Abstract
Purpose
Chronic lymphedema following breast cancer (BC) affects individuals physically, functionally, psychologically and financially. Despite national guidelines and evidence-based research supporting a prospective surveillance and early intervention model of care (PSM), bridging the gap between research and clinical practice has been difficult.
Method
As part of an international randomised controlled trial (RCT) , Australian women with BC from four hospitals were recruited, monitored for lymphedema at regular intervals over a 3-year period, and were provided a compression garment if intervention was triggered. The Reach, Effectiveness, Adoption, Implementation and Maintenance (RE-AIM) evaluation framework was used retrospectively to assess a PSM at the individual and organizational level for those who had completed at least two-year follow-up (N=219) in the RCT
Results
The application of the RE-AIM framework retrospectively demonstrated an extensive Reach to patients across public and private settings; the Effectiveness of prospective surveillance and early intervention was achieved through low progression rates to clinical lymphedema (1.8%); and all hospital sites initially approached Adopted the research study. Key Implementation strategies necessary for effectiveness of this model of care included education to health professionals and patients, staff acceptability, and development of a referral and care pathway. Maintenance dimensions were evaluated both at the individual level with 92-100% adherence rates for all non-optional study appointments over the 2-year period, and at the organisational-level PSM was sustained after recruitment ceased for the research study.
Conclusion
The PSM for lymphedema in BC can be successfully implemented using the RE-AIM framework applied retrospectively. The implementation of the PSM used in the RCT has assisted in changing clinical practices and improving the quality and effectiveness of the health care system.
Keywords: Lymphedema, bioimpedance spectroscopy (BIS), RE-AIM framework, Implementation, Prospective surveillance model (PSM)
INTRODUCTION
Australian breast cancer (BC) survival rates have steadily increased; however long-lasting side effects from treatment occur [1,2]. Breast cancer-related lymphedema (BCRL) affects an estimated 21% of patients [3] and their quality of life, and brings physical, functional, psychological and economic hardships [3-5]. The burden of BCRL is directly related to lymphedema severity, demonstrating a need for a prospective surveillance and early intervention model of care (PSM) [6].
Poor awareness of lymphedema symptoms usually results in treatment referral delays. Often, referral only occurs in the advanced non-reversible stages of lymphedema, resulting in managing and controlling symptoms rather than preventing further progression. Furthermore, lymphedema is often managed external to BC multidisciplinary clinics, resulting in time consuming medical referrals and delayed care [7].
Though evidence-based research shows that early identification and intervention is key [3,8-10], bridging the gap between research and clinical practice in health care systems internationally has been difficult. In Australia, a limited number of hospitals and multidisciplinary teams (MDT) use evidence-based practices to support the PSM. Implementation and dissemination of monitoring BC patients pre- and post-operatively is currently not standard practice.
There are several perceived barriers to implementing a PSM in BC. Lymphedema is often not regarded as important within the context of BC care and lack of MDT awareness of the benefits of early detection of sub-clinical lymphedema. Implementation is further limited by resource availability and infrastructure needs, access to equipment, staff training and time constraints, clinical protocols and referral pathways for ongoing clinical care.
Understanding the process of translating effective interventions into standard care practice is important for improving uptake and sustainability. Implementation science aims to facilitate the translation of clinically efficacious interventions into practice. RE-AIM is a widely used framework in implementation science [11-13] to evaluate implementation effectiveness and acknowledges the importance of other factors that influence the value of an intervention in real-world settings [14]. The RE-AIM framework consists of five dimensions: Reach, Effectiveness, Adoption, Implementation and Maintenance [11,12].
The aim of this study was to retrospectively examine the translational potential of a PSM in BC into the real-world setting through the application of the RE-AIM framework. This study used data and processes evaluated in an existing international randomized controlled research study (RCT) [15] as the influencing factor determining the integration of a PSM within the Australian health care system.
METHODS
Study Design and Sample
This was a retrospective analysis of data and processes from an ongoing multi-site, international RCT [15] with Human Research Ethics Committee (HREC) approval. Four Australian hospitals consisting of one public (government funded) (IW), and three private hospitals (private health insurance funded) (IM, IN and IS) were included and evaluated as part of the RCT [15].
Newly diagnosed women with BC (N= 1201) who met inclusion criteria [15] were enrolled in the RCT and randomized post-operatively to prospective surveillance at regular intervals via Bioimpedance Spectroscopy (BIS) or tape measure (TM) [16]. Patients underwent lymphedema prevention intervention when sub-clinical lymphedema (change of ≥6.5 L-Dex units, or ≥5% but <10% volume difference using TM) was identified with a compression sleeve and gauntlet worn for 4 weeks and then re-evaluated. The primary endpoint of the RCT was the rate of progression to clinical lymphedema requiring complex decongestive physiotherapy (CDP), with progression defined as a TM volume change in the at-risk arm ≥ 10% above the presurgical baseline. [15]. An interim analysis from the RCT included data from 508 participants who had completed 12-months of post-surgical follow up. This study includes the Australian site participants (N=219) evaluated in the interim analysis who have now completed at least 24-months of post-surgical follow-up.
