Abstract
We sought to assess changes in corneal biomechanical parameters in patients with diabetes mellitus (DM) in comparison with those among healthy controls using Corvis ST (CST). The study group included 209 eyes from healthy control subjects and 33 eyes from diabetic subjects, respectively. Following an ophthalmological examination, measurements with CST were taken. Additionally, hemoglobin A1c and blood glucose values were collected. Results were then compared to those of the control group after adjusting for potential confounding factors, including age-, intraocular pressure (IOP)-, central corneal thickness (CCT)-, spherical equivalent (SE)- and axial length (AL). After adjusting for potential confounding factors, including the age, IOP, CCT, SE, and AL, patients with DM presented significantly lower whole-eye movement (WEM) (ms) values than patients without DM (21.71 ± 0.84 vs. 22.15 ± 0.64 ms; P < .001). There was a significant and negative correlation between WEM (ms) and hemoglobin A1c in DM patients (r = −0.733; P = .001). In univariate and multivariate general linear mixed model (GLMM) analyses, IOP (P < .001 and P < .001, respectively) and the presence of DM (P = .001 and P < .001, respectively) significantly affected WEM (ms). In DM, significant changes in corneal biomechanical properties were detectable. The DM group showed significantly less deformable cornea and sclera than did the normal controls, even after adjusting for age, IOP, CCT, SE, and AL. These findings may cause misinterpretation of IOP measurements in diabetic patients. Therefore, the measurement of corneal biomechanics should be taken into consideration in clinical practice.
Keywords: biomechanics, corneal biomechanical properties, Corvis ST, diabetes mellitus, whole eye movement
1. Introduction
Glaucoma is progressive optic neuropathy, and elevated intraocular pressure (IOP) is the major risk factor for the development and progression of this disease.[1] The accuracy of IOP measurement is influenced by biomechanical characteristics of the cornea and central corneal thickness (CCT).[2] Although the correlation between CCT and glaucoma has been widely studied,[3,4] other factors, such as curvature or rigidity, may also affect the ocular response to the force applied during the IOP measurement process.[5–7] For this reason, new tonometers capable of accounting for biomechanical properties of the cornea are essential tools at present.
Evaluating corneal biomechanics is a challenge and could not be performed in vivo until the introduction of ocular response analyzers (ORAs).[8] The Corvis ST (CST) (Oculus Optikgeräte GmbH, Wetzlar, Germany) is an even newer instrument integrated with an ultra-high-speed Scheimpflug camera.[9] CST enables the direct visualization of corneal movement during the application of a rapid air puff, and CST measures biomechanical properties of the eye by recording the shape of the cornea as it becomes deformed in response to the application of an air pulse.[10]
Collagen-based body structures, including the joints, skin, and cornea, are more rigid in DM patients.[11–13] These pathophysiologic changes in DM may be the reason for inaccuracies and misinterpretations of IOP readings that occur in some diabetic cases.[14,15]
Although several studies have explored the relationship between corneal biomechanics and DM, it remains unclear whether corneal biomechanical properties change in diabetic patients.[14] Previous studies using ORAs have reported significant alterations of biomechanical properties in diabetic patients in relation to their metabolic state.[15–23] DM affected corneal biomechanics resulting in increased corneal hysteresis (CH) and corneal resistance factor (CRF).[15,17,19,22,23] CH was significantly higher in diabetic even after correcting for age, IOP, and CCT but was not related to the duration of diabetes.[16] In diabetes, CH and CRF are correlated to hemoglobin A1c (HbA1c) and blood glucose concentration.[17,19] Also, Primary open-angle glaucoma patients with diabetes have significantly higher CH values than those without diabetes.[20] On the contrary, other studies found that DM results in lower CH values than those in healthy control subjects.[18,21]
On the other hand, data of corneal parameters measured by CST in DM are limited to date.[21–23] Some CST parameters, such as, Deformation amplitude (DA), applanation 1 (A1) and applanation 2 (A2) times, A1 velocity, in the uncontrolled DM group eyes were found to be significantly different from healthy group eyes and controlled DM group eyes.[21] A1 and A2 deflection amplitudes were increased and highest concavity (HC) and A2 time were extended in DM.[22,23]
Therefore, it was the aim of the present observational, cross-sectional study to investigate corneal biomechanical properties in diabetic patients and compared with those of the healthy subjects after adjusting for age-, IOP-, CCT-, SE- and AL using CST. In addition, we sought to determine whether disease duration and levels of hyperglycemia and HbA1c influenced these changes. Finally, we aimed to test our study hypothesis that the diabetic cornea is stiffer than the healthy one.
2. Methods
In total, 180 patients and a total of 242 eyes were included in this retrospective observational study. The study subjects included 154 healthy control subjects and 26 diabetic subjects who visited the glaucoma clinic at Yeouido St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, between June and July 2018. This study followed the tenets of the Declaration of Helsinki for biomedical research and was approved by the Yeouido St. Mary’s Hospital Institutional Review Board (SC21RISI0085). Patients with previous corneal disease, surgical ocular interventions, systemic connective tissue diseases, contact lens use, or glaucoma were excluded. Current HbA1c and blood glucose values were collected from patients with DM. Median time interval between HbA1c assessment and CST measurement was 1.5 months. Furthermore, the time of DM diagnosis and current medication were precisely assessed.
Before study measurements, all subjects underwent a complete ophthalmologic examination, including best-corrected visual acuity measurement, slit-lamp biomicroscopy, Goldmann applanation tonometry IOP measurements and funduscopy, as well as, clinical assessment of presence, and severity of diabetic retinopathy and maculopathy. To exclude other influencing factors, ocular biometry (IOL Master; Carl Zeiss Meditec, Oberkochen, Germany) and corneal topography (Pentacam; Oculus, Wetzlar, Germany) were performed in each participant. Subsequently, measurements using CST (software ver. 102R1092; Oculus Optikgeräte GmbH, Wetzlar, Germany) were taken. All CST measurements were made between AM 8:30 and PM 4:30.
The inclusion criteria for the study were CCTs from 485 to 615 μm, less than 3 diopters of cylinder correction, and the presence of a normal anterior chamber and an open angle. The normal control group and DM group included patients with IOP-values of less than 22 mmHg with no history of using anti-glaucomatous eyedrops.
