Abstract
Background
In Japan, rituximab (RTX) for adult-onset frequently relapsing (FR)/steroid-dependent (SD) minimal change disease (MCD) is not explicitly reimbursed by insurance, and its standard regimen has not been established.
Methods
We conducted a cross-sectional web-based survey between November and December 2021. The participants were nephrologists certified by the Japanese Society of Nephrology and answered 7 items about RTX for adult MCD. Factors related to the experience of RTX administration at their facilities were estimated by generalized estimating equations.
Results
Of 380 respondents, 181 (47.6%) reported the experience of RTX use for adult MCD at their current facilities. Those who worked at university hospitals (vs. non-university hospitals, proportion difference 13.7%) and at facilities with frequent kidney biopsies (vs. 0 cases/year, 19.2% for 1–40 cases/year; 37.9% for 41–80 cases/year; 51.9% for ≥ 81 cases/year) used RTX more frequently. Of 181 respondents, 28 (15.5%) answered that there was no insurance coverage for RTX treatment. Of 327 respondents who had the opportunity to treat MCD, which was a possible indication for RTX, 178 (54.4%) indicated withholding of RTX administration. The most common reason was the cost due to lack of insurance coverage (141, 79.2%). Regarding RTX regimens for FR/SD MCD, introduction treatment with a single body surface area-based dose of 375 mg/m2 and maintenance treatment with a 6-month interval were the most common.
Conclusion
This survey revealed the nephrologists’ characteristics associated with RTX use, the barriers to RTX use, and the variation in the regimens for adult MCD in Japan.
Introduction
The medical treatment of minimal change disease (MCD) with frequent relapses and steroid dependency is a major challenge in clinical practice. As a result, achieving adequate control of MCD remains difficult even when combined therapy with corticosteroids and immunosuppressive agents is used [1]. Rituximab (RTX) is one of the currently accepted drugs as an effective treatment for frequently relapsing (FR)/steroid-dependent (SD) MCD. RTX has been successfully verified for its efficacy and safety in a randomized controlled trial (RCT) for childhood-onset FR/SD MCD [2] and has been positioned as one of the treatment options for FR/SD nephrotic syndrome in the latest Kidney Disease: Improving Global Outcomes (KDIGO) guidelines [3]. In the Japanese guidelines, RTX is listed in the treatment algorithm for MCD in adults [4, 5]. Nevertheless, the application of RTX in adult-onset FR/SD MCD in Japan remains uncertain due to the lack of supporting evidence for its efficacy and its exclusion from explicit medical reimbursement.
RTX for FR/SD MCD in adults was suggested to induce complete remission in more than 90% of cases and subsequently prevent relapse by a meta-analysis of several small observational studies [6]. However, the efficacy of RTX has not been established because no RCTs have been conducted for adult-onset cases. In addition, reimbursement of RTX in Japan is only approved for childhood-onset FR/SD MCD. Clarifying the current Japanese experience with RTX and the existing barriers to its use in adult cases may be useful in developing a treatment protocol and generating evidence for insurance reimbursement of RTX therapy for adult-onset FR/SD MCD.
Therefore, we conducted a web-based survey of Japanese nephrologists to analyze RTX treatment patterns and the reasons for barriers to its use for MCD in adults.
Materials and methods
Study design and setting
This was a cross-sectional web-based survey using Microsoft Forms (Microsoft, Redmond, WA, USA) conducted between November 15 and December 31, 2021. Detailed methods are summarized elsewhere [7]. This study was an anonymous survey of healthcare professionals to describe practices regarding primary nephrotic syndrome and was considered outside the scope of ethical review according to the Ethical Guidelines for Medical and Biological Research Involving Human Subjects [8]. The possibility of academic publication of the survey results was described at the beginning of the questionnaire, and only those who provided consent to complete the survey were included.
Participants
The target population was nephrologists certified by the Japanese Society of Nephrology (JSN). There were 5777 certified nephrologists at the time of the survey [9]. The sampling method used was convenience sampling, using the mailing list for JSN members or direct mailing by members of the working group to nephrologists of their acquaintance [7].We excluded respondents who were not currently involved in caring for patients with primary nephrotic syndrome in an outpatient setting and respondents who did not provide the identifiable zip code of their affiliation, based on their responses to relevant items in the survey form. The number of clinics (defined as medical facilities having ≤ 20 inpatient beds) and hospitals offering nephrology as a medical specialty in 2020 is 2154 and 1381, respectively [10]. As of July 18, 2023, there are 715 teaching facilities accredited by the JSN [11]. Although the exact number of facilities performing kidney biopsies is unavailable, we believe that the number is close to the number of these accredited teaching facilities.
