Abstract
Despite the availability of life-saving corticosteroids for 70 years, treatment for adrenal insufficiency is not able to recapitulate physiological diurnal cortisol secretion and results in numerous complications. Gene therapy is an attractive possibility for monogenic adrenocortical disorders such as congenital adrenal hyperplasia; however, requires further development of gene transfer/editing technologies and knowledge of the target progenitor cell populations. Vectors based on adeno-associated virus are the leading system for direct in vivo gene delivery but have limitations in targeting replicating cell populations such as in the adrenal cortex. One strategy to overcome this technological limitation is to deliver the relevant adrenocortical gene to a currently targetable organ outside of the adrenal cortex. To explore this possibility, we developed a vector encoding human 21-hydroxylase and directed expression to the liver in a mouse model of congenital adrenal hyperplasia. This extra-adrenal expression resulted in reconstitution of the steroidogenic pathway. Aldosterone and renin levels normalized, and corticosterone levels improved sufficiently to reduce adrenal hyperplasia. This strategy could provide an alternative treatment option for monogenic adrenal disorders, particularly for mineralocorticoid defects. These findings also demonstrate, when targeting the adrenal gland, that inadvertent liver transduction should be precluded as it may confound data interpretation.
Key words: gene therapy, congenital adrenal hyperplasia, steroidogenesis, adeno-associated virus, gene addition
Graphical abstract

Graves and colleagues demonstrate that intravenous administration of a liver-targeted AAV vector encoding human 21-hydroxylase cDNA restores steroidogenic function in 21-hydroxylase-deficient mice. Liver transduction has the potential to overcome the limitations imposed by adrenocortical cellular turnover. This also has implications for data interpretation in adrenally targeted gene transfer studies.
Introduction
Little has changed in the treatment for congenital adrenal hyperplasia (CAH) since exogenous steroids were first introduced 70 years ago.1,2,3 CAH encompasses seven monogenic disorders of the adrenal that disrupt enzymatic function and result in a deficiency of one or more adrenal steroids with compensatory adrenal hyperplasia secondary to adrenocorticotropic hormone (ACTH) stimulation.4 Deficiency of 21-hydroxylase is the most common cause of CAH with a global incidence of classical salt-wasting CAH estimated to be between 1 in 10,000 to 1 in 22,000.5,6,7 There is resultant deficiency of cortisol and aldosterone, and upstream precursor steroids are shunted along the 17-hydroxylase-facilitated pathway to form adrenal androgens in excess causing pre- and postnatal virilization.4 While exogenous corticosteroids are life-saving, treatment is far from perfect and cannot mimic the diurnal rhythm and physiological control of cortisol secretion, resulting in intervals of over- and undertreatment within a 24-h period. Adrenal crisis remains the most common cause of death and there is shorter life expectancy in those with CAH than the general population.8
Superior treatment options are needed to overcome the burden of disease in CAH.9 As current standard management is imperfect, alternative and adjunctive treatment options have been explored, including subcutaneous hydrocortisone pumps10,11 and modified-release once-daily hydrocortisone.12,13 Neither of these alternatives is ideal, as pump management is complex and the modified-release hydrocortisone has not been shown to be superior to standard management.14 Recent success with gene therapy for conditions such as spinal muscular atrophy has paved the way for other genetic treatments.15 There is a paucity of gene therapy studies targeting the adrenal cortex, despite publication of the first pre-clinical gene therapy study for CAH over 20 years ago.9 The murine adrenal cortex lacks 17-hydroxylase expression so does not produce adrenal androgens, and the major glucocorticoid in the mouse is corticosterone16: thus in the 21-hydroxylase-deficient mouse model, serum progesterone levels are elevated rather than 17-hydroxyprogesterone (17OHP).17 While there is no hyperandrogenism, the model otherwise appropriately recapitulates the human classical salt-wasting CAH phenotype with inadequate glucocorticoid and mineralocorticoid production to sustain neonatal life. Murine studies using intra-adrenal or intravenous delivery of a viral vector with 21-hydroxylase cDNA has demonstrated short-term improvement in progesterone.18,19,20 Based on these pre-clinical studies a human clinical trial is now under way using a gene addition strategy with rAAV5-CYP21A2 (ClinicalTrials.gov: NCT04783181).
However, these studies do not address the rapid cellular turnover in the adrenal cortex. The recombinant adeno-associated virus (rAAV) genome is maintained predominantly as extra-chromosomal episomes that are lost during cellular replication. Therefore, a gene addition strategy using rAAV will not provide a lasting effect.20,21 Furthermore, the development of neutralizing antibodies currently limits the use of rAAV therapy to a single treatment.22,23 For durable adrenal-directed gene therapy, the new genetic material must be integrated into the genome of adrenocortical progenitor cells, a feat that is beyond current technology due to difficulties in targeting this cell population.9 One strategy that could overcome this technological limitation is to deliver ectopic expression of 21-hydroxylase in a readily targetable organ outside of the adrenal cortex. We explored this theoretical possibility by developing an rAAV encoding human 21-hydroxylase (CYP21A2) and directing expression specifically to the liver in a mouse model of CAH. We hypothesized that hepatically expressed human CYP21A2 could participate in steroidogenesis and facilitate downstream adrenal production of corticosterone and aldosterone (Figure 1A). We found that extra-adrenal expression of a deficient adrenocortical enzyme could indeed co-operatively reconstitute adrenal steroidogenesis.
Figure 1.
Study set up to assess hepato-adrenal cooperativity in steroidogenesis
(A) Hypothesis: in 21-hydroxylase deficiency, the precursor steroid (progesterone in the mouse, 17-hydroxyprogesterone in the human) accumulates and enters the systemic circulation where it will circulate to the liver. Recombinant AAV-derived human 21-hydroxylase expressed in the liver will convert progesterone to deoxycorticosterone, which will then enter the systemic circulation, reaching the adrenal gland. Enzymes downstream of 21-hydroxylase will be unaffected and will be able to complete steroidogenesis. (B) Recombinant AAV vector genome; liver-specific enhancer-promoter (ApoE-hAAT), human 21-hydroxylase cDNA (hCYP21A2), and bovine growth hormone poly-adenylated tail (polyA). The scale bar represents 500 base pairs. (C) Prior to treatment, dried whole blood was collected on to filter paper. Mice (n = 5 male, n = 5 female) were administered the purified vector intravenously via the tail vein and harvested 4 weeks later. AAV, adeno-associated virus.
