Abstract
Background:
Exposure to perfluoroalkyl substances (PFAS) has been shown to be neurotoxic in experimental studies, but epidemiological evidence linking prenatal PFAS exposure to child neurodevelopment is equivocal and scarce.
Objective:
To quantify associations between prenatal exposure to legacy PFAS and children’s intelligence (IQ) and executive functioning (EF) in a Canadian pregnancy and birth cohort and to determine if these associations differ by child sex.
Methods:
We measured first-trimester plasma concentrations of perfluorooctanoic acid (PFOA), perfluorooctanesulfonic acid (PFOS), and perfluorohexanesulfonic acid (PFHxS) in the Maternal-Infant Research on Environmental Chemicals (MIREC) study and assessed children’s full-scale (n = 522), performance (n = 517), and verbal (n = 519) IQ using the Wechsler Preschool and Primary Scale of Intelligence (WPPSI-III). Children’s working memory (n = 513) and ability to plan and organize (n = 514) were assessed using a parent-reported questionnaire, the Behavior Rating Inventory of Executive Function - Preschool Version (BRIEF-P). We quantified associations between individual log2-transformed PFAS exposure and children’s IQ and EF using multiple linear regression analyses and evaluated effect modification by child sex. We also used Repeated Holdout Weighted Quantile Sum (WQS) regression models with effect modification by child sex to quantify the effect of combined exposure to all three PFAS chemicals on IQ and EF. All models were adjusted for key sociodemographic characteristics.
Results:
Geometric mean plasma concentrations (IQR) for PFOA, PFOS and PFHxS were 1.68 (1.10-2.50), 4.97 (3.20-6.20) and 1.09 (0.67-1.60) μg/L respectively. We found evidence of effect modification by child sex in all models examining performance IQ (p < .01). Specifically, every doubling of PFOA, PFOS, and or PFHxS was inversely associated with performance IQ, but only in males (PFOA: B = −2.80, 95% CI: −4.92, −0.68; PFOS: B = −2.64, 95% CI: −4.77, −0.52; PFHxS: B = −2.92, 95% CI: −4.72, −1.12). Similarly, every quartile increase in the WQS index was associated with poorer performance IQ in males (B = −3.16, 95% CI: −4.90, −1.43), with PFHxS contributing the largest weight to the index. In contrast, no significant association was found for females (B = 0.63, 95% CI: −0.99, 2.26). No significant associations were found for EF in either males or females.
Conclusions:
Higher prenatal PFAS exposure was associated with lower performance IQ in males, suggesting that this association may be sex- and domain-specific.
Keywords: PFAS, Neurodevelopment, Cognition, IQ, Preschool, Prenatal
Introduction
Perfluoroalkyl substances (PFAS) are a broad class of synthetic compounds made up of carbon and fluorine (Rogers et al., 2021). Due to their unique amphipathic properties, they have been widely used in industrial and consumer products to increase durability and for their heat, water, and stain resistant properties (Buck et al., 2011). PFAS are intentionally added to several products, such as firefighting foams, non-stick cookware, food packaging, textiles, and cosmetics (Glüge et al., 2020). Often called “forever chemicals”, PFAS are highly stable and persistent in the environment and the biota, and can contaminate drinking water and foods, further exposing human populations (Glüge et al., 2020).
The most widely used and well-studied PFAS are perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) (USEPA, 2017). Another PFAS is perfluorohexane sulfonic acid (PFHxS), which is a member of the same chemical category as PFOS. These compounds were voluntarily phased-out by their major manufacturers in the early 2000s due to concerns about their potential toxicity and ability to bioaccumulate (3M, 2000; EPA, 2000; USEPA, 2017). Still, due to their persistent nature (Fenton et al., 2021), exposures to PFOA, PFOS, and PFHxS are ubiquitous and often considered as “legacy” PFAS, with detections rates over 95% in many studies of pregnant women, including in Canada (Chang et al., 2021; Fisher et al., 2016; Shu et al., 2018; Tsai et al., 2018).
In both epidemiological and experimental studies, PFAS have been found to be associated with wide-ranging adverse health effects, including immune and thyroid dysfunction, liver disease, lipid dysregulation, kidney disease, and adverse reproductive and developmental outcomes (Fenton et al., 2021). Less is known, however, about the potential neurotoxicity of prenatal PFAS exposure.
Pregnancy represents an especially vulnerable period of exposure for nervous system development. The developing brain undergoes rapid changes and critical processes, such as neural migration, proliferation, and synaptogenesis (Rice & Barone, 2000). Fetuses can be exposed to PFAS via placental exposure (Cai et al., 2020; Mamsen et al., 2019a; Wang et al., 2019). Thus, prenatal exposure to PFAS could have deleterious effects on neurodevelopment.
Animal studies have found that neonatal exposure to PFAS can affect neuroproteins (Johansson et al., 2009; Lee & Viberg, 2013) and neurochemical signaling (Foguth et al., 2019, 2020), as well as induce oxidative stress and apoptosis (Lee et al., 2012; Liu et al., 2007). Further, neonatal or early life exposure to PFAS has been associated with neurobehavioural and cognitive deficits in mice (Johansson et al., 2008; Mshaty et al., 2020; Onishchenko et al., 2011; Viberg et al., 2013).
Despite consistent evidence of PFAS’s developmental neurotoxicity in laboratory animals, the neurodevelopmental effects of prenatal PFAS exposure in humans are conflicting. Some investigators have found significant inverse associations between different PFAS and neurodevelopmental outcomes such as early learning and intelligence in early childhood (Goudarzi et al., 2016; Oh et al., 2021; Wang et al., 2015; Yao et al., 2022). In contrast, other investigators have found positive or null associations (Carrizosa et al., 2021; Harris et al., 2018; Liew et al., 2018; Skogheim et al., 2020; Spratlen et al., 2020a; Stein et al., 2013; Vuong et al., 2019).
Sex may influence the relation between prenatal PFAS exposure and child neurodevelopment. In certain animal models, the renal transport of PFOA is regulated by sex hormones (Kudo et al., 2002) and some PFAS have longer half lives in males (Dzierlenga et al., 2020; Huang et al., 2019; Khazaee et al., 2020; Mamsen et al., 2019b). It is unclear whether a similar hormonal mechanism is at play in humans (Harada et al., 2005; Li et al., 2018) and there are mixed findings in the literature regarding a sex-specific association between prenatal PFAS and cognitive functioning (Carrizosa et al., 2021; Goudarzi et al., 2016c; Harris et al., 2018, 2021; Liew et al., 2018; Oh et al., 2021a; Ribas-Fitó et al., 2007; Spratlen et al., 2020a; Stein et al., 2013; Vuong et al., 2016b, 2019).
The limited and inconsistent results from previous studies warrant further research. Many studies to date recruited small sample sizes (i.e., n between 120–302 participants), thus limiting their statistical power (Goudarzi et al., 2016b; Oh et al., 2021a; Spratlen et al., 2020a; Vuong et al., 2019; Wang et al., 2015a). Some studies measured PFAS in samples collected later in pregnancy (Goudarzi et al., 2017a; Oh et al., 2021b; Spratlen et al., 2020b; Wang et al., 2015b) when physiological changes occur such as plasma volume expansion or changes in glomerular filtration rate which can result in exposure misclassification (Aguree & Gernand, 2019; Faupel-Badger et al., 2007; Savitz & Wellenius, 2018; Verner et al., 2015). Moreover, most studies have examined PFAS compounds in isolation, without consideration for the combined effect of exposure to multiple PFAS, which precludes the ability to account for co-occurring exposures.
To date, there has been no study of prenatal exposure to legacy PFAS and neurodevelopment conducted in Canada. Furthermore, as of April 2021, the government of Canada issued a notice of intent to address the broad class of PFAS through continued research and review of policy changes over the next two years (Canada Gazette Part 1, 2021). Given the high public health relevance, the purpose of the present study was to examine associations between prenatal exposure to legacy PFAS and children’s intelligence and EF, to assess the effects of combined exposure to legacy PFAS, and to examine sex-specific effects in a large prospective Canadian cohort.
Methods
Study Population
Participants were recruited from the prospective multi-site Maternal-Infant Research on Environmental Chemicals (MIREC) Study. A complete description of the MIREC Study can be found in the cohort profile (Arbuckle et al., 2013). Briefly, 2001 women in their first trimester of pregnancy were recruited from 11 sites across Canada between 2008 and 2011. Women were included if they were fluent in either English or French, were 18 years or older, and <14 weeks gestation. Women were excluded if they had any known fetal abnormalities or a history of medical complications. During the first trimester, trained research staff administered questionnaires to collect sociodemographic (e.g., maternal age, level of education, income, and country of birth) and behavioural information (e.g., smoking). Pre-pregnancy body mass index (BMI) was determined by dividing self-reported weight (kg) by measured height squared (m2).
