Abstract
Background
It is estimated that 32 million pregnant women suffer from anaemia worldwide. Due to increased metabolic demands, pregnant women are particularly vulnerable to anaemia and vitamin and mineral deficiencies, leading to adverse health effects in both the mother and her baby. Despite the demonstrated benefits of prenatal supplementation with iron and folic acid or multiple micronutrients, poor adherence to routine supplementation has limited the effectiveness of this intervention in many settings. Micronutrient powders for point‐of‐use fortification are packed, single‐dose sachets containing vitamins and minerals that can be added onto prepared food to improve its nutrient profile. The use of multiple micronutrient powders for point‐of‐use fortification of foods in pregnant women could be an alternative intervention to prenatal micronutrient supplementation.
Objectives
To assess the effects of prenatal home (point‐of‐use) fortification of foods with multiple micronutrient powders on maternal and newborn health.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 January 2015) and the International Clinical Trials Registry Platform (ICTRP) (31 January 2015). We also contacted relevant agencies to identify ongoing and unpublished studies.
Selection criteria
Randomised controlled trials (both individual and cluster randomisation) and quasi‐randomised trials, irrespective of language or publication status.
The intervention was micronutrient powders for point‐of‐use fortification of foods, containing at least three micronutrients with one of them being iron, provided to pregnant women of any gestational age and parity. Five comparison groups were considered: no intervention/placebo, iron and folic acid supplements, iron‐only supplements, folic‐acid only supplements, and multiple micronutrients in supplements.
Data collection and analysis
Two review authors independently assessed the eligibility of studies, extracted and checked data accuracy, and assessed the risk of bias of included studies.
Main results
Our search identified 12 reports (relating to six studies). We included two cluster‐randomised controlled trials (involving 1172 women) ‐ these trials were considered to be at a moderate to high risk of bias due to methodological limitations. One trial is ongoing, and three studies were excluded.
Micronutrient powders for point‐of‐use fortification of foods versus iron and folic acid supplements
One trial (involving 478 pregnant women attending 42 antenatal care centres) compared micronutrient powders containing iron, folic acid, vitamin C and zinc with iron and folic acid tablets provided daily from 14 to 22 weeks to 32 weeks' gestation. The trial did not report on any of this review's primary outcomes: maternal anaemia at or near term, maternal iron deficiency, maternal mortality, adverse effects, low birthweight, preterm births. Nor did the trial report on the majority of this review's secondary outcomes, with the exception of maternal adherence. Adherence to micronutrient powders was lower than adherence to iron and folic acid supplements (risk ratio (RR) 0.76, 95% confidence interval (CI) 0.66 to 0.87, one study, n = 405).
Micronutrient powders for point‐of‐use fortification of foods versus same multiple micronutrients in supplements
One study (involving 694 pregnant women from 18 communities), compared micronutrient powders containing iron, folic acid, vitamin C, zinc, iodine, vitamin E and vitamin B12 with tablets containing the same seven micronutrients. There was no difference in maternal anaemia at 37 weeks of gestation (RR 0.92, 95% CI 0.53 to 1.59, one study, n = 470, very low quality evidence). The trial did not report on any of this review's other primary outcomes in relation to maternal iron deficiency, maternal mortality, adverse effects, low birthweight, or preterm birth. In terms of this review's secondary outcomes, the included trial did not report on the majority of this review's prespecified secondary outcomes with one exception ‐ there was no clear difference in maternal haemoglobin Hb or near term (mean difference (MD) 1.0 g/L, 95% CI ‐1.77 to 3.77, one study, n = 470).
Authors' conclusions
Limited evidence suggests that micronutrient powders for point‐of‐use fortification of foods have no clear difference as multiple micronutrient supplements on maternal anaemia (very low quality evidence) and Hb at or near term. There is limited evidence to suggest that women were more likely to adhere to taking tablets than using micronutrient powders.
The overall quality of evidence was judged very low (due to methodological limitations), and no evidence was available for the majority of primary and secondary outcomes. Therefore, more evidence is needed to assess the potential benefits or harms of the use of micronutrient powders in pregnant women on maternal and infant health outcomes. Future trials should also assess adherence to micronutrient powders and be adequately powered to evaluate the effects on birth outcomes and morbidity.
Keywords: Female; Humans; Infant, Newborn; Pregnancy; Dietary Supplements; Food, Fortified; Anemia; Anemia/epidemiology; Anemia/therapy; Micronutrients; Micronutrients/administration & dosage; Powders; Pregnancy Complications, Hematologic; Pregnancy Complications, Hematologic/epidemiology; Pregnancy Complications, Hematologic/therapy; Prenatal Care; Prenatal Nutritional Physiological Phenomena; Prevalence; Randomized Controlled Trials as Topic
Plain language summary
Powders of iron plus other micronutrients for home (point‐of‐use) fortification of foods consumed by pregnant women
Pregnant women are particularly vulnerable to nutrient deficiencies due to the requirements of the growing baby during the pregnancy. In low‐income countries, many women have diets with low content of vitamins and minerals, and they participate in long hours of physical labour. They are also exposed to recurrent infections, which make nutritional deficiencies worse. Thus, lack of adequate nutrition can contribute to the poor health of these women their babies.
Iron and folic acid supplements in pregnancy are widely recommended. However, getting the supplements to the women and encouraging them to take them is particularly challenging. Micronutrient powders containing iron, vitamin A, zinc and other vitamins and minerals are usually packaged in single‐use sachets and can be sprinkled onto any semi‐solid food at home or in any other place where meals are to be consumed. The use of micronutrient powders to fortify foods may overcome this problem with micronutrient supplements. Micronutrient powders have already been shown to be effective in reducing anaemia and iron deficiency in young children and so may be useful for pregnant women.
This review looked at the use of micronutrient powders in pregnancy, compared with micronutrient supplements or with no additional micronutrients. We found only two studies involving a total of 1172 women, undertaken in Bangladesh and Mexico. The overall quality of evidence was judged very low (with a high risk of bias due to methodological limitations), and no evidence was available for the majority of primary and secondary outcomes of this review.
One trial compared micronutrient powders with iron and folate supplements, and the other trial compared micronutrient powders with the same nutrients but given as supplements. Overall, micronutrient powders fortification of foods had a similar effect as multiple micronutrient supplements on anaemia in mothers at or near term (very low quality evidence) or the mother's haemoglobin (Hb) at or near term. The study that compared micronutrient powders with iron and folic acid supplements reported that women were more likely to take iron and folic acid tablets than micronutrient powders. Nearly all of the review's important outcomes were not reported in the included studies. Therefore, more evidence is needed for both mother and infant health outcomes in order to adequately evaluate the use of micronutrient powders in pregnant women.
Summary of findings
Summary of findings for the main comparison. Micronutrient powders for point‐of‐use fortification of foods versus iron and folic acid supplements.
| Patient or population: women during pregnancy Settings: all settings Intervention: micronutrient powders for point‐of‐use fortification of foods versus iron and folic acid supplements | ||||
| Outcomes | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Maternal anaemia at term or near term (haemoglobin (Hb) less than 110 g/L at 34 weeks' gestation or more) | no data | 0 | no data | |
| Maternal iron deficiency at term or near term (as defined by authors of the trials, based on any indicator of iron status at 34 weeks' gestation or more) | no data | 0 | no data | |
| All‐cause maternal mortality (death while pregnant or within 42 days of termination of pregnancy) | no data | 0 | no data | |
| Adverse effects (any)* | no data | 0 | no data | |
| Low birthweight (birthweight less than 2500 g) | no data | 0 | no data | |
| Preterm births (births before 37 weeks of gestation) | no data | 0 | no data | |
| CI: confidence interval; RR: average risk ratio. | ||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||
Summary of findings 2. Micronutrient powders for point‐of‐use fortification of foods versus same multiple micronutrients in supplements.
| Patient or population: women during pregnancy Settings: all settings Intervention: micronutrient powders for point‐of‐use fortification of foods versus micronutrients in supplements | ||||
| Outcomes | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Maternal anaemia at term or near term (haemoglobin (Hb) less than 110 g/L at 34 weeks' gestation or more) | 0.92 (0.53, 1.59) |
470 | very low1 | |
| Maternal iron deficiency at term or near term (as defined by authors of the trials, based on any indicator of iron status at 34 weeks' gestation or more) | no data | 0 | no data | |
| All‐cause maternal mortality (death while pregnant or within 42 days of termination of pregnancy) | no data | 0 | no data | |
| Adverse effects (any)* | no data | 0 | no data | |
| Low birthweight (birthweight less than 2500 g) | no data | 0 | no data | |
| Preterm births (births before 37 weeks of gestation) | no data | 0 | no data | |
| CI: confidence interval; RR: average risk ratio. | ||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||
| 1 Only one trial with very serious methodological limitations (unclear method of randomisation, no blinding, and high loss to follow up). It is not possible to evaluate the consistency of the results (and thus it is considered a serious limitation). No serious imprecision. | ||||
Background
Description of the condition
It is estimated that 32 million pregnant women suffer anaemia, half being due to iron deficiency (Stevens 2013); an important proportion are also deficient in one or more vitamins and minerals (Muthayya 2013; The Micronutrient Initiative 2009). Pregnant women are particularly vulnerable to nutrient deficiencies due to increased metabolic demands to meet fetal requirements for growth and development (Christian 2010). Causes of poor maternal nutrition before and during pregnancy, particularly in low‐income countries, include diets low in energy and protein combined with inadequate intakes of micronutrients, cultural practices that discourage women from gaining weight, long hours of physical labour, and recurrent infections (Christian 2010; Dalmiya 2009). Inadequate intake and increased metabolic requirements during pregnancy further exacerbates pre‐existing maternal micronutrient deficiencies (Huffman 1998).
Deficiencies of vitamins and minerals in pregnancy are associated with adverse health outcomes for both the mother and the baby. Anaemia in pregnancy is a risk factor for maternal mortality (WHO 2009a) and is associated with adverse birth outcomes, including low birthweight and increased perinatal mortality (Black 2008; Christian 2010). Each 1 g/dL rise in mean haemoglobin (Hb) in late pregnancy reduces the odds of maternal deaths by 29% (CHERG 2013). An estimated 38% of pregnant women are anaemic, primarily due to iron deficiency, although the contribution of iron deficiency is fewer where other causes of anaemia exist (e.g. genetic blood disorders, malaria, HIV and non‐communicable diseases) (Stevens 2013). Iron‐deficiency anaemia affects approximately 19.2% of pregnant women, with the highest rates found in Africa and Asia (Black 2013).
Maternal vitamin A deficiency can cause visual impairment and increased risk of complications and death during pregnancy and in the postpartum period (Christian 2000; Tielsch 2008). Severe vitamin A deficiency in the mother can also lead to low amounts of vitamin A reserves in the baby, which can negatively affect lung development and the survival of the child in the first year of life (Checkley 2010; Tielsch 2008). An estimated 19.1 million pregnant women worldwide are vitamin A deficient (WHO 2009b). Iodine deficiency is the number one cause of preventable brain damage in childhood (WHO 1994) and leads to thyroid under‐function and goitrogenesis in adults (Glinoer 2007). Nearly two billion people have insufficient iodine intake, and even subclinical iodine deficiency during pregnancy increases the risk of miscarriage and fetal growth restriction (Zimmermann 2008).
