Abstract
Lung cancer carries one of the highest mortality rates among all cancers. It is often diagnosed at more advanced stages with limited treatment options compared to other malignancies. This study focuses on calnexin as a potential biomarker for diagnosis and treatment of lung cancer. Calnexin, a molecular chaperone integral to N-linked glycoprotein synthesis, has shown some associations with cancer. However, targeted therapeutic or diagnostic methods using calnexin have been proposed. Through 1D-LCMSMS, we identified calnexin as a biomarker for lung cancer and substantiated its expression in human lung cancer cell membranes using Western blotting, flow cytometry, and immunocytochemistry. Anti-calnexin antibodies exhibited complement-dependent cytotoxicity to lung cancer cell lines, resulting in a notable reduction in tumor growth in a subcutaneous xenograft model. Additionally, we verified the feasibility of labeling tumors through in vivo imaging using antibodies against calnexin. Furthermore, exosomal detection of calnexin suggested the potential utility of liquid biopsy for diagnostic purposes. In conclusion, this study establishes calnexin as a promising target for antibody-based lung cancer diagnosis and therapy, unlocking novel avenues for early detection and treatment.
Keywords: Antibody-based therapy, Biomarker, Calnexin, Lung cancer, Membrane protein
INTRODUCTION
Lung cancer presenting the highest mortality rates in both men and women worldwide, is the primary cause of cancer-related deaths (1). Data from the United States in 2022 revealed that lung cancer was the second most common type of cancer among newly registered cancer patients, constituting 12% and 13% of cancer cases in men and women, respectively (2). Despite its lower incidence compared to prostate cancer and breast cancer, mortality rate of lung cancer surpasses those of both prostate cancer and breast cancer, being approximately twice that of prostate cancer and 1.4 times that of breast cancer (2).
The high mortality rate associated with lung cancer is attributed to challenges in its early diagnosis (3) and limited availability of effective treatment options (4, 5). Recognizing that using antibody-based therapies is a successful strategy with lower toxicity than conventional chemotherapy (6, 7), the quest for biomarkers seeks to discern abnormalities in diseased tissues compared to healthy tissues (8). Particular membrane proteins often overexpressed on cancer cell membranes or uniquely expressed on certain cancer cells are potential targets for therapeutic agents, obviating the need for related drug delivery vehicles (9). A notable example is trastuzumab, which has demonstrated remarkable antitumor efficacy by targeting HER2 in breast cancer (10). Furthermore, membrane proteins can be directly utilized for tumor-targeted imaging. Antibodies binding to these proteins combined with radionuclides and/or near-infrared fluorescent dyes can facilitate direct tumor visualization before or during surgery (11). The recognition of exosomes has expanded liquid biopsy capabilities, allowing body fluids to be used for direct detection of proteins with increased expression in cancer cell membranes (12). Against this backdrop, our study focused on novel lung cancer-specific membrane proteins. While various efforts have been made to discover biomarkers for lung cancer treatment and diagnosis, existing methods face challenges such as effectiveness limited to specific patient groups or susceptibility to resistance (13). Our current investigation centers on calnexin as a potential biomarker for lung cancer treatment and diagnosis. This molecular chaperone is crucial for the synthesis of N-linked glycoproteins within the secretory pathway (14). It has been associated with tumor invasion and metastasis (15). Additionally, calnexin’s plasma membrane localization has been observed in cancerous tumors such as oral squamous cell carcinoma and melanoma (16). Another study has reported the secretion of calnexin in sera of lung cancer patients, suggesting its potential as a serum diagnostic marker (17).
Despite these findings, no studies on treatment and diagnosis of lung cancer using antibodies against calnexin have been reported. Thus, the present study aimed to evaluate the potential of calnexin as a target for lung cancer treatment and diagnosis.