2.1. CST measurements
The CST was used to obtain the measurements of the IOP and corneal biomechanical properties. When using this device, once the patient’s cornea was centered appropriately, the device automatically emitted an air impulse from a distance of 11 mm. An ultra-high-speed Scheimpflug camera recorded the corneal deformation process in response to the air impulse, collecting a total of 4330 images per second. The camera took 140 digital frames with a resolution of 640 × 480 pixels within 30 ms with an 8.5-mm horizontal coverage of the central cornea. In this context, due to the air impulse, the cornea evolves through three distinct phases (A1, HC, and A2); The recording begins with the cornea in the natural convex shape, at which time, the CCT is measured. As the air impulse is emitted, the cornea moves inwards through A1 or flattening of the cornea, into a concavity phase, until it reaches the HC. Then, the cornea gradually returns to its natural shape, passing through a second A2 stage. The IOP is measured based on the time to reach A1. In this study, measurements by CST were collected only once per eye because previous reports have indicated reliable and good-quality results may be obtained even after a single investigation.[24,25] The recorded CST parameters are listed in Table 1. The DA, which is the amount of corneal displacement at the HC, is made up of the deflection amplitude, which is the pure corneal component, and the whole-eye movement (WEM), which is the orbital component.[26,27]
Table 1.
Corvis ST parameters.
| Parameters | Description |
|---|---|
| A1 time | Time from air puff to A1 |
| A1 velocity | Inward velocity of the cornea at A1 |
| A2 time | Time from start to A2 |
| A2 velocity | Outward velocity of the cornea at A2 |
| HC time | Time of occurrence of the highest corneal concavity |
| Peak distance | Distance between the corneal peaks at maximal concavity |
| Radius of curvature | Radius of curvature of the cornea at highest concavity |
| A1 deformation amplitude | Sum of the deflection amplitude and the WEM at A1 |
| HC deformation amplitude | Corneal displacement at highest concavity |
| A2 deformation amplitude | Sum of the deflection amplitude and the WEM at A2 |
| A1 deflection amplitude | Moving distance of the corneal apex from the initial position to that at the A1 time without WEM |
| HC deflection amplitude | Distance of the corneal apex movement from the initiation of the deformation to the highest concavity without WEM |
| A2 deflection amplitude | Moving distance of the corneal apex from the initial position to that at the A2 time without WEM |
| Deflection amplitude maximum (mm) | The maximum amount of the corneal movement compensating for WEM |
| WEM (ms) | The duration of eye movement during the examination |
| WEM (mm) | The total amount of eye movement during the examination |
| Maximum inverse radius | Highest value of the reciprocal of radius curvature at highest concavity |
| DA ratio maximum (1, 2 mm) | Ratio between the deformation amplitude at the apex and 1 or 2 mm |
| Integrated radius | Represents the amount of the corneal concave state over the time between A1 and A2 |
| Stiffness parameter A1 (SP-A1) (mmHg/mm) | The resulting pressure on the cornea divided by the deflection amplitude at A1 |
A1 = applanation 1, A2 = applanation 2, DA = deformation amplitude, HC = highest concavity, WEM = whole-eye movement.
During the measurement, there is a slight but significant movement of the whole eye globe. The WEM is determined by the degree of the slow linear motion of eyeball in the anterior-posterior axis; the WEM is summarized by two measurements of the maximal displacement and the time taken to reach the maximal displacement. WEM reflects the absorbed portion of kinetic energy of the air puff by the extra-corneal tissues, which could reflect the mechanical conditions in orbit. The deflection amplitude and the WEM were retrospectively calculated by an external analysis of the CST data.
2.2 Statistical analyses
Levene’s test verified equality of variances for each of the variables. T-test was performed if equal variance was assumed and Welch’s T-test was performed where variances were unequal. A one-way analysis of variance and Scheffe’s multiple comparison test were used to compare data among the groups. Possible effects of potential confounding variables, including age, Goldmann applanation tonometry IOP, SE, and AL were controlled for by analyses of covariance. Pearson’s correlation and partial correlation were used to determine the relationships between CST parameters and DM profiles after adjusting for IOP and age at the time of CST measurement. To determine factors associated with the CST parameters, we conducted general linear mixed model (GLMM) analyses due to the inclusion of both eyes data using the Statistical Package for the Social Sciences (version 25.0; IBM Corporation, Armonk, NY, USA). In all analyses, P < .05 was taken to indicate statistical significance.
3. Results
The study was conducted involving a total of 242 eyes (including 209 eyes from normal controls and 33 eyes from DM patients). Baseline characteristics are shown in Table 2. There were 70 men (45.5%) and 84 women (54.5%) in the non-DM group, and 18 men (69.2%) and 8 women (30.8%) in the DM group. The age, IOP, refraction, keratometry, axial length, and CCT were similar in the DM and healthy groups.
Table 2.
Baseline characteristics of the normal control and diabetes mellitus groups.
| Baseline characteristic | Normal control (n = 209) | DM group (n = 33) | P Value* |
|---|---|---|---|
| Age (y) | 61.88 ± 13.47 | 64.85 ± 11.40 | .231 |
| Intraocular pressure (mmHg) | 14.45 ± 2.44 | 15.21 ± 3.52 | .236 |
| Spherical equivalent (D) | −1.49 ± 3.27 | −0.46 ± 2.49 | .115 |
| Keratometry (D) | 43.40 ± 1.83 | 43.42 ± 2.12 | .959 |
| Axial length (mm) | 24.20 ± 1.60 | 23.87 ± 0.98 | .399 |
| Central corneal thickness (μm) | 551.28 ± 31.00 | 560.55 ± 32.72 | .115 |
| Duration of DM (y) | – | 15.36 ± 11.25 | – |
| HbA1c (%) | – | 7.20 ± 0.96 | – |
| Blood glucose (mg/dL) | – | 164.13 ± 55.02 | – |
Values are presented as mean ± standard deviation.
DM = diabetes mellitus; HbA1c = hemoglobin A1c; – = not available.
Comparison between 2 groups completed using the student’s t test.
