Abstract
Natural Killer (NK) cells yield promise in therapy of hematologic malignancies. The clinical experience with adoptively transferred allogeneic NK cells over past two decades has revealed safety and minimal risk of CRS or ICANS. Unlike T cells which have to be genetically altered to avoid graft vs host disease (GVHD), HLA mismatched NK cells can be infused without GVHD risk. This makes them ideal for the development of off-the-shelf products. In this review we focus on NK biology relevant to the cancer therapy, the trajectory of NK therapeutics for leukemia, lymphoma, and myeloma; and advantages of the NK cell platform. We will also discuss novel methods to enhance NK cell targeting, persistence, and function in the tumor microenvironment. The future of NK cell therapy depends on novel strategies to realize these qualities.
Keywords: NK cell therapy, CAR-NK, Lymphodepletion, Myeloma, AML, Lymphoma
1. Introduction
Cellular therapy has revolutionized the treatment of cancer with chimeric antigen receptor (CAR)-expressing T cells having gained approval for non-Hodgkin lymphoma (NHL), acute lymphoblastic leukemia (ALL), and multiple myeloma (MM). Currently approved CAR-T cells rely on genetic modifications of a patient’s own T cells that have been removed by apheresis. While commercially available CAR-T products offer an available treatment strategy for many patients with relapsed and refractory hematologic malignancies, there are significant downsides to this approach such as logistical complexity, high clinical resource utilization, cost of individualized products, prolonged manufacturing times, and the need for bridging therapy in patients with rapid disease progression. Because CAR-T are not approved in the front-line setting, patients may have poor quality autologous T cells after chemotherapy that effects potency. While research is ongoing to develop safe, allogeneic CAR-T products, their application is limited by the potential for graft-versus-host disease and rapid clearance by the host immune system. The administration of CAR-T from any source can cause life-threatening side effects such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) [1].
More recently, cell therapy using Natural Killer (NK) cells has emerged as an alternative immunotherapeutic strategy which is rapidly evolving and advancing into clinical practice. NK cells are innate lymphocytes endowed with ability to kill virally infected or malignantly transformed cells without prior immunologic encounter. Because of their lack of HLA-restricted specificity, allogeneic NK cells have been administered across HLA barriers without GVHD that occurs with allogeneic T cells. NK cells possess diverse mechanisms to recognize and kill cancer cells which can be harnessed for cancer therapy (Fig. 1). In addition, novel NK cell development platforms yield the potential for allow a multi-dosing strategy that is not feasible with autologous CAR-T cells.
Fig. 1.
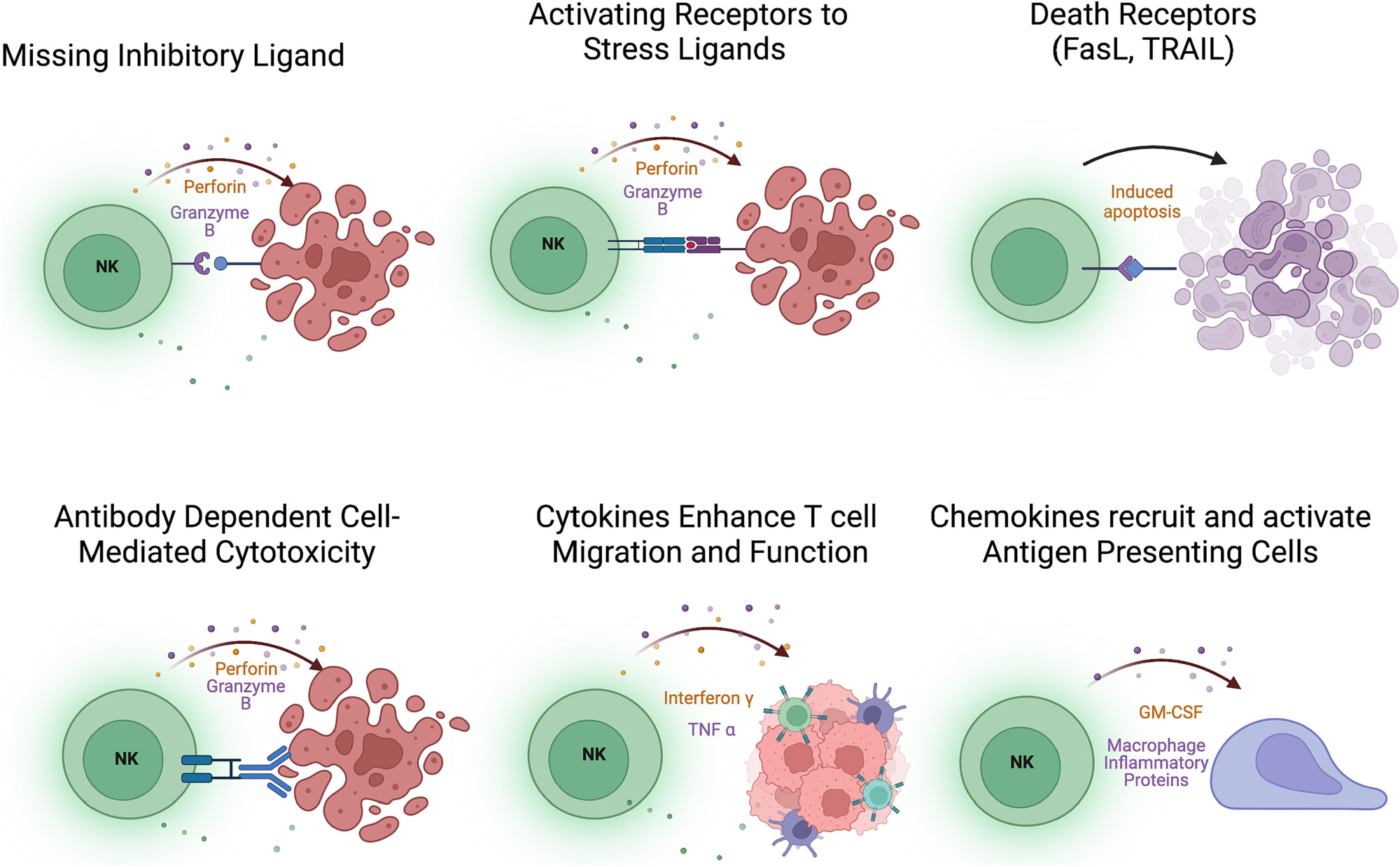
Mechanisms of NK cell activation. NK cells are skewed toward the release of the cytolytic granules perforin and granzyme by the loss of inhibition from “self” sensing receptors or up-regulation of stress ligands. NK cells also express death receptors that can induce apoptosis in target cells expressing death receptor ligands. Antibody-bound targets activate NK cells through CD16-binding of Fc portion of antibodies. NK cells also express cytokines that activate T cells and promote the recruitment of antigen presenting cells to the tumor microenvironment.
Our group was among the first to report the feasibility, safety and early efficacy of adoptively transferred allogeneic NK cells in acute myeloid leukemia (AML) in 2005 [2]. Since this early experience, further insights into NK cell biology and major technologic discoveries set the stage for remarkable advances in NK cell therapy for blood cancers. This review will discuss the current landscape of NK cell therapy in leukemia, lymphoma, and MM including cell sources and manufacturing, methods to enhance persistence and activity, as well as trafficking to the tumor microenvironment.
2. NK cell receptors
NK cells are part of innate immune system and constitute 5–15% of circulating lymphocytes in the peripheral blood. In contrast to T cells that require antigen presentation by major histocompatibility complex (MHC) molecules (termed HLA in humans) and priming through antigen-presenting cells, NK cells, are not antigen-specific [3]. Their activation occurs by integration of activating and inhibitory signals from germ-line encoded NK cell surface receptors (Fig. 2). Peripheral blood NK cells are recognized by expression of CD56 as CD56dim and CD56bright, CD56dim NK cells perform direct cell killing through degranulation of cytotoxic perforin and granzyme while CD56bright NK cells produce cytokines including interferon-γ, tumor necrosis factor-α to orchestrate other components of the immune system [4,5]).
Fig. 2.