Other micronutrients of concern during the gestational period include folic acid, calcium and vitamin D, zinc, and vitamin B12. Poor maternal folate status at conception increases the risk of neural tube and other congenital anomalies, and later in pregnancy is associated with haematological problems and possibly pre‐eclampsia (De‐Regil 2010; Tamura 2006). Calcium and vitamin D deficiency are also associated with gestational hypertension, which is the second leading cause of maternal morbidity and mortality (Duley 2009). Zinc insufficiency has been associated with pregnancy complications such as, growth retardation, congenital abnormalities and retarded neuro behavioural and immunological development in the fetus (Black 2001). Maternal B12 deficiency in some studies is also associated with neural tube defects and early fetal loss (Ray 2007).
Description of the intervention
Interventions to prevent and/or treat micronutrient malnutrition during pregnancy typically include dietary diversification to include foods with highly absorbable vitamins and minerals, industrial fortification of staple foods, and provision of supplements in the form of pills and tablets (Bhutta 2008). Since food fortification is unlikely to be sufficient to meet women's iron needs during pregnancy (WHO/FAO 2006), the most routinely used approach is daily iron and folic acid supplementation, which has been widely practiced in many countries (Beard 2000; Haws 2007; Villar 1997). Daily iron supplementation is generally recommended for pregnant worldwide, including high‐income countries such as the United States (CDC 1998) and the European Union (Commission of the European Communities 1993) to help meet the increased iron demands of pregnancy. However, in the most recent National Institute of Clinical Excellence Guidelines on Antenatal Care in the UK, universal iron supplementation was not advocated due to uncertain benefit and gastrointestinal side effects in iron‐replete women (NICE 2008).
The World Health Organization (WHO) currently recommends daily oral iron and folic acid supplementation as part of antenatal care to reduce the risk of low birthweight, maternal anaemia and iron deficiency (WHO 2011b). In addition to iron and folic acid supplements, additional vitamins and minerals can complement increased maternal micronutrient needs (UNICEF/WHO/UNU 1999; UNICEF 2004a; UNICEF 2004b; WHO 2011b). Formulated by UNICEF, WHO, and United Nations University, the international multiple micronutrient preparation contains one recommended dietary allowance of 15 micronutrients and provides much of the evidence base on multiple micronutrient supplementation in pregnancy (Dalmiya 2009; UNICEF/WHO/UNU 1999). In summary, multiple micronutrient supplementation in pregnancy has comparable benefits to iron‐folic acid supplementation in reducing anaemia, but may have additional benefits on intrauterine growth (particularly size for gestational age and birthweight) and outcomes in the neonatal period and in infancy (Fall 2009; Haider 2012; Huffman 1998; Shah 2009; Shankar 2008; Shrimpton 2009). In a large clinical trial in China, compared to daily prenatal folic acid alone, supplementation with iron‐folic acid and other micronutrients improved maternal Hb status, but did not affect the rate of perinatal mortality or other infant outcomes (Liu 2013). Maternal supplementation with vitamin A has been shown to reduce the risk of anaemia during pregnancy, but has no significant effects on maternal or perinatal mortality (Thorne‐Lyman 2012; van den Broek 2010).
Despite the demonstrated benefits of prenatal micronutrient supplementation, adherence to routine supplementation regimens during pregnancy is only about 50%, due to gastrointestinal side effects, particularly constipation and nausea, associated with supplemental iron (Nguyen 2008). Social determinants also play an important role as demonstrated in a randomised trial of prenatal supplementation in Bangladesh where higher adherence to food supplementation but lower adherence to micronutrient supplements was observed among women having fewer years of education (Shaheen 2014). Furthermore, poor adherence with supplementation programmes, low bioavailability and tolerance of various iron preparations, and the sparse implementation of food fortification in many countries have all likely limited the effectiveness of this intervention in many settings (Bhutta 2009; Zeng 2009).
In response to the operational constraints of micronutrient supplements, ‘point‐of‐use’ fortification of foods with micronutrient powders was developed as a novel approach for delivering iron and other micronutrients with foods to populations where supplementation has been difficult to implement or where the target group is difficult to reach through industrial fortification (de Pee 2008). Micronutrient powders are usually single‐dose packets of lipid‐encapsulated iron and other micronutrients that can be added to energy‐containing foods at home or in any other place where meals are to be consumed, such as schools, nurseries and refugee camps. Micronutrient powders are added to food without the explicit purpose of improving the flavour or colour. In some cases, point‐of‐use fortification is also known as home fortification (De‐Regil 2012). These powder mixes can be formulated with different amounts and combinations of vitamin and minerals. They are thought to have low production costs, and if provided in a single‐dose sachet, are unlikely to result in overdose (SGHI 2008).
Although the primary motivation behind the use of micronutrient powders for point‐of‐use fortification of foods has been to prevent and treat anaemia and iron deficiency in infants and young children, in some countries they are being used in other populations, including pre‐school and school‐aged children, women of reproductive age, emergency‐affected populations, and pregnant women (de Pee 2007; HFTAG 2013). To date, point‐of‐use fortification of foods with micronutrient powders have primarily been implemented in low‐ and middle‐income countries (UNICEF/CDC 2014).
How the intervention might work
The provision of vitamins and minerals to pregnant women through micronutrient powders for point‐of‐use fortification of foods may have higher acceptability and adherence to dosing regimens compared to routine supplements (De‐Regil 2011). Use of micronutrient powders for point‐of‐use fortification of foods is likely influenced by organoleptic (affecting the organs of sense) qualities, ease of preparation, side effects, ease of storage, and perceived positive effects, such as increased energy and strength. Because they are ingested with semi‐solid food, the absorption and utilisation of micronutrient powders for point‐of‐use fortification of foods may be different than supplements, depending on food habits and practices (e.g. reduced iron absorption because of high phytate in maize). Therefore, the effects and safety of micronutrient powders for point‐of‐use fortification of foods for women during pregnancy as an innovative intervention at the community level, or in comparison to routine iron and folic acid or multiple micronutrient supplementation provided through antenatal care, needs to be evaluated.
In malaria‐endemic areas, provision of iron has been a long‐standing controversy due to concerns that iron therapy may exacerbate the prevalence of severity of infections (Oppenheimer 2001). Approximately 40% of the world's population is exposed to malaria, and it is endemic in over 100 countries (WHO 2010). Of all the complications associated with malaria, anaemia is the most common and causes the highest number of malaria‐related deaths. In pregnant women, malaria increases the risk of maternal death, miscarriage, stillbirth and low birthweight with associated risk of neonatal death (WHO 2010). Although the mechanisms by which iron can contribute to adverse health outcomes from iron interventions in malaria‐endemic areas are not known, hypotheses include high peak concentrations of serum iron, in particular non‐transferrin‐bound iron, which enhance parasite growth; impaired host macrophage activation; and alteration in the gut microflora (Prentice 2007; Raiten 2011).
While there is limited research on the safety of iron supplementation among pregnant women in malarial areas, the use of prenatal iron and folic acid supplementation combined with intermittent preventive treatment of malaria during pregnancy has been shown to reduce neonatal mortality in malaria‐endemic regions (Titaley 2010). In this line, it has also been suggested that micronutrient powders for point‐of‐use fortification of foods may be less likely to increase the risk of malaria infection and thus be a safer alternative to iron supplements in malaria endemic regions. Micronutrient powders for point‐of‐use fortification are mixed with food and thus absorbed more slowly, yielding lower peak concentrations of unbound iron in circulation (Liyanage 2002).
The safety of this intervention in non‐endemic malaria areas has also been questioned, as a large clinical trial among children aged six to 18 months in Pakistan reported a sizeable 75% to 80% reduction in iron‐deficiency anaemia, but small unexpected increases in diarrhoea and respiratory morbidity (Soofi 2013). Whether these findings can be applied to pregnant women needs further assessment. More research is needed on the safety of iron interventions and micronutrient powder programs, including developing a standardised approach for monitoring and evaluation and exploring the role of integrated health interventions (Suchdev 2013).
Why it is important to do this review
The use of micronutrient powders for point‐of‐use fortification of complementary foods in young children has been shown to be effective in reducing anaemia and iron deficiency (De‐Regil 2011), and micronutrient powder projects targeting infants and children are being planned or implemented at national or regional scale in several countries globally (HFTAG 2013). The success of this intervention in infants and children has encouraged its use in pregnant women. An early non‐Cochrane systematic review (Suchdev 2011) concluded that despite the widespread implementation of interventions with micronutrient powders for point‐of‐use fortification of foods, there have been no studies evaluating the effects of this intervention in pregnant women on maternal and newborn health outcomes, highlighting the need for randomised controlled trials evaluating the effectiveness and safety of the use of micronutrient powders for point‐of‐use fortification of foods in pregnant women to inform policy‐making.
Various Cochrane reviews or protocols have evaluated the effects of supplementation with different vitamins and minerals for women during pregnancy and will complement the findings of this review. The effectiveness of daily and intermittent iron and folic acid supplements for women during pregnancy includes evaluations of these regimens with additional vitamins and minerals (Peña‐Rosas 2012a; Peña‐Rosas 2012b). These reviews include a range of interventions providing daily or intermittent oral supplementation (e.g. tablets, capsules) containing iron alone, iron + folic acid or iron + other vitamins and minerals. The impact of multiple micronutrient supplements during pregnancy, defined as supplementation with at least five micronutrients including the international multiple micronutrient preparation formulation (UNICEF/WHO/UNU 1999) or those with comparable composition in comparison with standard iron‐folic acid supplements, on specific maternal and pregnancy outcomes are reviewed elsewhere (Haider 2012). The latter review addresses the question of whether or not to give multiple vitamin and mineral supplements during pregnancy regardless of method of delivery (Haider 2012) and does not include subgroup analysis by delivery method (e.g. multiple micronutrient supplements versus micronutrient powders for point‐of‐use fortification of foods).
In 2011, the WHO stated that the routine use of micronutrient powders for point‐of‐use fortification of foods is not recommended as an alternative to iron and folic acid supplementation in pregnant women, as at that time there was no available evidence to directly assess the potential benefits or harms of the use of micronutrient powders for point‐of‐use fortification of foods in pregnant women for improving maternal and infant health outcomes (WHO 2011a). The information in this area needs to be reviewed with up‐to‐date evidence to better inform policy making.
Objectives
To assess the effects of prenatal home (point‐of‐use) fortification of foods with multiple micronutrient powders on newborn and maternal health.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (both individual and cluster‐randomisation) and quasi‐randomised trials, irrespective of language or publication status of the trials. Evidence from other trial designs are summarised in the discussion section but not included in the results or conclusions of this review.
Types of participants
Pregnant women of any gestational age and parity. Studies specifically targeting HIV‐positive women or pregnant women with any other pathology were excluded.
Types of interventions
Micronutrient powders for point‐of‐use fortification of semi‐solid foods containing at least three micronutrients, with one of them being iron, provided to women during pregnancy.
Five comparisons were considered.
Micronutrient powders for point‐of‐use fortification of foods versus no intervention/placebo
Micronutrient powders for point‐of‐use fortification of foods versus iron and folic acid supplements
Micronutrient powders for point‐of‐use fortification of foods versus iron‐only supplements
Micronutrient powders for point‐of‐use fortification of foods versus folic‐acid only supplements
Micronutrient powders for point‐of‐use fortification of foods versus same multiple micronutrients in supplements
Interventions that combined provision of micronutrient powders for point‐of‐use fortification with co‐interventions such as education or other approaches were included only if the other co‐interventions were the same in both the intervention and comparison groups. There were no limits on the duration of the intervention.
