Abstract
Background:
Delta-9-tetrahydrocannabinol (THC) is the main psychoactive component of cannabis. Historically, rodent studies examining the effects of THC have used intraperitoneal injection as the route of administration, heavily focusing on male subjects. However, human cannabis use is often through inhalation rather than injection.
Objective:
We sought to characterize the pharmacokinetic and phenotypic profile of acutely inhaled THC in female rats, compared to intraperitoneal injection, to identify any differences in exposure of THC between routes of administration.
Methods:
Adult female rats were administered THC via inhalation or intraperitoneal injection. Serum samples from multiple timepoints were analyzed for THC and metabolites 11-hydroxy-delta-9-tetrahydrocannabinol and 11-nor-9-carboxy-delta-9-tetrahydrocannabinol using ultra-performance liquid chromatography-tandem mass spectrometry. Rats were similarly treated for locomotor activity analysis.
Results:
Rats treated with 2 mg/kg THC intraperitoneally reached a maximum serum THC concentration of 107.7±21.9 ng/mL. Multiple THC inhalation doses were also examined (0.25 mL of 40 or 160 mg/mL THC), achieving maximum concentrations of 43.3±7.2 and 71.6±22.5 ng/mL THC in serum, respectively. Significantly reduced vertical locomotor activity was observed in the lower inhaled dose of THC and the intraperitoneal injected THC dose compared to vehicle treatment.
Conclusion:
This study established a simple rodent model of inhaled THC, demonstrating the pharmacokinetic and locomotor profile of acute THC inhalation, compared to an i.p. injected THC dose, in female subjects. These results will help provide support for future inhalation THC rat research which is especially important when researching behavior and neurochemical effects of inhaled THC as a model of human cannabis use.
Keywords: Delta-9-tetrahydrocannabinol (THC), cannabis, liquid-chromatography/mass-spectrometry, rodent, serum, locomotor activity
1. Introduction
Cannabis (Cannabis sativa L.) is the second most used psychoactive drug in the United States (US), with nearly 12 million young adults reporting cannabis use in 2018 (1). As legalization of cannabis for both medical and recreational use becomes more common, there is increased interest in understanding both the short- and long-term effects of cannabis use. According to a 2020 study, cannabis use among both adolescents and adults in the US has continuously increased since 2006, positively correlating with increasing medical marijuana legalization (2). Delta-9-tetrahydrocannabinol (THC), which is responsible for the psychoactive effects of cannabis, exerts its effects via interaction with the endocannabinoid system as a cannabinoid receptor 1 (CB1) agonist (3, 4). The endocannabinoid system is largely comprised of two G-protein coupled receptors, CB1 and cannabinoid receptor 2 (CB2), as well as endogenous ligands including anandamide (AEA) and 2-arachidonylglycerol (2-AG), and various other associated proteins and enzymes, responsible for synthesis, breakdown, and transportation of these ligands (5, 6). Cannabis use induces a host of physiological and cognitive changes in humans, both in acute exposure as well as after chronic use, resulting in altered physiology, executive function, nociception, and appetite, among others (7-12). These results have been observed in preclinical rodent models as well (13-15).
Route of administration is an important factor to consider in both clinical and preclinical investigations of cannabis use and its effects. Human cannabis use is primarily through inhalation, and previous research has demonstrated that the route of administration alters the pharmacokinetics of THC in humans (16, 17). A multitude of clinical studies investigating the pharmacokinetics of acute THC inhalation has been published, with diverse results (16, 18-22). Due to differences in and lack of standardization across studies, maximum concentration (Cmax) values of THC can differ drastically across studies, with mean Cmax values ranging from 4 ng/mL (in whole blood) (20) to 163 ng/mL (in plasma) (18). Preclinical studies offer opportunity to control for many factors that cannot be controlled easily in clinical studies (e.g. environment, diet, previous drug exposure). However, the majority of rodent THC studies (both pharmacokinetic and phenotypic) use intraperitoneal (i.p.) injected THC rather than inhalation administration. Just as in clinical models, this can alter the pharmacokinetic profile of the compound, as well as its phenotypic effects. Recent years have seen increased investigation of inhaled THC in preclinical models (see (23-26) among others) but there is limited knowledge of how pharmacokinetic and pharmacodynamic properties may differ depending on sex, age, strain, and equipment.
Route of administration must be considered when looking at behavioral responses to THC. I.p. injected THC is subject to first-pass metabolism while inhaled THC is not, so the time course the psychoactive drug and its metabolites take through the body are altered depending on the route of administration. This could potentially effect when phenotypic effects are observed, and the magnitude of such effects. Altered locomotion is a common phenotype in many drugs of abuse, including THC. High doses of THC have been demonstrated to produce suppressed locomotion in preclinical literature of both i.p. injected and inhaled THC (23, 27-30), while there is some evidence of low doses of THC resulting in increased locomotor activity (31). The cannabinoid tetrad test, an established paradigm used in preclinical work to test if compounds have CB1 agonist activity, includes locomotion as one of the four assessments (32, 33). Observing the effects of inhaled and i.p. injected THC on locomotion alongside the pharmacokinetic profiles offers a more substantial understanding of how different routes of administration may or may not lead to the same outcome.
The goal of the present study was to develop a simple THC inhalation model in rodents that produces similar levels of THC [Cmax/Dose] to what is observed after human use of cannabis (23, 24, 34, 35).
2. Material and Methods
2.1. Animals:
Adult female Sprague Dawley (Taconic, Rensselaer, NY) rats 9-12 weeks old, were used in this study. All animals were single housed in a temperature-controlled environment on a 12-hour reverse light cycle (lights off 0900-1800). Food and water were provided ad libitum except during drug exposure. All experiments were conducted in compliance with the National Academy of Sciences Guide for the Care and Use of Laboratory Animals and approved by the University at Buffalo Institutional Animal Care and Use Committee.
2.2. Drug:
THC was obtained from the NIDA Drug Supply Program. THC for inhalation was prepared in a 95% ethanol vehicle from a stock solution of 200 mg/mL. THC for i.p. injections was prepared in a vehicle solution of 1:1:18 ethanol:Kolliphor®:sterile saline, for a final concentration of 1 mg/mL THC, and i.p. injected at a volume of 2 μL/g.
2.3. Treatment:
2.3.1. Delta-9-tetrahydrocannabinol Inhalation:
Rats were placed in a sealed inhalation chamber (2.5 gallon; 16.5x11.3x5.5 inches) for a 5-minute habituation period. THC solution was vaporized using a Volcano vaporizer (Storz and Bickel, Germany). THC solution (0.25 mL) was dispensed onto steel pads for final amounts of 10 or 40 mg. After allowing ethanol to fully evaporate, the steel pad was placed into the vaporizer, and the drug was vaporized (at heat setting 9, approximately 226 °C). Vaporized THC was collected in an 8 L plastic balloon. THC vapor was administered into each airtight chamber through a fitted adaptor which was then sealed, and a 10-minute exposure period followed. Exposures were conducted under a fume hood to eliminate any risk of exposure to the investigators. Animals were continuously monitored throughout inhalation. All animals were returned to their home cages before the start of testing.
2.3.2. Delta-9-tetrahydrocannabinol Intraperitoneal Injections:
Animals were administered an i.p. injection of 2 mg/kg after body weight was taken and returned to their home cages before the start of testing.
2.4. Blood Collection:
Blood was collected at pre-determined timepoints of 5, 10, 20, 80, 160, 320, and 480 minutes. This time was either from the time of injection, or from the time the animals were removed from the 10-minute inhalation administration. Animals were rapidly anesthetized with 2% isoflurane, and blood was collected via tail vein. Each animal had blood collected at 1-2 timepoints, with no more than 1% of the animal’s body weight being collected in total (total n=36). Collected blood was placed into a 1.5 mL microcentrifuge tube and allowed to clot at room temperature for 30-45 minutes. The sample was then centrifuged for 15 minutes at 4°C and 604 x g. Serum was aliquoted and placed into a −80°C freezer for storage until analysis.
2.5. Analysis of test samples using UPLC-MS/MS
2.5.1. Materials and Reagents:
Commercially available standards (purity > 98%) for delta-9-tetrahydrocannabinol (THC), 11-nor-9-carboxy-delta-9-tetrahydrocannabinol (THC-COOH), and 11-hydroxy-delta-9-tetrahydrocannabinol (11-OH-THC) were obtained from Cerilliant (Round Rock, TX, USA). Additionally, deuterated internal standard (purity >98%) (IS) delta-9-tetrahydrocannabinol-d3 (THC-d3) was also obtained from Cerilliant (Round Rock, TX, USA). LC-MS grade water, acetonitrile, isopropanol, methanol, and formic acid were sourced from Fisher Scientific (Fair Lawn, NJ, USA). Blank rat serum was obtained from Innovative Research, Inc. (Novi, MI, USA).
