Abstract
A newly recognized parvovirus of laboratory rats, designated rat parvovirus type 1a (RPV-1a), was found to be antigenically distinct. It was cloned, sequenced, and compared with the University of Massachusetts strain of rat virus (RV-UMass) and other autonomous parvoviruses. RPV-1a VP1 identity with these viruses never exceeded 69%, thus explaining its antigenic divergence. In addition, RPV-1a had reduced amino acid identity in NS coding regions (82%), reflecting phylogenetic divergence from other rodent parvoviruses. RPV-1a infection in rats had a predilection for endothelium and lymphoid tissues as previously reported for RV. Infectious RPV-1a was isolated 3 weeks after inoculation of infant rats, suggesting that it, like RV, may result in persistent infection. In contrast to RV, RPV-1a was enterotropic, a characteristic previously associated with parvovirus infections of mice rather than rats. RPV-1a also differed from RV in that infection was nonpathogenic for infant rats under conditions where RV infection causes high morbidity and mortality. Thus, RPV-1a is the prototype virus of an antigenically, genetically, and biologically distinct rodent parvovirus serogroup.
Autonomous parvoviruses are small, nonenveloped DNA viruses. Molecular analysis of a prototype rodent parvovirus, minute virus of mice (MVM), indicates that the genome consists of a single-stranded DNA about 5 kb in length which is bracketed by short terminal palindromes involved in viral replication (13). Replication occurs through monomer- and dimer-length duplex DNA intermediates, resulting in encapsidation of monomer-length single-stranded DNA which is predominantly minus sense. The genome contains two large and several smaller open reading frames on the plus-sense strand which encode two nonstructural (NS) proteins and two capsid (VP) proteins. The NS proteins, NS1 and NS2, participate in transcription and replication and traditionally are highly conserved among rodent parvoviruses. The VP proteins, VP1, VP2, and VP3, confer species and tissue specificity and are less conserved. VP2 is the major capsid protein, and its coding sequences are contained within VP1. VP3 results from protease cleavage of VP2 and is present in various amounts in DNA-containing virions.
Viral replication requires host cell functions expressed during the S-phase transition. In addition, the state of host cell development and differentiation determine whether infection results in productive viral replication (13). These requirements correlate with the predilection of autonomous parvoviruses for mitotically active cells and contribute to their pathogenicity in vivo. Parvoviruses can cause severe infection in fetal or infant animals by killing mitotically active cells that are plentiful during prenatal and early postnatal development (20, 21, 25). Pathogenic infection is rare in more mature animals, where the complement of susceptible dividing cells is reduced.
For several decades, parvoviruses of laboratory mice and rats were grouped among three serotypes according to antigenic differences in viral capsid proteins detected by hemagglutination inhibition (HAI) or virus neutralization (VN) tests. MVM was the sole murine serotype, and two serotypes were recognized in rats: rat virus (RV) and H-1 virus. A second mouse serotype was established as a result of the isolation of mouse parvovirus type 1 (MPV-1) in 1993 (6, 30, 43). The prototypic strain, MPV-1a, is nonpathogenic in infant and adult mice but causes persistent lymphocytotrophic infection and immune dysfunction (23, 29).
The two identified parvoviruses of rats, RV and H-1 virus, are highly pathogenic for fetal and infant rats. Pathogenic infection causes severe damage to the liver, central nervous system, lymphoid system, and other tissues (19). In addition, endothelial infection results in hemorrhage and infarction, especially in the brain and spinal cord. RV also causes persistent infection in rats inoculated with virus by 6 days of age (14). Inoculation of juvenile rats causes subclinical infection of more limited duration but can perturb the host immune system (9, 24, 31).
Recent serological results provided evidence for a third parvovirus serotype in rats. Sera from clinically normal rats reacted with RV and H-1 virus by an immunofluorescence assay (IFA) which detects both NS and capsid antigens. However, the sera did not react with RV or H-1 capsid antigens by HAI. This report describes the isolation and characterization of the causative agent, the prototype virus of a new parvovirus serogroup. It has been named rat parvovirus type 1a (RPV-1a) following the recently suggested nomenclature (19). The results show that RPV-1a is antigenically, molecularly, and biologically distinct from RV and H-1 virus.
MATERIALS AND METHODS
Virus.
RPV-1a was isolated after amplification in vivo in a transplantable large granular lymphocyte (LGL) leukemia of the Fischer 344 (F344) rat (48) as described in Results. The leukemia line was obtained from Craig Reynolds, Frederick Cancer Research Center, Frederick, Md., and was propagated by intraperitoneal (i.p.) inoculation of young adult F344 rats with 5 × 107 LGL leukemia cells. The RPV-1a stock was subsequently amplified in vitro by two rounds of infection in 324K cells, a line of simian virus 40-transformed human embryo kidney cells (44), and the median infectious dose of RPV-1a in tissue culture (TCID50) was determined in 324K cells. A pathogenic strain of RV (RV-UMass) was obtained from Arthur Like (University of Massachusetts Medical Center, Worcester). It was propagated, and the TCID50 was determined in NRK cells, an established line of rat kidney cells (17). Virus replication was detected by cytopathic effect (CPE) or immunostaining for viral antigen, using convalescent sera from rats previously exposed to virus.
Preparation of viral DNA.
RPV-1a DNA was prepared from 324K cells infected at a multiplicity of infection (MOI) of 0.3 TCID50 per cell. RV-UMass DNA was prepared from NRK cells infected at an MOI of 0.001 TCID50 per cell. Both cultures were harvested when approximately 50% of the cell monolayer had developed CPE. Cells were pelleted and rinsed in phosphate-buffered normal saline, and low-molecular-weight DNA was isolated by using a modified Hirt protocol (18).
Cloning of the RPV-1a genome.
Hirt DNA from RPV-1a-infected cells was cleaved with EcoRI (bp 1074) and PstI (bp 3360) to release a large internal fragment of the genome, which was subsequently ligated into the EcoRI-PstI sites of the pGem3zf+ vector (Promega, Madison, Wis.). The vector was transfected into Escherichia coli JM109 by electroporation, and resultant colonies were screened by restriction enzyme analysis. The presence of RPV-1a in individual plasmids was confirmed by Southern analysis using the RV-Y probe.
The RPV-1a termini were cloned by bisecting the genome with HindIII to produce a 2.5-kb left terminus and a 2.59-kb right terminus. A Hirt preparation of RPV-1a DNA was treated with the Klenow fragment of DNA polymerase I to produce blunt ends for ligation. BamHI linkers were ligated to the ends, and the DNA was cut to completion with BamHI and HindIII. Digested DNA was electrophoresed through a 0.8% low-melting-point agarose gel. A 2.5- to 2.6-kb band was excised, and the DNA was purified by digestion with β-agarase (New England Bioloabs, Beverly, Mass.). The BluescriptII KS− vector (Stratagene, La Jolla, Calif.) was digested with HindIII and BamHI, treated with shrimp alkaline phosphatase (Amersham Life Sciences, Arlington Heights, Ill.), and ligated with the gel-purified RPV-1 DNA. The ligation mix was electroporated into E. coli JM109, and colonies were screened by filter hybridization (42) using a random-primed 32P-labeled probe made from a template of the RPV-1a EcoRI-PstI fragment. Positive clones were confirmed by restriction analysis, and two complete left termini were obtained. One right terminus was isolated, but approximately 150 terminal nucleotides were deleted compared to the published sequence of H-1 virus (39).
