Keywords: biopsy technique, histology, local anesthetic, muscle damage, skeletal muscle
Abstract
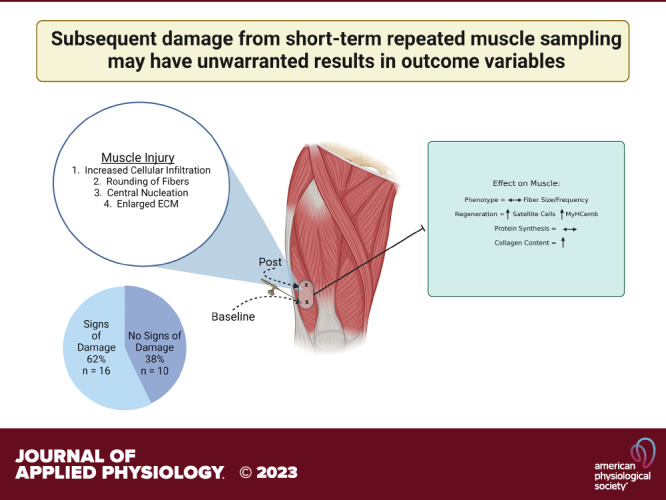
Changes in skeletal muscle are an important aspect of overall health. The collection of human muscle to study cellular and molecular processes for research requires a needle biopsy procedure which, in itself, can induce changes in the tissue. To investigate the effect of repeat tissue sampling, we collected skeletal muscle biopsy samples from vastus lateralis separated by 7 days. Cellular infiltrate, central nucleation, enlarged extracellular matrix, and rounding of muscle fibers were used as indices to define muscle damage, and we found that 16/26 samples (61.5%) revealed at least two of these symptoms in the secondary biopsy. The presence of damage influenced outcome measures usually obtained in human biopsies. Damaged muscle showed an increase in the number of small fibers even though average fiber and fiber type-specific cross-sectional area (CSA) were not different. This included higher numbers of embryonic myosin heavy chain-positive fibers (P = 0.001) as well as elevated satellite cell number (P = 0.02) in the damaged areas and higher variability in satellite cell count in the total area (P = 0.04). Collagen content was higher in damaged (P = 0.0003) as well as nondamaged areas (P = 0.05) of the muscle sections of the damaged compared with the nondamaged group. Myofibrillar protein and ribonucleic acid (RNA) fractional synthesis rates were not significantly different between the damaged compared with the nondamaged group. Results indicate that common outcomes as well as outcome variability in human muscle tissue are affected by previous biopsies. Therefore, the extent of potential damage should be assessed when performing repeated biopsies.
NEW & NOTEWORTHY Indices of damage can be found in repeated biopsy samples of nonintervened control legs. Variables, directly and not directly related to muscle damage or regeneration, were compromised in second biopsy. There is a need to determine potential damage within muscle tissue when repeated muscle sampling is part of the study design. Muscle biopsy sampling may be a source of increased heterogeneity in human muscle data.
INTRODUCTION
Skeletal muscle is an extremely plastic tissue that is able to adapt to various external stimuli such as nutrition and exercise. However, amid aging, underlying disease, and disuse, the ability of skeletal muscle to adapt becomes compromised ultimately impacting overall physical function and health status (1, 2). Although multiple techniques exist to quantify muscle, multiple biopsies are a feature of many studies that look at mechanistic and ultrastructural changes over time.
The introduction of a percutaneous muscle biopsy technique [first credited to Duchenne as early as the late 1800s (3)] has allowed for significant advancements in the field of muscle biology. Currently, a modified Bergström technique with applied suction is the most used method for obtaining human muscle allowing for muscle tissue to be readily and easily obtained for research (4). The procedure is minimally invasive, does not impact daily living, has short recovery times, and leaves a negligible scar. These features allow the procedure to be more repeatable when compared with invasive, open surgical muscle biopsies. On the other hand, limitations remain such as the requirement of a skilled operator and smaller sample size acquisition. These challenges may lead to repeated needle insertions and reduced muscle quality. Overall, the benefits of muscle biopsies far outweigh the risks as numerous immunohistochemical, as well as molecular, cellular, and functional outcomes can be measured from the collected tissue. To help reduce heterogeneity in the technical aspects of the procedure, a standardized step-by-step process with visual aids has been developed for obtaining muscle tissue from the vastus lateralis using the modified Bergström technique (5).
Repeated muscle biopsies may significantly impact study outcomes by introducing potential confounding factors and altering the physiological state of the muscle being examined when there is a measurable effect of the previous biopsy (or biopsies). The effect of various local anesthetics (6) and epinephrine (7–9) on gene expression (10) and muscle cellular signaling (11, 12) have been studied, in addition to comparisons between microbiopsies (smaller trochar opening) and the Bergström technique (13). These studies show that various concentrations of local anesthetics are toxic to skeletal muscle (6), epinephrine augments the damage (6, 8, 14), and permanent “microscarring” of the tissue may occur following repeated biopsies (7). It is currently speculative which variables measured in muscle are influenced by multiple biopsies due to differences in study design, exercise involvement, and variables of interest measured (15–17). It is possible that outcomes not related to damage may unknowingly be changed by the presence of injured or regenerating fibers from previous biopsies. Therefore, the effect of multiple biopsies within a relatively short time period in the same muscle should be evaluated to assess damage as a potentially confounding factor.
In our ongoing current clinical trial (NCT04131712), we implemented a within-subject repeated measurement design which required two muscle biopsies of the same leg serving as control over a 7-day period of time. We found evidence of damage in muscle sections in the control leg that did not receive any intervention, suggesting damage occurred as part of the previous muscle sampling procedure. This prompted the current secondary investigation with the purpose to carefully characterize the extent of the observed damage due to repeated sampling, and describe phenotypical abnormalities, both directly and indirectly related to damage. We also provide suggestions for the prevention of aberrant muscle outcomes.
MATERIALS AND METHODS
Experimental Design and Group Assignment
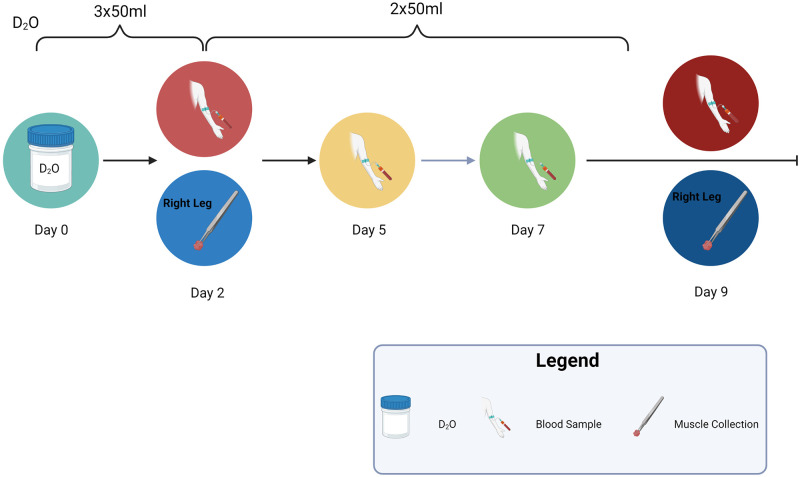
The goal of the original clinical trial (ClinicalTrials.gov, NCT04131712) was to determine the effect of a massage intervention on muscle atrophy during immobilization disuse. In our immobilization groups, our design allowed for within-subject control as only one leg was immobilized (left) and the other leg (right) remained active during crutch-assisted movement. Evidence of varying muscle damage was found within the second biopsy of untreated control limbs (nonimmobilized and nonmassaged limb). Data from the first n = 26 healthy young individuals of both sexes, aged 18–29 yr, and an average body mass index (BMI) of 23.5, were used for this secondary investigation unrelated to the original hypothesis. Participants consumed deuterium oxide [99% deuterium oxide (D2O)] 50 mL three times per day over the first 2 days and 50 mL two times per day for the final 7 days for a total of 1,000 mL. Muscle biopsies were performed on day 2 and day 9 from the same leg, while blood was drawn three to four times during the study to monitor body water D2O enrichment (Fig. 1). Participants were not to engage in additional physical activity such as resistance training but could remove the brace at night when going to sleep or taking a shower. The following operational definition was used to assign muscles into damaged versus nondamaged groups: muscle sections containing two or more of the four damage markers were designated as damaged and those with only one or zero markers as nondamaged. Damage markers were identified by two experienced blinded assessors on hematoxylin and eosin (H&E) sections using the following four criteria: 1) cellular infiltration, 2) rounding of fibers, 3) central nucleation, and 4) enlarged extracellular space. These criteria were included based on an injury scoring system using H&E sections adapted from Schmidt et al. (18). Areas from muscle sections containing edge effects, bubbles, wrinkles or folds were excluded from analyses.
