Abstract
Background:
Current guidelines recommend low-molecular-weight heparin for thromboprophylaxis after orthopaedic trauma. However, recent evidence suggests that aspirin is similar in efficacy and safety. To understand patients’ experiences with these medications, we compared patients’ satisfaction and out-of-pocket costs after thromboprophylaxis with aspirin versus low-molecular-weight heparin.
Methods:
This study was a secondary analysis of the PREVENTion of CLots in Orthopaedic Trauma (PREVENT CLOT) trial, conducted at 21 trauma centers in the U.S. and Canada. We included adult patients with an operatively treated extremity fracture or a pelvic or acetabular fracture. Patients were randomly assigned to receive 30 mg of low-molecular-weight heparin (enoxaparin) twice daily or 81 mg of aspirin twice daily for thromboprophylaxis. The duration of the thromboprophylaxis, including post-discharge prescription, was based on hospital protocols. The study outcomes included patient satisfaction with and out-of-pocket costs for their thromboprophylactic medication measured on ordinal scales.
Results:
The trial enrolled 12,211 patients (mean age and standard deviation [SD], 45 ± 18 years; 62% male), 9725 of whom completed the question regarding their satisfaction with the medication and 6723 of whom reported their out-of-pocket costs. The odds of greater satisfaction were 2.6 times higher for patients assigned to aspirin than those assigned to low-molecular-weight heparin (odds ratio [OR]: 2.59; 95% confidence interval [CI]: 2.39 to 2.80; p < 0.001). Overall, the odds of incurring any out-of-pocket costs for thromboprophylaxis medication were 51% higher for patients assigned to aspirin compared with low-molecular-weight heparin (OR: 1.51; 95% CI: 1.37 to 1.66; p < 0.001). However, patients assigned to aspirin had substantially lower odds of out-of-pocket costs of at least $25 (OR: 0.15; 95% CI: 0.12 to 0.18; p < 0.001).
Conclusions:
Use of aspirin substantially improved patients’ satisfaction with their medication after orthopaedic trauma. While aspirin use increased the odds of incurring any out-of-pocket costs, it protected against costs of ≥$25, potentially improving health equity for thromboprophylaxis.
Level of Evidence:
Therapeutic Level II. See Instructions for Authors for a complete description of levels of evidence.
Venous thromboembolism remains a common and potentially fatal complication following orthopaedic trauma1-4. Current guidelines recommend injectable low-molecular-weight heparin as chemoprophylaxis for patients with many fracture types5-8. However, research suggests that prescribed doses of injectable drugs are more likely than oral medications to be not administered by health-care providers to hospitalized patients, with non-administration usually due to patient refusal9,10. Furthermore, research has demonstrated that patients who have sustained a fracture strongly prefer oral thromboprophylactic medications over subcutaneous injection11. Patients are also sensitive to costs of medications that are assumed to provide similar protection against thromboembolic events11.
A recent randomized clinical trial of >12,000 patients found thromboprophylaxis with aspirin to be non-inferior to low-molecular-weight heparin for preventing fatal events following orthopaedic trauma12. The pulmonary embolism rates did not differ between treatment groups, and a similar between-group safety profile was observed. Low-molecular-weight heparin conferred a modest benefit against only distal deep vein thrombosis. A secondary aim of the trial was to compare aspirin and low-molecular-weight heparin with regard to the patients’ satisfaction with the medication and their out-of-pocket costs.
Understanding the patient’s experience with these commonly prescribed thromboprophylactic medications is essential to providing care responsive to patient preferences. In this secondary analysis of a randomized trial, we hypothesized that patients would have greater satisfaction and lower out-of-pocket costs with aspirin thromboprophylaxis than with low-molecular-weight heparin. We also sought to determine if the effect of the prescribed thromboprophylaxis on medication satisfaction and out-of-pocket costs differed based on the patient’s type of health insurance.
Materials and Methods
Study Design
This study was a secondary analysis of the PREVENTion of CLots in Orthopaedic Trauma (PREVENT CLOT) randomized clinical trial performed at 21 trauma centers in the U.S. and Canada13. Patients were enrolled from April 2017 through August 2021. The primary trial was registered with ClinicalTrials.gov (NCT02984384) and was co-led by the University of Maryland School of Medicine’s Department of Orthopaedics and the Major Extremity Trauma Research Consortium (METRC) Coordinating Center. The assessment of our study outcomes was prespecified in the trial protocol. The study was approved by the research ethics boards at the Coordinating Center and participating sites. In addition, we obtained informed consent from all study participants before enrollment.
Study Participants
The trial included adult patients with an operatively treated extremity fracture or a pelvic or acetabular fracture treated either operatively or nonoperatively. We excluded patients who presented to the hospital >48 hours after injury and those who had received ≥3 doses of thromboprophylaxis before enrollment. The full eligibility criteria are available in Appendix A.
Study Interventions
Patients were randomly allocated to receive aspirin or low-molecular-weight heparin thromboprophylaxis in a 1:1 ratio. The trial protocol dictated prescription of a 30-mg dose of low-molecular-weight heparin (enoxaparin) administered subcutaneously twice a day but permitted adjusted dosing for obese patients, patients with renal disease, or other medical indications based on each site’s protocols. An 81-mg dose of aspirin twice day was prescribed for the other treatment group. The duration of prophylaxis was based on each hospital’s clinical protocols, given the lack of consensus on the optimal duration6,8. The patient and the treating physician were aware of the treatment allocation.
For a patient to be considered treatment-adherent, 2 conditions had to be met. First, the patient had to have received at least 80% of their in-hospital study medication doses, allowing inpatient doses to be missed if the medication was temporarily stopped for surgery. Second, if the patient was prescribed thromboprophylaxis on discharge, their allocated study medication had to have been prescribed.
Study Outcomes
The 2 study outcomes for this secondary analysis were patient satisfaction with the medication and out-of-pocket medication cost. Research personnel collected these outcome data as part of routine study follow-up 90 days after randomization. Satisfaction with the medication was measured using a single question (“How satisfied or dissatisfied are you with your blood clot prevention medication?”) from the modified version of the Treatment Satisfaction Questionnaire for Medication (TSQM)14. The TSQM was modified based on patient stakeholders’ feedback and developer input. Only 1 question was used because scoring of the modified instrument has not been validated. The patients answered using a 7-point Likert scale ranging from “extremely dissatisfied” to “extremely satisfied” (see Appendix B).
Patients were asked specifically about the direct out-of-pocket cost for their thromboprophylactic medication, which was not to include other out-of-pocket costs incurred as part of their treatment. The data were self-reported by the patients at their final follow-up visit (90 days after randomization) on a 7-level ordinal scale, including “no out-of-pocket costs,” “<$25,” “$25 to $50,” “$51 to $100,” “$101 to $500,” $501 to $1000,” and “>$1000” (see Appendix C). The question was added as a protocol amendment 9 months after initiating the primary study based on feedback from our patient stakeholders and after the enrollment of approximately 2000 patients.
