Abstract
Background
Acute generalized eruptive pustulosis (AGEP) is a rare, but serious, drug-related adverse event. This study aimed to determine the relationships between AGEP and age, sex, primary disease, and prescription medications using a database of adverse drug events, namely, the Japanese Adverse Drug Event Report (JADER) database.
Methods
In this retrospective observational study, we analyzed AGEP reports extracted from the JADER database based on the preferred term for AGEP (code 10048799). We evaluated the effects of causative drugs, underlying diseases, age, and sex. The association between AGEP and prescription drugs was analyzed using the reporting odds ratio and adjusted for covariates using multiple logistic regression. Association rule mining was performed to evaluate the correlation between each combination of factors and AGEP.
Results
Between April 2004 and March 2023, 823,662 reports, including 869 reports on AGEP, were entered in the JADER database. The highest percentage of reports in each age group was in males aged <10 years, and clarithromycin was the most used drug in males aged <10 years. Nasopharyngitis was the most common reason for use, and Kawasaki disease was reported as a reason for use among males under 10 years of age, but not among females.
Conclusions
In boys aged <10 years, attention should be paid to the occurrence of AGEP when prescribing clarithromycin and treating Kawasaki disease.
1. Introduction
Acute generalized eruptive pustulosis (AGEP) is a severe cutaneous adverse event (AE) classified as a T cell-associated neutrophilic inflammatory reaction whose etiology has not been fully established [1]. The initial phase involves the stimulation and migration of drug-specific T cells into the skin. These T cells, together with natural killer T cells and natural killer cells, are activated in the skin and induce keratinocyte apoptosis through interactions between cytotoxic proteins and Fas/Fas ligands, forming subclassified vesicles. Various cytokines and chemokines are released from innate immune cells, acquired immune cells, and resident cells (keratinocytes, dendritic cells, mast cells, and neutrophils) that primarily cause neutrophilic inflammation and pustule formation [1]. AGEP is associated with drug intake in >90% of the cases, with the most common drugs being antibiotics, primarily beta-lactams and macrolides. Other causative agents include aminoglycosides, sulphonamides quinolones, antifungals, antimalarials, acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), calcium channel blockers, and antiepileptic drugs [2]. AGEP can occur within hours or days of ingesting the causative drug if the patient is already sensitized to it or 1–2 weeks after taking the drug if the patient had not taken the drug before [3].
AGEP is a rare AE with an estimated incidence of 1–5 cases per million patients per year and a mortality rate of <5% [1,4]. In general, AGEP spontaneously resolves within 2 weeks after discontinuation of the causative agent and has a favorable prognosis. However, organ involvement, mucosal involvement, secondary infection, delayed diagnosis, and comorbidities can lead to poor prognosis [5]. Therefore, healthcare professionals should be aware of the risk of developing AGEP. Therefore, early monitoring and appropriate intervention by healthcare providers are important. However, few studies have examined the relationships between AGEP and age, sex, primary disease, and prescription drugs. Therefore, analysis of the AGEP incidence profile using various factors as axes of analysis is justified.
Large-scale spontaneous reporting systems (SRSs) for AEs are important sources of information for post-marketing drug safety surveillance. Pharmaceutical regulatory authorities in various countries have established systems to record AEs voluntarily reported by drug manufacturers and distributors, healthcare professionals, and general users and to utilize them for safety monitoring. The Japanese Adverse Drug Event Report (JADER) is a database of drug reactions suitable for investigating rare AEs.
Several pharmacovigilance indices, such as reporting odds ratio (ROR), have been developed for drug-associated AEs [6]. The ROR is a powerful technique that allows for adjustments through multivariate logistic regression analysis and the partial control of confounding factors [7,8]. Time-to-onset analysis using the Weibull shape parameter (WSP) is useful in detecting AE signals [9].
Association rule mining (ARM) has been proposed as a new analytical approach to identify undetected associations between variables in large databases, such as potential risk factors [10,11]. This algorithm has been used to assess the associations between the AEs in the JADER database [11,12]. Here, we used pharmacovigilance analysis to determine the expression profile of AEGP and influencing factors, such as age, sex, comorbidities, and prescribed drugs.
2. Methods
2.1. Data source
The JADER database, containing data from April 2004 to March 2023, was obtained from the Pharmaceuticals and Medical Devices Agency website (www.pmda.go.jp). The structure of the JADER database is based on International Safety Reporting Guidelines. The database comprises four tables: 1) case list (demo), 2) drug information (drug), 3) drug reaction information table (reac), and 4) primary disease (hist). As the JADER database does not contain codes for identifying case reports (A1.11), we could not exclude duplicate case reports from the same patient (www.pmda.go.jp/files/000145474.pdf). Age was recorded in a “demo” table, which included patient demographic data. The following age-related items are entered in the “demo” table: <10, 10–19, 20–29, 30–39, 40–49, 50–59, 60–69, 70–79, 80–89, 90–99, or ≥100 years. In the drug table, the causality of each drug was assigned a code according to its association with AEs, such as “suspected drug,” “concomitant drug,” or “interacting drug.” In this study, data coded as “suspect drugs” were analyzed. Data from duplicate drugs were excluded from analysis.
2.2. Definition of AEs
AEs in the JADER database were defined as codes according to the terminology used in the Medical Dictionary for Regulatory Activities/Japanese version 23.1 (www.pmrj.jp/jmo/php/indexj.php). We used the preferred terms for AGEP (code 10048799).
2.3. Reporting percentage
To calculate the reporting rate, the number of reports in each age group (<10, 10–19, 20–29, 30–39, 40–49, 50–59, 60–69, 70–79, 80–89, and >90 years) listed in the JADER database and the number of reports recorded as AGEP in each age group were used.
2.4. Disproportionality assessment via multiple logistic regression analysis
The authorized pharmacovigilance index RORs [6] was calculated using a two-by-two contingency table for the presence/absence of specific drugs and AEs in case reports. The ROR is the ratio of the odds of reporting adverse events versus all other events associated with the drug of interest compared with the reporting odds for all other drugs present in the database. Disproportionality analysis was performed by calculating the ROR with corresponding 95% confidence intervals (95% CIs). A signal was considered when the lower limit of the 95% CI exceeded 1. Two or more cases were necessary to positively identify such signals.
Patient demographics and descriptive analyses of the suspected drugs suggested that Kawasaki disease, clarithromycin use, aspirin use, and famotidine use were prevalent factors in boys aged <10 years (Table 1, Table 2). Therefore, we refined the ROR signal using dedicated corrections to detect possible confounders in the database [13,14]. The ROR was adjusted using a multivariate logistic regression model. To calculate the adjusted ROR, only reports with complete information regarding the reporting year, sex, age, Kawasaki disease, aspirin use, and clarithromycin use were extracted from the JADER database. The following formula was used:
| Log (odds) = β0 + β1Y + β2S + β3A + β4K + β5Asp + β6Cla + β7Fa |
Table 1.