Figure 1.
Experimental timeline for study. Created with BioRender.com with permission. D2O, deuterium oxide.
Ethical Approval of Study
This study was approved by the University of Kentucky Institutional Review Board (IRB 43499) before any participant enrollment. Data and safety monitoring was conducted by the standing University of Kentucky Center for Clinical and Translational Science (CCTS) data and safety monitoring board (DSMB) on a semiannual basis.
Muscle Biopsy Procedure
Muscle biopsies were obtained from the untreated vastus lateralis within the CCTS. Muscle procurement was conducted using Bergström needles similarly to that reported by Shanely et al. (5) and according to procedures described in a previous study from our group (19). The principal operator is an experienced physician having conducted over 1,000 muscle biopsy procedures (20–22). The biopsy site was cleaned with Betadine followed by application of alcohol. A local anesthetic (1% Xylocaine HCl, 3 mL with buffered bicarbonate, without epinephrine) was used to numb an area the size of a quarter on the site of the outer thigh over the vastus lateralis muscle approximately 9–15 cm above the knee using a 23-gauge, 1-inch needle, subcutaneously. Anesthetic was further injected (5–10 cc) into the subcutaneous space from the dermis to the muscle fascia, with care to not penetrate deeper than the fascia. After 5 min, a 1-cm wide incision was made through the skin in the center of the anesthetized area with a sterile, single-use, #11 disposable scalpel. The fascia of the vastus lateralis muscle was then perforated to allow for entry of the biopsy needle. The depth of the incision was adjusted to accommodate estimated subcutaneous fat depth. A sterile 5-mm Bergström biopsy needle (Pelomi Industries, Denmark) was passed through the incision into the muscle, and a small piece (equal to 100–300 mg) of muscle was removed with applied suction using a Monoject 60 mL syringe attached via sterile tubing. Three to five clips, after a slight rotation, were made within each pass of the needle. Manual pressure was applied to the wound for approximately 5–7 min until bleeding stopped before steri-strips, gauze, and a pressure bandage were applied over the site after recleaning the leg. Muscle tissue was placed in cryovials and frozen in liquid nitrogen for analysis of protein and ribonucleic acid (RNA) synthesis and stored at −80°C. Muscle tissue for histological and immunohistochemical analysis was embedded in tragacanth gum, frozen in liquid nitrogen-cooled isopentane, and stored at −80°C.
Histological and Immunohistochemistry
Hematoxylin and eosin staining.
Muscle cross sections were cut at 7 µm, air-dried, and fixed in 100% methanol. Nuclei were stained using a hematoxylin (Shanden Instant #230022, Thermo Fisher) solution for 2 min, rinsed, and then put into an eosin solution for 2 min before dehydration through xylene and coverslipping.
Myosin heavy chain and cross-sectional area determination.
Muscle cross sections were cut at 7 µm, air-dried, and stored at −20°C before analysis. Frozen sections were incubated overnight with isotype-specific anti-mouse primary antibodies for myosin heavy chain (MyHC) 1 (1:75, IgG2B, BA.D5), MyHC 2a (supernatant, neat, IgG1, SC.71), and MyHC 2x (supernatant, neat, IgM, 6H1) from Developmental Studies Hybridoma Bank (DSHB, Iowa City, IA), along with laminin (1:100, Sigma-Aldrich, St. Louis, MO). For embryonic MyHC (MyHCemb) staining, SC.71 was replaced with F1.652 antibody (neat, DSHB). Sections were then incubated with secondary antibodies (1:250, goat anti-mouse IgG2B Alexa Fluor 647; 1:500, IgG1 Alexa Fluor 488; and 1:250, IgM Alexa Fluor 555) from Invitrogen, all diluted in phosphate-buffered saline (PBS), along with the secondary antibody for laminin (1:150, IgG AMCA (anti-mannobioside), Vector, Malvern, PA). Sections were mounted with VectaShield mounting medium (Vector), and images were captured at 20×. Fiber type distribution, mean fiber cross-sectional area (CSA), and fiber-type-specific CSA were quantified using MyoVision (23).
Satellite cell abundance.
Muscle cross sections were cut at 7 µm, air-dried, and stored at −20°C. Sections were fixed in 4% paraformaldehyde (PFA) followed by epitope retrieval using sodium citrate (10 mM, pH 6.5) at 92°C for 10 min. Sections were cooled to room temperature (RT), and endogenous peroxidase activity was quenched using 3% hydrogen peroxide in PBS. Sections were incubated overnight at 4°C with Pax7 primary antibody (1:100, DSHB). The next day, samples were incubated with an anti-mouse biotin-conjugated secondary antibody (1:1,000, Jackson ImmunoResearch, West Grove, PA) for 1 h. Signal amplification was performed using streptavidin-horseradish peroxidase (1:500, Thermo Fisher) and TSA Alexa Fluor 488 to visualize antibody binding. Finally, sections were mounted and nuclei were stained using Vectashield antifade mounting media with 4′,6-diamidino-2-phenylindole (DAPI, Vector). Sections were imaged at 20× magnification using a Zeiss upright fluorescent microscope (Zeiss AxioImager M1 Oberkochen, Germany). Satellite cells were identified as Pax7+/DAPI+ cells. Satellite cell number was expressed per myofiber.
Collagen staining.
Picrosirius red (PSR) staining was performed as previously described (24). Briefly, muscle sections were fixed with 4% PFA for 30 min, followed by placing the slides vertical to air dry for at least 1 h. Sections were incubated in PSR solution (0.1% PSR in saturated picric acid; Electron Microscopy Sciences, Hatfield, PA) for 1 h while rocking. Sections were washed, dehydrated with ethanol, and mounted with xylene-based mounting media. An average of five to six representative images (approximately 100–150 fibers per section encompassing about 70% of the entire cross section) were taken on an upright microscope (AxioImager M1, Oberkochen, Germany Zeiss), and PSR (red pixel area) was expressed as a percent of total pixel area (µm2).
Protein and RNA Synthesis Rates
Protein synthesis was determined according to methods described in Miller et al. (25). Briefly, skeletal muscle tissue was homogenized 1:10 in isolation buffer (100 mM KCl, 40 mM Tris HCl, 10 mM Tris Base, 5 mM MgCl2, 1 mM EDTA, and 1 mM ATP, pH = 7.5) with phosphatase and protease inhibitors (HALT, Thermo Fisher Scientific) using a bead homogenizer (Next Advance Inc., Averill Park, NY). After homogenization, subcellular fractions were isolated via differential centrifugation as previously described (26–28). Once protein pellets were isolated and purified, 250 µL 1 M NaOH was added, and pellets were incubated for 15 min at 50°C while slowly mixing. Protein was hydrolyzed by incubation for 24 h at 120°C in 6 N HCl. The pentafluorobenzyl-N,N-di(pentafluorobenzyl) derivative of alanine was analyzed on an Agilent 7890A GC coupled to an Agilent 5975C MS as previously described (26–28).
RNA isolation of ∼15–25 mg frozen muscle was performed for ribosomal turnover analysis according to our previously published procedures, using TRIzol (29–31). The isolated RNA was hydrolyzed overnight at 37°C with nuclease S1 and potato acid phosphatase. Hydrolysates were reacted with pentafluorobenzyl hydroxylamine and acetic acid and then acetylated with acetic anhydride and 1-methylimidazole. Dichloromethane extracts were dried, resuspended in ethyl acetate, and analyzed on an Agilent 7890A GC coupled to an Agilent 5975C MS.
To determine body water enrichment, 120 μL of plasma was placed in the inner well of an O-ring cap of inverted screw-capped tubes and placed in a heat block for overnight distillation at 80°C. Distilled samples were diluted 1:300 in ddH2O and analyzed on a liquid water isotope analyzer (Los Gatos Research, Los Gatos, CA) against a standard curve prepared with samples containing different concentrations of D2O.
The newly synthesized fraction (f) of proteins was calculated from the enrichment of alanine bound in muscle proteins over the entire labeling period, divided by the true precursor enrichment (p), using plasma D2O enrichment with mass isotope distribution analysis (MIDA) adjustment (32). Similarly, RNA synthesis (∼85% of total RNA exists as ribosomal RNA) was determined by deuterium incorporation into the purine ribose of RNA, as previously published (29, 30), with MIDA adjustment of the equilibration of the enrichment of body water pool with purine ribose.