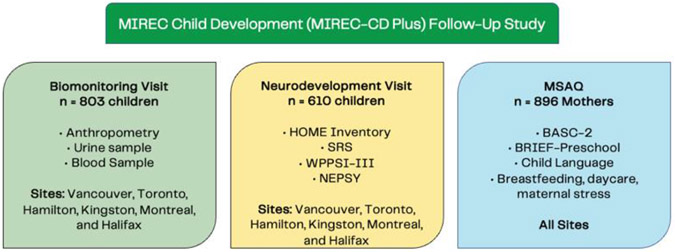
The present study also used data from the MIREC-Child Development (CD-Plus) follow-up study (Fisher et al., under review), which recruited MIREC families with children between the ages of 15 months and 5 years. The study included two home visits (biomonitoring; n = 803 and neurodevelopment; n = 610) and an online maternal self-administered questionnaire (MSAQ; n = 896; see Figure 1). The neurodevelopment Visit included mothers of singleton children between the ages of 3 and 4 years, who were born >28 weeks gestation, without congenital anomalies, a history of convulsions, or major neurologic disorders. Due to budgetary restrictions, the two home visits were limited to six of the recruitment sites from the original MIREC Study with the most participants: Vancouver, Toronto, Hamilton, Montreal, Kingston, and Halifax. However, participants from all sites were invited to complete the MSAQ in an online or paper format when their child was between 3 and 4 years of age.
Figure 1.
MIREC Child Development (MIREC-CD Plus) Follow-Up Study Visits (adapted from Fisher et al., under review) Abbreviations: SRS social responsiveness score, WPPSI-III Wechsler Preschool and Primary Scale of Intelligence, NEPSY Neuropsychological assessment, BASC-2 Behavior Assessment System, Brief-Preschool Behavior Rating Inventory of Executive Function - Preschool Version
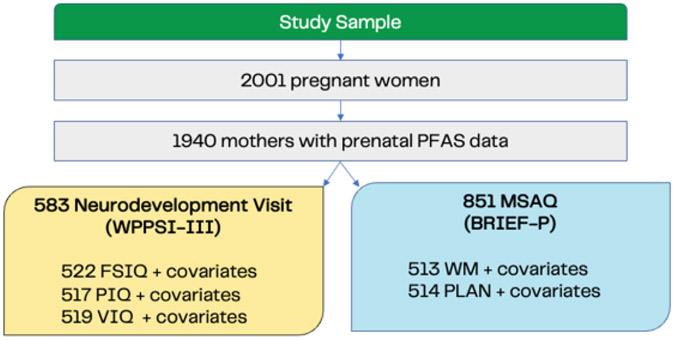
Of 1940 participants with prenatal PFAS exposure data, 851 had data on the Behavior Rating Inventory of Executive Function - Preschool Version (BRIEF-P), and 583 had data on the Weschler Preschool and Primary Scale of Intelligence (WPPSI-III). Of the 851 and 583 with complete BRIEF-P and WPPSI-III data, 514 and 522 had complete covariate data, respectively (See Figure 2).
Figure 2.
Study Sample
The MIREC Study was approved by the Health Canada Research Ethics Board and ethics committees at each of the participating sites. All women signed informed consent forms prior to participation.
PFAS Quantification
Blood samples were collected in the first trimester of pregnancy at a mean of 11.6 weeks gestation (range: 2-14). Samples were centrifuged and the plasma was aliquoted into smaller cryovials and stored at −80 °C within two hours of the blood draw. Chemical analyses were carried out at the Laboratoire de Toxicologie, Institut National de Santé Publique du Québec (Quebec City, Quebec, Canada), accredited by the Standards Council of Canada. Concentrations of PFAS, specifically, PFOA, PFOS, and PFHxS, were analyzed using ultra-high-pressure liquid chromatography (ACQUITY UPLC System; Waters Corporation, Milford, Massachusetts) coupled with tandem mass spectrometry, operated in the multiple reaction monitoring mode with an electrospray ion source in negative mode. The limits of detection (LODs) for PFOA, PFOS, and PFHxS were 0.1, 0.3, and 0.3 μg/L, respectively (Fisher et al., 2016).
Neurodevelopmental Outcomes
Intelligence (IQ).
Between ages 3 and 4, children’s intellectual abilities were assessed using the Wechsler Preschool and Primary Scale of Intelligence, Third Edition (WPPSI-III) (Wechsler, 2002). Trained researchers who were blinded to children’s PFAS exposure administered the test in children’s homes in either English or French. Age-standardized Canadian norms (WPPSI-III CDN Canadian Manual, 2004) were used to calculate the composite full-scale IQ (FSIQ), performance IQ (PIQ), and verbal IQ (VIQ) scores, with a mean of 100 and standard deviation of 15. FSIQ represents a measure of global intellectual functioning, PIQ represents nonverbal reasoning, spatial processing, and visual-motor skills, and VIQ represents verbal reasoning and comprehension
Executive Function (EF).
To assess children’s EF, mothers completed the BRIEF-P (Gioia et al., 2003) when their children were between 3 and 4 years of age. Mothers were unaware of their children’s PFAS exposure when completing the BRIEF-P. The inventory consists of 63 items and five subscales. For the purposes of the MIREC-CD Plus study, mothers completed 27 items pertaining to the working memory (WM) and planning/organizing (PLAN) subscales. The WM subscale represents difficulties retaining information needed to complete a task, and the PLAN subscale represents difficulties preparing for future events. Raw score totals for both subscales were converted to age- and sex-normed T-scores, with a mean of 50 and standard deviation of 10. Higher T-scores indicate poorer working memory or planning skills.
Covariates
A causal directed acyclic graph (Lipsky & Greenland, 2022) was used to choose covariates (see Suppl. Figure 1). We selected the following covariates: gestational week of blood sampling, maternal age, pre-pregnancy BMI, country of birth (Canadian born, foreign born), maternal level of education (trade school diploma or lower, bachelor’s degree or higher), parity (0, 1, 2+), maternal smoking during pregnancy (current smoker, former smoker, never smoked), study site, and the Home Observation Measurement of the Environment (HOME) score, a continuous measure of the quality of the child’s home environment (Caldwell & Bradley, 1979). A higher HOME score reflects a more caring and enriched home environment, with a maximum score of 55.
Statistical Analyses
We computed descriptive statistics for exposure and outcome variables, as well as model covariates. PFAS were log2-transformed to approximate a normal distribution and to interpret parameter estimates for each doubling of exposure, as well as to limit the possible effects of influential observations. Values below the limit of detection for PFOA (n = 1), PFOS (n = 1), and PFHxS (n = 26) were assigned a value of LOD/√2 (Hornung & Reed, 1990). Spearman correlations were used to examine the relationships among PFOA, PFOS, and PFHxS. Multiple linear regression analyses were run to quantify associations between log2-transformed PFAS exposure and children’s IQ and EF while controlling for relevant covariates (see Suppl. Figure 1).
We used weighted quantile sum (WQS) regression to examine the combined effect of PFOA, PFOS, and PFHxS on IQ and EF while controlling for relevant covariates (Carrico et al., 2015). Given that PFOA, PFOS, and PFHxS are correlated, linear regression models that assess the association of one PFAS compound with IQ or EF, while adjusting for the other compounds, are subject to dimensionality and collinearity. Further, linear regression models cannot quantify the combined impact of all PFAS compounds. The WQS approach addresses these issues by estimating a unidirectional weighted index from ranked chemical exposure data (Carrico et al., 2015). The estimation of weights, expressed as percentages that sum to one, indicate the relative contribution of each compound to the outcomes of interest (i.e., IQ and EF). A mean weight exceeding 33.3% (100%/3 compounds), indicates a larger contribution than expected by chance. We examined weights derived from 100 bootstrap models, with a 40:60 split of training and validation datasets. The mixture was set to have a negative association with our IQ outcomes, given our hypothesis that the mixture would be related to lower IQ scores. The mixture was set to have a positive association with our EF outcomes, given our hypothesis that the mixture would be related to higher scores on the BRIEF-P (i.e., higher scores indicate poorer performance). Further, the WQS index was created using PFAS quartiles, where a 1-unit increase in the WQS index was interpreted as a per-quartile increase in the log2-transformed PFAS mixture.
We ran the linear regression and WQS regression models with and without an interaction term between each PFAS or the WQS index and child sex to determine whether child sex modified the association between PFAS and child IQ or EF.
To improve the stability of the estimates and weights across training and validation data partitions, we implemented repeated holdout validation, which combines cross-validation and bootstrap resampling; repeating the WQS regression 100 times (Tanner et al., 2019). We report the mean effect estimate from the repeated holdout validation approach. In the case that the WQS coefficient is significantly different from zero, then we interpret and report the weights (Renzetti et al., 2021).
Regression diagnostics did not reveal any issues with collinearity, linearity, or homoscedasticity. However, the residuals for models run with FSIQ, VIQ, and WM as outcomes were negatively skewed with positive, excess kurtosis. Thus, all FSIQ, VIQ, and WM models were run with three outliers depicted in Q-Q plots removed. Further, diagnostics for all WQS models revealed slight deviations from linearity and homoscedasticity driven by the same three outliers. Therefore, WQS models were run with these outliers removed. Sensitivity analyses were run with the outliers included in all the linear regression and WQS models. Furthermore, additional analyses with maternal depression, measured via Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff, 1977), parental stress, measured via the Parenting Stress Index (Abidin, 1983), maternal alcohol consumption, and first trimester blood lead, were run for all WQS models that reached statistical significance.
All analyses were run using R software version 4.1.2 (R Core Team, 2021), and the gWQS package was used for WQS regression analysis (Renzetti et al., 2021). The p-value level of significance was .05.
Results
Descriptive Statistics
We tabulated the sociodemographic characteristics and outcome variables of the 522 mother-child dyads included in this study (Table 1). On average, mothers were 33 years old at delivery and had pre-pregnancy BMIs within the normal range. The majority of mothers were born in Canada (83%) and about two-thirds had a bachelor’s degree or higher (66%). Just under half (43%) were nulliparous and a minority (3%) smoked during pregnancy. Mother-child dyads had a mean HOME score of 47. Approximately half of the sample was male (~48%).
