Abstract
The classical amyloid cascade hypothesis postulates that the aggregation of amyloid plaques and the accumulation of intracellular hyperphosphorylated Tau tangles, together, lead to profound neuronal death. However, emerging research has demonstrated that soluble amyloid-β oligomers (SAβOs) accumulate early, prior to amyloid plaque formation. SAβOs induce memory impairment and disrupt cognitive function independent of amyloid-β plaques, and even in the absence of plaque formation. This work describes the development and characterization of a novel anti-SAβO (E3) nanobody generated from an alpaca immunized with SAβO. In-vitro assays and in-vivo studies using 5XFAD mice indicate that the fluorescein (FAM)-labeled E3 nanobody recognizes both SAβOs and amyloid-β plaques. The E3 nanobody traverses across the blood-brain barrier and binds to amyloid species in the brain of 5XFAD mice. Imaging of mouse brains reveals that SAβO and amyloid-β plaques are not only different in size, shape, and morphology, but also have a distinct spatial distribution in the brain. SAβOs are associated with neurons, while amyloid plaques reside in the extracellular matrix. The results of this study demonstrate that the SAβO nanobody can serve as a diagnostic agent with potential theragnostic applications in Alzheimer’s disease.
INTRODUCTION
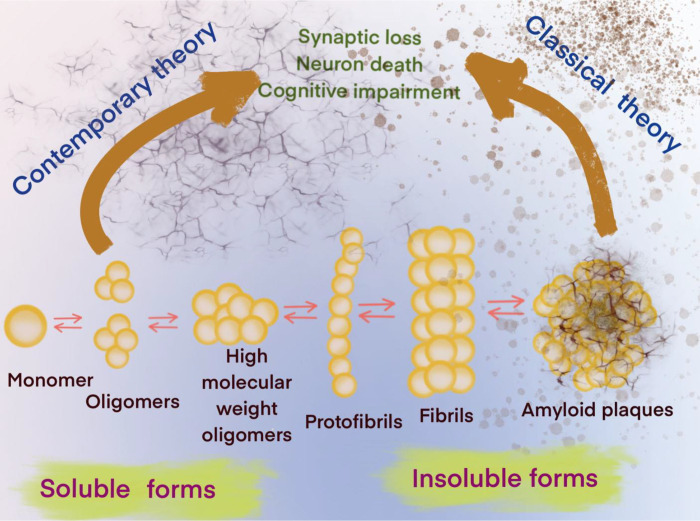
Alzheimer’s disease (AD) is one of only a few diseases that have been known for over a hundred years 1 without the development of diagnostic or disease-modifying therapies. In an aging population, AD will present one of the greatest challenges to medicine in this century. While the mechanisms underlying neuronal degeneration in AD remain elusive, the cytopathologic hallmarks of AD appear to be the formation of amyloid-β plaques between neurons and the intracellular accumulation of hyperphosphorylated Tau species. These processes are compounded by the reduction of amyloid-β clearance from the brain 2, which ultimately leads to profound neuronal toxicity and tissue atrophy 3. Originally, the amyloid cascade hypothesis considered soluble amyloid-β oligomers (SAβOs) as intermediates that would subsequently aggregate into protofibrils, insoluble fibrils and amyloid-β plaques, which were thought to be the main pathogenic cause of AD 4 (Fig. 1). This hypothesis indicates that targeting SAβO could be a beneficial early AD intervention.
Figure 1. Contemporary and classical hypotheses of the amyloid-β cascade mechanisms.
Experimental evidence ranging from cell studies to neuropathological and behavioral data suggests that elevated SAβO levels in the brain have pathological consequences 4. As more data have emerged, it is now accepted that SAβOs exist for an extended periods without conversion to fibrillar isoforms 5, and that SAβOs induce cognitive impairment in AD 6. Since SAβOs are determinants of the severity of neurodegeneration in AD 1, they could potentially serve as a biomarker for theragnostic applications 6–9. SAβO is a synaptotoxin found at elevated levels and associated with synapses in the brains of AD patients, but not in healthy elderly people 10–12. While several reports have implicated SAβOs as the cause of synaptic failure and neurodegeneration in AD 13–20, more detailed studies suggest that SAβOs cause neuronal death due to perturbation of normal membrane functions 21. SAβOs also induce memory impairment and disrupt cognitive function long before amyloid-β plaque deposition or even without plaque formation in a mouse model of AD 22–29 (Fig. 1). Together, these findings indicate that SAβOs act upstream of amyloid-β plaques and Tau to promote neuronal dysfunction. Thus, reagents that target SAβOs are needed, for the prevention of other pathological events, such as activation of Tau- and amyloid-β plaque-dependent pathways that lead to an increase in severity of the disease.
Two types of approaches have been applied to target pathological amyloid proteins. One is the use of small organic molecules that mimick known amyloid-binding molecules 30,31, and the other is the development and use of biologics 32–34. Organic molecules that bind to β-sheets within the amyloid fibrils or plaques inhibit growth by blocking binding sites for additional Aβ molecules. The chemical backbone of these organic molecules is restricted to a few structures of the benzothiazoles or stilbenes families. Many PET probes targeting amyloid fibrils/plaques were derived from these templates. For example, [11C]PIB and Flutemetamol are based on the benzothiazole structure, while Florbetapir and Florbetaben are derived from stilbenes. Because SAβOs lack the ordered beta solenoid structure of fibrils, developing small molecules to target SAβOs is more challenging.
In contrast, the development of biologics could serve as a promising strategy to target amyloid-β aggregates. Because SAβOs are made up of a few trimers or tetramers, they lack traditional binding surface for small molecules. Antibodies can recognize conformational epitopes unique to different oligomers. For instance, the mAb158 monoclonal antibody exhibits distinctive selectivity for soluble amyloid-β protofibrils compared to monomeric amyloid-β. This antibody binds preferentially to soluble protofibrils over mature, insoluble fibrils but has no affinity for amyloid precursor protein (APP) 35–38. M94 is a polyclonal antibody that was purified from antisera of host animals immunized with synthetic amyloid-β oligomers. M94 shows high selectivity for pathogenic amyloid-β oligomers but not for physiological monomers 8. Furthermore, ex-vivo staining of brain sections using the SAβO-specific antibodies revealed that SAβOs are distributed differently than neuritic plaques 39. Overall, these studies highlight the prospect of biologics for targeting dementia-associated amyloid oligomers.
In this work, we focused on the development of a nanobody targeting SAβOs. We also describe assays to validate the nanobody binding specificity using ex-vivo and in-vivo models. Nanobodies (sometimes referred to as VHH) are derived from heavy chain-only IgG isotypes found in animals of the camelid family 40. Nanobodies are approximately 1/10th the size of conventional antibodies (150 kDa). Despite their small size, nanobodies have unique specificities, potent affinities, and also exhibit high thermal stability and solubility 41. Nanobodies also exhibit relatively short serum half-lives of approximately 2 hours 42, making them ideal for imaging applications. Additionally, nanobodies have been shown to cross biological barriers, including the blood-brain barrier (BBB) 43,44, which makes them attractive for AD applications. Finally, the lack of antibody heavy chain constant regions would limit risk of Amyloid Related Imaging Abnormalities (ARIA) 45,46. The results obtained from this work suggest that the E3 nanobody can recognize both SAβOs and amyloid-β plaques in-vivo. This probe enables us to observe the distinct spatial distribution of SAβO compared to amyloid-β plaques.
RESULTS
Generation of SAβO as an antigen.
