Abstract
Due to the special property of food waste (FW), anaerobic digestion of food waste is facing many challenges like foaming, acidification, ammonia nitrogen and (NH4+-N) inhibition which resulted in a low biogas yield. A better understanding on the problems exiting in the FW anaerobic digestion would enhance the bio-energy recovery and increase the stable operation. Meanwhile, to overcome the bottle necks, pretreatment, co-digestion and additives is proposed as well as the solutions to improve biogas yield in FW digestion system. At last, future research directions regarding FW anaerobic digestion were proposed.
Keywords: Food waste, Anaerobic digestion, Utilization, Improvement, Additives
1. Introduction
Food waste includes food processing waste and edible residues produced by families, schools, canteens and catering industries. It is mainly composed by oil, meat, fruit peel, vegetables, bones, rice noodles, fish, waste tableware and other substances. In 2022, there was about 1.3 billion tons food waste produced. The amount of FW is also increased with the population growth which will reach to 2.1 billion tons by 2030.
FW is characteristics of high organic content, high moisture content, high fat, and high salt. It has dual attributes including environmental pollution and resource utilization. On the one hand, food waste is easy to rot, if it is not well treated, it will bring out serials of environmental problems. On the other hand, FW can be used to produce high value by-products through components extraction, anaerobic digestion, composting and pyrolysis [1,2]. Components like oil, flavonoids, phenolic acids, β-hydroxybenzoic acid and carobenoids et al. can be obtained by solvent-based extraction [3,4], supercritical carbon dioxide extraction, subcritical water extraction, pressurized liquid extraction [1,5]and thermal extraction [6]. Especially for the bioactive compounds like flavonoids and phenolic acids can be used to produce antioxidants with anticancer activities. While these extraction methods are characteristic of high operating cost, lower yield, high energy consumption and not environmental-friendly [7]. Composting can reduce the greenhouse gases (GHGs) emissions and improve soil properties. While composing of food waste also faced some challenges like odours control, process optimization and compost quality which requires be solved. Meanwhile the composting cycle should be shortened [8]. Considering the characteristic of the above two technologies, anaerobic digestion (AD) is proposed as an effective technology for the food waste treatment [[9], [10], [11]]. FW can be converted into high valuable products including biogas and organic fertilizer. Biogas can be used for power generation, heat energy and other fields, while organic fertilizers can improve the yield and quality of crop and vegetables.
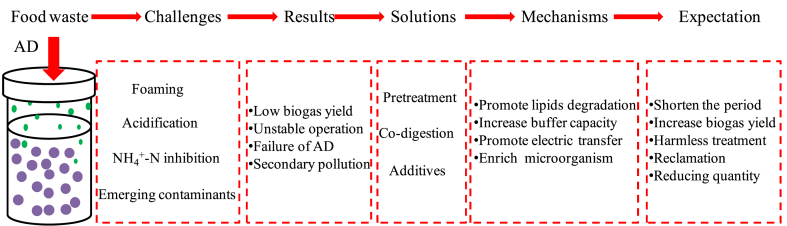
Although AD is widely used in the treatment of solid waste, there are still some key technical challenges such as the volatile fatty acids (VFAs) accumulation, process instability and foaming need be solved. How to realize effective treatment of food waste under the principle of “reducing quantity”, “harmless “and “reclamation” is an increasingly prominent topic for countries around the world. Hence, it is necessary to summarize the existing problems and development for the food waste treatment. This paper summarized the literature and reviews the technologies that related to the conversion of FW to biogas (Fig. 1). It concluded the challenges faced by the AD of food waste in details and provided the solutions as well as the properties of the solutions, which is helpful for the improvement of treatment level of food waste.
Fig. 1.
Main content of this work.
2. Characteristics of food waste
The main components of FW includes: (1) Food residue, such as leftovers, vegetable peels, fish and shrimp shells, bones and so on. (2) Food processing waste, such as leftover vegetable leaves after cutting, fruit pits, pulp, and so on. Table .1 displayed the composition of food waste around the world. It could be seen that there were obvious difference in the components of food waste due to the differences in eating habits. For example, the main staple food in China is rice and wheat which are the predominant composition in food waste, while the main staple food in Polish is meat, potatoes and cream which are the predominant composition in food waste. It is worth noting that vegetables has accounted for a considerable proportion in all the sources, which is one of the main source of bioactive compounds in the food waste.
Table 1.
Component of food waste.
| Sources | Main composition | Reference |
|---|---|---|
| Polish | Bread (23.9%), vegetables (23.3%), meat (15.1%) and potatoes (7.8%) | Jan.et al. [12] |
| Danish | Fresh vegetables and salads (30%), fresh fruit (17%), bakery (13%), drinks, confectionery and desserts (13%) | Maklawe et al. [13] |
| Korean | Fruits (29%), vegetables (30%), staple food (22%), egg (2%), Bakery products (2%), fish (4%), meat (3%), soy foods (4%) | Bashir et al. [14] |
| Finnish | Vegetables (19%), home cooked food (18), milk products (17 %), bakery and grain products (13%), fruits and berries (13%), meat, fish, and eggs (7%), convenience food (6%) | Kirsi et al. [15] |
| China | Wheat (25.78%), rice (20.36%) and vegetables (18.61%) | Long Qian et al. [16] |
| Greece | Vegetables (21%), cooked food without meat (20%), dairy products (17%), fruits (15%) and bread (10%) | Abeliotis et al. [17] |
| UK | Fruit (30%), vegetable (23%), bread and cereals (16%) | Joseph et al. [18] |
| Japan | Vegetable (22.9%), Fruit (13.7%), left towers (22.3%) | Tetsuji et al. [19] |
To further analyze the potential effect of food waste on the anaerobic digestion, examples of the chemical characteristic of food waste were displayed in Table .2. The total solid (TS) content of food waste ranges from 7.62% to 23.70%. The ratio of VS (volatile solids)/TS is more than 80% indicated a high organic content for biological treatment. While pH of the FW ranges from 3.6 to 6.6, which is not friendly for the anaerobic digestion and easy to cause acidification. Carbohydrate was the main component in TS, while the lipids also occupy a certain proportion which will cause negative effect on the anaerobic digestion. Though there is obvious difference among different sources of FW due to the eating habits, culture, climate, and seasons, the FW for anaerobic digestion will be facing many challenges like foaming, acidification, ammonia nitrogen (NH4+-N) inhibition and emerging contaminants.
Table 2.
Examples of food waste: chemical characteristic.
| Sources | TS (%) | VS (%) | pH | Protein (%TS) | Carbohydrate (%TS) | Lipids (%TS) | Reference |
|---|---|---|---|---|---|---|---|
| FW in a student canteen, China | 23.70 | 23.1 | 6.6 | 14 | 44.9 | 14.1 | Miao Yu et al. [20] |
| FW in a dining hall, Japan | 7.62 | 7.2 | 3.6 | 14.9 | 33.4 | 13.5 | Lijie Wu et al. [21] |
| FW in a restaurant, Korea | 18.1 | 17.1 | 6.5 | 18.2 | 61.7 | 12.9 | Lei Zhang et al. [22] |
| FW in a kitchen, India | 23.3 | 21 | 5.2 | / | / | / | Kranti.et al. [23] |
| FW in a kitchen, Singapore | 23.2 | 18.97 | 5.4 | 24.29 | 48.52 | 27.19 | Huanhuan Tong et al. [24] |
3. Problems exist in anaerobic digestion of FW
3.1. Foaming
Foaming is a common problem existed in the AD of FW [25], while the exact mechanism of foaming is still not well known. Qin He et al. found that the foaming was mainly caused by the growth of specifc bacteria and the production of surface active materials. Meanwhile, the accumulation of VFAs and total ammonia nitrogen (TAN) were also contributed to foaming by reducing the surface tension of sludge [26].Pingjin Yang et al. found that extracellular polymeric substances were the key factor to cause foaming in the AD of FW [27]. Foaming also relates to the operating parameter and microbial communities’ diversity [28]. The presence of oil, grease, lipid, proteins, lipoproteins, polysaccharide-lipid complexes in the feedstock and the accumulation of VFAs, ammonia during AD of FW may also contribute to foaming [29]. The negative effect of foaming, such as reduce mass transfer efficiency, affect microbial activity, lower biogas yield, cause the blockage of pipes and the overflow of biogas slurry, make operational challenges for AD operator. How to reduce to foaming during the anaerobic digestion of FW is required further study.
