Abstract

It is well-known that aqueous dispersions of phospholipids spontaneously assemble into bilayer structures. These structures have numerous applications across chemistry and materials science and form the fundamental structural unit of the biological membrane. The particular environment of the lipid bilayer, with a water-poor low dielectric core surrounded by a more polar and better hydrated interfacial region, gives the membrane particular biophysical and physicochemical properties and presents a unique environment for chemical reactions to occur. Many different types of molecule spanning a range of sizes, from dissolved gases through small organics to proteins, are able to interact with membranes and promote chemical changes to lipids that subsequently affect the physicochemical properties of the bilayer. This Review describes the chemical reactivity exhibited by lipids in their membrane form, with an emphasis on conditions where the lipids are well hydrated in the form of bilayers. Key topics include the following: lytic reactions of glyceryl esters, including hydrolysis, aminolysis, and transesterification; oxidation reactions of alkenes in unsaturated fatty acids and sterols, including autoxidation and oxidation by singlet oxygen; reactivity of headgroups, particularly with reactive carbonyl species; and E/Z isomerization of alkenes. The consequences of reactivity for biological activity and biophysical properties are also discussed.
1. Introduction
1.1. Overview
The structure of bilayers formed by amphipathic lipid molecules has been the subject of intense study since the pioneering work on the nature of the biological membrane by Danielli, Davson, and Robertson in the first half of the 20th century.1−3 However, it was not until the 1960s, with the realization by Bangham that lipids can spontaneously form liposomes that exhibit many of the properties of the biological membranes, that the properties of the lipid membrane began to be fully understood. Since then there has been an explosion of uses and applications of liposomes, from drug delivery agents and tools for studying the physical properties of membranes to models for biological membranes.4−7 Since 2010 there have been >36 000 primary articles (Web of Science search, Topic = liposom*, type = article, range 2010 to 2019; accessed 2022-05-25) and >14 000 patents (Espacenet search, https://worldwide.espacenet.com, worldwide search, Title, Abstract, or Claims contain “liposom*”, 2010 to present; accessed 2023-05-09) involving the use of liposomes. For almost as long as liposomes have been used, the chemical and physical stability of membranes, both biological and synthetic, has been examined in order to understand how the membrane responds to changes in the physical and chemical environment and how the products of reactions involving lipids can exert biological activity. Although many aspects of membrane stability are now well characterized, our understanding of lipid chemistry is still evolving.
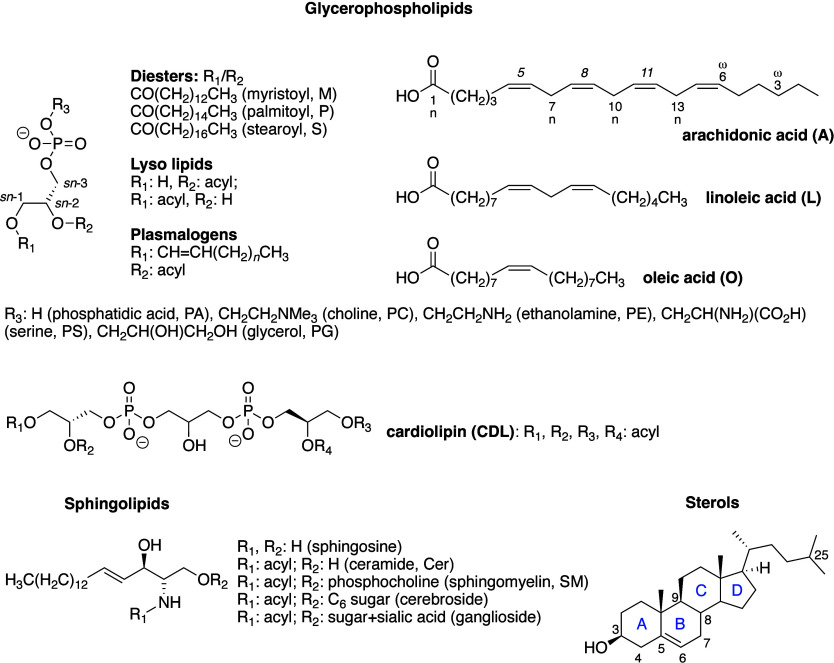
Biological membranes comprise a range of phospholipid species that are summarized in Figure 1.8,9 The major glycerophospholipids are diesters of glycerol and are primarily classified according to the identity of the headgroup and the fatty acyl chains. Related classes of lipids, grouped together under the term plasmalogens, contain ether-linked alkyl chains. Sphingolipids are based around a ceramide core and are notable for an amide-linked acyl group that significantly increases both their chemical stability and the mechanical stability of membranes that incorporate them. Cardiolipins (CDLs) are notable for containing two diacylglycerophosholipids linked through glycerol to form a structure with four acyl chains. These lipids are found in the membranes of prokaryotes and the mitochondrial membranes of eukaryotes. Sterols, most notably cholesterol, significantly change the fluidity and phase behavior of membranes.10−22
Figure 1.
Structures of phospholipids described in this review. Numbering corresponds to common nomenclature.
In biological membranes, which are rich in unsaturated lipids at the sn-2 position and are generally in a fluid liquid crystalline phase under physiological conditions, cholesterol incorporation increases their physical stability,23−25 decreases the lateral diffusion rate,19,26−28 and reduces the permeation of water and hydrophilic solutes across the bilayer,29−37 although the level of hydration in the interfacial region is increased to the depth of the acyl carbonyl groups.38−40 For amphiphilic organic molecules and peptides, the effects of cholesterol on permeability and partitioning vary with membrane composition and the nature of the partitioning molecule.41−44
Permeation and partitioning are of key significance for the reactivity of membrane lipids, as the regions around the glycerol esters and fatty acid olefin groups are major sites of reactivity. The bilayer permeability of hydrogen peroxide is low, but increases if the content of unsaturated lipids increases. Its concentration is greatest near the outer leaflet (extracellular space) and decreases across the membrane toward the inner leaflet.45 Oxygen partitions favorably into membranes to give higher steady state concentrations in the bilayer than the surrounding aqueous phase. The presence of cholesterol reduces the favorability of oxygen partitioning.46 In a similar vein, the permeability of dissolved gases, including oxygen47,48 and carbon dioxide,49 is decreased by the presence of cholesterol. In some systems, however, the presence of cholesterol has been found to increase oxygen permeability by an order of magnitude.50
In mixtures containing cholesterol that exhibit phase separation into liquid ordered (Lo) and liquid disordered (Ld) phases, the Lo phase has lower water permeability.29,51 However, the difference in water permeability between Lo and Ld is significantly lower than the difference between the permeability of gel and fluid phases of single-component lipid membranes. This difference is attributed to a permeation pathway that includes diffusion into the Ld regions at the Lo/Ld boundary with subsequent diffusion along the membrane midplane.52
The composition of biological membranes, in terms of lipid type, class, and acyl composition, varies significantly by organism, cell type, and organelle.8,9 Furthermore, some cells are able to adapt the lipid profile of their membranes in response to changes in the local environment.53−55 Within many membranes, the distribution of lipids is nonuniform, both laterally within the plane of membrane,56−59 and transversely between leaflets.20,26,60 For example, in eukaryotes the plasma membrane is enriched in phosphatidylethanolamine (PE), phosphatidylserine (PS), and phosphatidylinositol (PI) in the inner leaflet and phosphatidylcholine (PC), sphingolipids, and cholesterol in the outer plasma membrane leaflet.20,60−63 Mitochondrial membranes also exhibit lateral and transverse asymmetry.64,65 Synthetic liposomes, especially when prepared in bulk in the absence of extrinsic reagents, are transversely symmetric but can still exhibit lateral separation into domains with separate phases.19,66,67 The formation of more ordered phases, frequently termed lipid rafts, has been extensively studied in ternary systems, particularly those involving PC, sphingolipids, and cholesterol.56,58,68,69
Reactions of membrane lipids, such as oxidation and hydrolysis, that lead to significant chemical changes have been well reviewed.70−75 This is especially true in the field of food science,70−72 as oxidation of lipids can seriously impact the quality of commercial food and beverage products.
1.2. Biological Membranes
Liposomes have been valuable tools for relating the effects of chemical changes in membrane lipids to the corresponding changes in fundamental membrane properties, such as hydration, porosity, compressibility, lateral diffusion, and transverse diffusion (flip-flop).6,76−79 Studies in vitro have also proved useful for the development of assays to detect the same modifications in vivo, providing insights into the occurrence and consequences of lipid damage to biological membranes.80−86 The development of methods in mass spectrometry, particularly with regard to cellular lipidomic profiling,87−93 and matrix assisted laser desorption/ionization (MALDI) imaging94−96 is providing particularly powerful tools for revealing changes in lipid composition during aging and the progression of numerous diseases, including cardiovascular diseases, cancer, diabetes, and neurodegenerative diseases.80,97−100
Some of the chemical reactions that lipids undergo, most notably nonenzymatic hydrolysis, occur at significantly slower rates than most biological processes.101 As a result, in a healthy cell the products of lipid reactivity do not accumulate. The levels of lipid degradation products in vivo are intrinsically linked to homeostasis and cell signaling, and the levels are therefore controlled.54,102−111 This is particularly true for oxidation products that arise from reaction with reactive oxygen species (ROS). ROS are generated via the normal pathways of oxidative degradation that occur within mitochondria, mostly via reverse electron transport and flavin mononucleotide pathways.112−114 Under stressed conditions, such as during disease or following injury, increased levels of lipid degradation products are associated with the resulting physiological response.115 Elevated levels of lipid oxidation products have been implicated in degenerative neurological conditions such as Alzheimer’s116−120 and Parkinson’s diseases,118,121,122 as well as diseases that involve the overproduction of ROS such as type 2 diabetes123,124 and cancer,109,113,125 among others.115,126−128 Increased levels of oxidation products are found following traumatic brain injury96,129−131 and are also a marker of aging.117
Many lipid degradation products, particularly those arising from ROS, chemically modify other biological macromolecules such as proteins and nucleic acids.132−137 Such modifications have been implicated, for example, in the pathology of Parkinson’s disease through the modification of the protein α-synuclein at internal lysine residues by the byproducts of lipid peroxidation.138
The interplay between chemical damage to a particular class of lipid and homeostasis can be complex. For example, oxidative damage to glycerophosphocholine lipids by ROS changes the biophysical properties of the membrane, leading to activation of phospholipases that then catalyze lipid hydrolysis to form byproducts which themselves are involved in cell signaling.100,139,140 The purpose of this response, principally mediated by cPLA2, is to recycle arachidonic acid, which is particularly prone to oxidative damage.100,131,141 Conversely, loss of mitochondrial phospholipase activity leads to increased lipid peroxidation in this organelle.142 This complexity is not restricted to lipid class. Sphingomyelinases (SMases), for example, catalyze the formation of phosphatidylcholine from PA and sphingomyelin, producing ceramide as a byproduct.20,143,144 SMase activity increases in the presence of ROS.145,146 Ceramide and the related sphingolipid sphingosine-1-phosphate perform key roles in cellular homeostasis and apoptosis.147,148 The exchange between ceramide and sphingomyelin may also be used to modulate the lateral phase behavior of membranes.149 As a final point, redox-active metals such as Fe(II) produce radical initiators from ROS and facilitate the breakdown of lipid hydroperoxides. When the usual corrective enzyme for lipid hydroperoxides is absent, the generation of excess lipid hydroperoxides leads to cell death by a nonapoptotic route that is Fe(II)-dependent (ferroptosis).106
1.3. Drug Discovery and Delivery
Liposomes have been extensively examined as systems that can be used to deliver pharmaceutical agents150−153 and macromolecules such as proteins154,155 and nucleic acids4,156,157 that would otherwise be unstable. Due to the amphipathic nature of lipids, the principal uses of liposomes are to solubilize hydrophobic drugs or to encapsulate hydrophilic drugs that would otherwise be unstable in vivo. Liposomes can enhance tissue penetration and retention passively and can be decorated with groups that target them to specific tissues or cells,158−161 and many drugs become more biocompatible when incorporated in liposomes, reducing adverse reactions and improving their therapeutic index.151,162 For all these applications it is essential that lipids exhibit good chemical stability, both in vivo and during preparation, sterilization, and storage in the formulated form prior to administration,71,163 particularly since the byproducts of lipid breakdown can exhibit acute toxicity.164,165 The stability of encapsulated macromolecules can also be influenced by changes in the chemical composition of the membrane, especially if the macromolecule can bind hydrolysis products. For example, the stability of human serum albumin (HSA) is improved in aged 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC)/CDL liposomes, an effect attributed to the association of HSA with free fatty acids (FFAs) formed by hydrolysis.166
Liposomes are frequently modified with surface groups that improve bioavailability and in vivo stability159,160 Approaches used to improve stability include the use of modified lipids to change the surface activity,167,168 incorporation of polymeric materials, and the formation of colloidal dispersions such as solid lipid nanoparticles (SLNs).169,170 As biomembrane mimics, liposomes have been used to model drug-lipid interactions and pharmacokinetic behavior.5,171,172
1.4. Food
As membranes are ubiquitous in biology, many of the chemical processes that occur in liposomes can also occur during food processing. This is of particular concern because the usual cellular mechanisms for removing lipid byproducts no longer operate in many cases. This can lead to the accumulation of products that degrade taste, smell, visual appearance, and nutritional quality. In addition to the lipids found naturally in foods, liposomes are used in food processing to deliver molecules that enhance flavor and nutritional content, improve shelf life,173−176 or reduce exposure to proteolytic enzymes in the gastrointestinal tract.177 Consequently, a large body of literature has examined the chemical changes that occur in food during processing, packaging, and transport.70,72,153,178−181
1.5. Cosmetics
Liposomes are primarily used in cosmetics to improve the stability of components within the formulation or to enhance the delivery and skin penetration of materials such as antioxidants.182−185 As with drug delivery, this exploits the ability of liposomes to solubilize hydrophobic materials and entrap water-soluble actives. When applied topically, entrapment of actives with liposomes brings the dual advantages of increasing skin localization and reducing systemic distribution, as well as improving the bioavailability of the target drugs.186,187 Liposome incorporation into products such as skin creams often brings additional benefits, such as improvements in skin hydration.184,185,188,189
The FDA guidelines for industry, issued in April 2018 (https://www.fda.gov/media/70837/download, accessed 2023-06-23), make it a requirement that for any liposome formulation including synthetic lipids, the levels of products associated with lipid degradation are quantified, including lysophospholipids, free fatty acids, and peroxides. In addition, stress testing of the formulation is required to examine physicochemical stability under extremes of temperature, light, pH, and oxygen in order to determine optimal storage conditions and retest periods. From the extensive literature on lipid reactivity, it is apparent that, when stored at ambient or low temperatures at neutral pH, most liposomes are remarkably stable for lengthy periods, frequently exhibiting little chemical change over a period of several months.
1.6. Key Challenges for Studying the Reactivity of Membrane Lipids
There are two key obstacles to understanding the chemical reactivity of membrane lipids. First, lipids are challenging to manipulate due to their properties of limited solubility in many solvents, long retention times in both normal and reversed phase chromatography, and formation of heterogeneous phases in aqueous systems. Second, the products of lipid reactivity often have significantly different properties compared to lipids, generally being more polar and sometimes even volatile. In recent years there have been spectacular advances in the analysis of lipid reaction products due to advances in mass spectrometry (MS), together with liquid chromatography (LC), and direct infusion “shotgun” approaches,190−192 which have enabled the highly sensitive detection of trace components of lipid mixtures and thus revealed a wealth of diverse lipid chemistry.87,88 While synthetic systems such as liposomes and solid lipid nanoparticles are well described chemically when prepared fresh and therefore changes in composition in controlled conditions are easily related to chemical reactivity, particularly when instruments can be calibrated against authentic standards, the challenge is significantly greater in biological systems. The cell lipidome is complex and includes several examples of lipids of the same mass but differing fatty acid composition. Many of the more polar products of lipid reactivity also ionize much more readily than lipids themselves, leading to the detection of products of such low abundance that any chemical or biological activity is uncertain. Tandem mass spectrometry approaches can decipher the fatty acid composition of individual lipids, but it is not a trivial pursuit.
In most cells there is a continual turnover of lipids and therefore the composition of many cellular membranes, both in terms of lipid class and acyl group composition, can evolve over time. Consequences of this evolution are a changing reference point or control alongside changes in the distribution of reaction products. Some products of lipid chemical reactivity, such as fatty acids and lysolipids, are produced by processes that occur both with and without enzyme control, making it challenging to establish which mode of generation is most significant. Furthermore, some products of lipid lysis are themselves substrates for other enzymes and can change receptor activation, either by direct binding or indirectly through changes in membrane properties. Changes in the levels of these products are therefore challenging to monitor in order to establish cause and effect. Some products of lipid reactivity, such as oxidation products, trigger large physiological responses such as ferroptosis that produce significant additional complexity.
The purpose of this Review is to provide an update on our current understanding of the stability of all the classes of lipid commonly found in biological and synthetic membranes. It focuses on the chemical stability of lipids in their membrane form, as found in liposomes and biological membranes, with an emphasis on conditions in which the lipids are well hydrated in the form of bilayers.
2. Hydrolysis
This section is concerned with the chemical (i.e., non-enzyme-catalyzed) hydrolysis of glycerophospholipids, as this is by far the most significant class for which hydrolysis has been studied. Enzyme-catalyzed hydrolysis of glycerophospholipids is discussed for context. No significant reports exist of the chemical hydrolysis of sphingolipids or sterols.
2.1. Phospholipases
Before considering hydrolysis reactions of lipids in depth, it is useful to briefly consider the reactions catalyzed by phospholipases, as many of the reactions they catalyze are the same as hydrolysis reactions seen in vitro and, as mentioned earlier, the activity of these enzymes is often invoked in response to other types of chemical reactivity in vivo, such as oxidation. Many of the products of phospholipase activity are themselves biologically active, acting as second messengers in cell signaling.
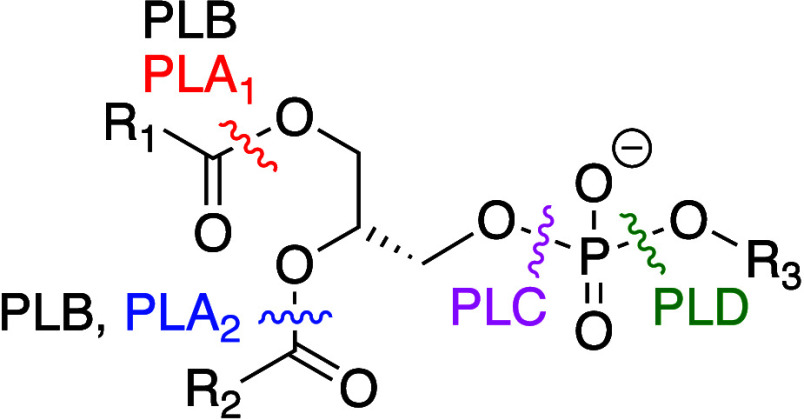
Phospholipases have been well reviewed.61,193−195 They act upon glycerophospholipids to generate hydrolysis products (Figure 2, Table 1). They are classified according to the bond hydrolyzed: phospholipases A (PLAs) hydrolyze the carboxyl esters, with PLA1 (frequently just termed “lipase”) and PLA2 selective for the sn-1 and sn-2 acyl groups, respectively; phospholipase C (PLC) hydrolyzes the phosphate ester on the glyceryl side to form a diacylglycerol and a phosphate ester of the headgroup; and phospholipase D (PLD) hydrolyzes the phosphate ester on the headgroup side to form a phosphatidic acid and the free headgroup (serine, ethanolamine, choline, glycerol, or inositol).
Figure 2.
Glycerophospholipid bonds cleaved by phospholipases A–D.
Table 1. Overview of the Chemical Reactivity of Phospholipases.
| enzyme | product(s) | major biological activity |
|---|---|---|
| PLA1 | free fatty acid | Saturated FFAs (typically found at the glycerol sn-1 position) increase lipid disorder and increase membrane permeability203,204 |
| 2-acyl-glycerophospholipid | changes in membrane fluidity, bending modulus, and barrier integrity;203,205−207 Signaling during cell growth (lyso-PI);110,207 and inflammation (lyso-PC)208 | |
| PLA2 | free fatty acid | polyunsaturated fatty acids (PUFAs) are involved in the inflammatory response and are precursors for second messengers in the CNS;209,210 unsaturated FFAs have a greater membrane perturbing effect than saturated FFAs203,204 |
| 1-acyl-glycerophospholipid | see 2-acyl-glycerophospholipid | |
| PLB | free fatty acid | see above |
| sn-glycero-3-phosphoalcohol | regulation of G-proteins (glycero-PI)207 | |
| PLC | diacylglycerol | activates protein kinase C211 |
| 3-phosphoalcohol | inflammatory response by activation of C-reactive protein (phosphocholine);212 cell signaling (inositol phosphates);213,214 others are involved in general metabolism or are inert | |
| PLD | phosphatidic acid | cell signaling (second messenger)197,215−217 |
| lipid headgroup (as alcohol) | general metabolism |
PLA1 and PLA2 both selectively form a FFA and a lysolipid. The lysolipid may itself be subject to further hydrolysis catalyzed by phospholipase B (PLB), which does not exhibit the sn-1/sn-2 selectivity of PLA. Some PLAs, such as cPLA2, also exhibit lysophospholipase activity.141,196 During chemical analysis of lipid membranes, the lysolipids formed by PLA1 and PLA2 activity typically give an equilibrium mixture of products that result from acyl migration between the sn-1 and sn-2 positions, with the sn-1 acyl being the major product. PLA1, PLA2, and PLD also exhibit transesterification activities, providing a means for exchanging the acyl groups in the case of the former two and the headgroup in the case of the latter.197 Non-PLA transesterifcation mechanisms have also been described for both glycerophospholipids198,199 and mitochondrial cardiolipins.200 Deacylation and reacylation reactions are controlled as part of the Lands’ cycle.198,201,202
2.2. Nonenzymatic Glycerophospholipid Hydrolysis
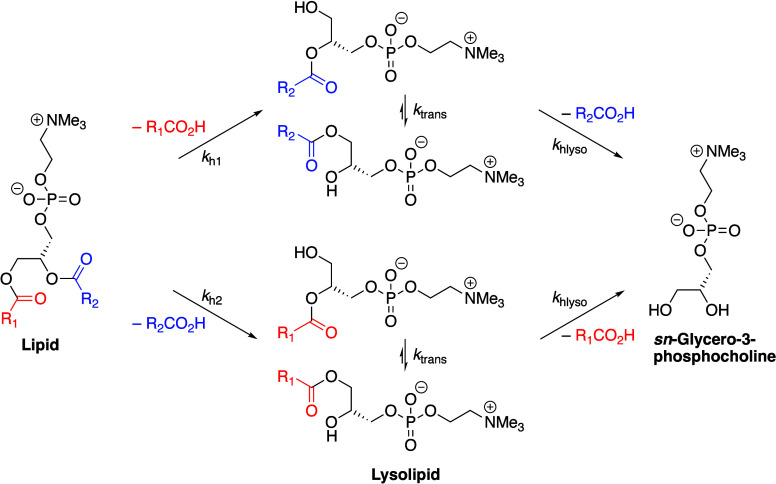
Extensive studies of lipid hydrolysis were reported in the 1980s and 1990s, most notably by the group of Crommelin and subsequently by Zhang, using PC liposomes as model systems.218−224 These studies, and many others, have demonstrated that phosphate ester hydrolysis in aqueous dispersions is so slow as to be insignificant on any reasonable time scale. Hydrolysis of the carboxylic esters (Scheme 1) is the most significant process for membrane lipids.
Scheme 1. Ester Hydrolysis Reactions of PCs.
2.2.1. Temperature and pH Effects
Hydrolysis of the glyceryl esters follows pseudo-first-order kinetics for the decrease in lipid concentration, down to 25% lipid remaining, with a V-shaped pH-rate profile.218,219,222,223,225−230 The slowest rates are at pH 5.8–6.5, with hydrolysis at the sn-1 and sn-2 positions having comparable rates (kh1 and kh2, respectively, Scheme 1), typically 8.5 × 10–4 h–1 in neutral single-component PC membranes. This rate of hydrolysis corresponds to a half-life of >30 days at room temperature. At low temperatures (4–6 °C), and in the absence of buffer additives, the half-life for hydrolysis is often >200 days (Table 2). The major contribution to hydrolysis is specific acid–base catalysis,218 but buffer catalysis also occurs, with acetate, tris, and citrate ions all providing general acid–base catalysis.223 During the course of the reaction, the production of free fatty acid (FFA) lowers the pH,231 and this pH change is frequently used as a means of monitoring hydrolysis rates. The lysolipid that initially forms following hydrolysis is subject to a transesterifcation reaction, resulting in acyl migration to form predominantly the 1-acyl species regardless of the initial site of hydrolysis. The rate of this transesterification (ktrans) has a minimum at a bulk pH of 4–5.232 Second-order rate constants for acid- and base-catalyzed transesterification have been estimated to be 4 × 10–4 and 160 M–1 s–1, respectively, with the first-order uncatalyzed reaction having a rate of 8 × 10–7 s–1.232−234 It is notable though, that the estimated rate of the acid-catalyzed reaction was determined at pH 1–2, which is close to the pKa of the phosphate group (0.8).235 The rate of the acid-catalyzed reaction may be faster at acidic pH values significantly greater than 0.8. As second-order rate constants for hydroxide-catalyzed hydrolysis are typically 0.3 M–1 s–1,222 base-catalyzed transesterification is faster than hydrolysis by three orders of magnitude. Conversely, in acidic conditions, hydrolysis (rate constant of 1.6 × 10–2 M–1 s–1) is faster by two orders of magnitude.
Table 2. A Summary of Studies to Examine the Rates of Lipid Hydrolysis in Model Systems.
| lipid | T (°C) | Phase | pH | notes | Ea (kJ mol–1)a | kobs (h–1)b | t1/2 (days) | ref |
|---|---|---|---|---|---|---|---|---|
| soybean PC | 72 | Lα | 4 | buffer-free | 7.7 × 10–3 | 3.7 | (223) | |
| 6.5 | 7.3 × 10–4 | 39.6 | ||||||
| 9 | 7.7 × 10–3 | 3.8 | ||||||
| soybean PC | 72 | Lα | 4 | buffer (acetate/citrate/tris), μ = 0.068, kw = 8.5 × 10–4 h–1 | 29.7 | 8.6 × 10–3 | 3.4 | (223) |
| 6.5 | kH = 0.8 × 102 M–1 h–1 | 57.2 | 1.7 × 10–3 | 17.2 | ||||
| 8 | kOH = 6.8 × 102 M–1 h–1 | 41.9 | 5.3 × 10–3 | 5.4 | ||||
| kbuff = 4.2 × 10–3 to 1.1 × 10–1 M–1 h–1 (except for AcOH) | ||||||||
| hydrogenated soy PC | 70 | Lα? | 4 | 50 mM buffer | 7.3 × 10–3 | 4.0 | (226) | |
| 6 | 3.9 × 10–4 | 74.5 | ||||||
| 9 | 4.6 × 10–3 | 7.1 | ||||||
| egg PC/chol (7:1) | 30 | Lo | 4 | citrate buffer (200 mM and 800 mM), Ea data are given here for 200 mM buffer, O2-free | 56.2 | 2.1 × 10–4 | 137.0 | (218) |
| 40 | 4 | 80.1 | 4.2 × 10–4 | 68.6 | ||||
| 50 | 4 | 4.8 | 7.4 × 10–4 | 39.1 | ||||
| 30 | 4.8 | 4.9 × 10–5 | 591.4 | |||||
| 40 | 4.8 | 1.2 × 10–4 | 235.8 | |||||
| 50 | 4.8 | 3.5 × 10–4 | 82.0 | |||||
| bovine heart plasmenylcholine (semisynthetic) | 38 | gel/Lα | 1.57 | NaCl (150 mM); buffers (20 mM): phosphate (pH 5.3), citrate (pH 4.3), unbuffered for pH 2.53 and 1.57 (pH adjusted with HCl); Tm = 37–38 °C | 1.8 | 0.02 | (242) | |
| 2.53 | 0.6 | 0.05 | ||||||
| 4.3 | 2.3 × 10–2 | 1.27 | ||||||
| 5.3 | 2.9 × 10–3 | 9.9 | ||||||
| DPPlsC | 37 | ? | 4.5 | DPPlsC is a di-O-((Z)-1′-hexadecenyl)-PCpl; citrate (20 mM), NaCl (150 mM). | 0.23 | 0.1 | (243) | |
| DPPC/DOPE (3:1) | 70 | Lα | 4 | DPPC hydrolysis; 20 mM lipid, 500 mM buffer (phosphate/acetate/HEPES) | 3.6 × 10–3 | 8.0 | (226) | |
| 5.8 | 1.3 × 10–3 | 22.7 | ||||||
| 9.1 | 1.5 × 10–2 | 1.9 | ||||||
| DPPC/DOPE (3:1) | 70 | Lα | 4 | DOPE hydrolysis; 20 mM lipid, 500 mM buffer (phosphate/acetate/HEPES) | 4.4 × 10–3 | 6.6 | (226) | |
| 5.8 | 2.4 × 10–3 | 12.3 | ||||||
| 9.1 | 3.3 × 10–2 | 0.9 | ||||||
| DOTAP/DOPE (1:1) | 70 | ? | 4 | DOTAP hydrolysis; 20 mM lipid, 500 mM buffer (phosphate/acetate/HEPES) | 4.4 × 10–3 | 6.6 | (226) | |
| 5.9 | 3.7 × 10–3 | 7.7 | ||||||
| 7.9 | 6.9 × 10–2 | 0.4 | ||||||
| DOTAP/DOPE (1:1) | 70 | ? | 4 | DOPE hydrolysis; 20 mM lipid, 500 mM buffer (phosphate/acetate/HEPES) | 4.4 × 10–3 | 6.6 | (226) | |
| 5.9 | 4.8 × 10–3 | 6.0 | ||||||
| 7.9 | 6.7 × 10–2 | 0.4 | ||||||
| DOTAP/chol/DPPC (5:10:85) | 50 | Lo? | DPPC hydrolysis; pH, buffers, and ionic strength not stated. | 3.7 × 10–3 | 7.7 | (244) | ||
| DOTAP/cholesterol/DPPC (5:10:85) | 50 | Lo? | DOTAP hydrolysis; pH, buffers, and ionic strength not stated. | 1.3 × 10–3 | (244) | |||
| Lipoid E80 | 40 | ? | 9.4 | containing itraconazole microcrystals; unbuffered, pH 9.4 at start, pH 4 at end | 7.2 × 10–4 | 40.1 | (231) | |
| Lipoid E80 | 40 | ? | 9.4 | containing itraconazole microcrystals; unbuffered, containing 0.22% (w/w) oleic acid, pH 9.46 at start, pH 7 at end | 2.9 × 10–4 | 143.2 | (231) | |
| DPPC/DSPE-PEG2000 (90:4) | 4 | ? | 2 | citrate buffer (300 mM); DPPC hydrolysis monitored | 3.1 × 10–4 | 94.4 | (229) | |
| 4 | 4 | 6.5 × 10–5 | 445.7 | |||||
| 22 | 2 | 1.8 × 10–3 | 16.2 | |||||
| 22 | 4 | 2.1 × 10–4 | 136.0 | |||||
| Lipoid E80 | 50 | n/a | oil in water emulsion following autoclaving (20 min, 121 °C); unbuffered, pH 6.1 before autoclaving, pH 5.0 after 3 months. | 6.9 × 10–4 (PC) | 42.0 | (236) | ||
| 5.6 × 10–4 (PE) | 51.3 |
Ea modeled by Arrhenius kinetics over a range of temperatures.
kobs is the observed rate. kobs =kw + kH[H+] + kOH[OH–] + kbuff[buffer], where kw is first-order rate constant for hydrolysis in water and kH, kOH, and kbuff are the second-order rate constants for acid-catalyzed, base-catalyzed, and buffer-catalyzed processes, respectively).
Further hydrolysis of the lysolipid ultimately forms sn-glycero-3-phosphocholine (GPC).220 Most hydrolysis studies focus on either direct measurement of lysolipid or FFA formation or indirect measurements of hydrolysis such as pH changes. The hydrolysis of the lysolipid to FFA and GPC is infrequently addressed. However, a number of studies have produced kinetic profiles that reveal an initial increase in lysolipid levels, with a subsequent decrease at longer time periods that can be attributed to GPC formation.229,236 GPC formation has been measured directly in a small number of cases.222,237
Above the temperature of the main gel to liquid crystalline phase transition (Tm), the temperature dependence of the rate exhibits Arrhenius kinetics, with activation energies (Ea) in the range 40–80 kJ mol–1.223,238,239 For PC lipids with saturated chains, activation energies increase in the gel phase, resulting in a discontinuity in plots of rate vs 1/T at the transition temperature of the lipid.219,240 For drug delivery applications, gel phase membranes tend to be more stable in blood plasma, with DPPC/1,2-dipalmitoyl-sn-glycero-3-phosphoglycerol (DPPG), for example, being more stable than hen egg phosphatidylcholine (EPC)/hen egg phosphatidylglycerol (EPG), as assessed by the retention of radiolabeled DPPC within the liposome fraction (49% of a tritium label in the acyl group was retained within EPC/EPG after 48 h compared to 80% in DPPC/DPPG). Inclusion of the drug temoporfin did not significantly affect stability.241
2.2.2. Chain Length Effects
In cholesterol-free fluid membranes above Tm, shorter acyl chains and increased levels of unsaturation both yield faster hydrolysis rates, effects probably related to water penetration into the interface.227 In bicelles composed of 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) and 1,2-dihexanoyl-sn-glycero-3-phosphocholine (DHPC), both saturated lipids, this is reflected by faster hydrolysis of the shorter chain lipid, which segregates to the edge of the bicelle.245,246 Cholesterol inclusion into fluid membranes slows hydrolysis,218 most likely as a consequence of an increased packing density in the liquid-ordered (Lo) phase relative to the fluid phase and therefore reduced water penetration. For membranes composed of saturated lipids, such as 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) and DPPC, chain length has little effect on rate for chain lengths between 12 and 18 carbons.219,247 Gel-phase membranes below Tm tend to exhibit slower hydrolysis than their counterparts that include cholesterol, particularly when the cholesterol content is high. For example, the activation energy for hydrolysis of DPPC/cholesterol (chol) (5:2) is lower than that of DPPC alone.219 In this case, the formation of the Lo phase has the potential to increase water penetration into the bilayer relative to the more densely packed gel phase. However, the effects of cholesterol incorporation on the hydrolysis kinetics of membranes composed of saturated lipids are small,222 and in many systems the inclusion of cholesterol imparts significant stability benefits with regard to hydrolysis. Some of these benefits may relate to specific interactions with components of the membrane such as PE,248 others to secondary effects resulting from the ability of cholesterol to decrease oxidative damage, particularly as the formation of oxidized species increases water penetration into the membrane and can thereby increases hydrolysis rates.249−251
In liposomes composed of DSPC/DPPC/1,2-dipalmitoyl-sn-glycero-3-phosphoglycerol (DSPG)/chol (35:35:20:10) and DSPC/DPPC/DSPG (38:38:24), qualitative detection of lysolipid was only possible in either system after 3 months at ambient temperatures.252 The commercial liposome preparation DOXIL, containing hydrogenated soybean PC (H-soyPC), cholesterol, and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine–polyethyleneglycol (DSPE-PEG), exhibited approximately 30% hydrolysis of the PE component on storage at 4 °C for 68 months.253
In a system composed of fully hydrogenated soy PC, investigated as a carrier for carboplatin, 5.4% degradation occurred after 6 months at 4 °C in the dark. Inclusion of cholesterol, ascorbyl palmitate (AP), or both (PC/chol/AP, 70:10:15 by mass), gave, respectively, 0%, 11%, or 12% lysolipid in the same conditions. At room temperature in daylight, the PC/chol system was remarkably stable, but the other systems all exhibited increased lysolipid formation, most notably the systems containing ascorbyl palmitate, where the level of lysolipid was >50%.254 In a study on the stability of egg PC liposomes, Samuni et al. also found that the presence of light had little impact on hydrolysis, observing ∼15% and ∼4% formation of FFA in EPC and EPC/chol (10:1) liposomes, respectively, after 16 months at room temperature and pH 7.4.255 In this study, the inclusion of vitamin E produced a slight reduction of 1–2% in the extent of hydrolysis.
2.2.3. Membrane Composition and Ionic Strength
In membranes composed of different classes of glycerophospholipids, those that are neutral (PC, PE) tend to exhibit slower rates of hydrolysis than those that are charged (PS, phosphatidylglycerol (PG), PI),256,257 with, for example, hydrolysis rates for lipid classes decreasing in rate in the order DPPG > DPPC > 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE) in comparable systems.219 The inclusion of charged species into membranes containing a saturated lipid, such as stearylamine, DPPG, cholesteryl sulfate, or dicetylphoshate, generally increases hydrolysis rates,219,227,244,258 an effect which has been ascribed to localized changes in the surface pH in accordance with Gouy–Chapman theory.219,227 Accordingly, hydrolysis rates in charged membranes are more sensitive to the ionic strength of the medium, with hydrolysis rates increasing at high ionic strength.259 The ionic strength of the medium has little effect on the reaction rate in many neutral membranes,218,219,227 although liposomes composed of PC and PE have been found to show an increase in size and decrease in retention rate with increasing ionic strength.260
2.2.4. The Effects of Membrane Additives
There are some notable deviations from predicted hydrolytic behavior, most notably for amphiphiles such as amines with titratable groups that are ionized at neutral pH. Increased rates in the presence of ascorbyl palmitate254 were noted above. Dialkylphosphates have a significant effect on the rate of lipid hydrolysis in a manner that is dependent on the chain length, giving faster rates when the length of the alkyl chain is similar to that of the lipid.237 For example, with H-soyPC/dipalmitoyl (10:1) liposomes, 77% of the lipid remained after 28 days (40 °C, pH 7.5), compared with ≥20% for didecyl and dieicosyl phosphate and 96% in the absence of the dialkylphosphate. Interestingly, in this system significant hydrolysis of the lysolipid was also observed. Incorporation of cholesterol increased hydrolysis rates below the Tm of DPPC and decreased rates above Tm. In both DPPC/DOPE (3:1) and 1,2-dioleoyl-3-trimethylammonium propane (DOTAP)/1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) (1:1) membranes, the rate of hydrolysis both components is almost pH-independent below pH ∼6.5, which is ascribed to predominant catalysis by the charged ammonium headgroup.226
It is increasingly becoming apparent that while many drugs, particularly uncharged compounds such as paclitaxel, have little effect on liposome stability,261 others, most notably cationic amphiphilic drugs (CADs), can markedly influence the hydrolytic stability of lipids. Incorporation of gemcitabine (dFdC) into DPPC/DSPC/DPPG2 (50:20:30) liposomes produced evidence for increased hydrolysis, with lysolipid levels between 0.6% and 1.3% in approximately a third of the samples following passive loading by incubating the vesicles for 30 min with dFdC at 60 °C and pH 7.4262 An earlier report suggests that the nature of the anion can influence the kinetics of hydrolysis promoted by dFdC. In liposomes composed of hydrogenated hen egg phosphatidylcholine (H-EPC)/chol (3:2), higher rates of hydrolysis were found in the presence of more lipophilic anions, decreasing in the order I– > Br– > Cl– > SO42– > F–, but only at dFdC concentrations ≥40 mM.256 At the highest concentration of dFdC (80 mM), in the presence of 50 mM NaI, 20% lysolipid formation was noted after 66 h at 60 °C, compared with <2.5% in the absence of dFdC. The precise role of the anion is uncertain, but it likely that it provides a counterion to the dFdC ammomium ion to facilitate partitioning of dFdC into the membrane.
More recent studies of the effects of CADs have identified a lipid dependence on the rate of hydrolysis. Casey et al. found that 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) liposomes incorporating 5 mol % raclopride were hydrolyzed at a rate of 0.1136 mol h–1 molRAC–1 over a 22 day period, producing 3.3 mol % at the end of the experiment. DOPC hydrolysis in the absence of raclopride was minimal over the same period.263 Hydrolysis in saturated lipid systems, or mixtures of DOPC with a high saturated lipid content, was significantly faster, with the rate increasing with at longer saturated chain lengths. A strong correlation was found between the rate of hydrolysis and Tm. Raclopride was proposed to act as a phase transfer catalyst, effectively promoting hydrolysis by acting as a general acid catalyst. Earlier research from the same group reached similar conclusions for the activity of haloperidol and spiperone in DOPC membranes.264 A total of 12 CADs was studied, and although the levels of activity varied, promotion of hydrolysis was found to be a general property of CADs. CADs will protonate at physiological pH, in turn initiating acid-catalyzed ester hydrolysis of the membrane. The rate will be determined by the mechanical state of the membrane (such as curvature elastic stress) as well as the chemistry of the CADs. Membranes made of 1,2-dilinoleoyl-sn-glycero-3-phosphocholine (DLPC; lower stored curvature stress) will hydrolyze at a slower rate than DOPC systems.
2.2.5. Physicochemical and Biological Effects of Hydrolysis
Hydrolysis of a single acyl chain yields equimolar FFA and lysolipid. Subsequent hydrolysis of the second acyl chain from the lysolipid, forming water-soluble GPC and another equivalent of FFA, will shift the balance of lysis products in favor of FFA. Studies show that on their own, both lysolipids and FFA increase disorder in the membrane, leading, for example, to the decreased retention of encapsulated drugs. However, the presence of both lysolipids (lyso-phopshatidylcholine, LPC) and FFA increases the stability of the bilayer, making the bilayer even less permeable than with phospholipids alone.203,227,265
In some systems, significant changes in morphology accompany lipid hydrolysis. In DPPC/DSPE-PEG2000 liposomes, lysis resulted in the formation of bilayer sheets and discs, but only upon heating and then recooling through the main gel to liquid crystalline phase transition, indicating that the initial mixture containing lysolipids and FFA is metastable.229 Strikingly, this effect was produced with <10% DPPC hydrolysis when the sample was stored at pH 4 and <5% when stored at pH 2. Disc formation was attributed to stabilization by lysolipids partitioning to the highly curved areas at the edge of the disc, an effect that has been noted elsewhere.266
The ability of hydrolysis reactions to produce morphological changes has recently been exploited by Kodama et al.267 They used the localized microinjection of hydroxide ions to the internal surface of a giant unilamellar vesicle (0.1 mol % rhodamine-labeled DOPE in DOPC) to drive lipid hydrolysis and generate movement of the vesicle. Related effects have been observed for the formation of complex 3D topologies from supported 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) bilayers, including the formation of protrusions from the bilayer surface, following treatment with hydroxide ions at pH ≥11 or acidic conditions at pH ≤1.268 In this case, the effects were not directly attributed to hydrolysis, as protrusions formed significantly faster than the predicted rate of hydrolysis, although the involvement of some lysolipid in stabilizing highly curved structures might still contribute to the observed changes.
The presence of lysolipids increases bilayer permeability and significantly disrupts bilayer structure and morphology at relatively low levels (<10 mol %). In most healthy cells the levels of lysolipids are regulated at <6% of total membrane lipids. Elevated levels of lysolipids are associated with diseases such as cancer and cardiovascular disease. Lysolipids have other roles in cell physiology, including signaling, reproduction and the inflammatory response.203,269
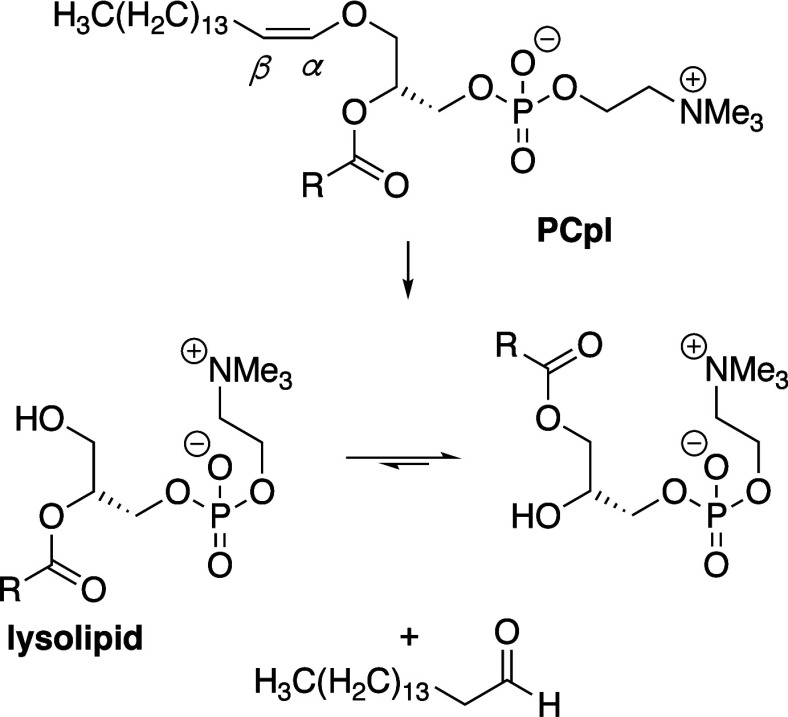
2.3. Hydrolysis of Plasmalogens
Vinyl ether linked lipids, such as plasmenylcholines, are found in many eukaryotic membranes and are generally more susceptible to hydrolysis than glycerophospholipids. This susceptibility arises from the electron-rich nature of the enol ether and results in preferential hydrolysis of this group over the adjacent ester (Scheme 2).242 First-order rate constants for hydrolysis of plasmenylcholines are 2–3× higher than those of glycerophospholipids under comparable conditions (Table 1).
Scheme 2. Hydrolysis of Plasmenyl Lipids.
