Abstract
The glycoproteins expressed by a Zaire species of Ebola virus were analyzed for cleavage, oligomerization, and other structural properties to better define their functions. The 50- to 70-kDa secreted and 150-kDa virion/structural glycoproteins (SGP and GP, respectively), which share the 295 N-terminal residues, are cleaved near the N terminus by signalase. A second cleavage event, occurring in GP at a multibasic site (RRTRR↓) that is likely mediated by furin, results in two glycoproteins (GP1 and GP2) linked by disulfide bonding. This furin cleavage site is present in the same position in the GPs of all Ebola viruses (R[R/K]X[R/K]R↓), and one is predicted for Marburg viruses (R[R/K]KR↓), although in a different location. Based on the results of cross-linking studies, we were able to determine that Ebola virion peplomers are composed of trimers of GP1-GP2 heterodimers and that aspects of their structure are similar to those of retroviruses, paramyxoviruses, and influenza viruses. We also determined that SGP is secreted from infected cells almost exclusively in the form of a homodimer that is joined by disulfide bonding.
Ebola (EBO) viruses are members of the Filoviridae and cause a severe, often fatal form of hemorrhagic fever disease in human and/or nonhuman primates (13). The disease is characterized by a widespread involvement of tissues and the presence of massive amounts of viral antigen in certain organs, such as the liver and spleen (12). An important feature of the infection is an immunosuppression of the host response, as evidenced by a lack of inflammation in infected tissues, degeneration and necrosis of the spleen, and a lack of humoral responses in fatal cases (13, 26). It has been conjectured that the glycoproteins expressed by filoviruses may have an important role in pathogenesis, possibly through immunosuppression of the host (17).
The glycoprotein (GP) gene of filoviruses is the fourth gene (of seven) from the 3′ end of the negative-strand RNA genome (16). All EBO viruses characterized thus far have the same unconventional type of GP gene organization that results in the expression of a secreted, nonstructural glycoprotein (SGP) in preference to the structural GP (17). The SGP is encoded in a single frame (0 frame), while the GP is encoded in two frames (0 and −1 frames). Expression of the GP occurs when the two frames are connected through a transcriptional editing event that results in the insertion of a single extra adenosine (added to a run of seven adenosines).
For Zaire species of EBO virus, the N-terminal 295 residues (including signal sequence) of the SGP (364 total residues) and GP (676 total residues) are identical, but the length and composition of their C-terminal sequences are unique. The GP, a type 1 transmembrane protein, is found on the surface of the infectious virion and functions in attachment structure in the binding and entry of the virus into susceptible cells. Comparisons of GP predicted amino acid sequences for all species of EBO virus show a general conservation in the N-terminal and C-terminal regions (each approximately one-third of the total sequence) and are separated by a highly variable middle section (17, 20). This protein is highly glycosylated, containing large amounts of N- and O-linked glycans, and for Marburg (MBG) virus (another type of filovirus) has been shown to form trimers (5). Just N terminal to the transmembrane anchor sequence of the GP (residues 650 to 672) is a motif (residues 585 to 609) that is highly conserved in filoviruses. This sequence also has a high degree of homology with a motif in the glycoproteins of oncogenic retroviruses that has been shown to be immunosuppressive in vitro (8, 17, 19, 23). Partially overlapping this motif is a heptad repeat sequence (53 residues; positions 541 to 593) that is thought to function in the formation of intermolecular coiled coils in the assembly of trimers, similar to structures predicted for the surface glycoproteins of other viruses (1, 2). Immediately N terminal to this sequence is a predicted fusion peptide (6) followed closely by a putative multibasic cleavage site for a subtilisin/kexin-like convertase, furin (11). Cleavage by furin has been indirectly demonstrated by use of specific inhibitors (21) and is predicted to occur at the last arginine in the sequence RRTRR↓ (position 501 from the beginning of the open reading frame [ORF]). Although the role of the SGP is less defined, recent studies have shown that SGP can bind to neutrophils, while GP binds to endothelial cells (24).
The different binding patterns of SGP and GP suggest that despite having identical N-terminal amino acid sequences (∼280 residues), these glycoproteins are structurally very distinct from one another. To better characterize the structures and roles of SGP and GP, we have biochemically examined these molecules expressed by an EBO virus isolated from the original 1976 outbreak in Zaire.
MATERIALS AND METHODS
Viruses and cell lines.
A Zaire species of EBO virus isolated from a fatal case during the original 1976 outbreak (Mayinga strain) (9) was examined. Low-passage stocks (isolated and cultured two to three times in Vero or Vero E6 cells) were used in all experiments. A low-passage stock of MBG virus (Musoke strain) was used as a control in GP cross-linking studies (4, 10). Vero E6 cells were cultured in Dulbecco’s minimal essential medium containing 10% fetal bovine serum and antibiotics (growth medium) as previously described (3). All work with infectious virions was performed under biosafety level 4 (maximum containment) conditions at the Centers for Disease Control and Prevention, Atlanta, Ga.
