Abstract
However, it is still difficult for clinicians to establish prognostic stratifications and therapeutic strategies because of the lack of tools for predicting the survival of triple-negative breast cancer patients with liver metastases (TNBC-LM). Based on clinical data from large populations, a sensitive and discriminative nomogram was developed and validated to predict the prognosis of TNBC patients with LM at initial diagnosis or at the later course.
Introduction/background
Liver metastasis (LM) in TNBC patients is associated with significant morbidity and mortality. The objective of this study was to construct a clinical model to predict the survival of TNBC-LM patients.
Materials and methods
Clinicopathologic data were retrieved from the Surveillance, Epidemiology, and End Results (SEER) database and the Fifth Affiliated Hospital of Sun Yat-Sen University (FAFSYU). Based on patients with newly diagnosed TNBC with LM (nTNBC-LM) from the SEER database, a predictive nomogram was established and validated. Its predictive effect on TNBC patients with LM at later disease course by enrolling TNBC patients from FAFSYU who developed LM later. The prognostic effect of different treatment for nTNBC-LM was further assessed.
Results
A prognostic model was developed and validated to predict the prognosis of TNBC-LM patients. For LM patients diagnosed at the initial or later treatment stage, the C-index (0.712, 0.803 and 0.699 in the training, validation and extended groups, respectively) and calibration plots showed the acceptable prognostic accuracy and clinical applicability of the nomogram. Surgical resection on the primary tumour and chemotherapy were found to be associated with significantly better overall survival (OS).
Conclusion
A sensitive and discriminative model was developed to predict OS in TNBC-LM patients both at and after initial diagnosis.
Keywords: Liver metastasis, Triple-negative breast cancer, Prognostic model, Survival
1. Introduction
Triple-negative breast cancer (TNBC) was named by lacking expression of oestrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor-2 (HER2) [1]. Due to its highly aggressive nature, patients with TNBC face a higher risk of relapse and mortality than patients with other subtypes [2,3]. During the disease course, almost 50% of TNBC patients will develop distant metastasis, and after metastasis, the median survival time is only 13.3 months [4].
Liver is one of the most common metastatic sites of BC and the first organ in nearly 30% of metastatic breast cancer (MBC) patients [5,6]. Liver metastasis (LM) is associated with significantly increased mortality, with an overall survival (OS) of only 3–6 months in untreated patients and a median survival time of 12–20 months, although great improvements in diagnosis and treatment have been made in recent decades [[7], [8], [9]]. TNBC-LM patients have an even worse prognosis than patients with other subtypes [5,[10], [11], [12], [13]]. A study including 4285 patients diagnosed with BC with LM (BCLM) revealed that TNBC patients had the worst OS, at only 8 months, while the OS of the entire cohort was 15 months [5].
Nomograms based on a large cohort were developed to predict the OS of patients with BCLM and showed acceptable sensitivity and specificity [10,11,14]. However, another study enrolled BCLM patients from all subtypes to construct a prognostic model, which may result in serious bias when applied to TNBC patients, as TNBC has distinct characteristics and comprises a relatively small proportion of cases. Therefore, to enhance the performance of the prognostic nomogram and guide treatment strategies for TNBC-LM patients, it is imperative to conduct studies and construct nomograms based exclusively on patients with this subtype, especially with a large population.
In present research, a clinical model for predicting OS at 6 months, 1 year, and 2 years was developed and showed great discriminative performance in predicting the prognosis of patients with TNBC with LM at diagnosis (nTNBC-LM) and later (lTNBC-LM). We hope our study will help in the risk stratification of patients with TNBC-LM and assist in making treatment strategy. We present the following article in accordance with the TRIPOD reporting checklist.
2. Materials and methods
2.1. Study population
BC patients diagnosed from 2010 to 2018 were searched in the SEER database, and clinicopathologic data were collected. The eligibility criteria included: (1) pathological confirmation of ER, PR and HER2 negativity and (2) complete follow-up information. The exclusion criteria were as follows (1) age < 18 years or > 100 years, (2) tumours undetected (T0) or in situ (Tis), and (3) unknown race, marital status, laterality, T/N stage, differentiation grade, or metastasis status/site.
A total of 615 nTNBC-LM patients were eligible for further analysis. Among them, 515 patients diagnosed from 2010 to 2016 were used to construct the model as the training cohort. The 100 patients from 2017 to 2018 were assigned to the validation cohort and validate the clinical model.
For the extended cohort, 88 lTNBC-LM patients, from 2002 to 2013, who met the same criteria as above were enrolled from the Fifth Affiliated Hospital of Sun Yat-Sen University (FAFSYU) database.
