Abstract
Brain networks are continuously modified throughout development, yet this plasticity can also make functional networks vulnerable to early life stress. Little is currently known about the effect of early life stress on the functional organization of the brain. The current study investigated the association between environmental stressors and network topology using data from the Adolescent Brain Cognitive DevelopmentSM (ABCD®) Study. Hierarchical modeling identified a general factor of environmental stress, representing the common variance across multiple stressors, as well as four subfactors including familial dynamics, interpersonal support, neighborhood SES deprivation, and urbanicity. Functional network topology metrics were obtained using graph theory at rest and during tasks of reward processing, inhibition, and affective working memory. The general factor of environmental stress was associated with less specialization of networks, represented by lower modularity at rest. Local metrics indicated that general environmental stress was also associated with less efficiency in the subcortical-cerebellar and visual networks while showing greater efficiency in the default mode network at rest. Subfactors of environmental stress were associated with differences in specialization and efficiency in select networks. The current study illustrates that a wide range of stressors in a child’s environment are associated with differences in brain network topology.
Keywords: Early life stress, Youth, Function, Networks, Topology
1. Introduction
Early life stress encompasses a wide variety of attributes in the environment that may impact the developing child, including socioeconomic status (SES; e.g., poverty), characteristics of the neighborhood (e.g., crime), interpersonal relationships (e.g., abuse, lack of support), and attributes of the physical environment (e.g., pollution levels). Early life stress has a substantial influence on shaping the developing brain, as brain structure and function are continuously modified based on inputs that enter the system and corresponding neural feedback in response to those inputs (Batista-García-Ramó and Fernández-Verdecia, 2018). Through this ongoing interaction with the environment, the brain goes through substantial remodeling. This plasticity allows the brain to acquire new functional properties and adapt to external demands (von Bernhardi et al., 2017). While structural networks are primarily established in the early phases of development, functional networks undergo heavy refinement and reorganization throughout development (Grayson and Fair, 2017, Ho et al., 2018). Therefore, functional networks can serve as a useful source of information regarding the brain’s response to environmental influences.
Prior research on the influence of early life stress on functional networks has primarily focused on specific stressors and examined functional connectivity. For example, using the Adolescent Brain Cognitive DevelopmentSM (ABCD®) Study, lower family income was associated with decreased functional connectivity in the default-mode network, inferior and superior parietal cortices, and posterior cerebellum as well as increased functional connectivity in motor, auditory, visual, and subcortical regions (Tomasi and Volkow, 2023). Using different levels of SES indices (neighborhood disadvantage, household income-to-needs, and parental education), others have found that SES measures were associated with differences in subcortical, somatosensory, and frontoparietal networks (Rakesh et al., 2021, Sripada et al., 2022). DeJoseph and colleagues examined three latent variables reflecting material/economic deprivation, caregiver social support, and psychosocial threat and found that deprivation and threat differed in associated functional connectivity differences in frontolimbic networks and threat moderated the relationship between income and dorsal and ventral attention network connectivity (DeJoseph et al., 2022). Additionally, Ellwood-Lowe et al. (2021) found that children living in poverty show different network connectivity while still achieving comparable cognitive performance compared to their peers with higher-income backgrounds, suggesting that differences in functional connectivity may represent an adaptative response to the environment. While this functional connectivity work has led to important insights, there is an increasing recognition that the functional organization of the brain may provide important additional information for understanding the effect of early life stress on brain development.
Network analysis using graph theory allows us to quantitatively characterize the functional organization of the brain. Using this approach, networks are composed of nodes (vertices) denoting brain regions that are linked by edges representing physical connections. Various metrics are used to describe characteristics of these networks, such as specialization (how modular or distinct the networks are) and efficiency (how many steps there are between regions). For a comprehensive introduction to network analysis using graph theory, see Bullmore and Sporns (2009). There are a wide variety of measures that can be used to describe network topology (for the metrics used in the present study, see Table 1). Over the course of development, the functional organization of the brain is refined, wherein modules generally become more distinct/specialized and efficient with age (Baum et al., 2017, Fair et al., 2009, Gu et al., 2015, Hagmann et al., 2010, Váša et al., 2018). Studies relating early life stress to functional network topology have shown that lower SES is associated with lower within-network functional segregation (less specialization and efficiency of functional networks) as early as 6 months of age (Gao et al., 2015), as well as in youth (Tooley et al., 2020) and adults (Chan et al., 2018). Thus, functional network topography can provide a more nuanced understanding of the effect of early life stress on the functional organization of the brain. However, these studies focused only on SES and resting state data.
Table 1.
Graph theory terminology.
| Term | Description |
|---|---|
| Nodes | Brain regions are represented by nodes (vertices). Nodes combine together to create modules. |
| Edges | Steps or connections between nodes. |
| Modularity | Degree to which a system subdivides into distinct communities or modules. Greater modularity indicates greater segregation (specialization) of modules and lower modularity indicates sparser segregation of modules. |
| Average shortest path length | Average number of edges along the shortest path for all possible node pairs. Lower average shortest path lengths indicate that neural signals travel a shorter distance (more efficient) and greater average shortest path lengths indicate neural signals travel a longer distance (less efficient). |
| Small-worldness | The degree to which a network exhibits both high local clustering and short average path lengths suggesting that most nodes can be reached from every other by a small number of steps or connections. Lower small-worldness indicates that the network is randomly connected. Greater small-worldness indicates that the network is highly interconnected (more lattice-like). |
| Diameter | Maximum distance between node pairs. Smaller diameter indicates the greatest length of any of the paths is shorter (more efficient). Larger diameter indicates that the greatest length of any of the paths is longer (less efficient). |
| Local efficiency | Characterizes how well information is exchanged by a node’s neighbors when the node is theoretically removed. Less local efficiency indicates that the network does not recover and exchange information as well if a node is removed. Greater local efficiency indicates that the network can recover and still efficiently exchange information if a node is removed. |
While SES is an important early life stressor, it does not fully account for all the highly co-occurring stressors that a youth may experience in daily life (Smith and Pollak, 2021). Hierarchical modeling can be used to identify both common and distinct dimensions of early life stress. In our previous work, we used hierarchical modeling to identify a general factor of environmental stress, representing the shared variance across various environmental stressors, as well as specific factors that highlight distinct subgroups of stressors that cluster together (Jeong et al., 2023). Specifically, we found subfactors for family dynamics (the dynamics of a child’s family environment such as history of mental illness, financial difficulty, or conflict in the family), interpersonal support (the support a child receives at school and at home), neighborhood SES deprivation (the availability of resources in a child’s neighborhood), and urbanicity (the quality of a child’s physical environment such as pollution levels and lead exposure risk). Using this model, we showed that the general factor of environmental stress was associated with globally smaller brain volumes as well as thinner cortices in several regions (Jeong et al., 2023). However, this study examined structure only and did not investigate network topology. Furthermore, prior studies examining the functional organization of the brain have focused on resting state data in the absence of active task demands, which is useful but misses the opportunity to examine functional organization in the context of cognitive, emotional, and reward processing (Stevens, 2016). Examining network topology during task performance in addition to the resting state can be informative for identifying network engagement across different contexts.
The purpose of the current study was to investigate the association between hierarchically defined environmental stressors and brain network topology at rest and during task-based functioning. To capture the functional organization of the brain, we used graph theory metrics to quantify the metrics of modularity, average shortest path length, local efficiency, diameter, and small-world omega (see Table 1 for definitions of these metrics; Rubinov and Sporns, 2010). These metrics were obtained in the context of resting state, reward processing (a monetary incentive delay task), affective working memory (an emotional n-back task), and inhibition (a stop signal task). We hypothesized that a broad range of environmental stressors represented by the general factor would be associated with less specialization (lower modularity) of functional networks. Based on previous research on SES, we predicted that greater neighborhood SES deprivation would be associated with less efficiency within functional networks, especially in subcortical, somatosensory, and frontoparietal networks. Examining the relationships between functional network topology and the dimensions of family dynamics, interpersonal support, and urbanicity was exploratory.