Data collection methods
The survey consisted of 34 questionnaire items, and 7 items were related to RTX treatment for MCD with/without frequent relapses in adults. All response options were multiple choice, with open-ended responses allowed for part of the items. Each question was asked only for participants who currently had the opportunity to treat patients with the relevant disease with RTX. Details of the 7 question items are shown in S1 Text. Briefly stated, these seven items asked about 1) the experience of RTX administration for adult MCD at the current affiliation, 2) the funding source of RTX treatment for adult MCD at the current affiliation, 3) the intention to withhold RTX for adult MCD that was supposedly to be responsive to RTX, and 4) the RTX regimen for FR cases of MCD (dose, intervals, and frequency during introduction/maintenance period, duration of the maintenance period). Information on participants’ backgrounds, years of experience as a physician, type of affiliation, number of kidney biopsies performed at the affiliation, number of patients with primary nephrotic syndrome treated in an outpatient setting, and zip codes of the affiliation was collected. According to the zip codes, the region and the population density of the affiliation location were identified.
Statistical analyses
Categorical variables are expressed as numbers and percentages. As all items were required to be answered, there were no missing values. Open-ended responses were reviewed by at least two nephrologists and classified into either existing choice categories or newly created categories. A comparison between groups with and without the experience of RTX use at the current affiliation (the RTX administration group and the non-administration group, respectively) was performed by Pearson’s chi-square test. To explore factors associated with the experience of RTX use at the current affiliation, a generalized estimating equation with robust variance estimation (with an identity link function and a Gaussian distribution family) was fitted with the following independent variables: years of experience as a physician, type of affiliation, number of kidney biopsies performed at the affiliation, number of patients with primary nephrotic syndrome treated in an outpatient setting, and population density of the location of the affiliation [12]. The proportion difference in usage experience was estimated as an effect measure. Sankey diagrams are presented to provide a graphical overview of the distribution of choices for the introduction and maintenance treatment protocol of FR/SD MCD in adults [13]. All analyses were performed using STATA version 17 (StataCorp LLC, College Station, TX, USA).
Results
Of the overall 434 respondents, 48 were not currently engaged in medical treatment of patients with primary nephrotic syndrome in an outpatient setting, and the zip codes of 6 respondents were not identified. As a result, the data of 380 respondents from 278 facilities were analyzed in this study. The locations of which the ZIP codes (assuming there was only one nephrology provider in that district) were correctly identified among the 278 facilities are shown in Fig 1. The characteristics of these 380 respondents are summarized in Table 1.
Fig 1. Nationwide map of the responding nephrologists’ workplace.
Of the 380 respondents, 278 unique, identifiable zip codes for their workplaces were provided, and 268 of which are mapped onto Google My Maps. To view the actual distribution via Google My Maps, please click on the following link: http://tinyurl.com/4j2ct6s9.
Table 1. Respondent characteristics (n = 380).
| n | (%) | |
|---|---|---|
| Experience | ||
| ≤ 10 years | 42 | (11.1%) |
| 11 to 20 years | 151 | (39.7%) |
| 21 to 30 years | 125 | (32.9%) |
| 31 years ≤ | 62 | (16.3%) |
| Affiliation | ||
| General hospital | 201 | (52.9%) |
| University hospital | 153 | (40.3%) |
| Clinic | 26 | (6.8%) |
| Number of kidney biopsies performed at the facility (per year) | ||
| None | 55 | (14.5%) |
| ≤ 40 | 118 | (31.1%) |
| 41 to 80 | 115 | (30.3%) |
| 81 ≤ | 92 | (24.2%) |
| Number of patients with primary nephrotic syndrome treated in an outpatient setting per participant (per month) | ||
| 1 to 4 | 106 | (27.9%) |
| 5 to 14 | 181 | (47.6%) |
| 15 ≤ | 93 | (24.5%) |
| Location of affiliation | ||
| Hokkaido | 8 | (2.1%) |
| Tohoku | 24 | (6.3%) |
| Kanto | 127 | (33.4%) |
| Chubu | 60 | (15.8%) |
| Kinki | 73 | (19.2%) |
| Chugoku | 37 | (9.7%) |
| Shikoku | 8 | (2.1%) |
| Kyushu/Okinawa | 43 | (11.3%) |
1. Characteristics of nephrologists with and without the experience of RTX use for adult MCD and its funding sources at the current facility
Among the respondents, 181 (47.6%) reported the experience of RTX use for adult MCD. Specifically, 33 (8.7%), 115 (30.3%), 24 (6.3%), and nine (2.4%) answered that they had an average number of less than one, 1 to 5, 6 to 20, and over 20 such cases per year, respectively. A total of 199 respondents (52.4%) had no experience with RTX use, and 35 (9.2%) referred their patients to other facilities where RTX was available. The participants’ characteristics grouped by the experience of RTX administration are summarized in Table 2.