RESULTS
Lung cancer cell-specific expression
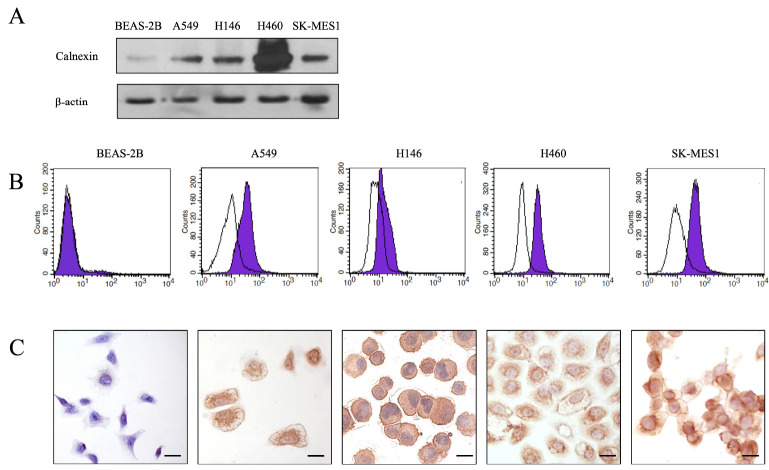
To validate the expression of calnexin on the membrane of human lung cancer cell lines, we employed a combination of techniques, including Western blotting, immunocytochemistry, and flow cytometry. First, SDS-PAGE was conducted to separate proteins from a normal human lung epithelial cell line (Beas2B) and four representative human lung cancer cell lines.
Primary lung cancer is typically classified into four major subtypes based on its size and morphology. For our study, we utilized representative model cells for each subtype, including A549 cell line (adenocarcinoma), H146 cell line (small cell carcinoma), H460 cell line (large cell carcinoma), and SK-MES1 (squamous cell carcinoma).
Subsequent Western blot analysis with an anti-calnexin antibody was performed to confirm specific expression of calnexin in human lung cancer cell lines. Calnexin was consistently detected at a size of 90 kDa in all four human lung cancer cell lines. In contrast, significantly lower expression was detected in normal lung epithelial cells (Fig. 1A). In addition, we utilized flow cytometry and immunocytochemistry to investigate surface expression of calnexin. In all four types of human lung cancer cell lines, the peak of cells incubated with the anti-alnexin antibody shifted to the right compared to control peaks, confirming the presence of calnexin on cell surfaces (Fig. 1B). However, Beas2B cells displayed almost the same histogram as the control, indicating minimal calnexin expression on the cell surface.
Fig. 1.
Calnexin protein expression in lung cancer cells. (A) Western blot analysis revealing elevated calnexin expression in A549, NCI-H146, NCI-H460, and SK-MES-1 lung cancer cell lines compared to a normal epithelial cell line Beas2B. (B) Flow cytometry histogram showing that the calnexin antibody can strongly bind to the surface of lung cancer cells (purple shaded area) with minimal binding to Beas2B (black curve). The experiment was performed in duplicate at least three times. (C) Lung cancer cells exhibit abundant membrane and ER binding, while Beas2B cells show minimal binding, particularly on the ER (× 400; scale bar, 20 μm).
Membrane expression of calnexin was further supported by immunocytochemistry, where cell membranes were stained brown due to reaction between DAB substrate and HRP (Fig. 1C). As depicted in the figure, cancer cells showed stronger overall staining, with expression predominantly concentrated on the cell membrane and endoplasmic reticulum (ER).
In vitro and in vivo tumor toxicity of the anti-calnexin antibody
The impact of the anti-calnexin antibody on cancer cells was assessed using four lung cancer cell lines previously identified as expressing calnexin. No significant effect of the anti-calnexin antibody on normal Beas2B cells was observed regarding the presence of complement (data not shown).
