Abstract
BACKGROUND:
Anemia in pregnancy is a major public health problem, especially in developing countries. The most common cause is nutritional deficiencies, especially iron deficiency. Adequate nutritional intake from food is essential during pregnancy. Therefore, this study aimed to investigate the relationship between food access and intake patterns with the incidence of iron deficiency among pregnant women living in the slum settlement in Makassar City.
MATERIALS AND METHODS:
This research is a sub-study of the Indonesian Birth Cohort Study based in Makassar City. This sub-study used a cross-sectional design and recruited 173 pregnant women in their second and third trimesters using total sampling. All data were collected using a structured questionnaire and recorded using KoboToolbox software. Serum ferritin levels were examined for iron status using the ELISA method at the Microbiology Laboratory Unit at Hasanuddin University Teaching Hospital. The statistical data were analyzed using STATA version 14 with Chi-square analysis and logistic regression.
RESULTS:
The prevalence of iron deficiency in pregnant women living in slum settlements in Makassar City was 78%. Logistic regression analysis showed that inadequate food diversity (AOR: 2.58; 95% CI: 1.17-5.69; P = 0.019) and food taboos (AOR: 2.81; 95% CI: 1.26-6.26; P = 0.011) were significantly associated with the incidence.
CONCLUSIONS:
Most pregnant women living in slum settlements in Makassar City experienced iron deficiency. Pregnant women who experience iron shortages have been connected to food taboos and dietary diversity.
Keywords: Food diversity, food taboo, iron deficiency, pregnant women, slum settlement
Introduction
Anemia is the most common complication in pregnancy, with a high incidence worldwide. According to the World Health Organization, in 2019, the global prevalence of anemia was 29.9% in women of reproductive age aged 15-49 years: 29.6% in non-pregnant women and 36.5% in pregnant women.[1] If a pregnant woman’s iron stores are significantly reduced, it may cause anemia, which can interfere with oxygen to the placenta and fetus and impact fetal health.[2] The most common causes of anemia include nutritional deficiencies, especially iron deficiency.[3] Iron deficiency anemia in pregnant women remains a health problem in Indonesia. Over the past 20 years, Indonesia has had the fourth highest prevalence of anemia in pregnant women among the ten Association of Southeast Asian Nations countries, reported at 44.2% in 2019. Iron deficiency anemia is a high-prevalence problem of malnutrition.[4]
According to the United Nations International Children’s Emergency Fund, anemia increases the risk of bleeding and sepsis during birth and causes an increased frequency of maternal death. Babies born to mothers with anemia are at risk of premature birth, low birth weight, infectious diseases, weakened immune systems, cognitive disorders, impaired physical development, and neonatal death.[5]
Anemia is more common in pregnancy because the need for iron and nutrients increases, especially in the second and third trimesters. It is associated with increased maternal blood volume, placental development, fetal growth, and bleeding during delivery. The fetal brain needs iron to develop.[6] Women with low iron stores at conception or who do not meet their iron requirements during pregnancy are at increased risk of developing iron deficiency anemia.[7]
The incidence of nutritional anemia is inseparable from access to food and the consumption patterns of the individual. Access to food indirectly affects anemia and also affects consumption patterns. Access to food is the ability of households and individuals with their resources to obtain sufficient food for their nutritional needs. This can be measured by examining the household or individual scope. Consumption patterns are related to eating habits, the availability of foods containing iron enhancers or inhibitors, food restrictions during pregnancy, meal frequency, balanced food types, and food diversity, which are essential to iron status during pregnancy.