Statistical Analysis
Results are expressed as means ± standard deviation (SD). For each outcome variable, normal distribution was assessed using the Shapiro–Wilk test, whereas the homogeneity of the variance was checked using Levene’s test. Student’s t tests (fiber CSA, fiber frequency, satellite cells, and collagen content) were run for normally distributed data, whereas nonparametric Wilcoxon testing (protein and RNA synthesis) was used to compare group (damaged vs. nondamaged) mean differences for non-normally distributed data. For CSA, secondary analyses were performed including sex and baseline type 1 fiber size as separate covariates to account for sex differences in muscle size as well as differences in group type 1 fiber size found at baseline. Statistical significance was assumed at P < 0.05. The JMP Pro statistical discovery assessment package (SAS, version 14.0) was used for statistical analyses.
RESULTS
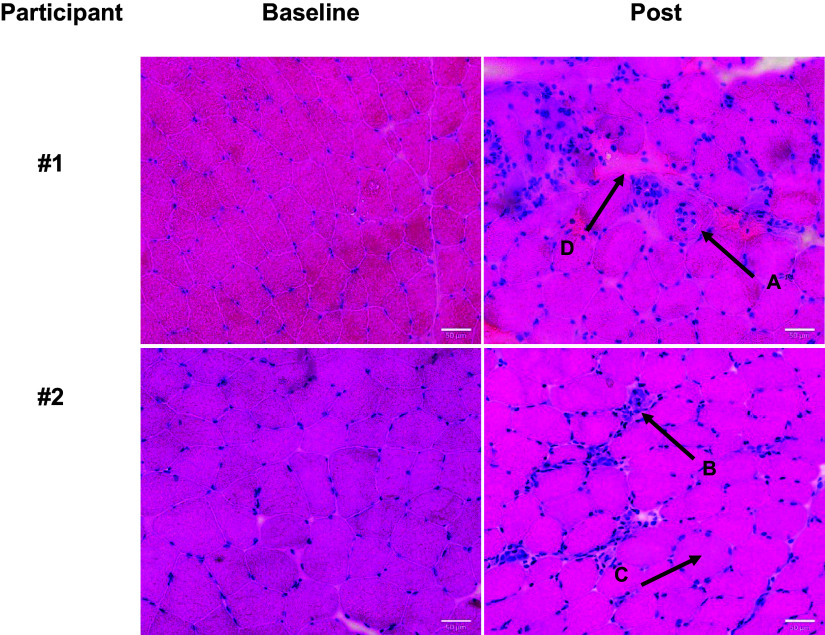
The majority of the second biopsies (61.5%) of human participants, performed on the control leg that did not receive an intervention, revealed evidence of damaged and/or regenerating muscle tissue as indicated by our operational definition, while only 7.6% of the initial biopsies included damage indicators. Representative images of baseline and second muscle biopsy samples show examples of centronucleation (Fig. 2, arrow A), cellular infiltration (Fig. 2, arrow B), rounding of fibers (Fig. 2, arrow C), and enhanced extracellular space (Fig. 2, arrow D). Raw data of analysis of the damage markers per muscle sample are listed in Supplemental Table S1 (see https://doi.org/10.6084/m9.figshare.23988921). At baseline, the outcome measures of interest in this study were not significantly different between the persons designated to the damaged and nondamaged groups, with the exception of type 1 fiber size that was larger in the nondamaged group (Table 1). Procedural characteristics of the muscle biopsies for the two groups are shown in Table 2. In most cases, only one pass was made with the Bergström needle with an average use of 13 mL of lidocaine before obtaining an average of 240 mg of muscle tissue (Table 2). No differences were observed between the groups for biopsy characteristics. Also, the assignment to nondamaged versus damaged groups was not operator specific as damage was found in second biopsies across samples obtained by three different trained operators with similar techniques. Furthermore, we found that biopsies were taken approximately 2 cm apart (Supplemental Fig. S1; see https://doi.org/10.6084/m9.figshare.23988921). Although we did not directly measure this distance for every participant exactly, the distance was comparable between samples showing damage versus no damage.
Figure 2.
Morphological signs of damage in repeated biopsy. Representative hematoxylin and eosin (H&E) pictures of baseline (left column) and second samples (right column) of two participants collected 7 days apart. Arrows indicate signs of muscle damage by which participant biopsies were grouped: A) central nucleation, B) cellular infiltration, C) rounding of fibers, and D) enlarged extracellular space. Scale bar is 50 µm.
Table 1.
Outcome measures for baseline muscle samples for damaged and nondamaged groups
| Baseline Mean ± SD |
P Value | ||
|---|---|---|---|
| Muscle Sample Characteristics | Nondamaged, n = 10 | Damaged, n = 16 | Between Group |
| Muscle phenotype (CSA and distribution) | |||
| Mean fiber CSA, µm2 | 4,598.01 ± 738.79 | 4,103.86 ± 845.17 | 0.13 |
| Type 1 fiber CSA, µm2 | 4,549.63 ± 802.56 | 3,858.34 ± 730.22 | 0.04* |
| Type 2a fiber CSA, µm2 | 4,659.04 ± 1,212.77 | 4,454.00 ± 1,093.99 | 0.67 |
| Type 2a/x fiber CSA, µm2 | 3,901.71 ± 668.10 | 3,770.16 ± 1040.07 | 0.75 |
| Type 1 fiber frequency, % | 50.57 ± 18.43 | 46.74 ± 11.93 | 0.57 |
| Type 2a fiber frequency, % | 47.22 ± 18.31 | 49.29 ± 11.85 | 0.76 |
| Type 2a/x fiber frequency, % | 2.22 ± 2.46 | 4.17 ± 7.33 | 0.35 |
| Satellite cell content (SC) | |||
| SC/fiber | 0.08 ± 0.04 | 0.07 ± 0.03 | 0.37 |
| Total collagen content | |||
| PSR/total area (%) | 5.53 ± 1.52 | 5.15 ± 1.33 | 0.53 |
| Fractional synthesis rate (FSR) | |||
| Protein synthesis, % per day | 2.18 ± 1.14 | 2.04 ± 1.68 | 0.80 |
| RNA synthesis, % per day | 0.03 ± 0.05 | 0.04 ± 0.05 | 0.58 |
CSA, cross-sectional area; PSR, Picrosirius red. *Significantly different between groups.
Table 2.
Procedural biopsy characteristics for nondamaged and damaged groups
| Group | Number (n) | Sex (F) | Amount of Lidocaine Used, mL | Biopsy Weight Collected, mg | Number of Passes (3–5 Clips) | Operatorsa (n) | Participant Notes |
|---|---|---|---|---|---|---|---|
| Baseline Sample (Right Leg) | |||||||
| Nondamaged | n = 10 | 7 | 13.2 ± 2.8 | 252.0 ± 71.4 | 1.2 ± 0.4 | 3 | Previously had COVID-19 (2) |
| Damaged | n = 16 | 11 | 12.6 ± 2.5 | 256.6 ± 78.4 | 1.1 ± 0.3 | 2 | Previously had COVID-19 (1) |
| Post 7 Day Sample (Right Leg) | |||||||
| Nondamaged | n = 10 | 7 | 13.0 ± 2.8 | 234.5 ± 50.5 | 1.0 ± 0.0 | 2 | None |
| Damaged | n = 16 | 11 | 13.8 ± 2.8 | 214.9 ± 71.8 | 1.0 ± 0.0 | 3 | Complaint of discomfort during Bergström needle insertion (4), dizziness following procedure (1) |
aSame operator was used 80%–85% of the time. COVID-19, coronavirus disease of 2019.
MyHC and laminin staining of a representative muscle of the nondamaged (Fig. 3A) and damaged group (Fig. 3B) shows the presence of smaller fibers, mainly type 1, in the damaged biopsy. However, mean fiber CSA was not different between the nondamaged versus damaged group (Fig. 3C). Mean fiber CSA for individual fiber types was also not different between the nondamaged and damaged groups (Fig. 3C). Likewise, type 1 (P = 0.37), type 2a (P = 0.49), and type 2a/x (P = 0.19) fiber frequencies were not significantly different between those with damage versus no damage (Fig. 3D). These results did not differ when including sex as a covariate for mean fiber size (P = 0.053); or when accounting for baseline type 1 fiber differences, there was no longer a trend for smaller type 1 fiber size (P = 0.68). However, the frequency distribution curves show a higher percentage of smaller fibers as well as lower percentages of larger fibers in the damaged compared with the nondamaged group (see arrows in Fig. 3E). Representative images of MyHCemb staining (Fig. 3F: nondamaged and Fig. 3G: damaged) indicate significantly more MyHCemb-positive fibers in damaged compared with nondamaged biopsies (P = 0.001, Fig. 3H), which accounts for the higher percentage of smaller fibers in the damaged group (Fig. 3E, arrow). A strong correlation between the number of MyHCemb-positive fibers and the percentage of fibers less than 1,000 μm2 (r = 0.79, P = 0.0003) was observed.