The Australian Healthcare System
Government (Medicare) insurance is accessible to all Australians, covering patients for treatment within the public health care hospital system. At times, this brings about large delays in lymphedema treatment. Patients can also pay for additional private health insurance, giving them access both to public and private hospitals. However, there can be large out of pocket expenses for a patient choosing to be treated privately.
Data collection
Data from the RCT [15] was analysed retrospectively to inform each RE-AIM dimension. Demographic information, physical measures informing lymphedema progression rates and assessment attendance helped inform RE-AIM dimensions. Discussions about the perceived efficacy of a PSM supported by the RCT, occurred between research personnel, MDT members, and organisational leaders throughout the study. Existing processes determined the most efficient way to introduce a large study with multiple measurements using TM and/or BIS without significantly affecting clinical flows or increasing patient or clinician delays.
Measures
Table 1 provides a description of the retrospective data collected from the RCT to assess the PSM and shows the use of these data to address each of the each RE-AIM dimensions
TABLE 1.
RE-AIM framework
| RE-AIM | Outcome measure | Methods of data collection |
|---|---|---|
| REACH | Representativeness of participants recruited to parent research study compared to the general population | Demographic information Comparison of public and private |
| EFFECTIVENESS | Lymphedema progression rates | Review study audit records |
| Can routine and regularly planned monitoring assessment appointments be achieved? | Monitoring assessments completed | |
| Hospital staff perception on the efficacy of early intervention | Discussions with members of breast cancer multidisciplinary teams and organisational leaders | |
| Impact on quality of life | LSID-A and FACT-B questionnaire | |
| ADOPTION | Factors contributing to the integration and barriers preventing implementation of the PSM within the public and private health systems | Discussions with organisational leaders identifying concerns prior to, during and post parent research study |
| Examining hospitals who did and did not integrate the PSM | Assessing public and private uptake | |
| IMPLEMENTATION | How, by whom and when was the PSM implemented. | Education of clinical staff, planning and monitoring and referral flow |
| How was the study modified over time to facilitate implementation | Observations during implementation to address barriers in real time | |
| Educating patients to become active participants in their monitoring and early intervention | Participants contacting research staff due to swelling concerns | |
| MAINTENANCE | Participant adherence/retention over 3year study time | Research study assessments completed and missed |
| Perceptions of maintaining a PSM | Discussions with multidisciplinary team members |
REACH
Reach reflects the absolute number and representativeness of the population of BC patients who could be involved in the PSM and RCT. Data collected included assessing the inclusion and exclusion criteria of the RCT to determine participation rates, dropouts and overall representativeness. Awareness strategies of lymphedema monitoring and treatment pre-study implementation were assessed through discussions with MDT members primarily BC surgeons, breast care nurses and lymphedema therapists.
EFFECTIVENESS
Effectiveness of the PSM refers to the number of women who progressed to clinical lymphedema despite regular monitoring and early intervention in the RCT. The proportion of assessments completed or missed across the 24-month period determined the rationale for the effectiveness of implementing a PSM.