Statistical Analysis
The trial’s overall sample size was determined based on the primary outcome of 90-day mortality13. However, for the analysis of the patients’ satisfaction with their thromboprophylactic medication, we assumed that half of the respondents would be at least “satisfied” based on previous studies15,16. With that assumption, 9000 respondents provided >90% power to detect a 15% change in the odds of increased satisfaction with the medication with an alpha of 5%. For the medication cost question, we assumed that 50% of the respondents would have no out-of-pocket costs and 10% would have >$500 in out-of-pocket costs. Therefore, 6000 respondents provided 80% power to detect a 15% difference in the odds of increased medication costs with an alpha of 5%.
Our primary analysis followed the intention-to-treat approach. We initially intended to fit cumulative logit models to compare the ordinal levels of patient satisfaction with the medication and out-of-pocket medication costs between treatment groups using complete-case data. We assessed the proportional odds assumption for these models with graphical summaries17. The patient satisfaction with the medication model met this assumption, and the reported odds ratio (OR) is interpreted as the odds of a higher level of satisfaction with the medication if a patient is assigned to receive aspirin rather than low-molecular-weight heparin. The cost model did not satisfy the proportional odds assumption, so we fit 2 logistic regression models. The first model dichotomized out-of-pocket costs as >$0 versus $0. The second model used a ≥$25 versus <$25 out-of-pocket cost outcome. In addition, we assessed differences in subcomponents of the ordinal outcomes with chi-square tests. To assess the heterogeneity of treatment effects, we added a health insurance indicator as an interaction term to the models.
We performed 2 sensitivity analyses. First, we restricted the sample to patients who had adhered to the assigned protocol. Second, given the high proportion of participants who refused to answer or were unable to provide an answer, we performed a sensitivity analysis using inverse probability of treatment weighting. In this analysis, we weighted each patient according to the inverse probability of responding to the satisfaction and cost outcome queries, as estimated based on 21 baseline and in-hospital factors. All analyses were performed with R, version 4.0.2 (R Project for Statistical Computing). Our threshold for significance was p = 0.05, and we did not adjust for multiple testing.
Results
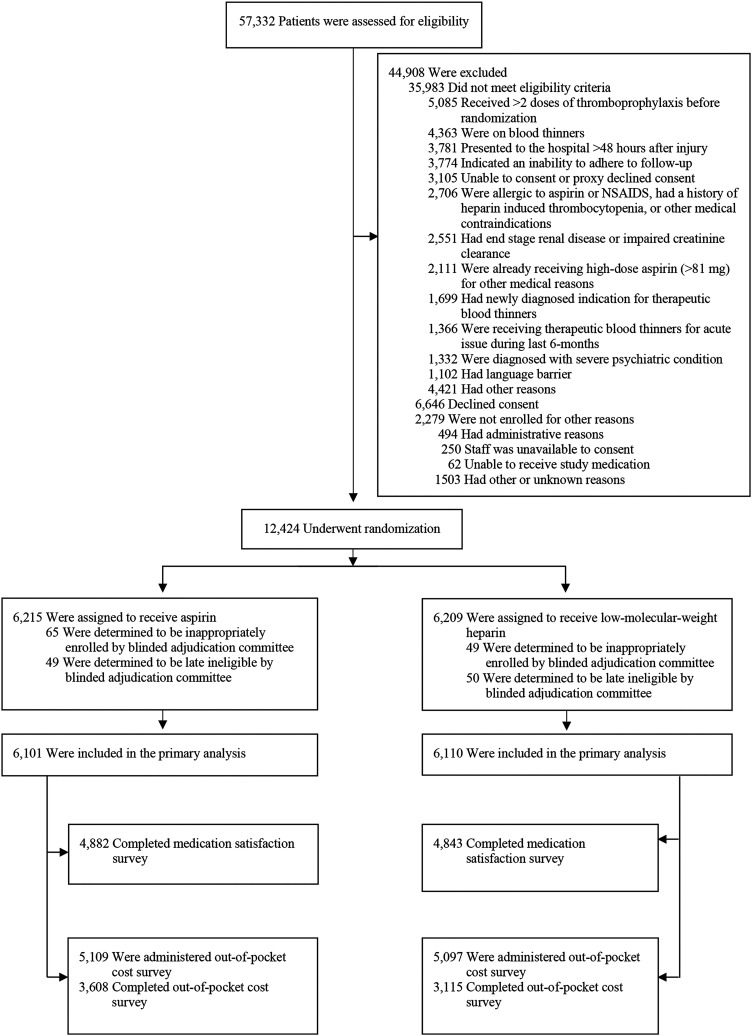
Of the 12,211 patients enrolled in the trial, 9725 (80%) completed the satisfaction-with-medication question. A total of 10,206 patients were enrolled after we introduced the out-of-pocket cost question to the case report forms, and 6723 (66%) of these patients agreed to report their out-of-pocket costs (Table I; Fig. 1). The mean age of the respondents (and standard deviation [SD]) was 45 ± 18 years, and 62% were male. Most patients (88%) had a fracture of a lower extremity. The median Injury Severity Score was 9 (interquartile range [IQR], 4 to 10). Nearly 40% of the patients had private health insurance; approximately 30% had coverage through Medicare, Medicaid, or Tricare; and 1 in 5 patients did not have health insurance at the time of injury. Thromboprophylaxis was prescribed at discharge for 91% of the patients for a median duration of 21 days in both treatment groups (aspirin: IQR, 19 to 21; low-molecular-weight heparin: IQR, 14 to 21). The median time from randomization to survey completion was 108 days (IQR, 95 to 127). Patients who responded to the 2 research questions were significantly more likely to be female, have private health insurance, and have a lower-extremity fracture compared with non-respondents (see Appendix Tables I and II).
Fig. 1.
CONSORT (Consolidated Standards of Reporting Trials) flowchart. NSAIDS = nonsteroidal anti-inflammatory drugs.
TABLE I.
