Abstract
We have tested triple and quadruple combinations of human monoclonal antibodies (MAbs), which are directed against various epitopes on human immunodeficiency virus type 1 (HIV-1) envelope glycoproteins, and a high-titer anti-HIV-1 human immunoglobulin (HIVIG) preparation for their abilities to neutralize a chimeric simian-human immunodeficiency virus (SHIV-vpu+). This virus encodes the HIV-1 strain IIIB env, tat, rev, and vpu genes. The quantitative nature of the Chou-Talalay method (Adv. Enzyme Regul. 22:27–55, 1984) allows ranking of various combinations under identical experimental conditions. Of all triple combinations tested, the most potent neutralization was seen with MAbs 694/98D plus 2F5 plus 2G12 (directed against domains on V3, gp41, and gp120, respectively) as measured by the total MAb concentration required to reach 90% neutralization (90% effective concentration [EC90], 2.0 μg/ml). All triple combinations involving MAbs and/or HIVIG that were tested yielded synergy with combination index values of <1; the dose reduction indices (DRIs) ranged from 3.1 to 26.2 at 90% neutralization. When four MAbs (the previous three plus MAb F105, directed against the CD4 binding site) were combined, higher neutralization potency (EC90, 1.8 μg/ml) and a higher degree of synergy compared to any triple combination were seen. The mean DRIs of the quadruple combination were approximately twice that of the most synergistic triple combination. We conclude that human MAbs targeting different HIV-1 envelope glycoprotein epitopes exhibit strong synergy when used in combination, a fact that could be exploited clinically for passive immunoprophylaxis against HIV-1.
Infection with the human immunodeficiency virus type 1 (HIV-1) will lead to AIDS in most cases if left untreated. During HIV-1 infection, neutralizing antibody responses that are directed against diverse epitopes on the HIV-1 envelope glycoprotein molecules gp120 and gp41 develop. In the initial stages of infection, the antibodies generated are mainly targeted against the linear neutralizing determinants in the third variable loop (V3) of gp120 (42). An early study showed that these antibodies neutralized a limited number of HIV-1 strains only (31), but further reports indicated that some anti-V3 antibodies reacted with less variable regions of V3 and exhibited a broader spectrum of HIV-1 neutralization (20, 23, 36). As HIV-1 infection progresses, antibodies directed against the CD4 binding site (CD4bd) and other complex epitopes develop that recognize discontinuous regions of gp120. These antibodies can neutralize diverse HIV-1 isolates (22, 25, 38, 44). Sera containing high-titer immunoglobulins to HIV type 2 (HIV-2) or simian immunodeficiency virus (SIV) have been used successfully for passive protection of monkeys against challenge by homologous viruses (39).
Extensive work has been performed to develop human monoclonal antibodies (MAbs) directed against divergent HIV-1 envelope antigens. Some human MAbs potently neutralized clinical HIV-1 isolates (4, 12, 20, 32, 35, 48). Combinations of human MAbs with different epitope specificities have shown additive or synergistic HIV-1 neutralization in vitro (2, 27, 45, 47, 50).
Animal models serve an important role in studying HIV pathogenesis and prophylaxis. In terms of clinical signs and laboratory findings, SIV infection of macaques mimics the natural course of HIV-1 infection in humans and thus is considered to be the best animal model (16). Owing to differences in envelope antigens between HIV-1 and SIV, human MAbs to HIV-1 cannot be studied in the SIV-macaque system. To overcome this barrier, SIV–HIV-1 chimeric viruses (SHIVs) were constructed that harbor HIV-1 env, tat, and rev genes in an SIV backbone. SHIVs replicate in macaque peripheral blood mononuclear cells (PBMC) (30, 40), infect monkeys, and, for some SHIV variants, cause lymphopenia or AIDS in infected animals (14, 24, 41).
In our previous report (29), we studied a panel of human MAbs and high-titer human anti-HIV-1 immunoglobulins (HIVIGs) for their abilities to neutralize SHIV-vpu+. The genome of this virus contains the tat, rev, vpu, and env genes of HIV-1 strain IIIB; the remainder of the genome is derived from the SIVmac239 backbone. SHIV-vpu+ grows well in human T-cell lines (CEMx174 and MT-2) and in macaque PBMC (29, 30). Thus, it can serve as an ideal candidate in the macaque model to study passive immunoprophylaxis both in vitro and in vivo. We have shown that several human MAbs neutralized SHIV-vpu+ and that combinations of two effective MAbs or MAb-HIVIG with different epitope specificities could act synergistically on the virus (29). Here, we report the interactions of human MAbs or HIVIG when used in triple and quadruple combinations against SHIV-vpu+. The most potent virus neutralization and the highest degree of synergy were seen with a quadruple combination of human MAbs.
MATERIALS AND METHODS
Human MAbs and HIVIG.
In this study, we tested the following human MAbs: F105, anti-CD4bd (37); 694/98D, anti-V3 domain (20); 2F5, anti-gp41 (35); and 2G12, directed against a complex gp120 epitope (49). All MAbs are of the immunoglobulin G1 (IgG1) subclass, including 2F5 which had been engineered to contain the constant region of IgG1 instead of that of IgG3. HIVIG2, produced by Abbott Laboratories (Abbott Park, Chicago, Ill.) was obtained from the National Institute of Allergy and Infectious Diseases. A human IgG MAb, 860-30D, with irrelevant specificity (860-30D is directed against human cytomegalovirus and shows no cross-reactivity to HIV-1 or SIV) was used as a negative, isotype-specific control as single agent. No neutralization of SHIV-vpu+ was seen (not shown).