ADOPTION
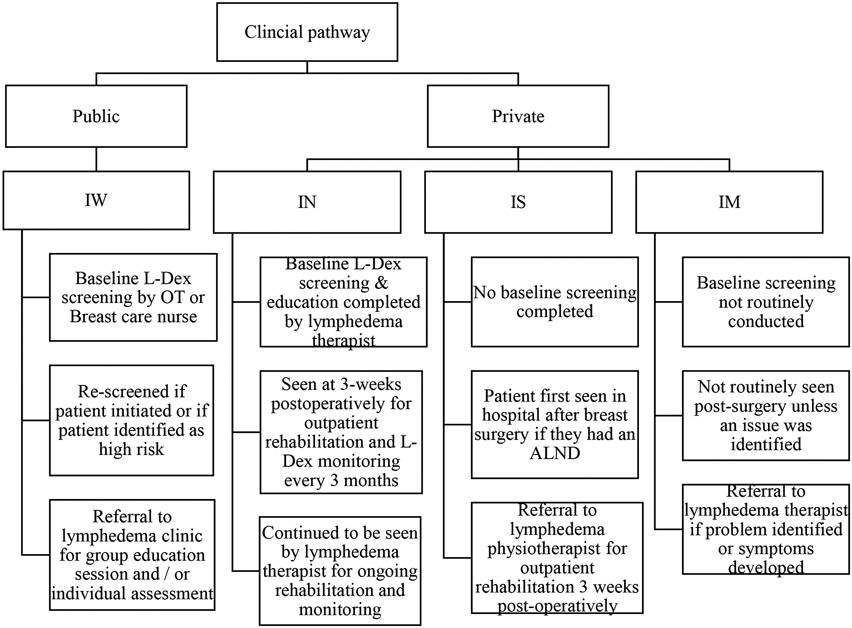
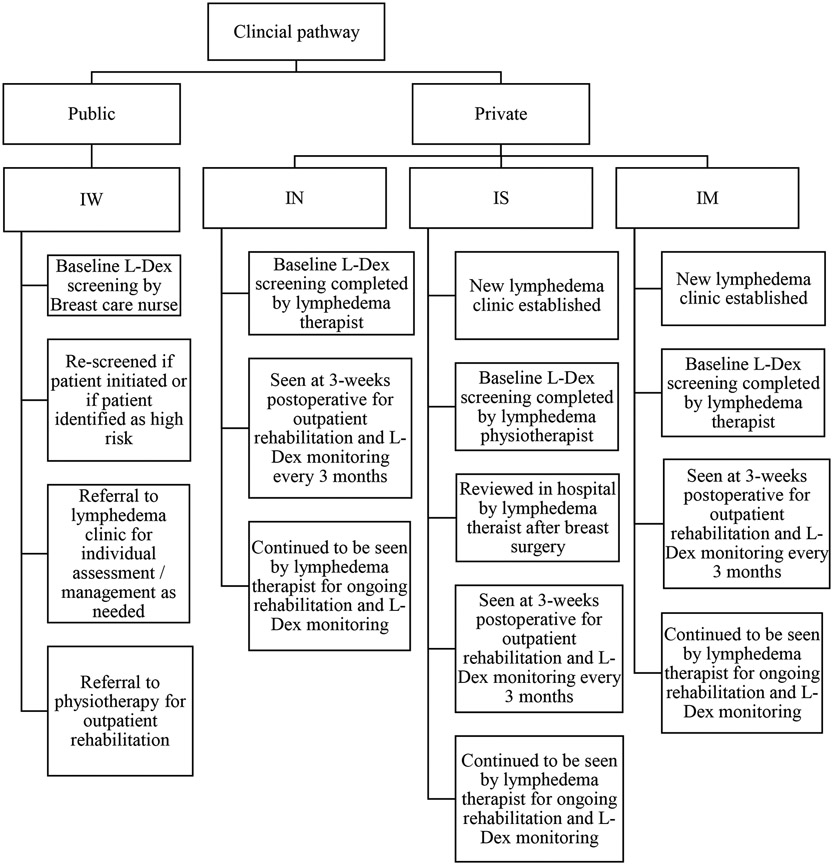
The characteristics of clinics and hospitals that were initially approached and participated in the RCT were compared with those who declined participation. Representativeness of both public and private health care systems were determined through assessing adoption strategies of where the RCT protocol was implemented. Standard protocols and procedures for early monitoring and intervention for lymphedema were assessed pre- and post-study integration. Figures 1 & 2 show the changes in PSM pre- and post-integration of the RCT.
FIG. 1.
Model of care prior to parent research study implementation
FIG. 2.
Model of care post parent research study implementation
IMPLEMENTATION
Implementation refers to the extent to which the PSM were delivered and integrated through the RCT protocols, including whether it was accepted and endorsed by the MDT and whether or not the team could adhere to continue monitoring for lymphedema once the RCT finished recruitment. Evaluating the implementation of the PSM using the RCT within already established clinical settings was a key component to the overall implementation assessment.
MAINTENANCE
Maintenance refers to the integration of a PSM through the implementation of the RCT protocols within public and private health care systems and the sustainability of these models over time. The early indicators of sustainability within clinical settings were determined using retention rates of recruited participants within the first year in the RCT. Maintenance was measured as the ongoing execution of a PSM versus return to the pre-existing models of care across the four sites.
RESULTS
REACH
The PSM in BC was demonstrated through the RCT’s extensive reach to both privately insured (n=130) and government-funded patients (n=89). The public hospital (IW) had a delay in commencing study recruitment due to slower ethics approval processes. As the public hospital comprises a large catchment area, patients occasionally declined participation in the RCT as a result of having their BC adjuvant treatment at a hospital closer to their home. However, individuals were similar across sites based on demographic descriptors.
