Abstract
As the use of endovascular aneurysm repair (EVAR) increases, anatomic constraints remain a challenge. In this case report, we describe the use of intravascular lithotripsy to facilitate EVAR in a patient with a severely calcified and stenotic aortic bifurcation. Future applications of intravascular lithotripsy could help expand the use of EVAR to patients with severely stenotic vasculature and optimize outcomes in the treatment of infrarenal abdominal aortic aneurysms.
Keywords: Abdominal aortic aneurysm (AAA), Aortic iliac occlusive disease (AIOD), Endovascular aortic repair (EVAR), Lithotripsy
Open abdominal aortic aneurysm (AAA) repair has been considered the gold standard treatment for infrarenal AAAs for many years. However, it remains a highly invasive surgery with significant surgical risk.1 Minimally invasive techniques, such as the use of endovascular aneurysm repair (EVAR), have been used to mitigate surgical risk in patients with poor cardiovascular and pulmonary reserve. However, EVAR has been associated with complications such as rupture, endoleak, device migration, and graft limb occlusion.2 The anatomic fit between the patient and endograft has been indicated as an important factor for reducing complications, with selection influenced by vessel diameter, tortuosity, and atherosclerotic plaque.3 Studies have demonstrated an association between calcification at the proximal attachment zone and an increased risk of endoleaks, migration, and thromboembolism.4 Additionally, a narrowed distal aortic bifurcation has been recognized as a risk factor for stent graft limb occlusion.5,6
In contrast to conventional angioplasty or atherectomy, Shockwave intravascular lithotripsy (IVL; Shockwave Medical) uses ultrasonic waves to induce micro-fracturing in calcified plaque. IVL allows for enhanced vessel compliance by fracturing calcifications of both intimal and medial layers without injuring the vessel.7 IVL has been proved effective in treating highly calcified occlusive disease in coronary and peripheral arteries and in facilitating transfemoral thoracic endovascular aortic repair.8, 9, 10 Through a kissing angioplasty technique, IVL has been used for EVAR with the device targeted toward narrowed distal aortas.11 However, the use of IVL in conjunction with the Gore Excluder AAA endoprosthesis (W.L. Gore & Associates) has not yet been described. We present the case of a patient with an infrarenal AAA, extending into and complicated by a heavily calcified aortic bifurcation, that was successfully managed with intravascular lithotripsy and EVAR. The patient provided written informed consent for the report of her case details and images associated with the case.
Case report
A 70-year-old woman presented to the clinic for evaluation for an asymptomatic infrarenal AAA. The AAA was discovered incidentally during a cardiac catheterization 7 years ago. The patient ultimately underwent coronary artery bypass grafting at that time. The aneurysm had been monitored by her cardiologist. Her only other prior surgical history was significant for a right carotid endarterectomy 4 years prior. The patient was a former 1 pack/day smoker without a family history of AAA.
Computed tomography angiography of the abdomen and pelvis demonstrated an infrarenal AAA with a maximum diameter of 5.8 cm that extended to the aortic bifurcation. The aortic bifurcation was stenotic to 6 mm. There was also severe calcification of the right common iliac artery origin with >75% stenosis. The left common iliac artery was also stenotic (Fig 1).
Fig 1.
Preprocedural computed tomography angiography with and without contrast enhancement of a 70-year-old woman with a history of an abdominal aortic aneurysm (AAA). A, Angiogram demonstrating fusiform AAA before stent graft placement. B, Computed tomography scan without contrast revealing a narrowed aorta measuring 6 mm at baseline. C, Computed tomography scan without contrast revealing severe stenosis (>75%) at the right common iliac artery and moderate stenosis (50%) at the left common iliac artery.
Procedure
Endovascular repair was recommended due to the patient's comorbidities. Ultrasound-guided access of the bilateral common femoral arteries was performed. The left side was upsized to an 8F sheath. The Perclose ProGlide system (Abbott Cardiovascular) was deployed in the left side of the groin as part of the preclosure technique. The right side required a cut down due to severe calcification that prevented percutaneous upsizing of the sheath. At this point, a Spartacore wire (Abbott Laboratories) was advanced into the abdominal aorta via the right side. An angiogram via a flush catheter from the left side of the groin revealed the location of the aortic bifurcation and confirmed the stenosis. Angioplasty with a 5-mm balloon was performed first. Despite angioplasty, there was still significant stenosis with a kinked vessel contour. Consideration was given to performing a kissing balloon technique but it was not believed to be of any added benefit given the residual stenosis. IVL was believed to give better vessel preparation by modifying the plaque structure without causing vessel damage. Lithotripsy with the Shockwave lithotripter was then performed using a 5-mm balloon (Fig 2).