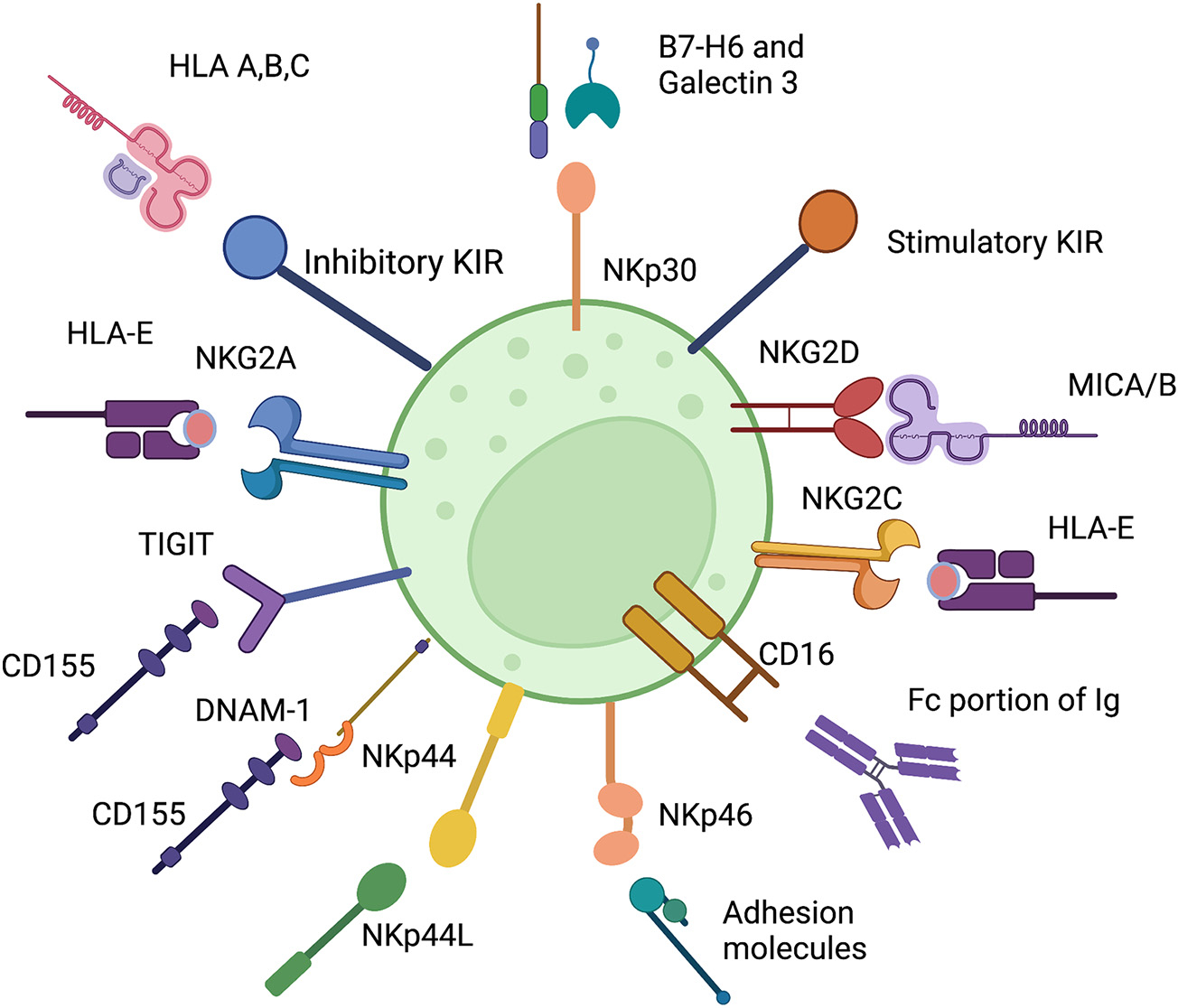
Select Inhibitory and Stimulatory NK cell receptors with their ligands. The inhibitory NK cell receptors (shown in blue) include KIR, NKG2A, and TIGIT. Activating receptors (in orange and yellow) bind a wide variety of ligands. NK cell activation and function is determined by balance of inhibitory and stimulatory signals. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Cells transformed by malignancy or viral infection often decrease expression of beta-2-microglobulin, a component of MHC class I molecules. This leads to impaired MHC presentation and allows the transformed cell to escape T cell recognition. One important role of NK cells is recognition of transformed cells with low or absent MHC (HLA in human) by the killer cell immunoglobulin–like receptors (KIR) by a process termed “missing self”. KIR can be inhibitory or activating based on their cytoplasmic tail. Long cytoplasmic tails have an immunoreceptor tyrosine-based inhibitory (ITIM) motif and short cytoplasmic tails associate with the adapter molecule DAP-12 that possesses an immunoreceptor tyrosine-based activation motif (ITAM). Inhibitory KIRs bind the classical HLA Class I and play a role in NK cell education. NK cells use inhibitory KIR to survey cells for normal expression of HLA, and loss of inhibitory KIR/HLA interactions leads to ‘missing self’ recognition of HLA-deficient cells and triggers NK killing function [6]. Direct cytotoxic killing of HLA-mismatched cancer cells represents a major mechanism underlying therapeutic potential of allogeneic NK cells for cancer therapy.
NK cell activation results from a complex and multifactorial interaction of many inhibitory and activating receptors with variable thresholds of activation. NK cell licensing occurs through interactions between MHC ligands on healthy cells and KIR on human NK cells [7] or Ly49 on murine NK cells [8]. This concept is supported by studies in animal models where mutation of critical binding residues in MHC abrogates NK cell function [9]. Licensed NK cells differ from unlicensed cells in metabolic pathways as they utilize glycolysis to sustain activation (similar to cytotoxic T cells) while unlicensed NK cells predominantly generate energy through oxidative phosphorylation. Licensed NK cells play a role in the control of MHC-deficient tumors [10]. Both licensed and unlicensed NK cells can be activated and manipulated to become immune effectors (reviewed [11]).
Activating receptors are not equally potent and the strength of their signal can be impacted by the surrounding cytokine milieu. Some activating KIRs also bind HLA molecules but with a lower affinity than their inhibitory counterparts. The ligands of some activating KIR are not yet fully elucidated [12]. The genes of the KIR locus are organized into either KIR B haplotypes, containing 1 or more activating KIR genes or KIR A haplotypes, which has mostly inhibitory KIRs and one potential activating form of KIR2DL4 and KIR2DS4 [13]). Analysis of unrelated donor (URD) HSCT), given to acute myeloid leukemia (AML) patients showed that KIR B haplotype donors were associated with better outcomes, primarily from relapse protection. [14]. Furthemre, the activating KIR2DS1 in allografts has been associated with reduced relapse but only when the recipient expressed HLA–C1. This suggests that high levels of HLA-C2 expression promote tolerance in the allografted NK cells [15].
NKG2D is an activating receptor that binds ligands up-regulated by cellular stress such as MICA and MICB [16]. However, NKG2D can also be down-regulated on NK cells from patients with digestive cancers [17], chronic lymphocytic leukemia (CLL) [18], breast cancer [19], chronic hepatitis B virus [20], and hepatitis C virus [21] and is thought to contribute to escape from NK cell recognition. Shedding of surface MICA/B ligands by the metalloprotease ADAM17 is another method to escape recognition by NK cells [22]. Higher levels of soluble MICA has been associated with progression in MM [23]. This evolutionary mechanism can be potentially exploited therapeutically by small molecule inhibitors of ADAM17 which can antagonize shedding of MICA to enhance NK cell function [24].
CD94 is an important NK cell receptor that can heterodimerize with NKG2A or NKG2C. Both of the resulting heterodimers recognize the non-classical HLA-E molecule which is often upregulated by malignantly transformed or virally infected cells to mediate NK cell inhibition. Signaling through NKG2A is inhibitory and signaling through NKG2C activates NK cell function through binding of DAP12 [25]. NKG2C+ NK cells play an important role in human viral infections [26–28] and may be useful to maximize missing-self activity against HLA-E positive tumors. The natural cytotoxicity receptors (NCR) include NKp46, NKp44, and NKp30. These bind a diverse set of host and pathogen-encoded ligands and their activation provokes both NK cells cytotoxicity and cytokine secretion (reviewed [29]).
Therapeutically relevant, NK cells achieve a level of killing specificity with antibody-dependent cell-mediated cytotoxicity (ADCC) by binding of their CD16 receptor to the Fc portion of antibodies. ADCC offers an opportunity for ‘targeting’ cancers with NK cell when combined with monoclonal antibodies against tumor antigens. The hypoxic environment of the bone marrow can downregulate activating receptors such as NKG2D and the NCR but CD16 expression and signaling remains intact [30], making ADCC an important method for control of cancer cells in the bone marrow. In addition to their role in direct cytotoxicity, the CD56bright subset of NK cells play an important role in the function and trafficking of other immune cells through secretion of cytokines (including interferon-γ, tumor necrosis factor-α), and chemokines (MIP-1α, MIP-1β, and RANTES) and cross-talk with the adaptive immune system through modulation of dendritic cells (reviewed [31]) and enhanced T cell function (reviewed [32]).
3. NK cell memory
While conventional NK cells are innate effectors, a unique subset of NK cells develop in response to cytomegalovirus (CMV) infection. These ‘adaptive’ NK cells possess memory recall and are antigen-specific for CMV derived proteins [33–36]. Their unique characteristics include significantly longer lifespan than conventional NK cells and in vivo persistence for months to years after the resolution of CMV viremia. Naturally derived adaptive NK cells are resistant to oxidative stress and exhibit low levels of CD38 [37]. Although the NK cell response to CMV is heterogenous, many adaptive NK cells express both the activating receptor NKG2C and the maturation marker CD57 as well as low expression of the inhibitory receptor NKG2A. Decreased expressions of the signaling molecules FcεR1γ, SYK, EAT-2, and the transcription factor PLZF are part of their signature [38]. Importantly, adaptive NK cells are endowed with potent anti-tumor activity and enhanced ADCC compared to conventional NK cells [39] and can been exploited therapeutically against hematologic malignancies.
Their anti-leukemia function is supported by clinical studies which showed that AML patients who underwent allogeneic HSCT and experienced CMV reactivation had a lower incidence of relapse and relapse protection correlated with adaptive NK cell expansion in the blood [40,41]. This finding served as proof of principle that NK cells are instrumental in maintaining remission in patients with AML. The impact of CMV and adaptive NK cells was explored in MM patients who underwent autologous stem cell transplant. While none of the patients experienced CMV viremia, some CMV seropositive patients exhibited robust expansion of adaptive NK cells by day 28 and experienced significantly delayed relapse of MM [42]. The underlying mechanism of adaptive NK cell proliferation remains to be determined, but these findings raise a possibility of using adaptive NK cells in relapse prevention.
Adaptive immunity has been recapitulated in vitro by activation of peripheral blood NK cells with interleukin-12 (IL-12), IL-15, and IL-18 to generate Cytokine-induced memory-like (CIML) NK cells. CIML NK cells exhibit enhanced responses to cytokine or activating receptor restimulation for weeks to months. These cells are considered ‘memory-like’ because of their ability to secrete significantly high levels of interferon γ when restimulated [43]. In the section on NK cell sources, we will review the current data on CIML NK cells in hematologic malignancies.
4. NK cell defects in hematologic malignancies
Recently, loss of NK cell function has been recognized to contribute to the development of MM from its non-malignant precursor state monoclonal gammopathy of undetermined significance (MGUS). The number of NK cells decrease as MM advances [44] and cytotoxicity is impaired [45]. MM down-regulates NK cell function through expression of checkpoint receptor ligands [46–48], TGF-β signaling from myeloid derived suppressor cells [49] and Treg cells [50] in the microenvironment, and chronic release of inflammatory cytokines that promote immune exhaustion [51,52].
In T-cell lymphomas, mouse models have shown that MYC-induced tumorigenesis was associated with a deficit in NK cell differentiation that correlated with suppression of Type I interferon in transcriptomic analyses [53]. Patients with T and B-ALL experience a deficit in both numbers and cytotoxic function of NK cells and an increase in CD56bright NK cells suggesting a deficit in NK cell differentiation [54]. Deficits in NK cell function have also been described in chronic myelogenous leukemia (CML) [55], chronic lymphocytic leukemia [56], AML [57] and classical Hodgkin lymphoma [58].