EFFECTIVENESS
Support for a PSM was seen with 25% of the 219 patients (n=55) triggering early intervention of compression therapy and only 1.8% progressing to clinical lymphedema (n=4). This could be attributed to ongoing patient education, self-awareness and self-monitoring of symptoms. MDT members and organisation leaders reported an overall positive perception on the efficacy of early intervention following BC treatment. Hospitals without routine pre-operative baseline BIS or TM saw the RCT as providing an ongoing PSM otherwise not offered to patients at their site. These clinicians typically knew the importance of prospective surveillance and early intervention however did not have the resources to offer this service. The intervention component of the RCT, providing two free arm-sleeves and gauntlets to patients who triggered, was a major contributor to the effectiveness of study implementation across the sites. The RCT also collected self-reported information from validated symptom and quality of life surveys. Study research personnel often found parallels between the objective measurements with survey responses.
ADOPTION
Factors to successful adoption of the PSM at all sites included administrative and clinical support staff who had an awareness of lymphedema and the perceived benefits of a PSM. Concerns such as room availability and usage, specifically in the public sector, were addressed early on by explaining study protocols and assessment requirements for the RCT with organizational leaders prior to study implementation. Research personnel presented the study protocol to MDT prior to initial recruitment to highlight the importance of pre-operative and post-operative measurements. This facilitated the early adoption of a PSM by all major hospitals initially contacted to participate in the RCT.
Adoption was enhanced through education of all MDT members and health managers across sites. Medical professionals especially advocated for study involvement at recruitment sites where TM and BIS measurements were not routinely completed. At these sites, MDT members educated patients about the importance of early monitoring for long-term outcomes. Patients’ willingness to consent after recommendations by medical professionals suggests the important role of information provided by treating physicians. Educating key personnel either individually or in teams is essential in overcoming barriers to implementing a PSM. Working with medical, nursing, allied health and administrative staff helped to facilitate recruitment and retention once the RCT was implemented. Coordinating study follow-up assessments to coincide with existing medical appointments made adoption of the PSM easier, as well as MDT members continually supporting and encouraging ongoing patient involvement.
IMPLEMENTATION
Implementation strategies adopted to support the PSM included education and training of clinical staff through regular presentations to MDT, scheduling of assessments including space requirements, as well as monitoring of study recruitment and referral flow. Ongoing education on the RCT protocol and latest evidence supporting a PSM was a key factor during implementation. In the public hospital, some patients were initially screened eligible, and baseline assessments were completed prior to MDT meetings. Eligibility criteria discussions with nursing staff ensured that potentially ineligible patients were not approached by research personnel until final treatment decisions were made. In the private hospitals, patients were screened by doctors, occupational therapists and nurses and often were already aware and interested in the study and eligible to participate.
The main concern across all recruitment sites was to alleviate issues surrounding disruption to clinic flow. Thus, during the RCT implementation, efforts focused on securing time and space for follow-up study assessments with minimal disruption. Research staff worked with administrative staff to facilitate room bookings outside of busy clinic schedules, during MDT meetings, or in other free clinic spaces within the hospital. The increased positive working relationships between team members facilitated the PSM implementation. In the public hospital where routine pre-operative measurements were done, implementation was facilitated by working with staff to input BIS study measures into the electronic medical records, or reducing the burden and time to take both measurements (TM and BIS). Positive relationships between research and clinical personnel facilitated communication of any ongoing concerns during the RCT implementation to enhance the PSM.
Buy-in from staff and MDTs were improved due to the ability of research personnel to see patients across the four study locations. For example, if a patient recruited to the RCT at one site worked closer to another study site, the patient was able to have follow-up assessments completed at the local site. Research personnel worked with patients to see them either prior to or after hospital appointments in busy clinics, allowing enough time for study assessments, which could take 15 minutes up to 1 hour to complete. Because BIS and TM were used in the RCT, staff from a variety of research backgrounds, were trained in both methods. Training for BIS took <30 minutes, training for TM took over an hour in addition to the time training on the protocol itself. This demonstrates a cost of implementation and a potential barrier to implementation. Despite these obstacles, the RCT demonstrated that thorough assessment as well as survey questionnaires while limiting disruption to normal clinic flow was possible. Routine integration of BIS and TM within clinical practice is possible by using patient reported outcomes presented in real-time to support patient care delivery as well as encouraging and empowering patients to become active participants in their care. Well-established referral pathways to lymphedema services allows patients to become active participants in the PSM.
MAINTENANCE
As part of the PSM assessments for the RCT protocol, were classified as non-optional (baseline, 3, 6, 12, 18, 24, 30 and 36-month) and optional (15- and 21-month) assessments. All optional visits could be missed without consequence to study involvement; however, patients who missed two consecutive non-optional visits were removed from the study. When possible, a window period allowed timing of assessments to coincide with clinical appointments. When accounting for study patients who were eligible to attend their assessments (i.e. were still enrolled in the study), across all four sites a 92%-100% attendance rate was seen for all non-optional study visits over the 3-year period (Table 2). This included patients who requested to not attend a study assessment for reasons including travel, work and family commitments. When examining 15-month and 21-month optional visits, where patients could elect to miss, patients were still motivated to attend (range, 41.3%-77.1%). Furthermore, no statistical difference was seen between public and private attendance rates. With research being voluntary and through a structured PSM such as the RCT, these results demonstrate patients taking ownership of their care. Once RCT recruitment ceased, a PSM was sustained at the two recruitment sites where a PSM were not part of routine clinical care (IM and IS).
