Abstract
Introduction:
Currently, the impact of body mass index (BMI) on the outcomes of laparoscopic liver resections (LLR) is poorly defined. This study attempts to evaluate the impact of BMI on the peri-operative outcomes following laparoscopic left lateral sectionectomy (L-LLS).
Methods:
A retrospective analysis of 2183 patients who underwent pure L-LLS at 59 international centers between 2004 and 2021 was performed. Associations between BMI and selected peri-operative outcomes were analyzed using restricted cubic splines.
Results:
A BMI of >27kg/m2 was associated with increased in blood loss (Mean difference (MD) 21mls, 95% CI 5 – 36), open conversions (Relative risk (RR) 1.13, 95% CI 1.03 – 1.25), operative time (MD 11 minutes, 95% CI 6–16), use of Pringles maneuver (RR 1.15, 95% CI 1.06−1.26) and reductions in length of stay (MD −0.2 days, 95% CI −0.3 to −0.1). The magnitude of these differences increased with each unit increase in BMI. However, there was a “U” shaped association between BMI and morbidity with the highest complication rates observed in underweight and obese patients.
Conclusion:
Increasing BMI resulted in increasing difficulty of L-LLS. Consideration should be given to its incorporation in future difficulty scoring systems in laparoscopic liver resections.
Keywords: laparoscopic liver, laparoscopic hepatectomy, minimally-invasive liver, minimally-invasive hepatectomy, body mass index, left lateral sectionectomy
Introduction
The adoption of laparoscopic liver resection (LLR) is steadily gaining global acceptance given its advantages of reduced blood loss, lower morbidity and faster recovery as compared to open liver resection (OLR) (1, 2). However, universal adoption is hampered by its steep learning curve, particularly for major hepatectomies or resections involving difficult postero-superior segments (3–7). In the 2nd International Consensus Conference on Laparoscopic Liver Resection held in Morioka, Japan, the experts stated the importance of the difficulty scoring systems (DSS) to predict the surgical difficulty of LLR, which guides patient selection according to the surgeon’s surgical experience and technical ability (8). Since then, several DSSs have been established, including the Iwate score (9), Southampton scoring system (10) and the Institut Mutualiste Montsouris (IMM) (11, 12).
One of the inherent drawbacks of the current DSSs is that it does not account for other variables which potentially influence difficulty in LLR. Obesity, a global health pandemic, has been associated with numerous co-morbidities, including non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), and liver cirrhosis (13). Recently, anthropometric measures such as Body Mass Index (BMI) have been found to influence peri-operative outcomes in LLR (14), however, such studies included all extents of liver resections and not specific types. It is likely that patient BMI may influence outcomes after LLR to differing extents depending on the type and extent of liver resection.
Amongst the different types of LLR, laparoscopic left lateral sectionectomy (L-LLS) is perhaps the more widely performed procedure. The left lateral lobe has several anatomic advantages, which render it amenable to LLR. Its proximity to the abdominal wall, ease of mobilization, and relatively consistent intra-hepatic biliovascular anatomy allows for a shorter learning curve. For these reasons, L-LLS has a generally lower difficulty score among DSS categories and has been advocated as a standard of care for all left lateral lobe lesions of the liver (15). Given the paucity of evidence evaluating the impact of BMI on L-LLS, our study aims to evaluate the association between BMI and perioperative outcomes following L-LLS.
Methods
This was a retrospective review of 2963 patients who underwent pure L-LLS at 59 international centers between 2004–2021. All institutions obtained their respective approvals according to their local center’s requirements. This study was approved by the Singapore General Hospital Institution Review Board, and the need for patient consent was waived. The anonymized data were collected in the individual centers. These were collated and analyzed centrally at the Singapore General Hospital.
Patients who underwent multiple liver resections and patients who had a history of liver resections were excluded. Additionally, patients who underwent concomitant major operations such as bilio-enteric anastomoses, lymph node dissection, colectomies, stoma reversal, gastrectomies, splenectomies, and vascular resections were excluded. Notably, patients who underwent concomitant minor operations such as hernia repair and ablations were included. After the exclusion criteria, there were 2363 cases. Of these, 180 had missing BMI data. Finally, 2183 L-LLS were included in this study.
Definitions
Liver resections were defined according to the 2000 Brisbane classification (16). LLS were classified as resection segments 2 and 3. Diameter of the largest lesion was used in the cases of multiple tumors. Difficulty of resections were graded according to the Iwate score. Post-operative complications were classified according to the Clavien-Dindo classification and recorded for up to 30 days or during the same hospitalization (17).
Statistical analyses
Body mass index was categorized into four categories: underweight (< 18.5kg/m2), normal BMI (18.5–24.9 kg/m2), overweight (25.0–29.9kg/m2), and obese (≥30.0kg/m2). Continuous and categorical variables were compared between BMI group using Kruskal Wallis and Fisher’s exact tests respectively, and omnibus P-values < 0.05 were interpreted to indicate statistically-significant differences between categories (Table 1). As it is conceivable that the dose-response relationships between BMI and perioperative outcomes could be non-linear or even non-monotonic, we also analyzed associations using restricted cubic splines. This approach makes fewer a priori assumptions in regards to model specification compared to standard regression approaches which typically assume linear or log-linear relationships. The number of knots, between 3 to 7, was chosen based on minimizing the Akaike information criterion (AIC) of robust regression or modified Poisson regression models for continuous and binary endpoints respectively, adjusted for all baseline characteristics (age, sex, year of surgery, previous abdominal surgery, ASA category, pathology, tumor size, multifocal tumors, and Iwate score) and centered at their means or point estimates for proportions. We analyzed the impact of geographical region (East vs West) using simple subgroup analysis, as we found that introduction of a covariate interaction term between BMI and region led to poor model fit and extreme effect sizes. Knot locations were placed at the recommended percentiles according to the Harrell and colleagues (18). Adjusted effect sizes (mean differences or relative risks for continuous and binary outcomes respectively) were visually rendered in Figure 1 and summarized in Table 2.
Table 1.