Number and reporting ratio of reported acute generalized eruptive pustulosis (AGEPa) under 10 years of age.
| Drugs | Case reports (%) | |
|---|---|---|
| Male | Cases in male AGEPa patients under 10 years of age | 113 (100.0) |
| Clarithromycin | 19 (16.8) | |
| Aspirin | 13 (11.5) | |
| Acetaminophen | 11 (9.7) | |
| Famotidine | 9 (8.0) | |
| Cefotaxime sodium | 7 (6.2) | |
| Azithromycin hydrate | 6 (5.3) | |
| Cefcapene pivoxil hydrochloride hydrate | 6 (5.3) | |
| l-Carbocisteine | 4 (3.5) | |
| Flomoxef sodium | 4 (3.5) | |
| Pranlukast hydrate | 4 (3.5) | |
| Combination cold remedy (OTCb Drugs) | 4 (3.5) | |
| Pabron | 3 (2.7) | |
| Tipepidine hibenzate | 2 (1.8) | |
| Freeze-dried sulfonated human normal immunoglobulin | 2 (1.8) | |
| Amoxicillin hydrate | 2 (1.8) | |
| Cyproheptadine hydrochloride hydrate | 2 (1.8) | |
| Chlorpheniramine maleate | 2 (1.8) | |
| Cefmetazole sodium | 1 (0.9) | |
| Tulobuterol | 1 (0.9) | |
| Amoxicillin hydrate/Potassium clavulanate | 1 (0.9) | |
| Carbinoxamine maleate | 1 (0.9) | |
| Aspirin and dialuminate | 1 (0.9) | |
| Antipyretic analgesic anti-inflammatory agent (OTCb Drugs) | 1 (0.9) | |
| Freeze-dried polyethylene glycol treated human normal immunoglobulin | 1 (0.9) | |
| Ophthalmic agent (OTCb Drugs) | 1 (0.9) | |
| Non pyrin cold remedy | 1 (0.9) | |
| Others | 4 (3.5) | |
| Female | Cases in female AGEPa patients under 10 years of age | 50 (100.0) |
| Amoxicillin hydrate | 6 (12.0) | |
| Saikokeishito | 5 (10.0) | |
| Tranexamic acid | 5 (10.0) | |
| Dextromethorphan hydrobromide hydrate | 5 (10.0) | |
| Cefditoren pivoxil | 5 (10.0) | |
| l-Carbocisteine | 5 (10.0) | |
| Clarithromycin | 4 (8.0) | |
| Acetaminophen | 3 (6.0) | |
| Antibiotics-resistant lactic acid bacteriae | 2 (4.0) | |
| Tosufloxacin tosilate hydrate | 2 (4.0) | |
| Cefcapene pivoxil hydrochloride hydrate | 2 (4.0) | |
| Cefotaxime sodium | 2 (4.0) | |
| Clemastine fumarate | 2 (4.0) | |
| Piperacillin sodium | 1 (2.0) | |
| Ampicillin sodium | 1 (2.0) |
Acute generalized eruptive pustulosis.
Over the counter.
Table 2.
Reasons for drug use in acute generalized eruptive pustulosis (AGEPa) patients under 10 years of age.
| Reasons for use | Case reports (%) | |
|---|---|---|
| Male | Cases in male AGEPa patients under 10 years of age | 83 (100.0) |
| Nasopharyngitis | 26 (31.3) | |
| Kawasaki disease | 21 (25.3) | |
| Fever | 10 (12.0) | |
| Bacterial infection | 8 (9.6) | |
| Sore throat | 4 (4.8) | |
| Upper airway inflammation | 2 (2.4) | |
| Lymphadenitis | 2 (2.4) | |
| Nasal inflammation | 1 (1.2) | |
| Wound | 1 (1.2) | |
| Influenza | 1 (1.2) | |
| Allergic rhinitis | 1 (1.2) | |
| Drug use for unknown indication | 4 (4.8) | |
| Heat treatment | 2 (2.4) | |
| Female | Cases in female AGEPa patients under 10 years of age | 46 (100.0) |
| Sore throat | 25 (54.3) | |
| Adenovirus test positive | 6 (13.0) | |
| Fever | 3 (6.5) | |
| Pneumonia | 3 (6.5) | |
| Bacterial infection | 3 (6.5) | |
| Rhinosinusitis | 1 (2.2) | |
| Non-tuberculous mycobacteria | 1 (2.2) | |
| Contact dermatitis | 1 (2.2) | |
| Fasciitis | 1 (2.2) | |
| Prevention of infection | 1 (2.2) | |
| Adenovirus infection | 1 (2.2) |
Acute generalized eruptive pustulosis.
The reporting year (Y), sex (S), stratified ages (<10, 10–19, 20–29, 30–39, 40–49, 50–59, 60–69, 70–79, 80–89, and >90 years) (A), Kawasaki disease (K), aspirin use (Asp), clarithromycin use (Cla), and famotidine use (Fa) were defined as independent variables. The dependent variable was a binary response to the absence or presence of AGEP in each report. To comparatively evaluate the effect of factors, we selected explanatory variables using a stepwise method [15,16] and a significance level of 0.25 (forward and backward). The contributions of the selected variables to the final model were evaluated. A likelihood ratio test was used to assess the influence of explanatory variables. Results were considered statistically significant at p < 0.05.
2.5. Time-to-onset analysis
The time-to-onset duration from the JADER database was calculated from the time of the patient's first prescription until the occurrence of AE. Median duration, quartiles, and WSP were used to evaluate the time-to-onset data. The scale parameter α of the Weibull distribution determines the scale of the distribution function. A larger-scale value (α) stretches the distribution, whereas a smaller scale value shrinks the data distribution. The shape parameter β of the Weibull distribution determines the shape of the distribution function. A larger shape value is indicated by a left-skewed curve, whereas a smaller shape value is indicated by a right-skewed curve. In the SRS analysis, the shape parameter β of the Weibull distribution was used to show the hazards without reference population as follows. When β was equal to 1, the hazard was estimated to be constant over time. If β was >1 and the 95% CI of β excluded the value 1, the hazard was considered to increase over time. Finally, if β was <1 and the 95% CI of β excluded the value 1, the hazard was considered to decrease over time [9].
Data analyses were performed using JMP Pro, version 16 (SAS Institute Inc., Cary, NC, USA).