Table 2.
Number and percentage of visits attended by those who were remained eligible
| Follow- up period |
IM (n = 17) | IN (n = 64) | IS (n = 49) | IW(n = 89) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Attended | Able | % | Attend | Able | % | Attended | Able | % | Attended | Able | % | |
| 3-month | 17 | 17 | 100.0% | 63 | 64 | 98.4% | 49 | 49 | 100.0% | 86 | 89 | 96.6% |
| 6-month | 17 | 17 | 100.0% | 64 | 64 | 100.0% | 49 | 49 | 100.0% | 88 | 89 | 98.9% |
| 12-month | 15 | 15 | 100.0% | 59 | 62 | 95.2% | 49 | 49 | 100.0% | 87 | 89 | 97.8% |
| 15-month * | 9 | 15 | 60.0% | 45 | 61 | 73.8% | 37 | 48 | 77.1% | 59 | 88 | 67.0% |
| 18-month | 13 | 15 | 86.7% | 59 | 60 | 98.3% | 41 | 47 | 87.2% | 75 | 82 | 91.5% |
| 21-month * | 7 | 15 | 46.7% | 35 | 58 | 60.3% | 22 | 47 | 46.8% | 33 | 80 | 41.3% |
| 24-month | 13 | 14 | 92.9% | 56 | 56 | 100.0% | 46 | 46 | 100.0% | 76 | 78 | 97.4% |
| 30-month | 12 | 13 | 92.3% | 54 | 56 | 96.4% | 42 | 45 | 93.3% | 72 | 76 | 94.7% |
| 36-month | 11 | 11 | 100.0% | 51 | 51 | 100.0% | 42 | 42 | 100.0% | 58 | 58 | 100.0% |
optional visits
DISCUSSION
A PSM in BC has been supported by international clinical and governance evidence. The retrospective evaluation of the RCT using the RE-AIM framework has shown that a PSM can be integrated and implemented effectively into routine clinical practice within the public and private sectors of the Australian Healthcare system. Although some BC studies have used the RE-AIM framework to investigate exercise or education-related interventions prospectively with recommendations made for individuals and organisations [17,13,18-20], this study is the first to use the RE-AIM framework in the context of BCRL and models of care.
REACH
The RE-AIM framework demonstrated, when compared to other studies, an extensive reach to patients across public and private settings through recruitment rates to the international RCT. For example, Pullen et al examined physical activity in BC survivors and demonstrated being able to reach only 2% of the number of BC survivors compared with the number of BC survivors living in the local region. Recruitment strategies and eligibility criteria in order to be able to statistically analyse groups of participants resulted in some challenge. Other recruitment challenges for the Pullen et al study was limited by the need for self-referral to participate in the study. Possible reasons for the lack of women to self-refer included lack of self-confidence about body image issues for women completing cancer treatment, the lack of social support, and the need to return to work or living a distance from where the program was carried out suggesting that physical presence by research personnel within local multidisciplinary cancer centres could increase study reach greater than recruitment flyers requiring participants to initiate initial study contact.
EFFECTIVENESS
Effectiveness of a PSM was evidenced by low progression rates to clinical lymphedema (1.8%) in the RCT. This finding aligns with previous clinical papers indicating that PSM can reduce progression to clinical lymphedema in patients with BC [15,12,3]. Moreover, a retrospective study examining 111 BC lymphedema patients demonstrated that early identification of lymphoedema and routine follow-up resulted in the maintenance of low edema levels for at least ten years[21]. Patients in the study were provided education, compression therapy, and recommended regular biannual visits at time of lymphedema diagnosis. [21]. The importance of early identification and management is supported in research by Casley-Smith who showed that in cases where early identification and management were not implemented, arm volume and thus lymphedema severity continually increased by 22% to 77% two- to eight-years post-diagnosis respectively[22].
ADOPTION
All hospital sites approached for participation in the PSM adopted the international RCT and subsequently implemented the PSM following ceasing of recruitment to the study.