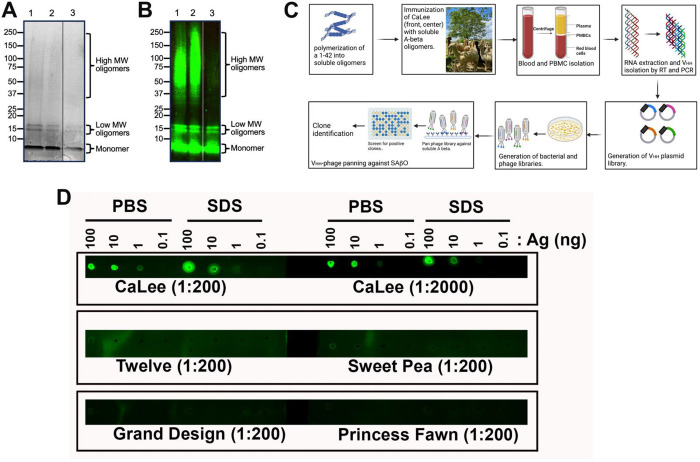
This work demonstrates the generation of batches of human SAβOs as an antigen for alpaca immunization. Based on prior work 47, the inclusion of 0.05% SDS in the preparation helped to induce stable oligomers. After confirmation of the generation of SAβOs by Coomassie Blue gel staining, and the material was stored at −20°C before use. The assembled amyloid-β preparation contained three major components that included monomers, low molecular weight oligomers (~16 kDa), and high molecular weight oligomers (Fig. 2A, B; original blots shown as Figs. S1, S2 are available in the Supplementary Information). The monomer concentration is inversely proportional to assembly time. At the beginning of the seeding process, the monomer dominates the solution and migrates at 4–4.5 kDa. However, overtime, this band gets lighter, indicating the formation of higher-order oligomers at the expense of their monomer counterparts. The low molecular weight oligomers are primarily composed of tetramers migrating at approximately 16 kDa. We also observed a small amount of higher molecular weight SAβO species in the 37–150 kDa range.
Figure 2. Production of nanobody from SAβO-injected alpaca.
(A) Coomassie stained SDS-polyacrylamide gel, and (B) Western analysis to confirm the production of the low molecular weight SAβOs (16 kDa) and some higher molecular species. Lanes 1 and 2 are from two different SAβO preparations; lane 3 is from free Abeta peptide. (C) General procedures for generating antibodies/nanobodies after immunization of alpaca with SAβO antigen. (D) Dot-blot analysis showed that the plasma from the alpaca immunized with SAβO (CaLee) recognizes SAβO antigen, while no response was observed for other non-immunized alpacas (Twelve, Sweety Pea, Grand Design, and Princess Fawn).
Development and characterization of SAβO nanobodies.
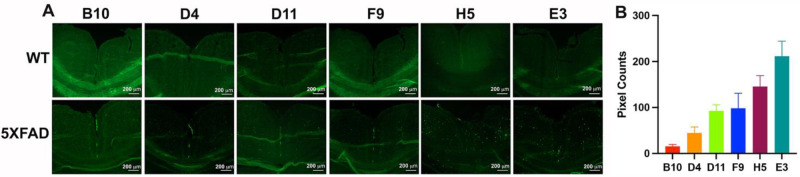
The overall procedure for generating anti-SAβO nanobodies is highlighted in Fig. 2C. We immunized an alpaca, CaLee, with SAβO antigen 7 times over the course of 84 days. Blood collections were performed 1 week after the 5th and 7th injections, and the plasma was tested for the presence of antibodies recognizing SAβO. The plasma obtained from CaLee, but not the control plasma from other alpacas, recognized the SAβO by dot-blot analysis (Fig. 2D). Nanobodies were isolated following an established protocol 48. We generated a phage display library using cDNA derived from the PBMC mRNA and performed affinity panning of the phage library. Positive clones were identified by ELISA and clones identified multiple times were prioritized (Fig. S3, Supplementary Information). Six clones were tested in 8-point dilution series and positive clones were prioritized. From this operation, we identified the 6 best clones that bind SAβO (D11, E3, F9, B10, D4 and H5).
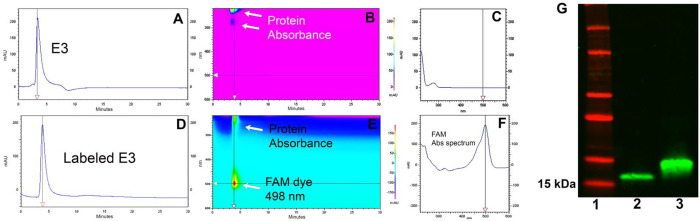
We also developed an ex-vivo assay using mouse brain tissue sections mounted onto glass slides to screen for nanobody binding specificity. To facilitate the screening process, the nanobody clones were all labeled with fluorescein-NHS (FAM) dye. After removal of excess dye using a desalting spin column (Zepa columns, 7K, MWCO, 2mL), confirmation of the labeling was done via 2D-HPLC (Fig. 3A–F). As shown in the representative data of the E3 nanobody, the chromatogram profile of FAM-labeled E3 nanobody is nearly identical to that of the unlabeled counterpart, albeit with a longer retention time. Aside from having similar absorbance characteristic of a protein at 220–280 nm, the FAM-labeled product has an additional absorbance of FAM dye at 498 nm (Fig. 3E, F). Western analysis also confirmed the FAM-labeled E3 product (Fig. 3G; original data shown as Fig.S4 is available in the Supplementary Information). The FAM-labeled nanobody clones were used to stain 5XFAD and WT brain slides. To compare the binding specificity of the clones, we incubated FAM-labeled nanobodies with consecutive brain sections for 1h at room temperature and imaged the slides using a fluorescent microscope (Fig. 4A). The screening data were exported to ImageJ to quantify the fluorescent signal and rank the nanobodies. Based on these data, we rank the nanobodies from best to worst: E3>H5>G11=F9>D4>B10 (Fig. 4B). This ex-vivo staining process was repeated using two different 5XFAD brains, and the data confirmed E3 is the best among the clones. From here, we selected the E3 nanobody clone for further characterization.
Figure 3. Representative data for the characterization of a dye-labeled clone.
(A,B,C) Two-dimensional HPLC assay using a Cytiva HiTrap Sepharose column to characterize E3 nanobody and its labeled product. Unlabeled E3 nanobody. (D,E,F) FAM-labeled E3 nanobody. (G) Western blot analysis to show the purity of E3 (lane 2) and confirm FAM-labeled labeled E3 (lane 3). Molecular weight markers are shown in lane 1.
Figure 4. Screening for the specificity of the nanobody using 5XFAD mouse brain slides.
(A) Ex-vivo evaluation of SAβO-binding specificity of the nanobody clones using consecutive brain slides obtained from a WT (6-month-old) and a 5XFAD mouse (11-month-old). (B) Ranking of nanobody clones after pixel quantification of 5XFAD brain slides. The threshold of each image was adjusted until the background disappeared using ImageJ. This process was repeated 3 times, and the data were presented as a mean ± SD.
FAM-labeled E3 nanobody recognizes SAβOs and amyloid-β plaques in an ex-vivo staining of brain sections.
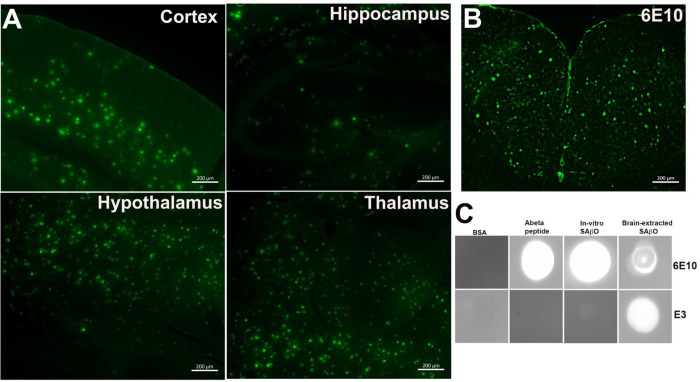
We used the FAM-labeled E3 nanobody to investigate the spatial distribution of SAβO in ex-vivo brain sections. The data in Fig. 5A demonstrate that the FAM-labeled E3 nanobody (1.8 μg/mL) stained several regions of the brains obtained from 5XFAD mice (n=3, 10-month-old). The stained amyloid-β species are heterogeneous and have distinct size, shape, and morphology features. More dense populations are found within the hypothalamus and thalamus, followed by cortex (Table 1). Aside from the smaller size species (~2 μm), the large-sized amyloid-β species (~14 μm) detected with FAM-labeled E3 are similar to those detected by Alexa Fluor-488-labeled 6E10 antibody (Fig. 5B). The 6E10 staining data are consistent with a past report that described amyloid-β plaques in 5XFAD mice to be approximately 20 μm 49. Using an in-house algorithm written in MatLab (Mathworks, Natick, MA), we analyzed the physical characteristics of amyloid species in the brain stained with FAM-labeled E3 nanobody. Overall, the density of amyloid-β species present in the thalamus and hypothalamus is higher than in the hippocampus or cortex (Table 1). It is also notable that the size of these SAβOs is nearly 7-fold smaller than that of amyloid-β plaques.
