Abstract
Intraepithelial lymphocytes (IEL) are a critical effector component of the gut-associated lymphoid tissue (GALT) and play an important role in mucosal immunity as well as in the maintenance of the epithelial cell integrity and barrier function. The objective of this study was to determine whether simian immunodeficiency virus (SIV) infection of rhesus macaques would cause alterations in the immunophenotypic profiles of IEL and their mitogen-specific cytokine (gamma interferon [IFN-γ] and MIP-1β) responses (by flow cytometry) and virus-specific cytotoxic T-cell (CTL) activity (by the chromium release assay). Virally infected IEL were detected through the entire course of SIV infection by in situ hybridization. Severe depletion of CD4+ single-positive and CD4+CD8+ double-positive T cells occurred early in primary SIV infection, which was coincident with an increased prevalence of CD8+ T cells. This was in contrast to a gradual depletion of CD4+ T cells in peripheral blood. The CD8+ IEL were the primary producers of IFN-γ and MIP-1β and were found to retain their potential to produce both IFN-γ and MIP-1β through the entire course of SIV infection. SIV-specific CTL activity was detected in primary IEL at 1, 2, and 4 weeks post-SIV infection. These results demonstrated that IEL may be involved in generating antiviral immune responses early in SIV infection and in suppressing viral infection thereafter. Alterations in homeostasis in epithelia due to severe CD4+ T-cell depletion accompanied by changes in the cytokine and chemokine production by IEL may play a role in the enteropathogenesis of SIV infection.
Gut-associated lymphoid tissue (GALT) is an important target organ of human immunodeficiency virus (HIV) and may serve as a potentially significant viral reservoir since it harbors the majority of lymphoid tissue in the body. The gastrointestinal immune system is extensively involved in the course of HIV infection, which is evidenced by commonly encountered intestinal complications in HIV-infected patients, including nutrient malabsorbtion, malnutrition, diarrhea, weight loss, and opportunistic enteric infections (17, 28). Histopathologic changes in intestinal mucosae encompass villous atrophy, hyperplastic crypts, and T-cell depletion (58). Mucosal surfaces of the gastrointestinal tract consist of a single layer of epithelial cells that provide a protective barrier against pathogenic microbes and other macromolecules. In addition, epithelial cells in the brush border, called enterocytes, are important in nutrient absorption. Several studies have shown that functional interrelationships exist between the gut epithelium and the mucosal immune system (33, 34). Hence, changes in the mucosal epithelium might reflect alterations in mucosal immunity. A distinct population of lymphocytes located between enterocytes in the epithelium above the basement membrane is called intraepithelial lymphocytes (IEL). These lymphocytes are phenotypically and functionally distinct from lymphocytes in the underlying lamina propria, lymph nodes, and peripheral blood. Due to their close and intimate contact with the epithelial cells and the environment, IEL play an important role in mucosal immunity. IEL possess a number of pathogen-specific and nonspecific functions enabling them to play a critical role in host defenses at mucosal surfaces. They have been shown to possess pathogen-specific cytotoxic T-cell (CTL) activity (10) and to be capable of mediating delayed-type hypersensitivity responses (48). Human IEL were demonstrated to have low natural killer (NK) activity in short-term assays (7). IEL also secrete various cytokines such as gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and interleukin-2 (IL-2) (12, 31), which may play an important role in immunomodulation at the mucosal surfaces. These cells can produce chemokines such as MIP-1β and RANTES, implicating a regulatory role in the inflammation process. Furthermore, IEL have been shown to secrete growth factors such as keratinocyte growth factor, which is involved in epithelial cell proliferation and maturation, and can be crucial in maintaining epithelial cell barrier integrity and function (4). These studies show that IEL constitute a unique population of cells which are capable of playing an important role in immunity in the gastrointestinal mucosae against pathogens as well as in the maintenance of the epithelium.
IEL are phenotypically diverse and contain both CD4+ and CD8+ T cells. A loss of mucosal CD4+ T cells has been reported for patients with advanced HIV infection and is associated with intestinal complications (17, 28). The effects of HIV infection on mucosal lymphocytes, specifically IEL, have not been fully elucidated. These studies have been limited by the inability to obtain sufficient amounts of intestinal tissue samples in early stages of HIV infection. The simian immunodeficiency virus (SIV)-infected rhesus macaques provide a suitable animal model to determine the role of IEL in the immunopathogenesis of HIV infection. SIV is a lentivirus that causes simian acquired immunodeficiency syndrome (SAIDS) in rhesus macaques. The clinical, immunological, and pathological changes in infected rhesus macaques are similar to those observed in HIV infection (19, 27, 49). The course of pathogenic SIVmac infection includes primary-acute, asymptomatic, and terminal stages of disease, as observed in HIV infection. Thus, SIVmac-infected macaques are well suited for the study of acute and chronic viral infection and its effect on intestinal dysfunction. The intestine is an early target organ of SIV, and a SIV-associated enteropathy syndrome was detected in primary SIV infection (18, 20, 50).
Since the functions carried out by IEL in the intestinal mucosae appear to be mediated in part by the cytokines or chemokines that they secrete, a better understanding of the phenotype and frequency of cytokine-producing cells within this compartment is critical to understand their function and role in viral pathogenesis. Cytokines such as IFN-γ and, recently, chemokines such as MIP-1β have been shown to play a role in immunity to HIV. However, perturbations in cytokine production as a consequence of the alterations in T-cell phenotypic profiles within the IEL could promote the breakdown of the intestinal immune system, thereby contributing to the pathogenesis of HIV infection.
IFN-γ, a proinflammatory secreted cytokine, has been shown to play a central role in the development of cellular immune responses leading to the elimination of intracellular pathogens (16, 46, 47). MIP-1β is a member of the C-C chemokine family of proteins secreted by T cells and other leukocytes which plays a role in the recruitment of subsets of T cells (55, 56) and macrophages (13) to the sites of infection. Furthermore, MIP-1β may contribute to tissue damage by stimulating the release of cytokines such as TNF-α, IL-1, and IL-6 (13), which perpetuate the inflammatory response.
Based on the above studies, we hypothesized that IEL may play an important role in the generation of antiviral responses and may exhibit immunophenotypic and functional alterations early in HIV infection. We examined the above hypothesis by determining the immunophenotypic and functional alterations in jejunal IEL subsets during primary-acute, asymptomatic, and SAIDS stages of SIV infection. The functional role of IEL was determined by measuring SIV-specific CTL activity by cytotoxicity assays. Their ability to produce IFN-γ and MIP-1β following short-term mitogenic stimulation was determined by three-color flow cytometry combined with intracellular staining for cytokines. The use of flow cytometry provided the ability to simultaneously determine the phenotype of the IEL and cytokine produced by them at a single cell level. Our results demonstrated that the prevalence of CD4+ subsets decreased dramatically early during the primary SIV infection, whereas the proportions of CD8+ IEL increased considerably. There was, however, no major change in the proportion of CD3+ IEL following SIV infection relative to SIV-negative animals. The dramatic depletion of CD4+ IEL was in contrast to the gradual depletion of CD4+ T cells observed in peripheral blood. SIV-specific CTL activity was found to be present in the primary-acute stage of infection. The proportion of CD8+ IEL producing IFN-γ and MIP-1β in response to mitogenic stimulation was found to increase following infection and remained high throughout the entire course of SIV infection, indicating that IEL in jejunal mucosae were primed in vivo following SIV infection.
MATERIALS AND METHODS
Animals, virus, and tissue collection.