This study was approved by the Ethics Committee of FAFSYU and was conducted in accordance with the principles of Helsinki Declaration. Since this is a retrospective analysis, we waived the requirement for patient's informed consent.
2.2. Selection of variables
Information on the following clinicopathologic variables was collected: age, marital status, race, T stage, N stage, differentiation grade, pathologic histology, laterality, extrahepatic metastasis (brain, lung and bone), surgery (on the breast tumour of primary site), chemotherapy, radiotherapy (on the primary tumour), and survival time. Specifically, the period between the time of detection of LM and the time of death was defined as survival time, for the extended cohort, and the age at diagnosis of LM was used for further analysis. The use of chemotherapy after LM, not the diagnosis of TNBC, was selected as a variable for further study.
2.3. Statistical analysis
In the present research, factors associated with the OS of TNBC patients with LM were determine by performing Cox regression analyses. Hazard ratios (HRs) with 95% confidence intervals (CIs) were also calculated. For visually present the model, a prognostic nomogram predicting the survival probability at 6 months, 1 year, and 2 years was developed, and its sensitivity and specificity was assessed by the C-index and calibration curves. The Kaplan–Meier method and log-rank tests were used to estimate survival time. R software was used for all the statistical analyses. Differences were considered significant at a two-sided p < 0.05.
3. Results
3.1. Population characteristics
In present study, 615 patients with nTNBC-LM from the SEER database and 88 patients with lTNBC-LM from the FAFSYU database were included to constructed and validated the prognostic nomogram. The baseline characteristics of the population are presented in Table 1, Table 2.
Table 1.
Demographics and clinicopathologic characteristics of invasive triple-negative breast cancer patients in SEER database.
| Variables | All patients, (n = 46,902) | 2010–2016 With Liver metastasis (Training) (n = 515) |
2017–2018 With Liver metastasis (validation) (n = 100) |
Without Liver metastasis (n = 46,287) |
|---|---|---|---|---|
| Age, median (IQR) | 59 (49, 69) | 59 (49.5, 69) | 62 (51,70) | 59 (49,69) |
| Race, n (%) | ||||
| White | 33674 (71.8) | 367 (71.2) | 69 (69.0) | 33238 (71.8) |
| Black | 9613 (20.5) | 123 (23.9) | 29 (29.0) | 9461 (20.4) |
| Asian&Pacific Islander | 3349 (7.1) | 22 (4.3) | 2 (2.0) | 3325 (7.2) |
| American Indian&Alaska Nativ | 266 (0.6) | 3 (0.6) | 0 (0) | 263 (0.6) |
| T stage, n (%) | ||||
| I | 21179 (45.2) | 60 (11.7) | 9 (9) | 21110 (45.6) |
| II | 19118 (40.8) | 156 (30.3) | 32 (32.0) | 18930 (40.9) |
| III | 3766 (8.0) | 97 (18.8) | 21 (21.0) | 3648 (7.9) |
| IV | 2839 (6.0) | 202 (39.2) | 38 (38.0) | 2599 (5.6) |
| Nodal status, n (%) | ||||
| N0/N1 | 42134 (89.8) | 352 (68.3) | 61 (61.0) | 41721 (90.1) |
| N2/N3 | 4768 (10.2) | 163 (31.7) | 39 (39.0) | 4566 (9.9) |
| Marital, n (%) | ||||
| Single | 20734 (44.2) | 284 (55.2) | 59 (59.0) | 20391 (44.1) |
| Married | 26168 (55.8) | 231 (44.8) | 41 (41.0) | 25896 (55.9) |
| Grade, n (%) | ||||
| I | 884 (1.9) | 4 (0.8) | 1 (1.0) | 879 (1.9) |
| II | 8344 (17.8) | 92 (17.8) | 12 (12.0) | 8240 (17.8) |
| III | 37419 (79.8) | 414 (80.4) | 87 (87.0) | 36918 (79.8) |
| IV | 255 (0.5) | 5 (1.0) | 0 (0.0) | 250 (0.5) |
| Laterality, n (%) | ||||
| Left | 24095 (51.37) | 268 (52.0) | 49 (49.0) | 23778 (51.37) |
| Right | 22797 (48.61) | 244 (47.4) | 50 (50.0) | 22503 (48.62) |
| Bilateral | 10 (0.02) | 3 (0.6) | 1 (1.0) | 6 (0.01) |
| Histologic Type, n (%) | ||||
| IDC | 43005 (91.7) | 475 (92.2) | 91 (91.0) | 42439 (91.7) |