Figure 3.
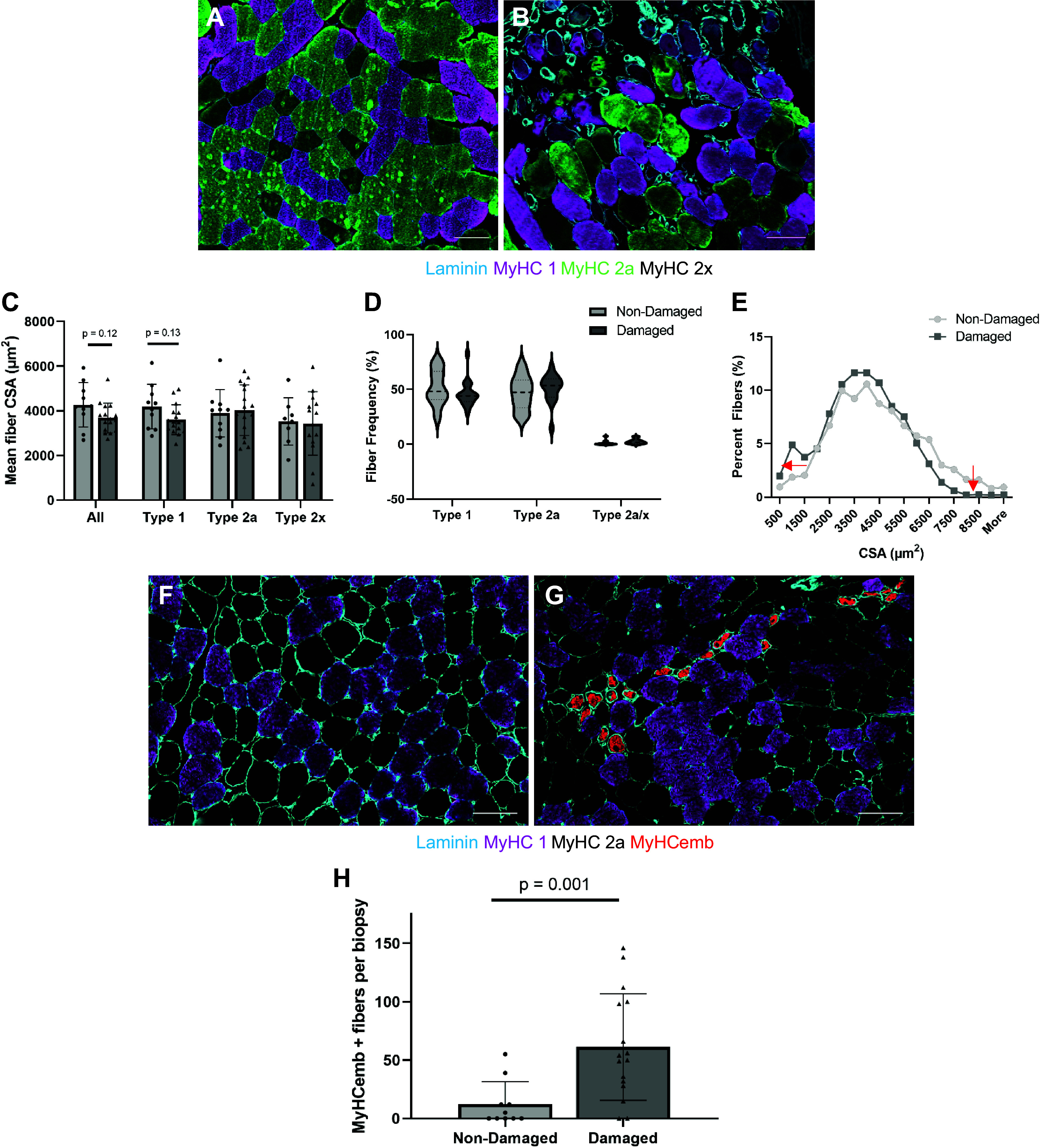
Differences in myosin heavy chain (MyHC) and cross-sectional area (CSA) between damaged and nondamaged biopsies. Representative images of muscle tissue immunoreacted with MyHC 1 (purple), 2a (green), 2x (black), and laminin (blue) with no signs of damage (A) or with damage/regeneration (B). Mean fiber CSA (C), fiber type frequencies (D), fiber size distribution (E) of nondamaged (n = 10, light gray) and damaged (n = 16, dark gray). Arrows in (E) indicate shift of distribution curve. Representative images of muscle sections with MyHC 1 (purple), 2a (black), and embryonic (red) for nondamaged (F) and damaged (G) groups. Number of embryonic MyHC (MyHCemb)-positive fibers in nondamaged (light gray, n = 10) and damaged (dark gray, n = 16) biopsies (H). Scale bars in all pictures are 100 µm. Values are means ± SD.
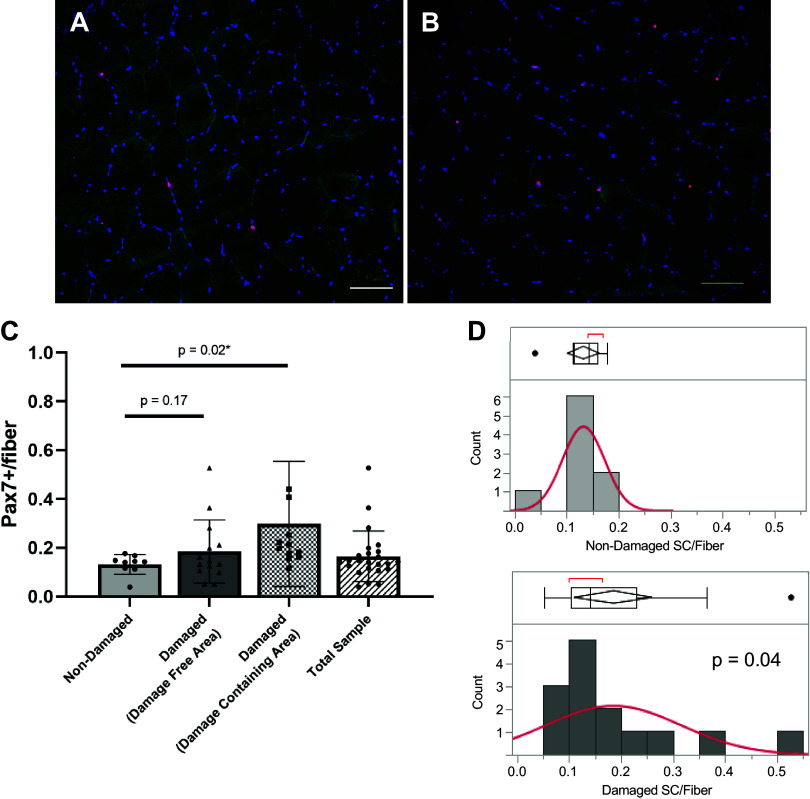
For satellite cell content, we analyzed muscle sections separate for areas containing obvious damage versus those areas that did not appear to have damage in the damaged group. Representative images free of obvious damage are shown in the nondamaged (Fig. 4A) and damaged (Fig. 4B) groups. Satellite cell density trended 40% higher (P = 0.17) in the damaged group in areas that did not show obvious signs of damage (Fig. 4C). In damaged specific sections of muscle tissue, satellite cells density was ∼60% higher than in muscle of the nondamaged group (Fig. 4C; P = 0.02). Levene’s test for variance revealed a significantly higher variability of satellite cell number in the damaged group (all areas together) compared with the nondamaged group (Fig. 4D, P = 0.04). In total, we show that damaged areas can influence the overall mean satellite cell count of a sample if all areas are included, even if damage is not known to be present (Fig. 4C, total sample).
Figure 4.
Higher satellite cell content and variability in damaged areas of repeat muscle biopsies. Representative images of Pax7 immunostaining of areas without obvious damage in nondamaged (A) and damaged (B) muscle biopsies. Pax7-positive cells are red. Pax7+ cells for nondamaged biopsies and damage-free area in damaged biopsies (labeled “damaged”) as well as areas specifically containing obvious damage (“damage containing area”) (C). Levene’s test for variance in nondamaged (D, top) and all areas of damaged (D, bottom) biopsies (P = 0.04). n = 10 for nondamaged and n = 16 for damaged groups. Scale bar 100 µm. Values are means ± SD. SC, satellite cell.
