S U M M A R Y
Hepatitis B Virus (HBV) was recognized many decades ago as an important occupational hazard for Health Care Workers (HCWs) globally. HCWs who are directly involved in patient care and are in continuous contact with blood or body fluids have an increased risk of occupationally acquiring the virus. The risk of HCWs in highly endemic areas is greater due to the greater prevalence of infection in the general population. Recommendations are available to guide HBV prevention activities or practices among HCWs. These include the use of the hepatitis B vaccine as a preexposure prophylaxis and the use of hepatitis B immunoglobulin alone or hepatitis B immunoglobulin plus the vaccine as postexposure prophylaxis. The uptake of preexposure prophylaxis has been observed to be low in resource-poor settings where the disease is highly endemic. Postexposure prophylaxis has become the remedy for preventing occupational transmission of HBV in these settings.
This review aimed to summarize the available evidence on the risk of transmission of HBV infection, the burden of infection and recommendations for pre- and postexposure prophylaxis for the prevention of occupational acquisition of HBV among HCWs. We conducted a narrative review to summarize the evidence available on the recommended steps of HBV exposure management and the utilization of post-exposure prophylaxis (PEP) for HBV. A comprehensive search was conducted in PubMed, Science Direct, Google Scholar, and Africa Journals Online (AJOL) databases. The keywords used were hepatitis B, hepatitis B virus postexposure prophylaxis, occupational exposures, and recommendations for postexposure to hepatitis B virus. We gleaned evidence from the literature sources and summarized the concepts related to exposure forms, postexposure prophylaxis management pathways and recommendations for the utilization of postexposure prophylaxis among exposed healthcare workers.
From the synthesis of evidence, we conclude that HBV infection is a life-threatening condition. However, the disease is preventable by using the HBV vaccine as a preexposure prophylaxis measure. An effective postexposure prophylaxis management program is also available, and the last resort to preventing occupational transmission of HBV among HCWs who non-responders are, or who fail to vaccinate completely against HBV. Irrespective of the availability of these lifesaving interventions, the use of pre- and post-exposure prophylaxis among HCWs in highly endemic regions is suboptimal. Many barriers operating at the individual HCW and health facility levels have been identified as impacting the successful use of HBV preventive measures.
Keywords: Exposures, Hepatitis B virus, Healthcare worker postexposure prophylaxis, Recommendations
Introduction
Health Care Workers (HCWs) are at risk of a variety of infectious pathogens that can be acquired through occupational exposure to blood or body fluids. Hepatitis B virus (HBV) is one of the numerous blood-borne pathogens known to be transmissible in healthcare settings. HBV is of particular importance because it is the most efficiently transmissible blood borne infection following occupational exposure [1].
For HCWs, the potential to acquire HBV via occupational exposure is of particular concern, as serologic studies among HCWs many decades ago reported a far greater seroprevalence of HBV infection than did the general population. Recently, studies have shown that HCWs are at increased risk of HBV infection, although the rates of seropositivity have steadily declined [2]. Having recognized HBV as a major occupational hazard for HCWs, the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) have recommended that all HCWs be vaccinated against HBV as preexposure prophylaxis against the virus. The vaccine is given in 3 doses over 6 months (0, 1, and 6 months). It is recommended that HCWs have their hepatitis B surface antibody (anti-HBs) level tested 1–2 months after completion of the series to demonstrate seroprotection against HBV. This strategy has been identified as the most cost-effective intervention and the mainstay of HBV prevention among HCWs. Unfortunately, poor adherence and suboptimal practices have been reported, especially in sub–Saharan Africa, where the disease is highly endemic, with the highest prevalence of HBV among HCWs compared to that in other developed regions of the world [3].
Among HCW populations in highly endemic regions, where HBV vaccination coverage is very low and there is continuous exposure to blood and body fluids with a risk of HBV infection, post exposure prophylaxis (PEP) for HBV has become the last resort and a lifesaving remedy for preventing occupational transmission and acquisition of HBV infection [4].
Post exposure prophylaxis (PEP) for HBV refers to the medical response given to prevent the transmission of blood-borne pathogens following potential exposure. In the context of HBV, PEP refers to the set of services that are provided to manage the specific aspects of exposure to HBV and to help prevent HBV infection in an HCW who is at risk of becoming infected. Studies performed among HCWs in many endemic countries revealed inadequate knowledge of PEP for HBV. Some HCWs did not know that PEP for HBV existed. In other instances, HCWs exposed to HBV were offered antiretroviral drugs recommended for prophylaxis for exposure to human immunodeficiency virus (HIV) [5]. This lack of information and awareness on PEP for HBV could promote HBV transmission among HCWs in the face of continuous exposure to contaminated blood and body fluids and increase the risk of HBV transmission.
This review examines the risk of HBV infection among HCWs in highly endemic areas and the recommendations for PEP for HBV infection for HCWs who face the constant risk of being exposed to blood and body fluids.
Review procedure
We conducted a narrative review to summarize the evidence available on the recommended steps for HBV exposure management and the utilization of PEP for HBV. A comprehensive search was conducted in PubMed, Science Direct, Google Scholar, and Africa Journals Online (AJOL) databases. The keywords used were hepatitis B, hepatitis B virus postexposure prophylaxis, occupational exposures, and recommendations for postexposure to hepatitis B virus. All the articles used were open access and written in English language. Articles published from 2010 to 2023 were included with few other classical information published before 2010.
Two authors namely L.A.O and D.A.W independently reviewed and screened articles by undertaking critical review of the titles, abstracts, and full manuscripts for their relevance to the review. When conflicts occurred, consensus was achieved after further discussion. A total of 84 articles were identified out of which 14 were excluded based on the year of publication and content that was misleading. 13 more articles were also excluded due to the unavailability of full texts. As a result, 57 articles were considered relevant and utilized for this for this narrative review.
S-D V.E. extracted data and synthesized evidence from the articles. Evidence was gleaned from all considered eligible in this study. There was careful synthesis of information such that important concepts related to exposure forms, postexposure prophylaxis management pathways and recommendations for the utilization of postexposure prophylaxis among exposed healthcare workers were extracted, documented, and stored electronically.