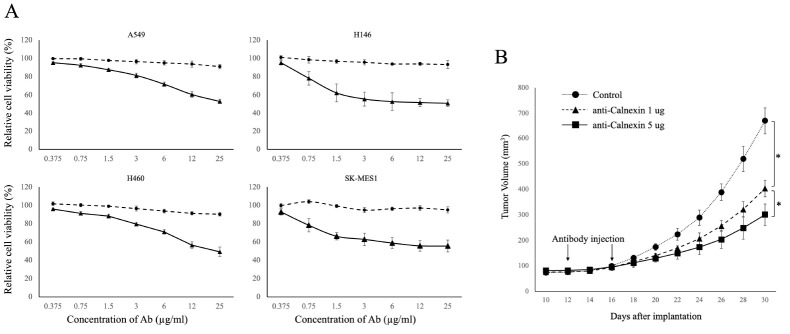
Treatment with the anti-calnexin antibody alone for 24 hours did not result in any significant changes compared to the control group (Fig. 2A, filled circles). However, when the antibody was co-administered with 5% serum, a reduction in cancer cell viability was observed in all lung cancer cell lines. Although the extent of the reduction varied depending on the specific cancer cell line, antibodies at a concentration of 10 μg/ml or higher reduced viability by approximately 60% when cells were treated with a serum containing a complement system.
Fig. 2.
In vitro and in vivo tumor toxicity of anti-calnexin antibody. (A) The dotted line illustrates concentration-dependent cytotoxicity, while the solid line represents complement-dependent cytotoxicity when the complement system is activated by adding goat serum to achieve a final concentration of 5% (vol/vol). Experiments were performed in duplicate at least three times. (B) Antitumor effect of anti-calnexin antibody. A549 cells were subcutaneously injected into backs of nude mice and treated intraperitoneally with 1 μg and 5 μg of anti-calnexin antibody on days 12 and 16 when the tumor size reached 70-150 mm3 after 10 days. *P-value < 0.05 (n = 9).
Next, effect of the anti-calnexin antibody on lung cancer in vivo was assessed. We subcutaneously injected 1 × 106 A549 cells into backs of BALB/c nude mice on day 0. Mice were divided into three groups. One group was treated with 1 μg of anti-calnexin antibody via intraperitoneal injection on the 12th and 16th days after cell injection. One group was treated with 5 μg of anti-calnexin antibody. The third group, a control group, received an equivalent amount of isotype antibody.
Tumor volumes were assessed every other day over a 30-day period. As illustrated in Fig. 2B, mice injected with calnexin antibodies exhibited significant (P < 0.05) inhibition of tumor growth compared to the control group. Furthermore, a dose-dependent effect was observed, with a more pronounced reduction in tumor size of mice injected with 5 μg of antibodies compared to those receiving 1 μg (P < 0.05).
Molecular imaging of calnexin expression in a xenograft model
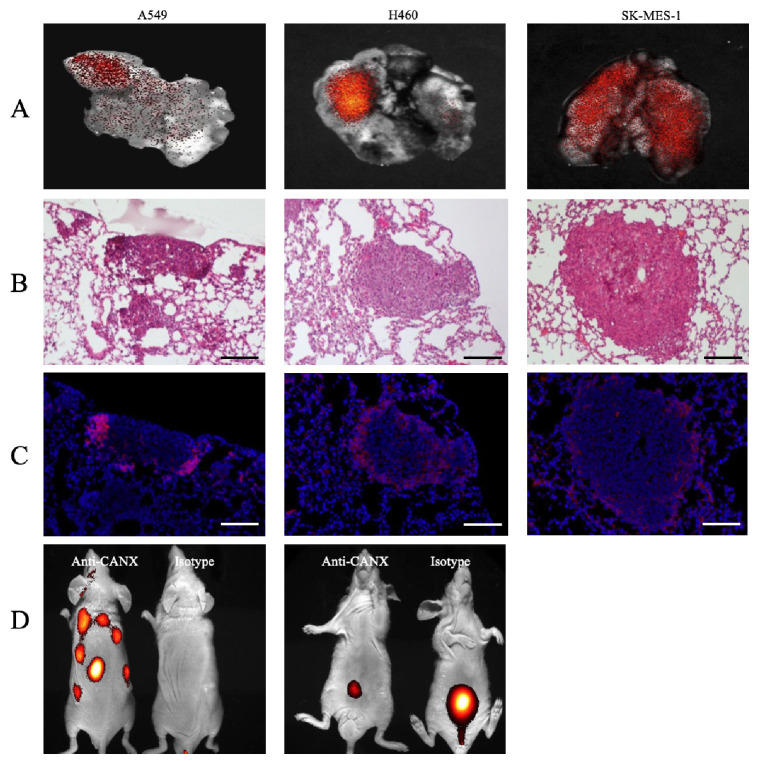
To assess the feasibility of the calnexin protein as a lung cancer marker, human lung cancer cell lines (A549, NCI-H460, and SK-mes1) were intravascularly injected into BALB/c nude mice to establish a xenograft model. Three weeks later, mice were injected intravenously with an antibody labeled with cy5.5 into the tail vein. When imaging animals while they were alive, little signal was observed (data not shown). However, upon sacrificing mice and removing their lungs for imaging, fluorescent images