The reactivity of vinyl ether lipids has been exploited for the development of drug delivery systems. Liposomes of a bis-vinyl ether lipid, 1,2-di-O-((Z)-1′-hexadecenyl)-sn-glycero-3-phosphocholine (DPPlsC), incorporating calcein, did not exhibit significant leakage at 37 °C and pH 7.4 over a 48 h period. At pH 4.5, significant calcein release was observed after a period of one hour, at which point the lipid was >20% hydrolyzed.243 This approach was used to successfully demonstrate targeted delivery of the drug Ara-C to KB cells using liposomes composed of DPPlsC /7-dehydrocholesterol (DHC)/DSPE-PEG3350-folate (9:1:0.05). Controlled release using liposomes containing vinyl ether groups has been reviewed.270
2.4. Other Hydrolyses
Few reports exist of other types of hydrolytic reactivity of lipids. Poznik et al. recently reported that lanthanide ions can act as Lewis acid catalysts in the decomposition of phosphodiesters.271 Bis-4-nitrophenyl phosphate (BNPP) was used to model the activity of La(III), Ce(III), Eu(III), Tb(III), and Yb(III) complexes in the presence of DOPC membranes. Although significant catalytic activity was found for complexes of these ions, no direct hydrolysis of lipid phosphates was reported. One report exists of a PC demethylation to form PE.254 This demethylation was reported for liposomes composed of H-soyPC and H-soyPC/chol containing ascorbyl palmitate (in a 7:1:1.5 ratio) that encapsulated carboplatin, where PE accounted for 5% of the material after 6 months at pH 3.5–4, regardless of temperature (4 °C/room temperature). Partially demethylated PC and lyso-PE were not detected.
2.5. Future Directions
While the fundamental aspects of membrane lipid hydrolysis are well established, there remain areas where further research is needed, most notably in better understanding the relationship between oxidation and hydrolysis and in predicting the stability of complex formulations containing lipid membranes alongside excipients and other active molecules. With regard to the latter, while there have been some studies that have found that lipid hydrolysis can be promoted by drugs and small organic molecules,263,264,272 others have found evidence to suggest that some compounds actually reduce the rate of background hydrolysis.273 The effects of drugs and related molecules on lipid stability remain underexplored.
3. Aminolysis and Transesterification
3.1. Intrinsic Lipidation
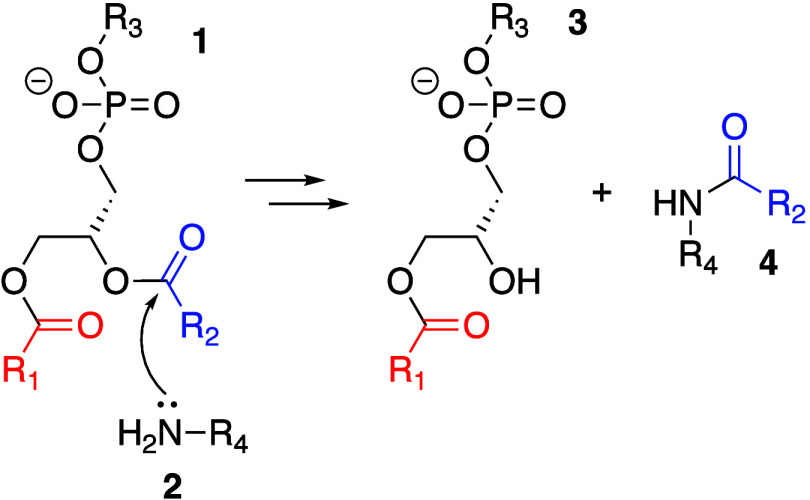
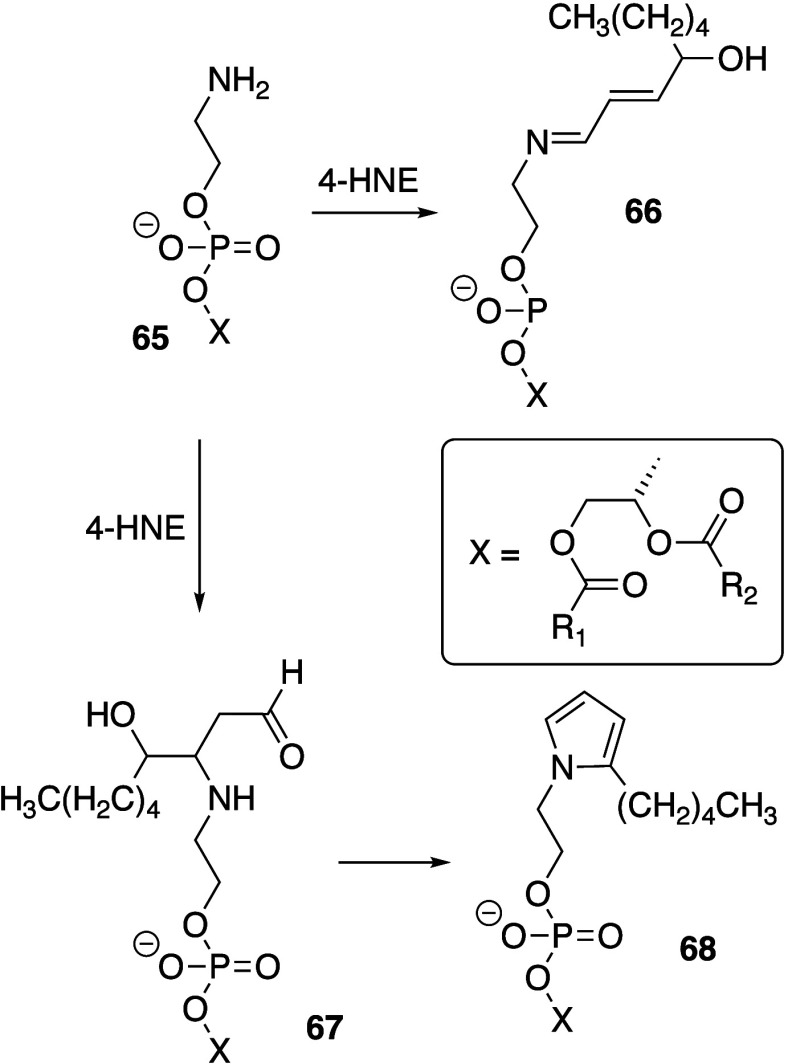
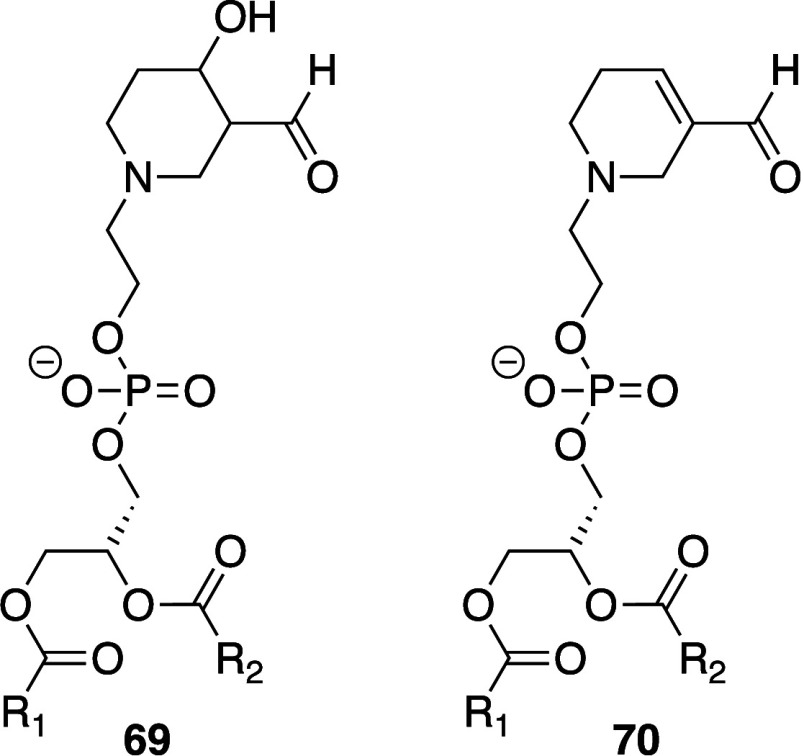
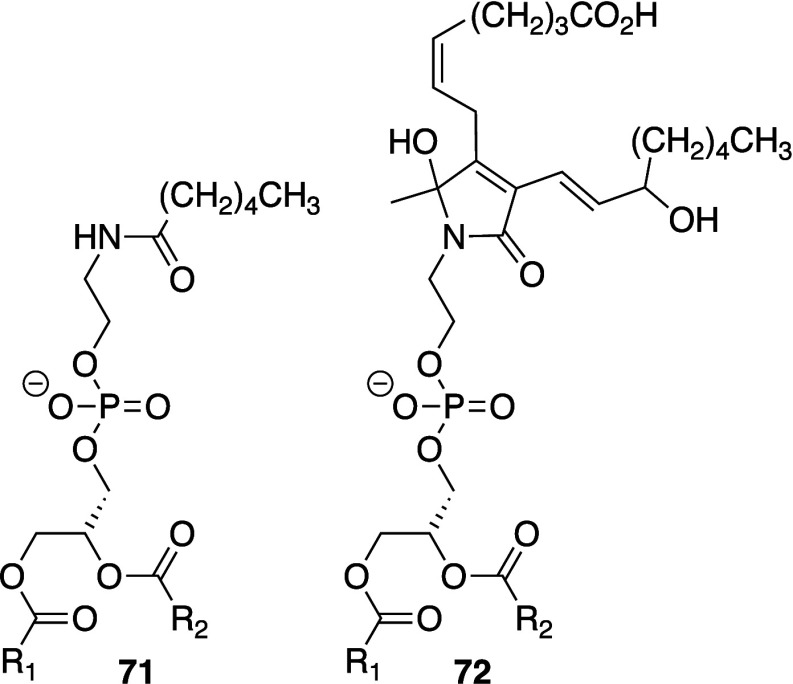
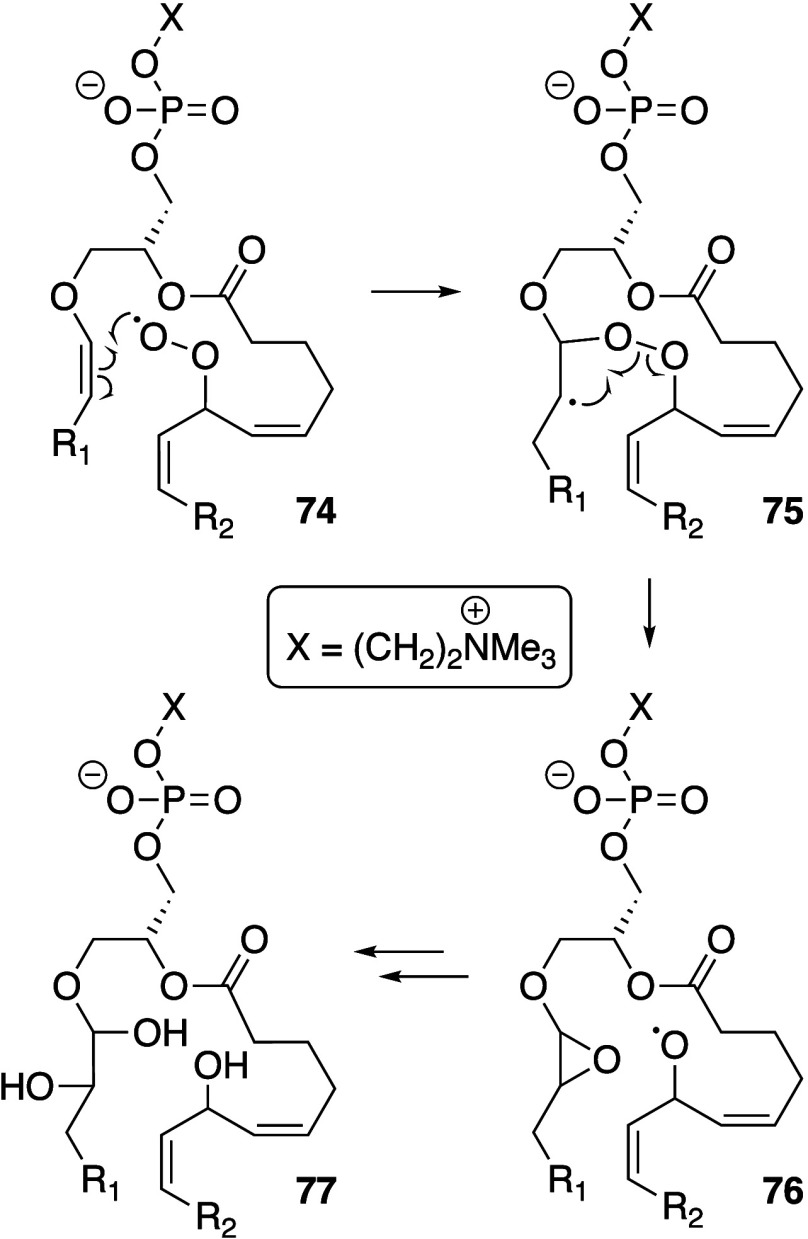
Direct reaction between membrane-embedded molecules and membrane lipids, termed “intrinsic lipidation”, has been little studied until relatively recently. As hydrolysis reactions at high pH involve nucleophilic attack of hydroxide ions on the ester carbonyl group, it should not be surprising that other nucleophilic groups within the membrane are capable of participating in a similar attack to form a lysolipid (3, Scheme 3) and a lipidated product, an amide in the case of an amine (4) or an ester should the nucleophilic group be an alcohol.
Scheme 3. Aminolysis Reactions of Glycerophospholipids.
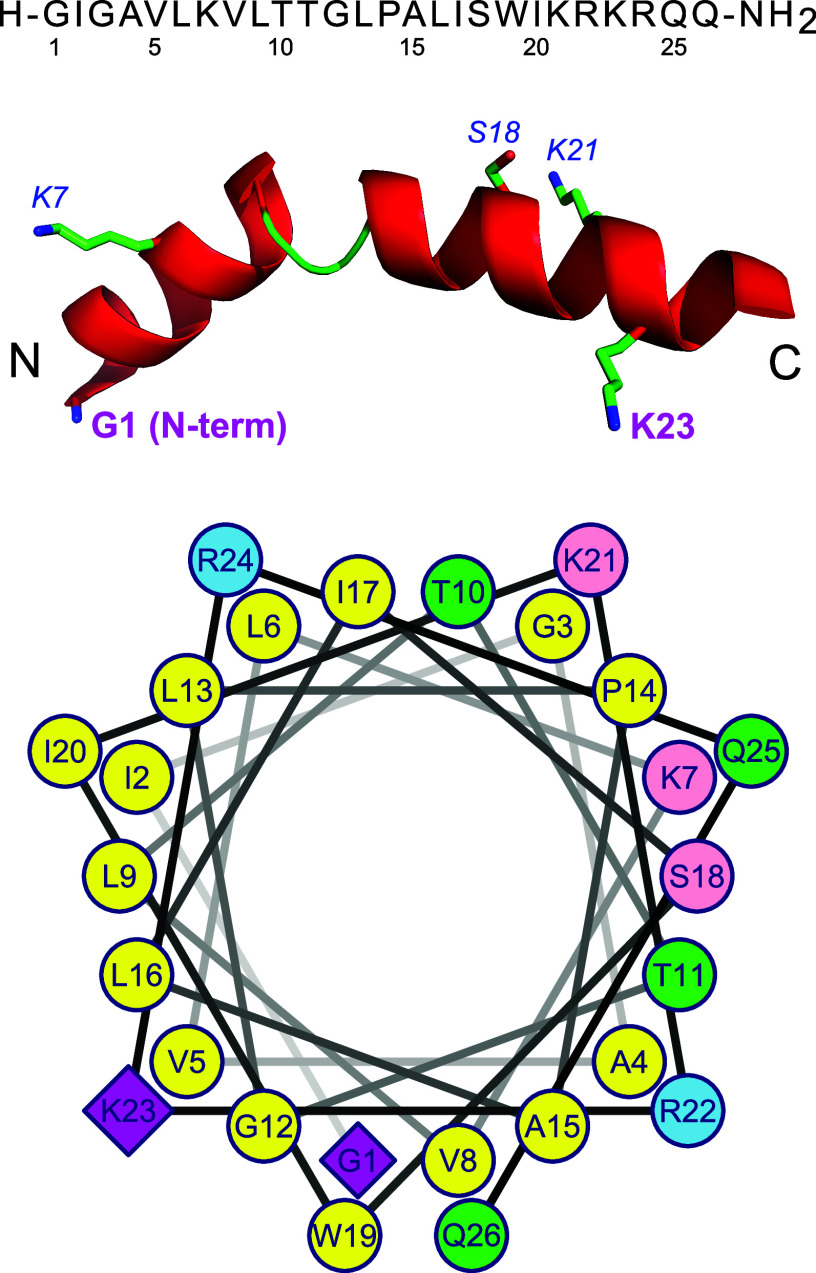
Membrane-embedded peptides have been shown to undergo acyl transfer reactions by direct reaction with lipids under physiological conditions (pH 7.4, 37 °C).274−276 These reactions, with a typical t1/2 of 20–40 h, are faster than hydrolysis reactions at this pH. The prototype for this reactivity is melittin. This 26-residue peptide (Figure 3) undergoes aminolysis reactions with diacylglycerophospholipids that involve the amino groups of internal lysine residues and the N-terminal amino group, and transesterification reactions involving an internal serine.275
Figure 3.
Sequence and structure of melittin: top, sequence; middle, crystal structure (PDB code 2MLT)277 with the major acylation sites shown in bold and the minor sites in italics; and bottom, helical wheel representation with the major acylation sites shown as diamonds, the minor sites shown as pink, charged residues shown as blue, polar uncharged residues shown as green, and apolar residues shown as yellow.
As with hydrolysis reactions, no selectivity is found for reaction at the acyl groups of sn-1 and sn-2 positions of the glycerol backbone, but selectivity is seen for the site of reaction on the peptide. The reactivity of the available nucleophilic centers on the peptide can be ranked in the order N-terminus > K23 ≫ K21 ≈ K7 > S18 (Figure 4).275 This ranking of reactivity is likely to be a consequence of a number of factors, including the pKa of the ammonium form of the amine and positioning of the reactive group within the membrane. The pKa of the N-terminal ammonium group of melittin is in the range 7.15–8.15 in a micelle-associated form, which is significantly lower than that of the internal lysine residues (9.2–10.2 for K21, K23, and K7)278,279 and consistent with the higher reactivity of the N-terminal amine, significant amounts of which will be in the neutral amine form within the membrane interface at physiological pH. The difference in reactivity between K23 and K7/K21, which cannot be accounted for simply in terms of pKa, is likely to reflect their different positioning within the membrane-embedded state of the peptide, with K23 being positioned close to the bilayer interface. Indeed, for other amphiphilic peptides, including magainin II and PGLa, reactivity is most frequently observed for residues that are located close to the interface between the polar and hydrophobic surfaces, which would position these residues in the interfacial regions of the bilayer.274 Peptides without interfacial reactive groups, such as penetratin, only undergo the reaction in very specific circumstances, such as high salt concentrations. Additional groups in melittin also undergo reaction with lipids, most notably the side chain hydroxyl of S18 to form ester-linked lipidated peptides, but the reactivity of these groups is lower than amines.275 Other products resulting from reactivity between melittin and membrane lipids have been detected, but they are extremely prone to in-source fragmentation during MS analysis and it has therefore been difficult to establish their identity. Potential sites for these reactions include the side chain of a histidine residue. In membrane models comprising two lipid classes with similar acyl chain compositions, such as POPC/1-stearoyl-2-linoleoyl-sn-glycero-3-phosphoethanolamine (SLPE), POPC/1-stearoyl-2-linoleoyl-sn-glycero-3-phosphoglycerol (SLPG), and POPC/1-stearoyl-2-stearoyl-sn-glycero-3-phosphoserine (SLPS) (4:1 in all cases), in which acyl transfer from the sn-1 and sn-2 positions of each lipid class can be distinguished, acyl transfer is seen from both the lipid classes in accordance with their relative abundance, with no appreciable selectivity between reactivity at the sn-1 and sn-2 positions (Figure 4). Differences are found in the overall reactivity of these systems, however, with POPC/SLPE being the most reactive, suggesting a role for the biophysical properties of the membrane in determining reactivity. Consistent with this, for lipid mixtures where the acyl chains are less well matched, differing reactivity patterns are seen, with transfer only found from DOPC in DOPC/1,2-distearoyl-sn-glycero-3-phosphoserine (DPPS) mixtures, but both lipid classes in DOPC/1,2-dimyristoyl-sn-glycero-3-phosphoglycerol (DMPG) mixtures.275
Figure 4.
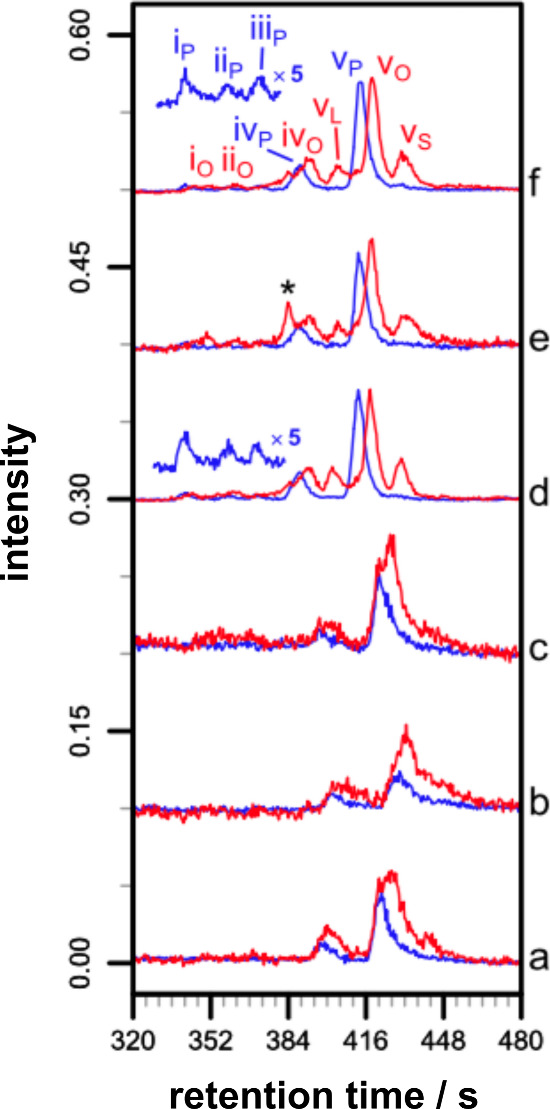
LC-MS analysis of melittin/liposome mixtures. Reaction conditions unless otherwise stated: [melittin], 26 μM; [POPC], 0.26 mM (P/L, 1:10), [NaCl], 90 mM; NaHCO3, 10 mM; pH 7.4; 37 °C. Key: rt, retention time. (a) No NaHCO3, 72 h. (b) 20 °C, 150 mM NaCl, 72 h. (c) Water (no NaHCO3 or NaCl), 48 h. (d) POPC/SLPS (4:1), 72 h. (e) POPC/SLPG (4:1), 48 h. (f) POPC/SLPE (4:1), 72 h. (a) Total ion chromatograms (TICs; area normalized). The broken box indicates the region in (b). (b) Extracted ion chromatograms (EICs; area normalized) for palmitoyl–melittin (blue) and oleoyl–melittin (red). For (d–f), combined EICs for oleoyl/stearoyl/linoleoyl–melittin are shown in red. The peak indicated by an asterisk is from a polymeric impurity. Chromatographic peak identities are annotated using a roman numeral to indicate the main site of peptide modification responsible for the peak, with a subscript to identify the acyl group; i–v correspond to S18, K21, K7, K23, and the N-terminus, respectively. Reprinted with permission from ref (274). Copyright 2013 Elsevier.
Other factors that could influence reactivity have been addressed, including temperature, the ionic strength of the medium, the role of the buffer, the counterion of the peptide, and the peptide/lipid ratio (in the range 1:10 to 1:100).274 Generally, both higher temperatures and increased salt concentrations favored the reaction, but the effects of other parameters were found to be small. It has also been demonstrated that peptides are able to undergo similar processes with lysolipids.280 A consequence of peptide lipidation in this manner is expected to be an increase in the membrane affinity of the lipidated peptide. This will be reflected by irreversible membrane binding. Examples exist in the literature where peptide association with membranes is not completely reversible, including association of the designed peptide TMX-1 to POPG membranes281 and melittin to PEG-stabilized POPC/palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol (POPG) nanodiscs.266 Although analytical speciation of these mixtures was not conducted at the end of these experiments to determine whether lipidation had occurred, it remains a strong possibility that this is the prime reason for irreversible binding in cases such as this.
Interestingly, the inclusion of cholesterol into PC membranes significantly increases reactivity, with in some cases up to 50% of melittin converted into lipidated products within 24 h.282 This higher reactivity occurs in spite of the reduced affinity of melittin for membranes containing cholesterol and is attributed to the change in disposition of the peptide in cholesterol containing membrane. Consistent with this change in disposition, the pattern of reactivity also shifts, with lipidation at K23 becoming the predominant product. In these conditions it is implicit that the rate-determining step is not associated with initial nucleophilic attack of reactive groups on melittin and as a consequence the membrane affinity is not a significant factor in predicting the rate of lipidation.
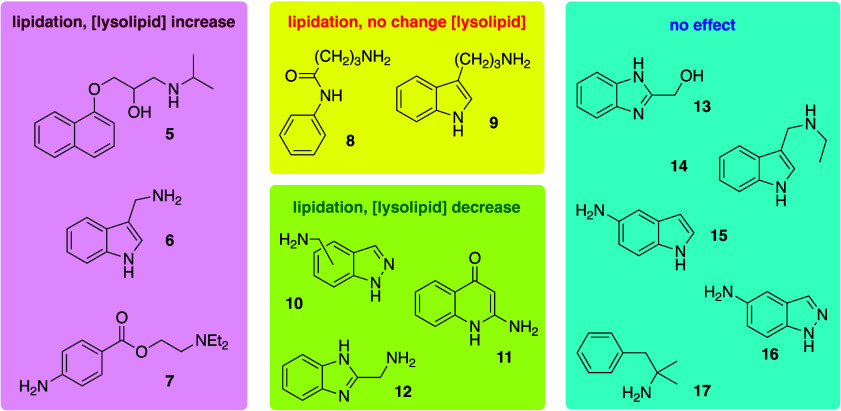
This nondependence of reactivity on membrane affinity is seen most strikingly for small organic molecules, many of which have low predicted membrane affinity based on their log P values and exhibit a spectrum of behavior from no effect on membrane stability, through effects on the rate of lipid hydrolysis, to participation in lipidation reactions (Figure 5).273 Notable examples are the drug propranolol (5), for which lipidation on the alcohol occurs almost exclusively rather than at the secondary amino group, both in model systems in vitro and HepG2 cells.272 Propranolol lipidation is likely driven by a combination of membrane partitioning of the predominant ammonium form (at physiological pH) to an appropriate depth to enable reaction on the oxygen, combined with disfavored reaction of the minor neutral form at the nitrogen center for steric reasons. Lipidation is generally disfavored by steric bulk near the reactive center, exemplified by the difference in reactivity of 3-aminomethylindole (6 and the corresponding N-ethyl analogue (14). Exchange of the nucleophilic group from an amino group to an alcohol can be sufficient to eliminate reactivity (e.g., 12 vs 13). Furthermore, many of the amino analogues of reactive aminoalkyl -substituted aromatics, exemplified by 16 (for 5-aminomethylindazole, 10), exhibit no apparent reactivity in membranes despite having lower pKa values than their aminoalkyl counterparts, which would be expected to favor the partitioning of the neutral form into the membrane. These examples further illustrate that the disposition of molecules in the membrane is likely to be of greater importance for reactivity than the overall affinity of a molecule for the membrane.
Figure 5.
Lipidation activity of exemplar low molecular weight organic molecules with neutral phospholipid membranes.272,273 The changes in lysolipid concentration are ≥24 h following the addition of the compounds to liposomes. Compounds with “no effect” yielded neither lipidated products nor changes in lysolipid levels relative to controls.
As lysolipids, whether formed by background hydrolysis or as byproducts of aminolysis or transesterification reactions, are potential sources of acyl groups for lipidation reactions, their concentration profiles can fluctuate during the progress of an intrinsic lipidation reaction. To add to this complexity, some compounds may be able to simultaneously participate in aminolysis or transesterification reactions and promote lipid hydrolysis. Indeed, after periods ≥24 h following compound addition to liposomes, compounds such as propranolol yield an increase in lysolipid concentration. Other compounds, however, most notably the aminomethylindazole family (10), yield decreased lysolipid concentrations, presumably reflecting either a greater reactivity with lysolipids than lipids or an activity to reduce the rate of background hydrolysis. It is noteworthy that some compounds (e.g., 8, 9) have no net effect on lysolipid concentrations, and others are able to increase the rate of background hydrolysis without any evidence of lipidation. In membranes with a negative charge, broadly similar patterns of intrinsic lipidation activity are found, with some variation in the nature of lysolipid changes observed for some compounds.
In principle, membrane proteins should undergo similar reactions in vivo. In many cases, however, the protein turnover rate will prevent the products from these reactions from accumulating. Protein degradation half-lives in mammals typically range from <1 h,283,284 through 2–4 days in the liver and blood, to >10 days in the brain.285,286 A small number of proteins have extremely low or zero degradation rates, including structural proteins such as collagen and proteins in postmitotic cells such as the nucleus of the lens, in which the normal cellular machinery is absent and as a consequence proteins are not recycled.283 It is reasonable, therefore, to expect the products of the intrinsic lipidation of membrane proteins to accrue in scenarios where the normal cellular machinery is absent, such as in extracellular fluids and postmitotic cells. In cases where intrinsic lipidation may occur in vivo, the observations from peptide lipidation in model membranes enable a prediction that it will result in incomplete lipidation at unusual residues that reside close to the membrane interface but do not conform to a consensus sequence, yielding an acyl group distribution that reflects the acyl composition of the host membrane.
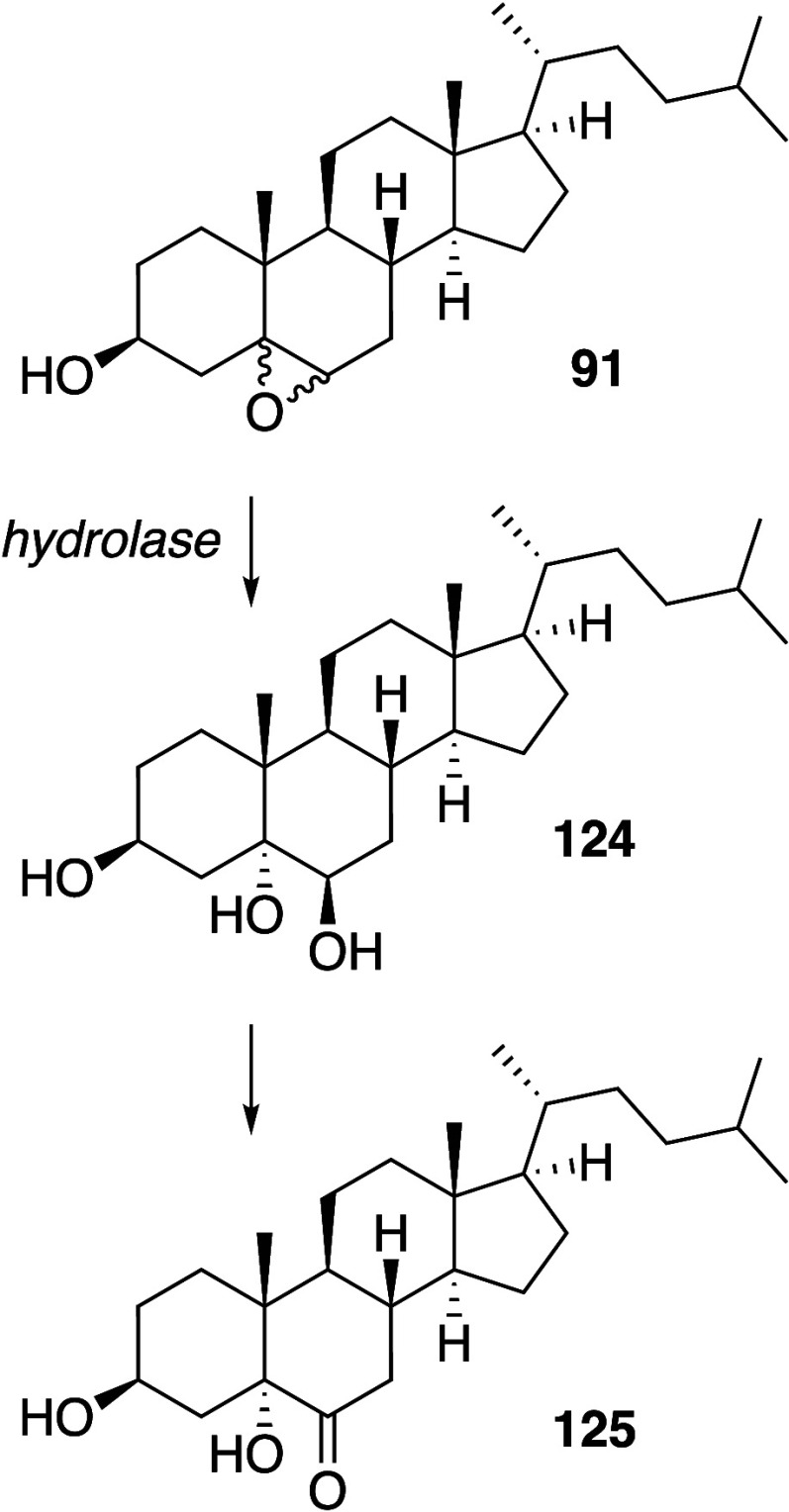
Lung surfactant protein C (SP-C) is initially synthesized as a precursor protein, proSP-C. Palmitoylation of proSP-C occurs at two internal cysteine residues with high efficiency287,288 under enzymatic control, occurring between the ER and the cis-Golgi.289,290 After processing to SP-C, the protein is incorporated into lamellar bodies (multilamellar vesicles) and transported to the plasma membrane, where fusion of the lamellar bodies releases SP-C into the liquid layer lining the airway epithelium. Examination of SP-C obtained from bronchoalveolar lavage has identified a third SP-C lipidation site. This site is palmitoylated at about 4% and has been identified as modification of an internal lysine, K11, by tandem mass spectrometry approaches.291,292 Lung surfactant is 78–90% phospholipid, with 50–70% of this being DPPC. The site of SP-C modification, the identification of the acyl group as palmitoyl and the incomplete conversion are all consistent with an intrinsic lipidation mechanism. Furthermore, the modified lysine is predicted to be close to the membrane interface in the transmembrane helical form of the protein and upon storage the extent of lysine modification was found to increase.292
Aquaporin-0 (AQP0, also termed the lens major intrinsic protein) is a membrane protein that, in fiber cells of the eye lens, has roles in water transport and the formation of gap junctions. Lens fiber cells are arranged concentrically, from the youngest cells in the outer cortex to the oldest (embryonic) cells in the lens nucleus.293 New fiber cells are added to the outer cortex from the epithelium that surrounds it, but during lens fiber cell differentiation cell organelles are removed and normal recycling of cellular components stops.294 As a consequence, fiber cells are postmitotic and contain some of the oldest proteins in the body. Post-translational modifications (PTMs) to AQP0 have been studied extensively by Schey and co-workers.295−302 They have documented a range of PTMs, including truncation, oxidation, deamidation, phosphorylation, and lipidation. The lipidation of AQP0 is unusual, occurring at the N-terminus and an internal lysine, K238, with the predominant modifications being oleoyl and palmitoyl in decreasing order of abundance.298 Recently, it has become apparent that the lipidation profile at both these sites includes a range of acyl modifications. For bovine AQP0, the acyl modifications at the N-terminus include, in decreasing order of magnitude, C18:1 ≫ C16:0 > C18:0 > C20:1 > C20:3 ≈ C16:1 > C20:2.303 Many of these modifications are also found at K238, with a similar relative abundance. Human AQP0 similarly has an extensive lipidation profile that differs by the inclusion of two different C20:4 and C20:3 modifications, as well as C22:4 and C20:5, although the latter two were only detected as oxidized species. For both human and bovine AQP0, but particularly the latter, there is a striking correlation between the abundance of the acyl modifications and the population of ester-linked acyl groups within the PE class of lipids (including plasmalogens). It is noteworthy that PE lipids are a major component of the cytoplasmic membrane leaflet to which the N-terminal amino group and K238 are proximal.20 Modification by intrinsic lipidation is consequently a distinct possibility, although lipidation by direct acyl transfer from membrane-associated Acyl-CoA has also been shown to be a viable process304−308 and cannot be completely ruled out as AQP0 passes through the ER, a major site of phospholipid biosynthesis.20 AQP0 lipidation is first detectable at about 20–30% of the distance from the outer cortex to the center of the lens nucleus and increases up to 60% of that distance before reaching a plateau.295,297 The value at this plateau increases with age from ∼30% in an 11 year old to ∼50% in a 32 year old.297 The increases in AQP0 lipidation both with age and proximity to the lens nucleus argue in favor of lipidation from the membrane.
Recently there has been a surge of interest in post-translational modifications to lysine. A range of modifications are known, many involving metabolic intermediates and most involving the formation of amides involving the lysine amino group.309 Concomitant with this has been increased interest in the sirtuins, a family of NAD+-dependent deacylase enzymes that catalyze the removal of these PTMs. Seven sirtuins are present in humans (SirT1–7), but they are present at varying levels in almost all organisms.310 The discovery that some sirtuins, including mammalian SirT6311 and Sir2A from Plasmodium falciparum(312) selectively hydrolyze long-chain acyl groups from the side chains of internal lysine residues suggests that this reaction is important enough in vivo for the evolution of protective mechanisms.313
Overall it is clear that molecules of any size that are able to interact with membranes have the potential to undergo direct acyl transfer reactions with the lipid to become lipidated, provided that there is a nucleophile suitably disposed in at least one bound configuration. This should be of some concern in instances where lipids are used to formulate potentially reactive drugs, such as in liposomes or solid lipid nanoparticles. Should protective mechanisms have evolved to recycle lipidated proteins or remove acyl modifications, the intrinsic lipidation process should also be of concern during the storage of foodstuffs. In biological systems, intrinsic lipidation has been proposed as one route by which amyloid formation could be triggered.101,314
3.2. Future Directions
The recognition that some molecules embedded in the membrane can be lipidated by direct acyl transfer from the lipid occurred relatively recently. There are still gaps in our understanding of this process with regard to both the molecular features and membrane dispositions that promote lipidation and the consequences of lipidation in vivo for the activities of biomolecules, as well as the distribution and clearance of drugs. It is likely that lipidation occurs in tissues or organelles where proteins have a slow turnover, but this is challenging to prove. There may also be cellular corrective mechanisms that are as yet unrecognized. In this regard, sirtuins with broad substrate specificity have been proposed as enzymes that may carry out this role.101 In some diseases, notably those involving amyloid formation, many of the long-lived deposited materials contain lipids alongside proteins, and it has been proposed that amyloid peptide lipidation may play a role in the nucleation process; however, this has yet to be proved.314 It will be interesting to examine whether lipidated peptides with diverse fatty acid profiles can be identified in proteomics studies. Currently, in many proteomics studies using LC-MS, the usable gradient stops short of mobile phase compositions where lipidated peptides would elute and be fragmented to permit identification.
4. Oxidation
The chemistry of lipid oxidation is very well documented due to its importance in food chemistry, liposome stability, and biological redox processes. By far the most important reactivity involves the alkene groups of unsaturated fatty acids, which are particularly liable to oxidation by atmospheric oxygen (autoxidation). Alkene reactivity is also a feature of the oxidative reactions undergone by plasmalogens, sphingolipids, and sterols. Oxidative processes involving free radical intermediates are known for other functional groups, particularly when reactive alkenes are not present, but these reactions only tend to occur following the formation of reactive intermediates during processes such radiative damage.
4.1. Free Radical Oxidation of Glycerophospholipids
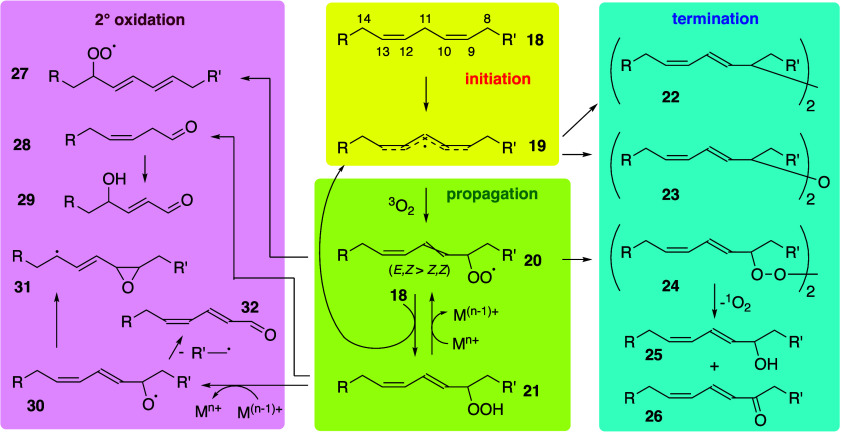
Oxidative reactions of fatty acids have been well reviewed by Spickett, Pratt, and Porter,74,315−317 plus a number of other recent reviews.108,112,181,318−326 An overview of the main processes is given in Scheme 4.
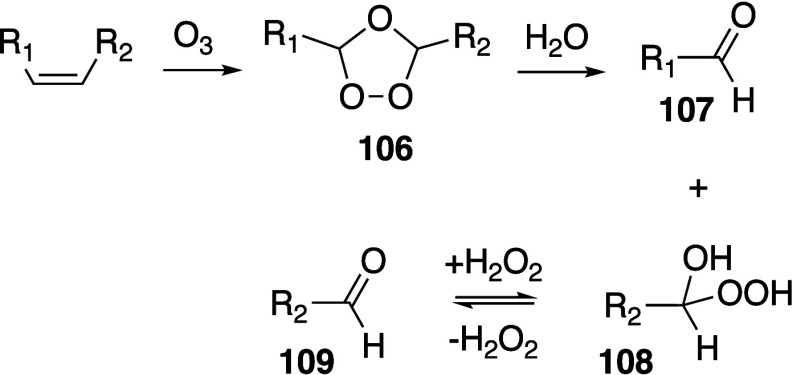
Scheme 4. Overview of Processes Involved in the Autoxidation of Homoconjugated Dienes.
The atom numbering of 18 corresponds to the carbon atom numbers of linoleic acid.