Results
Exposure forms and the risk of HBV infection transmission among healthcare workers
Hepatitis B virus (HBV) is a blood-borne pathogen that afflicts humans and is known to be readily transmitted via blood or body fluid exposure. This is the reason behind its identification as a biological hazard and therefore an occupational risk for HCWs worldwide. The infectivity of HBV is enhanced by it being a hard organism that can endure on environmental surfaces for seven days in blood or body fluid visible and even invisible.
The risk of transmission and infection with HBV is high among HCWs for two main reasons. First, among the 60 or more blood-borne pathogens responsible for transmissible infectious diseases, HBV is the most frequently and efficiently transmitted infection to HCWs (1). The rate of transmission of HBV is 6–30%, compared to 0.3 for Human Immunodeficiency Virus (HIV) and 1.8% for Hepatitis C Virus (HCV) [6,7]. (2) HCWs are constantly exposed to human blood and other potentially infectious biological materials more than the general population is. The risk of HBV acquisition has long been assessed to be 4–10 times greater in the HCW population than in the general population. Among non-immune HCWs, the occupational transmission of blood-borne infections may principally occur through parenteral, mucous membrane, or non-intact skin exposure. In mucosal-cutaneous exposure, a patient's blood, or other potentially infected biological material accidentally comes into contact with the mucous membranes of the eyes or mouth or with the skin, healthy or non-intact skin, of a HCW. Percutaneous exposure, on the other hand, occurs when a HCW sustains an injury from a contaminated sharp object or needle [8]. Among these three transmitting channels, percutaneous exposures carry the highest risk of HBV transmission [1,8]. These exposure forms are described in Table I.
Table I.
Forms of exposure to blood and body fluids among HCWs
| No | Type of exposure | Examples of exposure |
|---|---|---|
| 1 | Percutaneous Exposure | Exposure through needlesticks, cuts, punctures, and other injuries from sharp objects |
| 2 | Per-mucous exposure | Exposure through mucous membrane contacts (e.g., eyes, nose, or mouth) with blood, fluids, tissues, or specimens contaminated with HBV |
| 3 | Non-Intact Skin Exposure | Exposure through areas of the skin that have been opened by cuts, abrasions, dermatitis, chapped skin, etc |
Among all the biological materials or body fluids, blood contains the highest HBV titres and hence is considered the most important channel for transmitting infection in healthcare facilities compared to other body fluids, such as breast milk, bile, cerebrospinal fluid, faeces, nasopharyngeal washings, saliva, semen, sweat, and synovial fluid [9,10].
The risk of seroconversion increases with deep injury when the sharp device or instrument is visibly contaminated with the source patient's blood or when an injury involving a needle or sharp placed in the source patient's artery or vein occurs.
Another important factor that allows for transmission of HBV to HCWs is the hepatitis B e antigen (HBeAg) status of the source patient [8]. Hepatitis B e antigen (HBeAg) is a marker for high HBV replication and infectivity. The risk of occupational infection with HBV is particularly high when HCWs are exposed to blood or body fluids from source patients who are positive for both HBsAg and HBeAg [6,8]. Serologic studies performed in the past revealed a 22%–31% risk of developing clinical infection and a 37–62% risk of developing serologic evidence of HBV infection upon exposure to the blood of a source patient with positive hepatitis B surface antigen (HBsAg) and positive hepatitis B E antigen (HBeAg) [11].
The HBV load of the source patient is another important determinant of HBV transmission following exposure in nonimmune HCWs. In these cases, the risk of HBV transmission is estimated to be 19%–30% if the source patient has an HBV load greater than 106 IU/mL and 5% if the source patient is HBeAg negative or has a lower HBV load [1].
The burden and distribution of HBV among healthcare workers from different regions of the world
Healthcare workers (HCWs) form an important building block of the health system, and their collective function is to provide curative, preventive, and promotive activities toward improving and maintaining the health and well-being of all individuals [12]. At the global level; however, Prüss-Üstün et al. (2005) reported an estimated 66,000 HBV infections among HCWs in 2005 [13].
Babanejad et al.(2019), in their meta-analysis, reported pooled incidences of 2.84% and 2.22%, respectively, across 43 studies involving HCWs in the Eastern Mediterranean and Middle East regions of the world [14]. Another systematic review of studies performed among HCWs from the European Union and European Economic Regions reported a prevalence of 0.6–2.2% [15]. Available reports from the American region indicate prevalence rates between 0.1% and 0.8% [[16], [17], [18]]. Additionally, in Asia, a recent systematic review estimated a pooled prevalence of 4.0% among HCWs [19].
In Africa, where HBV is highly endemic, a systematic review and meta-analysis reported a pooled prevalence of 6.81% across 44 studies among HCWs [20].
The above estimates reveal great heterogeneity in the prevalence of HBV among HCWs from different regions of the world, with the African region and Asia having the highest prevalence rate among HCWs. Early studies describing the global epidemiology of HBV established that HBV is highly endemic in the African region and Asia, with more than 8% of the population living with chronic infections [21,22]. Therefore, the regional estimates of HBV infection among HCWs presented above are not surprising. The higher disease burden observed in HCWs from the African region agrees with Pappas and Fisher's (1985) suggestion that the prevalence of HBV among HCWs parallels or closely mirrors the prevalence of the general population that they serve [23].
Recommendations for HBV prevention for healthcare workers
Before the availability of a potent vaccine against HBV, avoiding occupational blood exposure was the only primary strategy adopted to prevent the transmission of HBV in healthcare settings. Following the development of the HBV vaccine in 1982, hepatitis B immunization and post exposure management were identified as integral components of a complete program for HBV prevention in health facilities. The WHO, CDC, and other international organizations have strongly supported the implementation of these cost-effective recommendations by HCWs to reduce the risk and extent of infection with HBV globally [[24], [25], [26]]. Pappas and Fisher, (1985) classified these HBV preventive strategies under (1) simple hygiene, now referred to as Standard Precautions (SPs); (2) Passive Immunoprophylaxis; and (3) Active prophylaxis [23]. These strategies are solid pillars for any HBV prevention program in health facilities and hence have become the cornerstone of all recommendations for HBV prevention in health facilities globally. These strategies are summarized in Figure 1
Figure 1.
HBV prevention strategies for HCWs.