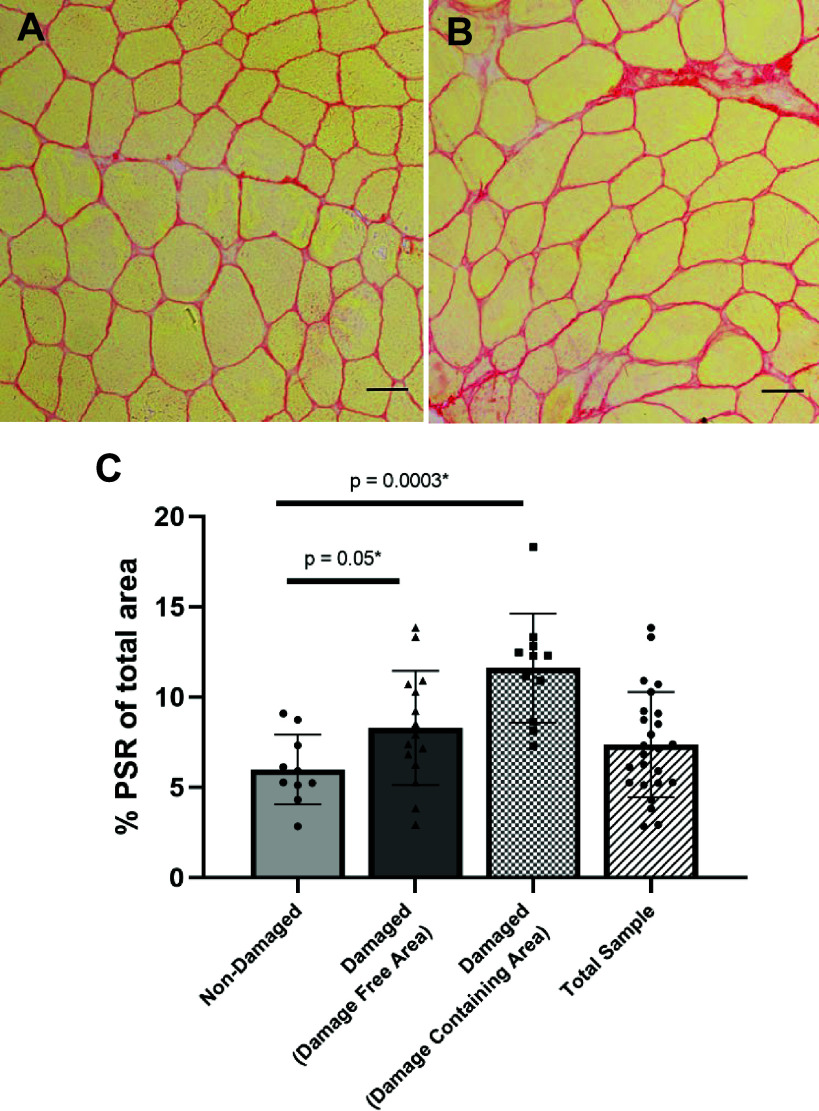
Representative images of PSR staining on damage-free areas in muscle biopsy sections from the nondamaged (Fig. 5A) and damaged (Fig. 5B) group and quantification of PSR (Fig. 5C) show collagen content (% PSR from total area) higher in damaged compared with nondamaged biopsy groups (P = 0.05) when measured in nondamaged areas in the group designated as damaged. When PSR was analyzed in damage-specific sections of muscle tissue, collagen content was 62% higher than in undamaged sections (P = 0.0003). In total, we show that damaged areas can influence the overall mean collagen content of a sample by approximately 25% if all areas are included, and damage is not known to be present (Fig. 5C, total sample).
Figure 5.
Collagen content is higher in muscle biopsies in the damaged group. Representative images of damage-free muscle areas of biopsies from the nondamaged (A) and damaged (B) groups. Percent picrosirius red (PSR) of biopsies (C) for nondamaged biopsies and damage-free area in damaged biopsies (labeled “damaged”) as well as areas specifically containing obvious damage (“damage-specific area”). n = 10 for nondamaged and n = 16 for damaged group. Scale bars 100 µm. Values are means ± SD.
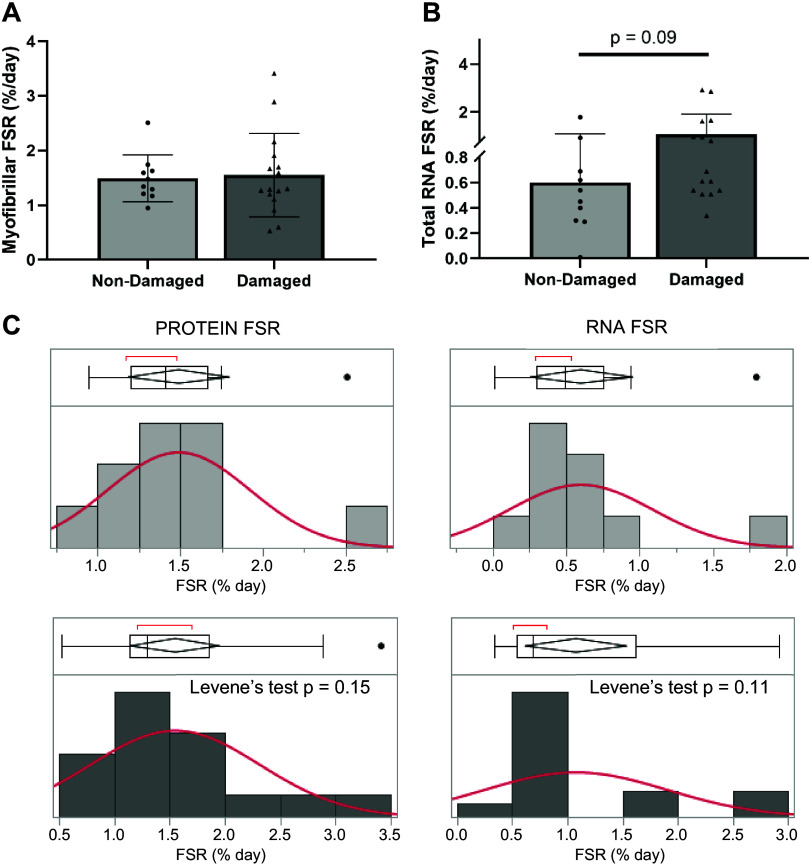
Fractional synthesis rates of myofibrillar protein (Fig. 6A; P = 0.68) was not different between nondamaged and damaged groups, while fractional synthesis rates of RNA (Fig. 6B; P = 0.09) trended to be higher in the damaged group. Using Levene’s test for equality of variances revealed that again, damaged tissue had a tendency for increased variability, but this did not reach significance for protein synthesis (P = 0.15) nor RNA synthesis (P = 0.11) (Fig. 6C).
Figure 6.
Fractional synthesis rate ribonucleic acid (RNA) trends to be higher in damaged muscle biopsies. Myofibrillar protein fractional synthesis rate (FSR; A) and total RNA FSR (B) of nondamaged (dark gray, n = 16) and damaged (light gray, n = 10) muscle biopsies. Values are means ± SD. Levene’s test for variance for protein (left) and RNA (right) FSR for nondamaged (light gray, top) and damaged (dark gray, bottom) groups (C).
DISCUSSION
The goal of this secondary analysis was to determine whether common analyses in human skeletal muscle are influenced by repeated biopsy procedures associated without any other intervention. We found that measurements directly (MyHCemb and satellite cells) and not directly related [collagen content and RNA fractional synthesis rate (FSR) trending] to damage were affected in the repeated biopsy, and the outcomes of these variables could be misinterpreted if the markers of injury are not assessed.