The mean duration of DM and HbA1c and blood glucose values in the DM group were 15.36 ± 11.25 years, 7.20 ± 0.96 %, and 164.13 ± 55.02 mg/dL, respectively. One patient received dietary therapy, 15 had oral antidiabetics, and 4 were treated by insulin alone or as a combination therapy. In 6 cases, data on current antidiabetic therapy were not available. Fourteen diabetic eyes had no funduscopic changes, whereas, in 19 eyes, diabetic retinopathy was present (7 mild nonproliferative, 4 moderate nonproliferative, 3 severe nonproliferative, and 5 proliferative cases of diabetic retinopathy). In 6 eyes, diabetic maculopathy was detectable.
CST parameters presented differences between the DM and healthy control groups. Because the device provides several parameters, a selection of certain CST values is given here and in Table 3. An unadjusted comparison between normal controls and the DM group revealed a significant difference in the WEM maximum (ms) (22.14 ± 0.64 vs. 21.71 ± 0.84 ms; P = .001). In an age-, IOP-, CCT-, SE- and AL-adjusted comparison, the DM group (0.45 ± 0.08 ms) displayed a significantly lower A2 DA (mm) compared to the normal group (0.48 ± 0.09 ms; P = .029). The WEM maximum (mm) of the DM group (0.34 ± 0.07 mm) was statistically significantly lower than that of the normal group (0.34 ± 0.07 mm; P = .04); in addition, the WEM maximum (ms) was statistically significantly lower in the DM group (21.71 ± 0.84 ms) than in the normal control group (22.15 ± 0.64 ms; P < .001) after adjusting for age, IOP, CCT, SE, and AL.
Table 3.
Unadjusted and age- and intraocular pressure-adjusted Corvis ST parameters in the normal control and diabetes mellitus groups.
| Corvis ST parameters | Unadjusted | Adjusted* | ||||
|---|---|---|---|---|---|---|
| Normal control (n = 209) | DM group (n = 33) | P value† | Normal control (n = 209) | DM group (n = 33) | P value† | |
| DA maximum (mm) | 1.13 ± 0.11 | 1.11 ± 0.13 | .297 | 1.13 ± 0.12 | 1.11 ± 0.13 | .632 |
| A1 time (ms) | 7.29 ± 0.31 | 7.39 ± 0.44 | .116 | 7.29 ± 0.31 | 7.39 ± 0.44 | .969 |
| A1 velocity (m/s) | 0.15 ± 0.02 | 0.15 ± 0.02 | .395 | 0.15 ± 0.02 | 0.15 ± 0.02 | .524 |
| A2 time (ms) | 22.09 ± 0.42 | 21.98 ± 0.53 | .188 | 22.09 ± 0.42 | 21.98 ± 0.53 | .925 |
| A2 velocity (m/s) | −0.26 ± 0.04 | −0.26 ± 0.04 | .993 | −0.26 ± 0.04 | −0.26 ± 0.04 | .489 |
| HC time (ms) | 16.94 ± 0.59 | 16.73 ± 0.65 | .067 | 16.93 ± 0.59 | 16.73 ± 0.65 | .057 |
| Peak distance (mm) | 5.11 ± 0.31 | 5.02 ± 0.33 | .129 | 5.10 ± 0.31 | 5.02 ± 0.33 | .411 |
| Radius (mm) | 7.58 ± 0.99 | 7.61 ± 0.89 | .852 | 7.58 ± 1.00 | 7.61 ± 0.89 | .518 |
| A1 DA (mm) | 0.14 ± 0.01 | 0.14 ± 0.01 | .281 | 0.14 ± 0.01 | 0.14 ± 0.01 | .924 |
| HC DA (mm) | 1.13 ± 0.12 | 1.11 ± 0.13 | .297 | 1.13 ± 0.12 | 1.11 ± 0.13 | .632 |
| A2 DA (mm) | 0.48 ± 0.09 | 0.45 ± 0.08 | .074 | 0.48 ± 0.09 | 0.45 ± 0.08 | .029 |
| A1 deflection amplitude (mm) | 0.10 ± 0.01 | 0.10 ± 0.01 | .546 | 0.10 ± 0.01 | 0.10 ± 0.01 | .949 |
| HC deflection amplitude (mm) | 0.92 ± 0.12 | 0.89 ± 0.12 | .189 | 0.92 ± 0.12 | 0.89 ± 0.12 | .475 |
| A2 deflection amplitude (mm) | 0.12 ± 0.02 | 0.12 ± 0.02 | .202 | 0.12 ± 0.02 | 0.12 ± 0.02 | .763 |
| Deflection amplitude maximum (mm) | 0.95 ± 0.11 | 0.91 ± 0.12 | .061 | 0.95 ± 0.11 | 0.91 ± 0.12 | .165 |
| WEM maximum (mm) | 0.37 ± 0.09 | 0.34 ± 0.07 | .061 | 0.37 ± 0.09 | 0.34 ± 0.07 | .040 |
| WEM maximum (ms) | 22.14 ± 0.64 | 21.71 ± 0.84 | .001 | 22.15 ± 0.64 | 21.71 ± 0.84 | <.001 |
| Maximum inverse radius (mm−1) | 0.17 ± 0.03 | 0.18 ± 0.04 | .351 | 0.17 ± 0.03 | 0.18 ± 0.04 | .079 |
| DA ratio maximum (2 mm) | 4.31 ± 1.70 | 4.20 ± 0.48 | .706 | 4.31 ± 1.71 | 4.20 ± 0.48 | .893 |
| DA ratio maximum (1 mm) | 1.55 ± 0.05 | 1.55 ± 0.05 | .420 | 1.55 ± 0.05 | 1.55 ± 0.05 | .963 |
| Integrated radius (mm−1) | 8.02 ± 1.09 | 7.87 ± 1.34 | .538 | 8.01 ± 1.09 | 7.87 ± 1.34 | .459 |
| SP-A1 (mmHg/mm) | 104.55 ± 17.14 | 111.21 ± 26.79 | .175 | 104.70 ± 17.11 | 111.21 ± 26.79 | .862 |
Values are presented as means ± standard deviation.
Significant results are indicated in bold.
A1 = applanation 1; A2 = applanation 2; DA = deformation amplitude; HC = highest concavity; SP-A1 = stiffness parameter A1; WEM = whole-eye movement.
Adjusted for age, IOP, CCT, spherical equivalent, axial length.
Comparison between two groups by Student’s t-test.
