Abstract
Aberrant DNA methylation plays a critical role in the development and progression of colorectal cancer (CRC), which has high incidence and mortality rates in Korea. Various CRC-associated methylation markers for cancer diagnosis and prognosis have been developed; however, they have not been validated for Korean patients owing to the lack of comprehensive clinical and methylome data. Here, we obtained reliable methylation profiles for 228 tumor, 103 adjacent normal, and two unmatched normal colon tissues from Korean patients with CRC using an Illumina Infinium EPIC array; the data were corrected for biological and experiment biases. A comparative methylome analysis confirmed the previous findings that hypermethylated positions in the tumor were highly enriched in CpG island and promoter, 5’ untranslated, and first exon regions. However, hypomethylated positions were enriched in the open-sea regions considerably distant from CpG islands. After applying a CpG island methylator phenotype (CIMP) to the methylome data of tumor samples to stratify the CRC patients, we consolidated the previously established clinicopathological findings that the tumors with high CIMP signatures were significantly enriched in the right colon. The results showed a higher prevalence of microsatellite instability status and MLH1 methylation in tumors with high CMP signatures than in those with low or non-CIMP signatures. Therefore, our methylome analysis and dataset provide insights into applying CRC-associated methylation markers for Korean patients regarding cancer diagnosis and prognosis.
Keywords: Colorectal cancer, CpG island methylator phenotype, DNA methylation, Microsatellite instability, MutL homolog 1 (MLH1)
INTRODUCTION
DNA methylation plays a crucial role in colorectal cancer (CRC) tumorigenesis as it regulates the methylation of regulatory elements of tumor suppressor genes or oncogenes and affects gene expression (1-3). For example, high microsatellite instability (MSI-H) resulting from MLH1 promoter hypermethylation has been linked to CRC prognosis and immune checkpoint inhibitors (4, 5). Furthermore, systematic methylation profiling of patients with CRC has revealed that a CpG island methylator phenotype (CIMP) can be reflected in the widely accepted molecular classification of CRC (6-10). This classifies the cancer type into four consensus molecular subtypes, characterized by overall hypermethylation, intermediate methylation, and low methylation throughout the genome (8).
The Cancer Genome Atlas (TCGA) provides multi-omics data, including methylome data on CRC. However, methylation markers have common or race-specific characteristics (11), making it challenging to understand the CRC characteristics in the Korean population from TCGA alone, which primarily contains data from Caucasian patients. Nevertheless, several studies on Korean patients with CRC have generated genome data from the tumor and adjacent normal tissues of the patients (12, 13). Thus, an unmet need for methylome data exists to confirm the effectiveness of methylation profiling-based subtyping in Korean patients with CRC.
To this end, we aimed to establish a comprehensive methylome dataset from Korean patients with CRC and systematically investigated the characteristics of methylation profiles in these patients. The associated data provide detailed groups with distinct clinicopathological and molecular characteristics, which will help determine the optimal therapy and develop more effective CRC prevention and treatment strategies.
RESULTS
Correction of sex- and batch-related biases provides highly harmonized data
We collected data from 228 Korean patients with CRC and obtained clinical information, such as sex, age, tumor location, and MSI status (Supplementary Table 1 and Supplementary Fig. 1). For methylation profiles of Korean patients with CRC, we evaluated the quality of the EPIC array data by inspecting the overall distribution of beta values and control strip plots, including the bisulfite conversion efficiency, extension quality, and specificity (Supplementary Fig. 2). We acquired 609,046 probe methylation beta values from 228 tumors and 105 normal samples for downstream analysis. Through statistical data exploration, including normalization and filtering, we obtained high-quality harmonized data and effectively eliminated technical noise and sex-based biases (Supplementary Figs. 3 and 4).