2. Methods
2.1. Participants
We used baseline data from release 4.0 of the Adolescent Brain Cognitive Development (ABCD) Study with a total of 11,876 9- to 10-year-old children who were recruited at 21 sites across the United States (see supplement for additional details on the representativeness of the sample). The ABCD Study researchers obtained parental consent and child assent from all participants. Vanderbilt University’s Institutional Review Board approved the use of this deidentified dataset. The following final sample sizes were included for each condition: rest (N = 3998), monetary incentive delay (N = 1872), emotional n-back (N = 1836), and stop signal task (N = 1864). See Table 1 for the demographics of the sample.
2.2. Measures
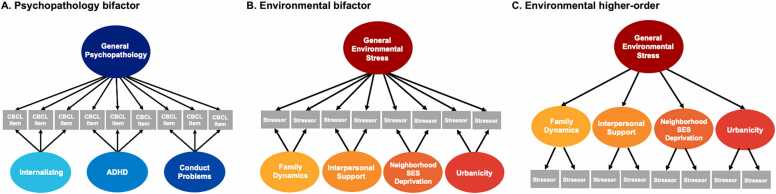
We performed a comprehensive review of the data available in the ABCD Study to identify items covering aspects of the child’s environment including neglect, abuse, resource availability, attributes of the physical environment such as population density and pollution, and interpersonal factors such as the availability of supportive relationships. Additional details on item selection can be found in our prior work (Jeong et al., 2023). Our previous work also showed that psychopathology is related to these network metrics (Reimann et al., 2022). To determine what environmental stress contributes above and beyond its associations with psychopathology, we used the Child Behavior Checklist (CBCL) to create psychopathology factors (Fig. 1a) which were added as covariates in all analyses (see the supplement for details). Descriptions of all measures included in our analyses can be found in the supplement.
Fig. 1.
Schematics of the Bifactor and Higher-Order Models Used to Delineate Psychopathology and Environmental Stress Factors. A) A confirmatory bifactor model of the Child Behavioral Checklist (CBCL) data yielded four orthogonal factors of psychopathology: general psychopathology, which represents symptoms across all domains, as well as specific factors for internalizing symptoms, attention-deficit/hyperactivity disorder (ADHD) symptoms, and conduct problems. B) A confirmatory bifactor model identified a general environmental stress factor, representing commonalities across all stressors, as well as four specific factors: family dynamics, interpersonal support, neighborhood socioeconomic status (SES) deprivation, and urbanicity. All factors in a bifactor model are uncorrelated with each other. C) A confirmatory higher-order model identifies the same four lower-order factors and then derives a general factor from the lower-order factors resulting in factors that are allowed to correlate with one another.
2.3. Deriving factors reflecting environmental stress
The hierarchical model of environmental stress used in this study is based on our previously published work (Jeong et al., 2023). Exploratory structural equation modeling with over 100 environmental stressors revealed four factors. The “family dynamics” factor consisted of items related to the dynamics of a child’s family environment such as a history of mental illness in the family, traumatic events experienced by the child, the presence of conflict within the family, and financial difficulty experienced by the immediate family. The “interpersonal support” factor consisted of items reflecting the support a child receives at home and school, such as the child’s perception of his or her connectedness to school and teachers, the primary caregiver’s warmth, and parental involvement in monitoring the child. The “neighborhood SES deprivation” factor was made up of items reflecting the availability of resources in a child’s neighborhood such as median rent and home value, the percentage of families living below or close to the poverty level, income disparity, median family income, and the percentage of the population with at least a high school diploma. Finally, the “urbanicity” factor was made up of variables related to the quality of a child’s physical environment which closely mapped onto urban living. Items included population density, perceptions of neighborhood safety, pollution levels, lead exposure risk, and walkability.
We used two different hierarchical models to identify a general factor of environmental stress: a bifactor model and a higher-order (second-order) model. In a bifactor model, the total variance in each item is partitioned between the general and specific factors so that they are orthogonal or uncorrelated with one another (Lahey et al., 2021). In a higher-order model, each item loads onto one of several correlated lower-order factors, which then load onto a second-order general factor (Lahey et al., 2021). As shown in our previous work, the general and specific or lower-order factors in these models exhibited adequate construct reliability and estimated replicability (Jeong et al., 2023). These models are shown in Figs. 1b and 1c. Further details on the modeling of the environmental factors can be found in our previous work (Jeong et al., 2023) and in the supplement.
2.4. Functional magnetic resonance imaging tasks
We examined functional network topology during a resting state condition in addition to three functional tasks (Casey et al., 2018). The monetary incentive delay task examines brain function during reward processing, motivation, and anticipation of rewards. Behavioral performance is quantified by total monetary earnings. The emotional n-back task examines working memory and emotion regulation processes. Performance is measured by the rate of accuracy for 2-back trials. The stop-signal task examines functional activity during inhibition conditions. Performance is quantified as the proportion correct on “Go” trials and the mean stop-signal delay subtracted from the mean reaction time on correct “Go” trials. Stop signal reaction times were reverse scored so that higher scores reflect better performance.
2.5. Image acquisition, processing, and quality assurance
Details on the image acquisition, processing, and quality assurance procedures have been previously published (Casey et al., 2018, Hagler et al., 2019; Stier et al., 2023) and are described in the supplement. Due to the need to ensure adequately clean data to derive the graph theory network metrics, stringent quality assurance and motion parameters were applied (see supplement). Data exclusion criteria included missing data, failed quality assurance measures, and individual runs with greater than .2 mm mean and 2 mm max framewise displacement to ensure suitable data for graph theory calculations.
2.6. Graph theory metrics
Atlas. We derived networks using the Shen-268 atlas. Partitions are grouped into eight established networks: subcortical-cerebellar, motor, medial frontal, frontoparietal, default mode, visual 1, visual 2, and visual association networks (Finn et al., 2015, Shen et al., 2013).
Connectivity Matrix Thresholding. We derived functional networks based on the correlation matrices between brain region signals. As done previously (Reimann et al., 2022, Stier et al., 2023), we retained the strongest 10%, 16.67%, 23%, and 30% of positive and negative connections within each network and within each task. This approach sought to remove noisy edges and allow for sparse networks that are still largely connected. To combat potential bias stemming from this approach, results are reported as significant only if significance is retained across at least three consecutive thresholds. This approach is similar to those used in prior literature (Fornito et al., 2010). Connections were binarized prior to graph theory calculations.
Measures of Network Efficiency. We used the python package networkX to compute graph theory metrics of modularity, average shortest path length, local efficiency, diameter, and small world omega (Hagberg et al., 2008). Calculations and descriptions of these metrics have been well-documented and are well-suited to characterize network-wide attributes (Rubinov and Sporns, 2010, Sporns, 2018). See Table 1 for definitions of the network metrics used and the supplement for the metric calculations.
2.7. Statistical analysis
Analyses were performed in Mplus version 8.4 using structural equation modeling to test associations between network topology metrics and environmental stress factors while controlling for covariates in the model (described below). Data were stratified based on site to control for site differences and clustered based on family membership to account for siblings and multiple births (twins and triplets). See the supplement for additional details on accounting for dependencies in the sample. Data were weighted by post-stratification weights to make the sample more representative of the U.S. population, per ABCD Study recommendations (Heeringa and Berglund, 2020).