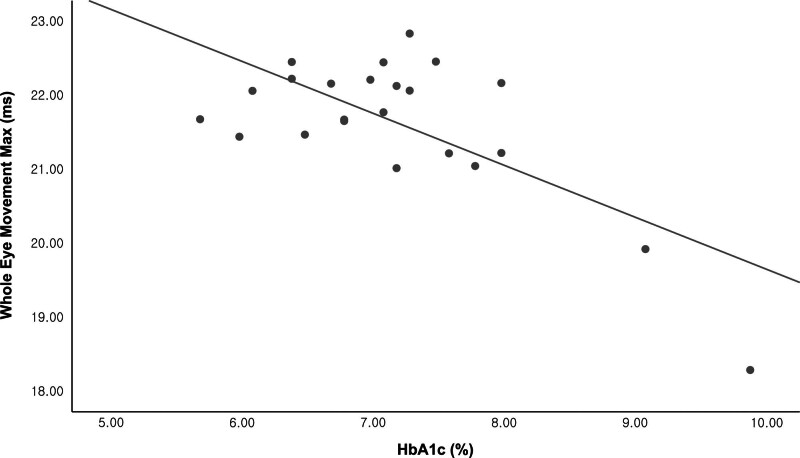
Table 4 and Figure 1 show the relationship between HbA1c and CST parameters in the DM group using unadjusted data and after adjusting for IOP alone and IOP, age, CCT, SE, and AL, respectively. The HbA1c showed a statistically significant relationship with the WEM maximum (ms) at when considering the unadjusted data (r = −0.694; P < ..001) and after adjusting for IOP (r = −0.708; P < .001) and IOP, age, CCT, SE and AL (r = −0.733; P = .001). The HC deflection amplitude (mm) showed negative after adjusting for IOP (r = −0.491; P = .02) and IOP, age, CCT, SE and AL (r = −0.501; P = .034). Separately, integrated radius (mm−1) showed a negative correlation with HbA1c (r = −0.453; P = .034) after adjusting for IOP and Deflection amplitude maximum (mm) showed negative correlation with HbA1c after adjusting for IOP, age, CCT, SE and AL (r = −0.501; P = .034).
Table 4.
Relationship between Corvis ST parameters and hemoglobin A1c in the diabetes mellitus group.
| Corvis ST parameters | Model 1* | Model 2† | Model 3‡ | |||
|---|---|---|---|---|---|---|
| r | P value | r | P value | r | P value | |
| DA maximum (mm) | −0.052 | .815 | −0.043 | .851 | −0.133 | .598 |
| A1 time (ms) | 0.052 | .813 | 0.286 | .196 | 0.192 | .446 |
| A1 velocity (m/s) | −0.278 | .199 | −0.478 | .024 | −0.483 | .042 |
| A2 time (ms) | −0.067 | .763 | −0.070 | .758 | -0.27 | .915 |
| A2 velocity (m/s) | 0.284 | .190 | 0.384 | .078 | 0.373 | .127 |
| HC time (ms) | 0.062 | .778 | 0.057 | .802 | 0.084 | .739 |
| Peak distance (mm) | −0.311 | .149 | −0.498 | .018 | −0.459 | .056 |
| Radius (mm) | 0.154 | .483 | 0.170 | .450 | 0.073 | .773 |
| A1 DA (mm) | −0.319 | .138 | −0.375 | .085 | −0.404 | .096 |
| HC DA (mm) | -0.052 | .815 | -0.043 | .851 | −0.133 | .598 |
| A2 DA (mm) | 0.061 | .783 | 0.054 | .811 | −0.068 | .789 |
| A1 deflection amplitude (mm) | −0.245 | .261 | −0.247 | .268 | −0.306 | .217 |
| HC deflection amplitude (mm) | −0.329 | .125 | −0.491 | .020 | −0.501 | .034 |
| A2 deflection amplitude (mm) | −0.181 | .409 | −0.186 | .407 | −0.176 | .484 |
| Deflection amplitude maximum (mm) | −0.337 | .116 | −0.488 | .021 | −0.501 | .034 |
| WEM maximum (mm) | 0.265 | .221 | 0.268 | .228 | 0.121 | .632 |
| WEM maximum (ms) | −0.694 | <.001 | −0.708 | <.001 | −0.733 | .001 |
| Maximum inverse radius (mm−1) | −0.051 | .816 | −0.042 | .854 | −0.033 | .898 |
| DA ratio maximum (2 mm) | −0.176 | .421 | −0.248 | .266 | −0.445 | .064 |
| DA ratio maximum (1 mm) | −0.057 | .796 | −0.050 | .826 | −0.149 | .554 |
| Integrated radius (mm−1) | −0.287 | .185 | −0.453 | .034 | −0.366 | .135 |
| SP-A1 (mmHg/mm) | 0.297 | .169 | 0.383 | .078 | 0.290 | .244 |
Significant results are shown in bold.
A1 = applanation 1; A2 = applanation 2; DA = deformation amplitude; HC = highest concavity; SP-A1 = Stiffness parameter A1; WEM = whole-eye movement.
Model 1: unadjusted, Pearson’s correlation coefficient.
Model 2: adjusted for IOP, partial correlation.
Model 3: adjusted for IOP, age, CCT, spherical equivalent and axial length, partial correlation.
Figure 1.
Scatterplot showing the relationship between WEM (ms) and HbA1c (Pearson’s r = −0.677; P < .001).