Iron and vitamin A intake are particularly low in urban slum-dwelling pregnant women, whereas demand for fatty and unhealthy foods is strong due to low prices and appealing flavors. Inadequate access to ANC is another factor that can be reflected in consumption patterns, making nutrition counseling for expectant women less effective.[8]
Iron status during pregnancy is significantly influenced by eating habits linked to food variety, meal frequency, accessibility to foods containing iron inhibitors, and dietary restrictions during pregnancy. One of the key elements affecting pregnancy and the fetus is a pregnant woman’s food intake. The personal health of the expectant mother is negatively impacted by an undernourished and unbalanced diet, which also impact the fetus’s growth.[5]
The amount of various food categories consumed over a specific reference period is referred to as dietary variety. For a mother’s health and development, a proper diet is crucial. Pregnancy nutrition is different from nutrition in the non-pregnant condition; eating enough food throughout pregnancy is essential for healthy delivery outcomes and the mother’s well-being.[9]
Limiting the type and amount of food ingested is a problem that frequently affects eating patterns in pregnant women, one of which is brought on by cultural factors or food taboos.[10] Meat, fish, potatoes, fruit, nuts, eggs, butternut, and pumpkin are the foods that expectant women should avoid eating the most. The majority of forbidden foods are excellent sources of vitamins, protein, and carbohydrates that are vital for the health of the mother and the growth of her unborn child.[11] If nourishing foods are utilized as a food taboo in this behavior, pregnant women run the danger of developing chronic energy shortage and anemia.[12]
To examine the prevalence of iron insufficiency in pregnant women, this study was done and inadequate food diversity and food taboos associated with maternal iron deficiency among pregnant women living in slum settlements in Makassar City, Indonesia.
Materials and Methods
Study design and subject
This sub-study of the Indonesian Birth Cohort Study was based in the Tallo sub-district, one of the high-density informal settlements in Makassar City, Indonesia. This sub-study used a cross-sectional design and recruited 173 pregnant women in their second and third trimesters using total sampling. Figure 1 shows a flowchart of participant recruitment.
Figure 1.
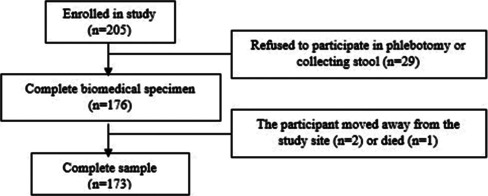
Flowchart of the participant recruitment
Data collection and tool
This study used a structured questionnaire that had been tested previously, with closed and open questions to obtain information about the characteristics of the participants and variable research data. The data were collected and recorded using KoboToolbox, an Android-based application. The independent variables in this study were physical food access, economical food access, social food access, food diversity, meal frequency, coffee consumption, tea consumption, milk consumption, and food taboos.
The variable of access to food used measurements based on the Food Security and Vulnerability Atlas 2020. Physical food access was measured as the distance from the market to the participant’s house (<3 km), economic food access was measured as consumption expenditure per month (<Indonesian GKM = IDR 299,433.00/month), and access to social food was measured as the consumption of four types of staple food every day. Food intake diversity was measured by the Food Frequency Questionnaire, which the researchers modified according to the consumption patterns of the Indonesian people. Using a validated questionnaire, the measurement of meal frequency (3 times/day), intake of coffee, tea, and milk, as well as food taboos and respondent characteristics were evaluated. Income measurement used the South Sulawesi UMR (IDR 3,385,145).
Serial ferritin levels were examined at the Microbiology Laboratory Unit at Hasanuddin University Teaching Hospital using the ELISA method (Ferritin Elisa Kit, DBC-Diagnostics Biochem Canada Inc). Pregnant women were classified as having adequate iron reserves in the body if their serum ferritin level was 25-283 ng/mL and experiencing iron deficiency if their serum ferritin was <25 ng/mL. The ELISA reader used was a Thermo Scientific-Multiskan FC with a 95-well plate. The examination results were read using a standard curve in Skanit 3.1 software.
Data analysis
The data analysis used STATA software version 14. Bivariate analysis of the relationship between iron deficiency and other variables using the Chi-square test was determined to be significant if the P value was <0.05. Variables with P values of <0.25 were included in the logistic regression model for multivariate analysis.
Results
The characteristics of the participants are seen in Table 1. A total of 137 pregnant women participated in this study. Of these, 113 (75.7%) were aged 20-35 years. Most (119; 68.8%) had completed secondary education. Regarding the participants’ occupation, 148 (85.6%) were housewives. More than half (100; 57.8%) had low income. Concerning gestational age, 96 (55.5%) participants were in the third trimester. As many as 125 (72.3%) had a multigravida history, and 110 (63.3%) were nulliparous. A total of 31 (17.9%) participants had experienced an abortion, and 91 (52.6%) had a birth interval of <2 years. Most participants (135; 78%) experienced iron deficiency during pregnancy.