Preparation of SHIV-vpu+ for neutralization.
An SHIV-vpu+ stock was prepared in macaque PBMC (New England Regional Primate Research Center, Southboro, Mass.) as described elsewhere (29). The virus titer was 8,185 50% tissue culture infectious doses/ml.
Virus neutralization assay.
We used an MT-2 cell viability assay (33) to measure the antibodies’ capacities to neutralize SHIV-vpu+ as described elsewhere (29). Briefly, antibodies at various dilutions (ranging from 8 to 16 μg/ml at the highest concentration), and combinations in triplicate wells were incubated with SHIV-vpu+ at 37°C for 45 min. MT-2 cells were then added to the mixture. After incubation at 37°C with 5% CO2 for 7 days, the degree of neutral red absorption, an indication of cell viability, was measured spectrophotometrically. The mean of two or three independent experiments was used as the final result.
The neutralization profiles of some MAbs in PBMC from specific-pathogen-free (SPF) rhesus macaques (bred at the Yerkes Regional Primate Research Center, Atlanta, Ga.) were also tested. Reverse transcriptase activity (19) in cell culture supernatants was measured to define the percent neutralization.
Determination of synergy and DRI.
The complex interactions of MAbs and HIVIGs in triple and quadruple combinations were analyzed by computer software in a stepwise fashion, beginning with single agent dose-response curves, followed by dose-response curves involving combinations of two antibodies. Finally, the triple combination was calculated as a combination of antibody no. 1 in a two-component combination with antibody no. 2 plus antibody no. 3. A further step according to the same principle was used to calculate the quadruple combination of MAbs. The analytical method of Chou-Talalay (7, 8) yields two parameters that describe the interactions among antibodies in a given combination: the combination index (CI) and the dose reduction index (DRI). A CI of <1 indicates synergism, a CI of 1 or close to 1 indicates additive effects, and a CI of >1 indicates antagonism.
DRI measures by what factor the dose of each drug in a combination may be reduced at a given effect level compared with the dose when each drug is used alone (10, 11). DRI may be influenced by the combination ratio and the number of drugs. Toxicity toward the host may be avoided or reduced when the dose is reduced.
The advantage of this method is that it takes into account not only the potency (median effect dose values [Dm] or antibody concentration at 50% neutralization [EC50]), but also the shape (sigmoidicity) of the dose effect curve, based on the median effect equation of Chou (10):
 |
1 |
Rearrangement of equation 1 gives
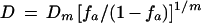 |
2 |
The logarithmic form of equation 1 gives
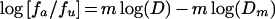 |
3 |
where D is dose (concentration of antibody), and fa and fu are the fractions of SHIV-vpu+ affected (neutralized) and unaffected, respectively. Equation 2 allows the calculation of D for x% neutralization (Dx) when the Dm and m values are determined from the median effect plot (10).
A plot of x = log (D) versus y = log [fa/(1 − fa)] (i.e., the median effect plot) yields the Dm values as x intercept antilog and the m value as the slope. Computer software developed by Chou (9) and Chou and Hayball (11), based on the median effect equation and the classic isobologram of Chou-Talalay (8), was used for automated analysis. Thus
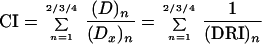 |
4 |
and the specified equation 2 gives
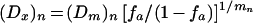 |
5 |
The correlation coefficient (r) was also obtained from the dose effect analysis for the antibody combinations by using Calcusyn (11). Based on the experimental data, r values close to 1 indicate the conformity of the assays.
RESULTS
SHIV-vpu+ neutralization in MT-2 cells by triple combinations of MAbs.
We wished to extend the scope of our previous studies involving combinations of two antibodies (29) to test if triple or quadruple combinations of these MAbs and HIVIG could further potentiate virus neutralization and increase synergy. Four human MAbs with different epitope specificities were used in this experiment. The results of individual triple combinations are shown in Table 1. All four combinations showed significant synergy as judged by the low CI and high DRI values. The most potent neutralization was seen with the combination 694/98D plus 2F5 plus 2G12 (Table 1). At 90% neutralization, the total amount of MAbs required was only 2.0 μg/ml and the CI reached 0.4. The combination of F105 plus 694/98D plus 2F5 reached the highest degree of synergy, as evidenced by a CI90 of 0.3. These two combinations also yielded remarkable DRIs. The average DRI90s were 13.1 and 14.8, respectively (ranging from 4.6 to 26.2 [Table 1]). The other triple MAb combinations, namely, F105 plus 694/98D plus 2G12 and F105 plus 2F5 plus 2G12, exhibited similar synergy with lower potency. At 90% neutralization, the CIs were 0.4 and 0.5 and the mean DRIs were 8.9 and 14.8, respectively (Table 1).
TABLE 1.