4.1.1. Initiation
Unsaturated fatty acids are liable to oxidation by the initial formation of a bis-allylic radical by hydrogen abstraction. Allylic radical formation is particularly prevalent in naturally occurring polyunsaturated fatty acids (PUFAs) as the double bonds are homoconjugate, being separated by a single methylene group. For example, cis,cis-Δ9,Δ12-octadecadienoic acid (lineoleic acid), commonly used as a model for oxidation reactions, has double bonds between carbon atoms 9/10 and 12/13 of the acyl chain, separated by a single methylene at carbon 11. Respective C–H homolytic bond dissociation energies for allyl and bis-allyl hydrogens are 65 and 77 kcal mol–1.181 Rate constants (ki) for initiation (typically 10–6 s–1)327 are significantly lower than those for propagation or termination. For studies of liposome aging in vitro and oxidative processes in food, initiation is frequently enhanced by the addition of free radical initiators to increase the overall initiation rate (ki[In•]). These have been well reviewed.317,326,328 Commonly used examples include water-soluble agents such as 2,2′-azobis(2-methylpropionamidine)dihydrochloride (AAPH),329 and lipophilic agents such as di-tert-butylhyponitrite (DBHN),330t-butylhydroperoxide,139 and 2,2′-azobis(4-methoxy-2,4-dimethylvaleronitrile) (MeO-AMVN).331
Photoinitiators react under light irradiation (UV or visible) to generate intermediates capable of initiation. Caution is needed when referring to photoinitiator types, with differing conventions sometimes used according to the circumstances of the process. For reactions involving oxygen, type I photoinitiators produce reactive radical intermediates that combine with oxygen or the superoxide anion radical (O2•–) to form oxygenated products. Type II photoinitiators induce energy transfer to oxygen to form singlet oxygen.332−334 In the absence of oxygen, photoinitiators that form radical intermediates capable of direct initiation are referred to as type I, and those that are able to extract hydrogen from a co-initiator are referred to as type II.335,336 Further caution is needed as some lipophilic photoinitiatiors may be mixed mode, which while being type I in principle give product distributions more typical of a type II photoinitiator within the hydrophobic core of the membrane.337
In the absence of organic free radical initiators, the most common sources of in vitro initiation are high valence metal ions,338 hydroxyl radicals generated by the action of redox-active metal ions on hydrogen peroxide (such as the Fenton reaction), and reactive oxygen species (ROS) generated photochemically,339 or by ionizing radiation.74 Irradiation of O2 by light of wavelength <240 nm generates a range of ROS, including singlet oxygen (O(1D)), hydroxyl radicals, ozone, and hydrogen peroxide.340
In some cases, agents that are able to mediate the generation of lower valence metal ions and act as hydrogen atom acceptors accelerate the initiation process. For example, ascorbate significantly enhances the oxidation of methyl linoleate by Fe(III).341 The proposed mechanism for this enhancement involves the formation of a trinuclear Fe(III) cluster with ascorbate that undergoes internal electron transfer to form an Fe(II) ascorbyl radical pair. The ascorbyl radical in this complex abstracts an allylic hydrogen from the fatty acid to release Fe(II) and ascorbic acid (pKa 4.8), which deprotonates to regenerate ascorbate.341,342 This process is therefore cyclic with respect to ascorbate and gives optimal initiation rates with 3–5 equiv of ascorbate per Fe(III) due to the stoichiometry of the active clusters. The Fe(II) released is then available to participate in peroxide decomposition reactions to form peroxyl or hydroxyl radicals and reform Fe(III).343 At higher levels, ascorbate reverts to acting as an antioxidant through radical scavenging. This difference in activity, being pro-oxidant at low levels and antioxidant at high levels, is one example of the complex behaviors that many antioxidants exhibit in different concentration ranges or in the presence of metals.344 The PC demethylation over a period of 6 months in H-soyPC and H-soyPC/chol (7:1) liposomes encapsulating carboplatin and incorporating ascorbyl palmitate (AP),254 discussed earlier in this Review, is potentially accounted for by formation of reactive ascorbyl intermediates in a similar manner to the Fe(III)/ascorbate oxidation. The reduction of Pt(IV) to Pt(II) by ascorbate is a known process,345 although being a net 2 electron transformation, the generation of an alkyl radical would necessitate the intermediacy of a Pt(III) species, as well as the generation of Pt(IV). Pt(III) intermediates, alongside ascorbyl radical formation, have been proposed during ascorbate reduction of Pt(IV),346 and in some cases the electron paramagnetic resonance (EPR) spectrum of the ascorbyl radical is observable during the reduction.347 The formation of dihydroxo Pt(IV) species from hydrogen peroxide is well-known348−350 and has been demonstrated for carboplatin.351 Hydrogen peroxide formation could occur in the early stages of autoxidation to facilitate Pt(II) oxidation. Oxidation to Pt(III) can also be mediated by the hydroxyl radical.352 The C–H bond dissociation enthalpy of the choline methyl is of the order of 100 kcal mol–1,353 so it is unlikely that the ascorbyl radical will be sufficient to form a methyl radical. However, crystal structures of ascorbate complexes with Pt reveal the presence of very strong Pt–C bonds that could provide sufficient energy to effect hydrogen abstraction.354,355
Inorganic phosphorus and sulfur radicals are not generally well studied as initiators, but some are sufficiently hydrophobic that their reactivity with lipids could be significant. Both the S-centered bisulfite radical and the P-centered dihydrophosphite radical should prefer to undergo addition reactions with lipids,356 as has been proposed for carbonate radicals.357
In biological systems, the principal ROS involved in hydroxyl radical formation are superoxide and hydrogen peroxide, formed by mitochondria as a consequence of electron transfer to oxygen during oxidative phosphorylation.114,358 A good review of atmospheric ROS and reactive nitrogen (RNS) generation is given by Pöschl and Shiraiwa.359 Hydrogen peroxide can also be generated by the oxidation of superoxide by redox-active metal ions (the first half of the Haber–Weiss reaction). In terms of oxidation, hydrogen peroxide itself is relatively inert toward lipids, but photochemical or metal-induced dissociation of hydrogen peroxide generates hydroxyl radicals. Peroxynitrite (ONO2–) exists in equilibrium with its conjugate acid (pKa 6.8) and is formed in vivo from nitric oxide (NO•) and superoxide. The acid form decomposes thermally and under metal catalysis to form, among other species, hydroxyl radicals and NO2•. The anionic form can combine with carbon dioxide to form an adduct that can decompose to form NO2• and the carbonate radical. The reaction of superoxide with hypochlorous acid is an additional method for hydroxyl radical generation in vivo, although this method may only occur in a few special cases.81,360
The production of ROS (and RNS334) in vivo by ionizing radiation leads to the activation of cellular mechanisms to regulate oxidative stress that can amplify the response in relation to the actual level of ROS generated.360 Although the ability of ionizing radiation at environmental levels to produce oxidative stress in vivo has been questioned,361 the production of ROS at higher radiation doses, such as those used in medical and biotechnology applications, is still potentially significant. Irradiation of deoxygenated water leads to the formation of a solvated electron, the water cation (H2O+), and electronically excited water (H2O*). The water cation combines with water to from a hydroxyl radical and a hydronium ion (H3O+). Electronically excited water decomposes to H• and OH•. In oxygenated solutions, the free electron can additionally reduce oxygen to form superoxide.362
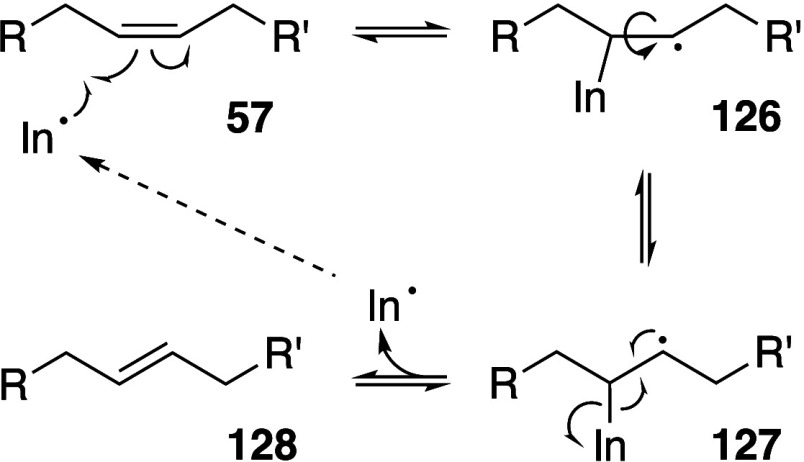
4.1.2. Propagation
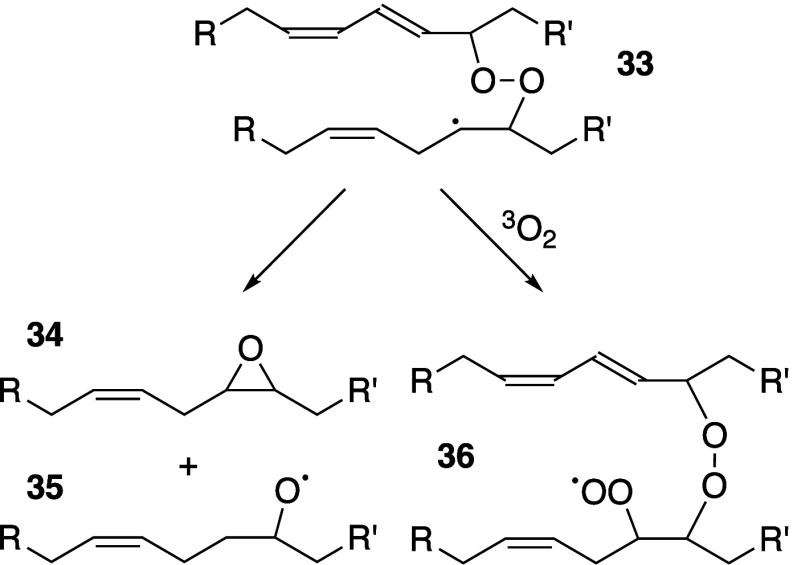
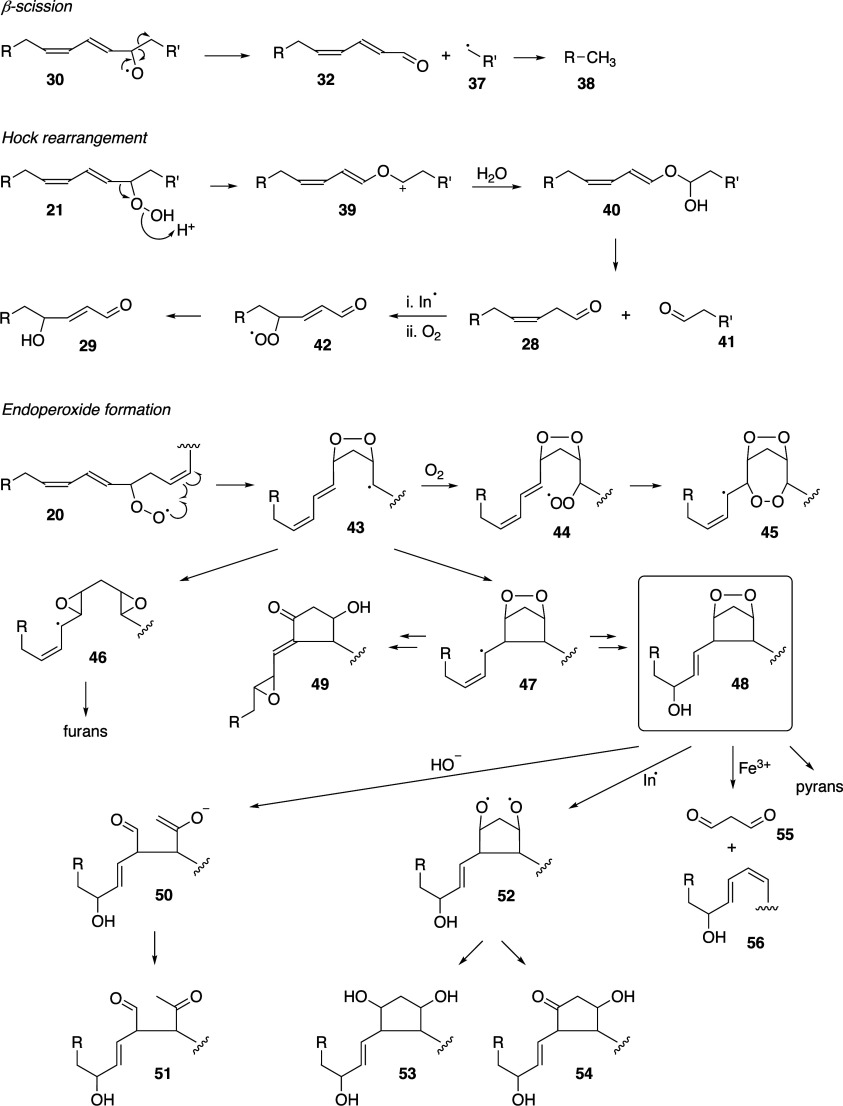
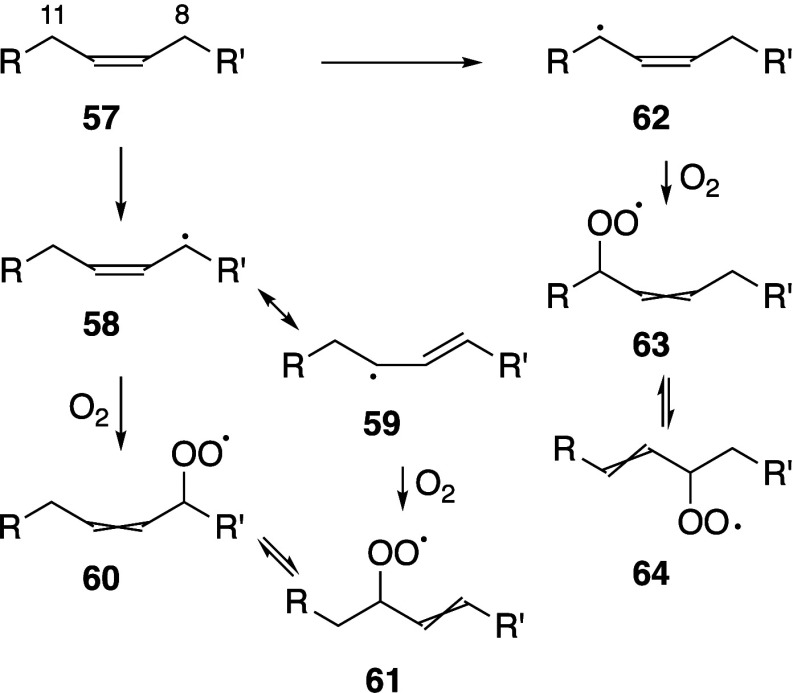
Reaction of the allylic radical 19 with triplet oxygen forms a peroxyl radical (20). Abstraction of a hydrogen atom by the peroxyl radical from the diene (18) forms a hydroperoxide (21), plus a further equivalent of 19, and is usually the rate limiting step in autoxidation.316,363 Evidence for this step being rate-limiting in membranes arises from observations that deuteration of the allylic positions of the PUFAs in cell models significantly reduces the overall rates of peroxidation in response to oxidative challenge by Fe(III),364 Cu(II),365 and hydroxyl radicals generated from Cu(II)/ascorbate.366 The magnitude of the kinetic isotope effects in these examples also indicate that tunneling is involved in the hydrogen transfer process.316 In the presence of ions such as Fe3+, hydroperoxide 21 can be converted back to the peroxyl radical 20, although this is a slow process.341 Hydroperoxide formation in colloidal dispersions favors the formation of the (E,Z)-configuration in 21; in homogeneous solutions, formation of the (Z,Z)-configuration predominates.367 The carbon-centerd bis-allylic radical that initially forms is resonance stabilized and can react in principle at any of three carbon centers (carbons 9, 11, and 13). In practice, in most cases the majority of oxidation products that are isolated arise from reaction at the 9- and 13-positions. This selectivity arises because β-scission of the 11-peroxyl radical, the kinetic product, regenerates 20 and O2 and is more rapid than trapping of this radical by the diene during propagation. Reaction at the 11-position is only detectable when the initially formed 11-peroxyl radical is trapped by a process that is comparable to or faster than β-scission, such as transfer from a phenolic antioxidant like α-tocopherol (α-TOC). The rate constant for peroxyl radical quenching by α-TOC is 3.5 × 106 M–1 s–1.368 Pratt and Porter have used the α-TOC quenching rate as a radical clock to determine the relative rates of other processes in the propagation pathway and account for the selectivity of peroxyl radical formation. For methyl linoleate at 37 °C, the rate constants for β-scission and trapping of peroxyl radicals by 18 are 2.6 × 106 s–1 and 62 M–1 s–1, respectively.368,369 The authors calculated the C–O bond strength for the 11-peroxyl radical to be 8 kcal mol–1 weaker than the 9- and 13-peroxy species.73,368 At the limit, with very high α-TOC concentrations, the product ratio for 9- vs 11- vs 13-peroxyl radical formation is approximately 1:2:1. This ratio is accounted for by the formation of two energetically equivalent complexes between the pentadienyl radical and O2, each complex involving carbon atom 11 and either carbon 9 or carbon 13. The preferred (E,Z)-configuration of the product 20 in colloidal dispersions may be accounted for by the minimization of steric (gauche) interactions in the transition state of the dienyl radical–oxygen complex formed during O2 addition to carbon 9 or 13 and secondary orbital interactions between the π molecular orbitals of ground state 3O2 and the dienyl radical (Figure 6).
Figure 6.
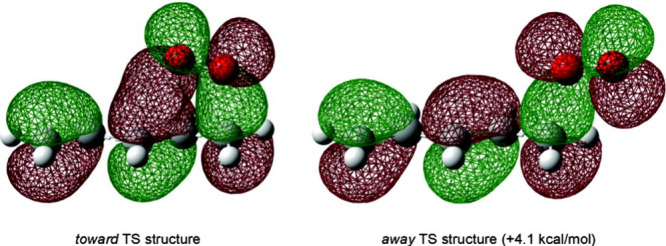
Secondary orbital interactions lead to a preferred geometry in the transition state structure for the reaction between pentadienyl radicals and molecular oxygen. From ref (368). Copyright 2011 American Chemical Society.
Another propagation mechanism that is suggested from studies on the oxidation of methyl linoleate in micelles is the fragmentation of the peroxyl radical 20 to release a hydroperoxyl radical (HO2•).327,367 Evidence for the formation of HO2•, which is almost completely dissociated to superoxide at neutral pH, comes from the observation that the enzyme superoxide dismutase (SOD), which catalyzes the conversion of superoxide to oxygen and hydrogen peroxide, significantly inhibits oxidation in these heterogeneous phases. Although peroxyl radical fragmentation to HO2• only occurs for PUFAs and is rather slow, with an estimated rate constant of 0.04 s–1, it may be significant for the transfer of the propagating chain between vesicles and micelles, a phenomenon that has been observed experimentally.327 Simulations suggest that the polar regions of the oxidation intermediates migrate toward the ester regions of the lipids to adopt a disposition closer to the membrane interface, a behavior that reduces the rate of propagation reactions when compared with these reactions in homogeneous phases.367
The peroxyl radical 20 may also undergo a peroxyl radical addition to a neighboring alkene to form a new peroxyalkyl radical adduct (33, Scheme 5) that can either then undergo further reaction with triplet oxygen to furnish a new peroxyl radical (36) or break down via an intramolecular homolytic reaction to form an epoxide (34) and an alkoxyl radical (35). The peroxyl radical addition can make a significant contribution to propagation for PUFAs but is of lower significance for less unsaturated systems.370
Scheme 5. Reactions of Peroxyl Radicals with Neighboring Alkenes.
4.1.3. Termination
Radical combination to form neutral species is the main termination mechanism in the absence of extrinsic agents such as antioxidants.317 For membrane lipids, processes involving only fatty acyl groups are not exceptionally well studied, mostly because the lipid dimers and polymeric species that result are challenging to characterize, but are well-known as part of the curing process for drying oils.371,372 Radical combination reactions with smaller inorganic radicals, such as those involving the nitrogen dioxide radical to form nitroallyl products and nitroalkenes or nitric oxide to form nitrosoalkenes, have received some attention.315,373,374 Other processes contribute to termination, including the Russell mechanism, a disproportionation involving the formation of a tetroxy species (24) by recombination of two peroxyl radicals, followed by breakdown of this unstable intermediate to form an alcohol (25), a ketone (26), and 1O2.375
Radical propagation can also be effectively terminated by redox processes involving nonlipid molecules, including antioxidants such as α-tocopherol,376−382 squalenes,383 hydropersulfides,380 and tryptophan and tyrosine residues in transmembrane proteins.384
Secondary Processes
Hydroperoxides (21) constitute the major primary products of fatty acid oxidation. A plethora of other oxidized species result from subsequent reactions and rearrangements of these hydroperoxides and their peroxyl radical precursors (Scheme 6). Many of these species are radicals; quenching of these by hydrogen transfer from 18 will also contribute to propagation, and their combination or disproportionation contribute to termination. As with the initially formed 11-peroxy species, β-scission of 20 and recombination of the dienyl radical with O2 eventually generates the thermodynamic diene 27, with an (E,E)-configuration. The C–O bond strength of the (E,Z)-peroxyl radical 20 has been calculated to be 1.5 kcal mol–1 weaker than that of the (E,E)-peroxyl radical 27.368
Scheme 6. Overview of Key Reactions Involved in Secondary Oxidation.
Hydroperoxides are subject to a number of further transformations. In the presence of a reducing metal ion, such as Fe2+, disproportionation of the hydroperoxide generates an alkoxy radical (30) and hydroxide (analogous to the Fenton reaction). This process, combined with the conversion of 21 to 20 by reduction of Fe3+ described above, provides a cycle for hydroperoxide degradation that is metal-catalyzed. In the absence of a metal ion, homolytic hydroperoxide fragmentation can be achieved photochemically to generate 30 and a hydroxyl radical.385 This alkoxy radical can then either form the corresponding alcohol by hydrogen transfer or undergo β-scission to form the conjugated aldehyde 32, with loss of an alkyl radical. Hydrogen transfer to the alkyl radical ultimately forms either an alkane or a truncated acyl group, depending on which side of the double bond the alkoxy radical is generated. Alternatively, intramolecular SHi reaction of the alkoxy radical generates the epoxide 31. Hydrolysis of one epoxide of 31 to form the alcohol and subsequent attack of this alcohol on the other epoxide generates a tetrahydrofuran.
Under acidic conditions, the Hock rearrangement generates a vinyl oxonium species 39 (Scheme 6) through alkyl migration to the proximal peroxyl oxygen with concomitant loss of water.386 Reaction of this cation with water generates a hemiacetal (40), which after hydrolysis produces the aldehyde 41via its acetal, and the homoconjugated aldehyde 28. Further reaction of 28via allyl radical formation and reaction with O2 generates the peroxyl radical 42, which ultimately forms either the corresponding hydroperoxide or alcohol 29 by peroxyl cleavage. γ-Hydroxy-α,β-unsaturated aldehydes 29 are key products of fatty acid oxidation, with 4-hydroxynonenal (4-HNE), formed by cleavage of Ω-6 fatty acids at carbon n-9, being one of the most abundant and most well studied.387 If an additional homoconjugated double bond is present in the R′ group of 20, intramolecular reaction of the peroxyl radical generates an endoperoxide and the alkyl radical 43. This requirement for homoconjugation in R′ makes this 5-exo cyclization reaction of particular importance for PUFAs. The rate constant for this process is comparable to that of β-fragmentation to generate the (E,E)-diene (described above), and as a consequence (E,E)-isomers are infrequently observed.317 Alkyl radical 43 is a key intermediate that can react further via a number of alternative pathways depending on conditions. Reaction with O2 generates peroxyl radical 44, which can subsequently from further macrocyclic endoperoxides such as 45. With additional unsaturation in R′, the process can be repeated to form products with multiple endoperoxyl groups. Ultimately, rearrangements of these endoperoxides generate isofurans and isoprostanes (Figure 7).111
Figure 7.
Isoprostanes and isofurans formed by fragmentation of endoperoxides. R and R′ correspond respectively to the groups proximal to the carboxyl and methyl ends of the parent fatty acid.
Under less oxidizing conditions, intramolecular rearrangement of 43 generates the bis-epoxy radical 46. Ring closing of 43 generates spirocyclopentane 47 with an exocyclic allyl radical center. Compound 47 is a precursor for the D2-isoprostane 49 (and corresponding J2-isoprostane, not shown). Reaction of the allyl center of 47 with O2 and reductive cleavage of the hydroperoxide generates allyl alcohol 48. This alcohol is another key intermediate in the generation of complex oxidation products.388 Under basic conditions, 48 undergoes ring opening to generate enolate 50 and ultimately the highly toxic325,389 γ-ketoaldehyde 51. Under suitable conditions for radical initiation, homolytic cleavage of the O–O bond of 48 generates the bis-oxy radical 52. This ultimately forms the diol 53 by hydrogen transfer under or the D2-type isoprostane 54 under more oxidizing conditions. The corresponding E2-isoprostane is also formed. Both D2- and E2-isoprostanes are further modified by dehydration to form the corresponding unsaturated ketones (J2- and A2-isoprostanes, respectively). In the presence of metal ions, 48 undergoes a ring opening reaction to generate an alkene (56) and one equivalent of malondialdehyde (55). Along with 4-HNE, malondialdehyde is one of the key tracers used to monitor fatty acid degradation in liposomes and foodstuffs. Given the variety of naturally occurring fatty acids, it is unsurprising that a range of other volatile products have been characterized following lipid oxidation, including aldehydes, carboxylic esters, alcohols, and hydrocarbons.390,391 Many of these contribute to the generation of bad aromas and flavors during oxidative processes in foodstuffs70,178,392,393 or the generation of distinctive colors and aromas during cooking.390,394−396 As apparent by the reactions in Schemes 4 and 6 that involve metals, as well as the role of metals in the generation of ROS, oxidative reactions are a particular issue for foodstuffs containing high levels of iron.338,397−399
Lipid oxidation in foodstuffs, when measured by a range of indices, such as the polyene index, peroxide value (PV), or thiobarbituric acid reactive substances (TBARS), occurs to a more significant extent than hydrolysis.255 While there are obvious variations in oxidation rate according to the nature of the material and how it is treated, the level of oxidized PUFAs tends to increase steadily up to 15 days or more.400,401 For example, recent studies using bogues found a ∼10% decrease in PUFA content after 16 days at 0 °C compared to 3% FFA formation.401 Oxidation rates decrease at low temperatures and can be further decreased by the exclusion of oxygen or the inclusion of antioxidants.402 Other treatments such as high-pressure processing (>300 MPa) have been found to increase rates of oxidation, an effect that may be a consequences of changes to the speciation of metal ions in the system.72
The aldehydes produced by secondary oxidation are significant because of their reactions as electrophiles with other macromolecules, including DNA, proteins, and lipids.81,132−134,389,403−405 Such reactions occur either directly through the carbonyl group (imine formation) or via the Michael reaction for α,β-unsaturated aldehydes. Reactions with aldehydes are responsible for the formation of pyrroles through processes such as the Maillard and Strecker reaction.394,406−409 An alternative breakdown pathway from hydroperoxide 21 leads to the formation of the keto equivalent of 4-HNE, 4-oxononenal (4-ONE), which also undergoes facile pyrrole formation.410 Although these reactions are usually assumed to require significant heating (e.g., during food cooking), their products have been detected at lower temperature (25–37 °C).411 Other aromatic species such as thiazolidines, furans, and thiophenes can be formed by reaction of aldehydes with peptides bearing an N-terminal cysteine, such as the Cys-Gly sequence derived from glutathione.412 In addition, thiol-containing species such as glutathione are able to form adducts with cyclic peroxides such as 48 by direct nucleophilic attack, as well as their α,β-unsaturated counterparts (e.g., 54) by conjugate addition.413 Nitroalkenes, formed by radical combination reactions, are reactive electrophiles that may also undergo similar reactions with the thiol group of cysteine as a potential nucleophile.414
The relationship between the Maillard reaction and lipid oxidation products is not straightforward. In the first instance, the thermal degradation of monounsaturated fatty acids (MUFAs) via hydroperoxides varies with lipid class, being markedly slower and giving a different product profile for PC and PE when compared with the free fatty acids or triglycerides. Volatile carbonyl compounds derived from lipid oxidation provide additional sources of reactive carbonyl species to those formed by the Amadori rearrangement for the final stages of the Maillard process. However, radical intermediates in the oxidation of lipids also influence the balance of carbonyl compounds formed by the Amadori rearrangements415 and the overall rate of the Maillard process. Lipid oxidation and the Maillard reaction exhibit and interdependency where the rate of one influences the rate of the other, either positively or negatively depending on the circumstances.394
Monounsaturated fatty acids (MUFAs) are significantly less reactive toward autoxidation than PUFAs. Indeed, when the fatty acyl composition of liposomes is enriched in oleoyl groups, the corresponding rate of oxidation of PUFAs in the same systems decreases, pointing to an antioxidant activity.416 Nonetheless, MUFAs are able to undergo similar autoxidation reactions to PUFAs, involving the abstraction of a hydrogen atom during initiation to form a radical at either of the allylic positions (58 and 62, Scheme 7, corresponding to carbon atoms 8 and 11 of oleic acid) that then form (Z)-peroxyl radicals by reaction with 3O2 as kinetic peroxide products (60 and 63).339,417−419 Both of these allylic radicals have corresponding resonance forms, exemplified by 59 for 58, that can also form peroxyl radical products, giving the corresponding (E)-peroxyl radicals as additional kinetic products (61 and 64, corresponding to carbon atoms 10 and 9 for oleic acid, respectively). Allylperoxyl radicals can undergo radical mediated [2,3] rearrangements, so that the initially formed peroxyl radicals can interconvert reversibly between 60/63 and 61/64 to form the (E)-isomers of peroxyl radicals 60 and 63 and the (Z)-isomers of 61 and 64. Ultimately, considering the geometric isomers of the alkene, this gives rise to eight potential peroxyl radicals (and corresponding hydroperoxides). The distribution of products is dependent on experimental conditions, including temperature, substrate choice, and the mode of oxidation. In model reactions with methyl oleate at 25 °C, formation of the 8-peroxyl product is marginally favored over formation of the 10-peroxyl product, with the 8-peroxyl product comprising approximately equal amounts of (E)- and (Z)-isomers and the 10-peroxyl radical almost entirely E. At higher temperatures the stereochemical fidelity of the rearrangement is reduced, which along with ab initio calculations has led a proposed mechanism involving dissociation of the peroxyl radical to from an allyl radical–oxygen complex.368 Escape of oxygen from this complex competes with collapse to the rearranged peroxyl radical. Ultimately, the peroxyl radicals 60, 61, 63, and 64 undergo similar trapping and termination reactions to those in Scheme 4. On the other hand, in another study, air oxidation of methyl oleate at 60 °C gave predominantly (E)-hydroperoxides at the 8-, 9-, and 10-positions as the major products (although the 9- and 10-isomers could not be separated).417 The product profile may can give information on the mode of oxidation to which a sample has been subjected. For example, in senescent cells, type II photosensitized oxidation of MUFAs at UV wavelengths forms all eight potential hydroperoxides; similar oxidation at visible wavelengths forms predominantly the (E)-isomers corresponding to oxidation at the 9- and 10-positions of oleic acid ((E)-61 and (E)-64, respectively), and autoxidation favors E/Z mixtures corresponding to oxidation at the 8- and 11-positions of oleic acid (60/63) alongside the (E)-isomers of 61/64.418 Thus, the (Z)-isomers of 61/64 are indicators of photooxidation at UV wavelengths, and the (Z)-isomers of 60/63 are markers for either autoxidation or photoxidation at UV wavelengths.
Scheme 7. Reactions of Isolated Alkenes.
The numbering corresponds to the carbon atom numbering of oleic acid.
4.1.4. Diacylglycerophospholipids Other than Phosphatidylcholine
Similar oxidation reactions to those of PC fatty acyl groups are seen with the acyl groups of PE, PS, PG, phosphatidylinositols (PIs), and PA.74,420,421 The amino groups of PE and PS are also potential reaction sites. For example, N-chlorination of PE via reaction with hypochlorous acid has been detected.81 Recently, reactions of PE, and to a lesser extent PS, amino groups with the reactive carbonyls produced by fatty acid oxidation have been described with increasing detail.325,421−426 For example, incubation of PE with 4-HNE in a two-phase ether/water system at 30 °C for 2 h led to the formation of the Schiff base adduct 66, together with the Michael adduct 67 and the pyrrole 68 formed from the latter (Scheme 8). Product abundances decreased in the order 68 > 67 > 65 ≫ 66. PS underwent similar Michael reactions with 4-HNE but was less reactive; consequently, the pyrrole and Schiff base products were not detected.426 Under similar conditions, γ-ketoaldehydes (a28) undergo Schiff base formation with PE, also leading to the formation of pyrroles as an end point.427,428
Scheme 8. Products Formed by the Reaction of PE Lipids with 4-HNE.
In a human retinal pigment epithelial cell line, 4-hydroxy-7-oxohept-4-enoic acid is formed from α,β-unsaturated aldehyde equivalent to 29, where the R group is a glyceryl ester. The lactone form of this hydroxy acid is able to modify PE and protein amino groups to form 2-carboxyethyl pyrroles.427 The products of alkenal addition to PE have been detected as trace products (0.01–0.02% of total PE) in rat retinas, increasing to 0.4–1% under conditions of diabetes-associated oxidative stress.429 Piperidines 69 and 70 (Figure 8), formed by consecutive Michael reactions of PE with acrolein followed by ring closure, have been detected in membrane extracts of HP-60 cells following their exposure to acrolein.430 Other experiments have detected amino-functionalized ethanolamines, breakdown products of N-modified PE, in red blood cell lipid extracts,431 mitochondrial membranes,432 and urine.433
Figure 8.
Amino-modified PE lipids isolated from HP-60 cells after exposure to acrolein.
Model experiments using liposomes composed of 1-stearoyl-2-arachidonyl-sn-glycero-3-phosphocholine (SAPC) and DPPE in the presence of t-butylhydroperoxide or Cu(II)/H2O2 initiators led to the formation of a number of new species resulting from PE amino group reactions with intermediates generated from arachidonate.423 Along with compounds of the type described above (Figure 8 and Scheme 8) and cross-linked products arising from reaction with malondialdehyde (53), N-acyl compounds such as N-hexanoyl-PE (71, Figure 9)434 and products resulting from reaction with prostaglandin-type oxidation products (72) could also be identified. Recent experiments suggest that exogenously added prostaglandins are more reactive toward PE than proteins.435
Figure 9.
Products formed by the reaction of PE with reactive oxygen carbonyl compounds derived from fatty acid oxidation.
In a model system comprising dipalmitoyl-sn-glycero-3-phosphocholine (DPPE), glucose, and the Fenton reagent, the hydroxyl radical was found to oxidize glucose to glyoxal and methyl glyoxal, which subsequently combined with the lipid to form carboxymethyl PE and carboxyethyl PE. Carboxymethyl PE was also formed by oxidation of the Amadori products by the hydroxyl radical.436 A number of other experiments have provided secondary evidence for the presence of amine-modified PE lipids, such as the development of fluorescent chromophores following peroxidation of both model lipid mixtures and biological membranes in vitro (reviewed in ref (424)). Oxidation of hemiketal intermediates formed by the reaction of amino groups with reactive aldehydes formed from cholesterol437 and fatty acids438 have recently been described, leading to the formation of amides. It is reasonable to expect that the amino groups of PE lipids may undergo similar reactions.
The products of lipid amine reactivity with carbohydrates and their autoxidation products have been detected in vivo. In a similar fashion to the Maillard reaction, reducing sugars such as glucose form a Schiff base with the PE amino group. Subsequent Amadori rearrangement of this imine leads to the formation of a ketosamine (73, Scheme 9). This amine is a precursor for several subsequent transformations, including oxidative degradation to form N-carboxymethyl- and N-carboxyethyl-PE.424,439 Autoxidation of glucose (Wolff pathway) or 73 (Namiki pathway) leads to the formation of a number of carbonyl species that are reactive toward aminophospholipids, including glyceraldehyde, glyoxal, and methyl glyoxal.439 Reactions of carbohydrate- and lipid-derived aldehydes and ketones with proteins and lipids ultimately leads to the formation of advanced glycation end products (AGEs) and advanced lipoxidation end products (ALEs), which are indicators of aging and are implicated in age-related diseases.440 In the case of lipids, AGEs are potential markers for diabetes.439
Scheme 9. Amadori Rearrangement Product Formed by the Reaction of Glucose with PE.
A study of the lyso-PE and PE lipids (including plasmanyl and plasmenyl) in human plasma that have been modified by the Amadori reaction identified 33 lyso-PEs and 142 PE products. Of these, the majority of lyso-PE- and PE -derived products were present at sub-nM levels, including all plasmanyl-PEs (PE O). The most abundant species detected, with plasma concentrations >5 nM, were the plasmalogens PE P-16:0/20:4, PE P-18:0/20:4, and PE P-18:1/20:4 and the lipids PE 18:0/20:4, PE 18:0/18:2, and PE16:0/22:6. By comparison with the corresponding unmodified PE lipids in the sample, the glycation rate was estimated at approximately 0.07%.441,442
Given the role of mitochondria in respiration and the generation of ROS, it is unsurprising that attention had been given to the oxidative stability of mitochondrial lipids. Particular attention has focused on PE lipids and cardiolipins (CDLs), which are major components of mitochondrial membranes, and their reaction with perhydroxyl radicals formed in the proximity of the inner membrane from the superoxide radical.443 CDLs are found predominantly in the inner mitochondrial membrane, with an asymmetric distribution skewed to the leaflet that faces the mitochondrial matrix. The fatty acid content of CDLs is complex. In many organs, CDLs contain a broad range of fatty acids, including PUFAs, in which each of the four acyl chains is different, with tetra-C20:4 being the predominant CDL. In others, including the heart, muscles, and the liver, the fatty acid distribution is remodeled to favor mostly C18:2 fatty acids. During aging and diabetes, the fatty acid composition of CDL in heart tissues is remodeled in favor of chains with increasing levels of unsaturation.443 Even though CDLs are of relatively low abundance compared to other mitochondrial glycerophospholipids, their fatty acids are significantly more susceptible to oxidation and are often the first lipids oxidized under oxidative stress.358,443 This susceptibility is attracting attention as it potentially indicates a role for CDL in cell regulatory responses and signaling. Following trauma such as brain injury, for example, >100 different oxidized CDLs can be formed.444 The range of species found includes many of those in Scheme 4, including hydroperoxides, alcohols, epoxides and aldehydes.445 While much of this oxidation is under enzymatic control, the proportion of the array of resulting oxidized CDLs that has physiological relevance is still unclear. Oxidation leads to CDL redistribution, with a loss of membrane asymmetry, and is sometimes accompanied by increased phospholipase-mediated ester hydrolysis to form monolyso CDLs.64
4.2. Plasmalogens
Plasmalogens are distinguished by the presence of an enol ether at the sn-1 position of the lipid. Oxidation of this alkene to form an epoxide occurs when a PUFA is present at the sn-2 position (Scheme 10).446 The peroxyl radical formed in the sn-2 chain (74) adds to the enol ether via alkylperoxy intermediate 75 to form the alkoxy radical 76. Trapping of the alkoxy radical and hydrolysis of the epoxide forms the acetal 77, which subsequently hydrolyzes to form an α-hydroxyaldehyde and a lyso-PC.122 The aldehyde product is able to form Schiff base with the amino group of PE and PEpl.447,448 Oxidation with Fe2+ and ascorbate provides an alternative route to the epoxide of the enol ether for plasmalogens without a PUFA at the sn-2 position.447,449
Scheme 10. Oxidation of Plasmalogens with a PUFA at the sn-2 Position.
The double bond pattern shown here is that of arachidonic acid.
4.3. Sphingolipids
The alkene of the sphingosyl group in these lipids is significantly more resistant to radical oxidation than the alkenes of plasmalogens and sterols. In recent work, oxidation of lactosyl and galactosyl ceramides using hydroxyl radicals generated by the Fenton reaction gave oxidation products on unsaturated fatty acyl chains, forming allyl hydroperoxides, allyl alcohols, and α,β-unsaturated ketones.450 If the fatty acyl chain was saturated, the only products were loss of the sugar group to form ceramide. Under similar conditions, ceramide was found to be unreactive, whereas sphingomyelin underwent acyl cleavage to the amine (sphingosylphosphorylcholine, SPC), and subsequent oxidation of SPC around the sphingosyl alkene to form 78–80 (Figure 10), as well as cleavage between C2 and C3 (81).451
Figure 10.
Products formed by the oxidation of sphingolipids.
4.4. Cholesterol
The nonenzymatic oxidation of cholesterol has been comprehensively reviewed.452 Due to the location of the alkene at the bridgehead between the A and B rings, cholesterol is marginally more reactive toward oxidation than the isolated double bond of monounsaturated fatty acids.453 In saturated lipid membranes, cholesterol undergoes slow oxidation, and cholesterol inclusion in most unsaturated membranes leads to a reduction in the rate of oxidation. Reactions at sites on the alkyl side chain are known, notably at the 24- and 25-positions, but are usually enzyme mediated and of little significance in autoxidation.319,382,454−457 Abstraction at more esoteric positions, such as C15, can be promoted in constrained environments, but it remains to be seen whether this can occur in membranes.458 Autoxidation of cholesterol leads to hydroperoxylation and hydroxylation, predominantly at the 7-position around the sterol core. The α- and β-isomers of 7-hydroxycholesterol (82, Figure 11) can be converted into 7-ketocholesterol (83) enzymatically in the liver, or nonenzymatically by a disproportionation reaction between peroxyl radicals that leads to the formation of 83, an alcohol, and 1O2 (Russell mechanism).
Figure 11.
Predominant products arising from the autoxidation of cholesterol.
The predominant product forms by reaction at the 7-position to form the hydroperoxide (84, Figure 12) via an intermediate 3-centered allylic radical involving carbon atoms C5–C7. In model reactions in chlorobenzene, the C4 oxidation product (87), arising via the alternative 3-centered radical involving C4–C6, is formed with a lower abundance to the C7 product, with the C6 oxidation product (86) having the lowest abundance of those observed.459
Figure 12.
Oxidation products arising from the autoxidation of cholesterol at the 7-position of the sterol ring.
The 5-hydroperoxides (85) are not normally observed because β-scission of the 5-peroxyl radical is faster than trapping during the propagation step. In a similar fashion to their trapping experiments described above for methyl linoleate, Zielinski and Pratt have recently demonstrated that reactions of cholesterol in chlorobenzene in the presence of either a truncated α-TOC analogue or 4-tert-butyl-2,6-dimethylphenol (BDMP) enable trapping of C5-peroxyl radical to form the corresponding hydroperoxide as a mixture of α- and β-isomers. The amounts of the 5-hydroperoxide formed were small but increased linearly with the concentration of BDMP and enabled the rate constant for β-scission of the 5-peroxyl radical to be determined as 5.6 × 105 s–1.459 The equivalent rate constant for fragmentation of the 4-peroxyl radical was 8.6 × 103 s–1. The ratio of products formed via abstraction at C4 vs C7 was found to depend on the concentration of BDMP. Density functional theory (DFT) calculations suggest that abstraction of the α-H at C7 is favored by steric hindrance with the β-methyl at C10 and secondary orbital interactions between the π-bond of the alkene and the part of the SOMO centered on the internal oxygen (89, Figure 13).460 Abstraction of β-H at C4 is favored by hydrogen bonding between the C3-OH and the incoming peroxyl radical during the propagation step (88). This hydrogen bonding lowers the barrier for hydrogen atom abstraction at C4 to a value not hugely greater than that for abstraction at C7. At high BDMP concentrations, the BDMP radical becomes the predominant radical species present, preventing the hydrogen bonding mechanism that supports abstraction of the β-H from C4 from operating.
Figure 13.
Transition states for hydrogen abstraction by peroxyl radicals during the propagation stage of cholesterol autoxidation.459,460
An alternative free radical route to oxidized sterols is via reaction with a lipid peroxyl radical to form the 5-peroxyl radical intermediate 90 (Scheme 11), which is subsequently transformed into 5,6-epoxycholesterol (91).461 Peroxyl radical addition at C5 occurs preferentially on the α-face of the structure.460
Scheme 11. Generation of 5,6-Epoxycholesterol (91).
Higher-order oxidation products also form (Figure 14), such as secosterol (92) and its aldol condensation product 93. These can be formed by a number of routes, including ozonolysis, reaction of cholesterol with 1O2, and Hock rearrangement of the α-isomer of 85.462 Amides such as 94 have been detected during the photosensitized oxidation of cholesterol and are proposed to form via oxidation of the hemiaminal intermediate formed by the reaction of the terminal amino group of lysine with the aldehyde group of 93.437
Figure 14.
Higher-order cholesterol oxidation products.
Formation of Schiff bases between cholesterol aldehydes and proteins leads to changes in protein folding that are potentially important in the development of disease.453,463 Ketosterols are generally unreactive toward Schiff base formation.
Cholestadienes are metabolic precursors to cholesterol and vitamin D3. Their levels are usually low in vivo, but as they are metabolic precursors to cholesterol and vitamin D3 they can accumulate in some circumstances. Incorporation of a second alkene into the sterol core, particularly when conjugated or homoconjugated to the C5–C6 alkene, significantly increases reactivity toward autoxidation.453 For example, the propagation rate constant for 7-dehydrocholesterol (DHC, 95) is 2260 M–1. Autoxidation of DHC leads to the formation of numerous (>10) oxysterol species (Scheme 12), mostly through reaction at C5 and C9, of which keto-dehydrocholesterols 96, 98, and 99 are significant examples. The latter two are formed via the cyclic peroxide 97. The 6-alcohol corresponding to 97 is also detectable as a reaction product.127,464,465
Scheme 12. Products Arising from the Oxidation of Cholestadiene.
Sterols 96, 98 and 99, formed by initial hydrogen atom transfer at the 9-position, are favored in the presence of α-tocopherol in solution. Initial hydrogen atom abstraction at the 14-position leads to 100 and 101 (Figure 15).466
Figure 15.
Products arising from the oxidation of cholestadiene following hydrogen atom abstraction at C14.
4.5. Nonradical Oxidation
Hypochlorous acid adds to double bonds via an electrophilic mechanism to form a mixture of 1,2-dichlorides and chlorohydrins.467 The latter can subsequently form epoxides by the elimination of chloride.81 The activity of myeloperoxidase (MPO) in cells of the immune system is primarily responsible for the formation of hypochlorous acid in vivo,468 whereas in vitro it is formed by the hydrochloric acid-catalyzed decomposition of hydrogen peroxide. Other hypohalous acids give similar processes. Electrophilic HOCl addition has been documented for sterols,452 sphingomyelin,469 and plasmalogens. In the latter case, the initial product (103) hydrolyzes to form a lyso-PC (104/105, Scheme 13).470 If the sn-2 chain is unsaturated, further HOCl addition can occur. This subsequent HOCl addition to the C=C of the lysolipid is faster than HOCl addition to the parent lipid (102).471 Ultimately, the chlorohydrin addition product with the lysolipid undergoes hydrolysis to the fatty acid and GPC.
Scheme 13. Reaction of PC Lipids with Hypochlorous Acid.
Hypochlorous acid also forms N-chloro species by reaction with the amino group of PE.81,467
The reaction of ozone with the unsaturated alkenes of lipid fatty acids leads, as expected, to the formation of the Criegee intermediate 106 (Scheme 14). Breakdown of 106 occurs by hydrolysis to form one equivalent of aldehyde (107) and one equivalent of the hydroxyhydroperoxide (108). The latter loses hydrogen peroxide to form a second equivalent of aldehyde (109). In the presence of excess ozone, aldehydes 107 and 109 can be further oxidized to the corresponding carboxylic acids.472,473 Good overviews of the sources of ozone359 and the volatile products that form via ozonolysis have been presented by Pöschl and Shiraiwa and Wisthaler and Weschler.474 Ozone is of interest because of its effects on the lungs, particularly the phospholipid-rich epithelial lining fluid (ELF), which is the first point of exposure following ozone inhalation. ELF is rich in saturated lipids, particularly DPPC, but contains a number of unsaturated PCs, including POPC, 1-palmitoyl-2-palmitoleoyl-sn-glycero-3-phosphocholine and 1-palmitoyl-2-linoleoyl-sn-glycero-3-phosphocholine (PLPC), alongside other lipid classes: PG, PE, PI, and plasmanyl and plasmenyl versions of PC and PE. The PG lipids are mostly monounsaturated in the sn-2 chain.475 Reaction of ozone with POPC in ELF leads to the formation of a number of oxidation products, most notably 1-palmitoyl-2-(9′-oxo-nonanoyl)-sn-glycero-3-phosphocholine (PoxnoPC) (116, Figure 17), which elicits a physiological response in the epithelial cells in contact with the surfactant,475,476 including the activation of phospholipases.139,477 Modern improvements in instrumentation have allowed a series of products formed by ozonolysis of the PG lipids to also be detected at ozone levels that are environmentally realistic.475 Interestingly, PLA2 recognizes the Criegee intermediate with the same efficiency as arachidonic acid,140 whereas the hydroxyhydroperoxide activates PLC.139 Aldehyde formation has also been noted following exposure of red blood cells to low levels of ozone in vitro,478 direct ozone permeation to the blood in vivo is unlikely because most reactions occur in the ELF.479 The sphingosyl alkene of sphingolipids undergoes ozonolysis to from the expected aldehyde.480
Scheme 14. Ozonolysis Reactions of Fatty Acid Alkenes.
Figure 17.
Examples of oxidized PCs.89,499−501 All examples here have palmitoyl at the sn-1 position.