2.6. ARM
The ARM is a useful technique for inferring the relationship between drugs and their possible risk factors [10,11]. Given a set of transactions T (each transaction is a set of items), an association rule can be expressed as X [lhs: left-hand-side, the antecedent of the rule] → Y [rhs: right-hand-side, the consequent of the rule], where X and Y are mutually exclusive sets of items [10,11]. Support determines how often a rule is observed in a database; in this case, the rule is a combination of X and Y. Support was calculated using the following formula:
| Support = P (X∩Y) = {X∩Y} / {D} |
where D is the total number of transactions in the database.
The confidence of the association rule demonstrated its strengths and was calculated using the following equation:
| Confidence = P (X∩Y) / P(X) |
Lift represents probability ratio. Given a rule that X and Y occur together with multiples of their two individual probabilities:
| Lift = P (X∩Y) / P(X) P(Y) |
Lift evaluates the independence of X and Y, with higher lift values indicating stronger relationships. If X and Y are independent, the lift is 1. If X and Y are positively or negatively correlated, the lift is > 1 or <1, respectively. ARM was performed using the arules package in R software (version 4.1.2). The parameter maxlen (i.e., the maximum length of itemset/rule, a parameter in the arules package) is the maximum size of the mined frequent itemsets. To efficiently extract association rules, thresholds for optimized support, confidence, and maxlen were defined depending on factors such as the size of the data, number of items, and research purpose. In this study, we defined the minimum support and confidence thresholds as 0.00001 and 0.001, respectively, and the maxlen threshold was restricted to 5. ARM was performed using the apriori function of the arules package in R version 4.1.2 software [17].
3. Results
3.1. Patient demographic
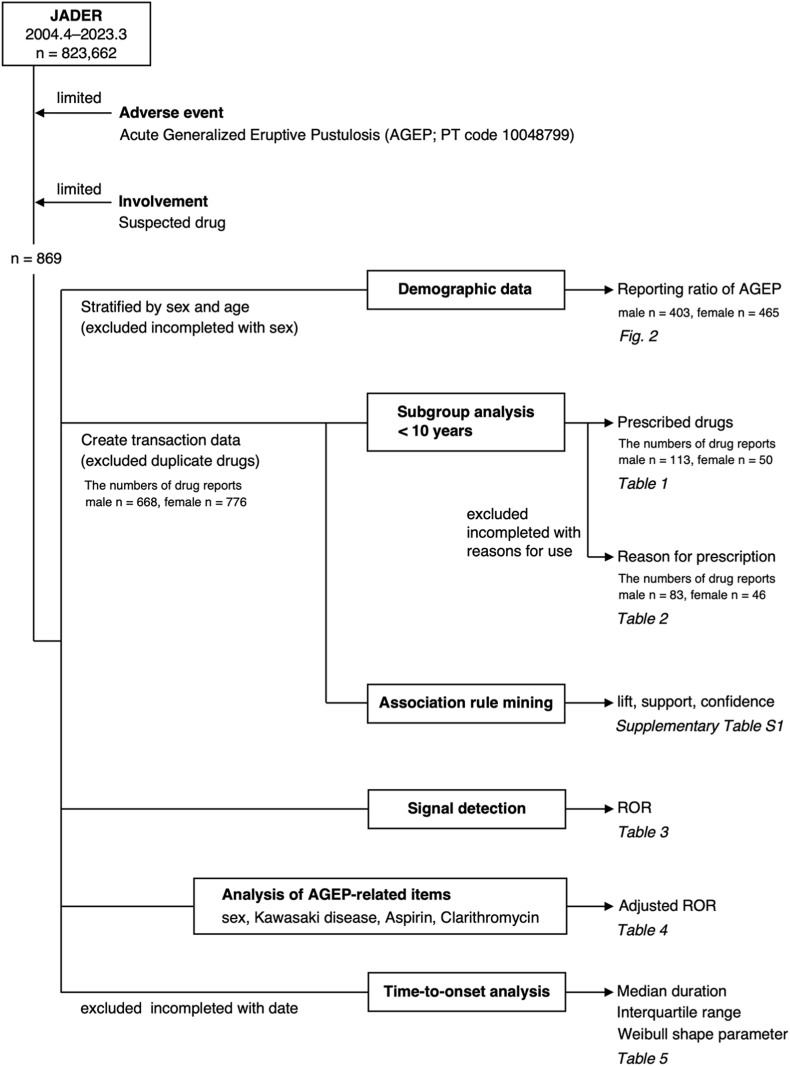
The JADER database contains 823,662 reports published between April 2004 and March 2023. Of these, 869 were AGEP reports. The number of AGEP reports for males and females were 403 (46.4%) and 465 (53.6%), respectively (Fig. 1).
Fig. 1.
Flow chart of data analysis.
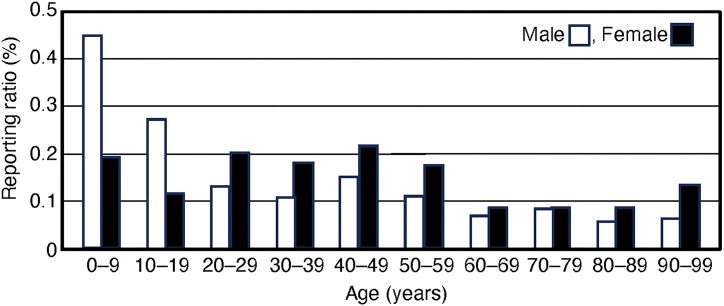
The percentage of AGEP reports in males and females is shown in Fig. 2. According to the 403 reports, the number of AGEP cases reported in males aged <10, 10–19, 20–29, 30–39, 40–49, 50–59, 60–69, 70–79, 80–89, and >90 years were 65 (0.4%), 28 (0.3%), 14 (0.1%), 18 (0.1%), 40 (0.2%), 53 (0.1%), 62 (0.07%), 91 (0.09%), 27 (0.06%), and 3 (0.06%), respectively. Among females, according to the 465 reports, the number of cases reported in the same age groups was 22 (0.2%), 13 (0.1%), 33 (0.2%), 46 (0.2%), 70 (0.2%), 81 (0.2%), 62 (0.09%), 72 (0.09%), 44 (0.09%), and 13 (0.1%), respectively. The number of reported cases was higher in the group of males aged <10 years.
Fig. 2.
Reporting ratio of acute generalized eruptive pustulosis (AGEP).
The mean patient age was 52.1 years. Moreover, the mean ages of male and female patients were 49.6 and 54.3 years, respectively.