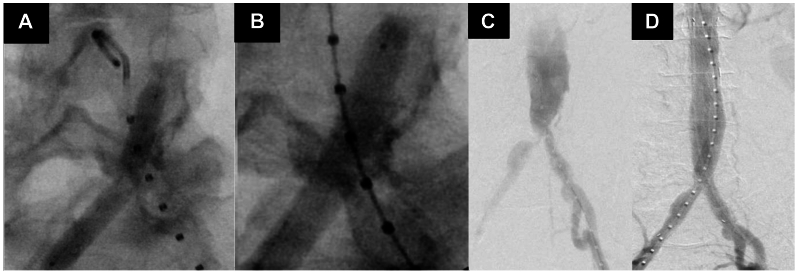
Fig 2.
Intravascular lithotripsy (IVL)-enabled endovascular repair of infrarenal abdominal aortic aneurysm (AAA). A, Pre-IVL angiogram demonstrating kinked vessel contour despite angioplasty. B, Angiogram revealing vessel expansion with angioplasty in conjunction with IVL. C, Fluoroscopy before IVL demonstrating a narrowed aortic bifurcation and stenotic right and left iliac arteries. D, Fluoroscopy after IVL revealing vessel expansion with angioplasty in conjunction with IVL.
A total of 180 pulses of lithotripsy were delivered to the right common iliac artery. Imaging after IVL revealed more favorable vessel expansion. A 12F sheath was then placed directly into the infrarenal aorta without difficulty. From the left side, the 8F sheath was then upsized to a 16F sheath. The main body device of a Gore Excluder endoprosthesis (23 × 12 × 16-mm device) was inserted. On the contralateral side, a 12 × 14 × 100-mm limb was placed, landing just proximal to the iliac bifurcation. Two 8-mm balloons were simultaneously inflated at the device flow divider and the proximal portions of the common iliac arteries bilaterally. A completion angiogram revealed the absence of type I and III endoleaks. However, there was evidence of a delayed type II endoleak. The left side was closed using the previously placed ProGlide sutures. After sheath removal, the right common femoral artery was closed surgically. The patient was taken to the intensive care unit for monitoring after the procedure.
Follow-up and outcomes
The postoperative course was uncomplicated. She was discharged from the hospital on postoperative day 2. She had no complaints of claudication, ischemic rest pain, nonhealing wounds, abdominal discomfort, or back discomfort. At 1 month, computed tomography angiography confirmed appropriate location of the device. The aneurysm sac diameter decreased to 5.6 cm from 5.8 cm. The common, internal, and external iliac arteries were widely patent bilaterally without evidence of aneurysmal dilatation or significant stenoses. Additionally, there was no evidence of an endoleak (Fig 3).
Fig 3.
One-month follow-up post-procedure images. A, Computed tomography scan without contrast demonstrating a widely patent inserted bifurcated aortic stent graft. B, Computed tomography scan without contrast revealing widely patent right and left common iliac arteries with decreased stenoses. C, Frontal view of preprocedure computed tomography scan with contrast revealing the narrowed aortic bifurcation with severe stenosis in the right common iliac artery and moderate stenosis in the left common iliac artery. D, Frontal view of postprocedure computed tomography scan with contrast demonstrating widely patent aortic bifurcation and right and left common iliac arteries.
Discussion
In the short term, EVAR has been associated with decreased morbidity and mortality compared with open surgery.9,12 Major challenges toward the use of EVAR include unfavorable anatomic features such as excess aortic and/or iliac artery tortuosity, excessive mural thrombus, and extremely small caliber access vessels.12,13 Before IVL, the options for vascular access were limited to the creation of surgical or endovascular conduits and retroperitoneal common iliac access.14 Due to the increased morbidity and mortality associated with these options, IVL can be considered as a potentially safer option for vessel preparation before stent placement. Although lithotripsy has commonly been used for the treatment of nephrolithiasis, cholelithiasis, and joint calcifications, the Shockwave lithotripter (Shockwave Medical) is capable of generating sonic waves to fracture the calcifications associated with atherosclerosis. In this case, we demonstrate that IVL can be safely used to facilitate treatment of infrarenal AAAs with hostile aortic morphology. The luminal gain achieved with IVL could also contribute to mitigating the risk of limb occlusion and improving patency of the limbs. To the best of our knowledge, this is the first described use of IVL to facilitate delivery of a Gore Excluder endovascular stent graft in a patient with significant stenoses of the aortic bifurcation and iliac arteries.
There is a case report that describes IVL being used in an EVAR using the Endologix AFX II device (Endologix).11 This device is known to be a lower profile device compared with the Gore Excluder device. Furthermore, the Gore device is more widely used for the treatment of infrarenal EVAR. Our report shows this technology and technique can be more broadly applied to facilitate EVAR in a wider range of patients.