Figure 5. Specificity of E3 nanobody in different regions of the 5XFAD brains.
(A) Representative ex-vivo imaging of different regions of the brains of 5XFAD mice (n=3, 10-month-old) after staining with FAM-labeled E3 nanobody. (B) Ex-vivo staining of AF488-labeled 6E10 antibody on a 5XFAD brain slide. (C) Dot-blot analysis of E3 nanobody binding. Lanes 1–3: blots to test the binding specificity of E3 nanobody against different negative control protein and amyloid-β isoforms. Lane 4: immunoblot using the 6E10 antibody to confirm the presence of SAβO in the supernatant of ultracentrifuge 5XFAD brain sample. All ex-vivo staining of brain slides were incubated for 1 h at room temperature.
Table 1.
Quantitative analysis of the amyloid-β species in the coronal brain sections of 5XFAD mice, depicted by the FAM-labeled E3 nanobody.
| Brain Regions | Number of Animals | Number of observed brain slides | Total number of Abeta populations | Density (number/mm2) | Abeta Area (μm2) | Average Size in Diameter of Abeta-associated FAM-E3a (μm) | Eccentricity | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average | S.D. | Average | S.D. | Average | S.D. | SAβO | Plaques | Average | S.D. | |||
| Cortex | 3 | 21 | 131.86 | 51.69 | 89.72 | 38.28 | 68.23 | 123.96 | 2 | 14 | 0.52 | 0.28 |
| Hippocampus | 3 | 6 | 63.00 | 62.61 | 38.87 | 34.74 | 41.01 | 87.95 | 0.57 | 0.27 | ||
| Hypothalamus | 3 | 6 | 296.50 | 7.78 | 170.90 | 3.10 | 47.69 | 77.34 | 0.63 | 0.23 | ||
| Thalamus | 3 | 6 | 207.75 | 78.49 | 122.44 | 46.68 | 43.92 | 78.02 | 0.53 | 0.29 | ||
Determined using ImageJ.
Next, we developed a dot-blot assay to test the specific recognition of E3 nanobody versus 6E10 antibody for different Abeta species, including free Abeta peptide, extracted SAβO from 5XFAD brains, or the in-vitro produced counterparts (Fig. 5C). For this study, the Abeta peptide was prepared fresh using human amyloid-beta (1–42) peptide, while extracted SAβOs were obtained from the supernatant of homogenized 5XFAD mouse brains after ultracentrifugation. The in-vitro SAβO was generated as described in Fig. 2. All three samples of the amyloid isoforms were quantified by a BCA assay, enabling all isomers to be “spotted” at equal concentrations. The dot-blot data (cropped and magnified for presentation) suggests that the E3 nanobody recognizes in-vitro SAβOs weakly. In contrast, it recognizes extracted SAβOs from 5XFAD brains strongly. The original dot blots are shown in Fig. S5 (Supplementary Information). This study also showed that the 6E10 antibody is less specific compared to the E3 nanobody as the antibody recognizes all three amyloid species.
FAM-labeled E3 nanobody can cross the BBB and detect SAβOs and amyloid-β plaques in the brain after intravenous injection.
To determine if the E3 nanobody could be used to image SAβO in-vivo, we injected the FAM-labeled E3 nanobody (1.8 mg/mL, 150 μL) into 5XFAD mice (n=5) and WT (n=3) mice. To ensure the fluorescent signal detected was not due to free dye diffusion to the brain or autofluorescence generated from the tissue or nanobody, two additional control cohorts of animals were included in the study. These controls included WT and 5XFAD mice that were intravenously injected with either free FAM dye (1 mg/mL, 150 μL) or unlabeled E3 nanobody (1.8 mg/mL, 150 μL) (n=3, each), respectively.
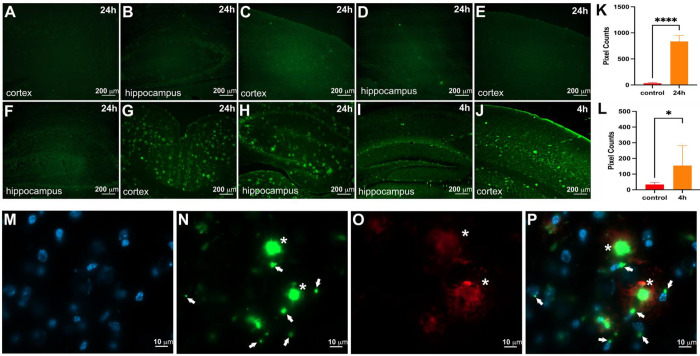
We imaged many different areas of the brain, but chose to present the cortex and hippocampal regions for each cohort since they are relevant to AD. As shown in Fig. 6, no fluorescent signals were detected in 5XFAD mice injected with E3 nanobody alone (Fig. 6A,B) or in WT mice injected with either free FAM dye (Fig. 6C,D) or FAM-labeled E3 nanobody (Fig. 6E,F). In contrast, FAM-labeled E3 nanobody detected copious amyloid-β deposits in the brain of 5XFAD mice at 24h (Fig. 6G,H) and 4h (Fig. 6 I,J) post-injection. Pixel quantification the signals demonstrated that the 24h-post-injection group was significantly higher than that of control mice (Fig. 6K) (p<0.0001). Although by a smaller amount than the 24h-cohort, the accumulation of signal in the 4h-post-injection cohort (n=3) was significantly higher than control counterparts (p<0.05) (Fig. 6L).
Figure 6. The E3 nanobody can cross the BBB and stain amyloid targets.
Representative data of hippocampus and cortex per cohort. All treatments were performed by intravenous injection via the tail veins. 5XFAD mice (n=3) were treated with E3 nanobody (A,B) resulting in no labeling. WT mice (n=3) were treated with free FAM fluorescent dye (C,D) resulting in no labeling. WT mice (n=3) were treated with FAM-labeled E3 nanobody (E,F) resulting in no labeling. 5XFAD mice (n=5) were treated with FAM-labeled E3 nanobody 24 h before brain collection (G,H) resulting in significant numbers of bright green fluorescent profiles in cortex and hippocampus. 5XFAD mice (n=3) were treated with FAM-labeled E3 Nb 4 h before brain collection (I,J) resulting in fewer bright green fluorescent profiles. The fluorescent pixels were quantified and compared between (A,B) (n=3) versus (G,H) (n=5) at ****p<0.0001 (K). And between (A,B) (n=3) versus (I,J) (n=3) at *p<0.005. Confocal imaging of ex-vivo 5XFAD hippocampus slides: DAPI staining of the nucleus (M). FAM-labeled E3 recognized SAβO (white arrows) and larger amyloid-β plaques (asterisks) (N). Amyloid-β plaques stained by Alexa 647-labeled 6E10 antibody (O). Merging of M, N and O (P) indicates that E3-positive staining around the nuclei (white arrows).
With the superior resolution provided by confocal microscopy, we also showed that SAβO and amyloid-β plaques recognized by the E3 nanobody not only differ in size, shape, and morphology, but they have distinct spatial distributions in the brain. As shown in Fig. 6.M–P, SAβOs are associated with the neuronal nuclei (white arrows). This observation agrees with a previous report showing the association of oligomers with neuronal nuclei 50. In contrast, the E3 nanobody-positive amyloid-β plaques (asterisks) reside in the cellular matrix. These results indicate that E3 nanobody, similar to other nanobodies, crosses the BBB, most likely due to its small size and relatively high isoelectric point (calculated to be 9.2 using Expasy 43,44,51,52).
Quantitative analysis.
The in-vivo data obtained using FAM-E3 nanobody revealed the spatial distribution of SAβOs in 5XFAD brains. In contrast, no registered fluorescence signal was detected in WT brains. While SAβOs and amyloid-β plaques were distributed across many regions of the brain of 5XFAD mice, they appeared to be more concentrated in some brain regions than others. Thus, we were interested in quantifying these differences using our algorithms developed in MATLAB. The data are summarized in Table 1 and show that the presence and the density of amyloid-β species in the hypothalamus (170.90/mm2) was surprisingly high, followed by the thalamus (122.44/mm2), compared to other regions. On the other hand, the hippocampus had the least number of amyloid-β species with a recorded density of 38.87/mm2). On average, the diameter of SAβO-associated FAM-E3 was about 2 μm, with average measured eccentricities of approximately 0.5.