Radiolabeling of EBO virus proteins.
E6 cells, cultured in either T-25 flasks or 24-well plates, were infected at a multiplicity of infection of approximately 1.0. Cells were incubated at 37°C for 48 h, washed twice with Hanks’ balanced salt solution, and washed once with cysteine- or glucose-deficient medium. Then labeling medium (cysteine- or glucose-deficient Eagle’s minimal essential medium, 2% dialyzed fetal bovine serum, antibiotics, 100 μCi of either [35S]cysteine or [3H]glucosamine per ml) was added. For purified virion preparations, cultures were incubated for 3 h, an equal volume of growth medium was added, and incubation was continued for an additional 21 h. Virions were purified by pelleting through a cushion of 20% sucrose as previously described (5), and the supernatant fluids were used for certain immunoprecipitation experiments. Pelleted virions were resuspended in 10 mM Tris-HCl (pH 7.6) containing 150 mM NaCl and 3 mM EDTA (TNE buffer) and either used immediately or stored in a nitrogen vapor freezer until needed.
RIP and Western blot assays.
Radioimmunoprecipitation (RIP) assays were performed on Triton X-100 (TX100)-treated, radiolabeled glycoproteins present in tissue culture fluid (TCF) as described elsewhere (17). High-titer polyclonal antisera reactive with EBO virus glycoproteins were mixed with TCF and incubated for 1 h at room temperature; typically 1 μl of antiserum was added for every 100 μl of TCF. Antigen-antibody complexes were isolated by binding to staphylococcal protein A bacterial absorbent (Boehringer Mannheim). Labeled proteins were subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) as previously described (5). Unless otherwise stated, immunoprecipitations were performed with a high-titer anti-EBO virus SGP/GP serum derived from rabbits immunized with glycoproteins from TCF of EBO virus-infected RK13 cells (cultured in Dulbecco’s minimal essential medium containing antibiotics and 2% rabbit serum) extracted by concanavalin A-agarose chromatography. In one experiment, pooled anti-SGP and pooled anti-GP sera (enzyme-linked immunosorbent assay titers of ∼1:12,000 each), derived from separate groups of five guinea pigs genetically immunized by genetic immunization (plasmid DNA injections) of guinea pigs (25), were used in RIP and Western blot assays. Western blot analysis was performed on virion glycoproteins (5) that were either mock treated or digested with a mixture of endoglycosidase F and N-glycosidase F to remove all N-linked glycans, as previously described (15). A chemiluminescence assay system (Kirkegaard & Perry Laboratories or Pierce) was used to visualize protein bands.
Structural analysis of GP and SGP.
A preparation of [35S]cysteine-labeled virions was made 1.7% in TX100 (from 10% in 1× TNE) and incubated at room temperature briefly; then nucleocapsids were pelleted by centrifugation in a microcentrifuge at 14,000 rpm for 5 min at 4°C. The supernatant fluid was removed, and the pellet (nucleocapsids) was resuspended in TNE (same volume as supernatant fluid). Both pellet and supernatant fluid, together with an untreated aliquot of virions, were mixed with an equal volume of 10% SDS, divided equally, and subjected to SDS-PAGE analysis under nonreducing or reducing (addition of 2-mercaptoethanol) conditions.
Oligomers of EBO virion glycoproteins were stabilized by using bis(succinimidylproprionate) (Sigma) to cross-link untreated and TX100-treated [3H]glucosamine-labeled virion preparations as previously reported (4). Cross-linked and untreated virion GP (nonreduced) were separated on 3.5% acrylamide gels (large-format slab gel). Cross-linked forms of phosphorylase b (Sigma) were included for molecular weight markers. Electrophoresis was performed with an SDS-NaPO4 buffer system according to a protocol provided by the manufacturer of the markers (18a).
Low-pH cleavage of immunoprecipitated SGP at a single Asp-Pro sequence (residues 208 and 209) was performed by releasing the protein from bacterial absorbent (by boiling for 3 min in 1% SDS), pelleting bacterial cells, vacuum drying aliquots of the supernatant fluid, resuspending the dried protein in 50 μl of 70% formic acid (freshly prepared from 89%; Sigma) or 50 μl of water (untreated), and incubating the material at 37°C for 40 h. Samples were vacuum dried and analyzed by SDS-PAGE.
N-terminal sequencing was performed on GP isolated from unlabeled virion preparations that had been treated with TX100 and pelleted to remove nucleocapsids or from SDS-disrupted virions digested with endoglycosidases. Proteins were separated by SDS-PAGE and electroblotted (semidry) onto polyvinylidene difluoride membranes. Proteins were stained with a solution of 0.1% Ponceau S in 50% methanol–10% acetic acid; then specific bands were excised and used in determining N-terminal sequences. Sequencing was performed by the Edman degradation technique, using a model PI 2090 Integrated Microsequencing System (Beckman/Porton Instruments, Inc.). Blotting and sequencing procedures were performed according to the manufacturer’s recommendations.