Singlet oxygen generation most commonly occurs trough the action of photosensitizers, in which the triplet state of the sensitizer (3T1), formed by intersystem crossing from the excited singlet state (1S1), transfers energy to 3O2. In some cases, sensitization through the singlet state can occur, although this mostly operates by 3O2 increasing the rate of intersystem crossing to 3T1.481 A range of organic molecules are capable of acting as photosensitizers, sharing the common property of being highly conjugated aromatic systems. In some cases, electron transfer competes with energy transfer, leading to the formation of superoxide.482 Dismutation of the superoxide anion generates hydrogen peroxide, which is a precursor for 1O2 through reactions with hypochlorous acid.483 The generation of 1O2 from the tetroxy species formed by combination of peroxyl radicals (Russell mechanism) has been described earlier in this Review. Agents that convert hydroperoxides to peroxyl radicals, including metal ions and peroxynitrite (via its radical decomposition products) can therefore participate in the generation of 1O2 by this route.483 Recent model experiments involving UVA irradiation of ethanolic solutions of fatty acids demonstrated significant generation of 1O2 by this mechanism.484 Recent work has demonstrated that the thermolysis of dioxetanes leads to the generation of excited triplet state ketones that transfer energy to 3O2 to form 1O2.485 Thermal decomposition of the endoperoxides of cyclohexa-1,4-dienes via a concerted pericyclic mechanism is another route that has been used to generate 1O2.486,487
Singlet oxygen reacts with alkenes to form dioxetanes via a 1,2-cycloaddition (110) and allyl hydroperoxides via the ene reaction (Scheme 15). In homoconjugated systems, the ene reaction forms both conjugated (111) and nonconjugated (112) products, with the former being the dominant product. In model reactions with linoleic acid, the ratio of conjugated to nonconjugated products is 66:34, with no preference for the alkene that reacts.391,488 As the first excited state of oxygen, oxidations with 1O2 are generally 3–4 orders of magnitude faster than those of 3O2 and are therefore of particular significance for monounsaturated fatty acids with low reactivity toward autoxidation by free radical mechanisms.
Scheme 15. Reactions of Singlet Oxygen with Alkenes.
Cardiolipin oxidation by singlet oxygen is influenced by lipid packing. The presence of Ca2+ ions, for example, increases CDL’s susceptibility to oxidation by decreasing the headgroup volume of CDL and increasing the volume of the acyl chains, leading to the observation of structures with high curvature.489
Cholesterol reacts with singlet oxygen to form 5-hydroperoxide (85) and the dioxetane 113 (Figure 16). The former undergoes rearrangement to form the more stable 7-hydroperoxide, and the latter is a precursor for the formation of secosterol 92(452) and cholesterol-5,6-epoxides. These epoxides are themselves reactive toward nucleophiles such as thiols and amines, forming 5-hydroxycholesterols substituted on the 6-position.490
Figure 16.
Dioxetane formed by the reaction of cholesterol with singlet oxygen.
Plasmalogens react with 1O2 to form dioxetanes that hydrolyze to form a lyso-PC and a long-chain aldehyde.447,491
The oxidation of sulfatides (sulfated galactocerebrosides) has been examined under ambient conditions in the presence of light. Oxidation of both sphingosyl and fatty acyl alkenes was observed over 24 h, with the former reacting slower than the latter. In the absence of light, no reaction was observed, leading the authors to conclude that the oxidation was mediated by photogenerated 1O2. In both cases, C=C bond cleavage occurred to form the corresponding aldehydes.480 In an earlier study, the oxidation of lactosyl and galactosyl ceramides was studied under UVA irradiation (320–400 nm). Oxidation of both sphingosyl and fatty acyl alkenes was observed alongside products resulting from oxidation in the sugar group.492 Oxidation in the sugar moiety is also a feature of glycated PEs, with cleavage of C–C bonds within the sugar moiety following UVA irradiation.493
4.6. Reactivity towards Oxidation of Membrane Lipids
Autoxidation reactions in membranes follow the same rate laws as their counterparts in homogeneous solution. All of the radical processes are slower in membranes, which may be attributed to the higher viscosity of the membrane (relative to solution), particularly below Tm.317 For initiation, this leads to increased radical recombination during initiation due to cage effects and low initiator efficiency.330 Propagation and termination reactions of methyl linoleate at 37 °C in DMPC vesicles are approximately 10- and 100-fold slower, respectively, compared to the same processes in homogeneous solution.330 Slower termination reactions are also found for methyl linoleate in micelles.329
It is generally believed that the slower rates for propagation and termination in micelles and bilayers are a consequence of partitioning of the polar peroxyl radical centers to the interfacial region with the bulk phase.330 Simulations have given conflicting results, with some suggesting that while hydroperoxides may partition preferably to the membrane interface, this is not the case for the peroxyl radical, which remains within the hydrophobic core of the membrane,494 whereas others indicate that formation of the peroxyl radical generates more coiled conformations of the hydrocarbon chain that place the reacting carbon atoms closer to the interface.367 A recent study of the relative oxidation rates of a range of lipids with varying levels of unsaturation, both in solution and in bilayers, revealed that the reaction rates in bilayer are half those obtained in solution. This was attributed to reaction sites, particularly for initiation, only being accessible in the outer membrane leaflet. This study also demonstrated lipids with higher levels of unsaturation (C20:4, C22:6) oxidize faster than their less unsaturated counterparts.495 Interestingly, this study also demonstrated that, for highly unsaturated acyl groups, hydrogen transfer during the propagation step is an intramolecular process, with the consequence that individual acyl chains are either unmodified or highly oxidized.
Unlike the situation in solution for linoleate, in bilayers there is a preference for the formation of peroxides at C9 over C13, which may be attributed to the closer proximity of C9 to the membrane interface or to differences in availability of hydrogen atom donors at different depths within the bilayer.317 The rate constant for β-scission of peroxyl radicals at C9 is lower than that of C13, which may be attributed to differences in polarity at these depths in the membrane.
For PUFAs such as arachidonate at the sn-2 position, reaction similarly occurs more readily via the bis-allyl radical center that is more proximal to the membrane surface. It is currently unclear why this is the case, but a likely reason is that this site is more proximal to the location from which water-soluble radical initiators diffuse.74 Unsaturated fatty acyl groups are rarely found at the sn-1 position of glycerophospholipids. This is the prime reason for the activation of PLA2 as a response to oxidative stress, as most of the acyl chain damage is on the sn-2 chain. For unsaturated sn-2 acyl chains, particularly if they are polyunsaturated, free radical initiation generally results in either the formation of hydroperoxides and their alcohol counterparts (120, 121, Figure 17), the products of endoperoxide formation (122), or the products of acyl chain truncation, which produces species such as 114–119. The range of truncated acyl groups found following lipid oxidation has been reviewed.74,496
Cholesterol inclusion slows the rate of oxidation.255 An interesting synergism exists between cholesterol and PUFA oxidation, however. Cholesterol is resistant to oxidation in saturated membranes but susceptible to oxidation in membranes containing MUFAs497 or PUFAs. In the latter, suppression of PUFA oxidation also suppresses cholesterol oxidation. The reason for this most likely lies in hydrogen transfer from cholesterol C7 to a PUFA peroxyl radical as a key initiation step in cholesterol oxidation.73,452,498 This transfer is particularly made apparent by the high tendency toward oxidation of cholesterol esters of PUFAs, where the abstraction at C7 becomes intramolecular and therefore more facile.122
Many of the effects of γ radiation are mediated through the ROS described above. In some instances, however, unexpected bond cleavages have been found, particularly when systems composed of saturated lipids are exposed to high radiation doses. For example, irradiation of DPPC and DPPG/DPPC liposomes led to the formation of small amounts of 1,2-dipalmitoyl-sn-glycero-3-phosphatidic acid (DPPA) by headgroup cleavage, 1/2-palmitoyl-sn-propanediol-3-phosphorylcholine, 1/2-palmitoyl-sn-propanediol-3-phosphorylglycerol, and dipalmitoyl-sn-glycerol-3-phosphorylethanol, alongside lysolipids (Figure 18).502 These cleavages tend to occur adjacent to heteroatoms in the headgroup and glycerol sections of the lipid, which have recently been calculated to be sites most amenable to hydrogen abstraction in this part of the lipid.503
Figure 18.
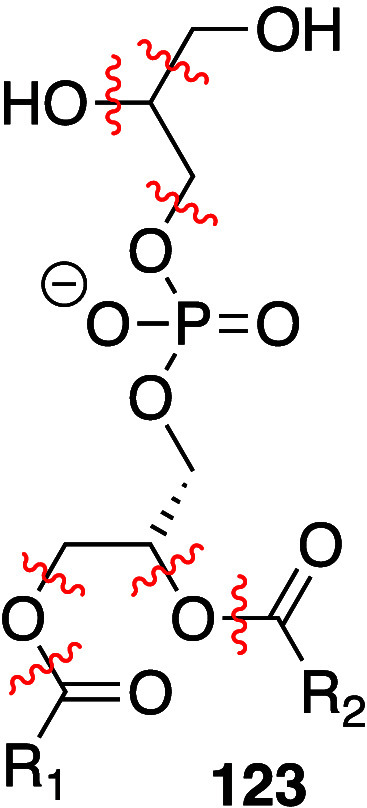
Bond cleavages, indicated by wavy lines, following γ-irradiation of DPPG (5.8 × 104 Gy).
A recent study investigated the oxidative stability of lipids during electroformation of giant unilamellar vesicles (GUVs). Typical conditions for GUV preparation (0.3–1.2 V, 5–20 h) led to the formation of significant levels of oxidized lipids for 1,2-didocosahexaenoyl-sn-glycero-3-phosphocholine (DHAPC) and DLPC, whereas DOPC was significantly more stable. Ultimately, the optimum conditions were a trade-off between lipid oxidation and vesicle size and yield.504
The oxidative instability of lipids has been exploited to develop drug delivery systems that can be triggered using near-infrared (NIR) radiation. Luo et al. included DOPC in liposomes composed of DSPC, a porphyrin-modified lipid and cholesterol. Under NIR, the production of singlet oxidation resulted in DOPC and cholesterol oxidation and the release of entrapped doxorubicin within 1–2 min.505
4.7. Biophysical Consequences of Oxidation
A key consequence of lipid oxidation is the introduction of polar groups, including alcohols and hydroperoxides for full-length lipids and aldehydes for truncated lipids, into the normally low-dielectric hydrocarbon interior of the membrane. This presence of polar groups leads to an increase in the water penetration of the membrane.109,506,507 As the level of oxidized lipids becomes very high, water penetration increases to the extent that continuous pores span both leaflets of the membrane.508−510 The presence of cholesterol increases the pore lifetime.508 Simulations suggest that at levels of oxidized lipids up to 25 mol % there is little or no perturbation to headgroup conformations and lipid mixing by the presence of full length oxidized chains, but the polar groups of the chains tend to migrate toward the polar regions of the lipid interface.373,511,512 This presence of oxidized fatty acyl chains on the surface of the membrane has led to the development of the whisker model, in which oxidized chain ends project away from the surface of the membrane into the aqueous medium.249,250 The presence of whiskers is an important marker for cell recognition to trigger apoptosis or membrane recycling.250,513
Truncated chains, on the other hand, induce lipid disordering.514−516 Decreased chain ordering, lowering of phase transition temperatures, increased permeability and rates of lipid flip-flop, membrane thinning, and decreased bending rigidity have also been observed experimentally at these levels of oxidized lipids.108,322,507,517−521 When primary amines are present in the membrane, in lipids such as sphingosine, for example, cross-linking can occur through imine formation with reactive aldehydes, which can subsequently drive vesicle aggregation.421
Low molecular weight oxidized hydrocarbon fragments such 4-HNE (29) similarly orient themselves with the polar groups oriented toward the lipid interface. Eventually, these molecules are able to diffuse out of the membrane.511,512 The enhanced water penetration, and the partitioning of polar groups of oxidation products toward the interface lead to an increase in the area per lipid. Oxidized lipids have also been found to desorb from the membrane.512,522 In contrast to the products of oxidation, some simulations indicate that the intermediate peroxyl radical groups move toward the lipid interface,367 whereas others suggest they may remain more deeply buried.494
In three-component mixtures containing cholesterol and sphingomyelin that exhibit microphase separation into a sphingomyelin-rich liquid-ordered (Lo) phase, the presence of oxidatively truncated lipids has been observed to stabilize Lo domains in a both monolayer523 and bilayer507 models.
Hydroxyl radicals, produced by the Fenton reaction, have been observed to lead to the formation of more condensed phases in PC monolayers and bilayers. This increased chain ordering has been attributed to loss of the choline ammonium group after reaction with the hydroxyl radical, which forms a negatively charged lipid that can coordinate Fe(II) ions, leading to cross-linking.515,524
4.8. Biological Consequences of Lipid Oxidation
Oxidative damage to lipids and sterols is associated with numerous diseases, particularly in conditions of oxidative stress, where the normal balance between the formation of reactive oxygen and nitrogen species and their removal by cellular defense mechanisms is perturbed in favor of the former.525,526 In many of these diseases, oxidized lipids arising from both enzymatic and nonenzymatic oxidation are typically present. Nonenzymatic processes include the reactions described in the previous sections, but it is worth noting that in some cases linked to disease the oxidative processes occurred during food preparation or storage, with subsequent dietary uptake of the oxidized products.423,527,528 Enzymatic processes include ferroptosis and reactions catalyzed by lipoxygenases, cyclooxygenases, and cytochrome P450. Lipoxygenases and cyclooxygenases act on fatty acids released from lipids by the action of phospholipases.529 Ferroptosis is a key enzyme-mediated process for cell death and can be triggered, among other methods, by elevated levels of lipid peroxides in cells that are under reactive oxygen stress. Ferroptosis involves an iron-mediated oxidation of lipids that leads to plasma membrane rupture, mitochondrial membrane breakdown, and ultimately cell unviability.321,530−535 Lipoxygenases selectively form hydroperoxides of PUFAs as precursors for bioactive fatty acid mediators such as leukotrienes and lipoxins. Cyclooxygenases (prostaglandin-endoperoxide synthases) catalyze the formation of secondary oxidation products such as endoperoxides and their subsequent transformation into prostaglandins.356 Cytochrome P450 enzymes constitute a family of monooxygenases that oxidize hydrophobic materials selectively to form alcohols or epoxides. In the case of sterols, sites of oxidation notably include the hydrocarbon chain (e.g., at C24). For lipids, notable substrates are PUFAs, to form lipoxins, and leukotrienes. Many of the oxidized lipids and secondary fatty acid oxidation products formed as a consequence of the activity of these enzymes are involved in the inflammatory response, some with pro-inflammatory effects and others with anti-inflammatory effects depending on the route and timing of their formation.529,536 Thus, several of the oxidized lipids presented in section 4.1.3 that are generated by nonenzymatic methods have a biological activity themselves; others can serve as substrates for additional transformation by enzymes. CYP is also involved in an overproduction of reactive oxygen species in mitochondria, associated with disease conditions where normal homeostasis has been disrupted.537,538
Given the potential molecular diversity offered by both enzymatic and nonenzymatic oxidation of the natural lipidome, it is unsurprising that the potential scale of the oxidatively modified lipidome, the epilipidome, is vast, with up to 106 different lipids with an oxidation product in a single chain and 1012 lipids with an oxidation product in both chains. Many members of the epilipidome are involved in cellular responses such as inflammation, apoptosis, and the triggering of the innate immune response.315,539
Oxidized lipids and sterols are particularly associated with a number of diseases, including cardiovascular diseases, most notably atherosclerosis,126,321,382,453,540−542 nonalcoholic fatty liver disease,382 lysosomal storage disorders,461 and Alzheimer’s disease.321,543−545 In general, the presence of oxidized lipids can lead to biological consequences either through changes to the material properties of the membrane or through a direct biological activity of the oxidized lipids as enzyme substrates or receptor ligands. Examples of the effects of oxidized lipids on membrane structure include the release of cytochrome c from mitochondrial membranes following oxidative damage by CYP activity538 and the loss of membrane integrity seen during ferroptosis. The presence of oxidized lipids within the membrane can also influence the activity of membrane-embedded receptors, as evidenced by the increased activity of the human serotonin 1A receptor in the presence of oxidized lipids such as PoxnoPC (116).546 In some cases, membrane remodeling, mediated by desaturase enzymes, has been observed in response to an oxidative challenge, suggesting that cells are able to adapt their lipidome in order to maintain membrane function.547
Useful indicators for the presence of fatty acid oxidation arising by autoxidation, as opposed to enzymatic methods, can be obtained by examination of the stereochemistry of the hydroperoxide and hydroxyl products and the balance between (E,E)- and (Z,E)-isomers for di-unsaturated fatty acids. Oxidation by lipoxygenases favors the (S)-isomer of hydroperoxides and their corresponding alcohols, whereas autoxidation produces a racemic mixture. The ratios of (E,E)- and (Z,E)-isomers of hydroxylinoleate varies subtly between controls and samples under oxidative stress. In controls and samples subjected to lipoxygenase treatment and samples exposed to singlet oxygen, the (Z,E)-isomers predominate; in samples under stress by autoxidation, or chemical oxidation by hypochlorite, the ratio of (E,E)- to (Z,E)-isomers is closer to 1:1.382 Tracking these indicators through the progress of a disease can provide useful information on the relative importance of environmental stresses and enzymatic oxidation. For example, in the early stages of atherosclerosis, the S/R enantiomer ratio of 13-hydroxylinoleate is high, while in the later stages it tends toward 1:1, indicating the activity of lipoxygenases in the early stages, and increases the level of in 13-hydroxylinoleate have also been found to be higher in plaques than in plasma.382 Altered levels of these lipid biomarkers have been associated with a number of other diseases, including nonalcoholic fatty liver disease and glaucoma.382
Oxidized lipids have been associated with binding to a number of receptors, including G-protein coupled receptors associated with the inflammatory response (platelet-activating factor receptor), nuclear receptors associated with lipid metabolism (peroxisome proliferator-activated receptors, PPARα), and receptors associated with innate immunity (CD14 and Toll-like receptors).536 Lipids derived from PUFAs are capable of effecting vascular relaxation, L-type calcium channel activity, and glucose homeostasis.356 Truncated PCs, such as 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine (POVPC) (114), are able to mediate the binding of oxidized low density lipoprotein to receptors involved in low density lipoprotein internalization and have a key role in the formation of foam cells and the progression of atherosclerosis.126 Oxysterols have been found to induce nitric oxide (NO) production and the release of pro-inflammatory cytokines.548
Reactive carbonyl species such as 4-hydroxynonenal (4-HNE, 29) and 4-hydroxyhexenal are sufficiently long-lived and amphiphilic to enter general circulation during food digestion or following their formation in tissues. Elevated blood plasma levels of 4-HNE are associated with Alzheimer’s disease, some cardiovascular diseases, and rheumatoid arthritis. The effects of 4-HNE are concentration- and cell-type-dependent, with effects on cell differentiation at low concentrations and cell apoptosis or necrosis at high concentrations.549 4-Hydroxyhexenal is also markedly cytotoxic537 and mutagenic as a consequence of adduct formation with the imidazole ring of guanine. Furthermore, epoxides formed from these unsaturated aldehydes are also reactive toward DNA bases.550 Malondialdehyde (53) levels have been found to be higher in other diseases, including Alzheimer’s disease, diabetes, chronic obstructive pulmonary disorder, and cirrhosis.382 Glycated lipids have been shown to have cellular toxicity and are found at elevated levels in patients with diabetes. Cell integrity can also be negatively impacted by their accumulation.422 Secosterol aldehydes (92 and 93) have been detected in the brains of patients with Alzheimer’s disease or Lewy body disease and in atherosclerotic tissues. In all of these cases, secosterol aldehyde-modified proteins are detectable.453 Protein derivatization by lipid-derived reactive carbonyl species has been shown to affect protein–protein, protein–DNA, and protein–membrane interactions, as well as the cellular location of the modified molecule. Protein lipoxidation is site- and protein-selective. The predominant sites for modification are the side chains of cysteine and lysine, and to a lesser extent, histidine. Notable targets include albumin (circulation), chaperones, and some cytoskeletal proteins (tubulin, actin, and vimentin). In some cases, such as Ras, lipoxidation activates the receptor to trigger a secondary messenger cascade.403,526
In the case of cholesterol, the 5,6-epoxy derivatives such as 91 (Scheme 16) are able to serve as substrates for cholesterol epoxide hydrolase that stereoselectively forms the corresponding 5α,6β-diol (124), which is subsequently transformed by with catalysis by a dehydrogenase into ketone 125.461 The 7β-isomer of hydroxycholesterol (82) is also a substrate for a dehydrogenase to form the corresponding 7-oxo compound (83).
Scheme 16. Sterols Formed by Enzymatic Processing of 5,6-Epoxycholesterol.
Compound 125 is found in elevated levels in breast cancer tissue, and high levels of 125 and 83 are associated with several types of Niemann–Pick disease. These latter two sterols are also precursors for oxidation by CYP to form ligands for oncoproteins in the hedgehog pathway.454,461,551 All of the sterols in Scheme 16 are found at elevated levels in atherosclerotic plaques relative to blood plasma. Raised levels of the 7β-isomer of hydroxycholesterol (82) are a notable marker for several cardiovascular diseases.454,537,552 Some oxidized sterols, notably 7-ketocholesterol (82) in the context of autoxidation, are inverse agonists of retinoid-related orphan receptors, are able to bind to cytoplasmic transport proteins, and are able to up-regulate enzymes involved in cholesterol biosynthesis.537 24(S)-Hydroxycholesterol (24S-OHC) and 25-hydroxycholesterol (25-OHC) are acylated by an Acyl-CoA:cholesterol acyltransferase 1 (ACAT1) and are then incorporated into lipid droplet-like structures that are implicated in a process leading to cell death in some cell types.553
4.9. Future Directions
Significant advances have been made in characterizing the vast range of products resulting from lipid oxidation, including oxidized lipid and fatty acid fragments and biological molecules modified by these fragments. However, there remain some significant obstacles to understanding the links between oxidative stress and disease. The complexity of the product profile under oxidative stress makes it challenging to determine which products have significant biological activity of their own and which are relatively inert oxidation byproducts.133,475,544 While it may be possible to correlate complex oxidation profiles to particular diseases, moving from demonstrating correlations to establishing molecular mechanisms is difficult. As evidenced by the recent strides in understanding ferroptosis, in the biological context the formation of oxidized lipids can trigger processes that significantly change the profile of oxidation products. It is now established that some oxidized lipids regulate inflammation and have particular effects on macrophages, inducing behavior characterized by reduced phagocytotic capability and reduced motility.126 It is likely that other macrophage phenotypes linked to disease are induced by particular lipid oxidation products. Establishing which oxidation products produce particular patterns of immune cell activity, and establishing the molecular mechanisms underpinning this activity, will be vital. Understanding the roles of oxidation products from cardiolipins will also be crucial. Given their proximity to cellular sites of ROS generation in mitochondria and their susceptibility to oxidation, cardiolipins are capable of yielding a vast array of oxidized products, several derivatives of which are active in regulating the inflammatory response and apoptosis.444,496
For applications that do not involve living systems with active antioxidant processes, there remain ongoing challenges to improve stability and thereby shelf life. In the case of formulations with liposomes for drug delivery applications, particular obstacles to development are presented by excipients and active components that are either prone to oxidation themselves or increase the sensitivity of lipids to oxidation.257,381 Currently, our understanding of how these types of molecules affect stability toward oxidation (and ester lysis) is insufficient to predict their effects de novo. In food technology there is a need to better relate the formation of glycated products to stages in food processing and attain a better understanding of mechanisms by which glycation occurs, as the product profiles can vary significantly with changes in temperature and pressure.422
From an analytical standpoint, challenges remain in identifying some oxidation products for which the fragmentation pathways are not well-known. It is also hard to determine the location of a chain at the glycerol sn-1 or sn-2 position if it is oxidized. Part of the solution to these issues will involve improvements in software.539 Mechanistically, there is a desire to clarify which hydroperoxides arise by direct oxidation, and which by isomerization,417 and the extent to which each of these mechanisms operates in vivo.
5. Isomerization
In addition to the free radical reactions that lead to autoxidation, free radical addition of sulfur- and nitrogen-centered electrophilic radicals forms adducts that, if not trapped by hydrogen abstraction, subsequently eliminate the electrophilic radical by β-scission. These radical addition–elimination reactions proceed with isomerization of the alkene from Z to E and formally represent the propagation step of a radical reaction (Scheme 17), with formation of the electrophilic radical as the initiation step and dimerization of the electrophilic radical as the termination step. These reactions have been well reviewed by Chatgilialoglu et al.554
Scheme 17. Alkene Isomerization Mediated by Electrophilic Radical Addition.
5.1. Sulfur Radicals
Thiols are a significant source of electrophilic radicals, forming thiyl radicals via single electron oxidation. Initiation is typically mediated by γ-radiation, azo initiators, hydrogen transfer to an allylic radical, hydrogen transfer to a photochemically excited ketone, or electron transfer from a reducing metal ion. Model reactions using 2-mercaptoethanol with methyl oleate have enabled the rate constants for each of the steps in Scheme 17 to be determined.555,556 At equilibrium, all MUFAs comprise an E/Z ratio of 84:16. Rate constants for thiyl radical addition to (Z)- and (E)-alkenes (57 and 128) are broadly similar (1.6 × 105 and 2.9 × 105 M–1 s–1 respectively), but the fragmentation of the alkyl radical 127 occurs with a rate constant that is an order of magnitude greater than that of 126 (1.6 × 108 and 1.7 × 107 s–1). This rate difference is attributed to the transition state for the elimination of the thiyl radical from 127 being lower in energy than that for elimination from 126. For but-2-ene, this energy difference has been calculated as 1 kcal mol–1, attributed to a late transition state where steric interactions between the alkene substituents are more significant during the elimination from 126.555 Similar small differences in the transition state free energy barriers for elimination from the intermediate radical have been suggested by theoretical studies of the reaction of CH3S• with homoconjugated dienes. The transition state free energy for the decomposition to the (E,Z)-alkene of the radical intermediate (corresponding to 126), formed by the addition of CH3S• to C2 of (2Z,5Z)-hepta-2,5-diene, was lower in energy by 0.2 kcal mol–1 than the transition state for (Z,Z)-alkene formation (Figure 19).557 Addition of CH3S• to C2 had a lower energy barrier to addition than C3. The results were similar for linoleic acid, with a transition state energy difference for elimination to E,Z vs Z,Z of 0.1 kcal mol–1. Reactions of thiyl radicals with PUFAs are more complex because hydrogen abstraction to form bis-allyl radicals competes with radical addition, with the ratio of the two reactions being approximately 1:1. Isomerization following radical addition proceeds in a stepwise manner, forming mixtures with combinations of (E)- and (Z)-alkenes at each position.554 The critical free energy barrier for thiyl radical addition has been calculated to be lower than that for hydrogen abstraction by 1.3 kcal mol–1.557 Recent modeling studies indicate that the radical addition step has a low energy barrier due to stabilizing interactions between the unpaired electrons of the sulfur and the π* orbitals of the alkene.558
Figure 19.
Free energy landscapes of radical additions. Only the lowest barrier path is shown; the reaction sites of lowest barrier are shown in parentheses. From reference (557). Copyright 2014 American Chemical Society.
Cysteine, methionine and glutathione are potential sources of sulfur-centered radicals in vivo.559,560 Cysteine has a low abundance in the transmembrane regions of proteins, and it has been suggested that its presence may act to accelerate propagation,384 noting that lipophilic thiols generally accelerate peroxidation and isomerization reactions.561 Aside from thiyl radical formation by hydrogen transfer to allylic radicals, pulse radiolysis experiments with Cys suggest that the intermediate thiyl radical (130, Scheme 18) undergoes 1,2- and 1,3-hydrogen shifts.562,563 In the latter case, subsequent β-scission generates dehydroalanine (132) and a sulfhydryl radical (133). Sulfhydryl radicals have been shown to induce E/Z isomerization in liposomes.564 Thiyl radicals generated from penicillamine and cysteamine undergo similar hydrogen migrations, although the former is restricted to 1,3- and the latter to 1,2-migration.565 Carbon centered radicals can also be formed at various sites in peptides by hydrogen transfer reactions with the thiyl radical formed from glutathione.562 These hydrogen transfer reactions occur under physiological conditions and are tolerant of oxygen.560 Single electron reduction of disulfides via γ-irradiation provides another route to thiyl radicals.565,566 For example, dimethydisulfide-mediated isomerization of the oleoyl group in POPC liposomes leads to >90% of the trans isomer at a γ-irradiation dose of 126 Gy. Carbon dioxide radical anions, produced by photodegradation of citrate in the presence of Fe(II), are able to react with disulfides to form thiyl radicals that can induce fatty acid isomerization in a synthetic surfactant, although this has not yet been demonstrated for a protein disulfide in membranes.567
Scheme 18. Generation of the Sulfhydryl Radical from Cys.
Thioethers such as methionine can potentially act as hydrogen atom acceptors to form thiouranyl radicals (R–S•(H)–R′) that subsequently fragment to a thiol (methyl mercaptan in the case of methionine) and a carbon-centered radical. The thiol is then available for subsequent thiyl radical formation.554
There is some evidence for thiyl radical-mediated isomerization in vivo, although the levels of (E)-isomers that accumulate are small unless conditions are used that favor the formation of thiyl radicals. Incubation of human monocytic leukemia cell membranes with thiols (2-mercaptoethanol, glutathione, and the biologically active thiol 3-(2-mercapthoethyl)quinazolin-2,4(1H,3H)-dione (MECH)) produced small increases in the levels of (E)-alkenes, typically 1–2% of total fatty acids, for 2-mercaptoethanol and glutathione but not for MECH.568 Increases were most notable for PUFAs, with little change in the E/Z ratio of MUFAs. Similar results were obtained for normal and cells cultured under oxidative stress. On the other hand, incubation of NTera-2 testicular cancer cells with bleomycin (BLM) led to significant increases the in proportion of both saturated fatty acids and (E)-isomers of MUFAs and PUFAs.569 The respective proportions of MUFAs and PUFAs with at least one (E)-alkene (% of total FA) in these experiments were 2.9% and 0.38% compared to 0.14% and 0.05% in controls without BLM, respectively.
Thiyl radical-induced isomerization produced by BLM, in combination with iron, has been well studied. Oxidation of the Fe(II) complex with BLM in the presence of O2 leads to the formation of the BLM–Fe(III) complex and ROS, most notably superoxide.570,571 The production of ROS in the BLM–iron system is associated with lipid peroxidation.572 In the presence of thiols, reduction of BLM–Fe(III) to BLM–Fe(II) occurs with concomitant formation of thiyl radicals (Scheme 19). In model POPC liposomes, E/Z isomerization of the oleoyl group to elaidoyl, promoted by BLM–Fe(II) complexes in the presence of oxygen and an amphiphilic thiol, occurs without the formation of significant oxidation products. Isomerization yields are typically 25–30% E after 24 h at 5% oxygen, with the amount of (E)-isomer decreasing at higher oxygen levels. In more complex models, E/Z isomerization of both MUFAs and PUFAs occurs, but the predominant products arise by PUFA oxidation.573
Scheme 19. Generation of Thiyl Radicals by Fe(III) Complexes of Bleomycin (BLM).
Sulfonyl radicals (RSO2•) form by rearrangement of thioperoxyl radicals (RSOO•), which themselves form reversibly through the reaction of thiyl radicals with oxygen, although the equilibrium favors the thiyl radical in all but saturating oxygen conditions. As a consequence, sulfonyl radical reactivity is only likely to be significant in exceptional circumstances, but these radicals nevertheless produce similar E/Z ratios to thiyl radicals by the general process outlined in Scheme 17.554
5.2. Nitrogen Radicals
Isomerization of isolated alkenes by NO2• has been demonstrated in the gas phase, where radical addition competes with allylic hydrogen abstraction, but generally NO2• reactivity with these alkenes is low.554 In PUFAs, hydrogen abstraction to form allylic radicals predominates over NO2• addition in the absence of oxygen and water, with ∼5% isomerization (vs addition) products at low concentrations of NO2 (6.8 ppm), which increases to ∼40% at higher NO2 levels (81 ppm). In aqueous or aerated systems at these NO2 levels, no isomerization products are detectable.574 This contrasts with ab initio predictions that addition reactions through the nitrogen should be favored in polar media, although the difference in the free energy barriers for hydrogen abstraction and free radical addition is small.557 The involvement of NO2• in isomerization reactions in vivo has been questioned.575 Reduction of NO2• radicals by thiols such as glutathione to generate thiyl radicals is potentially a more important mechanism for producing isomerization in vivo.
5.3. Isomerization of Membrane Lipids
Isomerization reactions of membrane lipids with thiyl radicals are tolerant to oxygen (up to 0.2 mM) and follow the same trends in reactivity as model reactions in homogeneous solutions, with each double bond of PUFAs behaving independently irrespective of chain position. Differences are seen for reactions in micelles and liposomes that depend on the ability of the parent thiol to penetrate the membrane, with lipophilic or amphiphilic thiols such as 2-mercaptoethanol being much more effective at producing isomerization than polar thiols like cysteine.554 With amphiphilic thiols, double bonds closer to the membrane interface isomerize ahead of those buried deeper within the membrane, reflecting the increased encounter rate between the corresponding thiyl radicals and surface-proximal alkenes.573,576
5.4. Biophysical Effects of Isomerization
Because of the detrimental effects of dietary “trans fats”, particularly in relation to cardiovascular disease, the effects of the (E)-alkenes on the biophysical properties of the membrane have been well studied. In general, for membranes containing monounsaturated fatty acyl groups, replacing the (Z)-alkene with an (E)-alkene leads to properties that are intermediate between the (Z)-alkene and corresponding fully saturated systems.577 Examples are given in Table 3.
Table 3. Selected Properties of Lipids Containing (E)- and (Z)-Alkenes.
| lipid | configuration | Tm (°C)a | ΔHm (kJ mol–1)a | –SCDd |
|---|---|---|---|---|
| POPC | 9Z (sn-2) | –3.4–2.4 | 19.7–32.9 | 0.091 (46 °C) |
| PEPC | 9E (sn-2) | 26b, 35c | 0.156 (46 °C) | |
| PSPC | 48.3–52 | 35.7–43.1 | ||
| SOPC | 9Z (sn-2) | –0.1–8.3 | 27.5–28.5 | |
| SEPC | 9E (sn-2) | 26 | 35.1 | |
| DSPC | 54.3–58.2 | 38.1–45.4 | 0.173 (65 °C)e | |
| DOPC | 9Z (sn-1/2) | –24–21 | 31.8–46.9 | 0.098 (51 °C) |
| DEPC | 9E (sn-1/2) | 12f | 33.1f | 0.13 (57 °C) |
Other properties of membranes containing (E)-isomers of fatty acids are consistent with tighter lipid packing compared to the corresponding (Z)-isomers, including decreases in fluidity,580 lateral581,582 and transverse mobility (“flip-flop”),580 and permeability.580,583
Binary mixtures containing lipids with (E)-fatty acids exhibit phase behavior that is broadly similar to their (Z)-counterparts, but with subtle differences resulting from the higher Tm of (E)-fatty acids and the affinity of (E)-fatty acyl groups for cholesterol. For example, as with DOPC, binary mixtures of 1,2-dielaidoyl-sn-glycero-3-phosphocholine (DEPC) with DPPC have a miscibility gap, with coexisting gel and fluid phases between 20 and 70 mol % DPPC at room temperature.584,585 As the Tm of DEPC is 12 °C, DEPC/DPPC binary mixtures are always in the gel phase below this temperature, regardless of composition, whereas at this temperature DOPC/DPPC mixtures, for example, are in the gel phase at >90 mol % DPPC, the fluid phase at <20 mol % DPPC, and in a mixed gel/fluid phase at compositions in between. Binary DEPC/DPPE mixtures exhibit fluid–fluid immiscibility.585 Cholesterol has a higher affinity for PCs with (E)-fatty acids than their (Z)-counterparts, as demonstrated by cholesterol partitioning experiments. The E/Z difference in cholesterol affinity becomes more pronounced with increasing chain length.583 Ternary POPC/1-palmitoyl-2-elaidoyl-sn-glycero-3-phosphocholine (PEPC)/cholesterol (60:30:10) membranes exhibit properties that are consistent with the formation of liquid ordered domains enriched in cholesterol and PEPC. These domains melt at a lower temperature than the corresponding system in which PEPC is replaced with 1-palmitoyl-2-stearoyl-sn-glycero-3-phosphocholine (PSPC).586
Modeling experiments have suggested that the conformational and packing behaviors of (E)-fatty acyl chains closely resemble those of saturated acyl chains.582,587 This has led to predictions that (E)-FFAs should increase membrane rigidity in a similar manner to palmitic acid.588 It has also recently been suggested that fatty acids with a (E)-alkene exhibit greater conformational flexibility at the C–C bonds adjacent to alkene than their (E)-counterparts. The increased flexibility allows for better packing, accounting for the increased order and affinity for cholesterol of (Z)-fatty acids.589
5.5. Future Directions
While many of the chemical details of the isomerization process are well described, there is still much to learn considering the propensity of individual fatty acids to undergo isomerization in the context of a complex biological membranes and the effects of (E)-fatty acid generation on membrane protein activity, particularly as the levels of (E)-fatty acid isomers increase with aging.590 Whether the presence of particular endogenous (E)-isomers, or combinations of (E)-isomers, are biomarkers for specific conditions is still not well characterized.591
6. Concluding Remarks
6.1. The Biological and Biophysical Effects of Chemical Reactivity
The products of membrane lipid reactivity have significant, and usually detrimental, effects on membrane integrity. Lysolipids cause perturbations to the bending rigidity and the morphology of lipid membranes and increase bilayer permeability. Oxidation of fatty acyl chains introduces polar groups into previously apolar regions of the lipid. While in some cases these polar groups migrate to more polar interfacial regions, the general effect of their presence is to disrupt bilayer integrity and allow water penetration to greater depths. As a consequence, the barrier to the diffusion of polar molecules across the membrane is reduced and the transbilayer relocation of lipids can occur more easily. This oxidative damage has a particular chance to accumulate when tissue turnover is reduced, which occurs notably in the deposits associated with cardiovascular disease and amyloid diseases. Several of the oxidized fragments resulting from fatty acid oxidation are able to modify other molecules, notably proteins. In some cases this can lead to loss of function. The distribution and diversity of protein modifications by electrophiles, the “adductome”, is complex and likely to be characteristic for particular diseases.404 The sensitivity of mass spectrometric techniques, exemplified by the work of He et al.,441 also revealed a similar complexity for lipid modifications by glycation. While the levels of these modifications may not have a significant physiological effect, they may offer up the potential for early detection of disease conditions. Similar complexity is also found in the “oxylipidome” of PUFAs, including their secondary oxidation products.388,536
It will be interesting to see whether modern advances in artificial intelligence can be used to detect trends in the distributions of lipoxidated proteins, fatty acid oxidation products, and glycated lipids for the detection of disease conditions at very early stages. Such detection would enable early interventions in these diseases.
6.2. The Time Scales of Lipid Chemical Reactivity
With respect to the consequences of lipid reactivity in vivo, it is worthwhile to consider the relative rates of some of the relevant processes from the perspective of both the reactions themselves and the typical rates of turnover for lipids and proteins. While the details vary significantly according to cell (and stage of the cell cycle, as well as subcellular location), tissue, and organism, there are some general trends in protein and lipid turnover. Protein half-lives vary from minutes to thousands of hours. Higher turnover rates are found in cells with short cell cycles or those that are dividing. In mice for example, proteins in the liver and brain have average half-lives in the range of 3–9 days. Some proteins are very long-lived (years or the entire lifespan). These long-lived proteins include proteins in postmitotic cells such as crystallins and aquaporins of lens fiber cells, but also some proteins in active cells such as components of the nuclear pore complex, where the half-life can be over a year.283,592 Lipids have half-lives that tend to be approximately 30% shorter than proteins.593 In many mammalian cells, degradation of approximately half of the total phospholipid occurs every one or two cell divisions,594 although as with proteins lipids in some postmitotic cells are not turned over during the entire lifespan of the organism.595 In eurkaryotic cells, there is a continuous transport of lipids from their site of biogenesis (ER for glycerophospholipids, peroxisomes for plasmalogens) to their site of activity and then onward to their site of degradation (often in lysosomes). Recent studies have highlighted the key roles of membrane contact sites, which permit the movement of lipids between organelles, as key mediators of lipid synthesis and degradation.596
Overall, the continuous turnover and trafficking of lipids occurs on a similar time scale to lipid hydrolysis (Section 2) in the absence of enzymes, with a half-life of hours to days. Lipidation reactions (Section 3), which also generate lysolipids, are typically marginally faster than hydrolysis reactions. Cellular lysolipid levels are under tight regulation, and in addition to recycling by degradation, lysolipids are also recycled by reacylation to the lipid under enzyme control.597 Consequently, under normal circumstances in a healthy cell, the products of hydrolysis should not accumulate. However, in circumstances where hydrolysis is accelerated, or turnover is restricted, lysolipid levels may increase to become physiologically significant. A good example occurs during the inflammatory response, where the generation of lysolipids is accelerated by PLA2. Drugs that reduce lysolipid processing, accelerate lipid hydrolysis, or undergo lipidation reactions may increase lysolipid levels sufficiently to trigger a physiological response. It is notable in this context that some of the drugs known to cause drug-induced phospholipidosis (DIPL), a lysosomal storage disorder characterized by the accumulation of lipids in multilamellar arrays, are also known to promote lysolipid formation.272,598 Although a direct causal link between lipid hydrolysis and DIPL has not been established, the molecular mechanisms behind DIPL are still the subject of debate.
At face value, many of the key steps in lipid oxidation (Section 4) occur with rates that are significantly higher than lipid turnover, and consequently one might expect that the levels of lipid oxidation products would be continuously high. However, there are two key points to note. First, many of the experiments to determine the mechanistic details of oxidation are conducted under forcing conditions, with levels of oxidants far higher than those that occur in most normal circumstances.599,600 Second, oxidative damage in vivo is limited in a healthy cell by the presence of defense mechanisms that operate with faster kinetics than oxidative reactions. This is the case for membrane antioxidants such as tocopherol;368 detoxification by aldoketoreductases, glutathione peroxidases, and glutathione S-transferases;133,601 and quenching of hydroperoxyl radicals by superoxide dismutase.327
In model systems in vitro, oxidation products accumulate on a time scale of minutes to hours.397,455,489 This is a similar time scale to cell death mediated by ferroptosis, giving a clear indicator of the rate by which toxic levels of oxidized lipid products can accrue when unchecked.602 However, the progression of diseases where lipid oxidation and an accumulation of damaged lipids is a factor, such as atherosclerosis,126,603 can be months to years. Alongside the generation of lipid oxidation products in disease conditions, the increased formation of reactive aldehydes leads to the generation of protein adducts that are both detectable and characteristic of the disease in question.133,404 In postmitotic cells, such as lens fiber cells, adaptations to reduce oxidative damage include a high cholesterol level and increased levels of saturated acyl chains.604 Oxidative damage to lipids does accrue in these cells, but even in aged individuals the levels can be surprisingly low.
As outlined above, some proteins have very slow turnover rates, and it may be expected that these proteins will accumulate modifications by reactive electrophiles derived from lipids over time. In this context it is notable that aquaporin-0 from both human and bovine lenses is lipidated with acyl chains in two positions that present a fatty acid profile that is similar to the membrane leaflet in which the protein resides, suggesting that these post-translational modifications have occurred by direct transfer from the lipid.303
Many of the processes by which membrane lipids react exhibit an interdependence. For example, reactive oxygen species, including hypochlorous acid, are able to cause the formation of lysolipids, which can then trigger an inflammatory response.597 By contrast, an accumulation of oxidatively damaged lipids facilitates water penetration to greater depths in the membrane, with subsequent increases in the rates of transbilayer lipid diffusion and hydrolysis and greater penetration of soluble oxidants.506
6.3. Final Comments
From the large body of work available on lipid reactivity, we now have a better understanding of the many chemical processes that impact membrane lipids.
First, lipids are susceptible to lysis reactions at the glyceryl ester groups involving nucleophilic attack on the ester carbonyl by water (hydrolysis) or organic nucleophiles. The byproducts are a lysolipid and either a free fatty acid or a lipidated product. Lysolipids can in turn be subjected to further hydrolysis to form sn-glycero-3-phosphocholine (GPC). Hydrolysis of lipids and membrane lipids can be affected by temperature (slower rates at low temperatures), pH (slowest rates at pH 5.8–6.5), and their chain length (higher rates for shorter chains and increased unsaturation levels). Some membrane additives, such as ascorbyl palmitate, dialkylphosphates, or cationic amphiphilic drugs (CADs), can also influence hydrolysis rates. In the case of aminolysis involving amine nucleophiles, reactivity is impacted by the pKa of the ammonium form of the amine. For both aminolysis and transesterification reactions, the disposition of the reactive group in the membrane-bound form of the nucleophile has a significant effect on reactivity. These lytic reactions, which potentially lead to the formation of multiple byproducts, can severely impact the biophysical propertied and long-term stability of lipid membranes.
Second, lipids are well documented to undergo oxidation processes, the most common being autoxidation of the alkene groups of unsaturated fatty acids and sterols through a multistep process involving triplet oxygen. Oxidation can also occur with singlet oxygen and by reaction with other oxidizing agents such as hypochlorous acid. A key biophysical effect of these oxidation reactions is to increase the depth of water penetration into lipid membranes. Biological effects are associated with disturbances to lipid homeostasis and the triggering of apoptotic pathways such as ferroptosis. Oxidative damage is particularly associated an increased risk of cardiovascular disease.
Finally, lipids are affected by the isomerization of the alkene from Z to E through a radical addition–elimination reaction. This happens in the presence of sulfur- and nitrogen-centered electrophilic radicals, with sources such as cysteine, methionine, or glutathione. Isomerization through thiyl radicals has also been demonstrated in membrane lipids. (E)-Alkenes have been shown to decrease the fluidity and increase the transmembrane mobility and permeability of membrane lipids.
Acknowledgments
J.M.S. and G.D. thank the EPSRC for funding (EP/V048155/1).