For each of the eight networks, four tasks, and four thresholds, we investigated the associations between our environmental stress factors and the graph theory metrics. Due to the orthogonality of factors derived from the bifactor model, the general environmental stress factor and four specific dimensions can be included in the same model without multicollinearity issues. Since the general environmental stress factor in a higher-order model is defined by loadings on the lower-order factors, the general and lower-order factors must be tested in separate models due to their being a perfectly collinear system. Equations for each model can be found in the supplement. We tested all eight networks in one structural equation model simultaneously. The false discovery rate was controlled (q < 0.05) using the stats package in R version 3.6.1 (http://www.r-project.org/). Specifically, the false discovery rate was used to control for multiple comparisons for each stressor across the eight networks, resulting in eight tests for each false discovery rate correction. Results were considered reliable if we obtained significant p-values across at least three consecutive thresholds. We performed a follow-up analysis examining associations between behavioral performance on the task and the environmental dimensions while covarying for sex, race/ethnicity, and psychopathology factors. Lastly, we conducted sensitivity analyses with current medication use added as an additional covariate.
2.8. Data and code availability
ABCD Study data are available through the National Institute of Mental Health Data Archive (https://nda.nih.gov/abcd). The code and a corresponding wiki for the analytic procedures can be found at https://github.com/VU-BRAINS-lab/ABCD_Stressor_Network.
3. Results
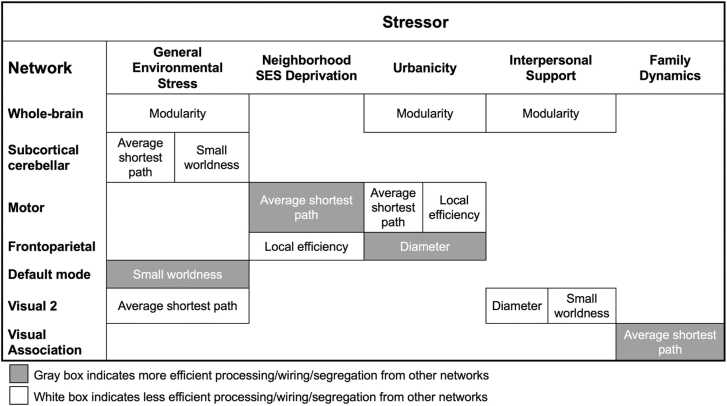
Results reported are from the bifactor model. Consistencies with and differences from the results obtained from the higher-order model are noted for each stressor. Refer to Table 1 for definitions of each network metric and Fig. 2 for the summary of results across bifactor and higher-order models.
Fig. 2.
Summary of the Results for the Association Between Environmental Stressors and Graph Theory Metrics by Network. This figure shows the metrics that were significantly associated with stressor factors obtained from bifactor modeling and higher-order modeling across three-consecutive thresholds. The results are aggregated across resting state and three task conditions.
3.1. Sample comparison
See supplemental Tables 1a-1d for comparisons between the included and excluded samples. The included sample was slightly older than the excluded sample and had a higher proportion of females and individuals identifying as non-Hispanic White, whereas the excluded sample had more males and more individuals identifying as Black, Hispanic, or other race/ethnicity. The included sample also had a slightly higher average household income and more years of parental education. Finally, the included sample showed lower general environmental stress and psychopathology scores than the excluded sample.
3.2. General environmental stress
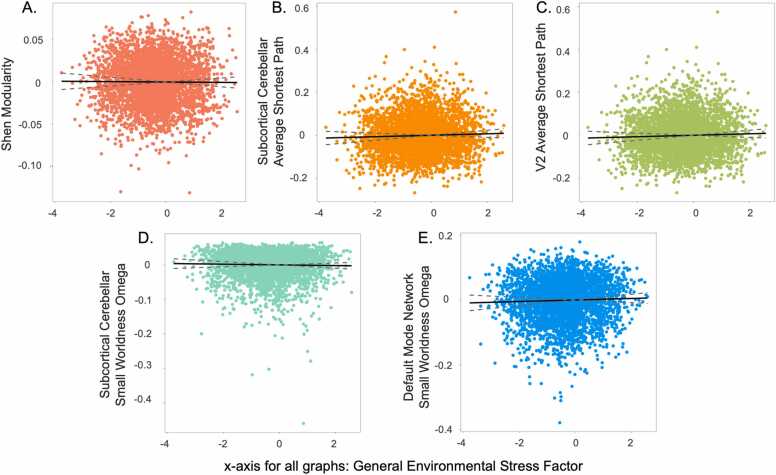
Modularity. The general environmental stress factor was associated with lower whole-brain modularity during rest (Fig. 3; supplemental Table 2a), which indicates denser connections between modules and sparser connections within modules. Such a pattern may suggest less specialization of modules (Vogel et al., 2010).
Fig. 3.
Correlations Between the General Environmental Stress Factor and Network Connectivity during Rest. General environmental stress was associated with a) lower whole-brain modularity, b) greater average shortest path in the subcortical-cerebellar network, c) greater average shortest path in the visual 2 network, d) lower small-worldness in the subcortical-cerebellar network, and e) greater small-worldness in the default mode network. Dotted lines indicate 95% confidence intervals.
Average Shortest Path. The general environmental stress factor was associated with greater average shortest path values (in other words, longer paths suggesting less efficiency) in the subcortical cerebellar and visual 2 networks during rest (Fig. 3; supplemental Table 3a).
Small-Worldness. The general environmental stress factor was also associated with lower small-worldness in the subcortical cerebellar network during rest (Fig. 3; supplemental Table 4a). Small-worldness reflects “wiring costs” and is important for efficient segregation and integration of information (Liao et al., 2017). Lower small-worldness may suggest less efficient wiring or composition in this network. In contrast, greater scores on the general environmental stress factor were associated with higher small-worldness values in the default-mode network at rest (Fig. 3; supplemental Table 4a), which suggests more efficient wiring of this network.
Other Network Metrics. No results between general environmental stress and the diameter or local efficiency metrics met our criteria for significance during rest (supplemental Tables 5a and 6a). No associations between general environmental stress and average shortest path, small world omega, diameter, or local efficiency met our criteria for significance during the emotional n-back, monetary incentive delay, or stop-signal tasks (supplement Tables 7a-18a).
Higher-Order Modeling. The results from the higher-order model were the same as the bifactor model in terms of associations with network properties (supplemental Tables 2b-18b), except for the loss of the small-worldness finding in the default mode network at rest (supplemental Table 4b). Additionally, the general environmental stress factor defined by higher-order modeling was associated with lower whole brain modularity during the emotional n-back task in addition to rest (Table 2b).
Table 2.