Pulse-chase analysis of SGP secretion.
Cultures of EBO virus-infected and uninfected E6 cells in 24-well plates were pulsed for 10 min with labeling medium ([35S]cysteine) and then chased by being washed twice and incubated with prewarmed (37°C) growth medium. TCF was harvested at 20, 40, 60, 120, and 240 min following the initial addition of labeling medium. Fluids were immediately treated to make them 1% in TX100, 1× in TNE, and 1 mM in phenylmethylsulfonyl fluoride and used immediately in immunoprecipitation assays.
Tunicamycins and brefeldin A treatments.
Infected E6 cells in 24-well plates were treated with cysteine-deficient medium containing 1.0, 0.5, 0.25, 0.125, 0.06, 0.3, and 0.015 μg of tunicamycin or brefeldin A (Sigma) per ml for 30 min. Untreated wells were included as a negative control. Medium containing [35S]cysteine and a corresponding amount of inhibitor was added to appropriate wells and incubated for 3 h, after which time the fluid was removed and treated with TX100 (as above) and used in RIP assays.
Expression of GP and SGP by recombinant DNA techniques.
Recombinant retroviruses (GP pseudotyped) were produced in 293T cells transfected with plasmids pLZRs-Luc-Gfp, pNGVL-MLVgag-pol, and pVR1012-EBO-GP as described elsewhere (24). Supernatants from 24 to 48 h posttransfection were clarified by low-speed centrifugation and either used immediately or stored at −80°C until needed. Production of SGP was performed by transfection of plasmid pVR1012-EBO-SGP (25) into 293 cells and then harvesting TCF containing the secreted SGP.
RESULTS
Glycoproteins present in EBO virions.
From SDS-PAGE analysis of detergent-disrupted EBO virions under reducing and nonreducing conditions (Fig. 1), it was concluded that (i) two glycoproteins are present, (ii) they are membrane associated, and (iii) they are linked by disulfide bonding. These two glycoproteins, named GP1 (∼130 kDa) and GP2 (∼24 kDa), are completely stripped from the virion by 1.7% TX100 in TNE, while the L (polymerase), NP, VP35, and VP30 are strongly associated with the nucleocapsid (pellet). The VP40 and VP24 appear to be incompletely stripped from virions by this treatment. Lane 3 of Fig. 1B shows the two glycoproteins dissociated by 2-mercaptoethanol treatment, and lane 6 shows a ∼150-kDa band corresponding to disulfide-bonded GP1 and GP2 (nonreduced; shown more clearly in Fig. 1A). A small (∼10-kDa) unknown protein band is seen associated with membrane and was not characterized.
FIG. 1.
Effects of nonionic detergent treatment on a purified virion preparation of EBO virus. Shown are autoradiograms of [35S]cysteine-labeled virion proteins run on 6% (A) and 10% (B) acrylamide gels (lanes contain the same samples). Lanes 1 to 3 contain reduced samples of untreated purified EBO virions, pelleted nucleocapsids derived from detergent (TX100) treatment of the same preparation, and membrane-associated proteins (supernatant fluid from the TX100 treatment), respectively; lanes 4 to 6 contain the same samples and in the same order, except that 2-mercaptoethanol was omitted from treatment (nonreduced). Identified in the left margin are the migration positions for the structural proteins; L (polymerase) protein is putative. The question mark in panel B identifies an unknown membrane-associated protein. Asterisks identify glycoprotein bands absent from the nucleocapsid pellet and present in the supernatant fluid.
N-terminal sequences of secreted and virion glycoproteins.
The N-terminal sequences for SGP (isolated from culture supernatants) and GP1 and GP2 (isolated from purified virions) were determined. The predicted sites for signalase cleavage of SGP and GP and the furin cleavage of GP0 to give GP1 and GP2 were all confirmed and are shown in Fig. 2.
FIG. 2.
Diagrammatic representation of SGP and GP molecules of EBO virus (Zaire species isolated in 1976) showing important structural features. The white N-terminal regions of SGP and GP correspond to identical (shared) sequences, while the black C termini identify sequences unique to GP or SGP molecules. The common signalase cleavage sites for both SGP and GP and the furin cleavage site for GP0 (uncleaved form of GP) (↓) were determined by N-terminal sequencing. Also shown are cysteine residues (S), predicted N-linked glycosylation sites (Y-shaped projections), a predicted fusion peptide, a heptad repeat sequence, and a transmembrane anchor sequence. In EBO viruses, the positions of these structures are conserved and their sequences are very similar or, in the case of N-linked glycosylation sites, are at least concentrated in the central region of GP.
Reactivities of antisera to GP and SGP.