Glossary
Abbreviations
- 4-HNE
4-hydroxynonenal
- 4-ONE
4-oxononenal
- AAPH
2,2′-azobis(2-methylpropionamidine)dihydrochloride
- AGE
advanced glycation end product
- AP
ascorbyl palmitate
- AQP0
aquaporin-0
- BDMP
4-tert-butyl-2,6-dimethylphenol
- BLM
bleomycin
- CAD
cationic amphiphilic drug
- CDL
cardiolipin
- Cer
ceramide
- chol
cholesterol
- DBHN
di-tert-butylhyponitrite
- DEPC
1,2-dielaidoyl-sn-glycero-3-phosphocholine
- dFdC
gemcitabine
- DFT
density functional theory
- DHC
7-dehydrocholesterol
- DHPC
1,2-dihexanoyl-sn-glycero-3-phosphocholine
- DMPC
1,2-dimyristoyl-sn-glycero-3-phosphocholine
- DMPG
1,2-dimyristoyl-sn-glycero-3-phosphoglycerol
- DOPC
1,2-dioleoyl-sn-glycero-3-phosphocholine
- DOPE
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine
- DOTAP
1,2-dioleoyl-3-trimethylammonium propane
- DPPA
1,2-dipalmitoyl-sn-glycero-3-phosphatidic acid
- DPPC
1,2-dipalmitoyl-sn-glycero-3-phosphocholine
- DPPE
1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine
- DPPG
1,2-dipalmitoyl-sn-glycero-3-phosphoglycerol
- DPPlsC
1,2-di-O-((Z)-1′-hexadecenyl)-sn-glycero-3-phosphocholine
- DSPC
1,2-distearoyl-sn-glycero-3-phosphocholine
- DSPE
1,2-distearoyl-sn-glycero-3-phosphoethanolamine
- DSPG
1,2-distearoyl-sn-glycero-3-phosphoglycerol
- EIC
extracted ion current
- ELF
epithelial lining fluid
- EPC
hen egg phosphatidylcholine
- EPG
hen egg phosphatidylglycerol
- EPR
electron paramagnetic resonance
- FFA
free fatty acid
- GPC
sn-glycero-3-phosphocholine
- GUV
giant unilamellar vesicle
- In
initiator
- In•
initiating radical
- H-EPC
hydrogenated hen egg phosphatidylcholine
- H-soyPC
hydrogenated soybean PC
- HEPES
hydroxyethylpiperazine ethanesulfonic acid
- LPC
lyso-phopshatidylcholine
- Ld
liquid disordered
- Lo
liquid ordered
- MALDI
matrix-assisted laser desorption/ionization
- MECH
3-(2-mercapthoethyl)quinazolin-2,4(1H,3H)-dione
- MeO-AMVN
2,2′-azobis(4-methoxy-2,4-dimethylvaleronitrile)
- MPO
myeloperoxidase
- MUFA
monounsaturated fatty acid
- NIR
near-infrared
- OHC
hydroxycholesterol
- PA
phosphatidic acid
- PazePC
1-palmitoyl-2-azelaoyl-sn-glycero-3-phosphocholine
- PC
phosphatidylcholine
- PCpl
plasmenylcholine
- PE
phosphatidylethanolamine
- PEG
polyethylene glycol
- PEPC
1-palmitoyl-2-elaidoyl-sn-glycero-3-phosphocholine
- PEpl
plasmenylethanolamine
- PG
phosphatidylglycerol
- PGPC
1-palmitoyl-2-glutaryl-sn-glycero-3-phosphocholine
- PH-eggPC
partially hydrogenated hen egg PC
- PH-soyPC
partially hydrogenated soybean PC
- PI
phosphatidylinositol
- PLA/B/C/D
phospholipase A/B/C/D
- POPC
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- POVPC
1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine
- PoxnoPC
1-palmitoyl-2-(9′-oxo-nonanoyl)-sn-glycero-3-phosphocholine
- PS
phosphatidylserine
- PTM
post-translational modification
- PUFA
polyunsaturated fatty acid
- PV
peroxide value
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- SAPC
1-stearoyl-2-arachidonyl-sn-glycero-3-phosphocholine
- SM
sphingomyelin
- SMase
sphingomyelinase
- SOD
superoxide dismutase
- SLPE
1-stearoyl-2-linoleoyl-sn-glycero-3-phosphoethanolamine
- SLPG
1-stearoyl-2-linoleoyl-sn-glycero-3-phosphoglycerol
- SLPS
1-stearoyl-2-stearoyl-sn-glycero-3-phosphoserine
- SOMO
singly occupied molecular orbital
- SP-C
surfactant protein C
- SPC
sphingosylphosphorylcholine
- TBARS
thiobarbituric acid reactive substances
- Tm
gel to liquid crystalline phase transition temperature
- α-TOC
α-tocopherol
Biographies
John M Sanderson completed his B.Sc. (Hons.) at Leeds University in Chemistry and Biochemistry (combined), before completing a Ph.D. on the pore structure of potassium channels at Leeds University. Following postdoctoral spells in the laboratories of Prof. Chris Hunter FRS at the University of Sheffield, UK, and Prof. John-Marie Lehn at the Collège de France, Paris, he took up a position as a Chemistry lecturer at Sussex University in 1999. He moved to Durham in 2001, where he is currently an associate professor. He is a fellow of the Royal Society of Chemistry and a committee member of the British Biophysical Society. His research focuses on the interactions of molecules of all sizes with lipid membranes. His current work focuses on the effects that drugs, peptides, and proteins have on the chemical stability of lipids and how changes in chemical stability might impact health and disease.
Geneviève Duché is a chemist specialized in supramolecular chemistry, drug delivery systems, and lipid chemistry. While part of the ARC Centre of Excellence on Convergent Bionano Science & Technology, her research interest extended to STS and integrated research in the field of nanotechnology. She completed her B.Sc. and M.Phil. at the University of New South Wales, before completing a Ph.D. on encapsulating self-assembled peptide hydrogel in liposomes for topical drug delivery. She then moved to Durham University for a postdoctoral position working on exploiting membrane lipidation for advanced drug delivery. She is currently working as a research engineer at the Université Technologique de Compiègne on peptide selection using phage display in partnership with BASF.
Author Contributions
J.M.S. wrote the sections on isomerization and aminolysis. G.D. and J.M.S. wrote the sections on oxidation and hydrolysis. G.D. and J.M.S. contributed equally to other sections and to editing.
The authors declare no competing financial interest.
References
- Robertson J. D. The Molecular Structure and Contact Relationships of Cell Membranes. Prog. Biophys. Mol. Biol. 1960, 10, 343–418. 10.1016/S0096-4174(18)30194-X. [DOI] [PubMed] [Google Scholar]
- Robertson J. D. The Ultrastructure of Cell Membranes and Their Derivatives. Biochem. Soc. Symp. 1959, 16, 3–43. [PubMed] [Google Scholar]
- Danielli J. F.; Davson H. A Contribution to the Theory of Permeability of Thin Films. J. Cell. Comp. Physiol. 1935, 5, 495–508. 10.1002/jcp.1030050409. [DOI] [Google Scholar]
- Gregoriadis G. Liposomes and mRNA: Two Technologies Together Create a Covid-19 Vaccine. Med. Drug Discovery 2021, 12, 100104. 10.1016/j.medidd.2021.100104. [DOI] [Google Scholar]
- Le M. T.; Litzenberger J. K.; Prenner E. J.. Biomimetic Model Membrane Systems Serve as Increasingly Valuable in Vitro Tools. In Advances in Biomimetics; George A., Ed.; IntechOpen: London, UK, 2011; pp 215–276. [Google Scholar]
- Liposomes as Tools in Basic Research and Industry; Philippot J. R., Schuber F., Eds.; CRC Press: Boca Raton, FL, 1995. [Google Scholar]
- Lasic D. D.Liposomes: From Physics to Applications; Elsevier, 1993. [Google Scholar]
- Marsh D.Handbook of Lipid Bilayers; CRC Press: Boca Raton, FL, 2013. [Google Scholar]
- The Lipid Handbook, 3rd ed.; Gunstone F. D., Harwood J. L., Dijkstra A. J., Eds.; CRC Press: Boca Raton, FL, 2007. [Google Scholar]
- Bui T. T.; Suga K.; Kuhl T. L.; Umakoshi H. Melting-Temperature-dependent Interactions of Ergosterol With Unsaturated and Saturated Lipids in Model Membranes. Langmuir 2019, 35, 10640–10647. 10.1021/acs.langmuir.9b01538. [DOI] [PubMed] [Google Scholar]
- Bui T. T.; Suga K.; Umakoshi H. Ergosterol-Induced Ordered Phase in Ternary Lipid Mixture Systems of Unsaturated and Saturated Phospholipid Membranes. J. Phys. Chem. B 2019, 123, 6161–6168. 10.1021/acs.jpcb.9b03413. [DOI] [PubMed] [Google Scholar]
- Unwin N. Structure of a Cholinergic Cell Membrane. Proc. Natl. Acad. Sci. U. S. A. 2022, 119, e2207641119 10.1073/pnas.2207641119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandrei F.; Engberg O.; Opálka L.; Jančálková P.; Pullmannová P.; Steinhart M.; Kováčik A.; Vávrová K.; Huster D. Cholesterol Sulfate Fluidizes the Sterol Fraction of the Stratum Corneum Lipid Phase and Increases Its Permeability. J. Lipid Res. 2022, 63, 100177. 10.1016/j.jlr.2022.100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song F.; Yang G.; Wang Y.; Tian S. Effect of Phospholipids on Membrane Characteristics and Storage Stability of Liposomes. Innovative Food Sci. Emerging Technol. 2022, 81, 103155. 10.1016/j.ifset.2022.103155. [DOI] [Google Scholar]
- Pruchnik H.; Włoch A.; Gładkowski W.; Grudniewska A.; Chojnacka A.; Krzemiński M.; Rudzińska M. Effect of Distigmasterol-Modified Acylglycerols on the Fluidity and Phase Transition of Lipid Model Membranes. Membranes (Basel, Switz.) 2022, 12, 1054. 10.3390/membranes12111054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Martín F.; D’Amelio N. Biomembrane Lipids: When Physics and Chemistry Join to Shape Biological Activity. Biochimie 2022, 203, 118–138. 10.1016/j.biochi.2022.07.011. [DOI] [PubMed] [Google Scholar]
- Cebecauer M.; Amaro M.; Jurkiewicz P.; Sarmento M. J.; Šachl R.; Cwiklik L.; Hof M. Membrane Lipid Nanodomains. Chem. Rev. 2018, 118, 11259–11297. 10.1021/acs.chemrev.8b00322. [DOI] [PubMed] [Google Scholar]
- Schmid F. Physical Mechanisms of Micro- and Nanodomain Formation in Multicomponent Lipid Membranes. Biochim. Biophys. Acta, Biomembr. 2017, 1859, 509–528. 10.1016/j.bbamem.2016.10.021. [DOI] [PubMed] [Google Scholar]
- Róg T.; Vattulainen I. Cholesterol, Sphingolipids, and Glycolipids: What Do We Know About Their Role in Raft-Like Membranes?. Chem. Phys. Lipids 2014, 184, 82–104. 10.1016/j.chemphyslip.2014.10.004. [DOI] [PubMed] [Google Scholar]
- van Meer G.; Voelker D. R.; Feigenson G. W. Membrane Lipids: Where They Are and How They Behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao L.; Nielsen M.; Thewalt J.; Ipsen J. H.; Bloom M.; Zuckermann M. J.; Mouritsen O. G. From Lanosterol to Cholesterol: Structural Evolution and Differential Effects on Lipid Bilayers. Biophys. J. 2002, 82, 1429–1444. 10.1016/S0006-3495(02)75497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch S. L.; Keller S. L. Organization in Lipid Membranes Containing Cholesterol. Phys. Rev. Lett. 2002, 89, 268101. 10.1103/PhysRevLett.89.268101. [DOI] [PubMed] [Google Scholar]
- Róg T.; Pasenkiewicz-Gierula M.; Vattulainen I.; Karttunen M. Ordering Effects of Cholesterol and Its Analogues. Biochim. Biophys. Acta 2009, 1788, 97–121. 10.1016/j.bbamem.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Chong P. L.-G.; Zhu W.; Venegas B. On the Lateral Structure of Model Membranes Containing Cholesterol. Biochim. Biophys. Acta 2009, 1788, 2–11. 10.1016/j.bbamem.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Marsh D. Elastic Curvature Constants of Lipid Monolayers and Bilayers. Chem. Phys. Lipids 2006, 144, 146–159. 10.1016/j.chemphyslip.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Sanderson J. M. Resolving the Kinetics of Lipid, Protein and Peptide Diffusion in Membranes. Mol. Membr. Biol. 2012, 29, 118–143. 10.3109/09687688.2012.678018. [DOI] [PubMed] [Google Scholar]
- Scheidt H. A.; Huster D.; Gawrisch K. Diffusion of Cholesterol and Its Precursors in Lipid Membranes Studied By 1H Pulsed Field Gradient Magic Angle Spinning NMR. Biophys. J. 2005, 89, 2504–2512. 10.1529/biophysj.105.062018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov A.; Oradd G.; Lindblom G. The Effect of Cholesterol on the Lateral Diffusion of Phospholipids in Oriented Bilayers. Biophys. J. 2003, 84, 3079–3086. 10.1016/S0006-3495(03)70033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frallicciardi J.; Melcr J.; Siginou P.; Marrink S. J.; Poolman B. Membrane Thickness, Lipid Phase and Sterol Type Are Determining Factors in the Permeability of Membranes to Small Solutes. Nat. Commun. 2022, 13, 1605. 10.1038/s41467-022-29272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov S. S.; Popova M. M.; Pohl P.; Horner A.; Akimov S. A.; Kireeva N. A.; Knorre D. A.; Batishchev O. V.; Severin F. F. Structural Role of Plasma Membrane Sterols in Osmotic Stress Tolerance of Yeast Saccharomyces Cerevisiae. Membranes (Basel, Switz.) 2022, 12, 1278. 10.3390/membranes12121278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting R.; Finn P. W.; Bogard A.; McKinney F.; Pankratz D.; Smith A. R.; Gardner E. A.; Fologea D. Experimental Investigations on the Conductance of Lipid Membranes Under Differential Hydrostatic Pressure. Membranes (Basel, Switz.) 2022, 12, 479. 10.3390/membranes12050479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K.; Iwamoto M.; Koshiji T.; Oiki S. Visualizing the Osmotic Water Permeability of a Lipid Bilayer Under Measured Bilayer Tension Using a Moving Membrane Method. J. Membr. Sci. 2021, 627, 119231. 10.1016/j.memsci.2021.119231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannesschlaeger C.; Horner A.; Pohl P. Intrinsic Membrane Permeability to Small Molecules. Chem. Rev. 2019, 119, 5922–5953. 10.1021/acs.chemrev.8b00560. [DOI] [PubMed] [Google Scholar]
- Cao Z.; Zhang X.; Wang C.; Liu L.; Zhao L.; Wang J.; Zhou Y. Different Effects of Cholesterol on Membrane Permeation of Arginine and Tryptophan Revealed By Bias-Exchange Metadynamics Simulations. J. Chem. Phys. 2019, 150, 084106. 10.1063/1.5082351. [DOI] [PubMed] [Google Scholar]
- Galea A. M.; Brown A. J. Special Relationship Between Sterols and Oxygen: Were Sterols an Adaptation to Aerobic Life. Free Radicals Biol. Med. 2009, 47, 880–889. 10.1016/j.freeradbiomed.2009.06.027. [DOI] [PubMed] [Google Scholar]
- Liposome Methods and Protocols; Basu S. C., Basu M., Eds.; Humana Press: Totowa, NJ, 2002. [Google Scholar]
- Corvera E.; Mouritsen O. G.; Singer M. A.; Zuckermann M. J. The Permeability and the Effect of Acyl-Chain Length for Phospholipid Bilayers Containing Cholesterol: Theory and Experiment. Biochim. Biophys. Acta 1992, 1107, 261–270. 10.1016/0005-2736(92)90413-G. [DOI] [PubMed] [Google Scholar]
- Kučerka N.; Pencer J.; Nieh M. P.; Katsaras J. Influence of Cholesterol on the Bilayer Properties of Monounsaturated Phosphatidylcholine Unilamellar Vesicles. Eur. Phys. J. E: Soft Matter Biol. Phys. 2007, 23, 247–254. 10.1140/epje/i2007-10202-8. [DOI] [PubMed] [Google Scholar]
- Marsh D. Polarity and Permeation Profiles in Lipid Membranes. Proc. Natl. Acad. Sci. U. S. A. 2001, 98, 7777–7782. 10.1073/pnas.131023798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subczynski W. K.; Wisniewska A.; Yin J. J.; Hyde J. S.; Kusumi A. Hydrophobic Barriers of Lipid Bilayer Membranes Formed By Reduction of Water Penetration By Alkyl Chain Unsaturation and Cholesterol. Biochemistry 1994, 33, 7670–7681. 10.1021/bi00190a022. [DOI] [PubMed] [Google Scholar]
- Dahley C.; Garessus E. D. G.; Ebert A.; Goss K. U. Impact of Cholesterol and Sphingomyelin on Intrinsic Membrane Permeability. Biochim. Biophys. Acta, Biomembr. 2022, 1864, 183953. 10.1016/j.bbamem.2022.183953. [DOI] [PubMed] [Google Scholar]
- Nakhaei P.; Margiana R.; Bokov D. O.; Abdelbasset W. K.; Jadidi Kouhbanani M. A.; Varma R. S.; Marofi F.; Jarahian M.; Beheshtkhoo N. Liposomes: Structure, Biomedical Applications, and Stability Parameters With Emphasis on Cholesterol. Front. Bioeng. Biotechnol. 2021, 9, 705886. 10.3389/fbioe.2021.705886. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yang Y.; Dong H.; Zhou H. X. Effects of Cholesterol on the Partitioning of a Drug Molecule in Lipid Bilayers. J. Phys. Chem. B 2021, 125, 5338–5345. 10.1021/acs.jpcb.1c02436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikdar S.; Banerjee M.; Vemparala S. Effect of Cholesterol on the Membrane Partitioning Dynamics of Hepatitis a Virus-2B Peptide. Soft Matter 2021, 17, 7963–7977. 10.1039/D1SM01019K. [DOI] [PubMed] [Google Scholar]
- Orrico F.; Lopez A. C.; Saliwonczyk D.; Acosta C.; Rodriguez-Grecco I.; Mouro-Chanteloup I.; Ostuni M. A.; Denicola A.; Thomson L.; Möller M. N. The Permeability of Human Red Blood Cell Membranes to Hydrogen Peroxide is Independent of Aquaporins. J. Biol. Chem. 2022, 298, 101503. 10.1016/j.jbc.2021.101503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angles G.; Pias S. C. Discerning Membrane Steady-State Oxygen Flux By Monte Carlo Markov Chain Modeling. Adv. Exp. Med. Biol. 2021, 1269, 137–142. 10.1007/978-3-030-48238-1_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.; Dotson R. J.; Angles G.; Pias S. C. Simulation Study of Breast Cancer Lipid Changes Affecting Membrane Oxygen Permeability: Effects of Chain Length and Cholesterol. Adv. Exp. Med. Biol. 2021, 1269, 15–21. 10.1007/978-3-030-48238-1_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson R. J.; McClenahan E.; Pias S. C. Updated Evaluation of Cholesterol’s Influence on Membrane Oxygen Permeability. Adv. Exp. Med. Biol. 2021, 1269, 23–30. 10.1007/978-3-030-48238-1_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michenkova M.; Taki S.; Blosser M. C.; Hwang H. J.; Kowatz T.; Moss F. J.; Occhipinti R.; Qin X.; Sen S.; Shinn E.; et al. Carbon Dioxide Transport Across Membranes. Interface Focus 2021, 11, 20200090. 10.1098/rsfs.2020.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Samir S.; Itel F.; Hegermann J.; Gros G.; Tsiavaliaris G.; Endeward V. O2 Permeability of Lipid Bilayers is Low, But Increases With Membrane Cholesterol. Cell. Mol. Life Sci. 2021, 78, 7649–7662. 10.1007/s00018-021-03974-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek J. M.; Jung W. H.; Yu E. S.; Ahn D. J.; Ryu Y. S. In Vitro Membrane Platform for the Visualization of Water Impermeability Across the Liquid-Ordered Phase Under Hypertonic Conditions. J. Am. Chem. Soc. 2022, 144, 21887–21896. 10.1021/jacs.2c06626. [DOI] [PubMed] [Google Scholar]
- Ghysels A.; Krämer A.; Venable R. M.; Teague W. E.; Lyman E.; Gawrisch K.; Pastor R. W. Permeability of Membranes in the Liquid Ordered and Liquid Disordered Phases. Nat. Commun. 2019, 10, 5616. 10.1038/s41467-019-13432-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr J. A. Lipid Metabolism in Mitochondrial Membranes. J. Inherited Metab. Dis. 2015, 38, 137–144. 10.1007/s10545-014-9748-x. [DOI] [PubMed] [Google Scholar]
- Biochemistry of Lipids, Lipoproteins and Membranes; Ridgway N., McLeod R., Eds.; Elsevier, 2015. [Google Scholar]
- Hölttä-Vuori M.; Salo V. T.; Nyberg L.; Brackmann C.; Enejder A.; Panula P.; Ikonen E. Zebrafish: Gaining Popularity in Lipid Research. Biochem. J. 2010, 429, 235–242. 10.1042/BJ20100293. [DOI] [PubMed] [Google Scholar]
- Lipid Rafts; McIntosh T. J., Ed.; Humana Press: Totowa, NJ, 2010. [Google Scholar]
- Coskun U.; Simons K. Membrane Rafting: From Apical Sorting to Phase Segregation. FEBS Lett. 2010, 584, 1685–1693. 10.1016/j.febslet.2009.12.043. [DOI] [PubMed] [Google Scholar]
- Lingwood D.; Simons K. Lipid Rafts as a Membrane-Organizing Principle. Science 2010, 327, 46–50. 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- Lipid Rafts and Caveolae: From Membrane Biophysics to Cell Biology; Fielding C. J., Ed.; Wiley-VCH, 2006. [Google Scholar]
- Transmembrane Dynamics of Lipids; Devaux P., Herrmann A., Eds.; John Wiley & Sons, 2011. [Google Scholar]
- Chap H. Forty Five Years With Membrane Phospholipids, Phospholipases and Lipid Mediators: A Historical Perspective. Biochimie 2016, 125, 234–249. 10.1016/j.biochi.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Marquardt D.; Geier B.; Pabst G. Asymmetric Lipid Membranes: Towards More Realistic Model Systems. Membranes (Basel, Switz.) 2015, 5, 180–196. 10.3390/membranes5020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G. Dynamic Transbilayer Lipid Asymmetry. Cold Spring Harbor Perspect. Biol. 2011, 3, a004671. 10.1101/cshperspect.a004671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan V. E.; Chu C. T.; Tyurina Y. Y.; Cheikhi A.; Bayir H. Cardiolipin Asymmetry, Oxidation and Signaling. Chem. Phys. Lipids 2014, 179, 64–69. 10.1016/j.chemphyslip.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro J. P.; Oliveira P. J.; Jurado A. S. Mitochondrial Membrane Lipid Remodeling in Pathophysiology: A New Target for Diet and Therapeutic Interventions. Prog. Lipid Res. 2013, 52, 513–528. 10.1016/j.plipres.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Ionova I. V.; Livshits V. A.; Marsh D. Phase Diagram of Ternary Cholesterol/Palmitoylsphingomyelin/Palmitoyloleoyl-Phosphatidylcholine Mixtures: Spin-Label EPR Study of Lipid-Raft Formation. Biophys. J. 2012, 102, 1856–1865. 10.1016/j.bpj.2012.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch S. L.; Keller S. L. Separation of Liquid Phases in Giant Vesicles of Ternary Mixtures of Phospholipids and Cholesterol. Biophys. J. 2003, 85, 3074–3083. 10.1016/S0006-3495(03)74726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberle F. A.; Feigenson G. W. Phase Separation in Lipid Membranes. Cold Spring Harbor Perspect. Biol. 2011, 3, a004630. 10.1101/cshperspect.a004630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K.; Vaz W. L. C. Model Systems, Lipid Rafts, and Cell Membranes. Annu. Rev. Biophys. Biomol. Struct. 2004, 33, 269–295. 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- Johnson D. R.; Decker E. A. The Role of Oxygen in Lipid Oxidation Reactions: A Review. Annu. Rev. Food Sci. Technol. 2015, 6, 171–190. 10.1146/annurev-food-022814-015532. [DOI] [PubMed] [Google Scholar]
- Kittipongpittaya K.; Salcedo L.; McClements D. J.; Decker E. A.. Impact of Delivery Systems on the Chemical Stability of Bioactive Lipids. In Nanotechnology and Functional Foods: Effective Delivery of Bioactive Ingredients; Sabliov C., Chen H., Yada R., Eds.; Wiley-Blackwell: Chichester, UK, 2015; pp 130–141. [Google Scholar]
- Medina-Meza I. G.; Barnaba C.; Barbosa-Cánovas G. V. Effects of High Pressure Processing on Lipid Oxidation: A Review. Innovative Food Sci. Emerging Technol. 2014, 22, 1–10. 10.1016/j.ifset.2013.10.012. [DOI] [Google Scholar]
- Porter N. A. A Perspective on Free Radical Autoxidation: The Physical Organic Chemistry of Polyunsaturated Fatty Acid and Sterol Peroxidation. J. Org. Chem. 2013, 78, 3511–3524. 10.1021/jo4001433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis A.; Spickett C. M. Chemistry of Phospholipid Oxidation. Biochim. Biophys. Acta 2012, 1818, 2374–2387. 10.1016/j.bbamem.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Heurtault B. Physico-Chemical Stability of Colloidal Lipid Particles. Biomaterials 2003, 24, 4283–4300. 10.1016/S0142-9612(03)00331-4. [DOI] [PubMed] [Google Scholar]
- Liposomes, Lipid Bilayers and Model Membranes: From Basic Research to Application; Pabst G., Kučerka N., Nieh M.-P., Katsaras J., Eds.; CRC Press: Boca Raton, FL, 2014. [Google Scholar]
- Mouritsen O. G. Model Answers to Lipid Membrane Questions. Cold Spring Harbor Perspect. Biol. 2011, 3, a004622. 10.1101/cshperspect.a004622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley C. M.; Ryman B. E. The Liposome: From Membrane Model to Therapeutic Agent. Trends Biochem. Sci. 1976, 1, 203–205. 10.1016/S0968-0004(76)80030-8. [DOI] [Google Scholar]
- Sessa G.; Weissmann G. Phospholipid Spherules (Liposomes) as a Model for Biological Membranes. J. Lipid Res. 1968, 9, 310–318. 10.1016/S0022-2275(20)43097-4. [DOI] [PubMed] [Google Scholar]
- Spickett C. M.; Pitt A. R. Oxidative Lipidomics Coming of Age: Advances in Analysis of Oxidized Phospholipids in Physiology and Pathology. Antioxid. Redox Signal. 2015, 22, 1646–1666. 10.1089/ars.2014.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs B.; Bresler K.; Schiller J. Oxidative Changes of Lipids Monitored By MALDI MS. Chem. Phys. Lipids 2011, 164, 782–795. 10.1016/j.chemphyslip.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Adachi J.; Yoshioka N.; Sato M.; Nakagawa K.; Yamamoto Y.; Ueno Y. Detection of Phosphatidylcholine Oxidation Products in Rat Heart Using Quadrupole Time-of-flight Mass Spectrometry. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2005, 823, 37–43. 10.1016/j.jchromb.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Chatterjee S.; Banerjee D. K. Preparation, Isolation, and Characterization of Liposomes Containing Natural and Synthetic Lipids. Methods Mol. Biol. 2002, 199, 3–16. 10.1385/1-59259-175-2:03. [DOI] [PubMed] [Google Scholar]
- Adlercreutz D.; Wehtje E. A Simple HPLC Method for the Simultaneous Analysis of Phosphatidylcholine and Its Partial Hydrolysis Products 1-and 2-Acyl Lysophosphatidylcholine. J. Am. Oil Chem. Soc. 2001, 78, 1007–1011. 10.1007/s11746-001-0379-8. [DOI] [Google Scholar]
- Kates M.Techniques of Lipidology: Isolation, Analysis and Identification of Lipids, 2nd ed.; Burdon R. H., van Knippenberg P. H., Eds.; Elsevier, 1986. [Google Scholar]
- Hollmann A.; Martinez M.; Maturana P.; Semorile L. C.; Maffia P. C. Antimicrobial Peptides: Interaction With Model and Biological Membranes and Synergism With Chemical Antibiotics. Front. Chem. 2018, 6, 204. 10.3389/fchem.2018.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paglia G.; Kliman M.; Claude E.; Geromanos S.; Astarita G. Applications of Ion-Mobility Mass Spectrometry for Lipid Analysis. Anal. Bioanal. Chem. 2015, 407, 4995–5007. 10.1007/s00216-015-8664-8. [DOI] [PubMed] [Google Scholar]
- Zhao Y. Y.; Miao H.; Cheng X. L.; Wei F. Lipidomics: Novel Insight Into the Biochemical Mechanism of Lipid Metabolism and Dysregulation-Associated Disease. Chem.-Biol. Interact. 2015, 240, 220–238. 10.1016/j.cbi.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Gruber F.; Bicker W.; Oskolkova O. V.; Tschachler E.; Bochkov V. N. A Simplified Procedure for Semi-Targeted Lipidomic Analysis of Oxidized Phosphatidylcholines Induced By UVA Irradiation. J. Lipid Res. 2012, 53, 1232–1242. 10.1194/jlr.D025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanksby S. J.; Mitchell T. W. Advances in Mass Spectrometry for Lipidomics. Annu. Rev. Anal. Chem. 2010, 3, 433–465. 10.1146/annurev.anchem.111808.073705. [DOI] [PubMed] [Google Scholar]
- Postle A. D.; Hunt A. N. Dynamic Lipidomics With Stable Isotope Labelling. J. Chromatogr. B 2009, 877, 2716–2721. 10.1016/j.jchromb.2009.03.046. [DOI] [PubMed] [Google Scholar]
- Roberts L. D.; McCombie G.; Titman C. M.; Griffin J. L. A Matter of Fat: An Introduction to Lipidomic Profiling Methods. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2008, 871, 174–181. 10.1016/j.jchromb.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Zhang D.; Duan X.; Shang B.; Hong Y.; Sun H. Analysis of Lipidomics Profile of Rice and Changes During Storage By UPLC-Q-extractive Orbitrap Mass Spectrometry. Food Res. Int. 2021, 142, 110214. 10.1016/j.foodres.2021.110214. [DOI] [PubMed] [Google Scholar]
- Xi Y.; Tu A.; Muddiman D. C. Lipidomic Profiling of Single Mammalian Cells By Infrared Matrix-Assisted Laser Desorption Electrospray Ionization (IR-MALDESI). Anal. Bioanal. Chem. 2020, 412, 8211–8222. 10.1007/s00216-020-02961-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson N. H.; Thomas A.; Chaurand P. Monitoring Time-Dependent Degradation of Phospholipids in Sectioned Tissues By MALDI Imaging Mass Spectrometry. J. Mass Spectrom. 2014, 49, 622–627. 10.1002/jms.3382. [DOI] [PubMed] [Google Scholar]
- Sparvero L. J.; Amoscato A. A.; Kochanek P. M.; Pitt B. R.; Kagan V. E.; Bayir H. Mass-Spectrometry Based Oxidative Lipidomics and Lipid Imaging: Applications in Traumatic Brain Injury. J. Neurochem. 2010, 115, 1322–1336. 10.1111/j.1471-4159.2010.07055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok H. J.; Shin H.; Lee J. W.; Lee G. K.; Suh C. S.; Kim K. P.; Lim H. J. Age-Associated Lipidome Changes in Metaphase II Mouse Oocytes. PLoS One 2016, 11, e0148577 10.1371/journal.pone.0148577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L.; Li M.; Shan Y.; Shen S.; Bai Y.; Liu H. Recent Advances in Lipidomics for Disease Research. J. Sep. Sci. 2016, 39, 38–50. 10.1002/jssc.201500899. [DOI] [PubMed] [Google Scholar]
- Li M.; Yang L.; Bai Y.; Liu H. Analytical Methods in Lipidomics and Their Applications. Anal. Chem. 2014, 86, 161–175. 10.1021/ac403554h. [DOI] [PubMed] [Google Scholar]
- Yang J.; Yang S.; Gao X.; Yuan Y. J. Integrative Investigation of Lipidome and Signal Pathways in Human Endothelial Cells Under Oxidative Stress. Mol. BioSyst. 2011, 7, 2428–2440. 10.1039/c1mb00002k. [DOI] [PubMed] [Google Scholar]
- Sanderson J. M. Far From Inert: Membrane Lipids Possess Intrinsic Reactivity That Has Consequences for Cell Biology. BioEssays 2020, 42, e1900147 10.1002/bies.201900147. [DOI] [PubMed] [Google Scholar]
- Yoon H.; Shaw J. L.; Haigis M. C.; Greka A. Lipid Metabolism in Sickness and in Health: Emerging Regulators of Lipotoxicity. Mol. Cell 2021, 81, 3708–3730. 10.1016/j.molcel.2021.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agmon E.; Stockwell B. R. Lipid Homeostasis and Regulated Cell Death. Curr. Opin. Chem. Biol. 2017, 39, 83–89. 10.1016/j.cbpa.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman H. J. Redox Signaling: An Evolution From Free Radicals to Aging. Free Radicals Biol. Med. 2016, 97, 398–407. 10.1016/j.freeradbiomed.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiurchiù V.; Maccarrone M. Bioactive Lipids as Modulators of Immunity, Inflammation and Emotions. Curr. Opin. Pharmacol. 2016, 29, 54–62. 10.1016/j.coph.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Yang W. S.; Stockwell B. R. Ferroptosis: Death By Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb C. A.; Cole M. P. Oxidative and Nitrative Stress in Neurodegeneration. Neurobiol. Dis. 2015, 84, 4–21. 10.1016/j.nbd.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee S. Membrane Lipid Peroxidation and Its Conflict of Interest: The Two Faces of Oxidative Stress. Curr. Sci. 2014, 107, 1811–1823. [Google Scholar]
- Volinsky R.; Kinnunen P. K. Oxidized Phosphatidylcholines in Membrane-Level Cellular Signaling: From Biophysics to Physiology and Molecular Pathology. FEBS J. 2013, 280, 2806–2816. 10.1111/febs.12247. [DOI] [PubMed] [Google Scholar]
- Piñeiro R.; Falasca M. Lysophosphatidylinositol Signalling: New Wine From an Old Bottle. Biochim. Biophys. Acta 2012, 1821, 694–705. 10.1016/j.bbalip.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Niki E. Lipid Peroxidation: Physiological Levels and Dual Biological Effects. Free Radicals Biol. Med. 2009, 47, 469–484. 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- Checa J.; Aran J. M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflammation Res. 2020, 13, 1057–1073. 10.2147/JIR.S275595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Meo S.; Reed T. T.; Venditti P.; Victor V. M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longevity 2016, 2016, 1245049. 10.1155/2016/1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. P. How Mitochondria Produce Reactive Oxygen Species. Biochem. J. 2009, 417, 1–13. 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt U.; Sharif O.; Martins R.; Knapp S. Accumulating Evidence for a Role of Oxidized Phospholipids in Infectious Diseases. Cell. Mol. Life Sci. 2015, 72, 1059–1071. 10.1007/s00018-014-1780-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew H.; Solomon V. A.; Fonteh A. N. Involvement of Lipids in Alzheimer’s Disease Pathology and Potential Therapies. Front. Physiol. 2020, 11, 598. 10.3389/fphys.2020.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naudí A.; Cabré R.; Jové M.; Ayala V.; Gonzalo H.; Portero-Otín M.; Ferrer I.; Pamplona R. Lipidomics of Human Brain Aging and Alzheimer’s Disease Pathology. Int. Rev. Neurobiol. 2015, 122, 133–189. 10.1016/bs.irn.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Kim G. H.; Kim J. E.; Rhie S. J.; Yoon S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. 10.5607/en.2015.24.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamba P.; Testa G.; Gargiulo S.; Staurenghi E.; Poli G.; Leonarduzzi G. Oxidized Cholesterol as the Driving Force Behind the Development of Alzheimer’s Disease. Front. Aging Neurosci. 2015, 7, 119. 10.3389/fnagi.2015.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun S.; Datta S.; Wang L.; Pegany R.; Cano M.; Handa J. T. The Impact of Lipids, Lipid Oxidation, and Inflammation on AMD, and the Potential Role of miRNAs on Lipid Metabolism in the RPE. Exp. Eye Res. 2019, 181, 346–355. 10.1016/j.exer.2018.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xicoy H.; Wieringa B.; Martens G. J. M. The Role of Lipids in Parkinson’s Disease. Cells 2019, 8, 27. 10.3390/cells8010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiteller G. Peroxyl Radicals: Inductors of Neurodegenerative and Other Inflammatory Diseases. Their Origin and How They Transform Cholesterol, Phospholipids, Plasmalogens, Polyunsaturated Fatty Acids, Sugars, and Proteins Into Deleterious Products. Free Radicals Biol. Med. 2006, 41, 362–387. 10.1016/j.freeradbiomed.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Ito F.; Sono Y.; Ito T. Measurement and Clinical Significance of Lipid Peroxidation as a Biomarker of Oxidative Stress: Oxidative Stress in Diabetes, Atherosclerosis, and Chronic Inflammation. Antioxidants 2019, 8, 72. 10.3390/antiox8030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon A. B. Beyond Diabetes: Does Obesity-Induced Oxidative Stress Drive the Aging Process. Antioxidants 2016, 5, 24. 10.3390/antiox5030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakiet A.; Kobiela J.; Stepnowski P.; Sledzinski T.; Mika A. Changes in Lipids Composition and Metabolism in Colorectal Cancer: A Review. Lipids Health Dis. 2019, 18, 29. 10.1186/s12944-019-0977-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S.; Li L.; Shen X.; Li Q.; Xu W.; Wang X.; Tao Y.; Yin H. An Update on Lipid Oxidation and Inflammation in Cardiovascular Diseases. Free Radicals Biol. Med. 2019, 144, 266–278. 10.1016/j.freeradbiomed.2019.03.036. [DOI] [PubMed] [Google Scholar]
- Xu L.; Porter N. A. Free Radical Oxidation of Cholesterol and Its Precursors: Implications in Cholesterol Biosynthesis Disorders. Free Radical Res. 2015, 49, 835–849. 10.3109/10715762.2014.985219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiecien S.; Jasnos K.; Magierowski M.; Sliwowski Z.; Pajdo R.; Brzozowski B.; Mach T.; Wojcik D.; Brzozowski T. Lipid Peroxidation, Reactive Oxygen Species and Antioxidative Factors in the Pathogenesis of Gastric Mucosal Lesions and Mechanism of Protection Against Oxidative Stress - Induced Gastric Injury. J. Physiol. Pharmacol. 2014, 65, 613–622. [PubMed] [Google Scholar]
- Anthonymuthu T. S.; Kenny E. M.; Lamade A. M.; Kagan V. E.; Bayır H. Oxidized Phospholipid Signaling in Traumatic Brain Injury. Free Radicals Biol. Med. 2018, 124, 493–503. 10.1016/j.freeradbiomed.2018.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearn M. L.; Niesman I. R.; Egawa J.; Sawada A.; Almenar-Queralt A.; Shah S. B.; Duckworth J. L.; Head B. P. Pathophysiology Associated With Traumatic Brain Injury: Current Treatments and Potential Novel Therapeutics. Cell. Mol. Neurobiol. 2017, 37, 571–585. 10.1007/s10571-016-0400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthonymuthu T. S.; Kenny E. M.; Bayır H. Therapies Targeting Lipid Peroxidation in Traumatic Brain Injury. Brain Res. 2016, 1640, 57–76. 10.1016/j.brainres.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng S. C.; Maier C. S. Label-Free Proteomics Assisted By Affinity Enrichment for Elucidating the Chemical Reactivity of the Liver Mitochondrial Proteome Toward Adduction By the Lipid Electrophile 4-Hydroxy-2-nonenal (HNE). Front. Chem. 2016, 4, 2. 10.3389/fchem.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldini G.; Domingues M. R.; Spickett C. M.; Domingues P.; Altomare A.; Sánchez-Gómez F. J.; Oeste C. L.; Pérez-Sala D. Protein Lipoxidation: Detection Strategies and Challenges. Redox Biol. 2015, 5, 253–266. 10.1016/j.redox.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera G.; Pizzimenti S.; Ciamporcero E. S.; Daga M.; Ullio C.; Arcaro A.; Cetrangolo G. P.; Ferretti C.; Dianzani C.; Lepore A.; et al. Role of 4-Hydroxynonenal-protein Adducts in Human Diseases. Antioxid. Redox Signal. 2015, 22, 1681–1702. 10.1089/ars.2014.6166. [DOI] [PubMed] [Google Scholar]
- Vasil’ev Y. V.; Tzeng S.-C.; Huang L.; Maier C. S. Protein Modifications By Electrophilic Lipoxidation Products: Adduct Formation, Chemical Strategies and Tandem Mass Spectrometry for Their Detection and Identification. Mass Spectrom. Rev. 2014, 33, 157–182. 10.1002/mas.21389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullery J. C.; Marnett L. J. Protein Modification By Oxidized Phospholipids and Hydrolytically Released Lipid Electrophiles: Investigating Cellular Responses. Biochim. Biophys. Acta 2012, 1818, 2424–2435. 10.1016/j.bbamem.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmer U.; Hermetter A. Protein Modification By Aldehydophospholipids and Its Functional Consequences. Biochim. Biophys. Acta 2012, 1818, 2436–2445. 10.1016/j.bbamem.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotegher N.; Bubacco L. Lysines, Achilles’ Heel in Alpha-Synuclein Conversion to a Deadly Neuronal Endotoxin. Ageing Res. Rev. 2016, 26, 62–71. 10.1016/j.arr.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Kafoury R. M.; Pryor W. A.; Squadrito G. L.; Salgo M. G.; Zou X.; Friedman M. Lipid Ozonation Products Activate Phospholipases A2, C, and D. Toxicol. Appl. Pharmacol. 1998, 150, 338–349. 10.1006/taap.1998.8418. [DOI] [PubMed] [Google Scholar]
- Salgo M. G.; Squadrito G. L.; Pryor W. A. Activation of Phospholipase A2 in 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine Liposomes Containing Lipid Ozonation Products. Chem. Res. Toxicol. 1994, 7, 458–462. 10.1021/tx00039a026. [DOI] [PubMed] [Google Scholar]
- Fujimori Y.; Kudo I.; Fujita K.; Inoue K. Characteristics of Lysophospholipase Activity Expressed By Cytosolic Phospholipase A2. Eur. J. Biochem. 1993, 218, 629–635. 10.1111/j.1432-1033.1993.tb18416.x. [DOI] [PubMed] [Google Scholar]
- Kinghorn K. J.; Castillo-Quan J. I.; Bartolome F.; Angelova P. R.; Li L.; Pope S.; Cochemé H. M.; Khan S.; Asghari S.; Bhatia K. P.; et al. Loss of PLA2G6 Leads to Elevated Mitochondrial Lipid Peroxidation and Mitochondrial Dysfunction. Brain 2015, 138, 1801–1816. 10.1093/brain/awv132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienias K.; Fiedorowicz A.; Sadowska A.; Prokopiuk S.; Car H. Regulation of Sphingomyelin Metabolism. Pharmacol. Rep. 2016, 68, 570–581. 10.1016/j.pharep.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Adada M.; Luberto C.; Canals D. Inhibitors of the Sphingomyelin Cycle: Sphingomyelin Synthases and Sphingomyelinases. Chem. Phys. Lipids 2016, 197, 45–59. 10.1016/j.chemphyslip.2015.07.008. [DOI] [PubMed] [Google Scholar]