Eleven colony-bred rhesus macaques (Macaca mulatta) from the California Regional Primate Research Center, Davis, were used in this study. The animals were housed in accordance with American Association for Accreditation of Laboratory Animal Care guidelines. Animals were seronegative for simian retrovirus type 1 and simian T-cell leukemia virus type 1. Nine adult animals were infected intravenously with 10 to 100 animal infectious doses of uncloned pathogenic SIVmac 251. SIV-infected animals were necropsied at various time points during the primary-acute stage at 1, 2, and 4 weeks postinfection (p.i.) (n = 3) and during the clinically asymptomatic stage at 36 to 44 weeks p.i. (n = 3) and the SAIDS stage (n = 3) of SIV infection. Two uninfected animals served as negative controls. Jejunal tissue samples were collected at necropsy and used for the isolation of IEL. Tissue samples were also formalin fixed and paraffin embedded for in situ hybridization analysis.
Detection and localization of SIV-infected cells by in situ hybridization.
SIV-infected cells in intestinal tissues were detected by in situ hybridization with digoxigenin-UTP-labeled SIV riboprobes according to the previously reported procedure with minor modifications (20). Riboprobes were specific for SIV gag, env, and pol regions. Formalin-fixed and paraffin-embedded intestinal tissue sections were deparaffinized, hydrated, and treated with proteinase K for 10 min at 37°C, followed by prehybridization for 1 h at 50°C in hybridization solution containing 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 5× Denhardt’s solution, 50% deionized formamide, 10% dextran sulfate, and 1 mg of tRNA per ml. The tissue sections were placed in a humidification chamber at 50°C for 16 to 18 h to hybridize with the labeled SIV probe. The unhybridized SIV RNA probe was removed by washing the slides at 50°C in the wash buffer containing 50% formamide and 2× SSC, followed by 1× SSC and 0.5× SSC. The slides were reacted with RNase A for 30 min to hydrolyze the remaining unhybridized SIV RNA probe. The tissue sections were incubated for 1 h with a monoclonal antibody to digoxigenin conjugated to alkaline phosphatase, and SIV-infected cells were visualized with nitroblue tetrazolium–X-Phos (5-bromo-4-chloro-3-indolylphosphate toluidinium salt [BCIP]). The cells were detected by counterstaining with hematoxylin. The positive controls included previously characterized SIV-infected intestinal tissues. The negative controls included intestinal tissues and peripheral blood mononuclear cells (PBMC) from uninfected healthy animals. The slides were examined with a microscope for the presence of SIV-positive cells.
Isolation of IEL.
IEL were isolated by previously published procedures with minor modifications (11, 21, 53, 64). Briefly, jejunum was cut into small pieces (0.5 cm2) and rinsed in cold phosphate-buffered saline (PBS). The tissue samples were placed into IEL isolation medium (Hanks balanced salt solution [HBSS] supplemented with 0.75 mM EDTA [Sigma], 100 U of penicillin [Gibco] per ml, 100 U of streptomycin [Gibco] per ml, and 5% fetal calf serum [FCS; Gibco]) at pH 7.2 and subjected to rapid shaking at 37°C for 30 min. This procedure was repeated three times. Liberated cells were centrifuged and washed with complete RPMI 1640 (Gibco) supplemented with 100 U of penicillin per ml, 100 U of streptomycin per ml, and 10% FCS. Lymphocytes were isolated through a 35%-60% (vol/vol) isotonic discontinuous Percoll (Sigma) density gradient. Lymphocytes were found to band at the interface between the 35% and 60% gradients. Isolated lymphocytes were washed in HBSS, and viability was assessed by trypan blue exclusion.
Antibodies.
Monoclonal antibodies (MAb) to CD3 (Biosource International, Camarillo, Calif.), CD4 (OKT4, Ortho Diagnostic Systems, Inc., Raritan, N.J.), CD8 (Caltag Laboratories, South San Francisco, Calif.), Vδ1 (Endogen, Woburn, Calif.), MIP-1β (Molecular Probes, Inc., Eugene, Oreg.), and IFN-γ (Pharmingen, San Diego, Calif.) were used in this study. Matched isotype controls were obtained from Caltag Laboratories.
Immunophenotyping of IEL.
Isolated cryopreserved IEL (2 × 106 cells) from jejunum were stained with biotinylated anti-CD3 followed by streptavidin conjugated to Tricolor (TC) for 30 min at 4°C. After being washed in PBS, the cells were stained with anti-CD4 conjugated to phycoerythrin (PE) and anti-CD8 conjugated to fluorescein isothiocyanate (FITC) for 30 min at 4°C, washed with PBS, and suspended in PBS for analysis. T cells positive for gamma delta (γδ+) T-cell receptor were detected with MAb to Vδ1. Negative-control samples were stained with matched isotype control antibodies.
Flow cytometric detection of intracellular IFN-γ and MIP-1β production.
Intracellular IFN-γ and MIP-1β production was detected in IEL following a short-term stimulation with phorbol myristate acetate (PMA) and ionomycin at a single cell level. Monensin was used to disaggregate the Golgi complex to arrest the proteins from being transported. Isolated cryopreserved IEL were incubated overnight at 37°C in humidified atmosphere of 5% CO2 at a concentration of 2 × 106 cells/ml in complete RPMI 1640 supplemented with 2 mM l-glutamine, 100 U of penicillin per ml, 100 U of streptomycin per ml, and 10% fetal bovine serum (FBS). Four hours before harvesting, the cells were stimulated with 10 ng of PMA (Sigma) per ml, 500 ng of ionomycin (Calbiochem, La Jolla, Calif.) per ml, and 2 μM monensin (Sigma). The cells were incubated with 2 μM monensin only to determine whether they were producing MIP-1β and IFN-γ in the absence of stimulation. After incubation, the cells were harvested and washed in cytoflow buffer (PBS with 1% BSA) and prepared for reacting with fluorescent antibody.
To determine the capacity of IEL to produce IFN-γ and MIP-1β, IEL were subjected to flow cytometric analysis according to methods previously described (14). Briefly, 4 × 106 cells were reacted with either MAb to Vδ1 conjugated to FITC or biotinylated MAb to CD3 followed by streptavidin conjugated to FITC or PE and were incubated for 30 min at 4°C. The cells were washed with PBS and reacted with CD8 MAb conjugated to TC. Negative controls were stained with matched isotype control MAb. After being washed in PBS, the cells were fixed with 100 μl of solution A (Cell Perm & Fix Kit; Caltag) for 15 min at room temperature in the dark, washed, and reacted with either FITC-conjugated anti-human IFN-γ or PE-conjugated anti-human MIP-1β MAb resuspended in 50 μl of permeabilizing solution B (Cell Perm & Fix Kit) for 15 min. Negative controls included samples reacted with matched isotype control MAb suspended in 50 μl of permeabilizing solution B. After being washed, the cells were resuspended in PBS and prepared for analysis. The cells were also fixed and reacted with anti-IFN-γ, anti-MIP-1β, and matched isotype control MAb without permeabilizing to ensure that only intracellular proteins were being reacted.
The cells were analyzed with a FACScan flow cytometer (Becton Dickinson, Mountainview, Calif.). Ten thousand events were collected in list mode after simultaneously gating on lymphocytes based upon their forward- and light-scatter characteristics and FL3 (CD3, CD8, or Vδ1) and forward scatter. Collected data were analyzed with the Cell Quest software (Becton Dickinson).
Measurement of SIV-specific CTL activity.