Comparison between baseline characteristics and perioperative outcomes of L-LLS stratified by BMI
| All N = 2183 | Underweight (BMI < 18.5 kg/m2) N = 84 | Normal BMI (BMI 18.5–24.9 kg/m2) N = 1114 | Overweight (BMI 25.0–29.9kg/m2) N = 722 | Obese (BMI ≥ 30.0 kg/m2) N = 263 | P-value | |
|---|---|---|---|---|---|---|
| Median age (IQR), yrs | 61 (50–70) | 59 (42–72) | 59 (49–69) | 63 (53–71) | 61 (53–71) | <0.001 |
| Male sex, n (%) | 1266 (58.0%) | 31 (36.9%) | 643 (57.7%) | 459 (63.6%) | 133 (50.6%) | <0.001 |
| Year of surgery, n (%) 2004–2009 2010–2015 2016–2021 |
182 (8.3%) 666 (30.5%) 1335 (61.2%) |
7 (8.3%) 35 (41.7%) 42 (50.0%) |
88 (7.9%) 328 (29.4%) 698 (62.7%) |
62 (8.6%) 217 (30.1%) 443 (61.3%) |
25 (9.5%) 86 (32.7%) 152 (57.8%) |
0.262 |
| Previous abdominal surgery, n/total (%) | 610/2104 (29.0%) | 16/82 (19.5%) | 277/1078 (25.7%) | 216/691 (31.3%) | 101/253 (39.9%) | <0.001 |
| ASA score, n/total (%) 1/2 3/4 |
1716/2182 (78.6%) 466/2182 (21.4%) |
64/84 (76.2%) 20/84 (23.8%) |
930/1113 (83.6%) 183/1113 (16.4%) |
539/722 (74.6%) 183/722 (25.4%) |
183/263 (69.6%) 80/263 (30.4%) |
<0.001 |
| Pathology HCC/ICC/cholangiohepatoma CRLM Other metastases Other primary malignancy Benign |
1076/2179 (49.4%) 378/2179 (17.3%) 106/2179 (4.9%) 13/2179 (0.6%) 606/2179 (27.8%) |
39/84 (46.4%) 8/84 (9.5%) 6/84 (7.2%) 0/84 (0.0%) 31/84 (36.9%) |
586/1113 (52.7) 172/1113 (15.5) 47/1113 (4.2) 8/1113 (0.7) 300/1113 (26.9) |
342/719 (47.6) 146/719 (20.3) 38/719 (5.2) 5/719 (0.7) 188/719 (26.2) |
109/263 (41.4) 52/263 (19.8) 15/263 (5.7) 0/263 (0.0) 87/263 (33.1) |
0.007 |
| Median tumor size, mm (IQR) | 35 (22–60) | 40 (23–60) | 32 (20–57) | 38 (24–60) | 40 (25–70) | 0.002 |
| Multiple tumors, n (%) | 319 (14.6%) | 6 (7.1%) | 149 (13.4%) | 111 (15.4%) | 53 (20.2%) | 0.008 |
| Median Iwate difficulty score, (IQR) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 0.579 |
| Iwate difficulty, n (%) Low Intermediate High Expert |
136 (6.2%) 2025 (92.8%) 22 (1.0%) 0 (0.0%) |
2 (2.4%) 81 (96.4%) 1 (1.2%) 0 (0.0%) |
71 (6.4%) 1033 (92.7%) 10 (0.9%) 0 (0.0%) |
50 (6.9%) 663 (91.8%) 9 (1.3%) 0 (0.0%) |
13 (4.9%) 248 (94.3%) 2 (0.8%) 0 (0.0%) |
0.608 |
| Open conversion, n (%) | 74 (3.4%) | 5 (6.0%) | 26 (2.3%) | 31 (4.3%) | 12 (4.6%) | 0.025 |
| Mean operating time (SD), min | 173 (89) | 177 (181) | 161 (79) | 183 (84) | 192 (94) | <0.001 |
| Mean blood loss (SD), ml | 161 (251) | 178 (408) | 143 (223) | 168 (222) | 212 (350) | <0.001 |
| Intraoperative blood transfusion, n (%) | 75 (3.4%) | 3 (3.6%) | 37 (3.3%) | 21 (2.9%) | 14 (5.3%) | 0.325 |
| Pringle maneuver applied, n/total (%) | 417/2102 (19.8%) | 16/79 (20.3%) | 197/1074 (18.3%) | 139/697 (19.9%) | 65/252 (25.8%) | 0.072 |
| Mean postoperative stay (SD), days | 6 (5) | 6 (4) | 6 (5) | 6 (5) | 5 (4) | 0.005 |
| Postoperative morbidity, n (%) | 273 (12.5%) | 12 (14.3%) | 127 (11.4%) | 90 (12.5%) | 44 (16.7%) | 0.124 |
| Major morbidity (Clavien-Dindo grade ≥ 3) | 63 (2.9%) | 1 (1.2%) | 38 (3.4%) | 18 (2.5%) | 6 (2.3%) | 0.556 |
| 30-day mortality, n (%) | 7 (0.3%) | 0 (0.0%) | 4 (0.4%) | 2 (0.3%) | 1 (0.4%) | 1.000 |
| 90-day mortality, n (%) | 13 (0.6%) | 1 (1.2%) | 8 (0.7%) | 3 (0.4) | 1 (0.4%) | 0.581 |
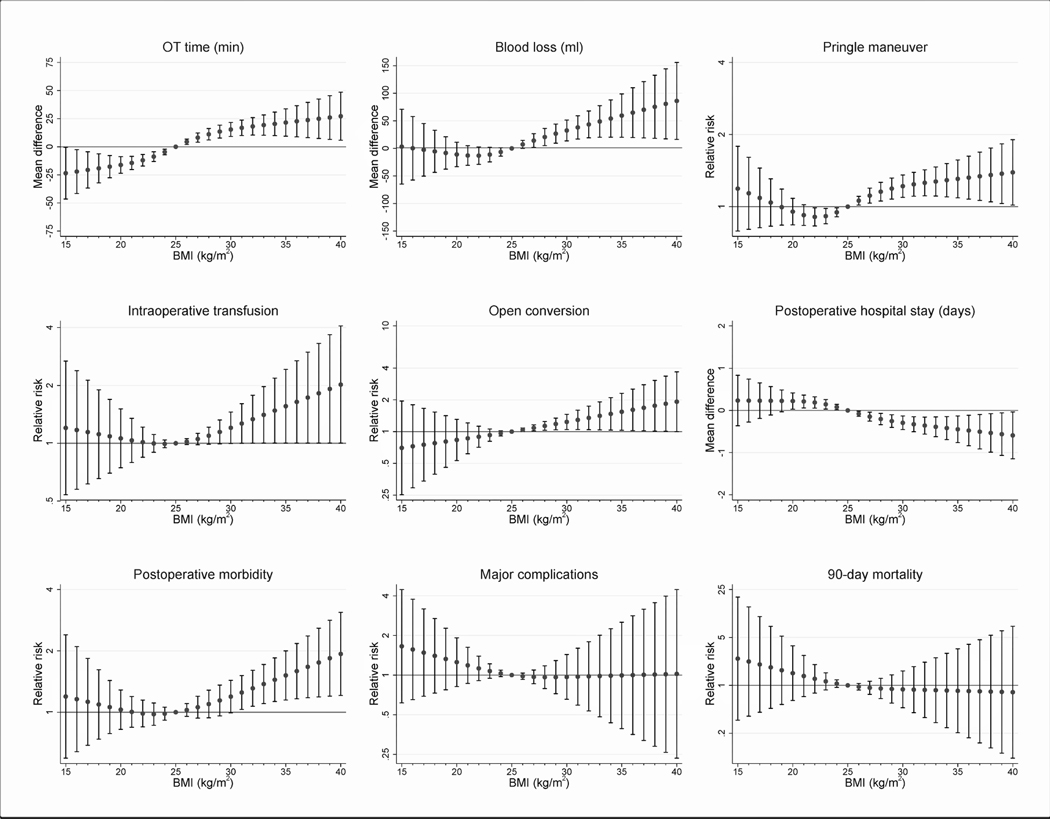
Figure 1.