Table 1.
Participant characteristics of 173 pregnant women
| Characteristic | n | % |
|---|---|---|
| Age (years) | ||
| <20 | 23 | 13.3 |
| 20-35 | 131 | 75.7 |
| >35 | 19 | 11.0 |
| Education | ||
| Completed elementary | 36 | 20.8 |
| Completed secondary | 119 | 68.8 |
| Completed tertiary | 18 | 10.4 |
| Occupation | ||
| Housewife | 148 | 85.6 |
| Employee | 5 | 2.9 |
| Self-employed | 17 | 9.8 |
| Other | 3 | 1.7 |
| Family income | ||
| Low | 100 | 57.8 |
| High | 73 | 42.2 |
| Gestational age | ||
| Trimester II | 77 | 44.5 |
| Trimester III | 96 | 55.5 |
| Gravida | ||
| Primigravida | 48 | 27.7 |
| Multigravida | 125 | 72.3 |
| Parity | ||
| Nullipara | 110 | 63.6 |
| Multipara | 63 | 36.4 |
| Abortus | ||
| Yes | 31 | 17.9 |
| No | 142 | 82.1 |
| Pregnancy interval (years) | ||
| <2 | 91 | 52.6 |
| ≥2 | 82 | 47.4 |
| Ferritin serum level | ||
| Iron deficiency | 135 | 78.0 |
| Normal | 38 | 23.0 |
Table 2 shows an analysis of characteristics related to the incidence of iron deficiency in pregnant women. The characteristics with a significant relationship were age 20-35 years (P = 0.058), gestational age in the third trimester (P = 0.000), and a pregnancy interval of <2 years (P = 0.028). The characteristics with no significant relationship to the incidence of iron deficiency were education (P = 0.480), occupation (P = 0.702), income (P = 0.065), gravida (P = 0.146), parity (P = 0.409), and history of abortion (P = 0.569).
Table 2.
Bivariate analysis between participant characteristics and iron status
| Characteristic | Iron status |
OR | 95% CI | P a | |||
|---|---|---|---|---|---|---|---|
| Deficient |
Normal |
||||||
| n | % | n | % | ||||
| Age (years) | |||||||
| <20 | 20 | 14.8 | 3 | 7.9 | 0.20 | 0.04-0.94 | 0.058* |
| 20-35 | 104 | 77.0 | 27 | 71.0 | 0.35 | 0.13-0.97 | |
| >35 | 11 | 8.2 | 8 | 21.1 | 1 | 1 | |
| Education | |||||||
| Completed elementary | 27 | 20.0 | 9 | 23.7 | 2.66 | 0.52-13.9 | 0.480 |
| Completed secondary | 92 | 68.2 | 27 | 71.0 | 2.34 | 0.50-10.8 | |
| Completed tertiary | 16 | 11.8 | 2 | 5.3 | 1 | 1 | |
| Occupation | |||||||
| Housewife | 116 | 85.9 | 32 | 84.2 | 1.10 | 0.11-10.2 | 0.702 |
| Employee | 4 | 3.0 | 1 | 2.6 | 1 | 1 | |
| Self-employed | 12 | 8.9 | 5 | 13.2 | 1.66 | 0.14-18.8 | |
| Other | 3 | 2.2 | 0 | 0 | 1 | 1 | |
| Family income | |||||||
| Low | 83 | 61.5 | 17 | 44.7 | 0.50 | 0.24-1.04 | 0.065 |
| High | 52 | 38.5 | 21 | 55.3 | 1 | 1 | |
| Gestational age | |||||||
| Trimester II | 50 | 37.0 | 27 | 71.0 | 1 | 1 | 0.000* |
| Trimester III | 85 | 63.0 | 11 | 29.0 | 0.23 | 0.10-0.52 | |
| Gravida | |||||||
| Primigravida | 42 | 30.4 | 7 | 18.4 | 1 | 1 | 0.146 |
| Multigravida | 94 | 69.6 | 31 | 81.6 | 1.93 | 0.78-4.74 | |
| Parity | |||||||
| Nullipara | 88 | 65.2 | 22 | 57.9 | 1 | 1 | 0.409 |
| Multipara | 47 | 34.8 | 16 | 42,1 | 1.36 | 0.65-2.83 | |