Results
Expression of human CYP21A2 in murine liver following rAAV-mediated delivery
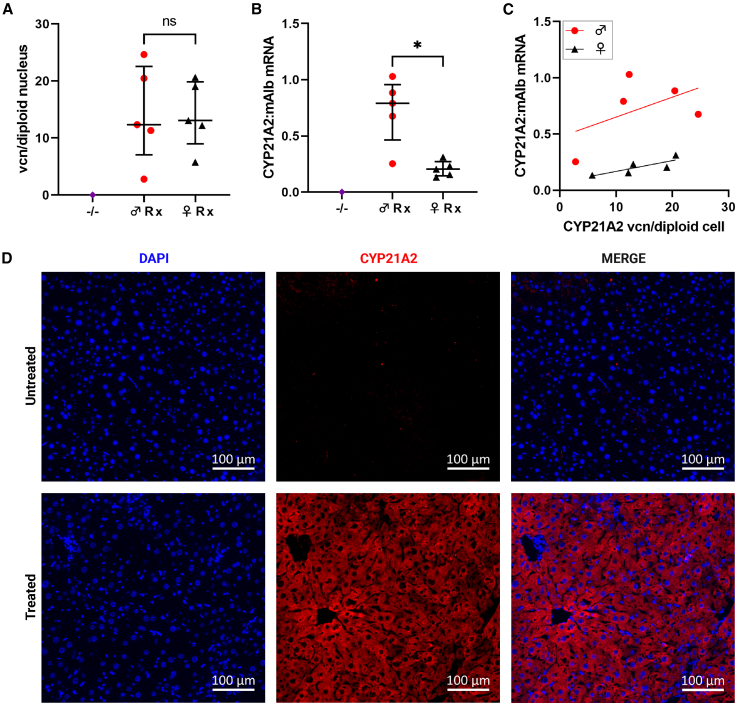
An rAAV2/8 vector utilizing a liver-specific promoter with the human CYP21A2 cDNA (Figure 1B) was intravenously delivered to 21-hydroxylase-deficient (C57BL/10SnSlc-H-2aw18; Cyp21a1−/−) adult mice, homozygous for a pathogenic variant in Cyp21a1, at a dose of 5 × 1011 vector genomes per mouse (Figure 1C). Four weeks after administration, liver transduction was confirmed by vector copy number (vcn) per diploid nucleus and vector encoded mRNA detected in the livers of treated Cyp21a1−/− mice (Figures 2A and 2B, respectively; Table S1). The median vcn/diploid nucleus was similar in males and females; however, there was 3-fold higher vector transcription into mRNA in males than females, as expected.24 In both sexes, the mouse with the lowest vcn/diploid nucleus had the lowest mRNA transcription (Figure 2C). Protein translation was demonstrated by immunohistochemical staining for CYP21A2 (Figure 2D). While human CYP21A2 mRNA was detectable in the treated Cyp21a1−/− mouse adrenals, levels were 300- to 800-fold lower than native Cyp21a1 mRNA in wild-type (Cyp21a1+/+) adrenals (Figure S1A).
Figure 2.
Robust delivery to and expression of human CYP21A2 in the murine liver
(A) Vector DNA detected in the livers of treated mice. (B) The ratio of vector-derived mRNA transcripts to murine albumin transcripts. (C) Vector copy number vs. vector expression in males (red) and females (black). (D) IHC staining demonstrated CYP21A2 protein (red) in the liver from a representative treated male −/− mouse with 20.5 vcn/diploid nucleus and transcript mRNA ratio 0.88. Scale bar represents 100 µm. DAPI nuclear staining in blue. vcn, vector copy number; IHC, immunohistochemistry; −/−, homozygous 21-hydroxylase deficiency; ♂, male; ♀, female; Rx, homozygous 21-hydroxylase deficient mice that were treated with vector; CYP21A2, human 21-hydroxylase; mAlb, murine albumin; ns, not significant. Individual data points are shown, and error bars represent median and interquartile range. ∗p < 0.05 on Mann-Whitney U test.
Human CYP21A2 expression in the liver co-operatively improved adrenal steroidogenesis
Serum aldosterone in the vector-treated Cyp21a1−/− mice was restored to Cyp21a1+/+ levels (Figure 3A; Table S1). Serum corticosterone in the treated Cyp21a1−/− mice was increased 4- to 5-fold compared with untreated Cyp21a1−/− controls; however, Cyp21a1+/+ serum corticosterone levels were not achieved following vector administration (Figure 3B; Table S1). Cyp21a1+/+ females had 2-fold higher serum corticosterone than their Cyp21a1+/+ male counterparts, as has been previously demonstrated by other researchers.25 The change in individual dried whole blood corticosterone improved in all but one Cyp21a1−/− mouse (Figure 3C). Although this mouse had the lowest vcn/diploid nucleus, lowest vector expression among the males, and lowest serum corticosterone (69.8 nmol/L), vcn/diploid nucleus did not always correlate with steroidogenic effect in other treated mice. Progesterone improved in the treated Cyp21a1−/− female mice but there was no change in the Cyp21a1−/− male progesterone levels (Figure 3D; Table S1). The progesterone remained 17- and 35-fold higher in Cyp21a1−/− females and males than Cyp21a1+/+ levels, respectively.
Figure 3.
Improvement in steroidogenesis following hepatic CYP21A2 expression
(A) Serum aldosterone. (B) Serum corticosterone. In the call-out box, +/+ controls were removed to demonstrate more clearly the change in serum corticosterone levels in −/− treated vs. −/− untreated. (C) Dried whole blood corticosterone before treatment and at harvest in males (red) and females (black). (D) Serum progesterone. (E) Renin expression demonstrated as the ratio of renal renin (Ren1) mRNA transcripts to Tbp transcripts. +/+, wild-type 21-hydroxylase sufficiency; −/−, homozygous 21-hydroxylase deficiency; ♂, male; ♀, female; Rx, homozygous 21-hydroxylase deficient mice that were treated with vector; Ren1, renin; Tbp, TATA-box binding protein; ns, not significant. Individual data points are shown, and error bars represent median and interquartile range. ∗p < 0.05; ∗∗p < 0.01 on Mann-Whitney U test. ∗∗∗p < 0.001 on two-way ANOVA.
Renin expression normalized and adrenal hyperplasia reduced following human CYP21A2 expression in the liver
Given that renin increases in response to reduced renal perfusion and reduced tubular sodium content to activate the renin-angiotensin-aldosterone system,26,27 renin (Ren1) expression was measured as a marker of mineralocorticoid function. Ren1 mRNA expression in the kidneys was normalized to TATA-box binding protein (Tbp) mRNA as the chosen reference housekeeping transcript.19,28,29,30 Renin expression was 25- to 30-fold higher in untreated Cyp21a1−/− mice compared with Cyp21a1+/+. Renin expression in Cyp21a1−/− mice was restored to Cyp21a1+/+ levels following treatment with the vector (Figure 3E; Table S1).