Graphical representation of the modelled effect sizes from restricted cubic splines (RCS) analyses, depicting adjusted association between BMI and perioperative outcomes.
Table 2.
Modelled effect sizes from restricted cubic splines (RCS) analyses, depicting adjusted association between BMI and perioperative outcomes of laparoscopic liver resection. Reference BMI is set as 25kg/m2 and treatment effects were adjusted using covariance adjustment for baseline covariates
| BMI (kg/m2) | Operation time (mins) MD (95% CI) | Estimated blood loss (ml) MD (95% CI) | Pringle maneuver RR (95% CI) | Blood transfusion RR (95% CI) | Open conversion RR (95% CI) | Post-op complications RR (95% CI) | Major complications RR (95% CI) | Post-op length of stay MD (95% CI) | 90-day mortality RR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| 15 | −24 (−46 to −1) | 3 (−65 to 71) | 1.19 (0.79 to 1.79) | 1.2 (0.54 to 2.68) | 0.7 (0.25 to 1.95) | 1.19 (0.59 to 2.4) | 1.66 (0.61 to 4.46) | 0.2 (−0.4 to 0.8) | 2.46 (0.31 to 19.42) |
| 16 | −22 (−42 to −3) | 0 (−57 to 58) | 1.14 (0.8 to 1.61) | 1.17 (0.58 to 2.39) | 0.73 (0.29 to 1.8) | 1.16 (0.64 to 2.1) | 1.57 (0.65 to 3.77) | 0.2 (−0.3 to 0.7) | 2.23 (0.36 to 13.99) |
| 17 | −21 (−37 to −4) | −3 (−50 to 45) | 1.09 (0.82 to 1.45) | 1.14 (0.61 to 2.13) | 0.75 (0.34 to 1.66) | 1.12 (0.69 to 1.84) | 1.48 (0.69 to 3.18) | 0.2 (−0.2 to 0.7) | 2.02 (0.41 to 10.08) |
| 18 | −19 (−32 to −6) | −5 (−44 to 33) | 1.04 (0.83 to 1.31) | 1.12 (0.65 to 1.9) | 0.78 (0.39 to 1.53) | 1.09 (0.74 to 1.62) | 1.4 (0.73 to 2.69) | 0.2 (−0.1 to 0.6) | 1.83 (0.46 to 7.26) |
| 19 | −18 (−28 to −8) | −8 (−38 to 21) | 1 (0.84 to 1.18) | 1.09 (0.7 to 1.69) | 0.81 (0.46 to 1.42) | 1.06 (0.79 to 1.43) | 1.32 (0.77 to 2.27) | 0.2 (0 to 0.5) | 1.66 (0.53 to 5.24) |
| 20 | −16 (−24 to −9) | −11 (−33 to 11) | 0.95 (0.84 to 1.08) | 1.06 (0.74 to 1.51) | 0.83 (0.53 to 1.31) | 1.03 (0.82 to 1.29) | 1.25 (0.82 to 1.92) | 0.2 (0 to 0.4) | 1.51 (0.6 to 3.78) |
| 21 | −14 (−20 to −9) | −13 (−31 to 5) | 0.92 (0.83 to 1.02) | 1.03 (0.79 to 1.35) | 0.86 (0.62 to 1.21) | 1 (0.84 to 1.19) | 1.19 (0.86 to 1.63) | 0.2 (0.1 to 0.4) | 1.37 (0.69 to 2.74) |
| 22 | −12 (−17 to −7) | −13 (−29 to 3) | 0.91 (0.83 to 0.99) | 1.01 (0.84 to 1.21) | 0.89 (0.71 to 1.13) | 0.99 (0.85 to 1.15) | 1.12 (0.91 to 1.39) | 0.2 (0.1 to 0.3) | 1.25 (0.77 to 2.01) |
| 23 | −9 (−13 to −5) | −11 (−24 to 2) | 0.91 (0.85 to 0.98) | 1 (0.9 to 1.11) | 0.93 (0.81 to 1.06) | 0.98 (0.86 to 1.11) | 1.07 (0.95 to 1.22) | 0.2 (0 to 0.3) | 1.14 (0.86 to 1.53) |
| 24 | −5 (−7 to −2) | −7 (−14 to 1) | 0.95 (0.91 to 0.99) | 0.99 (0.95 to 1.04) | 0.96 (0.91 to 1.02) | 0.98 (0.91 to 1.06) | 1.03 (0.97 to 1.09) | 0.1 (0 to 0.1) | 1.06 (0.93 to 1.21) |
| 25 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| 26 | 4 (2 to 7) | 7 (0 to 15) | 1.06 (1.02 to 1.1) | 1.02 (0.98 to 1.06) | 1.04 (1 to 1.08) | 1.03 (0.95 to 1.1) | 0.98 (0.93 to 1.04) | −0.1 (−0.1 to 0) | 0.96 (0.86 to 1.07) |
| 27 | 8 (4 to 12) | 14 (2 to 27) | 1.11 (1.03 to 1.19) | 1.05 (0.98 to 1.13) | 1.09 (1.01 to 1.16) | 1.06 (0.93 to 1.2) | 0.97 (0.86 to 1.09) | −0.2 (−0.3 to 0) | 0.92 (0.74 to 1.15) |
| 28 | 11 (6 to 16) | 21 (5 to 36) | 1.15 (1.06 to 1.26) | 1.09 (0.99 to 1.21) | 1.13 (1.03 to 1.25) | 1.1 (0.94 to 1.28) | 0.96 (0.79 to 1.18) | −0.2 (−0.3 to −0.1) | 0.9 (0.64 to 1.27) |
| 29 | 13 (8 to 19) | 27 (9 to 44) | 1.19 (1.08 to 1.32) | 1.15 (0.99 to 1.32) | 1.18 (1.04 to 1.34) | 1.14 (0.96 to 1.36) | 0.96 (0.72 to 1.29) | −0.3 (−0.4 to −0.1) | 0.89 (0.55 to 1.42) |
| 30 | 15 (9 to 22) | 32 (14 to 51) | 1.22 (1.09 to 1.36) | 1.2 (1 to 1.45) | 1.24 (1.05 to 1.46) | 1.19 (0.99 to 1.43) | 0.97 (0.65 to 1.43) | −0.3 (−0.5 to −0.1) | 0.88 (0.47 to 1.63) |
| 31 | 17 (10 to 24) | 38 (17 to 59) | 1.24 (1.11 to 1.39) | 1.27 (1 to 1.61) | 1.29 (1.05 to 1.59) | 1.25 (1.03 to 1.51) | 0.97 (0.59 to 1.6) | −0.3 (−0.5 to −0.2) | 0.87 (0.4 to 1.88) |