3.2. Acidification
The accumulation of VFAs is easily occurred in the anaerobic digestion of high solid concentration substrate [30]. As mentioned above, the TS concentration of FW can reach to 23.70%, in which VS is accounted for more than 80%. Moreover, food waste has a relative high content of lipid which may cause low carbon nitrogen ratio and lead acidification when FW is the sole substrate [31]. During the AD of food waste, lipid is first hydrolyzed to long-chain fatty acids (LCFAs) which will adsorb on the surface of microorganisms and hinder the mass transfer process resulting in a decrease of microorganism's activity and instability of anaerobic digestion. Meanwhile, the metabolites of LCFAs, especially the accumulation of VFAs can lead to the decrease of microorganism's activity and cause the imbalance between the acid stage and the methanogenic stage, resulting in the “acidification” in the anaerobic digestion system. The acidification will greatly decrease the pH and the activity of methane-forming bacteria due to its highly sensitive to the pH changes. Finally, it will lead to a low biogas yield and unstable of anaerobic digestion system. Hence, it is strongly demand for develop efficient technologies to avoid VFA inhibition in AD of FW and keep the stable operation.
3.3. NH4+-N inhibition
NH4+-N inhibition is a well-known problem in the AD of nitrogen-rich organic substrates such as food waste. The content of protein in the food waste will bring in the release of NH4+-N during the anaerobic digestion, which will limit biogas production and even cause digestion failure [32]. Kuichuan Sheng et al. found that an excessive inhibition of methanogenesis was appeared when the TAN concentrations was reached to 3.78 g/L in the anaerobic digestion of food waste [33]. Hong Chen et al. found that the ammonia inhibition was appeared when the TAN concentration in the AD system was exceeded 2 g/L. Ammonia inhibition will cause the accumulation of acetate and propionate and decrease of pH. Meanwhile, the activity of hydrogenotrophic methanogens was inhibited [34]. There was no uniform conclusion for the influence of ammonia on the methanogens. Some studies showed that hydrogenotrophic methanogens were more sensitive to ammonia than acetoclastic methanogens [34,35], while other literatures reported that the acetoclastic methanogens was more easily affected by ammonia than that of the hydrogenotrophic methanogens [36,37]. Though many methods like air stripping [32,38], nitrification and denitrification [33], chemical precipitation and ammonia-binding had been carried out for alleviating the ammonia inhibition in AD process, how to reduce operational cost and complexity, promote the efficiency and put in full-scale practical application of those technologies is still required study.
3.4. Emerging contaminants
Except traditional pollutants like COD and NH4+-N, emerging contaminants like antibiotic, plasticizer and microplastics are obtained much attention in recent years. Antibiotics is widely used as an antimicrobial drug in the medical field and also used as food additives for live stock anti-bacteria purposes [39]. It will cause some pathogenic bacteria to produce antibiotic resistance genes (ARGs) when discharged into environment. The ARGs in the anaerobic digestion of food waste mainly come from two aspects: (1) Direct sources. The ARGs in food sources like meat, eggs, vegetables and fruits contaminated by the ARGs left in crops, soil and groundwater ultimately leave in FW [40,41]. Amount of ARGs had been detected in meats, fruits and vegetables. Kärt Kanger et al. found that there were more than 300 kinds of ARGs in FW,especially for sulfonamide and tetracycline resistance genes [42]. Liao hanpeng et al. also found that more than 20 kinds of ARGs including ermB, ermF, mefA, sul1, sul2, tetX and tetW in FW [43]. (2) Indirect sources. During the anaerobic digestion of FW, ARGs can be brought in by the added sludge or manure [44]. Piggery manure was an important reservoir for ARGs with a high level of residual antibiotics (∼105 ng/g dry manure) [45].
ARGs can pollute the natural environment and cause risks to human health. Recent researches showed that AD could reduce the abundance of ARGs and cut down ARGs transferring from AD system into the environment [46,47]. However, there are still many key problems need further study: first, the mechanism of antibiotic degradation in anaerobic digestion is not clear; Current research on the evaluation of antibiotics and their resistance genes mainly focuses on the change of abundance; the influence of anaerobic digestion performance on the reduction of antibiotic and the “co-metabolism” between antibiotics and organic matter are still unknown; second, how to optimize the process parameters of anaerobic digestion to strengthen the efficiency of antibiotic removal and ARGs reduction; third, the migration and transformation pathway of antibiotics and ARGs in biogas slurry and biogas residue during anaerobic digestion is lack of study. Fourth, the research on the behavior of different kinds of antibiotics in the anaerobic digestion needs to be further strengthened.
Microplastics, as a <5 mm plastic, has been proven existing in food waste [48]. It mainly comes from food packaging, food containers, and plastic-coated paper. Microplastics also present in food itself like shellfish, table salts, honey, canned food and sugar [49,50]. Studies have been showed that microplastics could inhibit the anaerobic digestion processes resulting in a decrease of methane yield and microbial abundance [[51], [52], [53]]. Jingjing Zhang et al. found that microplastics could inhibit acidification and methanogenesis process when its concentration was exceed 0.25 g/L [54].Wei Wei et al. found that the side effect of microplastics on the AD was due to the induction of reactive oxygen species [53]. However, the mechanism of microplastics on the AD process is still lack of researches.
Plasticizer, as a kind of polymer additive, is widely used in the production of plastic products. Plasticizers like bisphenols, phthalates and parabens can be used to synthesize a variety of products, such as plastic bottles, food packaging, metal food cans and cosmetic plastic pellets. Plasticizers can be dissolved in plastic packaging and direct contact with food. It has been reported that the concentration of plasticizer in food can reach to 0.84 mg/kg. Part of plasticizers can be entered into environment accompanied with food waste. Plasticizer exposure can cause cardiovascular diseases and diabetes and make threats to human health. At present, most of the studies on plasticizers focus on degradation or removal, and their effects on anaerobic digestion process are still unknown.
4. Solutions
In order to increase biogas yield, alleviate the inhibitions of the hazardous substances and promote the removal rate of emerging contaminants, some strategies are developed like pretreatment, co-digestion and additives. The following are the details of the strategy.
4.1. Pretreatment
Lipid, as one of major contents in food waste, is difficult to degrade which will become the rate-limiting step during the anaerobic digestion of food waste. Pretreatment is considered as an effective method to promote lipid degradation and the efficiency of anaerobic digestion. The main purposes of pretreatment are: (1) to improve the surface characteristics of substrate and enhance the adaptability of microorganisms; (2) Reduce/eliminate potentially toxic and harmful substances; (3) Improve the biodegradability of complex compounds.
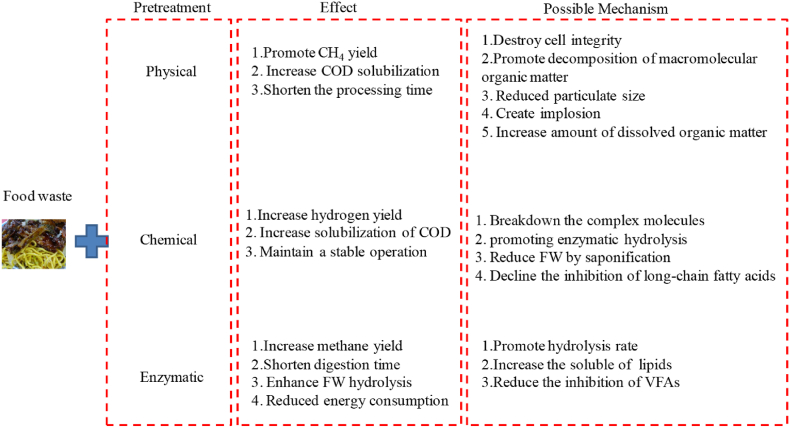
The commonly used pretreatment technologies are mainly divided into physical pretreatment [[55], [56], [57], [58]], chemical pretreatment [59,60] and enzymatic pretreatment [61,62].The effect and possible mechanism of food waste with different pretreatments was shown in Fig. 2. Thermal, ultrasound and irradiation radiation were the typical way of physical pretreatment. Javkhlan Ariunbaatar et al. investigated the thermal pretreatment on the anaerobic digestion of food waste and found that methane yield was increased by 40% by thermal pretreatment at 50 °C for 6–12 h due to the higher solubilization of chemical oxygen demand (COD) after thermal pretreatment [55]. Y. El Gnaoui et al. also found that soluble COD was increased by 43.41% after thermal pretreatment resulting in an increase of 23.68 % on the methane yield [63]. Similar effects were obtained by other physical pretreatment on the anaerobic digestion of food waste. Xionghui Fei et al. found that biogas yield was increased by 14.3% due to the soluble chemical oxygen demand (SCOD) increasing by 70.6% after irradiation pretreatment [64]. Liangchen Yue et al. found that soluble COD of lipid waste pretreated with ultrasound was higher than that with microwave under the same energy input resulting in a higher methane yield [65]. The mechanism of physical pretreatment is to change the morphological structure of substances and convert solid organic matter into the soluble COD which can be easily utilized by the microorganisms.
Fig. 2.
Effect and possible mechanism of food waste under different pretreatment.