Female mice are known to have larger adrenal glands than males.25 This pattern remained consistent in the Cyp21a1−/− mice with the bilateral adrenal mass 20% higher in Cyp21a1−/− females than Cyp21a1−/− males. Cyp21a1−/− females had adrenal mass 2.6-fold larger than Cyp21a+/+ females and Cyp21a1−/− male adrenal mass was 3.2-fold larger than Cyp21a1+/+ male adrenal mass. While the effect on corticosterone was partial, there was a reduction in size of the bilateral adrenal mass by one-third following treatment in both Cyp21a1−/− males and females (Figure 4A; Table S1). The bilateral adrenal mass was also measured as a percentage of the total body mass to account for any variation in body size and the reduction in adrenal size persisted (Figure S1B). Upon direct macroscopic examination, the adrenal glands from the treated Cyp21a1−/− animals were collapsed and flat, consistent with a reduction in volume, compared with the round untreated Cyp21a1−/− glands. The difference was visible macroscopically (Figure 4B). There was no change in the histological architecture of the adrenal cortex following treatment (data not shown).
Figure 4.
Reduction in adrenal hyperplasia after hepatic CYP21A2 expression
(A) Absolute bilateral adrenal mass. (B) Macroscopic photographs of representative adrenal glands after fat dissection. +/+, wild-type 2-hydroxylase sufficiency; −/−, homozygous 21-hydroxylase deficiency; ♂, male; ♀, female; Rx, homozygous 21-hydroxylase-deficient mice that were treated with vector. Individual data points are shown, and error bars represent as median and interquartile range. ∗∗p < 0.01 on Mann-Whitney U test.
Blood was collected during exsanguination causing physiological stress. The difference in maximal ACTH secretion in the treated Cyp21a1−/− mice was not statistically significant compared with the untreated Cyp21a1−/− mice (Figure S1C). Due to the short half-life of ACTH, expression of the ACTH receptor (Mc2r) was also examined. Although the expression of Mc2r did not change in the female mice following treatment, expression was reduced by 28% in treated Cyp21a1−/− male mice compared with untreated Cyp21a1−/−(Figure S1D).
Discussion
The adrenal cortex is an attractive gene therapy target, but its challenging biological properties have yet to be surmounted by contemporary gene delivery technology. Adeno-associated virus has natural tropism for the liver and is a logical target for gene therapies. Our approach exploits this characteristic with good clinical effect. This study demonstrated that extra-adrenal expression of human CYP21A2 facilitated by rAAV gene delivery to the liver successfully conferred hepato-adrenal co-operativity in steroidogenesis resulting in significant phenotypic correction in a 21-hydroxylase-deficient mouse. In adults with completed liver growth, this strategy overcomes the limitations imposed by adrenocortical cellular turnover, rAAV gene delivery, and targeting stem/progenitor cell populations that are not fully characterized.9,20 Mineralocorticoid function was restored and glucocorticoid production improved. This is the first study that has looked at the effect of rAAV-mediated adrenocortical gene delivery to the liver. Based on these data, this strategy could provide a treatment option for mineralocorticoid disorders such as aldosterone synthase deficiency; however, requires further development for use in disorders that impact glucocorticoid production. This study also highlights a need for caution in the interpretation of the effects of systemically administered therapies that may result from inadvertent liver transduction.
The clinically relevant outcomes of our strategy include improved steroidogenesis with subsequent phenotypic benefit. Restoration of aldosterone production would likely result in resolution of the salt-wasting component of the phenotype and therefore could improve the clinical phenotype from severe salt-wasting to simple virilizing CAH. This is important as the most severe form, salt-wasting CAH, has a higher mortality rate than milder phenotypes.31 While blood pressure and urine sodium were unable to be measured, renin expression was considered an appropriate surrogate marker for mineralocorticoid function. Following normalization of aldosterone production, renin expression in the kidney also normalized. The aldosterone likely allowed appropriate salt retention, which removed stimulation on the renin-angiotensin-aldosterone system and thus increased renin expression was no longer required.
Aldosterone is produced in picomolar serum concentrations, whereas corticosterone is produced in nanomolar concentrations. This means the amount of aldosterone required for physiological normalization is 1,000-fold less than corticosterone and is therefore more achievable. Corticosterone production improved 4- to 5-fold in the treated Cyp21a1−/− mice compared with untreated Cyp21a1−/− mice. However, it was still only 40% of wild-type level in males and 22% of wild-type level in females. Despite the corticosterone production not reaching wild-type levels, the increased corticosterone production was sufficient to reduce the hypothalamic-pituitary-adrenal axis stimulation, demonstrated by a reduction in adrenal size. As the serum was collected during a terminal procedure, the ACTH level detected was the maximum secretory capacity. When the pituitary is subject to chronic corticotropic releasing hormone stimulation from the hypothalamus, the pituitary ACTH-producing corticotrophic cells undergo hyperplasia.32 ACTH secretion is biphasic with the immediate release of stored ACTH followed by sustained release of newly synthesized ACTH.33 Untreated Cyp21a1−/− mice had a greater maximal ACTH secretory capacity than the treated Cyp21a1−/− mice. This indicates that either the storage or secretory capacity of ACTH by pituitary corticotrophs reduced following the vector treatment, likely due to reduced hypothalamic stimulation upon the pituitary as a direct result of increased corticosterone production by the adrenal cortex. Improvement in expression of the ACTH receptor in our Cyp21a1−/− male treatment group was similar to that of a previous study in which males and females were combined into a single treatment group, although the proportion of males and females in that study was not described.19 The finding was not detected in our treated Cyp21a1−/− female group demonstrating the importance of documenting both sexes separately as results are not generalizable between the sexes.
Despite these improvements, and some reduction in progesterone in the treated Cyp21a1−/− females, progesterone remained elevated and if recapitulated in humans would be associated with persistent androgen elevation. This could contribute to morbidity, particularly in women with CAH, and anti-androgen treatment may be required. Elevated progesterone may be required for the continual corticosterone production as increased progesterone production by the adrenal cortex allows spill-over into the systemic circulation, allowing the precursor to reach the newly expressed 21-hydroxylase in the liver, and therefore conversion to corticosterone. Should progesterone normalize, it is unclear whether that would still provide enough circulating substrate for hepatic 21-hydroxylation.