Micronutrient powders for home fortification refers to the addition of powders containing vitamins and minerals to energy‐containing foods at home or in any other place where meals are to be consumed, such as schools, nurseries and refugee camps. Micronutrient powders can be added to foods either during or after cooking or immediately before consumption without the explicit purpose of improving the flavour or colour.
We did not include other point‐of‐use fortification interventions such as lipid‐based supplements or crushed tablets and also excluded studies examining the effect of centrally‐processed fortified foods. We also excluded oral supplements forms such as tablets or capsules (WHO 2008).
Types of outcome measures
Primary outcomes
Maternal outcomes
Maternal anaemia at term or near term (haemoglobin (Hb) less than 110 g/L at 34 weeks' gestation or more)
Maternal iron deficiency at term or near term (as defined by authors of the trials, based on any indicator of iron status at 34 weeks' gestation or more)
All‐cause maternal mortality (death while pregnant or within 42 days of termination of pregnancy)
Adverse effects (any)*
Infant outcomes
Low birthweight (birthweight less than 2500 g)
Preterm births (births before 37 weeks of gestation)
Secondary outcomes
Maternal outcomes
Maternal iron‐deficiency anaemia at term or near term (defined as Hb less than 110 g/L and at least one other laboratory indicator at 34 weeks' gestation or more)
Maternal Hb at term or near term (in g/L at 34 weeks' gestation or more)
Infection during pregnancy (including urinary tract infections and others as specified by trialists)
Antepartum haemorrhage (as defined by trial authors)
Postpartum haemorrhage (intrapartum and postnatal, as defined by trial authors)
Pre‐eclampsia (as defined by trial authors)
Folate level at term or near term (serum or erythrocyte folate, nmol/L at 34 weeks' gestation or more)
Serum retinol concentrations at term or near term (μmol/L at 34 weeks' gestation or more)
Miscarriage (as defined by trial authors)
Maternal adherence with micronutrient powders for point‐of‐use fortification of foods (as defined by trial authors)
Infant outcomes
Serum ferritin concentration within the first three months (μg/L)
Stillbirths (as defined by trial authors)
Hb concentration within the first three months (g/L)
Neonatal death (death occurring in days 0 to 28 of life)
Stunting at any time within the first six months (‐2 Z‐score or lower)
Small‐for‐gestational‐age (birthweight less than 10% of weight in reference population of same age)
Early initiation of breastfeeding (put to breast within one hour of birth)
Exclusive breastfeeding (infants fed exclusively with breast milk)
For populations in malarial endemic areas, the following outcomes were reported.
Malaria incidence
Malaria severity
Placental malaria
* Adverse effects may include gastrointestinal side effects such as vomiting or metallic taste. For trials reporting individual adverse effects separately but not specifying the number of women reporting any adverse effects, for our primary outcome, we selected the adverse effect with the greatest number of women (in the intervention and control groups combined) reporting that particular problem. We did this to avoid double counting any women reporting more than one adverse effect. We recorded other relevant outcomes reported by trial authors and labelled them as 'non pre‐specified'.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (31 January 2015).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched the WHO International Clinical Trials Registry Platform (ICTRP) for any ongoing or planned trials (see: Appendix 1).
Searching other resources
We searched through the bibliographies of included studies and asked authors of included studies for lists of other studies that should be considered for inclusion.
For assistance in identifying ongoing or unpublished studies, we contacted the Sprinkles Global Health Initiative, the Home Fortification Technical Advisory Group, the nutrition section of the United Nations Children's Fund (UNICEF), the World Food Programme (WFP), the Micronutrient Initiative (MI), the Global Alliance for Improved Nutrition (GAIN), Helen Keller International (HKI), Sight and Life, the Departments of Nutrition for Health and Development from the World Health Organization (WHO), WHO regional offices and the U.S. Centers for Disease Control and Prevention (CDC).
We did not apply any language or date restrictions.
Data collection and analysis
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion.
If studies were published only as abstracts, or study reports containing little information on methods, we attempted to contact the authors to obtain further details on the study design, population, and intervention to properly assess the eligibility.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, consulted the other author. We entered data into Review Manager software (RevMan 2014) and checked for accuracy.
We used narrative synthesis, guided by the data extraction form, in terms of the ways in which studies are grouped and summarised in this review to explore intervention implementation (using information about resource use and findings from process evaluations), and to describe the impact of interventions by socio‐demographic characteristics known to be important from a health equity perspective based on the place of residence, race/ethnicity/culture/language, occupation, gender/sex, religion, education, socioeconomic status, and social capital “PROGRESS” framework, where this information was available (O'Neil 2014).
When information regarding the methods and results was unclear, we contacted the authors of the original reports for further details. If there was insufficient information for us to be able to assess risk of bias, studies were placed under "awaiting assessment" until further information is published or made available to us.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence. We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence, and we assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
We classified blinding as 'high risk of bias' if the blinding status of a trial was unclear or the trial was open.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
We classified blinding as 'high risk of bias' if the blinding status of a trial was unclear or the trial was open.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We assessed losses to follow‐up and post‐randomisation exclusions systematically for each trial. We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. We assessed methods as:
low risk of bias;
high risk of bias; or
unclear.
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review had been reported);
high risk of bias (where not all the study’s pre‐specified outcomes had been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported); or
unclear.
(6) Other sources of bias
We assessed whether each study was free of other problems that could put it at risk of bias: We noted for each included study any important concerns we had about other possible sources of bias.We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of further bias;
high risk of further bias; or
unclear whether there is a risk of further bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
The main findings of the review were set out in 'Summary of findings' tables prepared using GRADE profiler software (GRADEpro 2014). The primary outcomes for each comparison were listed with estimates of relative effects along with the number of participants and studies contributing data for those outcomes. For each individual primary outcome, the quality of the evidence was assessed independently by two review authors using the GRADE approach (Balshem 2010), which involves consideration of within‐study risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias and results in one out of four levels of quality (high, moderate, low or very low). This assessment was limited only to randomised clinical trials, as we did not expect any serious risk of indirectness or publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measure the same outcome, but used different methods. We presented results with 95% confidence intervals.
Unit of analysis issues
Cluster‐randomised trials
Only trials with cluster randomisation met our inclusion criteria. Thus, It was not possible to meta‐analyse them together with individually‐randomised trials or asses any heterogeneity attributable to the unit of randomisation. Both included trials were adjusted by the authors to account for clustering effect. Choudhury 2012, reported to use a linear mixed effects regression model for this purpose.
In future updates of this review, if appropriate, we will include cluster‐randomised trials in the analyses along with individually‐randomised trials. If they have not been adjusted, we will adjust their sample sizes using the methods described in the Handbook using an estimate of the intra cluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity and subgroup analysis to investigate the effects of the randomisation unit.
Multiple pregnancies
None of the included studies reported the inclusion of women with multiple pregnancies. If such data are included in future updates of this review we will consider for each outcome whether the appropriate denominator is the number of babies or the number of women. For all infant outcomes, the number of babies will be the appropriate denominator, and for maternal outcomes, the number of women will be used.
Multi‐armed trials
We included multi‐armed trials in the analyses along with individually‐randomised trials. We included the relevant intervention groups in a pair‐wise comparison of intervention groups that meet the criteria for including studies in the review. We combined groups to create a single pair‐wise comparison using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis. We attempted to contact authors for clarification on missing data.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
In future updates of this review, if more data become available, we will assess statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We will regard heterogeneity as substantial if an I² is greater than 30% and either the T² is greater than zero, or there is a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates of this review, if there were 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We did not combine data in meta‐analysis due to insufficient data. In future, updates of this review, we will carry out statistical analysis using the Review Manager software (RevMan 2014). We will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials.
If we use random‐effects analyses, the results will be presented as the average treatment effect with its 95% confidence interval, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
There were insufficient data to conduct meta‐analysis or assess heterogeneity. In future updates of this review, if we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We plan to carry out the following subgroup analyses.
By baseline nutritional status of women at the start of intervention (including anaemia and iron deficiency): anaemic or iron‐deficient versus non‐anaemic or non iron‐deficient versus mixed/unknown/unreported.
By malaria status in the study area: malaria present at the time of the trial versus absent/unknown/unreported malaria at the time of the trial.
By dosage of elemental iron in the micronutrient powder (per portion added or individual sachet): 30 mg or less versus more than 30 mg.
By provision scheme of the micronutrient powder intervention: daily versus weekly versus flexible.
For the comparisons related to malaria endemic areas we plan to conduct a subgroup analysis by use or not of concurrent antimalarial measures (treatment and prevention).
Subgroup analysis will be restricted to the review's primary outcomes.
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the χ2 statistic and P value, and the interaction test I² value. We will explore the forest plots visually and identify where confidence intervals do not overlap to identify differences between subgroup categories. We will also formally investigate differences between two or more subgroups (Borenstein 2009).
Sensitivity analysis
Planned sensitivity analyses were not performed due to insufficient data. In future updates, we will carry out sensitivity analysis (where appropriate) to examine the effects of removing trials at high risk of bias (trials with poor or unclear allocation concealment and either blinding or high/imbalanced loss to follow‐up) from the analysis. In future updates, we will examine the effects of different intra‐cluster correlation values for cluster trials (if these are included). We will attempt to identify sources of funding for all included studies and will note this in the 'Characteristics of included studies' table; sensitivity analysis by source of funds (industry versus non‐industry) will be conducted to highlight if there are any patterns in the findings. Sensitivity analysis will be restricted to the review's primary outcomes.
Results
Description of studies
Results of the search
The search of the Cochrane Pregnancy and Childbirth Group's Trials Register retrieved two reports of trials which involved 1172 women. Additional searching retrieved a further 11 reports. Three were extra reports of Hernández Cabrera 2008; we excluded three trials (six reports), and one study (two reports) was identified as ongoing (see: (Richter 2013)(Figure 1).
1.
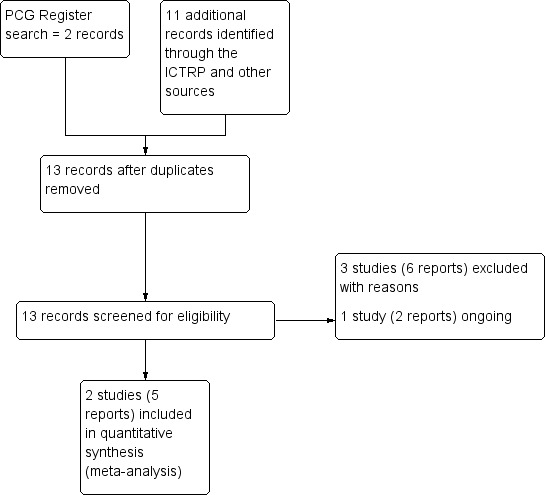
Study flow diagram.
Included studies
We included two trials (Choudhury 2012; Hernández Cabrera 2008) involving 478 and 694 pregnant women, respectively. The outcomes were defined differently in these two trials, as Choudhury 2012 only followed women to 32 weeks' gestation (not to term), while Hernández Cabrera 2008 reported outcomes at 37 weeks' gestation (near term). Both trials took place in rural settings, one in Bangladesh and the other in Mexico. None of the trial settings were endemic for malaria.
1) Micronutrient powders for point‐of‐use fortification of foods versus no intervention/placebo
Neither trial assessed this comparison.
2) Micronutrient powders for point‐of‐use fortification of foods versus iron and folic acid supplements
One trial (Choudhury 2012) involving 478 pregnant women assessed this comparison. Anaemia and haemoglobin (Hb) concentrations at 32 weeks' gestation were reported, as well as adherence to the intervention. No other primary or secondary outcomes were reported.