Anti-SGP and anti-GP sera were reacted with purified virus preparations in Western blot assays or infected culture supernatant fluids in RIP assays. Western blot assays showed strong cross-reactivity to untreated and endoglycosidase-digested virion GP (Fig. 3A), with some subtle differences in their binding patterns. The anti-GP serum reacts with certain products in the endoglycosidase-treated lane (below the prominent band) that are not detected by anti-SGP. Similarly, anti-SGP antisera reacts with contaminating SGP protein in the preparations that was not detected by the anti-GP serum. The strength of the anti-SGP reaction with the glycosylated SGP is greater than that with the deglycosylated form (amounts blotted were the same), suggesting that N-linked glycans may be important components in the antigenicity of this molecule. Anti-GP serum failed to react with the transmembrane-anchored GP2 molecule, which may reflect a lack of antigenicity and/or a skewing of humoral responses to more dominant epitopes on GP1. In RIP assays using infected culture fluid from which virions had been removed by pelleting, anti-GP showed a much stronger reactivity with GP1, despite the fact that much (>50-fold [unpublished data]) greater amounts of SGP than of free GP are found in the medium. Surprisingly, anti-SGP serum did not react with GP1, while SGP was strongly bound. This result suggests that part of the humoral response to SGP may have been directed to conformational epitopes that are absent from GP; the folding of the highly glycosylated GP1 may also affect the binding of anti-SGP antibodies reactive to GP1 in Western blots. Little or no GP2 was immunoprecipitated with either serum, indicating that GP2 is primarily associated with virions (or membranous particulate matter) in the medium that were removed by centrifugation.
FIG. 3.
Reactivities of polyclonal antibodies to GP and SGP in Western blot and RIP assays. (A) Paired lanes of untreated (−) and endoglycosidase F/N-glycosidase F-digested (+) virion proteins labeled with [35S]cysteine, run on 10% gels, and blotted onto nitrocellulose membranes. Blots were directly exposed to X-ray film (35S) to identify locations of structural proteins and reacted in enzyme immunoassays (chemiluminescent) with guinea pig antisera. Arrows indicate the decreased size of virion GP1 and GP2 glycoproteins due to removal of N-linked glycans. Sera were derived from animals immunized with naked plasmid DNA which directed the expression of GP (α-GP), SGP (α-SGP), or vector-only (α-VEC) products. Asterisks identify SGP and deglycosylated SGP detected by α-SGP. Shown in panel B are lanes containing [35S]cysteine-labeled proteins from an EBO virion preparation (marker) and RIP products immunoprecipitated from supernatant fluids depleted of virions (pelleted through 20% sucrose cushion) with the same antisera as used for panel A.
Oligomerization of GP and SGP.
Results of cross-linking experiments using purified EBO and MBG virions are shown in Fig. 4A. Monomeric, dimeric, and trimeric forms of GP were detected when either untreated or TX100-dissociated virions were cross-linked. The observed approximate molecular mass of the trimeric form of the EBO virus spike structure (450 kDa) is consistent with the predicted size of three GP1-GP2 molecules (150 kDa). The larger size of the MBG spike (∼500 kDa) is also consistent with previous calculations (4), given the greater number of predicted N-linked glycosylation sites (monomer = 170 kDa). Multimers of GP are not held together by disulfide bonding, as nonreducing SDS-PAGE dissociates them into their monomeric form (Fig. 4B).
FIG. 4.
Autoradiograms of multimeric forms of GP and SGP separated by SDS-PAGE. Numbers next to bands indicate monomeric, dimeric, and trimeric forms of GP or monomeric and dimeric forms of SGP. (A) EBO and MBG virion preparations that were untreated (lane 1), cross-linked (lane 2), and TX100 disrupted and then cross-linked (lane 3). Virion glycoproteins were labeled with [3H]glucosamine and separated on a 3.5% gel. Identified on the left are the migration positions and sizes (in kilodaltons) of cross-linked phosphorylase b marker proteins (195 kDa = dimer, 292 kDa = trimer, etc.; note that in 3.5% gels, proteins under 200 kDa migrate anomalously). (B) [3H]glucosamine-labeled EBO virion GP1 (lanes 1 and 3) and SGP immunoprecipitated from TCF (lanes 2 and 4) separated on a 3.5% gel. Samples were run under reducing (lanes 1 and 2) or nonreducing (lanes 3 and 4) conditions. The GP1 band in lane 3 corresponds to the monomeric form seen in lane 1 of panel A. GP and SGP bands in panels A and B were also verified by Coomassie blue staining of gels prior to autoradiography to identify the locations of purified virion proteins. (C) Ten percent gel containing immunoprecipitated [35S]cysteine-labeled SGPs that were either untreated or cleaved with formic acid. Lanes 1 and 3 contain untreated samples of SGP; lanes 2 and 4 contain SGP digested with formic acid (Asp-Pro cleavage; asterisks identify cleavage products). Samples in lanes 1 and 2 were run under reducing conditions, while those in lanes 3 and 4 were nonreduced.