| Abortus | |||||||
| Yes | 23 | 17.0 | 8 | 21.0 | 1.29 | 0.52-3.19 | 0.569 |
| No | 112 | 83.0 | 30 | 79.0 | 1 | 1 | |
| Pregnancy interval (years) | |||||||
| <2 | 77 | 57.0 | 14 | 36.8 | 0.43 | 0.20.0.92 | 0.028* |
| ≥2 | 58 | 43.0 | 24 | 63.2 | 1 | 1 | |
*Significant. Pa the value of the difference between groups obtained from the Chi-square test
Table 3 shows the independent variables that had a significant relationship with the incidence of iron deficiency in pregnant women: inadequate economic food access (P = 0.037), unbalanced social food access (P = 0.020), lack of food diversity (P = 0.006), frequency of eating <3 times/day (P = 0.037), consuming coffee (P = 0.045), consuming tea (P = 0.041), and food taboos (P = 0.046). The variables that were not related to the incidence of iron deficiency were physical food access (P = 0.585) and milk consumption habits (P = 0.720).
Table 3.
Bivariate analysis of variables associated with the incidence of iron deficiency in pregnant women
| Variable | Iron status |
OR | 95% CI | P | |||
|---|---|---|---|---|---|---|---|
| Deficient | Normal | ||||||
| n | % | n | % | ||||
| Physical food access | |||||||
| Inadequate | 75 | 55.6 | 23 | 60.5 | 1.22 | 0.58-2.55 | 0.585 |
| Adequate | 60 | 44.4 | 15 | 39.5 | 1 | 1 | |
| Economic food access | |||||||
| Inadequate | 63 | 46.7 | 25 | 65.8 | 0.45 | 0.21-0.96 | 0.037* |
| Adequate | 72 | 53.3 | 13 | 34.2 | 1 | 1 | |
| Social food access | |||||||
| Inadequate | 71 | 52.6 | 28 | 73.7 | 0.39 | 0.17-0.87 | 0.020* |
| Adequate | 64 | 47.4 | 10 | 26.3 | 1 | 1 | |
| Food intake diversity | |||||||
| Not diverse | 96 | 71.1 | 18 | 47.4 | 2.73 | 1.30-5.71 | 0.006* |
| Varied | 39 | 28.9 | 20 | 52.6 | 1 | 1 | |
| Daily intake frequency | |||||||
| <3 times/day | 61 | 45.2 | 10 | 26.3 | 2.30 | 1.03-5.12 | 0.037* |
| ≥3 times/day | 74 | 54.8 | 28 | 73.7 | 1 | 1 | |
| Coffee consumption | |||||||
| Yes | 56 | 41.5 | 9 | 23.7 | 2.28 | 1.00-5.19 | 0.045* |
| No | 79 | 58.5 | 29 | 76.3 | 1 | 1 | |
| Tea consumption | |||||||
| Yes | 95 | 70.4 | 20 | 52.6 | 2.13 | 1.02-4.46 | 0.041* |
| No | 40 | 29.6 | 18 | 47.4 | 1 | 1 | |
| Milk consumption | |||||||
| Yes | 56 | 41.5 | 17 | 44.7 | 0.87 | 0.42-1.80 | 0.720 |
| No | 79 | 58.5 | 21 | 55.3 | 1 | 1 | |
| Food taboo | |||||||
| Yes | 78 | 57.8 | 15 | 39.5 | 2.09 | 1.00-4.37 | 0.046* |
| No | 57 | 42.2 | 23 | 60.5 | 1 | 1 | |
*Significant. Pa the value of the difference between groups obtained from the Chi-square test
The multivariate analysis of independent variables with the incidence of iron deficiency in this study used a logistic regression model. The variables of food diversity (AOR: 2.58; 95% CI: 1.17-5.69; P = 0.019) and food taboos (AOR: 2.81; 95% CI: 1.26-6.26; P = 0.011) were significantly related to the incidence of iron deficiency in pregnant women [Table 4].