| ILC | 633 (1.3) | 12 (2.3) | 0 (0.0) | 621 (1.3) |
| Other | 3264 (7.0) | 28 (5.5) | 9 (9.0) | 3227 (7.0) |
| With Brain Metastasis, n (%) | ||||
| No | 46689 (99.5) | 457 (88.7) | 92 (92.0) | 46140 (99.7) |
| Yes | 213 (0.5) | 58 (11.3) | 8 (8.0) | 147 (0.3) |
| With Lung Metastasis, n (%) | ||||
| No | 46051 (98.2) | 319 (61.9) | 64 (64.0) | 45668 (98.7) |
| Yes | 851 (1.8) | 196 (38.1) | 36 (36.0) | 619 (1.3) |
| With Bone Metastasis, n (%) | ||||
| No | 45949 (98.0) | 268 (52.0) | 43 (43) | 45638 (98.6) |
| Yes | 953 (2.0) | 247 (48.0) | 57 (57) | 649 (1.4) |
| Surgery, n (%) | ||||
| No | 3211 (6.9) | 324 (62.9) | 69 (69.0) | 2818 (6.1) |
| Yes | 43691 (93.1) | 191 (37.1) | 31 (31.0) | 43469 (93.9) |
| Radition, n (%) | ||||
| No/unkown | 24237 (51.7) | 385 (74.8) | 74 (74.0) | 23778 (51.4) |
| Yes | 22665 (48.3) | 130 (25.2) | 26 (26) | 22509 (48.6) |
| Chemotherapy, n (%) | ||||
| No/unkown | 12198 (26.0) | 121 (23.5) | 22 (22.0) | 12055 (26.0) |
| Yes | 34704 (74.0) | 394 (76.5) | 78 (78.0) | 34232 (74.0) |
SEER: Surveillance, Epidemiology, and End Results; IDC: invasive ductal carcinoma; ILC: invasive lobular carcinoma; IQR: interquartile range.
Table 2.
Demographics and clinicopathologic characteristics of invasive triple-negative breast cancer patients in FAFSYU database.
| Variables | Patients with Liver metastasis latter (Extended group) (n = 88) |
|---|---|
| Age, median (IQR) | 50 (41.8, 56) |
| Marital, n(%) rowhead | |
| Single | 35 (39.8) |
| Married | 53 (60.2) |
| With Brain Metastasis, n(%) rowhead | |
| No | 78 (88.6) |
| Yes | 10 (11.4) |
| With Lung Metastasis, n(%) rowhead | |
| No | 55 (62.5) |
| Yes | 33 (37.5) |
| With Bone Metastasis, n(%) rowhead | |
| No | 48 (54.5) |
| Yes | 40(45.5) |
| Surgery, n(%) rowhead | |
| No | 9 (10.2) |
| Yes | 79 (89.8) |
| Chemotherapy, n(%) rowhead | |
| No/unkown | 26 (29.5) |
| Yes | 62 (70.5) |
FAFSYU: Fifth Affiliated Hospital of Sun Yat-Sen University; IDC: invasive ductal carcinoma; ILC: invasive lobular carcinoma; IQR: interquartile range.
3.2. Prognostic analysis of TNBC patients with LM at initial diagnosis
Potential prognostic factors in nTNBC-LM patients were analysed by Cox regression analysis. More advanced age (HR:1.014, 95% CI:1.008–1.021, p < 0.001) and extrahepatic metastases (including brain, bone and lung metastases) (HR:1.698, 95% CI:1.286–2.243, p < 0.001; HR:1.323, 95% CI:1.102–1.589, p < 0.001; HR:1.526, 95% CI:1.275–1.827, p < 0.001, respectively) were found to be significantly associated with an earlier death. Prolonged survival was also detected in patients who were married (HR = 0.725, 95% CI = 0.605–0.869, p < 0.001). Regarding anticancer treatment, surgery (HR = 0.575, 95% CI = 0.477–0.693, p < 0.001) or chemotherapy (HR = 0.309, 95% CI = 0.249–0.382, p < 0.001) confer a significantly extended survival duration for nTNBC-LM patients; however, no significant association was found between patients who received radiation on primary tumour and those who did not (HR = 0.832, 95% CI = 0.677–1.023, p=0.081) (Fig. 1A). Multivariate Cox regression analysis was performed to further analyse the associations between significant factors above and the survival of nTNBC-LM patients. Age (older age: HR = 1.007, 95% CI = 1.000–1.014, p = 0.039), marital status (married: HR = 0.791, 95% CI = 0.658–0.950, p = 0.012), extrahepatic metastasis (including brain and bone) (brain: HR = 1.488, 95% CI = 1.111–1.992, p=0.008; bone: HR = 1.224, 95% CI = 1.015–1.476, p = 0.034), surgery (HR = 0.637, 95% CI = 0.524–0.773, p < 0.001) and chemotherapy (HR = 0.352, 95% CI = 0.280–0.443, p < 0.001) were further confirmed to be independently associated with the prognosis of nTNBC-LM patients (Fig. 1B). The results are shown in Table 3.