To our knowledge, this is the first study that has examined the effect of rAAV-mediated adrenocortical gene delivery to the liver. This could provide an alternative treatment option for CAH. The adrenal cortex can regenerate itself when only the capsule remains,34 from populations of cells located in the capsule and subcapsular region.35 Cells from the peripheral layers differentiate into zona glomerulosa cells and then undergo lineage conversion as they migrate centripetally to populate the deeper zones until they reach the cortico-medullary junction where they apoptose.36,37,38 While rAAV is the most popular vector-based gene delivery system in vivo,39,40 and has shown long-term durability of expression in the liver of adults,41 one caveat is that it is predominantly episomal. Thus, during pediatric organ growth or in organs with life-long cellular turnover such as the adrenal cortex, the effect of gene therapy will be transient as the episomal vector genomes will be lost during cellular division.20,21,42 Moreover, repeated doses of rAAV therapy are not currently feasible due to the development of neutralizing antibodies and immunomodulatory technology has not yet been clinically validated.22,23 Thus, effective treatment must be durable after a single dose. We have demonstrated that hepatically expressed adrenal enzymes are able to contribute to adrenal steroidogenesis, and this gene addition approach is directly applicable to adults with monogenic adrenocortical disorders. Conventional gene addition strategies using rAAV do not address hepatocellular proliferation, which is a particular challenge in the pediatric liver.21,43 However, this could be surmounted in the pediatric CAH population, by use of a gene editing approach whereby the newly introduced genetic material is stably integrated into the host genome, which has been shown to be an effective method to provide durable hepatic transgene expression in the growing liver.44
Our study also provides evidence supporting the possibility that enzyme activity from inadvertent extra-adrenal organ transduction could contribute to the biochemical effects seen when rAAV is used to deliver adrenocortical genes systemically, particularly when ubiquitously expressing enhancer-promoters are used. This unrecognized effect may lead to misinterpretation of results, attributing delivery of vector to the adrenal cortex as the cause of the phenotypic effect rather than vector expressed outside the adrenal gland. A persistent effect may be seen despite anticipated adrenal turnover. Despite this, a phase I/II clinical trial is under way whereby rAAV5 is used to deliver CYP21A2 with a ubiquitous promoter to adults with 21-hydroxylase deficiency (ClinicalTrials.org: NCT04783181), and at least four adults with CAH have been treated thus far.45 Use of the rAAV5 capsid serotype and a ubiquitous promoter means that the CYP21A2 will be expressed extensively throughout the body, including the liver, and not just in the adrenal cortex. Indeed the rAAV5 capsid is used for liver-targeted delivery of factor IX in the approved product Hemgenix.46 There is some caution regarding the potential prematurity of this clinical trial in CAH, given concerns about safety and durability, particularly when traditional inexpensive steroid treatment (although not perfect) can suffice.47
rAAV-delivered extra-adrenal 21-hydroxylase has been previously attempted; however, did not achieve statistically significant results.48 To date, most of the pre-clinical gene therapy studies for CAH have focused on gene delivery to the adrenal cortex and none has proven durability beyond the adrenocortical cellular turnover period.18,19,20,49 While one group demonstrated a positive effect on the Cyp21a1−/− phenotype for at least 15 weeks despite very low levels of residual vector DNA in the adrenal glands,19 another demonstrated the effect was transient, lasting only 8 weeks in female Cyp21a1−/− mice.20 Use of a ubiquitous promoter can allow persistent expression of vector-derived DNA delivered to stable organs such as liver and muscle, implicating expression of vector from sites external to the target organ.50 In non-human primates that were treated with rAAV5-CYP21A2, the vector DNA in adrenal glands waned with time and there was more vector DNA and transgene expression in the liver than adrenal glands.51 While our CYP21A2 vector had a liver-specific promoter, a very small amount of CYP21A2 mRNA was expressed in the adrenal gland at detectable levels. However, it was up to 800-fold lower than native Cyp21a1 expression in wild-type adrenal glands and therefore unlikely to have exerted a clinical effect. Our study demonstrated that CYP21A2 specifically expressed in the liver can exert a steroidogenic effect, implying that some of the effect seen in other studies may have been due to 21-hydroxylase expressed extra-adrenally. Female mice have a more rapid adrenocortical cellular turnover rate than males (3 months vs. 9 months).52 Use of predominantly male mice may also prolong the duration of effect of rAAV gene delivery to the adrenal cortex. Ours is the first pre-clinical adrenocortical gene therapy study to report male and female data separately. It is known that female mice have larger adrenal cortices due to a larger zona fasciculata and as such, have higher corticosterone levels at baseline.25 Therefore, we considered it important to demonstrate the changes following gene therapy in both sexes.
While human clinical translation is plausible, the approach described would require refinement. From a gene delivery perspective, this would include pseudo-serotyping the rAAV vector with a highly human liver tropic capsid such as the AAV-SYDs53 or AAV-LK03,54 rather than the murine liver tropic AAV8 capsid serotype. This in turn would facilitate the use of lower vector doses with an associated reduction in unwanted immune-mediated adverse events currently being observed in high-dose rAAV trials, such as thrombotic microangiopathy. These are being increasingly well managed with short courses of glucocorticoids and other immune-modulating drugs.55,56 No other changes to the vector construct are likely to be required, such as inclusion of an inducible promoter-enhancer, given that upstream and downstream glucocorticoid production remains under physiological control. In women with CAH, as already discussed, the only other refinement would be inclusion of strategies to manage ongoing elevation of androgens and related sequelae with or without stress precautions in both sexes.
With current technology, simple gene delivery to the adrenal cortex using rAAV will not have a durable effect. We have demonstrated an alternative whereby adrenocortical genes can be delivered to a stable organ outside of the adrenal cortex using contemporary technology. The future of adrenal-directed gene therapy is to stably integrate new genetic material into the genome of adrenocortical progenitor cells such that the daughter cells maintain the correction.9 This method is impeded by the difficulty in identifying adrenocortical progenitor cells, which is required for the development or discovery of rAAV capsids that can transduce this cell population. Alternatively, a method that allows rAAV to evade the immune system would allow repeated administration of rAAV without the need to target the elusive progenitor population.
We have demonstrated that specifically expressing human CYP21A2 in the livers of 21-hydroxylase-deficient mice can correct the CAH phenotype through hepato-adrenal cooperativity in steroidogenesis with resultant normalization of aldosterone production and renin expression, and sufficient improvement in corticosterone production to allow reduction in adrenal gland hyperplasia. This strategy has the potential to overcome the current technological limitations of direct adrenal cortex targeting with rAAV. While this could be directly applied to an adult population with completed liver growth, it would need to be adapted to a gene editing approach for a durable effect in the pediatric CAH population. This work also demonstrates that extra-adrenal 21-hydroxylation has a meaningful clinical effect and any biochemical effect seen when utilizing systemically delivered rAAV with a ubiquitous promoter-enhancer cannot be definitively attributed to adrenal 21-hydroxylase expression.
Materials and methods
Animal procedures
All animal care and experimental procedures were evaluated and approved by the joint Children’s Medical Research Institute and The Children’s Hospital at Westmead Animal Care and Ethics Committee (Project C381). The C57BL/10SnSlc-H-2aw18 mouse model was kindly provided by Toshihiko Shiroishi (National Institute of Genetics) and the RIKEN BRC through the National BioResource Project of the Ministry of Education, Culture, Sports, Science and Technology, Japan. The H-2aw18 mouse model has 21-hydroxylase deficiency, which is neonatally lethal.57 Heterozygous (H-2b/aw18; Cyp21a1+/−) mice were bred to produce homozygous (H-2aw18, Cyp21a1−/−) offspring. Exogenous steroid rescue was required for survival of the homozygous offspring. Dams were examined daily to determine the presence of a vaginal plug, indicating E0.5. All dams received 5 μg of dexamethasone (Ilium Dexason, Troy Animal Healthcare) subcutaneously daily from gestational day 18 until the birth. Day 19 was the most common day of delivery in this colony. All pups received 10 μg corticosterone and 0.05 μg fludrocortisone subcutaneously from birth second daily until 3 weeks of age. Once genotype was available, steroid treatment was ceased prior to 3 weeks of age in non-homozygous pups. Genotype was determined using a PCR-based assay with genomic DNA from toe removal during identification at 9 days of age. Dams, pups, and weaned Cyp21a1−/− mice were provided with table rock salt thrice weekly. Cyp21a1−/− pups were weaned to small groups with other Cyp21a1−/− animals from the same litter or a single non-homozygous littermate to avoid resource competition. Animals were maintained with 12-h light/dark cycles with free access to standard mouse chow and water.