Demographics of the Sample.
| Rest |
EN-Back |
MID |
SST |
|||||
|---|---|---|---|---|---|---|---|---|
| (N = 3998) |
(N = 1836) |
(N = 1872) |
(N = 1864) |
|||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 9.96 | 0.64 | 10.03 | 0.63 | 10.02 | 0.63 | 10.01 | 0.63 |
|
N |
% |
N |
% |
N |
% |
N |
% |
|
| Sex | ||||||||
| Female | 2061 | 51.55 | 1013 | 55.17 | 1061 | 56.68 | 1034 | 55.47 |
| Male | 1937 | 48.44 | 823 | 44.83 | 811 | 43.32 | 830 | 44.53 |
| Race-Ethnicity | ||||||||
| White | 2301 | 57.55 | 1157 | 63.02 | 1145 | 61.16 | 1139 | 61.11 |
| Hispanic | 790 | 19.76 | 323 | 17.59 | 327 | 17.47 | 330 | 17.70 |
| Black | 411 | 10.28 | 146 | 7.95 | 163 | 8.71 | 163 | 8.74 |
| Other | 496 | 12.41 | 210 | 11.44 | 237 | 12.66 | 232 | 12.45 |
| Household Annual Income | ||||||||
| < $5,000 | 94 | 2.35 | 26 | 1.42 | 31 | 1.66 | 34 | 1.82 |
| $5,000-$11,999 | 96 | 2.40 | 28 | 1.53 | 29 | 1.55 | 29 | 1.56 |
| $12,000-$15,999 | 83 | 2.08 | 25 | 1.36 | 27 | 1.44 | 35 | 1.88 |
| $16,000-$24,999 | 149 | 3.73 | 57 | 3.10 | 61 | 3.26 | 59 | 3.17 |
| $25,000-$34,999 | 201 | 5.03 | 83 | 4.52 | 83 | 4.43 | 85 | 4.56 |
| $35000-$49,999 | 320 | 8.00 | 134 | 7.30 | 133 | 7.10 | 138 | 7.40 |
| $50,000-$74,999 | 518 | 12.96 | 248 | 13.51 | 248 | 13.25 | 232 | 12.45 |
| $75,000-$99,999 | 605 | 15.13 | 305 | 16.61 | 289 | 15.44 | 278 | 14.91 |
| $100,000-$199,999 | 1239 | 30.99 | 594 | 32.35 | 636 | 33.97 | 614 | 32.94 |
| > $200,000 | 441 | 11.03 | 217 | 11.82 | 226 | 12.07 | 243 | 13.04 |
| Missing | 252 | 6.30 | 119 | 6.48 | 109 | 5.82 | 117 | 6.28 |
| Parental Education | ||||||||
| No degree | 153 | 3.83 | 43 | 2.34 | 60 | 3.21 | 54 | 2.90 |
| High school degree/GED | 405 | 10.13 | 160 | 8.71 | 144 | 7.69 | 165 | 8.85 |
| Some college | 630 | 15.76 | 257 | 14.00 | 270 | 14.42 | 277 | 14.86 |
| Associate's degree | 515 | 12.88 | 234 | 12.75 | 229 | 12.23 | 214 | 11.48 |
| Bachelor's degree | 1257 | 31.44 | 652 | 35.51 | 675 | 36.06 | 622 | 33.37 |
| Master's degree | 779 | 19.48 | 365 | 19.88 | 369 | 19.71 | 398 | 21.35 |
| Professional/ Doctoral degree |
259 | 6.48 | 125 | 6.81 | 125 | 6.68 | 134 | 7.19 |
Note. EN-Back = emotional n-back; MID = monetary incentive delay; SST = stop signal task; the “Other” Race/Ethnicity category includes those who were identified by their parent as American Indian/Native American, Alaska Native, Native Hawaiian, Guamanian, Samoan, Other Pacific Islander, Asian Indian, Chinese, Filipino, Japanese, Korean, Vietnamese, Other Asian, or Other Race
Behavioral Performance. Analyses of behavioral measures indicated that the general environmental stress factor was associated with poorer performance during the emotional n-back task (lower proportion and lower rates of correct responses to 2-back stimuli) and the stop signal task (lower rates of correct go trials; supplemental Table 19a). The results from the higher-order model were mostly consistent with the bifactor model with an additional finding of poorer performance during the monetary incentive delay task (lower total earnings; supplemental Table 19b).
3.3. Neighborhood SES deprivation
Average Shortest Path. Greater neighborhood SES deprivation scores were associated with lower average shortest path length values (i.e., shorter, more efficient paths) in motor areas during rest (supplemental Table 3a).
Local Efficiency. Greater neighborhood SES deprivation scores from the bifactor model were associated with less local efficiency in the frontoparietal network during the emotional n-back task (supplemental Table 10a).
Other Network Metrics. No other associations with neighborhood SES deprivation were significant (supplemental Tables 2a-18a).
Higher-Order Modeling. The results from the higher-order model showed that, in contrast to the bifactor model, there were no significant associations obtained with neighborhood deprivation from the higher-order model and average shortest path length at rest or local efficiency during the emotional n-back task (supplemental Tables 3b, 10b). All other associations remained the same as the bifactor model (supplemental Tables 2b-18b).
Behavioral Performance. Neighborhood SES deprivation was associated with poorer performance during the monetary incentive delay task (less total earnings), the emotional n-back task (lower proportion of correct responses and lower rates of correct responses to 2-back stimuli), and the stop signal task (lower rates of correct go trials; supplemental Tables 19a and 19b).
3.4. Urbanicity
Modularity. Greater urbanicity scores from the bifactor model were associated with lower whole-brain modularity during rest (supplemental Table 2a).
Average Shortest Path. Greater urbanicity was associated with greater average shortest path length values (longer, less efficient paths) in the motor network during rest (supplemental Table 3a).
Local Efficiency. Greater urbanicity was associated with lower local efficiency in the motor network during rest (supplemental Table 6a) and during the emotional n-back task (supplemental Table 10a).
Diameter. Greater urbanicity was associated with smaller diameter (smaller distance between nodes suggesting more efficiency) in the frontoparietal network during the stop-signal task (supplemental Table 17a).
Other Network Metrics. No other associations were significant for urbanicity (supplemental Tables 2a-18a).
Higher-order Modeling. The associations obtained with urbanicity were consistent with those obtained from bifactor modeling (supplemental Tables 2b-18b); however, associations found with diameter during the stop-signal task were no longer significant (supplemental Table 17b).
Behavioral Performance. Greater scores on urbanicity were associated with enhanced performance during the monetary incentive delay task (i.e., greater total earnings), the emotional n-back task (higher rates of correct responses to 2-back stimuli), and the stop signal task (faster response times; supplemental Table 19a). Higher order modeling revealed additionally that greater urbanicity was associated with a greater proportion of correct responses on the emotional n-back task and a higher rate of correct go trials on the stop-signal task (supplemental Table 19b).
3.5. Interpersonal support
Modularity. Greater scores on interpersonal support were associated with lower whole-brain modularity during the stop-signal task (supplemental Table 2a).
Diameter. Greater interpersonal support was associated with greater diameter (greater distance between nodes suggesting less efficiency) in the visual 2 network during the stop-signal task (supplemental Table 17a).
Small-Worldness. Greater interpersonal support was associated with lower small-worldness (less efficient wiring) in the visual 2 network during the stop-signal task (supplemental Table 16a).
Other Network Metrics. No results between interpersonal support and any of the other network metrics were significant (supplemental Tables 2a-18a).
Higher-Order Modeling. The associations obtained with interpersonal support were consistent with those from bifactor modeling (supplemental Tables 2b-18b).
Behavioral Performance. There were no associations between interpersonal support and the behavioral indices (supplemental Tables 19a and 19b).
3.6. Family dynamics
Bifactor Modeling. There were no consistent significant associations between family dynamics obtained from bifactor modeling and any of the network metrics (supplemental Tables 2a-18a).
Higher-Order Modeling. The higher-order model showed that greater scores on family dynamics were associated with lower average shortest path length values (shorter, more efficient paths) in the visual association network during rest (supplemental Table 3b). All other associations remained non-significant (supplemental Tables 2b-18b).
Behavioral Performance. No associations between family dynamics and behavioral indices were observed (Table 19a and 19b).