Fig. 1.
Forest plot for the potential prognostic factors for overall survival in triple-negative breast cancer patients with liver metastasis at diagnosis by (A) univariate cox analysis and (B) multivariate cox analysis.
Table 3.
Univariate and multivariate cox analysis of overall survival of the training cohort.
| Variables | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95%Cl | p-value | HR | 95%Cl | p-value | |
| Age | 1.02 | 1.012–1.028 | <0.001 | 1.009 | 1.000–1.017 | 0.029 |
| Race | ||||||
| White | 1.000 | |||||
| Black | 1.178 | 0.937–1.481 | 0.162 | |||
| Asian&Pacific Islander | 1.319 | 0.754–2.309 | 0.332 | |||
| American Indian&Alaska Nativ | 1.689 | 0.236–12.065 | 0.602 | |||
| T stage | ||||||
| I ∼ III | 1.000 | |||||
| IV | 1.354 | 1.104–1.660 | 0.004 | 1.348 | 1.090–1.667 | 0.006 |
| Nodal status | ||||||
| N0 | 1.000 | |||||
| N+ | 0.771 | 0.600–0.992 | 0.043 | |||
| Marital status | ||||||
| Single | 1.000 | 1.000 | ||||
| Married | 0.700 | 0.57–0.860 | <0.001 | 0.856 | 0.690–1.061 | 0.155 |
| Laterality | ||||||
| Left | 1.000 | |||||
| Right | 1.152 | 0.943–1.408 | 0.165 | |||
| Bilateral | 0.954 | 0.134–6.817 | 0.963. | |||
| Histologic type | ||||||
| IDC | 1.000 | |||||
| ILC | 1.729 | 0.714–4.185 | 0.225 | |||
| Other | 0.830 | 0.539–1.278 | 0.396 | |||
| Extrahepatic metastasis sites | ||||||
| 0 | 1.000 | 1.000 | ||||
| 1 | 1.383 | 1.092–1.752 | 0.007 | 1.396 | 1.096–1.778 | 0.007 |
| 2 | 2.293 | 1.720–3.058 | <0.001 | 2.095 | 1.556–2.821 | <0.001 |
| 3 | 3.350 | 2.076–5.406 | <0.001 | 3.729 | 2.234–6.223 | <0.001 |
| Surgery | ||||||
| No | 1.000 | 1.000 | ||||
| Yes | 0.615 | 0.498–0.760 | <0.001 | 0.733 | 0.586–0.916 | 0.006 |
| Radiation | ||||||
| N/unknown | 1.000 | |||||
| Yes | 0.873 | 0.692–1.101 | 0.251 | |||
| Chemotherapy | ||||||
| NO/unknown | 1.000 | 1.000 | ||||
| Yes | 0.200 | 0.156–0.257 | <0.001 | 0.248 | 0.188–0.327 | <0.001 |
IDC: invasive ductal carcinoma; ILC: invasive lobular carcinoma; IQR: interquartile range; CI: confidence interval; HR: hazard ratios.
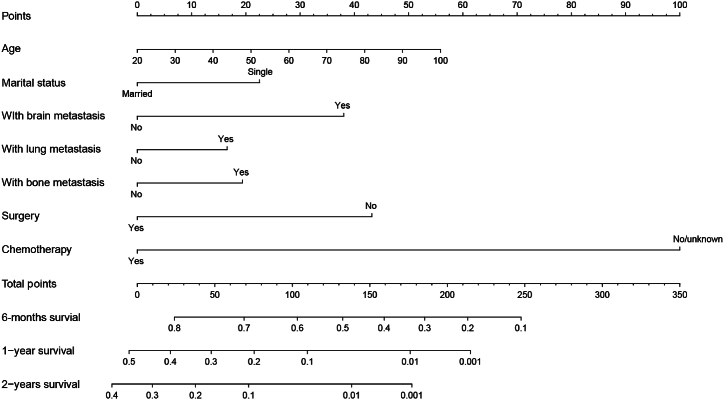
Based on the significant factors above, a prognostic model was developed to predict the 6-month, 1-year, and 2-year OS of nTNBC-LM patients (Fig. 2). The weighted contribution of each predictive factor was assessed to calculate the OS probabilities at 6 months, 1 year, and 2 years.
Fig. 2.
Nomograms for predicting 6-month, 1-year, and 2-year overall survival of triple-negative breast cancer patients with liver metastasis at diagnosis.