Cloning of the RV-UMass genome.
Hirt DNA from RV-UMass-infected cells was digested with HincII and EcoRI to release a 3.2-kb internal fragment. Digested DNA was ligated into BluescriptII KS+ vector and electroporated into E. coli JM109. Colonies were screened for insertion of the correct RV-UMass fragment.
To clone the left terminus, BamHI linkers were ligated to RV-UMass DNA as described above. The DNA was digested to completion with BamHI and EcoRI, ligated into pGem7zf+, and electroporated into E. coli JM109. Colonies were screened by filter hybridization using the left end of RPV-1a as a probe. The single left terminus isolate was 283 nucleotides shorter than the published sequence for H-1 virus and did not contain the hairpin sequences.
Sequencing and analysis of viral DNA.
Sequencing was performed either by using modified T7 DNA polymerase (Sequenase version 2.0; Amersham) as instructed by the manufacturer for dideoxy sequencing of double-stranded DNA or by the DNA Sequencing Facility at the W. M. Keck Foundation Biotechnology Laboratory at Yale School of Medicine, using PCR sequencing and an automated sequencer apparatus. Sequencing encompassed 97% of the RPV-1a genome and 77% of the RV-UMass genome, in both directions.
The sequences were analyzed with Genetics Computer Group (GCG) analysis programs on the VAX at Yale School of Medicine. The programs used were Pileup, Pretty, Gap, and Best Fit, and the parameters were default settings. The sequences used for comparison were those of MVMi (an immunosuppressive variant of MVM) (2, 41), MPV-1a (6), H-1 virus (39), canine parvovirus (CPV) (38), and porcine parvovirus (PPV) (47). A small region of RV-UMass was previously published (9), and there are three nucleotide differences from the RV sequence published here.
Animals.
Pregnant and 3-week-old, specific-pathogen-free (SPF) F344 rats were obtained from the Animal Genetics and Production Branch, National Cancer Institute, Bethesda, Md. Pregnant, SPF Sprague-Dawley (SD) rats were obtained from Taconic Farms, Germantown, N.Y. Rats were housed in Micro-isolator cages (Lab Products, Maywood, N.J.) as previously described (21). Sera from randomly selected rats were tested before inoculation, and individual rats were tested at necropsy for antibodies to Mycoplasma pulmonis and to common rodent viruses, including parvoviruses of rats, using an IFA (21). Noninoculated rats were uniformally seronegative. Inoculated rats were seropositive by IFA in all experiments, and antibodies specific for RPV-1a were confirmed by HAI during initial experiments.
Determination of infectious dose for RPV-1a.
The infectious dose at which 50% of the animals seroconverted (ID50) to RPV-1a was determined in neonatal F344 rats, and titers for ID50 and LD50, the dose lethal for 50% of inoculated animals, for RV-UMass were determined in neonatal SD rats. A minimum of seven neonates per dilution were inoculated oronasally (o.n.) with serial 10-fold dilutions of RPV-1a stock virus. Three weeks later, the rats were tested for RPV antibody by IFA using RPV-1a-infected cells as antigen.
Pathogenesis of RPV infection. (i) Infants.
Three litters (n = 27) of 2-day-old F344 rats were inoculated o.n. with 8 × 102 TCID50 of RPV-1a. Four to eight rats were euthanized with carbon dioxide gas on postinoculation days (PIDs) 5, 7, 10, and 20. The kidney, liver, heart, lung, small intestine, large intestine, submandibular, axillary, and inguinal lymph nodes, mesenteric lymph nodes, thymus, spleen, gonads, and pancreas were immersed in freshly prepared periodate-lysine-paraformaldehyde fixative (32), embedded in paraffin, sectioned, and examined by routine microscopy and by in situ hybridization (ISH) as described below.
(ii) Weanlings.
Sixteen 3-week-old female F344 rats were inoculated o.n. with 3 × 104 TCID50 of RPV-1a. Four rats were euthanized on each of PIDs 5, 7, 10, and 20, and tissues were processed as described for infant rats.
Detection of infectious virus in tissues.
Explant cultures of spleen, lung, and kidney were prepared at necropsy (37). Briefly, multiple small pieces of each organ were cultured in 25-cm2 culture flasks until outgrowths were confluent. Cultures were frozen and thawed three times, and the clarified supernatants were used to infect 324K cell monolayers. If no characteristic CPE was observed in the indicator cultures after 6 days, monolayers were washed three times with phosphate-buffered saline, fixed in cold acetone, and stained by indirect immunofluorescence using convalescent sera from RPV-1 infected rats.
ISH.
An RPV-1a-specific probe was made by digesting plasmid with EcoRI and PstI to release a 2.3-kb fragment (bp 1074 to 3360). RPV-1a sequences were separated from plasmid by electrophoresis in a 1% low-melting-point agarose gel. The 2.3-kb band was excised and melted, and aliquots containing 25 ng of RPV-1 DNA were used as templates to prepare 32P-labeled random-primed probes, using a commercial kit (Gibco-BRL, Gaithersburg, Md.), with a specific activity of 8 × 108 to 1.2 × 109 cpm/μg of DNA. Plus-sense-specific 35S-labeled riboprobes, with a specific activity of 2 × 107 cpm/μg of RNA, were synthesized according to the manufacturer’s protocol (Riboprobe System–T7; Promega, Madison, Wis.), using PstI linearized DNA as the template for T7 RNA polymerase.
Five-micrometer paraffin sections were mounted on RNase-free slides coated with aminoalkylsaline, deparaffinized, rehydrated, and digested with proteinase K (1 μg/ml in 100 mM Tris–50 mM EDTA [pH 8.0]) for 30 min at 37°C. Slides were acetylated in 0.25% acetic anhydride in 0.1 M triethanolamine at 22°C (twice for 5 min each time), heated at 65°C for 15 min to denature the DNA in 95% formamide in 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), immersed in ice-cold 0.1× SSC, and dehydrated. Hybridizations were performed in a 50-μl mixture containing RPV-1a probe (5 × 105 cpm/slide; specific activity, 108 to 109 cpm/μg of DNA or RNA), 0.6 M NaCl, 50% formamide, 10% dextran sulfate, 200 μg of salmon sperm DNA per ml, 10 mM Tris (pH 8.0), 1 mM EDTA, 2 mg of bovine serum albumin per ml, 0.2% Ficoll, and 0.2% polyvinyl pyrrolidone. Slides were coverslipped, immersed in mineral oil, and incubated for 3 days at 42°C. Oil was removed by three washes in chloroform, and coverslips were removed during four washes in 4× SSC. Unbound probe was removed by washes for 30-min wash in 2× SSC at 22°C, 30 min in 0.1× SSC at 65°C, and 15 min in 0.1× SSC at 22°C. Following dehydration in ethanol containing 0.3 M ammonium acetate, slides were air dried, dipped in Kodak NTB-3 liquid emulsion, and stored at 4°C for 1 to 14 days. Slides were developed for 5 min in a 1:1 dilution of Kodak D19, fixed, and counterstained in hematoxylin and eosin. Sections were evaluated by light microscopy and scored for the prevalence of signal-positive cells per low-power field as follows: negative, trace (1 to 5 cells), low (6 to 10 cells), moderate (11 to 20 cells), or high (>20 cells). Tissue sections of known ISH-positive samples were used as positive control slides, and tissue sections from uninfected animals served as negative control slides for each hybridization.