2.5.2. Instrumentation and Analytical Conditions:
The ultraperformance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) bioanalytical method was developed based on a previously validated method for use of detection of cannabinoids in low THC varieties of cannabis (36). A Waters Acquity Class-I UPLC coupled with a Xevo TQ-S Micro triple quadrupole mass spectrometer was used for separation and detection of cannabinoids and their metabolites. The system was controlled by MassLynx 4.2 with data processed using TargetLynx XS™ (Waters, Milford, MA, USA). Chromatographic separation was achieved as previously described (36), with some modifications; namely, the gradient elution started at 18% A which was linearly decreased to 0% over 4.5 minutes before a steep return to the initial conditions for re-equilibration of the column. The sample injection volume was 4 μL.
2.5.3. Preparation of calibration and quality control samples:
Two mix stock solutions (10 and 1 μg/mL) were prepared by combining the appropriate volume of 100 μg/mL primary stock of each compound to get the final concentrations, per compound. These mix stocks were then used to make eight working stock solutions (25, 50, 100, 250, 500, 1000, 1500, and 2500 ng/mL). Each calibration standard was prepared by spiking 18 μL of blank serum with 2 μL of the associated working stock to yield serum concentrations in the range of 2.5-250 ng/mL. The quality control samples were prepared from a different primary stock using the same method at the four following concentrations: 2.5 (lower limit of quantification (LLOQ)), 6 (low quality control (LQC)), 80 (medium quality control (MQC)), and 180 ng/mL (high quality control (HQC)).
2.5.4. Sample Preparation:
Samples were thawed to room temperature prior to analysis. Each sample was mixed by vortex and 20 μL was subjected to a simple and fast protein precipitation method for the removal of endogenous substances and extraction. Test samples, blank, calibration, and quality control standards were quenched with 100 μL of methanol containing 0.05% formic acid and 10 ng/mL IS. The samples were vortex mixed for 5 minutes at 650 rpm before being transferred to a 96-well Millipore (Burlington, MA, USA) multiscreen Solvinert 0.45 μm filter plates. The samples were filtered by centrifugation at 850×g for 2 minutes at 4°C. The filtrate was then subjected to UPLC-MS/MS analysis.
2.5.5. Bioanalytical Method Validation:
The Food and Drug Administration (FDA) guidelines for validation of bioanalytical methods were used to assess sensitivity, selectivity, linearity, carryover, accuracy, precision, recovery, and stability (37). The sensitivity of the method was determined by measuring the limit of detection (LOD) and the LLOQ. Selectivity was assessed by analyzing six different samples of blank rat serum to ensure the absence of interference at retention times of the analytes and IS. Calibration curves were created by plotting the analyte to internal standard peak area ratio against the nominal concentration in serum. The linear range of this method was 2.5-250 ng/mL and the linear fit was determined by 1/X weighing method for all sample runs. Carryover was assessed by running blank serum samples immediately following HQCs and comparing the peak analyte area in the blank to that of the LLOQ. Inter- and intra-day accuracy and precision were evaluated on three different days using QC samples at all four concentrations (n=6, each). Accuracy was evaluated by comparing the spiked concentration of the QC to the curve and precision was evaluated by comparing the observed values to one another. Recovery was assessed by comparing the analyte area in serum spiked with the compound before protein precipitation (pre-spike) or after protein precipitation (post-spike). The recovery was calculated as the ratio of the analyte area under the curve in the pre- versus post-spike samples. The possible matrix effect of serum was assessed by spiking the quencher solution (methanol containing 0.05% formic acid and 10 ng/mL IS) with the working stock solutions used to generate the calibration curve and adding either 20 μL of water or blank serum. The ratio of the mean analyte area under the curve of the calibration standards was compared to determine the percent matrix effect. Stability was assessed for conditions that were most likely to occur during sample collection, storage, and analysis. This included benchtop, autosampler, freeze-thaw, and stock stability.
2.6. Locomotor Activity:
A separate cohort of adult female Sprague Dawley rats (n=15) were tested for locomotor activity in an OpenField (OF) arena (Coulbourn Instruments, Holliston, MA). All animals underwent a habituation session, followed by five THC (or vehicle) administration days, each separated by a 3-day abstinence period. OF testing and analysis was conducted as previously described (38). On experimental days, animals first underwent either the i.p. injection of vehicle or THC, or 10 minutes of vehicle or THC exposure. Immediately following vehicle or drug administration, animals were placed into the OF chamber for 45 minutes.
2.7. Data Analysis:
All graphs and statistical analyses were generated using GraphPad Prism 8 (San Diego CA). A p value <0.05 was deemed significant.
2.7.1. Pharmacokinetic Analyses:
For the calculations of pharmacokinetic parameters, serum concentration-time data was subjected to non-compartmental analysis using Phoenix Version 8.3 (Certara, Princeton, NJ, USA). Maximum serum concentration (Cmax) and time to reach Cmax (Tmax) were identified directly from concentration-time data for THC and its metabolites. A linear trapezoidal method was used the calculation of area under the serum concentration-time profile (AUC), and clearance (CL/F) was calculated as Dose/AUC. Serum concentrations found below the LLOQ were excluded from the pharmacokinetic analysis (39).
2.7.2. Locomotor Analyses:
Two-way RM Analyses of the Variance (ANOVAs) were performed in GraphPad Prism 8 to analyze the open field data over time. One-way RM ANOVAs and paired t-tests were performed to analyze the totaled locomotor data. Post-hoc analyses were done with Tukey’s multiple comparisons or Sidak’s multiple comparisons tests if applicable.
3. Results
3.1. UPLC-MS/MS method validation:
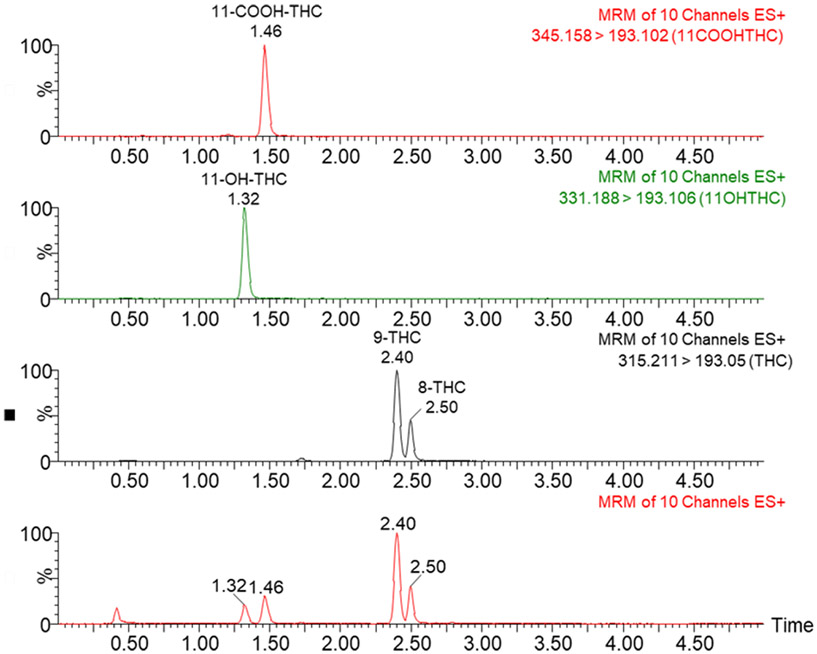
The bioanalytical method was successfully validated for sensitivity, selectivity, linearity, carryover, accuracy, precision, recovery, matrix effect, and stability in serum. A representative chromatogram is shown in Figure 1.
Figure 1:
Representative chromatograms for THC and its metabolites in positive ionization mode. Retention times (in minutes) are labeled above each peak.
3.2. Sensitivity and selectivity:
The LOD was found to be 1 ng/mL for each analyte as the response at this concentration was consistently a signal to noise ratio > 3, while the LLOQ was selected to be 2.5 ng/mL for each analyte as it showed a signal to noise ratio > 10 with accuracy and precision within 20 %. When six different blank rat serum samples were analyzed, no endogenous substances were eluted at the retention time of the analytes or IS.
3.3. Linearity and carryover:
Calibration curves for the range of 2.5-250 ng/mL were found to be linear for all runs with the coefficient of determination value greater than 0.99 for all runs, indicating an adequate linear fit. Carryover analysis of a blank sample immediately following an HQC of 180 ng/mL produced an analyte peak area <20% of the LLOQ for the analytes and <5% of IS, demonstrating negligible carryover.