5. NK cells as immunotherapy
The therapeutic feasibility of NK cells was first explored in the 1980s in the form of lymphokine activated killer (LAK) cells, an autologous mixture of T and NK cells expanded ex vivo with high-dose interleukin 2 (IL-2) in patients with refractory, metastatic solid tumors [59]. The NK therapy field was mostly catalyzed by a seminal clinical observation by Ruggeri, et al which demonstrated that in T cell depleted CD34 selected haploidentical donor HSCT, NK cells [60,61] exhibited a protective effect against AML with reduced leukemia relapse in the presence of KIR/HLA mismatch between the donor and recipient. Notably, relapse protection was not associated with increase in GVHD [60,61]. This concept led to more studies which further explored the safety and feasibility of using NK cell infusion after allogeneic and autologous transplant setting to consolidate responses [62,63] and encourage trials using NK cell adoptive transfer [2].
6. Sources of allogeneic NK cells for cancer therapy
Sources of NK cells for adoptive transfer have steadily increased with the advanced in cellular genetic engineering technologies (Fig. 3). Historically, the most frequently utilized source of NK cells was the peripheral blood of HLA-haploidentical donors, typically a patient’s sibling or child [2,51]. Partial HLA matching theoretically delays rejection by the recipient’s T cell compartment.
Fig. 3.
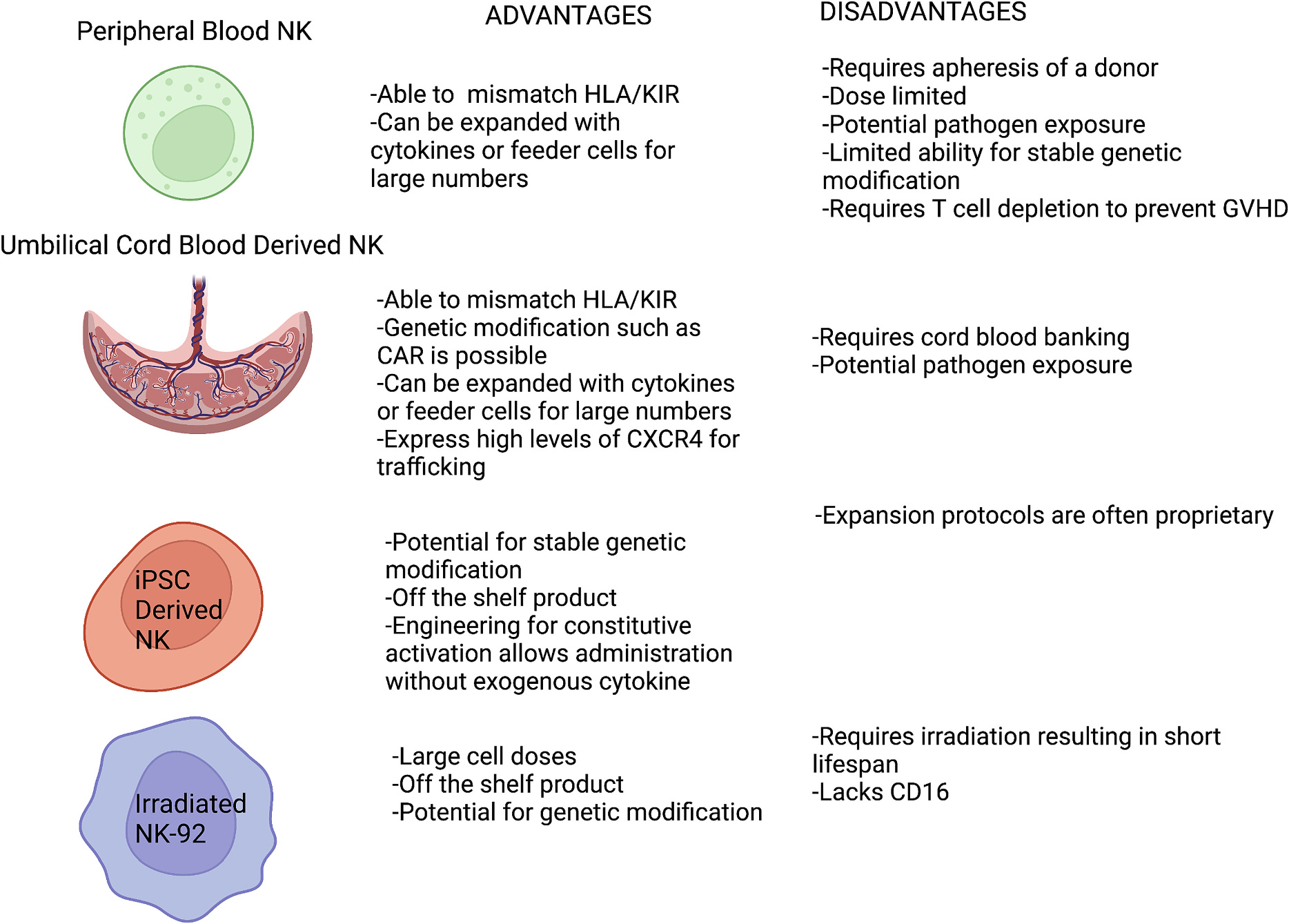
Sources of NK cells. NK cells can be derived from a variety of sources with variable benefits and disadvantages.
In 2005, our group at the University of Minnesota reported a pioneering clinical trial using adoptive transfer of haploidentical NK cells for patients with refractory cancer followed by IL-2 in a non-transplant setting [2]. NK cells were collected from related HLA Class I-haploidentical donors, CD3 depleted by GMP-grade CliniMacs device, incubated with high dose IL-2 overnight and infused to patients fresh in a dose escalation fashion. In the cohort of 43 patients with metastatic melanoma, renal cell carcinoma, Hodgkin’s lymphoma, or refractory AML, the trial demonstrated feasibility, safety and no immune associated toxicities along with short term NK cell persistence in blood and promising clinical efficacy in AML [2]. Subsequently, NK cells from haploidentical donors were combined with rituximab/IL-2 and administered to patients with refractory NHL [61]. While the efficacy was modest (27%), circulating donor NK cells persisted for 7–20 days after infusion in either study [2,61]. The NHL study also explored biological factors associated with response and found that baseline low levels of host Tregs and myeloid derived suppressor cells (MDSC), low IL-10 levels and low PD-1 on host T cells were associated with clinical response to NK cell therapy [64].
CIML NK cells exploit adaptive NK cell properties and have shown enhanced interferon-γ production and cytotoxicity against leukemia in vitro and enhanced survival in murine models of AML. In a phase 1 clinical trial in AML patients, adoptively transferred memory-like NK cells demonstrated clinical activity with four complete remissions in nine patients. Memory like NK cells proliferated extensively, were detectable in blood for weeks and assessments of bone marrow at day 8 after infusion revealed large percentage of interferon-gamma producing donor memory line NK cells [65]. The use of these cells is being further explored in clinical trials for relapse after HSCT for myeloid cancers (NCT05580601) and head and neck cancers (NCT04290546).
Among major drawbacks of peripheral blood as a source of NK cells are limited cell dose from the apheresed product, heterogenous cellular content, and variable NK cell function. CD3 depletion is required to ensure removal of T cells that can cause GVHD; selection for CD56 can yield greater purity of NK cells but further limits the cell dose. Further highlighting the importance of NK cell dose, a phase I study of HLA-haploidentical NK cells given as part of the preparatory regimen for allogeneic HSCT for high-risk myeloid malignancies revealed a significant association between CD56+ NK cells infused and overall survival [66].
To improve the NK cell dose, one strategy used peripheral blood, haploidentical NK cells expansion with cytokines IL-2 and IL-15 to generate large enough doses to perform multiple infusions on days 1 to 30 after autologous transplant. The clinical outcomes were negative, potentially due to NK cell exhaustion which occurs after prolonged in vitro culture with IL-15 and IL-2 or poor in vivo persistence [67]. Another study used IL-2 ex vivo expansion of NK cells from unrelated donors (MG4101) to treat patients with advanced lymphoma or solid tumor. An enhanced progression free survival (PFS) was noted in KIR-mismatched NK cells recipients however clinical efficacy was limited and NK persistence was short (between 1 and 4 days) likely due to lack of lymphodepleting chemotherapy prior to NK infusion [68]. These limitations prompted the search for more efficient expansion methods and new sources of NK cells.
NK cells from all sources can be expanded ex vivo utilizing irradiated feeder cells. Feeder cells from the erythroleukemic cell line, K562 can be transduced to express a variety of costimulatory molecules such as 4–1BBL and membrane-bound cytokines including IL-15 [69] and IL-21 [70]. While in vitro studies have shown that continuous IL-15 exposure induces an exhausted phenotype [71], the IL-21 expanded NK cells retain functionality against target cells and had longer telomeres than NK cells expanded with IL-15 [67]. These results suggest that the development of exhaustion is context dependent. To address potential safety concerns of feeder cell infusion with NK cells, feeder cell–free expansion systems have been developed, including the use of particles derived from the membranes of K562 cells, which retain expression of 4–1BBL and IL-21 [72].
The unique capacity of nicotinamide (NAM), a vitamin B derivative and allosteric inhibitor of NAD dependent metabolic pathways to expand hematopoietic cells has been recently explored for blood derived NK cells. In vitro expansion of peripheral blood healthy donor NK cells with NAM and IL-15 (14 days culture) resulted in a highly active, uniform product (GDA-201) characterized by expression of CD16, tissue homing receptor CD62L, preserved cytotoxicity, cytokine production and the resistance against oxidative stress. Results of a Phase 1 trial showed safety, NK cell persistence for 7–14 days, homing to bone marrow and tumor sites and promising clinical efficacy (74% overall response rate) in patients with B-cell NHL (NCT03019666) [73].
Umbilical cord blood (UCB)-derived NK cells are readily available and have the advantage of allowing KIR/HLA mismatching. In a first reported CAR-NK trial, UCB-derived NK cells were expanded with membrane bound IL-21-expressing feeder cells. The CAR against CD19 was transduced with a retrovirus along with IL-15 (for persistence) and an inducible caspase-9 that could be used to trigger NK death in case of intolerable side effects. These CD19-CAR NK cells showed promising efficacy in a phase 1 and 2 trial against CD19-expressing lymphomas. The large dose of CAR-NK cells (1 ×1 07 cells/kg) were tolerated without CRS, GVHD, or neurotoxicity and notably the CAR-NK cells persisted for up to 12 months after infusion. Out of 11 enrolled patients with CLL and NHL treated with a single dose, the objective response rate was 73% [74].