SGP molecules were determined to form homodimers stabilized by disulfide bonding (Fig. 4B), unlike the GP (GP1-GP2) multimers. It can also be seen from Fig. 4B and C that SGP is secreted from infected cells exclusively in the form of a dimer. In an attempt to characterize the orientation of the SGP molecules in the homodimer, low-pH cleavage of a single Asp-Pro linkage was performed and products were analyzed by SDS-PAGE under reducing and nonreducing conditions (Fig. 4C). The N-terminal cleavage fragment (166 residues) would have a peptide predicted to be 19.2 kDa with two predicted N-linked glycans, and the C-terminal fragment (156 residues) would have a 18.1-kDa peptide with four N-linked glycans. The predicted sizes of these fragments should migrate closely, and as shown in Fig. 4C (lane 2), the reduced cleavage products can be seen as a single band (∼25 to 26 kDa) that is absent from the nonreduced preparation (lane 4). Nonreduced formic acid-cleaved SGP yielded two bands (lane 4), the smaller of which corresponds in size to uncleaved SGP. However, we believe that this band contains SGP dimers in which both molecules have been cleaved but migrate at the same position as uncleaved SGP due to disulfide bonding on both sides of these cuts. The possible orientation for the SGP molecules in the dimer would be either a parallel or an antiparallel alignment.
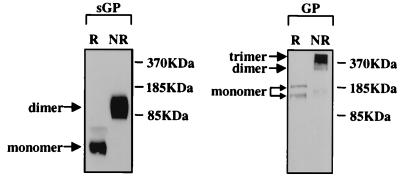
Trimer formation of GP and dimer formation of SGP were also determined to occur when these glycoproteins were expressed separately through recombinant DNA techniques (Fig. 5). This finding demonstrates that folding and assembly of multimers occurs independently of other EBO virus proteins.
FIG. 5.
Biochemical analysis of SGP and GP expressed separately through recombinant DNA techniques. Shown are the results of Western blot assays (chemiluminescent) detecting proteins separated on SDS–4 to 15% gradient polyacrylamide gels. SGP was derived from cell cultures transfected with plasmid DNA containing an unedited ORF sequence, and GP was derived from partially concentrated GP-pseudotyped retrovirus particles (GP expressed from an edited ORF sequence). Preparations were cross-linked as for Fig. 3 and analyzed under reducing (R) and nonreducing (NR) conditions. Migration positions of size markers are shown on the right; monomeric and multimeric forms are indicated on the left.
Secretion of SGP.
A pulse-chase experiment was performed to determine the time required for SGP to be expressed and secreted. Figure 6A shows that SGP is detected in the culture medium after only 20 min (10-min pulse and 10-min chase). After 60 to 80 min, significant amounts of GP1 were detected, while GP2 did not appear until approximately 80 min. Since GP2 is associated with membranes of virions, the earlier appearance of GP1 in the TCF is probably due to its release from the cell surface.
FIG. 6.
Production of EBO virus SGP in pulse-chase and tunicamycin treatment experiments. (A) RIP products obtained from the TCFs of EBO-infected (+) and uninfected (−) E6 cells. Following a 10-min pulse ([35S]cysteine) and chasing with cold medium, samples were taken from 20 to 240 min after the moment the radiolabel was added. (B) RIP products from the TCFs of cultures treated with tunicamycin at 1.0, 0.5, 0.25, 0.125, 0.062, 0.031, or 0.015 μg/ml (lanes 1 to 7, respectively) or untreated (lane 8).
Figure 6B shows the effect of tunicamycin secretion of SGP, and levels of 1 μg/ml or greater clearly inhibit secretion. A small band seen below SGP may be a secreted form lacking N-linked glycans, but the amount of this protein is insignificant compared with the glycosylated SGP produced in untreated cells. Treatment with brefeldin A completely inhibited the secretion of SGP when as little as 0.015 μg/ml was added to cultures (data not shown).
From analyses of amino acid sequences and cleavage events, we have produced a diagrammatic structure for the virion GP heterodimer (Fig. 7), which is based on information obtained from this study and results of prior predictions (6, 16, 17).
FIG. 7.
Diagrammatic representation of the structural GP. Shown is the predicted orientation of the GP1-GP2 heterodimer linked by undetermined disulfide bonding (indicated by the question mark). Intramolecular disulfide bonds that are shown come from prior predictions based on similarities to retrovirus glycoprotein structures (6). See Fig. 2 for other features of the amino acid sequence.
DISCUSSION
SDS-PAGE studies of EBO virion glycoproteins (Fig. 1 and 3A) and N-terminal sequencing have provided definitive evidence that GP is cleaved (from GP0 form) into two molecules, GP1 and GP2. Cleavage of the structural GP gene product of EBO virus was expected for the N-terminal signal sequence and occurs at the predicted site. Cleavage of GP by furin had been predicted from the RRTRR sequence, but identification of the C-terminal fragment in virions was complicated by its comigration with VP24 in SDS-PAGE. However, prior studies detected GP2 in EBO-infected tissue culture fluids (17, 21), and it was suggested that this uncharacterized glycoprotein might be derived from a cleavage event involving GP. Indirect evidence for furin cleavage of EBO virus GP was recently demonstrated through the use of specific inhibitors of furin activity (21).