Synergy of double and triple combinations of human IgG MAbs 694/98D, 2F5, 2G12, F105, and HIVIG2 for SHIV-vpu+ neutralization in MT-2 cells
| Single antibody (specificity) or antibody combination (ratio) | Concn (μg/ml)a for neutralization at:
|
CI90b | DRIc at EC90d
|
Mean DRI90 | |||
|---|---|---|---|---|---|---|---|
| 50% | 90% | MAb A | MAb B | MAb C | |||
| Single agents | |||||||
| 694/98D (anti-V3) | 1.7–1.9 | NAe | |||||
| 2F5 (anti-gp41) | 0.5–0.6 | 3.5–3.9 | |||||
| 2G12 (anti-gp120) | 0.4–0.5 | 1.6–3.2 | |||||
| F105 (anti-CD4bd) | 1.3–1.5 | NA | |||||
| HIVIG2 (multiple) | 33.6 | NA | |||||
| Combinations of two MAbs | |||||||
| 694/98D + 2F5 (2:1) | 0.5–0.7 | 2.3–3.0 | 0.3 | 12.3–14.3 | 4.0–4.7 | ||
| 694/98D + 2G12 (2:1) | 0.7 | 2.9–3.2 | 0.5–0.7 | 12.3 | 1.5–2.1 | ||
| 2F5 + 2G12 (1:1) | 0.3–0.4 | 1.7–2.1 | 0.7 | 2.6–3.9 | 2.1–2.7 | ||
| F105 + 2F5 (2:1) | 0.6–0.8 | 3.1–3.8 | 0.4–0.6 | 9.1–11.4 | 2.1–3.8 | ||
| F105 + 2G12 (2:1) | 0.6–0.7 | 2.9–3.2 | 0.6–0.7 | 8.6–9.4 | 1.7–2.2 | ||
| F105 + 694/98D (1:1) | 1.0–1.3 | 6.4–8.2 | 0.3–0.5 | 4.2–5.9 | 3.6–8.1 | ||
| Combination of MAbs and HIVIG2 | |||||||
| F105 + HIVIG2 (1:25) | 12.1 | 140.1 | 0.7 | 3.6 | 3.1 | ||
| 694/98D + HIVIG2 (1:25) | 13.4 | 84.1 | 0.4 | 5.1 | 5.2 | ||
| F105 + 694/98D + HIVIG2 (1:1:25) | 8.2 | 67.0 | 0.4 | 7.8 | 6.7 | 6.7 | 7.1 |
| Combinations of three MAbs | |||||||
| 694/98D + 2F5 + 2G12 (2:1:1) | 0.4 | 2.0 | 0.4 | 26.2 | 8.6 | 4.6 | 13.1 |
| F105 + 2F5 + 2G12 (2:1:1) | 0.5 | 2.3 | 0.5 | 18.4 | 4.2 | 4.2 | 8.9 |
| F105 + 694/98D + 2F5 (2:2:1) | 0.7 | 3.0 | 0.3 | 19.1 | 18.9 | 6.3 | 14.8 |
| F105 + 694/98D + 2G12 (2:2:1) | 0.7 | 2.8 | 0.4 | 15.9 | 25.4 | 3.1 | 14.8 |
The dose for neutralization by combination of two or three antibodies was the sum of that for each antibody used in the combination regimen.
Synergy is indicated when the CI is <1.0. CIs were calculated based on the classical isobol equation (8, 10).
DRIs were measured by comparing the doses required to reach a given degree of virus neutralization (i.e., 90%) when the antibody was used alone and in combination with other antibodies.
Antibodies A, B, and C indicate the antibodies in first, second, or third position, respectively.
NA, not achieved at the highest concentration tested.
SHIV neutralization in MT-2 cells by a triple combination of MAbs and HIVIG2.
The combination of F105 plus 694/98D plus HIVIG2 resulted in synergy (Table 1). HIVIG2, as a single agent, was significantly less potent than any neutralizing MAb tested. Consequently, a higher dose of this polyclonal preparation was needed in the combination, and, as a result, the total amount of antibodies required to produce significant neutralization was significantly higher compared to other triple MAb combinations. In this combination, 67 μg of antibodies per ml was needed to yield 90% neutralization, whereas in other MAb combinations, a mean total MAb amount of only 2.5 μg/ml was needed to yield the same degree of neutralization.
Neutralization of SHIV-vpu+ in macaque PBMC.
Since we intend to use the MAb combination regimens in vivo in macaques, we tested one MAb combination (F105 plus 2F5 plus 2G12) in PBMC of an SPF rhesus macaque. The results are shown in Table 2. Low CIs were observed, as shown previously for MT-2 cells. At 90% viral neutralization, the mean DRI was 10 (ranging from 4.5 to 20.3). The results obtained for macaque PBMC are consistent with those obtained for MT-2 cells (Table 1).
TABLE 2.
Synergy of human IgG MAbs F105, 2F5, and 2G12 for SHIV-vpu+ neutralizationa in macaque PBMC
| Single antibody (specificity) or combination (ratio) | Concn (μg/ml) for neutralization at:
|
CL90b | DRI at EC90c
|
|||
|---|---|---|---|---|---|---|
| 50% | 90% | MAb A | MAb B | MAb C | ||
| F105 (anti-CD4bd) | 1.9 | NAd | ||||
| 2F5 (anti-gp41) | 0.6 | 2.9 | ||||
| 2G12 (anti-gp120) | 0.5 | 2.5 | ||||
| F105 + 2F5 (2:1) | 1.0 | 4.3 | 0.6 | 7.7 | 2.0 | |
| F105 + 2G12 (2:1) | 0.6 | 3.1 | 0.5 | 10.5 | 2.3 | |
| 2F5 + 2G12 (1:1) | 0.4 | 2.0 | 0.8 | 2.7 | 2.3 | |
| F105 + 2F5 + 2G12 (2:1:1) | 0.4 | 2.1 | 0.5 | 20.3 | 5.3 | 4.5 |
The dose for neutralization by combination of two or three antibodies was the sum of that for each antibody used in the combination regimen.
Synergy is indicated when the CI is <1.0. CIs were calculated based on the classical isobol equation (8, 10).