Table 2. Characteristics of respondents with and without experience of rituximab use for adult minimal change disease in the current facility (n = 380).
| Administration group | Non-administration group | p value | |
|---|---|---|---|
| (n = 181) | (n = 199) | ||
| Experience | 0.25 | ||
| ≤ 10 years | 19 (10.5%) | 23 (11.6%) | |
| 11 to 20 years | 81 (44.8%) | 70 (35.2%) | |
| 21 to 30 years | 52 (28.7%) | 73 (36.7%) | |
| 31 years ≤ | 29 (16.0%) | 33 (16.6%) | |
| Affiliation | < 0.001 | ||
| Non-university hospital | 77 (42.5%) | 124 (62.3%) | |
| University hospital | 102 (56.4%) | 51 (25.6%) | |
| Clinic | 2 (1.1%) | 24 (12.1%) | |
| Number of kidney biopsies performed at the facility (per year) | < 0.001 | ||
| None | 4 (2.2%) | 51 (25.6%) | |
| ≤ 40 | 38 (21.0%) | 80 (40.2%) | |
| 41 to 80 | 70 (38.7%) | 45 (22.6%) | |
| 81 ≤ | 69 (38.1%) | 23 (11.6%) | |
| Number of patients with primary nephrotic syndrome treated in an outpatient setting per participant (per month) | < 0.001 | ||
| 1 to 4 | 27 (14.9%) | 79 (39.7%) | |
| 5 to 14 | 92 (50.8%) | 89 (44.7%) | |
| 15 ≤ | 62 (34.3%) | 31 (15.6%) | |
| Location of affiliation | < 0.001 | ||
| Hokkaido | 5 (2.8%) | 3 (1.5%) | |
| Tohoku | 19 (10.5%) | 5 (2.5%) | |
| Kanto | 71 (39.2%) | 56 (28.1%) | |
| Chubu | 34 (18.8%) | 26 (13.1%) | |
| Kinki | 29 (16.0%) | 44 (22.1%) | |
| Chugoku | 5 (2.8%) | 32 (16.1%) | |
| Shikoku | 3 (1.7%) | 5 (2.5%) | |
| Kyushu/Okinawa | 15 (8.3%) | 28 (14.1%) | |
| Population density of the location of the affiliation (per square kilometer) | < 0.001 | ||
| ≤ 999 | 52 (28.7%) | 85 (42.7%) | |
| 1000 to 5000 | 55 (30.4%) | 55 (27.6%) | |
| 5001 to 9999 | 20 (11.0%) | 30 (15.1%) | |
| 10000 ≤ | 54 (29.8%) | 29 (14.6%) |
From the multivariable analysis (Table 3), the respondents who worked at university hospitals used RTX more frequently than those at non-university hospitals (proportion difference 13.7%, 95% confidence interval [CI] 1.7–25.7%, p = 0.03). Those who belonged to a facility where more kidney biopsies were performed also used RTX more frequently (with 0 cases/year as the reference, 19.2% [95% CI 3.9–34.5%, p = 0.01] for 1–40 cases/year; 37.9% [21.2–54.6%, p < 0.01] for 41–80 cases/year; 51.9% [33.9–69.9%, p < 0.01] for ≥ 81 cases/year).
Table 3. Analysis of factors associated with the usage experience of rituximab in the current facility (n = 380).