3.4. Accuracy and precision:
Table 1 shows the results of intra- and inter-day accuracy and precision evaluations carried out on three different days using n=6 at each QC level. All values were within the acceptable limits of 15% of the target concentration, or 20% for the LLOQ.
Table 1.
Accuracy and precision of the assay in rat serum. n=6 at each concentration
| Rat Serum | ||||||
|---|---|---|---|---|---|---|
| THC | ||||||
| Concentration (ng/mL) |
Intra-day | Inter-day | ||||
| Measured Concentration (mean ± SD; ng/mL) |
Precision (% RSD) |
Accuracy (% bias) |
Measured Concentration (mean ± SD; ng/mL) |
Precision (% RSD) |
Accuracy (% bias) |
|
| 2.5 | 3.0 ± 0.5 | 15.3 | 19.4 | 2.8 ± 0.4 | 12.7 | 13.4 |
| 6 | 5.2 ± 0.6 | 11.5 | −14.2 | 5.6 ± 0.6 | 11.3 | −7.3 |
| 80 | 84.4 ± 5.6 | 6.6 | 5.5 | 82.3 ± 8.1 | 9.8 | 2.9 |
| 180 | 161.6 ± 10.5 | 6.5 | −10.2 | 159.3 ± 14.8 | 9.3 | −11.5 |
| THC-COOH | ||||||
| Concentration (ng/mL) |
Intra-day | Inter-day | ||||
| Measured Concentration (mean ± SD; ng/mL) |
Precision (% RSD) |
Accuracy (% bias) |
Measured Concentration (mean ± SD; ng/mL) |
Precision (% RSD) |
Accuracy (% bias) |
|
| 2.5 | 2.9 ± 0.3 | 8.6 | 17.3 | 2.7 ± 0.4 | 13.0 | 9.1 |
| 6 | 5.3 ± 0.5 | 9.7 | −11.6 | 5.4 ± 0.5 | 8.9 | −10.4 |
| 80 | 85.6 ± 4.3 | 5.0 | 7.0 | 81.5 ± 4.1 | 5.1 | 1.8 |
| 180 | 164.3 ± 8.7 | 5.3 | −8.8 | 164.2 ± 10.9 | 6.7 | −8.8 |
| 11-OH-THC | ||||||
| Concentration (ng/mL) |
Intra-day | Inter-day | ||||
| Measured Concentration (mean ± SD; ng/mL) |
Precision (% RSD) |
Accuracy (% bias) |
Measured Concentration (mean ± SD; ng/mL) |
Precision (% RSD) |
Accuracy (% bias) |
|
| 2.5 | 2.2 ± 0.3 | 12.3 | −10.4 | 2.1 ± 0.2 | 7.8 | −14.7 |
| 6 | 5.3 ± 0.5 | 8.6 | −11.7 | 5.5 ± 0.5 | 9.3 | −8.6 |
| 80 | 79.5 ± 10.7 | 13.5 | −0.7 | 82.7 ± 10.2 | 12.4 | 3.4 |
| 180 | 153.5 ± 7.7 | 5.0 | −14.7 | 154.6 ± 14.3 | 9.3 | −14.1 |
3.5. Recovery and matrix effect:
The mean recovery from serum was 95.6 ± 6.2, 91.1 ± 8.4, and 90.4 ± 12.0% for THC, THC-COOH, and 11-OH-THC, respectively. The percent recovery was consistent across QCs of different concentrations and always within 15%. The mean analyte response in serum across the various concentrations was 100.4 ± 8.8, 98.6 ± 10.8, and 94.4 ± 11.6% of the response in water for THC, THC-COOH, and 11-OH-THC, respectively, indicating the absence of matrix effects in serum.
3.6. Stability:
The stability of the analytes in serum was assessed in conditions most likely to occur during sample preparation and storage. The benchtop, autosampler, freeze-thaw, and long-term stock stability were assessed. The analytes were stable in serum for up to 4 hr at room temperature. Extracted samples were stable in the autosampler for 48 hr at 10° C. The compounds were stable in serum for up to two freeze-thaw cycles (thawed to room temperature after storage at −80° C). Stocks were stable after two months storage in −20°C.
3.7. Dose Calculation:
Dose was estimated according to the equation (34):
where is the concentration of drug in chamber (mg/L), is the time of exposure (min), is the respiratory minute volume (L/min), is the deposition factor, and is the body weight (kg). The for female Sprague Dawley rats weighing approximately 0.225 kg is 0.075 L/min (40). The deposition factor for rodents is 0.10. The average body weight of the female rats was 0.225 kg. To calculate the concentration of drug in the chamber the following equation was used:
The % recovery after vaporization was selected from (41). For the 40 mg/mL solution this value would be 0.6 mg/L. For the 160 mg/mL solution the calculated concentration value would be 2.5 mg/L. Once the concentration of drug in the chamber was calculated the estim0.75ated dose delivered was determined to be 0.19 mg/kg for the 40 mg/mL solution and 0.76 mg/kg for the 160 mg/mL solution.
To determine the human equivalent dose (HED) the following equation was used (42):
The value used for animal weight was 0.225 kg and for human weight was 75 kg. The HED for 0.19 mg/kg dose would be 0.028 mg/kg and for the 0.76 mg/kg dose would be 0.11 mg/kg.
3.8. Pharmacokinetics:
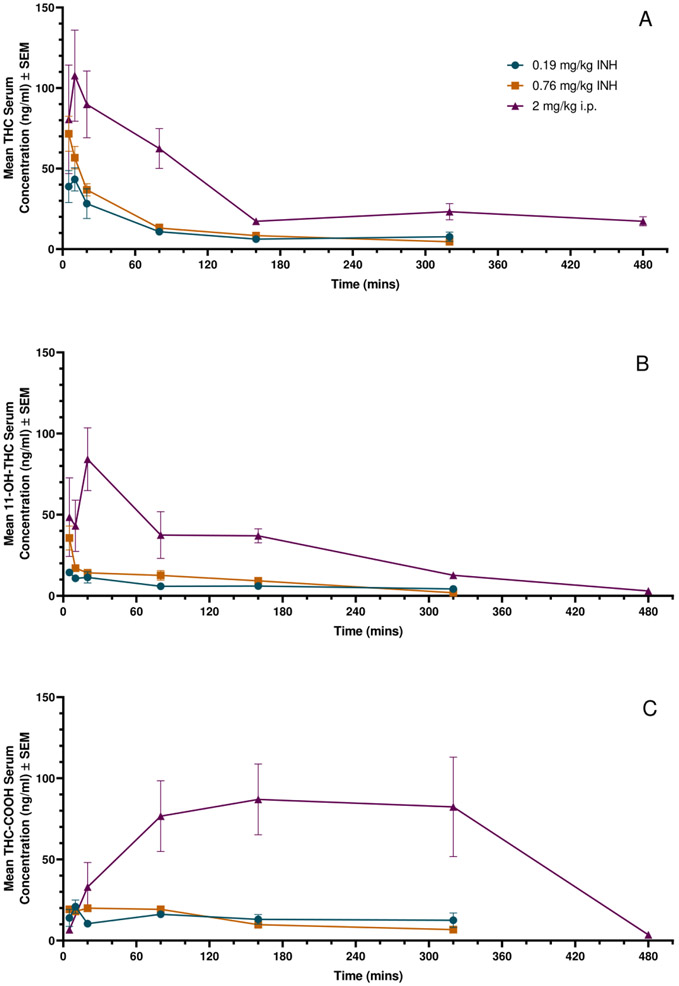
Concentration-time profiles for THC, 11-OH-THC, and THC-COOH after inhalation or i.p. administration of THC are shown in Figure 2. The 2 mg/kg i.p. dose of THC resulted in an average Cmax of 107.7±21.9 ng/mL after 10 minutes post-injection, while 0.19 mg/kg inhaled THC resulted in an average Cmax of 43.3±7.2 ng/mL at 10 minutes after the conclusion of inhalation, and 0.76 mg/kg inhaled THC resulted in an average Cmax of 71.6±22.5 ng/mL 5 minutes after inhalation was completed. Increasing the inhaled THC dose from 0.19 mg/kg to 0.76 mg/kg resulted in a 1.6 fold-change of Cmax values. For each timepoint on the THC curves, n=3-4 (Figure 2A).
Figure 2: Mean serum concentration-time profiles of THC and its metabolites after inhalation (INH) and i.p. administration of THC in rats.