Table 1.
Demographic Characteristics of the 522 Mother-Child Dyads Included in the Present Study
| Demographic Characteristic | Mean ± SD or N (%) |
|---|---|
| Mothers | |
| Maternal Age (yrs) | 32.6 ± 4.5 |
| Pre-pregnancy BMI (kg/m2) | 25.1 ± 5.9 |
| Country of Birth | |
| Canadian born | 434 (83.1) |
| Foreign born | 88 (16.9) |
| Level of Education | |
| Bachelor’s Degree or Higher | 345 (66.1) |
| Trade School Diploma/High School | 177 (33.9) |
| Parity | |
| 0 | 226 (43.3) |
| 1 | 218 (41.8) |
| 2+ | 78 (14.9) |
| Smoked During Pregnancy | |
| Current | 13 (2.5) |
| Former | 143 (27.4) |
| Never | 337 (64.6) |
| Quit During Pregnancy | 29 (5.6) |
| Children | |
| Sex | |
| Male | 254 (48.7) |
| Female | 268 (51.3) |
| HOME Score | 47.4 ± 4.3 |
| Age at Neurodevelopmental Testing | 3.4 ± 0.3 |
Abbreviations: BMI = body mass index; HOME = Home Observation for Measurement of the Environment
Children were between 3 and 4 years old at the time of neurodevelopmental testing. Descriptive statistics and bivariate associations between, PFOA, PFOS, and PFHxS are shown in Table 2. PFOS had the highest geometric mean concentration, followed by PFOA, and PFHxS. There were no significant differences in the concentrations of PFOA, PFOS, or PFHxS by child sex (p > .05; see Table 3). PFOA, PFOS, and PFHxS were all positively, moderately correlated.
Table 2.
Descriptive statistics of PFAS (N = 522)
| PFAS (μg/L) | Descriptive Statistics |
Spearman Correlation
Matrix |
||||||
|---|---|---|---|---|---|---|---|---|
| GM | Median | IQR | Minimum | Maximum | % <LOD | PFOA | PFOS | |
| PFOA | 1.68 | 1.70 | 1.10-2.50 | 0.16 | 11.00 | 0.19 | -- | -- |
| PFOS | 4.97 | 4.40 | 3.20-6.20 | 0.50 | 19.00 | 0.19 | .565*** | -- |
| PFHxS | 1.09 | 1.00 | 0.67-1.60 | 0.30 | 24.00 | 4.98 | .498*** | .569*** |
Abbreviations: GM = geometric mean; IQR = interquartile range; PFAS = perfluoroalkyl substances; PFOA = perfluorooctanoic acid; PFOS = perfluorooctanesulfonic acid, PFHxS = perfluorohexanesulfonic acid
p < .001
Table 3.
Descriptive Statistics of PFAS by Sex
| Boys (n = 254) | GM | Median | IQR | Minimum | Maximum |
|---|---|---|---|---|---|
| PFOA | 1.64 | 1.70 | 1.10-2.50 | 0.16 | 11.00 |
| PFOS | 4.22 | 4.35 | 3.20-6.20 | 0.50 | 19.00 |
| PFHxS | 1.07 | 0.93 | 0.65-1.60 | 0.32 | 9.30 |
| Girls (n = 268) | GM | Median | IQR | Minimum | Maximum |
| PFOA | 1.70 | 1.80 | 1.15-2.50 | 0.41 | 11.00 |
| PFOS | 4.41 | 4.50 | 3.15-6.30 | 1.00 | 16.00 |
| PFHxS | 1.10 | 1.00 | 0.70-1.60 | 0.30 | 24.00 |
Abbreviations: GM = geometric mean; IQR = interquartile range; PFAS = perfluoroalkyl substances; PFOA = perfluorooctanoic acid; PFOS = perfluorooctanesulfonic acid, PFHxS = perfluorohexanesulfonic acid
p < .001
Descriptive statistics and bivariate associations between our outcome variables are shown in Table 4. Mean FSIQ, PIQ, VIQ, WM, and PLAN scores were within the average range. Nevertheless, three scores (0.6%) were in the extremely low range (below 70) for FSIQ, PIQ, and VIQ. Females had significantly higher FSIQ, PIQ, and VIQ scores compared to males (FSIQ: 110 vs. 105, p < 0.01; PIQ: 105 vs. 102, p = .01; VIQ: 112 vs 107, p < .01). Males and females did not significantly differ on their WM or PLAN scores. FSIQ was strongly, positively correlated with PIQ and VIQ, whereas PIQ and VIQ were moderately, positively correlated. WM and PLAN scores were strongly, positively correlated. Both WM and PLAN scores were weakly, negatively correlated with FSIQ, PIQ, and VIQ.
Table 4.
Descriptive Statistics of the Outcome Measurements
| Outcome | Descriptive Statistics | Pearson Correlation Matrix | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | Minimum | Maximum | FSIQ | PIQ | VIQ | WM | |
| FSIQ | 522 | 107.23 | 13.55 | 51 | 143 | -- | -- | -- | -- |
| PIQ | 517 | 103.31 | 14.81 | 55 | 144 | .831*** | -- | -- | -- |
| VIQ | 519 | 109.69 | 13.36 | 58 | 144 | .824*** | .381*** | -- | -- |
| WM | 513 | 53.03 | 10.64 | 36 | 93 | −.266*** | −.184*** | −.274*** | -- |
| PLAN | 514 | 50.88 | 10.33 | 32 | 87 | −.150*** | −.115*** | −.139*** | .801*** |
Abbreviations: FSIQ = Full-Scale IQ; PIQ = Performance IQ; VIQ = Verbal IQ; WM = Working Memory subscale; PLAN = Planning/organizing subscale.
p<0.001
Associations between PFAS and IQ
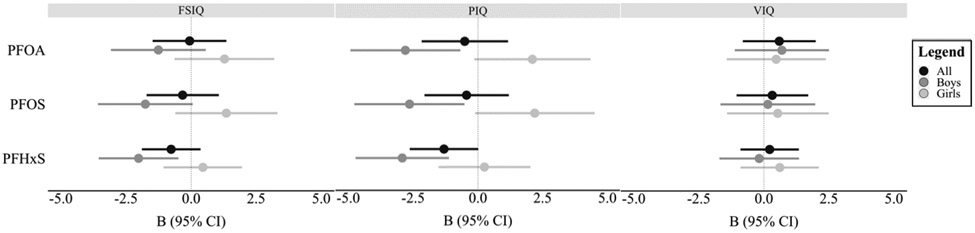
We observed evidence of effect modification by child sex in all models examining FSIQ and PIQ while controlling for relevant covariates (p for interaction term < .05; Supplemental Table 1, Figure 3). For males, every doubling in PFHxS was associated with 2.0-point (95% −3.6, −0.5) and 2.9-point (95% CI: −4.7, −1.1) decrements in FSIQ and PIQ, respectively. In contrast, for females, PFHxS was not associated with FSIQ or PIQ. PFOA and PFOS were also negatively associated with PIQ (PFOA: B = −2.8, 95% CI: −4.9, −0.7; PFOS: B = −2.6, 95% CI: −4.8, −0.5), but not FSIQ, in males, and were marginally positively associated with PIQ in females. No significant associations nor evidence of effect modification by sex were observed for VIQ.
Figure 3.
Visual representation of the covariate-adjusted associations between log2-transformed PFAS chemicals and IQ outcomes by sex. Abbreviations: PFAS = perfluoroalkyl substances; PFOA = perfluorooctanoic acid; PFOS = perfluorooctanesulfonic acid, PFHxS = perfluorohexanesulfonic acid; FSIQ = Full-Scale IQ; PIQ = Performance IQ; VIQ = Verbal IQ
Associations between PFAS and EF
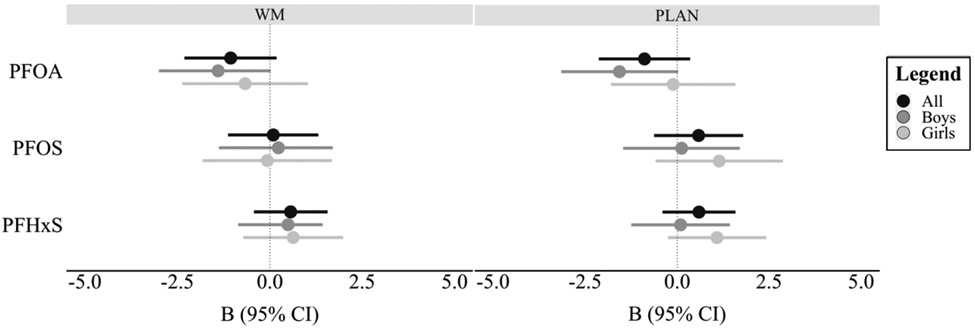
No significant associations were found between any of the PFAS and the WM or PLAN subscales while controlling for relevant covariates.. Further, no evidence of effect modification by child sex was found in any of the models (Supplemental Table 2, Figure 4); however, marginal inverse associations were found between PFOA and the WM and PLAN subscales in males.
Figure 4.
