins involved in CMV entry is still debated (13, 47), and it can be envisioned that US28 has a direct role in CMV entry, through its ability to enhance membrane fusion. This hypothesis could be addressed by testing the replicative ability and efficiency of cell entry of a US28-defective CMV. We did not observe enhancement of HIV-1 infectivity when US28 was expressed in HIV-1-producing cells or in CD4+ target cells (besides HIV-1 coreceptor activity). However, the experimental systems used did not ensure that US28 was actually borne by HIV-1 particles, and the stable expression of US28 at a high level in target cells could not be achieved. Further experiments are therefore necessary to define whether US28 can activate virus-cell fusion and play a role in CMV entry.
Although numerous herpesvirus proteins display features of GCRs, few were shown to have signal-transducing activity. Some might be activated through interaction with unknown ligands. Others might be devoid of GCR activity, in particular if cellular GCRs were captured by viruses for their ability to bind certain ligands or for other properties, such as their transmembrane topology. Our results with US28 suggest that these possibilities deserve further investigation.
ACKNOWLEDGMENTS
We thank N. Davis-Poynter, M. N. Gershengorn, J. Richardson, O. Schwartz, M. Sitbon, and N. Sol for generous gifts of reagents; I. Bouchaert and F. Letourneur for help with flow cytometry and sequencing; and L. Picard and N. Heveker for critical reading of the manuscript.
This work was supported by the Agence Nationale de Recherches sur le SIDA and by a fellowship to O.P. from Ensemble contre le SIDA.
REFERENCES
- 1.AbuBakar S, Boldogh I, Albrecht T. Human cytomegalovirus stimulates arachidonic acid metabolism through pathways that are affected by inhibitors of phospholipase-A2 and protein kinase-2. Biochem Biophys Res Commun. 1990;166:953–959. doi: 10.1016/0006-291x(90)90903-z. [DOI] [PubMed] [Google Scholar]
- 2.Ahuja S K, Murphy P M. Molecular piracy of mammalian interleukin-8 receptor type B by herpesvirus saimiri. J Biol Chem. 1993;268:20691–20694. [PubMed] [Google Scholar]
- 3.Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn M C, Cesarman E. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature. 1997;385:347–350. doi: 10.1038/385347a0. [DOI] [PubMed] [Google Scholar]
- 4.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 5.Bais C, Santomasso B, Coso O, Arvanitakis L, Geras-Raaka E, Gutkind J S, Asch A S, Cesarman E, Gershengorn M C, Mesri E A. G-protein-coupled receptor of Kaposi’s sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391:86–89. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- 6.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolard J. How do the polyene macrolides antibiotics affect the cellular membrane properties? Biochem Biophys Acta. 1986;864:257–304. doi: 10.1016/0304-4157(86)90002-x. [DOI] [PubMed] [Google Scholar]
- 8.Cesarman E, Nador R G, Bai F, Bohenzky R A, Russo J J, Moore P S, Chang Y, Knowles D M. Kaposi’s sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi’s sarcoma and maligant lymphoma. J Virol. 1996;70:8218–8231. doi: 10.1128/jvi.70.11.8218-8223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chee M S, Satchwell S C, Preddie E, Weston K M, Barrell B G. Human cytomegalovirus encodes three G protein-coupled receptor homologues. Nature. 1990;344:774–777. doi: 10.1038/344774a0. [DOI] [PubMed] [Google Scholar]
- 10.Chesebro B, Buller R, Portis J, Wehrly K. Failure of human immunodeficiency virus entry and infection in CD4-positive human brain and skin cells. J Virol. 1990;64:215–221. doi: 10.1128/jvi.64.1.215-221.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 12.Clavel F, Charneau P. Fusion from without directed by human immunodeficiency virus particles. J Virol. 1994;68:1179–1185. doi: 10.1128/jvi.68.2.1179-1185.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Compton T, Nowlin D M, Coper N R. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology. 1993;193:834–841. doi: 10.1006/viro.1993.1192. [DOI] [PubMed] [Google Scholar]
- 14.Davis-Poynter N J, Lynch D M, Vally H, Shellam G R, Rawlinson W D, Barrell B G, Farrell H E. Identification and characterization of a G protein-coupled receptor homolog encoded by murine cytomegalovirus. J Virol. 1997;71:1521–1529. doi: 10.1128/jvi.71.2.1521-1529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denesvre C, Sonigo P, Corbin A, Ellerbrok H, Sitbon M. Influence of transmembrane domains on the fusogenic abilities of human and murine leukemia retrovirus envelopes. J Virol. 1995;69:4149–4157. doi: 10.1128/jvi.69.7.4149-4157.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng H, Unutmaz D, KewalRamani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 17.Doms R W, Peiper S C. Unwelcomed guests with master keys: how HIV uses chemokine receptors for cellular entry. Virology. 1997;235:179–190. doi: 10.1006/viro.1997.8703. [DOI] [PubMed] [Google Scholar]