Because of the small volume of UCB per unit, another method to generate UCB-derived NK cells involves differentiation of CD34+ hematopoietic stem cells with a cytokine-based culture system [75]. NK cells isolated from UCB and subsequently expanded were compared to UCB stem cell-derived NK cells; expanded NK cells exhibited more KIR expression and higher cytotoxicity against cancer cell lines [76]. A potential benefit of UCB derived NK cells is that they exhibit higher levels of the chemokine CXCR4, which may enhance homing to the bone marrow space [77].
In a phase I study for MM, UCB derived NK cells were administered after conditioning with lenalidomide and melphalan. The patients then received an autologous transplant five days after NK cell infusion. As exhibited neither graft failure nor GVHD occurred, and most of the patients achieved at least a very good partial response or complete response. Although these results suggest that NK cell infusion may deepen the response to autologous transplant, the lack of a control arm and lack of information on remission duration limits interpretation of these findings. [78]
NK cells can be also generated from induced pluripotent stem cells (iPSC) which can differentiate into mature cell types of all three germ layers including functional NK cells. This strategy allows complex genetic modification and potentially yields NK cells with augmented functionality. Although current expansion protocols require 4–6 weeks to expand mature NK cells from iPSC, the resulting cellular product can be cryopreserved and available “off the shelf” in large quantities for multidose administration. An iPSC-derived NK cell line was engineered to express a high affinity, version of the Fc receptor CD16a resistant to ADAM17 cleavage (non-cleavable), a membrane-bound interleukin (IL)-15/IL-15R fusion protein and a knockout of the ecto-enzyme CD38. CD16 is subject to cleavage by the metalloprotease ADAM17 [24], leading to reduced ADCC so mutation of the ADAM17 cleavage site was introduced to prevent clipping. The IL-15/IL-15R fusion promotes persistence and expansion of these NK cells in vivo without off-target effects on T cells. Naturally-derived adaptive NK cells are resistant to oxidative stress and exhibit low levels of CD38, an ecto-enzyme that controls intracellular nicotinamide adenine dinucleotide (NAD) [79]. The genetic deletion of CD38 in iNK cells was shown to prevent NK cell-mediated fratricide in the presence of anti-CD38 antibodies and enhance metabolic functions. These triple engineered NK cells are termed iADAPT and were more cytotoxic against HL-60 leukemia line and MM1S myeloma line in immunodeficient mice than peripheral blood-derived NK cells [37]. Tumor targeting of iNK cells can further augmented by expression of CAR with a tumor specific domain.
Immortalized cell lines such as NK-92, derived from a patient with NHL, can yield large numbers of NK cells and allow for genetic manipulation to render cells with tumor antigen specificity. The clinical application of NK-92 requires irradiation to prevent tumorigenesis in recipient blood which limits proliferation and persistence [80]. NK-92 also cells do not express CD16 but can be engineered to express CD16 and perform ADCC [81].
7. Preparatory lymphodepleting (LD) regimens prior to NK cell therapy
The purpose of lymphodepleting chemotherapy is two-fold: elimination of host T cells that reject allogeneic NK cells based on HLA mismatch and induction of endogenous cytokines IL-15 and IL-7 that promote expansion of infused lymphocytes [82]. In a trial od haploidentical NK cells, lymphodepleting chemotherapy regimens were compared with high dose cyclophosphamide 50 g/kg × 2 doses and fludarabine 25 mg/m2 × 4 doses (Hi-Cy/Flu), low dose cyclophosphamide with methylprednisone, or cyclophosphamide alone. Lymphodepletion was followed by NK cell infusion and 10 million units of subcutaneous IL-2 every other day for six doses. [2] Only patients who had received the Hi-Cy/Flu experienced an expansion of greater than 100 donor NK cells/μL in the blood. Importantly, lymphodepleting chemotherapy led to an endogenous surge in IL-15 and a subsequent short-term persistence of adoptively transferred, unmodified NK cells in vivo. This therapy resulted in complete hematologic remission in five patients out of 19 with relapsed or refractory AML. Efficacy of Hi-Cy/Flu to support adoptively transferred allogeneic NK cells expansion in patients with refractory AML has been corroborated using memory-like CIML NK cells [65].
Lee, et al. reported a phase I study of haploidentical donor-derived NK cell infusion as a component of the preparative regimen for allotransplantation for myeloid malignancies from a separate HLA-identical donor. NK cells were chosen with HLA/KIR mismatch when possible. This trial used busulfan and fludarabine conditioning and gave subcutaneous IL-2 for 5 days after NK cell infusion [66].
In patients with refractory MM, allogeneic NK cells were infused after high-dose melphalan and fludarabine and supported by endogenous IL-15 surge. Each patient was infused with NK cells with at least one inhibitory KIR that lacked its cognate ligand (HLA-C1/C2 or HLA-Bw4). This study showed persistence of donor NK cells until day 7–9 after infusion and 50% rate of near complete remission suggesting the potential of NK cell mediated myeloma control after transplant [83].
In other NK trials for MM, melphalan has been used as a conditioning regimen alone [67] or combined with lenalidomide [78] but these regimens were myeloablative and not feasible for adoptive transfer of NK cells without stem cell infusion. Differences in trial design and the source of NK cells used makes it difficult to compare the efficacy of these regimens and work remains to determine the optimal lymphodepleting regimen for each indication.
The recent shortage of fludarabine and risk of prolonged cytopenias led to examining alternatives to fludarabine/cytarabine (flu/cy). Bendamustine emerged as a viable substitute at least for some autologous CAR-T products. In a pivotal trial of tisagenleucleucel (CD19 CAR-T cell) for relapsed/refractory large B cell lymphoma, there was no difference in CAR-T efficacy using flu/cy or bendamustine lymphodepletion. Flu/cy was associated with worse cytopenias, higher transfusion needs and more frequent admissions for febrile neutropenia [84]. The efficacy of bendamustine to enhance NK cell persistence is currently unknown. Importantly, while lymphodepletion is critical for native NK cells, it’s requirement may be obviated by NK cell engineering and intrinsic manipulations to enhance self-sustained proliferation, persistence, and resistance to rejection.
As cellular therapies continue to improve treatment options for hematologic malignancies and move into the solid tumor space, numerous questions remain regarding the ideal lymphodepletion for a particular patient with a particular disease. One promising approach is to make the cellular product less visible to host immune system. CD54 and CD58 are adhesion molecules required for the formation of an immune synapse [85,86]. Recent work has shown that deletion of CD58 and CD54 leads to enhanced persistence of iPSC-derived NK cells in immunodeficient (NSG15) mice without the need for lymphodepletion [87]. Another exciting, albeit early in development, mechanism to allow allogeneic cells to persist in the host without significant lymphodepletion is the alloimmune defense receptor (ADR). ADR recognizes 4–1BB which is temporarily up-regulated on activated lymphocytes and leads to elimination of activated, but not resting T and NK cells [88]. Recent data demonstrated that ADR expressing CD19 CAR-NK cells resist immune alloreactivity, have enhanced functional persistence and can eliminate tumor in the presence of alloreactive T cells [89].
8. Cytokines to maximize NK cell activity and persistence
NK cell persistence in blood for greater than 7 days has been correlated with favorable clinical outcomes and better disease control in leukemia [2,90,91]. Lymphocyte persistence has also been correlated with long-term efficacy in CAR T-cell therapy to treat blood cancers [92].
Exogenous cytokines such as IL-2 promote NK cell activation and supports short term NK cell persistence after infusion. One of limitations of IL-2 is that it also activates T regulatory cells, which may blunt NK cell function and contribute to allorejection in a dose-dependent manner. IL-2 increased the frequency of Tregs in blood; elevated Tregs in the blood was correlated with lower NK cell proliferation [64]. To mitigate this effect, the strategy of Treg cell depletion with IL-2-diphtheria toxin fusion protein was combined with allogeneic NK cell therapy for relapsed/refractory AML. At day fourteen, NK cell proliferation was 20% higher in patients treated with IL-2-diphtheria toxin fusion protein and was correlated with peripheral blood Treg depletion. In addition, AML clearance was significantly enhanced [90]. This strategy has been abandoned due to lack of reliable Treg-depleting pharmacotherapy.
IL-15 is produced by activated macrophages and monocytes and, like IL-2, induces the proliferation of CD56bright NK cells in a dose-dependent fashion. IL-15 activates cytotoxicity and antibody-dependent cellular cytotoxicity (ADCC) by NK cells [93]. Unlike IL-2, Tregs do not expand with administration of IL-15. In a clinical trial in AML, recombinant IL-15 was administered following Hi-Cy/Flu and peripheral blood NK cell infusion. Of the 26 patients, 32% achieved complete remission but the trial was hampered by significant rates of cytokine release syndrome from subcutaneous IL-15 [94].
An IL-15 superagonist, ALT-803, was developed to have more favorable pharmacokinetics. ALT-803 is a complex containing two molecules of an optimized amino acid–substituted (N72D) IL-15 and two molecules of the IL-15α receptor “sushi” domain fused to a dimeric human IgG1 Fc that confers stability and prolongs the half-life of the overall complex with NK cells product and monoclonal antibodies [95]. Clinical experience in allogeneic transplant recipients with relapsed hematologic malignancy showed safety but limited efficacy. ALT-803 stimulated expansion of NK cells and CD8+ T cells but did not increase T regulatory cells [96].
Although cytokines promote NK cell activation and persistence, continuous exposure to IL-15 leads to an exhausted phenotype [71]. Another relevant strategy to augment NK cell function relates to alteration of the cytokine-inducible Src homology 2-containing protein (CIS) which acts as an intracellular checkpoint in NK cells and a key negative regulator of IL-15 signaling [97]. Knock out of CISH (the gene encoding CIS) was utilized in cord blood-derived NK cells engineered to express a CD19 CAR and IL-15. CISH deletion improved cytokine production and degranulation of CD19 CAR/IL-15 expressing NK cells in a Raji lymphoma model [98]. Future research is needed to determine if CISH deletion impacts NK cell persistence in patients.