Adapted from Pappas and Fisher (1985).
Standard Precautions (SPs) (Simple hygiene)
Simple hygiene practices, now called Standard Precautions (SPs), are guidelines for preventing exposure to blood and other potentially infectious materials [27,28]. SPs are the minimum infection prevention practices that apply to all patient care activities, regardless of the patient's suspected or confirmed infection status, in any healthcare setting. These practices are designed to protect both HCWs and patients and prevent them from transmitting infections among patients and HCWs [29]. The specifics of the SPs for HBV prevention include hand hygiene, personal protective equipment, sharps safety (engineering and work practice controls), safe injection practices (i.e., aseptic techniques for parenteral medications), and the use of sterile instruments and devices. Over the years, these measures have been proven to decrease the rate of exposure to HBV and other blood-borne pathogens among HCWs and even patients in the healthcare setting [30].
Pre exposure prophylaxis (Active immunoprophylaxis)
The CDC, WHO, and other international organizations have recommended that all HCWs who have the potential to be exposed to blood and body fluids undergo pre-vaccination testing for HBV infection and receive three (3) doses of the HBV vaccine at schedules of 0, 1, and 6 months early in their career. It is also recommended that the vaccine be administered intramuscularly into the deltoid muscle. Additionally, HCWs must undergo post vaccination serological testing to evaluate or assess the development of antibodies against HBV antigen 1–2 months postvaccination [24,25]. The protective efficacy of HBV vaccination is directly related to the induction of anti-HBs antibodies. An antibody titre of >10 mlU/ml indicates long-term protection against HBV infection. Individuals who comply with the three-dose series of the vaccine are said to be wholly or completely vaccinated. Multiple studies have shown that following the standard 3-dose schedule in immunocompetent populations, close to 90–95% of vaccinees who are <40 years old at the time of vaccination develop neutralizing antibodies against HBV at levels ≥10 mlU/mL. This means that close to 5–10% of vaccinees who are immunocompetent fail to respond to the primary HBV vaccine series by failing to elicit detectable specific antibodies and therefore remain susceptible to HBV [31,32]. These individuals need to be revaccinated in a secondary vaccination series. Only true vaccine non-responders are declared after failing to respond to the secondary vaccination series and when HBV infection has been ruled out [7]. Non-responders to HBV vaccination who are evaluated and found to be HBsAg negative are considered susceptible to HBV infection, and there is a need to counsel them to avoid exposure and take the necessary measures to prevent HBV infection. HCWs with evidence of a complete, ≥3-dose HBV vaccine series and postvaccination anti-HBs ≥10 mIU/mL are considered immune and have long-term protection against HBV and do not need further anti-HBs level assessments [31].
Barriers to uptake of preexposure prophylaxis for HBV in highly endemic settings
The low uptake of HBV vaccination among HCWs in many HBV endemic settings is a widespread phenomenon. In central Africa, where the prevalence of HBV infection is above 8%, Auta et al., in a systematic review, reported an HBV vaccination coverage of 13.4% [33]. Specifically, in Somalia, 5.3% of HBV vaccinations were reported, whereas 20.5% were reported in Ethiopia [34,35]. In all these settings, the main barriers reported for poor vaccine uptake include the unavailability of vaccines, the high cost of acquiring the vaccine, and a lack of time or busy schedules [36]. Other studies have reported a lack of confidence in the vaccine as well as fear of injection as reasons for vaccine refusal [35]. A lack of oversight and poor governance for HBV prevention; lack of protocols and guidelines to follow; and unavailability of occupational health and safety committees and their respective focal persons to drive HBV prevention programmes are some of the health facility barriers identified as major factors affecting HBV vaccination among HCWs.
Low HCW vaccination levels and health system barriers require new management strategies and policies to maximize hepatitis B vaccination in high-risk professional groups in highly endemic settings.
Postexposure prophylaxis (PEP)
Postexposure Prophylaxis (PEP) for HBV refers to the medical response given to prevent the transmission of blood-borne pathogens following potential exposure. In the context of HBV, PEP refers to the set of services that are provided to manage the specific aspects of exposure to HBV and to help prevent HBV infection in a HCW who is at risk of becoming infected with HBV. These services include first aid treatment, assessment of the risk of exposure to HBV infection and counselling, HBV and antibody testing, and depending on the outcome of the exposure assessment, the prescription of Hepatitis B Immune Globulin (HBIG) and the HBV vaccine followed by appropriate support and follow-up [37,38].
HBIG is a gamma globulin product that contains a high titre of anti-HBs. HBIG is prepared from plasma preselected for a high titre of antibody against HBsAg. HBIG is recommended for use as a PEP for susceptible HCWs who have been exposed to blood or other body fluids containing HBV either by the percutaneous or mucous membrane route. The dose recommended is 0.06 mL/kg given immediately after exposure in the health care setting. [39]. HBIG is administered when the source patient is known to have HBV infection, when the HBV status is unknown, or when the HCW is not immune to HBV (not vaccinated or not a responder to the HBV vaccine).
It is recommended that HBIG be administered as soon as possible, preferably within 12–24 hours following percutaneous or mucosal exposure. PEP for HBV is highly unlikely to be effective if it is initiated 7 days after exposure [40]. HBIG is recommended in addition to the HBV vaccine when additional protection is needed. Table II presents a summary of PEP prescriptions for HBsAg-positive or HBsAg-negative patients and for vaccinated and unvaccinated HCWs.
Table II.
Summary of recommended steps in PEP management of HCWs exposed to HBV
| Vaccination & anti-body status | Treatment of source is: |
Treatment if the source patient is unknown or unavailable for testing | |
|---|---|---|---|
| HBsAg +VE | HBsAg-VE | ||
| 1. Unvaccinated/partially vaccinated | HBIGa1 & initiate/complete vaccine seriesb | Initiate/complete. vaccination series |
Initiate/complete vaccination series |
| 2. Completely vaccinated & responder | No treatment required | No treatment required | No treatment required |
| 3. Completely vaccinated and nonresponder 4. True nonresponder (6 doses of HBV vaccine) |
1 dose of HBIG & initiate 2nd vaccination series 2 doses of HBIG given one month apart |
No treatment is needed. No Treatment is needed. |
Treat as if the source were HBsAg +VE Treat as if the source were HBsAg +VE |
| 5. Antibody response is unknown | Test for antibodies. If adequate, no treatment is needed. If inadequate, HBIGa1 and a vaccine booster are required |
No Treatment Required | If Anti-HBs are adequate, no treatment is needed. If inadequate, HBIGa1 and a vaccine booster are required Recheck titre I 1–2 months |
Adapted from Beekmann & Henderson, 2015; CDC, 2001.