Cytotoxic assays were performed according to methods described previously (29). CTL activity was measured with primary IEL cultured overnight in RPMI 1640 medium supplemented with 10% FCS, antibiotics, and 5% human lymphocyte-conditioned medium (human IL-2; Schiaparelli Biosystems, Columbia, Md.) and 20 U of recombinant human IL-2 (donated by Cetus Corp., Emeryville, Calif.) per ml. Autologous B lymphocytes were transformed by the herpesvirus papio (594S × 1055) producer cell line (provided by M. Sharp, Southwest Foundation for Biomedical Research, San Antonio, Tex.) and infected overnight with wild-type vaccinia virus or recombinant vaccinia virus expressing the SIV major core protein (p55gag) or envelope glycoprotein (gp160env) of SIVmac239 (provided by L. Giavedoni and T. Yilma, University of California, Davis, Calif.) and then labeled with 50 μCi of 51chromium (Na2CrO4; Amersham, Arlington Heights, Ill.) per 106 cells. Effector and target (E and T) cells were added together at multiple E/T ratios in a 4-h chromium release assay, and percent specific lysis values were calculated from supernatant chromium measured in a liquid scintillation counter (Micro-Beta 1450; Wallac Biosystems, Gaithersberg, Md.). Specific lysis was considered positive when it was greater than twofold (± 3 standard deviations) of the lysis of wild-type vaccinia virus targets and when it was at least 10%.
RESULTS
Detection of SIV-infected IEL in jejunal mucosae.
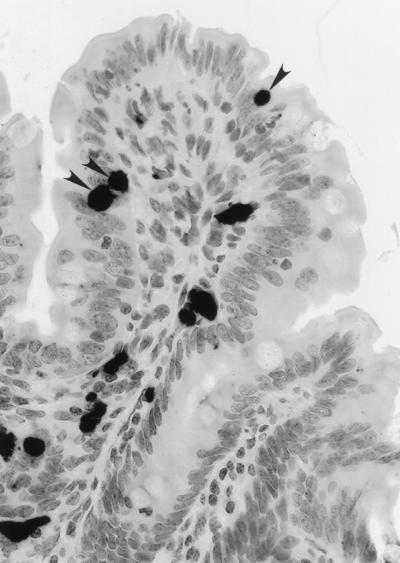
Dissemination and localization of SIV-infected cells in intestinal mucosae of SIV-infected animals were determined by in situ hybridization. The virus-positive cells were detectable through the entire course of SIV infection, but the numbers were variable. Higher numbers of virally infected cells were observed during the primary-acute and terminal stages of infection compared to the asymptomatic stage (data not shown). The SIV-positive cells were found in lamina propria, Peyer’s patches, and lymphoid aggregates. A higher number of IEL strongly positive for SIV nucleic acids were readily detected during the primary-acute (Fig. 1) and terminal stages of SIV infection. In asymptomatic animals, fewer SIV-infected IEL were detected. In terminally ill animals, SIV-infected cells were found to be more widely disseminated in the intestinal mucosae compared to the animals in primary-acute or asymptomatic stages.
FIG. 1.
The presence of SIV-infected IEL in the jejunum of an SIV-infected rhesus macaque. SIV-infected cells were detected by in situ hybridization with digoxigenin-labeled RNA probes during the primary acute stage of infection. Arrows indicate SIV-positive IEL.
Severe depletion of CD4+ IEL occurred in primary SIV infection and persisted till the terminal stage of infection.
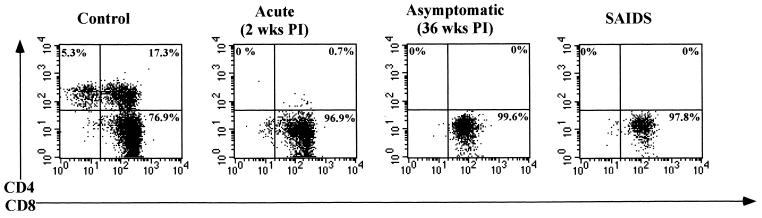
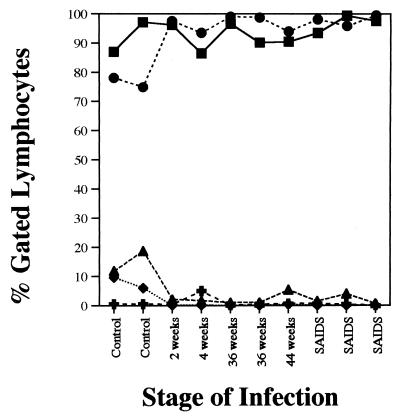
Alterations in immunophenotypic profiles of isolated IEL from SIV-infected jejunal tissues were examined by flow cytometric analysis and compared with the uninfected controls (Fig. 2). The IEL were predominantly CD3+ T cells in both uninfected and SIV-infected animals (∼86 to 98%). In uninfected animals, ∼74 to 78% of the CD3+ IEL expressed the CD8+ phenotype, whereas ∼12 to 19% expressed the double-positive (CD4+CD8+) phenotype and ∼6 to 10% expressed the CD4+ phenotype. Fewer than 1% of CD3+ IEL expressed the double-negative (CD4−CD8−) phenotype. The proportion of CD3− cells as a percentage of gated lymphocytes was found to range from 3 to 13%, of which CD3−CD8+ cells were less than 2%. The remaining CD3− cell population may harbor non-B–non-T cells or null cells. Dramatic alterations in the phenotypic profiles of IEL were detected following SIV infection (Fig. 3). The proportions of CD3+ IEL which were CD4+ single-positive (∼6 to 10%) and CD4+CD8+ double-positive (∼12 to 19%) T cells declined precipitously as early as 2 weeks p.i. (<0.5%) and remained low throughout the course of SIV infection. In contrast, the proportion of CD3+CD8+ T cells increased sharply at 2 weeks from ∼74 to 78% to ∼98% and remained high throughout the course of infection. No major changes were observed in the proportion of CD3+ IEL following SIV infection relative to uninfected animals. The γδ TCR+ cells constituted a minor subpopulation (5% or lower) of IEL in both SIV-infected and uninfected animals.
FIG. 2.
Flow cytometric analysis of CD3+ IEL from jejunal tissues from uninfected rhesus macaques and from the primary-acute (2 weeks p.i.), aymptomatic (36 weeks p.i.), and SAIDS phases of SIV-infected rhesus macaques. The IEL were stained for cell surface expression of CD3, CD4, and CD8 antigens and analyzed by flow cytometry. Negative-control samples were stained with matched isotype control antibodies.
FIG. 3.
SIV infection leads to a severe depletion of CD3+CD4+ and CD3+CD4+CD8+ IEL and an increase in the proportion of CD3+CD8+ IEL, without a major change in the proportions of CD3+ IEL. Isolated IEL were stained for cell surface expression of CD3, CD4, and CD8 antigens and were analyzed by flow cytometry. Negative-control samples were stained with matched isotype control antibodies. CD3+ (▪), CD3+CD4+ (⧫), CD3+CD8+ (•), CD3+CD4+CD8+ (▴), and CD3+CD4−CD8− (✚) IEL are indicated as percentages of gated lymphocytes.
The severe depletion of CD4+ IEL in primary SIV infection was not reflected in peripheral blood.
In contrast to the severe depletion of CD4+ IEL, the proportion of CD4+ T cells in peripheral blood was found to decline gradually at 2 weeks p.i. (∼34%) and at 4 weeks p.i. (∼31%) compared to uninfected controls (∼47%). The proportion of CD8+ T cells increased from ∼23% in uninfected animals to ∼45% at 2 weeks p.i. and ∼38% at 4 weeks p.i. No apparent changes were observed in the proportions of CD4+CD8+ T cells in peripheral blood following SIV infection relative to uninfected controls.
Intestinal CD8+ IEL retain a high capacity to produce IFN-γ through the entire course of SIV infection.
In order to determine the capacity of CD8+ or CD8− IEL to produce IFN-γ during the course of SIV infection, CD3+ IEL from SIV-infected animals were analyzed at a single-cell level following short-term mitogenic stimulation and compared with uninfected controls.