DRIs were measured by comparing the doses required to reach a given degree of virus neutralization (i.e., 90%) when the antibody was used alone and in combination with other antibodies. Antibodies A, B, and C indicate the antibodies in first, second, and third positions, respectively.
NA, not achieved at the highest concentration tested.
SHIV neutralization in MT-2 cells by a quadruple combination of MAbs.
Since any of the four MAbs tested in various triple combinations acted synergistically, we postulated that a combination involving all four MAbs would yield even higher synergy. The results of the F105 plus 694/98D plus 2F5 plus 2G12 combination are shown in Table 3. This quadruple combination yielded CIs even lower than those obtained from the triple combinations examined simultaneously. The CI90 reached 0.2, and the mean DRI was 27.6 (ranging from 16 to 39.9), which is considerably better than the values obtained from any of the triple combinations tested; the average DRI value of all of the MAbs in the four triple combinations tested was 11.7 in this assay (Table 3).
TABLE 3.
Synergy of human IgG MAbs F105, 694/98D, 2F5, and 2G12 for SHIV-vpu+ neutralizationa in MT-2 cells
| Single MAb (specificity) or combination (ratio) | Concn (μg/ml) for neutralization at:
|
CI90b | DRI at EC90c
|
||||
|---|---|---|---|---|---|---|---|
| 50% | 90% | MAb A | MAb B | MAb C | MAb D | ||
| F105 (anti-CD4bd) | 1.3 | NAd | |||||
| 694/98D (anti-V3) | 1.8 | NA | |||||
| 2F5 (anti-gp41) | 0.6 | 5.1 | |||||
| 2G12 (anti-gp120) | 0.5 | 4.9 | |||||
| F105 + 694/98D (1:1) | 1.4 | 8.9 | 0.4 | 5.2 | 5.5 | ||
| F105 + 2F5 (2:1) | 0.8 | 5.3 | 0.5 | 6.5 | 2.9 | ||
| F105 + 2G12 (2:1) | 0.8 | 4.7 | 0.5 | 7.4 | 3.1 | ||
| 694/98D + 2F5 (2:1) | 0.7 | 3.7 | 0.4 | 9.9 | 4.1 | ||
| 694/98D + 2G12 (2:1) | 0.8 | 4.0 | 0.4 | 9.1 | 3.7 | ||
| 2F5 + 2G12 (1:1) | 0.4 | 1.8 | 0.4 | 5.6 | 5.5 | ||
| F105 + 694/98D + 2F5 (2:2:1) | 0.8 | 3.6 | 0.3 | 15.4 | 16.3 | 6.7 | |
| F105 + 694/98D + 2G12 (2:2:1) | 0.9 | 3.5 | 0.3 | 16.7 | 17.6 | 7.1 | |
| F105 + 2F5 + 2G12 (2:1:1) | 0.6 | 2.8 | 0.4 | 16.1 | 7.0 | 6.8 | |
| 694/98D + 2F5 + 2G12 (2:1:1) | 0.5 | 2.7 | 0.3 | 17.2 | 7.1 | 6.9 | |
| F105 + 694/98D + 2F5 + 2G12 (2:2:1:1) | 0.5 | 1.8 | 0.2 | 37.9 | 39.9 | 16.5 | 16 |
The dose for neutralization by combination of two, three, or four antibodies was that for the sum of each antibody used in the combination regimen.
Synergy is indicated when the CI is <1.0. CIs were calculated based on the classical isobol equation (8, 10).
DRIs were measured by comparing the doses required to reach a given degree of virus neutralization (i.e., 90%) when the antibody was used alone and in combination with other antibodies. Antibodies A, B, C, and D indicate the antibodies in first, second, third, and fourth positions, respectively.
NA, not achieved at the highest concentration tested.
The mean r value for all of the triple and quadruple combinations was 0.983, which confirmed the consistency and reproducibility of the combination tests.
DISCUSSION
In the present study, we have shown that combinations of three or four effective human MAbs or HIVIG acted synergistically and completely neutralized SHIV-vpu+, even in rhesus macaque PBMC. For the first time, the quantitative interaction of MAbs in a quadruple combination has been calculated; the latter was the most potent among all regimens tested and revealed the highest degree of synergy.
The MAbs chosen for this experiment have different characteristics. MAb F105 is directed against the CD4bd (37) and neutralized laboratory and primary isolates of HIV-1 (38). The lack of native antibodies which shared the binding epitopes with F105 in the serum of HIV-infected patients correlated with disease progression (5). The anti-V3 MAb 694/98D not only neutralized several laboratory isolates of HIV-1 effectively (20, 21) but also activated complement (44). MAb 2G12 defines a distinctive but discontinuous neutralization epitope on HIV-1 gp120. It recognizes domains in the C2 and C3 regions near the base of the V3 loop and domains in the V4 loop and C4 region (49). In cross-competition experiments, 2G12 neither cross-blocked nor was cross-blocked by 45 other MAbs which recognized continuous or discontinuous gp120 epitopes, including the V2 and V3 loops; the C1, C4, and C5 regions; discontinuous epitopes overlapping the CD4bd; and CD4-induced epitopes (34). Moreover, some MAbs against V3, C4, and CD4bd-related epitopes modestly increased the binding of 2G12 to gp120 (34). Another MAb tested in this series of experiments was 2F5, which interacts with the amino acid sequence ELDKWA on the ectodomain of gp41 (35). Since this sequence is present in 72% of HIV-1 isolates of different clades, 2F5 is broadly reactive against various HIV-1 isolates (35). Both 2G12 and 2F5 have shown a potent ability to neutralize divergent clinical isolates of HIV-1 in vitro (12, 48). MAb 2F5 delayed the appearance of viremia and reduced virus load when given passively to chimpanzees before HIV-1 challenge (13).