Characteristics of All Patients Enrolled in the Trial, Patients Who Answered the Medication Satisfaction Question, and Patients Who Answered the Medication Cost Question*
| Characteristic | Overall Study Group (N = 12,211) | Patients Answering Medication Satisfaction Question (N = 9725) | Patients Answering Medication Cost Question (N = 6723) | |||
|---|---|---|---|---|---|---|
| Aspirin (N = 6101) | Low-Molecular- Weight Heparin (N = 6110) | Aspirin (N = 4882) | Low-Molecular- Weight Heparin (N = 4843) | Aspirin (N = 3608) | Low-Molecular- Weight Heparin (N = 3115) | |
| Age† (yr) | 44.5 ± 18.0 | 44.7 ± 17.6 | 44.4 ± 17.6 | 44.5 ± 17.2 | 44.5 ± 17.2 | 44.1 ± 16.7 |
| Male sex‡ | 3832 (62.8) | 3769 (61.7) | 3033 (62.1) | 2962 (61.2) | 2215 (61.4) | 1879 (60.3) |
| Body mass index§ (kg/m2) | 27.1 (23.6, 31.8) | 27.5 (23.8, 32.8) | 27.4 (23.8, 32.0) | 27.8 (23.9, 33.1) | 27.4 (23.9, 32.2) | 28.0 (24.2, 33.0) |
| Risk factors‡ | ||||||
| Previous VTE | 43 (0.7) | 46 (0.8) | 32 (0.7) | 40 (0.8) | 25 (0.7) | 26 (0.8) |
| Cancer | 140 (2.3) | 166 (2.7) | 122 (2.5) | 135 (2.8) | 86 (2.4) | 84 (2.7) |
| Smoking status‡ | ||||||
| Never smoked | 3012 (49.4) | 2935 (48.0) | 2506 (51.3) | 2375 (49.0) | 1850 (51.3) | 1502 (48.2) |
| Former smoker | 986 (16.2) | 1031 (16.9) | 797 (16.3) | 832 (17.2) | 593 (16.4) | 541 (17.4) |
| Current smoker | 2099 (34.4) | 2139 (35.0) | 1579 (32.3) | 1636 (33.8) | 1165 (32.3) | 1072 (34.4) |
| Medications before injury‡ | ||||||
| Prior aspirin | 496 (8.1) | 476 (7.8) | 402 (8.2) | 362 (7.5) | 297 (8.2) | 227 (7.3) |
| OCP/estrogen | 112 (1.8) | 107 (1.8) | 94 (1.9) | 91 (1.9) | 67 (1.9) | 57 (1.8) |
| Clopidogrel/other antiplatelet medication | 55 (0.9) | 52 (0.9) | 42 (0.9) | 41 (0.8) | 31 (0.9) | 25 (0.8) |
| Health insurance‡ | ||||||
| Private | 2093 (35.8) | 2140 (36.5) | 1810 (38.5) | 1817 (39.1) | 1342 (38.5) | 1145 (38.3) |
| Medicare/Medicaid/Tricare | 1784 (30.5) | 1826 (31.2) | 1348 (28.7) | 1357 (29.2) | 1020 (29.3) | 874 (29.3) |
| Other public options | 417 (7.1) | 403 (6.9) | 350 (7.4) | 335 (7.2) | 256 (7.4) | 222 (7.4) |
| Workers’ Compensation | 202 (3.5) | 202 (3.4) | 173 (3.7) | 174 (3.7) | 135 (3.9) | 131 (4.4) |
| No insurance | 1355 (23.2) | 1288 (22.0) | 1017 (21.6) | 965 (20.8) | 729 (20.9) | 614 (20.6) |
| Injury severity score§ | 9 (4 to 10) | 9 (4 to 10) | 9 (4 to 10) | 9 (4 to 10) | 9 (4 to 10) | 9 (4 to 10) |
| Injury regions‡# | ||||||
| Lower extremity | 5346 (88.1) | 5336 (87.8) | 4334 (89.1) | 4308 (89.2) | 3252 (90.5) | 2833 (91.2) |
| Upper extremity | 1655 (27.3) | 1688 (27.8) | 1324 (27.2) | 1344 (27.8) | 936 (26.0) | 825 (26.6) |
| Abdomen | 758 (12.5) | 808 (13.3) | 596 (12.3) | 652 (13.5) | 424 (11.8) | 393 (12.7) |
| Spine | 608 (10.0) | 655 (10.8) | 473 (9.7) | 526 (10.9) | 332 (9.2) | 327 (10.5) |
| Thorax | 1083 (17.8) | 1163 (19.1) | 846 (17.4) | 941 (19.5) | 580 (16.1) | 581 (18.7) |
| Neck | 59 (1.0) | 74 (1.2) | 54 (1.1) | 60 (1.2) | 36 (1.0) | 36 (1.2) |
| Face | 816 (13.4) | 875 (14.4) | 651 (13.4) | 705 (14.6) | 437 (12.2) | 442 (14.2) |
| Head | 778 (12.8) | 783 (12.9) | 610 (12.5) | 612 (12.7) | 411 (11.4) | 371 (11.9) |
VTE = venous thromboembolism, OCP = oral contraceptive pill.
The values are given as the mean and standard deviation.
The values are given as the number with the percentage in parentheses.
The values are given as the median with the interquartile range in parentheses.
Some patients sustained >1 injury.
Patient Satisfaction with Medication
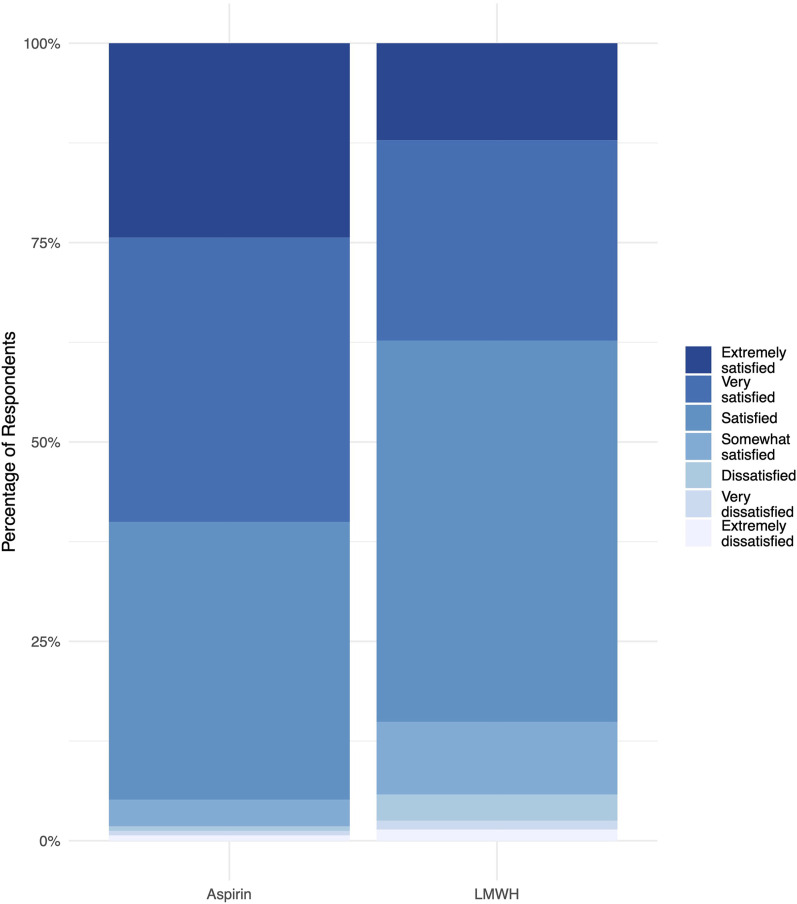
The odds of greater satisfaction with the medication were 2.6 times higher for patients assigned to aspirin relative to those assigned to low-molecular-weight heparin (OR: 2.59; 95% confidence interval [CI]: 2.39 to 2.80; p < 0.001) (Fig. 2). Specifically, 95% (4631) of the patients assigned to receive aspirin reported being satisfied, very satisfied, or extremely satisfied with their thromboprophylaxis medication. In contrast, 85% (4122) of the patients assigned to low-molecular-weight heparin were satisfied or better (p < 0.001).