DISCUSSION
The accumulation of amyloid-β aggregates in the brain is a hallmark of AD. In human AD, pathological amyloid-β proteins are composed of different assemblies that included senile plaques, protofibrils, fibrils, and SAβOs. Increasing evidence suggests that SAβOs serve as toxic species responsible for the pathogenesis and spread of AD 50. Prior to this work, a number of antibodies 50,53 or antibody fragments 54 have been used for the specific recognition of SAβOs. These reagents have helped examine the roles of SAβOs in AD brain, leading to the hypothesis that the disruption of particular synapses by SAβOs could be the culprit behind the memory loss in early AD 8. According to the contemporary model of the amyloid-β cascade hypothesis (Fig.1), SAβOs are considered stable and independent components, affecting AD pathology without conversion to fibrillar assemblies 5. Thus, targeting SAβOs is beneficial due to their early role in AD pathogenesis and disease progression. In this work, we report the development, production, and characterization of a novel SAβO-specific nanobody. Aside from being thermally stable, which makes it amenable for PET labeling chemistry, a nanobody has the added advantage of being able to traverse biological barriers. In fact, it has been reported that SAβOs possess a higher diffusion coefficient compared to large assemblies 55,56. With a mere size of ~15 kDa, 1/10th the size of conventional antibodies (150 kDa), nanobodies are smaller than any antibodies and antibody fragments described to date. For example, Fab (~50 kDa), F(ab’)2 (~110 kDa fragments), single chain (monovalent) Fv (scFv, ~25 kDa) with one antigen-binding site, bivalent scFv dimmers (50 kDa), and scFv-fusion proteins (sometimes called minibodies, ~80 kDa) 57, all are larger than nanobodies. We anticipate that using nanobodies for targeting SAβOs could serve as a gambit for responding to the delivery solution for biologics. These molecules could be used either as therapeutics or diagnostic pharmaceuticals for AD applications.
Nanobody production is relatively simple, robust, and economical. The quality of the SAβO antigen used for immunization was confirmed by Coomassie staining and Western blot analysis (Fig. 2A,B). We prepared relatively fresh SAβOs for alpaca injections, thus the material had a low propensity to grow larger aggregates. The VHH library was constructed, and a single panning step was performed against the immobilized SAβOs. From the enriched SAβO nanobody-expressing phage, we identified 61 clones by screening for binding to SAβOs via ELISA.
We also developed a robust assay to screen nanobody FAM-labeled nanobody clones using consecutive 5XFAD brain slides. Data in Fig. 4 showed that no signals were detected in the WT brain sections. In stark contrast, all the clones stained oligomers in the hippocampus and cortex of a 5XFAD mouse, although the degree of staining varied between clones in the brain slices. Among the six best clones, D11, F9, H5 and E3 showed considerable signal with at least 100 pixel counts per cortex, as we quantified using ImageJ. From these results, we picked the E3 nanobody for full characterization. We repeated the ex-vivo staining with FAM-labeled E3 nanobody using brain slides from different 5XFAD mice (n=3). The data showed that the FAM-E3 nanobody stained amyloid-β in the 5XFAD brains with a mixed morphology (Fig. 5A). It is apparent that two populations of amyloid-β isoforms dominate many parts of the brain, with the highest abundance found in the hypothalamus, followed by the thalamus, cortex, and then hippocampal regions (Table 1). The size, shape, and morphology of the smaller population are similar to what has been seen with anti-SAβO ACU193 and NU4 antibodies 58. Meanwhile, the larger population observed in the 6E10 antibody-treated 5XFAD brain slides resembles amyloid-β plaques (Fig. 5 B). To explain the mixed results of our in-vivo amyloid-β staining, we tested the specificities of E3 nanobody against amyloid-β isomers that included monomers, SAβOs, in-vitro generated SAβO, and SAβO, which were isolated from 5XFAD brain homogenates using ultracentrifugation. The results of this work confirmed the ex-vivo staining data showing that E3 nanobody is more specific than 6E10 antibody for SAβO in that it can recognize SAβOs extracted from the brain of 5XFAD mice but not the free Abeta peptide monomer (Fig. 5C). The data suggest that the nanobody recognizes the SAβOs not only based on the specific epitope but depending also on the secondary structure. A complete understanding of the molecular mechanism by which E3 nanobody preferentially recognizes oligomeric forms of Abeta will require detailed structural analysis. However, an enticing possibility is suggested by AlphaFold models of E3. The complementary determining regions of E3 may make a large, relatively flat surface, capable of binding across multiple rungs of the beta-solenoid structure of amyloid oligomers (Fig. S6, Supplementary Information). Such a binding mechanism would lead to strong preference for oligomers over monomers.
Importantly, the E3 nanobody could localize in the brain after intravenous injection. The in-vivo application of FAM-labeled nanobody (via intravenous injection) indicated that it could cross the BBB and stain amyloid-β in the brain of 5XFAD mice (Fig. 6). The FAM-labeled E3 nanobody is distributed significantly in the brain of 5XFAD mice, 4h post-intravenous injection (Fig. 6I,J). These data point to an advantage of nanobodies compared to larger antibodies. It has been reported that an uptake time of 4h post-intravenous injection of anti-SAβO antibody labeled with 64Cu PET radioisotope in 5XFAD mice resulted in poor signal distribution and was not robust for signal detection 58. In our work, we observed increased signal in the 24h-post-injection cohort (Fig. 6G,H). Based on the pharmacokinetics of the probe, it appears that the probe enters the brain of injected mice, binds SAβO targets and accumulate, leading to increased signal. The confocal imaging data showed the distinct locations between SAβOs (white arrows) and amyloid-β plaques (asterisks) (Fig. 6M–P). The former is associated with neurons, while the latter resides in the extracellular matrix. This regiospecific distribution may explain the distinct role of how they contribute to AD pathology.
In conclusion, we report a robust, large-scale, and reproducible generation of a novel SAβO nanobody. Extensive in-vitro and in-vivo characterization demonstrated that the nanobody recognizes SAβOs and amyloid-β plaques in a preclinical mouse model of AD. Since FAM-labeled E3 can penetrate the BBB and effectively stain amyloid-β in the brain, work is currently being undertaken to utilize this nanobody for diagnostic applications and potential therapies. Because SAβOs are involved in the early stage of AD pathology 21, we anticipate that this nanobody can be utilized to develop a novel and powerful platform for precision in-vivo molecular fingerprinting of SAβOs for AD early detection.
METHODS
Materials
All commercially-available reagents and solvents were used as received without further purification. FAM-NHS was purchased from Lumiprobe Corp (Hunt Valley, Maryland, USA), sealed with parafilm and stored at −20°C. The purity and stability of the dye were monitored every 6 months using liquid chromatography mass spectrometry (LC-MS). Human amyloid-β peptide (1–42) was obtained from Anaspec (Fremont, CA, Cat. AS-20276). Ultrapure water (18.2 MΩ) was obtained from a Millipore Direct Q-5 water purification system. A Hitachi HPLC system (Lachrom Elite) equipped with a diode array detector was used for purification, and employed a Cytiva HiTrap Desalting column with a mobile phase of 25 mM sodium phosphate, 150 mM sodium chloride, pH7.4. Three fluorescence microscopes were used in our studies, including a manual Zeiss Axio Observer, an automated Zeiss Axio Observer, and a Zeiss LSM 710 confocal laser scanning microscope. MALDI-TOF analysis was performed on a Bruker Rapiflex with sinapinic acid matrix.