Combinations of two human MAbs with different epitope specificities have shown additive or synergistic effects on laboratory-adapted as well as clinical isolates of HIV-1 (3, 27, 29, 45, 47, 50). Combining anti-CD4bd MAbs with anti-V3 MAbs synergistically neutralized HIV-1 (27, 45, 47). A recent study also reported synergy in the triple combination of anti-V2, anti-V3, and anti-CD4bd MAbs (50). These results all indicate that MAbs with different epitope specificities and at appropriate concentrations act synergistically on HIV-1, leading to more potent neutralization. The results of our study are in accordance with these findings. All triple MAb combinations reached significant synergy and DRIs. The quadruple MAb combination showed even better CIs and DRIs.
In comparison to the potency of the neutralizing human MAbs tested, the performance of HIVIG2 was somewhat disappointing in our assays, which could be explained by the relatively low abundance of highly neutralizing antibodies, the possible presence of infection-enhancing antibodies, or the presence of antibodies that interfere with neutralizing antibodies. Given the polyclonal nature of HIVIG2, we can only measure the overall neutralization capacity but not that of individual components contained in this mixture. Our strategy of combining human MAbs with known neutralization profiles is designed to avoid the problem of antibody interference and to potentiate virus neutralization.
The mechanism for the synergy in MAb-HIVIG combinations has not been fully elucidated. However, MAb binding studies have shown that conformational changes of the antigens during antigen-antibody interaction may make antigenic domains on the envelope glycoprotein more accessible to MAbs, thus facilitating MAb-antigen interaction (6, 47, 49, 51). We postulate that the antibody-virus interactions in our neutralization experiments also induced conformational changes in the envelope glycoprotein of SHIV-vpu+, which may explain the synergy observed.
Neutralizing human MAbs against HIV-1 have been studied extensively, given their potential application for passive prevention of infection in neonates born to HIV-1-positive mothers and healthcare workers or researchers accidentally exposed to the virus. Some MAbs with potent neutralizing effects have been tested extensively in vitro (4, 12, 46, 48, 49) and could be candidates for passive immunoprophylaxis.
Passive immunoprophylaxis has been tested in several animal models, with conflicting outcomes. Some studies have yielded promising results, whereas others failed to show protection against virus challenge. Sera with high titers of antibodies against the V3 loop of HIV-2 or SIV protected monkeys against infection after challenge with HIV-2 or SIVsm, respectively (39). When an anti-HIV-1 V3 MAb was given to chimpanzees before or shortly after challenge with HIV-1, the animals were protected (15). Administration of serum from a cynomolgus monkey, which had been immunized with inactivated whole HIV-2 and resisted homologous virus challenge, protected some but not all monkeys against challenge with HIV-2 or SIVsm (1), and three of four monkeys given SIVsm antiserum remained uninfected after challenge with homologous cell-free virus (1). Lewis et al. (28) tested passive prophylaxis with inactivated plasma derived from SIV-infected rhesus macaques or from animals given a peptide vaccine; half of the recipients of the SIV-peptide vaccine plasma and 82% of the animals treated with plasma from infected monkeys did not seroconvert after SIV challenge. When the protected monkeys were challenged a second time, a third of them remained uninfected (28). Gardner et al. (17) reported the success of passive immunization with plasma from a monkey protected after vaccination with inactivated whole SIVmac. The recipient monkeys were either completely or partially protected against intravenous challenge with 10 50% animal infectious doses of homologous cell-free virus. In contrast to these studies, inactivated plasma or purified immunoglobulins derived from monkeys chronically infected with SIVmac not only failed to protect but may have even facilitated infection and accelerated disease progression (18). Passive administration of MAb 2F5, which is directed against an epitope on gp41, failed to protect recipient chimpanzees against challenge with primary HIV-1 strains (13). However, the peak viral RNA production was delayed in one recipient and remained significantly lower in a second one compared to controls, indicating that passive immunoprophylaxis may have altered the course of viremia in these chimpanzees (13). Failure of passive immunoprophylaxis with either neutralizing MAbs or sera against SIV challenge was reported in a number of studies (26, 43). Our strategy to use combinations of effective neutralizing MAbs may eliminate the unpredictable influence of infection-enhancing antibodies of polyclonal sera from immunized or infected individuals and result in synergistic virus neutralization.
Our data and those of others raise the hope that maternal-infant transmission of HIV-1 could be blocked with synergistic combinations of effective human MAbs. Indeed, all antibodies tested in our series of experiments are of the IgG isotype and are expected to cross the placenta. Theoretically, the neutralizing MAb levels could be achieved in the fetus by passive immune therapy of the pregnant woman. We plan to test this hypothesis in our SHIV-rhesus macaque model of neonatal mucosal challenge, in which we have not only achieved reproducible oral infection but also observed disease (unpublished data).
ACKNOWLEDGMENTS
We thank Chris Gallegos for preparation of the manuscript.
This work was supported in part by NIH grants RO1-AI34266 to R.M.R., RO1-AI26926 to M.R.P. and L.C., RO1-AI36085 to S.Z.-P., and 1KO8-AI01327 to T.W.B. and was also supported by the Center for AIDS Research (CFAR) Core grants no. IP30 28691, awarded to the Dana-Farber Cancer Institute, and IP30 27742 TO S.Z-P.