With exogenous steroid rescue, 35%–50% of Cyp21a1−/− offspring survived the neonatal period with the higher survival rate seen with experienced dams. This is higher than the previously published 12% survival rate.19 Cyp21a1−/− males outnumbered Cyp21a1−/− females in the surviving offspring approximately 2:1, implying a survival advantage in males or disadvantage in females. Female mice are known to have higher serum corticosterone levels than males25 so the corticosterone deficiency may have had a more profound effect on female mice due to an increased absolute requirement. The median bilateral adrenal mass was 2.6 times greater in Cyp21a1−/− females and 3.4 times greater in Cyp21a1−/− males compared with wild-type adrenals.
Prior to injection, a whole blood spot was collected on filter paper between 2 and 4 p.m. from a conscious submandibular bleed. Adult Cyp21a1−/− mice received the rAAV8-CYP21A2 vector at a dose of 5 × 1011 vgc/mouse intravenously via the tail vein. Four weeks later the treated Cyp21a1−/− animals were harvested between 2 and 4 p.m. The same method was used to harvest untreated Cyp21a1−/− and wild-type (H-2b, Cyp21a1+/+) controls. The mice were anesthetized using isoflurane (3%–5%) to facilitate open cardiac puncture for blood collection. Blood was collected on filter paper and the remaining spun down to collect serum that was stored at −80°C until analysis. Following exsanguination, the animal was terminated with cervical dislocation and tissues harvested. Tissues for biochemical and molecular analysis were snap frozen in liquid nitrogen and stored at −80°C. Samples for immunohistochemistry were fixed in 4% (w/v) PFA overnight at 4°C and then underwent a sucrose gradient until embedding in optimal cutting temperature compound and snap frozen in isopentane chilled in liquid nitrogen.
Vector construction and production
The human CYP21A2 cDNA (GenBank: NM_000500.9) was cloned into an AAV2 backbone that had an ApoE-hAAT enhancer-promoter (liver-specific) that was provided by S. Cunningham21 (Figure 1B). A Kozak sequence was included immediately upstream of the hCYP21A2 cDNA. The vector was packaged by triple transfection of human embryonic kidney 293 cells, as previously described,21 and was pseudo-serotyped with the AAV8 capsid (rAAV2/8). Vector particles were purified from cell lysate using standard cesium chloride gradient centrifugation. Titer was assigned using digital droplet PCR (C1000 Touch Thermal Cycler #1851196 and QX200 Droplet Reader #1864003, BioRad) using primers specific for the bovine growth hormone polyadenylation tail signal (Table S2) with QX200 ddPCR EvaGreen Supermix (BioRad 1864034). The conditions were 95°C for 5 min, 40 cycles of 95°C for 30 s and 60°C for 1 min, followed by 4°C for 5 min, 90°C for 5 min.
Immunohistochemistry
Frozen liver sections (5 μm) were permeabilized in methanol and then 0.1% (v/v) Triton X-100. They were then blocked with 10% (v/v) donkey serum and 10% (v/v) fetal bovine serum in phosphate buffered saline without magnesium or calcium (PBS). After washing with 0.1% (v/v) Tween 20, the sections were incubated overnight with the rabbit primary CYP21A2 antibody (Abcam ab230327) diluted 1:50. After washing with 0.1% (v/v) Tween 20, the sections were incubated with the secondary antibody (AlexaFluor Donkey anti-rabbit 594, Invitrogen A32754) diluted 1:500, and then counterstained with DAPI, diluted to 0.03 μg/mL.
Vector copy number determination
Genomic DNA was extracted from frozen liver using a standard phenol-chloroform extraction method. It was digested with restriction enzyme HindIII-HF (New England BioLabs R3104L).
Vector copy number (vcn) was determined using digital droplet PCR (C1000 Touch Thermal Cycler #1851196 and QX200 Droplet Reader #1864003, BioRad) using primers specific for human CYP21A2 and murine albumin (Table S2) with QX200 ddPCR EvaGreen Supermix (BioRad 1864034). The conditions were 95°C for 5 min, 40 cycles of 95°C for 30 s and 60°C for 1 min, followed by 4°C for 5 min, 90°C for 5 min. The CYP21A2 count was normalized to two copies of murine albumin to determine vcn/diploid nucleus.
Quantitative real-time PCR
RNA was extracted using the Purelink RNA Mini Kit (Invitrogen 12183018A) and stored at −80°C. cDNA was generated from extracted RNA using the SuperScript IV First-Strand Synthesis System (Invitrogen 18091050) using the Oligo d(T)20 primer and stored at −20°C. Primers were used to detect gene expression for CYP21A2 (normalized to murine albumin as the reference transcript) in liver, Ren1 (normalized to Tbp as the reference transcript) in kidney and Mc2r, the ACTH receptor (normalized to Tbp) in adrenal cDNA, (Table S2). TB Green Premix Ex Taq II (Takara RR82WR) was used in the reaction with conditions as follows: 95°C for 30 s, 40 cycles of 95°C for 5 s, 60°C for 20 s, and 76°C for 10 s followed by melt 60°C–95°C (Qiagen Rotor-Gene Q).
Steroid profiles
Serum aldosterone, corticosterone, and progesterone
The steroid calibrators, controls, and isotopically labeled internal standard mix were obtained from Chromsystems Instruments & Chemicals Gmbh, Germany. The Optima LC-MS grade solvents including water, methanol, methyl t-butyl ether, acetonitrile, and formic acid were obtained from Fisher Chemicals, UK. Internal standard solution was added to calibrators, controls, and test samples and organic solvents were used for extraction. The extract supernatants were evaporated to dryness under nitrogen in a 37°C water bath. After evaporation, the extracted steroids were reconstituted in 50% methanol. Steroids were analyzed by ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MSMS) using validated in-house developed methods (Waters Acquity UPLC and Xevo TQ-S mass spectrometer).
Whole blood corticosterone
The dried blood spot samples were collected onto filter paper and were extracted using an organic solvent (95% methanol) containing deuterated internal standards for each steroid. Whole blood corticosterone was measured using liquid chromatography-tandem mass spectrometry (Waters Xevo TQ-S with Acquity UPLC system).
ACTH ELISA
ACTH was measured after thawing snap frozen serum using the Mouse/Rat ACTH SimpleStep ELISA Kit as per manufacturer instructions (Ab263880, Abcam).