Compared with physical pretreatment, chemical pretreatment mainly relies on chemical agents to change the properties of food waste. Commonly used chemical agents includes NaOH, Ca(OH)2, CaO and NH3·H2O. Alkali pretreatment can promote the solubilization of proteins and lignin as well as lipid saponification. Erdenebayar Naran found that methane yield could be increased by 25% after NaOH pretreatment due to the increase of soluble organic matter [60]. Chen Linyi et al. compared NaOH, KOH and CaO pretreatment on the anaerobic digestion of food waste and found that 1% CaO showed the best effect on the improvement of biogas yield [59]. Meanwhile, microbial analysis results showed that Methanosaeta and Methanospirillum were the predominant bacterial. However, alkali pretreatment could bring out other problems like salt formation and the inhibition of Na+/Ca2+/K+ on the anaerobic digestion. It was reported that the AD process would be inhibited when Na+ concentration was 3500–5500 mg/L. Moreover, surfactant was proven to increase the SCOD in anaerobic system. Jian Sun investigated the anionic surfactants SDBS and non-ionic surfactants APG on the anaerobic co-digestion of sludge, food wastes and green wastes [66]. The results showed that biogas production was inhibited by SDBS while promoted by APG. However, the effect of surfactants on anaerobic digestion depended on the type and dosage of surfactants.
Different from the above pretreatments, enzymatic pretreatment not only can promote the dissolution of solid organic matter and enhance biogas yield [67], but also cannot cause secondary pollution. Ying Meng et al. found that the methane yield from the anaerobic digestion of animal fat, vegetable oil and floatable grease after lipase pretreatment was increased by 80.8–157.7%, 26.9–53.8% and 37.0–40.7%, respectively, and the anaerobic digestion period was shortened by 10∼40 days [61]. The commonly used enzymes include carbohydrases, cellulase, β-glucanase, hemicellulase, proteases and lipases which are costly. Moreover, enzymatic pretreatment may also bring in other problems like accumulation of LCFAs and VFAs, which can cause the “acidification” of anaerobic system.
Although physical pretreatment can increase the hydrolysis efficiency and shorten the digestion time, it requires relatively high pretreatment cost and extra energy input. Chemical pretreatment can rapidly promote the degradation of complex organic matter with high efficiency and low cost, while the effect of chemical pretreatment is greatly affected by the concentration of chemical agents, and even inhibits anaerobic digestion at high concentrations. Enzymatic pretreatment is more efficient and environment friendly while it is costly. However, there is no research proven that pretreatment can alleviate the NH4+-N inhibition and remove the emerging contaminants. Meanwhile, Life cycle assessment for AD of food waste under different pretreatments is lack of research.
4.2. Co-digestion
Anaerobic co-digestion of food waste and other materials is considered as an effective way to realize more balanced nutrient levels, alleviate the ammonia inhibition, dilute toxic contaminants and increase biogas yield which is attracted more and more attentions. The commonly used additional substrates co-digestion with food waste includes algae, sludge, manure, agricultural straw and other organic waste. Chuenchart et al. observed that the methane yield could be increased by 10.4–89.9% in co-digestion with chicken manure compared with mono-digestion of food waste [68]. Kamonwan et al. found that the accumulation of VFAs and decrease of pH could be overcome during the co-digestion of food waste and algae and a stable CH4 production was also obtained [69]. Jingxin Zhang et al. evaluated an increase of 24.6% in methane yield and 10.1% in the VS reduction from the co-digestion of food waste and waste active sludge compared with control substrate [70].
Except the kind of substrate, the process parameters like bioreactor, temperature, substrate composition and organic loading rate also make great impact on the performance of co-digestion. LiJie Wu et al. found that biogas yield was increased by 19% in the mesophilic temperature when co-digesting food waste with de-oiled grease trap waste [71]. Wanli Zhang et al. investigated the performance of co-digestion of food waste, cattle manure and corn straw. The results showed that co-digestion of food waste and corn straw was inhibited by organic acids, while co-digestion of food waste and cattle manure showed a greatest synergistic effect resulting in a relative abundance of hydrogenotrophic methanogens (68.9%) and Methanosarcina (14.6%) [72]. While 388 mL·g−1VS methane production was obtained which was increased by 41.1% at a substrate ratio of 2 on the co-digestion of FW and cattle manure [73]. Xinxin Ma et al. found that the highest biogas yield (640 mL g−1 VS) was obtained at the substrate ratios of 7(food waste):3(sophora flavescens residues) [74], while 334 mL CH4/gCODInput was obtained in the co-digestion of food waste and algae at a mixture ratio of 8:2 [69]. While Du et al. found that methane yield could be increased at a ratio of 1.6:98.4 (weight basis) on the co-digestion of algal biomass and FW [75].
Though co-digestion with other substrates can promote the performance of anaerobic digestion, its mechanisms were different. Yun Peng et al. found that landfill leachate could alleviate the ammonia inhibition leading to a stable methane generation in the co-digestion system of food waste and landfill leachate. Meanwhile, microbial analysis showed that the relative abundance of Pelotomaculum was promoted [76]. Lei Zhang et al. found that trace element (cobalt, molybdenum, nickel and ion) in the piggery wastewater was the most important factor to enhance co-digestion performances of food waste and piggery wastewater [22]. Shangsong Jiang et al. found that the accumulation of VFAs was inhibited on the anaerobic digestion of food waste and fruit, vegetable waste and the relative abundances of microbial (Proteiniphilum Methanosaet, Candidatus and Methanofastidiosum) was enhanced resulting in the increase of biogas yield [77]. Bao-Shan Xing et al. found that a relative high abundance of Clostridia and Methanosarcina caused a high hydrolysis rate and VFA conversion rate during the co-digestion of food waste and waste activated sludge. Methanosarcina was responsible for the well performance of the co-digestion [78]. While Cunsheng Zhang et al. found that co-digestion food waste with cattle manure could enhance the buffer capacity without pH control even under high organic load. The increased biogas yield by co-digestion was caused by C/N ratio and high biodegradation rate of lipids [73]. The possible mechanism of food waste co-digestion with other substrate was displayed in Fig. 3.
Fig. 3.
Effect and possible mechanism of food waste co-digestion with other substrate.
Though there are many benefits for co-digestion of food waste with other substrates like alleviate the NH4+-N accumulation and VFAs, adjust C/N ratio and increase biogas yield, the addition of other substrates may also bring in addition pollutants like heavy mental, antibiotic and microplastic, which will make negative effect on the anaerobic digestion. Antibiotics together with antibiotic resistant bacteria, microplastic have been detected in livestock [79] and sludge [80]. Though some studies proven that co-digestion with food waste also could remove emerging contaminants like antibiotic [81], the effect of pollutants brought out by the addition substrate on the co-digestion and the research on emerging contaminants removal during the co-digestion are relatively scarce which is required further study.
4.3. Additives
To promote the performance of AD, trace elements, carbon-based materials (activated carbon and biochar) and iron-based materials (iron oxides, and magnetite) are used as the additive in the AD of food waste to enrich microorganisms, increase biogas yield and shorten the digestion period. However, both trace elements and activated carbon are expensive which is difficult for a large scale application.
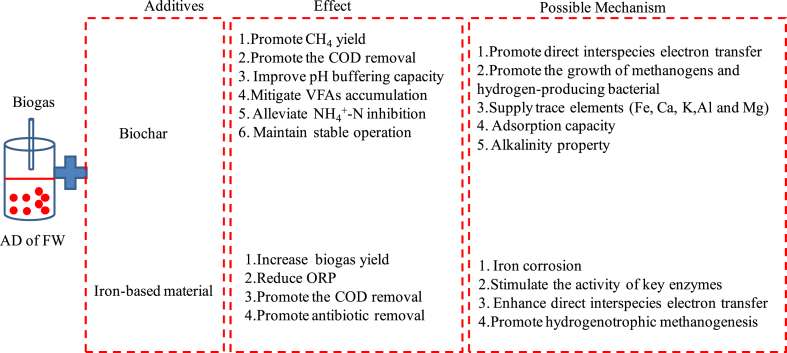
Biochar, as an environment friendly and inexpensive material substituted for activated carbon, can be produced from the pyrolysis of biomass in the absence of oxygen. It is characteristic of porous structure, large specific surface area and good conductive performance which is attracted most attention [[82], [83], [84], [85]]. The functions of biochar in the AD of food waste can be concluded as following: (1) Improve the pH buffering capacity. Carbohydrates in food waste can be easily converted to VFAs resulting in a decrease of pH. Biochar can effectively promote the buffering capacity of anaerobic digestion and neutralize the generated VFAs [86]. Debnath Ovi found that pH value was maintained in a slightly alkaline range (7.88–7.45) compared with that of the control groups (6.31–7.01) after the addition of biochar [84]. Le Zhang et al. also found that pH values was maintained in the range of 6.9–8.4 during the whole AD process of food waste under high substrate by biochar, while pH value in the control group was decreased to 6.7 [87]. (2) Mitigate VFAs accumulation. Acid accumulation is a common phenomenon in the AD of food waste, since amount of VFAs can be produced from carbohydrates in food waste. Promoting the VFA degradation is crucial for the system stability. Yahui Zhu et al. investigated the biochar addition on alleviating acid inhibition in anaerobic digestion of food waste and the results showed that biochar effectively accelerated the consumption of VFAs resulted in a lower VFAs concentration in biochar groups compared with that in groups without biochar [88]. Guneet Kaur et al. found that biochar not only promoted the degradation of both acetic and butyric acids, but also facilitated propionic acid oxidation during the co-digestion of food waste with sludge mediated by biochar [89]. Meanwhile, biochar was also proven to promote the degradation of LCFAs especially valeric acid, caproic acid and iso-valeric acid. (3) Alleviate ammonia inhibition. Ammonia nitrogen is produced by the protein degradation contained in food waste, which is in the form of free ammonium nitrogen (NH3) and ionized ammonium nitrogen (NH4+) [90]. Both forms directly or indirectly inhibit the AD process. It is strongly required to reduce the ammonia concentration for improving the performance of the AD of food waste. Yun Peng et al. investigated the effect of biochar on alleviating NH4+-N inhibition during long-term anaerobic digestion of food waste [91]. The results indicated that the methane yield was decreased sharply without biochar when TAN concentration was higher than 2000 mg/L, while only a slight decline in methane yield was appeared with biochar addition. It was due to the adsorption of NH4+/NH3 by biochar which reduced the TAN concentration and ammonia toxicity. Chengyuan Su et al. proved that biochar could alleviate the NH4+inhibition in the anaerobic digestion of food waste due to facilitate the microbes grow, promote the formation of biofilms and increase the relative abundance of Methanoregulaceae [90]. (4)Increase biogas yield. Jiao Cai et al. observed that the maximum methane production rate was increased by 33.3%–275% after the addition of different biochar dosages in the AD of food waste [92]. Debnath Ovi et al. also found that methane yield was improved from 333.32 ± 14.9 mL/gVSadd to 455.83 ± 17.9 mL/gVSadd by the assistance of biochar [84].