Hepatitis B immunoglobulin.
Given at different sites.
Steps for managing HCWs with occupational exposures to HBV
The steps for managing HCWs exposed to HBV have been clearly outlined below.
First aid treatment and care of the exposure site
Immediately after exposure, the site is allowed to bleed freely by holding it under running water. The injury site should be washed with soap and water for 15 minutes. The site should not be sucked or scrubbed during the process. For mucous membrane exposure, the mouth or eyes should be rinsed, flushed, or irrigated with water several times. For non-intact skin exposures, the area is thoroughly rinsed with running water if contaminated with blood, body fluids, secretions, or excretions. The use of bleach and antiseptics is not recommended [41].
Exposure reporting
The affected HCWs need to use the facility or hospital's existing structures to promptly report their exposure per institutional/facility policy and protocols. Reporting exposures not only helps in postexposure management but also helps shape guidelines for preventing future injuries and exposures in health facilities; hence, it is important for HCWs to be knowledgeable and understand the pathways for exposure reporting. Important components of the report should include the date and time of exposure and the type and nature of exposure (percutaneous, mucocutaneous, nonintact skin, etc.). The type of instrument or sharp object causing the injury, its state, and the procedure being carried out by the HCW were recorded. If available, information on the source patient and where exactly the exposure took place should be included in the report [10].
Healthcare worker risk assessment and counselling
This stage is performed by a physician, an emergency room physician, an occupational health physician, an occupational health nurse, or another health care professional who is knowledgeable in the assessment of blood or bodily fluid exposures. The risk assessment stage is aimed at determining the potential of exposure to transmit HBV to HCWs and determining which HCW factors are protective. The assessment involves determining the type of body fluid involved in the exposure, the nature of the exposure (e.g., percutaneous, mucous membrane, or contact with non-intact skin), and the HBsAg status of the source patient. Additionally, the HBsAg, immunization, and anti-HBs status of the exposed HCWs were determined. Laboratory tests may be required to make these determinations if the HBsAg status of the source patient and the anti-HBs status of the HCW are not known or documented. The source patient should be informed of the incident and tested for serologic evidence of bloodborne virus infection. Procedures should be followed for testing source persons, including providing informed consent, per recommended protocols [8].
If the source patient's HBsAg status cannot be determined because of refusal or because the source is unknown, the highest risk of HBsAg-positive status is assumed. Based on the outcome of the risk assessment, counselling of the affected HCWs is initiated to help reduce anxiety and fear and to promote adherence to postexposure prophylaxis regimen.
PEP prescription and administration
The prescription for PEP against HBV is given based on the outcome of the exposed HCW and the source patient assessment. The main drivers of the PEP regimen for exposure to HBV are immunization history, the anti-HBs levels of the exposed HCW and the HBsAg status of the source patient.
The major features of PEP management for exposed HCWs recommended by the CDC [8] are outlined as follows:
-
•
For unvaccinated HCWs, with an HBsAg-positive source, one (I) dose of HBIG is given, followed by an HBV vaccination series (3 doses, at 0, 1, and 6), with postvaccination serological testing performed 1–2 months after completion of the vaccination series.
-
•
For unvaccinated HCWs with HBsAg-negative source patients, the HBV vaccination series (3 doses, at 0, 1, and 6) is also initiated if the source patient is unknown. Postvaccination serological testing is recommended 1–2 months after the vaccination series.
-
•
For a completely vaccinated HCW with documented anti-HBs levels ≥10 mIU/mL, no action is required even if the source is HBsAg positive or unknown.
-
•
For completely vaccinated HCWs (true non responders) with documented anti-HBs levels <10 mIU/mL and source patients with a positive HBsAg status, two doses of HBIG at 0.06 ml/kg are recommended one month apart, and anti-HBs determination is recommended after 6 months. No action is required if the source patient is HBsAg negative or unknown.
For completely vaccinated HCWs (those who failed to respond after a single vaccination series) with documented anti-HB levels <10 mIU/mL and source patients with a positive HBsAg status, one dose of HBIG at 0.06 mL is recommended, followed by the initiation of a second vaccination series (3 doses at 0, 1, and 6). The same protocol was followed if the source was unknown.
A summary of the guidelines for the prescription of postexposure prophylaxis for exposed HCWs is presented in Table II.
Follow-up visits and post-PEP evaluation
A follow-up visit after PEP administration for HBV is aimed at helping the affected HCWs adhere to the PEP regimen, determine the effectiveness of PEP and detect any seroconversion, if possible. Follow-up testing is highly recommended for HCWs in these categories:
-
•
Exposed HCWs with anti-HB levels less than 10 mlU/mL at the time of exposure
-
•
Unvaccinated HCWs
-
•
Partially vaccinated (less than 3 doses of the HBV vaccine)
-
•
Non responders
In this case, HBsAg and total antiHBc should be assessed to rule out seroconversion exactly 6 months after exposure. Then, anti-HBs titre levels should be assessed 1–2 months after completion of the PEP HBV vaccination series to help determine seroprotection against HBV and to determine anti-HB status. For exposed HCWs who are true non responders (HCWs who failed to respond after 6 doses of the HBV vaccine), post-PEP evaluation includes testing for HBsAg and total anti-HBc [8,10].