| Proportion difference, point estimate | 95% confidence interval | p value | |
|---|---|---|---|
| Experience | |||
| ≤ 10 years | Reference | ||
| 11 to 20 years | 0.065 | (-0.066, 0.197) | 0.33 |
| 21 to 30 years | -0.078 | (-0.220, 0.064) | 0.28 |
| 31 years ≤ | 0.051 | (-0.107, 0.209) | 0.53 |
| Affiliation | |||
| Non-university hospital | Reference | ||
| University hospital | 0.137 | (0.017, 0.257) | 0.03 |
| Clinic | 0.017 | (-0.153, 0.187) | 0.85 |
| Number of kidney biopsies performed at the facility (per year) | |||
| None | Reference | ||
| ≤ 40 | 0.192 | (0.039, 0.345) | 0.01 |
| 41 to 80 | 0.379 | (0.212, 0.546) | < 0.01 |
| 81 ≤ | 0.519 | (0.339, 0.699) | < 0.01 |
| Number of patients with primary nephrotic syndrome treated in an outpatient setting per participant (per month) | |||
| 1 to 4 | Reference | ||
| 5 to 14 | 0.093 | (-0.001, 0.187) | 0.05 |
| 15 ≤ | 0.136 | (-0.0003, 0.273) | 0.05 |
| Population density of the location of the affiliation (per square kilometer) | |||
| ≤ 999 | Reference | ||
| 1000 to 5000 | 0.007 | (-0.113, 0.127) | 0.91 |
| 5001 to 9999 | -0.088 | (-0.249, 0.073) | 0.29 |
| 10000 ≤ | 0.105 | (-0.030, 0.241) | 0.13 |
A generalized estimating equation was fit to estimate the proportionate difference in the experience while considering the clustering of nephrologists in the same facility with the independent variables of years of experience as a physician, type of affiliation, number of kidney biopsies performed at the affiliation, number of patients with primary nephrotic syndrome treated in an outpatient setting, and the population density of the location of the affiliation.
Among 181 who had experience with RTX use, the funding source for RTX expenses was as follows: 140 (77.3%) reported that the costs were paid by patients with coverage by medical insurance, 24 (13.3%) reported that the costs were covered by patient self-payment or hospital payment without coverage by insurance, and 4 (2.2%) reported that the costs were covered by research funding from the clinical departments (Table 4).
Table 4. Funding sources of rituximab treatment for adult minimal change disease cases (n = 181).
| n | (%) | |
|---|---|---|
| Patient payment (covered by insurance) | 140 | (77.3%) |
| Unknown | 16 | (8.8%) |
| Hospital payment (not covered by insurance) | 15 | (8.3%) |
| Patient payment (not covered by insurance) | 9 | (5.0%) |
| Research funding from the clinical department | 4 | (2.2%) |
| Research funding from an individual doctor | 0 | (0%) |
| Others | 4 | (2.2%) |
2. Withholding of RTX administration for MCD in adults
Of 327 respondents in charge of adults with MCD that was supposedly responsive to RTX treatment, 178 (54.4%) indicated that they either had withheld or would withhold RTX administration. The most common reason was the inability to afford the financial costs due to lack of medical insurance coverage (141, 79.3%), followed by limited experience in the usage of RTX (31, 17.4%) (Table 5).
Table 5. Reasons for withholding rituximab for minimal change disease in adults (n = 178).
| n | (%) | |
|---|---|---|
| Inability to afford financial costs due to lack of medical insurance coverage | 141 | (79.3%) |
| Limited experience in its usage | 31 | (17.4%) |
| Prohibition of its usage by the facility or the ethical committee due to lack of medical insurance coverage | 19 | (10.7%) |
| Inadequate medical care system in the facility for possible complications of RTX treatment | 12 | (6.7%) |
| Others | 5 | (2.8%) |
3. Regimens of RTX for FR nephrotic syndrome
Of 380 respondents, 124 were excluded because they had limited experience in using RTX and could not answer the questions about the regimens; the data from the remaining 256 respondents were analyzed.
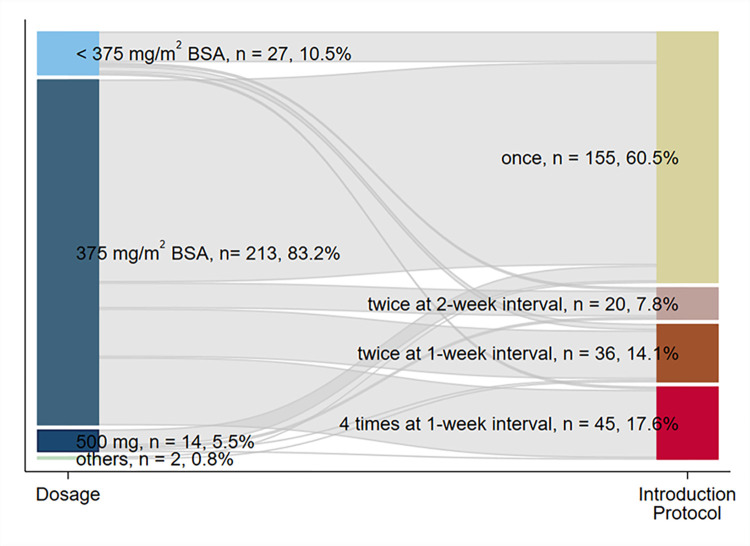
The relationship between the dose and the treatment protocol during the introduction period is illustrated in Fig 2. During the introduction period, the majority of respondents used a body surface area (BSA)-based dosage of 375 mg/m2 with an upper threshold of 500 mg (213, 83.2%). Regarding the administration protocols, 155 (60.5%) responded that they used single-dose administration, followed by four times at one-week intervals (45, 17.6%) and twice at one-week intervals (36, 14.1%). Of the 213 respondents who used a BSA-based dose of 375 mg/m2, 125 used single-dose administration, followed by four times at one-week intervals (42 respondents) and twice at one-week intervals (30 respondents).