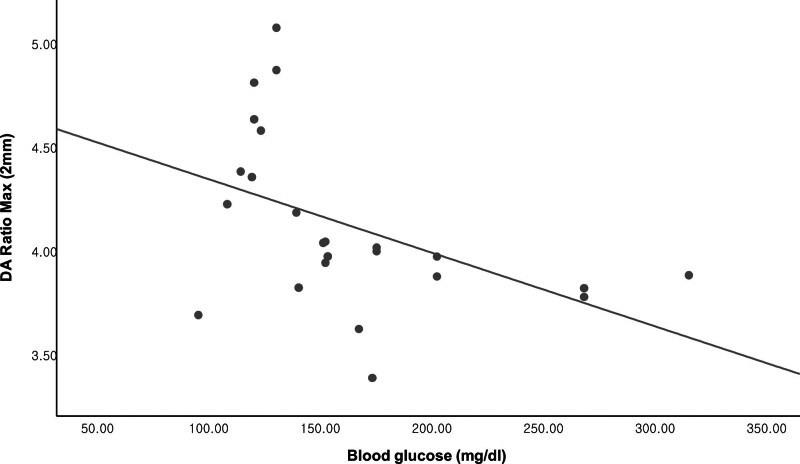
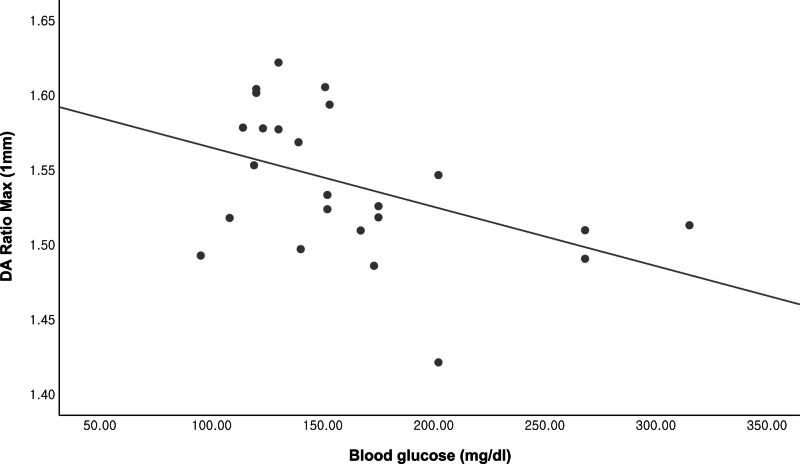
Table 5 and Figures 2 and 3 show the relationship between blood glucose level and CST parameters in the DM group using unadjusted data and after adjusting for IOP alone and IOP, age, CCT, SE, and AL, respectively. Blood glucose level had a statistically significant relationship with the DA ratio maximum (2 mm) and DA ratio maximum (1 mm) according to raw data (r = −0.464; P = .022 and r = −0.447; P = .029, respectively) and after adjusting for IOP (r = −0.498; P = .016 and r = −0.447; P = .033, respectively) and IOP, age, CCT, SE and AL (r = −0.583; P = .009 and r = −0.475; P = .04, respectively), respectively.
Table 5.
Relationship between deformation parameters and blood glucose level (mg/dL) in the diabetes mellitus group.
| Corvis ST parameters | Model 1* | Model 2† | Model 3‡ | |||
|---|---|---|---|---|---|---|
| r § | P value | r Π | P value | r Π | P value | |
| DA maximum (mm) | −0.163 | .445 | −0.055 | .802 | 0.041 | .868 |
| A1 time (ms) | 0.148 | .491 | −0.251 | .248 | −0.192 | .430 |
| A1 velocity (m/s) | −0.277 | .190 | −0.263 | .225 | −0.331 | .166 |
| A2 time (ms) | −0.184 | .390 | −0.088 | .691 | −0.046 | .853 |
| A2 velocity (m/s) | 0.191 | .371 | 0.105 | .634 | 0.142 | .562 |
| HC time (ms) | 0.158 | .462 | 0.148 | .501 | 0.179 | .463 |
| Peak distance (mm) | −0.160 | .456 | −0.035 | .873 | −0.152 | .535 |
| Radius (mm) | 0.005 | .982 | −0.052 | .814 | −0.009 | .970 |
| A1 DA (mm) | −0.012 | .955 | −0.094 | .669 | -0.069 | .780 |
| HC DA (mm) | −0.163 | .445 | -0.055 | .802 | 0.041 | .868 |
| A2 DA (mm) | 0.054 | .804 | 0.052 | .815 | 0.141 | .566 |
| A1 deflection amplitude (mm) | −0.144 | .502 | −0.153 | .485 | −0.152 | .534 |
| HC deflection amplitude (mm) | −0.267 | .207 | −0.235 | .280 | −0.277 | .251 |
| A2 deflection amplitude (mm) | −0.024 | .911 | −0.036 | .870 | 0.046 | .850 |
| Deflection amplitude maximum (mm) | −0.249 | .240 | −0.202 | .356 | −0.229 | .346 |
| WEM maximum (mm) | 0.070 | .745 | 0.073 | .742 | 0.182 | .456 |
| WEM maximum (ms) | −0.157 | .464 | −0.189 | .387 | −0.277 | .251 |
| Maximum inverse radius (mm−1) | −0.122 | .570 | −0.054 | .807 | -0.055 | .824 |
| DA ratio maximum (2 mm) | −0.464 | .022 | −0.498 | .016 | −0.583 | .009 |
| DA ratio maximum (1 mm) | −0.447 | .029 | −0.447 | .033 | −0.475 | .040 |
| Integrated radius (mm−1) | −0.251 | .237 | −0.201 | .357 | −0.322 | .179 |
| SP-A1 (mmHg/mm) | 0.172 | .422 | 0.079 | .721 | 0.273 | .259 |
Significant results are shown in bold.
A1 = applanation 1; A2 = applanation 2; DA = deformation amplitude; HC = highest concavity; SP-A1 = Stiffness parameter A1; WEM = whole-eye movement.
Model 1: unadjusted, Pearson’s correlation coefficient.
Model 2: adjusted for IOP, partial correlation.
Model 3: adjusted for IOP, age, CCT, spherical equivalent and axial length, partial correlation.
Pearson’s correlation coefficient.
Partial correlation coefficient.
Figure 2.
Scatterplot showing the relationship between the DA ratio maximum (2 mm) and blood glucose level (Pearson’s r = −0.403; P = .046).
Figure 3.
Scatterplot showing the relationship between the DA ratio maximum (1 mm) and blood glucose level (Pearson’s r = −0.435; P = .030).
Table 6 shows the relationship between DM duration and CST parameters in the DM group using unadjusted data and after adjusting for IOP and IOP, age, CCT, SE, and AL, respectively. Notably, DM duration exhibits a statistically significant relationship with A2 time (ms) after adjusting for IOP, age, CCT, SE, and AL (r = −0.411; P = .03).
Table 6.