Recommended pathway for postexposure prophylaxis for HBV
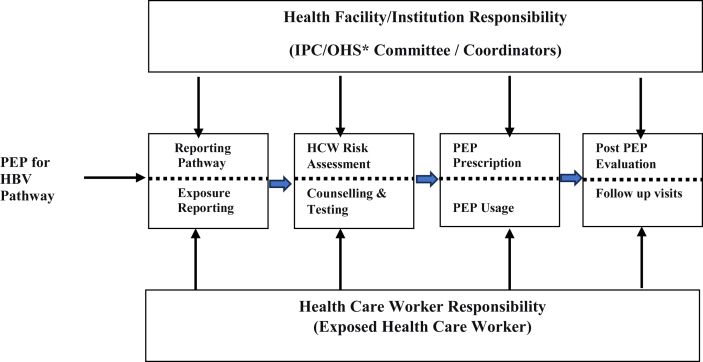
The successful implementation of the PEP management programme for HBV infection depends on the interplay between health facility responsibilities and the responsibilities of the exposed HCW [42]. The health facility has the role of instituting an exposure reporting pathway or system with trained individuals who would respond to HCWs with exposures promptly. The availability of facility-level policies and guidelines regarding the implementation of PEP coupled with an appropriate stock of recommended laboratory testing supplies and other PEP logistics have all been identified as major components of an effective PEP program. Positive knowledge and good attitudes of HCWs toward exposure reporting and the use of PEP are other important components of an effective PEP program [43].
For a PEP program to be successful, a clear pathway is needed for the effective management of exposed HCWs. First and foremost, exposed HCWs are responsible for reporting injury or exposure by existing occupational health and safety structures within health facilities. HCWs need to be knowledgeable about exposure reporting procedures. The report is forwarded immediately for documentation and surveillance. The next step is the comprehensive assessment of both the exposed HCW and the source patient (if known). A comprehensive evaluation was performed to review previous immunization and antibody level records of the exposed HCWs. Laboratory testing is performed when documentation of these parameters is unavailable. The risk of the source patient transmitting HBV to the HCW is also determined using the patient's medical records or history and laboratory tests performed after the source patient has consented to the evaluations.
PEP prescription and subsequent PEP initiation follow promptly depending on the outcome of both the exposed HCW and the source patient assessment. Appointments are then given for follow-up after PEP use [42,37]. Any break in this sequence of events may lead to failure or suboptimal adherence to a successful PEP programme [42] (Figure 1).
Figure 2 illustrates the sequence of events in a successful PEP program and the actions and responsibilities of HCWs and health facilities toward successful PEP implementation program.
Figure 2.
Recommended steps in the PEP program for HBV-exposed HCW. ∗IPC/OHS – Infection prevention/occupational health and safety.
Documented evidence on the effectiveness of postexposure prophylaxis for HBV
The effectiveness of HBIG and the HBV vaccine for the prevention of HBV infection when used as PEP was documented many years ago [44,45]. Recently, Gong et al. (2018) reported the effectiveness of HBIG in preventing new HBV infections [46]. Evidence from many controlled trials revealed the protective efficacy of HBIG alone and HBIG with the HB vaccine in the prevention of vertical transmission as well as transmission via blood and body fluid exposure [44,47]. Specifically, a study reported that PEP for HBV was very effective and prevented disease transmission even after it was administered a little after 24 hours after occupational exposure in HCWs [48].
Postexposure prophylaxis knowledge among HCWs in endemic regions
Knowledge of PEP for HBV is essential for effective PEP management of exposed HCWs [4]; despite this observation, not all HCWs who are at risk of HBV infection are knowledgeable about PEP for HBV. Many studies among HCWs have revealed suboptimal knowledge of PEP for HBV. In regions highly endemic for HBV, a profound to moderate lack of knowledge has been reported. For example, in Uganda, Isunju et al. (2022) revealed that only one-tenth of HCWs were aware of PEP for HBV [49]. In Ghana, two studies from the northern and southern sectors of the country both reported undesirable knowledge of PEP for HBV among HCWs [50]. A low level of PEP knowledge was equally reported among dentists and their interns in Pakistan and Nigeria [51,52].
The low level of awareness of HBV PEP reported in these studies is alarming, considering the high risk of HBV infection that characterizes healthcare settings in endemic settings. Ironically, the lack of awareness or low and undesirable level of knowledge reported among HCWs is rather prevalent in countries where HBV is highly endemic and where HBV vaccination coverage is very low [33]. This observation is an indication that HBV prevention using the PEP strategy is not an integral part of infection prevention and control education and training for HCWs, especially in low-resource and high-HBV endemic settings.
Barriers to PEP uptake among HCWs in HBV endemic settings
Unlike for HIV, only a few studies have reported on the uptake of PEP for HBV. This may be due to the low level of awareness of PEP for HBV among HCWs. Again, reporting occupational exposure, which is the bedrock of PEP use among HCWs, is suboptimal in many health facilities in HBV-endemic regions. For example, similar to other studies in Africa and some parts of Asia where the HBV disease burden is very high, a study on HCWs recruited from 3 Botswanan hospitals reported a low exposure reporting rate of 37% [53]. In contrast, a study performed in southern Ghana among 6 cadres of HCWs revealed an exposure reporting rate of 76.3% [54]. It has been established that, in many endemic settings, HCWs rely on undocumented procedures as PEP for HBV. Kumar et al. (2015) reported that HCWs exposed to HBV in India relied on local measures and undocumented practices as PEP for HBV [55]. Surprisingly, a study in Ghana reported that HCWs who were exposed to HBV and needed PEP were wrongfully offered antiretroviral agents as PEP for HBV (5). Many factors have been identified as barriers to effective management of HCWs exposed to HBV in endemic settings. Senoo-Dogbey et al. identified the lack of logistics, such as immunoglobulin and HBV vaccines, in health facilities in Ghana as major barriers [56]. Another major barrier is the widespread unavailability of protocols and guidelines specific to HBV prevention to provide direction for PEP in health facilities [5,56]. Studies have also reported poor leadership and governance for HBV prevention in health facilities, as indicated by the absence or unavailability of occupational health and safety committees and focal persons to drive HBV prevention efforts in health facilities [56]. The unsatisfactory knowledge and profound lack of awareness of PEP for HBV among HCWs reveals the poor integration of HBV prevention education and training into general in-service training of HCWs, which could have contributed to the use of undocumented and ineffective measures for PEP for HBV among exposed individuals [57].
The lack of awareness created as a result of inadequacies in training of HCWs in sharps safety and safe disposal of sharps and medical waste in general has also contributed to frequent exposures necessitating the use of PEP for HBV.