3.3. Calibration and validation of the nomogram for predicting the prognosis of nTNBC-LM patients
To assess the predictive accuracy of the prognostic nomogram, we employed both the C-index and calibration curves in both training and validation cohorts. In the training cohort, the C-index for predicting prognosis using the clinical model reached 0.712 (95% CI: 0.688–0.736). Furthermore, calibration curves demonstrated good alignment between predicted and observed outcomes at 6 months, 1 year, and 2 years (Fig. 3A–C, E). In the validation group, the C-index of the model was 0.803 (95% CI, 0.744–0.862). Visually, the calibration curves for survival probabilities at 6 months, 1 year, and 2 years showed a precise overlap between the predicted OS probabilities and the actual outcomes for nTNBC-LM patients (Fig. 3B–D, F). These findings underscore the clinical relevance and reliability of this model in prognosticating the outcomes of nTNBC-LM patients.
Fig. 3.
The calibration curve for predicting patient survival at (A) 6 months, (C) 1 year, and (E) 2 years in the training cohort and at (B) 6 months, (D)1 year, and (F)2 years in the validation cohort. Nomogram-predicted probability of overall survival is plotted on the x-axis; actual overall survival is plotted on the y-axis.
3.4. Validation of the nomogram for predicting the prognosis of lTNBC-LM patients
Eighty-eight lTNBC-LM patients were also included in our study as an extension group to explore whether the nomogram was able to predict the OS of TNBC patients diagnosed LMs at later disease course. The model showed acceptable predictive accuracy and clinical applicability, as indicated by a C index of 0.699 (95% CI = 0.642–0.756), and the calibration curves also demonstrated a favorable consistency between the nomogram predictions and the observed outcomes of 6-month, 1-year, and 2-year OS in lTNBC-LM patients (Fig. 4A, B, C).
Fig. 4.
The calibration curve for predicting patient survival at (A) 6 months, (B) 1 year, and (C) 2 years in the extended cohort (TNBC patients with liver metastasis during latter disease course).
3.5. Survival analysis based on treatment in TNBC patients with LM at initial diagnosis
To further evaluate the survival advantage of the treatment, survival curves were plotted to show the prognostic efficacy of surgery, chemotherapy and radiotherapy directly in 615 nTNBC-LM patients. The results showed that patients who underwent surgery (median OS: 13 months vs. 7 months) on primary tumour or chemotherapy (median OS: 12 months vs. 2 months) exhibited significantly longer survival time. However, no significant difference in OS was found between TNBC-LM patients who received radiotherapy and those who did not (median OS: 10 months vs. 8 months) (Fig. 5A, B, C).
Fig. 5.
Kaplan–Meier survival curve for overall survival in triple-negative breast cancer patients with liver metastasis at diagnosis, stratified by (A) surgery, (B) chemotherapy, (C) radiotherapy.
4. Discussion
Due to its high invasiveness and aggressiveness, TNBC is a distinct entity in BC [15,16]. Among BCLM patients, TNBC patients with LM have the worst prognosis, and it is still a challenge for clinicians to stratify patients according to prognosis and predict the outcome of TNBC-LM patients, although great efforts have been made by researchers [5,[10], [11], [12], [13]]. Therefore, a clinical model based on a large population for predicting the survival of TNBC-LM patients is warranted. Hence, in our study, a prognostic nomogram was developed and validated to predict OS at 6 months, 1 year, and 2 years in nTNBC-LM patients. Moreover, our model was validated in an extended cohort and showed acceptable accuracy in predicting the OS of lTNBC-LM patients.
Age, marital status, extrahepatic metastasis (including brain and bone), surgery and systemic chemotherapy were found to be associated with better prognosis in nTNBC-LM patients according to multivariate Cox regression analysis significantly and be independent prognostic factors. Advanced age has been found to be associated with poorer prognosis in BC patients with LM.10-13Among the MBC patients with a dismal prognosis, those who were younger tended to have better Karnofsky performance scores and fewer complications [17], which allowed them to receive more and/or more aggressive systemic treatment (e.g. chemotherapy) after the diagnosis of LM. Marital status was another independent variable correlated with improved prognosis in nTNBC-LM patients. This may be influenced by economic and social psychological variables. More stable financial support from spouses or partners of married patients may be an important reason for patients’ better treatment adherence. A study enrolling 5709 patients revealed that more patients in the married group had medical insurance [18]. Psychosocially, single patients face more distress, depression and anxiety due to less companionship and comfort [19], and these negative emotions have been suggested to facilitate tumour progression and influence treatment efficacy [20,21]. Extrahepatic metastasis (including brain, lung and bone metastasis) was also found to be an independent prognostic variable. TNBC patients with more organs metastases have a heavier tumour burden and greater tumour heterogeneity and thus are more susceptible to treatment resistance. Unexpectedly, lung metastasis was no longer significant according to multivariate Cox regression analysis, which may be due to the sample size.