- 18.Dragic T, Charneau P, Clavel F, Alizon M. Complementation of murine cells for human immunodeficiency virus envelope/CD4-mediated fusion in human/murine heterokaryons. J Virol. 1992;66:4794–4802. doi: 10.1128/jvi.66.8.4794-4802.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dragic T, Hazan U, Alizon M. Detection of cell fusion mediated by the envelopes of human retroviruses by transactivation of a reporter gene. In: Adolph K W, editor. Methods in molecular genetics. 7. Viral gene techniques. Orlando, Fla: Academic Press; 1995. pp. 218–236. [Google Scholar]
- 20.Endres M J, Clapham P R, Marsh M, Ahuja M, Davis Turner J, McKnight A, Thomas J F, Stoebenau-Haggarty B, Choe S, Vance P J, Wells T N C, Power C A, Sutterwala S S, Doms R W, Landau N R, Hoxie J A. CD4-independent infection by HIV-2 is mediated by fusion/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 21.Farzan M, Choe H, Martin K, Marcon L, Hofmann W, Karlsson G, Sun Y, Barrett P, Marchand N, Sullivan N, Gerard N, Gerard C, Sodroski J. Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med. 1997;186:405–411. doi: 10.1084/jem.186.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao J-L, Murphy P M. Human cytomegalovirus open reading frame US28 encodes a functional β chemokine receptor. J Biol Chem. 1994;269:28539–28542. [PubMed] [Google Scholar]
- 23.Gompels U A, Nicholas J, Lawrence G, Jones M, Thomson B J, Martin M E D, Efstathiou S, Craxton M, Macaulay H A. The DNA sequence of human herpesvirus-6: structure, coding content and genome evolution. Virology. 1992;209:29–51. doi: 10.1006/viro.1995.1228. [DOI] [PubMed] [Google Scholar]
- 24.Harrington R D, Geballe A P. Cofactor requirements for human immunodeficiency virus type 1 entry into a CD4-expressing human cell line. J Virol. 1993;67:5939–5947. doi: 10.1128/jvi.67.10.5939-5947.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones J S, Risser R. Cell fusion induced by the murine leukemia virus envelope glycoprotein. J Virol. 1993;67:67–74. doi: 10.1128/jvi.67.1.67-74.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kielan M C, Helenius A. Role of cholesterol in fusion of Semliki Forest virus with membranes. J Virol. 1984;52:281–283. doi: 10.1128/jvi.52.1.281-283.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lapham C K, Ouyang J, Chandrasekhar B, Nguyen N Y, Dimitrov D S, Golding H. Evidence for cell-surface association between fusin and the CD4-gp 120 complex in human cell lines. Science. 1996;274:602–605. doi: 10.1126/science.274.5287.602. [DOI] [PubMed] [Google Scholar]
- 28.Lindau M, Almers W. Structure and function of fusion pores in exocytosis and ectoplasmic membrane fusion. Curr Opin Cell Biol. 1995;7:509–517. doi: 10.1016/0955-0674(95)80007-7. [DOI] [PubMed] [Google Scholar]
- 29.Margulies B J, Browne H, Gibson W. Identification of the human cytomegalovirus G protein-coupled receptor homologue encoded by UL33 in infected cells and enveloped virus particles. Virology. 1996;225:111–125. doi: 10.1006/viro.1996.0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marsh M, Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michelson S, Dal Monte P, Zipeto D, Bodaghi B, Laurent L, Oberlin E, Arenzana-Seisdedos F, Virelizier J-L, Landini M P. Modulation of Rantes production by human cytomegalovirus infection of fibroblasts. J Virol. 1997;71:6495–6500. doi: 10.1128/jvi.71.9.6495-6500.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monck J R, Fernandez J M. The fusion pore and mechanisms of biological membrane fusion. Curr Opin Cell Biol. 1996;8:524–533. doi: 10.1016/s0955-0674(96)80031-7. [DOI] [PubMed] [Google Scholar]
- 33.Moore J P, Trkola A, Dragic T. Co-receptors for HIV-1 entry. Curr Opin Immunol. 1997;9:551–562. doi: 10.1016/s0952-7915(97)80110-0. [DOI] [PubMed] [Google Scholar]
- 34.Murphy P M. The molecular biology of leucocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 35.Neote K, DiGregorio D, Mak J Y, Horuk R, Schall T J. Molecular cloning, functional expression, and signaling characteristics of a C-C chemokine receptor. Cell. 1993;72:415–425. doi: 10.1016/0092-8674(93)90118-a. [DOI] [PubMed] [Google Scholar]
- 36.Nicholas J. Determination and analysis of the complete nucleotide sequence of human herpesvirus 6. J Virol. 1996;70:5975–5989. doi: 10.1128/jvi.70.9.5975-5989.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicholas J, Cameron K R, Honess R W. Herpesvirus saimiri encodes homologues of G protein-coupled receptors and cyclins. Nature. 1992;355:362–365. doi: 10.1038/355362a0. [DOI] [PubMed] [Google Scholar]