IMPLEMENTATION
Key strategies used for effectiveness of the PSM included education of health professionals and patients, staff buy-in and acceptability, and development of a referral and clinical care pathway that is supported by adequate resources and expertise to sustain the PSM . These implementation strategies align with previous reports of implementation strategies that may enhance implementation [23] and studies of the effective implementation of models of care [24]. Adherence to the latest research [15,9,3] and key position statements from Australian and US peak professional associations and government agencies [25-27] is recommended. Specifically, individuals at risk of lymphedema due to lymph node involvement or treatment should receive education and baseline measurements to monitor for normal limb variance and regular monitoring that could enhance the adoption and implementation of a PSM.
Important implementation considerations.
There are important considerations when implementing a lymphedema PSM program. These include: a) decisions surrounding who should be responsible for patient monitoring; b) timeliness and follow-up of monitoring; c) method of measurement (BIS or TM); d) what to do when sub-clinical lymphedema becomes evident; e) data storage and ownership; and e) who should provide patient education and early intervention [4,28]. Frequency of lymphedema monitoring guidelines along with standardised diagnostic criteria cut-off points, as adopted by this study’s protocol, helped to overcome previous barriers to implementing PSM of care as standard clinical practice[29]. Adoption could be facilitated by training any health professional to take routine measurements and fit compression garments during patient monitoring visits, as was done in this study. Where BIS devices are accessible, BIS measurements, which can be performed by administrative staff, might be preferable in busy clinics. Moreover, with an agreed clinical pathway and appropriate referral pathways in place, suitable follow-up care can occur.
Because of the RCT implementation, changes in referral flow within two of the four hospitals were made (Figure 2). Patients were referred to a lymphedema trained therapist pre-implementation of the RCT only if symptoms were reported. Following the RCT implementation, and as a result of continually educating surgeons and allied health professionals within the four sites, practice changes implementing a PSM were made to ensure patients were being monitored for sub-clinical lymphedema from time of diagnosis.
MAINTENANCE
Dimensions were evaluated both at the individual level with 92-100% adherence rates for all non-optional study appointments over the 2-year period, and at the organisational-level where PSM was sustained after recruitment ceased for the RCT. The high adherence rate of this study (92-100% for non-optional visits) is different to other studies who have found lower adherence rates to interventions such as physical activity in BC patients and sustainment of physical activity following study completion [13]. A national report published by Breast Cancer Network Australia in 2018 identified lymphedema as one of the top five issues of concern and priority for women following BC treatment [30]. Therefore, reasons for high adherence to follow-up study assessment visits may be due to: 1) ease of visits, 2) desire to identify early onset lymphedema, and 3) reduction of psychological distress regarding potential development of lymphedema. Patients also reported feeling empowered by participating in the RCT. They were pleased to know that they were being monitored for lymphedema and were often disappointed when the three-year study monitoring period ended. Others may have adhered to the RCT as they were aware that if they triggered early intervention whilst on the study that they were provided with two free compression arm-sleeves and gauntlets.
Study limitations
As women are more likely to be diagnosed with BC than men, and though not excluded, the RCT has little male representation. Additional research is needed to better understand whether a PSM can be expanded into the male population and other cancer groups. As a result of BIS sensitivity not being established in the presence of certain other medical conditions, the research study had strict exclusion criteria for patients with medical conditions that were known to cause swelling. With patients having neo-adjuvant chemotherapy and patients with bilateral metal in their hips, knees or ankles being excluded at one-point in the study, the overall reach for a PSM may be larger than that of the RCT. Most women declining the study because they felt overwhelmed at the time of diagnosis. However, implementing early monitoring as part of routine clinical care with fewer follow-up visits, particularly for lower risk patients, could potentially overcome this.
Sustainability issues which need to be overcome for successful implementation include out of pocket expenses in the private sector for lymphedema services and lack of resources and time delays for treatment in the public sector. Educating healthcare systems around the greater costs that advanced BCRL has at both the individual and organisational level compared with early BCRL can provide support for a PSM[31]. Furthermore, training administrative and allied health staff to conduct BIS measurements, a quick, reliable and objective measure that both clinicians and patients can use to monitor ongoing care can contribute to the sustainability of a PSM. Resourcing of staff and having monitoring clinical pathways in place with emphasis on what occurs when a trigger for early intervention occurs is an important consideration and should be undertaken in the MDT setting. Health insurance rebates or Medicare funding would further allow the rapid update of a PSM of care modelled on this RCT into real-world settings.
Conclusion
Using the RE-AIM framework as a guide, the PSM for lymphedema in BC can be successfully implemented in both public and private multidisciplinary breast cancer centres. The implementation of the PSM used in the RCT has assisted in changing clinical practices and improving the quality and effectiveness of the health care system.
Acknowledgements:
All multidisciplinary team members and administrative support staff at each site who supported the implementation of this study
Competing interests & financial support:
This study was funded by ImpediMed, medi, and by the United States National Institutes of Health (NIH/NCATS UL1 TR000445).