Fig. 2.
Patient satisfaction with their thromboprophylactic medication stratified by treatment group. LMWH = low-molecular-weight heparin.
Our 2 sensitivity analyses produced a similar result. Among patients who adhered to the protocol (n = 8559), the odds of increased satisfaction with the medication were 2.8 times higher in the aspirin group (OR: 2.75; 95% CI: 2.54 to 2.99; p < 0.001). After adjusting for the probability of responding to the question, we obtained an OR of 2.57 (95% CI: 2.40 to 2.74, p < 0.001) favoring aspirin.
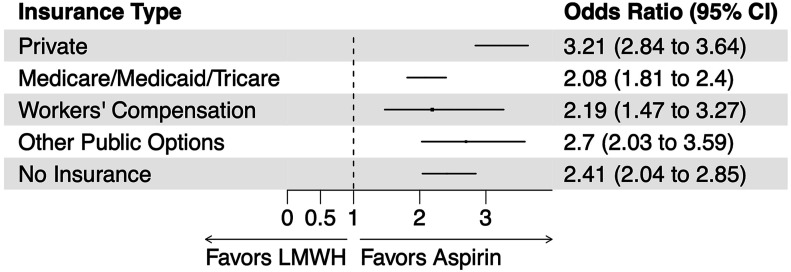
The effect of thromboprophylaxis on satisfaction with the medication did not differ in direction based on the type of health insurance, but the magnitude of the effect did vary significantly (interaction p < 0.001) (Fig. 3; see also Appendix Fig. 1). Specifically, aspirin conferred the greatest increase in satisfaction when used by patients with private health insurance (OR: 3.21; 95% CI: 2.84 to 3.64; p < 0.001). The effect was smallest for patients insured by Medicare, Medicaid, or Tricare (OR: 2.08; 95% CI: 1.81 to 2.40; p < 0.001).
Fig. 3.
Variation in the effect of aspirin versus low-molecular-weight heparin (LMWH) thromboprophylaxis on patient satisfaction with the medication by type of health insurance. CI = confidence interval.
Medication Out-of-Pocket Costs
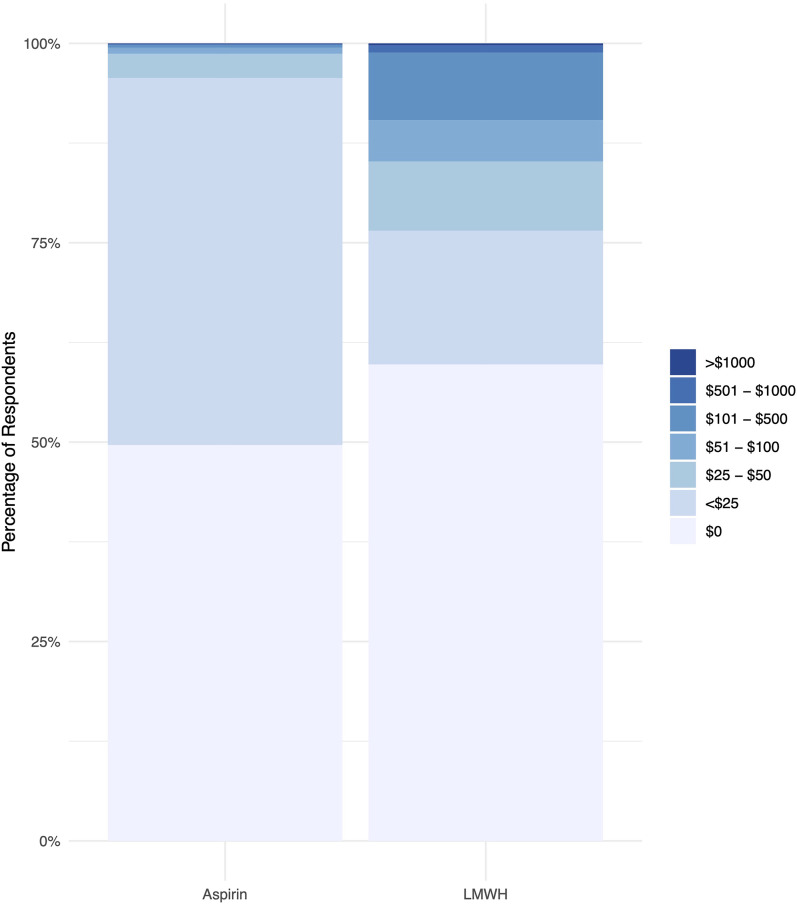
Patients assigned to aspirin had increased odds of incurring any out-of-pocket costs for their thromboprophylactic medication compared with patients assigned to low-molecular-weight heparin (OR: 1.51; 95% CI: 1.37 to 1.66; p < 0.001) (Fig. 4). However, patients assigned to aspirin were significantly less likely to incur out-of-pocket costs of ≥$25 (OR: 0.15; 95% CI: 0.12 to 0.18; p < 0.001). In addition, more patients in the low-molecular-weight heparin arm reported that they did not incur out-of-pocket costs because they did not take any thromboprophylactic medication after it was prescribed at discharge than in the aspirin arm (3% versus 2%, p < 0.001).
Fig. 4.
Patient-reported out-of-pocket thromboprophylactic medication costs stratified by treatment group. LMWH = low-molecular-weight heparin.
Analysis of the subset of patients who adhered to their assigned treatment (n = 5926) showed consistent results with regard to incurring any out-of-pocket costs (OR: 1.56; 95% CI: 1.41 to 1.73; p < 0.001) and out-of-pocket costs of ≥$25 (OR: 0.13; 95% CI: 0.11 to 0.16; p < 0.001). In addition, when we weighted the entire sample by the inverse probability of responding to the question, we observed a similar effect of the assigned treatment group on incurring any out-of-pocket costs (OR: 1.51; 95% CI: 1.37 to 1.67; p < 0.001) and out-of-pocket costs of ≥$25 (OR: 0.15; 95% CI: 0.13 to 0.18; p < 0.001).