- 38.Osborne R, Rigby M, Siebelink K, Neil J C, Jarrett O. Virus neutralization reveals antigenic variation among feline immunodeficiency virus isolates. J Gen Virol. 1984;75:3641–3645. doi: 10.1099/0022-1317-75-12-3641. [DOI] [PubMed] [Google Scholar]
- 39.Pinter A, Chn T-E, Lowy A, Cortez N G, Silagi S. Ecotropic murine leukemia virus-induced fusion of murine cells. J Virol. 1986;57:1048–1054. doi: 10.1128/jvi.57.3.1048-1054.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pleskoff O, Seman M, Alizon M. Amphotericin B derivative blocks human immunodeficiency virus type 1 entry after CD4 binding: effect on virus-cell fusion but not on cell-cell fusion. J Virol. 1995;69:570–574. doi: 10.1128/jvi.69.1.570-574.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pleskoff O, Sol N, Labrosse B, Alizon M. Human immunodeficiency virus strains differ in their ability to infect CD4+ cells expressing the rat homolog of CXCR-4 (fusin) J Virol. 1997;71:3259–3262. doi: 10.1128/jvi.71.4.3259-3262.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pleskoff O, Tréboute C, Brelot A, Heveker N, Seman M, Alizon M. Identification of a chemokine receptor encoded by human cytomegalovirus as a cofactor for HIV-1 entry. Science. 1997;276:1874–1878. doi: 10.1126/science.276.5320.1874. [DOI] [PubMed] [Google Scholar]
- 43.Power C A, Meyer A, Nemeth K, Bacon K B, Hoogewerf A J, Proudfoot A E, Wells T N. Molecular cloning and functional expression of a novel CC chemokine receptor cDNA from a human basophilic cell line. J Biol Chem. 1995;270:19495–19500. doi: 10.1074/jbc.270.33.19495. [DOI] [PubMed] [Google Scholar]
- 44.Rawlinson W D, Farrell H E, Barrel B G. Analysis of the complete DNA sequence of murine cytomegalovirus. J Virol. 1996;70:8833–8849. doi: 10.1128/jvi.70.12.8833-8849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rucker J, Edinger A L, Sharron M, Samson M, Lee B, Berson J F, Yi Y, Margulies B, Doranz B J, Collman R G, Parmentier M, Doms R W. Utilization of chemokine receptors, orphan receptors, and herpesvirus-encoded receptors by diverse human and simian immunodeficiency viruses. J Virol. 1997;71:8999–9007. doi: 10.1128/jvi.71.12.8999-9007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz O, Alizon M, Heard J M, Danos O. Impairment of T cell receptor-dependent stimulation in CD4+ lymphocytes after contact with membrane-bound HIV-1 envelope glycoprotein. Virology. 1994;198:360–365. doi: 10.1006/viro.1994.1042. [DOI] [PubMed] [Google Scholar]
- 47.Söderberg C, Giugni T D, Zaia J A, Larsson S, Wahlberg J M, Möller E. CD13 (human aminopeptidase N) mediates human cytomegalovirus infection. J Virol. 1993;67:6576–6585. doi: 10.1128/jvi.67.11.6576-6585.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sol N, Ferchal F, Braun J, Pleskoff O, Tréboute C, Ansard I, Alizon M. Usage of the coreceptors CCR-5, CCR-3, and CXCR-4 by primary and cell line-adapted human immunodeficiency virus type 2. J Virol. 1997;71:8237–8244. doi: 10.1128/jvi.71.11.8237-8244.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sommerfelt M A, Weiss R A. Receptor interference groups of 20 retroviruses plating on human cells. Virology. 1990;176:58–69. doi: 10.1016/0042-6822(90)90230-o. [DOI] [PubMed] [Google Scholar]
- 50.Strader C D, Fong T M, Tota M R, Underwood D, Dixon R A. Structure and function of G protein-coupled receptors. Annu Rev Biochem. 1994;63:101–132. doi: 10.1146/annurev.bi.63.070194.000533. [DOI] [PubMed] [Google Scholar]
- 51.Telford E A M, Watson M W, Aird H A, Perry J, Davison A J. The DNA sequence of equine herpesvirus 2. J Mol Biol. 1994;249:520–528. doi: 10.1006/jmbi.1995.0314. [DOI] [PubMed] [Google Scholar]
- 52.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G, Martin S R, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its coreceptor CCR5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 53.Welch A R, McGregor L M, Gibson W. Cytomegalovirus homologs of cellular G-protein coupled receptor genes are transcribed. J Virol. 1991;65:3915–3918. doi: 10.1128/jvi.65.7.3915-3918.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White J. Membrane fusion. Science. 1992;258:917–924. doi: 10.1126/science.1439803. [DOI] [PubMed] [Google Scholar]
- 55.White J, Martin K, Helenius A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J Cell Biol. 1981;89:674–679. doi: 10.1083/jcb.89.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson C A, Marsh J W, Eiden M V. The requirements for viral entry differ from those for virally induced syncytium formation in NIH 3T3/DTras cells exposed to Moloney murine leukemia virus. J Virol. 1992;66:7262–7269. doi: 10.1128/jvi.66.12.7262-7269.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 58.Yee J K, Miyanohara A, LaPorte P, Bouic K, Burns J C, Friedmann T. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc Natl Acad Sci USA. 1994;91:9564–9568. doi: 10.1073/pnas.91.20.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]