REFERENCES
- 1.Biberfeld G, Putkonen P, Thorstensson R, Norrby E. Prevention of HIV-2 and SIVsm infection in cynomolgus monkeys by active or passive immunization. AIDS Res Hum Retroviruses. 1992;8:1511–1513. doi: 10.1089/aid.1992.8.1511. [DOI] [PubMed] [Google Scholar]
- 2.Buchbinder A, Karwowska S, Gorny M K, Burda S T, Zolla-Pazner S. Synergy between human monoclonal antibodies to HIV extends their effective biologic activity against homologous and divergent strains. AIDS Res Hum Retroviruses. 1992;8:425–427. doi: 10.1089/aid.1992.8.425. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 3.Burkly L C, Mulrey N, Blumenthal R, Dimitrov D S. Synergistic inhibition of human immunodeficiency virus type 1 envelope glycoprotein-mediated cell fusion and infection by an antibody to CD4 domain 2 in combination with anti-gp120 antibodies. J Virol. 1995;69:4267–4273. doi: 10.1128/jvi.69.7.4267-4273.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton D R, Pyati J, Koduri R, Sharp S J, Thornton G B, Parren P W H I, Sawyer L S W, Hendry R M, Dunlop N, Nara P L, Lamacchia M, Garratty E, Stiehm E R, Bryson Y J, Cao Y, Moore J P, Ho D D, Barbas C F., III Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 5.Cavacini L A, Emes C I, Power J, Unerdahl J, Goldstein R, Mayer K K, Posner M R. Loss of serum antibodies to a conformational epitope of HIV-1/gp 120 identified by a human monoclonal antibody is associated with disease progression. J Acquired Immune Defic Syndr. 1993;6:1093–1102. [PubMed] [Google Scholar]
- 6.Cavacini L A, Emes C L, Power J, Buchbinder A, Zolla-Pazner S, Posner M R. Human monoclonal antibodies to the V3 loop of HIV-1 gp120 mediate variable and distinct effects on binding and viral neutralization by a human monoclonal antibody to the CD4 binding site. J Acquired Immune Defic Syndr. 1993;6:6353–6358. [PubMed] [Google Scholar]
- 7.Chou T-C, Talalay P. Generalized equations for the analysis of inhibitors of Michaelis-Menten and higher order kinetic systems with two or more mutually exclusive and nonexclusive inhibitors. Eur J Biochem. 1981;115:207–216. doi: 10.1111/j.1432-1033.1981.tb06218.x. [DOI] [PubMed] [Google Scholar]
- 8.Chou T-C, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 9.Chou J, Chou T-C. Dose-effect analysis with microcomputers: quantitation of ED50, LD50, synergism, antagonism, low-dose risk, receptor-ligand binding and enzyme kinetics. Manual and software. Cambridge, United Kingdom: Biosoft; 1987. [Google Scholar]
- 10.Chou T-C. The median-effect principle and the combination index for quantitation of synergism and antagonism. In: Chou T-C, Rideout D C, editors. Synergism and antagonism in chemotherapy. San Diego, Calif: Academic Press; 1991. pp. 61–102. [Google Scholar]
- 11.Chou T-C, Hayball M. CalcuSyn for Windows. Multiple-drug dose-effect analyzer and manual. Cambridge, United Kingdom: Biosoft; 1996. [Google Scholar]
- 12.Conley A J, Kessler II J A, Boots L J, Tung J-S, Arnold B A, Keller P M, Shaw A R, Emini E A. Neutralization of divergent human immunodeficiency virus type 1 variants and primary isolates by IAM-41-2F5, an anti-gp41 human monoclonal antibody. Proc Natl Acad Sci USA. 1994;91:3348–3352. doi: 10.1073/pnas.91.8.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conley A J, Kessler II J A, Boots L J, Mckenna P M, Schleif W A, Emini E A, III Mark G E, Katinger H, Cobb E K, Lunceford S M, Rouse S R, Murthy K K. The consequence of passive administration of an anti-human immunodeficiency virus type 1 neutralizing monoclonal antibody before challenge of chimpanzees with a primary virus isolate. J Virol. 1996;70:6751–6758. doi: 10.1128/jvi.70.10.6751-6758.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunn C S, Beyer C, Kieny M P, Gloeckler L, Schmitt D, Gut J P, Kirn A, Aubertin A M. High viral load and CD4 lymphopenia in rhesus and cynomolgus macaques infected by a chimeric primate lentivirus constructed using the env, rev, tat, and vpu genes from HIV-1 Lai. Virology. 1996;223:351–361. doi: 10.1006/viro.1996.0486. [DOI] [PubMed] [Google Scholar]
- 15.Emini E A, Schleif W A, Nunberg J H, Conley A J, Eda Y, Tokiyoshi S, Putney S D, Matsushita S, Cobb K E, Jett C M, Eichberg J W, Murthy K K. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature. 1992;355:728–730. doi: 10.1038/355728a0. [DOI] [PubMed] [Google Scholar]
- 16.Franchini G, Gurgo C, Guo H G. Sequence of simian immunodeficiency virus and its relationship to the human immunodeficiency virus. Nature. 1987;328:539–543. doi: 10.1038/328539a0. [DOI] [PubMed] [Google Scholar]
- 17.Gardner M, Rosenthal A, Jennings M, Yee J A, Antipa L, Mackenzie M. Passive immunization of macaques against SIV infection. J Med Primatol. 1994;23:164–174. doi: 10.1111/j.1600-0684.1994.tb00118.x. [DOI] [PubMed] [Google Scholar]