Animals
All animal experiments performed complied with institutional guidelines and were conducted according to the protocol approved by the Vanderbilt Institutional Animal Care and Use Committee. Furthermore, all work performed in this study complies with the Animal Research Reporting In Vivo Experiments (ARRIVE) guidelines. The animals were maintained at Vanderbilt University under standard conditions, in a 12h-light/dark cycle, and with free access to food and water, as we described in the past 59. The 5XFAD mice overexpress both mutant human APP and PS1 genes and exhibit high APP levels correlating with high burden and accelerated accumulation of amyloid-β 59. The mice were genotyped by a standard polymerase chain reaction (PCR) using DNA isolated from tail tips and the following primers provided by Jackson Laboratory (Bar Harbor, Maine): Mutant reverse, 5’-CGG GCC TCT TCG CTA TTA C −3’; Common, 5’-ACC CCC ATG TCA GAG TTC CT −3’; Wild Type reverse, 5’-TAT ACA ACC TTG GGG GAT GG-3’. Amplified PCR products were then analyzed by size fractionation using agarose (1.5%) gel electrophoresis; band sizes for wild type = 216bp, 5XFAD heterozygous = 129bp and 216bp, and 5XFAD homozygous = 129 bp. The 5XFAD mice were maintained as heterozygotes and all mice used in these experiments were approximately 6- to 11-month-old.
Alpaca immunizations and bleeds were performed at Turkey Creek Biotechnology, LLC (Waverly, TN) in accordance with their Institutional Animal Care and Use Committee protocol 18–02.
Generation of SAβO as an antigen
This work was performed using a reported procedure with some modifications 47. Human amyloid-β peptide (1–42) (5.0 mg, Anaspec, Fremont, CA, Cat. AS-20276) was dissolved in DMSO to a final concentration of 5 mM. An equivalent volume of DMSO served as a negative control. The materials were sonicated at room temperature for 10 minutes in Eppendorf tubes. A small portion of the solution (20 μL) was set aside and stored at −20°C for future monomer analysis. The remaining solution was diluted further to 100 μM with cold PBS (1x) containing 0.05% SDS followed by a brief vortex (30 seconds). At the same time, the DMSO negative control was diluted with an equivalent of volume of cold PBS (1x) containing 0.05% SDS. The resulting stock solution of 100 μM peptide solution and control DMSO were then incubated overnight at 4°C. Approximately 24h later, the stock solution was further diluted to 25 μM with PBS (1x), and an equivalent volume of PBS (1x) was again added to the DMSO negative control. The samples were incubated for two weeks at 4°C to enable amyloid-β oligomerization.
Following incubation, the amyloid-β concentration was found to be ~150 μg/mL as determined by the BCA protein assay (Thermo Fisher Scientific, Waltham, MA, Cat. 23225). The amyloid-β was then concentrated using Amicon Ultra-4 Centrifugal Filter Units with a cutoff of 3 kDa NMWL (Millipore Sigma, Burlington, MA, Cat. UFC800324) to reach a final concentration of 500 μg/mL. The purity of the SAβO was confirmed by SDS-PAGE using 12% precast polyacrylamide gels (BioRad, Hercules, CA, Cat. 4561044) followed by Coomassie staining (Fig. 2A). The data showed that with this method, we could generate a mixture of low and high molecular weight oligomers. Freshly prepared samples were made prior to each animal immunization, and the quality of each preparation was confirmed by SDS-PAGE and Coomassie staining of the gels.
Coomassie staining
Soluble amyloid-β oligomer preparations were solubilized in Laemmli Sample buffer (1X final concentration), boiled for 5 minutes at 96°C, and analyzed by SDS-PAGE. The prepared samples (20 μL) were loaded into a 12-well 4–20% gradient gel (BioRad Laboratories, Hercules CA, Cat No. 4561095) along with BioRad Precision Plus Protein Kaleidoscope Prestained Protein Standards (BioRad Laboratories, Hercules CA, Cat No. 1610375) and electrophoresed at 170V for 50 minutes. The gel was washed three times for 5 minutes each with deionized water, stained with BioSafe Coomassie G-250 (BioRad Laboratories, Hercules CA, Cat No. 1610786) with gentle shaking for 1h, destained overnight, and imaged on a LiCOR Odyssey M (LiCOR Biosciences, Lincoln NE). Images were collected and extracted using Empiria software (LiCOR Biosciences, Lincoln NE).
Western analysis
Samples were prepared for immunoblotting by adding Laemmli Sample buffer (1X final concentration) and 20 μL of each sample was loaded onto a 12-well 4–20% gradient gel (BioRad Laboratories, Hercules CA, Cat No. 4561095) along with BioRad Precision Plus Protein Kaleidoscope Prestained Protein Standards (BioRad Laboratories, Hercules CA, Cat No. 1610375). Following electrophoresis at 170V for 50 minutes, the gel proteins were transferred onto a 0.2 μm supported nitrocellulose membrane (BioRad Laboratories, Hercules CA, Cat No. 1620097) using the TransBlot Turbo System (BioRad Laboratories, Hercules CA, Cat. No. 1704150) and TransBlot Turbo Buffer (BioRad Laboratories, Hercules CA, Cat No. 10026938) at 2.5A, 25V, for 10 minutes. The blots were rinsed in deionized water for 5 minutes, then placed between two pieces of Whatman filter paper (Cytiva, Malborough MA, Cat No. 10427818) to dry for 15 minutes at 37°C. Dried blots were rehydrated in TTBS for 5 minutes and blocked for 1h in LiCOR Odyssey Intercept Buffer TBS (LiCOR Biosciences, Lincoln NE, Cat No. 27–60001) at room temperature. Blots were heat-sealed in ULine Poly Tubing bags containing 5 mL of 1:1000 (v/v) 6E10 antibody (BioLegend, San Diego CA, Cat No. 803004) in ULine Poly Tubing and incubated overnight at 4°C. Blots were then washed 4 times with large volumes of TTBS, and incubated with 1:5000 (v/v) LiCOR Donkey anti-Mouse IRDye800CW (LiCOR Biosciences, Lincoln NE, 926–32212) for 1h at room temperature. Blots were washed 4 times with TTBS and imaged on a LiCOR Odyssey M LiCOR Odyssey M (LiCOR Biosciences, Lincoln NE) at 800nm and 700nm channels, and images were collected and processed using LiCOR Empiria Software (LiCOR Biosciences, Lincoln NE).
Alpaca immunization
The SAβO preparation (300 μg) was emulsified in Gerbu adjuvant for the initial injections (day 0), followed by boosters at days 14, 28, 42, 56, 70 and 84 with 150 μg SAβO in Gerbu adjuvant. Blood was drawn for analysis on day 63 (first bleed) and day 91 (second bleed). The plasma was monitored by dot-blot analysis to test for the presence of antibodies recognizing SAβO. Peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation from 17.5 mL of alpaca blood using SepMate centrifugal devices, following the manufacturer’s protocol (Stemcell Technologies, Vancouver, CA).
Phage library construction
A cDNA library was made by reverse transcription with oligo dT primers and Superscript IV (ThermoScientific). A nested PCR strategy was used to amplify VHH coding regions of fragments, as described 48,60. The resulting PCR fragments were ligated into pBBR3, a modified pADL22 vector (Antibody Design Labs). All clones contain in-frame, C-terminal, HA and hexahistadine tags. The library was electroporated into high-efficiency TG1 cells (Lucent), and phage were produced using CM13 helper phage. Phage were harvested from the supernatant by PEG precipitation and suspended in PBS.
Panning for SAβO nanobodies and isolation of individual clones
A single round of panning against SAβO immobilized on a Nunc MaxiSorp (Nalge Nunc Intl., Rochester, NY) plates was performed. Three wells of a 96-well MaxiSorp plate were coated overnight with 10 μg of SAβO in PBS (100 μL); three PBS-coated wells served as controls. After removing unbound antigen, the wells were blocked with 2% nonfat milk in PBS for 2h, washed, and incubated with 2×1012 phage particles in blocking buffer for 1h. After extensive alternating washes with PBS and PBS + 0.5% Tween 20, phage were eluted with 100 μL of 100 mM glycine pH2.2 and then immediately neutralized with 100 μL of 1 M Tris pH8. Recovered phage were used to infect TG1 E. coli, and single clones were picked and inoculated in deep-well 96 well blocks containing 1 mL Terrific Broth and 100 μg/mL ampicillin. Plates were grown at 37°C for 5h, followed by 28°C overnight. Then, 1 mM IPTG was added, and the growth was continued for 3h. Bacteria were pelleted and lysed by two freeze-thaw cycles with a total of 400 μL of PBS. After centrifugation, the periplasmic extracts were analyzed by ELISA for the presence of nanobody-recognizing SAβO.