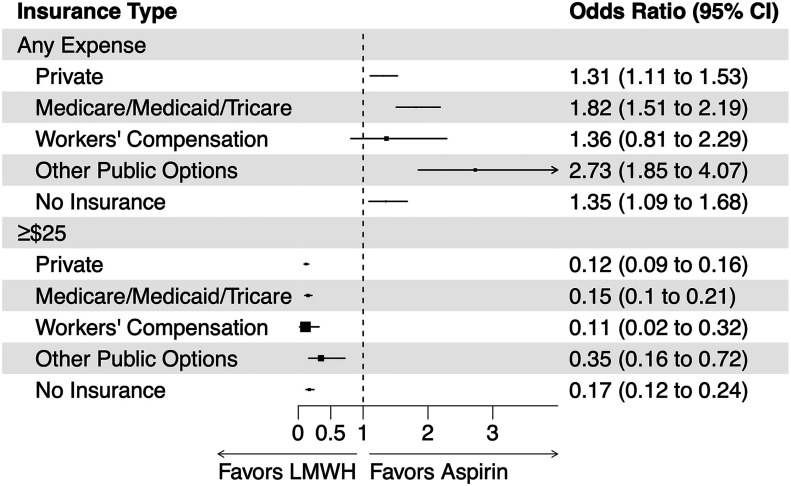
The effect of the thromboprophylactic medication on patients incurring any out-of-pocket costs varied significantly in magnitude based on their health insurance (interaction p = 0.001) (Fig. 5; see also Appendix Fig. 2). However, the direction of the treatment effect on incurring any out-of-pocket costs was consistent across all health insurance types. The effect of the thromboprophylactic medication on patients incurring ≥$25 in out-of-pocket costs did not significantly vary based on their type of health insurance (interaction p = 0.08).
Fig. 5.
Variation in the effect of aspirin versus low-molecular-weight heparin (LMWH) thromboprophylaxis on any out-of-pocket costs (versus no out-of-pocket costs) and on out-of-pocket costs of ≥$25 (versus <$25) by type of health insurance. CI = confidence interval.
Discussion
In this secondary analysis of a large, randomized trial comparing aspirin with low-molecular-weight heparin in patients who had sustained orthopaedic trauma, we found patient satisfaction with their thromboprophylactic medication to be significantly greater for those assigned to receive aspirin. These patients were more likely than those assigned to low-molecular-weight heparin to incur some out-of-pocket costs for thromboprophylaxis. However, aspirin thromboprophylaxis reduced the odds of incurring ≥$25 in out-of-pocket costs by 85%. The magnitude of patient satisfaction with the medication and protection against out-of-pocket costs varied based on the type of health insurance. However, the direction of these effects was consistent across all health insurance types in favor of aspirin.
Our finding that patients assigned oral thromboprophylaxis have greater satisfaction with the medication than patients assigned a subcutaneous injection is consistent with previous research15,16,18,19. In a study of patients with pelvic or lower-extremity fractures, John et al. observed greater satisfaction with orally administered rivaroxaban than with low-molecular-weight heparin15. This finding of more satisfaction with oral thromboprophylaxis than with an injectable alternative has been replicated in studies of fracture patients and other patient populations16,18,19. Our large sample adds substantial precision to these previous estimates and informs how the treatment effect varies based on insurance type.
Increased satisfaction with medication has been previously linked to increased medication adherence20. While we did not systematically measure post-discharge medication adherence, previously inpatient adherence was not found to be meaningfully different between aspirin (95%) and low-molecular-weight heparin (97%)12. However, adherence at the time of discharge, based on whether those prescribed thromboprophylaxis on discharge were prescribed the medication assigned to them in the study, was greater for patients assigned to aspirin (94%) compared with low-molecular-weight heparin (89%)12. Also, 3% of the patients in our low-molecular-weight heparin group reported they did not incur out-of-pocket costs because they did not take either medication after discharge, compared with 2% in the aspirin arm. While patients assigned to aspirin had 2.6 times higher odds of satisfaction with the medication, a high proportion (85%) of the patients in the low-molecular-weight heparin group reported at least mid-level satisfaction on the 7-point Likert scale. Research suggests that lower regimen complexity is an important driver of satisfaction with medication20, and this presumably contributed to our observed result.
To our knowledge, this is the first study to compare patient out-of-pocket costs for these commonly prescribed thromboprophylactic regimens. Several studies have compared the cost-effectiveness of aspirin with that of low-molecular-weight heparin for thromboprophylaxis. However, those studies involved patients who had undergone joint replacement and were from the perspective of a national health-care payer21-23. None reported or included the costs incurred by patients, only the direct health-care system costs.
The list price of $1076 to $1435 for a course of treatment with low-molecular-weight heparin is substantially higher than the $5 to $10 for a bottle of low-dose aspirin. However, we found that most patients in the study paid <$25 for their thromboprophylaxis, irrespective of their treatment allocation or health insurance coverage, suggesting that most patients are shielded from these high costs. Our finding was consistent with the low median out-of-pocket costs for low-molecular-weight heparin reported in a previous study of patients with gynecological cancer24. While incurring out-of-pocket costs of ≥$25 was uncommon, thromboprophylaxis with aspirin substantially reduced the odds of incurring a considerable thromboprophylaxis expense.
This study had several limitations. First, the out-of-pocket cost question was added to the trial’s case report forms 9 months after the trial began. Despite this delay, which meant that there were 2015 fewer eligible respondents, the sample size provided sufficient power to detect between-group differences. Second, 2486 patients did not report their satisfaction with the medication and 5488 patients did not report their out-of-pocket costs. While we did observe significant demographic differences between respondents and non-respondents, our sensitivity analyses suggest that these differences were immaterial and the estimates were robust to non-responsiveness. Third, we did not explicitly measure post-discharge adherence and thus cannot assess if satisfaction with the medication and out-of-pocket costs are associated with such adherence. However, previous research has demonstrated higher post-discharge adherence with aspirin compared with low-molecular-weight heparin25. Finally, patients can receive many hospital bills after a fracture and incur many costs. Recall bias and a lack of awareness of which expenses are related to thromboprophylaxis are other limitations but were likely non-differential between treatment arms.
This study also had a number of strengths. It was performed at 21 trauma centers in the U.S. and Canada, increasing the generalizability of the findings. In conjunction with the trial’s clinical results12, these findings indicate that aspirin appears to be a reasonable choice for thromboprophylaxis in terms of clinical outcomes and likely has advantages in patient satisfaction and out-of-pocket costs.
Previous research demonstrated that thromboprophylaxis with aspirin is similar to the more commonly prescribed low-molecular-weight heparin with respect to efficacy and safety12. Based on our results, we concluded that patients assigned to aspirin were also substantially more satisfied with their medication. Furthermore, the lower odds of out-of-pocket costs of ≥$25 associated with aspirin might help mitigate a potential health equity issue related to thromboprophylaxis in this patient population. Clinicians should consider these advantages of aspirin when selecting thromboprophylaxis after orthopaedic trauma. Future research is required to determine if these satisfaction and cost benefits apply to other patient populations.
Appendix
Supporting material provided by the authors is posted with the online version of this article as a data supplement at jbjs.org (http://links.lww.com/JBJS/H894).