Visual representation of the covariate-adjusted associations between log2-transformed PFAS chemicals and EF outcomes by sex. Abbreviations: PFAS = perfluoroalkyl substances; PFOA = perfluorooctanoic acid; PFOS = perfluorooctanesulfonic acid, PFHxS = perfluorohexanesulfonic acid; WM = Working Memory subscale; PLAN = Planning/organizing subscale.
WQS Models
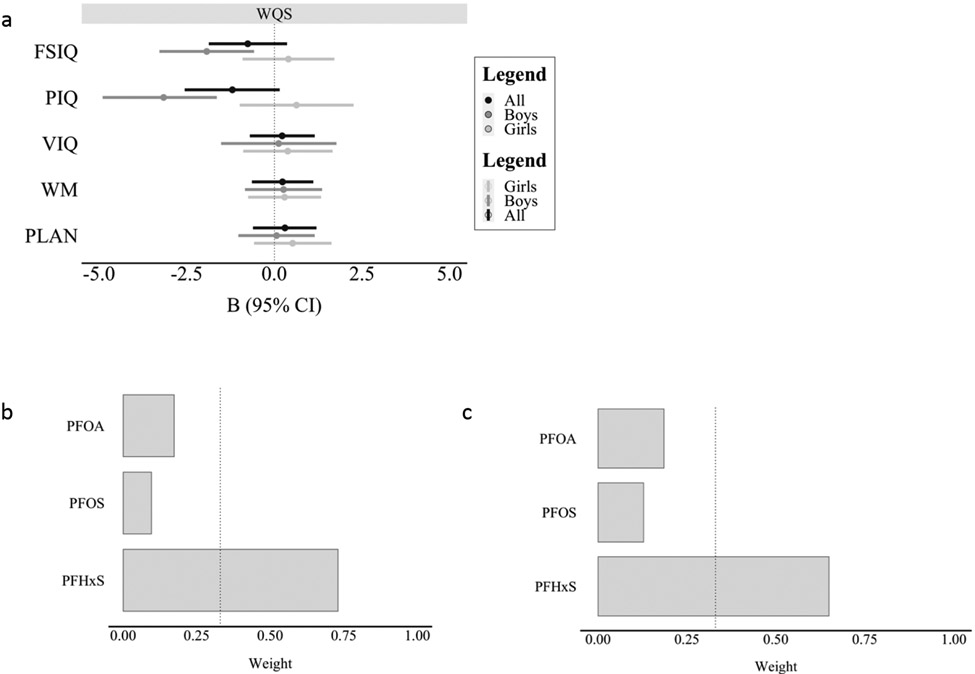
We observed a 1.9-point (95% CI: −3.3, −0.6) decrease in FSIQ only in males, for a quartile increase in the WQS index; however, the interaction term between sex and the WQS index was not significant (p = .13). In the model examining the effect of the WQS index on FSIQ in males, PFHxS was the most highly weighted compound and contributed 73% of the effect of the index on FSIQ, followed by PFOA (17%), and PFOS (9%) (see Figure 5b). We observed evidence of effect modification by child sex in the model examining PIQ (p = .04; Supplemental Table 3, Figure 5a). Specifically, every quartile increase in the WQS index was associated with a 3.1-point (95% CI: −4.9, −1.4) decrease in PIQ only in males. In the model examining the effect of the WQS index on PIQ in males, PFHxS was the most highly weighted compound and contributed 69% of the effect of the index on PIQ, followed by PFOA (19%), and PFOS (14%) (see Figure 5c). The WQS index was not significantly associated with VIQ, WM and PLAN subscales.
Figure 5.
a) Visual representation of the associations between the WQS index and IQ and EF outcomes by sex; b) relative weight of PFOA, PFOS, and PFHxS within the WQS index on FSIQ in males, dotted vertical line represents 33.3 percent (100/3 PFAS chemicals). c) relative weight of PFOA, PFOS, and PFHxS within the WQS index on PIQ in males, dotted vertical line represents 33.3 percent (100/3 PFAS chemicals). Abbreviations: PFAS = perfluoroalkyl substances; PFOA = perfluorooctanoic acid; PFOS = perfluorooctanesulfonic acid, PFHxS = perfluorohexanesulfonic acid; FSIQ = Full-Scale IQ; PIQ = Performance IQ; VIQ = Verbal IQ; WM = Working Memory subscale; PLAN = Planning/organizing subscale.
Sensitivity Analyses
Results of the multiple linear regression models or the WQS models did not change appreciably with the three outliers included in the model (see Supplemental Table 4 and 5). Adjusting for maternal depression, parental stress, alcohol consumption, and blood lead did not substantially impact the results estimating associations with males’ FSIQ or PIQ from the WQS model (Supplemental Table 6).
Discussion
This is the first Canadian pregnancy and birth cohort study to examine prenatal PFAS exposure and child sex in relation to preschool children’s intelligence and executive function. Overall, we found higher maternal PFAS concentrations were consistently associated with lower performance IQ in males, suggesting that this association may be sex- and domain-specific. We further used WQS regression to estimate the overall impact of the PFAS mixture. Consistent with our linear regression models, higher levels of the PFAS mixture were associated with decreased performance IQ only in males. Further evaluation of chemical weights within the WQS index identified PFHxS as a chemical of concern.
Our finding of male vulnerability to prenatal PFAS exposure was consistent with research examining sex-specific effects of PFAS (Carrizosa et al., 2021; Yao et al., 2022), the literature from the MIREC cohort (Azar et al., 2021; Desrochers-Couture et al., 2018; Green et al., 2019), and the broader literature on developmental neurotoxicity of some metals (Arbuckle, 2006; Gade et al., 2021; Singh et al., 2018). For example, in the INMA cohort in Spain, prenatal exposure to PFHxS and PFOA was associated with lower scores on the McCarthy Scales of Children’s Abilities only in males (Carrizosa et al., 2021). Although in a different developmental domain than those of interest in this study, in the Laizhou Wan Birth Cohort in China, prenatal PFBS exposure was significantly associated with lower gross motor development in infancy, but only in males (Yao et al., 2022). In contrast, the Hokkaido Study found a significant inverse association between PFOA and mental development only in females (Goudarzi et al., 2016a). Furthermore, other studies have found no consistent pattern between PFOS or PFOA and sex-specific neurodevelopmental effects (Harris et al., 2018; Liew et al., 2018).
Inconsistencies in results from previous studies may reflect differences in PFAS concentrations, study design, methodology, and population characteristics. For example, the geometric mean concentrations of PFOA (1.7 μg/L) and PFOS (4.3 μg/L) in the present study were generally lower than levels reported in pregnant women in the United States (Harris et al., 2021), Spain (Carrizosa et al., 2021), Denmark (Liew et al., 2018), and Taiwan (Wang et al., 2015a), where concentrations of PFOA range from 2.4 to 5.6 μg/L, and PFOS range from 6.1 to 28.1 μg/L. In contrast, the geometric mean concentration of PFHxS (1.1 μg/L) is generally consistent with levels previously reported in pregnant women (Carrizosa et al., 2021; Harris et al., 2018; Liew et al., 2018; Yan Wang et al., 2015). Nonetheless, the trends in the concentration levels appear to be in line with those observed in the general Canadian population (Pollock et al., 2021) and other pregnancy cohorts (Carrizosa et al., 2021; Harris et al., 2018; Liew et al., 2018; Wang et al., 2015).
PFAS toxicity may result in a sex-specific effect by one or more mechanisms. First, animal studies have consistently shown that PFAS have longer half-lives and slower clearance in males than females (Dzierlenga et al., 2020; Huang et al., 2019; Khazaee et al., 2020). In humans, the placenta is sexually-dimorphic (Martin et al., 2017) and PFAS accumulates at higher concentrations in the placenta of pregnancies with male fetuses than female fetuses (Mamsen et al., 2019b). Secondly, PFAS are known to be endocrine disruptors, with thyroid- (Coperchini et al., 2021) as well as gonadal-disrupting effects (Goudarzi et al., 2017; Itoh et al., 2016; Kobayashi et al., 2021; Nian et al., 2020). Research suggests that there is an interaction between gonadal and thyroid hormones and that certain neurotoxicants may change thyroid physiology in a sex-specific manner (Batista & Hensch, 2019). Since thyroid hormones are essential for neurodevelopment (Prezioso et al., 2018), it is possible that gonadal steroids interact with thyroid hormones to increase male susceptibility to IQ deficits. Lastly, sex differences in epigenetic transmission have been proposed as another mechanism to explain prenatal sex differential vulnerability. While epigenetic changes occur across the lifespan, the prenatal period is expected to be particularly vulnerable to epigenetic alterations (Kundakovic & Jaric, 2017). Epigenetic processes, such as DNA methylation, are known to impact neurodevelopment (Lister et al., 2013) and some studies have found that PFAS can impact DNA methylation differently in males and females. For example, in a Faroese birth cohort, prenatal PFOS exposure was associated with cord blood DNA methylome changes in males but not females; these changes were predicted to alter expression of genes that are involved in embryonic and nervous system development (Leung et al., 2018).
The observed association between PFAS exposure during early brain development and lower PIQ but not VIQ in males may be explained by the impact of PFAS on the hippocampus. The hippocampus is one of the dominant areas of PFAS accumulation in the brain (Cao & Ng, 2021), and neuropsychological and functional neuroimaging studies have shown that the hippocampus, in addition to learning and memory, plays a direct role in higher-order spatial perception (Lee et al., 2012; Lisman et al., 2017). Another reason why non-verbal intelligence may be more sensitive than verbal intelligence is that a more enriched home environment in early childhood (i.e., 0-6 years) is associated with greater verbal intelligence (Rodriguez & Tamis-Lemonda, 2011). Since maternal-child dyads in the MIREC study had relatively high scores on the HOME tool, it is possible that the richness of their environment buffered against the potential adverse effects of PFAS on verbal intelligence. Moreover, the brain regions associated with visual functioning may be more vulnerable to exposures in early pregnancy because they develop earlier than regions associated with language functioning. (Yin et al., 2019).