Table 4.
Logistic regression analysis
| Variables | Model I |
Model II |
Model III |
|||
|---|---|---|---|---|---|---|
| COR (95% CI) | P | COR (CI 95%) | P | AOR (CI 95%) | P | |
| Physical Food Access | ||||||
| Inadequate | 0,46 (0,20-1,06) | 0.069 | ||||
| Adequate | 1 | |||||
| Economic Food Access | ||||||
| Inadequate | 0,46 (0,19-1,09) | 0.081 | ||||
| Adequate | 1 | |||||
| Access to Social Food | ||||||
| Inadequate | 2.31 (1,00-5,28) | 0.047* | 2,72 (1,25-5,91) | 0.011** | 3,05 (1,42-6,54) | 0.004** |
| Adequate | 1 | 1 | 1 | |||
| Daily intake frequency | ||||||
| <3 times/day | 2,57 (1,05-6,28) | 0.038* | 2,28 (0,98-5,29) | 0.054 | ||
| ≥3 times/day | 1 | 1 | ||||
| Coffee Consumption | ||||||
| Yes | 1,71 (0,69-4,27) | 0.245 | ||||
| No | 1 | |||||
| Tea Consumption | ||||||
| Yes | 1,64 (0,71-3,78) | 0.246 | ||||
| No | 1 | |||||
| Food Taboo | ||||||
| Yes | 2,56 (1,10-5,91) | 0.028* | 2,65 (1.21-5,81) | 0.014** | 2,40 (1,11-5,16) | 0.025** |
| No | 1 | 1 | 1 | |||
AOR=Adjusted odds ratio. *significant <0.05; ** significant <0.05
Discussion
This study found that 75% of pregnant women experienced iron deficiency. The prevalence of iron deficiency in pregnant women in this study was higher than in previous studies, such as in Lagos (12.3%), using a standard concentration of <15 μg/L;[13] Ghana (trimester I: 15.6%, trimester II: 20%, and trimester III: 38.3%), with a standard level of <15 μg/L2; Sri Lanka (41.9%), with a standard level of ≥30 ng/mL for pregnant women in the first trimester[14]; and Latvia (trimester I: 2.8%, trimester II: 7.9%, and trimester III: 27%) with a standard level of <30 μg/L.[6] This is because no previous studies have examined the incidence of iron deficiency among pregnant women in slums, using different serum ferritin standards and having different characteristics.
Pregnant women who do not eat a variety of foods are more likely to experience iron deficiency than those who do. Pregnant women need a variety of foods to increase nutrition, meet their increased nutritional needs during pregnancy, and prevent adverse effects on their fetuses.[15] This research is in line with that conducted on Unguja Island, Tanzania, which found that pregnant women with inadequate food consumption were more likely to experience anemia due to the need for adequate nutrition for the women and the development and growth of the fetus.[16]
A study conducted in Ghana showed that more than 50% of pregnant women were at risk of deficiency of vitamins A, E, B2, B3, and B6, folic acid, iron, protein, calcium, and zinc daily. The consumption of various foods provides essential nutrients and phytochemicals to the body for growth, development, and the prevention of various diseases, whereas low dietary diversity causes nutritional deficiencies. Improving diet and nutritional status both before and during pregnancy are essential to prevent unwanted effects of malnutrition during pregnancy.[15] The main risk factors for anemia are insufficient protein and iron intake.[17]
Previous research in Tigray, Ethiopia, showed that a low food diversity score was statistically significantly related to the incidence of anemia in pregnant women. Low dietary diversity causes mineral and vitamin deficiencies that can affect iron status. This is because pregnant women experience an increased need for energy and nutrition, and their eating frequency also increases.[18] Another study in Nepal showed that pregnant women with low food diversity during pregnancy risked micronutrient deficiencies and various problems such as nutritional anemia. Low food diversity is strongly influenced by the economy, knowledge of pregnant women’s nutrition, work, and family support.[19]
Research in southern Ethiopia shows that a low diet diversity score in pregnant women is significantly associated with anemia. Diversity of food refers to the nine groups of staple foods and cereals, vegetables, fruits, grains, meat, fish, organ meats, milk, and eggs.[20] Women who are expecting are more likely to have inadequate food diversity if they lack understanding about it. Adequate food diversity exists if pregnant women consume at least five food groups, such as vegetables, animal food sources, fruits, and dairy products.[9]
According to the study, the prevalence of iron insufficiency is correlated with pregnant women’s attitudes about food taboos. Food taboos or myths may affect the fetus, birthing process, or newborn. Crabs, shrimp, squid, shellfish, moringa leaves, eggplant, snake fruit, pineapple, durian, and cassava are foods to avoid. These restrictions are enforced by parents or in-laws, other relatives, and the local community.