When GP sequences of all known EBO viruses are aligned, one finds that the furin cleavage site is conserved but differs slightly from one EBO species to another. The furin cleavage sites of Zaire species of EBO viruses that caused outbreaks in Zaire in 1976 (RRTRR) and 1995 (RRARR) and in Gabon in 1994 and 1996 (RRTRR) are identical or very similar, while those of the Ivory Coast (RRKRR) and Sudan (RKRSRR) species have some extra positively charged residues. The site for the Reston species, however, deviates from the other EBO virus sequences in that a lysine occupies the −4 position (RKQKR), which has been viewed as requiring an arginine for efficient cleavage (11), but cleavage at (R/K)X(K/R)R or (R/K)XXX(K/R)R sequences in other proteins has been reported (22). Although the Reston species replicates more slowly than some of the other EBO viruses in tissue culture, ultimately large amounts of infectious virions are produced and when inoculated into monkeys are quite capable of causing severe disease (7). If the cleavage site is less than optimal, it does not deviate greatly from the more commonly seen sequence (RRXRR), and its slower growth in culture may be due to other factors. Further, the Ivory Coast EBO virus (whose GP should be easily cleaved by furin) replicates as slowly as or more slowly than the Reston species. A predicted furin cleavage site for MBG viruses (residues 432 to 435; R[R/K]KR) is found much further N terminal to the fusion peptide (68 residues) than those of EBO viruses; another potential cleavages site is found in the alpha-helix region (residues 558 to 561; RLRR), though it may not be easily cleaved after trimer formation.
From cross-linking studies of EBO virion GP (Fig. 4A), we conclude that it forms trimers that are ∼450 kDa in size. This is in agreement with predictions based on three 150-kDa GP1-GP2 heterodimers and is consistent with trimer formation by MBG virus. We have also determined that SGP is secreted from infected cells almost exclusively in the form of a dimer (Fig. 4); together with structural differences from GP (Fig. 3), this finding suggests that it does not act to decoy the immune response away from infected cells by mimicking the structure of GP. Analysis of SGP fragments cleaved by formic acid treatment (Asp-Pro bond) (Fig. 4C) has led us to conclude that disulfide bonding occurs between cysteines (conserved in all EBO viruses) at both ends of the SGP molecule. This close association of two SGP molecules would undoubtedly confer a unique conformation and may explain why animals genetically immunized to SGP produce antibodies that are not very reactive with GP in RIP assays (Fig. 3B). We have not determined the orientation in which dimerized SGP molecules are held together, but it appears that relative to their N and C termini, they are aligned in a parallel or antiparallel orientation.
The formations of trimers by GP and dimers by SGP occur independently of one another, as was demonstrated by expressing them separately (Fig. 5), and likely occur when these glycoproteins are folded in the endoplasmic reticulum. It is not known if GP1 molecules (released from GP2) that are free in the culture medium maintain a trimerized structure, but given the fact that no disulfide bonding occurs between GP1 molecules, they are probably dislodged from their association with GP2 as monomers. SGP dimers are very rapidly transported through the endoplasmic reticulum and Golgi stacks and secreted into the medium, as quickly as 20 min or less (Fig. 6A), and secretion is inhibited by tunicamycin and brefeldin A. The rapid movement of SGP through the glycosidic pathway may affect processing and could account for the heterogeneity of this glycoprotein, as evidenced by its wide range in size (50 to 70 kDa).
The disulfide bond(s) that links the GP1 and GP2 molecules is yet to be identified (as are those holding the SGP dimer together). However, if the predicted intramolecular disulfide bonds of GP2 are correct, and assuming that the cysteines located in the transmembrane anchor sequence are unavailable for bonding to GP1, then only one cysteine in GP2 should be available for bonding with one of the five cysteines in GP1 (Fig. 7). The association of GP1 and GP2 in a heterodimer would position the hydrophobic N terminus of GP1 close to the hydrophobic GP2. The C terminus of GP1 is hydrophilic and highly glycosylated and has mucin-like characteristics (due to O-linked glycans). An extended mucin-like property would tend to project this end of the molecule away from the membrane-anchored GP2, where it can interact with receptors in an aqueous environment. The major role of the GP trimer is thus one of receptor binding, but it could also function to protect the virion from immune responses through its extensive glycosylation, as filovirus virions are resistant to neutralization by antibody (13). The role of GP2 appears to be one of trimer assembly, positioning of GP1 for receptor binding and virus entry. GP2 contains the putative fusion peptide, and in vitro studies have shown that in the presence of calcium, a synthetic version of the fusion peptide (GAAIGLAWIPYFGPAAE) is efficiently inserted into membranes containing phosphatidylinositol and fuses them (14). The GP2 molecule is predicted to form disulfide bonds between cysteine residues immediately flanking the fusion peptide, which would position the fusion peptide in front of the coiled coils in the trimer and, following some conformational change, would enter into a fusogenic state (2).