- de Faria Poloni J.; Chapola H.; Feltes B. C.; Bonatto D. The Importance of Sphingolipids and Reactive Oxygen Species in Cardiovascular Development. Biol. Cell 2014, 106, 167–181. 10.1111/boc.201400008. [DOI] [PubMed] [Google Scholar]
- Alessenko A. V.; Bugrova A. E.; Dudnik L. B. Connection of Lipid Peroxide Oxidation With the Sphingomyelin Pathway in the Development of Alzheimer’s Disease. Biochem. Soc. Trans. 2004, 32, 144–146. 10.1042/bst0320144. [DOI] [PubMed] [Google Scholar]
- Arish M.; Husein A.; Kashif M.; Saleem M.; Akhter Y.; Rub A. Sphingosine-1-phosphate Signaling: Unraveling Its Role as a Drug Target Against Infectious Diseases. Drug Discovery Today 2016, 21, 133–142. 10.1016/j.drudis.2015.09.013. [DOI] [PubMed] [Google Scholar]
- Alessenko A.V.; Shupik M.A.; Gutner U.A.; Bugrova A.E.; Dudnik L.B.; Shingarova L.N.; Mikoyan A.; Vanin A.F. The Relation Between Sphingomyelinase Activity, Lipid Peroxide Oxidation and NO-Releasing in Mice Liver and Brain. FEBS Lett. 2005, 579, 5571–5576. 10.1016/j.febslet.2005.08.085. [DOI] [PubMed] [Google Scholar]
- Silva L. C.; Futerman A. H.; Prieto M. Lipid Raft Composition Modulates Sphingomyelinase Activity and Ceramide-Induced Membrane Physical Alterations. Biophys. J. 2009, 96, 3210–3222. 10.1016/j.bpj.2008.12.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daraee H.; Etemadi A.; Kouhi M.; Alimirzalu S.; Akbarzadeh A. Application of Liposomes in Medicine and Drug Delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. 10.3109/21691401.2014.953633. [DOI] [PubMed] [Google Scholar]
- Pattni B. S.; Chupin V. V.; Torchilin V. P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. 10.1021/acs.chemrev.5b00046. [DOI] [PubMed] [Google Scholar]
- de Araújo D. R.; da Silva D. C.; Barbosa R. M.; Franz-Montan M.; Cereda C. M.; Padula C.; Santi P.; de Paula E. Strategies for Delivering Local Anesthetics to the Skin: Focus on Liposomes, Solid Lipid Nanoparticles, Hydrogels and Patches. Expert Opin. Drug Delivery 2013, 10, 1551–1563. 10.1517/17425247.2013.828031. [DOI] [PubMed] [Google Scholar]
- Maherani B.; Arab-Tehrany E.; Mozafari M. R.; Gaiani C.; Linder M. Liposomes: A Review of Manufacturing Techniques and Targeting Strategies. Curr. Nanosci. 2011, 7, 436–452. 10.2174/157341311795542453. [DOI] [Google Scholar]
- Jahadi M.; Khosravi-Darani K. Liposomal Encapsulation Enzymes: From Medical Applications to Kinetic Characteristics. Mini-Rev. Med. Chem. 2017, 17, 366–370. 10.2174/1389557516666160801111507. [DOI] [PubMed] [Google Scholar]
- Ng H. L.; Lu A.; Lin G.; Qin L.; Yang Z. The Potential of Liposomes With Carbonic Anhydrase IX to Deliver Anticancer Ingredients to Cancer Cells in Vivo. Int. J. Mol. Sci. 2015, 16, 230–255. 10.3390/ijms16010230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenchov R.; Bird R.; Curtze A. E.; Zhou Q. Lipid Nanoparticles–from Liposomes to Mrna Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. 10.1021/acsnano.1c04996. [DOI] [PubMed] [Google Scholar]
- Movahedi F.; Hu R. G.; Becker D. L.; Xu C. Stimuli-Responsive Liposomes for the Delivery of Nucleic Acid Therapeutics. Nanomedicine 2015, 11, 1575–1584. 10.1016/j.nano.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Nogueira E.; Gomes A. C.; Preto A.; Cavaco-Paulo A. Design of Liposomal Formulations for Cell Targeting. Colloids Surf., B 2015, 136, 514–526. 10.1016/j.colsurfb.2015.09.034. [DOI] [PubMed] [Google Scholar]
- Marqués-Gallego P.; de Kroon A. I. Ligation Strategies for Targeting Liposomal Nanocarriers. Biomed. Res. Int. 2014, 2014, 129458. 10.1155/2014/129458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble G. T.; Stefanick J. F.; Ashley J. D.; Kiziltepe T.; Bilgicer B. Ligand-Targeted Liposome Design: Challenges and Fundamental Considerations. Trends Biotechnol. 2014, 32, 32–45. 10.1016/j.tibtech.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Musacchio T.; Torchilin V. P. Recent Developments in Lipid-Based Pharmaceutical Nanocarriers. Front. Biosci. 2011, 16, 1388–1412. 10.2741/3795. [DOI] [PubMed] [Google Scholar]
- Lim S. B.; Banerjee A.; Önyüksel H. Improvement of Drug Safety By the Use of Lipid-Based Nanocarriers. J. Controlled Release 2012, 163, 34–45. 10.1016/j.jconrel.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Pharmaceutical Stress Testing: Predicting Drug Degradation; Baertschi S. W., Alsante K. M., Reed R. A., Eds.; Informa Healthcare: London, UK, 2011. [Google Scholar]
- Lutz J.; Augustin A. J.; Jäger L. J.; Bachmann D.; Brandl M. Acute Toxicity and Depression of Phagocytosis in Vivo By Liposomes: Influence of Lysophosphatidylcholine. Life Sci. 1994, 56, 99–106. 10.1016/0024-3205(94)00930-9. [DOI] [PubMed] [Google Scholar]
- Tereshkina Y. A.; Torkhovskaya T. I.; Tikhonova E. G.; Kostryukova L. V.; Sanzhakov M. A.; Korotkevich E. I.; Khudoklinova Y. Y.; Orlova N. A.; Kolesanova E. F. Nanoliposomes as Drug Delivery Systems: Safety Concerns. J. Drug Targeting 2022, 30, 313–325. 10.1080/1061186X.2021.1992630. [DOI] [PubMed] [Google Scholar]
- Frahm G. E.; Cameron B. E.; Smith J. C.; Johnston M. J. W. Generation of Fatty Acids From 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine/cardiolipin Liposomes That Stabilize Recombinant Human Serum Albumin. J. Liposome Res. 2013, 23, 101–109. 10.3109/08982104.2012.747535. [DOI] [PubMed] [Google Scholar]
- Nag O. K.; Awasthi V. Surface Engineering of Liposomes for Stealth Behavior. Pharmaceutics 2013, 5, 542–569. 10.3390/pharmaceutics5040542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stealth Liposomes; Lasic D., Martin F., Eds.; CRC Press: Boca Raton, FL, 1995. [Google Scholar]
- Naseri N.; Valizadeh H.; Zakeri-Milani P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. 10.15171/apb.2015.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S.; Baboota S.; Ali J.; Khan S.; Narang R. S.; Narang J. K. Nanostructured Lipid Carriers: An Emerging Platform for Improving Oral Bioavailability of Lipophilic Drugs. Int. J. Pharm. Invest. 2015, 5, 182–191. 10.4103/2230-973X.167661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon A. M.; Casey D.; Law R. V.; Gee A.; Templer R. H.; Ces O. Drug Interactions With Lipid Membranes. Chem. Soc. Rev. 2009, 38, 2509–2519. 10.1039/b813853m. [DOI] [PubMed] [Google Scholar]
- Peetla C.; Stine A.; Labhasetwar V. Biophysical Interactions With Model Lipid Membranes: Applications in Drug Discovery and Drug Delivery. Mol. Pharmaceutics 2009, 6, 1264–1276. 10.1021/mp9000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanniri E.; Bagheripoor-Fallah N.; Sohrabvandi S.; Mortazavian A. M.; Khosravi-Darani K.; Mohammad R. Application of Liposomes in Some Dairy Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 484–493. 10.1080/10408398.2013.779571. [DOI] [PubMed] [Google Scholar]
- Borel T.; Sabliov C. M. Nanodelivery of Bioactive Components for Food Applications: Types of Delivery Systems, Properties, and Their Effect on ADME Profiles and Toxicity of Nanoparticles. Annu. Rev. Food Sci. Technol. 2014, 5, 197–213. 10.1146/annurev-food-030713-092354. [DOI] [PubMed] [Google Scholar]
- Reza Mozafari M.; Johnson C.; Hatziantoniou S.; Demetzos C. Nanoliposomes and Their Applications in Food Nanotechnology. J. Liposome Res. 2008, 18, 309–327. 10.1080/08982100802465941. [DOI] [PubMed] [Google Scholar]
- Taylor T. M.; Davidson P. M.; Bruce B. D.; Weiss J. Liposomal Nanocapsules in Food Science and Agriculture. Crit. Rev. Food Sci. Nutr. 2005, 45, 587–605. 10.1080/10408390591001135. [DOI] [PubMed] [Google Scholar]
- Liu W.; Ye A.; Liu W.; Liu C.; Han J.; Singh H. Behaviour of Liposomes Loaded With Bovine Serum Albumin During in Vitro Digestion. Food Chem. 2015, 175, 16–24. 10.1016/j.foodchem.2014.11.108. [DOI] [PubMed] [Google Scholar]
- Bernardi D. M.; Bertol T. M.; Pflanzer S. B.; Sgarbieri V. C.; Pollonio M. A. Ω-3 in Meat Products: Benefits and Effects on Lipid Oxidative Stability. J. Sci. Food. Agric. 2016, 96, 2620–2634. 10.1002/jsfa.7559. [DOI] [PubMed] [Google Scholar]
- Edris A. E. Formulation and Shelf Life Stability of Water-Borne Lecithin Nanoparticles for Potential Application in Dietary Supplements Field. J. Diet. Suppl. 2012, 9, 211–222. 10.3109/19390211.2012.708717. [DOI] [PubMed] [Google Scholar]
- Henna Lu F. S.; Nielsen N. S.; Timm-Heinrich M.; Jacobsen C. Oxidative Stability of Marine Phospholipids in the Liposomal Form and Their Applications. Lipids 2011, 46, 3–23. 10.1007/s11745-010-3496-y. [DOI] [PubMed] [Google Scholar]
- Shahidi F.; Zhong Y. Lipid Oxidation and Improving the Oxidative Stability. Chem. Soc. Rev. 2010, 39, 4067–4079. 10.1039/b922183m. [DOI] [PubMed] [Google Scholar]
- Van Tran V.; Moon J. Y.; Lee Y. C. Liposomes for Delivery of Antioxidants in Cosmeceuticals: Challenges and Development Strategies. J. Controlled Release 2019, 300, 114–140. 10.1016/j.jconrel.2019.03.003. [DOI] [PubMed] [Google Scholar]
- Chou T.-H. Current Application of Lipid- and Surfactant-Based Vesicles for Cosmeceuticals: A Review. Curr. Pharm. Biotechnol. 2015, 16, 1035–1044. 10.2174/1389201016666150907113849. [DOI] [PubMed] [Google Scholar]
- Mu L.; Sprando R. L. Application of Nanotechnology in Cosmetics. Pharm. Res. 2010, 27, 1746–1749. 10.1007/s11095-010-0139-1. [DOI] [PubMed] [Google Scholar]
- Delivery System Handbook for Personal Care and Cosmetic Products: Technology, Applications, and Formulations; Rosen M. R., Ed.; William Andrew Publishing: Norwich, N.Y., 2005. [Google Scholar]
- Zhan C.; Santamaria C. M.; Wang W.; McAlvin J. B.; Kohane D. S. Long-Acting Liposomal Corneal Anesthetics. Biomaterials 2018, 181, 372–377. 10.1016/j.biomaterials.2018.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragagni M.; Mennini N.; Maestrelli F.; Cirri M.; Mura P. Comparative Study of Liposomes, Transfersomes and Ethosomes as Carriers for Improving Topical Delivery of Celecoxib. Drug Delivery 2012, 19, 354–361. 10.3109/10717544.2012.724472. [DOI] [PubMed] [Google Scholar]
- Souto E. B.; Müller R. H. Cosmetic Features and Applications of Lipid Nanoparticles (SLN, NLC). Int. J. Cosmet. Sci. 2008, 30, 157–165. 10.1111/j.1468-2494.2008.00433.x. [DOI] [PubMed] [Google Scholar]
- Betz G.; Aeppli A.; Menshutina N.; Leuenberger H. In Vivo Comparison of Various Liposome Formulations for Cosmetic Application. Int. J. Pharm. 2005, 296, 44–54. 10.1016/j.ijpharm.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Schuhmann K.; Srzentić K.; Nagornov K. O.; Thomas H.; Gutmann T.; Coskun D. C.; Tsybin Y. O.; Shevchenko A. Monitoring Membrane Lipidome Turnover By Metabolic 15N Labeling and Shotgun Ultra-High-Resolution Orbitrap Fourier Transform Mass Spectrometry. Anal. Chem. 2017, 89, 12857–12865. 10.1021/acs.analchem.7b03437. [DOI] [PubMed] [Google Scholar]
- Han X.; Gross R. W. Shotgun Lipidomics: Electrospray Ionization Mass Spectrometric Analysis and Quantitation of Cellular Lipidomes Directly From Crude Extracts of Biological Samples. Mass Spectrom. Rev. 2005, 24, 367–412. 10.1002/mas.20023. [DOI] [PubMed] [Google Scholar]
- Hsu F. F. Mass Spectrometry-Based Shotgun Lipidomics - a Critical Review From the Technical Point of View. Anal. Bioanal. Chem. 2018, 410, 6387–6409. 10.1007/s00216-018-1252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filkin S. Y.; Lipkin A. V.; Fedorov A. N. Phospholipase Superfamily: Structure, Functions, and Biotechnological Applications. Biochemistry (Moscow) 2020, 85, 177–195. 10.1134/S0006297920140096. [DOI] [PubMed] [Google Scholar]
- Cerminati S.; Paoletti L.; Aguirre A.; Peirú S.; Menzella H. G.; Castelli M. E. Industrial Uses of Phospholipases: Current State and Future Applications. Appl. Microbiol. Biotechnol. 2019, 103, 2571–2582. 10.1007/s00253-019-09658-6. [DOI] [PubMed] [Google Scholar]
- Ramrakhiani L.; Chand S. Recent Progress on Phospholipases: Different Sources, Assay Methods, Industrial Potential and Pathogenicity. Appl. Biochem. Biotechnol. 2011, 164, 991–1022. 10.1007/s12010-011-9190-6. [DOI] [PubMed] [Google Scholar]
- Loo R. W.; Conde-Frieboes K.; Reynolds L. J.; Dennis E. A. Activation, Inhibition, and Regiospecificity of the Lysophospholipase Activity of the 85-kDa Group IV Cytosolic Phospholipase A2. J. Biol. Chem. 1997, 272, 19214–19219. 10.1074/jbc.272.31.19214. [DOI] [PubMed] [Google Scholar]
- Nelson R. K.; Frohman M. A. Thematic Review Series: Phospholipases: Central Role in Lipid Signaling and Disease Physiological and Pathophysiological Roles for Phospholipase D. J. Lipid Res. 2015, 56, 2229–2237. 10.1194/jlr.R059220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindou H.; Hishikawa D.; Harayama T.; Eto M.; Shimizu T. Generation of Membrane Diversity By Lysophospholipid Acyltransferases. J. Biochem. 2013, 154, 21–28. 10.1093/jb/mvt048. [DOI] [PubMed] [Google Scholar]
- Shohet S. B. The Apparent Transfer of Fatty Acid From Phosphatidylcholine to Phosphatidylethanolamine in Human Erythrocytes. J. Lipid Res. 1971, 12, 139–142. 10.1016/S0022-2275(20)39522-5. [DOI] [PubMed] [Google Scholar]
- Malhotra A.; Xu Y.; Ren M.; Schlame M. Formation of Molecular Species of Mitochondrial Cardiolipin. 1. A Novel Transacylation Mechanism to Shuttle Fatty Acids Between sn-1 and sn-2 Positions of Multiple Phospholipid Species. Biochim. Biophys. Acta 2009, 1791, 314–320. 10.1016/j.bbalip.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moessinger C.; Klizaite K.; Steinhagen A.; Philippou-Massier J.; Shevchenko A.; Hoch M.; Ejsing C. S.; Thiele C. Two Different Pathways of Phosphatidylcholine Synthesis, the Kennedy Pathway and the Lands Cycle, Differentially Regulate Cellular Triacylglycerol Storage. BMC Cell Biol. 2014, 15, 43. 10.1186/s12860-014-0043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S.; Castillo C.; Stevens T. Phospholipid Remodeling/generation in Giardia: The Role of the Lands Cycle. Trends Parasitol. 2001, 17, 316–319. 10.1016/S1471-4922(01)01901-8. [DOI] [PubMed] [Google Scholar]
- Arouri A.; Mouritsen O. G. Membrane-Perturbing Effect of Fatty Acids and Lysolipids. Prog. Lipid Res. 2013, 52, 130–140. 10.1016/j.plipres.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Muranushi N.; Takagi N.; Muranishi S.; Sezaki H. Effect of Fatty Acids and Monoglycerides on Permeability of Lipid Bilayer. Chem. Phys. Lipids 1981, 28, 269–279. 10.1016/0009-3084(81)90013-X. [DOI] [PubMed] [Google Scholar]
- Leidy C.; Ocampo J.; Duelund L.; Mouritsen O. G.; Jørgensen K.; Peters G. H. Membrane Restructuring By Phospholipase A2 is Regulated By the Presence of Lipid Domains. Biophys. J. 2011, 101, 90–99. 10.1016/j.bpj.2011.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen J. R.; Andresen T. L.; Feldborg L. N.; Duelund L.; Ipsen J. H. Understanding Detergent Effects on Lipid Membranes: A Model Study of Lysolipids. Biophys. J. 2010, 98, 2199–2205. 10.1016/j.bpj.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corda D.; Iurisci C.; Berrie C. P. Biological Activities and Metabolism of the Lysophosphoinositides and Glycerophosphoinositols. Biochim. Biophys. Acta 2002, 1582, 52–69. 10.1016/S1388-1981(02)00137-3. [DOI] [PubMed] [Google Scholar]
- Dinkla S.; van Eijk L. T.; Fuchs B.; Schiller J.; Joosten I.; Brock R.; Pickkers P.; Bosman G. J. Inflammation-Associated Changes in Lipid Composition and the Organization of the Erythrocyte Membrane. BBA Clin. 2016, 5, 186–192. 10.1016/j.bbacli.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Caterina R. n-3 Fatty Acids and the Inflammatory Response - Biological Background. Eur. Heart J. Suppl. 2001, 3, D42–D49. 10.1016/S1520-765X(01)90118-X. [DOI] [Google Scholar]
- Tilley S. L.; Coffman T. M.; Koller B. H. Mixed Messages: Modulation of Inflammation and Immune Responses By Prostaglandins and Thromboxanes. J. Clin. Invest. 2001, 108, 15–23. 10.1172/JCI200113416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal C. E.; Newton A. C. Tuning the Signalling Output of Protein Kinase C. Biochem. Soc. Trans. 2014, 42, 1477–1483. 10.1042/BST20140172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marnell L.; Mold C.; Du Clos T. W. C-Reactive Protein: Ligands, Receptors and Role in Inflammation. Clin. Immunol. 2005, 117, 104–111. 10.1016/j.clim.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Livermore T. M.; Azevedo C.; Kolozsvari B.; Wilson M. S.; Saiardi A. Phosphate, Inositol and Polyphosphates. Biochem. Soc. Trans. 2016, 44, 253–259. 10.1042/BST20150215. [DOI] [PubMed] [Google Scholar]
- Singh A.; Bhatnagar N.; Pandey A.; Pandey G. K. Plant Phospholipase C Family: Regulation and Functional Role in Lipid Signaling. Cell Calcium 2015, 58, 139–146. 10.1016/j.ceca.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Liang D.; Wu K.; Tei R.; Bumpus T. W.; Ye J.; Baskin J. M. A Real-Time, Click Chemistry Imaging Approach Reveals Stimulus-Specific Subcellular Locations of Phospholipase D Activity. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 15453–15462. 10.1073/pnas.1903949116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S.; Yao S.; Wang G.; Guo L.; Zhou Y.; Hong Y.; Wang X. Phospholipase Dε Enhances Braasca napus Growth and Seed Production in Response to Nitrogen Availability. Plant Biotechnol. J. 2016, 14, 926–937. 10.1111/pbi.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. A.; Salloum D.; Menon D.; Frias M. A. Phospholipase D and the Maintenance of Phosphatidic Acid Levels for Regulation of Mammalian Target of Rapamycin (mTOR). J. Biol. Chem. 2014, 289, 22583–22588. 10.1074/jbc.R114.566091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. A.; Pawelchak J. Effect of pH, Ionic Strength and Oxygen Burden on the Chemical Stability of EPC/cholesterol Liposomes Under Accelerated Conditions. Part 1: Lipid Hydrolysis. Eur. J. Pharm. Biopharm. 2000, 50, 357–364. 10.1016/S0939-6411(00)00127-2. [DOI] [PubMed] [Google Scholar]
- Zuidam N. J.; Crommelin D. J. A. Chemical Hydrolysis of Phospholipids. J. Pharm. Sci. 1995, 84, 1113–1119. 10.1002/jps.2600840915. [DOI] [PubMed] [Google Scholar]
- Grit M.; Crommelin D. J. The Effect of Aging on the Physical Stability of Liposome Dispersions. Chem. Phys. Lipids 1992, 62, 113–122. 10.1016/0009-3084(92)90089-8. [DOI] [PubMed] [Google Scholar]
- Zuidam N. J.; Lee S. S.; Crommelin D. J. Sterilization of Liposomes By Heat Treatment. Pharm. Res. 1993, 10, 1591–1596. 10.1023/A:1018916518515. [DOI] [PubMed] [Google Scholar]
- Grit M.; Zuidam N. J.; Underberg W. J. M.; Crommelin D. J. A. Hydrolysis of Partially Saturated Egg Phosphatidylcholine in Aqueous Liposome Dispersions and the Effect of Cholesterol Incorporation on Hydrolysis Kinetics. J. Pharm. Pharmacol. 2011, 45, 490–495. 10.1111/j.2042-7158.1993.tb05585.x. [DOI] [PubMed] [Google Scholar]
- Grit M.; de Smidt J. H.; Struijke A.; Crommelin D. J. A. Hydrolysis of Phosphatidylcholine in Aqueous Liposome Dispersions. Int. J. Pharm. 1989, 50, 1–6. 10.1016/0378-5173(89)90173-7. [DOI] [Google Scholar]
- Crommelin D. J. A. Influence of Lipid Composition and Ionic Strength on the Physical Stability of Liposomes. J. Pharm. Sci. 1984, 73, 1559–1563. 10.1002/jps.2600731118. [DOI] [PubMed] [Google Scholar]
- Saetern A. M.; Skar M.; Braaten A.; Brandl M. Camptothecin-Catalyzed Phospholipid Hydrolysis in Liposomes. Int. J. Pharm. 2005, 288, 73–80. 10.1016/j.ijpharm.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Vernooij E. A.; Kettenes-van den Bosch J. J.; Underberg W. J.; Crommelin D. J. Chemical Hydrolysis of DOTAP and DOPE in a Liposomal Environment. J. Controlled Release 2002, 79, 299–303. 10.1016/S0168-3659(01)00534-X. [DOI] [PubMed] [Google Scholar]
- Grit M.; Crommelin D. J. Chemical Stability of Liposomes: Implications for Their Physical Stability. Chem. Phys. Lipids 1993, 64, 3–18. 10.1016/0009-3084(93)90053-6. [DOI] [PubMed] [Google Scholar]
- Guo X.; Andrew MacKay J.; Szoka F. C. Mechanism of pH-Triggered Collapse of Phosphatidylethanolamine Liposomes Stabilized By an Ortho Ester Polyethyleneglycol Lipid. Biophys. J. 2003, 84, 1784–1795. 10.1016/S0006-3495(03)74986-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ickenstein L. M.; Sandström M. C.; Mayer L. D.; Edwards K. Effects of Phospholipid Hydrolysis on the Aggregate Structure in DPPC/DSPE-PEG2000 Liposome Preparations After Gel to Liquid Crystalline Phase Transition. Biochim. Biophys. Acta 2006, 1758, 171–180. 10.1016/j.bbamem.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Schnorenberg M. R.; Yoo S. P.; Tirrell M. V.; LaBelle J. L. Synthesis and Purification of Homogeneous Lipid-Based Peptide Nanocarriers By Overcoming Phospholipid Ester Hydrolysis. ACS Omega 2018, 3, 14144–14150. 10.1021/acsomega.8b01772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werling J.; Graham S.; Owen H.; Nair L.; Gonyon T.; Carter P. W. Physicochemical Stability of Phospholipid-Dispersed Suspensions of Crystalline Itraconazole. Eur. J. Pharm. Biopharm. 2008, 69, 1104–1113. 10.1016/j.ejpb.2008.01.030. [DOI] [PubMed] [Google Scholar]
- Plückthun A.; Dennis E. A. Acyl and Phosphoryl Migration in Lysophospholipids: Importance in Phospholipid Synthesis and Phospholipase Specificity. Biochemistry 1982, 21, 1743–1750. 10.1021/bi00537a007. [DOI] [PubMed] [Google Scholar]
- Sugasini D.; Subbaiah P. V. Rate of Acyl Migration in Lysophosphatidylcholine (LPC) is Dependent Upon the Nature of the Acyl Group. Greater Stability of sn-2 Docosahexaenoyl LPC Compared to the More Saturated LPC Species. PLoS One 2017, 12, e0187826 10.1371/journal.pone.0187826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K. K.-C.; Nozaki K.; Wong C.-H. Problems of Acyl Migration in Lipase-Catalyzed Enantioselecttve Transformation of Meso-1,3-diol Systems. Biocatalysis 1990, 3, 169–177. 10.3109/10242429008992059. [DOI] [Google Scholar]
- Moncelli M. R.; Becucci L.; Guidelli R. The Intrinsic pKa Values for Phosphatidylcholine, Phosphatidylethanolamine, and Phosphatidylserine in Monolayers Deposited on Mercury Electrodes. Biophys. J. 1994, 66, 1969–1980. 10.1016/S0006-3495(94)80990-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich-Guilatt L.; Dubernet C.; Gaudin K.; Lambert G.; Couvreur P.; Chaminade P. Phospholipid Hydrolysis in a Pharmaceutical Emulsion Assessed By Physicochemical Parameters and a New Analytical Method. Eur. J. Pharm. Biopharm. 2005, 61, 69–76. 10.1016/j.ejpb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Hayashi K.; Arakane K.; Naito N.; Nagano T.; Hirobe M. Alkyl Chain Length Dependency in Hydrolysis of Liposomal Phosphatidylcholine By Dialkylphosphate. Chem. Pharm. Bull. 1995, 43, 1751–1754. 10.1248/cpb.43.1751. [DOI] [Google Scholar]
- Weschayanwiwat P.; Scamehorn J. F.; Reilly P. J. Surfactant Properties of Low Molecular Weight Phospholipids. J. Surfactants Deterg. 2005, 8, 65–72. 10.1007/s11743-005-0332-8. [DOI] [Google Scholar]
- Kensil C. R.; Dennis E. A. Alkaline Hydrolysis of Phospholipids in Model Membranes and the Dependence on Their State of Aggregation. Biochemistry 1981, 20, 6079–6085. 10.1021/bi00524a025. [DOI] [PubMed] [Google Scholar]
- Grit M.; Underberg W. J.; Crommelin D. J. Hydrolysis of Saturated Soybean Phosphatidylcholine in Aqueous Liposome Dispersions. J. Pharm. Sci. 1993, 82, 362–366. 10.1002/jps.2600820405. [DOI] [PubMed] [Google Scholar]
- Holzschuh S.; Kaeß K.; Fahr A.; Decker C. Quantitative in Vitro Assessment of Liposome Stability and Drug Transfer Employing Asymmetrical Flow Field-Flow Fractionation (AF4). Pharm. Res. 2016, 33, 842–855. 10.1007/s11095-015-1831-y. [DOI] [PubMed] [Google Scholar]
- Gerasimov O. V.; Schwan A.; Thompson D. H. Acid-Catalyzed Plasmenylcholine Hydrolysis and Its Effect on Bilayer Permeability: A Quantitative Study. Biochim. Biophys. Acta, Biomembr. 1997, 1324, 200–214. 10.1016/S0005-2736(96)00220-9. [DOI] [PubMed] [Google Scholar]
- Rui Y.; Wang S.; Low P. S.; Thompson D. H. Diplasmenylcholine-Folate Liposomes: An Efficient Vehicle for Intracellular Drug Delivery. J. Am. Chem. Soc. 1998, 120, 11213–11218. 10.1021/ja9742949. [DOI] [Google Scholar]
- Zhong Z.; Ji Q.; Zhang J. A. Analysis of Cationic Liposomes By Reversed-Phase HPLC With Evaporative Light-Scattering Detection. J. Pharm. Biomed. Anal. 2010, 51, 947–951. 10.1016/j.jpba.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Ottiger M.; Bax A. Characterization of Magnetically Oriented Phospholipid Micelles for Measurement of Dipolar Couplings in Macromolecules. J. Biomol. NMR 1998, 12, 361–372. 10.1023/A:1008366116644. [DOI] [PubMed] [Google Scholar]
- Gabriel N. E.; Roberts M. F. Short-Chain Lecithin/long-Chain Phospholipid Unilamellar Vesicles: Asymmetry, Dynamics, and Enzymatic Hydrolysis of the Short-Chain Component. Biochemistry 1987, 26, 2432–2440. 10.1021/bi00383a006. [DOI] [PubMed] [Google Scholar]
- Tinker D. O.; Purdon A. D.; Wei J.; Mason E. Kinetics of Hydrolysis of Dispersions of Saturated Phosphatidylcholines By Crotalus atrox Phospholipase A2. Can. J. Biochem. 1978, 56, 552–558. 10.1139/o78-084. [DOI] [PubMed] [Google Scholar]
- Grzybek M.; Kubiak J.; Łach A.; Przybyło M.; Sikorski A. F. A Raft-Associated Species of Phosphatidylethanolamine Interacts With Cholesterol Comparably to Sphingomyelin. A Langmuir-Blodgett Monolayer Study. PLoS One 2009, 4, e5053 10.1371/journal.pone.0005053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalá Á. Lipid Peroxidation Modifies the Assembly of Biological Membranes “the Lipid Whisker Model. Front. Physiol. 2015, 5, 520. 10.3389/fphys.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalá A. Lipid Peroxidation Modifies the Picture of Membranes From the “Fluid Mosaic Model” to the “Lipid Whisker Model. Biochimie 2012, 94, 101–109. 10.1016/j.biochi.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Simon S. A.; McIntosh T. J. Depth of Water Penetration Into Lipid Bilayers. Methods Enzymol. 1986, 127, 511–521. 10.1016/0076-6879(86)27041-X. [DOI] [PubMed] [Google Scholar]
- Jeschek D.; Lhota G.; Wallner J.; Vorauer-Uhl K. A Versatile, Quantitative Analytical Method for Pharmaceutical Relevant Lipids in Drug Delivery Systems. J. Pharm. Biomed. Anal. 2016, 119, 37–44. 10.1016/j.jpba.2015.11.020. [DOI] [PubMed] [Google Scholar]
- Shibata H.; Yomota C.; Okuda H. Simultaneous Determination of Polyethylene Glycol-Conjugated Liposome Components By Using Reversed-Phase High-Performance Liquid Chromatography With UV and Evaporative Light Scattering Detection. AAPS PharmSciTech 2013, 14, 811–817. 10.1208/s12249-013-9967-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietzyk B.; Henschke K. Degradation of Phosphatidylcholine in Liposomes Containing Carboplatin in Dependence on Composition and Storage Conditions. Int. J. Pharm. 2000, 196, 215–218. 10.1016/S0378-5173(99)00425-1. [DOI] [PubMed] [Google Scholar]
- Samuni A. M.; Lipman A.; Barenholz Y. Damage to Liposomal Lipids: Protection By Antioxidants and Cholesterol-Mediated Dehydration. Chem. Phys. Lipids 2000, 105, 121–134. 10.1016/S0009-3084(99)00136-X. [DOI] [PubMed] [Google Scholar]
- Moog R.; Brandl M.; Schubert R.; Unger C.; Massing U. Effect of Nucleoside Analogues and Oligonucleotides on Hydrolysis of Liposomal Phospholipids. Int. J. Pharm. 2000, 206, 43–53. 10.1016/S0378-5173(00)00497-X. [DOI] [PubMed] [Google Scholar]
- Fan Y.; Marioli M.; Zhang K. Analytical Characterization of Liposomes and Other Lipid Nanoparticles for Drug Delivery. J. Pharm. Biomed. Anal. 2021, 192, 113642. 10.1016/j.jpba.2020.113642. [DOI] [PubMed] [Google Scholar]
- Duplessis J.; Ramachandran C.; Weiner N.; Muller D. The Influence of Lipid Composition and Lamellarity of Liposomes on the Physical Stability of Liposomes Upon Storage. Int. J. Pharm. 1996, 127, 273–278. 10.1016/0378-5173(95)04281-4. [DOI] [Google Scholar]
- Claessens M. M.; van Oort B. F.; Leermakers F. A.; Hoekstra F. A.; Cohen Stuart M. A. Charged Lipid Vesicles: Effects of Salts on Bending Rigidity, Stability, and Size. Biophys. J. 2004, 87, 3882–3893. 10.1529/biophysj.103.036772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Swing C. J.; Feng T.; Xia S.; Yu J.; Zhang X. Effects of Environmental pH and Ionic Strength on the Physical Stability of Cinnamaldehyde-Loaded Liposomes. J. Dispersion Sci. Technol. 2020, 41, 1568–1575. 10.1080/01932691.2019.1627887. [DOI] [Google Scholar]
- Wang Z.-Y.; Zhang H.; Yang Y.; Xie X.-Y.; Yang Y.-F.; Li Z.; Li Y.; Gong W.; Yu F.-L.; Yang Z.; et al. Preparation, Characterization, and Efficacy of Thermosensitive Liposomes Containing Paclitaxel. Drug Delivery 2016, 23, 1222–1231. 10.3109/10717544.2015.1122674. [DOI] [PubMed] [Google Scholar]
- Limmer S.; Hahn J.; Schmidt R.; Wachholz K.; Zengerle A.; Lechner K.; Eibl H.; Issels R. D.; Hossann M.; Lindner L. H. Gemcitabine Treatment of Rat Soft Tissue Sarcoma With Phosphatidyldiglycerol-Based Thermosensitive Liposomes. Pharm. Res. 2014, 31, 2276–2286. 10.1007/s11095-014-1322-6. [DOI] [PubMed] [Google Scholar]
- Casey D.; Charalambous K.; Gee A.; Law R. V.; Ces O. Amphiphilic Drug Interactions With Model Cellular Membranes Are Influenced By Lipid Chain-Melting Temperature. J. R. Soc., Interface 2014, 11, 20131062. 10.1098/rsif.2013.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baciu M.; Sebai S. C.; Ces O.; Mulet X.; Clarke J. A.; Shearman G. C.; Law R. V.; Templer R. H.; Plisson C.; Parker C. A.; et al. Degradative Transport of Cationic Amphiphilic Drugs Across Phospholipid Bilayers. Philos. Trans. R. Soc., A 2006, 364, 2597–2614. 10.1098/rsta.2006.1842. [DOI] [PubMed] [Google Scholar]
- Zuidam N. J.; Gouw H. K.; Barenholz Y.; Crommelin D. J. Physical (in) Stability of Liposomes Upon Chemical Hydrolysis: The Role of Lysophospholipids and Fatty Acids. Biochim. Biophys. Acta 1995, 1240, 101–110. 10.1016/0005-2736(95)00180-5. [DOI] [PubMed] [Google Scholar]
- Agmo Hernández V.; Reijmar K.; Edwards K. Label-Free Characterization of Peptide-Lipid Interactions Using Immobilized Lipodisks. Anal. Chem. 2013, 85, 7377–7384. 10.1021/ac4012842. [DOI] [PubMed] [Google Scholar]
- Kodama A.; Sakuma Y.; Imai M.; Oya Y.; Kawakatsu T.; Puff N.; Angelova M. I. Migration of Phospholipid Vesicles in Response to OH- Stimuli. Soft Matter 2016, 12, 2877–2886. 10.1039/C5SM02220G. [DOI] [PubMed] [Google Scholar]
- Goertz M. P.; Goyal N.; Montano G. A.; Bunker B. C. Lipid Bilayer Reorganization Under Extreme pH Conditions. Langmuir 2011, 27, 5481–5491. 10.1021/la2001305. [DOI] [PubMed] [Google Scholar]
- Tan S. T.; Ramesh T.; Toh X. R.; Nguyen L. N. Emerging Roles of Lysophospholipids in Health and Disease. Prog. Lipid Res. 2020, 80, 101068. 10.1016/j.plipres.2020.101068. [DOI] [PubMed] [Google Scholar]
- Kim J.-M.; Shin J.; Shum P.; Thompson D. H. Acid-and Oxidatively-Labile Vinyl Ether Surfactants: Synthesis and Drug Delivery Applications. J. Dispersion Sci. Technol. 2001, 22, 399–407. 10.1081/DIS-100107849. [DOI] [Google Scholar]
- Poznik M.; Maitra U.; König B. The Interface Makes a Difference: Lanthanide Ion Coated Vesicles Hydrolyze Phosphodiesters. Org. Biomol. Chem. 2015, 13, 9789–9792. 10.1039/C5OB01265A. [DOI] [PubMed] [Google Scholar]
- Britt H.; García-Herrero C. A.; Denny P. W.; Mosely J. A.; Sanderson J. M. Lytic Reactions of Drugs With Lipid Membranes. Chem. Sci. 2019, 10, 674–680. 10.1039/C8SC04831B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt H. M.; Prakash A. S.; Appleby S.; Mosely J. A.; Sanderson J. M. Lysis of Membrane Lipids Promoted By Small Organic Molecules: Reactivity Depends on Structure But Not Lipophilicity. Sci. Adv. 2020, 6, eaaz8598 10.1126/sciadv.aaz8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dods R. H.; Bechinger B.; Mosely J. A.; Sanderson J. M. Acyl Transfer From Membrane Lipids to Peptides is a Generic Process. J. Mol. Biol. 2013, 425, 4379–4387. 10.1016/j.jmb.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Dods R. H.; Mosely J. A.; Sanderson J. M. The Innate Reactivity of a Membrane Associated Peptide Towards Lipids: Acyl Transfer to Melittin Without Enzyme Catalysis. Org. Biomol. Chem. 2012, 10, 5371–5378. 10.1039/c2ob07113d. [DOI] [PubMed] [Google Scholar]
- Pridmore C. J.; Mosely J. A.; Rodger A.; Sanderson J. M. Acyl Transfer From Phosphocholine Lipids to Melittin. Chem. Commun. 2011, 47, 1422–1424. 10.1039/C0CC04677A. [DOI] [PubMed] [Google Scholar]
- Terwilliger T. C.; Eisenberg D. The Structure of Melittin. I. Structure Determination and Partial Refinement. J. Biol. Chem. 1982, 257, 6010–6015. 10.1016/S0021-9258(20)65097-9. [DOI] [PubMed] [Google Scholar]
- Zhu L.; Kemple M. D.; Yuan P.; Prendergast F. G. N-Terminus and Lysine Side Chain pKa Values of Melittin in Aqueous Solutions and Micellar Dispersions Measured By 15N NMR. Biochemistry 1995, 34, 13196–13202. 10.1021/bi00040a035. [DOI] [PubMed] [Google Scholar]
- Yuan P.; Fisher P. J.; Prendergast F. G.; Kemple M. D. Structure and Dynamics of Melittin in Lysomyristoyl Phosphatidylcholine Micelles Determined By Nuclear Magnetic Resonance. Biophys. J. 1996, 70, 2223–2238. 10.1016/S0006-3495(96)79788-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail V. S.; Britt H. M.; Mosely J. A.; Sanderson J. M. Peptide Lipidation in Lysophospholipid Micelles and Lysophospholipid-Enriched Membranes. Faraday Discuss. 2021, 232, 282–294. 10.1039/D1FD00030F. [DOI] [PubMed] [Google Scholar]
- Wimley W. C.; White S. H. Designing Transmembrane α-Helices That Insert Spontaneously. Biochemistry 2000, 39, 4432–4442. 10.1021/bi992746j. [DOI] [PubMed] [Google Scholar]
- Britt H. M.; Mosely J. A.; Sanderson J. M. The Influence of Cholesterol on Melittin Lipidation in Neutral Membranes. Phys. Chem. Chem. Phys. 2019, 21, 631–640. 10.1039/C8CP06661B. [DOI] [PubMed] [Google Scholar]
- Toyama B. H.; Hetzer M. W. Protein Homeostasis: Live Long, Won’t Prosper. Nat. Rev. Mol. Cell Biol. 2013, 14, 55–61. 10.1038/nrm3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden E.; Geva-Zatorsky N.; Issaeva I.; Cohen A.; Dekel E.; Danon T.; Cohen L.; Mayo A.; Alon U. Proteome Half-Life Dynamics in Living Human Cells. Science 2011, 331, 764–768. 10.1126/science.1199784. [DOI] [PubMed] [Google Scholar]
- Rahman M.; Previs S. F.; Kasumov T.; Sadygov R. G. Gaussian Process Modeling of Protein Turnover. J. Proteome Res. 2016, 15, 2115–2122. 10.1021/acs.jproteome.5b00990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J. C.; Guan S.; Burlingame A.; Prusiner S. B.; Ghaemmaghami S. Analysis of Proteome Dynamics in the Mouse Brain. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 14508–14513. 10.1073/pnas.1006551107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harayama T.; Shindou H.; Kita Y.; Otsubo E.; Ikeda K.; Chida S.; Weaver T. E.; Shimizu T. Establishment of LC-MS Methods for the Analysis of Palmitoylated Surfactant Proteins. J. Lipid Res. 2015, 56, 1370–1379. 10.1194/jlr.D060236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Brinke A.; van Golde L. M.; Batenburg J. J. Palmitoylation and Processing of the Lipopeptide Surfactant Protein C. Biochim. Biophys. Acta 2002, 1583, 253–265. 10.1016/S1388-1981(02)00248-2. [DOI] [PubMed] [Google Scholar]
- ten Brinke A.; Batenburg J. J.; Haagsman H. P.; van Golde L. M.; Vaandrager A. B. Differential Effect of Brefeldin a on the Palmitoylation of Surfactant Protein C Proprotein Mutants. Biochem. Biophys. Res. Commun. 2002, 290, 532–538. 10.1006/bbrc.2001.6223. [DOI] [PubMed] [Google Scholar]
- ten Brinke A.; Batenburg J. J.; Gadella B. M.; Haagsman H. P.; Vaandrager A. B.; van Golde L. M. The Juxtamembrane Lysine and Arginine Residues of Surfactant Protein C Precursor Influence Palmitoylation Via Effects on Trafficking. Am. J. Respir. Cell Mol. Biol. 2001, 25, 156–163. 10.1165/ajrcmb.25.2.4423. [DOI] [PubMed] [Google Scholar]
- Griffiths W. J.; Gustafsson M.; Yang Y.; Curstedt T.; Sjövall J.; Johansson J. Analysis of Variant Forms of Porcine Surfactant Polypeptide-C By Nano-Electrospray Mass Spectrometry. Rapid Commun. Mass Spectrom. 1998, 12, 1104–1114. . [DOI] [PubMed] [Google Scholar]
- Gustafsson M.; Curstedt T.; Jörnvall H.; Johansson J. Reverse-Phase HPLC of the Hydrophobic Pulmonary Surfactant Proteins: Detection of a Surfactant Protein C Isoform Containing Nε-Palmitoyl-lysine. Biochem. J. 1997, 326, 799–806. 10.1042/bj3260799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester J. V.; Dick A. D.; McMenamin P. G.; Roberts F.; Pearlman E.. The Eye: Basic Sciences in Practice; Saunders Ltd.: Edinburgh, UK, 2016. [Google Scholar]
- Bassnett S.; Shi Y.; Vrensen G. F. Biological Glass: Structural Determinants of Eye Lens Transparency. Philos. Trans. R. Soc., B 2011, 366, 1250–1264. 10.1098/rstb.2010.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenke J. L.; Rose K. L.; Spraggins J. M.; Schey K. L. MALDI Imaging Mass Spectrometry Spatially Maps Age-Related Deamidation and Truncation of Human Lens Aquaporin-0. Invest. Ophthalmol. Vis. Sci. 2015, 56, 7398–7405. 10.1167/iovs.15-18117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schey K. L.; Grey A. C.; Nicklay J. J. Mass Spectrometry of Membrane Proteins: A Focus on Aquaporins. Biochemistry 2013, 52, 3807–3817. 10.1021/bi301604j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez D. B.; Garland D.; Schey K. L. Spatial Analysis of Human Lens Aquaporin-0 Post-Translational Modifications By MALDI Mass Spectrometry Tissue Profiling. Exp. Eye Res. 2011, 93, 912–920. 10.1016/j.exer.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schey K. L.; Gutierrez D. B.; Wang Z.; Wei J.; Grey A. C. Novel Fatty Acid Acylation of Lens Integral Membrane Protein Aquaporin-0. Biochemistry 2010, 49, 9858–9865. 10.1021/bi101415w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korlimbinis A.; Berry Y.; Thibault D.; Schey K. L.; Truscott R. J. W. Protein Aging: Truncation of Aquaporin 0 in Human Lens Regions is a Continuous Age-Dependent Process. Exp. Eye Res. 2009, 88, 966–973. 10.1016/j.exer.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.; Schey K. L. Proteolysis and Mass Spectrometric Analysis of an Integral Membrane: Aquaporin 0. J. Proteome Res. 2004, 3, 807–812. 10.1021/pr049945w. [DOI] [PubMed] [Google Scholar]
- Ball L. E.; Garland D. L.; Crouch R. K.; Schey K. L. Post-Translational Modifications of Aquaporin 0 (AQP0) in the Normal Human Lens: Spatial and Temporal Occurrence. Biochemistry 2004, 43, 9856–9865. 10.1021/bi0496034. [DOI] [PubMed] [Google Scholar]