Detection of positive SAβO nanobody clones
A MaxiSorp plate was coated overnight at 4°C with 1 μg/well of SAβO in PBS (100 μL), washed, blocked with LI-COR Intercept buffer (PBS), and then incubated with 50 μL of periplasmic extracts lysate from above and 50 μL of PBS for 2h at room temperature or overnight at 4°C. Wells were washed with TTBS and incubated with a HRP-conjugated anti-HA tag (Roche, Basel, CH) antibody for 1h at room temperature. After washing, the plate was developed with TMB ultra (Sigma, St. Louis, MO) to identify positive clones.
In an alternative procedure, a 96-well Costar plate (black with clear bottom) was coated overnight at 4°C with 0.5 μg/well of SAβO (100 μL), washed with TTBS, blocked with LI-COR Intercept buffer (TBS), and then incubated with 50 μL of periplasmic extracts from above and 50 μL of PBS for 2h at room temperature or overnight at 4°C. The wells were washed with TTBS and incubated with fluorophore 800-conjugated anti-HA antibody for 1h at room temperature. After washing with TTBS, the plate was imaged using the LI-COR Odyssey Imager to identify positive clones.
Clone identification
A total of 4 96-well plates of clones were picked from two different nanobody libraries. All clones showing signal above background were sequenced. Six clones were both identified multiple times and found to be positive in multiple assays. These six clones were expressed and purified from 3 L bacterial cultures. A portion of each purified nanobody was biotinylated with 20-fold molar excess of NHS-biotin (Pierce) and 8 point, 5-fold, binding titrations were done for each clone using streptavidin-HRP or anti-HA-HRP (Thermo Fisher).
Nanobody purification
For large scale purifications of E3, the plasmid was isolated and used to transform T7 shuffle bacteria. An overnight culture was used to inoculate fresh media which was grown at 37°C until a OD600 of ~1.2 was reached. At this point the temperature was lowered to 18°C, 1mM IPTG was added, and protein expression was continued overnight. Cells were harvested by centrifugation and lysed with an Emulsiflex. Nanobodies were purified using Talon affinity resin (Takara Bio, Ann Arbor, MI). Aliquots of E3 were chemically biotinylated using NHS-Biotin (Thermo Fisher, Waltham, MA).
Labeling E3 nanobody with FAM-NHS dye
The E3 nanobody (1.8 mg/mL, 57 nmol) in PBS (1x, 50 μL) was treated with Na2CO3 (1 M, 6 μL, pH8) prior to the addition of FAM-NHS dye (20 eq., 1140 nmol, 0.54 mg) in argon-purged anhydrous DMSO (12 μL). The reaction vial was rotated overnight at 4°C. Excess of activated dye was neutralized with Tris-HCl buffer (10 μL, pH7), and the labeled product was separated from the excess dye using a 7k MWCO Zepa spin column and reconstituted in PBS (1x, pH7). The resulting FAM-E3 conjugate was characterized by 2D-HPLC using a Cytiva HiTrap desalting column (5 mL, cat. 29048684) under the isocratic conditions of 25 mM sodium phosphate, 150 mM sodium chloride, pH7.4, and a flow rate of 0.5 mL/min.
Immunohistochemistry staining
Brains embedded in OCT were cut into coronal sections (10 μm) using a Tissue-Tek (Sakura Finetek USA, Torrance, CA) cryostat and mounted onto charged glass slides. Prior to staining, slides were washed with PBS (10 minutes) and then treated with blocking buffer (5% normal goat serum, 0.2% Triton X-100, 0.5% bovine albumin in PBS) for 1h at room temperature. The treated sections were then incubated overnight at 4°C or 1h at room temperature with the E3-labeled nanobody diluted 1:100 in PBS. The sections were then washed with PBS twice for 10 minutes and once for 30 minutes, and cover-slipped with Vectashield plus antifade mounting medium containing DAPI (Vector Laboratories, Burlingame, CA, Cat. H2000–2) before observation using fluorescence microscopy.
For slides that required the use of secondary antibodies, the process was performed the same way as above except that after incubation with nanobody, the sections were subsequently washed in PBS 3 times for 10 minutes each and then incubated for 30 minutes at room temperature with a secondary antibody diluted in PBS containing 3% NGS (normal goat serum), 0.2% Triton X-100 and 0.5% bovine albumin. The sections were then washed with PBS twice for 10 minutes and once again for 30 minutes before being cover-slipped with Vectashield plus and examined by fluorescence microscopy.
Isolation of amyloid-β fibrils/plaques from 5XFAD mouse brains
This procedure was modified based on the Abcam “Extraction of amyloid-β plaques from mouse brain” protocol (https://www.abcam.com/protocols/extraction-of-amyloid-beta-from-mouse-brain). The brain of a 5XFAD mouse (n=2, 6-month-old) was homogenized in PBS (5 mL), followed by the addition of formic acid (10 mL). The mixture was sonicated for 1 minute on ice and centrifuged at 135,000 g for 1h at 4°C. The collected supernatant was neutralized with a neutralizing buffer composed of 1M Tris base, 0.5M Na2HPO4, and 0.05% NaN3. The solution was aliquoted, and flash frozen on dry ice before storage at −80°C. Aliquots were thawed prior to loading onto nitrocellulose membranes for dot-blot analysis.
Dot-blot assays
For testing the plasma, one microliter of 0.1, 0.01, 0.001, and 0.0001 mg/mL stock solutions of SAβO in SDS sample buffer or PBS were spotted on Amersham Protran Supported 0.2 mm Nitrocellulose (GE Healthcare Life Sciences). The membranes were allowed to dry, wetted in Tris-buffered saline (TBS), blocked in Intercept Blocking Buffer (LI-COR), and then incubated overnight with alpaca plasma diluted 1:200 in Intercept Blocking Buffer. After washing with TBS/0.1% Tween, the membranes were incubated with an 800 fluorophore-labeled goat anti-alpaca secondary antibody, washed with TBS/0.1% Tween, and then imaged on the Odyssey Infrared Imaging system (LI-COR).
For testing the specificity of the E3 nanobody, samples of bovine serum albumin (BSA), amyloid-b monomer, in-vitro-generated SAbO, and mouse brain SAbO extract were spotted onto a nitrocellulose membrane and allowed to dry at room temperature. The membrane was then blocked for 30 minutes using Intercept (TBS) blocking buffer (LI-COR Bioscience, Lincoln, Nebraska) before three 5-minute washes with TTBS. Next, the blot was incubated for 30 minutes in a solution containing E3 nanobody (10 mg/mL) in TTBS before three 5-minute washes with TTBS. The membrane was then incubated with goat anti-VHH antibody (12.5 mg/mL in TTBS; Jackson ImmunoResearch, West Grove, PA) for 30 minutes and washed with TTBS three times for 5 minutes each again. Finally, the blot was incubated with donkey anti-goat AF488 (2 mg/mL in TTBS; Abcam, Cambridge, UK) and washed three more times for 5 minutes each with TTBS before imaging. All incubations and washes were performed at room temperature with gentle shaking. A separate control blot to test for non-specific binding was prepared likewise as above, except that no E3 nanobody was used as a primary antibody against the samples. Imaging was performed on a ChemiDoc MP Imaging System (Bio-Rad, Hercules, CA).
Quantitative analysis program written on MATLAB
Semi-automated quantification of amyloid-β burden was performed for each slide using a custom image analysis script written in MATLAB (Mathworks, Natick, MA). First, an image was loaded into the program. Second, adaptive threshold was used to decrease the impact of background noise. Third, a tissue mask was created based on that adaptive threshold and then cleaned up using a morphological opening. Finally, regions-of-interest (ROIs) were manually drawn, enabling us to avoid histological artifacts. From the ROI plaque masks, plaque morphologic parameters can be calculated using the function “regionprops”. Plaque density was reported in plaques per mm2 and was defined as the number of plaques divided by the ROI area.