- 18.Gardner M, Rosenthal A, Jennings M, Yee J A, Antipa L, Robinson E., Jr Passive immunization of rhesus macaques against SIV infection and disease. AIDS Res Hum Retroviruses. 1995;11:843–854. doi: 10.1089/aid.1995.11.843. [DOI] [PubMed] [Google Scholar]
- 19.Goff S, Traktman P, Baltimore D. Isolation and properties of Moloney murine leukemia virus: use of a rapid assay for release of virion reverse transcriptase. J Virol. 1981;38:239–248. doi: 10.1128/jvi.38.1.239-248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorny M K, Conley A J, Karwowska S, Buchbinder A, Xu J Y, Emini E A, Koenig S, Zolla-Pazner S. Neutralization of diverse HIV-1 variants by an anti-V3 human monoclonal antibody. J Virol. 1992;66:7538–7542. doi: 10.1128/jvi.66.12.7538-7542.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorny M K, Xu J Y, Karwowska S, Buchbinder A, Zolla-Pazner S. Repertoire of neutralizing human monoclonal antibodies specific for the V3 domain of HIV-1 gp120. J Immunol. 1993;150:635–643. [PubMed] [Google Scholar]
- 22.Ho D D, McKeating J A, Li X L, Moudgil T, Daar E S, Sun N C, Robinson J E. Conformational epitope on gp120 important in CD4 binding and human immunodeficiency virus type 1 neutralization identified by a human monoclonal antibody. J Virol. 1991;65:489–493. doi: 10.1128/jvi.65.1.489-493.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jahaverian K, Langlois A J, LaRosa F J, Profy A T, Bolognesi D P, Herlihy W C, Putney S D, Matthews T J. Broadly neutralizing antibodies elicited by the hypervariable neutralizing determinant of HIV-1. Science. 1990;250:1590–1593. doi: 10.1126/science.1703322. [DOI] [PubMed] [Google Scholar]
- 24.Joag S V, Foresman L, Stephens E B, Zhao L-J, Adany I, Pinson D M, McClure H M, Narayan O. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed macaques. J Virol. 1996;70:3189–3197. doi: 10.1128/jvi.70.5.3189-3197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karwowska S, Gorny M K, Buchbinder A, Gianakakos V, Williams C, Fuerst T, Zolla-Pazner S. Production of human monoclonal antibodies specific for conformational and linear non-V3 epitopes of gp120. AIDS Res Hum Retroviruses. 1992;8:1099–1106. doi: 10.1089/aid.1992.8.1099. [DOI] [PubMed] [Google Scholar]
- 26.Kent K A, Kitchin P, Mills K H, Page M, Taffs F, Corcoran T, Silvera P, Flanagan B, Powell C, Rose J, Ling C, Aubertin A M, Stott E J. Passive immunization of cynomolgus macaques with immune sera or a pool of neutralizing monoclonal antibodies failed to protect against challenge with SIVmac251. AIDS Res Hum Retroviruses. 1994;10:189–194. doi: 10.1089/aid.1994.10.189. [DOI] [PubMed] [Google Scholar]
- 27.Laal S, Burda S, Gorny M K, Karwowska S, Buchbinder A, Zolla-Pazner S. Synergistic neutralization of human immunodeficiency virus type 1 by combinations of human monoclonal antibodies. J Virol. 1994;68:4001–4008. doi: 10.1128/jvi.68.6.4001-4008.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis M G, Elkins W R, McCutchan F E, Benverniste R E, Lai C Y, Montefiori D C, Burke D S, Eddy G A, Shafferman A. Passively transferred antibodies directed against conserved regions of SIV envelope protect macaques from SIV infection. Vaccine. 1993;11:1347–1355. doi: 10.1016/0264-410x(93)90106-8. [DOI] [PubMed] [Google Scholar]
- 29.Li A, Baba T W, Sodroski J, Zolla-Pazner S, Gorny M K, Robinson J, Posner R, Katinger H, Barbas III C F, Burton D R, Chou T-C, Ruprecht R M. Synergistic neutralization of a chimeric SIV/HIV-1 virus with combinations of human anti-HIV-1 envelope monoclonal antibodies or hyper immune globulins. AIDS Res Hum Retroviruses. 1997;13:647–656. doi: 10.1089/aid.1997.13.647. [DOI] [PubMed] [Google Scholar]
- 30.Lu Y, Salvato M S, Pauza C D, Li J T, Sodroski J, Manson K, Wyand M, Letvin N L, Jenkins S, Touzjian N, Chutkowski C, Kushner N, LeFaile M, Payne L G, Roberts B. Utility of SHIV for testing HIV-1 vaccine candidates in macaques. J Acquired Immune Defic Synd Hum Retrovirol. 1996;12:99–106. doi: 10.1097/00042560-199606010-00001. [DOI] [PubMed] [Google Scholar]
- 31.Matthews T, Langlois A, Robey W, Chang N, Gallo R, Fischinger R, Bolognesi D. Restricted neutralization of divergent human T-lymphotropic virus type III isolated by antibodies to the major envelope glycoprotein. Proc Natl Acad Sci USA. 1986;83:9709–9713. doi: 10.1073/pnas.83.24.9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mioe, C. E., S. Su, P. Chigurupati, S. Burda, C. Williams, M. K. Gorny, and S. Zolla-Pazner. Neutralization of HIV-1 primary isolates by polyclonal and monoclonal human antibodies. Int. Immunol., in press. [DOI] [PubMed]