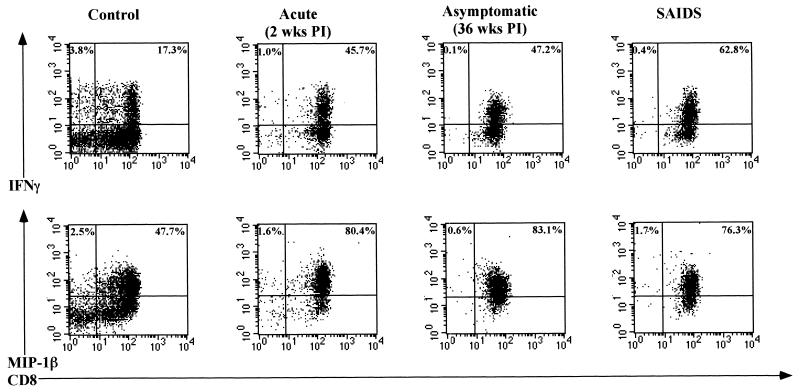
The CD8+ IEL were the primary producers of IFN-γ in uninfected and SIV-infected animals (Fig. 4). Following in vitro mitogenic stimulation, the proportion of CD8+ IEL producing IFN-γ was found to increase at 2 weeks p.i. (∼42%) and then to decline at 4 weeks p.i. (∼20%) relative to uninfected animals (∼12 to 21%). The proportion of CD8+ IEL producing IFN-γ was variable and ranged from ∼6 to 42% during the asymptomatic stage of infection. A higher proportion of CD8+ IEL (∼42 to 64%) produced IFN-γ during the SAIDS stage of infection, indicating that many CD8+ IEL were primed in vivo during the SAIDS stage of infection. In uninfected animals, ∼3% of CD3+CD8− IEL (consisting of the CD4+ and γδ+ subsets) produced IFN-γ. The proportion of CD3+CD8− IEL capable of producing IFN-γ decreased considerably following SIV infection (<1%), which could be attributed to the depletion of CD4+ T cells in SIV infection. The proportion of CD8+CD3− IEL (presumably NK cells) producing IFN-γ was found to be negligible (∼0 to 0.5%) throughout the course of infection. No IFN-γ production was detected in IEL in the absence of mitogenic stimulation.
FIG. 4.
IEL display an increased capacity to produce IFN-γ and MIP-1β following SIV infection. The capacity of CD3+CD8+ and CD3+CD8− IEL to produce IFN-γ and MIP-1β following short-term in vitro stimulation with PMA and ionomycin was determined by flow cytometry. IEL were stained for cell surface expression of CD3 and CD8 antigens, fixed, permeabilized, and stained for intracellular production of IFN-γ and MIP-1β. Negative-control samples were stained with matched isotype control antibodies.
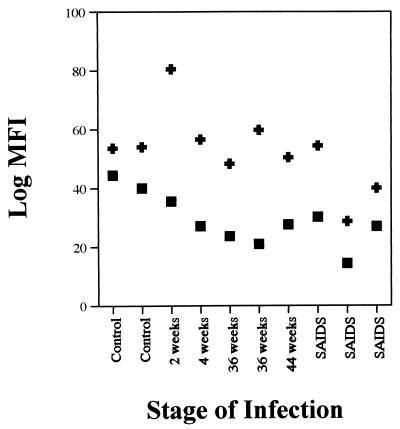
The amount of IFN-γ produced per cell (intensity) was determined and is presented as log mean fluorescence intensity in Fig. 5. The intensity of IFN-γ expression in IEL was found to decline following SIV infection as early as 2 weeks p.i. and continued to remain low throughout the course of infection relative to uninfected animals.
FIG. 5.
Amount of IFN-γ produced per cell (intensity) decreased following SIV infection without any change in the intensity of MIP-1β expression. Log mean fluorescence intensities (log MFI) of IFN-γ (▪) and MIP-1β (✚) expression by CD3+CD8+ IEL from uninfected and SIV-infected animals are shown. The capacity of CD3+ IEL to produce IFN-γ and MIP-1β following short-term in vitro stimulation was determined by using IEL from uninfected and SIV-infected rhesus macaques at the primary-acute (2 and 4 weeks p.i.), asymptomatic (36 to 44 weeks p.i.), and SAIDS phases. The IEL were stained for cell surface expression of CD3 and CD8 antigens, fixed, permeabilized, and stained for intracellular production of IFN-γ and MIP-1β. Negative-control samples were stained with matched isotype control antibodies.
Intestinal CD8+ IEL retain a high capacity to produce MIP-1β throughout the entire course of SIV infection.
To examine the capacity of CD8+ and CD8− IEL to produce MIP-1β following short-term mitogenic stimulation, CD3+ IEL from SIV-infected animals were examined at the single-cell level and were compared with uninfected controls. The ability of IEL to constitutively produce MIP-1β was determined with IEL from uninfected and SIV-infected animals in the absence of mitogenic stimulation.
The CD8+ IEL (in the absence of mitogenic stimulation) were the primary producers of MIP-1β. The frequency of CD8+ IEL producing MIP-1β was found to decline early in SIV infection at 2 weeks to ∼16% compared to uninfected animals (∼25 to 31%) and remained low throughout the course of infction (data not shown). This suggested that SIV infection suppressed the ability of CD8+ IEL to produce MIP-1β in vivo.
In mitogen-activated IEL, the CD3+CD8+ cells were the primary producers of MIP-1β in both uninfected and SIV-infected animals (Fig. 4). In contrast to the decline in constitutive expression of MIP-1β by unstimulated IEL, the frequency of short-term mitogen-stimulated CD8+ IEL producing MIP-1β increased considerably in SIV-infected animals. This increase was detectable as early as 2 weeks p.i. (∼80%) and remained high during the asymptomatic (∼77 to 94%) and SAIDS (∼75 to 83%) stages of infection compared to that of uninfected animals. This would indicate that a higher proportion of CD8+ IEL from SIV-infected animals was primed in vivo to produce MIP-1β. A minor proportion of CD3+CD8− IEL were found to make MIP-1β in SIV-negative animals (∼3.2 to 7.9%), which declined at 2 weeks p.i. (∼2 to 3.6%) and remained low (Fig. 5) throughout the course of infection (<1.8%). The proportions of CD8+CD3− IEL producing MIP-1β were found to be negligible (∼0 to 1.7%) throughout the entire course of SIV infection. No major differences were noticed in the amount of MIP-1β expression per cell in SIV-infected animals relative to uninfected animals (Fig. 5).
SIV-specific CTL activity was detected in IEL during primary SIV infection.
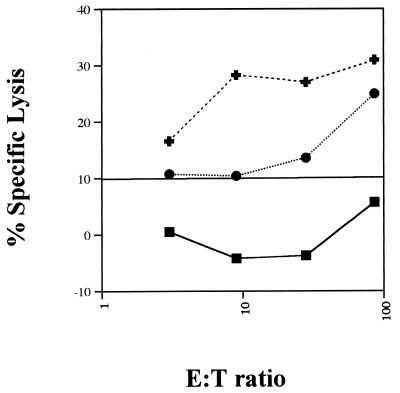
The IEL from macaques at 1, 2, and 4 weeks post-SIV infection were examined for the presence of SIV gag- and env-specific CTL activity in primary cultures, which was presented as percent specific lysis. SIV-specific CTL activity against env-expressing target cells at 1 and 2 weeks p.i. (data not shown) was found, whereas at 4 weeks p.i. CTL activity against both gag- and env-expressing target cells was found (Fig. 6). With IEL at 1 and 2 weeks p.i., significant target cell lysis was observed at E:T ratios of 9:1 and 3:1, respectively, against SIV env-expressing target cells. At 4 weeks p.i., significant target cell lysis was observed at an E:T ratio of 28:1 against SIV gag-expressing target cells and at an E:T ratio of 3:1 against SIV env-expressing target cells.