3) Micronutrient powders for point‐of‐use fortification of foods versus iron‐only supplements
Neither trial assessed this comparison.
4) Micronutrient powders for point‐of‐use fortification of foods versus folic‐acid only supplements
Neither trial assessed this comparison.
5) Micronutrient powders for point‐of‐use fortification of foods versus same multiple micronutrients in supplements
One trial Hernández Cabrera 2008 involving 694 participants (470 pregnant women in two of the three arms of this study) assessed this comparison in which maternal anaemia and Hb concentration at term or near term (37 weeks' gestation) were assessed. At the three‐month postpartum visit, the authors reported acceptability data among 268 women who remained in the trial (174 in the micronutrient powders for point‐of‐use fortification and tablet arms). No other primary or secondary outcomes were reported.
Excluded studies
Three trials were excluded from the review.
One study (Hartman‐Craven 2009) assessed absorption and bioavailability of an iron + folic acid micronutrient powder for point‐of‐use fortification versus tablet in pregnant women, but no primary or secondary outcomes for determination of safety or effectiveness were assessed. Another study (Khambalia 2009) evaluated the effects of an iron and folic acid‐containing micronutrient powder for point‐of‐use fortification in pregnant women, but the comparison group was also given a micronutrient powder for point‐of‐use fortification containing folic acid (not a tablet). The third study (Rim 2014) was a pre‐post design (no control or comparison group) of micronutrient powder containing iron, folic acid, zinc and vitamin C in pregnant and lactating women delivered weekly for six months. See the Characteristics of excluded studies table for more details.
Risk of bias in included studies
The included trials had a moderate to high risk of bias. The methods for randomisation were not adequately described in either trial. Neither the participants not the outcome assessors were blinded to the treatment allocation. A 'Risk of bias' graph (review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies) is presented in Figure 2. Figure 3 presents the 'Risk of bias' summary (review authors' judgements about each 'Risk of bias' item for the two included studies).
2.
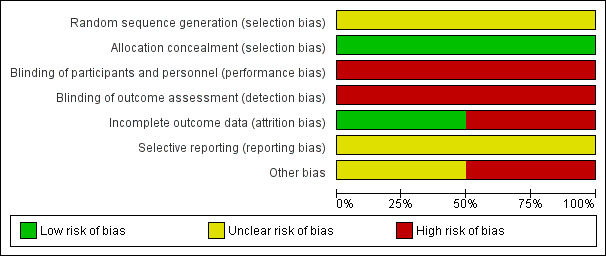
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
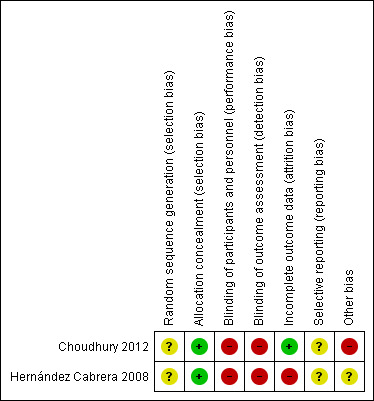
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Neither of the trials reported the methods for generating the randomisation sequence (Choudhury 2012; Hernández Cabrera 2008).
Allocation concealment
As they were cluster‐randomised studies, we judged the two included trial as having low risk of selection bias at the individual level (Choudhury 2012; Hernández Cabrera 2008).
Blinding
In the two included trials (Choudhury 2012. Hernández Cabrera 2008), we judged that the performance and detection risk was high as neither participants nor outcome assessors were blinded to the interventions.
Incomplete outcome data
We judged that trials with more than 20% loss to follow‐up, or with imbalanced loss to follow‐up in different arms of trials, were inadequate in terms of completeness of outcome data. The Choudhury 2012 trial was judged to be at low risk of bias as the rate of loss to follow‐up was 15%, without any differences between trial arms, whereas Hernández Cabrera 2008's trial was judged to be at high risk of bias, since the loss to follow‐up was 24.3% in the micronutrient powders for point‐of‐use fortification group, higher than that in the other trial arms.
Selective reporting
We could not formally assess outcome reporting bias as we did not have access to study protocols of the included trials, and assessing outcome reporting bias from published reports alone can be difficult. Thus, it is unclear if other outcomes were assessed and not reported.
Other potential sources of bias
In the Choudhury 2012 trial, there was a higher prevalence of baseline anaemia among participants receiving micronutrient powders (50%) compared to the participants receiving iron and folic acid tablets (39.1%).
Effects of interventions
Micronutrient powders for point‐of‐use fortification of foods versus iron and folic acid supplements (comparison 1)
Primary outcomes
Maternal outcomes
Maternal anaemia at term or near term (haemoglobin (Hb) less than 110 g/L at 34 weeks' gestation or more)
No trials reported on this outcome
Maternal iron deficiency at term or near term (as defined by authors of the trials, based on any indicator of iron status at 34 weeks' gestation or more)
No trials reported on this outcome
All‐cause maternal mortality (death while pregnant or within 42 days of termination of pregnancy)
No trials reported on this outcome
Adverse effects (any)
No trials reported on this outcome
Infant outcomes
Low birthweight (birthweight less than 2500 g)
No trials reported on this outcome
Preterm births (births before 37 weeks of gestation)
No trials reported on this outcome
Secondary outcomes
Maternal outcomes
The following maternal secondary outcomes were not reported in the included studies.
Maternal iron‐deficiency anaemia at term or near term (defined as Hb less than 110 g/L and at least one other laboratory indicator at 34 weeks' gestation or more)
Maternal Hb at term or near term (in g/L at 34 weeks' gestation or more)
Infection during pregnancy (including urinary tract infections and others as specified by trialists)
Antepartum haemorrhage (as defined by trial authors)
Postpartum haemorrhage (intrapartum and postnatal, as defined by trial authors)
Pre‐eclampsia (as defined by trial authors)
Folate level at term or near term (serum or erythrocyte folate, nmol/L at 34 weeks' gestation or more)
Serum retinol concentrations at term or near term (μmol/L at 34 weeks' gestation or more)
Miscarriage (as defined by trial authors)
Maternal adherence with micronutrient powders for point‐of‐use fortification of foods (as defined by trial authors)
One study reported on this outcome (Choudhury 2012). Maternal adherence was lower in the micronutrient powders for point‐of‐use fortification group, compared to the iron and folic acid supplementation group (risk ratio (RR) 0.76; 95% confidence interval (CI) 0.66 to 0.87, one study n = 405 (Analysis 1.1)).
1.1. Analysis.
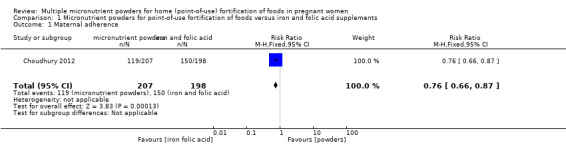
Comparison 1 Micronutrient powders for point‐of‐use fortification of foods versus iron and folic acid supplements, Outcome 1 Maternal adherence.
Infant outcomes
The following infant secondary outcomes were not reported in the included studies:
Serum ferritin concentration within the first three months (μg/L)
Stillbirths (as defined by trial authors)
Hb concentration within the first three months (g/L)
Neonatal death (death occurring in days 0 to 28 of life)
Stunting at any time within the first six months (‐2 Z‐score or lower)
Small‐for‐gestational‐age (birthweight less than 10% of weight in reference population of same age)
Early initiation of breastfeeding (put to breast within one hour of birth)
Exclusive breastfeeding (infants fed exclusively with breast milk)
Non‐prespecified outcomes
Choudhury 2012 reported on several non‐prespecified outcomes. At 32 weeks' gestation, there were no clear differences between micronutrient powders for point‐of‐use fortification and iron and folic acid supplementation on mild anaemia, defined as Hb 100 to 109 g/L (RR 1.03; 95% CI 0.75 to 1.42 (Analysis 1.2)) or any anaemia, defined as Hb < 110 g/L (RR 1.25; 95% CI 1.00 to 1.57; one study; (Analysis 1.3)). Compared to iron and folic acid supplementation, micronutrient powders for point‐of‐use fortification use resulted in a higher rate of moderate anaemia at 32 weeks' gestation, defined as Hb 70 to 99 g/L (RR 1.75; 95% CI 1.11 to 2.77; one study; (Analysis 1.4)) and a 2.5 g/L lower Hb concentration at 32 weeks' gestation (mean difference (MD) ‐2.50 g/L; 95% CI ‐4.85 to ‐0.15 (Analysis 1.5)).
1.2. Analysis.
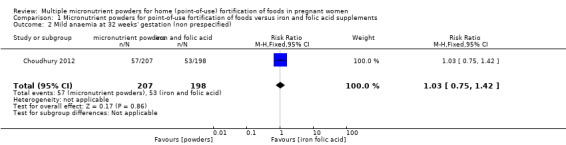
Comparison 1 Micronutrient powders for point‐of‐use fortification of foods versus iron and folic acid supplements, Outcome 2 Mild anaemia at 32 weeks' gestation (non prespecified).
1.3. Analysis.
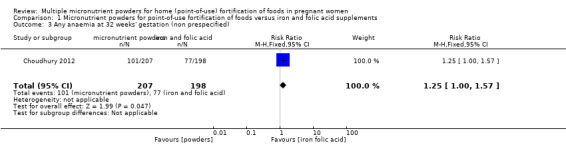
Comparison 1 Micronutrient powders for point‐of‐use fortification of foods versus iron and folic acid supplements, Outcome 3 Any anaemia at 32 weeks' gestation (non prespecified).
1.4. Analysis.
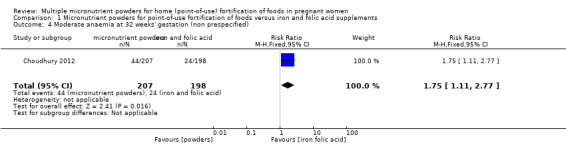
Comparison 1 Micronutrient powders for point‐of‐use fortification of foods versus iron and folic acid supplements, Outcome 4 Moderate anaemia at 32 weeks' gestation (non prespecified).
1.5. Analysis.
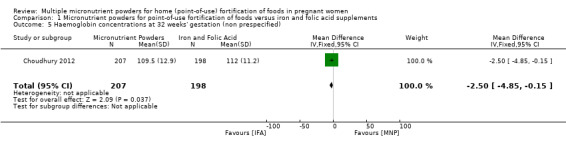
Comparison 1 Micronutrient powders for point‐of‐use fortification of foods versus iron and folic acid supplements, Outcome 5 Haemoglobin concentrations at 32 weeks' gestation (non prespecified).
Micronutrient powders for point‐of‐use fortification of foods versus the same multiple micronutrients in supplements (comparison 2)
Primary outcomes
Maternal outcomes
Maternal anaemia at term or near term (haemoglobin (Hb) less than 110 g/L at 34 weeks' gestation or more)
One study reported on this outcome (Hernández Cabrera 2008). When compared to multiple micronutrient supplementation, the same micronutrients but in the form of powders for point‐of‐use fortification, there was no clear difference in the prevalence of maternal anaemia at term or near term (RR 0.92; 95% CI 0.53 to 1.59, very low quality evidence (Analysis 2.1)).
2.1. Analysis.
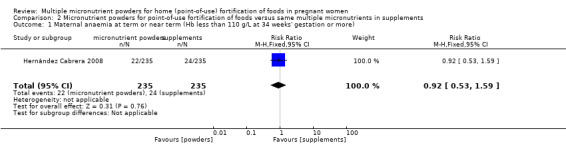
Comparison 2 Micronutrient powders for point‐of‐use fortification of foods versus same multiple micronutrients in supplements, Outcome 1 Maternal anaemia at term or near term (Hb less than 110 g/L at 34 weeks' gestation or more).