The possible reasons for biochar improving the performance of anaerobic digestion are: (1) Property of biochar. The surface area and pore volume of biochar ranges from 2.7 m2/g-315.2 m2/g and from 0.01 cm3/g to 1.06 cm3/g respectively which makes its high adsorption capacity through physical adsorption, surface precipitation and complexation and pore-filling for the removal of contaminants [93]. Meanwhile, inorganic alkalis and organic alkalis functional groups contained in biochar can neutralize the VFAs which promote the buffering capacity of AD. (3) Facilitate direct interspecies electron transfer (DIET) between methanogens and other bacteria. Moreover, phenolic functional groups on the biochar can be acted as electron donors, while quinone and polycondensation aromatic functional groups can be acted as electron acceptors [94]. Biochar could reduce the need for cytochromec as an interspecific electronic linkage and improved the DIET pathway among Geobacter species, Defluviitoga, Thermovirga, Cloacibacillus and methanogenic archaea [95]. Yafan Cai found that biochar alleviate the ammonia inhibition mainly by accelerating of the DIET, promoting the growth of microorganisms, providing nutrients and adsorb ammonia nitrogen [96]. (4) Accelerate the growth of methanogens and hydrogen-producing bacterial. Yuxuan Cui found that biochar could enrich syntrophomonas and methanogens resulting in the methanogenic pathways shifting from acetoclastic/hydrogenotrophic methanogenic pathways to diverse methanogenic pathways [97].Similar results were obtained by Le Zhang et al. who also found that biochar simultaneously enriched Methanothermobacter and Methanosarcina and lead a synergy of hydrogenotrophic and acetoclastic methanogenic pathways [87].Meanwhile, the iron (Fe), calcium (Ca), potassium (K), Aluminium (Al) and Magnesium (Mg) contained in the biochar were proven to promote the growth of methanogens in AD [83].
Another cheaper material commonly used as the additive in the anaerobic digestion is iron-based materials like zero-valent iron (ZVI), magnetite (such as Fe3O4) and hematite (α-Fe2O3). Panliang Wang investigated the effect of ZVI, ferric oxide and magnetite nanoparticles on the AD of food waste and the results showed that those three iron-based materials could stimulate electron transfer by enriching syntrophic genera, primary acetate dependent methanogens resulting in an increase of biogas yield [98].The function of iron-based materials on the AD of food waste can be reflected as following aspects: (1)Reduce oxidation-reduction potential (ORP) and create a favorable environment to microbes [99]. Luo et al. found that the ORP was decreased from −145.42 mV to-272.7 mV after the addition of ZVI [99]. Xin Kong et al. found that acidogenesis in food waste digestion under a high OLR was butyric-type fermentation. While after addition of nZVI, butyric acid was converted to acetic acid resulted in an increase of methanogen activity [31]. (2)Increase biogas yield and promote COD removal rate. An increase of 67% and 36% respectively for COD removal rate and methane percentage were obtained in the 10 g/L ZVI addition in the AD of food waste leachate [100]. Tugui Yuan et al. also found that the average methane generation rate was increased by 44.2%–54.3% and the energy yield was promoted by 15.6% after the addition of ZVI on the AD of FW [101]. (3) Remove emerging contaminants. Pan Wang et al. found that the abundance of Firmicutes was reduced with nZVI addition resulting in the reduction of tetracycline genes in the anaerobic digestion of food waste [102]. Pin Gao also found that Fe0 could promote the reduction of tetracycline resistance genes during the anaerobic digestion of waste sludge and kitchen waste [80].
The potential mechanisms of iron-based materials on the anaerobic digestion mainly include: (1)Promote hydrogenotrophic methanogenesis by generating H2 from corrosion. During the corrosion of ZVI, hydrogen can be produced (Fe0+2H2O→Fe2++2OH− + H2) and further used as an electron donor for hydrogenotrophic methanogens to convert CO2 into CH4 [101].Meanwhile, ZVI can consume H+ through redox reaction and convert CO2 to produce methane (8H++4Fe0+CO2→CH4+4Fe2++2H2O). (2) Stimulate the activity of key enzymes. Fe2+ was proven to improve enzymatic activity like acetate kinase, phosphotrans acetylase, butyrate kinase [103] and phosphotrans butyrylase enzymes. Fe2+ can also increase the function of coenzyme F420 further promoting the conversion of CO2 and H2 into CH4 [104]. (3) Enhance DIET for methane production. α-Fe2O3-bentonite was proven to increase the abundance of hydrolytic acidification bacteria and hydrogenotrophic methanogens and promote DIET between syntrophic bacteria and methanogens [105]. However, ZVI was easily agglomerated which could reduce the activity of ZVI. Meanwhile, exceed nZVI dosage might increase H2 partial pressure which could inhibit the anaerobic digestion [106].
Some researchers investigated the biochar loaded ZVI and sulfidated nanoscale zero-valent iron (S-nZVI) on the AD of food waste. Pan Wang et al. investigated the biochar supported nano zero-valent iron (BC/nZVI) on two-phase anaerobic digestion of food waste and the results showed that BC/nZVI could enrich Defluviitoga in acidogenic phase and Methanothrix in methanogenic phase. Meanwhile, BC/nZVI improved the abundance of acetate kinase, butyrate kinase and other related enzymes [107]. Xinzi Wang found that BC-nZVI could promote hydrolysis process, reduce the inhibition of NH4+-N and avoid VFAs accumulation through enriching the methanogenesis and activating the related enzymes [108]. Dejin Zhang et al. found that S-nZVI could further strengthen the effect of nZVI in promoting the hydrogenotrophic methanogenesis and ammonia-tolerant hydrogenotrophic methanogenesis [109]. Dejin Zhang et al. invesitigated the effect of S-nZVI on the anaerobic digestion of food waste under high ammonia-stressed conditions and concluded that S-nZVI displayed a better performance in promoting the DIET than nZVI resulting in an increase of methane yield under high ammonia-stressed conditions [110].
The possible mechanism of biochar and iron-based materials were shown in Fig. 4.Till now, most of the researches are focused on the biogas yield, intermediate inhibition, system stable mediated by biochar and iron-based materials, few studies are focused the removal of emerging contaminants using biochar and ZVI and their mechanisms. Meanwhile, whether the energy consumed by the preparation of biochar and iron-based materials can compensate the biogas increased by the AD is worthy to study. Moreover, a moderate dose of iron-based materials can be easily controlled in the batch experiment at given operation conditions, while how to realize the iron recycling and avoid the accumulation of iron in a continuous experiment is required further study. Meanwhile, the further treatment of iron in biogas residue or biogas slurry also needs study.
Fig. 4.
Effect and possible mechanism of additives on the AD of FW.
5. Conclusions
Anaerobic digestion is an effective technology for the treatment of food waste, while it is often suffered by foaming, acidification and NH4+-N inhibition induced by FW property. Pretreatment can promote the hydrolysis and improve the biodegradability of complex compounds, co-digestion can balance nutrient levels, alleviate the NH4+-N inhibition, dilute toxic contaminants; the addition of additives can enrich microorganisms and shorten the digestion period. Though pretreatment, co-digestion and additives can improve the performance of FW anaerobic digestion based on the different mechanisms, there are still some works need further study to realize the “reducing quantity”, “harmless “and “reclamation” of the FW.