showed partial cy5.5 labeling (Fig. 3A). When we sectioned the lung tissue to confirm correct labeling of the cancerous area, at 3 weeks after injecting the lung cancer cell line into the tail vein, tumor masses exceeding 200 μm in diameter were observed in the lung tissue (Fig. 3B). Under fluorescence microscopy, the anti-calnexin antibody primarily labeled edges of tumor masses, while little antibody binding was visible in the center of the tumor as shown in Fig. 3C.
Fig. 3.
In vivo and ex vivo imaging of a tumor xenograft model. (A) Ex vivo imaging of lungs in a tumor xenograft mouse model. (B) Lung tissues stained with H&E for ex vivo imaging. (C) Serial sections stained with DAPI and observed under a fluorescence microscope (× 100; scale bar, 100 μm). (D) In vivo imaging of a subcutaneous tumor xenografted model. Imaging results were obtained at 3 hours after intravenous injection of labeled antibodies (n = 6).
To investigate the potential use of anti-calnexin antibodies for tumor imaging, we established a subcutaneously implanted lung cancer xenograft model. In this model, a 3 μg dose of cy5.5-labeled anti-calnexin antibody and an equivalent dose of cy5.5-labeled goat IgG isotype control were injected intravenously into the tail vein. Fluorescence signals were recorded at 3 hours after injection. A significant signal was detected around the tumor in mice injected with the cy5.5-labeled anti-calnexin antibody, whereas no significant signal was observed at the tumor site in mice injected with the goat IgG isotype control. In ventral lateral images without tumor implantation, all mice showed a signal in the bladder area. However, the signal was relatively weak in anti-calnexin antibody-treated mice but significantly stronger in goat IgG isotype control mice (Fig. 3D).
Detection of exosomal proteins for lung cancer diagnosis
Subcutaneous murine xenograft models were established using four lung cancer cell lines. Due to variations in growth rates among these cell lines, blood samples were collected when the largest tumor reached a diameter of 1 cm.
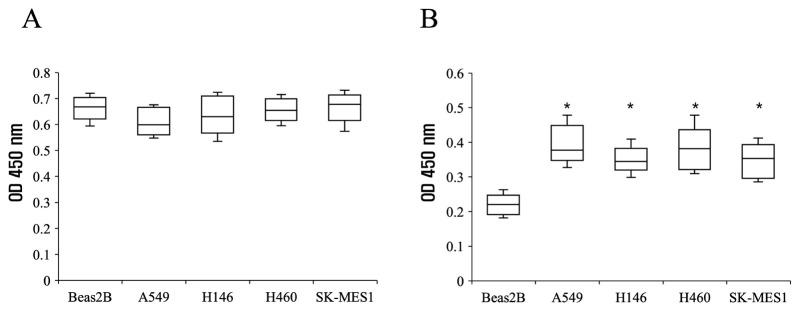
Fig. 4A displays ELISA results using CD9, a marker of exosomes. Comparable levels of CD9 were detected in Beas2B (close to normal cells) and all four lung cancer cell lines. This finding confirmed that nearly equivalent amounts of exosomes were isolated from all xenograft models. In contrast, an ELISA utilizing an anti-calnexin antibody revealed significantly higher levels of calnexin in all four lung cancer cell lines compared to Beas2B, suggesting a distinctive expression pattern of calnexin in transplanted mice. These observations contribute valuable insights for potential diagnostic applications in lung cancer detection.