PFOA, PFOS, and PFHxS were all individually inversely associated with PIQ in males, but the WQS mixture was dominated by PFHxS. These findings may be explained, in part, by co-exposure confounding among the PFAS chemicals in the single PFAS-analyses and emphasize the importance of using novel mixture methods to assess the effect of co-exposure to multiple PFAS. The finding that PFHxS was found to be a chemical of concern for males over and above PFOA and PFOS is not unique to this study. Authors of a cohort study conducted in Boston reported that higher prenatal concentrations of a PFAS mixture (i.e., PFOA, PFNA, PFOS, PFHxS, EtFOSAA, and MeFOSAA) were associated with lower T4 levels in male infants; PFHxS and MeFOSAA were the most highly weighted for lower T4 levels in the WQS index (Preston et al., 2020). As previously mentioned, thyroid hormones are essential for neurodevelopment (Prezioso et al., 2018) and may mediate the relationship between prenatal PFHxS exposure and cognitive development in males. This suggests that PFHxS – which had the lowest concentration of all the PFAS – may have a lower benchmark dose of toxicity than PFOA and PFOS.
Our observed null association between prenatal PFAS exposure and EF is consistent with results from the Project Viva cohort in Boston (Harris et al., 2021) that found no significant associations between prenatal PFAS exposure on executive functioning difficulties between the ages of 6 and 10. In contrast, in the Danish national birth cohort, prenatal exposure to PFOA, perfluoroheptane sulfonic acid (PFHpS), PFHxS, PFOS, and perfluorooctanesulfonamide (PFOSA) were all associated with greater executive function difficulties at age 5, specifically, with greater issues with meta-cognition as assessed by the Meta-Cognition Index on the BRIEF-P (Bach et al., 2022). Similarly, the HOME Study in Cincinnati found that prenatal PFOS exposure was associated with greater meta-cognitive difficulties, as assessed by the Meta-Cognition Index and global executive functioning deficits, as assessed by the Global Executive Composite at ages 5 and 8 (Vuong et al., 2016) Inconsistencies in results may be due to the fact that our study had data on only two of the subscales of the BRIEF-P (WM and PLAN), which have lower reliabilities than composite or index scores, and thus, may have been less sensitive outcome measures (Sherman & Brooks, 2010). Our null findings may also be due to the age at testing, since EF deficits (especially planning and organizing) are more pronounced in older children (Best et al., 2009).
Strengths and Limitations
Strengths of this study include the prospective design and the large sample size (n = 522), which is in contrast with many previous studies with smaller sample sizes that range between 120 and 302 participants (Goudarzi et al., 2016b; Oh et al., 2021a; Spratlen et al., 2020a; Vuong et al., 2019; Wang et al., 2015a). We also controlled for a wide array of potential confounding factors including the quality of the home environment, which was lacking in many previous studies (Carrizosa et al., 2021; Goudarzi et al., 2016; Liew et al., 2018; Oh et al., 2021; Stein et al., 2013; Vuong et al., 2019). Furthermore, this study used individual-level prenatal PFAS plasma concentrations, which given their long half-lives, are likely not variable across pregnancy (Fenton et al., 2021). Indeed, the intraclass correlation between trimester one and trimester three PFAS samples ranges from 0.6 to 0.8 (Fisher et al., 2016). Finally, we used weighted quantile sum technique to estimate the relative contributions of PFOA, PFOS, and PFHxS on IQ and EF.
Compared to the general Canadian population giving birth in the same time period, women in the MIREC cohort were older, predominantly Caucasian, and had higher household incomes and education levels (Arbuckle et al., 2013). Future studies are needed to determine if the obtained results are generalizable to other populations. On the other hand, the narrower sociodemographic profile of the MIREC cohort also diminished potential confounding by socioeconomic status and ethnicity. Furthermore, we were unable to control for parental IQ or pregnancy physiology such as glomerular filtration rate or plasma volume expansion which can impact prenatal PFAS concentrations (Sagiv et al., 2015). However, given that our measures of PFAS were from trimester one of pregnancy, these physiological changes of pregnancy are less likely to be major confounders (Aguree & Gernand, 2019). Moreover, there may be multiple comparisons issues due to the number of regressions run. Nevertheless, we observed consistent associations across the multiple models. Further, by using the WQS approach, we were able to account for all exposures in one regression and found consistent associations.
Future Directions
This study focused on legacy PFAS, which is important given their long half-lives and ubiquitous exposure. Even though they are being phased out, exposures will persist for decades (OECD, 2006). More research is needed on the potential neurotoxicity and health effects of exposure to replacement PFAS (Brase et al., 2021). Further, we had only one measure of children’s IQ and EF scores at preschool age between 3 and 4 years. Longitudinal studies are needed to examine the associations of PFAS exposure during early brain development on cognitive abilities and in older children.
Conclusion
Subtle alterations in brain development can have wide-ranging impacts on both an individual and a population-based level (Lanphear, 2015). Higher prenatal exposure to PFAS was associated with lower non-verbal intelligence in males with PFHxS as the chemical of concern. This study is the first of its kind in Canada and may inform risk assessment and management of these chemicals.
Supplementary Material
Acknowledgements:
We would like to acknowledge the MIREC Study Group, as well as the MIREC study participants and staff. The MIREC Study was funded by the Chemicals Management Plan of Health Canada, the Canadian Institutes for Health Research (MOP-81285), and the Ontario Ministry of the Environment.
References
- 3M. (2000). Voluntary use and exposure information profile: perfluorooctane sulfonic acid and various salt forms (226-0928). 1–34. [Google Scholar]
- Abidin RR (1983). Parenting Stress Index. Pediatric Psychology. [DOI] [PubMed] [Google Scholar]
- Aguree S, & Gernand AD (2019). Plasma volume expansion across healthy pregnancy: A systematic review and meta-analysis of longitudinal studies. BMC Pregnancy and Childbirth, 19(1), 1–11. 10.1186/s12884-019-2619-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuckle TE (2006). Are there sex and gender differences in acute exposure to chemicals in the same setting? Environmental Research, 101(2), 195–204. 10.1016/j.envres.2005.08.015 [DOI] [PubMed] [Google Scholar]
- Arbuckle TE, Fraser WD, Fisher M, Davis K, Liang CL, Lupien N, Bastien S, Velez MP, Von Dadelszen P, Hemmings DG, Wang J, Helewa M, Taback S, Sermer M, Foster W, Ross G, Fredette P, Smith G, Walker M, … Ouellet E (2013). Cohort profile: The maternal-infant research on environmental chemicals research platform. Paediatric and Perinatal Epidemiology. 10.1111/ppe.12061 [DOI] [PubMed] [Google Scholar]
- Azar N, Booij L, Muckle G, Arbuckle TE, Séguin JR, Asztalos E, Fraser WD, Lanphear BP, & Bouchard MF (2021). Prenatal exposure to polybrominated diphenyl ethers (PBDEs) and cognitive ability in early childhood. Environment International, 146, 106296. 10.1016/j.envint.2020.106296 [DOI] [PubMed] [Google Scholar]
- Bach CC, Liew Z, Matthiesen NB, Henriksen TB, Bech BH, Nohr EA, Bonefeld-Jørgensen EC, & Olsen J (2022). In utero exposure to perfluoroalkyl and polyfluoroalkyl substances and attention and executive function in the offspring: A study in the Danish National Birth Cohort. Environmental Research, 212(April), 113262. 10.1016/j.envres.2022.113262 [DOI] [PubMed] [Google Scholar]
- Batista G, & Hensch TK (2019). Critical period regulation by thyroid hormones: Potential mechanisms and sex-specific aspects. Frontiers in Molecular Neuroscience, 12(April), 1–9. 10.3389/fnmol.2019.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JR, Miller PH, & Jones LL (2009). Executive functions after age 5: Changes and correlates. In Developmental Review (Vol. 29, Issue 3, pp. 180–200). 10.1016/j.dr.2009.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brase RA, Mullin EJ, & Spink DC (2021). Legacy and emerging per-and polyfluoroalkyl substances: Analytical techniques, environmental fate, and health effects. International Journal of Molecular Sciences, 22(3), 1–30. 10.3390/ijms22030995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, De Voogt P, Jensen AA, Kannan K, Mabury SA, & van Leeuwen SPJ (2011). Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integrated Environmental Assessment and Management, 7(4), 513–541. 10.1002/ieam.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Li QQ, Chu C, Wang SZ, Tang YT, Appleton AA, Qiu RL, Yang BY, Hu LW, Dong GH, & Zeng XW (2020). High trans-placental transfer of perfluoroalkyl substances alternatives in the matched maternal-cord blood serum: Evidence from a birth cohort study. Science of the Total Environment, 705, 135885. 10.1016/j.scitotenv.2019.135885 [DOI] [PubMed] [Google Scholar]