Differentially methylated positions in tumor tissues show distinct distribution in genic and CpG island-associated regions
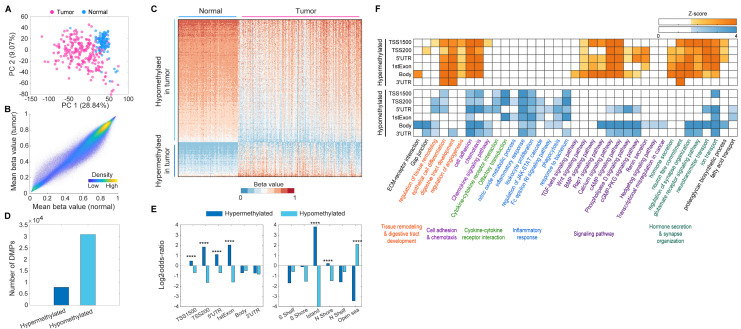
In the principal component analysis plot (Fig. 1A), a clear separation between tumor and normal samples was observed while analyzing the 609,046 processed methylation datasets. Notably, the grand mean methylation level of 609,046 probes across all normal samples was slightly higher than that across tumor samples (0.5824 and 0.5570 for normal and tumor samples, respectively; Fig. 1B). Subsequently, we identified 38,607 differentially methylated positions (DMPs) between the tumor and normal samples (Supplementary Table 2) and found approximately four times more hypomethylated positions (30,783 probes) than hypermethylated positions (7,824 probes) in the tumor samples (Fig. 1C, D). For each DMP group, we calculated the odds ratio of enrichment for hyper- and hypomethylated positions for various genomic annotations, including gene promoter-like regions, body regions, and islands or shores. In promoter-like regions (TSS1500, TSS200, 5’-untranslated region [UTR], and first exon), the odds ratios between the number of observed and expected hypermethylated positions were 1.3972, 3.5963, 4.1059, and 2.1671, respectively (P < 0.0001; Supplementary Table 3, top and Fig. 1E, left). Hypermethylated positions were highly enriched in CpG island and N shore regions (Supplementary Table 4, bottom and Fig. 1E, right), with an odds ratio of 13.9850 for CpG island regions (P < 0.0001). In contrast, hypomethylated positions in tumor samples were primarily found in open-sea (odds ratio: 4.2877, P < 0.0001) regions (Supplementary Table 4, bottom and Fig. 1E), which are considerably distant from the CpG island regions.
Fig. 1.
Identification of differentially methylated positions between tumor and normal tissues. (A) Principal component (PC) analysis of 228 tumors (red) and 105 normal tissue samples (blue) using the preprocessed 609,046 methylation probes. The plot shows the PC1 (x-axis) and PC2 (y-axis) with their explained variances. (B) Scatter plot showing mean beta values of the 609,046 methylation probes across normal (x-axis) and tumor samples (y-axis). (C) Heat map describing hypo- and hypermethylated positions in the tumor tissue samples, compared with normal tissue samples. For better visualization, we randomly selected 5% of the differentially methylated positions (DMPs) and performed a hierarchical clustering with the application of Euclidean distance and the average linkage method. The color bar denotes the gradient of beta values as a measurement of methylation level. (D) Number of total DMPs between the tumor and normal tissue samples. (E) Bar graph of log2-odds-ratios representing the enrichment significance of the DMPs for each of the genic (left) and CpG-island-associated regions (right). ****P < 0.0001 by the empirical statistical test as described in Supplementary Methods and Materials. (F) Heat map showing the functional enrichment patterns of GOBPs and KEGG pathways by hyper- and hypomethylated positions at genomic regions (TSS1500, TSS200, 5’-UTR, first exon, body, and 3’-UTR). Color bar, gradient of Z-score for the enrichment P-value computed by using DAVID software.
To understand cellular processes and signaling pathways epigenetically regulated by the DMPs, we performed a functional enrichment analysis of Gene Ontology Biological Processes (GOBP) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways for the hyper- and hypomethylated positions located at TSS1500, TSS200, 5’-UTR, first exon, body, and 3’-UTR regions (Fig. 1F). We found that (i) angiogenesis, signaling pathways (TGF-beta, Wnt, and BMP signaling pathways), hormone secretion, and neuron fate commitment were significantly (P < 0.05) enriched by only the hypermethylated positions; (ii) cytokine-cytokine receptor interaction (olfactory transduction), inflammatory response (nitric oxide metabolic process, leukocyte proliferation, JAK-STAT cascade, Fc epsilon RI signaling pathway, phagocytosis, and response to bacterium), Hedgehog signaling pathway, transcriptional misregulation in cancer, and fatty acid transport were enriched by only the hypomethylated positions; (iii) ECM-receptor interaction, gap junction, tissue remodeling and digestive tract development (epithelial cell differentiation), cell adhesion and chemotaxis (chemokine signaling pathway), signaling pathways (Rap1, calcium, cAMP, phospholipase D, and cGMP-PKG signaling pathways and renin secretion), synapse organization (glutamate receptor signaling pathway and neurotransmitter and ion transport), and proteoglycan biosynthetic process were enriched by hyper- and hypomethylated positions. Moreover, the comparison with the enrichment P-values for negative control positions (see details in Supplementary Materials and Methods) implied that the enriched GOBPs and KEGG pathways were predominantly regulated by the DMPs (Fig. 1F and Supplementary Fig. 5). Notably, among the GOBPs and KEGG pathways enriched by hyper- and hypomethylated positions, ECM-receptor interaction, TGF-beta and Hedgehog signaling pathways, and transcriptional misregulation in cancer were enriched by only the DMPs located at body regions. This finding supports that methylation-associated epigenetic regulation of the body region plays a distinct role in CRC development compared to the promoter-like region, as described in previous studies (2, 14). Collectively, these results suggest that our DNA methylome data may provide valuable insights into CRC biology.