Similar studies on pregnant Ethiopian women revealed that anemia can occur in pregnant women with dietary limitations. This can lead to poor quality and variety of food and impact pregnant women’s health, including causing anemia. Avoided foods include dark green leafy vegetables, which contain iron.[21] Research conducted in Tajikistan showed that food taboos can affect the development of the fetus, children’s health (including low birth weight), and malnutrition in pregnant women, which are considered significant drivers of neonatal mortality. Food taboos among pregnant women are primarily due to restrictions from their parents or in-laws.[22]
Several food restrictions exist in Indonesia, including prohibitions on seafood (squid and shrimp), despite their high protein and cholesterol content. Eggplant is also regarded as a taboo food, despite its vitamin A, folic acid, and iron content, which are essential during pregnancy. Pineapple is also avoided, although it contains vitamins A, B, and C and minerals such as calcium, phosphorus, and iron.[23] Moringa leaves are rich in a variety of essential amino acids and necessary minerals, including vitamins A, C, and E and iron.[24] Salak is rich in folic acid. Proteins found in shrimp, crab, squid, and shellfish stimulate the formation of fetal organs during pregnancy.[25] However, nutritionists do not recommend that pregnant women consume excessive seafood due to concerns that it may contain mercury and cholesterol, which may harm their health.[26]
Pregnancy-related iron deficiency might increase the risk of morbidity, including peripartum cardiomyopathy,[13] postpartum infections, and decreased oxygen supply to the placenta and infant,[2] which can result in premature birth and low birth weight.[27] Pregnant women are encouraged to attend antenatal care appointments, and pregnant women are monitored to regularly take iron supplement tablets.[13] Interventions to reduce iron deficiency include providing nutritional education on choosing appropriate healthy foods.[15]
This study examines Ferritin levels in pregnant women living in Makassar urban slums. It is known that until now, no similar study has been conducted in Makassar city with identical settlement and population characteristics. Thus, the findings of this study can be preliminary evidence to see a description of the incidence of deficiency in pregnant women. However, this study has several limitations, as well as evaluating dietary diversity depending on the accuracy of the ’respondent’s memory and using simply the food frequency questionnaire instead of a 24-hour recall. This study only identified a single biomarker, namely serum ferritin levels, without examining hemoglobin levels that can also be used as an indicator of anemia.
Conclusion
Variables statistically related to the incidence of iron deficiency in pregnant women are inadequate food intake, diversity of pregnant women, and food taboos. These two variables are related to the incidence of iron deficiency in pregnant women because pregnant women experience increased nutritional needs during pregnancy such as calories, iron, protein, and vitamins.
Financial support and sponsorship
This sub-study was supported by the Center for Epidemiology and Population Health Studies (CEPHS) at the Faculty of Public Health, Hasanuddin University.
Conflict of interest
There are no conflicts of interest.
Acknowledgment
The authors acknowledge the contribution of the phlebotomist who collected blood samples, all participants in this study, and their families. The biological samples analyzed in the study are the same samples that were collected with the support of the PMDSU program. This sub-study was conducted after obtaining written consent from each participant, clearly explaining the research purposes and the measurements used. Ethical approval was obtained from the Hasanuddin University Health Research Ethics Committee, with ethical approval recommendation number 13984/UN4.14.1/TP. 01.02.2022.