Data represented as Mean ± SEM. A: THC in serum after 0.2 or 0.76 mg/kg inhaled THC or 2 mg/kg i.p. injected THC, n=3-4 at each time point. B: 11-OH-THC in serum after 0.2 or 0.76 mg/kg inhaled THC or 2 mg/kg i.p. injected THC, n=1-4 at each time point. C: THC-COOH after 0.2 or 0.76 mg/kg inhaled THC or 2 mg/kg i.p. injected THC, n=1-4 at each time point.
The results of the serum analysis were utilized to estimate the pharmacokinetic parameters for each of the three THC doses administered, with results presented in Table 2. The 2 mg/kg i.p. THC dose had a greater Cmax than either the 0.19 mg/kg or 0.76 mg/kg vaporized THC dose, as well as greater total drug exposure. While both the 2 mg/kg i.p. and 0.19 mg/kg vaporized doses had peak concentrations 10 minutes post administration, the 0.76 mg/kg vaporized dose observed peak concentrations at 5 minutes after exposure. Following the i.p. dose, a slightly lower elimination rate was observed than the vaporized doses, and a longer half-life as well. While the apparent volume of distribution with the 2 mg/kg i.p. dose was higher than the 0.19 mg/kg vaporized dose, it was lower than the 0.76 mg/kg vaporized dose. Similarly, the volume of distribution at steady state was much higher in the 0.76 mg/kg vaporized dose than the 0.19 mg/kg vaporized dose. Again, clearance of the 2 mg/kg i.p. dose was greater than in the 0.19 mg/kg vaporized dose, but lower than the 0.76 mg/kg vaporized dose.
Table 2:
Pharmacokinetics parameters of THC in female Sprague Dawley rats. Abbreviations: Cmax, maximum serum concentration; AUCinf, area under the curve from 5 minutes post-exposure to infinity; ke, elimination rate constant; t1/2, terminal half-life; Vd/F, volume of distribution; Vss, volume of distribution at steady state; CL or CL/F, clearance; **, indicating apparent volume of distribution or clearance.
| Parameter | 2 mg/kg i.p. | 0.19 mg/kg vaporized | 0.76 mg/kg vaporized |
|---|---|---|---|
| Cmax (ng/mL) | 107.7 | 43.3 | 71.6 |
| Tmax (min) | 10 | 10 | 5 |
| AUCinf (hr*ng/mL) | 364.4 | 87.0 | 94.4 |
| ke (1/hr) | 0.2 | 0.3 | 0.3 |
| t1/2 (hr) | 3.4 | 2.2 | 2.7 |
| Vd/F** (L/kg) | 26.9 | 7.4 | 33.0 |
| Vss (L/kg) | - | 8.6 | 24.4 |
| CL or CL/F** (L/hr/kg) | 5.5** | 2.3 | 8.5 |
The primary metabolite, 11-OH-THC, had an average Cmax of 84.2 ± 19.3 ng/mL 20 minutes after a 2 mg/kg i.p. injection, while 0.19 mg/kg inhaled THC resulted in an average Cmax of 14.4±1.4 ng/mL 5 minutes after the conclusion of inhalation, and 0.76 mg/kg inhaled THC resulted in an average Cmax of 35.6±7.4 ng/mL at 5 minutes after inhalation was completed. For each time point on the 11-OH-THC curves, n=1-3; 11-OH-THC in multiple test samples fell below the LLOQ, and were therefore excluded from the pharmacokinetic data analysis (Figure 2B).
Another phase I metabolite, THC-COOH had an average Cmax of 87.0 ± 21.8 ng/mL 160 minutes after the 2 mg/kg i.p. injection, while 0.19 mg/kg inhaled THC resulted in an average Cmax of 20.9 ± 4.1 ng/mL 10 minutes after inhalation, and 0.76 mg/kg inhaled THC resulted in an average Cmax of 19.9 ng/mL 20 minutes after inhalation was completed. For each time point on the THC-COOH curves, n=0-4; plasma concentrations fell below LLOQ and were excluded from the data analysis (Figure 2C). Cmax values for all compounds analyzed are summarized in Table 3.
Table 3:
Mean peak serum concentrations (Cmax) of THC and its metabolites after inhalation (INH) and i.p. administration of THC in rats (n=3 for each value, mean ± SEM).
| THC Dose and route of administration |
Cmax (ng/mL) | ||
|---|---|---|---|
| THC | 11-OH-THC | THC-COOH | |
| 2 mg/kg (i.p.) | 107.7 ± 21.9 | 84.2 ± 19.3 | 87.0 ± 21.8 |
| 0.19 mg/kg (INH) | 43.3 ± 7.2 | 14.4 ± 1.4 | 20.9 ± 4.1 |
| 0.76 mg/kg (INH) | 71.6 ± 10.9 | 35.6 ± 7.4 | 19.9 ± 2.2 |
3.9. Locomotor Activity:
The effect of i.p. injected and inhaled THC on locomotor activity was tested in an OpenField arena. Four parameters were measured: floor plane distance, margin distance, center distance, and vertical plane entries (rearing events). For each parameter, a Two-Way RM ANOVA was performed, with Time and Treatment as factors, and the appropriate post-hoc analysis (Sidak’s multiple comparison for the i.p. test, and Tukey ‘s multiple comparison for the inhalation test). Total distances/entries were analyzed with One-way RM ANOVAs or paired T-tests.
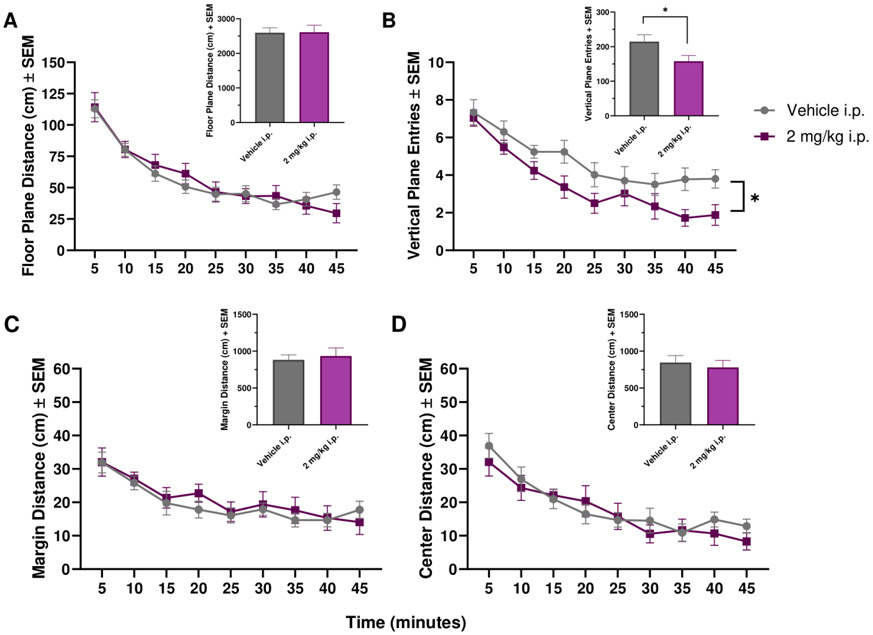
For i.p. injected THC, there was a significant effect of Time on floor plane distance [F(3.978,71.61)=33.76, p<0.0001], margin distance [F(3.688,66.38)=12.00, p<0.0001], and center distance [F(4.672,84.09)=18.88, p<0.0001; Figure 3A, C-D]. For vertical plane entries, there was a significant effect of Time [F(4.782,86.07)=27.43, p<0.0001] and Treatment [F(1,18)=4.898, p=0.0400; Figure 3B]. Analyzing total entries, there was a significant reduction in vertical plane entries after THC administration when compared to vehicle [t(9)=2.700, p=0.0244; Figure 3B].
Figure 3: Mean locomotor activity of THC after i.p. administration in female Sprague Dawley rats.
Mean floor plane distance, vertical plane entries, margin distance, and center distance after administration of i.p. vehicle or 2 mg/kg i.p. THC over a 45 minute period. n=10 for each group. * indicates p<0.05.