3.7. Sensitivity analyses
Medication. The results after controlling for medication use were highly similar (supplemental Tables 20a-26b). The general environmental stress factor continued to be associated with lower whole-brain modularity during rest and the emotional n-back task, greater average shortest path lengths in the subcortical-cerebellar and visual 2 networks at rest, lower small-worldness in the subcortical-cerebellar network at rest, and greater small-worldness in the default mode network at rest. A new association between general environmental stress and lower average shortest path lengths in the default mode network was found during rest but only for the bifactor model and not the higher-order model. Neighborhood SES deprivation continued to be associated with lower average shortest path lengths in the motor network at rest for the bifactor but not the higher-order model. Urbanicity continued to be associated with lower whole-brain modularity during rest, greater average shortest path lengths in the motor network at rest, and less local efficiency in the motor network at rest and during the emotional n-back task. Urbanicity was also associated with smaller diameter in the frontoparietal network during the stop-signal task but only for the bifactor model and not the higher-order model. Interpersonal support continued to be associated with lower whole-brain modularity during the stop-signal task, lower small-worldness in the visual 2 network during the stop-signal task, and greater diameter in the visual 2 network during the stop-signal task. A new association between family dynamics and lower average shortest path lengths in the visual association network was uncovered during rest.
3.8. Individual items from neighborhood SES deprivation and urbanicity
The neighborhood SES deprivation and urbanicity factors are comprised of heterogeneous variables and while these variables were shown to “hang together” using data-driven approaches, it is also useful to see how these factors compare to select individual items. We selected a variable representative of each of the neighborhood SES deprivation and urbanicity factors (i.e., neighborhood median income and pollution measure (NO2 level)) and performed analyses with the network metrics.
Income. Neighborhood median income (supplemental Tables 27a-27q) was related to lower average shortest path lengths and greater small-worldness in the subcortical-cerebellar network during rest. In contrast to the neighborhood SES deprivation factor, neighborhood median income was not associated with lower average shortest path lengths in the motor network during rest or less local efficiency in frontoparietal network during the emotional n-back task.
Pollution. Consistent with findings with urbanicity, pollution levels (supplemental Tables 29a-28q) were associated with lower modularity as well as greater average path lengths and less local efficiency in the motor network during rest, with the addition of lower modularity during the stop-signal task as well. Pollution levels were also associated with greater average shortest path lengths in the motor network during the emotional n-back task and less local efficiency in the motor network during both the emotional n-back task and the monetary incentive delay task.
4. Discussion
The current study leveraged a large sample of youth to examine the associations between multiple environmental stressors and the functional topology of brain networks. The results demonstrated that various environmental stressors, represented by general and specific factors of environmental stress, were related to divergent patterns of functional network topology. At the broadest level, a wide variety of stressors represented by the general environmental stress factor were associated with less specialization of networks globally as well as divergent patterns in specific networks (subcortical-cerebellar, visual, and default mode network). At a more refined level, the specific subfactors of neighborhood SES deprivation and urbanicity were associated with distinct variations in the specialization and efficiency of networks, while interpersonal support and family dynamics showed few differences in network topology. Overall, the present findings illustrate that various forms of environmental stress are associated with divergent patterns in the functional organization of the developing brain.
4.1. Synthesis of the current results with prior work in human and animal studies
The results of the current study are consistent with previous research showing that individuals exposed to early life stress have distinguishable neural activation in similar brain regions (Cohodes et al., 2021, Dannlowski et al., 2012, Dannlowski et al., 2013, Pechtel and Pizzagalli, 2011, Rosen et al., 2018). This is in line with animal studies showing that early life stress (e.g., maternal deprivation in rodent models) is associated with changes in brain areas related to emotion and cognitive functioning (for an overview, see Marco et al., 2015). Additionally, prior work using the ABCD Study sample has shown that facets of early life stress, such as low SES, are associated with differences in functional connectivity in the same networks as found in the current study: the subcortical cerebellar, motor, frontoparietal, default mode, and visual networks (DeJoseph et al., 2022, Ellwood-Lowe et al., 2021, Rakesh et al., 2021, Sripada et al., 2022, Tomasi and Volkow, 2023). However, studies using the ABCD Study sample examined functional connectivity and did not examine the functional organization of the brain through network topology defined with graph theory. The present study expands on prior work by using network topology metrics to describe characteristics of brain networks, such as specialization and efficiency. Our findings that environmental stress dimensions are associated with differences in modularity (specialization) and various metrics that index efficiency are in line with prior network topology work in non-ABCD Study samples showing that lower SES is associated with less specialization and efficiency in functional networks from infancy through adulthood (Chan et al., 2018, Gao et al., 2015, Tooley et al., 2020). The present study replicates and builds upon this prior work by extending these findings beyond the resting state and showing similar associations during three tasks in the ABCD Study data.
4.2. The positive and negative influences associated with urbanicity
Interestingly, the urbanicity factor showed divergent results. Urbanicity was associated with less specialization of networks at the whole-brain level and less efficiency in the motor network while at the same time showing evidence of greater efficiency in the frontoparietal network and enhanced performance during the reward processing, affective working memory, and inhibition tasks. In our previous work using this factor, we also found that urbanicity showed unexpected results. We showed that urbanicity was associated with larger brain volumes in a number of regions, in contrast to the globally smaller volumes associated with the general environmental stress factor (Jeong et al., 2023). Urban living has both positive and negative influences. Negative aspects of urban living (such as safety concerns, toxin exposure, and crowding) could make urban living detrimental to the developing brain. In support of this, prior work has suggested that urban living may be associated with differences in neural activation within brain regions implicated in affective and stress processes (Lederbogen et al., 2011), as well as with differences in brain structure and inflammation (Calderón-Garcidueñas et al., 2008). Likewise, the results of the current study found that urban living is related to less specialization and efficiency in some brain networks. However, urban living has positive aspects as well including greater social support, more opportunities, and easier access to resources. The positive influences of urban living may be reflected by the finding of greater efficiency in the frontoparietal network and better task performance in our results. However, the benefits of urban living are disproportionate based on income (Dye, 2008), which suggests that taking into account other factors like SES may help explain the apparently divergent findings for urbanicity. Taken together, the results of the current study suggest that urban living likely involves a complicated interaction of influences, which can impact children in both positive and negative ways.
4.3. Adaptation and resilience
Over the course of development in childhood, it is thought that the brain will become more specialized at the local level as shown by an increase in modularity, which allows for more efficient processing and specialization of networks (Gu et al., 2015). However, the children included in these “normative” samples are not necessarily representative of the general population. Therefore, care must be taken when interpreting the associations between environmental stress and network topology. While the results of the current study showed that environmental stress was associated with less specialization at the whole brain level, these differences may reflect context-appropriate adaptation, rather than deficits (Ellis et al., 2017, Nketia et al., 2021, Noble et al., 2021, Taylor et al., 2023). Lower modularity associated with greater environmental stress may indicate greater utilization of the whole brain rather than relying on specific networks, which could allow individuals to be more flexible in the highly unpredictable environments associated with early life stress (Ellis et al., 2009). Similarly, higher efficiency in the default mode network may suggest that children in adverse environments rely on self-referential thoughts and future-oriented thinking associated with this network (Raichle, 2015), which is an adaptive strategy to deal with stressful environments that could indicate resilience (Ellwood-Lowe et al., 2021, Ellwood-Lowe et al., 2022). Although we did not examine how these network characteristics associated with environmental stress relate to real-life outcomes, future work would benefit from considering the adaptive perspective when interpreting the characteristics of neural networks in the context of early life stress.