Integrative analysis of CIMP reveals DNA methylation-associated clinicopathological implications for Korean patients with CRC
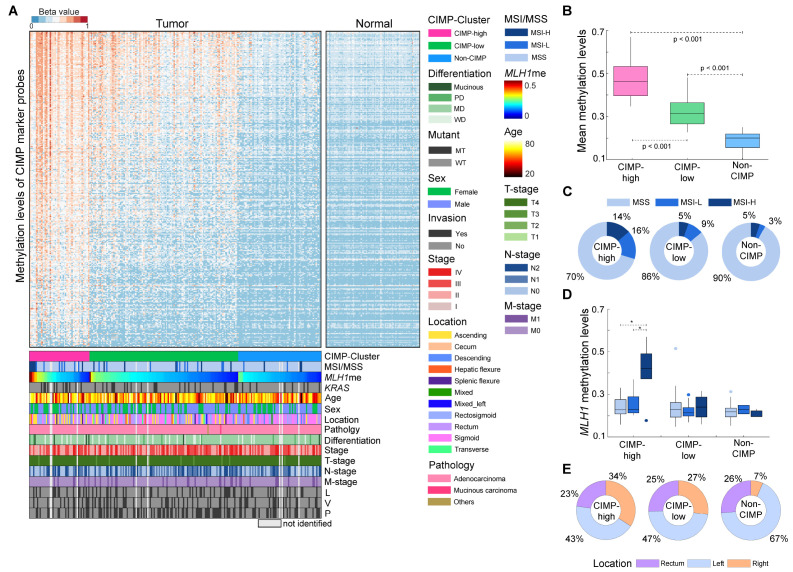
To interpret the clinicopathological characteristics of Korean patients with CRC based on their methylation profiles, we assessed the prevalence of CIMP. The 228 tumor samples were clustered into three groups with respect to the methylation levels in the 1,470 CIMP probe set derived from 258 previously identified CIMP gene markers (15) (see details in Supplementary Materials and Methods) (Fig. 2A). We confirmed the significant (ANOVA with a post hoc test, P < 0.001) changes in the mean methylation levels of the CIMP marker probes between the three groups (Fig. 2B). We then investigated whether there were clinicopathological differences across the three groups (Supplementary Table 1). There were no significant differences in the age (ANOVA, P = 0.448), sex (Chi-square test, P = 0.517), tumor AJCC stage (Fisher’s exact test, P = 0.641), T (Fisher’s exact test, P = 0.109), N (Chi-square test, P = 0.514), or M (Chi-square test, P = 0.956) stages or the differentiation status (poorly, moderately, and well-differentiated states and mucinous status; Fisher’s exact test, P = 0.174) distributions between the CIMP groups. Additionally, we examined the association between survival and CIMP status by conducting a relapse-free survival analysis for the patients stratified by CIMP status. We ascertained that the patients in the CIMP-H exhibited a significantly (Log-rank test, hazard ratio = 2.215 and P-value < 0.05) poorer outcome than their counterparts (i.e., CIMP-L and non-CIMP; Supplementary Fig. 6).
Fig. 2.