FIG. 6.
SIV-specific cytotoxic activities against SIV gag- and env-expressing target cells were detected in the primary IEL. Primary IEL from a rhesus macaque at 4 weeks p.i. were cultured overnight in RPMI 1640 medium supplemented with 10% FCS, antibiotics, and 5% human lymphocyte-conditioned medium and 20 U of recombinant human IL-2 per ml. Autologous B lymphocytes were transformed by herpesvirus papio and infected overnight with wild-type vaccinia virus (vvWR) or recombinant vaccinia virus expressing the SIV major core protein, p55gag (vvgag), or envelope glycoprotein, gp160env (vvenv), of SIVmac239. Effector and target cells were added together at multiple E:T ratios in a 4-h chromium release assay, and percent specific lysis against WR (▪), gag (•)-, and env (✚)-expressing target cells was calculated.
DISCUSSION
Our studies have demonstrated that IEL were infected early in SIV infection and that their phenotypic profile and function were significantly altered following SIV infection. A higher number of SIV-positive cells were detected during the primary-acute and terminal stages of SIV infection compared to the asymptomatic stage of SIV infection. These IEL were strongly positive for SIV nucleic acids, indicating that they could support active viral replication (Fig. 1). The presence of SIV-infected IEL may have several implications. Due to their intimate contact with epithelial cells, it is possible that epithelial cells acquire viral genomes from infected IEL. In in vitro culture studies, horizontal transmission of HIV to epithelial cells from infected lymphocytes has been previously demonstrated (54). Infected IEL may release virus into the lumen or lamina propria and spread infection to available susceptible targets such as macrophages and activated T cells. Many macrophages have been found in the vicinity of the tip of villi in the small intestine (20). Furthermore, viral infection may cause functional defects in the IEL.
Alterations in the immunophenotypic profiles of jejunal IEL occurred during primary SIV infection. A dramatic decline in the proportion of the CD3+CD4+ single-positive and CD3+CD4+CD8+ double-positive T-cell subsets which was detectable at 2 weeks p.i. occurred (Fig. 2). The depletion of these subsets was consistent throughout the asymptomatic and terminal stages of infection. An increase in the proportion of CD3+CD8+ T cells coincided with the CD4+ T-cell depletion. Schieferdecker et al. (43) reported that the majority of the intestinal T cells are memory T cells and are in an activated state. Furthermore, studies have shown that the activation of CD4+ T cells is important for HIV infection and replication (39, 42). Hence, a higher affinity for activated T cells in the intestinal mucosae may explain the rapid decline in the proportions of CD4+ single-positive and CD4+CD8+ double-positive T cells in the jejunal IEL compartment. Studies of the duodenal mucosae of long-term HIV-infected patients have shown loss of CD4+ T cells (28) which was more pronounced in the mucosae than in the peripheral blood (44). Heise et al. (19) detected T-cell depletion in the intestinal mucosae of SIV-infected rhesus macaques, and Veazey et al. (59) have shown that infection with a molecularly cloned virus, SIVmac239, resulted in CD4+ T-cell depletion within the intestinal lamina propria lymphocytes. However, these investigators did not present any data on the immunophenotypic alterations in IEL following SIV infection. The role of CD4+ IEL depletion in intestinal dysfunction and viral pathogenesis is not fully known, but the loss of this subset may have several implications. The loss of CD4+ IEL could lead to disruption of the epithelial barrier and decreased nutrient absorptive functions. Since the various subsets of T cells interact to maintain immune homeostasis at the intestinal epithelium, a severe depletion of CD4+ and CD4+CD8+ IEL may result in altered homeostasis in epithelia and have an adverse effect on the integrity of the intestinal epithelium. The CD4+ IEL may provide unknown growth factors for epithelial cell maturation, and the loss of these cells may contribute to partial villous atrophy and crypt hyperplasia. The functions of the CD3+CD4+CD8+ double-positive T-cell subset in the intestine are not fully defined. Therefore, it is difficult to speculate on the functional significance of the depletion of these T cells in HIV and SIV infection. However, normal CD4+CD8+ double-positive T cells have been shown to express activation antigens (3) and the memory phenotype (65), which may explain their preferential depletion in the intestinal mucosae following SIV infection. The depletion of these subsets of IEL will further impair the ability of intestinal lymphoid tissue to respond to pathogens. The CD4+ T-cell depletion in peripheral blood was found to be gradual following SIV infection, which was in contrast to the severe CD4+ IEL depletion during primary SIV infection. Previous studies have shown that the depletion of CD4+ T cells in peripheral blood was gradual in both SIV and HIV infections (24, 27, 37, 61) and became more apparent in advanced stages of infection. The above findings demonstrated that peripheral blood did not adequately reflect the CD4+ T-cell changes in the intestinal mucosae. Therefore, it will be important to examine T-cell dynamics in the intestinal mucosae during viral infection independent of peripheral blood.
Our results demonstrated that the onset of CD8+ T-cell increase in IEL occurred in primary SIV infection and was coincident with CD4+ T-cell depletion. An increased prevalence of CD8+ T cells in the intestinal mucosae of long-term HIV-infected patients has been previously reported (45). Veazey et al. (59) have shown that CD4+ T-cell depletion within the intestinal lamina propria lymphocytes was accompanied by an increase in the prevalence of CD8+ T cells. The majority of IEL from human and mouse intestines were found to be activated and positive for the cytotoxic phenotype (22, 26, 32, 51). Thus, they may be playing an important role in the recognition of virally infected cells. The presence of SIV-specific cytotoxic activity in the small intestinal IEL from SIV-infected rhesus macaques with terminal disease has been reported elsewhere (10). The activated CD8+ T cells with CTL activity may play an important role in the control of infection. On the other hand, these cells may also cause damage to the intestinal epithelium by secreting cytokines which contribute to the immunopathogenesis of SIV/HIV, as observed in HIV-associated alveolitis (1).
Immunophenotypic alterations in the IEL of SIV-infected animals could have an impact on the local cytokine network which may modify the immune homeostasis at the intestinal epithelium. The CD8+ IEL were the primary producers of IFN-γ following SIV infection (Fig. 4). The frequency of CD8+ IEL producing IFN-γ was found to increase during the SAIDS stage of infection, indicating that a higher proportion of this subset were primed in vivo following SIV infection to secrete IFN-γ. Although the frequency of IFN-γ-producing cells increased, the intensity of expression (amount of IFN-γ produced per cell) was found to decrease following SIV infection (Fig. 5) and remained low throughout the course of infection, indicating a functional dysregulation in these subsets. This decrease in the level of IFN-γ expression may have implications for downregulating cellular immune functions, thereby contributing to pathogenesis, since IFN-γ has been shown to play an important role in antiviral immunity by lysis of infected cells by CTL activity (38, 62). On the other hand, an increase in the proportion of IFN-γ-producing cells may indicate an abnormal production of IFN-γ which may directly alter the integrity of the intestinal epithelium by increasing the permeability of epithelial cell-cell tight junctions (35). Furthermore, IFN-γ has been reported to have a potentiating effect on macrophages and neutrophils and to stimulate the synthesis and release of reactive oxygen metabolites (2, 57), which may lead to the development of inflammatory conditions in the intestinal mucosae. There is no information available on cytokine expression in IEL at a single-cell level in HIV infection. However, a few studies have reported cytokine production in PBMC by flow cytometric analysis (23, 36, 52, 60). Using flow cytometric analysis, Klein et al. (23) have shown that the proportion of IFN-γ-producing T cells decreased among the CD4+ T cells in peripheral blood from long-term HIV-infected patients, whereas the proportion of CD8+ T cells producing IFN-γ strongly increased. An attempt to delineate the potential of γδ TCR+ subset of IEL to produce IFN-γ was not very successful, since only a few events could be gated to arrive at a definitive conclusion. Taguchi et al. (52) have shown that both γδ and αβ TCR+ murine IEL were capable of producing cytokines such as IFN-γ and IL-5. Bouillier et al. (5) demonstrated at a single-cell level that Vδ1 T cells in peripheral blood from HIV-infected patients were primed to produce IFN-γ and TNF-α compared to control donors, and these T-cell subsets were found to express high levels of perforin and to display in vitro cytotoxic activity. Furthermore, γδ T cells in the peripheral blood of HIV-infected patients were found to rapidly expand and produce IFN-γ in response to nonpeptide antigens relative to HIV-uninfected subjects (15). Based on the above studies, it is suggested that γδ IEL may have a potentially functional role in SIV infection and may have the ability to produce IFN-γ following infection.