Conclusion
Hepatitis B virus infection is a life-threatening condition. However, the disease is preventable by using the HBV vaccine as a preexposure prophylaxis measure. An effective postexposure prophylaxis management program is also available, and preventing occupational transmission of HBV among HCWs who fail to vaccinate completely or who fail to respond to the HBV vaccine has become a lifesaving strategy. The recommended pathway for PEP management includes exposure reporting, HBV transmission risk assessment, PEP prescription and utilization, and follow-up. The responsibility of an effective PEP management programme lies on the shoulders of the exposed HCW and the facility representatives in charge of PEP management. Adherence to each component of the PEP management pathway is essential, and immediate or timely exposure reporting is the cornerstone of a PEP management program. Effective risk assessment and timely initiation of PEP are crucial, and follow-ups are equally essential in evaluating the process. HCWs, especially those in highly endemic regions where the prevalence of the infection is high in the general population and where HBV vaccination coverage is suboptimal, should take advantage of the availability of PEP for HBV and avoid occupational transmission of HBV, which is a life-threatening disease that has the potential to cause hepatocellular carcinoma and liver cirrhosis.
Implications for practice
In settings where the risk of exposure to blood and body fluids is high and hepatitis B vaccination coverage among HCWs remains very low, there is a need for health facility managers to activate safety and infection prevention teams and equip them with knowledge and skills. There is a need for facilities to have effective systems for exposure reporting and PEP management for healthcare workers who are at risk of hepatitis B infection, especially in highly endemic areas.
What the study adds:
-
•
In highly endemic areas where hepatitis B vaccination coverage is low, healthcare workers need to follow strict postexposure management recommendations to protect themselves from acquiring HBV infection.
-
•
The recommended steps include exposure reporting, healthcare worker and source patient assessment, prescription and utilization of PEP and, ultimately, post-PEP evaluation.
Acknowledgement
We are thankful to all who provided diverse support in writing this review article.
Funding support
The authors did not receive any external financial support.
Competing interest
The authors declare that there are no known competing interests.
Ethical clearance
This is a narrative review; hence, no human experimentation was undertaken; therefore, ethical clearance was not applicable.
References
- 1.Coppola N., De Pascalis S., Onorato L., Calò F., Sagnelli C., Sagnelli E. Hepatitis B virus and hepatitis C virus infection in healthcare workers. World J Hepatol. 2016;8(5):273–281. doi: 10.4254/wjh.v8.i5.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis J.D., Enfield K.B., Sifri C.D. Hepatitis B in healthcare workers: Transmission events and guidance for management. World J Hepatol. 2015;7(3):488–497. doi: 10.4254/wjh.v7.i3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahamat G., Kenmoe S., Akazong E.W., Ebogo-Belobo J.T., Mbaga D.S., Bowo-Ngandji A., et al. Global prevalence of hepatitis B virus serological markers among healthcare workers: A systematic review and meta-analysis. World J Hepatol. 2021;13(9):1190–1202. doi: 10.4254/wjh.v13.i9.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Senoo-Dogbey V.E. The knowledge of postexposure prophylaxis for hepatitis B virus and related factors among health care workers in Accra, Ghana. Arch Occup Heal. 2021;6(1):1164–1170. [Google Scholar]
- 5.Tiko M. University of Ghana; 2023. Self efficacy for hepatitis B prevention among midwives. MPhil Thesis. [Google Scholar]
- 6.Beltrami E.M., Williams I.T., Shapiro C.N., Chamberland M.E. Risk and management of blood-borne infections in health care workers. Clin Microbiol Rev. 2000;13(3):385–407. doi: 10.1128/cmr.13.3.385-407.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hepatitis B Foundation. Hepatitis B facts and figures [Internet]. Hepatitis B. 2023 [cited 2023 Jun 26]. Available from: https://www.hepb.org/what-is-hepatitis-b/what-is-hepb/facts-and-figures/.
- 8.Centers for Disease Control and Prevention Updated U.S. public health service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. Mortal Morb Wkly Rep [Internet] 2001;(1–42):50. http://www.cdc.gov/MMWR/preview/mmwrhtml/rr5011a1.htm Available from: [PubMed] [Google Scholar]
- 9.Bond W.W., Petersen N.J., Favero M.S. Viral hepatitis B: aspects of environmental control. Health Lab Sci. 1977;14(4):235–252. [PubMed] [Google Scholar]
- 10.Nemr N., Kishk R., Mandour M., Ragheb M. Occupational risk of hepatitis B virus exposure: Overview and recommendations. Suez Canal Univ Med J. 2018;21(2):59–70. [Google Scholar]
- 11.Werner B.G., Grady G.F. Accidental hepatitis-B-surface-antigen-positive inoculations. Use of e antigen to estimate infectivity. Ann Intern Med. 1982;97(3):367–369. doi: 10.7326/0003-4819-97-3-367. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization Health Workers. World Heal Rep 2006 [Internet] 2006:1–15. http://www.who.int/whr/2006/06_chap1_en.pdf Available from: [Google Scholar]
- 13.Prüss-Üstün A., Rapiti E., Hutin Y. Estimation of the global burden of disease attributable to contaminated sharps injuries among health-care workers. Am J Ind Med [Internet] 2005;48(6):482–490. doi: 10.1002/ajim.20230. http://www.ncbi.nlm.nih.gov/pubmed/16299710 Available from: [DOI] [PubMed] [Google Scholar]