| 32 | 18 (10 to 26) | 43 (19 to 68) | 1.26 (1.11 to 1.42) | 1.33 (1 to 1.78) | 1.35 (1.04 to 1.75) | 1.31 (1.07 to 1.61) | 0.98 (0.53 to 1.79) | −0.4 (−0.6 to −0.2) | 0.86 (0.34 to 2.18) |
| 33 | 19 (10 to 28) | 49 (20 to 77) | 1.27 (1.11 to 1.47) | 1.41 (1 to 1.97) | 1.41 (1.04 to 1.91) | 1.38 (1.1 to 1.72) | 0.98 (0.48 to 2.01) | −0.4 (−0.6 to −0.2) | 0.85 (0.29 to 2.53) |
| 34 | 20 (10 to 31) | 54 (20 to 88) | 1.29 (1.1 to 1.51) | 1.48 (1 to 2.19) | 1.47 (1.03 to 2.1) | 1.44 (1.13 to 1.85) | 0.99 (0.43 to 2.25) | −0.4 (−0.7 to −0.2) | 0.84 (0.24 to 2.94) |
| 35 | 22 (9 to 34) | 59 (20 to 99) | 1.31 (1.09 to 1.57) | 1.56 (1 to 2.43) | 1.54 (1.03 to 2.31) | 1.52 (1.15 to 2) | 0.99 (0.39 to 2.52) | −0.5 (−0.8 to −0.1) | 0.83 (0.2 to 3.42) |
| 36 | 23 (9 to 37) | 65 (20 to 110) | 1.32 (1.08 to 1.63) | 1.64 (1 to 2.69) | 1.61 (1.02 to 2.53) | 1.59 (1.16 to 2.17) | 1 (0.35 to 2.82) | −0.5 (−0.8 to −0.1) | 0.83 (0.17 to 3.98) |
| 37 | 24 (8 to 39) | 70 (19 to 121) | 1.34 (1.06 to 1.69) | 1.73 (1 to 2.98) | 1.68 (1.02 to 2.78) | 1.67 (1.18 to 2.37) | 1 (0.32 to 3.16) | −0.5 (−0.9 to −0.1) | 0.82 (0.14 to 4.63) |
| 38 | 25 (7 to 42) | 75 (18 to 133) | 1.36 (1.05 to 1.76) | 1.82 (1 to 3.31) | 1.76 (1.01 to 3.06) | 1.75 (1.19 to 2.59) | 1.01 (0.29 to 3.55) | −0.5 (−1 to −0.1) | 0.81 (0.12 to 5.39) |
| 39 | 26 (7 to 45) | 81 (17 to 144) | 1.37 (1.03 to 1.83) | 1.92 (1 to 3.67) | 1.84 (1.01 to 3.36) | 1.84 (1.2 to 2.83) | 1.02 (0.26 to 3.98) | −0.6 (−1.1 to −0.1) | 0.8 (0.1 to 6.27) |
| 40 | 27 (6 to 49) | 86 (16 to 156) | 1.39 (1.02 to 1.9) | 2.02 (1 to 4.08) | 1.92 (1 to 3.69) | 1.93 (1.21 to 3.09) | 1.02 (0.23 to 4.46) | −0.6 (−1.2 to 0) | 0.8 (0.09 to 7.3) |
Results
The baseline characteristics of the 2183 patients are summarized in Table 1. Of the 2183 patients, a total of 985 patients (45.2%) had a BMI of ≥25kg/m2. The median age was 61 years (range, 50–70) and males accounted for 58.0% of this cohort. A total of 1716 patients (78.6%) had an ASA score of 1 or 2. Malignant neoplasms accounted for 72.2% (n=1573) of all liver resections. The median tumour size was 35mm (range, 22–66mm) with a median Iwate difficulty score of 5 (range, 4–5), while approximately 14.6% (n=319) had surgery performed for multiple tumours. The overall mean postoperative length of stay was 6 days. Operative conversions accounted for 3.4% of this series (n=74), with major morbidity of 2.9% (n=63). The overall 30- and 90-day mortality was 0.3% (n=7) and 0.6% (n=13), respectively. Both mean blood loss (178 vs. 143 vs. 168 vs. 212 ml in underweight, normal weight, overweight and obese patients, respectively; p < 0.001) and operating time (177 vs. 161 vs. 183 vs. 192 minutes in underweight, normal weight, overweight and obese patients, respectively; p < 0.001) were greater in patients with higher BMI. Patients who were obese (BMI ≥30kg/m2) had a lower post-operative length of stay as compared to those who were not obese (5 vs 6 days; p =0.005).
Comparison between patients stratified by BMI
The modeled effect sizes from restricted cubic splines analyses between BMI and peri-operative outcomes among patients undergoing L-LLS are summarized in Table 2 and visually represented in Figure 1. A BMI of >27kg/m2 was associated with a reduction in post-operative stay (MD −0.2 days, 95% CI −0.3 to −0.1) and an increased risk of blood loss (Mean difference (MD) 21mls, 95% CI 5 – 36), open conversions (Relative risk (RR) 1.13, 95% CI 1.03 – 1.25), operative time (MD 11 minutes, 95% CI 6–16) and use of pringles maneuver (RR 1.15, 95% CI 1.06−1.26).
In addition, with every unit increase in BMI, there was an increase in the magnitude of MD and RR. At a BMI of 40, the MD in operative time was 27 minutes (95% CI 6–49) and the MD in blood loss was 86mls (95% CI 15–156) while the RR of need for pringles and post-operative complications was 1.39 (95% CI 1.02–1.90) and 1.93 (95% CI 1.21–3.09) respectively. The magnitude of change observed in open conversions (RR 1.84, 95% CI 1.01–3.36) and postoperative length of stay (MD −0.6 days, 95% CI −1.1 to −0.1) reached a peak when the BMI was 39.