- Caldwell BM, & Bradley RH . (1979). Home observation for measurement of the environment. Little Rock: University of Arkansas at Little Rock. [Google Scholar]
- Cao Y, & Ng C (2021). Absorption, distribution, and toxicity of per- And polyfluoroalkyl substances (PFAS) in the brain: A review. Environmental Science: Processes and Impacts, 23(11), 1623–1640. 10.1039/d1em00228g [DOI] [PubMed] [Google Scholar]
- Carrico C, Gennings C, Wheeler DC, & Factor-Litvak P (2015). Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. Journal of Agricultural, Biological, and Environmental Statistics, 20(1), 100–120. 10.1007/s13253-014-0180-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrizosa C, Murcia M, Ballesteros V, Costa O, Manzano-Salgado CB, Ibarluzea J, Iñiguez C, Casas M, Andiarena A, Llop S, Lertxundi A, Schettgen T, Sunyer J, Ballester F, Vrijheid M, & Lopez-Espinosa MJ (2021). Prenatal perfluoroalkyl substance exposure and neuropsychological development throughout childhood: The INMA Project. Journal of Hazardous Materials, 416(January). 10.1016/j.jhazmat.2021.125185 [DOI] [PubMed] [Google Scholar]
- Chang CJ, Ryan PB, Smarr MM, Kannan K, Panuwet P, Dunlop AL, Corwin EJ, & Barr DB (2021). Serum per- and polyfluoroalkyl substance (PFAS) concentrations and predictors of exposure among pregnant African American women in the Atlanta area, Georgia. Environmental Research, 198(November 2020), 110445. 10.1016/j.envres.2020.110445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coperchini F, Croce L, Ricci G, Magri F, Rotondi M, Imbriani M, & Chiovato L (2021). Thyroid Disrupting Effects of Old and New Generation PFAS. Frontiers in Endocrinology, 11(January). 10.3389/fendo.2020.612320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrochers-Couture M, Oulhote Y, Arbuckle TE, Fraser WD, Séguin JR, Ouellet E, Forget-Dubois N, Ayotte P, Boivin M, Lanphear BP, & Muckle G (2018). Prenatal, concurrent, and sex-specific associations between blood lead concentrations and IQ in preschool Canadian children. Environment International, 121(Pt 2), 1235–1242. 10.1016/j.envint.2018.10.043 [DOI] [PubMed] [Google Scholar]
- Dzierlenga AL, Robinson VG, Waidyanatha S, DeVito MJ, Eifrid MA, Gibbs ST, Granville CA, & Blystone CR (2020). Toxicokinetics of perfluorohexanoic acid (PFHxA), perfluorooctanoic acid (PFOA) and perfluorodecanoic acid (PFDA) in male and female Hsd:Sprague dawley SD rats following intravenous or gavage administration. Xenobiotica, 50(6), 722–732. 10.1080/00498254.2019.1683776 [DOI] [PubMed] [Google Scholar]
- EPA. (2000). EPA and 3M ANNOUNCE PHASE OUT OF PFOS.
- Faupel-Badger JM, Hsieh CC, Troisi R, Lagiou P, & Potischman N (2007). Plasma volume expansion in pregnancy: Implications for biomarkers in population studies. Cancer Epidemiology Biomarkers and Prevention, 16(9), 1720–1723. 10.1158/1055-9965.EPI-07-0311 [DOI] [PubMed] [Google Scholar]
- Fenton SE, Ducatman A, Boobis A, DeWitt JC, Lau C, Ng C, Smith JS, & Roberts SM (2021). Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environmental Toxicology and Chemistry, 40(3), 606–630. 10.1002/etc.4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Arbuckle TE, Liang CL, Leblanc A, Gaudreau E, Foster WG, Haines D, Davis K, & Fraser WD (2016). Concentrations of persistent organic pollutants in maternal and cord blood from the maternal-infant research on environmental chemicals (MIREC) cohort study. Environmental Health: A Global Access Science Source, 15(1), 1–14. 10.1186/s12940-016-0143-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Muckle G, Lanphear B, Arbuckle TE, Braun JM, Zidek A, Velez MP, Lupien N, Bastien S, Ashley-Martin J, Oulhote Y, Borghese MM, Walker M, Asztalos E, Bouchard M, Booij L, Seal K, & Fraser W (under review). The Canadian Maternal-Infant Research on Environmental Chemicals Child Development Study (MIREC CD-PLUS). BMJ Open. [DOI] [PubMed] [Google Scholar]
- Foguth RM, Flynn RW, de Perre C, Iacchetta M, Lee LS, Sepúlveda MS, & Cannon JR (2019). Developmental exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) selectively decreases brain dopamine levels in Northern leopard frogs. Toxicology and Applied Pharmacology, 377(June), 114623. 10.1016/j.taap.2019.114623 [DOI] [PubMed] [Google Scholar]
- Foguth RM, Hoskins TD, Clark GC, Nelson M, Flynn RW, de Perre C, Hoverman JT, Lee LS, Sepúlveda MS, & Cannon JR (2020). Single and mixture per- and polyfluoroalkyl substances accumulate in developing Northern leopard frog brains and produce complex neurotransmission alterations. Neurotoxicology and Teratology, 81(June), 106907. 10.1016/j.ntt.2020.106907 [DOI] [PubMed] [Google Scholar]
- Gade M, Comfort N, & Re DB (2021). Sex-specific neurotoxic effects of heavy metal pollutants: Epidemiological, experimental evidence and candidate mechanisms. Environmental Research, 201(July), 111558. 10.1016/j.envres.2021.111558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glüge J, Scheringer M, Cousins IT, Dewitt JC, Goldenman G, Herzke D, Lohmann R, Ng CA, Trier X, & Wang Z (2020). An overview of the uses of per- And polyfluoroalkyl substances (PFAS). Environmental Science: Processes and Impacts, 22(12), 2345–2373. 10.1039/d0em00291g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudarzi H, Araki A, Itoh S, Sasaki S, Miyashita C, Mitsui T, Nakazawa H, Nonomura K, & Kishi R (2017a). The Association of Prenatal Exposure to Perfluorinated Chemicals. Environmental Health Perspectives, 125(1), 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudarzi H, Araki A, Itoh S, Sasaki S, Miyashita C, Mitsui T, Nakazawa H, Nonomura K, & Kishi R (2017b). The Association of Prenatal Exposure to Perfluorinated Chemicals. Environmental Health Perspectives, 125(1), 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudarzi H, Nakajima S, Ikeno T, Sasaki S, Kobayashi S, Miyashita C, Ito S, Araki A, Nakazawa H, & Kishi R (2016a). Prenatal exposure to perfluorinated chemicals and neurodevelopment in early infancy: The Hokkaido Study. Science of the Total Environment, 541, 1002–1010. 10.1016/j.scitotenv.2015.10.017 [DOI] [PubMed] [Google Scholar]
- Goudarzi H, Nakajima S, Ikeno T, Sasaki S, Kobayashi S, Miyashita C, Ito S, Araki A, Nakazawa H, & Kishi R (2016b). Prenatal exposure to perfluorinated chemicals and neurodevelopment in early infancy: The Hokkaido Study. Science of the Total Environment, 541, 1002–1010. 10.1016/j.scitotenv.2015.10.017 [DOI] [PubMed] [Google Scholar]
- Goudarzi H, Nakajima S, Ikeno T, Sasaki S, Kobayashi S, Miyashita C, Ito S, Araki A, Nakazawa H, & Kishi R (2016c). Prenatal exposure to perfluorinated chemicals and neurodevelopment in early infancy: The Hokkaido Study. The Science of the Total Environment, 541, 1002–1010. 10.1016/j.scitotenv.2015.10.017 [DOI] [PubMed] [Google Scholar]
- Green R, Lanphear B, Hornung R, Flora D, Martinez-Mier EA, Neufeld R, Ayotte P, Muckle G, & Till C (2019). Association between Maternal Fluoride Exposure during Pregnancy and IQ Scores in Offspring in Canada. JAMA Pediatrics. 10.1001/jamapediatrics.2019.1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K, Inoue K, Morikawa A, Yoshinaga T, Saito N, & Koizumi A (2005). Renal clearance of perfluorooctane sulfonate and perfluorooctanoate in humans and their species-specific excretion. Environmental Research, 99(2), 253–261. 10.1016/j.envres.2004.12.003 [DOI] [PubMed] [Google Scholar]
- Harris MH, Oken E, Rifas-Shiman SL, Calafat AM, Bellinger DC, Webster TF, White RF, & Sagiv SK (2021). Prenatal and childhood exposure to per- and polyfluoroalkyl substances (PFAS) and child executive function and behavioral problems. Environmental Research, 202. 10.1016/j.envres.2021.111621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MH, Oken E, Rifas-Shiman SL, Calafat AM, Ye X, Bellinger DC, Webster TF, White RF, & Sagiv SK (2018). Prenatal and childhood exposure to per- and polyfluoroalkyl substances (PFASs) and child cognition. Environment International, 115(March), 358–369. 10.1016/j.envint.2018.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, & Reed LD (1990). Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene, 5(1), 46–51. 10.1080/1047322X.1990.10389587 [DOI] [Google Scholar]