9. Enhancing NK cell targeting
The most powerful activating signal on NK cells is mediated by triggering CD16 receptor and unlike other activating receptors, it does not require a combination of activating signals or cytokines [99] (Fig. 4). Germ-line polymorphisms of CD16 influence the affinity of binding the Fc portion of antibodies and has been associated with differential benefit to the monoclonal antibody trastuzumab in breast cancer [100] and rituximab in NHL [101]. This biology inspired the development of an off-the-shelf NK cell product from iPSC genetically manipulated to express higher affinity CD16 [102]. The strong signaling mediated by CD16 indicates that adoptive NK cellular therapy can synergize with the administration of tumor targeting antibodies. Another approach to enhance CD16 directed NK cell activation utilizes engager molecules. These bi-specific killer engagers (BiKE) combine two single chain variable fragment antibody-targeting domains, with one domain targeting an activating receptor such as CD16 and the other targeting a tumor antigen [103,104].
Fig. 4.
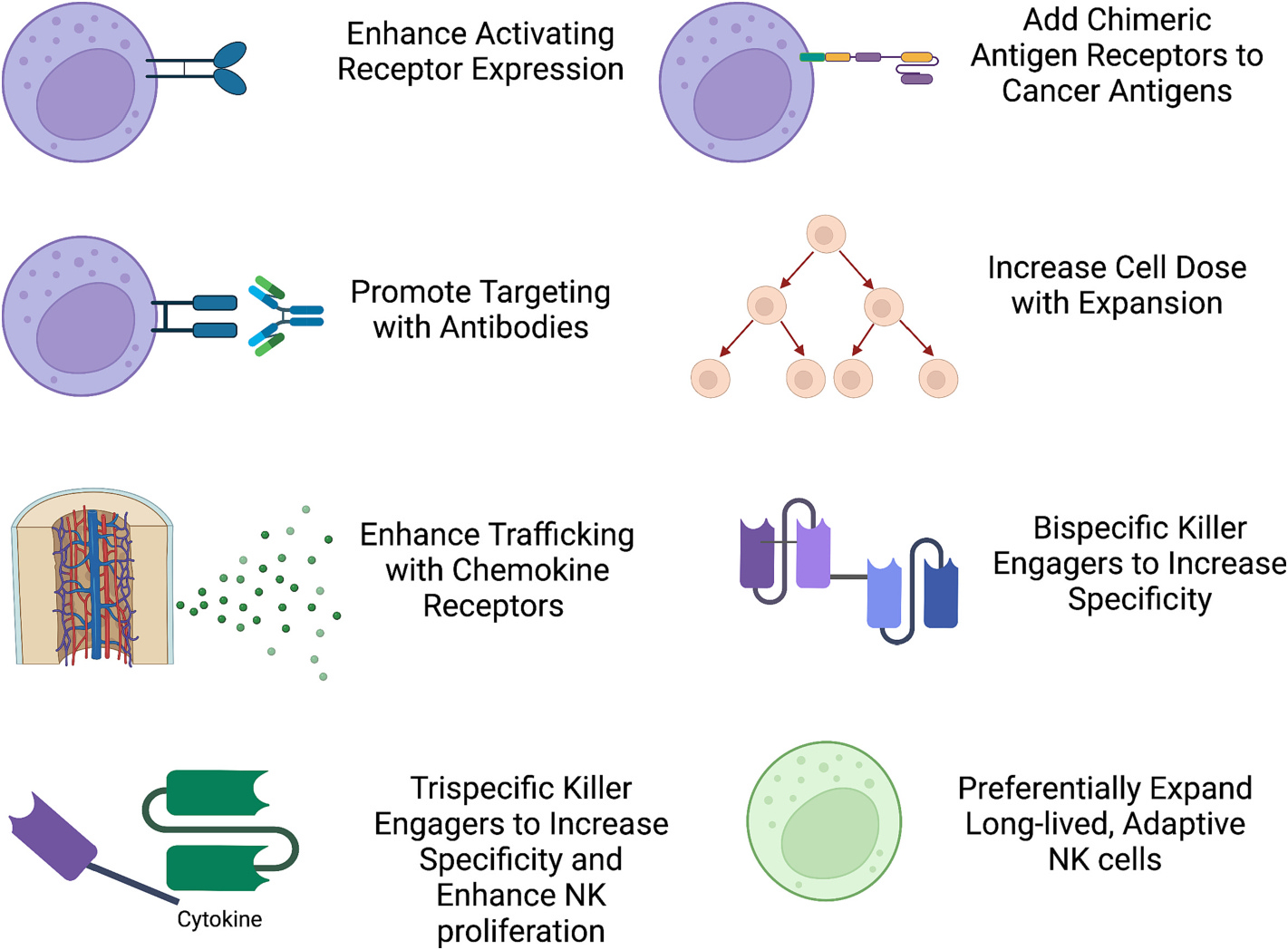
Methods to enhance NK cell therapy. NK cell targeting can be enhanced with antibodies, engagers, and chimeric antigen receptors. Other functionalities that can be enhanced include chemokine receptors to promote trafficking or use of specific NK cell subsets such as adaptive NK cells or cytokine induced memory-like (CIML) NK cells.
BiKEs have also been linked with a modified IL-15 molecule to induce NK cell proliferation, forming a tri-specific killer engager (TriKE). TriKEs have shown in vivo anti-tumor activity against chronic lymphocytic leukemia [105] and AML [106]. In mouse models TriKEs against CD19, CLEC12A or CD33 reduced leukemia tumor burden [107,108] and a TriKE against B7-H3 reduced ovarian cancer tumor burden [109]. TriKEs augment NK cell proliferation and cytokine production compared to monoclonal antibodies or BiKE constructs without IL-15. These are an attractive addition to current cancer treatments because they can reinvigorate and target a patient’s own NK cells or can be combined with allogeneic NK cell infusion (Fig. 4).
To further enhance binding, a tetravalent, bispecific antibody AFM13 was designed with two binding domains against CD30 and two against CD16A to treat CD30+ hematologic malignancies such as Hodgkin lymphoma. AFM13 was tested with conventional peripheral blood NK cells or UCB expanded NK cells. The authors generated CIML NK from UCB NK cells with pre-incubation with IL-12, IL-15, and IL-18 and found significantly enhanced AFM13 triggered cytotoxicity against CD30+ cell lines compared to conventional peripheral blood NK cells where the response was more heterogenous between donors. This study highlights the importance of the development of more standardized and active NK cell products for optimal efficacy [110]. AFM13 and modified NK cells are now being used in a Phase I/II trial for patients with refractory Hodgkin lymphoma, mycosis fungoides, and anaplastic large cell lymphoma (NCT04074746).
Chimeric antigen receptors (CAR) are a potent method of targeting that can be engineered for expression on autologous or allogeneic lymphocytes. iPSC-derived NK cells (iNK) are particularly amenable to complex genetic modification with viral and non-viral methods to yield NK cells with desired characteristics [111,112] Preclinical studies of CAR-iNK show activity against colorectal cancer [113], glioblastoma [114], and CD19-expressing cell lines [115]. Clinical CAR-NK cell trials targeting antigens such as human epidermal growth factor receptor 2 (HER2), CD33, Mucin-1 (MUC1), CD7, BCMA, and NKG2D ligands are currently underway [116].
In addition to choosing the right tumor target, the efficacy of a CAR construct also depends on the selection of the optimal transmembrane and stimulatory domains to enhance effector cell response. A study of iPSC-derived NK cells against ovarian cancer cells using various transmembrane domains demonstrated that the transmembrane domain of NKG2D, co-stimulatory domain of 2B4, and CD3ζ signaling domain led to the best cancer control in a xenograft model [117].
Another important consideration in CAR-mediated targeting is trogocytosis. This phenomenon occurs when living cells come in contact and the immune effector takes a ‘bite’ of cellular membrane from the target cell. Trogocytosis leads to loss of antigen expression on tumor cells and low level expression of CAR ligands on CAR-T [118] and CAR-NK cells [119]. CD19 CAR-NK acquired CD19 expression within minutes of incubation with Raji lymphoma cells. An F-actin inhibitor that blocks immunological synapse formation prevented the transfer of CD19 to CAR-NK cells. The authors also showed that CAR NK cells that underwent trogocytosis exhibited an exhausted phenotype, were less cytotoxic, and were cleared more quickly in vivo than NK cells that had not undergone trogocytosis. To reduce fratricide of CAR-NK cells that were expressing products of trogocytosis, the authors engineered the cells to express the ectodomain of CS1 (which binds to other CS1 molecules) and the inhibitory internal domain of KIR2DL1. Fratricide occurs when an NK cell recognizes another NK cell and mediates cytotoxicity, such as the CD19 molecule that has been obtained by trogocytosis in this case. CS1 is constitutively expressed by NK cells and the CS1/KIR2DL1 construct decreased fratricide of CD19 CAR-NK cells that had undergone trogocytosis. CD19 CAR-NK cells were compared against CD19 CAR-NK with the CS1/KIR2DL1 receptor in an immunodeficient mouse model with lymphoma and there was significantly lower tumor burden and longer NK cell persistence when the inhibitory receptor was added [119].
CAR-NK can also be combined with BiKE to enhance or diversify targeting. NK-92 cells were engineered to express a NKG2D-CD3ζ chimeric antigen receptor. Because many tumors shed NKG2D ligands, the NKG2D CAR-NK were given with a bispecific antibody against NKG2D and HER2. When given together, control of HER2+ glioblastoma was controlled in mice and even cured some of the animals [120].
10. Improving NK Cell Trafficking into the Tumor Microenvironment
The frequency of endogenous NK cells is extremely low in tumors. Nevertheless, higher numbers of host NK cells in the tumor microenvironment have been associated with improved outcomes in solid tumors including lung, esophageal, and colon (reviewed [121]). One major limitation of adoptive NK cell therapy is getting the infused cells to traffic to the tumor sites. Strategies to enhance NK cell homing have predominantly focused on cell extrinsic factors such as chemokines, selectins, integrins and their receptors as well as manipulating the immunosuppressive microenvironment.