Post-operative complications were more frequent when patients had a BMI of >30kg/m2 (RR 1.25, 95% CI 1.03 – 1.51) or < 18.5kg/m2.
Subset analyses of patients stratified by Eastern and Western centers
There were 1009 procedures performed in Eastern centers and 1174 in Western centers. The distribution of patients in Eastern centers were 46 (4.6%) underweight, 632 (62.6%) normal weight, 278 (27.6%) overweight and 53 (5.3%) overweight. In Western centers, there were 38 (3.2%) underweight, 482 (41.1%) normal weight), 444 (37.8%) overweight and 210 (17.9%) overweight.
Subset analyses in Eastern centers demonstrated similar results to the overall cohort whereby a higher BMI was associated with increased blood loss, intraoperative blood transfusion rate and operation time (Supplemental Figures 1–3). Similarly, a “U” shaped association was observed between BMI and postoperative morbidity (Supplemental Figure 4). However, there was no linear correlation between postoperative length of stay and BMI (Supplemental Figure 5).
Similarly, subset analyses in Western centers demonstrated linear correlation between BMI and blood loss, blood transfusion rate, open conversion rate and operation time (Supplemental Figures 6–9) and a “U” shaped association with postoperative morbidity (Supplemental Figure 10). Postoperative length of stay did not correlate with an increasing BMI (Supplemental Figure 11).
Discussion
In this study, we demonstrated that increasing BMI was associated with increasing difficulty of L-LLS (Figure 1) as evidenced by the increasing operation time, blood loss, blood transfusion, open conversion rate and frequency of use of the Pringle Maneuver. The magnitude of these adverse outcomes increased with every unit increase in an individual’s BMI. However notably, postoperative morbidity demonstrated a “U” shaped association with BMI whereby underweight and obese patients experienced the highest morbidity rate.
The L-LLS is one of the most commonly performed minimally invasive liver resection and has been advocated as the procedure of choice for new surgeons embarking on LLR (19) owing to its anatomically favorable location, ease of glissonian and outflow control and predictable parenchymal transection line, allowing for a safe and efficient dissection with good outcomes even in patients with cirrhosis (20–22). Presently, the Iwate difficulty scoring systems assessing the technical difficulty of L-LLS will score with a difficulty ranging from low (11) to intermediate (9). However notably, BMI was not included in the Iwate system. Presently, there is limited data on the influence of obesity on MILR and its effect on operative outcomes remains contentious. Moreover, previous studies included all extends of liver resections but not specific types although intuitively, the impact of BMI would differ between different types of liver resections (14).
One possibility for the observed results in our study may revolve around the presence of non-alcoholic fatty liver disease (NAFLD), a disease spectrum ranging from isolated fatty infiltration of the liver to non-alcoholic steatohepatitis (NASH) and progressive liver cirrhosis (23). NAFLD has an estimated prevalence of between 15–30% in the general population (24, 25), with figures increasing in the presence of obesity. In fact, studies in obese patients reveal that NAFLD complicates over 80% of patients undergoing bariatric surgery, with over 25% of patients demonstrating biopsy proven NASH (26).
The parenchymal changes of NAFLD influence difficulty in L-LLS in various ways, depending on where it rests on the disease spectrum. On one end, livers with NAFLD have excessive intra-hepatic triglyceride accumulation, resulting in large, soft friable livers prone to intra-operative fracture and bleeding. The resultant hepatomegaly also renders liver mobilization and liver manipulation more challenging, particularly in instances when maneuvres are required to control hemorrhage. On the other hand, livers with NASH related cirrhosis present with difficulties inherent to any patient with cirrhosis. The resultant fibrosis often renders parenchymal breakage and dissection of small bilovascular elements challenging. The presence of portal hypertension and thrombocytopenia among cirrhotic patients also further elevates the risk of bleeding during parenchymal transection. In addition, the presence of visceral adiposity may compound difficulty by affording a smaller operative space and poor visual exposure in addition to an already enlarged liver, the amalgamation of these reasons possibly accounting for the increased operative time, blood loss, morbidity, and operative conversions (27, 28).
Beyond the adverse peri-operative outcomes associated with BMI, we also found that post-operative length of stay appeared to decrease with an increasing BMI, suggesting that overweight and obese patients had a shorter postoperative length of stay following L-LLS compared to their normal or underweight counterparts. This may be in part due to the protective effects of better nutrition and adequate fat stores among patients with a higher BMI (29, 30). However, a more likely explanation would be due to the confounding effect of policy differences between healthcare systems around the world. In Western centers, there is a consensus for greater independent care, functional recovery, and early discharge in order to ease the overall medical costs. On the contrary, Asian centers see patients rely heavily on external assistance for self-care, leading to prolonged hospital stay till an appropriate caregiver or step-down facility is available, particularly in an aging population. As shown in this study, whereby patients with higher BMI were more likely to be from Western centers while those with lower BMI were more likely from Asian centers, it is possible that the observed lower post-operative stay may be due to this confounding factor. This hypothesis is further supported by our findings on subset analyses whereby there was no correlation between BMI and length of stay in either Western or Eastern centers.
Another important observation which should be highlighted was with regards to major morbidity and overall morbidity which showed a “U” shaped association with BMI (Figure 1). Patients undergoing L-LLS experienced the highest rates of morbidity and major complications at the extreme ends of BMI. Patients at the lower extreme of BMI may have cachexia which is a well-known predisposing factor to increased postoperative morbidity. Similarly on the other extreme of BMI, obese patients are predisposed to postoperative complications resulting in the “U” shaped curve.
Prior studies evaluating the role of laparoscopic liver resections in overweight or obese patients have established its safety and benefit through reductions in overall morbidity as opposed to its open counterpart (27, 31), reflecting the recognized benefits of a minimally invasive approach. Among patients solely undergoing laparoscopic liver resections, the impact of BMI has remained contentious. A large retrospective series by Yu et al (29) demonstrated an increase in operative conversions among patients with higher BMI but not mortality or blood loss. It is noteworthy that a large proportion Yu’s series included patients undergoing hepatectomy for benign pathologies. Moreover, obese patients (defined as a BMI ≥28kg/m2) only accounted for approximately 5% of the overall study population. In contrast, the predominant indication for L-LLS in this series was malignancy. Consequently, variables including tumour size and the anatomic proximity to major inflow or hepatic vein outflow theoretically predisposes to an increased risk of bleeding. Additionally, the proportion of obese patients (defined as BMI ≥30kg/m) accounted for approximately 12% of this series, possibly contributing to a higher proportion of NALFD-related parenchymal changes, which could potentially explain why an increase in blood loss was noted in our series.