- Huang MC, Dzierlenga AL, Robinson VG, Waidyanatha S, DeVito MJ, Eifrid MA, Granville CA, Gibbs ST, & Blystone CR (2019). Toxicokinetics of perfluorobutane sulfonate (PFBS), perfluorohexane-1-sulphonic acid (PFHxS), and perfluorooctane sulfonic acid (PFOS) in male and female Hsd:Sprague Dawley SD rats after intravenous and gavage administration. Toxicology Reports, 6(June), 645–655. 10.1016/j.toxrep.2019.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Interstate Technology and Regulatory Council (ITRC). (2020). History and use of per- and poly-fluoroalkyl substances (PFAS) [Fact sheet]. https://pfas-1.itrcweb.org/fact_sheets_page/PFAS_Fact_Sheet_History_and_Use_April2020.pdf [Google Scholar]
- Itoh S, Araki A, Mitsui T, Miyashita C, Goudarzi H, Sasaki S, Cho K, Nakazawa H, Iwasaki Y, Shinohara N, Nonomura K, & Kishi R (2016). Association of perfluoroalkyl substances exposure in utero with reproductive hormone levels in cord blood in the Hokkaido Study on Environment and Children’s Health. Environment International, 94, 51–59. 10.1016/j.envint.2016.05.011 [DOI] [PubMed] [Google Scholar]
- Johansson N, Eriksson P, & Viberg H (2009). Neonatal exposure to PFOS and PFOA in mice results in changes in proteins which are important for neuronal growth and synaptogenesis in the developing brain. Toxicological Sciences, 108(2), 412–418. 10.1093/toxsci/kfp029 [DOI] [PubMed] [Google Scholar]
- Johansson N, Fredriksson A, & Eriksson P (2008). Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. NeuroToxicology, 29(1), 160–169. 10.1016/j.neuro.2007.10.008 [DOI] [PubMed] [Google Scholar]
- Khazaee M, Guardian MGE, Aga DS, & Ng CA (2020). Impacts of Sex and Exposure Duration on Gene Expression in Zebrafish Following Perfluorooctane Sulfonate Exposure. Environmental Toxicology and Chemistry, 39(2), 437–449. 10.1002/etc.4628 [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Sata F, Ikeda-Araki A, Miyashita C, Itoh S, Goudarzi H, Iwasaki Y, Mitsui T, Moriya K, Shinohara N, Cho K, & Kishi R (2021). Associations among maternal perfluoroalkyl substance levels, fetal sex-hormone enzymatic gene polymorphisms, and fetal sex hormone levels in the Hokkaido study. Reproductive Toxicology, 105(May), 221–231. 10.1016/j.reprotox.2021.09.003 [DOI] [PubMed] [Google Scholar]
- Kudo N, Katakura M, Sato Y, & Kawashima Y (2002). Sex hormone-regulated renal transport of perfluorooctanoic acid. Chemico-Biological Interactions, 139(3), 301–316. 10.1016/S0009-2797(02)00006-6 [DOI] [PubMed] [Google Scholar]
- Kundakovic M, & Jaric I (2017). The epigenetic link between prenatal adverse environments and neurodevelopmental disorders. Genes, 8(3). 10.3390/genes8030104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ACH, Yeung LK, & Barense MD (2012). The hippocampus and visual perception. Frontiers in Human Neuroscience, 6(APRIL 2012), 1–17. 10.3389/fnhum.2012.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HG, Lee YJ, & Yang JH (2012). Perfluorooctane sulfonate induces apoptosis of cerebellar granule cells via a ROS-dependent protein kinase C signaling pathway. NeuroToxicology, 33(3), 314–320. 10.1016/j.neuro.2012.01.017 [DOI] [PubMed] [Google Scholar]
- Lee I, & Viberg H (2013). A single neonatal exposure to perfluorohexane sulfonate (PFHxS) affects the levels of important neuroproteins in the developing mouse brain. NeuroToxicology, 37, 190–196. 10.1016/j.neuro.2013.05.007 [DOI] [PubMed] [Google Scholar]
- Leung YK, Ouyang B, Niu L, Xie C, Ying J, Medvedovic M, Chen A, Weihe P, Valvi D, Grandjean P, & Ho SM (2018). Identification of sex-specific DNA methylation changes driven by specific chemicals in cord blood in a Faroese birth cohort. Epigenetics, 13(3), 290–300. 10.1080/15592294.2018.1445901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Fletcher T, Mucs D, Scott K, Lindh CH, Tallving P, & Jakobsson K (2018). Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occupational and Environmental Medicine, 75(1), 46–51. 10.1136/oemed-2017-104651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew Z, Ritz B, Bach CC, Asarnow RF, Bech BH, Nohr Ellen A., B. R, Henriksen TB, Bonefeld-Jørgensen EC, & Olsen J (2018). Prenatal exposure to perfluoroalkyl substances and birth outcomes; An updated analysis from the Danish National Birth Cohort. International Journal of Environmental Research and Public Health, 15(9), 1–7. 10.3390/ijerph15091832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew Z, Ritz B, Bach CC, Asarnow RF, Bech BH, Nohr EA, Bossi R, Henriksen TB, Bonefeld-Jørgensen EC, & Olsen J (2018). Prenatal Exposure to Perfluoroalkyl Substances and IQ Scores at Age 5; a Study in the Danish National Birth Cohort. Environmental Health Perspectives, 126(6), 067004. 10.3390/ijerph15091832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky AM, & Greenland S (2022). Causal Directed Acyclic Graphs. JAMA - Journal of the American Medical Association, 327(11), 1083–1084. 10.1001/jama.2022.1816 [DOI] [PubMed] [Google Scholar]
- Lisman, Buzsaki G, Eichenbaum, Nadel L, Ranganath, & Redish D (2017). How the HPC contributes to memory, navigation and cognition. 20(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R, Mukamel EA, Nery JR, Urich M, Puddifoot CA, Johnson ND, Lucero J, Huang Y, Dwork AJ, Schultz MD, Yu M, Tonti-Filippini J, Heyn H, Hu S, Wu JC, Rao A, Esteller M, He C, Haghighi FG, … Ecker JR (2013). Global epigenomic reconfiguration during mammalian brain development. Science, 341(6146). 10.1126/science.1237905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Yu K, Shi X, Wang J, Lam PKS, Wu RSS, & Zhou B (2007). Induction of oxidative stress and apoptosis by PFOS and PFOA in primary cultured hepatocytes of freshwater tilapia (Oreochromis niloticus). Aquatic Toxicology, 82(2), 135–143. 10.1016/j.aquatox.2007.02.006 [DOI] [PubMed] [Google Scholar]
- Mamsen LS, Björvang RD, Mucs D, Vinnars MT, Papadogiannakis N, Lindh CH, Andersen CY, & Damdimopoulou P (2019a). Concentrations of perfluoroalkyl substances (PFASs) in human embryonic and fetal organs from first, second, and third trimester pregnancies. Environment International, 124(November 2018), 482–492. 10.1016/j.envint.2019.01.010 [DOI] [PubMed] [Google Scholar]
- Mamsen LS, Björvang RD, Mucs D, Vinnars MT, Papadogiannakis N, Lindh CH, Andersen CY, & Damdimopoulou P (2019b). Concentrations of perfluoroalkyl substances (PFASs) in human embryonic and fetal organs from first, second, and third trimester pregnancies. Environment International, 124(January), 482–492. 10.1016/j.envint.2019.01.010 [DOI] [PubMed] [Google Scholar]
- Martin E, Smeester L, Bommarito PA, Grace MR, Boggess K, Kuban K, Karagas MR, Marsit CJ, O’Shea TM, & Fry RC (2017). Sexual epigenetic dimorphism in the human placenta: Implications for susceptibility during the prenatal period. Epigenomics, 9(3), 267–278. 10.2217/epi-2016-0132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mshaty A, Haijima A, Takatsuru Y, Ninomiya A, Yajima H, Kokubo M, Khairinisa MA, Miyazaki W, Amano I, & Koibuchi N (2020). Neurotoxic effects of lactational exposure to perfluorooctane sulfonate on learning and memory in adult male mouse. Food and Chemical Toxicology, 145(August), 111710. 10.1016/j.fct.2020.111710 [DOI] [PubMed] [Google Scholar]
- Nian M, Luo K, Luo F, Aimuzi R, Huo X, Chen Q, Tian Y, & Zhang J (2020). Association between Prenatal Exposure to PFAS and Fetal Sex Hormones: Are the Short-Chain PFAS Safer? Environmental Science and Technology, 54(13), 8291–8299. 10.1021/acs.est.0c02444 [DOI] [PubMed] [Google Scholar]
- OECD. (2006). Preliminary Lists of PFOS, PFAS, PFOA and Related Compounds and Chemicals that May Degrade to PFCA. 10.1787/oecd_papers-v6-art38-en [DOI] [Google Scholar]
- Oh J, Schmidt RJ, Tancredi D, Calafat AM, Roa DL, Hertz-Picciotto I, & Shin HM (2021a). Prenatal exposure to per- and polyfluoroalkyl substances and cognitive development in infancy and toddlerhood. Environmental Research, 196(February), 110939. 10.1016/j.envres.2021.110939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Schmidt RJ, Tancredi D, Calafat AM, Roa DL, Hertz-Picciotto I, & Shin HM (2021b). Prenatal exposure to per- and polyfluoroalkyl substances and cognitive development in infancy and toddlerhood. Environmental Research, 196(February), 110939. 10.1016/j.envres.2021.110939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishchenko N, Fischer C, Wan Ibrahim WN, Negri S, Spulber S, Cottica D, & Ceccatelli S (2011). Prenatal exposure to PFOS or PFOA alters motor function in mice in a sex-related manner. Neurotoxicity Research, 19(3), 452–461. 10.1007/s12640-010-9200-4 [DOI] [PubMed] [Google Scholar]
- Pollock T, Karthikeyan S, Walker M, Werry K, & St-Amand A (2021). Trends in environmental chemical concentrations in the Canadian population: Biomonitoring data from the Canadian Health Measures Survey 2007–2017. Environment International, 155, 106678. [DOI] [PubMed] [Google Scholar]