CXCR4 promotes migration of NK cells to the bone marrow where it binds its ligand stromal-derived factor 1α (SDF-1 α). A study of labeled, adoptively transferred NK cells in Rhesus macaques showed that NK cells first migrated to the lungs, followed by the liver and spleen and only low levels of NK cells migrated to the bone marrow (3.4% of total NK dose one day following infusion) [122]. A gain of function mutation in CXCR4 enhances homing to the bone marrow and can be genetically engineered into NK cells by transfection with mRNA. This method significantly improves bone marrow homing in mice [123]. Some glioblastomas secrete SDF-1a and genetic engineering of NK cells to overexpress CXCR4 enhances trafficking into the tumor and is associated with improved glioblastoma control [124].
CXCR2 is another chemokine receptor that is involved in NK cell trafficking to renal cell carcinoma (RCC) and osteosarcomas. Only about 10% of peripheral blood NK cells express CXCR2. To overcome this limitation, over-expression of CXCR2 in NK-92 (NK-92-CXCR2) cells promoted migration into osteosarcoma lung metastases in vivo [125]. RCC secretes CXCL5, the ligand for CXCR2, but NK cells exposed to RCC in vitro, rapidly lose CXCR2 expression. When NK cells were transduced to over-express CXCR2, they exhibited improved trafficking along a gradient of RCC tumor supernatant and enhanced tumor killing [126].
Several tumor types including brain, breast, cervical, colon, gastric, lung, melanoma, mesothelioma, ovarian, prostate, renal, and thyroid, AML, chronic lymphocytic leukemia, and Hodgkin’s lymphoma secrete IL-8, the ligand for the chemokine receptor CXCR1. Electroporation of NK cells with CXCR1 mRNA enhanced trafficking in a murine ovarian cancer model and enhanced tumor control [127].
CD62L (L-selectin) also facilitates trafficking to marrow and tumor sites but does not rely on a chemokine gradient. Rather it mediates the initial attachment and slow rolling of these cells along high endothelial venules allowing extravasation from the blood into tissues [128]. Nicotinamide expanded NK cells were recently shown to yield high expression of CD62L and also exhibited improved trafficking to marrow, spleen and tumor site in a murine lymphoma model and phase 1 clinical trial [73]. In the future, the combination of engineered NK cells with chemokine alterations should be tested to improve tissue migration.
11. Turning off immune suppression in the tumor microenvironment
Even once NK cells traffic to the tumor microenvironment, they face a significant hurdle from immunosuppressive elements that include inhibitory cytokines, regulatory cells, hypoxia, and metabolic byproducts (reviewed [129]). Even the relative hypoxia of the bone marrow environment compared to the peripheral blood significantly alters the gene expression profile and chemotactic ability of NK cells [130]. In in vitro experiments, NK cell cytotoxicity is significantly reduced at low oxygen tension but IL-2 at least partially restores cytotoxic function [131].
Tumors themselves can secrete suppressive cytokines. Tregs and myeloid derived suppressor cells (MDSC) directly inhibit NK cell function through TGF-β signaling. In contrast to conventional NK cells, adaptive NK cells are relatively resistant to the suppressive effects of Tregs and MDSCs. Pre-clinical models of adaptive NK cells improved responses in tumors with highly suppressive microenvironments [132,133].
Another method to abrogate NK cell suppression in the tumor microenvironment is to interrupt TGF-β signaling. SMAD3 is a downstream factor in the TGF-β signaling pathway and it plays an essential role in TGF-β-mediated immune suppression. SMAD3 knock-down NK-92 cells showed enhanced cancer killing activities, enhanced IFN-γ production in vitro, and better anticancer effects than NK-92 empty vector control in non-obese diabetic severe combined immunodeficiency mice with xenografted hepatoma or melanoma [134].
Utilizing a dominant negative receptor for TGF-β, UCB-derived NK cells retained their ability to kill glioblastoma cells in the presence of TGF-β [135]. Another method to antagonize TGF-β signaling, NK-92 cells engineered to express a chimeric receptor with TGF-β extracellular domain and the intracellular domain of NKG2D were resistant to TGF-β-induced suppressive signaling and exhibited higher killing capacity and IFN-γ production against tumor cells compared with the control NK cells [136]. Several methods now exist to engineer NK cells to resist suppression by TGF-β, which may increase the utility of NK cell therapy in the tumor microenvironment.
12. Future directions
Novel NK cell sources, enhanced knowledge of NK cell biology, and improved methods in genetic engineering have enabled the NK cell therapeutic field to progress significantly. Among critical advantages compared to CAR-T cell therapies are minimal risk of CRS and neurotoxicity, and lack of GVHD. These advantages may make NK cell therapy preferable for older and frailer patients who cannot tolerate the side effects of CAR-T. It is also possible that NK cells therapeutics may be safer to give at community sites that lack the capability to administer CAR-T cells. Because of the variety of mechanisms by which NK cells can recognize tumor targets, antigen escape is less likely with NK cell therapy compared to CAR-T. Multiple targeting or flexible targeting is also more feasible with NK cell products because of their off-the-shelf capabilities and combinations with multiple antibodies concomitantly or sequentially. Antigen loss is an important mechanism of escape from CAR-T cell therapy [137,138] so the ability to target multiple antigens may prevent selection for low-expressing clones. In order to make NK cell therapy widely available and accessible, the field will depend on methods that consistently yield an off-the-shelf, cryopreserved product that is both efficacious and economical to produce. Methodologies utilizing peripheral blood, UCB, or iPSC platforms and various combinations of engineered receptors to improve NK cell function and targeting have already established this proof of concept. Over the next decade clinical trials data will help determine which of these alterations are most clinically effective and safe for specific malignancies and may lead to major paradigm shifts in cancer treatment.
Better understanding of NK cell trafficking, function in the tumor microenvironment and interaction with adaptive immune system is necessary to maximize the clearance of cancer cells that survive in the tumor sites. These future insights will help NK cell therapy to gain a foothold in the solid tumor setting as well. The development of novel in vivo NK cell engagers and combinatory therapies with activators and inhibitors of checkpoint receptors can further enhance NK cell potency. While questions regarding the optimal source, dose, schedule, number of infusions and conditioning regimen for specific disease applications continue to be addressed both experimentally and clinically, NK cell therapy is gaining a solid ground as an important source of cellular therapy for blood cancers.
Practice points.
Clinical experience using allogeneic NK cell infusions for patients with hematologic malignancies suggests minimal risk for cytokine release syndrome, neurotoxicity or GVHD.
Novel NK cell therapies exhibit early promise in patients with relapsed/refractory AML, lymphoma and myeloma and could be investigated for patients who are not suitable for standard of care therapies
Research agenda.
NK cells derived from varied sources including blood, umbilical cord blood and induced pluripotent stem cells are exploitable for genetic engineering, cryopreservation and multiple infusions in early phase clinical trials.
Novel NK cell products have to be optimized to improve persistence, target multiple cancer cell receptors and exhibit enhanced tumor homing.
NK cell combination strategies need to be explored to overcome the suppressive tumor microenvironment.
Funding
This research did not receive any specific grant from funding agencies.
in the public, commercial, or not-for-profit sectors.
Footnotes
Declaration of Competing Interest
Veronika Bachanova: research funding from Gamida Cell, Incyte, BMS and Citius, serves as DMSB member for Miltenyi Biotec and on advisory board for Kite, Caribou, Takeda, Astra Zeneca, ADC Therapeutic and Amgen.
References
- [1].Siegler EL, Kenderian SS. Neurotoxicity and cytokine release syndrome after chimeric antigen receptor T cell therapy: insights into mechanisms and novel therapies. Front Immunol 2020;11:1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Miller JS, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005;105(8):3051–7. [DOI] [PubMed] [Google Scholar]
- [3].Kiessling R, Klein E, Wigzell H. “natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol 1975;5(2):112–7. [DOI] [PubMed] [Google Scholar]
- [4].Cooper MA, Caligiuri MA. Isolation and characterization of human natural killer cell subsets. Curr Protoc Immunol 2004;7:34. Chapter 7: p. Unit. [DOI] [PubMed] [Google Scholar]
- [5].Caligiuri MA. Human natural killer cells. Blood 2008;112(3):461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sentman CL, Olsson MY, Karre K. Missing self recognition by natural killer cells in MHC class I transgenic mice. A ‘receptor calibration’ model for how effector cells adapt to self. Semin Immunol 1995;7(2):109–19. [DOI] [PubMed] [Google Scholar]
- [7].Anfossi N, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity 2006;25(2):331–42. [DOI] [PubMed] [Google Scholar]
- [8].Kim S, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature 2005;436(7051):709–13. [DOI] [PubMed] [Google Scholar]
- [9].Kielczewska A, et al. Critical residues at the Ly49 natural killer receptor’s homodimer interface determine functional recognition of m157, a mouse cytomegalovirus MHC class I-like protein. J Immunol 2007;178(1):369–77. [DOI] [PubMed] [Google Scholar]
- [10].Karre K, et al. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 1986;319(6055):675–8. [DOI] [PubMed] [Google Scholar]
- [11].Tu MM, Mahmoud AB, Makrigiannis AP. Licensed and unlicensed NK cells: differential roles in Cancer and viral control. Front Immunol 2016;7:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Campbell KS, Purdy AK. Structure/function of human killer cell immunoglobulin-like receptors: lessons from polymorphisms, evolution, crystal structures and mutations. Immunology 2011;132(3):315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hsu KC, et al. The killer cell immunoglobulin-like receptor (KIR) genomic region: gene-order, haplotypes and allelic polymorphism. Immunol Rev 2002;190:40–52. [DOI] [PubMed] [Google Scholar]
- [14].Cooley S, et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood 2009;113(3):726–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Venstrom JM, et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med 2012;367(9):805–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bauer S, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 1999;285(5428):727–9. [DOI] [PubMed] [Google Scholar]
- [17].Peng YP, et al. Comprehensive analysis of the percentage of surface receptors and cytotoxic granules positive natural killer cells in patients with pancreatic cancer, gastric cancer, and colorectal cancer. J Transl Med 2013;11:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Parry HM, et al. NK cell function is markedly impaired in patients with chronic lymphocytic leukaemia but is preserved in patients with small lymphocytic lymphoma. Oncotarget 2016;7(42):68513–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mamessier E, et al. Human breast tumor cells induce self-tolerance mechanisms to avoid NKG2D-mediated and DNAM-mediated NK cell recognition. Cancer Res 2011;71(21):6621–32. [DOI] [PubMed] [Google Scholar]