Clustering of 228 colon tumor samples according to the CpG island methylator phenotype (CIMP). (A) Clustering of 228 colon tumor and 105 normal tissue samples based on the methylation levels of the CIMP marker probes. For the visualization of the heatmap, the samples (column) in each of the CIMP groups were sorted according to MLH1 methylation level. For each of the 16 clinicopathological characteristics (CIMP cluster, microsatellite instability [MSI] status, MLH1 methylation, KRAS mutation, age, sex, location, pathology, differentiation, stage, and T-, N-, and M-stages, and lymphatic [L], venous [V], and perineural [P] invasions), the samples without relevant information (“not identified”) appear bright gray in the heatmap. (B) Boxplot showing mean beta values of CIMP marker probes for the patient groups classified according to their CIMP status. (C) Proportion of patients with MSI-low and -high, and microsatellite stability (MSS) statuses for each of the CIMP groups. (D) Boxplot showing the distribution of MLH1 beta values for the patient groups classified according to their CIMP and MSI statuses. (E) Distribution of tissue location for each of the CIMP groups. The boxes in (B) and (D) display the lower, median, and upper quartiles; the whiskers represent the minimum and maximum values. *P < 0.05 by one-way ANOVA with a post hoc test (Sidak correction).
Among the 228 patients with CRC, 220 were tested for genetic mutations (Supplementary Table 1), 44 (20.0%) of them had KRAS mutations. However, there were no significant differences in the distribution of KRAS (Chi-square test, P = 0.175) mutations among the CIMP groups. Microsatellite instability (MSI) status was available for 220 patients; the frequency of MSI-H tumors in the CIMP-H group (13.6%) was significantly (Fisher’s exact test, P = 0.041) higher than that in the CIMP-L (5.3%) and non-CIMP (4.8%) groups (Fig. 2C and Supplementary Table 1). To address the shared features of the patients with MSI-H status in the CIMP-H group, we investigated the methylation levels of MLH1—a DNA mismatch repair gene in which the hypermethylation of the promoter is associated with MSI in CRC (4). The MLH1 methylation levels were higher in the CIMP-H group (mean methylation: 0.26) than in the CIMP-L (mean methylation: 0.23) and non-CIMP (mean methylation: 0.22) groups (T-test [CIMP-H vs. non-CIMP-H], P < 0.05, see MLH1 methylation levels in Supplementary Fig. 7A). The CIMP-H group exhibited a significant association between MLH1 methylation and MSI-H status (Fig. 2D). The mean MLH1 methylation level of patients with CIMP-H and MSI-H status (0.41) was significantly (ANOVA with a post hoc test, P < 0.001) higher than that of patients with CIMP-H and MSS (0.25) or MSI-L status (0.24); however, the mean methylation levels of all CIMP markers did not significantly differ with the MSI status of CIMP-H patients (Supplementary Fig. 8).
Tumor location data were available for 212 patients; the distribution was as follows: 113 (53.3%) on the left side (descending, rectosigmoid, sigmoid, and splenic flexure) of the colon, 55 (25.9%) in the rectum, and 44 (20.8%) on the right side (ascending, cecum, and hepatic flexure) of the colon (Fig. 2E and Supplementary Table 1). In the CIMP-H group, 15 tumor samples (34%) were found on the right side of the colon and 29 (66%) on the left side. In contrast, only 25 (22%) and 4 (7%) right colon tumors were observed in the CIMP-L and non-CIMP groups, respectively. These results showed significant enrichment (Chi-square test, P = 0.006) of tumor samples on the right colon in the CIMP-H group, suggesting that the CIMP status was correlated with the anatomical location within the large intestine. Collectively, DNA methylation profiling of CIMP markers and MLH1 was highly associated with MSI status and tumor location as well as relapse-free survival, rather than other clinicopathological features in CRC patients.
Previously known CIMP markers were associated with the clinicopathological implications of Korean CRC methylome data. However, the markers did not fully represent our DMPs and vice versa. For example, when we compared the methylation levels of the probes annotated with the promoter-like regions of 11 previously identified CIMP marker genes (7), the methylation levels of the ten genes were significantly (T-test, P < 0.05) higher in the CIMP-H group than in the CIMP-L and non-CIMP groups. However, the methylation levels of SOCS1 were not significantly changed (Supplementary Fig. 7A). Thus, we sought to identify a set of novel CIMP marker candidates representing the DMPs. To this end, we first selected 680 of the 7,824 hypermethylated positions in the tumor tissues (see details in Supplementary Materials and Methods). These positions could stratify patients into three discrete categories, which concurred with our pre-established CIMP classifications (Supplementary Fig. 7B, C). After the refining process for the 680 positions, we prioritized 16 positions as novel CIMP marker candidates, showing significant (P-value < 0.0001) changes in mean methylation levels between the patients with CIMP-H, CIMP-L, and non-CIMP status (Supplementary Fig. 9 and Supplementary Table 5; see details in Supplementary Materials and Methods).