Nucleotide sequence accession numbers.
The RPV-1a and RV-UMass sequences have been submitted to GenBank and assigned accession no. AF036710 and AF036711, respectively.
RESULTS
Isolation of RPV-1a.
Evidence for a previously unrecognized parvovirus of rats arose during pathogenesis studies of RV-Y, the Yale strain of Kilham rat virus (21). Uninoculated weanling SD rats obtained from a commercial breeding colony were seropositive for RV in an IFA assay, and rats inoculated with RV-Y in the same experiment were prematurely seropositive. In contrast, sera from both groups of rats were negative for antibodies to RV-Y and H-1 virus by HAI assay. An additional group of rats from a commercial source was tested on arrival and had an identical serological profile. These results implied that the rats had been infected with an antigenically distinct parvovirus prior to shipment.
Initial attempts to amplify and isolate the virus involved inoculation of neonatal athymic and euthymic rats, both of which are highly susceptible to RV infection. A clarified homogenate, prepared by pooling spleen fragments from 15 seropositive F344 rats, was inoculated i.p. and o.n. into 2-day-old F344 rats. The euthymic rats were seropositive for the agent by PID 15. Immunostaining revealed a few viral antigen-positive cells in thymus and spleen and in explant cultures of brain, kidney, and thymus collected on PIDs 7, 10, and 15.
The detection of positive cells in lymphoid tissues led to an alternative strategy for in vivo amplification of virus which exploited the predilection of parvoviruses for mitotically active cells. The LGL leukemia of F344 rats induces lymphocytic tumors in the abdomen and thymus after i.p. inoculation of tumor cells and progresses to severe leukemia with hepatosplenomegaly and anemia within 6 weeks (48). Eight juvenile F344 rats were inoculated i.p. with LGL leukemia cells and 3 weeks later inoculated i.p. with the RPV splenic homogenate or RV-UMass. At 1 and 2 weeks after inoculation, rats were euthanized and tissues were examined by ISH using a 32P-labeled probe for RV-Y. Numerous positive cells were detected in abdominal and thymic tumors and in vascular endothelium after infection with either virus, but the hybridization signal was less intense over individual RPV-infected cells compared with RV infection. Additionally, tumor-bearing rats infected with RV developed liver necrosis coincident with infection, whereas tumor-bearing rats inoculated with the RPV splenic homogenate did not develop liver necrosis (28).
A clarified 10% homogenate was made from tumors harvested from several RPV-infected rats and was used to infect monolayer cultures of NRK and 324K cells. After 4 days, only a few antigen-positive cells were seen in the NRK cultures. In contrast, CPE was evident in 324K cells at PID 4, and by PID 5 more than 50% of the cells were virus antigen positive by immunostaining. The 324K cells were harvested, and the cell lysate was passaged a second time in 324K cells to generate a higher titer viral stock. The cell lysate of the second passage was used for subsequent infectivity and pathogenesis experiments.
The antigenic dissimilarity between RPV-1a and RV or H-1 virus was confirmed by HAI and VN. Sera from RPV-1a-infected animals did not inhibit hemagglutination of guinea pig erythrocytes by RV or H-1 virus (Table 1). The RPV-1a P1 stock, which had a titer 1,000-fold lower than that of the RV-UMass stock, had low hemagglutination activity. As a result, RPV-1a antigen in HAI assays was nonspecifically inhibited by normal rat sera (36). However, sera from RPV-1a-infected animals neutralized the infectivity of RPV-1a but not RV-UMass, and sera from RV-infected rats neutralized the infectivity of RV-UMass but not RPV-1a (36). Thus, RPV-1a is antigenically distinct from RV and H-1 virus.
TABLE 1.
HAI results
| Antiserum | No. of positive sera/total tested
|
|
|---|---|---|
| RV | H-1 | |
| RV | 14/14 | 0/14 |
| RPV | 0/32 | 0/32 |
| H-1 | 0/3 | 3/3 |
Comparison of molecular characteristics of RPV-1a, RV-UMass, and H-1 virus.
Restriction digests of RPV-1a and RV-UMass Hirt DNA were compared by using enzymes expected to cleave rodent parvovirus DNA at a limited number of sites. Most enzymes either did not cleave or resulted in a different restriction pattern for RPV-1a DNA compared to RV-UMass, whereas restriction patterns for RV-UMass and RV-Y DNA were virtually identical (5). In addition, the RV probe hybridized to RV-UMass DNA more efficiently than to RPV-1a DNA, even though approximately 10 times more RPV-1a DNA was loaded on the gel. This result correlated with the comparatively weak ISH signal obtained when the RV probe was hybridized to RPV-1a-infected rat tissues. Defined restriction sites were used to isolate and clone large segments of the RPV-1a and RV-UMass genomes for sequence analysis and for use as strain-specific probes.
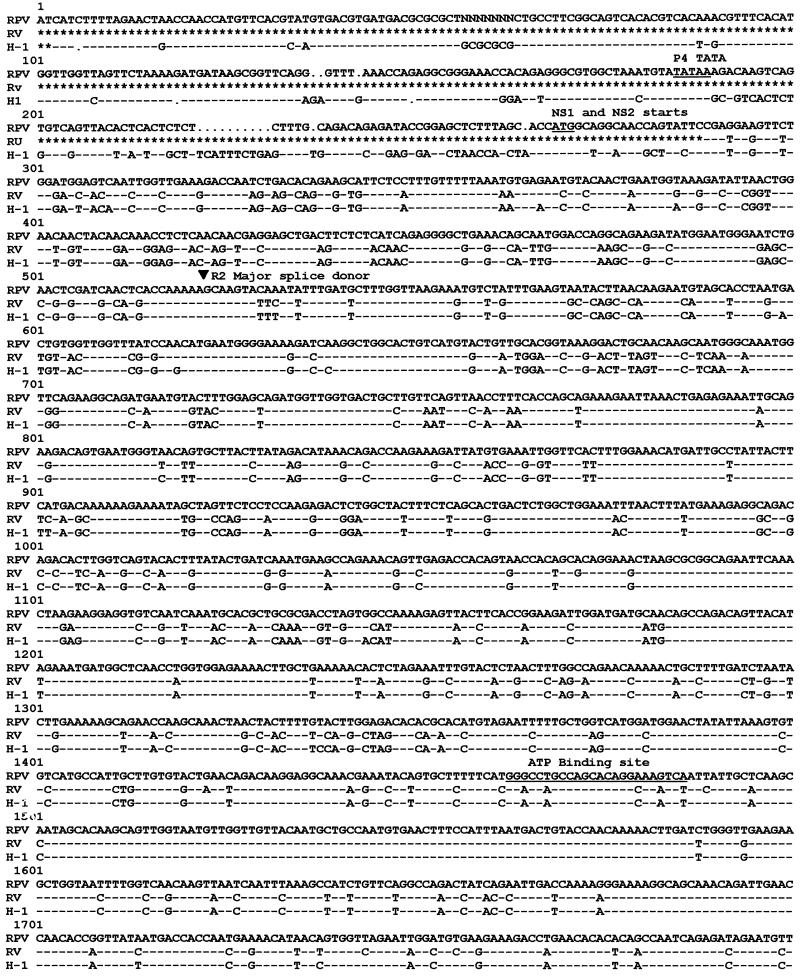
Alignment of nucleotide sequences of RPV-1a, RV-UMass, and H-1 virus (Fig. 1) revealed sequence variation between RPV-1a and RV-UMass or H-1 virus, although genomic organization and transcriptional signals were conserved. Locations of major open reading frames in the RPV-1a genome closely resembled those previously established for MVM and H-1 virus (4, 39). RPV-1a sequence around splice donor sites was the same as that used in MVM for generating R2 transcript from R1 transcript (33), but RPV-1a differed from MVM in the polypyrimidine track of the major and minor splice acceptors. When the P4 promoter region of RPV-1a was compared with that of H-1 virus, it was found to be conserved through the TATAA box, after which the number of nucleotide changes from H-1 virus increased. The TATAA box also was conserved in the P38 promoter region, but the TAR element had two nucleotide changes from H-1 virus and RV-UMass. The cloned left terminus of RPV-1a was intact and differed from that of H-1 virus (39) at 7 positions and from that of RV (3) at 12 positions. The cloned right terminus of RPV-1a lacked approximately 150 nucleotides compared to the right terminus of H-1 virus, including the hairpin sequences. A repeat sequence that was duplicated at the right end of H-1 virus genome was present as one copy in RPV-1a.