The majority of the MIP-1β-producing IEL were found to express the CD3+CD8+ phenotype in uninfected and SIV-infected animals (Fig. 4). It was interesting to note that almost ∼25 to 30% of the isolated CD8+ IEL from uninfected controls had constitutive levels of intracellular MIP-1β in the absence of mitogenic stimulation (data not shown). However, the level of constitutive MIP-1β expression decreased in SIV-infected animals, which was evident as early as 2 weeks p.i., indicating that viral infection might have led to a downmodulation of MIP-1β expression by IEL in the jejunal mucosae (data not shown). MIP-1β along with other chemokines such as RANTES and MIP-1α has been shown to play an important role in controlling HIV infection (9). Thus, a downregulation of constitutive MIP-1β production by IEL in primary SIV infection may have significant implications for the progression of SIV infection, since this may lead to an increasing viral burden in gastrointestinal tissues. In contrast to these observations, short-term mitogen-stimulated CD8+ IEL from SIV-infected animals were found to exhibit a dramatically high potential to produce MIP-1β, indicating that these subsets retained a high potential to produce MIP-1β following activation. There were no major differences in the intensity of MIP-1β expression per individual cell in SIV-infected animals compared to uninfected animals. Thus, the increase was limited to the frequency of MIP-1β-producing IEL and not to the amount of MIP-1β per cell. MIP-1β has been shown to chemoattract and activate specific subsets of T cells and macrophages and to stimulate the secretion of other proinflammatory cytokines such as TNF-α, IL-1, and IL-6 (13) in murine peritoneal macrophages. High levels of circulating TNF-α have been demonstrated in serum from patients with AIDS (25, 41) and in rhesus macaques infected with SIVmac251 (8). These proinflammatory cytokines may stimulate the production of various inflammatory mediators by macrophages and neutrophils that can potentially damage the intestinal mucosae, leading to symptoms of nutrient malabsorbtion, chronic diarrhea, dehydration, and wasting that is frequently observed in SIV-infected animals (18, 50) and increase the susceptibility to opportunistic pathogens associated with AIDS.
Cytotoxic T cells have been shown to be central to the development of immune responses to viral infections (6, 30, 40, 63). SIV-specific CTL activity was previously detected in IEL from the small intestine and vaginal epithelium from long-term SIV-infected animals (10, 29). Our results for the first time have detected SIV-specific CTL activity in IEL during primary SIV infection. The CTL activity was detected in the jejunal IEL at 1 and 2 weeks p.i. against env-expressing target cells (data not shown) and at 4 weeks p.i. against both gag- and env-expressing target cells (Fig. 6). The presence of SIV-infected cells in the intestine during early stages of infection may contribute to the recruitment or development of SIV-specific CTL responses. In our studies, the majority of jejunal IEL (>90%) were shown to be of the CD8+ phenotype in SIV-infected animals (Fig. 3), indicating that CD8+ IEL may be the primary source of SIV-specific CTL activity. The proportions of CD8+CD3− (which presumably comprises NK cells) were found to decline following SIV infection, suggesting that these subsets of CD8+ IEL may not be responsible for CTL-mediated lysis of target cells. It is possible that some γδ subsets of IEL may exhibit SIV-specific CTL activity. However, further experiments need to be performed with γδ subsets to determine their potential for SIV-specific CTL activity.
In conclusion, the role of IEL in the immunopathogenesis of SIV at gastrointestinal mucosal surfaces was evaluated. For the first time, we have identified and delineated changes in the immunophenotype of IEL and their capacity to produce IFN-γ and MIP-1β following SIV infection. Severe CD4+ IEL depletion was observed in primary SIV infection, which may lead to altered homeostasis in the intestinal epithelia. Our results demonstrated that SIV-specific CTL activity was observed in the jejunal IEL in primary SIV infection. An increased capacity of CD8+ IEL to produce IFN-γ and MIP-1β was observed throughout the course of SIV infection. This increased capacity of CD8+ IEL to produce IFN-γ and MIP-1β and the presence of CTL activity may reflect an active role for IEL in the generation and maintenance of immune responses to viral infection as well as opportunistic infections. However, altered homeostasis due to phenotypic changes in the jejunal IEL accompanied by increased cytokine and chemokine production may contribute to SIV-associated enteropathy.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (R01-DK43183 and RR-00169) and the Universitywide AIDS Research Program, University of California.
We thank Chris Miller, Linda Antipa, Ross Tarara, and Don Canfield at the California Regional Primate Research Center for their valuable assistance in this project. We are grateful to Jeanette Rheinhardt for her invaluable expertise in performing in situ hybridization analysis.
REFERENCES
- 1.Autran B, Mayaud C M, Raphael M, Plata F, Denis M, Bourguin A, Guillon J M, Debre P, Akoun G. Evidence for a cytotoxic T-lymphocyte alveolitis in human immunodeficiency virus-infected patients. AIDS. 1988;2:179–183. [PubMed] [Google Scholar]
- 2.Bielefeldt Ohmann H, Babiuk L A. In vitro generation of hydrogen peroxide and of superoxide anion by bovine polymorphonuclear neutrophilic granulocytes, blood monocytes, and alveolar macrophages. Inflammation. 1984;8:251–275. doi: 10.1007/BF00916415. [DOI] [PubMed] [Google Scholar]
- 3.Blue M L, Daley J F, Levine H, Schlossman S F. Coexpression of T4 and T8 on peripheral blood T cells demonstrated by two-color fluorescence flow cytometry. J Immunol. 1985;134:2281–2286. [PubMed] [Google Scholar]
- 4.Boismenu R, Havran W L. Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science. 1994;266:1253–1255. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- 5.Boullier S, Dadaglio G, Lafeuillade A, Debord T, Gougeon M L. V delta 1 T cells expanded in the blood throughout HIV infection display a cytotoxic activity and are primed for TNF-alpha and IFN-gamma production but are not selected in lymph nodes. J Immunol. 1997;159:3629–3637. [PubMed] [Google Scholar]
- 6.Byrne J A, Oldstone M B. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus: clearance of virus in vivo. J Virol. 1984;51:682–686. doi: 10.1128/jvi.51.3.682-686.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carman P S, Ernst P B, Rosenthal K L, Clark D A, Befus A D, Bienenstock J. Intraepithelial leukocytes contain a unique subpopulation of NK-like cytotoxic cells active in the defense of gut epithelium to enteric murine coronavirus. J Immunol. 1986;136:1548–1553. [PubMed] [Google Scholar]