Statistical analysis
GraphPad Prism 9 was used for statistical analysis and graph production. Non-parametric statistical methods including the Mann-Whitney U test were used.
Data and code availability
All relevant data are included in the manuscript or supplementary materials. Any additional details of the studies not clearly described can be obtained by contacting Lara E. Graves.
Acknowledgments
The authors would like to acknowledge and thank Sharon C Cunningham for providing the liver-specific vector backbone, and Sharntie Christina for assistance with some laboratory techniques.
L.E.G. was supported by the University of Sydney Post-Graduate Award (UPA) scholarship and the Yass Memorial Scholarship from the Children’s Medical Research Institute. This research was supported by the Australasian Paediatric Endocrine Group (APEG)/Pfizer Industry Research Grant 2019. The APEG Research Grants, supported by industry, are funded through an unsolicited institutional research grant. Companies outside of APEG are not involved in the program design and selection of awardees.
Figures were created with BioRender.com.
Author contributions
L.E.G. contributed to conceptualization, data curation and analysis, funding acquisition, investigation, project administration, visualization, and writing – original draft, reviewing and editing. E.B.v.D. and C.Z. contributed to investigation and writing – reviewing and editing. S.K. and T.W. contributed to investigation, resources and writing the relevant methodology. S.S. contributed to resources, supervision, and writing – reviewing and editing. S.G. contributed to conceptualization, methodology, project administration, supervision, and writing – reviewing and editing. I.E.A. contributed to conceptualization, methodology, project administration, resources, supervision, and writing – reviewing and editing.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtm.2024.101232.
Supplemental information
References
- 1.Wilkins L., Lewis R.A., Klein R., Rosemberg E. The suppression of androgen secretion by cortisone in a case of congenital adrenal hyperplasia. Bull. Johns Hopkins Hosp. 1950;86:249–252. [PubMed] [Google Scholar]
- 2.Wilkins L., Lewis R.A., Klein R., Gardner L.I., Crigler J.F., Jr., Rosemberg E., Migeon C.J. Treatment of congenital adrenal hyperplasia with cortisone. J. Clin. Endocrinol. Metab. 1951;11:1–25. doi: 10.1210/jcem-11-1-1. [DOI] [PubMed] [Google Scholar]
- 3.Bartter F.C., Albright F., Forbes A.P., Leaf A., Dempsey E., Carroll E. The effects of adrenocorticotropic hormone and cortisone in the adrenogenital syndrome associated with congenital adrenal hyperplasia: an attempt to explain and correct its disordered hormonal pattern. J. Clin. Invest. 1951;30:237–251. doi: 10.1172/JCI102438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Maouche D., Arlt W., Merke D.P. Congenital adrenal hyperplasia. Lancet. 2017;390:2194–2210. doi: 10.1016/S0140-6736(17)31431-9. [DOI] [PubMed] [Google Scholar]
- 5.Lai F., Srinivasan S., Wiley V. Evaluation of a Two-Tier Screening Pathway for Congenital Adrenal Hyperplasia in the New South Wales Newborn Screening Programme. Int. J. Neonatal Screen. 2020;6:63. doi: 10.3390/ijns6030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Navarro-Zambrana A.N., Sheets L.R. Ethnic and National Differences in Congenital Adrenal Hyperplasia Incidence: A Systematic Review and Meta-Analysis. Horm. Res. Paediatr. 2023;96:249–258. doi: 10.1159/000526401. [DOI] [PubMed] [Google Scholar]
- 7.Berglund A., Ornstrup M.J., Lind-Holst M., Dunø M., Bækvad-Hansen M., Juul A., Borch L., Jørgensen N., Rasmussen Å.K., Andersen M., et al. Epidemiology and diagnostic trends of congenital adrenal hyperplasia in Denmark: a retrospective, population-based study. Lancet Reg. Health. Eur. 2023;28 doi: 10.1016/j.lanepe.2023.100598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claahsen-van der Grinten H.L., Speiser P.W., Ahmed S.F., Arlt W., Auchus R.J., Falhammar H., Flück C.E., Guasti L., Huebner A., Kortmann B.B.M., et al. Congenital Adrenal Hyperplasia-Current Insights in Pathophysiology, Diagnostics, and Management. Endocr. Rev. 2022;43:91–159. doi: 10.1210/endrev/bnab016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graves L.E., Torpy D.J., Coates P.T., Alexander I.E., Bornstein S.R., Clarke B. Future Directions for Adrenal Insufficiency: Cellular Transplantation and Genetic Therapies. J. Clin. Endocrinol. Metab. 2023;108:1273–1289. doi: 10.1210/clinem/dgac751. [DOI] [PubMed] [Google Scholar]
- 10.Nella A.A., Mallappa A., Perritt A.F., Gounden V., Kumar P., Sinaii N., Daley L.A., Ling A., Liu C.Y., Soldin S.J., Merke D.P. A Phase 2 Study of Continuous Subcutaneous Hydrocortisone Infusion in Adults With Congenital Adrenal Hyperplasia. J. Clin. Endocrinol. Metab. 2016;101:4690–4698. doi: 10.1210/jc.2016-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mallappa A., Nella A.A., Sinaii N., Rao H., Gounden V., Perritt A.F., Kumar P., Ling A., Liu C.Y., Soldin S.J., Merke D.P. Long-term use of continuous subcutaneous hydrocortisone infusion therapy in patients with congenital adrenal hyperplasia. Clin. Endocrinol. 2018;89:399–407. doi: 10.1111/cen.13813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mallappa A., Sinaii N., Kumar P., Whitaker M.J., Daley L.A., Digweed D., Eckland D.J.A., Van Ryzin C., Nieman L.K., Arlt W., et al. A phase 2 study of Chronocort, a modified-release formulation of hydrocortisone, in the treatment of adults with classic congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 2015;100:1137–1145. doi: 10.1210/jc.2014-3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones C.M., Mallappa A., Reisch N., Nikolaou N., Krone N., Hughes B.A., O'Neil D.M., Whitaker M.J., Tomlinson J.W., Storbeck K.H., et al. Modified-Release and Conventional Glucocorticoids and Diurnal Androgen Excretion in Congenital Adrenal Hyperplasia. J. Clin. Endocrinol. Metab. 2017;102:1797–1806. doi: 10.1210/jc.2016-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Speiser P.W. Emerging medical therapies for congenital adrenal hyperplasia. F1000Res. 2019;8:363. doi: 10.12688/f1000research.17778.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pascual-Morena C., Cavero-Redondo I., Lucerón-Lucas-Torres M., Martínez-García I., Rodríguez-Gutiérrez E., Martínez-Vizcaíno V. Onasemnogene Abeparvovec in Type 1 Spinal Muscular Atrophy: A Systematic Review and Meta-Analysis. Hum. Gene Ther. 2023;34:129–138. doi: 10.1089/hum.2022.161. [DOI] [PubMed] [Google Scholar]