DISCUSSION
We investigated alterations in the DNA methylation levels in 228 patients with CRC and their potential associations with clinicopathological features. Our analysis of a large methylation dataset comprising 609,046 probes revealed a clear distinction between tumor and normal samples, indicating significant differences in methylation levels between the groups. We also identified 38,607 DMPs between the tumor and normal samples, with more hypomethylated positions in the tumor samples. Hypermethylated positions were primarily found in the gene promoter-like and CpG island regions, and hypomethylated positions were exclusively detected in the open-sea regions.
Our study embarked on a comprehensive exploration of DMPs to elucidate their biological relevance in CRC. While we initially highlighted the significance of overrepresented genes in GOBP and KEGG pathways, we expanded our analysis by investigating specific genes pivotal to CRC progression (Supplementary Table 6). A salient finding from our study is the hypermethylation of genes such as SFRP1, SFRP2, SOX17, and WIF1, shedding light on their antagonistic roles in the WNT signaling pathway and their consequent significance in CRC (16). Of particular note is the SMAD family, which plays a central role in the signal transduction of the TGF-β superfamily (17, 18). This family encompasses TGF-βs, bone morphogenetic proteins, and activins. The observed hypermethylation of SMAD1 and SMAD2 in CRC underscores a potential dysregulation of the TGF-β signaling pathway (19, 20), warranting further investigation. Our findings concerning ADAMTSs resonate with existing literature, emphasizing their nuanced roles across various cancers (21-23). The diverse expression of ADAMTSs, particularly the involvement of ADAMTS1 in colon cancer cachexia, emphasizes their relevance in CRC (24). Their multifunctional roles, from tumor-protective effects to interactions with the extracellular matrix, are pivotal. Observations of genetic aberrations, whether mutations or epigenetic silencing in ADAMTS genes, align with broader oncological research (23). CDH13 emerged as another critical gene in our study. Given its established role in cell recognition and adhesion, its methylation pattern in CRC could herald early oncogenic processes (25). Our findings on TMEFF2 (26), ADCY1 (27), and ADCY4 (28) hypermethylation complement and extend the existing cancer research, linking them to cell adhesion and cAMP signaling pathways. By placing these findings with other omics-based studies, we aim to cultivate a holistic understanding of the molecular intricacies underpinning CRC progression.
We also examined the prevalence of CIMP and stratified the patients into three groups (CIMP-H, CIMP-L, and non-CIMP) based on their mean methylation levels for a set of previously known CIMP marker probes. We observed a significant enrichment of patients with MSI-H within the CIMP-H group, and the MLH1 methylation levels were higher in the CIMP-H group than in the other two groups. This significant association between MLH1 methylation level and MSI-H status in the CIMP-H group supports a combinatorial assessment of CIMP status and MLH1 methylation level as a promising measurement predicting MSI status of CRC, as suggested by previous studies (29).
The CIMP status was correlated with not only patient relapse-free survival but also the anatomical location of the large intestine, with an enrichment of right colon tumors in the CIMP-H group. Our results are similar to those of previous studies (6, 30). In particular, right-sided colon cancer has a poorer oncological prognosis than left-sided colon cancer (31). Considering the distinct cancer prognosis as per the tumor location, a methylation profiling of tumor samples may be necessary to infer the location and establish a proper treatment plan. Collectively, these results support the previous findings on CRC-associated methylation characteristics, which may facilitate a personalized approach to cancer diagnosis and prognosis.
Our findings provide novel insights into the epigenetic alterations associated with CRC and their potential clinical implications. For example, we suggested potential CRC diagnostic markers commonly used for other ethnic groups as well as the novel CIMP marker candidates, which may be associated with several clinicopathological characteristics. The enrichment of hypermethylated positions in the gene promoter-like and CpG island regions suggests that these regions play a crucial role in the development of CRC. The observed associations among CIMP status, MSI status, and MLH1 methylation level suggest that these biomarkers have prognostic value in patients with CRC. We also highlight the importance of considering the anatomical location of tumors when evaluating epigenetic alterations in colon cancer, as we observed a significant association between the CIMP status and tumor location in the large intestine. In comparison with TCGA CRC dataset, although our cohort contained a disproportionate amount of samples from later stages than early stages (Supplementary Table 7, P < 0.001), this profiling could provide an opportunity to investigate markers of poor prognosis. Further studies are required to validate our findings and elucidate the mechanisms underlying these epigenetic alterations in CRC.