In terms of therapy, we found that nTNBC-LM patients who received palliative surgery or systemic chemotherapy exhibited significantly longer survival (in months), but no difference was found in radiotherapy. Previously, palliative surgery on the primary tumour in MBC patients was performed only to relieve symptoms. When diagnosed with distant metastasis, patients are believed to have lost the opportunity for surgery, and the only option is receiving systemic therapy [22]. However, a prospective clinical trial recently reported a 34% lower risk of death and a 17.2% greater 5-year OS in patients with MBC at initial diagnosis who underwent surgical resection of the primary tumour than in patients who did not [23]. Moreover, based on large cohorts, multiple retrospective studies have showed that BCLM patients may achieve significantly better prognoses after primary tumour resection.10,12,13Moreover, chemotherapy was significantly correlated with improved OS in LM patients in our study. According to the latest clinical guidelines, chemotherapy, including BCLM, remains a cornerstone treatment for metastatic breast cancer [24]. In addition, studies have shown that TNBC patients show significantly greater response than patients with the luminal subtype after receiving neoadjuvant chemotherapy, and this treatment is associated with a better survival in patients with TNBC [25,26]. Thus, future researches should explore the appropriate chemical drugs and optimize chemotherapy strategy to improve outcomes and prognosis in TNBC patients with LM.
Here, a model was developed to predict the prognosis of TNBC-LM patients, leveraging significant variables identified by Cox regression analysis. To assess the predictive power of our model, the C-index was computed, and calibration plots were generated. Our prognostic nomogram achieved C-index values of 0.707 and 0.801 in the training and internal validation cohorts, respectively. According to previous studies, a nomogram with a C-index exceeding 0.7 is considered to possess satisfactory sensitivity and specificity [27]. Furthermore, the reliability of our nomogram was evident from the calibration curves, which demonstrated excellent alignment between predicted and observed outcomes in both cohorts. In the extended group, although the C-index of 0.685 was slightly below 0.7, this may be attributed to the relatively small sample size. Nevertheless, the calibration curves still exhibited favorable consistency. Therefore, the model developed in our present study performs well in predicting the survival of patients with TNBC-LM at initial diagnosis or later. This is a rare study involving the construction of a visual clinical model aimed at improving the prognosis of both nTNBC-LM and lTNBC-LM patients, and we hope that this model can aid in risk stratifying and clinical decision making.
Limitations exist in our present study. First, this study was retrospective, inherently carrying some unavoidable bias. Second, the study focused on a relatively small cohort from a single centre in the extended cohort, and larger population from multiple centres are needed. Third, clinicopathological factors, such as the number and size of liver metastases, which have been previously identified as prognostic variables in BCLM patients [28], may potentially impact the survival outcomes of TNBC-LM patients and therefore deserve consideration in our future studies. Unfortunately, these crucial details were not available in the SEER database, thus limiting their inclusion in our current model. However, the prognostic effects of these factors in TNBC-LM patients deserve further research. Fourth, there have been a tendency of precision and individualization in the treatment of patients with cancer, and multiple mechanism was found to be involved in the BCLM and these may be potential target of patients [[29], [30], [31], [32]]. Our present study was a clinical trial without the further exploration of the mechanism, so more efforts are needed in experimental research.
5. Conclusions
In conclusion, age, marital status, extrahepatic metastasis (including brain, lung and bone), surgery and systemic chemotherapy were found to be associated with prognosis in nTNBC-LM patients; of these factors, all but lung metastasis were independent prognostic factors. Based on these results, our study are the first to established and validate a model for predicting prognosis in TNBC-LM patients, including those with LM at initial treatment or later, and this model can help stratify patients for further treatment and develop a more appropriate treatment strategy.
Clinical practice points
For lacking a tool to predict the survival of TNBC patients with LM, it is difficult for clinicians to stratify patients according to prognosis and make therapeutic strategies.
We are the first to construct and validate a sensitive and discriminative model for overall survival at 6 months, 1 year, and 2 years in TNBC patients who were diagnosed with liver metastasis at initial treatment or later.
This model may provide a valuable reference for clinicians to stratify patients for further treatment and develop a more appropriate treatment strategy.
Ethics statement
The ethical review was approved by the Ethics Committee of the Fifth Affiliated Hospital of Sun Yat-Sen University(K135-1).
Data availability statement
No. Data will be made available on request.
The data in training and internal validation cohort is available at https://seer.Cancer.gov/. The data from FAFSYU database are available on reasonable request. The data are not publicly available due to privacy or ethical restrictions.