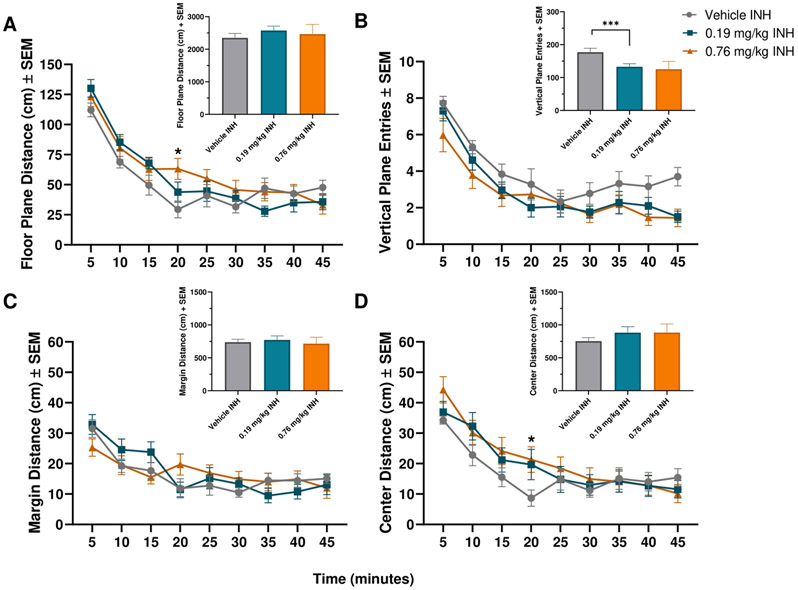
For the vaporized THC, there was a significant effect of Time [F(5.280, 169.0)=89.89, p<0.0001] and an interaction of Time x Treatment [F(16, 256)=3.059, p<0.0001] on floor plane distance. Post-hoc comparisons showed that at 20 minutes, the 0.76 mg/kg THC dose subjects had significantly higher floor plane distance compared to the vehicle group (p=0.0161; Figure 4A). For vertical plane entries, there was a significant effect of Time [F(3.662,113.5)=50.10, p<0.0001; Figure 4B]. There was a significant effect of Time [F(5.159,165.1)=20.32, p<0.0001] and a significant interaction of Time x Treatment [F(16,256)=2.430, p=0.0020] for margin distance (Figure 4C). There were no significant post-hoc comparisons. For center distance there was a significant effect of Time [F(5.325,170.4)=43.16, p<0.0001] and a significant interaction of Time x Treatment [F(16,256)=2.148, p=0.0071]. Post hoc analysis found that at 20 minutes, the 0.76 mg/kg vaporized THC group had significantly higher center distance travelled compared to the vehicle group (p=0.0459; Figure 4D). Analyzing total entries, there was a significant effect of treatment on vertical plane entries [F(1.177,16.48)=4.648, p=0.0410]. Tukey’s multiple comparisons test showed that 0.19 mg/kg INH resulted in significantly decreased vertical plane entries compared to vehicle (p=0.0003).
Figure 4: Mean locomotor activity after inhaled THC in female Sprague Dawley rats.
Mean floor plane distance, vertical plane entries, margin distance, and center distance after administration of inhaled vehicle, 0.19 mg/kg, or 0.76 mg/kg inhaled THC. n=10-15 for each group. * indicates p<0.05, *** indicates p<0.001. Panels A and D, * indicates significant difference between vehicle and 0.76 mg/kg THC groups.
4. Discussion
Our results demonstrate a model for THC inhalation in female rats at two different doses, in comparison to an i.p. injected dose within the well-established range used in preclinical THC research (24, 27, 43-45). The pharmacokinetic results obtained for our i.p. study are comparable to previous i.p. THC pharmacokinetic studies in rodents (46, 47). However, few comprehensive time-course studies of i.p. injected THC in rats exist in the literature, which further emphasizes the need for understanding the pharmacokinetic properties of THC in preclinical models, regardless of route of administration. Compared to our i.p. reference dose of THC, we found that both inhaled doses of THC resulted in more rapid absorption and metabolism of THC, as well as lower Cmax values. This is supported by our dose calculations, which found that the low dose of inhaled THC is approximate to a 0.19 mg/kg dose of THC, while the higher dose of inhaled THC is approximate to a 0.76 mg/kg dose of THC. While the two different doses of inhaled THC resulted in a 1.6-fold change of Cmax values, the total THC exposure was largely equivalent L (87.0 hr*ng/mL vs. 94.4 hr*ng/mL). This may reflect a limitation to controlling the amount of drug delivered in this route of administration compared to i.p. injection in a rodent model, as body weight and respiratory rate can vary in individual animals. As this paradigm delivers vapor to the exposure chamber rather than directly to the rat, this may be a small limitation but avoids the stress of restraining a subject to directly infuse the vapor in the nose/mouth. This approach therefore provides important data on the pharmacokinetic and pharmacodynamic response to vapor THC in female rats without the confound of restraint.
In addition to quantifying THC levels in serum, we also quantified two metabolites, 11-OH-THC and THC-COOH. The primary metabolite of THC, 11-OH-THC, has similar psychoactive properties as THC, and is therefore important to consider when investigating the behavioral effects of THC in preclinical models. Another metabolite THC-COOH, while inactive, and therefore having no psychotropic activity to consider in behavior analysis, is often used in cannabis drug testing in clinical settings. THC-COOH remains detectable in the bloodstream for longer time periods in humans, often 7 days or more after administration (18, 48). Metabolism of THC is mediated by the Cytochrome P450 (CYP) family of enzymes in both humans and rats (49); differences in CYP-mediated metabolism have been observed across species (50, 51), which must be considered when comparing preclinical THC inhalation models with the available clinical publications. Our inhaled THC serum concentration-time curves show rapid absorption of THC into the systemic circulation with a rapid decline in both THC and 11-OH-THC levels, while THC-COOH concentrations remain steady throughout (Figure 2). Previous THC inhalation models in rats have seen similar results, with peak THC concentrations immediately following vapor exposure with rapid elimination (23, 26, 34, 52). Our results for the 0.76 mg/kg vaporized THC specifically produced very similar Cmax and Tmax values as Baglot et al., one of the only other studies looking at THC inhalation in female Sprague Dawley rats; similarities in time course and Tmax continued when comparing results for 11-OH-THC levels as well (26). Differences may therefore be attributed to difference in inhalation paradigm. Additionally, our results are similar to those seen in a 2018 clinical pharmacokinetic study, which also observed in their acute inhalation analysis that the primary metabolite, 11-OH-THC, was up to 30% of THC, and there was a greater exposure to the THC-COOH metabolite than to THC (22).
To accompany the pharmacokinetic data collected, we additionally tested the effect of each THC dose on locomotor activity. Locomotion is a commonly tested parameter to measure the phenotypic effect of THC, and is grouped into the classic “tetrad” test, which is commonly used to determine if compounds have CB1 agonist activity (32, 33). We observed minimal effect of the 0.76 mg/kg inhaled THC on horizontal distance traveled or altered thigmotaxis over a 45-minute period, but we did see reduced rearing activity for both the i.p. injected THC dose (Figure 3), as well as the lower inhaled THC dose (Figure 4). Previous studies looking at comparable doses of i.p. injected THC have seen similar results: horizontal locomotion is often not effected until doses at or exceeding 15 mg/kg (27-29). Other studies looking at acute inhaled THC have described inconsistent results in horizontal locomotion (23-25, 30). Suppressed locomotion was observed in studies with higher blood concentration levels of THC (30), or in paradigms that did not also determine blood concentration values of THC (23), but additional studies with varying doses and blood concentration levels of THC have observed little to no effect of inhaled THC on locomotor activity (24, 25).
There is evidence of THC having a biphasic effect in rodents, including on locomotor measures, where low doses can actually increase locomotor activity, while high doses will create the hypolocomotor effect more commonly observed (31). This may explain the slightly heightened locomotion seen 20 minutes into the open field session for the 0.76 mg/kg cohort, in comparison to vehicle (Figure 4A). This effect could reflect max THC and/or 11-OH-THC levels in the brain; Hložek et al. saw peak THC brain levels 15 minutes after THC inhalation in males (35), while Baglot et al. saw peak THC brain levels at 30 minutes (26). The study from Katsidoni et al. also saw increased rearing activity at the low dose of THC (31). Conversely, Bruijnzeel and colleagues found that after inhaled cannabis, rearing activity was decreased in rats, along with decreased horizontal locomotion and higher blood plasma levels of THC than the present study (30). Thus, the doses used in our study showed decreased rearing activity, and minimal effects on floor plane activity. Our results suggest that rearing activity is more sensitive to acute vaporized THC administration than horizontal locomotion (Figures 3 and 4).
Additionally, THC administration has been shown to produce anxiolysis at low doses in rodents (30, 53-55). Altered thigmotaxic activity, the tendency for rodents to avoid unsafe spaces (i.e., the center of the open field), can indicate anxiolytic effects of administered drugs (56). Our inhaled 0.76 mg/kg THC dose did see increased center distance travelled at 20 minutes into the session compared to the vehicle group, which could indicate a small anxiolytic effect corresponding to this dose (Figure 4D). Our i.p. injected THC dose of 2 mg/kg may fall into a window that does not affect anxiety, or it could speak to a difference due to route of administration.