- 16.Vinson G.P. Functional Zonation of the Adult Mammalian Adrenal Cortex. Front. Neurosci. 2016;10:238. doi: 10.3389/fnins.2016.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gotoh H., Sagai T., Hata J., Shiroishi T., Moriwaki K. Steroid 21-hydroxylase deficiency in mice. Endocrinology. 1988;123:1923–1927. doi: 10.1210/endo-123-4-1923. [DOI] [PubMed] [Google Scholar]
- 18.Tajima T., Okada T., Ma X.M., Ramsey W., Bornstein S., Aguilera G. Restoration of adrenal steroidogenesis by adenovirus-mediated transfer of human cytochrome P450 21-hydroxylase into the adrenal gland of 21-hydroxylase-deficient mice. Gene Ther. 1999;6:1898–1903. doi: 10.1038/sj.gt.3301018. [DOI] [PubMed] [Google Scholar]
- 19.Perdomini M., Dos Santos C., Goumeaux C., Blouin V., Bougnères P. An AAVrh10-CAG-CYP21-HA vector allows persistent correction of 21-hydroxylase deficiency in a Cyp21(-/-) mouse model. Gene Ther. 2017;24:275–281. doi: 10.1038/gt.2017.10. [DOI] [PubMed] [Google Scholar]
- 20.Markmann S., De B.P., Reid J., Jose C.L., Rosenberg J.B., Leopold P.L., Kaminsky S.M., Sondhi D., Pagovich O., Crystal R.G. Biology of the Adrenal Gland Cortex Obviates Effective Use of Adeno-Associated Virus Vectors to Treat Hereditary Adrenal Disorders. Hum. Gene Ther. 2018;29:403–412. doi: 10.1089/hum.2017.203. [DOI] [PubMed] [Google Scholar]
- 21.Cunningham S.C., Dane A.P., Spinoulas A., Alexander I.E., Alexander I.E. Gene delivery to the juvenile mouse liver using AAV2/8 vectors. Mol. Ther. 2008;16:1081–1088. doi: 10.1038/mt.2008.72. [DOI] [PubMed] [Google Scholar]
- 22.Boutin S., Monteilhet V., Veron P., Leborgne C., Benveniste O., Montus M.F., Masurier C. Prevalence of Serum IgG and Neutralizing Factors Against Adeno-Associated Virus (AAV) Types 1, 2, 5, 6, 8, and 9 in the Healthy Population: Implications for Gene Therapy Using AAV Vectors. Hum. Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 23.Li A., Tanner M.R., Lee C.M., Hurley A.E., De Giorgi M., Jarrett K.E., Davis T.H., Doerfler A.M., Bao G., Beeton C., Lagor W.R. AAV-CRISPR gene editing is negated by pre-existing immunity to Cas9. Mol. Ther. 2020;28:1432–1441. doi: 10.1016/j.ymthe.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dane A.P., Cunningham S.C., Graf N.S., Alexander I.E. Sexually dimorphic patterns of episomal rAAV genome persistence in the adult mouse liver and correlation with hepatocellular proliferation. Mol. Ther. 2009;17:1548–1554. doi: 10.1038/mt.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bielohuby M., Herbach N., Wanke R., Maser-Gluth C., Beuschlein F., Wolf E., Hoeflich A. Growth analysis of the mouse adrenal gland from weaning to adulthood: time- and gender-dependent alterations of cell size and number in the cortical compartment. Am. J. Physiol. Endocrinol. Metab. 2007;293:E139–E146. doi: 10.1152/ajpendo.00705.2006. [DOI] [PubMed] [Google Scholar]
- 26.Page I.H., Helmer O.M. A Crystalline Pressor Substance (Angiotonin) Resulting from the Reaction between Renin and Renin-Activator. J. Exp. Med. 1940;71:29–42. doi: 10.1084/jem.71.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braun-Menendez E., Fasciolo J.C., Leloir L.F., Muñoz J.M. The substance causing renal hypertension. J. Physiol. 1940;98:283–298. doi: 10.1113/jphysiol.1940.sp003850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radonić A., Thulke S., Mackay I.M., Landt O., Siegert W., Nitsche A. Guideline to reference gene selection for quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2004;313:856–862. doi: 10.1016/j.bbrc.2003.11.177. [DOI] [PubMed] [Google Scholar]
- 29.Costello H.M., Krilis G., Grenier C., Severs D., Czopek A., Ivy J.R., Nixon M., Holmes M.C., Livingstone D.E.W., Hoorn E.J., et al. High salt intake activates the hypothalamic-pituitary-adrenal axis, amplifies the stress response, and alters tissue glucocorticoid exposure in mice. Cardiovasc. Res. 2023;119:1740–1750. doi: 10.1093/cvr/cvac160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witt A., Mateska I., Palladini A., Sinha A., Wölk M., Harauma A., Bechmann N., Pamporaki C., Dahl A., Rothe M., et al. Fatty acid desaturase 2 determines the lipidomic landscape and steroidogenic function of the adrenal gland. Sci. Adv. 2023;9 doi: 10.1126/sciadv.adf6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Falhammar H., Frisén L., Norrby C., Hirschberg A.L., Almqvist C., Nordenskjöld A., Nordenström A. Increased mortality in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 2014;99:E2715–E2721. doi: 10.1210/jc.2014-2957. [DOI] [PubMed] [Google Scholar]
- 32.Scheithauer B.W., Kovacs K., Randall R.V. The pituitary gland in untreated Addison's disease. A histologic and immunocytologic study of 18 adenohypophyses. Arch. Pathol. Lab Med. 1983;107:484–487. [PubMed] [Google Scholar]
- 33.DeBold C.R., DeCherney G.S., Jackson R.V., Sheldon W.R., Alexander A.N., Island D.P., Rivier J., Vale W., Orth D.N. Effect of synthetic ovine corticotropin-releasing factor: prolonged duration of action and biphasic response of plasma adrenocorticotropin and cortisol. J. Clin. Endocrinol. Metab. 1983;57:294–298. doi: 10.1210/jcem-57-2-294. [DOI] [PubMed] [Google Scholar]
- 34.Ingle D.J., Higgins G.M. Autotransplantation and Regeneration of the Adrenal Gland. Endocrinology. 1938;22:458–464. doi: 10.1210/endo-22-4-458. [DOI] [Google Scholar]
- 35.Zwemer R.L., Wotton R.M., Norkus M.G. A study of corticoadrenal cells. Anat. Rec. 1938;72:249–263. doi: 10.1002/ar.1090720210. [DOI] [Google Scholar]