Funding
This study was supported by the Guangdong Basic and Applied Basic Research Foundation (Grant Number: 2021A1515010421).
CRediT authorship contribution statement
Liguo Zhang: Writing – original draft, Visualization, Validation, Formal analysis, Data curation. Zhen Qiao: Visualization, Validation, Data curation, Conceptualization. Yinsheng Yao: Validation, Formal analysis. Zhiqiang Li: Validation, Formal analysis. Lingzhi Hu: Visualization, Validation, Formal analysis, Data curation. Yinyan Mao: Data curation. Xiuling Liu: Validation. Weirong Chen: Supervision, Data curation, Conceptualization. Qing'an Zeng: Writing – review & editing, Supervision, Funding acquisition. Hong Zhao: Writing – review & editing, Writing – original draft, Supervision, Project administration, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Weirong Chen, Email: 924743255@qq.com.
Qing'an Zeng, Email: Zengqa123@163.com.
Hong Zhao, Email: Zhaoh59@mail.sysu.edu.cn.
References
- 1.Wolff A.C., Hammond M.E., Hicks D.G., et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline update. J. Clin. Oncol. 2013;31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984. Nov 1. [DOI] [PubMed] [Google Scholar]
- 2.O G., C L., N G., L P., U N., Oncology HNJAooojotESfM Triple-negative breast cancer--current status and future directions. 2009;20(12):1913–1927. doi: 10.1093/annonc/mdp492. [DOI] [PubMed] [Google Scholar]
- 3.Dent R., Trudeau M., Pritchard K.I., et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin. Cancer Res. 2007;13(15):4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. 10.1158/1078-0432.CCR-06-3045 %J Clinical Cancer Research. [DOI] [PubMed] [Google Scholar]
- 4.Lin N.U., Claus E., Sohl J., Razzak A.R., Arnaout A., Winer E.P. 2008. pp. 2638–2645. (Sites of Distant Recurrence and Clinical Outcomes in Patients with Metastatic Triple-Negative Breast Cancer). 113(10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie J., Xu Z. A population-based study on liver metastases in women with newly diagnosed breast cancer. Cancer Epidemiol. Biomarkers Prev. 2019;28(2):283–292. doi: 10.1158/1055-9965.EPI-18-0591. [DOI] [PubMed] [Google Scholar]
- 6.Pentheroudakis G., Fountzilas G., Bafaloukos D., et al. Metastatic breast cancer with liver metastases: a registry analysis of clinicopathologic, management and outcome characteristics of 500 women. Breast Cancer Res. Treat. 2006;97(3):237–244. doi: 10.1007/s10549-005-9117-4. [DOI] [PubMed] [Google Scholar]
- 7.Zhao H.-Y., Gong Y., Ye F.-G., Ling H., Hu X. Incidence and prognostic factors of patients with synchronous liver metastases upon initial diagnosis of breast cancer: a population-based study. Cancer Manag. Res. 2018;10:5937–5950. doi: 10.2147/CMAR.S178395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Largillier R., Ferrero J.M., Doyen J., et al. Prognostic factors in 1,038 women with metastatic breast cancer. Ann. Oncol. 2008;19(12):2012–2019. doi: 10.1093/annonc/mdn424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldhirsch A., Gelber R.D., Castiglione M. Relapse of breast cancer after adjuvant treatment in premenopausal and perimenopausal women: patterns and prognoses. J. Clin. Oncol. : Official Journal of the American Society of Clinical Oncology. 1988;6(1):89–97. doi: 10.1200/JCO.1988.6.1.89. [DOI] [PubMed] [Google Scholar]
- 10.Xiong Y., Shi X., Hu Q., Wu X., Long E., Bian Y. A nomogram for predicting survival in patients with breast cancer liver metastasis: a population-based study. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.600768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian C., Liu S., Wang Y., Song X. Prognosis and genomic landscape of liver metastasis in patients with breast cancer. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.588136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji L., Fan L., Zhu X., Gao Y., Wang Z. Prognostic score for de novo metastatic breast cancer with liver metastasis and its predictive value of locoregional treatment benefit. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.651636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji L., Cheng L., Zhu X., Gao Y., Fan L., Wang Z. Risk and prognostic factors of breast cancer with liver metastases. BMC Cancer. 2021;21(1):238. doi: 10.1186/s12885-021-07968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruiz A., Wicherts D.A., Sebagh M., et al. Predictive profile-nomogram for liver resection for breast cancer metastases: an aggressive approach with promising results. Ann. Surg Oncol. 2017;24(2):535–545. doi: 10.1245/s10434-016-5522-7. [DOI] [PubMed] [Google Scholar]