3.2. Descriptive analysis of suspect drugs
In males who developed AGEP, the top three drugs used were acetaminophen (46/668 cases, 6.9%), amoxicillin hydrate (38/668 cases, 5.7%), and clarithromycin (29/668 cases, 4.3%). Among females, acetaminophen was used in 57/776 cases (7.3%), amoxicillin hydrate in 44/776 (5.7%), terbinafine hydrochloride in 20/776 (2.6%), and loxoprofen sodium hydrate in 20/776 (2.6%).
Table 1 shows the percentage of drugs used in males and females under 10 years of age; clarithromycin was the most used drug in males under 10 years of age in 19/113 cases (16.8%). Of the 19 reports involving clarithromycin, the reason for its use was stated in 13 cases, 12 of which involved nasopharyngitis.
The percentages of the reasons for use in males and females under 10 years of age are shown in Table 2. Nasopharyngitis was reported most frequently in males under 10 years of age, accounting for 26/83 cases (31.3%), whereas Kawasaki disease was reported in 21/83 males (25.3%) under 10 years of age; however, no females reported Kawasaki disease as a reason for use. The most frequently used drugs for treating Kawasaki disease are aspirin and famotidine.
3.3. Disproportionality assessment via multiple logistic regression analysis
The RORs of the 20 most frequently used drugs are shown in Table 3. Signals were detected for all 20 drugs. For aspirin, the signal was detected only in males. For diclofenac sodium, tosufloxacin tosilate hydrate and cefazolin sodium, the signal was detected in females.
Table 3.
Number of reports and RORa of acute generalized eruptive pustulosis (AGEP).
| Drugs | Male and Female |
Male |
Female |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Case | Crude RORa (95% CIb) | Total | Case | Crude RORa (95% CIb) | Total | Case | Crude RORa (95% CIb) | |
| Acetaminophen | 4552 | 103 | 24.7 (20.1–30.4) | 2058 | 46 | 25.4 (18.6–34.6) | 2398 | 57 | 22.9 (17.3–30.3) |
| Amoxicillin hydrate | 2718 | 83 | 32.9 (26.1–43.1) | 1507 | 38 | 28.1 (20.1–39.4) | 1566 | 44 | 26.4 (19.2–36.1) |
| Terbinafine hydrochloride | 1553 | 44 | 29.0 (21.3–39.5) | 742 | 24 | 35.1 (23.1–53.3) | 761 | 20 | 23.3 (14.8–36.7) |
| Clarithromycin | 3815 | 44 | 11.6 (8.5–15.7) | 1653 | 29 | 18.9 (12.9–27.7) | 2086 | 15 | 6.2 (3.7–10.3) |
| Loxoprofen sodium hydrate | 8247 | 39 | 4.7 (3.4–6.4) | 3989 | 19 | 4.9 (3.1–7.8) | 4217 | 20 | 4.1 (2.6–6.4) |
| Ampicillin sodium/Sulbactam sodium | 1678 | 35 | 21.0 (14.9–29.5) | 952 | 18 | 19.9 (12.3–32.0) | 700 | 17 | 21.4 (13.1–34.9) |
| L-Carbocisteine | 1788 | 30 | 16.7 (11.6–24.1) | 784 | 17 | 22.8 (14.0–37.3) | 993 | 13 | 11.3 (6.5–19.7) |
| Cefcapene pivoxil hydrochloride hydrate | 1865 | 26 | 13.8 (9.3–20.4) | 904 | 14 | 16.1 (9.4–27.5) | 945 | 12 | 10.9 (6.1–19.4) |
| Combination cold remedy (OTCc Drugs) | 1642 | 24 | 14.4 (9.6–21.7) | 836 | 18 | 22.7 (14.1–36.6) | 790 | 6 | 6.4 (2.9–14.4) |
| Celecoxib | 4364 | 20 | 4.4 (2.8–6.9) | 1493 | 7 | 4.7 (2.2–10.0) | 2703 | 13 | 4.1 (2.4–7.1) |
| Cefdinir | 761 | 20 | 26.1 (16.7–41.0) | 341 | 6 | 18.0 (8.0–40.5) | 411 | 14 | 30.1 (17.5–51.7) |
| Fexofenadine hydrochloride | 774 | 20 | 25.7 (16.4–40.2) | 358 | 3 | 8.4 (2.7–26.3) | 393 | 17 | 38.9 (23.7–63.7) |
| Diclofenac sodium | 4030 | 19 | 4.6 (2.9–7.2) | 1841 | 3 | 1.6 (0.5–5.0) | 2083 | 16 | 6.6 (4.0–10.9) |
| Diltiazem hydrochloride | 606 | 19 | 31.3 (19.7–49.7) | 334 | 10 | 31.2 (16.5–59.1) | 269 | 9 | 29.2 (15.0–57.2) |
| Tazobactam sodium/Piperacillin sodium | 2369 | 18 | 7.4 (4.6–11.8) | 1449 | 13 | 9.2 (5.3–16.0) | 871 | 5 | 4.8 (2.0–11.7) |
| Cefditoren pivoxil | 999 | 18 | 17.7 (11.1–28.4) | 435 | 4 | 9.3 (3.4–24.9) | 548 | 14 | 22.4 (13.1–38.3) |
| Aspirin | 9535 | 18 | 1.8 (1.1–2.9) | 5633 | 15 | 2.7 (1.6–4.5) | 3478 | 3 | 0.7 (0.2–2.2) |
| Tosufloxacin tosilate hydrate | 785 | 17 | 21.4 (13.1–34.7) | 367 | 0 | – | 411 | 17 | 37.1 (22.6–60.8) |
| Cefazolin sodium | 1759 | 17 | 9.4 (5.8–15.2) | 896 | 1 | – | 842 | 16 | 16.6 (10.0–27.5) |
| Ceftriaxone sodium hydrate | 3358 | 16 | 4.6 (2.8–7.6) | 1734 | 6 | 3.5 (1.5–7.8) | 1585 | 10 | 5.4 (2.9–10.0) |
Reporting Odds Ratio.
Confidence Interval.
Over the counter.
Using a stepwise logistic regression model, we examined and selected significant AGEP-related variables among the demographic factors (sex, stratified age, and drug use) and reporting year (Table 4). Famotidine was not incorporated into the model equation because it was not a statistically significant term according to results of the stepwise method. The results in the final model indicated significant contributions to AGEP in the reporting year, stratified age (except for ages 80–89 years), Kawasaki disease and clarithromycin. Adjusted RORs for the analyzed groups were as follows: 1.1 for the female group (95% CI: 0.99–1.3); 3.5 for the <10 years group (2.7–4.5); 7.7 for Kawasaki disease (4.1–14.4); and 6.6 for clarithromycin (5.2–8.5).