Confocal imaging
The brain sections obtained from 5XFAD mice (n=3) were incubated with a mixture of FAM-labeled E3 (1.8 mg/mL) or mouse 6E10 (BioLegend, San Diego, CA, USA) for 1h at room temperature with gentle rocking. Then, the slides were washed with PBS three times before being incubated with goat anti-mouse IgG cross-adsorbed secondary antibody, Alexa Fluor 647 (Invitrogen, Waltham, MA, USA). The slides were then counter-stained with DAPI incorporated in Vectashield (Vector Laboratories, Newark, CA, USA). The slides were sealed with a coverslip before microscopy. The images were obtained with 20x and 63x objectives using a Zeiss LSM 710 confocal laser scanning microscope. Then, the images were reconstructed with Zeiss Zen Blue software.
Protein structure analysis
AlphaFold 61 as implemented through the AlphaFold Colab was used to calculate 20 models of E3 using 4 different starting seeds. The predicted value of the local distance difference test (pLDTT), a per residue confidence value output by AlphaFold, was used for the coloring scheme in panel C in supplemental Fig. S6, as described in 62.
Statistical analysis
We used t-tests to compare the differences between two groups. The data were expressed as means ± SE of the difference. The statistical significance of the differences between sample means was determined via unpaired t-tests, the results of which were considered significant when P < 0.05 as reported with GraphPad Prism software.
ACKNOWLEDGMENTS
We thank Anuj AJ Rastogi at the Vanderbilt Cell & Developmental Biology (CDB) Equipment Resource Core for training and assistance with the use of an ultracentrifuge. We also thank Miles Carter for contribution to the data collection. Confocal microscopy was performed through the use of the Vanderbilt Cell Imaging Shared Resource (CISR) supported by NIH grantsCA68485, DK20593, DK58404, DK59637 and EY08126. The Zeiss LSM710 confocal microscope was acquired through NIH S10 RR027396 equipment grant.
FUNDING
This work was supported partially by Vanderbilt CTSA grant UL1TR002243 from NCATS/NIH, Vanderbilt Brain Institute TIPS funding, the Vanderbilt Ingram Cancer Center shared Resource Scholarships, and the NIA R01 AG061138.
Footnotes
BEW and BWS are co-founders of Turkey Creek Biotechnology LLC and have equity ownership. The authors declare no other competing conflicts of interest with the contents of this articles.
ASSOCIATED CONTENT
Supporting Information
Figure S1. Original Coomassie staining data.
Figure S2. Original Western analysis data.
Figure S3. ELISA data.
Figure S4. Original Western blot data.
Figure S5. dot blot data to confirm the specificity of E3 nanobody.
Figure S6. Alpha-Fold modeling to demonstrate potential binding mode of E3 nanobody.
DATA AVAILABILITY.
The complete amino acid sequence of the E3 nanobody is available in GenBank, with the accession number BankIt2800678 SABO_E3 PP401722. The data will be available after March 13th, 2024.
REFERENCES
- 1.Nguyen P. H. et al. Amyloid Oligomers: A Joint Experimental/Computational Perspective on Alzheimer’s Disease, Parkinson’s Disease, Type II Diabetes, and Amyotrophic Lateral Sclerosis. Chem Rev 121, 2545–2647 (2021). 10.1021/acs.chemrev.0c01122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De-Paula V. J., Radanovic M., Diniz B. S. & Forlenza O. V. Alzheimer’s disease. Subcell Biochem 65, 329–352 (2012). 10.1007/978-94-007-5416-4_14 [DOI] [PubMed] [Google Scholar]
- 3.Selkoe D. J. Alzheimer’s disease. Cold Spring Harb Perspect Biol 3, 1–16 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cline E. N., Bicca M. A., Viola K. L. & Klein W. L. The Amyloid-beta Oligomer Hypothesis: Beginning of the Third Decade. J Alzheimers Dis 64, S567–S610 (2018). 10.3233/JAD-179941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chromy B. A. et al. Self-assembly of Abeta(1–42) into globular neurotoxins. Biochemistry 42, 12749–12760 (2003). 10.1021/bi030029q [DOI] [PubMed] [Google Scholar]
- 6.Habashi M. et al. Early diagnosis and treatment of Alzheimer’s disease by targeting toxic soluble Abeta oligomers. Proc Natl Acad Sci U S A 119, e2210766119 (2022). 10.1073/pnas.2210766119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein W. L. Synaptic targeting by A beta oligomers (ADDLS) as a basis for memory loss in early Alzheimer’s disease. Alzheimers Dement 2, 43–55 (2006). 10.1016/j.jalz.2005.11.003 [DOI] [PubMed] [Google Scholar]
- 8.Lacor P. N. et al. Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J Neurosci 24, 10191–10200 (2004). 10.1523/JNEUROSCI.3432-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLean C. A. et al. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol 46, 860–866 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Bjorklund N. L. et al. Absence of amyloid beta oligomers at the postsynapse and regulated synaptic Zn2+ in cognitively intact aged individuals with Alzheimer’s disease neuropathology. Mol Neurodegener 7, 23 (2012). 10.1186/1750-1326-7-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koffie R. M. et al. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci U S A 106, 4012–4017 (2009). 10.1073/pnas.0811698106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez-Nievas B. G. et al. Dissecting phenotypic traits linked to human resilience to Alzheimer’s pathology. Brain 136, 2510–2526 (2013). 10.1093/brain/awt171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esparza T. J. et al. Amyloid-beta oligomerization in Alzheimer dementia versus high-pathology controls. Ann Neurol 73, 104–119 (2013). 10.1002/ana.23748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fowler S. W. et al. Genetic modulation of soluble Abeta rescues cognitive and synaptic impairment in a mouse model of Alzheimer’s disease. J Neurosci 34, 7871–7885 (2014). 10.1523/JNEUROSCI.0572-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukumoto H. et al. High-molecular-weight beta-amyloid oligomers are elevated in cerebrospinal fluid of Alzheimer patients. FASEB J 24, 2716–2726 (2010). 10.1096/fj.09-150359 [DOI] [PubMed] [Google Scholar]
- 16.Klyubin I. et al. Amyloid beta protein dimer-containing human CSF disrupts synaptic plasticity: prevention by systemic passive immunization. J Neurosci 28, 4231–4237 (2008). 10.1523/JNEUROSCI.5161-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nilsberth C. et al. The ‘Arctic’ APP mutation (E693G) causes Alzheimer’s disease by enhanced Abeta protofibril formation. Nat Neurosci 4, 887–893 (2001). 10.1038/nn0901-887 [DOI] [PubMed] [Google Scholar]
- 18.Sehlin D. et al. Large aggregates are the major soluble Abeta species in AD brain fractionated with density gradient ultracentrifugation. PLoS One 7, e32014 (2012). 10.1371/journal.pone.0032014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shankar G. M. et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med 14, 837–842 (2008). 10.1038/nm1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh D. M. et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539 (2002). 10.1038/416535a [DOI] [PubMed] [Google Scholar]
- 21.Pham T. & Cheng K. H. Exploring the binding kinetics and behaviors of self-aggregated beta-amyloid oligomers to phase-separated lipid rafts with or without ganglioside-clusters. Biophys Chem 290, 106874 (2022). 10.1016/j.bpc.2022.106874 [DOI] [PubMed] [Google Scholar]
- 22.Cleary J. P. et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci 8, 79–84 (2005). 10.1038/nn1372 [DOI] [PubMed] [Google Scholar]
- 23.Figueiredo C. P. et al. Memantine rescues transient cognitive impairment caused by high-molecular-weight abeta oligomers but not the persistent impairment induced by low-molecular-weight oligomers. J Neurosci 33, 9626–9634 (2013). 10.1523/JNEUROSCI.0482-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsia A. Y. et al. Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc Natl Acad Sci U S A 96, 3228–3233 (1999). 10.1073/pnas.96.6.3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ledo J. H. et al. Amyloid-beta oligomers link depressive-like behavior and cognitive deficits in mice. Mol Psychiatry 18, 1053–1054 (2013). 10.1038/mp.2012.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lourenco M. V. et al. TNF-alpha mediates PKR-dependent memory impairment and brain IRS-1 inhibition induced by Alzheimer’s beta-amyloid oligomers in mice and monkeys. Cell Metab 18, 831–843 (2013). 10.1016/j.cmet.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 27.Mucke L. et al. High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci 20, 4050–4058 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poling A. et al. Oligomers of the amyloid-beta protein disrupt working memory: confirmation with two behavioral procedures. Behav Brain Res 193, 230–234 (2008). 10.1016/j.bbr.2008.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westerman M. A. et al. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci 22, 1858–1867 (2002). https://doi.org:22/5/1858 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ni R., Gillberg P. G., Bergfors A., Marutle A. & Nordberg A. Amyloid tracers detect multiple binding sites in Alzheimer’s disease brain tissue. Brain 136, 2217–2227 (2013). 10.1093/brain/awt142 [DOI] [PubMed] [Google Scholar]