We conclude from our structural data that SGP has a function separate from that of GP, a view supported by the results of binding studies using SGP produced from plasmid-transfected cells and a GP-pseudotyped murine retrovirus (24). These studies demonstrated that SGP binds to neutrophils (via CD16b, the Fcγ receptor) and not to endothelial cells, whereas the opposite pattern of cell binding was found for the GP-pseudotyped vector. In addition, there were indications that SGP binding may also inhibit neutrophil activation, which may influence inflammatory responses that normally provide innate immunity. Such an effect in EBO virus disease in human and nonhuman primates would likely contribute to pathogenesis, especially in light of the large amount of SGP circulating in the blood during acute infections and the lack of inflammation in tissues (26). It is also conceivable that in addition of neutrophils, SGP also binds to other cell types and/or soluble factors. One cannot overlook a possible role for GP in immunosuppression, however, since it contains a potential immunosuppressive motif in GP2 (17, 19).
It is clear from our study and prior investigations that the GP genes of EBO viruses have evolved to express two glycoproteins that have distinctly separate roles and that this aspect of their evolution has occurred independently of the MBG virus lineage (18). Aspects of the EBO virion spike structure are comparable to the attachment/glycoprotein structures of viruses in the families Retroviridae, Paramyxoviridae, and Orthomyxoviridae, indicating similar functions in virus entry. Thus, the role for GP is one of targeting EBO virions into specific cell types that allow replication and spread of the virus and entry via membrane fusion. The role of SGP, on the other hand, appears to be more subtle. The large amount of SGP produced by EBO viruses may be linked to some process in the infection of their natural hosts, but it might also have a pathogenic role outside of the natural host. By studying the mechanisms EBO viruses have evolved to circumvent immune defenses, investigators not only can discover aspects of their biology but also can use these viruses (or virus genes) as tools in the study of human immune responses. In the future, we hope to further characterize the in vivo and in vitro effects of these molecules and gain clearer insights into their three-dimensional structures.
ACKNOWLEDGMENTS
We thank John P. O’Connor for editing the text of the manuscript, Danny L. Jue for assistance in protein chemistry, and Stuart T. Nichol for valued discussions.
REFERENCES
- 1.Chambers P, Pringle C R, Easton A J. Heptad repeat sequences are located adjacent to hydrophobic regions in several types of virus fusion glycoproteins. J Gen Virol. 1990;71:3075–3080. doi: 10.1099/0022-1317-71-12-3075. [DOI] [PubMed] [Google Scholar]
- 2.Chan D C, Fass D, Berger J M, Kim P S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 3.Elliott L H, Kiley M P, McCormick J B. Descriptive analysis of Ebola virus proteins. Virology. 1985;147:169–176. doi: 10.1016/0042-6822(85)90236-3. [DOI] [PubMed] [Google Scholar]
- 4.Feldmann H, Will C, Schikore M, Slenczka W, Klenk H-D. Glycosylation and oligomerization of the spike protein of Marburg virus. Virology. 1991;182:353–356. doi: 10.1016/0042-6822(91)90680-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldmann H, Nichol S T, Klenk H-D, Peters C J, Sanchez A. Characterization of filoviruses based on differences in structure and antigenicity of the virion glycoprotein. Virology. 1994;199:469–473. doi: 10.1006/viro.1994.1147. [DOI] [PubMed] [Google Scholar]
- 6.Gallaher W R. Similar structural models of the transmembrane proteins of Ebola and avian sarcoma viruses. Cell. 1996;85:477–478. doi: 10.1016/s0092-8674(00)81248-9. [DOI] [PubMed] [Google Scholar]
- 7.Jahrling P B, Geisbert T W, Jaax N K, Hanes M A, Ksiazek T G, Peters C J. Experimental infection of cynomolgus macaques with Ebola-Reston filoviruses from the 1989–1990 U.S. epizootic. Arch Virol. 1996;11:115–134. doi: 10.1007/978-3-7091-7482-1_11. [DOI] [PubMed] [Google Scholar]
- 8.Kadota J-I, Cianciolo G J, Snyderman R. A synthetic peptide homologous to retroviral transmembrane envelope proteins depresses protein kinase C mediated lymphocyte proliferation and directly inactivated protein kinase C: a potential mechanism for immunosuppression. Microbiol Immunol. 1991;35:443–459. doi: 10.1111/j.1348-0421.1991.tb01575.x. [DOI] [PubMed] [Google Scholar]
- 9.Kiley M P, Regnery R L, Johnson K M. Ebola virus: identification of virion structural proteins. J Gen Virol. 1980;49:333–341. doi: 10.1099/0022-1317-49-2-333. [DOI] [PubMed] [Google Scholar]