To our knowledge, this study is the largest series evaluating the role of BMI on outcomes following L-LLS. The addition of rigorous statistical analysis using restricted cubic splines increases the robustness of these findings by recognizing non-linear, non-monotonic relationships between exposure and outcome, thereby uncovering more precise correlations. However, there are several limitations which should be acknowledged. Firstly, the retrospective nature of this study renders it prone to confounding and bias. Secondly, as an international multi-center study, there is invariably heterogeneity across centers with respect to patient selection, surgeon experience, and perioperative management. Furthermore, as this study included patients from both Eastern and Western population, this may have an effect on outcomes due to the different implications of BMI between both populations. Hence some the BMI cutoffs for obesity differs between Eastern and Western countries. It has been shown that Asians tend to have a higher body fat ratio and higher percentages of visceral as opposed to peripheral adiposity when compared to their western counterparts for the same age, gender, and BMI (27, 32).
Conclusion
L-LLS in patients with an increasing BMI is associated with higher open conversion rates, operative morbidity, greater intraoperative blood loss, use of pringles and longer operation time. Postoperative morbidity however demonstrated a “U”-shaped association with increasing BMI. The significance of BMI as a predictor of difficulty should therefore be given consideration as an important variable in future difficulty scores.
Supplementary Material
Supplemental Figure 1. Association between BMI and blood loss in Eastern centers.
Supplemental Figure 2. Association between BMI and blood transfusion rate in Eastern centers
Supplemental Figure 3. Association between BMI and operation time in Eastern centers
Supplemental Figure 4. Association between BMI and postoperative morbidity in Eastern centers
Supplemental Figure 5. Association between BMI and postoperative length of stay in Eastern centers
Supplemental Figure 6. Association between BMI and blood loss in Western centers
Supplemental Figure 7. Association between BMI and blood transfusion rate in Western centers
Supplemental Figure 8. Association between BMI and open conversion rate in Western centers
Supplemental Figure 9. Association between BMI and operation time in Western centers
Supplemental Figure 10. Association between BMI and postoperative morbidity in Western centers
Supplemental Figure 11. Association between BMI and postoperative length of stay in Western centers
Study funding
Dr T. P. Kingham was partially supported by the US National Cancer Institute MSKCC Core Grant number P30 CA008748 for this study
Dr M. Yin was partially funded by the Research Project of Zhejiang Provincial Public Welfare Fund project in the Field of Social development (LGF20H160028)
Dr Brian K. P. Goh was partially supported by a grant from the Intuitive Foundation for this study. All research findings, conclusions or recommendations expressed in this work are those of the authors and not of the Intuitive Foundation.
Declarations
We confirm all the authors are accountable for all aspects of the work
i) Dr Goh BK has received travel grants and honorarium from Johnson and Johnson, Olympus and Transmedic the local distributor for the Da Vinci Robot.
ii) Dr Marino MV is a consultant for CAVA robotics LLC.
iii) Johann Pratschke reports a research grant from Intuitive Surgical Deutschland GmbH and personal fees or non-financial support from Johnson & Johnson, Medtronic, AFS Medical, Astellas, CHG Meridian, Chiesi, Falk Foundation, La Fource Group, Merck, Neovii, NOGGO and Promedicis.
iv) Moritz Schmelzle reports personal fees or other support outside of the submitted work from Merck, Bayer, ERBE, Amgen, Johnson & Johnson, Takeda, Olympus, Medtronic, Intuitive.
v) Asmund Fretland reports receiving speaker fees from Bayer.
Fernando Rotellar reports speaker fees and support outside the submitted work from Integra, Medtronic, Olympus, Corza, Sirtex and Johnson & Johnson.
International robotic and laparoscopic liver resection study group investigators are coauthors of this study
1. Mikel Gastaca (Hepatobiliary Surgery and Liver Transplantation Unit, Biocruces Bizkaia Health Research Institute, Cruces University Hospital, University of the Basque Country, Bilbao, Spain)
2. Juul Meurs (Department of Digestive and Hepatobiliary/Pancreatic Surgery, Groeninge Hospital, Kortrijk, Belgium)
3. Celine De Meyere (Department of Digestive and Hepatobiliary/Pancreatic Surgery, Groeninge Hospital, Kortrijk, Belgium
4. Kit-Fai Lee (Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, Prince of Wales Hospital, The Chinese University of Hong Kong, New Territories, Hong Kong SAR, China)
5. Kelvin K. Ng (Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, Prince of Wales Hospital, The Chinese University of Hong Kong, New Territories, Hong Kong SAR, China)
6. Diana Salimgereeva (Department of Hepato-Pancreato-Biliary Surgery, Moscow Clinical Scientific Center, Moscow, Russia)
7. Ruslan Alikhanov (Department of Hepato-Pancreato-Biliary Surgery, Moscow Clinical Scientific Center, Moscow, Russia)
8. Lip-Seng Lee (Hepatopancreatobiliary Unit, Department of Surgery, Changi General Hospital, Singapore)
9. Jae-Young Jang (Department of General Surgery, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea)
10. Masayuki Kojima (Department of Surgery, Fujita Health University School of Medicine, Aichi, Japan)
11. Kruger Jaime Arthur Pirola (Liver Surgery Unit, Department of Gastroenterology, University of Sao Paulo School of Medicine, Sao Paulo, Brazil)
12. Fabricio Ferreira Coelho (Liver Surgery Unit, Department of Gastroenterology, University of Sao Paulo School of Medicine, Sao Paulo, Brazil)
13. Victor Lopez-Lopez (Department of General, Visceral and Transplantation Surgery, Clinic and University Hospital Virgen de la Arrixaca, IMIB-ARRIXACA, El Palmar, Murcia, Spain)
14. Margarida Casellas I Robert (Hepatobiliary and Pancreatic Surgery Unit, Department of Surgery, Dr. Josep Trueta Hospital, IdIBGi, Girona, Spain)
15. Roberto Montalti (Department of Clinical Medicine and Surgery, Division of HPB, Minimally Invasive and Robotic Surgery, Federico II University Hospital Naples, Naples, Italy)