In 2014, Manwell and colleagues utilized a similar approach as used here, with a vaporizer and THC solution diluted in ethanol, albeit at lower doses and looking at male rats rather than females (24). Information on female pharmacokinetics is important for future prenatal THC inhalation research. This study collected blood samples at two timepoints, therefore not covering the entire absorption, distribution, metabolism, and elimination process but rather capturing a small window of data, and each timepoint only has data from a single subject. Their data suggests that there may be some sex differences in how the inhaled THC is metabolized, but without more data it is difficult to make an exact comparison. Another study from 2017 used the vaporizer as well, adapting it to expel directly into the animal chamber rather than using the balloons that are used with the vaporizer in humans (35). This alters the amount of vapor being expelled into the chamber compared with our results and Manwell et al.’s, but is otherwise comparable from an apparatus viewpoint. This modification seems to have allowed for greater Cmax of THC in serum, but the pharmacokinetic profile is comparable. Other studies have begun to use e-cigarette vaporizers (23, 26, 57, 58), which may be advantageous given the rise in consuming THC with “vapes,” but are more complex and require significantly more variables to be tested. These custom apparatuses often have multiple factors to consider when comparing across studies, including the duration of each puff, the temperature at which the e-cigarette vaporizes the THC, and the effect the vehicle solution may have on phenotypic response. Overall, the pharmacokinetic data from an e-cigarette mode of delivery shows similar trends to that seen from our results and others (23, 26, 34, 52, 57, 58). One notably different study from Nguyen and colleagues achieved mean Cmax levels of 303 ng/mL THC in female Sprague Dawley rats at 5 minutes post-30 minute inhalation of 200 mg/mL THC (59). This may be attributed to the high concentration of THC solution coupled with the extended period of exposure compared to our model and other models. Here, we present an alternative inhalation paradigm that is advantageous to modified e-cigarette models which require more complex design (multiple components and custom software are often necessary to ran these apparatuses) and/or more costly equipment, while still capturing the full plasma concentration-time profile of THC in serum out to 320 minutes post-inhalation at multiple doses, as well as representative phenotypic data, in female rats. To our knowledge, this is the first study examining the use of the Volcano Vaporizer for a THC inhalation paradigm in female rats.
The most common form of cannabis consumption in human users is inhalation, both for recreational and medicinal purposes (60, 61). Multiple clinical studies have looked at the pharmacokinetics of acute THC use in humans, both administered as cannabis or as an isolated compound (16, 18-22). These studies tend to vary regarding important demographic factors such as subject age, sex, and cannabis use history, as well as drug formulation (smoking vs vaping), dose of THC administered, and duration of exposure. This results in a wide variety of THC Cmax values in blood, plasma, and/or serum, from 4 ng/mL (20) to 162 ng/mL (18) just in these studies mentioned. While this may reflect the differences in smoking activity in the general population, it can make it difficult to make any solid conclusions from a clinical research standpoint. In addition to different smoking habits, cannabis in the United States, as well as other countries, has seen increased potency in recent years, which can further complicate research focused on specific doses of THC, both preclinical and clinical (62, 63). Finally, individual subjects can have vastly different pharmacokinetic results within the same study conditions; one study found that 6 subjects all administered the same dose of THC under the same regime had plasma Cmax values ranging from 70-280 ng/mL, further emphasizing the large variability between not only studies, but individuals (18). The authors concluded that this variation may have been due to individual differences in inhalation (i.e., inhalation volume, duration, and frequency). A clinical study performed at a similar dose to those used here showed plasma concentration Cmax ranging from 73.81 ± 63.09 ng/mL comparable to the Cmax values achieved in this study (64).
Use of preclinical rodent models allows for control of some factors that may complicate clinical research, such as route of administration. As previously mentioned, a large majority of current THC preclinical research is focused on i.p. injection of THC, which is not representative of the common clinical route of administration. Use of inhaled THC can instead control for the effect that route of administration can have on the pharmacokinetics of and phenotypic response to the drug. Drugs i.p. injected are subject to first-pass metabolism via the liver (65, 66), whereas inhaled drugs bypass this step. It therefore is advantageous to match the route of administration as best as possible in preclinical models of cannabis use to the prevalent mode of use in humans, inhalation. While oral consumption is another common route of administration, it was not the focus of this study. It has recently been demonstrated in a preclinical model that the route of administration can indeed influence the phenotypic response to the drug. A 2021 publication (67) found that administering THC in multiple different routes of administration had an effect on the potency of drug and the onset and duration of effect in a drug discrimination paradigm, further supporting the notion that preclinical THC research would benefit from matching the inhalation route of administration. Our serum THC concentration results clearly demonstrate the effect route of administration has on the pharmacokinetic properties of THC; both inhaled doses of THC saw increased rates of absorption and quicker clearance from the bloodstream as compared to the i.p. injected THC (Figure 2A).
As with all research studies, there are some limitations in the work presented here. This paper primarily focuses on female rats, and it has been previously established that the pharmacological properties of THC in rodents may be dependent on both sex and strain (68-70). While our focus for this study was examining female rats as a first step for additional work, investigating the same paradigm in male rats may be valuable in the future. Additionally, it may be useful to investigate the effects of chronic THC administration prior to collecting test blood samples, to elucidate the consequences chronic use may have on the pharmacokinetic properties of THC in rats. There has been clinical research demonstrating that chronic THC consumption can result in detection of THC and/or metabolites for a significantly longer time period than seen in infrequent users (48, 71, 72), even though prolonged psychological effects may not be seen. As previously discussed, clinical research investigating inhaled THC has used multiple formulations (vaped as an isolated compound or smoked within the cannabis plant); there is evidence to suggest the pharmacokinetics of these two methods of inhalation are comparable (73), but more attention to possible differences is still needed. As more preclinical work with inhaled THC is reported, distinguishing differences will become clearer. The present study included locomotor testing for 45-minute periods after THC administration; it may be beneficial to look at time periods extending out multiple hours after exposure, as there may be longer term effects of THC and metabolites as they remain in the bloodstream for multiple hours. Finally, this rodent model of passive inhalation cannot be directly compared with active inhalation models in clinical research, as each animal will have unique breathing rates within the inhalation chamber. A similar limitation does exist in human models, however, where each subject may have different inhalation volumes and hold times in even the most regulated testing regimes.
5. Conclusion
Here we present a validated UPLC-MS/MS method for the quantification of THC, 11-OH-THC, and THC-COOH in rat serum, as well as a preclinical inhalation model for further THC research. This model is a simple and safe method for the administration of inhaled THC which could be implemented in both acute and chronic preclinical THC research going forward. The serum Cmax values for both our low and high dose of inhaled THC (43.3 ng/mL and 71.6 ng/mL, respectively) were found to be within a range observed in clinical studies investigating smoked or vaped cannabis (mean Cmax ranges from 4-162 ng/mL) at similar doses (16, 18-21), and could be utilized in future phenotypic and neurological studies investigating the effects of THC consumption in rats.
Acknowledgements:
We wish to thank the NIDA Drug Supply Program for providing us with the THC.
Funding:
This research was supported by NIDA [grant number 045640]; the University of Florida Clinical and Translational Science Institute, which is supported in part by the NIH National Center for Advancing Translational Sciences [grant number UL1TR001427].
Footnotes
Conflicts of Interest: Dr. Panayotis Thanos is the Section Editor for the journal Current Pharmaceutical Design.
Research Involving Animals: All experiments were conducted in compliance with the National Academy of Sciences Guide for the Care and Use of Laboratory Animals and approved by the University at Buffalo Institutional Animal Care and Use Coimnittee.
Availability of Data and Materials:
The data that support the findings of this study are available from the corresponding author, PT, on special request.