Acknowledgments
Note:
Members of the Major Extremity Trauma Research Consortium (METRC) include:
Methods Center at Johns Hopkins Bloomberg School of Public Health: Anthony R. Carlini, MS, Brianna E. Fowler, MS, BS (no longer affiliated), Tara J. Taylor, MPH, Stephen T. Wegener, PhD, Elias Weston-Farber, BS
Patient Stakeholder Advisory Committee Members: Steven Craig Herndon Jr., Debra Marvel, MA
Adjudication Committee Members: Gregory J. Jurkovich, MD, Christopher Lee, MD, Ajai K. Malhotra, MD, FACS, Matthew D. Riedel, MD
Data and Safety Monitoring Board Members: Thomas A. DeCoster, MD, Gregory M. Vercellotti, MD, Jeffrey L. Wells, AA
Allegheny General Hospital: Edward R. Westrick, MD
Atrium Health-Carolinas Medical Center: Michael J. Bosse, MD, A. Britton Christmas, MD, MBA, Kyle William Cunningham, MD, MPH, Joseph R. Hsu, MD, Toan Huynh, MD, David George Jacobs, MD, Laurence B. Kempton, MD, Stephen H. Sims, MD, Christine Churchill, MA
Atrium Health-Wake Forest Baptist: Eben A. Carroll, MD, Sharon Babcock, MD, Preston R. Miller III, MD, Holly T. Pilson, MD, James Brett Goodman, MBA, Martha B. Holden, AAS, AA
Brigham and Women’s Hospital and Massachusetts General Hospital at Harvard Medical School: Michael J. Weaver, MD, John G. Esposito, MD, FRCSC, Samuel Z. Goldhaber, MD, Marilyn Heng, MD, MPH (now affiliated with University of Miami Ryder Trauma Center), Madeline M. McGovern, MD, George C. Velmahos, MD, PhD, Arvind von Keudell, MD, MPH
Brooke Army Medical Center: Jessica C. Rivera, MD, PhD (now affiliated with Louisiana State University)
Dartmouth-Hitchcock Medical Center: Ida Leah Gitajn, MD, MS
Foothills Medical Centre: Prism S. Schneider, MD, PhD, Richard E. Buckley, MD, FRCS
Hamilton General Hospital: Jodi Gallant, MSc, Paula McKay, BSc
Harborview Medical Center at the University of Washington: Conor P. Kleweno, MD, Julie Agel, MA
Indiana University Health Methodist Hospital: Roman M. Natoli, MD, PhD, Greg E. Gaski, MD (now affiliated with Inova Fairfax Medical Campus), Carrie L. Heincelman, MD (now affiliated with Beth Israel Deaconess Medical Center), Yohan Jang, DO, Luke A. Lopas, MD, Todd O. McKinley, MD, Raveesh Daniel Richard, MD, FAAOS (now affiliated with Denver Health and Hospital Authority), Anthony T. Sorkin, MD, Walter Virkus, MD, Lauren C. Hill, BS, CCRC
Inova Fairfax Medical Campus: Robert A. Hymes, MD, Michael Holzman, MD, Farhanaz Panjshiri, MD, MS (no longer affiliated), Jeff E. Schulman, MD, Lolita Ramsey, PhD, RN, Jaslynn A.N. Cuff, MA (no longer affiliated)
Johns Hopkins Bloomberg School of Public Health: Elliott R. Haut, MD, PhD
McGovern Medical School at The University of Texas Health Science Center at Houston: Stephen J. Warner, MD, PhD, Bryan A. Cotton, MD, MPH, Keyla D. Guevara, CCRC
MetroHealth Medical Center: Jeffrey A. Claridge, MD, MS, Heather A. Vallier, MD, Mary A. Breslin, MPH (no longer affiliated)
R Adams Cowley Shock Trauma Center, University of Maryland School of Medicine: Daniel Connelly, MD, Qasim M. Ghulam, DO, Bryce E. Haac, MD (now affiliated with Intermountain Health), Christopher T. LeBrun, MD (now affiliated with Riverside Community Hospital), Theodore T. Manson, MD, MS, Jason Nascone, MD, Raymond A. Pensy, MD, Andrew N. Pollak, MD, Ugochukwu N. Udogwu, MD, Cynthia Elaine Burke, BS (now affiliated with Penn State Health Milton S. Hershey Medical Center), Yasmin Degani, MPH, Genaro A. DeLeon, MS, Zachary D. Hannan, BS, Kathleen M. Healey, BA, Andrea L. Howe, BS, Dimitrius P. Marinos, BS (no longer affiliated), Phillip C. McKegg, MS, Natasha S. McKibben, BS, Nicolas H. Zingas, BS (now affiliated with Sidney Kimmel Medical College at Thomas Jefferson University)
Rhode Island Hospital at Brown University: Andrew R. Evans, MD
The University of Arizona: Brad M. Askam, MD, Christina Boulton, MD
The University of Tennessee: John C. Weinlein, MD
University of Mississippi Medical Center: Patrick F. Bergin, MD, Eldrin Bhanat, MD, MPH, Matthew E. Kutcher, MD, MS, John Morellato, MBBS, FRCSC, Priyanka V. Nehete, BDS, MPH, Ugur Yener, MD
University of Wisconsin: Paul S. Whiting, MD, Christopher Domes, MD, Gabrielle R. Kuhn, MD
Vanderbilt University Medical Center: Vamshi Gajari, MBBS (no longer affiliated), Andres Fidel Moreno-Diaz, MD, Elsa B. Rodriguez-Baron, MD, Andres Rodriguez-Buitrago, MD (now affiliated with Division of Orthopaedics, Fundacion Santa Fe de Bogota), Daniel J. Stinner, MD, PhD, Karen M. Trochez, BA
The authors thank Peter DePalo Sr., BS, Dartmouth-Hitchcock Medical Center, for patient consent and data entry; Leah C. Kennedy, RN, BScN, Foothills Medical Centre, for patient interaction and data acquisition; Karin Lienhard, PhD, Foothills Medical Centre, for research management; Ryan Martin, MD, Foothills Medical Centre, for patient enrollment; Stephanie C. Yee, BSc, Foothills Medical Centre, for patient enrollment, follow-up, and data collection; Jordan Leonard, BScKin, Hamilton General Hospital, for patient screening, recruitment, data collection, and data entry; Hikmatullah Arif, BS, Harborview Medical Center at the University of Washington, for work as the lead clinical research coordinator; Krista Brown, MS, Indiana University Health Methodist Hospital (no longer affiliated), for work as a research coordinator; Lakye Lenee Deeter, BS, Indiana University Health Methodist Hospital (no longer affiliated), for work as a research coordinator; Neil Sardesai, MD, Indiana University Health Methodist Hospital (now affiliated with Kaiser Permanente), for patient enrollment and data acquisition; Abdulai T. Bangura, BS, R Adams Cowley Shock Trauma Center, University of Maryland School of Medicine (now affiliated with University of Missouri), for patient enrollment and follow up; Haley K. Demyanovich, MS, R Adams Cowley Shock Trauma Center, University of Maryland School of Medicine, for patient enrollment, data acquisition, data entry, and data cleaning; W. Andrew Eglseder, MD, R Adams Cowley Shock Trauma Center, University of Maryland School of Medicine, for patient enrollment; Jayesh Gupta, MD, R Adams Cowley Shock Trauma Center, University of Maryland School of Medicine (now affiliated with Temple University Hospital), for patient enrollment and interaction; Marckenley Isaac, MD, MS, R Adams Cowley Shock Trauma Center, University of Maryland School of Medicine (now affiliated with Mount Sinai Hospitals), for patient enrollment and follow-up; Alexandra Mulliken, BS, R Adams Cowley Shock Trauma Center, University of Maryland School of Medicine (no longer affiliated), for patient screening, enrollment, and data collection; M.J. Crisco, RN, CRC, Rhode Island Hospital at Brown University, for patient enrollment, data collection, and follow-up; Stephanie N. Lueckel, MD, ScM, Rhode Island Hospital at Brown University, for patient enrollment; Gregory A. Brown, MD, PhD, for work as a Data Safety Monitoring Board member; Stephen Breazeale, PhD, CRNP, for work as a study stakeholder group member with compensation; Stephen N. Fisher, MD, PhD, for work as a study stakeholder group member; Jason R. Wild, MD, The University of Arizona (no longer affiliated), for patient interaction; Michael J. Beebe, MD, The University of Tennessee, for patient enrollment and data collection; Rajinder M. Khanna, MD, University of Mississippi Medical Center, for patient enrollment, data entry, and data collection; Deborah Brauer, MS, University of Wisconsin (no longer affiliated), for data acquisition; and Robert H. Boyce, MD, Vanderbilt University Medical Center, for patient interaction.