Fig 2. The distribution and combination of the rituximab treatment protocol in the introduction period presented by a Sankey diagram.
BSA, body surface area.
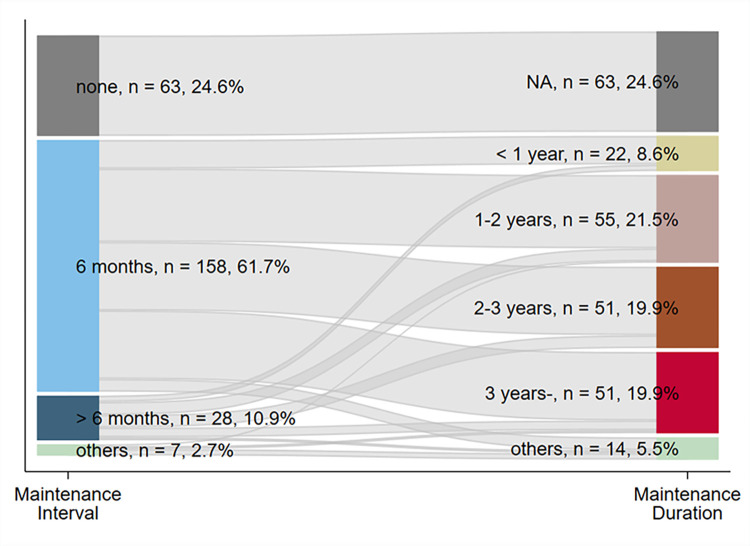
The relationship between the treatment interval and the treatment duration is illustrated in Fig 3. During the maintenance period with sustained remission, 158 respondents (61.7%) indicated that the interval of RTX administration was 6 months, and 63 (24.6%) had no plan for RTX treatment. Four respondents (1.6%, categorized into the “others” category in Fig 2) reported administration based on the CD19/20-positive cell count. The most common total duration of treatment was 1 to 2 years (55, 21.5%), followed by 2 to 3 years and more than 3 years (51, 19.9%, both). Three respondents (1.2%, categorized into the “others” category in Fig 2) determined the duration of treatment based on the CD19/20-positive cell count. The treatment duration had high variability, independent of the treatment interval.
Fig 3. The distribution and combination of the rituximab treatment protocol in the maintenance period presented by a Sankey diagram.
NA, not applicable.
Discussion
In this web-based questionnaire study among nephrologists, we reported the actual situation of RTX treatment for MCD in adults in Japan. Approximately half of the respondents had experience with RTX use for adult MCD at their current affiliation, and many of them belonged to university hospitals or medical institutions where kidney biopsies were commonly performed. While medical insurance covered the majority of RTX treatment expenses, 15.5% of the respondents reported that the cost was not paid by insurance. Moreover, more than half of the respondents had either withheld or would withhold RTX treatment for MCD in adults, mainly due to the inability to afford the financial cost. As for RTX for FR/SD MCD in adults, although there was substantial variability among the treatment protocols, the most common response was introduction treatment with a single BSA-based dose of 375 mg/m2 and maintenance treatment with a 6-month interval.
Several factors may account for the significantly higher RTX utilization among nephrologists affiliated with university hospitals or facilities with a high frequency of kidney biopsies. First, such facilities tend to have specialized medical departments and well-organized treatment provision systems, making access to RTX easier. As such, a protocol for managing infusion reactions that may occur during RTX treatment is also well established. Second, nephrologists working at such facilities are more likely to encounter refractory MCD cases for which RTX is a good treatment indication and, as a result, are more likely to gain experience in RTX use.
The present findings of a nonnegligible percentage of full payment for RTX therapy by institutions or patients and over half of the respondents withholding RTX due to the inability to afford RTX expenses underscore the need for insurance reimbursement for FR/SD MCD in adults. Indeed, an excellent cost-effectiveness of RTX therapy has been reported. RTX therapy with a total of four doses every 6 months with 500 mg has been shown to not only reduce relapse but also cut 56% of medical costs [14]. RTX therapy based on CD19 monitoring has also been shown to have superior benefits in medical costs [15]. On the other hand, this study also revealed that the majority of RTX use in adult FR/SD MCD cases was approved for reimbursement without being assessed as inappropriate by the authorities.