- Schey K. L.; Fowler J. G.; Schwartz J. C.; Busman M.; Dillon J.; Crouch R. K. Complete Map and Identification of the Phosphorylation Site of Bovine Lens Major Intrinsic Protein. Invest. Ophthalmol. Vis. Sci. 1997, 38, 2508–2515. [PubMed] [Google Scholar]
- Ismail V. S.; Mosely J. A.; Tapodi A.; Quinlan R. A.; Sanderson J. M. The Lipidation Profile of Aquaporin-0 Correlates With the Acyl Composition of Phosphoethanolamine Lipids in Lens Membranes. Biochim. Biophys. Acta 2016, 1858, 2763–2768. 10.1016/j.bbamem.2016.06.026. [DOI] [PubMed] [Google Scholar]
- Bizzozero O. A.; Bixler H. A.; Pastuszyn A. Structural Determinants Influencing the Reaction of Cysteine-Containing Peptides With Palmitoyl-Coenzyme A and Other Thioesters. Biochim. Biophys. Acta 2001, 1545, 278–288. 10.1016/S0167-4838(00)00291-0. [DOI] [PubMed] [Google Scholar]
- Dunphy J. T.; Schroeder H.; Leventis R.; Greentree W. K.; Knudsen J. K.; Silvius J. R.; Linder M. E. Differential Effects of Acyl-CoA Binding Protein on Enzymatic and Non-Enzymatic Thioacylation of Protein and Peptide Substrates. Biochim. Biophys. Acta 2000, 1485, 185–198. 10.1016/S1388-1981(00)00060-3. [DOI] [PubMed] [Google Scholar]
- Leventis R.; Juel G.; Knudsen J. K.; Silvius J. R. Acyl-CoA Binding Proteins Inhibit the Nonenzymic S-Acylation of Cysteinyl-Containing Peptide Sequences By Long-Chain Acyl-CoAs. Biochemistry 1997, 36, 5546–5553. 10.1021/bi963029h. [DOI] [PubMed] [Google Scholar]
- Schroeder H.; Leventis R.; Shahinian S.; Walton P. A.; Silvius J. R. Lipid-Modified, Cysteinyl-Containing Peptides of Diverse Structures Are Efficiently S-Acylated At the Plasma Membrane of Mammalian Cells. J. Cell Biol. 1996, 134, 647–660. 10.1083/jcb.134.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesnel S.; Silvius J. R. Cysteine-Containing Peptide Sequences Exhibit Facile Uncatalyzed Transacylation and Acyl-CoA-dependent Acylation At the Lipid Bilayer Interface. Biochemistry 1994, 33, 13340–13348. 10.1021/bi00249a021. [DOI] [PubMed] [Google Scholar]
- Lin H.; Su X.; He B. Protein Lysine Acylation and Cysteine Succination By Intermediates of Energy Metabolism. Chem. Biol. 2012, 7, 947–960. 10.1021/cb3001793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauve A. A.; Wolberger C.; Schramm V. L.; Boeke J. D. The Biochemistry of Sirtuins. Annu. Rev. Biochem. 2006, 75, 435–465. 10.1146/annurev.biochem.74.082803.133500. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Yang T.; Li X.; Peng T.; Hang H. C.; Li X. D. Integrative Chemical Biology Approaches for Identification and Characterization of “Erasers” for Fatty-Acid-acylated Lysine Residues Within Proteins. Angew. Chem., Int. Ed. Engl. 2015, 54, 1149–1152. 10.1002/anie.201408763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu A. Y.; Zhou Y.; Khan S.; Deitsch K. W.; Hao Q.; Lin H. Plasmodium Falciparum Sir2A Preferentially Hydrolyzes Medium and Long Chain Fatty Acyl Lysine. ACS Chem. Biol. 2012, 7, 155–159. 10.1021/cb200230x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman J. L.; Baeza J.; Denu J. M. Activation of the Protein Deacetylase SIRT6 By Long-Chain Fatty Acids and Widespread Deacylation By Mammalian Sirtuins. J. Biol. Chem. 2013, 288, 31350–31356. 10.1074/jbc.C113.511261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson J. M. The Association of Lipids With Amyloid Fibrils. J. Biol. Chem. 2022, 298, 102108. 10.1016/j.jbc.2022.102108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spickett C. M. Formation of Oxidatively Modified Lipids as the Basis for a Cellular Epilipidome. Front. Endocrinol. 2020, 11, 602771. 10.3389/fendo.2020.602771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski Z. A.; Pratt D. A. Lipid Peroxidation: Kinetics, Mechanisms, and Products. J. Org. Chem. 2017, 82, 2817–2825. 10.1021/acs.joc.7b00152. [DOI] [PubMed] [Google Scholar]
- Yin H.; Xu L.; Porter N. A. Free Radical Lipid Peroxidation: Mechanisms and Analysis. Chem. Rev. 2011, 111, 5944–5972. 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- Wölk M.; Prabutzki P.; Fedorova M. Analytical Toolbox to Unlock the Diversity of Oxidized Lipids. Acc. Chem. Res. 2023, 56, 835–845. 10.1021/acs.accounts.2c00842. [DOI] [PubMed] [Google Scholar]
- Demirci-Çekiç S.; Özkan G.; Avan A. N.; Uzunboy S.; Çapanoğlu E.; Apak R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. 10.1016/j.jpba.2021.114477. [DOI] [PubMed] [Google Scholar]
- Faroux J. M.; Ureta M. M.; Tymczyszyn E. E.; Gómez-Zavaglia A. An Overview of Peroxidation Reactions Using Liposomes as Model Systems and Analytical Methods as Monitoring Tools. Colloids Surf., B 2020, 195, 111254. 10.1016/j.colsurfb.2020.111254. [DOI] [PubMed] [Google Scholar]
- Foret M. K.; Lincoln R.; Do Carmo S.; Cuello A. C.; Cosa G. Connecting the “Dots”: From Free Radical Lipid Autoxidation to Cell Pathology and Disease. Chem. Rev. 2020, 120, 12757–12787. 10.1021/acs.chemrev.0c00761. [DOI] [PubMed] [Google Scholar]
- Bacellar I. O. L.; Baptista M. S. Mechanisms of Photosensitized Lipid Oxidation and Membrane Permeabilization. ACS Omega 2019, 4, 21636–21646. 10.1021/acsomega.9b03244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadyro O.; Samovich S.; Edimecheva I. Free-Radical and Biochemical Reactions Involving Polar Part of Glycerophospholipids. Free Radicals Biol. Med. 2019, 144, 6–15. 10.1016/j.freeradbiomed.2019.02.033. [DOI] [PubMed] [Google Scholar]
- Collin F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. 10.3390/ijms20102407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S. S.; Guo L. Lipid Peroxidation Generates Biologically Active Phospholipids Including Oxidatively N-Modified Phospholipids. Chem. Phys. Lipids 2014, 181, 1–33. 10.1016/j.chemphyslip.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel E. N.Lipid Oxidation, 2nd ed.; Oily Press, 2005. [Google Scholar]
- Roginsky V.; Barsukova T. Superoxide Dismutase Inhibits Lipid Peroxidation in Micelles. Chem. Phys. Lipids 2001, 111, 87–91. 10.1016/S0009-3084(01)00148-7. [DOI] [PubMed] [Google Scholar]
- Niki E. Free Radical Initiators as Source of Water- or Lipid-Soluble Peroxyl Radicals. Methods Enzymol. 1990, 186, 100–108. 10.1016/0076-6879(90)86095-D. [DOI] [PubMed] [Google Scholar]
- Pliss E. M.; Loshadkin D. V.; Grobov A. M.; Kuznetsova T. S.; Rusakov A. I. Kinetic Study and Simulation of Methyl Linoleate Oxidation in Micelles. Russ. J. Phys. Chem. B 2015, 9, 127–131. 10.1134/S1990793115010091. [DOI] [Google Scholar]
- Barclay L. R. C.; Locke S. J.; MacNeil J. M.; Vankessel J. Quantitative Studies of the Autoxidation of Linoleate Monomers Sequestered in Phosphatidylcholine Bilayers. Absolute Rate Constants in Bilayers. Can. J. Chem. 1985, 63, 2633–2638. 10.1139/v85-437. [DOI] [Google Scholar]
- Noguchi N.; Yamashita H.; Gotoh N.; Yamamoto Y.; Numano R.; Niki E. 2,2’-Azobis (4-Methoxy-2,4-dimethylvaleronitrile), a New Lipid-Soluble Azo Initiator: Application to Oxidations of Lipids and Low-Density Lipoprotein in Solution and in Aqueous Dispersions. Free Radicals Biol. Med. 1998, 24, 259–268. 10.1016/S0891-5849(97)00230-X. [DOI] [PubMed] [Google Scholar]
- Baptista M. S.; Cadet J.; Di Mascio P.; Ghogare A. A.; Greer A.; Hamblin M. R.; Lorente C.; Nunez S. C.; Ribeiro M. S.; Thomas A. H.; et al. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919. 10.1111/php.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezende L. G.; Tasso T. T.; Candido P. H. S.; Baptista M. S. Assessing Photosensitized Membrane Damage: Available Tools and Comprehensive Mechanisms. Photochem. Photobiol. 2022, 98, 572–590. 10.1111/php.13582. [DOI] [PubMed] [Google Scholar]
- Anglada J. M.; Martins-Costa M. T. C.; Francisco J. S.; Ruiz-López M. F. Photoinduced Oxidation Reactions At the Air-Water Interface. J. Am. Chem. Soc. 2020, 142, 16140–16155. 10.1021/jacs.0c06858. [DOI] [PubMed] [Google Scholar]
- Kowalska A.; Sokolowski J.; Bociong K. The Photoinitiators Used in Resin Based Dental Composite-a Review and Future Perspectives. Polymers (Basel, Switz.) 2021, 13, 470. 10.3390/polym13030470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allushi A.; Kutahya C.; Aydogan C.; Kreutzer J.; Yilmaz G.; Yagci Y. Conventional Type II Photoinitiators as Activators for Photoinduced Metal-Free Atom Transfer Radical Polymerization. Polym. Chem. 2017, 8, 1972–1977. 10.1039/C7PY00114B. [DOI] [Google Scholar]
- Vignoni A.; Layana C.; Junqueira H. C.; Thomas A. H.; Itri R.; Baptista M. S.; Vignoni M. Alkylation of a Hydrophilic Photosensitizer Enhances the Contact-Dependent Photo-Induced Oxidation of Phospholipid Membranes. Dyes Pigm. 2021, 187, 109131. 10.1016/j.dyepig.2020.109131. [DOI] [Google Scholar]
- Schaich K. M. Metals and Lipid Oxidation. Contemporary Issues. Lipids 1992, 27, 209–218. 10.1007/BF02536181. [DOI] [PubMed] [Google Scholar]
- Cavallini G.; Sgarbossa A.; Parentini I.; Bizzarri R.; Donati A.; Lenci F.; Bergamini E. Dolichol: A Component of the Cellular Antioxidant Machinery. Lipids 2016, 51, 477–486. 10.1007/s11745-016-4137-x. [DOI] [PubMed] [Google Scholar]
- Sanii B.; Parikh A. N. Patterning Fluid and Elastomeric Surfaces Using Short-Wavelength UV Radiation and Photogenerated Reactive Oxygen Species. Annu. Rev. Phys. Chem. 2008, 59, 411–432. 10.1146/annurev.physchem.58.032806.104644. [DOI] [PubMed] [Google Scholar]
- Miccichè F.; van Haveren J.; Oostveen E.; Laven J.; Ming W.; Okan Oyman Z.; van der Linde R. Oxidation of Methyl Linoleate in Micellar Solutions Induced By the Combination of Iron(II)/ascorbic Acid and Iron(II)/H2O2. Arch. Biochem. Biophys. 2005, 443, 45–52. 10.1016/j.abb.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Casalino E.; Sblano C.; Landriscina C. A Possible Mechanism for Initiation of Lipid Peroxidation By Ascorbate in Rat Liver Microsomes. Int. J. Biochem. Cell Biol. 1996, 28, 137–149. 10.1016/1357-2725(95)00131-X. [DOI] [PubMed] [Google Scholar]
- Kawakatsu M.; Terao J.; Matsushita S. Phospholipid Oxidation Catalyzed By Ferrous Ion and Ascorbic Acid. Agric. Biol. Chem. 1984, 48, 1275–1279. 10.1271/bbb1961.48.1275. [DOI] [Google Scholar]
- Laguerre M.; Lecomte J.; Villeneuve P. Evaluation of the Ability of Antioxidants to Counteract Lipid Oxidation: Existing Methods, New Trends and Challenges. Prog. Lipid Res. 2007, 46, 244–282. 10.1016/j.plipres.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Choi S.; Filotto C.; Bisanzo M.; Delaney S.; Lagasee D.; Whitworth J. L.; Jusko A.; Li C.; Wood N. A.; Willingham J.; et al. Reduction and Anticancer Activity of Platinum (IV) Complexes. Inorg. Chem. 1998, 37, 2500–2504. 10.1021/ic971047x. [DOI] [Google Scholar]
- Wexselblatt E.; Gibson D. What Do We Know About the Reduction of Pt(IV) Pro-Drugs. J. Inorg. Biochem. 2012, 117, 220–229. 10.1016/j.jinorgbio.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Weaver E. L.; Bose R. N. Platinum(II) Catalysis and Radical Intervention in Reductions of Platinum(IV) Antitumor Drugs By Ascorbic Acid. J. Inorg. Biochem. 2003, 95, 231–239. 10.1016/S0162-0134(03)00136-3. [DOI] [PubMed] [Google Scholar]
- Murray P.; Koch K. R.; van Eldik R. Mechanism of Tetrachloroplatinate(II) Oxidation By Hydrogen Peroxide in Hydrochloric Acid Solution. Dalton Trans. 2014, 43, 6308–6314. 10.1039/C3DT53057D. [DOI] [PubMed] [Google Scholar]
- Wilson J. J.; Lippard S. J. Synthetic Methods for the Preparation of Platinum Anticancer Complexes. Chem. Rev. 2014, 114, 4470–4495. 10.1021/cr4004314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippert B. Impact of Cisplatin on the Recent Development of Pt Coordination Chemistry: A Case Study. Coord. Chem. Rev. 1999, 182, 263–295. 10.1016/S0010-8545(98)00192-1. [DOI] [Google Scholar]
- Johnstone T. C.; Alexander S. M.; Wilson J. J.; Lippard S. J. Oxidative Halogenation of Cisplatin and Carboplatin: Synthesis, Spectroscopy, and Crystal and Molecular Structures of Pt(IV) Prodrugs. Dalton Trans. 2015, 44, 119–129. 10.1039/C4DT02627F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swancutt K. L.; Mezyk S. P.; Kiddle J. J. Free Radical-Induced Redox Chemistry of Platinum-Containing Anti-Cancer Drugs. Radiat. Res. 2007, 168, 423–427. 10.1667/RR1054.1. [DOI] [PubMed] [Google Scholar]
- Song K. S.; Liu L.; Guo Q. X. Effects of Alpha-Ammonium, α-Phosphonium, and α-Sulfonium Groups on C-H Bond Dissociation Energies. J. Org. Chem. 2003, 68, 4604–4607. 10.1021/jo020709j. [DOI] [PubMed] [Google Scholar]
- Arendse M. J.; Anderson G. K.; Rath N. P. Synthesis and Characterization of Platinum (II) Complexes of L-Ascorbic Acid. Crystal Structure of Ascorbato-C2, O5-ethylenediamineplatinum (II) Dihydrate. Inorg. Chem. 1999, 38, 5864–5869. 10.1021/ic990152z. [DOI] [Google Scholar]
- Davies M. B. Reactions of L-Ascorbic Acid With Transition Metal Complexes. Polyhedron 1992, 11, 285–321. 10.1016/S0277-5387(00)83175-7. [DOI] [Google Scholar]
- Weiny J. A.; Boeglin W. E.; Calcutt M. W.; Stec D. F.; Brash A. R. Monolayer Autoxidation of Arachidonic Acid to Epoxyeicosatrienoic Acids as a Model of Their Potential Formation in Cell Membranes. J. Lipid Res. 2022, 63, 100159. 10.1016/j.jlr.2021.100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisby R. H.; Johnson S. A.; Parker A. W.; Tavender S. M. Time-Resolved Resonance Raman Spectroscopy of the Carbonate Radical. J. Chem. Soc., Faraday Trans. 1998, 94, 2069–2072. 10.1039/a801239c. [DOI] [Google Scholar]
- Lokhmatikov A. V.; Voskoboynikova N. E.; Cherepanov D. A.; Sumbatyan N. V.; Korshunova G. A.; Skulachev M. V.; Steinhoff H. J.; Skulachev V. P.; Mulkidjanian A. Y. Prevention of Peroxidation of Cardiolipin Liposomes By Quinol-Based Antioxidants. Biochemistry (Moscow) 2014, 79, 1081–1100. 10.1134/S0006297914100101. [DOI] [PubMed] [Google Scholar]
- Pöschl U.; Shiraiwa M. Multiphase Chemistry At the Atmosphere-Biosphere Interface Influencing Climate and Public Health in the Anthropocene. Chem. Rev. 2015, 115, 4440–4475. 10.1021/cr500487s. [DOI] [PubMed] [Google Scholar]
- Mikkelsen R. B.; Wardman P. Biological Chemistry of Reactive Oxygen and Nitrogen and Radiation-Induced Signal Transduction Mechanisms. Oncogene 2003, 22, 5734–5754. 10.1038/sj.onc.1206663. [DOI] [PubMed] [Google Scholar]
- Smith J. T.; Willey N. J.; Hancock J. T. Low Dose Ionizing Radiation Produces Too Few Reactive Oxygen Species to Directly Affect Antioxidant Concentrations in Cells. Biol. Lett. (London, U. K.) 2012, 8, 594–597. 10.1098/rsbl.2012.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinks J. W. T.; Woods R. J.. An Introduction to Radiation Chemistry, 3rd ed.; Wiley, 1990. [Google Scholar]
- Kunath S.; Moosmann B. What is the Rate-Limiting Step Towards Aging? Chemical Reaction Kinetics Might Reconcile Contradictory Observations in Experimental Aging Research. GeroScience 2020, 42, 857–866. 10.1007/s11357-019-00058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotticelli M. G.; Crabbe A. M.; Wilson R. B.; Shchepinov M. S. Insights Into the Role of Oxidative Stress in the Pathology of Friedreich Ataxia Using Peroxidation Resistant Polyunsaturated Fatty Acids. Redox Biol. 2013, 1, 398–404. 10.1016/j.redox.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S.; Lamberson C. R.; Xu L.; To R.; Tsui H. S.; Shmanai V. V.; Bekish A. V.; Awad A. M.; Marbois B. N.; Cantor C. R.; et al. Small Amounts of Isotope-Reinforced Polyunsaturated Fatty Acids Suppress Lipid Autoxidation. Free Radicals Biol. Med. 2012, 53, 893–906. 10.1016/j.freeradbiomed.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S. C. W.; Furman R.; Axelsen P. H.; Shchepinov M. S. Free Radical Chain Reactions and Polyunsaturated Fatty Acids in Brain Lipids. ACS Omega 2022, 7, 25337–25345. 10.1021/acsomega.2c02285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloviev M.; Moskalenko I.; Pliss E. Quantum Chemical Evaluation of the Role of HO2• Radicals in the Kinetics of the Methyl Linoleate Oxidation in Micelles. React. Kinet., Mech. Catal. 2019, 127, 561–581. 10.1007/s11144-019-01613-w. [DOI] [Google Scholar]
- Pratt D. A.; Tallman K. A.; Porter N. A. Free Radical Oxidation of Polyunsaturated Lipids: New Mechanistic Insights and the Development of Peroxyl Radical Clocks. Acc. Chem. Res. 2011, 44, 458–467. 10.1021/ar200024c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brash A. R. Autoxidation of Methyl Linoleate: Identification of the Bis-Allylic 11-Hydroperoxide. Lipids 2000, 35, 947–952. 10.1007/s11745-000-0604-0. [DOI] [PubMed] [Google Scholar]
- Do Q.; Lee D. D.; Dinh A. N.; Seguin R. P.; Zhang R.; Xu L. Development and Application of a Peroxyl Radical Clock Approach for Measuring Both Hydrogen-Atom Transfer and Peroxyl Radical Addition Rate Constants. J. Org. Chem. 2021, 86, 153–168. 10.1021/acs.joc.0c01920. [DOI] [PubMed] [Google Scholar]
- Soucek M. D.; Khattab T.; Wu J. Review of Autoxidation and Driers. Prog. Org. Coat. 2012, 73, 435–454. 10.1016/j.porgcoat.2011.08.021. [DOI] [Google Scholar]
- Miccichè F.; van Haveren J.; Oostveen E.; Ming W.; van der Linde R. Oxidation and Oligomerization of Ethyl Linoleate Under the Influence of the Combination of Ascorbic Acid 6-Palmitate/iron-2-ethylhexanoate. Appl. Catal., A 2006, 297, 174–181. 10.1016/j.apcata.2005.09.008. [DOI] [Google Scholar]
- Oliveira M. C.; Yusupov M.; Bogaerts A.; Cordeiro R. M. How Do Nitrated Lipids Affect the Properties of Phospholipid Membranes. Arch. Biochem. Biophys. 2020, 695, 108548. 10.1016/j.abb.2020.108548. [DOI] [PubMed] [Google Scholar]
- Mastrogiovanni M.; Trostchansky A.; Rubbo H. Fatty Acid Nitration in Human Low-Density Lipoprotein. Arch. Biochem. Biophys. 2020, 679, 108190. 10.1016/j.abb.2019.108190. [DOI] [PubMed] [Google Scholar]
- Howard J. A.; Ingold K. U. Self-Reaction of sec-Butylperoxy Radicals. Confirmation of the Russell Mechanism. J. Am. Chem. Soc. 1968, 90, 1056–1058. 10.1021/ja01006a037. [DOI] [Google Scholar]
- Helberg J.; Pratt D. A. Autoxidation vs. Antioxidants - the Fight for Forever. Chem. Soc. Rev. 2021, 50, 7343–7358. 10.1039/D1CS00265A. [DOI] [PubMed] [Google Scholar]
- Khallouki F.; Saber S.; Bouddine T.; Hajji L.; Elbouhali B.; Silvente-Poirot S.; Poirot M. In Vitro and in Vivo Oxidation and Cleavage Products of Tocols: From Chemical Tuners to “Vitamineome” Therapeutics. A Narrative Review. Food Biosci. 2022, 49, 101839. 10.1016/j.fbio.2022.101839. [DOI] [Google Scholar]
- Losada-Barreiro S.; Sezgin-Bayindir Z.; Paiva-Martins F.; Bravo-Díaz C. Biochemistry of Antioxidants: Mechanisms and Pharmaceutical Applications. Biomedicines 2022, 10, 3051. 10.3390/biomedicines10123051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouh N.; Bourlieu-Lacanal C.; Figueroa-Espinoza M. C.; Durand E.; Villeneuve P. Tocopherols as Antioxidants in Lipid-Based Systems: The Combination of Chemical and Physicochemical Interactions Determines Their Efficiency. Compr. Rev. Food Sci. Food Saf. 2022, 21, 642–688. 10.1111/1541-4337.12867. [DOI] [PubMed] [Google Scholar]
- Wu Z.; Khodade V. S.; Chauvin J. R.; Rodriguez D.; Toscano J. P.; Pratt D. A. Hydropersulfides Inhibit Lipid Peroxidation and Protect Cells From Ferroptosis. J. Am. Chem. Soc. 2022, 144, 15825–15837. 10.1021/jacs.2c06804. [DOI] [PubMed] [Google Scholar]
- Musakhanian J.; Rodier J. D.; Dave M. Oxidative Stability in Lipid Formulations: A Review of the Mechanisms, Drivers, and Inhibitors of Oxidation. AAPS PharmSciTech 2022, 23, 151. 10.1208/s12249-022-02282-0. [DOI] [PubMed] [Google Scholar]
- Niki E. Lipid Oxidation That is, and is Not, Inhibited By Vitamin E: Consideration About Physiological Functions of Vitamin E. Free Radicals Biol. Med. 2021, 176, 1–15. 10.1016/j.freeradbiomed.2021.09.001. [DOI] [PubMed] [Google Scholar]
- Assi A.; Michael-Jubeli R.; Jacques-Jamin C.; Duplan H.; Baillet-Guffroy A.; Tfayli A. Skin Surface Lipids Photo-oxidation: A Vibrational Spectroscopy Study. J. Raman Spectrosc. 2023, 54, 487–500. 10.1002/jrs.6504. [DOI] [Google Scholar]
- Hajieva P.; Abrosimov R.; Kunath S.; Moosmann B. Antioxidant and Prooxidant Modulation of Lipid Peroxidation By Integral Membrane Proteins. Free Radical Res. 2023, 57, 105. 10.1080/10715762.2023.2201391. [DOI] [PubMed] [Google Scholar]
- Costanzo L. L.; De Guidi G.; Giuffrida S.; Sortino S.; Condorelli G. Antioxidant Effect of Inorganic Ions on UVC and UVB Induced Lipid Peroxidation. J. Inorg. Biochem. 1995, 59, 1–13. 10.1016/0162-0134(94)00048-F. [DOI] [PubMed] [Google Scholar]
- Schneider C.; Tallman K. A.; Porter N. A.; Brash A. R. Two Distinct Pathways of Formation of 4-Hydroxynonenal. Mechanisms of Nonenzymatic Transformation of the 9- and 13-Hydroperoxides of Linoleic Acid to 4-Hydroxyalkenals. J. Biol. Chem. 2001, 276, 20831–20838. 10.1074/jbc.M101821200. [DOI] [PubMed] [Google Scholar]
- Poli G.; Schaur R. J. 4-Hydroxynonenal in the Pathomechanisms of Oxidative Stress. IUBMB Life 2000, 50, 315–321. 10.1080/713803726. [DOI] [PubMed] [Google Scholar]
- Dyall S. C.; Balas L.; Bazan N. G.; Brenna J. T.; Chiang N.; da Costa Souza F.; Dalli J.; Durand T.; Galano J. M.; Lein P. J.; et al. Polyunsaturated Fatty Acids and Fatty Acid-Derived Lipid Mediators: Recent Advances in the Understanding of Their Biosynthesis, Structures, and Functions. Prog. Lipid Res. 2022, 86, 101165. 10.1016/j.plipres.2022.101165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschel A.; Zahedi R. P.; Ahrends R. Protein Lipid Modifications-More Than Just a Greasy Ballast. Proteomics 2016, 16, 759–782. 10.1002/pmic.201500353. [DOI] [PubMed] [Google Scholar]
- Shahidi F.; Hossain A. Role of Lipids in Food Flavor Generation. Molecules 2022, 27, 5014. 10.3390/molecules27155014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe E.; Min D. B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. 10.1111/j.1541-4337.2006.00009.x. [DOI] [Google Scholar]
- Huang X.; Ahn D. U. Lipid Oxidation and Its Implications to Meat Quality and Human Health. Food Sci. Biotechnol. 2019, 28, 1275–1285. 10.1007/s10068-019-00631-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez R.; Pateiro M.; Gagaoua M.; Barba F. J.; Zhang W.; Lorenzo J. M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. 10.3390/antiox8100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y.; Yu M.; Li D.; Zhao G.; Zhang M.; Wang Z.; Liu Y.; Zhou D. Effects of Non-Enzymatic Browning and Lipid Oxidation on Color of Ready-to-eat Abalone During Accelerated Storage and Its Control. Foods 2023, 12, 1514. 10.3390/foods12071514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohail A.; Al-Dalali S.; Wang J.; Xie J.; Shakoor A.; Asimi S.; Shah H.; Patil P. Aroma Compounds Identified in Cooked Meat: A Review. Food Res. Int. 2022, 157, 111385. 10.1016/j.foodres.2022.111385. [DOI] [PubMed] [Google Scholar]
- Nooshkam M.; Varidi M.; Bashash M. The Maillard Reaction Products as Food-Born Antioxidant and Antibrowning Agents in Model and Real Food Systems. Food Chem. 2019, 275, 644–660. 10.1016/j.foodchem.2018.09.083. [DOI] [PubMed] [Google Scholar]
- Zhou Z.; Zhang Y. Y.; Xin R.; Huang X. H.; Li Y. L.; Dong X.; Zhou D.; Zhu B.; Qin L. Metal Ion-Mediated Pro-Oxidative Reactions of Different Lipid Molecules: Revealed By Nontargeted Lipidomic Approaches. J. Agric. Food Chem. 2022, 70, 10284–10295. 10.1021/acs.jafc.2c02402. [DOI] [PubMed] [Google Scholar]
- Mozuraityte R.; Kristinova V.; Rustad T.; Storrø I. The Role of Iron in Peroxidation of PUFA: Effect of pH and Chelators. Eur. J. Lipid Sci. Technol. 2016, 118, 658–668. 10.1002/ejlt.201400590. [DOI] [Google Scholar]
- Kristinová V.; Mozuraityte R.; Storrø I.; Rustad T. Antioxidant Activity of Phenolic Acids in Lipid Oxidation Catalyzed By Different Prooxidants. J. Agric. Food Chem. 2009, 57, 10377–10385. 10.1021/jf901072t. [DOI] [PubMed] [Google Scholar]
- Vidal N. P.; Goicoechea E.; Manzanos M. J.; Guillén M. D. 1H NMR Study of the Changes in Brine- and Dry-Salted Sea Bass Lipids Under Thermo-Oxidative Conditions: Both Salting Methods Reduce Oxidative Stability. Eur. J. Lipid Sci. Technol. 2015, 117, 440–449. 10.1002/ejlt.201400329. [DOI] [Google Scholar]
- Šimat V.; Bogdanović T.; Poljak V.; Petričević S. Changes in Fatty Acid Composition, Atherogenic and Thrombogenic Health Lipid Indices and Lipid Stability of Bogue (Boops boops Linnaeus, 1758) During Storage on Ice: Effect of Fish Farming Activities. J. Food Compos. Anal. 2015, 40, 120–125. 10.1016/j.jfca.2014.12.026. [DOI] [Google Scholar]
- Li X.; Luo Y.; You J.; Shen H. Stability of Papain-Treated Grass Carp (Ctenopharyngodon idellus) Protein Hydrolysate During Food Processing and Its Ability to Inhibit Lipid Oxidation in Frozen Fish Mince. J. Food Sci. Technol. 2015, 52, 542–548. 10.1007/s13197-013-1031-x. [DOI] [Google Scholar]
- Zorrilla S.; Mónico A.; Duarte S.; Rivas G.; Pérez-Sala D.; Pajares M. A. Integrated Approaches to Unravel the Impact of Protein Lipoxidation on Macromolecular Interactions. Free Radicals Biol. Med. 2019, 144, 203–217. 10.1016/j.freeradbiomed.2019.04.011. [DOI] [PubMed] [Google Scholar]
- Shibata T.; Uchida K. Protein Adductomics: A Comprehensive Analysis of Protein Modifications By Electrophiles. Free Radicals Biol. Med. 2019, 144, 218–222. 10.1016/j.freeradbiomed.2019.02.034. [DOI] [PubMed] [Google Scholar]
- Campos-Pinto I.; Méndez L.; Schouten J.; Wilkins J.; Fedorova M.; Pitt A. R.; Davis P.; Spickett C. M. Epitope Mapping and Characterization of 4-Hydroxy-2-nonenal Modified-Human Serum Albumin Using Two Different Polyclonal Antibodies. Free Radicals Biol. Med. 2019, 144, 234–244. 10.1016/j.freeradbiomed.2019.05.008. [DOI] [PubMed] [Google Scholar]
- de Oliveira F. C.; Coimbra J. S.; de Oliveira E. B.; Zuñiga A. D.; Rojas E. E. Food Protein-Polysaccharide Conjugates Obtained Via the Maillard Reaction: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1108–1125. 10.1080/10408398.2012.755669. [DOI] [PubMed] [Google Scholar]
- Hidalgo F. J.; Zamora R. Amino Acid Degradations Produced By Lipid Oxidation Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 1242–1252. 10.1080/10408398.2012.761173. [DOI] [PubMed] [Google Scholar]
- Smuda M.; Glomb M. A. Fragmentation Pathways During Maillard-Induced Carbohydrate Degradation. J. Agric. Food Chem. 2013, 61, 10198–10208. 10.1021/jf305117s. [DOI] [PubMed] [Google Scholar]
- Rizzi G. P. The Strecker Degradation of Amino Acids: Newer Avenues for Flavor Formation. Food Rev. Int. 2008, 24, 416–435. 10.1080/87559120802306058. [DOI] [Google Scholar]
- Mohan A.; Roy A.; Duggirala K.; Klein L. Oxidative Reactions of 4-Oxo-2-nonenal in Meat and Meat Products. LWT 2022, 165, 113747. 10.1016/j.lwt.2022.113747. [DOI] [Google Scholar]
- Lu F. S.; Nielsen N. S.; Baron C. P.; Diehl B. W.; Jacobsen C. Oxidative Stability of Dispersions Prepared From Purified Marine Phospholipid and the Role of Α-Tocopherol. J. Agric. Food Chem. 2012, 60, 12388–12396. 10.1021/jf303560f. [DOI] [PubMed] [Google Scholar]
- Wang T.; Zhen D.; Tan J.; Xie J.; Cheng J.; Zhao J. Characterization of Initial Reaction Intermediates in Heated Model Systems of Glucose, Glutathione, and Aliphatic Aldehydes. Food Chem. 2020, 305, 125482. 10.1016/j.foodchem.2019.125482. [DOI] [PubMed] [Google Scholar]
- Chaves-Filho A. B.; Yoshinaga M. Y.; Dantas L. S.; Diniz L. R.; Pinto I. F. D.; Miyamoto S. Mass Spectrometry Characterization of Thiol Conjugates Linked to Polyoxygenated Polyunsaturated Fatty Acid Species. Chem. Res. Toxicol. 2019, 32, 2028–2041. 10.1021/acs.chemrestox.9b00199. [DOI] [PubMed] [Google Scholar]
- Grippo V.; Mojovic M.; Pavicevic A.; Kabelac M.; Hubatka F.; Turanek J.; Zatloukalova M.; Freeman B. A.; Vacek J. Electrophilic Characteristics and Aqueous Behavior of Fatty Acid Nitroalkenes. Redox Biol. 2021, 38, 101756. 10.1016/j.redox.2020.101756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.; Zhang X.; Ye B.; Yan H.; Zhao Y.; Liu L. Effect of Unsaturated Fatty Acids on Glycation Product Formation Pathways. Food Res. Int. 2021, 143, 110288. 10.1016/j.foodres.2021.110288. [DOI] [PubMed] [Google Scholar]
- Lee C.; Barnett J.; Reaven P. D. Liposomes Enriched in Oleic Acid Are Less Susceptible to Oxidation and Have Less Proinflammatory Activity When Exposed to Oxidizing Conditions. J. Lipid Res. 1998, 39, 1239–1247. 10.1016/S0022-2275(20)32548-7. [DOI] [PubMed] [Google Scholar]
- Ding C.; Wang L.; Yao Y.; Li C. Mechanism of the Initial Oxidation of Monounsaturated Fatty Acids. Food Chem. 2022, 392, 133298. 10.1016/j.foodchem.2022.133298. [DOI] [PubMed] [Google Scholar]
- Rontani J. F. Use of Gas Chromatography-Mass Spectrometry Techniques (GC-MS, GC-MS/MS and GC-QTOF) for the Characterization of Photooxidation and Autoxidation Products of Lipids of Autotrophic Organisms in Environmental Samples. Molecules 2022, 27, 1629. 10.3390/molecules27051629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter N. A.; Caldwell S. E.; Mills K. A. Mechanisms of Free Radical Oxidation of Unsaturated Lipids. Lipids 1995, 30, 277–290. 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- Ni Z.; Milic I.; Fedorova M. Identification of Carbonylated Lipids From Different Phospholipid Classes By Shotgun and LC-MS Lipidomics. Anal. Bioanal. Chem. 2015, 407, 5161–5173. 10.1007/s00216-015-8536-2. [DOI] [PubMed] [Google Scholar]
- Jiménez-Rojo N.; Viguera A. R.; Collado M. I.; Sims K. H.; Constance C.; Hill K.; Shaw W. A.; Goñi F. M.; Alonso A. Sphingosine Induces the Aggregation of Imine-Containing Peroxidized Vesicles. Biochim. Biophys. Acta 2014, 1838, 2071–2077. 10.1016/j.bbamem.2014.04.028. [DOI] [PubMed] [Google Scholar]
- Han L.; Lin Q.; Liu G.; Han D.; Niu L. Review of the Formation and Influencing Factors of Food-Derived Glycated Lipids. Crit. Rev. Food Sci. Nutr. 2022, 62, 3490–3498. 10.1080/10408398.2020.1867052. [DOI] [PubMed] [Google Scholar]
- Choroszyński M.; Barcikowska M.; Barczak A. Metabolism and the Effect of Animal-Derived Oxysterols in the Diet on the Development of Alzheimer’s Disease. Ann. Nutr. Metab. 2022, 78, 125–132. 10.1159/000520514. [DOI] [PubMed] [Google Scholar]
- Naudí A.; Jové M.; Ayala V.; Cabré R.; Portero-Otín M.; Pamplona R. Non-Enzymatic Modification of Aminophospholipids By Carbonyl-Amine Reactions. Int. J. Mol. Sci. 2013, 14, 3285–3313. 10.3390/ijms14023285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guichardant M.; Bernoud-Hubac N.; Chantegrel B.; Deshayes C.; Lagarde M. Aldehydes From N-6 Fatty Acid Peroxidation. Effects on Aminophospholipids. Prostaglandins, Leukotrienes Essent. Fatty Acids 2002, 67, 147–149. 10.1054/plef.2002.0412. [DOI] [PubMed] [Google Scholar]
- Guichardant M.; Taibi-Tronche P.; Fay L. B.; Lagarde M. Covalent Modifications of Aminophospholipids By 4-Hydroxynonenal. Free Radicals Biol. Med. 1998, 25, 1049–1056. 10.1016/S0891-5849(98)00149-X. [DOI] [PubMed] [Google Scholar]
- Linetsky M.; Guo J.; Udeigwe E.; Ma D.; Chamberlain A. S.; Yu A. O.; Solovyova K.; Edgar E.; Salomon R. G. 4-Hydroxy-7-oxo-5-heptenoic Acid (HOHA) Lactone Induces Apoptosis in Retinal Pigment Epithelial Cells. Free Radicals Biol. Med. 2020, 152, 280–294. 10.1016/j.freeradbiomed.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernoud-Hubac N.; Fay L. B.; Armarnath V.; Guichardant M.; Bacot S.; Davies S. S.; Roberts L. J.; Lagarde M. Covalent Binding of Isoketals to Ethanolamine Phospholipids. Free Radicals Biol. Med. 2004, 37, 1604–1611. 10.1016/j.freeradbiomed.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Bacot S.; Bernoud-Hubac N.; Chantegrel B.; Deshayes C.; Doutheau A.; Ponsin G.; Lagarde M.; Guichardant M. Evidence for in Situ Ethanolamine Phospholipid Adducts With Hydroxy-Alkenals. J. Lipid Res. 2007, 48, 816–825. 10.1194/jlr.M600340-JLR200. [DOI] [PubMed] [Google Scholar]
- Zemski Berry K. A.; Murphy R. C. Characterization of Acrolein-Glycerophosphoethanolamine Lipid Adducts Using Electrospray Mass Spectrometry. Chem. Res. Toxicol. 2007, 20, 1342–1351. 10.1021/tx700102n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requena J. R.; Ahmed M. U.; Fountain C. W.; Degenhardt T. P.; Reddy S.; Perez C.; Lyons T. J.; Jenkins A. J.; Baynes J. W.; Thorpe S. R. Carboxymethylethanolamine, a Biomarker of Phospholipid Modification During the Maillard Reaction in Vivo. J. Biol. Chem. 1997, 272, 17473–17479. 10.1074/jbc.272.28.17473. [DOI] [PubMed] [Google Scholar]
- Pamplona R.; Requena J. R.; Portero-Otín M.; Prat J.; Thorpe S. R.; Bellmunt M. J. Carboxymethylated Phosphatidylethanolamine in Mitochondrial Membranes of Mammals-evidence for Intracellular Lipid Glycoxidation. Eur. J. Biochem. 1998, 255, 685–689. 10.1046/j.1432-1327.1998.2550685.x. [DOI] [PubMed] [Google Scholar]
- Hadley M.; Draper H. H. Identification of N-(2-propenal)ethanolamine as a Urinary Metabolite of Malondialdehyde. Free Radicals Biol. Med. 1989, 6, 49–52. 10.1016/0891-5849(89)90159-7. [DOI] [PubMed] [Google Scholar]
- Tsuji K.; Kawai Y.; Kato Y.; Osawa T. Formation of N-(hexanoyl)ethanolamine, a Novel Phosphatidylethanolamine Adduct, During the Oxidation of Erythrocyte Membrane and Low-Density Lipoprotein. Biochem. Biophys. Res. Commun. 2003, 306, 706–711. 10.1016/S0006-291X(03)01038-6. [DOI] [PubMed] [Google Scholar]
- Sullivan C. B.; Matafonova E.; Roberts L. J.; Amarnath V.; Davies S. S. Isoketals Form Cytotoxic Phosphatidylethanolamine Adducts in Cells. J. Lipid Res. 2010, 51, 999–1009. 10.1194/jlr.M001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L.; Lin Q.; Liu G.; Han D.; Niu L.; Su D. Lipids Promote Glycated Phospholipid Formation By Inducing Hydroxyl Radicals in a Maillard Reaction Model System. J. Agric. Food Chem. 2019, 67, 7961–7967. 10.1021/acs.jafc.9b02771. [DOI] [PubMed] [Google Scholar]
- Wanjala G. W.; Onyango A. N.; Abuga D. R.; Muchuna J. K.; Onyango C.; Makayoto M. Lysine Reacts With Cholesterol Hydroperoxide to Form Secosterol Aldehydes and Lysine-Secosterol Aldehyde Adducts. J. Chem. 2020, 2020, 1–8. 10.1155/2020/5862645. [DOI] [Google Scholar]
- Wanjala G. W.; Onyango A. N.; Abuga D.; Onyango C.; Makayoto M. Does Lysine Drive the Conversion of Fatty Acid Hydroperoxides to Aldehydes and Alkyl-Furans. Sci. Afr. 2021, 12, e00797 10.1016/j.sciaf.2021.e00797. [DOI] [Google Scholar]
- Solís-Calero C.; Ortega-Castro J.; Frau J.; Muñoz F. Nonenzymatic Reactions Above Phospholipid Surfaces of Biological Membranes: Reactivity of Phospholipids and Their Oxidation Derivatives. Oxid. Med. Cell. Longevity 2015, 2015, 319505. 10.1155/2015/319505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vistoli G.; De Maddis D.; Cipak A.; Zarkovic N.; Carini M.; Aldini G. Advanced Glycoxidation and Lipoxidation End Products (AGES and ALES): An Overview of Their Mechanisms of Formation. Free Radical Res. 2013, 47 (Suppl 1), 3–27. 10.3109/10715762.2013.815348. [DOI] [PubMed] [Google Scholar]
- He X.; Li Z.; Zhang Q. A UPLC-MRM-MS Method for Comprehensive Profiling of Amadori Compound-Modified Phosphatidylethanolamines in Human Plasma. Anal. Bioanal. Chem. 2021, 413, 431–443. 10.1007/s00216-020-03012-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X.; Chen G. Y.; Zhang Q. Comprehensive Identification of Amadori Compound-Modified Phosphatidylethanolamines in Human Plasma. Chem. Res. Toxicol. 2019, 32, 1449–1457. 10.1021/acs.chemrestox.9b00158. [DOI] [PubMed] [Google Scholar]
- Panov A. V.; Dikalov S. I. Cardiolipin, Perhydroxyl Radicals, and Lipid Peroxidation in Mitochondrial Dysfunctions and Aging. Oxid. Med. Cell. Longevity 2020, 2020, 1323028. 10.1155/2020/1323028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan V. E.; Tyurina Y. Y.; Tyurin V. A.; Mohammadyani D.; Angeli J. P.; Baranov S. V.; Klein-Seetharaman J.; Friedlander R. M.; Mallampalli R. K.; Conrad M.; et al. Cardiolipin Signaling Mechanisms: Collapse of Asymmetry and Oxidation. Antioxid. Redox Signal. 2015, 22, 1667–1680. 10.1089/ars.2014.6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciel E.; Domingues P.; Marques D.; Simões C.; Reis A.; Oliveira M. M.; Videira R. A.; Peixoto F.; Domingues M. R. M. Cardiolipin and Oxidative Stress: Identification of New Short Chain Oxidation Products of Cardiolipin in in Vitro Analysis and in Nephrotoxic Drug-Induced Disturbances in Rat Kidney Tissue. Int. J. Mass Spectrom. 2011, 301, 62–73. 10.1016/j.ijms.2010.06.036. [DOI] [Google Scholar]
- Khaselev N.; Murphy R. C. Susceptibility of Plasmenyl Glycerophosphoethanolamine Lipids Containing Arachidonate to Oxidative Degradation. Free Radicals Biol. Med. 1999, 26, 275–284. 10.1016/S0891-5849(98)00211-1. [DOI] [PubMed] [Google Scholar]
- Stadelmann-Ingrand S.; Pontcharraud R.; Fauconneau B. Evidence for the Reactivity of Fatty Aldehydes Released From Oxidized Plasmalogens With Phosphatidylethanolamine to Form Schiff Base Adducts in Rat Brain Homogenates. Chem. Phys. Lipids 2004, 131, 93–105. 10.1016/j.chemphyslip.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Bacot S.; Bernoud-Hubac N.; Baddas N.; Chantegrel B.; Deshayes C.; Doutheau A.; Lagarde M.; Guichardant M. Covalent Binding of Hydroxy-Alkenals 4-HDDE, 4-HHE, and 4-HNE to Ethanolamine Phospholipid Subclasses. J. Lipid Res. 2003, 44, 917–926. 10.1194/jlr.M200450-JLR200. [DOI] [PubMed] [Google Scholar]
- Jira W.; Spiteller G. Plasmalogens and Their Oxidative Degradation Products in Low and High Density Lipoprotein. Chem. Phys. Lipids 1996, 79, 95–100. 10.1016/0009-3084(95)02511-1. [DOI] [PubMed] [Google Scholar]
- Couto D.; Santinha D.; Melo T.; Ferreira-Fernandes E.; Videira R. A.; Campos A.; Fardilha M.; Domingues P.; Domingues M. R. Glycosphingolipids and Oxidative Stress: Evaluation of Hydroxyl Radical Oxidation of Galactosyl and Lactosylceramides Using Mass Spectrometry. Chem. Phys. Lipids 2015, 191, 106–114. 10.1016/j.chemphyslip.2015.08.014. [DOI] [PubMed] [Google Scholar]
- Melo T.; Maciel E.; Oliveira M. M.; Domingues P.; Domingues M. R. M. Study of Sphingolipids Oxidation By ESI Tandem MS. Eur. J. Lipid Sci. Technol. 2012, 114, 726–732. 10.1002/ejlt.201100328. [DOI] [Google Scholar]
- Iuliano L. Pathways of Cholesterol Oxidation Via Non-Enzymatic Mechanisms. Chem. Phys. Lipids 2011, 164, 457–468. 10.1016/j.chemphyslip.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Miyamoto S.; Lima R. S.; Inague A.; Viviani L. G. Electrophilic Oxysterols: Generation, Measurement and Protein Modification. Free Radical Res. 2021, 55, 416–440. 10.1080/10715762.2021.1879387. [DOI] [PubMed] [Google Scholar]