References
- 1.WHO Prevalence of anaemia in pregnant women (aged 15-49) (%) Prevalence 2021 Apr. 2021 Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/prevalence-of-anaemia-in-pregnant-women-(-) [Google Scholar]
- 2.Pobee RA, Setorglo J, Klevor M, Murray-Kolb LE. The prevalence of anemia and iron deficiency among pregnant Ghanaian women, a longitudinal study. PLoS One. 2021;16:e0248754. doi: 10.1371/journal.pone.0248754. doi: 10.1371/journal.pone.0248754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO Health Topics Anaemia 2022. Available from: https://www.who.int/health-topics/anaemia#tab=tab_1 . [Google Scholar]
- 4.Bresani CC, Braga MC, Felisberto DF, Tavares-de-Melo CE, Salvi DB, Batista-Filho M. Accuracy of erythrogram and serum ferritin for the maternal anemia diagnosis (AMA): A phase 3 diagnostic study on prediction of the therapeutic responsiveness to oral iron in pregnancy. BMC Pregnancy Childbirth. 2013;13:13.. doi: 10.1186/1471-2393-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bivolarska AV, Gatseva PD, Maneva AI. The role of eating habits on the iron status of pregnant women. J Am Coll Nutr. 2016;35:118–24. doi: 10.1080/07315724.2015.1014946. [DOI] [PubMed] [Google Scholar]
- 6.Rezgale R, Pudule I, Cauce V, Klaramunta Antila K, Bule V, Lazdane G, et al. Iron status in pregnant women in Latvia: An epidemiological, cross-sectional, multicenter study according to WHO and UK criteria. Medicina (Kaunas) 2022;58:955.. doi: 10.3390/medicina58070955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Løvschal LB, Høgh S, Bergholt T, Maslin K, Shawe J, Hegaard HK, et al. Iron supplementation during the first trimester of pregnancy after a national change of recommendation: A Danish cross-sectional study. J Nutr Sci. 2022;11:e19.. doi: 10.1017/jns.2022.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh-Jerath S, Devasenapathy N, Singh A, Shankar A, Zodpey S. Ante natal care (ANC) utilization, dietary practices and nutritional outcomes in pregnant and recently delivered women in urban slums of Delhi, India: An exploratory cross-sectional study. Reprod Health. 2015;12:20.. doi: 10.1186/s12978-015-0008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nigussie E, Ferede A, Markos M. Diversified dietary intake and associated factors among pregnant mothers attending antenatal care follow-up in public health facilities of Dire Dawa, Eastern Ethiopia. PLOS Glob Public Health. 2022;2:e0000002.. doi: 10.1371/journal.pgph.0000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chahyanto BA, Wulansari A. Aspek Gizi Dan Makna Simbolis Tabu Makanan Ibu Hamil Di Indonesia. J Ekol Kesehat. 2018;17:52–63. [Google Scholar]
- 11.Zerfu TA, Umeta M, Baye K. Dietary habits, food taboos, and perceptions towards weight gain during pregnancy in Arsi, rural central Ethiopia: A qualitative cross-sectional study. J Health Popul Nutr. 2016;35:22. doi: 10.1186/s41043-016-0059-8. doi: 10.1186/s41043-016-0059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kristya AM, Laras Sitoayu, Rachmanida Nuzrina PR, Sa’pang M. Perilaku Food Taboo pada Ibu Hamil dan Faktor yang Mempengaruhinyan di Puskesmas Pamarayan Kabupaten Serang, Banten. J Ekol Kesehat. 2021;20:139–51. [Google Scholar]