Fig. 4.
ELISA of exosomes from lung cancer xenograft mouse serum. ELISA utilized lung cancer xenograft mouse serum exosomes with normal and Beas2B-injected mouse sera as controls. Comparable OD values were observed with anti-CD9 (A). However, the anti-calnexin antibody revealed higher OD values in cancer cell-injected mouse serum compared to controls (n = 6, *P < 0.05) (B).
DISCUSSION
The discovery of biomarkers through membrane proteomics presents the possibility of diagnostic or therapeutic targets in various cancers. To this end, we used 1D-LCMSMS to identify membrane proteins specifically overexpressed in four human lung cancer cell lines but not in normal lung tissues (unpublished data).
In this research, we investigated how calnexin could be utilized in the treatment and diagnosis of lung cancer. Calnexin is a calcium-binding endoplasmic reticulum-associated protein that has been reported to transiently interact with newly synthesized N-linked glycoproteins to facilitate folding and assembly (18). Regarding its physiological function, it has been reported that it is associated with a partial T-cell antigen receptor complex and that it might act as a signaling complex to regulate thymocyte maturation (19). It has also been reported to play a role in receptor-mediated endocytosis at synapses (20). Furthermore, plasma membrane localization of calnexin has been detected in cancerous tumors such as oral squamous cell carcinoma and melanoma (16). Another study has reported that calnexin is secreted in the sera of lung cancer patients, suggesting that calnexin could be a possible serum diagnostic marker (17).
However, there are no reports on how this protein could be specifically used for diagnosis or treatment. We first recognized that omics data might contain errors and conducted experiments to validate our findings. Western blot, flow cytometry, and immunocytochemistry confirmed that calnexin was upregulated in the four lung cancer cell lines (Fig. 1). Calnexin expression confirmed by immunocytochemistry was upregulated and predominantly located on the plasma membrane of the four lung cancer cell lines. Minimal expression was observed in Beas2B, mainly on the endoplasmic reticulum. It has been reported that calnexin, an endoplasmic reticulum protein, is detected on the cell surface, hinting its controlled redistribution between the endoplasmic reticulum and plasma membrane (21).
The expression of calnexin in both ER and plasma membrane suggests its potential as an accessible therapeutic target. Antibodies against this protein could be directly used as therapeutic agents without requiring additional delivery tools. To investigate the therapeutic potential of the anti-calnexin antibody, its effects on four lung cancer cell lines were evaluated. This antibody alone effectively inhibited the growth of all four lung cancer cell lines. Notably, when combined with the complement system (Fig. 2A), the antibody exhibited cytotoxicity against all cancer cell lines. Failure of this antibody to induce death of cancer cells after it was administered to cancer cells in vitro suggests the involvement of cellular mechanisms or complement-dependent cytotoxicity. Therefore, our initial investigation focused on measuring complement-dependent cytotoxicity.
This therapeutic effect was further confirmed in a xenograft model. Since the previously used goat-derived antibody was unsuitable for xenograft models, a new mouse-derived antibody was produced using the same peptide as a hapten to successfully elicit an immune response. Results presented in Fig. 2B showed that the mouse anti-calnexin antibody significantly inhibited tumor growth in a dose-dependent manner.
While imaging of animals with induced lung tumors did not provide a clear signal, ex vivo imaging of harvested organs revealed a strong signal throughout lungs (Fig. 3A). Subsequent tissue processing including slicing and staining confirmed the presence of a distinct tumor mass labeled by the calnexin antibody. This suggests its potential as a promising target for lung cancer imaging with an optimized chromophore.