In introduction therapy for FR/SD MCD in adults, a BSA-based dose of 375 mg/m2 was most frequently employed, but its frequency varied, with a single dose being the most common. This Japanese practice pattern differed from the four weekly administrations indicated on the RTX label. First, the four weekly doses indicated on the label were merely extrapolated from the dosing for malignant lymphoma [16]. Second, although studies have reported the effectiveness of this dosing protocol of RTX for MCD in adults [17, 18], there has been growing interest in treatment protocols with less frequent dosing. Indeed, treatment outcomes with BSA-based doses of 375 mg/m2 and single or double doses have been reported for childhood- and adult-onset FR/SD MCD [19–21]. On the other hand, it is noteworthy that several respondents chose a substandard dosage. This reality may reflect findings from both Japanese and foreign studies that reported the effectiveness of a single 200-mg dose of RTX [22, 23].
Regarding maintenance therapy, the large proportion of choices for regular dosing at 6-month intervals may reflect those intervals employed in clinical trials conducted in Japan [20, 21, 24]. In contrast, a certain proportion of the respondents reported no periodic dosing. This may reflect nephrologists’ concerns about the increased incidence of adverse events such as infections, the production of anti-chimeric antibodies to RTX, and hypogammaglobulinemia reported in the pediatric setting [25]. Alternatives to periodic RTX dosing include mycophenolate mofetil [26] and RTX administration based on CD19-positive cell monitoring [27]. In this study, a minority of the respondents opted for the latter option. For the identification of the optimal treatment strategy for adult FR/SD MCD, further studies with patient data to analyze the effectiveness of those RTX regimens and doses are warranted. If patient-reported outcomes could be added to such studies, differences in treatment adherence, satisfaction, and quality of life would also be revealed.
The strength of this study was our enrollment of nephrologists engaged in the management of nephrotic syndrome nationwide to describe the real-world practice of RTX therapy for MCD in adults. In addition, the variation in introduction protocols and the intervals and durations of the maintenance period were straightforwardly demonstrated by Sankey diagrams. At the same time, there are several limitations. First, web-based convenience sampling may have introduced selection bias, and the responses in this study may not accurately reflect actual nationwide patterns. In addition, the total number of individuals who were invited to complete the survey was unavailable. Second, because the survey was anonymous, individuals may have provided more than one response. However, the dedication required for participation makes multiple responses unlikely. Third, there may have been information bias due to self-reporting on the percentages of RTX use, such as recall bias and social desirability bias. To determine a more accurate actual status of RTX use, a large database study linking patient data from medical records with physician data is necessary. Finally, we were not able to examine the regulations regarding RTX use on a facility basis. However, we believe that physician-level summary data would also be useful, for example, the funding sources of RTX treatment in Table 4, because discretionary authority may vary by job position even among nephrologists at the same facility.
Conclusion
This nationwide survey of Japanese nephrologists on RTX practice patterns for MCD in adults revealed the characteristics associated with the treatment propensity, variation in the protocol during the introduction and maintenance periods, and the actual burden of the treatment costs and withholding of RTX in adult MCD cases. Currently, an RCT of RTX for MCD in adults (A-TEAM study) is underway in Japan, and the efficacy of a protocol of two BSA-based doses of 375 mg/m2 weekly during the introduction phase, followed by a single dose during the maintenance phase at 6 months after the introduction, will be clarified [28]. Together with future results from the clinical trial, the present findings are expected to contribute to the establishment of a standard of care with RTX for FR/SD MCD in adults.
Supporting information
(DOCX)
Acknowledgments
We would like to thank Springer Nature Author Services for English language editing.
Data Availability
The minimal data can be found within the article and its accompanying Supporting Information files. However, as the survey results may contain potentially identifiable participant information, they cannot be openly shared. For inquiries concerning access to this data, please reach out to the administrative office of General Incorporated Association PeDAL at admin@pedal.or.jp.