-
(1)
The optimization of pretreatments. The ideal pretreatment is characteristic of low energy input and low treatment cost while high energy obtained and high treatment efficiency. Future researches should focus on cutting down the energy consumption and operation cost through improving pretreatment efficiency, reducing enzymatic cost or combination of pretreatments.
-
(2)
Further screening for suitable substrates for co-digestion with food waste to decrease the risk of new pollutants brought in by the substrate. Meanwhile, further analyze the microbial composition and adaptability is required to increase the efficiency and stability of co-digestion system.
-
(3)
The utilization of biogas residue and biogas slurry as well as their impact on the environment after the addition of additives is also required further investigation. Meanwhile, the emerging contaminants removal during the AD of FW is crucial for harmless treatment.
-
(4)
Evaluate the life cycle assessment of FW anaerobic digestion to analyze the environmental and economic benefits. Meanwhile, the carbon balance during the whole AD process is also required.
Data availability statement
The data that support the findings of this study are available from the corresponding author (Qunpeng Cheng, cqp627@126.com), upon reasonable request.
CRediT authorship contribution statement
Kefang He: Writing – original draft. Ying Liu: Conceptualization. Longjin Tian: Investigation. Wanyou He: Writing – review & editing. Qunpeng Cheng: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would acknowledge the financial support provided by Hubei Jiebang science and technology project (2021BEC026), Science and technology association system deepening reform pilot demonstration and Research project (2023092101), and Hubei Important Project of Technological Innovation (2022BCA081).
References
- 1.Carmona-Cabello M., Leiva-Candia D., Castro-Cantarero J.L., Pinzi S., Dorado M.P. Valorization of food waste from restaurants by transesterification of the lipid fraction. Fuel. 2018;215:492–498. [Google Scholar]
- 2.Längauer D., Lin Y.-Y., Chen W.-H., Wang C.-W., Šafář M., Čablík V. Simultaneous extraction and emulsification of food waste liquefaction bio-oil. Energies. 2018;11:3031. [Google Scholar]
- 3.Pavlović N., Jokić S., Jakovljević M., Blažić M., Molnar M. Green extraction methods for active compounds from food waste-cocoa bean shell. Foods. 2020;9 doi: 10.3390/foods9020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.More P.R., Jambrak A.R., Arya S.S. Green, environment-friendly and sustainable techniques for extraction of food bioactive compounds and waste valorization. Trends Food Sci. Technol. 2022;128:296–315. [Google Scholar]
- 5.Arafat Y., Altemimi A., Ibrahim S.A., Badwaik L.S. Valorization of sweet lime peel for the extraction of essential oil by solvent free microwave extraction enhanced with ultrasound pretreatment. Molecules. 2020;25 doi: 10.3390/molecules25184072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freitas de Medeiros E., da Silva Afonso M., Ziemann dos Santos M.A., Bento F.M., Quadro M.S., Andreazza R. Physicochemical characterization of oil extraction from fishing waste for biofuel production. Renew. Energy. 2019;143:471–477. [Google Scholar]
- 7.Alvi T., Asif Z., Iqbal Khan M.K. Clean label extraction of bioactive compounds from food waste through microwave-assisted extraction technique-A review. Food Biosci. 2022;46 [Google Scholar]
- 8.Cerda A., Artola A., Font X., Barrena R., Gea T., Sánchez A. Composting of food wastes: status and challenges. Bioresour. Technol. 2018;248:57–67. doi: 10.1016/j.biortech.2017.06.133. [DOI] [PubMed] [Google Scholar]
- 9.Zhang R., El-Mashad H.M., Hartman K., Wang F., Liu G., Choate C., Gamble P. Characterization of food waste as feedstock for anaerobic digestion. Bioresour. Technol. 2007;98:929–935. doi: 10.1016/j.biortech.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 10.Wang J.Y., Xu H.L., Zhang H., Tay J.H. Semi-continuous anaerobic digestion of food waste using a hybrid anaerobic solid-liquid bioreactor. Water Sci. Technol. 2003;48:169–174. [PubMed] [Google Scholar]
- 11.Zhang C., Su H., Wang Z., Tan T., Qin P. Biogas by semi-continuous anaerobic digestion of food waste. Appl. Biochem. Biotechnol. 2015;175:3901–3914. doi: 10.1007/s12010-015-1559-5. [DOI] [PubMed] [Google Scholar]
- 12.den Boer J., Kobel P., den Boer E., Obersteiner G. Food waste quantities and composition in Polish households. Waste Manag. Res. 2023;41:1318–1330. doi: 10.1177/0734242X231155095. [DOI] [PubMed] [Google Scholar]
- 13.Edjabou M.E., Petersen C., Scheutz C., Astrup T.F. Food waste from Danish households: generation and composition. Waste Manag. 2016;52:256–268. doi: 10.1016/j.wasman.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 14.Adelodun B., Kim S.H., Choi K.-S. Assessment of food waste generation and composition among Korean households using novel sampling and statistical approaches. Waste Manag. 2021;122:71–80. doi: 10.1016/j.wasman.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Silvennoinen K., Katajajuuri J.-M., Hartikainen H., Heikkilä L., Reinikainen A. Food waste volume and composition in Finnish households. Br. Food J. 2014;116:1058–1068. [Google Scholar]
- 16.Qian L., Li F., Cao B., Wang L., Jin S. Determinants of food waste generation in Chinese university canteens: evidence from 9192 university students. Resour. Conserv. Recycl. 2021;167 [Google Scholar]
- 17.Abeliotis K., Lasaridi K., Boikou K., Chroni C. Food waste volume and composition in households in Greece. GlobalNEST International Journal. 2019;21:399–404. [Google Scholar]
- 18.Langley J., Yoxall A., Heppell G., Rodriguez E.M., Bradbury S., Lewis R., Luxmoore J., Hodzic A., Rowson J. Food for Thought? — a UK pilot study testing a methodology for compositional domestic food waste analysis. Waste Manag. Res. 2009;28:220–227. doi: 10.1177/0734242X08095348. [DOI] [PubMed] [Google Scholar]
- 19.Yamada T., Asari M., Miura T., Niijima T., Yano J., Sakai S-i. Municipal solid waste composition and food loss reduction in Kyoto City. J. Mater. Cycles Waste Manag. 2017;19:1351–1360. [Google Scholar]
- 20.Yu M., Wu C., Wang Q., Sun X., Ren Y., Li Y.-Y. Ethanol prefermentation of food waste in sequencing batch methane fermentation for improved buffering capacity and microbial community analysis. Bioresour. Technol. 2018;248:187–193. doi: 10.1016/j.biortech.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Wu L.-J., Kobayashi T., Li Y.-Y., Xu K.-Q. Comparison of single-stage and temperature-phased two-stage anaerobic digestion of oily food waste. Energy Convers. Manag. 2015;106:1174–1182. [Google Scholar]
- 22.Zhang L., Lee Y.-W., Jahng D. Anaerobic co-digestion of food waste and piggery wastewater: focusing on the role of trace elements. Bioresour. Technol. 2011;102:5048–5059. doi: 10.1016/j.biortech.2011.01.082. [DOI] [PubMed] [Google Scholar]
- 23.Kuruti K., Begum S., Ahuja S., Anupoju G.R., Juntupally S., Gandu B., Ahuja D.K. Exploitation of rapid acidification phenomena of food waste in reducing the hydraulic retention time (HRT) of high rate anaerobic digester without conceding on biogas yield. Bioresour. Technol. 2017;226:65–72. doi: 10.1016/j.biortech.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Tong H., Shen Y., Zhang J., Wang C.-H., Ge T.S., Tong Y.W. A comparative life cycle assessment on four waste-to-energy scenarios for food waste generated in eateries. Appl. Energy. 2018;225:1143–1157. [Google Scholar]
- 25.Kougias P.G., Boe K., Ot S., Kristensen L.A., Angelidaki I. Anaerobic digestion foaming in full-scale biogas plants: a survey on causes and solutions. Water Sci. Technol. : a journal of the International Association on Water Pollution Research. 2014;69:889–895. doi: 10.2166/wst.2013.792. [DOI] [PubMed] [Google Scholar]