4.4. Considerations when using latent factors
The present study used hierarchical modeling to derive latent factors to capture the shared and unique variance across various environmental stressors. While this approach is akin to the hierarchical models used to define the general psychopathology factor (p factor) and subfactors widely used in the literature, the practice of deriving latent variables requires careful consideration (McLaughlin et al., 2023, VanderWeele, 2022). Reflective and formative measurement models are two approaches that are commonly used in measurement and scale development. The common distinction between the two models relates to the assumptions they hold regarding the relationship between constructs and measurement items; in the reflective model, the indicator of causality is from constructs to measurement items while in the formative model, causality is from measurement items to constructs (Coltman et al., 2008; Hanafiah, 2020). McLaughlin and colleagues suggest that formative models are more appropriate for understanding early life stress because they derive underlying dimensions of adversity from the tendency for adversities to cumulatively predict certain outcomes (McLaughlin et al., 2023). However, the authors also note that reflective models could have some utility for identifying the underlying structural sources of co-occurring adversities (McLaughlin et al., 2023).
The use of one measurement model over the other depends on whether the goal is to estimate adversity co-occurrence (if so, then reflective models are appropriate) or to operationalize dimensions of adversity (in which case formative models are optimal). In the present study, our goal was the former – to identify environmental stressors that co-occur – not to claim that the factors we found are the definitive representation of how early life stressors should be operationalized. A strength of reflective measurement models is that they are typically simpler in structure making them easier to interpret. In the context of these considerations, our reflective approach is appropriate for our goal: to simplify a large number of variables into co-occurring adversities to create a more manageable and interpretable framework. However, we acknowledge that to achieve the ultimate goal of operationalizing dimensions of adversity, formative measurement models will remain the ideal approach in future work.
4.5. Strengths and limitations
While this study has a number of strengths, including using a large, well-characterized sample of youth with extensive assessment of environmental stressors, modeling environmental stress hierarchically, and generating network metrics not currently available in the ABCD Study data release, there are also a number of limitations to consider. First, because graph theory metrics require stringent handling of motion artifacts, this inevitably results in a sample with less variation in environmental stress and psychopathology symptoms, which is not as representative of the population and may mask the true strength of these effects. However, the ability of the current study to find significant results in a sample with less variance in the environmental stress predictors suggests that additional work on the effects of environmental stress on brain development is warranted.
Second, while our approach for modeling factors of environmental stress has the strength of revealing which constructs “hang together,” such an approach can also make it difficult to interpret the contribution of individual constructs. At the same time, the clustering of items together may suggest that variables share commonalities which could suggest a meaningful pattern in the data. For example, the urbanicity factor is comprised of heterogeneous items (such as lead pollution and walkability) and although it is unlikely these items influence the brain through similar mechanisms, the clustering of these items together suggests that there exists a broader construct of urban living that collectively has important influences on brain development. In this way, latent constructs provide insight into the interrelationships between seemingly unrelated items which helps us understand complex relationships among individual observed variables. Thus, there is merit to using both factor analytic approaches and research on specific constructs going forward. Third, the current study included psychopathology as a covariate based on our prior work showing an association between psychopathology and these network metrics (Reimann et al., 2022). While useful for revealing the unique contribution environmental stress has on network topology, this approach will also inevitably result in the loss of some relevant information as psychopathology and environmental stress are intertwined. Fourth, while we included race/ethnicity as a covariate in the statistical analyses to capture sociopolitical influences not fully represented by income and parental education, there are important implications to consider when race/ethnicity is used in neuroimaging research, most importantly that race/ethnicity is socially constructed, not biologically innate (for an in-depth discussion, see Cardenas-Iniguez and Gonzalez, 2023).
Fifth, the neural metrics used in the current analyses are averaged across each fMRI task. Thus, although the metrics are measured in the context of inhibition, affective working memory, and reward processing, they are not able to differentiate between dynamic cognitive states within each task. For example, during the stop-signal task, participants either successfully engage in inhibition or fail to inhibit a response and our metrics are not able to differentiate between those different outcomes within the same task. To address this, functional network metrics can be analyzed at the event-related level. Sixth, the present analyses employ a cross-sectional design and, therefore, only capture one moment in time. Finally, the emotional n-back task in the ABCD Study combines affective processing (emotional faces) with working memory (n-back task), making it difficult to parse apart the relative contribution of each to the results found in the current study.
5. Conclusions and future directions
Future work on the effects of early life stress on brain development would benefit from a number of refinements. First, there is merit in continuing to use both latent factors and individual items going forward. Data-driven approaches that uncover the underlying structure of environmental stressors are important for understanding how these constructs relate to one another, which may provide insight into classes of stressors that group together in important ways. At the same time, we still need research on individual environmental stressors in isolation (e.g., income) to reveal the mechanisms underlying specific constructs. Second, future work that examines more focal measures of brain function using functional connectivity and task-based fMRI will help us to develop of fuller understanding of the influence of environmental stressors on brain development. Third, longitudinal research is needed to delineate the relationships between environmental stress and functional network topology over time and throughout different stages of brain development. Finally, we need to consider the translational impacts of this work. Our understanding of the association between environmental stress and network topology is still in its infancy; therefore, it may be premature to consider potential biomarkers of risk or using these metrics to track symptom progression. However, the results of the current study found promising signal that needs to be 1) replicated in additional samples, 2) examined in a variety of age ranges, and 3) compared across groups (e.g., across mental health disorders).
CRediT authorship contribution statement
Tyler M Moore: Writing – review & editing, Methodology, Formal analysis. Andrew J Stier: Resources, Methodology, Formal analysis, Data curation. Camille Archer: Writing – review & editing, Writing – original draft. E. Leighton Durham: Writing – review & editing, Writing – original draft. Marc G Berman: Writing – review & editing, Supervision, Resources, Methodology, Funding acquisition, Formal analysis. Julia R Pines: Writing – review & editing, Formal analysis. Antonia Kaczkurkin: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Investigation, Funding acquisition. Gabrielle E Reimann: Writing – review & editing, Writing – original draft. Hee Jung Jeong: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Data curation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by grants R01MH117014 (TMM), R00MH117274 (ANK), and T32-MH18921 (ELD is a trainee on this grant) from the National Institute of Mental Health, the NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation (ANK), the Sloan Research Fellowship (ANK), the David H. and Beverly A. Barlow Grant from the American Psychological Foundation (ANK), a National Science Foundation grant NSF-S&CC 1952050 (MGB), and the Lifespan Brain Institute of the University of Pennsylvania and the Children’s Hospital of Philadelphia (TMM). This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. 1937963 (GER). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive DevelopmentSM (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9–10 and follow them over 10 years into early adulthood. The ABCD Study® is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortium_members/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in the analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH, NSF, or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from RRID: SCR_015769, DOI 10.15154/1523041 (data release 4.0) and NDA study DOI: 10.15154/1528453. DOIs can be found at https://nda.nih.gov/abcd/study-information.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2024.101367.
Appendix A. Supplementary material
Supplementary material
Data Availability
ABCD Study data are available through the National Institute of Mental Health Data Archive (https://nda.nih.gov/abcd). The code and wiki for the analytic procedures can be found on GitHub.