Conclusions
IVL seems to be a safe and effective option for endovascular treatment of infrarenal AAAs complicated by aortoiliac occlusive disease. In this case report, IVL enabled EVAR in a patient with heavily calcified iliac arteries and aortic bifurcation, without resulting in rupture, perforation, or dissection. Future studies are still necessary to determine whether IVL is sufficient enough to expand the use of EVAR to patients with severely calcified vascular access vessels.
Disclosures
R.M. is a consultant for Cydar, Endospan, Medtronic, Silk Road, W.L. Gore & Associates. S.O., J.O., and X.L. have no conflicts of interest.
Footnotes
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Sun Z.H. Abdominal aortic aneurysm: treatment options, image visualizations and follow-up procedures. J Geriatr Cardiol. 2012;9:49–60. doi: 10.3724/SP.J.1263.2012.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang G., Zhai S., Li T., et al. Limb graft occlusion following endovascular aortic repair: incidence, causes, treatment and prevention in a study cohort. Exp Ther Med. 2017;14:1763–1768. doi: 10.3892/etm.2017.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katzen B.T., Dake M.D., MacLean A.A., Wang D.S. Endovascular repair of abdominal and thoracic aortic aneurysms. Circulation. 2005;112:1663–1675. doi: 10.1161/CIRCULATIONAHA.105.541284. [DOI] [PubMed] [Google Scholar]
- 4.Wyss T.R., Dick F., Brown L.C., Greenhalgh R.M. The influence of thrombus, calcification, angulation, and tortuosity of attachment sites on the time to the first graft-related complication after endovascular aneurysm repair. J Vasc Surg. 2011;54:965–971. doi: 10.1016/j.jvs.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Chaikof E.L., Fillinger M.F., Matsumura J.S., et al. Identifying and grading factors that modify the outcome of endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1061–1066. doi: 10.1067/mva.2002.123991. [DOI] [PubMed] [Google Scholar]
- 6.Briggs C., Babrowski T., Skelly C., Milner R. Anatomic and clinical characterization of the narrow distal aorta and implications after endovascular aneurysm repair. J Vasc Surg. 2018;68:1030–1038.e1. doi: 10.1016/j.jvs.2017.12.073. [DOI] [PubMed] [Google Scholar]
- 7.Adams G., Shammas N., Mangalmurti S., et al. Intravascular lithotripsy for treatment of calcified lower extremity arterial stenosis: initial analysis of the disrupt PAD III study. J Endovasc Ther. 2020;27:473–480. doi: 10.1177/1526602820914598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tepe G., Brodmann M., Werner M., et al. Intravascular lithotripsy for peripheral artery calcification: 30-day outcomes from the randomized disrupt PAD III trial. JACC Cardiovasc Interv. 2021;14:1352–1361. doi: 10.1016/j.jcin.2021.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Wong C.P., Chan L.P., Au D.M., Chan H.W.C., Chan Y.C. Efficacy and safety of intravascular lithotripsy in lower extremity peripheral artery disease: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2022;63:446–456. doi: 10.1016/j.ejvs.2021.10.035. [DOI] [PubMed] [Google Scholar]
- 10.Rosseel L., De Backer O., Søndergaard L., Bieliauskas G. Intravascular iliac artery lithotripsy to enable transfemoral thoracic endovascular aortic repair. Catheter Cardiovasc Interv. 2020;95:E96–E99. doi: 10.1002/ccd.28379. [DOI] [PubMed] [Google Scholar]
- 11.Khalid N., Iantorno M., Shlofmitz E., Hashim H., Waksman R., Bernardo N. Kissing intravascular lithotripsy facilitated endovascular repair of a complex saccular abdominal aortic aneurysm with narrowed distal aorta: a first-in-human report. JACC Cardiovasc Interv. 2019;12:e97–e99. doi: 10.1016/j.jcin.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Jaunoo S. Endovascular aneurysm repair (EVAR) Int J Surg. 2008;6:266–269. doi: 10.1016/j.ijsu.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Daye D., Walker T.G. Complications of endovascular aneurysm repair of the thoracic and abdominal aorta: evaluation and management. Cardiovasc Diagn Ther. 2018;8(Suppl 1):S138–S156. doi: 10.21037/cdt.2017.09.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vatakencherry G., Molloy C., Sheth N., Liao M., Lam C.K. Percutaneous access planning, techniques and considerations for endovascular aortic repair (EVAR) Cardiovasc Diagn Ther. 2018;8(Suppl 1):S184–S190. doi: 10.21037/cdt.2018.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]