Table 4.
Multiple logistic regression analysis.
| Variablea | Total (n) | Case(n) | Crude RORb (95% CIc) | Adjusted RORb (95% CIc) | Likelihood ratio test |
|---|---|---|---|---|---|
| P-value | |||||
| Reporting year | – | – | – | 1.04 (1.03–1.1)d | <0.0001e |
| <10 years | 27478 | 87 | 2.9 (2.4–3.7) | 3.5 (2.7–4.5) | <0.0001e |
| 10–19 years | 21635 | 41 | 1.7 (1.2–2.3) | 2.2 (1.6–3.1) | <0.0001e |
| 20–29 years | 26810 | 47 | 1.5 (1.2–2.1) | 2.1 (1.5–2.9) | <0.0001e |
| 30–39 years | 42030 | 64 | 1.3 (1.04–1.7) | 1.8 (1.4–2.4) | <0.0001e |
| 40–49 years | 59534 | 110 | 1.7 (1.4–2.1) | 2.2 (1.7–2.7) | <0.0001e |
| 50–59 years | 93280 | 134 | 1.3 (1.1–1.5) | 1.8 (1.4–2.2) | <0.0001e |
| 80–89 years | 99377 | 71 | 0.6 (0.5–0.7) | 0.9 (0.7–1.1) | 0.2161 |
| Sex (female) | 385950 | 465 | 1.2 (1.04–1.4) | 1.1 (0.99–1.3) | 0.0633 |
| Kawasaki disease | 861 | 14 | 15.9 (9.3–27.1) | 7.7 (4.1–14.4) | <0.0001e |
| Aspirin | 44224 | 40 | 0.9 (0.6–1.2) | 0.8 (0.6–1.2) | 0.2306 |
| Clarithromycin | 10895 | 75 | 7.1 (5.6–9.0) | 6.6 (5.2–8.5) | <0.0001e |
Significant variables selected with stepwise method.
Reporting Odds Ratio.
Confidence Interval.
Unit odds ratio when the continuous variable changes by only one unit.
P < 0.05.
3.4. Time-to-onset analysis
For the time-to-onset analysis, we extracted combinations for which complete information regarding the dates of treatment initiation and AGEP onset were available. The 20 most frequently used drugs were evaluated (Table 5). The median duration (interquartile range) of AGEP onset was 2.0 (1.0–7.0). The median (inter quartile range) for the onset day of AGEP after treatment with clarithromycin in males were 1.5 (0.3–2.0). The lower limit of the 95% CI of the WSP β value for clarithromycin in males was >1.
Table 5.
Time-to-onset profiles of acute generalized eruptive pustulosis of top 20 most frequently used drugs in the Japanese Adverse Event Report database.
| Drugs | Male and Female |
Male |
Female |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Casesa(n) | Median (interquartile range, day) | Scale parameter, α (95% confidence interval) | Shape parameter, β (95% confidence interval) | Casesa(n) | Median (interquartile range, day) | Scale parameter, α (95% confidence interval) | Shape parameter, β (95% confidence interval) | Casesa(n) | Median (interquartile range, day) | Scale parameter, α (95% confidence interval) | Shape parameter, β (95% confidence interval) | |
| Total | 572 | 2.0 (1.0–7.0) | 6.3 (5.6–7.0) | 0.9 (0.8–0.9) | 250 | 2.0 (1.0–6.0) | 6.2 (5.2–7.3) | 0.9 (0.8–1.0) | 322 | 2.0 (1.0–7.0) | 6.3 (5.5–7.3) | 0.8 (0.8–0.9) |
| Acetaminophen | 23 | 2.0 (1.0–3.0) | 2.5 (1.5–3.9) | 1.2 (0.8–1.7) | 8 | 2.0 (1.0–3.5) | 2.1 (1.1–3.6) | 2.0 (0.9–3.7) | 15 | 2.0 (1.0–3.0) | 2.7 (1.4–4.9) | 1.1 (0.7–1.7) |
| Amoxicillin hydrate | 20 | 1.0 (1.0–2.8) | 2.3 (1.6–3.3) | 1.6 (1.1–2.2) | 10 | 1.0 (0.0–1.0) | 1.6 (0.9–2.8) | 1.6 (0.9–2.5) | 10 | 2.0 (1.0–3.5) | 2.9 (1.9–4.4) | 1.8 (1.01–2.9) |
| Terbinafine hydrochloride | 15 | 17.0 (8.0–23.0) | 17.0 (12.3–23.0) | 1.9 (1.2–2.8) | 7 | 9.0 (8.0–20.0) | 6.3 (2.3–16.2) | 1.04 (0.5–1.9) | 8 | 21.0 (9.5–29.0) | 22.1 (15.6–30.8) | 2.7 (1.3–4.8) |
| Clarithromycin | 10 | 1.0 (0.0–2.3) | 2.6 (1.4–4.6) | 1.5 (0.8–2.5) | 4 | 1.5 (0.3–2.0) | 1.8 (1.2–2.8) | 4.6 (1.3–11.4) | 6 | 1.0 (0.0–3.8) | 3.0 (1.1–7.9) | 1.4 (0.6–2.7) |
| Loxoprofen sodium hydrate | 16 | 1.0 (0.0–3.8) | 3.8 (2.1–6.6) | 1.2 (0.7–1.8) | 6 | 2.5 (0.8–5.3) | 4.2 (1.9–9.1) | 1.5 (0.7–2.6) | 10 | 1.0 (0.0–2.8) | 3.4 (1.2–8.8) | 1.02 (0.5–1.8) |
| Ampicillin sodium/Sulbactam sodium | 15 | 2.0 (1.0–3.0) | 2.9 (1.9–4.4) | 1.4 (0.9–2.0) | 5 | 1.0 (0.5–1.5) | 1.4 (0.9–2.2) | 3.0 (1.2–5.6) | 10 | 2.5 (1.0–5.3) | 3.5 (1.9–6.2) | 1.6 (0.8–2.6) |
| L-Carbocisteine | 13 | 2.0 (1.0–4.0) | 2.9 (1.9–4.3) | 1.7 (1.001–2.6) | 9 | 2.0 (1.0–5.0) | 3.1 (1.8–5.1) | 1.6 (0.9–2.7) | 4 | 1.5 (0.3–2.8) | 2.3 (1.2–4.3) | 2.7 (0.9–6.1) |
| Cefcapene pivoxil hydrochloride hydrate | 14 | 2.0 (0.8–4.3) | 4.2 (2.3–7.3) | 1.2 (0.7–1.8) | 5 | 0.0 (0.0–2.5) | 2.8 (0.6–14.0) | 1.7 (0.4–4.7) | 9 | 2.0 (2.0–6.5) | 3.6 (1.5–8.2) | 0.96 (0.5–1.5) |
| Combination cold remedy (OTCb Drugs) | ||||||||||||
| Celecoxib | 12 | 2.0 (0.0–9.8) | 6.9 (4.0–11.7) | 1.6 (0.8–2.8) | 3 | 6.0 (0.0–9.0) | 8.1 (5.2–13.0) | 5.9 (1.3–16.0) | 9 | 2.0 (0.0–10.0) | 6.3 (2.8–13.6) | 1.3 (0.6–2.4) |
| Cefdinir | 7 | 5.0 (1.0–5.0) | 8.0 (2.5–24.1) | 0.9 (0.4–1.5) | 4 | 3.0 (0.3–5.0) | 4.1 (1.6–10.6) | 2.0 (0.6–4.9) | 3 | 5.0 (2.0–34.0) | 17.6 (1.3–271.7) | 1.01 (0.2–2.7) |