Maternal iron deficiency at term or near term (as defined by authors of the trials, based on any indicator of iron status at 34 weeks' gestation or more)
No trials reported on this outcome.
All‐cause maternal mortality (death while pregnant or within 42 days of termination of pregnancy)
No trials reported on this outcome.
Adverse effects (any)
No trials reported on this outcome.
Infant outcomes
Low birthweight (birthweight less than 2500 g)
No trials reported on this outcome.
Preterm births (births before 37 weeks of gestation)
No trials reported on this outcome.
Secondary outcomes
Maternal outcomes
Maternal iron‐deficiency anaemia at term or near term (defined as Hb less than 110 g/L and at least one other laboratory indicator at 34 weeks' gestation or more)
No trials reported on this outcome.
Maternal Hb at term or near term (in g/L at 34 weeks' gestation or more)
One study reported on this outcome (Hernández Cabrera 2008).There was a statistically non‐significant 1.0 g/L higher maternal Hb concentration at term in the micronutrient powders for point‐of‐use fortification compared to multiple micronutrient supplementation group (MD 1.00 g/L; 95% CI ‐1.77 to 3.77 (Analysis 2.2)).
2.2. Analysis.
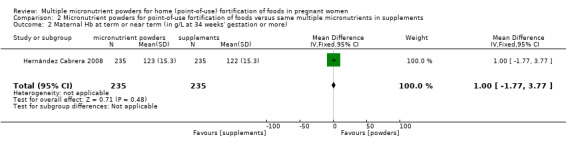
Comparison 2 Micronutrient powders for point‐of‐use fortification of foods versus same multiple micronutrients in supplements, Outcome 2 Maternal Hb at term or near term (in g/L at 34 weeks' gestation or more).
The following maternal secondary outcomes were not reported in the included studies.
Infection during pregnancy (including urinary tract infections and others as specified by trialists)
Antepartum haemorrhage (as defined by trial authors)
Postpartum haemorrhage (intrapartum and postnatal, as defined by trial authors)
Pre‐eclampsia (as defined by trial authors)
Folate level at term or near term (serum or erythrocyte folate, nmol/L at 34 weeks' gestation or more)
Serum retinol concentrations at term or near term (μmol/L at 34 weeks' gestation or more)
Miscarriage (as defined by trial authors)
Maternal adherence with micronutrient powders for point‐of‐use fortification of foods (as defined by trial authors)
Infant outcomes
The following infant secondary outcomes were not reported in the included studies:
Serum ferritin concentration within the first three months (μg/L)
Stillbirths (as defined by trial authors)
Hb concentration within the first three months (g/L)
Neonatal death (death occurring in days 0 to 28 of life)
Stunting at any time within the first six months (‐2 Z‐score or lower)
Small‐for‐gestational‐age (birthweight less than 10% of weight in reference population of same age)
Early initiation of breastfeeding (put to breast within one hour of birth)
Exclusive breastfeeding (infants fed exclusively with breast milk)
Discussion
Determining the optimal intervention to deliver iron and other micronutrients to women during pregnancy is a critical clinical and public health question due the importance of maternal nutrition on the health of the fetus and mother. Programmatic challenges to prenatal micronutrient supplementation, including poor adherence and low bioavailability and tolerance to various iron preparations, have resulted in the development and evaluation of point‐of‐use fortification with micronutrient powders in pregnancy. Programs providing micronutrient powders for point‐of‐use fortification of foods, particularly to children under five years of age, have been implemented to‐scale in more than 40 countries globally (distributed in more than 60 countries) and have been shown in programme evaluations to reduce iron‐deficiency anaemia in preschool children (HFTAG 2013; UNICEF/CDC 2014). While it may be reasonable from the biological perspective to assume that the effects of micronutrient powders for point‐of‐use fortification of foods are the same as multiple micronutrient supplementation in pregnant women, no countries have yet implemented large‐scale prenatal micronutrient powders programmes, due to lack of evidence to support policy guidance (WHO 2011a).
Only two trials (involving 1172 women) met the inclusion criteria of this review. One trial compared groups of pregnant women receiving micronutrient powders for point‐of‐use fortification of foods to iron and folic acid supplementation, and the study compared micronutrient powders for point‐of‐use fortification of foods to multiple micronutrient supplementation.
Summary of main results
Results from one included study showed that micronutrient powders for point‐of‐use fortification of foods had a similar effect on maternal anaemia and haemoglobin (Hb) at or near term when compared to multiple micronutrient supplements. None of the other prespecified clinically primary outcomes, such as maternal iron deficiency, maternal mortality, adverse effects, low birthweight, preterm births, and nutritional status of infants, could be assessed due to lack of data.
In the other trial, there were no data to compare the effects of micronutrient powders for point‐of‐use fortification of foods in comparison to iron and folic acid tablets for any of the primary outcomes. Non‐prespecified outcomes of anaemia and Hb concentrations at week 32 of gestation, showed that there were no clear differences between micronutrient powders and iron and folic acid supplements on haematological outcomes (e.g., anaemia and Hb concentration); however, there was lower maternal adherence to micronutrient powders when compared to iron and folic acid supplements. Authors hypothesised that low adherence was due to mixing the micronutrient powders in rice, which made it gritty or sour tasting; furthermore, non‐adherent women expressed concerns that adding micronutrient powders to their daily food would worsen pregnancy‐related nausea, and increase appetite (Choudhury 2012).
Overall completeness and applicability of evidence
The two included studies were conducted in a low‐income (Choudhury 2012) and upper‐middle‐income(Hernández Cabrera 2008) countries, which may increase their generalisability to women in low‐ and middle‐income countries with the highest rates of anaemia and undernutrition. One study was a non‐inferiority trial of prenatal micronutrient powders for point‐of‐use fortification versus iron and folic acid supplementation, using a daily dose of 60 mg iron in each product (Choudhury 2012), whereas the other study was an effectiveness trial which evaluated the impact of micronutrient powders for point‐of‐use fortification compared to multiple micronutrient supplementation, both containing 15 mg of iron, in a community setting (Hernández Cabrera 2008).
These findings are limited by a dearth of data. A recent systematic review on the effectiveness of micronutrient powders for point‐of‐use fortification in women and children identified no studies on women that met the authors' inclusion criteria (Salam 2013). Some ongoing trials, such as a trial in Guatemala comparing the impact of lipid‐based nutrient supplements, corn‐soy blend or micronutrient powders for point‐of‐use fortification among pregnant women and children (Richter 2013), may provide additional evidence once available. However, to our knowledge, no trials are currently registered or ongoing that assess the wide array of clinically important health outcomes of prenatal micronutrient supplementation, including maternal iron deficiency and mortality, as well as birth and neonatal outcomes.
Despite the lack of evidence, some public health programmes are distributing micronutrient powders for point‐of‐use fortification in pregnant women as part of pilot projects or for specific sub‐populations. For example, the World Food Programme (WFP) recommends micronutrient powders and other fortified blended and ready‐to‐use foods to meet the particular nutritional needs of young children, pregnant and lactating women and those infected with HIV, tuberculosis and malaria (WFP 2013). Other international agencies, such as United Nations High Commisioner for Refugees (UNHCR), and the non‐governmental organisation Hellen Keller International (HKI) have reported on experiences distributing micronutrient powders in emergency situations and in refugee camps, focused on young children, but adaptable to other populations including adolescents and pregnant women (HFTAG 2013b; UNHCR 2011). In North Korea, a Canadian non‐governmental organisation, First Steps, has been distributing micronutrient powders for point‐of‐use fortification to pregnant women and children six to 24 months of age through government health clinics on the east and west coast of the Democratic People's Republic of Korea (North Korea) since 2008 (Rim 2014).
Quality of the evidence
Although not all reports included detailed information on the methods followed in the studies, an effort was made to contact authors in order to obtain more data. Blinding of women and outcome assessors was not attempted in either of the included trials. However, blinding was unlikely to have had an impact on the reported results (e.g. anaemia). Attrition was not considered a serious problem in the included studies. For the Hernández Cabrera 2008 study, there is a risk for publication bias, since only two scientific abstracts and one peer‐reviewed publication (reporting on qualitative research only) have been published for a study that was completed in 2007.
It was possible to assess the overall quality of the evidence for only one primary outcome: maternal anaemia at term or near term. Due to the methodological limitations and the lack of studies, we considered the overall quality as very low.
Potential biases in the review process
We were aware of the possibility of introducing bias at every stage of the review process and therefore tried to minimise bias in a number of ways, including assigning two review authors to assess eligibility for inclusion, to carry out data extraction, and to assess risk of bias. Since this intervention is very recent and well‐known among implementing agencies, and they were contacted as part of the search strategy, we consider there is minimal risk of publication bias.
Agreements and disagreements with other studies or reviews
A recent systematic review on the effectiveness of micronutrient powders for point‐of‐use fortification in women and children identified no studies on women that met the authors' inclusion criteria (Salam 2013). In agreement with the trials included in this review which indicated lower adherence of prenatal micronutrient powders compared to supplements, a study from the University of Toronto recently reported that prenatal calcium delivered as conventional tablets was preferred and more acceptable than powdered calcium among pregnant women in Bangladesh (Baxter 2014). These findings may not be generalisable to standard formulations of prenatal supplements, since they contained 1500 mg of calcium to adhere to WHO dose recommendations, resulting in a large serving weight of 11 g to 19 g per unit for the powder, as compared to the 1.0 g micronutrient powders sachets typically used.
Authors' conclusions
Implications for practice.
There is limited evidence to suggest that micronutrient powders for point‐of‐use fortification of foods have a similar effect as multiple micronutrient supplements on maternal anaemia at or near term and haemoglobin (Hb) concentrations at or near term. The overall quality of evidence was judged very low, and no evidence was available for the majority of primary and secondary outcomes. Therefore, more evidence is needed to assess the potential benefits or harms of the use of micronutrient powders in pregnant women on maternal and infant health outcomes.
Data on adherence to the intervention suggest that the provision of micronutrient powders for point‐of‐use fortification of foods to pregnant women may be less acceptable to women than traditional tablets. This needs to be explored further in different settings, particularly in Africa where there is the highest burden of maternal 'undernutrition'.
Implications for research.
This review highlights the need for well‐conducted, randomised controlled trials evaluating the effectiveness and safety of micronutrient powders for point‐of‐use fortification consumed by women during pregnancy. Trials should measure population‐relevant health outcomes, including side effects of this intervention in pregnant women, and other maternal and infant health outcomes. Additionally, trials should assess the effects of variability between different micronutrient combinations, dosages, frequency and duration.
Acknowledgements
The World Health Organization, and Parminder Suchdev and Luz Maria De‐Regil, retain copyright and all other rights in their respective contributions to the manuscript of this review as submitted for publication.