Relationship between deformation parameters and diabetes mellitus duration in the diabetes mellitus group.
| Corvis ST parameters | Model 1* | Model 2† | Model 3‡ | |||
|---|---|---|---|---|---|---|
| r | P value | r | P value | r | P valueValue | |
| DA maximum (mm) | 0.047 | .795 | −0.134 | .464 | −0.223 | .253 |
| A1 time (ms) | −0.161 | .370 | 0.044 | .810 | 0.024 | .905 |
| A1 velocity (m/s) | 0.019 | .916 | −0.235 | .195 | −0.282 | .146 |
| A2 time (ms) | −0.083 | .647 | −0.414 | .018 | −0.411 | .030 |
| A2 velocity (m/s) | −0.004 | .982 | 0.185 | .310 | 0.208 | .288 |
| HC time (ms) | −0.048 | .790 | −0.045 | .806 | 0.018 | .926 |
| Peak distance (mm) | 0.090 | .620 | −0.055 | .766 | 0.008 | .966 |
| Radius (mm) | −0.071 | .696 | −0.005 | .976 | 0.116 | .557 |
| A1 DA (mm) | −0.167 | .354 | −0.115 | .532 | −0.096 | .627 |
| HC DA (mm) | 0.047 | .795 | −0.134 | .464 | −0.223 | .253 |
| A2 DA (mm) | 0.024 | .895 | 0.015 | .934 | −0.057 | .775 |
| A1 deflection amplitude (mm) | −0.082 | .651 | −0.079 | .666 | -0.042 | .833 |
| HC deflection amplitude (mm) | 0.075 | .679 | −0.088 | .631 | −0.094 | .633 |
| A2 deflection amplitude (mm) | −0.150 | .404 | −0.150 | .412 | −0.127 | .519 |
| Deflection amplitude maximum (mm) | 0.057 | .751 | −0.119 | .516 | −0.128 | .517 |
| WEM maximum (mm) | 0.088 | .625 | 0.080 | .664 | -0.013 | .949 |
| WEM maximum (ms) | 0.115 | .523 | 0.136 | .459 | 0.209 | .287 |
| Maximum inverse radius (mm−1) | 0.086 | .634 | 0.016 | .932 | -0.102 | .605 |
| DA ratio maximum (2 mm) | 0.190 | .291 | 0.108 | .555 | -0.033 | .868 |
| DA ratio maximum (1 mm) | 0.176 | .328 | 0.095 | .603 | -0.013 | .946 |
| Integrated radius (mm−1) | 0.133 | .460 | 0.021 | .909 | -0.093 | .638 |
| SP-A1 (mmHg/mm) | −0.083 | .646 | 0.060 | .743 | 0.075 | .705 |
Significant results are shown in bold.
A1 = applanation 1; A2 = applanation 2; DA = deformation amplitude; HC = highest concavity; SP-A1 = Stiffness parameter A1; WEM = whole-eye movement.
Model 1: unadjusted, Pearson’s correlation coefficient.
Model 2: adjusted for IOP, partial correlation.
Model 3: adjusted for IOP, age, CCT, spherical equivalent and axial length, partial correlation.
To identify the factors affecting the CST parameters, general linear mixed model (GLMM) analyses were performed. The WEM max.(ms) was associated with IOP and the presence of DM in both the univariate (P < .001 and P = .001, respectively) and multivariate (P < .001 and P < .001, respectively) regression analyses (Table 7).
Table 7.
General linear mixed model (GLMM) analyses using whole-eye movement maximum (ms) as the dependent variable.
| Univariate* | Multivariate† | |||
|---|---|---|---|---|
| Beta | P value | Beta | P value | |
| Age (years) | 0.007 | .07 | − | − |
| Sex, male:female | 0.12 | .240 | − | − |
| Intraocular pressure (mmHg) | −0.072 | <.001 | −0.067 | <.001 |
| Spherical equivalent (D) | −0.011 | .452 | - | − |
| Axial length (mm) | −0.019 | .598 | − | − |
| Central corneal thickness (μm) | 0.000 | .972 | − | − |
| Presence of DM | −0.479 | .001 | −0.529 | <.001 |
Significant results are shown in bold.
DM = diabetes mellitus.
Univariate GLMM analysis.
Multivariate GLMM analysis.
4. Discussion
In the current study, our results revealed significant differences existed in CST parameters between DM and healthy control eyes, particularly regarding A2 DA (age-, IOP-, SE-, and AL- adjusted), WEM (mm) (age-, IOP-, SE-, and AL- adjusted) and WEM (ms) (unadjusted and age-, IOP-, SE- and AL- adjusted). We found that A1 velocity (m/s), HC deflection amplitude (mm), deflection amplitude maximum (mm), WEM (ms), and integrated radius (mm−1) were inversely correlated with HbA1c, while the DA ratio maximum (1 mm) and DA ratio maximum (2 mm) were inversely correlated with blood glucose level and the A2 time (ms) was inversely correlated with the duration of DM. These results are in agreement with those of earlier CST studies of diabetic patients. Ramm et al reported that DA ratio Max and the integrated radius were smaller in uncontrolled than in controlled diabetic patients.[22] Pérez-Rico et al reported that diabetes leads to a significantly lower A1 velocity and A2 time.[21] These findings could indicate a stiffer cornea in DM and the elastic properties of the cornea may reflect the diabetic patients’ metabolic state and that diabetic patients with elevated HbA1c showed a significant decrease in the elasticity of their corneal substance.
To our knowledge, only a few studies to date have investigated corneal biomechanics using CST in diabetic patients; Further, these studies have covered other CST parameters but not WEM.
In this context, earlier CST studies reported a significant dependency of IOP on CST parameters.[28–30] In DM, an IOP elevation has been reported,[18,31] and possible reasons for this include reduced aqueous humor outflow caused by AGE (advanced glycation end products)-induced changes of the trabecular meshwork, an altered osmotic gradient of the aqueous humor, and an inaccuracy of IOP measurement caused by corneal biomechanical changes in DM.[21,32,33] Using CST measurements, Vinciguerra et al[30] revealed an age dependency for most of the parameters. Therefore, corrections for age and IOP seem to be indispensable in the analysis of CST results.