Additionally, we explored the diagnostic potential of exosomes. Serum from a xenograft model created by subcutaneously injecting human lung cancer cells and Beas2B cells into BALB/c nude mice was isolated for direct ELISA assays. However, data showed significant deviation, complicating analysis. To address this, samples were purified using an exosome purification kit and ELISA analysis was repeated. ELISA with an antibody against CD9, an exosome marker, detected some expression in all lung cancer cell lines and the Beas2B xenograft model (Fig. 4A). In contrast, ELISA with an antibody against calnexin only detected expression in lung cancer cell lines. The ability of the anti-calnexin antibody to detect calnexin protein via exosomes suggests its potential for use in liquid biopsies, even though it is a membrane protein.
In conclusion, this study investigated the significance of calnexin as a crucial target for both diagnostic and therapeutic applications in lung cancer. Notably, the anti-calnexin antibody demonstrated an ability to inhibit lung cancer growth therapeutically. Moreover, on the diagnostic front, we established the potential utility of this antibody in in vivo imaging and detection of calnexin via exosomes.
MATERIALS AND METHODS
Cell lines and cell culture
The human lung cancer cell lines A549, NCI-H460, NCI-H146, and SK-mes1 were sourced from the Korean Cell Line Bank, while the human bronchial epithelial cell line (BEAS-2B) was acquired from ATCC. The cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Flow cytometry
Anti-calnexin antibody and a goat IgG isotype control were incubated in 50 mM Na2HPO4 (pH 7.4) with cy5.5 N-hydroxysuccinimide (NHS) ester (Lumiprobe Corporation, USA) dissolved in dimethyl sulfoxide (DMSO) at 25°C for 4 hours. Subsequently, cy5.5-conjugated antibodies were isolated using a Sephadex G-25 column (Sigma-Aldrich, St. Louis, MO, USA).
The calnexin peptide (SKTPELNLDQFHDKT) and anti-calnexin antibody were incubated at a 20:1 molar ratio at 25°C for 2 hours. Cells were prepared in DPBS by detaching them with a cell scraper and washing them with blocking buffer (3% fetal bovine serum in DPBS). The cells were then incubated with anti-calnexin antibody or peptide-inhibited anti-calnexin antibody in staining buffer (blocking buffer with 0.1% sodium azide) at 4°C for 30 minutes in the dark. The antibodies were conjugated with cy5.5, and a flow cytometer (BD FASCalibur, Becton-Dickinson, CA, USA) was used for signal detection.
Immunocytochemistry
A549, NCI-H146, NCI-H460, and SK-MES-1 cells were cultured on chamber slides (SPL Life Sciences Co., Ltd, Korea). NCI-H146 cells in suspension were attached to glass slides using Cellspin (Hanil Science Industrial Co., Ltd., Korea).
The cells were fixed in 2% paraformaldehyde for 10 minutes and then incubated in 3% normal rabbit serum diluted in TBS at 25°C for 30 minutes to block non-specific binding. Subsequently, they were incubated with the anti-calnexin antibody at 25°C for 1 hour, followed by HRP-conjugated anti-goat IgG antibody for 30 minutes. The cells were stained with a 3,3’-diaminobenzidine (DAB) substrate, and nuclei were counterstained with hematoxylin (Bethyl Laboratories, Inc., Montgomery, TX, USA).
Complement-dependent cytotoxicity assay
Lung cancer cells were seeded in a 96-well plate and allowed to attach for 24 hours at 37°C in a 7% CO2 atmosphere. The cells were then exposed to a serial dilution ranging from 1.56 μg/ml to 100 μg/ml of the anti-calnexin antibody. To facilitate the CDC assay, goat serum was added to achieve a final concentration of 5% in each well. Control groups were established using goat isotype IgG under the same conditions as described above.
Following the designated incubation period under the specified cell culture conditions, cell viability was assessed using both crystal violet staining and an MTS assay. For the crystal violet staining, cells were treated with a staining solution (0.05% crystal violet in methanol), and the absorbance at 545 nm was measured for each well using a microplate reader (Spectramax M2, Molecular Devices, USA). The MTS assay was conducted using the Celltiter 96 Aqueous One Solution Cell Proliferation Assay kit (Promega, Madison, WI, USA), following the provided instructions.