As part of the pre‐publication editorial process, this review has been commented on by four peers (an editor and three referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. ICTRP search terms
micronutrient AND powder* AND pregnant
micronutrient AND powder* AND pregnancy
Data and analyses
Comparison 1. Micronutrient powders for point‐of‐use fortification of foods versus iron and folic acid supplements.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal adherence | 1 | 405 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.66, 0.87] |
| 2 Mild anaemia at 32 weeks' gestation (non prespecified) | 1 | 405 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.75, 1.42] |
| 3 Any anaemia at 32 weeks' gestation (non prespecified) | 1 | 405 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.00, 1.57] |
| 4 Moderate anaemia at 32 weeks' gestation (non prespecified) | 1 | 405 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.11, 2.77] |
| 5 Haemoglobin concentrations at 32 weeks' gestation (non prespecified) | 1 | 405 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐4.85, ‐0.15] |
Comparison 2. Micronutrient powders for point‐of‐use fortification of foods versus same multiple micronutrients in supplements.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal anaemia at term or near term (Hb less than 110 g/L at 34 weeks' gestation or more) | 1 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.53, 1.59] |
| 2 Maternal Hb at term or near term (in g/L at 34 weeks' gestation or more) | 1 | 470 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐1.77, 3.77] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Choudhury 2012.
| Methods | Cluster‐randomised non‐inferiority trial. 2‐arm study. | |
| Participants | 478 pregnant women (gestational age 14–22 weeks) attending 42 antenatal care centres (ANC) in the rural sub district of Kaliganj in central Bangladesh. Women with haemoglobin lower than 70 g/L or greater than 140 g/L, with more than 22 weeks of gestation, or who were already taking iron supplements prior to the start of the study were excluded. | |
| Interventions | Participants were randomised, at the cluster level (ANC), to 1 of 2 interventions: group 1 (21 clusters, n = 243) received micronutrient powders containing (60 mg of elemental iron as ferrous fumarate), 400 μg of folic acid, 30 mg of vitamin C, and 5 mg of zinc) daily or group 2 (21 clusters, n = 235) received tablets containing 60 mg elemental iron and 400 μg (0.4 mg) folic acid daily. Participants from group 1 were instructed to sprinkle the full content of a package of the powders into any semi‐solid or semi‐liquid food. | |
| Outcomes | Haemoglobin concentrations, mild and moderate anaemia at baseline, 24, 28 and 32 weeks of gestation, adherence to intervention. | |
| Notes | Baseline nutritional status of women at the start of intervention mixed group 1: 50% (n = 121) and group 2: (39.1%, n = 92). Malaria status in the study area: absent/unknown/unreported malaria at the time of the trial. Dosage of elemental iron in the micronutrient powder (per portion added or individual sachet): more than 30 mg. Provision scheme of the micronutrient powder intervention: daily. Source of funding: HJ Heinz Company Foundation. Clustering effect accounted in the original analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported. |
| Allocation concealment (selection bias) | Low risk | As this is a cluster‐randomised trial, the risk of selection bias at the individual level is low. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded to the interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the interventions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition of 15%, similar in both groups. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear if outcomes other than those reported were assessed and purposely not reported. |
| Other bias | High risk | Higher prevalence of baseline anaemia among participants from group 1 receiving micronutrient powders (50.0%) compared to the participants in group 2 receiving iron and folic acid tablets (39.1%). |
Hernández Cabrera 2008.
| Methods | Cluster‐randomised trial conducted Sept 2005 to Dec 2007, implemented in the context of Oportunidades programme, a conditional cash transfer programme implemented in rural areas in 1997 and urban areas in 2002 with authorisation of Oportunidades officials at the federal, state and local level, National Institute of Public Health Ethics Commission, in Mexico. Appropriate statistical analyses for cluster‐randomised trials were used and intention‐to‐treat analyses and comparison between groups at each time point using the Gllamm command in Stata clustered on woman and community. | |
| Participants | 694 Oportunidades beneficiary pregnant women were recruited before 24 weeks pregnancy and without severe anaemia. Supplemented until 3 months postpartum. Survey of acceptability implemented at the 3‐month postpartum visit. | |
| Interventions | Communities were randomly assigned (18 communities per supplement) to 1 of 3 interventions: group 1 (n = 235 women) received daily 1 g of multiple micronutrient powders (Sprinkles®) containing 15 mg elemental iron, 400 μg (0.4 mg) folic acid and 5 additional micronutrients (zinc, iodine, vitamin E, vitamin C, vitamin B12); group 2 (n = 235 women) received 1 daily tablet (Laboratorios Zerboni, S.A.) containing 15 mg elemental iron, 400 μg (0.4 mg) folic acid and the same 5 additional micronutrients; group 3 (n = 224 women) received 44 g daily of whole whole‐milk based, sweetened fortified beverage for pregnant and lactating women (Nutrivida®) containing 15 mg elemental iron, 400 μg (0.4 mg) folic acid and the same 5 additional micronutrients, and also provided energy, protein, lipids, carbohydrates and sodium. Supplements were delivered daily for 6 months then weekly. Follow up visits at 37 weeks of pregnancy and 1 and 3 months postpartum. | |
| Outcomes | Weight gain, programme retention, acceptability, anaemia, Hb concentration at 37 weeks, and 1 and 3 months postpartum. Acceptability was measured using a 5‐point Likert scale from 1 "I liked it very much" to 5 "I didn't like it at all". More detailed data on acceptability were collected from semi‐structured interviews of a convenience sample of pregnant and lactating beneficiaries of the Opportunidades program; however, since these were not the same women enrolled as part of the randomised trial, their data were not included in the analysis. | |
| Notes | Contact person: Dr Lynnette Neufeld, lneufeld@gainhealth.org Source of funding: Opportunidades, Mexican Secretary of Health Clustering effect accounted in the original analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported. |
| Allocation concealment (selection bias) | Low risk | As this is a cluster‐randomised trial, the risk of selection bias at the individual level is low. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded to the interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the interventions. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up higher in group 1 (24.3%), compared to group 2 (14.9%) and group 3 (13.8%). |
| Selective reporting (reporting bias) | Unclear risk | Outcomes assessed were reported. It is unclear if other outcomes were assessed and not reported. |
| Other bias | Unclear risk | There is insufficient information to permit judgement. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Hartman‐Craven 2009 | 18 healthy pregnant women (24‐32 weeks' gestation) recruited from staff and visitors at the Hospital for Sick Children, Toronto, Canada, were randomised (using a cross‐over design) 1 of 2 groups: group 1 received a powdered supplement containing 30 mg of elemental iron (as micronised dispersible ferric pyrophosphate with an emulsifier coating) and 600 μg (0.6 mg) folic acid or group 2: received a tablet containing 27 mg elemental iron (as ferrous fumarate and 1000 μg (1 mg) folic acid. Absorption and bioavailability were assessed by measuring changes in serum iron and folate over 8 hours. No primary or secondary outcomes for determination of safety or effectiveness were assessed. The powdered micronutrient supplement only contained 2 micronutrients (iron and folic acid) and does not meet our criteria for a ´multiple´ micronutrient powder, containing at least 3 micronutrients. The objective of this randomised controlled trial in pregnant women was to measure the relative bioavailability of iron and folic acid from a powdered supplement that can be sprinkled on semi‐solid foods or beverages compared to a traditional tablet supplement. The type of interventions do not allow for comparisons within the scope of this review. |
| Khambalia 2009 | 272 married, nulliparous non‐pregnant women in Kaliganj, central Bangladesh were randomly assigned to 1 of 2 groups: group 1 received Sprinkles® containing 60 mg elemental iron (as ferrous fumarate) and 400 μg (0.4 mg) folic acid periconceptionally daily; group 2 received Sprinkles® containing 400 μg (0.4 mg) folic acid alone daily in the form of a powdered supplement to be added to food. Women who were not married, had previously given birth, older than 40 years of age, not a permanent household member (living in household less than 6 months), not living in same household as their husband, using an implant form of birth control, previous surgery to prevent pregnancy, had used iron supplements within the previous 3 months, were known to be pregnant at enrolment, or were identified as severely anaemic at baseline (haemoglobin concentration less than 70 g/L) were excluded. Participants were followed up monthly for 9 months or until they became pregnant. Each woman was given 35 sachets at enrolment and at each monitoring visit (30 sachets for a 1‐month daily supply and 5 extra sachets). The primary outcome was haemoglobin concentration among women who became pregnant (iron and folic acid compared with folic acid). The type of participants and interventions do not allow for comparisons within the scope of this review. |
| Rim 2014 | 2 cross‐sectional surveys were conducted among 494 pregnant women with a mean gestational age of 26 weeks (and 448 lactating women and 1265 children 6‐24 months of age) in South Hwanghae Province, DRP Korea (North Korea) before and after distribution of weekly Sprinkles® for 6 months. The Sprinkles® formulation for pregnant women contained 60 mg iron, 400 μg (0.4 mg) folic acid, 30 mg vitamin C, and 6 mg zinc. Primary outcomes included haemoglobin concentration pre‐ and post‐intervention and compliance (defined as weekly Sprinkles use over the 6‐month intervention). This study was supported by First Steps Canada. The lack of a control or comparison group forbids inclusion in this review. |
Characteristics of ongoing studies [ordered by study ID]
Richter 2013.
| Trial name or title | Preventing malnutrition in children under 2 approach in Guatemala. |
| Methods | Cluster‐randomised trial with 6 study arms conducted by the International Food Policy Research Institute (IFPRI), in partnership with the United States Agency for International Development (USAID)‐funded Food and Nutrition Technical Assistance Project (FANTA) aimed to compare the impact of lipid‐based nutrient supplements, corn‐soy blend or micronutrient powders among pregnant women and children in a Title II maternal and child health and nutrition programme implemented by Mercy Corps in Guatemala. |
| Participants | Pregnant women enrolled who were 3–7 months pregnant in Alta Verapaz, Guatemala and followed along with their children until 24 months of age. Women were beneficiaries of the maternal and infant community food diversification (PROCOMIDA for Programa Comunitario Materno Infantil de Diversificación Alimentaria) programme. |
| Interventions | The intervention has 6 arms: 1) full family ration, corn‐soy blend, behavioural change communication and required health visits; 2) reduced family ration, corn‐soy blend, behavioural change communication and required health visits; 3) no family ration, corn‐soy blend, behavioural change communication and required health visits; 4) full family ration, lipid‐based nutrient supplements, behavioural change communication and required health visits; 5) full family ration, micronutrient powders, behavioural change communication and required health visits; 6) usual care. Composition of micronutrient powders as 2 2 g sachets includes 20 mg elemental iron, 400 μg (0.4 mg) folic acid, and 19 other micronutrients. |
| Outcomes | Questionnaires to assess women's health, participation in prenatal care and demographics; anthropometry; unclear if biochemical testing for anaemia or micronutrient status is being done. |
| Starting date | April 2010. |
| Contact information | Deanna Olney, PhD; d.olney@cgiar.org |
| Notes | Data not yet available. |
Differences between protocol and review
We changed the maternal secondary outcome from "maternal satisfaction" to "maternal adherence" since adherence is a broader and more useful outcome to measure for evaluation and comparison purposes.
In the main results (under comparison 1, secondary outcomes) we also report four outcomes that were not prespecified in our protocol.
mild anaemia, defined as haemoglobin (Hb) 100 to 109 g/L
any anaemia, defined as Hb < 110 g/L
moderate anaemia at 32 weeks' gestation, defined as Hb 70 to 99 g/L
Hb concentration at 32 weeks' gestation
Contributions of authors
Parminder Suchdev, Luz Maria De‐Regil and Juan Pablo Peña‐Rosas provided content and epidemiological support for the protocol and review. Luz Maria De‐Regil and Juan Pablo Peña‐Rosas provided methodological support. All authors contributed to writing the review, commented and provided extensive feedback. All authors discussed the document and provided edits and references for the review.
Disclaimer: Juan Pablo Peña‐Rosas is a full‐time staff member of the World Health Organization. Luz Maria De‐Regil is affiliated with the Micronutrient Initiative. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the official position, decisions, policy or views of the World Health Organization, or the Micronutrient Initiative.