In this study, WEM (ms), the duration of vertical displacement of the whole eye during the examination, was decreased in the DM group. The CST device reports corneal stiffness along with the extra-corneal tissue (including sclera, fat, and muscle) stiffness. The deformation amplitude signal needs to be divided into the deflection amplitude and WEM.[34,35] This is important because the kinetic energy of the air puff is absorbed by the cornea and the extra-corneal tissues. Thus, the decreased WEM (ms) could indicate a stiffer, more inflexible sclera resulting a shorter duration of the whole eye movement process.
Recently, clinical research papers on WEM have been published. First, Vinciguerra et al measured WEM in healthy subjects and obtained normative data, finding that WEM increases with age.[30] WEM has also been used to develop a new index for keratoconus detection.[28,35,36] In glaucoma research, Jung et al found that unadjusted WEM was smaller in the glaucoma group than the normal group, in agreement with our results.[37] Also, Akoi et al measured WEM in an open-angle glaucoma population and ascertained the effect of WEM on the relationship between corneal hysteresis and glaucomatous progression.[38]
In the current study, Table 7 shows that the IOP was significantly negatively correlated with the WEM, meaning that, with higher IOP values, there was less WEM. This indicated greater resistance to movement of the entire globe in eyes with a higher IOP. This could be because the supporting tissues such as the sclera become stiffer with increasing IOP, as reported in an experimental study.[39]
Why DM is associated with greater corneal stiffness remains unclear. However, DM can alter the cornea as well as other collagen-containing tissues in the eye, such as the sclera. The effects of these changes in the cornea, sclera, and orbit on WEM are undoubtedly complex; in DM patients, the hyperglycemic state leads to collagen cross-linking.[12,15,40] Bailey et al demonstrated that collagen-containing tissues became stiffer and more inflexible with age and especially in the presence of high blood glucose levels or diabetics.[41] The reason for these alterations might be the formation of AGEs, which arise from nonenzymatic glycation of reducing sugars with amino groups of collagen (i.e. Maillard reaction) and lead to cross-links with a consecutive increased stiffness in these tissues. Albon et al observed an age-related increase in pentosidine, a form of AGE, in the aging lamina cribrosa.[42,43]
Glucose-mediated corneal and scleral stiffening because of corneal and scleral collagen cross-linking might have been responsible for the lower WEM values found in these patients. This finding is in agreement with a review performed by Krueger and Ramos-Esteban.[31] A similar collagen cross-linking phenomenon seems to occur with aging, and older patients have higher corneal and scleral stiffening.[8,44] As there was no age difference between our study groups and we adjusted for age, it is unlikely that aging was responsible for the WEM differences observed in this study.
It is important to discuss the possible implication of our findings. First, it remains controversial whether the observed biomechanical changes in terms of an increase in the stiffness of the ocular tissues must be considered as a detrimental risk factor or whether the tissue stiffening might indicate a protective effect for IOP control in diabetic patients. As predicted by recent studies, a stiffer sclera would protect optic nerve head tissues from biomechanical insult.[45–47] Although, other research suggests that having a stiffer sclera may accelerate axonal damage.[48] The higher tissue stiffening we found in our DM patients could be either a protective factor or a risk factor for glaucoma. Nevertheless, our study did not evaluate whether such a difference could result in a lower or higher risk of visual field worsening. In addition, Tables 4, 5, and 6 and Figures 1, 2, and 3 show that these corneal biomechanical changes in DM eyes are influenced more so by levels of hyperglycemia or HbA1c than by disease duration.
Table 4 shows that only the maximum WEM velocity is correlated with HbA1c and Table 5 shows that DA ratio max (2mm) and DA ratio max (1mm) are correlated with blood glucose level. Although HbA1c is usually a dependent variable of long-term blood glucose levels, the results of Tables 4 and 5 seem inconsistent. The HbA1c and blood glucose level both have been well known to reflect patients’ general DM status. HbA1c is the product of a stable linkage of glucose to the N-terminal valine of the beta-chain of hemoglobin.[49] Since HbA1c is a particular kind of glycated hemoglobin in red blood cells, measured level of it is predominantly dominated by the life-span of red blood cells, that is, 120 days in average. Hence, it reflects treatment compliance and average blood glucose level of the patient during recent 8–12 weeks.
Therefore, blood glucose level reflects the short term, and HbA1c level indicates the degree of long-term glycemic control. In the short-term effect of hyperglycemia, high aqueous humor glucose level is also reported.[50] On the other hand, HbA1c can reflect ECM (Extracellular matrix) remodeling such as collagen cross-linking in the long-term effect of hyperglycemia. Therefore, there may be inconsistent results in Tables 4 and 5. However, further research is needed to determine the detailed mechanism.
Also, WEM max.(ms) is the duration of movement of the whole eyeball during the examination. This mainly reflects orbital factors, such as compliance of sclera, periorbital fat tissue, and orbital muscle. Whereas DA ratio maximum (1 or 2mm) is the ratio between the corneal deformation amplitude at the apex and at 1 or 2mm. This reflects pure corneal factor. The fact that each parameter is affected by different factors can cause difference of time needed for significant changes to occur in each parameter. Since the orbital factors include compliance of sclera, periorbital fat, and muscle, the time point at which a measurable degree of glycation occurs could be disparate between these extra-corneal tissues and cornea.
The present study has several limitations. First, disease-specific factors, such as DM presence and disease duration, were assessed by anamnestic questionnaires, which might be imprecise in elucidating the true results. Furthermore, DM type in some patients was defined based on patients’ interview only. Second, the values for the HbA1C and blood glucose level for the normal control patients were not possible to evaluate due to the retrospective nature of the study. Third, prognostic value of the Corvis parameters has not been clarified that restrict the use of these measures and can be a limitation to any study. Fourth, the small number of DM patients in this study restricted our investigation. Fifth, the CST measurements were not performed during the same office hours in all patients. Therefore, there might be potential effect of diurnal IOP changes which can confound CST measurements, although no significant diurnal changes in corneal biomechanical parameters were detected with CST previously.[51] Finally, the statistical power of our study may have decreased due to the difference in sample size of 2 groups. Future well-designed studies with larger sample size or meta-analysis of the available data in the current literature might help to clarify these issues.