- 14.Babanejad M., Izadi N., Alavian S.M. A systematic review and meta-analysis on the prevalence of HBsAg in health care workers from Eastern Mediterranean and Middle Eastern Countries. Int J Prev Med. 2019;10(144) doi: 10.4103/ijpvm.IJPVM_111_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tavoschi L., Mason L., Petriti U., Bunge E., Veldhuijzen I., Duffell E. Hepatitis B and C among healthcare workers and patient groups at increased risk of iatrogenic transmission in the European Union/European Economic Area. J Hosp Infect [Internet] 2019;102(4):359–368. doi: 10.1016/j.jhin.2019.03.004. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas D.L., Factor S.H., Kelen G.D., Antonio S., Washington E.T., Quinn T.C., et al. Viral hepatitis in health care personnel at the Johns Hopkins Hospital: Seroprevalence of and risk factors for hepatitis B virus and hepatitis C virus infection. Arch Intern Med [Internet] 1993;153(14):1705. http://archinte.jamanetwork.com/article.aspx?articleid=617609 Available from: [PubMed] [Google Scholar]
- 17.Calleja-Panero J.L., Llop-Herrera E., Ruiz-Moraga M., dela-Revilla-Negro J., Calvo-Bonacho E., Pons-Renedo F., et al. Prevalence of viral hepatitis (B and C) serological markers in healthy working population. Rev Esp Enfermedades Dig. 2013;105(5):249–254. doi: 10.4321/s1130-01082013000500002. [DOI] [PubMed] [Google Scholar]
- 18.Ciorlia L.A.S., Zanetta D.M.T. Hepatitis B in healthcare workers: prevalence, vaccination and relation to occupational factors. Brazilian J Infect Dis. 2005;9(5):384–389. doi: 10.1590/s1413-86702005000500005. [DOI] [PubMed] [Google Scholar]
- 19.Maamor N.H., Muhamad N.A., Mohd Dali N.S., Abdul Mutalip M.H., Leman F.N., Aris T., et al. Seroprevalence of hepatitis B among healthcare workers in Asia and Africa and its Association with their knowledge and awareness: A systematic review and meta-analysis. Front Public Heal. 2022;10(April):1–12. doi: 10.3389/fpubh.2022.859350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atlaw D., Sahiledengle B., Tariku Z. Hepatitis B and C virus infection among healthcare workers in Africa: a systematic review and meta-analysis. Environ Health Prev Med. 2021;26(1):1–14. doi: 10.1186/s12199-021-00983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou J., Liu Z., Gu F. Epidemiology and prevention of hepatitis B virus infection. Int J Med Sci. 2005;2(1):50–57. doi: 10.7150/ijms.2.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ott J.J., Stevens G.A., Groeger J., Wiersma S.T. Global epidemiology of hepatitis B virus infection: New estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine [Internet] 2012;30(12):2212–2219. doi: 10.1016/j.vaccine.2011.12.116. Available from: [DOI] [PubMed] [Google Scholar]
- 23.Pappas S.C., Fisher M.M. Preventing hepatitis B in health care workers. Can Fam Phy [Internet] 1985;31:1941–1944. http://www.ncbi.nlm.nih.gov/pubmed/21274208%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC2327899 Available from: [PMC free article] [PubMed] [Google Scholar]
- 24.Hepatitis W.H.O. B vaccines: WHO position paper-Recommendations. Wkly Epidemiol Rec [Internet] 2009;84(40):405–420. http://www.who.int/wer Available from: [Google Scholar]
- 25.World Health Organization (WHO) Weekly Epidemiological Record. Vol. 28. 2004. Hepatitis B vaccines : WHO position paper – July 2004. [Google Scholar]
- 26.World Health Organization Media centre [Internet] 2017:1–7. http://www.who.int/mediacentre/factsheets/fs204/en/ Available from: [Google Scholar]
- 27.Center for Disease Control and Prevention Recommendations for preventing transmission of infection with human T-lymphotropic virus type III/lymphadenopathy-associated virus during 1nvasive procedures. MMWR Morb Mortal Wkly Rep. 1987;34(45) [Google Scholar]
- 28.Center for Disease Control and Prevention Universal precautions for prevention of transmission of other bloodborne pathogens in health-care settings. MMWR Morb Mortal Wkly Rep. 1988;37(24):377–388. [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention . Centers for Disease Control and Prevention; 2013. Guide to infection prevention for outpatient settings: minimum expectations for safe care. [Google Scholar]
- 30.Yassin M., Gupta V. Role of infection control in prevention of hepatitis B virus in hemodialysis patients. Infect Disord: Drug Targets. 2013;13(3):162–168. doi: 10.2174/1871526511313030003. [DOI] [PubMed] [Google Scholar]
- 31.National Center for Immunization Research & Surveillance Hepatitis B vaccines for Australians : [Internet] NCIRS Fact Sheet. 2015:1–11. https://ncirs.org.au/ncirs-fact-sheets-faqs/hepatitis-b-vaccines-for-australians [cited 2017 Mar 1]. Available from: [Google Scholar]
- 32.Walayat S., Ahmed Z., Martin D., Puli S., Cashman M., Dhillon S. Recent advances in vaccination of nonresponders to standard dose hepatitis B virus vaccine. World J Hepatol. 2015;7(24):2503–2509. doi: 10.4254/wjh.v7.i24.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Auta A., Adewuyi E.O., Kureh G.T., Onoviran N., Adeloye D. Hepatitis B vaccination coverage among health-care workers in Africa: A systematic review and meta-analysis. Vaccine [Internet] 2018;36(32):4851–4860. doi: 10.1016/j.vaccine.2018.06.043. Available from: [DOI] [PubMed] [Google Scholar]
- 34.Ali A.S., Hussein N.A., Elmi E.O.H., Ismail A.M., Abdi M.M. Hepatitis B vaccination coverage and associated factors among medical students: a cross-sectional study in Bosaso, Somalia, 2021. BMC Public Health [Internet. 2023;23(1):1–8. doi: 10.1186/s12889-023-15992-2. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Awoke N., Mulgeta H., Lolaso T., Tekalign T., Samuel S., Obsa M.S., et al. Full-dose hepatitis B virus vaccination coverage and associated factors among health care workers in Ethiopia: A systematic review and meta-analysis. PLoS One [Internet. 2020;15(10 October):1–15. doi: 10.1371/journal.pone.0241226. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Machmud P.B., Glasauer S., Gottschick C., Mikolajczyk R. Knowledge, vaccination status, and reasons for avoiding vaccinations against hepatitis b in developing countries: A systematic review. Vaccines. 2021;9(6):1–26. doi: 10.3390/vaccines9060625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization . World Health Organization; 2014. Postexposure prophylaxis to prevent HIV infection [Internet] pp. 1–3.http://www.who.int/hiv/pub/prophylaxis/guidelines/en/ Available from: [Google Scholar]