References
- 1.NIDA. Marijuana DrugFacts 2019. [Available from: https://www.drugabuse.gov/publications/drugfacts/marijuana. [Google Scholar]
- 2.Yu B, Chen X, Chen X, Yan H. Marijuana legalization and historical trends in marijuana use among US residents aged 12–25: results from the 1979–2016 National Survey on drug use and health. BMC Public Health. 2020;20(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laaris N, Good CH, Lupica CR. Δ9-tetrahydrocannabinol is a full agonist at CB1 receptors on GABA neuron axon terminals in the hippocampus. Neuropharmacology. 2010;59(1):121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62(4):588–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacological reviews. 2006;58(3):389–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackie K. Cannabinoid receptors: where they are and what they do. J Neuroendocrinol. 2008;20 Suppl 1:10–4. [DOI] [PubMed] [Google Scholar]
- 7.Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med. 2011;5(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broyd SJ, van Hell HH, Beale C, Yücel M, Solowij N. Acute and Chronic Effects of Cannabinoids on Human Cognition—A Systematic Review. Biological Psychiatry. 2016;79(7):557–67. [DOI] [PubMed] [Google Scholar]
- 9.Elikkottil J, Gupta P, Gupta K. The analgesic potential of cannabinoids. J Opioid Manag. 2009;5(6):341–57. [PMC free article] [PubMed] [Google Scholar]
- 10.Farokhnia M, McDiarmid GR, Newmeyer MN, Munjal V, Abulseoud OA, Huestis MA, et al. Effects of oral, smoked, and vaporized cannabis on endocrine pathways related to appetite and metabolism: a randomized, double-blind, placebo-controlled, human laboratory study. Translational Psychiatry. 2020;10(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fearby N, Penman S, Thanos P. Effects of Δ9-Tetrahydrocannibinol (THC) on Obesity at Different Stages of Life: A Literature Review. Int J Environ Res Public Health. 2022;19(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clouse G, Penman S, Hadjiargyrou M, Komatsu DE, Thanos PK. Examining the role of cannabinoids on osteoporosis: a review. Arch Osteoporos. 2022;17(1):146. [DOI] [PubMed] [Google Scholar]
- 13.Cohen K, Weinstein A. The Effects of Cannabinoids on Executive Functions: Evidence from Cannabis and Synthetic Cannabinoids—A Systematic Review. Brain Sci. 2018;8(3):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris HM, Rousseau MA, Wanas AS, Radwan MM, Caldwell S, Sufka KJ, et al. Role of Cannabinoids and Terpenes in Cannabis-Mediated Analgesia in Rats. Cannabis Cannabinoid Res. 2019;4(3):177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farrimond JA, Mercier MS, Whalley BJ, Williams CM. Cannabis sativa and the Endogenous Cannabinoid System: Therapeutic Potential for Appetite Regulation. Phytotherapy Research. 2011;25(2):170–88. [DOI] [PubMed] [Google Scholar]
- 16.Ohlsson A, Lindgren J-E, Wahlen A, Agurell S, Hollister LE, Gillespie HK. Plasma delta-9-tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clinical Pharmacology & Therapeutics. 1980;28(3):409–16. [DOI] [PubMed] [Google Scholar]
- 17.Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4(8):1770–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huestis MA, Henningfield JE, Cone EJ. Blood Cannabinoids. I. Absorption of THC and Formation of 11-OH-THC and THCCOOH During and After Smoking Marijuana*. Journal of Analytical Toxicology. 1992;16(5):276–82. [DOI] [PubMed] [Google Scholar]
- 19.Lee D, Bergamaschi MM, Milman G, Barnes AJ, Queiroz RHC, Vandrey R, et al. Plasma Cannabinoid Pharmacokinetics After Controlled Smoking and Ad libitum Cannabis Smoking in Chronic Frequent Users. Journal of analytical toxicology. 2015;39(8):580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spindle TR, Cone EJ, Schlienz NJ, Mitchell JM, Bigelow GE, Flegel R, et al. Acute Pharmacokinetic Profile of Smoked and Vaporized Cannabis in Human Blood and Oral Fluid. Journal of analytical toxicology. 2019;43(4):233–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartman RL, Brown TL, Milavetz G, Spurgin A, Gorelick DA, Gaffney G, et al. Controlled Cannabis Vaporizer Administration: Blood and Plasma Cannabinoids with and without Alcohol. Clinical Chemistry. 2015;61(6):850–69. [DOI] [PubMed] [Google Scholar]
- 22.Meyer P, Langos M, Brenneisen R. Human Pharmacokinetics and Adverse Effects of Pulmonary and Intravenous THC-CBD Formulations. Medical Cannabis and Cannabinoids. 2018;1(1):36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen JD, Aarde SM, Vandewater SA, Grant Y, Stouffer DG, Parsons LH, et al. Inhaled delivery of Δ(9)-tetrahydrocannabinol (THC) to rats by e-cigarette vapor technology. Neuropharmacology. 2016;109:112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manwell LA, Charchoglyan A, Brewer D, Matthews BA, Heipel H, Mallet PE. A vapourized Δ(9)-tetrahydrocannabinol (Δ(9)-THC) delivery system part I: development and validation of a pulmonary cannabinoid route of exposure for experimental pharmacology studies in rodents. J Pharmacol Toxicol Methods. 2014;70(1):120–7. [DOI] [PubMed] [Google Scholar]
- 25.Taffe MA, Creehan KM, Vandewater SA, Kerr TM, Cole M. Effects of Δ9-tetrahydrocannabinol (THC) vapor inhalation in Sprague-Dawley and Wistar rats. Experimental and Clinical Psychopharmacology. 2021;29(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baglot SL, Hume C, Petrie GN, Aukema RJ, Lightfoot SHM, Grace LM, et al. Pharmacokinetics and central accumulation of delta-9-tetrahydrocannabinol (THC) and its bioactive metabolites are influenced by route of administration and sex in rats. Scientific Reports. 2021;11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiley JL, O'Connell MM, Tokarz ME, Wright MJ Jr., Pharmacological effects of acute and repeated administration of Delta(9)-tetrahydrocannabinol in adolescent and adult rats. J Pharmacol Exp Ther. 2007;320(3):1097–105. [DOI] [PubMed] [Google Scholar]
- 28.Harte-Hargrove LC, Dow-Edwards DL. Withdrawal from THC during adolescence: Sex differences in locomotor activity and anxiety. Behavioural Brain Research. 2012;231(1):48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taffe MA, Creehan KM, Vandewater SA. Cannabidiol fails to reverse hypothermia or locomotor suppression induced by Δ9-tetrahydrocannabinol in Sprague-Dawley rats. British Journal of Pharmacology. 2015;172(7):1783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruijnzeel AW, Qi X, Guzhva LV, Wall S, Deng JV, Gold MS, et al. Behavioral Characterization of the Effects of Cannabis Smoke and Anandamide in Rats. PLoS One. 2016;11(4):e0153327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katsidoni V, Kastellakis A, Panagis G. Biphasic effects of Δ9-tetrahydrocannabinol on brain stimulation reward and motor activity. International Journal of Neuropsychopharmacology. 2013;16(10):2273–84. [DOI] [PubMed] [Google Scholar]
- 32.Metna-Laurent M, Mondésir M, Grel A, Vallée M, Piazza P-V. Cannabinoid-Induced Tetrad in Mice. Current Protocols in Neuroscience. 2017;80(1):9.59.1–9..10. [DOI] [PubMed] [Google Scholar]
- 33.Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, et al. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav. 1991;40(3):471–8. [DOI] [PubMed] [Google Scholar]
- 34.Ravula A, Chandasana H, Setlow B, Febo M, Bruijnzeel AW, Derendorf H. Simultaneous quantification of cannabinoids tetrahydrocannabinol, cannabidiol and CB1 receptor antagonist in rat plasma: An application to characterize pharmacokinetics after passive cannabis smoke inhalation and co-administration of rimonabant. J Pharm Biomed Anal. 2018;160:119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hložek T, Uttl L, Kadeřábek L, Balíková M, Lhotková E, Horsley RR, et al. Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. European Neuropsychopharmacology. 2017;27(12):1223–37. [DOI] [PubMed] [Google Scholar]
- 36.Berthold EC, Yang R, Sharma A, Kamble SH, Kanumuri SR, King TI, et al. Regulatory sampling of industrial hemp plant samples (Cannabis sativa L.) using UPLC-MS/MS method for detection and quantification of twelve cannabinoids. Journal of Cannabis Research. 2020;2(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.FDA. Bioanalytical Method Validation: Guidance for Industry. 2018.
- 38.Robison LS, Michaelos M, Gandhi J, Fricke D, Miao E, Lam C-Y, et al. Sex Differences in the Physiological and Behavioral Effects of Chronic Oral Methylphenidate Treatment in Rats. Frontiers in Behavioral Neuroscience. 2017;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma A, Jaiswal S, Shukla M, Sharma M, Chauhan PMS, Rangaraj N, et al. HPLC–MS-MS Method Development and Validation of Antileishmanial Agent, S010-0269, in Hamster Serum. Journal of Chromatographic Science. 2015;53(9):1542–8. [DOI] [PubMed] [Google Scholar]
- 40.OEHHA. Calculation of Rat Breathing Rate Based on Bodyweight: California Environmental Protection Agency; 2018.