- 26.He Q., Li L., Zhao X., Qu L., Wu D., Peng X. Investigation of foaming causes in three mesophilic food waste digesters: reactor performance and microbial analysis. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-14258-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang P., Peng Y., Liu H., Wu D., Yuan R., Wang X., Li L., Peng X. Multi-scale analysis of the foaming mechanism in anaerobic digestion of food waste: from physicochemical parameter, microbial community to metabolite response. Water Res. 2022;218 doi: 10.1016/j.watres.2022.118482. [DOI] [PubMed] [Google Scholar]
- 28.Yang P., Peng Y., Tan H., Liu H., Wu D., Wang X., Li L., Peng X. Foaming mechanisms and control strategies during the anaerobic digestion of organic waste: a critical review. Sci. Total Environ. 2021;779 doi: 10.1016/j.scitotenv.2021.146531. [DOI] [PubMed] [Google Scholar]
- 29.Tanimu M.I., Mohd Ghazi Ti, Harun M.R., Idris A. Effects of feedstock carbon to nitrogen ratio and organic loading on foaming potential in mesophilic food waste anaerobic digestion. Appl. Microbiol. Biotechnol. 2015;99:4509–4520. doi: 10.1007/s00253-015-6486-4. [DOI] [PubMed] [Google Scholar]
- 30.Shiyan G., Wenyi Z., Huige X., Ruji W., Jiyang S., Min Z., Yi L. The effect of anaerobic co-fermentation on acidification performance of food waste and cardboard waste. Water Sci. Technol. 2022;85:839–850. doi: 10.2166/wst.2022.002. [DOI] [PubMed] [Google Scholar]
- 31.Kong X., Wei Y., Xu S., Liu J., Li H., Liu Y., Yu S. Inhibiting excessive acidification using zero-valent iron in anaerobic digestion of food waste at high organic load rates. Bioresour. Technol. 2016;211:65–71. doi: 10.1016/j.biortech.2016.03.078. [DOI] [PubMed] [Google Scholar]
- 32.Serna-Maza A., Heaven S., Banks C.J. Ammonia removal in food waste anaerobic digestion using a side-stream stripping process. Bioresour. Technol. 2014;152:307–315. doi: 10.1016/j.biortech.2013.10.093. [DOI] [PubMed] [Google Scholar]
- 33.Sheng K., Chen X., Pan J., Kloss R., Wei Y., Ying Y. Effect of ammonia and nitrate on biogas production from food waste via anaerobic digestion. Biosyst. Eng. 2013;116:205–212. [Google Scholar]
- 34.Chen H., Wang W., Xue L., Chen C., Liu G., Zhang R. Effects of ammonia on anaerobic digestion of food waste: process performance and microbial community. Energy & Fuels. 2016;30:5749–5757. [Google Scholar]
- 35.Yenigün O., Demirel B. Ammonia inhibition in anaerobic digestion: a review. Process Biochem. 2013;48:901–911. [Google Scholar]
- 36.Zhang C., Su H., Baeyens J., Tan T. Reviewing the anaerobic digestion of food waste for biogas production. Renew. Sustain. Energy Rev. 2014;38:383–392. [Google Scholar]
- 37.Walker M., Iyer K., Heaven S., Banks C.J. Ammonia removal in anaerobic digestion by biogas stripping: an evaluation of process alternatives using a first order rate model based on experimental findings. Chem. Eng. J. 2011;178:138–145. [Google Scholar]
- 38.Melgaço L.A.O., Meers E., Mota C.R. Ammonia recovery from food waste digestate using solar heat-assisted stripping-absorption. Waste Manag. 2020;113:244–250. doi: 10.1016/j.wasman.2020.05.047. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J., Zhang L., Loh K.-C., Dai Y., Tong Y.W. Enhanced anaerobic digestion of food waste by adding activated carbon: fate of bacterial pathogens and antibiotic resistance genes. Biochem. Eng. J. 2017;128:19–25. [Google Scholar]
- 40.He P., Yu Z., Shao L., Zhou Y., Lü F. Fate of antibiotics and antibiotic resistance genes in a full-scale restaurant food waste treatment plant: implications of the roles beyond heavy metals and mobile genetic elements. J. Environ. Sci. 2019;85:17–34. doi: 10.1016/j.jes.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Wang P., Qiao Z., Li X., Wu D., Xie B. Fate of integrons, antibiotic resistance genes and associated microbial community in food waste and its large-scale biotreatment systems. Environ. Int. 2020;144 doi: 10.1016/j.envint.2020.106013. [DOI] [PubMed] [Google Scholar]
- 42.Kanger K., Guilford N.G.H., Lee H., Nesbø C.L., Truu J., Edwards E.A. Antibiotic resistome and microbial community structure during anaerobic co-digestion of food waste, paper and cardboard. FEMS Microbiol. Ecol. 2020;96 doi: 10.1093/femsec/fiaa006. [DOI] [PubMed] [Google Scholar]
- 43.Liao H., Friman V.-P., Geisen S., Zhao Q., Cui P., Lu X., Chen Z., Yu Z., Zhou S. Horizontal gene transfer and shifts in linked bacterial community composition are associated with maintenance of antibiotic resistance genes during food waste composting. Sci. Total Environ. 2019;660:841–850. doi: 10.1016/j.scitotenv.2018.12.353. [DOI] [PubMed] [Google Scholar]
- 44.Ezzariai A., Hafidi M., Khadra A., Aemig Q., El Fels L., Barret M., Merlina G., Patureau D., Pinelli E. Human and veterinary antibiotics during composting of sludge or manure: global perspectives on persistence, degradation, and resistance genes. J. Hazard Mater. 2018;359:465–481. doi: 10.1016/j.jhazmat.2018.07.092. [DOI] [PubMed] [Google Scholar]
- 45.Liu W., Li J., Li X., Tian Y., Meng J., Zheng M., Yuan Z. Increasing the removal efficiency of antibiotic resistance through anaerobic digestion with free nitrous acid pretreatment. J. Hazard Mater. 2022;438 doi: 10.1016/j.jhazmat.2022.129535. [DOI] [PubMed] [Google Scholar]
- 46.Long S., Liu X., Chen J., Zhao L., Pavlostathis S.G. Effect of tetracycline on bio-electrochemically assisted anaerobic methanogenic systems: process performance, microbial community structure, and functional genes. Sci. Total Environ. 2022;837 doi: 10.1016/j.scitotenv.2022.155756. [DOI] [PubMed] [Google Scholar]
- 47.Cheng D., Ngo H.H., Guo W., Chang S.W., Nguyen D.D., Liu Y., Shan X., Nghiem L.D., Nguyen L.N. Removal process of antibiotics during anaerobic treatment of swine wastewater. Bioresour. Technol. 2020;300 doi: 10.1016/j.biortech.2019.122707. [DOI] [PubMed] [Google Scholar]
- 48.O'Connor J., Mickan B.S., Siddique K.H.M., Rinklebe J., Kirkham M.B., Bolan N.S. Physical, chemical, and microbial contaminants in food waste management for soil application: a review. Environ. Pollut. 2022;300 doi: 10.1016/j.envpol.2022.118860. [DOI] [PubMed] [Google Scholar]
- 49.Li Q., Ma C., Zhang Q., Shi H. Microplastics in shellfish and implications for food safety. Curr. Opin. Food Sci. 2021;40:192–197. [Google Scholar]
- 50.De-la-Torre G.E. Microplastics: an emerging threat to food security and human health. J. Food Sci. Technol. 2020;57:1601–1608. doi: 10.1007/s13197-019-04138-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li L., Geng S., Li Z., Song K. Effect of microplastic on anaerobic digestion of wasted activated sludge. Chemosphere. 2020;247 doi: 10.1016/j.chemosphere.2020.125874. [DOI] [PubMed] [Google Scholar]
- 52.Fu S.-F., Ding J.-N., Zhang Y., Li Y.-F., Zhu R., Yuan X.-Z., Zou H. Exposure to polystyrene nanoplastic leads to inhibition of anaerobic digestion system. Sci. Total Environ. 2018;625:64–70. doi: 10.1016/j.scitotenv.2017.12.158. [DOI] [PubMed] [Google Scholar]
- 53.Wei W., Huang Q.-S., Sun J., Dai X., Ni B.-J. Revealing the mechanisms of polyethylene microplastics affecting anaerobic digestion of waste activated sludge. Environ. Sci. Technol. 2019;53:9604–9613. doi: 10.1021/acs.est.9b02971. [DOI] [PubMed] [Google Scholar]
- 54.Zhang J., Zhao M., Li C., Miao H., Huang Z., Dai X., Ruan W. Evaluation the impact of polystyrene micro and nanoplastics on the methane generation by anaerobic digestion. Ecotoxicol. Environ. Saf. 2020;205 doi: 10.1016/j.ecoenv.2020.111095. [DOI] [PubMed] [Google Scholar]