Sources of support
Internal sources
-
Emory University, USA.
Parminder Suchdev works as Associate Professor of Pediatrics for Emory University and receives salary support from the US Centers for Disease Control and Prevention (CDC), Atlanta, GA
Evidence and Programme Guidance Unit, Department of Nutrition for Health and Development, World Health Organization, Switzerland.
Micronutrient Initiative (MI), Canada.
External sources
-
Micronutrient Initiative (MI), Canada.
WHO acknowledges Micronutrient Initiative (MI) for their financial support to the Evidence and Programme Guidance Unit for the development and update of systematic reviews on nutrition and nutrition‐sensitive interventions.
-
The Bill & Melinda Gates Foundation, USA.
WHO acknowledges The Bill & Melinda Gates Foundation for their financial support to the Evidence and Programme Guidance Unit for the development and update of systematic reviews of the evidence in nutrition and nutrition‐sensitive interventions.
Declarations of interest
Parminder S Suchdev receives salary support from the U.S. Centers for Disease Control & Prevention.
Luz Maria De‐Regil ‐ The Micronutrient Initiative supports the implementation of large‐scale research projects that provide multiple micronutrient powders to children six to 23 months of age as well as programmes that provide iron and folic acid supplements to pregnant women. None of them met the inclusion criteria of this review.
Juan Pablo Peña‐Rosas ‐ The Evidence and Programme Guidance Unit, Department of Nutrition for Health and Development receives financial support from the Government of Luxembourg, the Micronutrient Initiative, US Centers for Disease Control and Prevention and the Bill & Melinda Gates Foundation for its work in several projects.
New
References
References to studies included in this review
Choudhury 2012 {published data only}
- Choudhury N, Aimone A, Hyder SM, Zlotkin SH. Relative efficacy of micronutrient powders versus iron‐folic acid tablets in controlling anemia in women in the second trimester of pregnancy. Food & Nutrition Bulletin 2012;33(2):142‐9. [DOI] [PubMed] [Google Scholar]
Hernández Cabrera 2008 {published and unpublished data}
- Colchero A, Neufeld LM. Cost estimations of different types of micronutrient supplements for children and pregnant women. FASEB Journal 2008;22:Abstract No:678.21. [Google Scholar]
- Hernández Cabrera A, García Guerra A, Domínguez CP, García Feregrino R, Neufeld LM. Effect of three supplements with identical micronutrient content on anemia in pregnant Mexican women. FASEB Journal 2008;22:Abstract No: 677.8. [Google Scholar]
- Neufeld L. Efficacy of 3 nutritional supplements to improve diverse outcomes in children under 2 years of age and pregnant women. ClinicalTrials.gov (http://clinicaltrials.gov) [accessed 19 July 2013] 2007.
- Young SL, Blanco I, Hernandez‐Cordero S, Pelto GH, Neufeld LM. Organoleptic properties, ease of use, and perceived health effects are determinants of acceptability of micronutrient supplements among poor Mexican women. Journal of Nutrition 2010;140(3):605‐11. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Hartman‐Craven 2009 {published data only}
- Hartman‐Craven B, Christofides A, O'Connor DL, Zlotkin S. Relative bioavailability of iron and folic acid from a new powdered supplement compared to a traditional tablet in pregnant women. BMC Pregnancy Childbirth 2009;9:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khambalia 2009 {published data only}
- Khambalia A. Periconceptional iron supplementation and iron and folate status among pregnant and non‐pregnant women in rural Bangladesh: PhD thesis. Toronto: University of Toronto, 2009. [Google Scholar]
- Khambalia A, O'Connor D, Zlotkin S. Reduced anemia and improved iron and folate status before and after pregnancy among females in rural Bangladesh is related to length of and adherence to periconceptional iron and folic acid supplementation. FASEB Journal 2009;23 Suppl:917.6. [Google Scholar]
- Khambalia AZ, O'Connor DL, Macarthur C, Dupuis A, Zlotkin SH. Periconceptional iron supplementation does not reduce anemia or improve iron status among pregnant women in rural Bangladesh. American Journal of Clinical Nutrition 2009;90(5):1295‐302. [DOI] [PubMed] [Google Scholar]
Rim 2014 {unpublished data only}
- Rim HY. Use of microencapsulated iron sprinkles to control IDA in pregnant/lactating women and infants in DPR Korea. Institute of Child Nutrition, DPR Korea 2014.
- Ritchie S. First Steps Canada (personal communication) 2014.
References to ongoing studies
Richter 2013 {published data only}
- Leroy JL. Preventing malnutrition in children under two years of age approach. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 14 June 2014] 2010.
- Richter SM, Leroy JL, Olney D, Quinones E, Ruel M. Strengthening and Evaluating the Preventing Malnutrition in Children under 2 Approach in Guatemala: Report of the Enrollment Survey. Washington DC: FHI 360, 2013. [Google Scholar]
Additional references
Balshem 2010
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Baxter 2014
- Baxter J, Roth D, Al Mahmud A, Ahmed T, Islam M, Zlotkin SH. Tablets are preferred and more acceptable than powdered prenatal calcium supplements among pregnant women in Dhaka, Bangladesh. Journal of Nutrition 2014;144(7):1106‐12. [DOI] [PubMed] [Google Scholar]
Beard 2000
- Beard J. Effectiveness and strategies of iron supplementation during pregnancy. American Journal of Clinical Nutrition 2000;71(5 Suppl):1288S‐1294S. [DOI] [PubMed] [Google Scholar]
Bhutta 2009
- Bhutta ZA, Rizvi A, Raza F, Hotwani S, Zaidi S, Moazzam Hossain S, et al. A comparative evaluation of multiple micronutrient and iron‐folic acid supplementation during pregnancy in Pakistan: impact on pregnancy outcomes. Food and Nutrition Bulletin 2009;30(4 Suppl):S496‐S505. [DOI] [PubMed] [Google Scholar]
Black 2001
- Black RE. Micronutrients in pregnancy. British Journal of Nutrition 2001;85(Suppl 2):S193‐S197. [DOI] [PubMed] [Google Scholar]
Black 2008
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, Onis M, Ezzati M, et al. Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371(9608):243‐60. [DOI] [PubMed] [Google Scholar]
Black 2013
- Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, Onis M, et al. Maternal and child undernutrition and overweight in low‐income and middle‐income countries. Lancet 2013;382(9890):427‐51. [DOI] [PubMed] [Google Scholar]
Borenstein 2009
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐Analysis (Statistics in Practice). Oxford: John Wiley & Sons Inc, 2009. [Google Scholar]
CDC 1998
- Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. Morbidity & Mortality Weekly Report 1998;47:1‐29. [PubMed] [Google Scholar]
Checkley 2010
- Checkley W, West KP Jr, Wise RA, Baldwin MR, Wu L, LeClerq SC, et al. Maternal vitamin A supplementation and lung function in offspring. New England Journal of Medicine 2010;362(19):1784‐94. [DOI] [PubMed] [Google Scholar]
CHERG 2013
- Murray‐Kolb LE, Chen L, Chen P, Shapiro M, Caulfield L. CHERG iron report: maternal mortality, child mortality, perinatal mortality, child cognition, and estimates of prevalence of anemia due to iron deficiency. cherg.org/publications/iron‐report.pdf (accessed February 5 2014) 2013.
Christian 2000
- Christian P, West KP Jr, Khatry SK, Kimbrough‐Pradhan E, LeClerq SC, Katz J, et al. Night blindness during pregnancy and subsequent mortality among women in Nepal: effects of vitamin A and beta‐carotene supplementation. American Journal of Epidemiology 2000;152(6):542‐7. [DOI] [PubMed] [Google Scholar]
Christian 2010
- Christian P. Micronutrients, birth weight, and survival. Annual Review of Nutrition 2010;30:83‐104. [DOI] [PubMed] [Google Scholar]
Commission of the European Communities 1993
- Commission of the European Communities. Nutrient and Energy Intakes for the European Community. Reports of the Scientific Committee for Food. Luxembourg: Commission of the European Communities, 1993. [Google Scholar]
Dalmiya 2009
- Dalmiya D, Darnton‐Hill I, Schultink W, Shrimpton R. Multiple micronutrient supplementation during pregnancy: a decade of collaboration in action. Food and Nutrition Bulletin 2009;30(4 Suppl):S477‐479. [DOI] [PubMed] [Google Scholar]
de Pee 2007
- Pee S, Moench‐Pfanner R, Martini E, Zlotkin S, Darton‐Hill I, Bloem MW. Home‐fortification in emergency response and transition programming: experiences in Aceh and Nias, Indonesia. Food and Nutrition Bulletin 2007;28:189‐97. [DOI] [PubMed] [Google Scholar]
de Pee 2008
- Pee S, Kraemer K, Briel T, Boy E, Grasset C, Moench‐Pfanner R, et al. Quality criteria for micronutrient powder products. Food and Nutrition Bulletin 2008;29(3):232‐41. [DOI] [PubMed] [Google Scholar]
De‐Regil 2010
- De‐Regil LM, Fernández‐Gaxiola AC, Dowswell T, Peña‐Rosas JP. Effects and safety of periconceptional folate supplementation for preventing birth defects. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD007950.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
De‐Regil 2011
- De‐Regil LM, Suchdev PS, Vist GE, Walleser S, Peña‐Rosas JP. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD008959.pub2] [DOI] [PubMed] [Google Scholar]
De‐Regil 2012
- De‐Regil LM, Jefferds MED, Peña‐Rosas JP. Point‐of‐use fortification of foods with micronutrient powders containing iron in children of preschool and school age. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009666] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duley 2009
- Duley L. The global impact of pre‐eclampsia and eclampsia. Seminars in Perinatology 2009;33(3):130‐7. [DOI] [PubMed] [Google Scholar]
Fall 2009
- Fall C, Fisher DJ, Osmond C, Margetts BM. Multiple micronutrient supplementation during pregnancy in low‐income countries: A meta‐analysis of effects on birth size and length of gestation. Food and Nutrition Bulletin 2009;30(4):S533‐S546. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glinoer 2007
- Glinoer D. The importance of iodine nutrition during pregnancy. Public Health Nutrition 2007;10(12A):1542‐6. [DOI] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- McMaster University. GRADEpro. [Computer program on www.gradepro.org]. Version 2015. McMaster University, 2014.