FIG. 1.
Sequence comparison of RPV-1a, RV-UMass, and H-1. The right terminus of H-1 virus is not included, as this region was not cloned or sequenced for RPV-1a or RV-UMass. Dashes indicate nucleotides identical to those of RPV-1a, dots indicate spaces inserted for maximal sequence alignment, and asterisks indicate nonsequenced regions. Regions conserved among parvoviruses are underlined, and MVM splice donors and acceptors are indicated with arrows. GCG programs Pileup and Pretty were used to generate the alignment.
Nucleotide sequence alignment of RV-UMass and H-1 virus (Fig. 1) revealed higher identity throughout the genome than was evident for RPV-1a. Additionally, open reading frames and transcriptional control regions, including splice donors, splice acceptors, and the P38 promoter, were identical for the two viruses.
The alignment results were confirmed by using GCG sequence analysis programs to calculate nucleotide sequence identity of RPV-1a or RV-UMass with other autonomous parvoviruses (5). The extents of identity of RV-UMass were 90.4% for H-1 virus and progressively lower for MVMi (82%), MPV-1a (81.4%), and CPV (65.2%). In contrast, the extents of identity of RPV-1a were 74% for RV-UMass, 72% for H-1, 72% for MPV-1a and MVMi, and 66% for CPV.
Putative amino acid sequences for NS and VP proteins also were compared among the autonomous parvoviruses (5). RV-UMass NS1 was 99% identical to that encoded by H-1 virus and 91.6% identical to those encoded by MVMi and MPV-1a. RPV-1a NS1, however, was approximately 82% identical to those encoded by H-1, MVMi, and MPV-1a and 80% identical to that encoded by CPV. For RV-UMass NS2, the portion encoded prior to the minor splice was 98% identical to that of H-1 virus, but the comparable region of RPV-1a NS2 was only 66% identical to that encoded by H-1 virus (5). The three carboxy termini of NS2, formed by differential splicing at the minor splice of R2, also were compared (Fig. 2). The major NS2 form (PEITWF) was identical for RV-UMass and H-1 virus but differed from that of RPV-1a by two amino acids. The H-1 virus NS2 form (YNGTSS) had one coding difference in RV-UMass, whereas RPV-1a had two coding changes and an extension of 18 amino acids that included the 12 amino acids of the minor NS2 form. The minor form of NS2, LGASWL, had one coding change in RV-UMass, and RPV-1a was truncated by three amino acids and had four coding changes from H-1 virus. As expected for viruses belonging to different serogroups, the capsid proteins were more dissimilar than the NS proteins (5). Conservation in the unique N terminus resulted in higher percent identity for VP1 than for VP2. VP1 identity was highest between RV-UMass and H-1 virus (81.6%) and progressively lower for MVMi (75.2%), MPV-1a (74.2%), PPV (59.7%), and CPV (58.8%). As with the NS proteins, the extents of identity of RPV-1a VP1 protein were consistently low for RV-UMass (69.4%), H-1 (64.7%), MVMi (65.6%), MPV-1a (66.9%), CPV (58.6%), and PPV (55.7%).
FIG. 2.
Carboxy termini of NS2 in H-1, RV-UMass, and RPV-1a.
Pathogenesis of RPV-1a infection in infant rats.
The TCID50 and ID50 in neonatal F344 rats were compared to those defined for RV-UMass (Table 2). The TCID50 of RPV-1a was more than 2 logs lower than that of RV-UMass, but the ID50 was approximately equal. An LD50 was not obtained for RPV-1a because rats given the highest dose of RPV-1a, which was similar to the LD50 of RV-UMass, remained clinically normal.
TABLE 2.
Infectivity of RV-UMass and RPV-1a in cell culture and rats
| Virus | Value (infectious units/ml)
|
||
|---|---|---|---|
| TCID50 | ID50a | LD50a | |
| RV-UMass | 108 | 107 | 2 × 105 |
| RPV-1a | 3.2 × 105 | 2 × 106 | 0 |
Determined in neonatal SD (RV) or F344 (RPV) rats.
A preliminary study suggested that acute RPV-1a infection was not pathogenic for infant (2-day-old) F344 rats. Each of two groups of 11 infant rats was inoculated either i.p. or o.n. with 8 × 102 TCID50, and three of each groups were necropsied on PID 7. Spleen and kidney explants from all rats contained infectious virus, and ISH revealed numerous virus-positive cells in brain, lung, heart, thymus, lymph nodes, spleen, liver, kidney, and intestine. However, lesions were not detected after either route of inoculation, and remaining rats seroconverted by PID 21.
To further examine the pathogenesis of RPV-1a infection in neonates, 20 2-day-old F344 rats were inoculated o.n. with 8 × 102 TCID50 of virus. A minimum of four rats were assessed for infection and lesions on each of PIDs 5, 7, 10, and 20. Infectious virus was detected by explant cultures of spleen, lung, and kidney through PID 20, although the prevalence of positive tissues was highest during the first week of infection (Tables 3 and 4). Antibody to RPV-1a was detected in all rats tested on PIDs 10 and 20.
TABLE 3.
Isolation of RPV-1a after o.n. inoculation of F44 rats
| Tissue | No. from which virus was isolated/no. tested at indicated PID
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Infants
|
Weanlings
|
|||||||
| 5 | 7 | 10 | 20 | 5 | 7 | 10 | 20 | |
| Lung | 4/4 | 4/4 | 4/4 | 5/8 | 4/4 | 4/4 | 2/4 | 3/4 |
| Kidney | 4/4 | 4/4 | 4/4 | 5/8 | 4/4 | 2/4 | 0/4 | 0/4 |
| Spleen | 4/4 | 4/4 | 3/4 | 5/8 | 4/4 | 4/4 | 4/4 | 2/4 |
TABLE 4.