- 55.Ariunbaatar J., Panico A., Yeh D.H., Pirozzi F., Lens P.N.L., Esposito G. Enhanced mesophilic anaerobic digestion of food waste by thermal pretreatment: substrate versus digestate heating. Waste Manag. 2015;46:176–181. doi: 10.1016/j.wasman.2015.07.045. [DOI] [PubMed] [Google Scholar]
- 56.Ormaechea P., Castrillón L., Marañón E., Fernández-Nava Y., Negral L., Megido L. Influence of the ultrasound pretreatment on anaerobic digestion of cattle manure, food waste and crude glycerine. Environ. Technol. 2017;38:682–686. doi: 10.1080/09593330.2016.1208278. [DOI] [PubMed] [Google Scholar]
- 57.Fei X., Jia W., Chen T., Ling Y. Life cycle assessment of food waste anaerobic digestion with hydrothermal and ionizing radiation pretreatment. J. Clean. Prod. 2022;338 [Google Scholar]
- 58.Li J., Zhang W., Li X., Ye T., Gan Y., Zhang A., Chen H., Xue G., Liu Y. Production of lactic acid from thermal pretreated food waste through the fermentation of waste activated sludge: effects of substrate and thermal pretreatment temperature. Bioresour. Technol. 2018;247:890–896. doi: 10.1016/j.biortech.2017.09.186. [DOI] [PubMed] [Google Scholar]
- 59.Linyi C., Yujie Q., Buqing C., Chenglong W., Shaohong Z., Renglu C., Shaohua Y., Lan Y., Zhiju L. Enhancing degradation and biogas production during anaerobic digestion of food waste using alkali pretreatment. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109743. [DOI] [PubMed] [Google Scholar]
- 60.Naran E., Toor U.A., Kim D.-J. Effect of pretreatment and anaerobic co-digestion of food waste and waste activated sludge on stabilization and methane production. Int. Biodeterior. Biodegrad. 2016;113:17–21. [Google Scholar]
- 61.Meng Y., Luan F., Yuan H., Chen X., Li X. Enhancing anaerobic digestion performance of crude lipid in food waste by enzymatic pretreatment. Bioresour. Technol. 2017;224:48–55. doi: 10.1016/j.biortech.2016.10.052. [DOI] [PubMed] [Google Scholar]
- 62.Yin Y., Liu Y.-J., Meng S.-J., Kiran E.U., Liu Y. Enzymatic pretreatment of activated sludge, food waste and their mixture for enhanced bioenergy recovery and waste volume reduction via anaerobic digestion. Appl. Energy. 2016;179:1131–1137. [Google Scholar]
- 63.Gnaoui Y.E., Karouach F., Bakraoui M., Barz M., Bari H.E. Mesophilic anaerobic digestion of food waste: effect of thermal pretreatment on improvement of anaerobic digestion process. Energy Rep. 2020;6:417–422. [Google Scholar]
- 64.Fei X., Chen T., Jia W., Shan Q., Hei D., Ling Y., Feng J., Feng H. Enhancement effect of ionizing radiation pretreatment on biogas production from anaerobic fermentation of food waste. Radiat. Phys. Chem. 2020;168 [Google Scholar]
- 65.Yue L., Cheng J., Tang S., An X., Hua J., Dong H., Zhou J. Ultrasound and microwave pretreatments promote methane production potential and energy conversion during anaerobic digestion of lipid and food wastes. Energy. 2021;228 [Google Scholar]
- 66.Sun J., Zhang Y., Pan X., Zhu G. The effects of anionic and non-ionic surfactant on anaerobic co-digestion of sludge, food wastes and green wastes. Environ. Technol. 2019;40:2538–2547. doi: 10.1080/09593330.2018.1446457. [DOI] [PubMed] [Google Scholar]
- 67.Mendes A.A., Pereira E.B., de Castro H.F. Effect of the enzymatic hydrolysis pretreatment of lipids-rich wastewater on the anaerobic biodigestion. Biochem. Eng. J. 2006;32:185–190. [Google Scholar]
- 68.Chuenchart W., Logan M., Leelayouthayotin C., Visvanathan C. Enhancement of food waste thermophilic anaerobic digestion through synergistic effect with chicken manure. Biomass Bioenergy. 2020;136 [Google Scholar]
- 69.Khanthong K., Kadam R., Kim T., Park J. Synergetic effects of anaerobic co-digestion of food waste and algae on biogas production. Bioresour. Technol. 2023;382 doi: 10.1016/j.biortech.2023.129208. [DOI] [PubMed] [Google Scholar]
- 70.Zhang J., Li W., Lee J., Loh K.-C., Dai Y., Tong Y.W. Enhancement of biogas production in anaerobic co-digestion of food waste and waste activated sludge by biological co-pretreatment. Energy. 2017;137:479–486. [Google Scholar]
- 71.Wu L.-J., Kobayashi T., Kuramochi H., Li Y.-Y., Xu K.-Q. Improved biogas production from food waste by co-digestion with de-oiled grease trap waste. Bioresour. Technol. 2016;201:237–244. doi: 10.1016/j.biortech.2015.11.061. [DOI] [PubMed] [Google Scholar]
- 72.Zhang W., Wang X., Xing W., Li R., Yang T., Yao N., Lv D. Links between synergistic effects and microbial community characteristics of anaerobic co-digestion of food waste, cattle manure and corn straw. Bioresour. Technol. 2021;329 doi: 10.1016/j.biortech.2021.124919. [DOI] [PubMed] [Google Scholar]
- 73.Zhang C., Xiao G., Peng L., Su H., Tan T. The anaerobic co-digestion of food waste and cattle manure. Bioresour. Technol. 2013;129:170–176. doi: 10.1016/j.biortech.2012.10.138. [DOI] [PubMed] [Google Scholar]
- 74.Ma X., Yu M., Yang M., Gao M., Wu C., Wang Q. Synergistic effect from anaerobic co-digestion of food waste and Sophora flavescens residues at different co-substrate ratios. Environ. Sci. Pollut. Control Ser. 2019;26:37114–37124. doi: 10.1007/s11356-019-06399-x. [DOI] [PubMed] [Google Scholar]
- 75.Du X., Tao Y., Li H., Liu Y., Feng K. Synergistic methane production from the anaerobic co-digestion of Spirulina platensis with food waste and sewage sludge at high solid concentrations. Renew. Energy. 2019;142:55–61. [Google Scholar]
- 76.Peng Y., Li L., Yuan W., Wu D., Yang P., Peng X. Long-term evaluation of the anaerobic co-digestion of food waste and landfill leachate to alleviate ammonia inhibition. Energy Convers. Manag. 2022;270 [Google Scholar]
- 77.Jiang S., Yu D., Xiong F., Lian X., Jiang X. Enhanced methane production from the anaerobic co-digestion of food waste plus fruit and vegetable waste. Environ. Sci. Pollut. Control Ser. 2023;30:70592–70603. doi: 10.1007/s11356-023-27328-z. [DOI] [PubMed] [Google Scholar]
- 78.Xing B.-S., Wang X.C. High-rate mesophilic co-digestion with food waste and waste activated sludge through a low-magnitude increasing loading regime: performance and microorganism characteristics. Sci. Total Environ. 2021;777 [Google Scholar]
- 79.Kasumba J., Appala K., Agga G.E., Loughrin J.H., Conte E.D. Anaerobic digestion of livestock and poultry manures spiked with tetracycline antibiotics. Journal of Environmental Science and Health, Part B. 2020;55:135–147. doi: 10.1080/03601234.2019.1667190. [DOI] [PubMed] [Google Scholar]
- 80.Gao P., Gu C., Wei X., Li X., Chen H., Jia H., Liu Z., Xue G., Ma C. The role of zero valent iron on the fate of tetracycline resistance genes and class 1 integrons during thermophilic anaerobic co-digestion of waste sludge and kitchen waste. Water Res. 2017;111:92–99. doi: 10.1016/j.watres.2016.12.047. [DOI] [PubMed] [Google Scholar]
- 81.Gao M., Yang M., Ma X., Xie D., Wu C., Wang Q. Effect of co-digestion of tylosin fermentation dreg and food waste on anaerobic digestion performance. Bioresour. Technol. 2021;325 doi: 10.1016/j.biortech.2021.124693. [DOI] [PubMed] [Google Scholar]
- 82.Lee J.T.E., Ok Y.S., Song S., Dissanayake P.D., Tian H., Tio Z.K., Cui R., Lim E.Y., Jong M.-C., Hoy S.H., Lum T.Q.H., Tsui T.-H., Yoon C.S., Dai Y., Wang C.-H., Tan H.T.W., Tong Y.W. Biochar utilisation in the anaerobic digestion of food waste for the creation of a circular economy via biogas upgrading and digestate treatment. Bioresour. Technol. 2021;333 doi: 10.1016/j.biortech.2021.125190. [DOI] [PubMed] [Google Scholar]
- 83.Sugiarto Y., Sunyoto N.M.S., Zhu M., Jones I., Zhang D. Effect of biochar addition on microbial community and methane production during anaerobic digestion of food wastes: the role of minerals in biochar. Bioresour. Technol. 2021;323 doi: 10.1016/j.biortech.2020.124585. [DOI] [PubMed] [Google Scholar]