- Zmysłowski A.; Szterk A. Oxysterols as a Biomarker in Diseases. Clin. Chim. Acta 2019, 491, 103–113. 10.1016/j.cca.2019.01.022. [DOI] [PubMed] [Google Scholar]
- Diczfalusy U. On the Formation and Possible Biological Role of 25-Hydroxycholesterol. Biochimie 2013, 95, 455–460. 10.1016/j.biochi.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Leoni V.; Caccia C. Oxysterols as Biomarkers in Neurodegenerative Diseases. Chem. Phys. Lipids 2011, 164, 515–524. 10.1016/j.chemphyslip.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Canzoneri F.; Leoni V.; Rosso G.; Risso D.; Menta R.; Poli G. Oxysterols as Reliable Markers of Quality and Safety in Cholesterol Containing Food Ingredients and Products. Front. Nutr. 2022, 9, 853460. 10.3389/fnut.2022.853460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo F.; Andreu I.; Brunetti M.; Schmallegger M.; Gescheidt G.; Neshchadin D.; Miranda M. A. Hydrogen Abstraction From the C15 Position of the Cholesterol Skeleton. J. Org. Chem. 2019, 84, 15184–15191. 10.1021/acs.joc.9b02181. [DOI] [PubMed] [Google Scholar]
- Zielinski Z. A.; Pratt D. A. Cholesterol Autoxidation Revisited: Debunking the Dogma Associated With the Most Vilified of Lipids. J. Am. Chem. Soc. 2016, 138, 6932–6935. 10.1021/jacs.6b03344. [DOI] [PubMed] [Google Scholar]
- Zielinski Z. A. M.; Pratt D. A. H-Atom Abstraction vs Addition: Accounting for the Diverse Product Distribution in the Autoxidation of Cholesterol and Its Esters. J. Am. Chem. Soc. 2019, 141, 3037–3051. 10.1021/jacs.8b11524. [DOI] [PubMed] [Google Scholar]
- Griffiths W. J.; Yutuc E.; Abdel-Khalik J.; Crick P. J.; Hearn T.; Dickson A.; Bigger B. W.; Hoi-Yee Wu T.; Goenka A.; Ghosh A.; et al. Metabolism of Non-Enzymatically Derived Oxysterols: Clues From Sterol Metabolic Disorders. Free Radicals Biol. Med. 2019, 144, 124–133. 10.1016/j.freeradbiomed.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer E. L.; Zopyrus N.; Zielinski Z. A. M.; Facey G. A.; Pratt D. A. On the Products of Cholesterol Autoxidation in Phospholipid Bilayers and the Formation of Secosterols Derived Therefrom. Angew. Chem., Int. Ed. Engl. 2020, 59, 2089–2094. 10.1002/anie.201914637. [DOI] [PubMed] [Google Scholar]
- Cygan N. K.; Scheinost J. C.; Butters T. D.; Wentworth P. Adduction of Cholesterol 5,6-Secosterol Aldehyde to Membrane-Bound Myelin Basic Protein Exposes an Immunodominant Epitope. Biochemistry 2011, 50, 2092–2100. 10.1021/bi200109q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchalski H.; Xu L.; Porter N. A. Tunneling in Tocopherol-Mediated Peroxidation of 7-Dehydrocholesterol. Org. Biomol. Chem. 2015, 13, 1249–1253. 10.1039/C4OB02377C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.; Porter N. A. Reactivities and Products of Free Radical Oxidation of Cholestadienols. J. Am. Chem. Soc. 2014, 136, 5443–5450. 10.1021/ja5011674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter N. A.; Xu L.; Pratt D. A. Reactive Sterol Electrophiles: Mechanisms of Formation and Reactions With Proteins and Amino Acid Nucleophiles. Chemistry (Basel, Switz.) 2020, 2, 390–417. 10.3390/chemistry2020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spickett C. M.; Jerlich A.; Panasenko O. M.; Arnhold J.; Pitt A. R.; Stelmaszyńska T.; Schaur R. J. The Reactions of Hypochlorous Acid, the Reactive Oxygen Species Produced By Myeloperoxidase, With Lipids. Acta Biochim. Polym. 2000, 47, 889–899. 10.18388/abp.2000_3944. [DOI] [PubMed] [Google Scholar]
- Rayner B. S.; Love D. T.; Hawkins C. L. Comparative Reactivity of Myeloperoxidase-Derived Oxidants With Mammalian Cells. Free Radicals Biol. Med. 2014, 71, 240–255. 10.1016/j.freeradbiomed.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Nusshold C.; Kollroser M.; Köfeler H.; Rechberger G.; Reicher H.; Ullen A.; Bernhart E.; Waltl S.; Kratzer I.; Hermetter A.; et al. Hypochlorite Modification of Sphingomyelin Generates Chlorinated Lipid Species That Induce Apoptosis and Proteome Alterations in Dopaminergic PC12 Neurons in Vitro. Free Radicals Biol. Med. 2010, 48, 1588–1600. 10.1016/j.freeradbiomed.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessig J.; Schiller J.; Arnhold J.; Fuchs B. Hypochlorous Acid-Mediated Generation of Glycerophosphocholine From Unsaturated Plasmalogen Glycerophosphocholine Lipids. J. Lipid Res. 2007, 48, 1316–1324. 10.1194/jlr.M600478-JLR200. [DOI] [PubMed] [Google Scholar]
- Lessig J.; Fuchs B. HOCl-Mediated Glycerophosphocholine and Glycerophosphoethanolamine Generation From Plasmalogens in Phospholipid Mixtures. Lipids 2010, 45, 37–51. 10.1007/s11745-009-3365-8. [DOI] [PubMed] [Google Scholar]
- Santrock J.; Gorski R. A.; O’Gara J. F. Products and Mechanism of the Reaction of Ozone With Phospholipids in Unilamellar Phospholipid Vesicles. Chem. Res. Toxicol. 1992, 5, 134–141. 10.1021/tx00025a023. [DOI] [PubMed] [Google Scholar]
- Squadrito G. L.; Uppu R. M.; Cueto R.; Pryor W. A. Production of the Criegee Ozonide During the Ozonation of 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine Liposomes. Lipids 1992, 27, 955–958. 10.1007/BF02535571. [DOI] [PubMed] [Google Scholar]
- Wisthaler A.; Weschler C. J. Reactions of Ozone With Human Skin Lipids: Sources of Carbonyls, Dicarbonyls, and Hydroxycarbonyls in Indoor Air. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 6568–6575. 10.1073/pnas.0904498106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almstrand A. C.; Voelker D.; Murphy R. C. Identification of Oxidized Phospholipids in Bronchoalveolar Lavage Exposed to Low Ozone Levels Using Multivariate Analysis. Anal. Biochem. 2015, 474, 50–58. 10.1016/j.ab.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlson C.; Harrison K.; Allen C. B.; Ahmad S.; White C. W.; Murphy R. C. Oxidized Phospholipids Derived From Ozone-Treated Lung Surfactant Extract Reduce Macrophage and Epithelial Cell Viability. Chem. Res. Toxicol. 2002, 15, 896–906. 10.1021/tx010183i. [DOI] [PubMed] [Google Scholar]
- Pryor W. A.; Squadrito G. L.; Friedman M. A New Mechanism for the Toxicity of Ozone. Toxicol. Lett. 1995, 82–83, 287–293. 10.1016/0378-4274(95)03563-X. [DOI] [PubMed] [Google Scholar]
- Uppu R. M.; Cueto R.; Squadrito G. L.; Pryor W. A. What Does Ozone React With At the Air/lung Interface? Model Studies Using Human Red Blood Cell Membranes. Arch. Biochem. Biophys. 1995, 319, 257–266. 10.1006/abbi.1995.1290. [DOI] [PubMed] [Google Scholar]
- Ballinger C. A.; Cueto R.; Squadrito G.; Coffin J. F.; Velsor L. W.; Pryor W. A.; Postlethwait E. M. Antioxidant-Mediated Augmentation of Ozone-Induced Membrane Oxidation. Free Radicals Biol. Med. 2005, 38, 515–526. 10.1016/j.freeradbiomed.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Park H.; Kim P.; Jiang Y.; Costello C. E. Surface Oxidation Under Ambient Air-not Only a Fast and Economical Method to Identify Double Bond Positions in Unsaturated Lipids But Also a Reminder of Proper Lipid Processing. Anal. Chem. 2014, 86, 5697–5705. 10.1021/ac404214a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer C.; Schmidt R. Physical Mechanisms of Generation and Deactivation of Singlet Oxygen. Chem. Rev. 2003, 103, 1685–1757. 10.1021/cr010371d. [DOI] [PubMed] [Google Scholar]
- Hayyan M.; Hashim M. A.; AlNashef I. M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016, 116, 3029–3085. 10.1021/acs.chemrev.5b00407. [DOI] [PubMed] [Google Scholar]
- Miyamoto S.; Ronsein G. E.; Prado F. M.; Uemi M.; Corrêa T. C.; Toma I. N.; Bertolucci A.; Oliveira M. C.; Motta F. D.; Medeiros M. H.; et al. Biological Hydroperoxides and Singlet Molecular Oxygen Generation. IUBMB Life 2007, 59, 322–331. 10.1080/15216540701242508. [DOI] [PubMed] [Google Scholar]
- Regensburger J.; Maisch T.; Knak A.; Gollmer A.; Felgentraeger A.; Lehner K.; Baeumler W. Uva Irradiation of Fatty Acids and Their Oxidized Products Substantially Increases Their Ability to Generate Singlet Oxygen. Phys. Chem. Chem. Phys. 2013, 15, 17672–17680. 10.1039/c3cp51399h. [DOI] [PubMed] [Google Scholar]
- Mano C. M.; Prado F. M.; Massari J.; Ronsein G. E.; Martinez G. R.; Miyamoto S.; Cadet J.; Sies H.; Medeiros M. H.; Bechara E. J.; et al. Excited Singlet Molecular O2(1Δg) is Generated Enzymatically From Excited Carbonyls in the Dark. Sci. Rep. 2014, 4, 5938. 10.1038/srep05938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mascio P.; Martinez G. R.; Miyamoto S.; Ronsein G. E.; Medeiros M. H.; Cadet J. Singlet Molecular Oxygen: Düsseldorf - São Paulo, the Brazilian Connection. Arch. Biochem. Biophys. 2016, 595, 161–175. 10.1016/j.abb.2015.11.016. [DOI] [PubMed] [Google Scholar]
- Turro N. J.; Chow M. F.; Rigaudy J. Mechanism of Thermolysis of Endoperoxides of Aromatic Compounds. Activation Parameters, Magnetic Field, and Magnetic Isotope Effects. J. Am. Chem. Soc. 1981, 103, 7218–7224. 10.1021/ja00414a029. [DOI] [Google Scholar]
- Min D. B.; Boff J. M. Chemistry and Reaction of Singlet Oxygen in Foods. Compr. Rev. Food Sci. Food Saf. 2002, 1, 58–72. 10.1111/j.1541-4337.2002.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Miranda É. G. A.; Araujo-Chaves J. C.; Kawai C.; Brito A. M. M.; Dias I. W. R.; Arantes J. T.; Nantes-Cardoso I. L. Cardiolipin Structure and Oxidation Are Affected By Ca2+ At the Interface of Lipid Bilayers. Front. Chem. 2020, 7, 930. 10.3389/fchem.2019.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirot M.; Silvente-Poirot S. Cholesterol-5,6-epoxides: Chemistry, Biochemistry, Metabolic Fate and Cancer. Biochimie 2013, 95, 622–631. 10.1016/j.biochi.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Morand O. H.; Zoeller R. A.; Raetz C. R. Disappearance of Plasmalogens From Membranes of Animal Cells Subjected to Photosensitized Oxidation. J. Biol. Chem. 1988, 263, 11597–11606. 10.1016/S0021-9258(18)38001-3. [DOI] [PubMed] [Google Scholar]
- Santinha D.; Ferreira-Fernandes E.; Melo T.; Silva E. M.; Maciel E.; Fardilha M.; Domingues P.; Domingues M. R. Evaluation of the Photooxidation of Galactosyl- and Lactosylceramide By Electrospray Ionization Mass Spectrometry. Rapid Commun. Mass Spectrom. 2014, 28, 2275–2284. 10.1002/rcm.7020. [DOI] [PubMed] [Google Scholar]
- Melo T.; Silva E. M.; Simões C.; Domingues P.; Domingues M. R. Photooxidation of Glycated and Non-Glycated Phosphatidylethanolamines Monitored By Mass Spectrometry. J. Mass Spectrom. 2013, 48, 68–78. 10.1002/jms.3129. [DOI] [PubMed] [Google Scholar]
- Garrec J.; Monari A.; Assfeld X.; Mir L. M.; Tarek M. Lipid Peroxidation in Membranes: The Peroxyl Radical Does Not “float. J. Phys. Chem. Lett. 2014, 5, 1653–1658. 10.1021/jz500502q. [DOI] [PubMed] [Google Scholar]
- Else P. L.; Kraffe E. Docosahexaenoic and Arachidonic Acid Peroxidation: It’s a Within Molecule Cascade. Biochim. Biophys. Acta 2015, 1848, 417–421. 10.1016/j.bbamem.2014.10.039. [DOI] [PubMed] [Google Scholar]
- Tyurina Y. Y.; Tyurin V. A.; Anthonymuthu T.; Amoscato A. A.; Sparvero L. J.; Nesterova A. M.; Baynard M. L.; Sun W.; He R.; Khaitovich P.; et al. Redox Lipidomics Technology: Looking for a Needle in a Haystack. Chem. Phys. Lipids 2019, 221, 93–107. 10.1016/j.chemphyslip.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A.; Shibasaki-Kitakawa N.; Noda T.; Sukegawa Y.; Kimura Y.; Yonemoto T. Kinetic Analysis of Co-Oxidation of Biomembrane Lipids Induced By Water-Soluble Radicals. J. Am. Oil Chem. Soc. 2016, 93, 803–811. 10.1007/s11746-016-2821-x. [DOI] [Google Scholar]
- Chang Y. H.; Abdalla D. S.; Sevanian A. Characterization of Cholesterol Oxidation Products Formed By Oxidative Modification of Low Density Lipoprotein. Free Radicals Biol. Med. 1997, 23, 202–214. 10.1016/S0891-5849(96)00626-0. [DOI] [PubMed] [Google Scholar]
- Onyango A. N. Formation of Aldehydic Phosphatidylcholines During the Anaerobic Decomposition of a Phosphatidylcholine Bearing the 9-Hydroperoxide of Linoleic Acid. Biomed. Res. Int. 2016, 2016, 8218439. 10.1155/2016/8218439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A. H.; Catalá Á.; Vignoni M. Soybean Phosphatidylcholine Liposomes as Model Membranes to Study Lipid Peroxidation Photoinduced By Pterin. Biochim. Biophys. Acta 2016, 1858, 139–145. 10.1016/j.bbamem.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Stutts W. L.; Menger R. F.; Kiss A.; Heeren R. M.; Yost R. A. Characterization of Phosphatidylcholine Oxidation Products By MALDI MSn. Anal. Chem. 2013, 85, 11410–11419. 10.1021/ac402400f. [DOI] [PubMed] [Google Scholar]
- Zuidam N. J.; Versluis C.; Vernooy E. A.; Crommelin D. J. Gamma-Irradiation of Liposomes Composed of Saturated Phospholipids: Effect of Bilayer Composition, Size, Concentration and Absorbed Dose on Chemical Degradation and Physical Destabilization of Liposomes. Biochim. Biophys. Acta 1996, 1280, 135–148. 10.1016/0005-2736(95)00275-8. [DOI] [PubMed] [Google Scholar]
- Green M. C.; Nakata H.; Fedorov D. G.; Slipchenko L. V. Radical Damage in Lipids Investigated With the Fragment Molecular Orbital Method. Chem. Phys. Lett. 2016, 651, 56–61. 10.1016/j.cplett.2016.03.014. [DOI] [Google Scholar]
- Breton M.; Amirkavei M.; Mir L. M. Optimization of the Electroformation of Giant Unilamellar Vesicles (GUVs) With Unsaturated Phospholipids. J. Membr. Biol. 2015, 248, 827–835. 10.1007/s00232-015-9828-3. [DOI] [PubMed] [Google Scholar]
- Luo D.; Li N.; Carter K. A.; Lin C.; Geng J.; Shao S.; Huang W. C.; Qin Y.; Atilla-Gokcumen G. E.; Lovell J. F. Rapid Light-Triggered Drug Release in Liposomes Containing Small Amounts of Unsaturated and Porphyrin-Phospholipids. Small 2016, 12, 3039–3047. 10.1002/smll.201503966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte E.; Megli F. M.; Khandelia H.; Jeschke G.; Bordignon E. Lipid Peroxidation and Water Penetration in Lipid Bilayers: A W-Band EPR Study. Biochim. Biophys. Acta 2013, 1828, 510–517. 10.1016/j.bbamem.2012.09.026. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz P.; Olżyńska A.; Cwiklik L.; Conte E.; Jungwirth P.; Megli F. M.; Hof M. Biophysics of Lipid Bilayers Containing Oxidatively Modified Phospholipids: Insights From Fluorescence and EPR Experiments and From MD Simulations. Biochim. Biophys. Acta 2012, 1818, 2388–2402. 10.1016/j.bbamem.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Wiczew D.; Szulc N.; Tarek M. Molecular Dynamics Simulations of the Effects of Lipid Oxidation on the Permeability of Cell Membranes. Bioelectrochemistry 2021, 141, 107869. 10.1016/j.bioelechem.2021.107869. [DOI] [PubMed] [Google Scholar]
- Cwiklik L.; Jungwirth P. Massive Oxidation of Phospholipid Membranes Leads to Pore Creation and Bilayer Disintegration. Chem. Phys. Lett. 2010, 486, 99–103. 10.1016/j.cplett.2010.01.010. [DOI] [Google Scholar]
- Sankhagowit S.; Wu S. H.; Biswas R.; Riche C. T.; Povinelli M. L.; Malmstadt N. The Dynamics of Giant Unilamellar Vesicle Oxidation Probed By Morphological Transitions. Biochim. Biophys. Acta 2014, 1838, 2615–2624. 10.1016/j.bbamem.2014.06.020. [DOI] [PubMed] [Google Scholar]
- Khabiri M.; Roeselova M.; Cwiklik L. Properties of Oxidized Phospholipid Monolayers: An Atomistic Molecular Dynamics Study. Chem. Phys. Lett. 2012, 519–520, 93–99. 10.1016/j.cplett.2011.11.016. [DOI] [Google Scholar]
- Qiao L.; Ge A.; Liang Y.; Ye S. Oxidative Degradation of the Monolayer of 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) in Low-Level Ozone. J. Phys. Chem. B 2015, 119, 14188–14199. 10.1021/acs.jpcb.5b08985. [DOI] [PubMed] [Google Scholar]
- Valentine R. C.; Valentine D. L.. Lipid Whisker Model and Water-Wire Theory of Energy Uncoupling in Neurons. In Neurons and the DHA Principle; CRC Press: Boca Raton, FL, 2012; pp 126–135. [Google Scholar]
- Megli F. M.; Russo L. Different Oxidized Phospholipid Molecules Unequally Affect Bilayer Packing. Biochim. Biophys. Acta 2008, 1778, 143–152. 10.1016/j.bbamem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Knobloch J. J.; Nelson A. R.; Köper I.; James M.; McGillivray D. J. Oxidative Damage to Biomimetic Membrane Systems: In Situ Fe(II)/Ascorbate Initiated Oxidation and Incorporation of Synthetic Oxidized Phospholipids. Langmuir 2015, 31, 12679–12687. 10.1021/acs.langmuir.5b02458. [DOI] [PubMed] [Google Scholar]
- Mendes Ferreira T.; Sood R.; Bärenwald R.; Carlström G.; Topgaard D.; Saalwächter K.; Kinnunen P. K.; Ollila O. H. Acyl Chain Disorder and Azelaoyl Orientation in Lipid Membranes Containing Oxidized Lipids. Langmuir 2016, 32, 6524–6533. 10.1021/acs.langmuir.6b00788. [DOI] [PubMed] [Google Scholar]
- Makky A.; Tanaka M. Impact of Lipid Oxidization on Biophysical Properties of Model Cell Membranes. J. Phys. Chem. B 2015, 119, 5857–5863. 10.1021/jp512339m. [DOI] [PubMed] [Google Scholar]
- Lidman M.; Pokorná Š.; Dingeldein A. P.; Sparrman T.; Wallgren M.; Šachl R.; Hof M.; Gröbner G. The Oxidized Phospholipid PazePC Promotes Permeabilization of Mitochondrial Membranes By Bax. Biochim. Biophys. Acta 2016, 1858, 1288–1297. 10.1016/j.bbamem.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Runas K. A.; Acharya S. J.; Schmidt J. J.; Malmstadt N. Addition of Cleaved Tail Fragments During Lipid Oxidation Stabilizes Membrane Permeability Behavior. Langmuir 2016, 32, 779–786. 10.1021/acs.langmuir.5b02980. [DOI] [PubMed] [Google Scholar]
- Runas K. A.; Malmstadt N. Low Levels of Lipid Oxidation Radically Increase the Passive Permeability of Lipid Bilayers. Soft Matter 2015, 11, 499–505. 10.1039/C4SM01478B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volinsky R.; Cwiklik L.; Jurkiewicz P.; Hof M.; Jungwirth P.; Kinnunen P. K. Oxidized Phosphatidylcholines Facilitate Phospholipid Flip-Flop in Liposomes. Biophys. J. 2011, 101, 1376–1384. 10.1016/j.bpj.2011.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland M. C.; Parikh A. N. Model Studies of Membrane Disruption By Photogenerated Oxidative Assault. J. Phys. Chem. B 2010, 114, 6377–6385. 10.1021/jp102861v. [DOI] [PubMed] [Google Scholar]
- Volinsky R.; Paananen R.; Kinnunen P. K. Oxidized Phosphatidylcholines Promote Phase Separation of Cholesterol-Sphingomyelin Domains. Biophys. J. 2012, 103, 247–254. 10.1016/j.bpj.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröning A.; Ahrens H.; Ortmann T.; Lawrenz F.; Brezesinski G.; Scholz F.; Helm C. A. Molecular Mechanisms of Phosphatidylcholine Monolayer Solidification Due to Hydroxyl Radicals. Soft Matter 2011, 7, 6467. 10.1039/c1sm05312d. [DOI] [Google Scholar]
- Pootheri A.; Lopez W. M.; Saraswathy R. Action of Reactive Oxygen Species in Metabolic Diseases Employed as Biomarkers of Oxidative Stress. Res. J. Biotechnol. 2022, 17, 207–215. 10.25303/1712rjbt2070215. [DOI] [Google Scholar]
- Viedma-Poyatos Á.; González-Jiménez P.; Langlois O.; Company-Marín I.; Spickett C. M.; Pérez-Sala D. Protein Lipoxidation: Basic Concepts and Emerging Roles. Antioxidants 2021, 10, 295. 10.3390/antiox10020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Yang X.; Xiao F.; Jie F.; Zhang Q.; Liu Y.; Xiao H.; Lu B. Dietary Cholesterol Oxidation Products: Perspectives Linking Food Processing and Storage With Health Implications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 738–779. 10.1111/1541-4337.12880. [DOI] [PubMed] [Google Scholar]
- Brown R. B. Phospholipid Packing Defects and Oxysterols in Atherosclerosis: Dietary Prevention and the French Paradox. Biochimie 2019, 167, 145–151. 10.1016/j.biochi.2019.09.020. [DOI] [PubMed] [Google Scholar]
- Das U. N. Cell Membrane Theory of Senescence” and the Role of Bioactive Lipids in Aging, and Aging Associated Diseases and Their Therapeutic Implications. Biomolecules 2021, 11, 241. 10.3390/biom11020241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.; Tang L.; Zhang Y.; Ge G.; Jiang X.; Mo Y.; Wu P.; Deng X.; Li L.; Zuo S.; et al. Regulatory Pathways and Drugs Associated With Ferroptosis in Tumors. Cell Death Dis. 2022, 13, 544. 10.1038/s41419-022-04927-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Li X.; Zheng C.; Yang C.; Zhang R.; Wang A.; Feng J.; Hu X.; Chang S.; Zhang H. Ferroptosis, a New Form of Cell Death Defined After Radiation Exposure. Int. J. Radiat. Biol. 2022, 98, 1201–1209. 10.1080/09553002.2022.2020358. [DOI] [PubMed] [Google Scholar]
- Tang D.; Chen X.; Kang R.; Kroemer G. Ferroptosis: Molecular Mechanisms and Health Implications. Cell Res. 2021, 31, 107–125. 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.; Li X.; Xu X.; Li L.; Liang N.; Zhang L.; Lv J.; Wu Y. C.; Yin H. Ferroptosis and Cardiovascular Disease: Role of Free Radical-Induced Lipid Peroxidation. Free Radical Res. 2021, 55, 405–415. 10.1080/10715762.2021.1876856. [DOI] [PubMed] [Google Scholar]
- Stamenkovic A.; Pierce G. N.; Ravandi A. Phospholipid Oxidation Products in Ferroptotic Myocardial Cell Death. Am. J. Physiol.: Heart Circ. Physiol. 2019, 317, H156–H163. 10.1152/ajpheart.00076.2019. [DOI] [PubMed] [Google Scholar]
- Stoyanovsky D. A.; Tyurina Y. Y.; Shrivastava I.; Bahar I.; Tyurin V. A.; Protchenko O.; Jadhav S.; Bolevich S. B.; Kozlov A. V.; Vladimirov Y. A.; et al. Iron Catalysis of Lipid Peroxidation in Ferroptosis: Regulated Enzymatic or Random Free Radical Reaction. Free Radicals Biol. Med. 2019, 133, 153–161. 10.1016/j.freeradbiomed.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spickett C. M.Oxidized Phospholipid Signaling: Distress to Eustress. In Oxidative Stress: Eustress and Distress; Sies H., Ed.; Elsevier, 2020; pp 263–285. [Google Scholar]
- Sottero B.; Leonarduzzi G.; Testa G.; Gargiulo S.; Poli G.; Biasi F. Lipid Oxidation Derived Aldehydes and Oxysterols Between Health and Disease. Eur. J. Lipid Sci. Technol. 2019, 121, 1700047. 10.1002/ejlt.201700047. [DOI] [Google Scholar]
- Zangar R. C.; Davydov D. R.; Verma S. Mechanisms That Regulate Production of Reactive Oxygen Species By Cytochrome P450. Toxicol. Appl. Pharmacol. 2004, 199, 316–331. 10.1016/j.taap.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Ni Z.; Goracci L.; Cruciani G.; Fedorova M. Computational Solutions in Redox Lipidomics - Current Strategies and Future Perspectives. Free Radicals Biol. Med. 2019, 144, 110–123. 10.1016/j.freeradbiomed.2019.04.027. [DOI] [PubMed] [Google Scholar]
- Petrucci G.; Rizzi A.; Hatem D.; Tosti G.; Rocca B.; Pitocco D. Role of Oxidative Stress in the Pathogenesis of Atherothrombotic Diseases. Antioxidants 2022, 11, 1408. 10.3390/antiox11071408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhigbe R.; Ajayi A. The Impact of Reactive Oxygen Species in the Development of Cardiometabolic Disorders: A Review. Lipids Health Dis. 2021, 20, 23. 10.1186/s12944-021-01435-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhayaza R.; Haque E.; Karbasiafshar C.; Sellke F. W.; Abid M. R. The Relationship Between Reactive Oxygen Species and Endothelial Cell Metabolism. Front. Chem. 2020, 8, 592688. 10.3389/fchem.2020.592688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshtasbi H.; Pakchin P. S.; Movafeghi A.; Barar J.; Castejon A. M.; Omidian H.; Omidi Y. Impacts of Oxidants and Antioxidants on the Emergence and Progression of Alzheimer’s Disease. Neurochem. Int. 2022, 153, 105268. 10.1016/j.neuint.2021.105268. [DOI] [PubMed] [Google Scholar]
- Hassan W.; Noreen H.; Rehman S.; Kamal M. A.; da Rocha J. B. T. Association of Oxidative Stress With Neurological Disorders. Curr. Neuropharmacol. 2022, 20, 1046–1072. 10.2174/1570159X19666211111141246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.; Kukreti R.; Saso L.; Kukreti S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. 10.3390/molecules24081583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaradei A.; Wang Z.; Malmstadt N. Oxidation of Membrane Lipids Alters the Activity of the Human Serotonin 1a Receptor. Langmuir 2022, 38, 6798–6807. 10.1021/acs.langmuir.1c03238. [DOI] [PubMed] [Google Scholar]
- Ferreri C.; Sansone A.; Krokidis M. G.; Masi A.; Pascucci B.; D’Errico M.; Chatgilialoglu C. Effects of Oxygen Tension for Membrane Lipidome Remodeling of Cockayne Syndrome Cell Models. Cells 2022, 11, 1286. 10.3390/cells11081286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasoni M. G.; Benedetti S.; Crinelli R.; Palma F.; Canonico B.; Monittola F.; Zerbinati C.; Iuliano L.; Luchetti F. 3β-Hydroxy-5β-hydroxy-B-norcholestane-6β-carboxaldehyde (SEC-B) Induces Proinflammatory Activation of Human Endothelial Cells Associated With Nitric Oxide Production and Endothelial Nitric Oxide Synthase/caveolin-1 Dysregulation. Antioxidants 2022, 11, 1148. 10.3390/antiox11061148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaganjac M.; Cindrić M.; Jakovčević A.; Žarković K.; Žarković N. Lipid Peroxidation in Brain Tumors. Neurochem. Int. 2021, 149, 105118. 10.1016/j.neuint.2021.105118. [DOI] [PubMed] [Google Scholar]
- Choudhury S.; Pan J.; Amin S.; Chung F. L.; Roy R. Repair Kinetics of Trans-4-hydroxynonenal-induced Cyclic 1, N2-Propanodeoxyguanine DNA Adducts By Human Cell Nuclear Extracts. Biochemistry 2004, 43, 7514–7521. 10.1021/bi049877r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samadi A.; Sabuncuoglu S.; Samadi M.; Isikhan S. Y.; Chirumbolo S.; Peana M.; Lay I.; Yalcinkaya A.; Bjørklund G. A Comprehensive Review on Oxysterols and Related Diseases. Curr. Med. Chem. 2020, 28, 110–136. 10.2174/0929867327666200316142659. [DOI] [PubMed] [Google Scholar]
- Sottero B.; Rossin D.; Staurenghi E.; Gamba P.; Poli G.; Testa G. Omics Analysis of Oxysterols to Better Understand Their Pathophysiological Role. Free Radicals Biol. Med. 2019, 144, 55–71. 10.1016/j.freeradbiomed.2019.05.026. [DOI] [PubMed] [Google Scholar]
- Suzuki A.; Urano Y.; Ishida T.; Noguchi N. Different Functions of Vitamin E Homologues in the Various Types of Cell Death Induced By Oxysterols. Free Radicals Biol. Med. 2021, 176, 356–365. 10.1016/j.freeradbiomed.2021.10.008. [DOI] [PubMed] [Google Scholar]
- Chatgilialoglu C.; Ferreri C.; Melchiorre M.; Sansone A.; Torreggiani A. Lipid Geometrical Isomerism: From Chemistry to Biology and Diagnostics. Chem. Rev. 2014, 114, 255–284. 10.1021/cr4002287. [DOI] [PubMed] [Google Scholar]
- Chatgilialoglu C.; Samadi A.; Guerra M.; Fischer H. The Kinetics of Z/E Isomerization of Methyl Oleate Catalyzed By Photogenerated Thiyl Radicals. ChemPhysChem 2005, 6, 286–291. 10.1002/cphc.200400388. [DOI] [PubMed] [Google Scholar]
- Chatgilialoglu C.; Altieri A.; Fischer H. The Kinetics of Thiyl Radical-Induced Reactions of Monounsaturated Fatty Acid Esters. J. Am. Chem. Soc. 2002, 124, 12816–12823. 10.1021/ja027428d. [DOI] [PubMed] [Google Scholar]
- Tzeng Y. Z.; Hu C. H. Radical-Induced Cis-Trans Isomerization of Fatty Acids: A Theoretical Study. J. Phys. Chem. A 2014, 118, 4554–4564. 10.1021/jp502434t. [DOI] [PubMed] [Google Scholar]
- Degirmenci I.; Coote M. L. Comparison of Thiyl, Alkoxyl, and Alkyl Radical Addition to Double Bonds: The Unusual Contrasting Behavior of Sulfur and Oxygen Radical Chemistry. J. Phys. Chem. A 2016, 120, 1750–1755. 10.1021/acs.jpca.6b00538. [DOI] [PubMed] [Google Scholar]
- Trujillo M.; Alvarez B.; Radi R. One- and Two-Electron Oxidation of Thiols: Mechanisms, Kinetics and Biological Fates. Free Radical Res. 2016, 50, 150–171. 10.3109/10715762.2015.1089988. [DOI] [PubMed] [Google Scholar]
- Nauser T.; Koppenol W. H.; Schöneich C. Protein Thiyl Radical Reactions and Product Formation: A Kinetic Simulation. Free Radicals Biol. Med. 2015, 80, 158–163. 10.1016/j.freeradbiomed.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunath S.; Schindeldecker M.; De Giacomo A.; Meyer T.; Sohre S.; Hajieva P.; von Schacky C.; Urban J.; Moosmann B. Prooxidative Chain Transfer Activity By Thiol Groups in Biological Systems. Redox Biol. 2020, 36, 101628. 10.1016/j.redox.2020.101628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozziconacci O.; Williams T. D.; Schöneich C. Intramolecular Hydrogen Transfer Reactions of Thiyl Radicals From Glutathione: Formation of Carbon-Centered Radical At Glu, Cys, and Gly. Chem. Res. Toxicol. 2012, 25, 1842–1861. 10.1021/tx3000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozziconacci O.; Kerwin B. A.; Schöneich C. Reversible Hydrogen Transfer Reactions of Cysteine Thiyl Radicals in Peptides: The Conversion of Cysteine Into Dehydroalanine and Alanine, and of Alanine Into Dehydroalanine. J. Phys. Chem. B 2011, 115, 12287–12305. 10.1021/jp2070453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykakis I. N.; Ferreri C.; Chatgilialoglu C. The Sulfhydryl Radical (HS•/S•-): A Contender for the Isomerization of Double Bonds in Membrane Lipids. Angew. Chem., Int. Ed. Engl. 2007, 46, 1914–1916. 10.1002/anie.200604525. [DOI] [PubMed] [Google Scholar]
- Nauser T.; Koppenol W. H.; Schöneich C. Reversible Hydrogen Transfer Reactions in Thiyl Radicals From Cysteine and Related Molecules: Absolute Kinetics and Equilibrium Constants Determined By Pulse Radiolysis. J. Phys. Chem. B 2012, 116, 5329–5341. 10.1021/jp210954v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaljević B.; Bujak I. T. Lipid Modification Processes Induced By Thiyl Radicals. Radiat. Phys. Chem. 2016, 124, 99–103. 10.1016/j.radphyschem.2016.01.034. [DOI] [Google Scholar]
- Prajapati I.; Subelzu N.; Zhang Y.; Wu Y.; Schöneich C. Near UV and Visible Light Photo-Degradation Mechanisms in Citrate Buffer: One-Electron Reduction of Peptide and Protein Disulfides Promotes Oxidation and Cis/Trans Isomerization of Unsaturated Fatty Acids of Polysorbate 80. J. Pharm. Sci. 2022, 111, 991–1003. 10.1016/j.xphs.2022.01.026. [DOI] [PubMed] [Google Scholar]
- Ferreri C.; Kratzsch S.; Brede O.; Marciniak B.; Chatgilialoglu C. Trans Lipid Formation Induced By Thiols in Human Monocytic Leukemia Cells. Free Radicals Biol. Med. 2005, 38, 1180–1187. 10.1016/j.freeradbiomed.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Cort A.; Ozben T.; Melchiorre M.; Chatgilialoglu C.; Ferreri C.; Sansone A. Effects of Bleomycin and Antioxidants on the Fatty Acid Profile of Testicular Cancer Cell Membranes. Biochim. Biophys. Acta 2016, 1858, 434–441. 10.1016/j.bbamem.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Bailly C.; Beauvillain J. C.; Bernier J. L.; Hénichart J. P. Plasma Membrane Perturbations of KB3 Cells Induced By the Bleomycin-Iron Complex. Cancer Res. 1990, 50, 385–392. [PubMed] [Google Scholar]
- Antholine W. E.; Petering D. H. On the Reaction of Iron Bleomycin With Thiols and Oxygen. Biochem. Biophys. Res. Commun. 1979, 90, 384–389. 10.1016/0006-291X(79)91636-X. [DOI] [PubMed] [Google Scholar]
- Muliawan H.; Burkhardt A.; Scheulen M. E.; Kappus H. Minor Role of Lipid Peroxidation in Acute Bleomycin Toxicity in Rats. J. Cancer Res. Clin. Oncol. 1982, 103, 135–143. 10.1007/BF00409644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cort A.; Ozben T.; Sansone A.; Barata-Vallejo S.; Chatgilialoglu C.; Ferreri C. Bleomycin-Induced Trans Lipid Formation in Cell Membranes and in Liposome Models. Org. Biomol. Chem. 2015, 13, 1100–1105. 10.1039/C4OB01924E. [DOI] [PubMed] [Google Scholar]
- Gallon A. A.; Pryor W. A. The Reaction of Low Levels of Nitrogen Dioxide With Methyl Linoleate in the Presence and Absence of Oxygen. Lipids 1994, 29, 171. 10.1007/BF02536725. [DOI] [PubMed] [Google Scholar]
- Chatgilialoglu C.; Ferreri C.; Lykakis I. N.; Wardman P. Trans-Fatty Acids and Radical Stress: What Are the Real Culprits. Bioorg. Med. Chem. 2006, 14, 6144–6148. 10.1016/j.bmc.2006.05.052. [DOI] [PubMed] [Google Scholar]
- Ferreri C.; Samadi A.; Sassatelli F.; Landi L.; Chatgilialoglu C. Regioselective Cis-Trans Isomerization of Arachidonic Double Bonds By Thiyl Radicals: The Influence of Phospholipid Supramolecular Organization. J. Am. Chem. Soc. 2004, 126, 1063–1072. 10.1021/ja038072o. [DOI] [PubMed] [Google Scholar]
- Soni S. P.; Ward J. A.; Sen S. E.; Feller S. E.; Wassall S. R. Effect of Trans Unsaturation on Molecular Organization in a Phospholipid Membrane. Biochemistry 2009, 48, 11097–11107. 10.1021/bi901179r. [DOI] [PubMed] [Google Scholar]
- Seelig J.; Waespe-Sarcevic N. Molecular Order in Cis and Trans Unsaturated Phospholipid Bilayers. Biochemistry 1978, 17, 3310–3315. 10.1021/bi00609a021. [DOI] [PubMed] [Google Scholar]
- McElhaney R. N. The Use of Differential Scanning Calorimetry and Differential Thermal Analysis in Studies of Model and Biological Membranes. Chem. Phys. Lipids 1982, 30, 229–259. 10.1016/0009-3084(82)90053-6. [DOI] [PubMed] [Google Scholar]
- Moss R. A.; Fujita T.; Okumura Y. Effect of Unsaturation on Lipid Dynamics Within Synthetic Lipid Membranes. Langmuir 1991, 7, 440–441. 10.1021/la00051a002. [DOI] [Google Scholar]
- Ferreri C.; Pierotti S.; Chatgilialoglu C.; Barbieri A.; Barigelletti F. Probing the Influence of Cis-Trans Isomers on Model Lipid Membrane Fluidity Using Cis-Parinaric Acid and a Stop-Flow Technique. Chem. Commun. 2006, 529–531. 10.1039/B512812A. [DOI] [PubMed] [Google Scholar]
- Roach C.; Feller S. E.; Ward J. A.; Shaikh S. R.; Zerouga M.; Stillwell W. Comparison of Cis and Trans Fatty Acid Containing Phosphatidylcholines on Membrane Properties. Biochemistry 2004, 43, 6344–6351. 10.1021/bi049917r. [DOI] [PubMed] [Google Scholar]
- Niu S. L.; Mitchell D. C.; Litman B. J. Trans Fatty Acid Derived Phospholipids Show Increased Membrane Cholesterol and Reduced Receptor Activation as Compared to Their Cis Analogs. Biochemistry 2005, 44, 4458–4465. 10.1021/bi048319+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijck P. W.; Kaper A. J.; Oonk H. A.; de Gier J. Miscibility Properties of Binary Phosphatidylcholine Mixtures. A Calorimetric Study. Biochim. Biophys. Acta 1977, 470, 58–69. 10.1016/0005-2736(77)90061-X. [DOI] [PubMed] [Google Scholar]
- Wu S. H.; McConnell H. M. Phase Separations in Phospholipid Membranes. Biochemistry 1975, 14, 847–854. 10.1021/bi00675a032. [DOI] [PubMed] [Google Scholar]
- Björkbom A.; Ramstedt B.; Slotte J. P. Phosphatidylcholine and Sphingomyelin Containing an Elaidoyl Fatty Acid Can Form Cholesterol-Rich Lateral Domains in Bilayer Membranes. Biochim. Biophys. Acta 2007, 1768, 1839–1847. 10.1016/j.bbamem.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Róg T.; Murzyn K.; Gurbiel R.; Takaoka Y.; Kusumi A.; Pasenkiewicz-Gierula M. Effects of Phospholipid Unsaturation on the Bilayer Nonpolar Region: A Molecular Simulation Study. J. Lipid Res. 2004, 45, 326–336. 10.1194/jlr.M300187-JLR200. [DOI] [PubMed] [Google Scholar]
- Hishida M.; Yanagisawa R.; Usuda H.; Yamamura Y.; Saito K. Communication: Rigidification of a Lipid Bilayer By an Incorporated N-Alkane. J. Chem. Phys. 2016, 144, 041103. 10.1063/1.4941059. [DOI] [PubMed] [Google Scholar]
- Kulig W.; Pasenkiewicz-Gierula M.; Róg T. Cis and Trans Unsaturated Phosphatidylcholine Bilayers: A Molecular Dynamics Simulation Study. Chem. Phys. Lipids 2016, 195, 12–20. 10.1016/j.chemphyslip.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Zambonin L.; Prata C.; Cabrini L.; Maraldi T.; Fiorentini D.; Vieceli Dalla Sega F.; Hakim G.; Landi L. Effect of Radical Stress and Ageing on the Occurrence of Trans Fatty Acids in Rats Fed a Trans-Free Diet. Free Radic. Biol. Med. 2008, 44, 594–601. 10.1016/j.freeradbiomed.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Hung W.-L.; Sun Hwang L.; Shahidi F.; Pan M.-H.; Wang Y.; Ho C.-T. Endogenous Formation of Trans Fatty Acids: Health Implications and Potential Dietary Intervention. J. Funct. Foods 2016, 25, 14–24. 10.1016/j.jff.2016.05.006. [DOI] [Google Scholar]
- Mathieson T.; Franken H.; Kosinski J.; Kurzawa N.; Zinn N.; Sweetman G.; Poeckel D.; Ratnu V. S.; Schramm M.; Becher I.; et al. Systematic Analysis of Protein Turnover in Primary Cells. Nat. Commun. 2018, 9, 689. 10.1038/s41467-018-03106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura T.; Siekevitz P.; Palade G. E. Turnover of Constituents of the Endoplasmic Reticulum Membranes of Rat Hepatocytes. J. Biol. Chem. 1967, 242, 2389–2396. 10.1016/S0021-9258(18)95974-0. [DOI] [PubMed] [Google Scholar]
- Dawidowicz E. A. Dynamics of Membrane Lipid Metabolism and Turnover. Annu. Rev. Biochem. 1987, 56, 43–61. 10.1146/annurev.bi.56.070187.000355. [DOI] [PubMed] [Google Scholar]
- Hughes J. R.; Levchenko V. A.; Blanksby S. J.; Mitchell T. W.; Williams A.; Truscott R. J. No Turnover in Lens Lipids for the Entire Human Lifespan. eLife 2015, 4, e06003 10.7554/eLife.06003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.; Huang X. Lipid Metabolism At Membrane Contacts: Dynamics and Functions Beyond Lipid Homeostasis. Front. Cell. Dev. Biol. 2020, 8, 615856. 10.3389/fcell.2020.615856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel K. M.; Schiller J.; Galuska C. E.; Fuchs B. Phospholipases and Reactive Oxygen Species Derived Lipid Biomarkers in Healthy and Diseased Humans and Animals - a Focus on Lysophosphatidylcholine. Front. Physiol. 2021, 12, 732319. 10.3389/fphys.2021.732319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiden B.; Sandhoff K. Emerging Mechanisms of Drug-Induced Phospholipidosis. Biol. Chem. 2019, 401, 31–46. 10.1515/hsz-2019-0270. [DOI] [PubMed] [Google Scholar]
- Chatgilialoglu C.; Ferreri C.; Masi A.; Melchiorre M.; Sansone A.; Terzidis M. A.; Torreggiani A. Biomimetic Models of Radical Stress and Related Biomarkers. Chimia 2012, 66, 368–371. 10.2533/chimia.2012.368. [DOI] [PubMed] [Google Scholar]
- Savini F.; Bobone S.; Roversi D.; Mangoni M. L.; Stella L. From Liposomes to Cells: Filling the Gap Between Physicochemical and Microbiological Studies of the Activity and Selectivity of Host-defense Peptides. Pept. Sci. 2018, 110, e24041 10.1002/pep2.24041. [DOI] [Google Scholar]
- Ursini F.; Maiorino M.; Gregolin C. The Selenoenzyme Phospholipid Hydroperoxide Glutathione Peroxidase. Biochim. Biophys. Acta 1985, 839, 62–70. 10.1016/0304-4165(85)90182-5. [DOI] [PubMed] [Google Scholar]
- Yang W. S.; Stockwell B. R. Synthetic Lethal Screening Identifies Compounds Activating Iron-Dependent, Nonapoptotic Cell Death in Oncogenic-RAS-Harboring Cancer Cells. Chem. Biol. 2008, 15, 234–245. 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witztum J. L.; Steinberg D. Role of Oxidized Low Density Lipoprotein in Atherogenesis. J. Clin. Invest. 1991, 88, 1785–1792. 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widomska J.; Subczynski W. K.; Mainali L.; Raguz M. Cholesterol Bilayer Domains in the Eye Lens Health: A Review. Cell Biochem. Biophys. 2017, 75, 387–398. 10.1007/s12013-017-0812-7. [DOI] [PMC free article] [PubMed] [Google Scholar]