- 41.Hazekamp A, Ruhaak R, Zuurman L, van Gerven J, Verpoorte R. Evaluation of a vaporizing device (Volcano®) for the pulmonary administration of tetrahydrocannabinol. Journal of Pharmaceutical Sciences. 2006;95(6):1308–17. [DOI] [PubMed] [Google Scholar]
- 42.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miladinovic T, Manwell LA, Raaphorst E, Malecki SL, Rana SA, Mallet PE. Effects of chronic nicotine exposure on Δ9-tetrahydrocannabinol-induced locomotor activity and neural activation in male and female adolescent and adult rats. Pharmacology Biochemistry and Behavior. 2020;194:172931. [DOI] [PubMed] [Google Scholar]
- 44.Abela AR, Rahbarnia A, Wood S, Lê AD, Fletcher PJ. Adolescent exposure to Δ9-tetrahydrocannabinol delays acquisition of paired-associates learning in adulthood. Psychopharmacology. 2019;236(6):1875–86. [DOI] [PubMed] [Google Scholar]
- 45.Cha YM, White AM, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of delta9-THC on learning in adolescent and adult rats. Pharmacology Biochemistry and Behavior. 2006;83(3):448–55. [DOI] [PubMed] [Google Scholar]
- 46.Ruiz CM, Torrens A, Castillo E, Perrone CR, Cevallos J, Inshishian VC, et al. Pharmacokinetic, behavioral, and brain activity effects of Δ(9)-tetrahydrocannabinol in adolescent male and female rats. Neuropsychopharmacology. 2021;46(5):959–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vozella V, Zibardi C, Ahmed F, Piomelli D. Fast and Sensitive Quantification of Δ9-Tetrahydrocannabinol and Its Main Oxidative Metabolites by Liquid Chromatography/Tandem Mass Spectrometry. Cannabis Cannabinoid Res. 2019;4(2):110–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karschner EL, Schwilke EW, Lowe RH, Darwin WD, Pope HG, Herning R, et al. Do Δ9-tetrahydrocannabinol concentrations indicate recent use in chronic cannabis users? Addiction. 2009;104(12):2041–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grotenhermen F. Pharmacokinetics and Pharmacodynamics of Cannabinoids. Clinical Pharmacokinetics. 2003;42(4):327–60. [DOI] [PubMed] [Google Scholar]
- 50.Martignoni M, Groothuis GMM, De Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opinion on Drug Metabolism & Toxicology. 2006;2(6):875–94. [DOI] [PubMed] [Google Scholar]
- 51.Harvey DJ, Brown NK. Comparative in vitro metabolism of the cannabinoids. Pharmacol Biochem Behav. 1991;40(3):533–40. [DOI] [PubMed] [Google Scholar]
- 52.Nguyen JD, Creehan KM, Grant Y, Vandewater SA, Kerr TM, Taffe MA. Explication of CB(1) receptor contributions to the hypothermic effects of Δ(9)-tetrahydrocannabinol (THC) when delivered by vapor inhalation or parenteral injection in rats. Drug Alcohol Depend. 2020;214:108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fokos S, Panagis G. Effects of delta9-tetrahydrocannabinol on reward and anxiety in rats exposed to chronic unpredictable stress. J Psychopharmacol. 2010;24(5):767–77. [DOI] [PubMed] [Google Scholar]
- 54.Rubino T, Sala M, Viganò D, Braida D, Castiglioni C, Limonta V, et al. Cellular Mechanisms Underlying the Anxiolytic Effect of Low Doses of Peripheral Δ9-Tetrahydrocannabinol in Rats. Neuropsychopharmacology. 2007;32(9):2036–45. [DOI] [PubMed] [Google Scholar]
- 55.Berrendero F, Maldonado R. Involvement of the opioid system in the anxiolytic-like effects induced by Δ9-tetrahydrocannabinol. Psychopharmacology. 2002;163(1):111–7. [DOI] [PubMed] [Google Scholar]
- 56.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. European Journal of Pharmacology. 2003;463(1):3–33. [DOI] [PubMed] [Google Scholar]
- 57.Breit KR, Rodriguez CG, Lei A, Thomas JD. Combined vapor exposure to THC and alcohol in pregnant rats: Maternal outcomes and pharmacokinetic effects. Neurotoxicology and Teratology. 2020;82:106930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moore CF, Davis CM, Harvey EL, Taffe MA, Weerts EM. Appetitive, antinociceptive, and hypothermic effects of vaped and injected Δ-9-tetrahydrocannabinol (THC) in rats: exposure and dose-effect comparisons by strain and sex. Pharmacology Biochemistry and Behavior. 2021;202:173116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen JD, Grant Y, Kerr TM, Gutierrez A, Cole M, Taffe MA. Tolerance to hypothermic and antinoceptive effects of Δ9-tetrahydrocannabinol (THC) vapor inhalation in rats. Pharmacology Biochemistry and Behavior. 2018;172:33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lankenau SE, Fedorova EV, Reed M, Schrager SM, Iverson E, Wong CF. Marijuana practices and patterns of use among young adult medical marijuana patients and non-patient marijuana users. Drug and Alcohol Dependence. 2017;170:181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steigerwald S, Wong PO, Cohen BE, Ishida JH, Vali M, Madden E, et al. Smoking, Vaping, and Use of Edibles and Other Forms of Marijuana Among U.S. Adults. Annals of Internal Medicine. 2018;169(12):890–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McLaren J, Swift W, Dillon P, Allsop S. Cannabis potency and contamination: a review of the literature. Addiction. 2008;103(7):1100–9. [DOI] [PubMed] [Google Scholar]
- 63.ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC. Changes in Cannabis Potency Over the Last 2 Decades (1995-2014): Analysis of Current Data in the United States. Biological psychiatry. 2016;79(7):613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramaekers JG, van Wei JH, Spronk DB, Toennes SW, Kuypers KPC, Theunissen EL, et al. Cannabis and tolerance: acute drug impairment as a function of cannabis use history. Scientific reports. 2016;6:26843-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lukas G, Brindle SD, Greengard P. The route of absorption of intraperitoneally administered compounds. J Pharmacol Exp Ther. 1971;178(3):562–4. [PubMed] [Google Scholar]
- 66.Al Shoyaib A, Archie SR, Karamyan VT. Intraperitoneal Route of Drug Administration: Should it Be Used in Experimental Animal Studies? Pharmaceutical Research. 2019;37(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wiley JL, Taylor SI, Marusich JA. Δ(9)-Tetrahydrocannabinol discrimination: Effects of route of administration in rats. Drug Alcohol Depend. 2021;225:108827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wiley JL, Barrus DG, Farquhar CE, Lefever TW, Gamage TF. Sex, species and age: Effects of rodent demographics on the pharmacology of Δ9-tetrahydrocanabinol. Progress in NeuroPsychopharmacology and Biological Psychiatry. 2021;106:110064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wiley JL, Burston JJ. Sex differences in Δ9-tetrahydrocannabinol metabolism and in vivo pharmacology following acute and repeated dosing in adolescent rats. Neuroscience Letters. 2014;576:51–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Farquhar CE, Breivogel CS, Gamage TF, Gay EA, Thomas BF, Craft RM, et al. Sex, THC, and hormones: Effects on density and sensitivity of CB(1) cannabinoid receptors in rats. Drug Alcohol Depend. 2019;194:20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johansson E, Agurell S, Hollister LE, Halldin MM. Prolonged apparent half-life of Δ1-tetrahydrocannabinol in plasma of chronic marijuana users. Journal of Pharmacy and Pharmacology. 1988;40(5):374–5. [DOI] [PubMed] [Google Scholar]
- 72.Desrosiers NA, Himes SK, Scheidweiler KB, Concheiro-Guisan M, Gorelick DA, Huestis MA. Phase I and II Cannabinoid Disposition in Blood and Plasma of Occasional and Frequent Smokers Following Controlled Smoked Cannabis. Clinical Chemistry. 2014;60(4):631–43. [DOI] [PubMed] [Google Scholar]
- 73.Newmeyer MN, Swortwood MJ, Barnes AJ, Abulseoud OA, Scheidweiler KB, Huestis MA. Free and Glucuronide Whole Blood Cannabinoids' Pharmacokinetics after Controlled Smoked, Vaporized, and Oral Cannabis Administration in Frequent and Occasional Cannabis Users: Identification of Recent Cannabis Intake. Clinical Chemistry. 2016;62(12):1579–92. [DOI] [PubMed] [Google Scholar]