- 84.Ovi D., Chang S.W., Wong J.W.C., Johnravindar D., Varjani S., Hoon Jeung J., Chung W.J., Thirupathi A., Ravindran B. Effect of rice husk and palm tree-based biochar addition on the anaerobic digestion of food waste/sludge. Fuel. 2022;315 [Google Scholar]
- 85.Cheng Q., Xu C., Huang W., Jiang M., Song G. Improving anaerobic digestion of piggery wastewater by alleviating stress of ammonia using biochar derived from rice straw. Environ. Technol. Innovat. 2020;19 [Google Scholar]
- 86.Xu C., Ding Y., Liu J., Huang W., Cheng Q., Fan G., Yan J., Zhang S., Song G., Xiao B. Anaerobic digestion of sulphate wastewater mediated by biochar. Environ. Technol. 2021:1–12. doi: 10.1080/09593330.2021.2011428. [DOI] [PubMed] [Google Scholar]
- 87.Zhang L., Lim E.Y., Loh K.-C., Ok Y.S., Lee J.T.E., Shen Y., Wang C.-H., Dai Y., Tong Y.W. Biochar enhanced thermophilic anaerobic digestion of food waste: focusing on biochar particle size, microbial community analysis and pilot-scale application. Energy Convers. Manag. 2020;209 [Google Scholar]
- 88.Zhu Y., Jin Z., Yu Q., Zhao Z., Zhang Y. Alleviating acid inhibition in anaerobic digestion of food waste: coupling ethanol-type fermentation with biochar addition. Environ. Res. 2022;212 doi: 10.1016/j.envres.2022.113355. [DOI] [PubMed] [Google Scholar]
- 89.Kaur G., Johnravindar D., Wong J.W.C. Enhanced volatile fatty acid degradation and methane production efficiency by biochar addition in food waste-sludge co-digestion: a step towards increased organic loading efficiency in co-digestion. Bioresour. Technol. 2020;308 doi: 10.1016/j.biortech.2020.123250. [DOI] [PubMed] [Google Scholar]
- 90.Su C., Zhao L., Liao L., Qin J., Lu Y., Deng Q., Chen M., Huang Z. Application of biochar in a CIC reactor to relieve ammonia nitrogen stress and promote microbial community during food waste treatment. J. Clean. Prod. 2019;209:353–362. [Google Scholar]
- 91.Peng Y., Li L., Dong Q., Yang P., Liu H., Ye W., Wu D., Peng X. Evaluation of digestate-derived biochar to alleviate ammonia inhibition during long-term anaerobic digestion of food waste. Chemosphere. 2023;311 doi: 10.1016/j.chemosphere.2022.137150. [DOI] [PubMed] [Google Scholar]
- 92.Cai J., He P., Wang Y., Shao L., Lue F. Effects and optimization of the use of biochar in anaerobic digestion of food wastes. Waste Management & Research the Journal of the International Solid Wastes & Public Cleansing Association Iswa. 2016;34:409. doi: 10.1177/0734242X16634196. [DOI] [PubMed] [Google Scholar]
- 93.Beneficial role of biochar addition on the anaerobic digestion of food waste: a systematic and critical review of the operational parameters and mechanisms. Ambaye T.G., Rene E.R., Nizami A.-S., Dupont C., Vaccari M., van Hullebusch, editors. J. Environ. Manag. 2021;290 doi: 10.1016/j.jenvman.2021.112537. [DOI] [PubMed] [Google Scholar]
- 94.Zhao D., Yan B., Liu C., Yao B., Luo L., Yang Y., Liu L., Wu F., Zhou Y. Mitigation of acidogenic product inhibition and elevated mass transfer by biochar during anaerobic digestion of food waste. Bioresour. Technol. 2021;338 doi: 10.1016/j.biortech.2021.125531. [DOI] [PubMed] [Google Scholar]
- 95.Cai Y., Gallegos D., Zheng Z., Stinner W., Wang X., Pröter J., Schäfer F. Exploring the combined effect of total ammonia nitrogen, pH and temperature on anaerobic digestion of chicken manure using response surface methodology and two kinetic models. Bioresour. Technol. 2021;337 doi: 10.1016/j.biortech.2021.125328. [DOI] [PubMed] [Google Scholar]
- 96.Cai Y., Zhu M., Meng X., Zhou J.L., Zhang H., Shen X. The role of biochar on alleviating ammonia toxicity in anaerobic digestion of nitrogen-rich wastes: a review. Bioresour. Technol. 2022;351 doi: 10.1016/j.biortech.2022.126924. [DOI] [PubMed] [Google Scholar]
- 97.Cui Y., Mao F., Zhang J., He Y., Tong Y.W., Peng Y. Biochar enhanced high-solid mesophilic anaerobic digestion of food waste: cell viability and methanogenic pathways. Chemosphere. 2021;272 doi: 10.1016/j.chemosphere.2021.129863. [DOI] [PubMed] [Google Scholar]
- 98.Wang P., Li X., Li Y., Su Y., Wu D., Xie B. Enhanced anaerobic digestion performance of food waste by zero-valent iron and iron oxides nanoparticles: comparative analyses of microbial community and metabolism. Bioresour. Technol. 2023;371 doi: 10.1016/j.biortech.2023.128633. [DOI] [PubMed] [Google Scholar]
- 99.Luo J., Feng L., Chen Y., Li X., Chen H., Xiao N., Wang D. Stimulating short-chain fatty acids production from waste activated sludge by nano zero-valent iron. J. Biotechnol. 2014;187:98–105. doi: 10.1016/j.jbiotec.2014.07.444. [DOI] [PubMed] [Google Scholar]
- 100.Antwi P., Zhang D., Luo W., Kabutey F.T., Li J., Su H., Wu M., Liu Z. Response of hydrolysis, methanogenesis, and microbial community structure to iron dose during anaerobic digestion of food waste leachate. Biomass Conversion and Biorefinery. 2022;12:5959–5973. [Google Scholar]
- 101.Yuan T., Shi X., Sun R., Ko J.H., Xu Q. Simultaneous addition of biochar and zero-valent iron to improve food waste anaerobic digestion. J. Clean. Prod. 2021;278 [Google Scholar]
- 102.Wang P., Chen X., Liang X., Cheng M., Ren L. Effects of nanoscale zero-valent iron on the performance and the fate of antibiotic resistance genes during thermophilic and mesophilic anaerobic digestion of food waste. Bioresour. Technol. 2019;293 doi: 10.1016/j.biortech.2019.122092. [DOI] [PubMed] [Google Scholar]
- 103.Wei J., Hao X., van Loosdrecht M.C.M., Li J. Feasibility analysis of anaerobic digestion of excess sludge enhanced by iron: a review. Renew. Sustain. Energy Rev. 2018;89:16–26. [Google Scholar]
- 104.Zhao Z., Zhang Y., Li Y., Quan X., Zhao Z. Comparing the mechanisms of ZVI and Fe3O4 for promoting waste-activated sludge digestion. Water Res. 2018;144:126–133. doi: 10.1016/j.watres.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 105.Zhu R., Chen Y., Zhao T., Jiang Q., Wang H., Zheng L., Shi D., Zhai J., He Q., Gu L. Enhanced mesophilic anaerobic co-digestion of waste sludge and food waste by using hematite (α-Fe2O3) supported bentonite as additive. Bioresour. Technol. 2020;313 doi: 10.1016/j.biortech.2020.123603. [DOI] [PubMed] [Google Scholar]
- 106.Huang Y.-X., Guo J., Zhang C., Hu Z. Hydrogen production from the dissolution of nano zero valent iron and its effect on anaerobic digestion. Water Res. 2016;88:475–480. doi: 10.1016/j.watres.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 107.Wang P., Yu M., Lin P., Zheng Y., Ren L. Effects of biochar supported nano zero-valent iron with different carbon/iron ratios on two-phase anaerobic digestion of food waste. Bioresour. Technol. 2023;382 doi: 10.1016/j.biortech.2023.129158. [DOI] [PubMed] [Google Scholar]
- 108.Wang X., Wang P., Meng X., Ren L. Performance and metagenomics analysis of anaerobic digestion of food waste with adding biochar supported nano zero-valent iron under mesophilic and thermophilic condition. Sci. Total Environ. 2022;820 doi: 10.1016/j.scitotenv.2022.153244. [DOI] [PubMed] [Google Scholar]
- 109.Zhang D., Wei Y., Wu S., Zhou L. Consolidation of hydrogenotrophic methanogenesis by sulfidated nanoscale zero-valent iron in the anaerobic digestion of food waste upon ammonia stress. Sci. Total Environ. 2022;822 doi: 10.1016/j.scitotenv.2022.153531. [DOI] [PubMed] [Google Scholar]
- 110.Zhang D., Lu P., Zhang M., Wei Y., Liang J., Zhou L. Elucidating interactive effects of sulfidated nanoscale zero-valent iron and ammonia on anaerobic digestion of food waste. J. Biosci. Bioeng. 2023;135:63–70. doi: 10.1016/j.jbiosc.2022.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (Qunpeng Cheng, cqp627@126.com), upon reasonable request.