- 15.Bianchini G., Balko J.M., Mayer I.A., Sanders M.E., Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016;13(11):674–690. doi: 10.1038/nrclinonc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malorni L., Shetty P.B., De Angelis C., et al. Clinical and biologic features of triple-negative breast cancers in a large cohort of patients with long-term follow-up. Breast Cancer Res. Treat. 2012;136(3):795–804. doi: 10.1007/s10549-012-2315-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Im S., X L., Js W., et al. Validation and development of a modified breast graded prognostic assessment as a tool for survival in patients with breast cancer and. Brain Metastases. 2015;33(20):2239–2245. doi: 10.1200/jco.2014.58.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou C., Zhang Y., Hu X., Fang M., Xiao S. The effect of marital and insurance status on the survival of elderly patients with stage M1b colon cancer: a SEER-based study. BMC Cancer. 2021;21(1):891. doi: 10.1186/s12885-021-08627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitman A., Suleman S., Hyde N., Hodgkiss A. Depression and anxiety in patients with cancer. BMJ. 2018;361:k1415. doi: 10.1136/bmj.k1415. [DOI] [PubMed] [Google Scholar]
- 20.Schakel L., Veldhuijzen D.S., Crompvoets P.I., et al. Effectiveness of stress-reducing interventions on the response to challenges to the immune system: a meta-analytic review. Psychother. Psychosom. 2019;88(5):274–286. doi: 10.1159/000501645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell G., Lightman S. The human stress response. Nat. Rev. Endocrinol. 2019;15(9):525–534. doi: 10.1038/s41574-019-0228-0. [DOI] [PubMed] [Google Scholar]
- 22.Sa K., Ak S., Surgery M.M.J. Does aggressive local therapy improve survival in metastatic breast cancer? 2002;132(4):620–626. doi: 10.1067/msy.2002.127544. discussion 626-7. [DOI] [PubMed] [Google Scholar]
- 23.Soran A., Ozmen V., Ozbas S., et al. Randomized trial comparing resection of primary tumor with No surgery in stage IV breast cancer at presentation: protocol MF07-01. Ann. Surg Oncol. 2018;25(11):3141–3149. doi: 10.1245/s10434-018-6494-6. [DOI] [PubMed] [Google Scholar]
- 24.Gradishar W.J., Moran M.S., Abraham J., et al. Breast cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(6):691–722. doi: 10.6004/jnccn.2022.0030. [DOI] [PubMed] [Google Scholar]
- 25.Q L., Q L., P Z., et al. 2015. pp. 1746–1753. (A Phase II Study of Capecitabine Plus Cisplatin in Metastatic Triple-Negative Breast Cancer Patients Pretreated with Anthracyclines and Taxanes). 16(12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.W H., V V., S H., et al. 2018. Response Rates and Pathologic Complete Response by Breast Cancer Molecular Subtype Following Neoadjuvant Chemotherapy; pp. 559–567. 170(3) [DOI] [PubMed] [Google Scholar]
- 27.Yq H., Ch L., L H., et al. 2016. Development and Validation of a Radiomics Nomogram for Preoperative Prediction of Lymph Node Metastasis in Colorectal Cancer; pp. 2157–2164. 34(18) [DOI] [PubMed] [Google Scholar]
- 28.Chen L., Tan C., Li Q., et al. Assessment of the albumin-bilirubin score in breast cancer patients with liver metastasis after surgery. Heliyon. 2023;9(11) doi: 10.1016/j.heliyon.2023.e21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou Y., Ye F., Kong Y., et al. The single-cell landscape of intratumoral heterogeneity and the immunosuppressive microenvironment in liver and brain metastases of breast cancer. Adv. Sci. 2023;10(5) doi: 10.1002/advs.202203699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng Y., Du W., Huang Z., et al. Hsa_circ_0060467 promotes breast cancer liver metastasis by complexing with eIF4A3 and sponging miR-1205. Cell Death Discov. 2023;9(1):153. doi: 10.1038/s41420-023-01448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu P., Wang Z., Ou X., et al. The FUS/circEZH2/KLF5/feedback loop contributes to CXCR4-induced liver metastasis of breast cancer by enhancing epithelial-mesenchymal transition. Mol. Cancer. 2022;21(1):198. doi: 10.1186/s12943-022-01653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z., Yang L., Wu P., et al. The circROBO1/KLF5/FUS feedback loop regulates the liver metastasis of breast cancer by inhibiting the selective autophagy of afadin. Mol. Cancer. 2022;21(1):29. doi: 10.1186/s12943-022-01498-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No. Data will be made available on request.
The data in training and internal validation cohort is available at https://seer.Cancer.gov/. The data from FAFSYU database are available on reasonable request. The data are not publicly available due to privacy or ethical restrictions.