Haider 2012
- Haider BA, Bhutta ZA. Multiple‐micronutrient supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD004905.pub3] [DOI] [PubMed] [Google Scholar]
Haws 2007
- Haws RA, Thomas AL, Bhutta ZA, Darmstadt GL. Impact of packaged interventions on neonatal health: a review of the evidence. Health Policy and Planning 2007;22(4):193‐215. [DOI] [PubMed] [Google Scholar]
HFTAG 2013
- Home Fortification Technical Advisory Group. A Manual for Developing and Implementing Monitoring Systems for Home Fortification Interventions. Geneva: HFTAG, 2013. [Google Scholar]
HFTAG 2013b
- HFTAG. Home Fortification with Micronutrient Powders (MNP). Basel: Sight and Life, 2013. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Huffman 1998
- Huffmann SL, Baker J, Schumann J, Zehner ER. The Case for Promoting Multiple Vitamin/Mineral Supplements for Women of Reproductive Age in Developing Countries. Washington: The LINKAGES Project, 1998. [Google Scholar]
Liu 2013
- Liu JM, Mei Z, Ye R, Serdula MK, Ren A, Cogswell ME. Micronutrient supplementation and pregnancy outcomes: double‐blind randomized controlled trial in China. JAMA Internal Medicine 2013;173(4):276‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liyanage 2002
- Liyanage C, Zlotkin S. Bioavailability of iron from micro‐encapsulated iron sprinkle supplement. Food and Nutrition Bulletin 2002;23:133‐7. [PubMed] [Google Scholar]
Muthayya 2013
- Muthayya S, Rah JH, Sugimoto JD, Roos FF, Kraemer K, Black RE. The global hidden hunger indices and maps: an advocacy tool for action. PLoS ONE 2013;8(6):e67860. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nguyen 2008
- Nguyen P, Nava‐Ocampo A, Levy A, O'Connor DL, Einarson TR, Taddio A, et al. Effect of iron content on the tolerability of prenatal multivitamins in pregnancy. BMC Pregnancy and Childbirth 2008;8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2008
- National Institute for Health and Clinical Excellence. Antenatal Care: Routine Care for the Healthy Pregnant Woman. London: National Institute for Health and Clinical Excellence, 2008. [Google Scholar]
O'Neil 2014
- O'Neill J, Tabish H, Welch V, Petticrew M, Pottie K, Clarke M, et al. Applying an equity lens to interventions: using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. Journal of Clinical Epidemiology 2014;67(1):56‐64. [DOI] [PubMed] [Google Scholar]
Oppenheimer 2001
- Oppenheimer SJ. Iron and its relation to immunity and infectious disease. Journal of Nutrition 2001;131:616S‐635S. [DOI] [PubMed] [Google Scholar]
Peña‐Rosas 2012a
- Peña‐Rosas JP, De‐Regil LM, Dowswell T, Viteri FE. Daily oral iron supplementation during pregnancy. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD004736.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peña‐Rosas 2012b
- Peña‐Rosas JP, De‐Regil LM, Dowswell T, Viteri FE. Intermittent oral iron supplementation during pregnancy. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD009997] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prentice 2007
- Prentice AM, Ghattas H, Doherty C, Cox SE. Iron metabolism and malaria. Food and Nutrition Bulletin 2007;28(4):S524‐S539. [DOI] [PubMed] [Google Scholar]
Raiten 2011
- Raiten DJ, Namaste S, Brabin B. Considerations for the safe and effective use of iron interventions in areas of malaria burden ‐ executive summary. International Journal for Vitamin and Nutrition Research 2011;81(1):57‐71. [DOI] [PubMed] [Google Scholar]
Ray 2007
- Ray JG, Wyatt PR, Thompson MD, Vermeulen MJ, Meier C, Wong PY, et al. Vitamin B12 and the risk of neural tube defects in a folic‐acid‐fortified population. Epidemiology 2007;18(3):362‐6. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Salam 2013
- Salam RA, MacPhail C, Das JK, Bhutta ZA. Effectiveness of micronutrient powders (MNP) in women and children. BMC Public Health 2013;13(Suppl 3):S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
SGHI 2008
- Sprinkles Global Health Initiative (SGHI). Micronutrient sprinkles for use in infants and young children: guidelines on recommendations for use and program monitoring and evaluation. http://www.sghi.org/resource_centre/GuidelinesGen2008.pdf (Accessed September 10 2010) 2008.
Shah 2009
- Shah PS, Ohlsson A, for the Knowledge Synthesis Group on Determinants of Low Birth Weight and Preterm Births. Effects of prenatal multimicronutrient supplementation on pregnancy outcomes: a meta‐analysis. CMAJ 2009;180(12):E99‐E108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaheen 2014
- Shaheen R, Streatfield PK, Naved RT, Lindholm L, Persson LA. Equity in adherence to and effect of prenatal food and micronutrient supplementation on child mortality: results from the MINIMat randomized trial, Bangladesh. BMC Public Health 2014;14(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shankar 2008
- Supplementation with Multiple Micronutrients Intervention Trial (SUMMIT) Study Group, Shankar AH, Jahari AB, Sebayang SK, Aditiawarman, Apriatni M, Harefa B, et al. Effect of maternal multiple micronutrient supplementation on fetal loss and infant death in Indonesia: a double‐blind cluster‐randomised trial. Lancet 2008;371(9608):215‐27. [DOI] [PubMed] [Google Scholar]
Shrimpton 2009
- Shrimpton R, Huffman S, Zehner ER, Darnton‐Hill I, Dalmiya N. Multiple micronutrient supplementation during pregnancy in developing‐country settings: Policy and program implications of the results of a meta‐analysis. Food and Nutrition Bulletin 2009;30(4):S556‐S573. [DOI] [PubMed] [Google Scholar]
Soofi 2013
- Soofi S, Cousens S, Iqbal SP, Akhund T, Bhutta Z. Impact of provision of daily zinc and iron with multiple micronutrients on growth and morbidity among young children in Pakistan: a cluster RCT of micronutrient powders in Pakistan. Lancet 2013;382(9886):29‐40. [DOI] [PubMed] [Google Scholar]
Stevens 2013
- Stevens GA, Finucane MM, De‐Regil LM, Paciorek CJ, Flaxman SR, Branca F, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non‐pregnant women for 1995–2011: a systematic analysis of population‐representative data. Lancet Global Health 2013;1(1):e16‐e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Suchdev 2011
- Suchdev PS, De‐Regil LM, Walleser S, Vist GE, Peña‐Rosas JP. Multiple micronutrient powders for home (point of use) fortification of foods in pregnant women: a systematic review. WHO e‐Library of Evidence for Nutrition Actions (http://apps.who.int/iris/bitstream/10665/44748/1/9789241502559_eng.pdf) [accessed September 2014] 2011.
Suchdev 2013
- Suchdev PS, Neufeld LM. Micronutrient powders for young children. Lancet 2013;382(9899):1171. [DOI] [PubMed] [Google Scholar]
Tamura 2006
- Tamura T, Picciano MF. Folate and human reproduction. American Journal of Clinical Nutrition 2006;83:993‐1016. [DOI] [PubMed] [Google Scholar]
The Micronutrient Initiative 2009
- The Micronutrient Initiative (MI) Flour Fortification Initiative, USAID, GAIN, WHO, The World Bank, UNICEF. Investing in the Future: a United Call to Action on Vitamin and Mineral Deficiencies: Global Report 2009. Ottawa: The Micronutrient Initiative, 2009. [Google Scholar]
Thorne‐Lyman 2012
- Thorne‐Lyman AL, Fawzi WW. Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta‐analysis. Paediatric and Perinatal Epidemiology 2012;Suppl 1:36‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tielsch 2008
- Tielsch JM, Rahmathullah L, Katz J, Thulasiraj RD, Coles C, Sheeladevi S, et al. Maternal night blindness during pregnancy Is associated with low birthweight, morbidity, and poor growth in South India. Journal of Nutrition 2008;138(4):787‐92. [DOI] [PubMed] [Google Scholar]
Titaley 2010
- Titaley CR, Dibley MJ, Roberts CL, Agho K. Combined iron/folic acid supplements and malaria prophylaxis reduce neonatal mortality in 19 sub‐Saharan African countries. American Journal of Clinical Nutrition 2010;92(1):235‐43. [DOI] [PubMed] [Google Scholar]
UNHCR 2011
- UNHCR. UNHCR Operational Guidance on the Use of Special Nutritional Products to Reduce Micronutrient Deficiencies and Malnutrition in Refugee Populations. Geneva: UNHCR, 2011. [Google Scholar]
UNICEF 2004a
- UNICEF. Multiple micronutrient supplements to enhance foetal and infant survival, growth and development. Workshop to Review Effectiveness Trials; 2004 15‐18 June; Bangkok, Thailand. 2004.
UNICEF 2004b
- UNICEF. Multiple micronutrient supplementation during pregnancy (MMSDP): a review of progress of efficacy trials. UNICEF/UNU/WHO Study Team. Report of a Meeting Held 2004 21‐23 June; Bangkok, Thailand. Centre for International Child Health, Institute of Child Health, University College London and UNICEF, 2004.
UNICEF/CDC 2014
- UNICEF, CDC. Global assessment of home fortification interventions, 2011. Home Fortification Technical Advisory Group. Geneva: Home Fortification Technical Advisory Group, 2014.
UNICEF/WHO/UNU 1999
- UNICEF/WHO/UNU. Composition of a multi‐micronutrient supplement to be used in pilot programmes among pregnant women in developing countries. Report of an UNICEF/WHO/UNU workshop (http://www.idpas.org/pdf/059CompositionofMult‐MicronutrientSupplement.pdf). New York: UNICEF, 1999. [Google Scholar]
van den Broek 2010
- Broek N, Dou L, Othman M, Neilson JP, Gates S, Gülmezoglu AM. Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD008666.pub2] [DOI] [PubMed] [Google Scholar]
Villar 1997
- Villar J, Bergsjo P. Scientific basis for the content of routine antenatal care. I. Philosophy, recent studies and power to eliminate or alleviate adverse maternal outcomes. Acta Obstetricia et Gynecologica Scandinavica 1997;76(1):1‐14. [DOI] [PubMed] [Google Scholar]
WFP 2013
- World Food Programme. WFP Strategic Plan (2014–2017). WFP/EB.A/2013/5‐A/1, 2013. [Google Scholar]
WHO 1994
- World Health Organization. Iodine and Health: a Statement by the World Health Organization. WHO/NUT/94.4. Geneva: World Health Organization, 1994. [Google Scholar]
WHO 2008
- World Health Organization. The International Pharmacopoeia. (http://apps.who.int/phint/en/p/about/) (accessed 2008) 2008.
WHO 2009a
- World Health Organization. Global health risks: mortality and burden of disease attributable to selected major risks. http://www.who.int/healthinfo/global_burden_disease/ GlobalHealthRisks_report_full.pdf (accessed July 2011) 2009:1‐62.
WHO 2009b
- World Health Organization (WHO). Global Prevalence of Vitamin A Deficiency in Populations at risk 1995‐2005: WHO global Database on Vitamin A Deficiency. Geneva: World Health Organization, 2009. [Google Scholar]
WHO 2010
- World Health Organization. Malaria. In: Poumerol G, Wilder‐Smith A editor(s). Malaria. International Travel and Health. Situation as on 1 January 2010. Geneva: World Health Organization, 2010. [Google Scholar]
WHO 2011a
- World Health Organization. Guideline: Use of Multiple Micronutrient Powders for Home Fortification of Foods Consumed by Pregnant Women. Geneva: World Health Organization, 2011. [PubMed] [Google Scholar]
WHO 2011b
- World Health Organization. Guideline: Daily Iron and Folic Acid Supplements for Pregnant Women. Geneva: World Health Organization, 2011. [Google Scholar]
WHO/FAO 2006
- World Health Organization/Food and Agriculture Organization of the United Nations. Guidelines on Food Fortification with Micronutrients. Geneva: World Health Organization and Food and Agriculture Organization of the United Nations, 2006. [Google Scholar]
Zeng 2009
- Zeng L, Yan H, Cheng Y, Dang S, Dibley MJ. Adherence and costs of micronutrient supplementation in pregnancy in a double‐blind, randomized, controlled trial in rural western China. Food and Nutrition Bulletin 2009;30(4 Suppl):S480‐S487. [DOI] [PubMed] [Google Scholar]
Zimmermann 2008
- Zimmermann MB, Jooste PL, Pandav CS. Iodine‐deficiency disorders. Lancet 2008;372(9645):1251‐62. [DOI] [PubMed] [Google Scholar]