Funding Statement
This study was partly supported by a Grant-in-Aid for Intractable Renal Diseases Research, Research on Rare and Intractable Diseases, and Health and Labor Sciences Research Grants from the Ministry of Health, Labour and Welfare of Japan (ID: 20FC1045). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Vivarelli M, Massella L, Ruggiero B, Emma F. Minimal Change Disease. Clin J Am Soc Nephrol. 2017;12(2):332–45. Epub 2016/12/13. doi: 10.2215/CJN.05000516 ; PubMed Central PMCID: PMC5293332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iijima K, Sako M, Nozu K, Mori R, Tuchida N, Kamei K, et al. Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2014;384(9950):1273–81. Epub 2014/06/27. doi: 10.1016/S0140-6736(14)60541-9 . [DOI] [PubMed] [Google Scholar]
- 3.Kidney Disease: Improving Global Outcomes Glomerular Diseases Work G. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021;100(4S):S1–S276. Epub 2021/09/25. doi: 10.1016/j.kint.2021.05.021 . [DOI] [PubMed] [Google Scholar]
- 4.Narita I OH, Yasuda Y, Shibagaki Y, Wada T, Akiyama S, et al. Evidence-based clinical practice guidelines for nephrotic syndrome 2020: Tokyo Igakusha; 2020. [Google Scholar]
- 5.Wada T, Ishimoto T, Nakaya I, Kawaguchi T, Sofue T, Shimizu S, et al. A digest of the Evidence-Based Clinical Practice Guideline for Nephrotic Syndrome 2020. Clin Exp Nephrol. 2021;25(12):1277–85. Epub 2021/09/27. doi: 10.1007/s10157-021-02098-5 . [DOI] [PubMed] [Google Scholar]
- 6.Xue C, Yang B, Xu J, Zhou C, Zhang L, Gao X, et al. Efficacy and safety of rituximab in adult frequent-relapsing or steroid-dependent minimal change disease or focal segmental glomerulosclerosis: a systematic review and meta-analysis. Clin Kidney J. 2021;14(4):1042–54. Epub 2021/06/08. doi: 10.1093/ckj/sfaa191 ; PubMed Central PMCID: PMC8173623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wada T, Shimizu S, Koizumi M, Sofue T, Nishiwaki H, Sasaki S, et al. Japanese clinical practice patterns of primary nephrotic syndrome 2021: a web-based questionnaire survey of certified nephrologists. Clin Exp Nephrol. 2023. Epub 2023/06/13. doi: 10.1007/s10157-023-02366-6 . [DOI] [PubMed] [Google Scholar]
- 8.Eba J, Nakamura K. Overview of the ethical guidelines for medical and biological research involving human subjects in Japan. Jpn J Clin Oncol. 2022;52(6):539–44. Epub 2022/03/30. doi: 10.1093/jjco/hyac034 ; PubMed Central PMCID: PMC9157286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Japanese Medical Specialty Board. List of the numbers of members and certified physicians of each society (as of August 2021) (In Japanese) 2022 [cited 2023 19 Dec]. Available from: https://jmsb.or.jp/wp-content/uploads/2022/04/gaiho_2021.pdf.
- 10.Ministry of Health, Labour and Welfare. Summary of Static/Dynamic Survey of Medical Institutions and Hospital Report, 2020 (in Japanese) 2022 [cited 2023 19 Dec]. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/iryosd/20/dl/09gaikyo02.pdf.
- 11.Japanese Society of Nephrology. Teaching facilities accredited by the Japanese Society of Nephrology (715 facilities as of July 18, 2023) (In Japanese) 2023 [cited 2023 19 Dec]. Available from: https://jsn.or.jp/jsninfo/about/facilities/.
- 12.Naimi AI, Whitcomb BW. Estimating Risk Ratios and Risk Differences Using Regression. Am J Epidemiol. 2020;189(6):508–10. Epub 2020/03/29. doi: 10.1093/aje/kwaa044 . [DOI] [PubMed] [Google Scholar]
- 13.Lamer A, Laurent G, Pelayo S, El Amrani M, Chazard E, Marcilly R. Exploring Patient Path Through Sankey Diagram: A Proof of Concept. Stud Health Technol Inform. 2020;270:218–22. Epub 2020/06/24. doi: 10.3233/SHTI200154 . [DOI] [PubMed] [Google Scholar]
- 14.Takura T, Takei T, Nitta K. Cost-Effectiveness of Administering Rituximab for Steroid-Dependent Nephrotic Syndrome and Frequently Relapsing Nephrotic Syndrome: A Preliminary Study in Japan. Sci Rep. 2017;7:46036. Epub 2017/04/08. doi: 10.1038/srep46036 ; PubMed Central PMCID: PMC5384079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramachandran R, Bharati J, Rao I, Kashif AW, Nada R, Minz R, et al. Persistent CD-19 depletion by rituximab is cost-effective in maintaining remission in calcineurin-inhibitor dependent podocytopathy. Nephrology (Carlton). 2019;24(12):1241–7. Epub 2018/12/27. doi: 10.1111/nep.13554 . [DOI] [PubMed] [Google Scholar]