- [20].Sun C, et al. TGF-beta1 down-regulation of NKG2D/DAP10 and 2B4/SAP expression on human NK cells contributes to HBV persistence. PLoS Pathog 2012; 8(3):e1002594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bozzano F, et al. Activating NK cell receptor expression/function (NKp30, NKp46, DNAM-1) during chronic viraemic HCV infection is associated with the outcome of combined treatment. Eur J Immunol 2011;41(10):2905–14. [DOI] [PubMed] [Google Scholar]
- [22].Waldhauer I, et al. Tumor-associated MICA is shed by ADAM proteases. Cancer Res 2008;68(15):6368–76. [DOI] [PubMed] [Google Scholar]
- [23].Zingoni A, et al. MICA-129 dimorphism and soluble MICA are associated with the progression of multiple myeloma. Front Immunol 2018;9:926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Romee R, et al. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17). Blood 2013;121(18):3599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lanier LL, et al. Association of DAP12 with activating CD94/NKG2C NK cell receptors. Immunity 1998;8(6):693–701. [DOI] [PubMed] [Google Scholar]
- [26].Lopez-Verges S, et al. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A 2011;108(36):14725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Guma M, et al. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood 2006;107(9):3624–31. [DOI] [PubMed] [Google Scholar]
- [28].Rolle A, et al. IL-12-producing monocytes and HLA-E control HCMV-driven NKG2C+ NK cell expansion. J Clin Invest 2014;124(12):5305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Barrow AD, Martin CJ, Colonna M. The natural cytotoxicity receptors in health and disease. Front Immunol 2019;10:909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Balsamo M, et al. Hypoxia downregulates the expression of activating receptors involved in NK-cell-mediated target cell killing without affecting ADCC. Eur J Immunol 2013;43(10):2756–64. [DOI] [PubMed] [Google Scholar]
- [31].Ferlazzo G, Morandi B. Cross-talks between natural killer cells and distinct subsets of dendritic cells. Front Immunol 2014;5:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schuster IS, et al. “natural regulators”: NK cells as modulators of T cell immunity. Front Immunol 2016;7:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature 2009;457(7229):557–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rolle A, et al. CD2-CD58 interactions are pivotal for the activation and function of adaptive natural killer cells in human cytomegalovirus infection. Eur J Immunol 2016;46(10):2420–5. [DOI] [PubMed] [Google Scholar]
- [35].Lee J, et al. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity 2015;42(3):431–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sun JC, et al. Homeostatic proliferation generates long-lived natural killer cells that respond against viral infection. J Exp Med 2011;208(2):357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Woan KV, et al. Harnessing features of adaptive NK cells to generate iPSC-derived NK cells for enhanced immunotherapy. Cell Stem Cell 2021;28(12):2062–2075 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Schlums H, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity 2015;42(3):443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhang T, et al. Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRgamma deficiency. J Immunol 2013;190(4):1402–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Takenaka K, et al. Cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation is associated with a reduced risk of relapse in patients with acute myeloid leukemia who survived to day 100 after transplantation: the Japan Society for Hematopoietic Cell Transplantation Transplantation-related Complication Working Group. Biol Blood Marrow Transplant 2015;21(11):2008–16. [DOI] [PubMed] [Google Scholar]
- [41].Davis ZB, et al. Adaptive natural killer cell and killer cell immunoglobulin-like receptor-expressing T cell responses are induced by cytomegalovirus and are associated with protection against cytomegalovirus reactivation after allogeneic donor hematopoietic cell transplantation. Biol Blood Marrow Transplant 2015;21 (9):1653–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Merino AM, et al. Early adaptive natural killer cell expansion is associated with decreased relapse after autologous transplantation for multiple myeloma. Transplant Cell Ther 2021;27(4):310 e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cooper MA, et al. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A 2009;106(6):1915–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tienhaara A, Pelliniemi TT. Peripheral blood lymphocyte subsets in multiple myeloma and monoclonal gammopathy of undetermined significance. Clin Lab Haematol 1994;16(3):213–23. [DOI] [PubMed] [Google Scholar]
- [45].Carbone E, et al. HLA class I, NKG2D, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood 2005;105(1):251–8. [DOI] [PubMed] [Google Scholar]
- [46].Benson DM Jr, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 2010;116(13):2286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lozano E, et al. Nectin-2 expression on malignant plasma cells is associated with better response to TIGIT blockade in multiple myeloma. Clin Cancer Res 2020;26 (17):4688–98. [DOI] [PubMed] [Google Scholar]
- [48].Xu J, et al. Characterizing the tumor suppressor role of CEACAM1 in multiple myeloma. Cell Physiol Biochem 2018;45(4):1631–40. [DOI] [PubMed] [Google Scholar]
- [49].Van Valckenborgh E, et al. Multiple myeloma induces the immunosuppressive capacity of distinct myeloid-derived suppressor cell subpopulations in the bone marrow. Leukemia 2012;26(11):2424–8. [DOI] [PubMed] [Google Scholar]
- [50].Beyer M, et al. In vivo peripheral expansion of naive CD4+CD25high FoxP3+regulatory T cells in patients with multiple myeloma. Blood 2006;107(10):3940–9. [DOI] [PubMed] [Google Scholar]
- [51].Vacca A, et al. A disturbance of the IL-2/IL-2 receptor system parallels the activity of multiple myeloma. Clin Exp Immunol 1991;84(3):429–34. [PMC free article] [PubMed] [Google Scholar]
- [52].Lust JA, et al. Reduction in C-reactive protein indicates successful targeting of the IL-1/IL-6 axis resulting in improved survival in early stage multiple myeloma. Am J Hematol 2016;91(6):571–4. [DOI] [PubMed] [Google Scholar]
- [53].Swaminathan S, et al. MYC functions as a switch for natural killer cell-mediated immune surveillance of lymphoid malignancies. Nat Commun 2020;11(1):2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Duault C, et al. Activated natural killer cells predict poor clinical prognosis in high-risk B- and T-cell acute lymphoblastic leukemia. Blood 2021;138(16):1465–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Chen CI, et al. NK cells are dysfunctional in human chronic myelogenous leukemia before and on imatinib treatment and in BCR-ABL-positive mice. Leukemia 2012;26(3):465–74. [DOI] [PubMed] [Google Scholar]
- [56].MacFarlane AWT, et al. NK cell dysfunction in chronic lymphocytic leukemia is associated with loss of the mature cells expressing inhibitory killer cell Ig-like receptors. Oncoimmunology 2017;6(7):e1330235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Stringaris K, et al. Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica 2014;99(5):836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Chiu J, Ernst DM, Keating A. Acquired natural killer cell dysfunction in the tumor microenvironment of classic Hodgkin lymphoma. Front Immunol 2018;9:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].West EJ, et al. Immune activation by combination human lymphokine-activated killer and dendritic cell therapy. Br J Cancer 2011;105(6):787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ruggeri L, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002;295(5562):2097–100. [DOI] [PubMed] [Google Scholar]
- [61].Velardi A Natural killer cell alloreactivity 10 years later. Curr Opin Hematol 2012;19(6):421–6. [DOI] [PubMed] [Google Scholar]
- [62].Passweg JR, et al. Purified donor NK-lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia 2004;18 (11):1835–8. [DOI] [PubMed] [Google Scholar]
- [63].Koehl U, et al. Ex vivo expansion of highly purified NK cells for immunotherapy after haploidentical stem cell transplantation in children. Klin Padiatr 2005;217 (6):345–50. [DOI] [PubMed] [Google Scholar]
- [64].Bachanova V, et al. Haploidentical natural killer cells induce remissions in non-Hodgkin lymphoma patients with low levels of immune-suppressor cells. Cancer Immunol Immunother 2018;67(3):483–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Romee R, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med 2016;8(357):357ra123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lee DA, et al. Haploidentical natural killer cells infused before allogeneic stem cell transplantation for myeloid malignancies: a phase I trial. Biol Blood Marrow Transplant 2016;22(7):1290–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Tschan-Plessl A, et al. Cellular immunotherapy with multiple infusions of in vitro-expanded haploidentical natural killer cells after autologous transplantation for patients with plasma cell myeloma. Cytotherapy 2021;23(4):329–38. [DOI] [PubMed] [Google Scholar]
- [68].Yang Y, et al. Phase I study of random healthy donor-derived allogeneic natural killer cell therapy in patients with malignant lymphoma or advanced solid tumors. Cancer Immunol Res 2016;4(3):215–24. [DOI] [PubMed] [Google Scholar]
- [69].Fujisaki H, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res 2009;69(9):4010–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Denman CJ, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PloS One 2012;7(1):e30264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Felices M, et al. Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect. JCI Insight 2018;3(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Oyer JL, et al. Natural killer cells stimulated with PM21 particles expand and biodistribute in vivo: clinical implications for cancer treatment. Cytotherapy 2016;18(5):653–63. [DOI] [PubMed] [Google Scholar]
- [73].Bachanova M, Luo Brachya, Peled Miller. First-in-human phase I study of nicotinamide-expanded related donor natural killer cells for the treatment of relapsed/refractory non-Hodgkin lymphoma and multiple myeloma. Transplantation and Cellular Therapy 2019:25. [Google Scholar]
- [74].Liu E, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med 2020;382(6):545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Spanholtz J, et al. High log-scale expansion of functional human natural killer cells from umbilical cord blood CD34-positive cells for adoptive cancer immunotherapy. PloS One 2010;5(2):e9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Goldenson BH, et al. Umbilical cord blood and iPSC-derived natural killer cells demonstrate key differences in cytotoxic activity and KIR profiles. Front Immunol 2020;11:561553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Luevano M, et al. The unique profile of cord blood natural killer cells balances incomplete maturation and effective killing function upon activation. Hum Immunol 2012;73(3):248–57. [DOI] [PubMed] [Google Scholar]
- [78].Shah N, et al. Phase I study of cord blood-derived natural killer cells combined with autologous stem cell transplantation in multiple myeloma. Br J Haematol 2017;177(3):457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Graeff R, et al. Acidic residues at the active sites of CD38 and ADP-ribosyl cyclase determine nicotinic acid adenine dinucleotide phosphate (NAADP) synthesis and hydrolysis activities. J Biol Chem 2006;281(39):28951–7. [DOI] [PubMed] [Google Scholar]