- 8.Clayette P, Le G R, Noack O, Vaslin B, Le N R, Benveniste O, Theodoro F, Fretier P, Dormont D. Tumor necrosis factor-alpha in serum of macaques during SIVmac251 acute infection. J Med Primatol. 1995;24:94–100. doi: 10.1111/j.1600-0684.1995.tb00152.x. [DOI] [PubMed] [Google Scholar]
- 9.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 10.Couedel C A, Le G R, Tulliez M, Guillet J G, Venet A. Direct ex vivo simian immunodeficiency virus (SIV)-specific cytotoxic activity detected from small intestine intraepithelial lymphocytes of SIV-infected macaques at an advanced stage of infection. J Virol. 1997;71:1052–1057. doi: 10.1128/jvi.71.2.1052-1057.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies M D, Parrott D M. Preparation and purification of lymphocytes from the epithelium and lamina propria of murine small intestine. Gut. 1981;22:481–488. doi: 10.1136/gut.22.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dillon S B, Dalton B J, MacDonald T T. Lymphokine production by mitogen and antigen activated mouse intraepithelial lymphocytes. Cell Immunol. 1986;103:326–338. doi: 10.1016/0008-8749(86)90093-6. [DOI] [PubMed] [Google Scholar]
- 13.Fahey T D, Tracey K J, Tekamp O P, Cousens L S, Jones W G, Shires G T, Cerami A, Sherry B. Macrophage inflammatory protein 1 modulates macrophage function. J Immunol. 1992;148:2764–2769. [PubMed] [Google Scholar]
- 14.Ferrick D A, Schrenzel M D, Mulvania T, Hsieh B, Ferlin W G, Lepper H. Differential production of interferon-gamma and interleukin-4 in response to Th1- and Th2-stimulating pathogens by gamma delta T cells in vivo. Nature. 1995;373:255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 15.Garcia V E, Sieling P A, Gong J, Barnes P F, Uyemura K, Tanaka Y, Bloom B R, Morita C T, Modlin R L. Single-cell cytokine analysis of gamma delta T cell responses to nonpeptide mycobacterial antigens. J Immunol. 1997;159:1328–1335. [PubMed] [Google Scholar]
- 16.Heinzel F P, Sadick M D, Holaday B J, Coffman R L, Locksley R M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heise C, Dandekar S, Kumar P, Duplantier R, Donovan R M, Halsted C H. Human immunodeficiency virus infection of enterocytes and mononuclear cells in human jejunal mucosa. Gastroenterology. 1991;100:1521–1527. doi: 10.1016/0016-5085(91)90648-5. [DOI] [PubMed] [Google Scholar]
- 18.Heise C, Miller C J, Lackner A, Dandekar S. Primary acute simian immunodeficiency virus infection of intestinal lymphoid tissue is associated with gastrointestinal dysfunction. J Infect Dis. 1994;169:1116–1120. doi: 10.1093/infdis/169.5.1116. [DOI] [PubMed] [Google Scholar]
- 19.Heise C, Vogel P, Miller C J, Halsted C H, Dandekar S. Simian immunodeficiency virus infection of the gastrointestinal tract of rhesus macaques. Functional, pathological, and morphological changes. Am J Pathol. 1993;142:1759–1771. [PMC free article] [PubMed] [Google Scholar]
- 20.Heise C, Vogel P, Miller C J, Lackner A, Dandekar S. Distribution of SIV infection in the gastrointestinal tract of rhesus macaques at early and terminal stages of AIDS. J Med Primatol. 1993;22:187–193. [PubMed] [Google Scholar]
- 21.James S P, Graeff A S, Zeitz M. Predominance of helper-inducer T cells in mesenteric lymph nodes and intestinal lamina propria of normal nonhuman primates. Cell Immunol. 1987;107:372–383. doi: 10.1016/0008-8749(87)90245-0. [DOI] [PubMed] [Google Scholar]
- 22.Kawaguchi M, Nanno M, Umesaki Y, Matsumoto S, Okada Y, Cai Z, Shimamura T, Matsuoka Y, Ohwaki M, Ishikawa H. Cytolytic activity of intestinal intraepithelial lymphocytes in germ-free mice is strain dependent and determined by T cells expressing gamma delta T-cell antigen receptors. Proc Natl Acad Sci USA. 1993;90:8591–8594. doi: 10.1073/pnas.90.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein S A, Dobmeyer J M, Dobmeyer T S, Pape M, Ottmann O G, Helm E B, Hoelzer D, Rossol R. Demonstration of the Th1 to Th2 cytokine shift during the course of HIV-1 infection using cytoplasmic cytokine detection on single cell level by flow cytometry. AIDS. 1997;11:1111–1118. doi: 10.1097/00002030-199709000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Kneitz C, Kerkau T, Muller J, Coulibaly C, Stahl-Hennig C, Hunsmann G, Hunig T, Schimpl A. Early phenotypic and functional alterations in lymphocytes from simian immunodeficiency virus infected macaques. Vet Immunol Immunopathol. 1993;36:239–255. doi: 10.1016/0165-2427(93)90022-v. [DOI] [PubMed] [Google Scholar]
- 25.Lahdevirta J, Maury C P, Teppo A M, Repo H. Elevated levels of circulating cachectin/tumor necrosis factor in patients with acquired immunodeficiency syndrome. Am J Med. 1988;85:289–291. doi: 10.1016/0002-9343(88)90576-1. [DOI] [PubMed] [Google Scholar]
- 26.Lefrancois L, Goodman T. In vivo modulation of cytolytic activity and Thy-1 expression in TCR-gamma delta+ intraepithelial lymphocytes. Science. 1989;243:1716–1718. doi: 10.1126/science.2564701. [DOI] [PubMed] [Google Scholar]
- 27.Letvin N L, King N W. Immunologic and pathologic manifestations of the infection of rhesus monkeys with simian immunodeficiency virus of macaques. J Acquired Immune Defic Syndr. 1990;3:1023–1040. [PubMed] [Google Scholar]
- 28.Lim S G, Condez A, Lee C A, Johnson M A, Elia C, Poulter L W. Loss of mucosal CD4 lymphocytes is an early feature of HIV infection. Clin Exp Immunol. 1993;92:448–454. doi: 10.1111/j.1365-2249.1993.tb03419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lohman B L, Miller C J, McChesney M B. Antiviral cytotoxic T lymphocytes in vaginal mucosa of simian immunodeficiency virus-infected rhesus macaques. J Immunol. 1995;155:5855–5860. [PMC free article] [PubMed] [Google Scholar]
- 30.Lukacher A E, Braciale V L, Braciale T J. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J Exp Med. 1984;160:814–826. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lundqvist C, Baranov V, Soderstrom K, Athlin L, Kiessling R, Hammarstrom S, Hammarstrom M L. Phenotype and cytokine profile of intraepithelial lymphocytes in human small and large intestine. Ann N Y Acad Sci. 1995;756:395–399. doi: 10.1111/j.1749-6632.1995.tb44544.x. [DOI] [PubMed] [Google Scholar]
- 32.Lundqvist C, Melgar S, Yeung M M, Hammarstrom S, Hammarstrom M L. Intraepithelial lymphocytes in human gut have lytic potential and a cytokine profile that suggest T helper 1 and cytotoxic functions. J Immunol. 1996;157:1926–1934. [PubMed] [Google Scholar]
- 33.MacDonald T T, Spencer J. Evidence that activated mucosal T cells play a role in the pathogenesis of enteropathy in human small intestine. J Exp Med. 1988;167:1341–1349. doi: 10.1084/jem.167.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacDonald T T, Spencer J. Gut immunology. Bailliere’s Clin Gastroenterol. 1990;4:291–313. doi: 10.1016/0950-3528(90)90003-y. [DOI] [PubMed] [Google Scholar]
- 35.Madara J L, Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989;83:724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyaard L, Hovenkamp E, Keet I P, Hooibrink B, de Jong I H, Otto S A, Miedema F. Single cell analysis of IL-4 and IFN-gamma production by T cells from HIV-infected individuals: decreased IFN-gamma in the presence of preserved IL-4 production. J Immunol. 1996;157:2712–2718. [PubMed] [Google Scholar]