16. Mariano Giglio (Department of Clinical Medicine and Surgery, Division of HPB, Minimally Invasive and Robotic Surgery, Federico II University Hospital Naples, Naples, Italy)
17. Mizelle D’Silva (Department of Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seoul, Korea)
18. Boram Lee (Department of Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seoul, Korea)
19. Hao-Ping Wang (Department of Surgery, Chang Gung Memorial Hospital, Kaohsiung)
20. Franco Pascual (Department of Hepatobiliary Surgery, Assistance Publique Hopitaux de Paris, Centre Hepato-Biliaire, Paul-Brousse Hospital, Villejuif, France)
21. Mansour Saleh (Department of Hepatobiliary Surgery, Assistance Publique Hopitaux de Paris, Centre Hepato-Biliaire, Paul-Brousse Hospital, Villejuif, France)
22. Simone Vani (Hepatobiliary Surgery Unit, Fondazione Policlinico Universitario A. Gemelli, IRCCS, Catholic University of the Sacred Heart, Rome, Italy)
23. Francesco Ardito (Hepatobiliary Surgery Unit, Fondazione Policlinico Universitario A. Gemelli, IRCCS, Catholic University of the Sacred Heart, Rome, Italy)
24. Ugo Giustizieri (HPB Surgery, Hepatology and Liver Transplantation, Fondazione IRCCS Istituto Nazionale Tumori di Milano, Milan, Italy)
25. Davide Citterio (HPB Surgery, Hepatology and Liver Transplantation, Fondazione IRCCS Istituto Nazionale Tumori di Milano, Milan, Italy)
26. Federico Mocchegiani (HPB Surgery and Transplantation Unit, United Hospital of Ancona, Department of Experimental and Clinical Medicine Polytechnic University of Marche)
27. Giammauro Berardi (Division of General Surgery and Liver Transplantation, San Camillo Forlanini Hospital, Rome, Italy)
28. Marco Colasanti (Division of General Surgery and Liver Transplantation, San Camillo Forlanini Hospital, Rome, Italy)
29. Yoelimar Guzmán (General & Digestive Surgery, Hospital Clínic, Barcelona, Spain)
30. Kevin P. Labadie (Department of Surgery, University of Washington Medical Center. Seattle, USA)
31. Maria Conticchio (Unit of Hepato-Pancreatc-Biliary Surgery, “F. Miulli” General Regional Hospital, Acquaviva delle Fonti, Bari, Italy)
32. Epameinondas Dogeas (Department of Surgery, Division of Hepatobiliary and Pancreatic Surgery, University of Pittsburgh Medical Center, Pittsburgh, PA, USA)
33. Emanuele F. Kauffmann (Division of General and Transplant Surgery, University of Pisa, Pisa, Italy)
34. Mario Giuffrida (Hepatobiliary Surgery Unit, Department of Medicine and Surgery, University of Parma, Parma, Italy)
35. Daniele Sommacale (Department of Digestive and Hepatobiliary and Pancreatic Surgery, AP-HP, Henri-Mondor Hospital, Creteil, France)
36. Alexis Laurent (Department of Digestive and Hepatobiliary and Pancreatic Surgery, AP-HP, Henri-Mondor Hospital, Creteil, France)
37. Paolo Magistri (HPB Surgery and Liver Transplant Unit, University of Modena and Reggio Emilia, Modena, Italy)
38. Kohei Mishima (Center for Advanced Treatment of Hepatobiliary and Pancreatic Diseases, Ageo Central General Hospital, Saitama, Japan)
39. Moritz Schmelzle (Department of Surgery, Campus Charité Mitte and Campus Virchow-Klinikum, Charité-Universitätsmedizin, Corporate Member of Freie Universität Berlin, and Berlin Institute of Health, Berlin, Germany)
40. Felix Krenzien (Department of Surgery, Campus Charité Mitte and Campus Virchow-Klinikum, Charité-Universitätsmedizin, Corporate Member of Freie Universität Berlin, and Berlin Institute of Health, Berlin, Germany)
41. Prashant Kadam (Department of Hepatopancreatobiliary and Liver Transplant Surgery, University Hospitals Birmingham NHS Foundation Trust, Birmingham, United Kingdom)
42. Eric C. H. Lai (Department of Surgery, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China)
43. Jacob Ghotbi (The Intervention Centre and Department of HPB Surgery, Oslo University Hospital, Institute of Clinical Medicine, University of Oslo, Oslo, Norway)
44. Åsmund Avdem Fretland (The Intervention Centre and Department of HPB Surgery, Oslo University Hospital, Institute of Clinical Medicine, University of Oslo, Oslo, Norway)
45. Fabio Forchino (Department of General and Oncological Surgery. Mauriziano Hospital, Turin, Italy)
46. Alessandro Mazzotta (Department of Digestive, Oncologic and Metabolic Surgery, Institute Mutualiste Montsouris, Universite Paris Descartes, Paris, France)
47. Francois Cauchy (Department of HPB Surgery and Liver Transplantation, Beaujon Hospital, Clichy, France)
48. Chetana Lim (Department of Digestive, HBP and Liver Transplantation, Hopital Pitie-Salpetriere, Sorbonne Universite, Paris, France)
49. Bernardo Dalla Valle (General and Hepatobiliary Surgery, Department of Surgery, Dentistry, Gynecology and Pediatrics University of Verona, GB Rossi Hospital, Verona, Italy)
50. Junhao Zheng (Department of General Surgery, Sir Run-Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, China)
51. Phan Phuoc Nghia (Department of Hepatopancreatobiliary Surgery, University Medical Center, University of Medicine and Pharmacy, Ho Chi Minh City, Vietnam)
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schmelzle M, Krenzien F, Schoning W, Pratschke J. Laparoscopic liver resection: indications, limitations, and economic aspects. Langenbecks Arch Surg. 2020;405(6):725–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan Y, Cai X, Geller DA. Laparoscopic Liver Resection: A Review of Current Status. J Laparoendosc Adv Surg Tech A. 2017;27(5):481–6. [DOI] [PubMed] [Google Scholar]
- 3.Vigano L, Laurent A, Tayar C, Tomatis M, Ponti A, Cherqui D. The learning curve in laparoscopic liver resection: improved feasibility and reproducibility. Ann Surg. 2009;250(5):772–82. [DOI] [PubMed] [Google Scholar]
- 4.Goh BKP, Lee SY, Teo JY, Kam JH, Jeyaraj PR, Cheow PC, et al. Changing trends and outcomes associated with the adoption of minimally invasive hepatectomy: a contemporary single-institution experience with 400 consecutive resections. Surg Endosc. 2018;32(11):4658–65. [DOI] [PubMed] [Google Scholar]
- 5.Chua D, Syn N, Koh YX, Goh BKP. Learning curves in minimally invasive hepatectomy: systematic review and meta-regression analysis. Br J Surg. 2021;108(4):351–8. [DOI] [PubMed] [Google Scholar]