- 38.World Health Organization . World Health Organization; 2007. Postexposure prophylaxis to prevent HIV infection : Joint WHO/ILO guidelines on postexposure prophylaxis to prevent HIV infection [Internet] pp. 1–104.file:///C:/Users/Harrison/Desktop/Consult/Mubaric/A1/9789241596374_eng.pdf [cited 2023 Jul 3]. Available from: [Google Scholar]
- 39.Kroger A.T., Pickering L.K., Wharton M., Mawle A., Hinman A.R., Orenstein W.A. In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Bennett J.E., Dolin R., Blaser M., editors. 2014. Immunization; pp. 3516–3553. [Google Scholar]
- 40.Country of Los Angeles Public Health Immunization Program Hepatitis B immune globulin [Internet] 2015:5. http://publichealth.lacounty.gov/ip/providers/B71.htm –6 [cited 2023 Jun 10]. Available from: [Google Scholar]
- 41.Penalver J. Management of needlestick injuries in the health care setting. Ethics J Am Med Assoc. 2005;7(10):683–686. doi: 10.1001/virtualmentor.2005.7.10.cprl1-0510. [DOI] [PubMed] [Google Scholar]
- 42.Boden L.I., Petrofsky Y.V., Hopcia K., Wagner G.R., Hashimoto D. Understanding the hospital sharps injury reporting pathway. Am J Ind Med. 2016;118(24):6072–6078. doi: 10.1002/ajim.22392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Courtenay-Quirk C., Selenic D., Lahuerta M., Kassa G., Murrman M., Bock N. Development of an intervention to increase occupational postexposure prophylaxis in sub-Saharan Africa. J Assoc Nurses AIDS Care [Internet] 2016;27(5):727–730. doi: 10.1016/j.jana.2016.06.004. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krugman S., Giles J.P., Hammond J. Viral hepatitis, type B (MS-2 strain): prevention with specific hepatitis B immune serum globulin. J Am Med Assoc. 1971;218(11):1665–1670. [PubMed] [Google Scholar]
- 45.Winsnes R., Siebke J.C. Efficacy of post-exposure prophylaxis with hepatitis B immunoglobulin in Norway. J Infect. 1986;12(1):11–21. doi: 10.1016/s0163-4453(86)94746-8. [DOI] [PubMed] [Google Scholar]
- 46.Gong J., Liu X. Effect of HBIG combined with hepatitis B vaccine on blocking HBV transmission between mother and infant and its effect on immune cells. Exp Ther Med. 2018;15(1):919–923. doi: 10.3892/etm.2017.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beasley R.P., Hwang L.-Y., Stevens C.E., Lin C.-C., Hsieh F.-J., Wang K.-Y., et al. Efficacy of Hepatitis B Immune Globulin for Prevention of Perinatal Transmission of the Hepatitis B Virus Carrier State: Final Report of a Randomized Double-Blind, Placebo-Controlled Trial. Hepatology. 1983;3(2):135–141. doi: 10.1002/hep.1840030201. [DOI] [PubMed] [Google Scholar]
- 48.Chang H.H., Lee W.K., Moon C., Choi W.S., Yoon H.J., Kim J., et al. The acceptable duration between occupational exposure to hepatitis B virus and hepatitis B immunoglobulin injection: Results from a Korean nationwide, multicenter study. Am J Infect Control. 2016;44(2):189–193. doi: 10.1016/j.ajic.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 49.Isunju J.B., Wafula S.T., Ndejjo R., Nuwematsiko R., Bakkabulindi P., Nalugya A., et al. Awareness of hepatitis B postexposure prophylaxis among healthcare providers in Wakiso district, Central Uganda. PLoS One [Internet] 2022;17(6 Jun):1–14. doi: 10.1371/journal.pone.0270181. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Konlan K.D., Aarah-Bapuah M., Kombat J.M., Wuffele G.M. The level of nurses ’ knowledge on occupational postexposure to hepatitis B infection in the Tamale metropolis, Ghana. BMC Heal Serv Res. 2016;1(12):1–15. doi: 10.1186/s12913-017-2182-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okoh M., Saheeb B.D. Assessment of knowledge, attitude and practice of postexposure prophylaxis against blood-borne viral infection among dental surgeons in a teaching hospital. South African J Infect Dis [Internet] 2016;1(1):1–6. http://www.tandfonline.com/doi/full/10.1080/23120053.2016.1198079 Available from: [Google Scholar]
- 52.Ahmed Z., Zahra U., Saleem N. Knowledge about Postexposure Prophylaxis for Hepatitis B Virus among Dentists and Dental Students in Pakistan. Int Dent J Student’s Res. 2015;3(December):189–194. [Google Scholar]
- 53.Kassa G., Selenic D., Lahuerta M., Gaolathe T., Liu Y., Letang G., et al. Occupational exposure to bloodborne pathogens among health care workers in Botswana: Reporting and utilization of postexposure prophylaxis. Am J Infect Control [Internet] 2016;44(8):879–885. doi: 10.1016/j.ajic.2016.01.027. Available from: [DOI] [PubMed] [Google Scholar]
- 54.Senoo-Dogbey V.E. Adherence to post-exposure prophylaxis for Hepatitis B virus among health care workers in Accra, Ghana. IOSR J Nurs Heal Sci. 2021;10(5):52–59. [Google Scholar]
- 55.Kumar H.N.H., Nambiar R.P., Mohapatra S., Khanna A., Praveen R., Sai Bhawana D. A cross-sectional study on hepatitis B vaccination status and postexposure prophylaxis practices among health care workers in teaching hospitals of Mangalore. Ann Glob Heal [Internet] 2015;81(5):664–668. doi: 10.1016/j.aogh.2015.08.015. http://linkinghub.elsevier.com/retrieve/pii/S221499961501231X Available from: [DOI] [PubMed] [Google Scholar]
- 56.Senoo-Dogbey V.E., Armah D., Wuaku D.A. Hepatitis B infection prevention: Audit of selected healthcare facilities in the Greater Accra Region, Ghana. Infect Prev Pract [Internet] 2023;5(2) doi: 10.1016/j.infpip.2023.100284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beekmann S.E., Henderson D.K. Occupational exposures among healthcare workers: New prevention methods and recommended postexposure prophylaxis for HIV and Hepatitis B and C. Curr Treat Options Infect Dis [Internet] 2015;7(1):28–38. http://link.springer.com/10.1007/s40506-014-0036-y Available from: [Google Scholar]