Prevalence of infectious virus and viral DNA in tissues of F344 rats after o.n. inoculation with RPV-1a as determined by ISH
| Tissue | Results of ISH at indicated PIDa
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Infants
|
Weanlings
|
|||||||
| 5 | 7 | 10 | 20 | 5 | 7 | 10 | 20 | |
| Lung | − | − | • | • | ||||
| Kidney | •• | • | •• | • | − | |||
| Spleen | • | • | •• | • | − | |||
| Lymph nodes | •• | •• | •• | ••• | ••• | ••• | • | |
| Thymus | − | − | •• | − | − | |||
| Liver | •• | • | • | − | ||||
| Small intestine | •• | •• | •• | • | ••• | ••• | •• | − |
| Brain | − | • | • | − | ||||
| Heart | − | • | •• | − | − | |||
Number of cells per low-power field: −, negative; , trace (1 to 5 cells); •, low (6 to 10 cells); •• moderate (11 to 20 cells); •••, high (>20 cells). Apart from a few infected endothelial cells, salivary glands, pancreas, large intestine, gonads were negative in infants and weanlings.
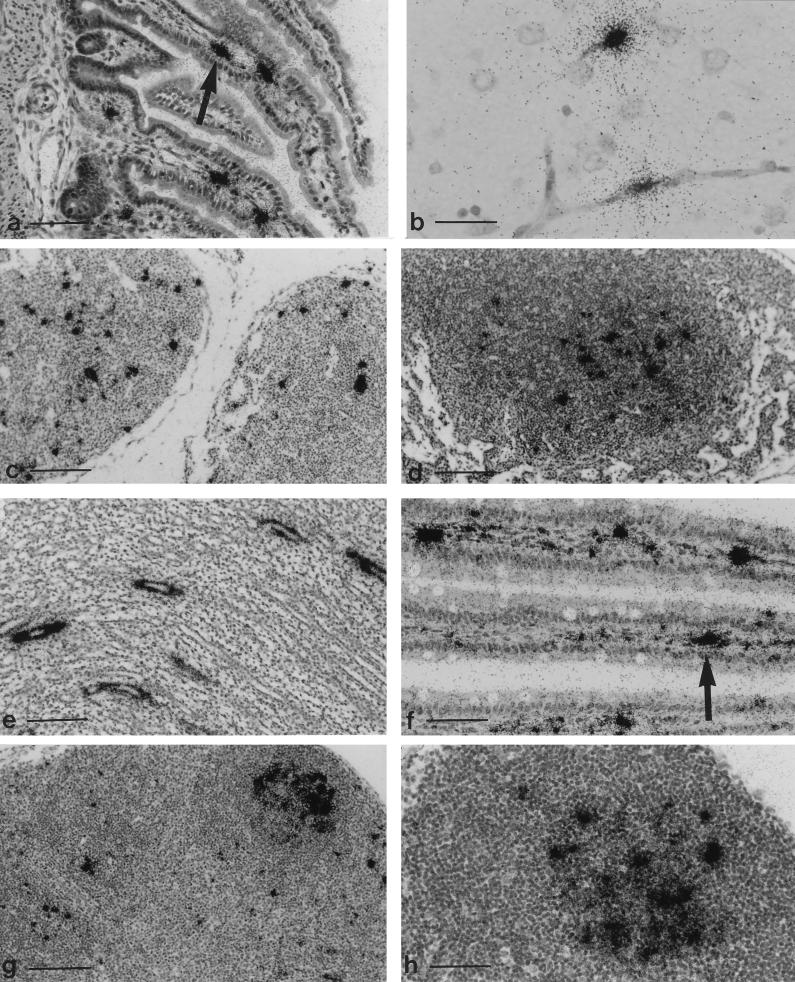
Viral DNA was detected by ISH at all time points. A moderate number of cells were viral DNA positive in the lamina propria of the small intestine by PID 5, but enterocytes were not labeled. A few positive cells also were found in liver, kidney, spleen, and mesenteric lymph node but not in lung, despite the fact that it yielded infectious virus by explant culture. By PID 7, the number of infected cells in liver, kidney, and lymph nodes (Fig. 3c) had increased, stayed the same in spleen and small intestine (Fig. 3a), and appeared among capillary endothelium in brain (Fig. 3b), lung, heart, and thymus. A similar distribution of labeled cells was seen on PID 10, with increased signal in spleen, brain, and heart. Additionally, endothelial infection was prominent in intestinal lamina propria, pulmonary alveoli, intrahepatic veins and sinusoids, renal glomerular tufts, and interstitial vessels. Despite widespread infection of capillary endothelium, there was no evidence of hemorrhage or necrosis as was found at comparable stages of RV infection (21). Intestinal and vascular smooth muscle, cardiac myofibers, hepatocytes, and renal convoluted tubules were variably viral DNA positive. The number of affected tissues remained high on PID 20, but the prevalence of positive cells had decreased except in lymph nodes (Fig. 3d) and kidneys, in which prominent, segmental labeling occurred among collecting tubules (Fig. 3e).
FIG. 3.
(a to d) Tissues from rats inoculated o.n. with RPV-1a at 2 days of age and hybridized with a plus-sense 35S-labeled riboprobe to detect minus-sense DNA. (a) Small intestine at PID 7, with an arrow indicating signal in the lamina propria. Bar, 150 μm. (b) Brain at PID 7. Capillary endothelium is labeled, but there is no hemorrhage. Bar, 37 μm. (c) Mesenteric lymph node at PID 7. Bar, 150 μm. (d) Mesenteric lymph node at PID 20. Signal is localized over germinal center, a typical pattern for persistent lymphocytotropic infection with rodent parvoviruses. Bar, 150 μm. (e) Kidney from a rat inoculated with RPV-1a at 2 days of age, collected at PID 20, and hybridized with a 32P-labeled random-primed RPV probe. Signal is localized over collecting tubules. Bar, 150 μm. (f to h). Tissues from rats inoculated o.n. with RPV-1a at 3 weeks of age and hybridized with a plus-sense 35S-labeled riboprobe for minus-sense DNA. (f) Small intestine at PID 7, with an arrow indicating signal localized in the lamina propria. Bar, 75 μm. (g) Mesenteric lymph node at PID 7. Positive cells are in paracortical zones, and localization of signal over germinal centers has begun. Bar, 150 μm. (h) Mesenteric lymph node at PID 20 with signal over a germinal center. Bar, 75 μm.
Pathogenesis of RPV-1a infection in juvenile rats.
Sixteen 4-week-old F344 rats were inoculated o.n. with 3 × 104 TCID50 of RPV-1a, and tissues from four rats were assessed at each of PIDs 5, 7, 10, and 20 (Tables 3 and 4). The kidney contained infectious virus through PID 7, whereas virus was detected in spleen and lung through PID 20. Tissue tropism resembled that seen in infants. The heaviest concentration of positive cells at PID 5 was the mesenteric lymph nodes and in the lamina propria of the small intestine, but enterocytes were rarely labeled. Infection of capillary endothelium in lung, brain, liver, and kidney also was noted on PID 5. The number of cells containing viral DNA increased to a maximum by PID 7 in the lamina propria of the small intestine (Fig. 3f), lymph nodes (Fig. 3g), and spleen. Signal in lymphoid tissue was concentrated in red pulp and periarteriolar lymphoid sheaths in spleen and in germinal centers in spleen and lymph nodes (Fig. 3g), a pattern reported previously for RV (15) and MPV (23) infection. Seroconversion also occurred by PID 7, and positive cells were sparse in all other indicated tissues. The mesenteric lymph nodes remained ISH positive through PID 20 (Fig. 3h).