- 16.Maloney DG, Grillo-Lopez AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997;90(6):2188–95. Epub 1997/10/06. . [PubMed] [Google Scholar]
- 17.Ren H, Lin L, Shen P, Li X, Xie J, Pan X, et al. Rituximab treatment in adults with refractory minimal change disease or focal segmental glomerulosclerosis. Oncotarget. 2017;8(55):93438–43. Epub 2017/12/08. doi: 10.18632/oncotarget.21833 ; PubMed Central PMCID: PMC5706808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenoglio R, Sciascia S, Beltrame G, Mesiano P, Ferro M, Quattrocchio G, et al. Rituximab as a front-line therapy for adult-onset minimal change disease with nephrotic syndrome. Oncotarget. 2018;9(48):28799–804. Epub 2018/07/11. doi: 10.18632/oncotarget.25612 ; PubMed Central PMCID: PMC6034752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruggenenti P, Ruggiero B, Cravedi P, Vivarelli M, Massella L, Marasa M, et al. Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol. 2014;25(4):850–63. Epub 2014/02/01. doi: 10.1681/ASN.2013030251 ; PubMed Central PMCID: PMC3968490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takei T, Itabashi M, Moriyama T, Kojima C, Shiohira S, Shimizu A, et al. Effect of single-dose rituximab on steroid-dependent minimal-change nephrotic syndrome in adults. Nephrol Dial Transplant. 2013;28(5):1225–32. Epub 2012/12/15. doi: 10.1093/ndt/gfs515 . [DOI] [PubMed] [Google Scholar]
- 21.Iwabuchi Y, Miyabe Y, Makabe S, Nakano M, Manabe S, Karasawa K, et al. Comparison of the response of frequently relapsing steroid-dependent minimal change nephrotic syndrome to rituximab therapy between childhood-onset and adult-onset disease. Medicine (Baltimore). 2018;97(42):e12704. Epub 2018/10/20. doi: 10.1097/MD.0000000000012704 ; PubMed Central PMCID: PMC6211879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujimoto K, Kagaya Y, Kumano S, Fujii A, Tsuruyama Y, Matsuura T, et al. Retrospective single-arm cohort study of steroid-dependent minimal change nephrotic syndrome treated with very low-dose rituximab. Clin Nephrol. 2021;95(1):29–36. Epub 2020/10/20. doi: 10.5414/CN110245 . [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Zhao H, Li X, Qian R, Gao P, Lu S, et al. Efficacy of low-dose rituximab in minimal change disease and prevention of relapse. BMC Nephrol. 2023;24(1):112. Epub 2023/04/27. doi: 10.1186/s12882-023-03092-7 ; PubMed Central PMCID: PMC10134665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyabe Y, Takei T, Iwabuchi Y, Moriyama T, Nitta K. Amelioration of the adverse effects of prednisolone by rituximab treatment in adults with steroid-dependent minimal-change nephrotic syndrome. Clin Exp Nephrol. 2016;20(1):103–10. Epub 2015/07/04. doi: 10.1007/s10157-015-1139-6 . [DOI] [PubMed] [Google Scholar]
- 25.Sinha A, Mathew G, Arushi A, Govindarajan S, Ghanapriya K, Grewal N, et al. Sequential rituximab therapy sustains remission of nephrotic syndrome but carries high risk of adverse effects. Nephrol Dial Transplant. 2023;38(4):939–49. Epub 2022/09/08. doi: 10.1093/ndt/gfac228 . [DOI] [PubMed] [Google Scholar]
- 26.Iijima K, Sako M, Oba M, Tanaka S, Hamada R, Sakai T, et al. Mycophenolate Mofetil after Rituximab for Childhood-Onset Complicated Frequently-Relapsing or Steroid-Dependent Nephrotic Syndrome. J Am Soc Nephrol. 2022;33(2):401–19. Epub 2021/12/10. doi: 10.1681/ASN.2021050643 ; PubMed Central PMCID: PMC8819987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramachandran R, Bharati J, Nada R, Minz R, Kohli HS. Rituximab in maintaining remission in adults with podocytopathy. Nephrology (Carlton). 2020;25(8):616–24. Epub 2020/04/17. doi: 10.1111/nep.13717 . [DOI] [PubMed] [Google Scholar]
- 28.Trials JRoC. IDEC-C2B8 clinical phase III trial (A-TEAM study): Japan Registry of Clinical Trials; 2020 [cited 2023 13 Aug]. Available from: https://jrct.niph.go.jp/en-latest-detail/jRCT2051200045.