- 6.Chiow AKH, Fuks D, Choi G-H, Syn N, Sucandy I, Marino MV, et al. International multicentre propensity score-matched analysis comparing robotic versus laparoscopic right posterior sectionectomy. Br J Surg. 2021;108(12):1513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goh BKP, Lee SY, Koh YX, Kam JH, Chan CY. Minimally invasive major hepatectomies: a Southeast Asian single institution contemporary experience with its first 120 consecutive cases. ANZ J Surg. 2020;90(4):553–7. [DOI] [PubMed] [Google Scholar]
- 8.Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg. 2015;261(4):619–29. [DOI] [PubMed] [Google Scholar]
- 9.Wakabayashi G What has changed after the Morioka consensus conference 2014 on laparoscopic liver resection? Hepatobiliary Surg Nutr. 2016;5(4):281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halls MC, Berardi G, Cipriani F, Barkhatov L, Lainas P, Harris S, et al. Development and validation of a difficulty score to predict intraoperative complications during laparoscopic liver resection. Br J Surg. 2018;105(9):1182–91. [DOI] [PubMed] [Google Scholar]
- 11.Kawaguchi Y, Fuks D, Kokudo N, Gayet B. Difficulty of Laparoscopic Liver Resection: Proposal for a New Classification. Ann Surg. 2018;267(1):13–7. [DOI] [PubMed] [Google Scholar]
- 12.Linn YL, Wu AG, Han HS, Liu R, Chen KH, Fuks D, et al. Systematic review and meta-analysis of difficulty scoring systems for laparoscopic and robotic liver resections. J Hepatobiliary Pancreat Sci. 2022. [DOI] [PubMed] [Google Scholar]
- 13.Acosta LF, Garcia CR, Dugan A, Marti F, Davenport D, Gedaly R. Impact of super obesity on perioperative outcomes after hepatectomy: The weight of the risk. Surgery. 2017;162(5):1026–31. [DOI] [PubMed] [Google Scholar]
- 14.Chua DW, Syn N, Koh YX, Teo JY, Cheow PC, Chung AYF, et al. Association of standardized liver volume and body mass index with outcomes of minimally invasive liver resections. Surg Endosc. 2022. [DOI] [PubMed] [Google Scholar]
- 15.Abu Hilal M, Aldrighetti L, Dagher I, Edwin B, Troisi RI, Alikhanov R, et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg. 2018;268(1):11–8. [DOI] [PubMed] [Google Scholar]
- 16.Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005;12(5):351–35. [DOI] [PubMed] [Google Scholar]
- 17.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo Classification of Surgical Complications. Annals of Surgery. 2009;250(2):187–96. [DOI] [PubMed] [Google Scholar]
- 18.Harrell FJ, Lee K, Pollock B. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 1988;80(15):1198–202. [DOI] [PubMed] [Google Scholar]
- 19.Wang HP, Yong CC, Wu AGR, Cherqui D, Troisi RI, Cipriani F, et al. Factors associated with and impact of open conversion on the outcomes of minimally invasive left lateral sectionectomies: An international multicenter study. Surgery. 2022;172(2):617–24. [DOI] [PubMed] [Google Scholar]
- 20.Goh BKP, Syn N, Lee SY, Koh YX, Teo JY, Kam JH, et al. Impact of liver cirrhosis on the difficulty of minimally-invasive liver resections: a 1:1 coarsened exact-matched controlled study. Surg Endosc. 2021;35(9):5231–8. [DOI] [PubMed] [Google Scholar]
- 21.Kabir T, Tan ZZ, Syn NL, Wu E, Lin JD, Zhao JJ, et al. Laparoscopic versus open resection of hepatocellular carcinoma in patients with cirrhosis: meta-analysis. Br J Surg. 2021;109(1):21–9. [DOI] [PubMed] [Google Scholar]
- 22.Troisi RI, Berardi G, Morise Z, Cipriani F, Ariizumi S, Sposito C, et al. Laparoscopic and open liver resection for hepatocellular carcinoma with Child-Pugh B cirrhosis: multicentre propensity score-matched study. Br J Surg. 2021;108(2):196–204. [DOI] [PubMed] [Google Scholar]
- 23.McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62(5):1148–55. [DOI] [PubMed] [Google Scholar]
- 24.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42(1):44–52. [DOI] [PubMed] [Google Scholar]
- 25.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34(3):274–85. [DOI] [PubMed] [Google Scholar]
- 26.Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45(4):600–6. [DOI] [PubMed] [Google Scholar]
- 27.Kwan B, Waters PS, Keogh C, Cavallucci DJ, O’Rourke N, Bryant RD. Body mass index and surgical outcomes in laparoscopic liver resections: a systematic review. ANZ J Surg. 2021;91(11):2296–307. [DOI] [PubMed] [Google Scholar]
- 28.Urdaneta Perez MG, Garwe T, Stewart K, Sarwar Z, Morris KT. Obesity is an Independent Risk Factor for Mortality in Otherwise Healthy Patients After Hepatectomy. J Surg Res. 2020;255:50–7. [DOI] [PubMed] [Google Scholar]
- 29.Yu X, Yu H, Fang X. The impact of body mass index on short-term surgical outcomes after laparoscopic hepatectomy, a retrospective study. BMC Anesthesiol. 2016;16(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao L, Wang J, Kong J, Zheng X, Yu X. The impact of body mass index on short-term and long-term surgical outcomes of laparoscopic hepatectomy in liver carcinoma patients: a retrospective study. World J Surg Oncol. 2022;20(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heise D, Bednarsch J, Kroh A, Schipper S, Eickhoff R, Coolsen M, et al. Laparoscopic hepatectomy reduces postoperative complications and hospital stay in overweight and obese patients. World J Gastrointest Surg. 2021;13(1):19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHOE C Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. The Lancet. 2004;363(9403):157–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Association between BMI and blood loss in Eastern centers.
Supplemental Figure 2. Association between BMI and blood transfusion rate in Eastern centers
Supplemental Figure 3. Association between BMI and operation time in Eastern centers
Supplemental Figure 4. Association between BMI and postoperative morbidity in Eastern centers
Supplemental Figure 5. Association between BMI and postoperative length of stay in Eastern centers
Supplemental Figure 6. Association between BMI and blood loss in Western centers
Supplemental Figure 7. Association between BMI and blood transfusion rate in Western centers
Supplemental Figure 8. Association between BMI and open conversion rate in Western centers
Supplemental Figure 9. Association between BMI and operation time in Western centers
Supplemental Figure 10. Association between BMI and postoperative morbidity in Western centers
Supplemental Figure 11. Association between BMI and postoperative length of stay in Western centers