- 31.Wu C., Bowers M. T. & Shea J. E. On the origin of the stronger binding of PIB over thioflavin T to protofibrils of the Alzheimer amyloid-beta peptide: a molecular dynamics study. Biophys J 100, 1316–1324 (2011). 10.1016/j.bpj.2011.01.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ostrowitzki S. et al. A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimers Res Ther 9, 95 (2017). 10.1186/s13195-017-0318-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panza F. et al. Bapineuzumab: anti-beta-amyloid monoclonal antibodies for the treatment of Alzheimer’s disease. Immunotherapy 2, 767–782 (2010). 10.2217/imt.10.80 [DOI] [PubMed] [Google Scholar]
- 34.Sevigny J. et al. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature 537, 50–56 (2016). 10.1038/nature19323 [DOI] [PubMed] [Google Scholar]
- 35.Englund H. et al. Sensitive ELISA detection of amyloid-beta protofibrils in biological samples. J Neurochem 103, 334–345 (2007). 10.1111/j.1471-4159.2007.04759.x [DOI] [PubMed] [Google Scholar]
- 36.Magnusson K. et al. Specific uptake of an amyloid-beta protofibril-binding antibody-tracer in AbetaPP transgenic mouse brain. J Alzheimers Dis 37, 29–40 (2013). 10.3233/JAD-130029 [DOI] [PubMed] [Google Scholar]
- 37.Sehlin D. et al. Heavy-chain complementarity-determining regions determine conformation selectivity of anti-abeta antibodies. Neurodegener Dis 8, 117–123 (2011). 10.1159/000316530 [DOI] [PubMed] [Google Scholar]
- 38.Tucker S. et al. The murine version of BAN2401 (mAb158) selectively reduces amyloid-beta protofibrils in brain and cerebrospinal fluid of tg-ArcSwe mice. J Alzheimers Dis 43, 575–588 (2015). 10.3233/JAD-140741 [DOI] [PubMed] [Google Scholar]
- 39.Kayed R. et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300, 486–489 (2003). 10.1126/science.1079469 [DOI] [PubMed] [Google Scholar]
- 40.Daley L. P., Gagliardo L. F., Duffy M. S., Smith M. C. & Appleton J. A. Application of monoclonal antibodies in functional and comparative investigations of heavy-chain immunoglobulins in new world camelids. Clin Diagn Lab Immunol 12, 380–386 (2005). 10.1128/CDLI.12.3.380-386.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanke L. et al. An alpaca nanobody neutralizes SARS-CoV-2 by blocking receptor interaction. Nat Commun 11, 4420 (2020). 10.1038/s41467-020-18174-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glassman P. M. et al. Molecularly Engineered Nanobodies for Tunable Pharmacokinetics and Drug Delivery. Bioconjug Chem 31, 1144–1155 (2020). 10.1021/acs.bioconjchem.0c00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abulrob A., Sprong H., Van Bergen en Henegouwen, P. & Stanimirovic, D. The blood-brain barrier transmigrating single domain antibody: mechanisms of transport and antigenic epitopes in human brain endothelial cells. J Neurochem 95, 1201–1214 (2005). 10.1111/j.1471-4159.2005.03463.x [DOI] [PubMed] [Google Scholar]
- 44.Li T. et al. Cell-penetrating anti-GFAP VHH and corresponding fluorescent fusion protein VHH-GFP spontaneously cross the blood-brain barrier and specifically recognize astrocytes: application to brain imaging. FASEB J 26, 3969–3979 (2012). 10.1096/fj.11-201384 [DOI] [PubMed] [Google Scholar]
- 45.Fuller J. P., Stavenhagen J. B. & Teeling J. L. New roles for Fc receptors in neurodegeneration-the impact on Immunotherapy for Alzheimer’s Disease. Front Neurosci 8, 235 (2014). 10.3389/fnins.2014.00235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sperling R. et al. Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol 11, 241–249 (2012). https://doi.org:S1474-4422(12)70015-7 [pii] 10.1016/S1474-4422(12)70015-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryan D. A., Narrow W. C., Federoff H. J. & Bowers W. J. An improved method for generating consistent soluble amyloid-beta oligomer preparations for in vitro neurotoxicity studies. J Neurosci Methods 190, 171–179 (2010). 10.1016/j.jneumeth.2010.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pardon E. et al. A general protocol for the generation of Nanobodies for structural biology. Nat Protoc 9, 674–693 (2014). 10.1038/nprot.2014.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oakley H. et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci 26, 10129–10140 (2006). https://doi.org:26/40/10129 [pii] 10.1523/JNEUROSCI.1202-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He P., Schulz P. & Sierks M. R. A conformation-specific antibody against oligomeric beta-amyloid restores neuronal integrity in a mouse model of Alzheimer’s disease. J Biol Chem 296, 100241 (2021). 10.1074/jbc.RA120.015327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bjellqvist B., Basse B., Olsen E. & Celis J. E. Reference points for comparisons of two-dimensional maps of proteins from different human cell types defined in a pH scale where isoelectric points correlate with polypeptide compositions. Electrophoresis 15, 529–539 (1994). 10.1002/elps.1150150171 [DOI] [PubMed] [Google Scholar]
- 52.Bjellqvist B. et al. The focusing positions of polypeptides in immobilized pH gradients can be predicted from their amino acid sequences. Electrophoresis 14, 1023–1031 (1993). 10.1002/elps.11501401163 [DOI] [PubMed] [Google Scholar]
- 53.Lambert M. P. et al. Vaccination with soluble Abeta oligomers generates toxicity-neutralizing antibodies. J Neurochem 79, 595–605 (2001). 10.1046/j.1471-4159.2001.00592.x [DOI] [PubMed] [Google Scholar]
- 54.Bitencourt A. L. B., Campos R. M., Cline E. N., Klein W. L. & Sebollela A. Antibody Fragments as Tools for Elucidating Structure-Toxicity Relationships and for Diagnostic/Therapeutic Targeting of Neurotoxic Amyloid Oligomers. Int J Mol Sci 21 (2020). 10.3390/ijms21238920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mannini B. et al. Molecular mechanisms used by chaperones to reduce the toxicity of aberrant protein oligomers. Proc Natl Acad Sci U S A 109, 12479–12484 (2012). 10.1073/pnas.1117799109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mannini B. et al. Toxicity of protein oligomers is rationalized by a function combining size and surface hydrophobicity. ACS Chem Biol 9, 2309–2317 (2014). 10.1021/cb500505m [DOI] [PubMed] [Google Scholar]
- 57.Olafsen T. & Wu A. W. Novel antibody vectors for imaging. Semin Nucl Med 40, 167–181 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Viola K. L. et al. The Therapeutic and Diagnostic Potential of Amyloid beta Oligomers Selective Antibodies to Treat Alzheimer’s Disease. Front Neurosci 15, 768646 (2021). 10.3389/fnins.2021.768646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Behof W. J. et al. A novel antioxidant ergothioneine PET radioligand for in vivo imaging applications. Scientific Reports 11, 18450 (2021). 10.1038/s41598-021-97925-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maass D. R., Sepulveda J., Pernthaner A. & Shoemaker C. B. Alpaca (Lama pacos) as a convenient source of recombinant camelid heavy chain antibodies (VHHs). J Immunol Methods 324, 13–25 (2007). 10.1016/j.jim.2007.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jumper J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021). 10.1038/s41586-021-03819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oeffner R. D. et al. Putting AlphaFold models to work with phenix.process_predicted_model and ISOLDE. Acta Crystallogr D Struct Biol 78, 1303–1314 (2022). 10.1107/S2059798322010026 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The complete amino acid sequence of the E3 nanobody is available in GenBank, with the accession number BankIt2800678 SABO_E3 PP401722. The data will be available after March 13th, 2024.