The percutaneous muscle biopsy technique has been used for many years, yet very little evidence exists for procedural effects of repeated biopsies on measurements in vastus lateralis muscle and variability in outcomes. However, it is suggested that the biopsy procedure itself could be a confounding factor when interpreting muscle-related data (33). Earlier studies showed clear indication of anesthetic-induced muscle damage within surrounding muscle fibers (6–10). This has led to the refinement of muscle collection procedures for research, such as the halting of combined epinephrine, the inclusion of bicarbonate infused lidocaine to reduce acidity and patient discomfort, and keeping lidocaine administration above the muscle fascia. Also, muscle injury created by the muscle sampling technique (incision/puncture) can affect the surrounding fibers (34), although these results are usually secondary to the original presented aims such as in the current report. Recommended distances of 2–3 cm between biopsy sites have, therefore, been included in the standardized operating procedures (5). A few studies specifically reported on changes in muscle tissue following closely timed collections alone or when compared with eccentric-damaging exercise (11, 12, 17). In alignment with these studies, we report that muscle damage was present in 61.5% of follow-up biopsies obtained 7 days after the baseline sampling. In addition, we show the effect of repeated sampling on important outcome measures frequently used to assess skeletal muscle adaptation in response to an intervention, such as muscle fiber phenotype and CSA, satellite cell number, collagen content, and myofibrillar protein and RNA synthesis. It is, therefore, important to rule out whether observed responses are due to an intervention or the experimental procedure of a previous biopsy.
While biopsies obtained for research are quick and produce relatively minor discomfort, proper precautions and sterile environments must be utilized for individualized safety concerns. We were unable to determine whether a specific operator, amount of lidocaine, or number of biopsy needle passes was responsible for the muscle damage seen in our second samples. In cases where a second pass may be needed due to participant complaints of discomfort or inadequate tissue amount, the operator must be careful not to inject lidocaine near the muscle fascia as it would have already been punctured allowing for lidocaine to potentially penetrate deeper into the muscle. Our average dose of lidocaine was approximately 13 mL, which is higher than what others reported (5), so the volume of lidocaine cannot be ruled out as a cause for muscle damage. In addition, while it was not measured directly, the distance between biopsy sites may have been less than the recommended 2–3 cm in remaining cases. Therefore, biopsy procedure documents should include possible tissue damage confounders including a measured distance between multiple biopsy sites, the amount of lidocaine used, as well as noting the angle of needle insertion (direction of trochar in the muscle) as an alternative confounder. Table 3 introduces steps that can be taken to reduce procedural damage in the muscle samples that can confound interpretation of the repeated biopsy when taken within the time period that includes regeneration of muscle.
Table 3.
Recommendations for repeated muscle collections during short time frames
| During Primary Vastus Lateralis Tissue Collection |
|
|
|
|
|
|
|
|
| During Secondary Vastus Lateralis Tissue Collection (on same leg) |
|
|
|
|
| Other Considerations |
|
|
|
Muscle injury invokes structural changes stimulating coordinated cellular reactions consisting of immune, inflammatory, and satellite cell responses to start regenerative repair processes (37). These processes include the infiltration of immune cells, the activation, proliferation, and fusion of satellite cells, and extracellular matrix (ECM) remodeling; these events are most likely affected by multiple biopsy procedures during the early regeneration phase of muscle (1–7 days). However, it is possible that outcomes are affected by multiple sampling at later time points, since the regeneration process is not complete until 14 days or later, depending on other variables, such as age (38, 39). Roth et al. (40) describe so-called “hypercontracted fibers” resulting as a consequence of muscle sampling and tissue processing versus extreme damage generated by eccentric exercise. They concluded that microscopic observations involving structural disruptions in sarcomere length without accompanied necrosis, mononuclear inflammatory cell invasion, or other signs of degeneration are suggestive of structural artifacts caused by the muscle sampling itself and is distinct from eccentric contraction-mediated responses. However, structural artifacts are not always measured and hard to identify in tissue that does not include overt damage. These artifacts could induce changes in muscle that induce a regenerative response that is not as severe as when overt damage is observed, but could confound findings of ECM remodeling or RNA synthesis, for example, as shown in our data. Two studies highlight different interpretations of damage induced by repeated muscle biopsies. Guerra et al. (11) report that multiple biopsies through a single 5–6 mm incision with a Bergström needle angled in different directions for each sample (>3 cm apart) did not elicit detectable changes in signaling pathways related to inflammation, stress, or reparation within the first 2 h, although the evidence of increased STAT3 phosphorylation and IL-6 mRNA abundance were observed. The authors also suggest that an injury to the skin does not result in this type of signaling in the underlying muscle; however, 2 h is relatively short compared with most study designs. On the contrary, Malm et al. (12) demonstrated that multiple biopsies cause changes in adult human skeletal muscle similar to eccentric cycling with respect to neutrophils, macrophages, and cytokine (IL-1) analyses, as well as satellite cell activation over a period of 7 days. Interestingly, significant changes related to the biopsy had disappeared by day 7, and the only variable still significant following eccentric exercise was CD15, a marker for granulocytes; measures related to ECM remodeling or protein and RNA homeostasis were not studied. However, in that study, biopsy sites were spaced at only 2 cm apart and a forceps biopsy technique was used that resulted in smaller amounts of muscle retrieved, which has been reported to affect intramuscular measures (13). Obvious differences exist between these conflicting studies such as the technique used for muscle sampling and the time-dependent phase of regeneration studied (41). Our study also used the 7-day time point for the repeated biopsy, and we chose to evaluate markers often assessed for interventions such as satellite cell number, MyHC distribution and CSA, as well as collagen content and myofibrillar protein and RNA synthesis (42). We found that markers of regeneration (MyHCemb and satellite cell number and variability), collagen content, and a trend for RNA synthesis were changed by the presence of damage in the tissue section, even when apparently nondamaged areas were analyzed.
Previous work showed that repeated sampling at greater than 3 cm does not affect muscle phenotype such as fiber type frequencies or fiber-specific CSA as long as more than 150 fibers are counted (16). Similarly, vascular-related indices such as blood flow and capillarization are not affected, but mitochondrial variables may be altered due to reduced tissue oxygen saturation (15, 16). We also found that mean fiber CSA and fiber type frequency were not different between our groups with a 2-cm spacing, but there was a trend for smaller mean fiber CSA which was mainly driven by type 1 fiber CSA and the presence of a subpopulation of fibers smaller than 1,500 µm2. We found that this subpopulation is mainly MyHCemb positive, indicating previous damage and regeneration. Also, satellite cell number was not different in the damaged group when areas of muscle sections were counted that did not show obvious damage, although there was a trend for higher numbers. Interestingly, the variability of satellite cell number was higher in the damaged group, which may confound measures of interventions when potential damage to the tissue is not obvious or adjacent to the section cut for analysis. We now show, for the first time, to our knowledge, the effect of previous biopsies on collagen abundance and myofibrillar protein and RNA synthesis. Collagen content was significantly higher in biopsies from damaged tissues, even in areas of the biopsy where no evident damage was observed. Similarly, total RNA synthesis trended higher within damaged biopsies labeled as damaged, even though myofibrillar protein FSR was not different between the damaged and the nondamaged groups. Since it is more difficult to identify damage when muscle tissue is homogenized for FSR determination (or other methods such as Westerns or RNA sequencing), care should be taken to identify potential injury in adjacent samples. Therefore, it is advisable to perform an assessment of potential damage, such as H&E staining, to ensure accurate findings.
This study is not without limitations; this is a secondary analysis that was not a priori powered to detect significant differences from the sample size and type of measurements used. It is possible that trending P values would change if a larger sample size were studied and concepts conceived beforehand. In addition, our statistical approach did not allow for balancing baseline demographic data as blinded assessors determined tissue damage after the participants were already enrolled and randomized. Human research is inevitably complicated by measurement variability. We unsurprisingly see higher variation in our microscopic measures of fiber cross-sectional area than we do at the macroscopic level when assessing whole muscle with dual-energy X-ray absorptiometry (DXA) or computed tomography (CT) measures (43). This leads to inconsistencies when associating various measures of muscle size with muscle-related outcomes (43). Since researchers cannot obtain muscle tissue from the exact same location due to the healing process (i.e., fibrosis) and muscle is not uniform with variations in fiber type and cross-sectional area, inherent variability exists between biopsies. Recent research has determined that the average within-subject coefficient of variation for muscle vastus lateralis phenotype can range anywhere from 13% to 18%, but that differences in leg or longitudinal orientation do not need to be considered when investigating characteristics (36). Here, we show that muscle tissue damage is possible in muscle samples taken adjacent to previous muscle biopsy sites, even when taking the proper precautions, and is another source for greater outcome variability, which could hinder finding significant results related to a proposed intervention. Further research to establish statistical techniques to account for inherent measurement error would be beneficial to address human heterogeneity and avoid misinterpreting results. In addition, knowledge of possible damage utilizing H&E staining for the exclusion of heavily damaged cryosections of muscle would help increase the accuracy of data. Finally, due to the unique study design, it is possible that immobilization may lower the generalizability of these findings as we do not know the behavioral or possible influence of overloading the nonimmobilized contralateral limb.