Footnotes
A list of the METRC members is included as a note at the end of the article.
Disclosure: This study was funded by the Patient-Centered Outcomes Research Institute (PCS-1511-32745 and DI-2022C3-29701). The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJS/H893).
Contributor Information
Collaborators: Anthony R. Carlini, Brianna E. Fowler, Tara J. Taylor, Stephen T. Wegener, Elias Weston-Farber, Steven Craig Herndon, Debra Marvel, Gregory J. Jurkovich, Christopher Lee, Ajai K. Malhotra, Matthew D. Riedel, Thomas A. DeCoster, Gregory M. Vercellotti, Jeffrey L. Wells, Edward R. Westrick, Michael J. Bosse, A. Britton Christmas, Kyle William Cunningham, Joseph R. Hsu, Toan Huynh, David George Jacobs, Laurence B. Kempton, Stephen H. Sims, Christine Churchill, Eben A. Carroll, Sharon Babcock, Preston R. Miller, Holly T. Pilson, James Brett Goodman, Martha B. Holden, Michael J. Weaver, John G. Esposito, Samuel Z. Goldhaber, Marilyn Heng, Madeline M. McGovern, George C. Velmahos, Arvind von Keudell, Jessica C. Rivera, Ida Leah Gitajn, Prism S. Schneider, Richard E. Buckley, Jodi Gallant, Paula McKay, Conor P. Kleweno, Julie Agel, Roman M. Natoli, Greg E. Gaski, Carrie L. Heincelman, Yohan Jang, Luke A. Lopas, Todd O. McKinley, Raveesh Daniel Richard, Anthony T. Sorkin, Walter Virkus, Lauren C. Hill, Robert A. Hymes, Michael Holzman, Farhanaz Panjshiri, Jeff E. Schulman, Lolita Ramsey, Jaslynn A.N. Cuff, Elliott R. Haut, Stephen J. Warner, Bryan A. Cotton, Keyla D. Guevara, Jeffrey A. Claridge, Heather A. Vallier, Mary A. Breslin, Daniel Connelly, Qasim M. Ghulam, Bryce E. Haac, Christopher T. LeBrun, Theodore T. Manson, Jason Nascone, Raymond A. Pensy, Andrew N. Pollak, Ugochukwu N. Udogwu, Cynthia Elaine Burke, Yasmin Degani, Genaro A. DeLeon, Zachary D. Hannan, Kathleen M. Healey, Andrea L. Howe, Dimitrius P. Marinos, Phillip C. McKegg, Natasha S. McKibben, Nicolas H. Zingas, Andrew R. Evans, Brad M. Askam, Christina Boulton, John C. Weinlein, Patrick F. Bergin, Patrick F. Bergin, Matthew E. Kutcher, John Morellato, Priyanka V. Nehete, Ugur Yener, Paul S. Whiting, Christopher Domes, Gabrielle R. Kuhn, Vamshi Gajari, Andres Fidel Moreno-Diaz, Elsa B. Rodriguez-Baron, Andres Rodriguez-Buitrago, Daniel J. Stinner, Karen M. Trochez, Peter DePalo, Leah C. Kennedy, Karin Lienhard, Ryan Martin, Stephanie C. Yee, Jordan Leonard, Hikmatullah Arif, Krista Brown, Lakye Lenee Deeter, Neil Sardesai, Abdulai T. Bangura, Haley K. Demyanovich, W. Andrew Eglseder, Jayesh Gupta, Marckenley Isaac, Alexandra Mulliken, MJ. Crisco, Stephanie N. Lueckel, Gregory A. Brown, Stephen Breazeale, Stephen N. Fisher, Jason R. Wild, Michael J. Beebe, Rajinder M. Khanna, Deborah Brauer, and Robert H. Boyce
Data Sharing
A data-sharing statement is provided with the online version of the article (http://links.lww.com/JBJS/H895).