DISCUSSION
Rodent parvovirus infections had been attributed to three serogroups: MVM in mice and RV and H-1 virus in rats. The recent discovery of MPV, a second serogroup in mice, and the isolation and characterization of RPV reported here indicate that a larger number of antigenically distinct parvoviruses infect laboratory rodents than previously recognized.
Initial evidence for MPV and RPV arose from the coordinated use of serologic methods that detect both NS and VP antigens (enzyme-linked immunosorbent assay and IFA) with those that detect only VP antigens (HAI, VN). Detection of RPV-1a infection by enzyme-linked immunosorbent assay or IFA for RV or H-1 virus infection is explained by cross-reactive antibodies directed against NS proteins, which are 80% identical among these viruses. Serogroup-specific tests, HAI or VN assays which use virions as antigen, reflect the low identity (60%) of RPV-1a VP2 with those of RV and H-1 virus. In these assays, VP amino acids accessible for immune recognition are those on the capsid surface, and specific interactions between the VP molecules in the virus capsid are likely to result in more complex antigen presentation and recognition than for NS proteins. Thus, the use of rodent parvovirus virions as antigen may enhance the serologic specificity of diagnostic tests.
The distinctiveness of RPV-1a was supported by molecular analysis. Although the genomic organization of RPV-1a was equivalent to that of RV and H-1 virus, RPV-1a differed substantially from RV and H-1 virus in nucleotide and putative amino acid sequence in both NS- and VP-encoding regions. Conservation of splice donor sites for generating R2 transcript from R1 transcript suggests that RPV-1a has a transcription pattern similar to that of MVM. However, differences from MVM in the polypyrimidine track of the major and minor splice acceptors of RPV-1a may indicate different ratios of viral transcripts from MVM. Furthermore, the RV sequence closely resembled that for H-1 virus, and it had progressively reduced identity for murine, canine, and porcine parvoviruses, whereas the RPV sequence had a similar and lower level of identity for all of these viruses. Numerous nucleotide changes throughout the RPV-1a genome explains the reduced signal obtained with RV probes when RPV-1a DNA is detected by molecular hybridization.
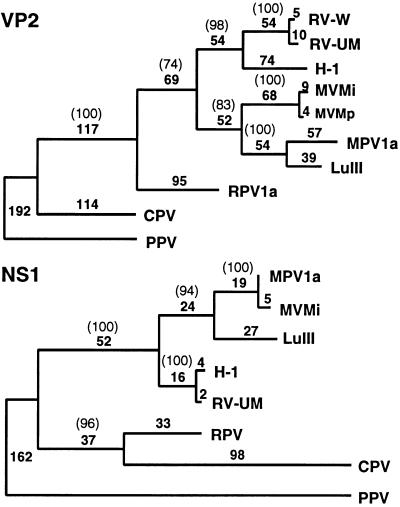
The divergence of RPV-1a nucleotide and amino acid sequences, especially for typically conserved NS proteins, suggests phylogenetic differences in the relationship of RPV-1a to other parvoviruses. Preliminary analysis of aligned amino acid sequences of NS1 and VP2 proteins shows that rodent parvoviruses form a clade that does not include RPV-1a (Fig. 4). This exclusion suggests that RPV-1a diverged from a common ancestor of rodent parvoviruses belonging to the clade. If this is the case, additional rodent parvovirus serogroups, with distinct and/or intermediate genotypes, may be infecting rodent populations. Initial evidence for this possibility, which includes limited sequence analysis of recent parvovirus isolates from rats that have coding differences from H-1 virus and RV in VP regions but not in NS regions, has been presented (40).
FIG. 4.
Phylogeny-derived using parsimony analysis of 10 VP2 (A) and 8 NS1 (B) amino acid sequences. RV-W refers to the unpublished sequence of an RV strain kindly provided by Rene Wicker. The phylogeny was rooted by using PPV as an outgroup. The number of nucleotide changes in each horizontal branch is given, and the number in parentheses is the bootstrap support for this analysis that was obtained in 1,000 repetitions.
The phylogenetic divergence of rodent parvoviruses also has implications for epizootiology of natural infections. Traditionally, parvoviruses were thought to be species specific. Studies of CPV and feline parvovirus (FPV) have determined, however, that minimal sequence changes in the capsid region of the genome can have dramatic consequences for host range (12, 46). Replacement of two residues in the FPV genome with two CPV-like residues enabled FPV to replicate in canine cells and dogs. While tropisms for mouse cells have been altered by minimal coding changes in the capsid genes of MVM (7), the effects of coding changes on the species-specific determinant of rodent parvoviruses has not been determined.
Experimental RPV-1a infection has important similarities to RV infection (21). For both viruses, infection in infants is widespread and includes lymphoid tissues, blood vessels, lung, and kidney. Renal involvement during RPV-1a infection also suggests that, as with RV, urinary excretion of virus contributes to the transmission of infection. Both viruses also are detectable after the onset of antiviral immunity in rats inoculated as infants or juveniles. However, it is too early to conclude that RPV-1a infection persists for extended periods because it was monitored for only 3 weeks in this study. Infant rats infected with RV under comparable conditions can harbor infectious virus for at least 6 months, and juveniles can remain infected for up to 8 weeks (24).
Substantive differences also were found between RPV-1a and RV infection. In vitro, RV-UMass replicates well in NRK cells, whereas RPV-1a replicates well in 324K cells. In vivo, the small intestine is a primary site of RPV infection after o.n. inoculation, whereas the respiratory tract is a primary site of RV infection and little or no infection occurs in the intestine. Intestinal infection with RPV-1a resembles MPV-1a and MVM infections of mice (10, 23), and early intestinal involvement suggests that the alimentary tract is the portal of viral entry. Enteric infection was found primarily in the lamina propria, which suggests that enterocytic infection is transient or that enteric infection results from viremia secondary to viral entry at another site. Although ISH detected no evidence for an alternative route, more exhaustive study of potential sites such as the oropharynx and nasopharynx must be considered.
A major difference between RPV and RV infection is their virulence for infant rats. Inoculation of 2-day-old rats with 2 × 103 TCID50 of RV causes high morbidity and mortality from multisystemic necrosis and hemorrhage (15, 21). Inoculation of 2-day-old rats with a comparable dose of RPV-1a did not cause perceptible disease, although increases in ISH signal over time indicate that productive infection occurred. The avirulence of RPV-1a infection also resembles MPV-1a infection of mice in which widespread infection occurs without noticeable histologic lesions. Additionally, high-dose RPV-1a inoculation of pregnant rats did not cause fetal infection or disease (28), as does occur with some pathogenic strains of RV (14, 22, 25). It is premature to conclude, however, that RPV-1a is completely avirulent because in vitro infection of 324K cells is cytolytic. Virus-induced lesions may escape histologic detection because cytolysis is only minimally greater than background apoptosis.