| Fexofenadine hydrochloride | 3 | 5.0 (5.0–85.0) | – | – | 2 | 5.0 (5.0–5.0) | – | – | 1 | 85.0 (85.0–85.0) | – | – |
| Diclofenac sodium | 9 | 2.0 (1.5–21.0) | 8.6 (2.2–30.9) | 0.7 (0.4–1.2) | 2 | 9.0 (2.0–16.0) | – | – | 7 | 2.0 (1.0–26.0) | 9.0 (1.4–50.3) | 0.7 (0.3–1.2) |
| Diltiazem hydrochloride | 5 | 4.0 (2.5–9.0) | 4.3 (1.2–15.4) | 1.03 (0.4–1.9) | 2 | 4.0 (4.0–4.0) | – | – | 3 | 3.0 (2.0–14.0) | 6.4 (0.4–113.3) | 0.97 (0.2–2.6) |
| Tazobactam sodium/Piperacillin sodium | 6 | 2.0 (0.8–5.3) | 4.1 (1.4–11.6) | 1.1 (0.5–1.9) | 3 | 3.0 (0.0–3.0) | – | – | 3 | 1.0 (1.0–12.0) | – | – |
| Cefditoren pivoxil | 9 | 3.0 (1.0–3.0) | 3.5 (2.0–6.0) | 1.5 (0.8–2.3) | 2 | 2.0 (1.0–3.0) | – | – | 7 | 3.0 (1.0–3.0) | 3.9 (2.0–7.4) | 1.5 (0.8–2.5) |
| Aspirin | 4 | 0.5 (0.0–3.3) | 2.8 (0.6–14.0) | 1.7 (0.4–4.7) | 3 | 0.0 (0.0–1.0) | – | – | 1 | 4.0 (4.0–4.0) | – | – |
| Tosufloxacin tosilate hydrate | 2 | 4.5 (1.0–8.0) | – | – | 0 | – | – | – | 2 | 4.5 (1.0–8.0) | – | – |
| Cefazolin sodium | 7 | 1.0 (1.0–13.0) | 5.1 (1.6–14.8) | 0.9 (0.4–1.6) | 0 | – | – | – | 7 | 1.0 (1.0–13.0) | 5.1 (1.6–14.8) | 0.9 (0.4–1.6) |
| Ceftriaxone sodium hydrate | 7 | 5.0 (0.0–11.0) | 11.6 (6.7–19.8) | 2.5 (0.98–4.9) | 4 | 5.5 (0.0–15.5) | 15.2 (9.4–25.2) | 5.5 (1.2–14.9) | 3 | 5.0 (0.0–8.0) | 7.1 (4.2–12.2) | 5.1 (1.1–13.8) |
The records with complete adverse event occurrence and prescription star date were used for the time-to-onset analysis.
Over the counter.
3.5. ARM
We evaluated the association between AGEP and demographic data. The mining algorithm identified 100 rules for AGEP (Supplementary Table S1). {<10 years, Kawasaki disease, male} → {AGEP} and {Kawasaki disease, male} → {AGEP} demonstrated high lift value (Supplementary Table S1 [48, 49]).
4. Discussion
4.1. Association of AGEP with age and sex
According to the results of the multiple logistic regression analysis, it has been suggested that females reported more AGEP cases than males. In the present study, AGEP occurred in all age groups and was reported more frequently in females than in males. This finding is consistent with those of other studies [[18], [19], [20]]. In the EuroSCAR study, a multinational case-control study, the mean age was 56 years [19]. In a study using the World Health Organization pharmacovigilance database, the mean age was 57.3 years, with females being older than males [20]. In the present study, the overall mean age was 52.1 years, which is slightly lower than that reported in previous studies. The higher mean age for females was consistent with that of a previous study (49.6 years for males and 54.3 years for females).
A more detailed examination revealed that males reported a higher percentage of AGEP cases in the <10 years and teenage age groups than in other age groups. The results of multiple logistic regression analysis indicated that age <10 years influenced AGEP development. However, a higher percentage of males aged 10–50 years reported AGEP than those aged 60–90 years. Unlike for males, the reporting rate for females aged <10 years was not higher than that for other age groups. The highest reporting rate was observed among females in their 40s. The highest reporting rate among males was observed in those aged <10 years. Some studies have suggested that AGEP occurs more frequently in individuals aged >25 years [18]. Some studies have reported that AGEP is rare in children [2]. Thus, the results of our study differ from those of previous studies involving males with AGEP.
4.2. Time-to-onset analysis
The median time of drug onset was within 5 days for all drugs, except terbinafine hydrochloride, cefdinir, fexofenadine hydrochloride and ceftriaxone sodium hydrate. (Table 5). Thus, the AGEP reported in the JADER database may represent a case of drug sensitization. However, the lack of detailed information precludes further discussion.
4.3. Association of AGEP with prescription drugs
The following drugs have been cited as causative agents of AGEP in other studies: antibiotics (beta-lactams, macrolides, aminoglycosides, quinolones), sulfonamides, antifungals, antimalarials, acetaminophen, NSAIDs, calcium channel blockers, and antiepileptic drugs [2,5,21]. In the EuroSCAR study, the use of pristinamycin (an antistaphylococcal drug used in Europe), aminopenicillin (ampicillin/amoxicillin), quinolone antibiotics, (hydroxy)chloroquine, sulfonamide antibiotics, terbinafine, and diltiazem is strongly associated with AGEP [19]. Many drugs reported in the present study are consistent with those listed above.