MATERIALS AND METHODS
Ethical statement
This study was approved by Seoul National University Bundang Hospital Institutional Review Board (approval number: B-1709-423-306), Uijeongbu St. Mary’s Hospital Institutional Review Board (approval number: XC17TNDI0068), and Yonsei University Institutional review board (approval number: 7001988-201910-BR-727-02).
Data availability
The raw IDAT files and processed methylation profiles are available in Korea BioData Station (K-BDS, https://kbds.re.kr/) with the accession ID PRJKA2086326 (32). The raw CRC methylome of TCGA was downloaded from The Genomic Data Commons portal (https://portal.gdc.cancer.gov/repository).
Funding Statement
ACKNOWLEDGEMENTS This research was supported by the Bio & Medical Technology Development Program (grant number: NRF-2017M3A9A7050614, NRF-2017M3A9A7050610 and NRF-2023M3A9A7009442) and the Korea BioData Station Program.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Das PM, Singal R. DNA methylation and cancer. J Clin Oncol. 2004;22:4632–4642. doi: 10.1200/JCO.2004.07.151. [DOI] [PubMed] [Google Scholar]
- 2.Lakshminarasimhan R, Liang G. The role of DNA methylation in cancer. Adv Exp Med Biol. 2016;945:151–172. doi: 10.1007/978-3-319-43624-1_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishiyama A, Nakanishi M. Navigating the DNA methylation landscape of cancer. Trends Genet. 2021;37:1012–1027. doi: 10.1016/j.tig.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Herman JG, Umar A, Polyak K, et al. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci U S A. 1998;95:6870–6875. doi: 10.1073/pnas.95.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao P, Li L, Jiang X, Li Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J Hematol Oncol. 2019;12:54. doi: 10.1186/s13045-019-0738-1.bd2eddabccb74f80bd688578a40e2f5d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zlobec I, Bihl M, Foerster A, Rufle A, Lugli A. Comprehensive analysis of CpG island methylator phenotype (CIMP)-high, -low, and -negative colorectal cancers based on protein marker expression and molecular features. J Pathol. 2011;225:336–343. doi: 10.1002/path.2879. [DOI] [PubMed] [Google Scholar]
- 7.Nazemalhosseini Mojarad E, Kuppen PJ, Aghdaei HA, Zali MR. The CpG island methylator phenotype (CIMP) in colorectal cancer. Gastroenterol Hepatol Bed Bench. 2013;6:120–128. doi: 10.32388/ezc1s6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh MP, Rai S, Pandey A, Singh NK, ivastava S., Sr Molecular subtypes of colorectal cancer: an emerging therapeutic opportunity for personalized medicine. Genes Dis. 2021;8:133–145. doi: 10.1016/j.gendis.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joe S, Kim J, Lee JY, et al. Epigenetic insights into colorectal cancer: comprehensive genome-wide DNA methylation profiling of 294 patients in Korea. BMB Rep. 2023;56:563–568. doi: 10.5483/BMBRep.2023-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galanter JM, Gignoux CR, Oh SS, et al. Differential methylation between ethnic sub-groups reflects the effect of genetic ancestry and environmental exposures. eLife. 2017;6:e20532. doi: 10.7554/eLife.20532.b0d45908dba14dbf845c1016fde9062b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeon SA, Ha YJ, Kim JH, et al. Genomic and transcriptomic analysis of Korean colorectal cancer patients. Genes Genomics. 2022;44:967–979. doi: 10.1007/s13258-022-01275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SH, Lee B, Shim JH, et al. Landscape of actionable genetic alterations profiled from 1,071 tumor samples in Korean cancer patients. Cancer Res Treat. 2019;51:211–222. doi: 10.4143/crt.2018.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X, Han H, De Carvalho DD, Lay FD, Jones PA, Liang G. Gene body methylation can alter gene expression and is a therapeutic target in cancer. Cancer Cell. 2014;26:577–590. doi: 10.1016/j.ccr.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McInnes T, Zou D, Rao DS, et al. Genome-wide methylation analysis identifies a core set of hypermethylated genes in CIMP-H colorectal cancer. BMC Cancer. 