- 37.Meyaard L, Miedema F. Immune dysregulation and CD4+ T cell loss in HIV-1 infection. Springer Semin Immunopathol. 1997;18:285–303. doi: 10.1007/BF00813499. [DOI] [PubMed] [Google Scholar]
- 38.Morris A G, Lin Y L, Askonas B A. Immune interferon release when a cloned cytotoxic T-cell line meets its correct influenza-infected target cell. Nature. 1982;295:150–152. doi: 10.1038/295150a0. [DOI] [PubMed] [Google Scholar]
- 39.Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells Nature 326:711–713. (Erratum, 1987;344:178. doi: 10.1038/326711a0. , 1990.) [DOI] [PubMed] [Google Scholar]
- 40.Quinnan G V, Jr, Kirmani N, Rook A H, Manischewitz J F, Jackson L, Moreschi G, Santos G W, Saral R, Burns W H. Cytotoxic t cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N Engl J Med. 1982;307:7–13. doi: 10.1056/NEJM198207013070102. [DOI] [PubMed] [Google Scholar]
- 41.Reddy M M, Sorrell S J, Lange M, Grieco M H. Tumor necrosis factor and HIV P24 antigen levels in serum of HIV-infected populations. J Acquired Immune Defic Syndr. 1988;1:436–440. [PubMed] [Google Scholar]
- 42.Rosenberg Z F, Fauci A S. Immunopathogenesis of HIV infection. FASEB J. 1991;5:2382–2390. doi: 10.1096/fasebj.5.10.1676689. [DOI] [PubMed] [Google Scholar]
- 43.Schieferdecker H L, Ullrich R, Hirseland H, Zeitz M. T cell differentiation antigens on lymphocytes in the human intestinal lamina propria. J Immunol. 1992;149:2816–2822. [PubMed] [Google Scholar]
- 44.Schneider T, Jahn H U, Schmidt W, Riecken E O, Zeitz M, Ullrich R. Loss of CD4 T lymphocytes in patients infected with human immunodeficiency virus type 1 is more pronounced in the duodenal mucosa than in the peripheral blood. Berlin Diarrhea/Wasting Syndrome Study Group. Gut. 1995;37:524–529. doi: 10.1136/gut.37.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schneider T, Ullrich R, Bergs C, Schmidt W, Riecken E O, Zeitz M. Abnormalities in subset distribution, activation, and differentiation of T cells isolated from large intestine biopsies in HIV infection. The Berlin Diarrhoea/Wasting Syndrome Study Group. Clin Exp Immunol. 1994;95:430–435. doi: 10.1111/j.1365-2249.1994.tb07014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scott P, Natovitz P, Coffman R L, Pearce E, Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988;168:1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scott P, Pearce E, Cheever A W, Coffman R L, Sher A. Role of cytokines and CD4+ T-cell subsets in the regulation of parasite immunity and disease. Immunol Rev. 1989;112:161–182. doi: 10.1111/j.1600-065x.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 48.Shields J G, Parrott D M. Appearance of delayed-type hypersensitivity effector cells in murine gut mucosa. Immunology. 1985;54:771–776. [PMC free article] [PubMed] [Google Scholar]
- 49.Simon M A, Chalifoux L V, Ringler D J. Pathologic features of SIV-induced disease and the association of macrophage infection with disease evolution. AIDS Res Hum Retroviruses. 1992;8:327–337. doi: 10.1089/aid.1992.8.327. [DOI] [PubMed] [Google Scholar]
- 50.Stone J D, Heise C C, Miller C J, Halsted C H, Dandekar S. Development of malabsorption and nutritional complications in simian immunodeficiency virus-infected rhesus macaques. AIDS. 1994;8:1245–1256. doi: 10.1097/00002030-199409000-00005. [DOI] [PubMed] [Google Scholar]
- 51.Sydora B C, Mixter P F, Holcombe H R, Eghtesady P, Williams K, Amaral M C, Nel A, Kronenberg M. Intestinal intraepithelial lymphocytes are activated and cytolytic but do not proliferate as well as other T cells in response to mitogenic signals. J Immunol. 1993;150:2179–2191. [PubMed] [Google Scholar]
- 52.Taguchi T, Aicher W K, Fujihashi K, Yamamoto M, McGhee J R, Bluestone J A, Kiyono H. Novel function for intestinal intraepithelial lymphocytes. Murine CD3+, gamma/delta TCR+ T cells produce IFN-gamma and IL-5. J Immunol. 1991;147:3736–3744. [PubMed] [Google Scholar]
- 53.Taguchi T, McGhee J R, Coffman R L, Beagley K W, Eldridge J H, Takatsu K, Kiyono H. Analysis of Th1 and Th2 cells in murine gut-associated tissues. Frequencies of CD4+ and CD8+ T cells that secrete IFN-gamma and IL-5. J Immunol. 1990;145:68–77. [PubMed] [Google Scholar]
- 54.Tan X, Pearce-Pratt R, Phillips D M. Productive infection of a cervical epithelial cell line with human immunodeficiency virus: implications for sexual transmission. J Virol. 1993;67:6447–6452. doi: 10.1128/jvi.67.11.6447-6452.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taub D D, Conlon K, Lloyd A R, Oppenheim J J, Kelvin D J. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 alpha and MIP-1 beta. Science. 1993;260:355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 56.Taub D D, Lloyd A R, Conlon K, Wang J M, Ortaldo J R, Harada A, Matsushima K, Kelvin D J, Oppenheim J J. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;177:1809–1814. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trinchieri G, Perussia B. Interferons and lymphocyte-mediated cytotoxicity. Tex Rep Biol Med. 1981;41:596–602. [PubMed] [Google Scholar]
- 58.Ullrich R, Zeitz M, Heise W, L’age M, Hoffken G, Riecken E O. Small intestinal structure and function in patients infected with human immunodeficiency virus (HIV): evidence for HIV-induced enteropathy. Ann Intern Med. 1989;111:15–21. doi: 10.7326/0003-4819-111-1-15. . (Erratum, 111:954.) [DOI] [PubMed] [Google Scholar]
- 59.Veazey R S, DeMaria M, Chalifoux L V, Shvetz D E, Pauley D R, Knight H L, Rosenzweig M, Johnson PR, Desrosiers RC, Lackner A A. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 60.Waldrop S L, Pitcher C J, Peterson D M, Maino V C, Picker L J. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J Clin Invest. 1997;99:1739–1750. doi: 10.1172/JCI119338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wolthers K C, Schuitemaker H, Miedema F. Rapid CD4+ T-cell turnover in HIV-1 infection: a paradigm revisited. Immunol Today. 1998;19:44–48. doi: 10.1016/s0167-5699(97)01188-2. [DOI] [PubMed] [Google Scholar]
- 62.Yamada Y K, Meager A, Yamada A, Ennis F A. Human interferon alpha and gamma production by lymphocytes during the generation of influenza virus-specific cytotoxic T lymphocytes. J Gen Virol. 1986;67:2325–2334. doi: 10.1099/0022-1317-67-11-2325. [DOI] [PubMed] [Google Scholar]
- 63.Yap K L, Ada G L, McKenzie I F. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature. 1978;273:238–239. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]
- 64.Zeitz M, Greene W C, Peffer N J, James S P. Lymphocytes isolated from the intestinal lamina propria of normal nonhuman primates have increased expression of genes associated with T-cell activation. Gastroenterology. 1988;94:647–655. doi: 10.1016/0016-5085(88)90235-1. [DOI] [PubMed] [Google Scholar]
- 65.Zuckermann F A, Gaskins H R. Distribution of porcine CD4/CD8 double-positive T lymphocytes in mucosa-associated lymphoid tissues. Immunology. 1996;87:493–499. [PMC free article] [PubMed] [Google Scholar]