Clarithromycin was reported in nearly 17% of males aged <10 years but in ≤10% of males of other generations. Clarithromycin is listed as a suspected drug of AGEP in the Ministry of Health, Labour and Welfare's “Serious Adverse Drug Reaction Manual” [22]. In this study, clarithromycin use was most frequently reported in males aged <10 years. Therefore, when prescribing clarithromycin to male patients aged <10 years, clinicians should consider the risk of AGEP. NSAIDs have been reported as drugs suspected to induce AGEP [2]. Therefore, when aspirin is used to treat Kawasaki disease, caution should be exercised against the development of AGEP. Since clarithromycin is not used for the treatment of Kawasaki disease, there is no relationship between the two, and each is considered an independent risk factor for AGEP. Famotidine is prescribed to prevent gastrointestinal disturbances when prednisolone is used to treat Kawasaki disease. Multiple regression analysis suggested that famotidine use had little effect on the occurrence of AGEP.
Previous studies have shown that certain drugs may be associated with severe drug eruptions and human leukocyte antigen (HLA) gene polymorphisms. Carbamazepine is one such drug that has been associated with HLA-B*15:02, HLA-A*31:01, and HLA-B*15:11 [23]. Although no clear mechanism has been elucidated, it is believed that the interaction of major histocompatibility complex molecules encoded by specific HLA alleles with carbamazepine or carbamazepine-derived molecules causes them to be recognized by T cells as “non-self” and trigger an immune response [24]. A previous study suggested an association between HLA-A*02:07 and clarithromycin-induced skin damage in Chinese patients [25]. This bonding is believed to cause T cells to recognize the drug as “non-self” similar to the case of carbamazepine, triggering an immune response that leads to drug rash. These findings may partially explain the high number of reports describing AGEP in males aged <10 years.
4.4. AGEP and disease in males aged <10 years
Kawasaki disease primarily occurs in infants under 5 years of age, with boys being at 1.5 times higher risk than girls [26]. In this study, aspirin signaling was detected only in RORs among males, and Kawasaki disease was identified as the reason for aspirin use in males aged <10 years. Clear differences in the incidence of Kawasaki disease have been observed among ethnic groups [26]. The incidence of Kawasaki disease is 2.5 times higher in Asians and Pacific Islanders than in Caucasians, with the Japanese population exhibiting the highest global incidence [26]. According to the ARM analysis, the combination of Kawasaki disease, male sex, and age <10 years showed relatively high lift values. Therefore, the incidence of AGEP should be considered when treating males aged <10 years with Kawasaki disease in Japan.
Males under 10 years of age treated with clarithromycin should be cautioned against the development of AGEP. Clinicians should note that aspirin, which is used to treat Kawasaki disease, is a possible causative agent of AGEP. We hope that the data from this study will be useful in the clinical treatment of AGEP.
4.5. Limitations
Analyses using SRSs, such as the JADER database, have several notable limitations. Intrinsic problems with SRS data include overreporting, underreporting, missing data, lack of detailed information on the patient's background, lack of a control population or reference group, and the presence of confounding factors. Considering the inherent problems of all SRSs, it should not be used for true risk assessment [6,27]. To date, no widely accepted method has been established to adjust for covariates in studies using datasets from the SRSs. Multiple logistic regression methods can be used to address confounders that affect the reliability of results. The adjusted ROR provides an approximate indicator of signal strength and can be used to formulate hypotheses. In ARM, the researcher determines the parameters (support, confidence, and maxlen) according to the dataset and research object. In several studies, the values of these parameters varied [10,11]. These parameters are not strict criteria. Further epidemiological studies are required to confirm these findings. The results of the analysis using SRSs should be interpreted with caution, keeping in mind existing clinical results.
In clinical practice, AGEP profiles from real-world post-marketing data have not yet been established. JADER is the primary tool available for pharmacovigilance, as it is the largest and most widely used database globally. We believe the results of the JADER database analysis to be valid because appropriate analytical methods were used.
Ethics approval
Ethical approval and informed consent were not sought for this study because the study was a database-related observational study without directly involving any research subjects. All results were obtained from data openly available online from the PMDA website (www.pmda.go.jp). All data from the JADER database were fully anonymized by the relevant regulatory authority before we accessed them.
Funding statement
This study was partially supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant numbers: 21K06646 and 21K11100). No additional external funding was received for this study.
Data availability statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request. Data used in this study are available from the following links: https://www.pmda.go.jp/safety/info-services/drugs/adr-info/suspected-adr/0003.html.
CRediT authorship contribution statement
Moe Yamashita: Writing – review & editing, Writing – original draft, Visualization, Formal analysis, Data curation. Mika Maezawa: Writing – review & editing, Data curation. Wataru Wakabayashi: Writing – review & editing, Data curation. Sakiko Hirofuji: Writing – review & editing, Data curation. Koumi Miyasaka: Writing – review & editing, Data curation. Nanaka Ichihara: Writing – review & editing, Visualization, Formal analysis. Yuka Nokura: Writing – review & editing, Visualization, Formal analysis. Kensuke Matsui: Writing – review & editing, Visualization, Formal analysis. Satoshi Nakao: Writing – review & editing, Data curation. Hiroyuki Tanaka: Writing – review & editing, Writing – original draft, Visualization, Formal analysis. Mitsuhiro Nakamura: Writing – review & editing, Writing – original draft, Visualization, Formal analysis.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Mitsuhiro Nakamura reports financial support was provided by Japan Society for the Promotion of Science. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e27800.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Feldmeyer L., Heidemeyer K., Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int. J. Mol. Sci. 2016;17(8):1214. doi: 10.3390/ijms17081214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lizardo-Castro G.A., Guillén-Mejía G.W. Acute generalized exanthematous pustulosis. Pustulosis exantemática generalizada aguda. Bol. Med. Hosp. Infant. Mex. 2022;79(4):268–273. doi: 10.24875/BMHIM.21000125. [DOI] [PubMed] [Google Scholar]