- [80].Klingemann H, Boissel L, Toneguzzo F. Natural killer cells for immunotherapy - advantages of the NK-92 cell line over blood NK cells. Front Immunol 2016;7:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Jochems C, et al. An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele. Oncotarget 2016;7(52):86359–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Hirayama AV, et al. The response to lymphodepletion impacts PFS in patients with aggressive non-Hodgkin lymphoma treated with CD19 CAR T cells. Blood 2019;133(17):1876–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Shi J, et al. Infusion of haplo-identical killer immunoglobulin-like receptor ligand mismatched NK cells for relapsed myeloma in the setting of autologous stem cell transplantation. Br J Haematol 2008;143(5):641–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Ghilardi G, et al. Bendamustine is safe and effective for lymphodepletion before tisagenlecleucel in patients with refractory or relapsed large B-cell lymphomas. Ann Oncol 2022;33(9):916–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Guedj C, et al. T cell adhesion triggers an early signaling pole distal to the immune synapse. J Cell Sci 2016;129(13):2526–37. [DOI] [PubMed] [Google Scholar]
- [86].Demetriou P, et al. A dynamic CD2-rich compartment at the outer edge of the immunological synapse boosts and integrates signals. Nat Immunol 2020;21(10):1232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Quirin Hammer KP, Mbofung Rina M, van Ooijen Hanna, Varady Erika, Pan Yijia, Jelcic Mark, et al. Combined genetic ablation of CD54 and CD58 in CAR engineered cytotoxic lymphocytes effectively averts allogeneic immune cell rejection. American Society of Hematology abstract; 2022. [Google Scholar]
- [88].Mo F, et al. Engineered off-the-shelf therapeutic T cells resist host immune rejection. Nat Biotechnol 2021;39(1):56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Williams AM, Pan Y, Groff B, Mbofung RM, Chang A, Chen CY, et al. Alloimmune defense receptor redirects host immune cell alloreactivity to potentiate functional persistence and anti-tumor activity of off-the-shelf cell-based Cancer therapy. American Society of Hematology abstract; 2022. [Google Scholar]
- [90].Bachanova V, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood 2014;123(25):3855–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Grzywacz B, et al. Natural killer cell homing and persistence in the bone marrow after adoptive immunotherapy correlates with better leukemia control. J Immunother 2019;42(2):65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Park JH, Geyer MB, Brentjens RJ. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood 2016;127(26):3312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Carson WE, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med 1994;180(4):1395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Cooley S, et al. First-in-human trial of rhIL-15 and haploidentical natural killer cell therapy for advanced acute myeloid leukemia. Blood Adv 2019;3(13):1970–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Kim PS, et al. IL-15 superagonist/IL-15RalphaSushi-fc fusion complex (IL-15SA/IL-15RalphaSu-fc; ALT-803) markedly enhances specific subpopulations of NK and memory CD8+ T cells, and mediates potent anti-tumor activity against murine breast and colon carcinomas. Oncotarget 2016;7(13):16130–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Romee R, et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood 2018;131(23):2515–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Delconte RB, et al. CIS is a potent checkpoint in NK cell-mediated tumor immunity. Nat Immunol 2016;17(7):816–24. [DOI] [PubMed] [Google Scholar]
- [98].Daher M, et al. Targeting a cytokine checkpoint enhances the fitness of armored cord blood CAR-NK cells. Blood 2021;137(5):624–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Bryceson YT, et al. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev 2006;214:73–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Gavin PG, et al. Association of Polymorphisms in FCGR2A and FCGR3A with degree of Trastuzumab benefit in the adjuvant treatment of ERBB2/HER2-positive breast Cancer: analysis of the NSABP B-31 trial. JAMA Oncol 2017;3(3):335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Veeramani S, et al. Rituximab infusion induces NK activation in lymphoma patients with the high-affinity CD16 polymorphism. Blood 2011;118(12):3347–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Zhu H, et al. Pluripotent stem cell-derived NK cells with high-affinity noncleavable CD16a mediate improved antitumor activity. Blood 2020;135(6):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Gauthier L, et al. Multifunctional natural killer cell engagers targeting NKp46 trigger protective tumor immunity. Cell 2019;177(7):1701–1713 e16. [DOI] [PubMed] [Google Scholar]
- [104].Marklin M, et al. Bispecific NKG2D-CD3 and NKG2D-CD16 fusion proteins for induction of NK and T cell reactivity against acute myeloid leukemia. J Immunother Cancer 2019;7(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Felices M, et al. Novel CD19-targeted TriKE restores NK cell function and proliferative capacity in CLL. Blood Adv 2019;3(6):897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Sarhan D, et al. 161533 TriKE stimulates NK-cell function to overcome myeloid-derived suppressor cells in MDS. Blood Adv 2018;2(12):1459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Arvindam US, et al. A trispecific killer engager molecule against CLEC12A effectively induces NK-cell mediated killing of AML cells. Leukemia 2021;35(6):1586–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Vallera DA, et al. IL15 Trispecific killer engagers (TriKE) make natural killer cells specific to CD33+ targets while also inducing persistence, in vivo expansion, and enhanced function. Clin Cancer Res 2016;22(14):3440–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Vallera DA, et al. NK-cell-mediated targeting of various solid tumors using a B7-H3 tri-specific killer engager in vitro and in vivo. Cancers (Basel) 2020;12(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Kerbauy LN, et al. Combining AFM13, a bispecific CD30/CD16 antibody, with cytokine-activated blood and cord blood-derived NK cells facilitates CAR-like responses against CD30(+) malignancies. Clin Cancer Res 2021;27(13):3744–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wilber A, et al. Efficient and stable transgene expression in human embryonic stem cells using transposon-mediated gene transfer. Stem Cells 2007;25(11):2919–27. [DOI] [PubMed] [Google Scholar]
- [112].Costa M, et al. A method for genetic modification of human embryonic stem cells using electroporation. Nat Protoc 2007;2(4):792–6. [DOI] [PubMed] [Google Scholar]
- [113].Zhang Q, et al. Combination therapy with EpCAM-CAR-NK-92 cells and Regorafenib against human colorectal Cancer models. J Immunol Res 2018;2018:4263520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Han J, et al. CAR-engineered NK cells targeting wild-type EGFR and EGFRvIII enhance killing of glioblastoma and patient-derived glioblastoma stem cells. Sci Rep 2015;5:11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Liu E, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 2018;32(2):520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Kloess S, et al. CAR-expressing natural killer cells for Cancer retargeting. Transfus Med Hemother 2019;46(1):4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Li Y, et al. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell 2018;23(2):181–192 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Hamieh M, et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature 2019;568(7750):112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Li Y, et al. KIR-based inhibitory CARs overcome CAR-NK cell trogocytosis-mediated fratricide and tumor escape. Nat Med 2022;28(10):2133–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Zhang C, et al. Bispecific antibody-mediated redirection of NKG2D-CAR natural killer cells facilitates dual targeting and enhances antitumor activity. J Immunother Cancer 2021;9(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Larsen SK, Gao Y, Basse PH. NK cells in the tumor microenvironment. Crit Rev Oncog 2014;19(1–2):91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Sato N, et al. In-vivo tracking of adoptively transferred natural killer-cells in rhesus macaques using (89)zirconium-oxine cell labeling and PET imaging. Clin Cancer Res 2020;26(11):2573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Levy E, et al. Enhanced bone marrow homing of natural killer cells following mRNA transfection with gain-of-function variant CXCR4(R334X). Front Immunol 2019;10:1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Muller N, et al. Engineering NK cells modified with an EGFRvIII-specific chimeric antigen receptor to overexpress CXCR4 improves immunotherapy of CXCL12/SDF-1alpha-secreting glioblastoma. J Immunother 2015;38(5):197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Kiany S, Huang G, Kleinerman ES. Effect of entinostat on NK cell-mediated cytotoxicity against osteosarcoma cells and osteosarcoma lung metastasis. Oncoimmunology 2017;6(8):e1333214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Kremer V, et al. Genetic engineering of human NK cells to express CXCR2 improves migration to renal cell carcinoma. J Immunother Cancer 2017;5(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Ng YY, Tay JCK, Wang S. CXCR1 expression to improve anti-Cancer efficacy of intravenously injected CAR-NK cells in mice with peritoneal xenografts. Mol Ther Oncolytics 2020;16:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Simon SI, et al. L-selectin (CD62L) cross-linking signals neutrophil adhesive functions via the mac-1 (CD11b/CD18) beta 2-integrin. J Immunol 1995;155(3):1502–14. [PubMed] [Google Scholar]
- [129].Hasmim M, et al. Critical role of tumor microenvironment in shaping NK cell functions: implication of hypoxic stress. Front Immunol 2015;6:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Parodi M, et al. Hypoxia modifies the transcriptome of human NK cells, modulates their Immunoregulatory profile, and influences NK cell subset migration. Front Immunol 2018;9:2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Sarkar S, et al. Hypoxia induced impairment of NK cell cytotoxicity against multiple myeloma can be overcome by IL-2 activation of the NK cells. PloS One 2013;8(5):e64835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Sarhan D, Miller JS. Assessing canonical and adaptive natural killer cell function in suppression assays in vitro. Methods Mol Biol 2019;1913:153–66. [DOI] [PubMed] [Google Scholar]
- [133].Sarhan D, et al. Adaptive NK cells resist regulatory T-cell suppression driven by IL37. Cancer Immunol Res 2018;6(7):766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Wang QM, et al. Enhanced Cancer immunotherapy with Smad3-silenced NK-92 cells. Cancer Immunol Res 2018;6(8):965–77. [DOI] [PubMed] [Google Scholar]
- [135].Yvon ES, et al. Cord blood natural killer cells expressing a dominant negative TGF-beta receptor: implications for adoptive immunotherapy for glioblastoma. Cytotherapy 2017;19(3):408–18. [DOI] [PubMed] [Google Scholar]
- [136].Wang Z, et al. Augmented anti-tumor activity of NK-92 cells expressing chimeric receptors of TGF-betaR II and NKG2D. Cancer Immunol Immunother 2017;66(4):537–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Sotillo E, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov 2015;5(12):1282–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Samur MK, et al. Biallelic loss of BCMA as a resistance mechanism to CAR T cell therapy in a patient with multiple myeloma. Nat Commun 2021;12(1):868. [DOI] [PMC free article] [PubMed] [Google Scholar]