References
- Batista-García-Ramó K., Fernández-Verdecia C.I. What we know about the brain structure-function relationship. Behav. Sci. 2018;8(4) doi: 10.3390/bs8040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum G.L., Ciric R., Roalf D.R., Betzel R.F., Moore T.M., Shinohara R.T., Kahn A.E., Vandekar S.N., Rupert P.E., Quarmley M., Cook P.A., Elliott M.A., Ruparel K., Gur R.E., Gur R.C., Bassett D.S., Satterthwaite T.D. Modular Segregation of Structural Brain Networks Supports the Development of Executive Function in Youth. Curr. Biol. 2017;27(11):1561–1572.e8. doi: 10.1016/j.cub.2017.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bernhardi R., Eugenín-von Bernhardi L., Eugenín J. In: The Plastic Brain. von Bernhardi R., Eugenín J., Mulleer K.J., editors. Springer International Publishing; 2017. What is neural plasticity? pp. 1–15. [Google Scholar]
- Bullmore E., Sporns O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009;10(3):186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L., Mora-Tiscareño A., Ontiveros E., Gómez-Garza G., Barragán-Mejía G., Broadway J., Chapman S., Valencia-Salazar G., Jewells V., Maronpot R.R., Henríquez-Roldán C., Pérez-Guillé B., Torres-Jardón R., Herrit L., Brooks D., Osnaya-Brizuela N., Monroy M.E., González-Maciel A., Reynoso-Robles R.…Engle R.W. Air pollution, cognitive deficits and brain abnormalities: A pilot study with children and dogs. Brain Cogn. 2008;68(2):117–127. doi: 10.1016/j.bandc.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Cardenas-Iniguez C., Gonzalez R.M. Recommendastions for the resonsible use and communication of race and ethnicity in neuroimaging research. PsyArXiv Prepr. 2023:1–29. doi: 10.1038/s41593-024-01608-4. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Cannonier T., Conley M.I., Cohen A.O., Barch D.M., Heitzeg M.M., Soules M.E., Teslovich T., Dellarco D.V., Garavan H., Orr C.A., Wager T.D., Banich M.T., Speer N.K., Sutherland M.T., Riedel M.C., Dick A.S., Bjork J.M., Thomas K.M.…Dale A.M. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev. Cogn. Neurosci. 2018;32(March):43–54. doi: 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M.Y., Na J., Agres P.F., Savalia N.K., Park D.C., Wig G.S. Socioeconomic status moderates age-related differences in the brain’s functional network organization and anatomy across the adult lifespan. Proc. Natl. Acad. Sci. USA. 2018;115(22):E5144–E5153. doi: 10.1073/pnas.1714021115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohodes E.M., Kitt E.R., Baskin-Sommers A., Gee D.G. Influences of early-life stress on frontolimbic circuitry: Harnessing a dimensional approach to elucidate the effects of heterogeneity in stress exposure. Dev. Psychobiol. 2021;63(2):153–172. doi: 10.1002/dev.21969. [DOI] [PubMed] [Google Scholar]
- Coltman T., Devinney T.M., Midgley D.F., Venaik S. Formative versus reflective measurement models: Two applications of formative measurement. J. Bus. Res. 2008;61(12):1250–1262. doi: 10.1016/j.jbusres.2008.01.013. [DOI] [Google Scholar]
- Dannlowski U., Stuhrmann A., Beutelmann V., Zwanzger P., Lenzen T., Grotegerd D., Domschke K., Hohoff C., Ohrmann P., Bauer J., Lindner C., Postert C., Konrad C., Arolt V., Heindel W., Suslow T., Kugel H. Limbic scars: Long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol. Psychiatry. 2012;71(4):286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Dannlowski U., Kugel H., Huber F., Stuhrmann A., Redlich R., Grotegerd D., Dohm K., Sehlmeyer C., Konrad C., Baune B.T., Arolt V., Heindel W., Zwitserlood P., Suslow T. Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Hum. Brain Mapp. 2013;34(11):2899–2909. doi: 10.1002/hbm.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJoseph M.L., Herzberg M.P., Sifre R.D., Berry D., Thomas K.M. Measurement matters: An individual differences examination of family socioeconomic factors, latent dimensions of children’s experiences, and resting state functional brain connectivity in the ABCD sample. Dev. Cogn. Neurosci. 2022;53(December 2021) doi: 10.1016/j.dcn.2021.101043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye C. Health and urban living. Science. 2008;319(5864):766–769. doi: 10.1126/science.1150198. [DOI] [PubMed] [Google Scholar]
- Ellis B.J., Figueredo A.J., Brumbach B.H., Schlomer G.L. Fundamental dimensions of environmental risk: The impact of harsh versus unpredictable environments on the evolution and development of life history strategies. Hum. Nat. 2009;Vol. 20(Issue 2) doi: 10.1007/s12110-009-9063-7. [DOI] [PubMed] [Google Scholar]
- Ellis B.J., Bianchi J.M., Griskevicius V., Frankenhuis W.E. Beyond Risk and Protective Factors: An Adaptation-Based Approach to Resilience. Perspect. Psychol. Sci. 2017;12(4):561–587. doi: 10.1177/1745691617693054. [DOI] [PubMed] [Google Scholar]
- Ellwood-Lowe M.E., Whitfield-Gabrieli S., Bunge S.A. Brain network coupling associated with cognitive performance varies as a function of a child’s environment in the ABCD study. Nat. Commun. 2021;12(1):1–14. doi: 10.1038/s41467-021-27336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood-Lowe M.E., Irving C.N., Bunge S.A. Exploring neural correlates of behavioral and academic resilience among children in poverty. Dev. Cogn. Neurosci. 2022;54(September 2021) doi: 10.1016/j.dcn.2022.101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Power J.D., Dosenbach N.U.F., Church J.A., Miezin F.M., Schlaggar B.L., Petersen S.E. Functional brain networks develop from a “local to distributed” organization. PLoS Comput. Biol. 2009;5(5) doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn E.S., Shen X., Scheinost D., Rosenberg M.D., Huang J., Chun M.M., Papademetris X., Constable R.T. Functional connectome fingerprinting: Identifying individuals using patterns of brain connectivity. Nat. Neurosci. 2015;18(11) doi: 10.1038/nn.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A., Zalesky A., Bullmore E.T. Network scaling effects in graph analytic studies of human resting-state fMRI data. Front. Syst. Neurosci. 2010;4(June):1–16. doi: 10.3389/fnsys.2010.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Alcauter S., Elton A., Hernandez-Castillo C.R., Smith J.K., Ramirez J., Lin W. Functional network development during the first year: Relative sequence and socioeconomic correlations. Cereb. Cortex. 2015;25(9):2919–2928. doi: 10.1093/cercor/bhu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson D.S., Fair D.A. Development of large-scale functional networks from birth to adulthood: A guide to the neuroimaging literature. NeuroImage. NeuroImage. 2017;160(February):15–31. doi: 10.1016/j.neuroimage.2017.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S., Satterthwaite T.D., Medaglia J.D., Yang M., Gur R.E., Gur R.C., Bassett D.S. Emergence of system roles in normative neurodevelopment. Proc. Natl. Acad. Sci. USA. 2015;112(44) doi: 10.1073/pnas.1502829112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg, A.A., Swart, P.J., & Schult, D. (2008). Exploring network structure, dynamics, and function using networkx. In G. Varoquaux, T. Vaught, & K.J. Millman (Eds.), 7th Python in Science Conference (SciPy 2008).