Animal experiments
A mouse anti-calnexin antibody was generated by conjugating a synthesized peptide to keyhole limpet hemocyanin (KLH). Antibodies were produced by intraperitoneally injecting mice with the KLH-conjugated peptide antigen, followed by their isolation and purification.
For in vivo testing, BALB/c nude mice were used in lung cancer metastasis and subcutaneous xenograft models. Lung cancer cells were injected, and imaging was performed approximately 3 weeks later. In the subcutaneous model, anti-tumor effects were confirmed by intraperitoneal injections of 1 μg and 5 μg of the mouse anti-calnexin antibody on days 12 and 16 after cell implantation compared to the control group receiving an equivalent amount of isotype antibody.
In vivo imaging
The Cy5.5-labeled anti-calnexin antibody or goat IgG isotype control was administered through tail vein injection into tumor-bearing mice. Subcutaneous xenograft mice were subjected to imaging at 1 hour, 3 hours, 18 hours, and 24 hours after injection using X-ray imaging (Kodak Image Station 400MM, Eastman Kodak Company, New Haven, CT). Subsequently, the mice were euthanized for ex vivo imaging. The tumor and various tissues, including the lung, liver, spleen, kidney, and bladder, were dissected and imaged. These dissected tissues were sectioned and analyzed using a fluorescence microscope (Eclipse TE2000, Nikon Instruments Inc., USA).
Purification of exosomes
Xenograft mouse sera were pre-cleared using a liporemoval column (Rosetta Exosomes, Korea). Exosomes were purified from the pre-cleared samples using ExoluteⓇ (Rosetta Exosomes, Korea). The final purified samples were used in experiments following a BCA protein assay. This purification process ensured the isolation of exosomes from biological fluid, enabling downstream analyses and experimentation.
ELISA
Enzyme-linked immunosorbent assay (ELISA) was conducted using Reagent Set A (BD Bioscience, USA). Exosome proteins were applied to a 96-well plate and incubated for 2 hours at 37°C. Subsequently, the plate underwent three rounds of washing, and a blocking step was carried out using 5% BCA in 1X TBS for 2 hours at 37°C. Antibodies (anti-calnexin, anti-CD9) were then incubated with a 5% BCA buffer for 2 hours at 37°C. After incubation with primary antibodies, the plate underwent five additional washing steps before HRP-conjugated secondary antibodies in 5% BCA buffer were incubated for 1 hour. Subsequently, the plate underwent seven rounds of washing. TMB (3,3’,5,5’ tetramethylbenzidine) solution was added, and the mixture was incubated for 5 minutes. The reaction was halted with the addition of a stop solution after 5 minutes, and the optical density was measured at 450 nm.
Statistical analysis
Mann-Whitney U-tests were conducted to analyze the anti-tumor effect of the anti-calnexin antibody and the difference in exosomal calnexin levels between cancer-bearing mice and healthy normal controls. A P-value less than 0.05 was considered statistically significant. IBM SPSS version 19 was used for statistical analysis.
Funding Statement
ACKNOWLEDGEMENTS This research was supported by the Korea Drug Development Fund funded by the Ministry of Science and ICT; the Ministry of Trade, Industry, and Energy; and the Ministry of Health and Welfare (RS-2021-DD121345, Korea).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Organization WHO. https://www.who.int/news-room/fact-sheets/detail/lung-cancer.