- 13.Ajepe AA, Okunade KS, Sekumade AI, Daramola ES, Beke MO, Ijasan O, et al. Prevalence and foetomaternal effects of iron deficiency anaemia among pregnant women in Lagos, Nigeria. PLoS One. 2020;15:e0227965. doi: 10.1371/journal.pone.0227965. doi: 10.1371/journal.pone. 0227965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amarasinghe GS, Agampodi TC, Mendis V, Malawanage K, Kappagoda C, Agampodi SB. Prevalence and aetiologies of anaemia among first trimester pregnant women in Sri Lanka; the need for revisiting the current control strategies. BMC Pregnancy Childbirth. 2022;22:16. doi: 10.1186/s12884-021-04341-z. doi: 10.1186/s12884-021-04341-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayensu J, Annan R, Lutterodt H, Edusei A, Peng LS. Prevalence of anaemia and low intake of dietary nutrients in pregnant women living in rural and urban areas in the Ashanti region of Ghana. PLoS One. 2020;15:e0226026. doi: 10.1371/journal.pone.0226026. doi: 10.1371/journal.pone. 0226026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibore NS, Ngowi AF, Munyogwa MJ, Ali MM. Dietary habits associated with anemia in pregnant women attending antenatal care services. Curr Dev Nutr. 2021;5:nzaa178. doi: 10.1093/cdn/nzaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asghari S, Mohammadzadegan-Tabrizi R, Rafraf M, Sarbakhsh P, Babaie J. Prevalence and predictors of iron-deficiency anemia: Women’s health perspective at reproductive age in the suburb of dried Urmia Lake, Northwest of Iran. J Educ Health Promot. 2020;9:332.. doi: 10.4103/jehp.jehp_166_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berhe K, Fseha B, Gebremariam G, Teame H, Etsay N, Welu G, et al. Risk factors of anemia among pregnant women attending antenatal care in health facilities of Eastern Zone of Tigray, Ethiopia, case-control study, 2017/18. Pan Afr Med J. 2019;34:121.. doi: 10.11604/pamj.2019.34.121.15999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shrestha V, Paudel R, Sunuwar DR, Lyman ALT, Manohar S, Amatya A. Factors associated with dietary diversity among pregnant women in the western hill region of Nepal: A community based cross-sectional study. PLoS One. 2021;16:e0247085. doi: 10.1371/journal.pone.0247085. doi: 10.1371/journal.pone.0247085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lebso M, Anato A, Loha E. Prevalence of anemia and associated factors among pregnant women in Southern Ethiopia: A community based cross-sectional study. PLoS One. 2017;12:e0188783.. doi: 10.1371/journal.pone.0188783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohammed SH, Taye H, Larijani B, Esmaillzadeh A. Food taboo among pregnant Ethiopian women: Magnitude, drivers, and association with anemia. Nutr J. 2019;18:19.. doi: 10.1186/s12937-019-0444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNamara K, Wood E. Food taboos, health beliefs, and gender: Understanding household food choice and nutrition in rural Tajikistan. J Health Popul Nutr. 2019;38:17.. doi: 10.1186/s41043-019-0170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diana R, Khomsan A, Anwar F, Christianti DF, Kusuma R, Rachmayanti RD. Dietary quantity and diversity among anemic pregnant women in Madura Island, Indonesia. J Nutr Metab. 2019;2019:2647230.. doi: 10.1155/2019/2647230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nadimin, Hadju V, As’ad S, Bukhari A, Arundhana AI, Imrawati A comparison between extract Moringa oleifera and iron tablet on prevention low birth weight in pregnant mothers in Makassar, Indonesia. Enferm Clin. 2020;30:26–30. [Google Scholar]
- 25.Jayadi YI, Dewi NU, Rahmawati, Hermiyanty, Herman, Syahrir S. Food taboo among pregnant women and children in the Kaili tribe Palu City, Indonesia. Enferm Clin. 2020;30:109–13. [Google Scholar]
- 26.Masruroh U. Identification of food avoidance myths in pregnant women, Kedungwringin village, Jatilawang district, Banyumas regency. Nusantara Raya International Conference. 2022;2022:213–6. [Google Scholar]
- 27.Majella MG, Sarveswaran G, Krishnamoorthy Y, Sivaranjini K, Arikrishnan K, Kumar SG. A longitudinal study on high risk pregnancy and its outcome among antenatal women attending rural primary health centre in Puducherry, South India. J Educ Health Promot. 2019;8:12.. doi: 10.4103/jehp.jehp_144_18. [DOI] [PMC free article] [PubMed] [Google Scholar]