- 3.Sidoroff S., Halevy S., Bavinck J.N.B., Vaillant L., Roujeau J.C. Acute generalized exanthematous pustulosis (AGEP) – a clinical reaction pattern. J. Cutan. Pathol. 2001;28(3):113–119. doi: 10.1034/j.1600-0560.2001.028003113.x. [DOI] [PubMed] [Google Scholar]
- 4.Sussman M., Napodano A., Huang S., Are A., Hsu S., Motaparthi K. Pustular psoriasis and acute generalized exanthematous pustulosis. Medicina. 2021;57(10):1004. doi: 10.3390/medicina57101004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De A., Das S., Sarda A., Pal D., Biswas P. Acute generalised exanthematous pustulosis: an update. Indian J. Dermatol. 2018;63(1):22–29. doi: 10.4103/ijd.IJD_581_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Puijenbroek E.P., Bate A., Leufkens H.G., Lindquist M., Orre R., Egberts A.C. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol. Drug Saf. 2002;11(1):3–10. doi: 10.1002/pds.668. [DOI] [PubMed] [Google Scholar]
- 7.van Puijenbroek E.P., Egberts A.C., Heerdink E.R., Leufkens H.G. Detecting drug-drug interactions using a database for spontaneous adverse drug reactions: an example with diuretics and non-steroidal anti-inflammatory drugs. Eur. J. Clin. Pharmacol. 2000;56(9–10):733–738. doi: 10.1007/s002280000215. [DOI] [PubMed] [Google Scholar]
- 8.Egberts A.C., Meyboom R.H., van Puijenbroek E.P. Use of measures of disproportionality in pharmacovigilance: three Dutch examples. Drug Saf. 2002;25(6):453–458. doi: 10.2165/00002018-200225060-00010. [DOI] [PubMed] [Google Scholar]
- 9.Sauzet O., Carvajal A., Escudero A., Molokhia M., Cornelius V.R. Illustration of the weibull shape parameter signal detection tool using electronic healthcare record data. Drug Saf. 2013;36(10):995–1006. doi: 10.1007/s40264-013-0061-7. [DOI] [PubMed] [Google Scholar]
- 10.Harpaz R., Chase H.S., Friedman C. Mining multi-item drug adverse effect associations in spontaneous reporting systems. BMC Bioinf. 2010;11:S7. doi: 10.1186/1471-2105-11-S9-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yildirim P. Association patterns in open data to explore ciprofloxacin adverse events. Appl. Clin. Inf. 2015;6(4):728–747. doi: 10.4338/ACI-2015-06-RA-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatahira H., Hasegawa S., Sasaoka S., Kato Y., Abe J., Motooka Y., et al. Analysis of fall-related adverse events among older adults using the Japanese Adverse Drug Event Report (JADER) database. J Pharm Health Care Sci. 2018;4:32. doi: 10.1186/s40780-018-0129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka M., Hasegawa S., Nakao S., Shimada K., Mukai R., Matsumoto K., et al. Analysis of drug-induced hearing loss by using a spontaneous reporting system database. PLoS One. 2019;14(10) doi: 10.1371/journal.pone.0217951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimada K., Hasegawa S., Nakao S., Mukai R., Matsumoto K., Tanaka M., et al. Adverse event profiles of ifosfamide-induced encephalopathy analyzed using the food and drug administration adverse event reporting system and the Japanese adverse drug event report databases. Cancer Chemother. Pharmacol. 2019;84(5):1097–1105. doi: 10.1007/s00280-019-03949-5. [DOI] [PubMed] [Google Scholar]
- 15.Nakao S., Hasegawa S., Shimada K., Mukai R., Tanaka M., Matsumoto K., et al. Evaluation of anti-infective-related Clostridium difficile-associated colitis using the Japanese adverse drug event report database. Int Med Sci. 2020;17(7):921–930. doi: 10.7150/ijms.43789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimada K., Hasegawa S., Nakao S., Mukai R., Sasaoka A., Ueda N., et al. Adverse reaction profiles of hemorrhagic adverse reactions caused by direct oral anticoagulants analyzed using the Food and Drug Administration Adverse Event Reporting System (FAERS) database and the Japanese Adverse Drug Event Report (JADER) database. Int Med Sci. 2019;16(9):1295–1303. doi: 10.7150/ijms.34629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agrawal R., Srikant R. Fast algorithms for mining association rules in large databases. Proceedings of the 20th International Conference on Very Large Data Bases. 1994;7:478–499. [Google Scholar]
- 18.Vallejo-Yagüe E., Martinez-De la Torre A., Mohamad O.S., Sabu S., Burden A.M. Drug triggers and clinic of acute generalized exanthematous pustulosis (AGEP): a literature case series of 297 patients. J. Clin. Med. 2022;11(2):397. doi: 10.3390/jcm11020397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sidoroff A., Dunant A., Viboud C., Halevy S., Bouwes Bavinck J.N., Naldi L., et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR) Br. J. Dermatol. 2007;157(5):989–996. doi: 10.1111/j.1365-2133.2007.08156.x. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-De la Torre A., van Weenen E., Kraus M., Weiler S., Feuerriegel S., Burden A.M. A network analysis of drug combinations associated with acute generalized exanthematous pustulosis (AGEP) J. Clin. Med. 2021;10(19):4486. doi: 10.3390/jcm10194486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miteva L., Kadurina M., Schwartz R.A. Childhood acute generalized exanthematous pustulosis induced by oral ketoconazole. Acta Dermatovenerol. Croat. 2010;18(4):267–270. [PubMed] [Google Scholar]
- 22.Ministry of Health, Labour and Welfare. Serious adverse drug reaction manual: acute generalized eruptive pustulosis. https://www.pmda.go.jp/files/000145283.pdf.
- 23.Dean L. In: Medical Genetics Summaries. Pratt V.M., Scott S.A., Pirmohamed M., Esquivel B., Kattman B.L., Malheiro A.J., editors. 2018. Carbamazepine therapy and HLA genotype. Bethesda (MD. [Google Scholar]
- 24.Amstutz U., Shear N.H., Rieder M.J., Hwang S., Fung V., Nakamura H., et al. Recommendations for HLA-B*15:02 and HLA-A*31:01 genetic testing to reduce the risk of carbamazepine-induced hypersensitivity reactions. Epilepsia. 2014;55(4):496–506. doi: 10.1111/epi.12564. [DOI] [PubMed] [Google Scholar]
- 25.Chen S.A., Zhang L.R., Yang F.P., et al. HLA-A*02:07 allele associates with clarithromycin-induced cutaneous adverse drug reactions in Chinese patients. Basic Clin. Pharmacol. Toxicol. 2018;123(3):308–313. doi: 10.1111/bcpt.13011. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal S., Agrawal D.K. Kawasaki disease: etiopathogenesis and novel treatment strategies. Expert Rev Clin Immunol. 2017;13(3):247–258. doi: 10.1080/1744666X.2017.1232165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poluzzi E., Raschi E., Piccinni C., Ponti F.D. Data mining techniques in pharmacovigilance: analysis of the publicly accessible FDA Adverse Event Reporting System (AERS) Data Mining Applications in Engineering and Medicine. 2012:265–302. doi: 10.5772/50095. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request. Data used in this study are available from the following links: https://www.pmda.go.jp/safety/info-services/drugs/adr-info/suspected-adr/0003.html.