The mechanisms of productive, nonpathogenic parvovirus infections in vivo are unknown, but virus strain, host age, and host immunocompetence at inoculation are influencing factors (10, 16, 21). As noted previously, the pathogenicity of virulent RV strains for young rats is high until 6 days postpartum, at which point it decreases markedly. Thus, it was surprising that high doses of RPV-1a were nonpathogenic for 2-day-old rats. An explanation for this difference may lie in the dissimilarity of RPV-1a NS proteins from those encoded by RV and H-1 virus. Cytotoxicity of MVM in vitro is primarily mediated by NS1 (11), whose expression correlates with a perturbation of the cell cycle (34, 35) and interferes with synthesis and phosphorylation of cell proteins (1). Thus, coding changes in RPV-1a NS proteins may lower the cytotoxicity of NS1, possibly by altering its interaction with host cell proteins during viral infection. Cytotoxicity also is influenced by the intracellular protein concentration of NS1 (1). Nucleotide changes around putative RPV-1a splice junctions may alter ratios of P4 transcripts, and as a consequence, levels of NS1 protein may be affected. Maximal MVM cytotoxicity in human cell lines requires NS2 (8, 26, 27), suggesting that extensive coding changes found in NS2 could reduce the cytotoxicity of RPV-1a for rat cells. In addition, one nucleotide change (T to G) in the region between the two minor splice donors encoding the carboxy terminus of the intermediate-level NS2 form (YDGTSS) results in conversion of the termination signal in RV-UMass to a glutamic acid in RPV-1a. As a result, translation continues through the minor splice and results in a terminus of LGTSGIQVPGTW, the carboxy terminus of the minor MVM NS2 form LGASWL. Thus, RPV-infected cells may contain a higher level of the minor carboxy-terminal form of NS2 and no NS2 with YDGTSS on the direct terminus.
Parvoviral pathogenicity also may be influenced by the susceptibility of specific cells or tissues to infection and cytotoxicity. Capsid genes have been shown to mediate cell tropisms of other parvoviruses (7, 12). Thus, coding differences between VP genes of RPV-1a and RV may correlate, albeit indirectly, with pathogenicity. These differences in virulence among parvoviruses of rats may be decipherable through development of replicating recombinants between RV and RPV-1a.
The potential impact of RPV-1a infection on research using laboratory rats is unknown, but at least two concerns are pertinent. First, RPV-1a is lymphocytotropic. It shares this property with RV, MPV-1, and MVMi, all of which cause immune dysfunction in vitro and/or in vivo (19). Furthermore, RPV-1a DNA persisted in lymphoid tissue of seropositive rats for at least 3 weeks. These findings suggest that RPV-1a may modulate immune function and disrupt immunologic research in rats. Second, rodent parvoviruses have often been associated with neoplasms (45), a property that was exploited for the isolation of RPV-1a. During experiments with the LGL leukemia, we noted that RPV-1a-infected, tumor-bearing rats often had milder disease (e.g., reduced hepatosplenomegaly) or a delayed onset of clinical signs and leukemia compared to uninfected rats (28). This preliminary finding suggests that RPV-1a could interfere with development or growth of neoplasms in oncologic research or anticancer drug testing.
ACKNOWLEDGMENTS
We thank Colin Parrish (Cornell University, Ithaca, N.Y.) for performing the phylogenetic analysis and Werner Nicklas for suggesting that a transplantable tumor be used as a substrate for viral replication.
This work was supported by Public Health Service grants RR00393 and RR11740 to R.O.J.
REFERENCES
- 1.Anouja F, Wattiez R, Mousset S, Caillet-Fauquet P. The cytotoxicity of the parvovirus minute virus of mice nonstructural protein NS1 is related to changes in the synthesis and phosphorylation of cell proteins. J Virol. 1997;71:4671–4678. doi: 10.1128/jvi.71.6.4671-4678.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Astell C R, Gardiner E M, Tattersall P. DNA sequence of the lymphotropic variant of minute virus of mice, MVM(i), and comparison with the DNA sequence of the fibrotropic prototype strain. J Virol. 1986;57:656–659. doi: 10.1128/jvi.57.2.656-669.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Astell C R, Smith M, Chow M B, Ward D C. Structure of the 3′ hairpin termini of four rodent parvovirus genomes: nucleotides sequence homology at origins of DNA replication. Cell. 1979;17:691–703. doi: 10.1016/0092-8674(79)90276-9. [DOI] [PubMed] [Google Scholar]
- 4.Astell C R, Thomson M, Merchlinsky M, Ward D C. The complete DNA sequence of minute virus of mice, an autonomous parvovirus. Nucleic Acids Res. 1983;11:999–1018. doi: 10.1093/nar/11.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ball-Goodrich, L. J. 1997. Unpublished results.
- 6.Ball-Goodrich L J, Johnson E A. Molecular characterization of a newly recognized mouse parvovirus. J Virol. 1994;68:6476–6486. doi: 10.1128/jvi.68.10.6476-6486.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ball-Goodrich L J, Tattersall P. Two amino acid substitutions within the capsid are coordinately required for the acquisition of fibrotropism by the lymphotropic strain of minute virus of mice. J Virol. 1992;66:3415–3423. doi: 10.1128/jvi.66.6.3415-3423.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandenburger A, Legendre D, Avalosse B, Rommelaere J. NS-1 and NS-2 proteins may act synergistically in the cytopathogenicity of parvovirus MVMp. Virology. 1990;174:576–584. doi: 10.1016/0042-6822(90)90110-d. [DOI] [PubMed] [Google Scholar]
- 9.Brown D W, Welsh R M, Like A A. Infection of peripancreatic lymph nodes but not islets precedes Kilham rat virus-induced diabetes in BB/Wor rats. J Virol. 1993;67:5873–5878. doi: 10.1128/jvi.67.10.5873-5878.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brownstein D G, Smith A L, Jacoby R O, Johnson E A, Hansen G, Tattersall P. Pathogenesis of infection with a virulent allotropic variant of minute virus of mice and regulation by host genotype. Lab Invest. 1991;65:357–364. [PubMed] [Google Scholar]
- 11.Caillet-Fauquet P, Perros M, Brandenburger A, Spegelaere P, Rommelaere J. Programmed killing of human cells by means of an inducible clone of parvoviral genes encoding non-structural proteins. EMBO J. 1990;9:2989–2995. doi: 10.1002/j.1460-2075.1990.tb07491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang S-F, Sgro J-Y, Parrish C R. Multiple amino acids in the capsid structure of canine parvovirus coordinately determine the canine host range and specific antigenic and hemagglutination properties. J Virol. 1992;66:6858–6867. doi: 10.1128/jvi.66.12.6858-6867.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotmore S F, Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- 14.Gaertner D, Smith A, Jacoby R. Efficient induction of persistent and prenatal parvovirus infection in rats. Virus Res. 1996;44:67–78. doi: 10.1016/0168-1702(96)01351-2. [DOI] [PubMed] [Google Scholar]