Footnotes
Conflicts of Interest:
Author L.K has acted as an Education Consultant to ImpediMed Limited. J.B is an ImpediMed stockholder. All other authors declare that they have no individual conflicts of interest or financial ties to disclose.
Ethics Committees approval: Macquarie University Human Research Ethics Committee Reference No: 5201400739
References
- 1.Eakin EG, Hayes SC, Haas MR, Reeves MM, Vardy JL, Boyle F, Hiller JE, Mishra GD, Goode AD, Jefford M, Koczwara B, Saunders CM, Demark-Wahnefried W, Courneya KS, Schmitz KH, Girgis A, White K, Chapman K, Boltong AG, Lane K, McKiernan S, Millar L, O’Brien L, Sharplin G, Baldwin P, Robson EL (2015) Healthy Living after Cancer: a dissemination and implementation study evaluating a telephone-delivered healthy lifestyle program for cancer survivors. BMC Cancer 15 (1):992. doi: 10.1186/s12885-015-2003-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laidley A, Anglin B (2016) The Impact of L-Dex(®) Measurements in Assessing Breast Cancer-Related Lymphedema as Part of Routine Clinical Practice. Front Oncol 6:192–192. doi: 10.3389/fonc.2016.00192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koelmeyer LA, Borotkanics RJ, Alcorso J, Prah P, Winch CJ, Nakhel K, Dean CM, Boyages J (2019) Early surveillance is associated with less incidence and severity of breast cancer–related lymphedema compared with a traditional referral model of care. Cancer 125 (6):854–862. doi: 10.1002/cncr.31873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stout NL, Pfalzer LA, Springer B, Levy E, McGarvey CL, Danoff JV, Gerber LH, Soballe PW (2012) Breast cancer-related lymphedema: comparing direct costs of a prospective surveillance model and a traditional model of care. Phys Ther 92 (1):152–163. doi: 10.2522/ptj.20100167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levenhagen K, Davies C, Perdomo M, Ryans K, Gilchrist L (2017) Diagnosis of Upper Quadrant Lymphedema Secondary to Cancer: Clinical Practice Guideline From the Oncology Section of the American Physical Therapy Association. Phys Ther 97 (7):729–745. doi: 10.1093/ptj/pzx050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basta MN, Fox JP, Kanchwala SK, Wu LC, Serletti JM, Kovach SJ, Fosnot J, Fischer JP (2016) Complicated breast cancer-related lymphedema: evaluating health care resource utilization and associated costs of management. The American Journal of Surgery 211 (1):133–141. doi: 10.1016/j.amjsurg.2015.06.015 [DOI] [PubMed] [Google Scholar]
- 7.Framework L (2006) Best practice for the management of lymphoedema. International consensus London: MEP Ltd:3–52 [Google Scholar]
- 8.Stout NL, Pfalzer LA, Springer B, Levy E, McGarvey CL, Danoff JV, Gerber LH, Soballe PW (2012) Breast cancer–related lymphedema: comparing direct costs of a prospective surveillance model and a traditional model of care. Physical therapy 92 (1):152–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilgore LJ, Korentager SS, Hangge AN, Amin AL, Balanoff CR, Larson KE, Mitchell MP, Chen JG, Burgen E, Khan QJ (2018) Reducing breast cancer-related lymphedema (BCRL) through prospective surveillance monitoring using bioimpedance spectroscopy (BIS) and patient directed self-interventions. Annals of surgical oncology 25 (10):2948–2952 [DOI] [PubMed] [Google Scholar]
- 10.Whitworth PW, Cooper A (2018) Reducing chronic breast cancer-related lymphedema utilizing a program of prospective surveillance with bioimpedance spectroscopy. The Breast Journal 24 (1):62–65. doi: 10.1111/tbj.12939 [DOI] [PubMed] [Google Scholar]
- 11.Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, Griffey R, Hensley M (2011) Outcomes for Implementation Research: Conceptual Distinctions, Measurement Challenges, and Research Agenda. Administration and Policy in Mental Health and Mental Health Services Research 38 (2):65–76. doi: 10.1007/s10488-010-0319-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell SA, Chambers DA (2017) Leveraging Implementation Science to Improve Cancer Care Delivery and Patient Outcomes. J Oncol Pract 13 (8):523–529. doi: 10.1200/JOP.2017.024729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pullen T, Bottorff JL, Sabiston CM, Campbell KL, Eves ND, Ellard SL, Gotay C, Fitzpatrick K, Sharp P, Caperchione CM (2018) Utilizing RE-AIM to examine the translational potential of Project MOVE, a novel intervention for increasing physical activity levels in breast cancer survivors. Translational Behavioral Medicine 9 (4):646–655. doi: 10.1093/tbm/iby081 [DOI] [PubMed] [Google Scholar]