- Hagler D.J., Hatton S., Cornejo M.D., Makowski C., Fair D.A., Dick A.S., Sutherland M.T., Casey B.J., Barch D.M., Harms M.P., Watts R., Bjork J.M., Garavan H.P., Hilmer L., Pung C.J., Sicat C.S., Kuperman J., Bartsch H., Xue F.…Dale A.M. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. NeuroImage. 2019;202 doi: 10.1016/j.neuroimage.2019.116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P., Sporns O., Madan N., Cammoun L., Pienaar R., Wedeen V.J., Meuli R., Thiran J.P., Grant P.E. White matter maturation reshapes structural connectivity in the late developing human brain. Proc. Natl. Acad. Sci. USA. 2010;107(44):19067–19072. doi: 10.1073/pnas.1009073107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafiah M.H. Formative vs. Reflective Measurement Model: Guidelines for Structural Equation Modeling Research. Int J Anal Appl. 2020;18(5):876–889. [Google Scholar]
- Heeringa S.G., Berglund P.A. A Guide for Population-based Analysis of the Adolescent Brain Cognitive Development (ABCD) Study Baseline Data. BioRxiv. 2020:1–36. [Google Scholar]
- Ho T.C., Dennis E.L., Thompson P.M., Gotlib I.H. Network-based approaches to examining stress in the adolescent brain. Neurobiol. Stress. 2018;8(April):147–157. doi: 10.1016/j.ynstr.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H.J., Moore T.M., Durham E.L., Reimann G.E., Dupont R.M., Cardenas-Iniguez C., Berman M.G., Lahey B.B., Kaczkurkin A.N. General and Specific Factors of Environmental Stress and Their Associations With Brain Structure and Dimensions of Psychopathology. Biol. Psychiatry Glob. Open Sci. 2023;3(3):480–489. doi: 10.1016/j.bpsgos.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey B.B., Moore T.M., Kaczkurkin A.N., Zald D.H. Hierarchical models of psychopathology: empirical support, implications, and remaining issues. World Psychiatry. 2021;20(1) doi: 10.1002/wps.20824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederbogen F., Kirsch P., Haddad L., Streit F., Tost H., Schuch P., Wüst S., Pruessner J.C., Rietschel M., Deuschle M., Meyer-Lindenberg A. City living and urban upbringing affect neural social stress processing in humans. Nature. 2011;474(7352):498–501. doi: 10.1038/nature10190. [DOI] [PubMed] [Google Scholar]
- Liao X., Vasilakos A.V., He Y. Small-world human brain networks: Perspectives and challenges. Neurosci. Biobehav. Rev. 2017;77(January):286–300. doi: 10.1016/j.neubiorev.2017.03.018. [DOI] [PubMed] [Google Scholar]
- Marco E.M., Llorente R., López-Gallardo M., Mela V., Llorente-Berzal Á., Prada C., Viveros M.P. The maternal deprivation animal model revisited. Neurosci. Biobehav. Rev. 2015;51:151–163. doi: 10.1016/j.neubiorev.2015.01.015. [DOI] [PubMed] [Google Scholar]
- McLaughlin K.A., Weissman D.G., Flournoy J. Correction to: Challenges with Latent Variable Approaches to Operationalizing Dimensions of Childhood Adversity ? a Commentary on Sisitsky et al. (2023) Res. Child Adolesc. Psychopathol. 2023;51:1809–1811. doi: 10.1007/s10802-023-01125-1. [DOI] [PubMed] [Google Scholar]
- Nketia J., Amso D., Brito N.H. Towards a more inclusive and equitable developmental cognitive neuroscience. Dev. Cogn. Neurosci. 2021;52 doi: 10.1016/j.dcn.2021.101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K.G., Hart E.R., Sperber J.F. Socioeconomic Disparities and Neuroplasticity: Moving Toward Adaptation, Intersectionality, and Inclusion. Am. Psychol. 2021;76(9):1486–1495. doi: 10.1037/amp0000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P., Pizzagalli D.A. Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology. 2011;214(1):55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E. The Brain’s Default Mode Network. Annu. Rev. Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Rakesh D., Zalesky A., Whittle S. Similar but distinct – Effects of different socioeconomic indicators on resting state functional connectivity: Findings from the Adolescent Brain Cognitive Development (ABCD) Study®. Dev. Cogn. Neurosci. 2021;51 doi: 10.1016/j.dcn.2021.101005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann G.E., Stier A.J., Moore T.M., Durham E.L., Jeong H.J., Cardenas-Iniguez C., Dupont R.M., Pines J.R., Berman M.G., Lahey B.B., Kaczkurkin A.N. Atypical Functional Network Properties and Associated Dimensions of Child Psychopathology During Rest and Task Performance. Biol. Psychiatry Glob. Open Sci. 2022;3(3):541–549. doi: 10.1016/j.bpsgos.2022.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen M.L., Sheridan M.A., Sambrook K.A., Meltzoff A.N., McLaughlin K.A. Socioeconomic disparities in academic achievement: A multi-modal investigation of neural mechanisms in children and adolescents. NeuroImage. 2018;173(November 2017):298–310. doi: 10.1016/j.neuroimage.2018.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: Uses and interpretations. NeuroImage. 2010;52(3) doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Shen X., Tokoglu F., Papademetris X., Constable R.T. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. NeuroImage. 2013;82:403–415. doi: 10.1016/j.neuroimage.2013.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.E., Pollak S.D. Rethinking Concepts and Categories for Understanding the Neurodevelopmental Effects of Childhood Adversity. Perspect. Psychol. Sci. 2021;16(1):67–93. doi: 10.1177/1745691620920725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. Graph theory methods: Applications in brain networks. Dialog-. Clin. Neurosci. 2018;20(2):111–120. doi: 10.31887/DCNS.2018.20.2/OSPORNS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada C., Gard A.M., Angstadt M., Taxali A., Greathouse T., McCurry K., Hyde L.W., Weigard A., Walczyk P., Heitzeg M. Socioeconomic resources are associated with distributed alterations of the brain’s intrinsic functional architecture in youth. Dev. Cogn. Neurosci. 2022;58(October) doi: 10.1016/j.dcn.2022.101164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M.C. The contributions of resting state and task-based functional connectivity studies to our understanding of adolescent brain network maturation. Neurosci. Biobehav. Rev. 2016;70:13–32. doi: 10.1016/j.neubiorev.2016.07.027. [DOI] [PubMed] [Google Scholar]
- Stier A.J., Cardenas-Iniguez C., Kardan O., Moore T.M., Meyer F.A.C., Rosenberg M.D., Kaczkurkin A.N., Lahey B.B., Berman M.G. A pattern of cognitive resource disruptions in childhood psychopathology. Netw. Neurosci. 2023:1–28. doi: 10.1162/netn_a_00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor E.K., Abdurokhmonova G., Romeo R.R. Socioeconomic Status and Reading Development: Moving from “Deficit” to “Adaptation” in Neurobiological Models of Experience-Dependent Learning. Mind, Brain, Educ. 2023;17(4):324–333. doi: 10.1111/mbe.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D. Effects of family income on brain functional connectivity in US children: associations with cognition. Mol. Psychiatry. 2023:1–8. doi: 10.1038/s41380-023-02222-9. (January) [DOI] [PubMed] [Google Scholar]
- Tooley U.A., MacKey A.P., Ciric R., Ruparel K., Moore T.M., Gur R.C., Gur R.E., Satterthwaite T.D., Bassett D.S. Associations between Neighborhood SES and Functional Brain Network Development. Cereb. Cortex. 2020;30(1):1–19. doi: 10.1093/cercor/bhz066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele T.J. Constructed measures and causal inference. Epidemiology. 2022;33(1):141–151. doi: 10.1097/EDE.0000000000001434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Váša F., Seidlitz J., Romero-Garcia R., Whitaker K.J., Rosenthal G., Vértes P.E., Shinn M., Alexander-Bloch A., Fonagy P., Dolan R.J., Jones P.B., Goodyer I.M., Sporns O., Bullmore E.T. Adolescent tuning of association cortex in human structural brain networks. Cereb. Cortex. 2018;28(1):281–294. doi: 10.1093/cercor/bhx249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A.C., Power J.D., Petersen S.E., Schlaggar B.L. Development of the brain’s functional network architecture. Neuropsychol. Rev. 2010;20(4):362–375. doi: 10.1007/s11065-010-9145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
ABCD Study data are available through the National Institute of Mental Health Data Archive (https://nda.nih.gov/abcd). The code and a corresponding wiki for the analytic procedures can be found at https://github.com/VU-BRAINS-lab/ABCD_Stressor_Network.
ABCD Study data are available through the National Institute of Mental Health Data Archive (https://nda.nih.gov/abcd). The code and wiki for the analytic procedures can be found on GitHub.