- 15.Gaertner D J, Jacoby R O, Johnson E A, Paturzo F X, Smith A L, Brandsma J L. Characterization of acute rat parvovirus infection by in situ hybridization. Virus Res. 1993;28:1–18. doi: 10.1016/0168-1702(93)90085-2. [DOI] [PubMed] [Google Scholar]
- 16.Gaertner D J, Jacoby R O, Smith A L, Ardito R B, Paturzo F X. Persistence of rat virus in athymic rats. Arch Virol. 1989;105:259–268. doi: 10.1007/BF01311362. [DOI] [PubMed] [Google Scholar]
- 17.Guberski D I, Thomas V A, Shek W R, Like A A, Handler E S, Rossini A A, Wallace J E, Welsh R M. Induction of type I diabetes by Kilham rat virus in diabetes-resistant BB/Wor rats. Science. 1991;254:1010–1013. doi: 10.1126/science.1658938. [DOI] [PubMed] [Google Scholar]
- 18.Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 19.Jacoby R O, Ball-Goodrich L J, Besselsen D G, McKisic M D, Riley L K, Smith A L. Rodent parvovirus infections. Lab Anim Sci. 1996;46:370–380. [PubMed] [Google Scholar]
- 20.Jacoby R O, Ball-Goodrich L J. Parvovirus infections of mice and rats. Semin Virol. 1995;6:329–337. [Google Scholar]
- 21.Jacoby R O, Bhatt P N, Gaertner D J, Johnson E A, Smith A L. The pathogenesis of rat virus infection in infant and juvenile rats after oronasal inoculation. Arch Virol. 1987;95:251–270. doi: 10.1007/BF01310784. [DOI] [PubMed] [Google Scholar]
- 22.Jacoby R O, Gaertner D J, Bhatt P N, Paturzo F X, Smith A L. Transmission of experimentally induced rat virus infection. Lab Anim Sci. 1988;38:11–14. [PubMed] [Google Scholar]
- 23.Jacoby R O, Johnson E A, Ball-Goodrich L J, Smith A L, McKisic M D. Characterization of mouse parvovirus infection by in situ hybridization. J Virol. 1995;69:3915–3919. doi: 10.1128/jvi.69.6.3915-3919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacoby R O, Johnson E A, Paturzo F X, Gaertner D J, Brandsma J L, Smith A L. Persistent rat parvovirus infection in individually housed rats. Arch Virol. 1991;117:193–205. doi: 10.1007/BF01310765. [DOI] [PubMed] [Google Scholar]
- 25.Kilham L, Ferm V. Rat virus (RV) infection of pregnant, fetal and newborn rats. Proc Soc Exp Biol Med. 1964;117:874–879. doi: 10.3181/00379727-117-29723. [DOI] [PubMed] [Google Scholar]
- 26.Legendre D, Rommelaere J. Targeting of promoters for trans activation by a carboxy-terminal domain of the NS-1 protein of the parvovirus minute virus of mice. J Virol. 1994;68:7974–7985. doi: 10.1128/jvi.68.12.7974-7985.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Legrand C, Rommelaere J, Caillet-Fauquet P. MVM(p) NS-2 protein expression is required with NS-1 for maximal cytotoxicity in human transformed cells. Virology. 1993;195:149–155. doi: 10.1006/viro.1993.1355. [DOI] [PubMed] [Google Scholar]
- 28.Leland, S., and R. Jacoby. 1997. Unpublished results.
- 29.McKisic M, Paturzo F, Smith A. Mouse parvovirus infection potentiates rejection of tumor allografts and modulates T cell effector functions. Transplantation. 1996;61:292–299. doi: 10.1097/00007890-199601270-00022. [DOI] [PubMed] [Google Scholar]
- 30.McKisic M D, Lancki D W, Otto G, Padrid P, Snook S, Cronin II D C, Lohmar P D, Wong T, Fitch F. Identification and propagation of a putative immunosuppressive orphan parvovirus in cloned T cells. J Immunol. 1993;150:419–428. [PubMed] [Google Scholar]
- 31.McKisic M D, Paturzo F X, Gaertner D J, Jacoby R O, Smith A L. A nonlethal rat parvovirus infection suppresses T lymphocyte effector functions. J Immunol. 1995;155:3979–3986. [PubMed] [Google Scholar]
- 32.McLean I W, Nakane P K. Periodate-lysine-paraformaldehyde fixative. A new fixative for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- 33.Morgan W R, Ward D C. Three splicing patterns are used to excise the small intron common to all minute virus of mice RNAs. J Virol. 1986;60:1170–1174. doi: 10.1128/jvi.60.3.1170-1174.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Op De Beeck A, Anouja F, Mousset S, Rommelaere J, Caillet-Fauquet P. The nonstructural proteins of the autonomous parvovirus minute virus of mice interfere with the cell cycle, inducing accumulation in G2. Cell Growth Differ. 1995;6:781–787. [PubMed] [Google Scholar]
- 35.Op De Beeck A, Caillet-Faquet P. The NS1 protein of the autonomous parvovirus minute virus of mice blocks cellular DNA replication: a consequence of lesions to the chromatin? J Virol. 1997;71:5323–5329. doi: 10.1128/jvi.71.7.5323-5329.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paturzo, F. 1997. Unpublished results.
- 37.Paturzo F X, Jacoby R O, Bhatt P N, Smith A L, Gaertner D J, Ardito R B. Persistence of rat virus in seropositive rats as detected by explant culture. Arch Virol. 1987;95:137–142. doi: 10.1007/BF01311341. [DOI] [PubMed] [Google Scholar]
- 38.Reed A P, Jones E V, Miller T J. Nucleotide sequence and genome organization of canine parvovirus. J Virol. 1988;62:266–276. doi: 10.1128/jvi.62.1.266-276.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhode S L, III, Paradiso P R. Parvovirus genome: nucleotide sequence of H-1 and mapping of its genes by hybrid-arrested translation. J Virol. 1983;45:173–184. doi: 10.1128/jvi.45.1.173-184.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riley L, Besselsen D, Knowles R, Franklin C, Hook R J, Resch-Williford C, Pintel D. Abstracts of the Annual Meeting of the American Association for Laboratory Animal Science. Minneapolis, Minn: American Association for Laboratory Animal Science; 1996. Characterization of two newly recognized rat parvovirus strains, abstract PS06; p. 2. [Google Scholar]
- 41.Sahli R, McMaster G K, Hirt B. DNA sequence comparison between two tissue-specific variants of the autonomous parvovirus, minute virus of mice. Nucleic Acids Res. 1985;13:3617–3633. doi: 10.1093/nar/13.10.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 43.Smith A L, Jacoby R O, Johnson E A, Paturzo F, Bhatt P N. In vivo studies with an “orphan” parvovirus of mice. Lab Anim Sci. 1993;43:175–182. [PubMed] [Google Scholar]
- 44.Tattersall P, Bratton J. Reciprocal productive and restrictive virus-cell interactions of immunosuppressive and prototype strains of minute virus of mice. J Virol. 1983;46:944–955. doi: 10.1128/jvi.46.3.944-955.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tattersall P, Cotmore S F. The rodent parvoviruses. In: Bhatt P N, Jacoby R O, Morse H C, News A E, editors. Viral and mycoplasmal infections of rodents: effects on biomedical research. Orlando, Fla: Academic Press; 1986. pp. 305–348. [Google Scholar]
- 46.Truyen U, Gruenberg A, Chang S-F, Obermaier B, Veijalainen P, Parrish C. Evolution of the feline-subgroup parvoviruses and the control of canine host range in vivo. J Virol. 1995;69:4702–4710. doi: 10.1128/jvi.69.8.4702-4710.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vasudevacharya J, Basak S, Srinivas R V, Compans R W. Nucleotide sequence analysis of the capsid genes and the right-hand terminal palindrome of porcine parvovirus, strain NADL-2. Virology. 1989;173:368–377. doi: 10.1016/0042-6822(89)90549-7. [DOI] [PubMed] [Google Scholar]
- 48.Ward J M, Reynolds C W. Large granular lymphocyte leukemia. A heterogeneous lymphocytic leukemia in F344 rats. Am J Pathol. 1983;111:1–10. [PMC free article] [PubMed] [Google Scholar]