Conclusions
Despite the expanding knowledge obtained to improve muscle biopsy sampling procedures, emphasis still needs to be placed on proper technique and precautions for unbiased data, especially when an intervention is applied, and sampling is to be repeated in short time periods. The amount of lidocaine administrated, the angle of needle insertion, the distance between consecutive biopsies, and time between procedures needs to be carefully documented. Our results show that at 7 days after the initial biopsy, injury induced by the first procedure affects outcomes related to regenerative responses and tissue remodeling and that this may be missed if damage is not specifically assessed and accounted for in standardized methods. In addition, the higher variability in outcome measures may make it harder to detect differences in the case of intervention studies.
ETHICAL APPROVALS
This study was approved by the University of Kentucky institutional review board (IRB 43499) before any participants enrolling. Data and safety monitoring were provided by the University of Kentucky Center for Clinical and Translational Science (CCTS) data and safety monitoring board (UK CCTS DSMB) on a semi-annual basis.
DATA AVAILABILITY
The datasets used and/or analyzed during the current study are available upon reasonable request.
SUPPLEMENTAL DATA
Supplemental Table S1 and Fig. S1: https://doi.org/10.6084/m9.figshare.23988921.
GRANTS
The study was funded by the National Institutes of Health – National Center for Complementary and Integrative Health grant R21AT010847 and supported by the NIH Clinical and Translational Science Awards (CTSA) (UL1TR001998) at the University of Kentucky. The NIH had no role in the collection, analysis, or interpretation of data, or in writing the manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Benjamin Miller and Esther Dupont-Versteegden are editors of Journal of Applied Physiology and were not involved and did not have access to information regarding the peer-review process or final disposition of this article. An alternate editor oversaw the peer-review and decision-making process for this article.
CONSENT TO PARTICIPATE
All participation was on a voluntary basis, and each participant was required to sign an approved IRB consent form and HIPAA authorization before any procedures taking place.
AUTHOR CONTRIBUTIONS
B.F.M., T.A.B., and E.E.D.-V. conceived and designed research; A.J.M., A.L.C., P.A.K., T.A.B., and E.E.D.-V. performed experiments; D.E.L., A.J.M., and A.L.C. analyzed data; D.E.L., A.L.C., B.F.M., P.A.K., T.A.B., and E.E.D.-V. interpreted results of experiments; D.E.L. and A.L.C. prepared figures; D.E.L. and E.E.D.-V. drafted manuscript; D.E.L., A.L.C., B.F.M., P.A.K., T.A.B., and E.E.D.-V. edited and revised manuscript; D.E.L., A.J.M., A.L.C., B.F.M., P.A.K., T.A.B., and E.E.D.-V. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors would like to thank each of our valuable research participants for their time, effort, and dedication given during the study. We would like to thank Sumya Elgumati and Dr. Martha Biddle for performing muscle biopsies. Additional thanks go to Hannah Bowlds for her technical expertise in the analysis of immunohistochemistry and Frederick Peelor III for gas chromatography/mass spectrometry analyses. The online graphical abstract was created with BioRender.com with permission.
The first author, D.E.L., is a research associate senior in the University of Kentucky College of Health Sciences, and an exercise physiologist in the UK CCTS Functional Assessment and Body Composition Core Lab.
REFERENCES
- 1. Cawthon PM, Fox KM, Gandra SR, Delmonico MJ, Chiun-Fang C, Anthony MS, Sewall A, Goodpaster B, Satterfield S, Cummings SR, Harris TB; Health, Aging and Body Composition Study. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc 57: 1411–1419, 2009. doi: 10.1111/j.1532-5415.2009.02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fyfe JJ, Hamilton DL, Daly RM. Minimal-dose resistance training for improving muscle mass, strength, and function: a narrative review of current evidence and practical considerations. Sports Med 52: 463–479, 2022. doi: 10.1007/s40279-021-01605-8. [DOI] [PubMed] [Google Scholar]
- 3. Duchenne GBA. Recherches sur la paralysie musculaire pseudohypertrophique ou paralysie myo-sclerosique. Arch Gen Med 11: 3–25, 1868. [Google Scholar]
- 4. Evans WJ, Phinney SD, Young VR. Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc 14: 101–102, 1982. [PubMed] [Google Scholar]
- 5. Shanely RA, Zwetsloot KA, Triplett NT, Meaney MP, Farris GE, Nieman DC. Human skeletal muscle biopsy procedures using the modified Bergström technique. J Vis Exp 91: e51812, 2014. doi: 10.3791/51812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Foster AH, Carlson BM. Myotoxicity of local anesthetics and regeneration of the damaged muscle fibers. Anesth Analg 59: 727–736, 1980. [PubMed] [Google Scholar]
- 7. Benoit PW. Microscarring in skeletal muscle after repeated exposures to lidocaine with epinephrine. J Oral Surg 36: 530–533, 1978. [PubMed] [Google Scholar]
- 8. Benoit PW. Reversible skeletal muscle damage after administration of local anesthetics with and without epinephrine. J Oral Surg 36: 198–201, 1978. [PubMed] [Google Scholar]
- 9. Yagiela JA, Benoit PW, Buoncristiani RD, Peters MP, Fort NF. Comparison of myotoxic effects of lidocaine with epinephrine in rats and humans. Anesth Analg 60: 471–480, 1981. [PubMed] [Google Scholar]
- 10. Trappe TA, Standley RA, Liu SZ, Jemiolo B, Trappe SW, Harber MP. Local anesthetic effects on gene transcription in human skeletal muscle biopsies. Muscle Nerve 48: 591–593, 2013. doi: 10.1002/mus.23860. [DOI] [PubMed] [Google Scholar]
- 11. Guerra B, Gómez-Cabrera MC, Ponce-González JG, Martinez-Bello VE, Guadalupe-Grau A, Santana A, Sebastia V, Viña J, Calbet JAL. Repeated muscle biopsies through a single skin incision do not elicit muscle signaling, but IL-6 mRNA and STAT3 phosphorylation increase in injured muscle. J Appl Physiol (1985) 110: 1708–1715, 2011. doi: 10.1152/japplphysiol.00091.2011. [DOI] [PubMed] [Google Scholar]
- 12. Malm C, Nyberg P, Engstrom M, Sjodin B, Lenkei R, Ekblom B, Lundberg I. Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J Physiol 529: 243–262, 2000. doi: 10.1111/j.1469-7793.2000.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hughes MC, Ramos SV, Turnbull PC, Nejatbakhsh A, Baechler BL, Tahmasebi H, Laham R, Gurd BJ, Quadrilatero J, Kane DA, Perry CGR. Mitochondrial bioenergetics and fiber type assessments in microbiopsy vs. Bergstrom percutaneous sampling of human skeletal muscle. Front Physiol 6: 360, 2015. doi: 10.3389/fphys.2015.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yagiela JA, Benoit PW, Fort NF. Mechanism of epinephrine enhancement of lidocaine-induced skeletal muscle necrosis. J Dent Res 61: 686–690, 1982. doi: 10.1177/00220345820610051301. [DOI] [PubMed] [Google Scholar]
- 15. Bubak MP. The effect of the muscle biopsy procedure on blood flow and tissue oxygenation. Med Sci Sports Exerc 47(5S): 292, 2015. [Google Scholar]
- 16. Nederveen JP, Ibrahim G, Fortino SA, Snijders T, Kumbhare D, Parise G. Variability in skeletal muscle fibre characteristics during repeated muscle biopsy sampling in human vastus lateralis. Appl Physiol Nutr Metab 45: 368–375, 2020. doi: 10.1139/apnm-2019-0263. [DOI] [PubMed] [Google Scholar]