2017;17:228. doi: 10.1186/s12885-017-3226-4.b983699b554c4626aa4eff65786a812f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva AL, Dawson SN, Arends MJ, et al. Boosting Wnt activity during colorectal cancer progression through selective hypermethylation of Wnt signaling antagonists. BMC Cancer. 2014;14:891. doi: 10.1186/1471-2407-14-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009;19:71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang F, Chen YG. Regulation of TGF-beta receptor activity. Cell Biosci. 2012;2:9. doi: 10.1186/2045-3701-2-9.ae9765e4295a4bbe90bbb6e251979f66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding N, Luo H, Zhang T, Peng T, Yao Y, He Y. Correlation between SMADs and colorectal cancer expression, prognosis, and immune infiltrates. Int J Anal Chem. 2023;2023:8414040. doi: 10.1155/2023/8414040.02a56baa25d44feebc2c45966eb03129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hata A, Chen YG. TGF-beta signaling from receptors to Smads. Cold Spring Harb Perspect Biol. 2016;8:a022061. doi: 10.1101/cshperspect.a022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagstaff L, Kelwick R, Decock J, Edwards DR. The roles of ADAMTS metalloproteinases in tumorigenesis and metastasis. Front Biosci (Landmark Ed) 2011;16:1861–1872. doi: 10.2741/3827. [DOI] [PubMed] [Google Scholar]
- 22.Choi JE, Kim DS, Kim EJ, et al. Aberrant methylation of ADAMTS1 in non-small cell lung cancer. Cancer Genet Cytogenet. 2008;187:80–84. doi: 10.1016/j.cancergencyto.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Lind GE, Kleivi K, Meling GI, et al. ADAMTS1, CRABP1, and NR3C1 identified as epigenetically deregulated genes in colorectal tumorigenesis. Cell Oncol. 2006;28:259–272. doi: 10.1155/2006/949506.f2e5e496853c4ee4bc4c055de4ccc494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cal S, López-Otín C. ADAMTS proteases and cancer. Matrix Biol 44- 2015;46:77–85. doi: 10.1016/j.matbio.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Hibi K, Kodera Y, Ito K, Akiyama S, Nakao A. Methylation pattern of CDH13 gene in digestive tract cancers. Br J Cancer. 2004;91:1139–1142. doi: 10.1038/sj.bjc.6602095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan H, He J, Guan Q, et al. Identifying CpG sites with different differential methylation frequencies in colorectal cancer tissues based on individualized differential methylation analysis. Oncotarget. 2017;8:47356–47364. doi: 10.18632/oncotarget.17647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Yang J, Wang X, Li X. GNG7 and ADCY1 as diagnostic and prognostic biomarkers for pancreatic adenocarcinoma through bioinformatic-based analyses. Sci Rep. 2021;11:20441. doi: 10.1038/s41598-021-99544-x.5210d2047b8e4074b25d615e6db7e969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan Y, Mu J, Huang M, et al. Epigenetic identification of ADCY4 as a biomarker for breast cancer: an integrated analysis of adenylate cyclases. Epigenomics. 2019;11:1561–1579. doi: 10.2217/epi-2019-0207. [DOI] [PubMed] [Google Scholar]
- 29.Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–130. doi: 10.1111/j.1365-2559.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 30.Sugai T, Habano W, Jiao YF, et al. Analysis of molecular alterations in left- and right-sided colorectal carcinomas reveals distinct pathways of carcinogenesis: proposal for new molecular profile of colorectal carcinomas. J Mol Diagn. 2006;8:193–201. doi: 10.2353/jmoldx.2006.050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrelli F, Tomasello G, Borgonovo K, et al. Prognostic survival associated with left-sided vs right-sided colon cancer: a systematic review and meta-analysis. JAMA Oncol. 2017;3:211–219. doi: 10.1001/jamaoncol.2016.4227. [DOI] [PubMed] [Google Scholar]
- 32.Lee B, Hwang S, Kim PG, et al. Introduction of the Korea BioData Station (K-BDS) for sharing biological data. Genomics Inform. 2023;21:e12. doi: 10.5808/gi.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw IDAT files and processed methylation profiles are available in Korea BioData Station (K-BDS, https://kbds.re.kr/) with the accession ID PRJKA2086326 (32). The raw CRC methylome of TCGA was downloaded from The Genomic Data Commons portal (https://portal.gdc.cancer.gov/repository).