- 10.Kiley M P, Cox N J, Elliott L H, Sanchez A, DeFries R, Buchmeier M J, Richman D D, McCormick J B. Physicochemical properties of Marburg virus: evidence for three distinct virus strains and their relationship to Ebola virus. J Gen Virol. 1988;69:1957–1967. doi: 10.1099/0022-1317-69-8-1957. [DOI] [PubMed] [Google Scholar]
- 11.Klenk H-D, Garten W. Host cell proteases controlling virus pathogenicity. Trends Microbiol. 1994;2:39–43. doi: 10.1016/0966-842x(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 12.Ksiazek T G, Rollin P E, Jahrling P B, Johnson E, Dalgard D W, Peters C J. Enzyme immunosorbent assay for Ebola virus antigens in tissues of infected primates. J Clin Microbiol. 1992;30:947–950. doi: 10.1128/jcm.30.4.947-950.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters C J, Sanchez A, Rollin P E, Ksiazek T G, Murphy G A. Filoviridae: Marburg and Ebola viruses. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven Press; 1996. pp. 1161–1176. [Google Scholar]
- 14.Ruiz-Argüello M B, Goñi F M, Pereira F B, Nieva J L. Phosphatidylinositol-dependent membrane fusion induced by a putative fusogenic sequence of Ebola virus. J Virol. 1998;72:1775–1781. doi: 10.1128/jvi.72.3.1775-1781.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez A, Frey T K. Vaccinia-vectored expression of the rubella virus structural proteins and characterization of the E1 and E2 glycosidic linkages. Virology. 1991;183:636–646. doi: 10.1016/0042-6822(91)90993-l. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez A, Kiley M P, Holloway B P, Auperin D D. Sequence analysis of the Ebola virus genome: organization, genetic elements, and comparison with the genome of Marburg virus. Virus Res. 1993;29:215–240. doi: 10.1016/0168-1702(93)90063-s. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez A, Trappier S G, Mahy B W J, Peters C J, Nichol S T. The virion glycoproteins of Ebola viruses are encoded in two reading frames and are expressed through transcriptional editing. Proc Natl Acad Sci USA. 1996;93:3602–3607. doi: 10.1073/pnas.93.8.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez A, Trappier S G, Ströher U, Nichol S T, Bowen M D, Feldmann H. Variation in the glycoprotein and VP35 genes of Marburg virus strains. Virology. 1998;240:138–146. doi: 10.1006/viro.1997.8902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Sigma Chemical Co. Technical bulletin no. MWS-877X. St. Louis, Mo: Sigma Chemical Co.; 1988. [Google Scholar]
- 19.Volchkov V E, Blinov V M, Netesov S V. The envelope glycoprotein of Ebola virus contains an immunosuppressive-like domain similar to oncogenic retroviruses. FEBS Lett. 1992;305:181–184. doi: 10.1016/0014-5793(92)80662-z. [DOI] [PubMed] [Google Scholar]
- 20.Volchkov V E, Becker S, Volchkova V A, Ternovoj V A, Kotov A N, Netesov S V, Klenk H-D. GP mRNA of Ebola virus is edited by the Ebola virus polymerase and by T7 and vaccinia virus polymerases. Virology. 1995;214:421–430. doi: 10.1006/viro.1995.0052. [DOI] [PubMed] [Google Scholar]
- 21.Volchkov, V. E., H. Feldman, V. A. Volchkova, and H.-D. Klenk. Processing of Ebola virus glycoprotein by proprotein convertase furin. Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- 22.Watanabe T, Murakami K, Nakayama K. Positional and additive effects of basic-amino-acids on processing of precursor proteins within the constitutive secretory pathway. FEBS Lett. 1993;320:215–218. doi: 10.1016/0014-5793(93)80589-m. [DOI] [PubMed] [Google Scholar]
- 23.Will C, Mühlberger E, Linder D, Slenczka W, Klenk H-D, Feldmann H. Marburg virus gene 4 encodes the virion membrane protein, a type I transmembrane glycoprotein. J Virol. 1993;67:1203–1210. doi: 10.1128/jvi.67.3.1203-1210.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang, Z.-Y., R. Delgado, L. Xu, R. F. Todd, E. G. Nabel, A. Sanchez, S. T. Nichol, and G. J. Nabel. Differential cellular interactions of the secreted and transmembrane Ebola virus glycoproteins: implication for viral pathogenesis. Science 279:1034–1037. [DOI] [PubMed]
- 25.Xu L, Sanchez A, Yang Z-Y, Zaki S R, Nabel E G, Nichol S T, Nabel G J. Genetic immunization for Ebola virus infection. Nat Med. 1997;4:37–42. doi: 10.1038/nm0198-037. [DOI] [PubMed] [Google Scholar]
- 26.Zaki R R, Greer P W, Goldsmith C S, Coffield L M, Rollin P E, Callain P, et al. Abstracts of the Proceedings of the International Colloquium on Ebola Virus Research, Antwerp, Belgium. 1996. Ebola virus hemorrhagic fever: pathologic, immunopathologic and ultrastructural studies; p. 35. [Google Scholar]