- Preston EV, Webster TF, Claus Henn B, McClean MD, Gennings C, Oken E, Rifas-Shiman SL, Pearce EN, Calafat AM, Fleisch AF, & Sagiv SK (2020). Prenatal exposure to per- and polyfluoroalkyl substances and maternal and neonatal thyroid function in the Project Viva Cohort: A mixtures approach. Environment International, 139(April), 105728. 10.1016/j.envint.2020.105728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prezioso G, Giannini C, & Chiarelli F (2018). Effect of thyroid hormones on neurons and neurodevelopment. Hormone Research in Paediatrics, 90(2), 73–81. 10.1159/000492129 [DOI] [PubMed] [Google Scholar]
- R Core Team. (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- Radloff LS (1977). The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement, 1(3), 385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Renzetti S, Curtin P, Just AC, Bello G, & Gennings C (2021). gWQS: Generalized Weighted Quantile Sum Regression. R Package Version 3.0.4. [Google Scholar]
- Ribas-Fitó N, Torrent M, Carrizo D, Júlvez J, Grimalt JO, & Sunyer J (2007). Exposure to hexachlorobenzene during pregnancy and children’s social behavior at 4 years of age. Environmental Health Perspectives, 115(3), 447–450. 10.1289/ehp.9314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, & Barone SJ (2000). Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environmental Health Perspectives, 108 Suppl(Suppl 3), 511–533. 10.1289/ehp.00108s3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez ET, & Tamis-Lemonda CS (2011). Trajectories of the home learning environment across the first 5years: Associations with children’s vocabulary and literacy skills at prekindergarten. Child Development, 82(4), 1058–1075. 10.1111/j.1467-8624.2011.01614.x [DOI] [PubMed] [Google Scholar]
- Rogers RD, Reh CM, & Breysse P (2021). Advancing per- and polyfluoroalkyl substances (PFAS) research: an overview of ATSDR and NCEH activities and recommendations. Journal of Exposure Science and Environmental Epidemiology. 10.1038/s41370-021-00316-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Rifas-Shiman SL, Webster TF, Mora AM, Harris MH, Calafat AM, Ye X, Gillman MW, & Oken E (2015). Sociodemographic and Perinatal Predictors of Early Pregnancy Per- and Polyfluoroalkyl Substance (PFAS) Concentrations. Environmental Science and Technology, 49(19), 11849–11858. 10.1021/acs.est.5b02489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz DA, & Wellenius GA (2018). Invited Commentary: Exposure Biomarkers Indicate More Than Just Exposure. American Journal of Epidemiology, 187(4), 803–805. 10.1093/aje/kwx333 [DOI] [PubMed] [Google Scholar]
- Sherman EMS, & Brooks BL (2010). Behavior rating inventory of executive function - Preschool version (BRIEF-P): Test review and clinical guidelines for use. Child Neuropsychology, 16(5), 503–519. 10.1080/09297041003679344 [DOI] [Google Scholar]
- Shu H, Lindh CH, Wikström S, & Bornehag CG (2018). Temporal trends and predictors of perfluoroalkyl substances serum levels in Swedish pregnant women in the SELMA study. PLoS ONE, 13(12), 1–11. 10.1371/journal.pone.0209255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G, Singh V, Sobolewski M, Cory-Slechta DA, & Schneider JS (2018). Sex-dependent effects of developmental lead exposure on the brain. Frontiers in Genetics, 9(MAR), 1–17. 10.3389/fgene.2018.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogheim TS, Villanger GD, Weyde KVF, Engel SM, Surén P, Øie MG, Skogan AH, Biele G, Zeiner P, Øvergaard KR, Haug LS, Sabaredzovic A, & Aase H (2020). Prenatal exposure to perfluoroalkyl substances and associations with symptoms of attention-deficit/hyperactivity disorder and cognitive functions in preschool children. International Journal of Hygiene and Environmental Health, 223(1), 80–92. 10.1016/j.ijheh.2019.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratlen MJ, Perera FP, Lederman SA, Rauh VA, Robinson M, Kannan K, Trasande L, & Herbstman J (2020a). The association between prenatal exposure to perfluoroalkyl substances and childhood neurodevelopment. Environmental Pollution, 263, 114444. 10.1016/j.envpol.2020.114444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratlen MJ, Perera FP, Lederman SA, Rauh VA, Robinson M, Kannan K, Trasande L, & Herbstman J (2020b). The association between prenatal exposure to perfluoroalkyl substances and childhood neurodevelopment. Environmental Pollution, 263, 114444. 10.1016/j.envpol.2020.114444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CR, Savitz DA, & Bellinger DC (2013). Perfluorooctanoate and neuropsychological outcomes in children. Epidemiology, 24(4), 590–599. 10.1097/EDE.0b013e3182944432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner EM, Bornehag C-G, & Gennings C (2019). Repeated holdout validation for weighted quantile sum regression. MethodsX, 6, 2855–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MS, Miyashita C, Araki A, Itoh S, Bamai YA, Goudarzi H, Okada E, Kashino I, Matsuura H, & Kishi R (2018). Determinants and temporal trends of perfluoroalkyl substances in pregnant women: The Hokkaido study on environment and children’s health. International Journal of Environmental Research and Public Health, 15(5). 10.3390/ijerph15050989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA. (2017). Technical Fact Sheet - Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA). EPA 505-F-17-001, United States Environmental Protection Agency. May, 6. [Google Scholar]
- Verner MA, Loccisano AE, Morken NH, Yoon M, Wu H, McDougall R, Maisonet M, Marcus M, Kishi R, Miyashita C, Chen MH, Hsieh WS, Andersen ME, Clewell HJ, & Longnecker MP (2015). Assfociations of perfluoroalkyl substances (PFAS) with lower birth weight: An evaluation of potential confounding by glomerular filtration rate using a physiologically based pharmacokinetic model (PBPK). Environmental Health Perspectives, 123(12), 1317–1324. 10.1289/ehp.1408837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viberg H, Lee I, & Eriksson P (2013). Adult dose-dependent behavioral and cognitive disturbances after a single neonatal PFHxS dose. Toxicology, 304, 185–191. 10.1016/j.tox.2012.12.013 [DOI] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Webster GM, Sjödin A, Calafat AM, Braun JM, Dietrich KN, Lanphear BP, & Chen A (2016a). Prenatal polybrominated diphenyl ether and perfluoroalkyl substance exposures and executive function in school-age children. Environmental Research, 147, 556–564. 10.1016/j.envres.2016.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Webster GM, Sjödin A, Calafat AM, Braun JM, Dietrich KN, Lanphear BP, & Chen A (2016b). Prenatal polybrominated diphenyl ether and perfluoroalkyl substance exposures and executive function in school-age children. Environmental Research, 147, 556–564. 10.1016/j.envres.2016.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Xie C, Dietrich KN, Braun JM, Webster GM, Calafat AM, Lanphear BP, & Chen A (2019). Prenatal and childhood exposure to poly- and perfluoroalkyl substances (PFAS) and cognitive development in children at age 8 years. Environmental Research, 172, 242–248. 10.1016/j.envres.2019.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Han W, Wang C, Zhou Y, Shi R, Bonefeld-Jørgensen EC, Yao Q, Yuan T, Gao Y, Zhang J, & Tian Y (2019). Efficiency of maternal-fetal transfer of perfluoroalkyl and polyfluoroalkyl substances. Environmental Science and Pollution Research, 26(3), 2691–2698. 10.1007/s11356-018-3686-3 [DOI] [PubMed] [Google Scholar]
- Wang Y, Rogan WJ, Chen HY, Chen PC, Su PH, Chen HY, & Wang SL (2015a). Prenatal exposure to perfluroalkyl substances and children’s IQ: The Taiwan maternal and infant cohort study. International Journal of Hygiene and Environmental Health, 218(7), 639–644. 10.1016/j.ijheh.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Rogan WJ, Chen HY, Chen PC, Su PH, Chen HY, & Wang SL (2015b). Prenatal exposure to perfluroalkyl substances and children’s IQ: The Taiwan maternal and infant cohort study. International Journal of Hygiene and Environmental Health, 218(7), 639–644. 10.1016/j.ijheh.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. (2002). WPPSI-III administration and scoring manual. Psychological Corporation. [Google Scholar]
- WPPSI-III CDN Canadian Manual. (2004). Harcourt Assessment. [Google Scholar]
- Yao Q, Vinturache A, Lei X, Wang Z, Pan C, Shi R, Yuan T, Gao Y, & Tian Y (2022). Prenatal exposure to per- and polyfluoroalkyl substances, fetal thyroid hormones, and infant neurodevelopment. Environmental Research, 206(October 2021), 112561. 10.1016/j.envres.2021.112561 [DOI] [PubMed] [Google Scholar]
- Yin W, Chen MH, Hung SC, Baluyot KR, Li T, & Lin W (2019). Brain functional development separates into three distinct time periods in the first two years of life. NeuroImage, 189(August 2018), 715–726. 10.1016/j.neuroimage.2019.01.025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.