References
- 1.Barrera LM, Perel P, Ker K, Cirocchi R, Farinella E, Morales Uribe CH. Thromboprophylaxis for trauma patients. Cochrane Database Syst Rev. 2013 Mar 28;(3):CD008303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geerts WH, Code KI, Jay RM, Chen E, Szalai JP. A prospective study of venous thromboembolism after major trauma. N Engl J Med. 1994 Dec 15;331(24):1601-6. [DOI] [PubMed] [Google Scholar]
- 3.Knudson MM, Ikossi DG, Khaw L, Morabito D, Speetzen LS. Thromboembolism after trauma: an analysis of 1602 episodes from the American College of Surgeons National Trauma Data Bank. Ann Surg. 2004 Sep;240(3):490-6, discussion 496-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haut ER, Chang DC, Pierce CA, Colantuoni E, Efron DT, Haider AH, Cornwell EE, 3rd, Pronovost PJ. Predictors of posttraumatic deep vein thrombosis (DVT): hospital practice versus patient factors-an analysis of the National Trauma Data Bank (NTDB). J Trauma. 2009 Apr;66(4):994-9, discussion 999-1001. [DOI] [PubMed] [Google Scholar]
- 5.Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, Ortel TL, Pauker SG, Colwell CW, Jr. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 Feb;141(2)(Suppl):e278S-325S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sagi HC, Ahn J, Ciesla D, Collinge C, Molina C, Obremskey WT, Guillamondegui O, Tornetta P, 3rd; Orthopaedic Trauma Association Evidence Based Quality Value and Safety Committee. Venous thromboembolism prophylaxis in orthopaedic trauma patients: a survey of OTA member practice patterns and OTA expert panel recommendations. J Orthop Trauma. 2015 Oct;29(10):e355-62. [DOI] [PubMed] [Google Scholar]
- 7.Rogers FB, Cipolle MD, Velmahos G, Rozycki G, Luchette FA. Practice management guidelines for the prevention of venous thromboembolism in trauma patients: the EAST practice management guidelines work group. J Trauma. 2002 Jul;53(1):142-64. [DOI] [PubMed] [Google Scholar]
- 8.Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 Feb;141(2)(Suppl):7S-47S. Errata in: Chest. 2012 Apr;141(4):1129, and Chest. 2012 Dec;142(6):1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Popoola VO, Lau BD, Tan E, Shaffer DL, Kraus PS, Farrow NE, Hobson DB, Aboagye JK, Streiff MB, Haut ER. Nonadministration of medication doses for venous thromboembolism prophylaxis in a cohort of hospitalized patients. Am J Health Syst Pharm. 2018 Mar 15;75(6):392-7. [DOI] [PubMed] [Google Scholar]
- 10.Popoola VO, Tavakoli F, Lau BD, Lankiewicz M, Ross P, Kraus P, Shaffer D, Hobson DB, Aboagye JK, Farrow NA, Haut ER, Streiff MB. Exploring the impact of route of administration on medication acceptance in hospitalized patients: Implications for venous thromboembolism prevention. Thromb Res. 2017 Dec;160:109-13. [DOI] [PubMed] [Google Scholar]
- 11.Haac BE, O’Hara NN, Mullins CD, Stein DM, Manson TT, Johal H, Castillo R, O’Toole RV, Slobogean GP. Patient preferences for venous thromboembolism prophylaxis after injury: a discrete choice experiment. BMJ Open. 2017 Aug 11;7(8):e016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Toole RV, Stein DM, O’Hara NN, Frey KP, Taylor TJ, Scharfstein DO, Carlini AR, Sudini K, Degani Y, Slobogean GP, Haut ER, Obremskey W, Firoozabadi R, Bosse MJ, Goldhaber SZ, Marvel D, Castillo RC; Major Extremity Trauma Research Consortium (METRC). Aspirin or Low-Molecular-Weight Heparin for Thromboprophylaxis after a Fracture. N Engl J Med. 2023 Jan 19;388(3):203-13. [DOI] [PubMed] [Google Scholar]
- 13.O’Toole RV, Stein DM, Frey KP, O’Hara NN, Scharfstein DO, Slobogean GP, Taylor TJ, Haac BE, Carlini AR, Manson TT, Sudini K, Mullins CD, Wegener ST, Firoozabadi R, Haut ER, Bosse MJ, Seymour RB, Holden MB, Gitajn IL, Goldhaber SZ, Eastman AL, Jurkovich GJ, Vallier HA, Gary JL, Kleweno CP, Cuschieri J, Marvel D, Castillo RC; METRC. PREVENTion of CLots in Orthopaedic Trauma (PREVENT CLOT): a randomised pragmatic trial protocol comparing aspirin versus low-molecular-weight heparin for blood clot prevention in orthopaedic trauma patients. BMJ Open. 2021 Mar 24;11(3):e041845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atkinson MJ, Sinha A, Hass SL, Colman SS, Kumar RN, Brod M, Rowland CR. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004 Feb 26;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.John MP, 2nd, Streufert BD, Downes K, Chase CB, Mir HR. A prospective randomized controlled trial comparing enoxaparin & rivaroxaban for venous thromboembolism prophylaxis in orthopaedic trauma. J Orthop Trauma. 2022 Dec 1;36(12):615-22. [DOI] [PubMed] [Google Scholar]
- 16.Hendriks T, McGregor S, Rakesh S, Robinson J, Ho KM, Baker R. Patient satisfaction after conversion from warfarin to direct oral anticoagulants for patients on extended duration of anticoagulation for venous thromboembolism - The SWAN Study. PLoS One. 2020 Jun 4;15(6):e0234048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.French B, Shotwell MS. Regression models for ordinal outcomes. JAMA. 2022 Aug 23;328(8):772-3. [DOI] [PubMed] [Google Scholar]
- 18.Guntupalli SR, Brennecke A, Behbakht K, Tayebnejad A, Breed CA, Babayan LM, Cheng G, Ramzan AA, Wheeler LJ, Corr BR, Lefkowits C, Sheeder J, Matsuo K, Flink D. Safety and efficacy of apixaban vs enoxaparin for preventing postoperative venous thromboembolism in women undergoing surgery for gynecologic malignant neoplasm: a randomized clinical trial. JAMA Netw Open. 2020 Jun 1;3(6):e207410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nam D, Nunley RM, Johnson SR, Keeney JA, Clohisy JC, Barrack RL. Thromboembolism prophylaxis in hip arthroplasty: routine and high risk patients. J Arthroplasty. 2015 Dec;30(12):2299-303. [DOI] [PubMed] [Google Scholar]
- 20.Barbosa CD, Balp MM, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schousboe JT, Brown GA. Cost-effectiveness of low-molecular-weight heparin compared with aspirin for prophylaxis against venous thromboembolism after total joint arthroplasty. J Bone Joint Surg Am. 2013 Jul 17;95(14):1256-64. [DOI] [PubMed] [Google Scholar]
- 22.Chauhan O, Skedgel C, Pleasance S, Thompson K, Anderson D. The cost analysis of aspirin versus low molecular weight heparin for thromboprophylaxis following total hip arthroplasty. Blood. 2013;122(21):1134. [Google Scholar]
- 23.Dawoud DM, Wonderling D, Glen J, Lewis S, Griffin XL, Hunt BJ, Stansby G, Reed M, Rossiter N, Chahal JK, Sharpin C, Barry P. Cost-utility analysis of venous thromboembolism prophylaxis strategies for people undergoing elective total hip and total knee replacement surgeries in the English National Health Service. Front Pharmacol. 2018 Nov 27;9:1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cain K, Harrison R, Marten C, Fleming N, Taylor J. Comparison of out-of-pocket cost between apixaban and enoxaparin for extended-duration venous thromboembolism prophylaxis in gynecologic oncology patients. Gynecol Oncol. 2021;162:S118-9. [Google Scholar]
- 25.Haac BE, Van Besien R, O’Hara NN, Slobogean GP, Manson TT, O’Toole RV, Johal H, Berger PZ, Reahl GB, Marinos D, Degani Y, Mascarenhas D, Connelly D, Scalea TM, Stein DM. Post-discharge adherence with venous thromboembolism prophylaxis after orthopedic trauma: Results from a randomized controlled trial of aspirin versus low molecular weight heparin. J Trauma Acute Care Surg. 2018 Apr;84(4):564-74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data-sharing statement is provided with the online version of the article (http://links.lww.com/JBJS/H895).