- 36.Chang S.P., Morrison H.D., Nilsson F., Kenyon C.J., West J.D., Morley S.D. Cell proliferation, movement and differentiation during maintenance of the adult mouse adrenal cortex. PLoS One. 2013;8 doi: 10.1371/journal.pone.0081865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freedman B.D., Kempna P.B., Carlone D.L., Shah M., Guagliardo N.A., Barrett P.Q., Gomez-Sanchez C.E., Majzoub J.A., Breault D.T. Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Dev. Cell. 2013;26:666–673. doi: 10.1016/j.devcel.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zwemer R.L. A study of adrenal cortex morphology. Am. J. Pathol. 1936;12:107–114.1. doi: 10.1097/00005053-193607000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang D., Tai P.W.L., Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019;18:358–378. doi: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barrett D., Nguyen-Jatkoe L., Foss-Campbell B., Micklus A., Wendland A. Gene, Cell, & RNA Therapy Landscape, Q2 2021 Quarterly Data Report. 2021. https://asgct.org/global/documents/asgct-pharma-intelligence-quarterly-report-july-20.aspx [DOI] [PMC free article] [PubMed]
- 41.Nathwani A.C., Reiss U.M., Tuddenham E.G.D., Rosales C., Chowdary P., McIntosh J., Della Peruta M., Lheriteau E., Patel N., Raj D., et al. Long-Term Safety and Efficacy of Factor IX Gene Therapy in Hemophilia B. N. Engl. J. Med. 2014;371:1994–2004. doi: 10.1056/nejmoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alexander I.E., Cunningham S.C., Logan G.J., Christodoulou J. Potential of AAV vectors in the treatment of metabolic disease. Gene Ther. 2008;15:831–839. doi: 10.1038/gt.2008.64. [DOI] [PubMed] [Google Scholar]
- 43.Cunningham S.C., Spinoulas A., Carpenter K.H., Wilcken B., Kuchel P.W., Alexander I.E. AAV2/8-mediated correction of OTC deficiency is robust in adult but not neonatal Spf(ash) mice. Mol. Ther. 2009;17:1340–1346. doi: 10.1038/mt.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ginn S.L., Amaya A.K., Liao S.H.Y., Zhu E., Cunningham S.C., Lee M., Hallwirth C.V., Logan G.J., Tay S.S., Cesare A.J., et al. Efficient in vivo editing of OTC-deficient patient-derived primary human hepatocytes. JHEP Rep. 2020;2 doi: 10.1016/j.jhepr.2019.100065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adrenas Trial Update from Adrenas Therapeutics. 2023. https://adrenastx.com/wp-content/uploads/CAH-Letter-to-Community-Q1-2023-Update-FINAL.pdf
- 46.Heo Y.A. Etranacogene Dezaparvovec: First Approval. Drugs. 2023;83:347–352. doi: 10.1007/s40265-023-01845-0. [DOI] [PubMed] [Google Scholar]
- 47.White P.C. Emerging treatment for congenital adrenal hyperplasia. Curr. Opin. Endocrinol. Diabetes Obes. 2022;29:271–276. doi: 10.1097/MED.0000000000000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naiki Y., Miyado M., Horikawa R., Katsumata N., Onodera M., Pang S., Ogata T., Fukami M. Extra-adrenal induction of Cyp21a1 ameliorates systemic steroid metabolism in a mouse model of congenital adrenal hyperplasia. Endocr. J. 2016;63:897–904. doi: 10.1507/endocrj.EJ16-0112. [DOI] [PubMed] [Google Scholar]
- 49.Naiki Y., Miyado M., Shindo M., Horikawa R., Hasegawa Y., Katsumata N., Takada S., Akutsu H., Onodera M., Fukami M. AAV-mediated gene therapy for patients' fibroblasts, iPS cells, and a mouse model of congenital adrenal hyperplasia. Hum. Gene Ther. 2022;33:801–809. doi: 10.1089/hum.2022.005. [DOI] [PubMed] [Google Scholar]
- 50.Pontoizeau C., Simon-Sola M., Gaborit C., Nguyen V., Rotaru I., Tual N., Colella P., Girard M., Biferi M.-G., Arnoux J.-B., et al. Neonatal gene therapy achieves sustained disease rescue of maple syrup urine disease in mice. Nat. Commun. 2022;13 doi: 10.1038/s41467-022-30880-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eclov R.J., Lewis T.E.W., Kapandia M., Scott D.W., Rouse J.L., Romero K.B., Mansfield G., Beard C.W. Durable CYP21A2 gene therapy in non-human primates for treatment of congenital adrenal hyperplasia. ESGCT 27th Annual Congress In collaboration with SETGyc Barcelona, Spain October 22-25, 2019 Abstracts. Hum. Gene Ther. 2019:A93–A94. doi: 10.1089/hum.2019.29095.abstracts. [DOI] [Google Scholar]
- 52.Grabek A., Dolfi B., Klein B., Jian-Motamedi F., Chaboissier M.C., Schedl A. The Adult Adrenal Cortex Undergoes Rapid Tissue Renewal in a Sex-Specific Manner. Cell Stem Cell. 2019;25:290–296.e2. doi: 10.1016/j.stem.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 53.Cabanes-Creus M., Navarro R.G., Zhu E., Baltazar G., Liao S.H.Y., Drouyer M., Amaya A.K., Scott S., Nguyen L.H., Westhaus A., et al. Novel human liver-tropic AAV variants define transferable domains that markedly enhance the human tropism of AAV7 and AAV8. Mol. Ther. Methods Clin. Dev. 2022;24:88–101. doi: 10.1016/j.omtm.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lisowski L., Dane A.P., Chu K., Zhang Y., Cunningham S.C., Wilson E.M., Nygaard S., Grompe M., Alexander I.E., Kay M.A. Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature. 2014;506:382–386. doi: 10.1038/nature12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Au H.K.E., Isalan M., Mielcarek M. Gene Therapy Advances: A Meta-Analysis of AAV Usage in Clinical Settings. Front. Med. 2021;8 doi: 10.3389/fmed.2021.809118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Merke D.P., Auchus R.J., Sarafoglou K., Geffner M.E., Kim M.S., Escandon R.D., Bharucha K.N., Shaywitz A.J., Eclov R., Beard C.W., et al. Design of a Phase 1/2 Open-Label, Dose-Escalation Study of the Safety and Efficacy of Gene Therapy in Adults With Classic Congenital Adrenal Hyperplasia (CAH) Due to 21-hydroxylase Deficiency Through Administration of an Adeno-Associated Virus (AAV) Serotype 5-Based Recombinant Vector Encoding the Human CYP21A2 Gene. J. Endocr. Soc. 2021;5:A82. doi: 10.1210/jendso/bvab048.165. [DOI] [Google Scholar]
- 57.Shiroishi T., Sagai T., Natsuume-Sakai S., Moriwaki K. Lethal deletion of the complement component C4 and steroid 21-hydroxylase genes in the mouse H-2 class III region, caused by meiotic recombination. Proc. Natl. Acad. Sci. USA. 1987;84:2819–2823. doi: 10.1073/pnas.84.9.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are included in the manuscript or supplementary materials. Any additional details of the studies not clearly described can be obtained by contacting Lara E. Graves.