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Oberije C, De Ruysscher D, Houben R, et al. A validated prediction model for overall survival from stage III non-small cell lung cancer: toward survival prediction for individual patients. Int J Radiat Oncol Biol Phys. 2015;92:935–944. doi: 10.1016/j.ijrobp.2015.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernabe-Caro R, Chen Y, Dowlati A, Eason P. Current and emerging treatment options for patients with relapsed small-cell lung carcinoma: a systematic literature review. Clin Lung Cancer. 2023;24:185–208. doi: 10.1016/j.cllc.2023.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell JM, Flight RM, Moseley HNB. Untargeted lipidomics of non-small cell lung carcinoma demonstrates differentially abundant lipid classes in cancer vs. non-cancer tissue. Metabolites. 2021;11:740. doi: 10.3390/metabo11110740.71d7cd8c2dff47edba9bb2a5e5e4c12f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin S, Sun Y, Liang X, et al. Emerging new therapeutic antibody derivatives for cancer treatment. Signal Transduct Target Ther. 2022;7:39. doi: 10.1038/s41392-021-00868-x.bd04cca7f670449f8fca16ce460475f7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sung HJ, Cho JY. Biomarkers for the lung cancer diagnosis and their advances in proteomics. BMB Rep. 2008;41:615–625. doi: 10.5483/BMBRep.2008.41.9.615. [DOI] [PubMed] [Google Scholar]
- 8.Dalton WS, Friend SH. Cancer biomarkers--an invitation to the table. Science. 2006;312:1165–1168. doi: 10.1126/science.1125948. [DOI] [PubMed] [Google Scholar]
- 9.Yin H, Flynn AD. Drugging membrane protein interactions. Annu Rev Biomed Eng. 2016;18:51–76. doi: 10.1146/annurev-bioeng-092115-025322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2023;41:1638–1645. doi: 10.1200/JCO.22.02516. [DOI] [PubMed] [Google Scholar]
- 11.Boonstra MC, de Geus SW, Prevoo HA, et al. Selecting targets for tumor imaging: an overview of cancer-associated membrane proteins. Biomark Cancer. 2016;8:119–133. doi: 10.4137/BIC.S38542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karami Fath M, Azami J, Masoudi A, et al. Exosome-based strategies for diagnosis and therapy of glioma cancer. Cancer Cell Int. 2022;22:262. doi: 10.1186/s12935-022-02642-7.f700db29a19a469696b91a422fbfb1ab [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giustini NP, Jeong AR, Buturla J, Bazhenova L. Advances in treatment of locally advanced or metastatic non-small cell lung cancer: targeted therapy. Clin Chest Med. 2020;41:223–235. doi: 10.1016/j.ccm.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Cameron PH, Chevet E, Pluquet O, Thomas DY, Bergeron JJ. Calnexin phosphorylation attenuates the release of partially misfolded alpha1-antitrypsin to the secretory pathway. J Biol Chem. 2009;284:34570–34579. doi: 10.1074/jbc.M109.053165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okayama A, Miyagi Y, Oshita F, et al. Proteomic analysis of proteins related to prognosis of lung adenocarcinoma. J Proteome Res. 2014;13:4686–4694. doi: 10.1021/pr4012969. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Ma D, Wang X, et al. Calnexin impairs the antitumor immunity of CD4(+) and CD8(+) T cells. Cancer Immunol Res. 2019;7:123–135. doi: 10.1158/2326-6066.CIR-18-0124. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi M, Nagashio R, Jiang SX, et al. Calnexin is a novel sero-diagnostic marker for lung cancer. Lung Cancer. 2015;90:342–345. doi: 10.1016/j.lungcan.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Schrag JD, Bergeron JJ, Li Y, et al. The structure of calnexin, an ER chaperone involved in quality control of protein folding. Mol Cell. 2001;8:633–644. doi: 10.1016/S1097-2765(01)00318-5. [DOI] [PubMed] [Google Scholar]
- 19.Wiest DL, Bhandoola A, Punt J, Kreibich G, McKean D, Singer A. Incomplete endoplasmic reticulum (ER) retention in immature thymocytes as revealed by surface expression of "ER-resident" molecular chaperones. Proc Natl Acad Sci U S A. 1997;94:1884–1889. doi: 10.1073/pnas.94.5.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li HD, Liu WX, Michalak M. Enhanced clathrin-dependent endocytosis in the absence of calnexin. PLoS One. 2011;6:e21678. doi: 10.1371/journal.pone.0021678.e96433f27eab4d9086ea02854af8ff5b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myhill N, Lynes EM, Nanji JA, et al. The subcellular distribution of calnexin is mediated by PACS-2. Mol Biol Cell. 2008;19:2777–2788. doi: 10.1091/mbc.e07-10-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]