- 33.Montefiori D C, Robinson W B, Jr, Schuffman S S. Evaluation of antiviral drugs and neutralizing antibodies to human immunodeficiency virus by a rapid and sensitive microtiter infection assay. J Clin Microbiol. 1988;26:231–235. doi: 10.1128/jcm.26.2.231-235.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore J P, Sodroski J. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J Virol. 1996;70:1863–1872. doi: 10.1128/jvi.70.3.1863-1872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muster T, Guinea R, Trkola A, Purtscher M, Klima A, Steindl F, Palese P, Katinger H. Cross-neutralizing activity against divergent human immunodeficiency virus type 1 isolates induced by the gp41 sequence ELDKEAS. J Virol. 1994;68:4031–4034. doi: 10.1128/jvi.68.6.4031-4034.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohno T, Terada M, Yoneda Y, Shea K W, Chambers R F, Stroka D M, Nakamura M, Kufe D W. A broadly neutralizing monoclonal antibody that recognizes the V3 region of human immunodeficiency virus type 1 glycoprotein gp120. Proc Natl Acad Sci USA. 1991;88:10726–10729. doi: 10.1073/pnas.88.23.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Posner M R, Hideshina T, Cannon T, Mukherjee M, Mayer K H, Byrn R A. An IgG human monoclonal antibody that reacts with HIV-1/gp120, inhibits virus binding to cells, and neutralizes infection. J Immunol. 1991;146:4325–4332. [PubMed] [Google Scholar]
- 38.Posner M R, Cavacini L A, Emes C L, Power J, Byrn R A. Neutralization of HIV-1 by F105, a human monoclonal antibody to the CD4 binding site of gp120. J Acquired Immune Defic Syndr. 1993;6:7–14. [PubMed] [Google Scholar]
- 39.Putkonen P, Thorstensson R, Ghavamzadeh L, Albert J, Hild K, Biberfeld G, Norrby E. Prevention of HIV-2 and SIVsm infection by passive immunization in cynomolgus monkeys. Nature. 1991;352:436–438. doi: 10.1038/352436a0. [DOI] [PubMed] [Google Scholar]
- 40.Reimann K A, Li J T, Voss G, Lekutis C, Tenner-Racz K, Racz P, Lin W, Montefiori D C, Lee-Parritz D E, Lu Y, Collman R G, Sodroski J, Letvin N L. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J Virol. 1996;70:3198–3206. doi: 10.1128/jvi.70.5.3198-3206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reimann K A, Li J T, Veazey R, Halloran M, Park I W, Karlsson G B, Sodroski J, Letvin N L. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996;70:6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rusche J R, Javaherian K, McDanal C, Petro J, Lynn D L, Grimaila R, Langlois A, Gallo R C, Arthur L O, Fischinger P J, Bolognesi D P, Putney S D, Matthews T J. Antibodies that inhibit fusion of human immunodeficiency virus-infected cells bind a 24-amino-acid sequence of the viral envelope gp120. Proc Natl Acad Sci USA. 1988;85:3198–3202. doi: 10.1073/pnas.85.9.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siegel F, Kurth R, Norley S. Neither whole inactivated virus immunogen nor passive immunoglobulin transfer protects against SIVagm infection in the African green monkey natural host. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;8:217–226. doi: 10.1097/00042560-199503010-00001. [DOI] [PubMed] [Google Scholar]
- 44.Spear G T, Takefman D M, Sullivan B L, Landay A L, Zollar-Pazner S. Complement activation by human monoclonal antibodies to human immunodeficiency virus. J Virol. 1993;67:53–59. doi: 10.1128/jvi.67.1.53-59.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thali M, Furman C, Wahren B, Posner M, Ho D D, Robinson J, Sodroski J. Cooperativity of neutralizing antibodies directed against the V3 and CD4 binding regions of the human immunodeficiency virus gp120 envelope glycoprotein. J Acquired Immune Defic Syndr. 1992;5:591–599. [PubMed] [Google Scholar]
- 46.Tilley S A, Honnen W J, Racho M E, Hilgartner M, Pinter A. A human monoclonal antibody against the CD4 binding site of HIV-1 gp120 exhibits potent, broadly neutralizing activity. Res Virol. 1991;142:247–259. doi: 10.1016/0923-2516(91)90010-z. [DOI] [PubMed] [Google Scholar]
- 47.Tilley S A, Honnen W J, Racho M E, Chou T-C, Pinter A. Synergistic neutralization of HIV-1 by human monoclonal antibodies against the V3 loop and the CD4-binding site of gp120. AIDS Res Hum Retroviruses. 1992;8:461–467. doi: 10.1089/aid.1992.8.461. [DOI] [PubMed] [Google Scholar]
- 48.Trkola A, Pomales A B, Yuan H, Korber B, Maddon P J, Allaway G P, Katinger H, Barbas III C F, Burton D R, Ho D D, Moore J P. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. J Virol. 1995;69:6609–6617. doi: 10.1128/jvi.69.11.6609-6617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore J P, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vijh-Warrier S, Pinter A, Honnen W J, Tilley S. Synergistic neutralization of human immunodeficiency virus type 1 by a chimpanzee monoclonal antibody against the V2 domain of gp120 in combination with monoclonal antibodies against the V3 loop and the CD4-binding site. J Virol. 1996;70:4466–4473. doi: 10.1128/jvi.70.7.4466-4473.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wyatt R, Thali M, Tilley S, Pinter A, Posner M R, Ho D D, Robinson J, Sodroski J. Relationship of the human immunodeficiency virus type 1 gp120 third variable loop to a component of the CD4 binding site in the fourth conserved region. J Virol. 1992;66:6997–7004. doi: 10.1128/jvi.66.12.6997-7004.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