- 17. van de Vyver M, Myburgh KH. Cytokine and satellite cell responses to muscle damage: interpretation and possible confounding factors in human studies. J Muscle Res Cell Motil 33: 177–185, 2012. doi: 10.1007/s10974-012-9303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schmidt CA, Amorese AJ, Ryan TE, Goldberg EJ, Tarpey MD, Green TD, Karnekar RR, Yamaguchi DJ, Spangenburg EE, McClung JM. Strain-dependent variation in acute ischemic muscle injury. Am J Pathol 188: 1246–1262, 2018. doi: 10.1016/j.ajpath.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Finlin BS, Memetimin H, Zhu B, Confides AL, Vekaria HJ, El Khouli RH, Johnson ZR, Westgate PM, Chen J, Morris AJ, Sullivan PG, Dupont-Versteegden EE, Kern PA. The β3-adrenergic receptor agonist mirabegron improves glucose homeostasis in obese humans. J Clin Invest 130: 2319–2331, 2020. doi: 10.1172/JCI134892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fry CS, Noehren B, Mula J, Ubele MF, Westgate PM, Kern PA, Peterson CA. Fibre type‐specific satellite cell response to aerobic training in sedentary adults. J Physiol 592: 2625–2635, 2014. doi: 10.1113/jphysiol.2014.271288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Long DE, Peck BD, Martz JL, Tuggle SC, Bush HM, McGwin G, Kern PA, Bamman MM, Peterson CA. Metformin to augment strength training effective response in seniors (MASTERS): study protocol for a randomized controlled trial. Trials 18: 192, 2017. doi: 10.1186/s13063-017-1932-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spencer M, Finlin BS, Unal R, Zhu B, Morris AJ, Shipp LR, Lee J, Walton RG, Adu A, Erfani R, Campbell M, McGehee RE Jr, Peterson CA, Kern PA. Omega-3 fatty acids reduce adipose tissue macrophages in human subjects with insulin resistance. Diabetes 62: 1709–1717, 2013. doi: 10.2337/db12-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wen Y, Murach KA, Vechetti IJ Jr, Fry CS, Vickery C, Peterson CA, McCarthy JJ, Campbell KS. MyoVision: software for automated high-content analysis of skeletal muscle immunohistochemistry. J Appl Physiol 124: 40–51, 2018. doi: 10.1152/japplphysiol.00762.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hettinger ZR, Wen Y, Peck BD, Hamagata K, Confides AL, Van Pelt DW, Harrison DA, Miller BF, Butterfield TA, Dupont-Versteegden EE. Mechanotherapy reprograms aged muscle stromal cells to remodel the extracellular matrix during recovery from disuse. Function (Oxf) 3: zqac015, 2022. doi: 10.1093/function/zqac015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miller BF, Hamilton KL, Majeed ZR, Abshire SM, Confides AL, Hayek AM, Hunt ER, Shipman P, Peelor FF 3rd, Butterfield TA, Dupont-Versteegden EE. Enhanced skeletal muscle regrowth and remodelling in massaged and contralateral non-massaged hindlimb. J Physiol 596: 83–103, 2018. doi: 10.1113/JP275089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Drake JC, Bruns DR, Peelor FF 3rd, Biela LM, Miller RA, Hamilton KL, Miller BF. Long-lived crowded-litter mice have an age-dependent increase in protein synthesis to DNA synthesis ratio and mTORC1 substrate phosphorylation. Am J Physiol Endocrinol Metab 307: E813–E821, 2014. doi: 10.1152/ajpendo.00256.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Drake JC, Bruns DR, Peelor FF 3rd, Biela LM, Miller RA, Miller BF, Hamilton KL. Long-lived Snell dwarf mice display increased proteostatic mechanisms that are not dependent on decreased mTORC1 activity. Aging Cell 14: 474–482, 2015. doi: 10.1111/acel.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Drake JC, Peelor FF 3rd, Biela LM, Watkins MK, Miller RA, Hamilton KL, Miller BF. Assessment of mitochondrial biogenesis and mTORC1 signaling during chronic rapamycin feeding in male and female mice. J Gerontol A Biol Sci Med Sci 68: 1493–1501, 2013. doi: 10.1093/gerona/glt047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mathis AD, Naylor BC, Carson RH, Evans E, Harwell J, Knecht J, Hexem E, Peelor FF 3rd, Miller BF, Hamilton KL, Transtrum MK, Bikman BT, Price JC. Mechanisms of in vivo ribosome maintenance change in response to nutrient signals. Mol Cell Proteomics 16: 243–254, 2017. doi: 10.1074/mcp.M116.063255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sieljacks P, Wang J, Groennebaek T, Rindom E, Jakobsgaard JE, Herskind J, Gravholt A, Møller AB, Musci RV, de Paoli FV, Hamilton KL, Miller BF, Vissing K. Six weeks of low-load blood flow restricted and high-load resistance exercise training produce similar increases in cumulative myofibrillar protein synthesis and ribosomal biogenesis in health males. Front Physiol 10: 649, 2019. doi: 10.3389/fphys.2019.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miller BF, Baehr LM, Musci RV, Reid JJ, Peelor FF 3rd, Hamilton KL, Bodine SC. Muscle-specific changes in protein synthesis with aging and reloading after disuse atrophy. J Cachexia Sarcopenia Muscle 10: 1195–1209, 2019. doi: 10.1002/jcsm.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Busch R, Kim YK, Neese RA, Schade-Serin V, Collins M, Awada M, Gardner JL, Beysen C, Marino ME, Misell LM, Hellerstein MK. Measurement of protein turnover rates by heavy water labeling of nonessential amino acids. Biochim Biophys Acta 1760: 730–744, 2006. doi: 10.1016/j.bbagen.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 33. Popov DV, Makhnovskii PA, Zgoda VG, Gazizova GR, Vepkhvadze TF, Lednev EM, Motanova ES, Lysenko EA, Orlov OI, Tomilovskaya ES. Rapid changes in transcriptomic profile and mitochondrial function in human soleus muscle after 3-day dry immersion. J Appl Physiol (1985) 134: 1256–1264, 2023. doi: 10.1152/japplphysiol.00048.2023. [DOI] [PubMed] [Google Scholar]
- 34. Aronson D, Wojtaszewski JFP, Thorell A, Nygren J, Zangen D, Richter EA, Ljungqvist O, Fielding RA, Goodyear LJ. Extracellular-regulated protein kinase cascades are activated in response to injury in human. Am J Physiol Cell Physiol 275: C555–C561, 1998. doi: 10.1152/ajpcell.1998.275.2.C555. [DOI] [PubMed] [Google Scholar]
- 35. O’Sullivan PJ, Gorman GM, Hardiman OM, Farrell MJ, Logan PM. Sonographically guided percutaneous muscle biopsy in diagnosis of neuromuscular disease: a useful alternative to open surgical biopsy. J Ultrasound Med 25: 1–6, 2006. doi: 10.7863/jum.2006.25.1.1. [DOI] [PubMed] [Google Scholar]
- 36. Horwath O, Envall H, Röja J, Emanuelsson EB, Sanz G, Ekblom B, Apró W, Moberg M. Variability in vastus lateralis fiber type distribution, fiber size, and myonuclear content along and between the legs. J Appl Physiol (1985) 131: 158–173, 2021. doi: 10.1152/japplphysiol.00053.2021. [DOI] [PubMed] [Google Scholar]
- 37. Tidball JG. Mechanisms of muscle injury, repair, and regeneration. Compr Physiol 1: 2029–2062, 2011. doi: 10.1002/cphy.c100092. [DOI] [PubMed] [Google Scholar]
- 38. Yang W, Hu P. Skeletal muscle regeneration is modulated by inflammation. J Orthop Translat 13: 25–32, 2018. doi: 10.1016/j.jot.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Qian T, Kou J, Tang S, Ruan Z, Guo P. Research progress of skeletal muscle injury repair and treatment strategies. Int J Health Pharm Med 4: 128–137, 2023. doi: 10.38007/IJHPM.2023.040113. [DOI] [Google Scholar]
- 40. Roth SM, Martel GF, Rogers MA. Muscle biopsy and muscle fiber hypercontraction: a brief review. Eur J Appl Physiol 83: 239–245, 2000. doi: 10.1007/s004210000287. [DOI] [PubMed] [Google Scholar]
- 41. Forcina L, Cosentino M, Musarò A. Mechanisms regulating muscle regeneration: insights into the interrelated and time-dependent phases of tissue healing. Cells 9: 1297, 2020. doi: 10.3390/cells9051297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hettinger ZR, Hamagata K, Confides AL, Lawrence MM, Miller BF, Butterfield TA, Dupont-Versteegden EE. Age-related susceptibility to muscle damage following mechanotherapy in rats recovering from disuse atrophy. J Gerontol A Biol Sci Med Sci 76: 2132–2140, 2021. doi: 10.1093/gerona/glab186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Long DE, Peck BD, Lavin KM, Dungan CM, Kosmac K, Tuggle SC, Bamman MM, Kern PA, Peterson CA. Skeletal muscle properties show collagen organization and immune cell content are associated with resistance exercise response heterogeneity in older persons. J Appl Physiol (1985) 132: 1432–1447, 2022. doi: 10.1152/japplphysiol.00025.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1 and Fig. S1: https://doi.org/10.6084/m9.figshare.23988921.
Data Availability Statement
The datasets used and/or analyzed during the current study are available upon reasonable request.