- 14.Glasgow RE, McKay HG, Piette JD, Reynolds KD (2001) The RE-AIM framework for evaluating interventions: what can it tell us about approaches to chronic illness management? Patient education and counseling 44 (2):119–127 [DOI] [PubMed] [Google Scholar]
- 15.Ridner SH, Dietrich MS, Cowher MS, Taback B, McLaughlin S, Ajkay N, Boyages J, Koelmeyer L, DeSnyder SM, Wagner J, Abramson V, Moore A, Shah C (2019) A Randomized Trial Evaluating Bioimpedance Spectroscopy Versus Tape Measurement for the Prevention of Lymphedema Following Treatment for Breast Cancer: Interim Analysis. Annals of Surgical Oncology. doi: 10.1245/s10434-019-07344-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ridner SH, Dietrich MS (2015) Development and validation of the lymphedema symptom and intensity survey-arm. Supportive Care in Cancer 23 (10):3103–3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips SM, Alfano CM, Perna FM, Glasgow RE (2014) Accelerating Translation of Physical Activity and Cancer Survivorship Research into Practice: Recommendations for a More Integrated and Collaborative Approach. Cancer Epidemiology Biomarkers & Prevention 23 (5):687. doi: 10.1158/1055-9965.EPI-13-1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belkora J, Volz S, Loth M, Teng A, Zarin-Pass M, Moore D, Esserman L (2015) Coaching patients in the use of decision and communication aids: RE-AIM evaluation of a patient support program. BMC Health Services Research 15 (1):209. doi: 10.1186/s12913-015-0872-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.lina A, Rebecca E (2011) A RE-AIM Evaluation of Theory-Based Physical Activity Interventions. Journal of Sport and Exercise Psychology 33 (2):198–214. doi: 10.1123/jsep.33.2.198 [DOI] [PubMed] [Google Scholar]
- 20.Leach H, Danyluk J, Culos–Reed S (2014) Design and implementation of a community-based exercise program for breast cancer patients. Current Oncology 21 (5):267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson K, Branje E (2010) Arm lymphoedema in a cohort of breast cancer survivors 10 years after diagnosis. Acta Oncol 49 (2):166–173. doi: 10.3109/02841860903483676 [DOI] [PubMed] [Google Scholar]
- 22.Casley-Smith JR (1995) Alterations of untreated lymphedema and it's grades over time. Lymphology 28 (4):174–185 [PubMed] [Google Scholar]
- 23.Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, Proctor EK, Kirchner JE (2015) A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implementation Science 10 (1):21. doi: 10.1186/s13012-015-0209-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scholl I, LaRussa A, Hahlweg P, Kobrin S, Elwyn G (2018) Organizational- and system-level characteristics that influence implementation of shared decision-making and strategies to address them — a scoping review. Implementation Science 13 (1):40. doi: 10.1186/s13012-018-0731-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dylke ES Early detection of breast cancer-related lymphoedema. [Internet], NSW: Australasian Lymphology Association; September 201. Available from https://www.lymphoedema.org.au/public/7/files/Position%20Statements/ALA%20Position%20StatementEarly%20Detection%20of%20BCRL.pdf. [Google Scholar]
- 26.ACI Chronic Care Network. Lymphoedema: A guide for clinical services. [Internet]. NSW: ACI Chronic Care Network; 10 February 2020. Available from: https://www.aci.health.nsw.gov.au/__data/assets/pdffile/0008/477998/lymphoedema-guide.pdf [Google Scholar]
- 27.National Lymphedema Network. Position Paper on Screening & Early Detection of Breast-Cancer-Related Lymphedema: The Imperative. [Internet]. New York; April 2011. Available from: https://lymphnet.org/position-papers. [Google Scholar]
- 28.Armer JM, Hulett JM, Bernas M, Ostby P, Stewart BR, Cormier JN (2013) Best-practice guidelines in assessment, risk reduction, management, and surveillance for post-breast cancer lymphedema. Current breast cancer reports 5 (2):134–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stout Gergich NL, Pfalzer LA, McGarvey C, Springer B, Gerber LH, Soballe P (2008) Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer 112 (12):2809–2819. doi: 10.1002/cncr.23494 [DOI] [PubMed] [Google Scholar]
- 30.Breast Cancer Network Australia. State of the Nation Report. [Internet]. Australia: BCNA; June 2018. Available from https://www.bcna.org.au/media/6656/sotn-report-lowres.pdf [Google Scholar]
- 31.Boyages J, Xu Y, Kalfa S, Koelmeyer L, Parkinson B, Mackie H, Viveros H, Gollan P, Taksa L (2017) Financial cost of lymphedema borne by women with breast cancer. Psycho-oncology 26 (6):849–855 [DOI] [PMC free article] [PubMed] [Google Scholar]