In addition, the results of our study demonstrate significant changes in some corneal biomechanical parameters by CST in diabetics. However, several parameters did not show significant changes. There are various parameters indicating corneal stiffness other than deflection amplitude in CST. The parameters that have been reported to show significant changes in DM were not in consistency among several previous studies. In addition, some of them reported that only a portion of the parameters showed significant changes, like in the present study.[21–23] Also, CST parameters are likely to be affected by multiple factors, such as IOP, age, axial length, and central corneal thickness. Therefore, we believe that the results may vary from study to study to some degree, according to the study settings and subject populations.
Despite these drawbacks, our results provide important information showing that some parameters, including A2DA and WEM, demonstrate significant changes in the DM group. Moreover, this is the first study analyzing WEM, a specific CST parameter, which showed significant change in DM eyes. Also, some CST parameters showed a significant correlation to HbA1c value and blood glucose level. Perhaps explaining, poor metabolic control in DM might crucially change corneal biomechanics and be related with collagen cross-linking.
5. Conclusions
In conclusion, changes in corneal biomechanical behaviors have been shown to exist in DM patients using CST. Besides other factors, the reasons might be an accumulation of advanced glycation end products and greater collagen and proteoglycan cross-linking in the corneal stroma. These changes might be of importance in IOP measurement, the role of different CST parameter values on glaucoma evaluation, and susceptibility. In addition, these findings may have implications for understanding the relationship between diabetes and glaucoma.
Author contributions
KO and JIM contributed to the design of the manuscript. YHN and KO collected the data. JIM and YJ performed the clinical examination and investigation. YJ and KO shared data analysis and interpretation and revised the intellectual content of the manuscript. YJ and JIM critically revised the manuscript. All authors read and approved the final manuscript.
Abbreviations:
- A1 =
- applanation 1
- A2 =
- applanation 2
- AGE =
- advanced glycation end products
- AL =
- axial length
- CCT =
- central corneal thickness
- CST =
- Corvis ST
- DA =
- deformation amplitude
- DM =
- diabetes mellitus
- HbA1c =
- hemoglobin A1c
- HC =
- highest concavity
- IOP =
- intraocular pressure
- ORAs =
- ocular response analyzers,
- SE =
- spherical equivalent
- WEM =
- whole-eye movement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No.2021R1H1A2095534).
This study followed the tenets of the Declaration of Helsinki for biomedical research and was approved by the Yeouido St. Mary’s Hospital Institutional Review Board (SC21RISI0085). Informed consent was waived under the provisions of IRB since this study was conducted in a retrospective manner.
The authors have no conflicts of interest.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Ohn K, Ho Noh Y, Moon, JI, Jung Y. Measurement of corneal biomechanical properties in diabetes mellitus using the Corvis ST. Medicine 2022;101:36(e30248).
Contributor Information
Kyoung Ohn, Email: kyoungohn@gmail.com.
Young Ho Noh, Email: sundo1185035@gmail.com.
Jung Il Moon, Email: jimoon@catholic.ac.kr.
References
- [1].Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the early manifest glaucoma trial. Arch Ophthalmol. 2002;120:1268–79. [DOI] [PubMed] [Google Scholar]
- [2].Brandt JD. The influence of corneal thickness on the diagnosis and management of glaucoma. J Glaucoma. 2001;10:S65–7. [DOI] [PubMed] [Google Scholar]
- [3].Gordon MO, Beiser JA, Brandt JD, et al. The ocular hypertension treatment study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–20. [DOI] [PubMed] [Google Scholar]
- [4].Jonas JB, Stroux A, Velten I, et al. Central corneal thickness correlated with glaucoma damage and rate of progression. Invest Ophthalmol Vis Sci. 2005;46:1269–74. [DOI] [PubMed] [Google Scholar]
- [5].Cook JA, Botello AP, Elders A, et al. Systematic review of the agreement of tonometers with goldmann applanation tonometry. Ophthalmology. 2012;119:1552–7. [DOI] [PubMed] [Google Scholar]
- [6].Bao F, Huang Z, Huang J, et al. Clinical evaluation of methods to correct intraocular pressure measurements by the goldmann applanation tonometer, ocular response analyzer, and corvis st tonometer for the effects of corneal stiffness parameters. J Glaucoma. 2016;25:510–9. [DOI] [PubMed] [Google Scholar]
- [7].McCafferty S, Lim G, Duncan W, et al. Goldmann tonometer error correcting prism: clinical evaluation. Clin Ophthalmol. 2017;11:835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Luce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg. 2005;31:156–62. [DOI] [PubMed] [Google Scholar]
- [9].Koprowski R. Automatic method of analysis and measurement of additional parameters of corneal deformation in the corvis tonometer. Biomed Eng Online. 2014;13:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ambrosio R, Ramos I, Luz A, et al. Dynamic ultra high speed Scheimpflug imaging for assessing corneal biomechanical properties. Rev Bras Oftalmol. 2013;72:99–102. [Google Scholar]
- [11].Hajrasouliha AR, Tavakoli S, Esteki A, et al. Abnormal viscoelastic behaviour of passive ankle joint movement in diabetic patients: an early or a late complication? Diabetologia. 2005;48:1225–8. [DOI] [PubMed] [Google Scholar]
- [12].Sady C, Khosrof S, Nagaraj R. Advanced maillard reaction and crosslinking of corneal collagen in diabetes. Biochem Biophys Res Commun. 1995;214:793–7. [DOI] [PubMed] [Google Scholar]
- [13].Dyer DG, Dunn JA, Thorpe SR, et al. Accumulation of maillard reaction products in skin collagen in diabetes and aging. J Clin Invest. 1993;91:2463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Del Buey MA, Casas P, Caramello C, et al. An update on corneal biomechanics and architecture in diabetes. J Ophthalmol. 2019;2019:7645352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Goldich Y, Barkana Y, Gerber Y, et al. Effect of diabetes mellitus on biomechanical parameters of the cornea. J Cataract Refract Surg. 2009;35:715–9. [DOI] [PubMed] [Google Scholar]
- [16].Hager A, Wegscheider K, Wiegand W. Changes of extracellular matrix of the cornea in diabetes mellitus. Graefes Arch Clin Exp Ophthalmol. 2009;247:1369–74. [DOI] [PubMed] [Google Scholar]
- [17].Kotecha A, Oddone F, Sinapis C, et al. Corneal biomechanical characteristics in patients with diabetes mellitus. J Cataract Refract Surg. 2010;36:1822–8. [DOI] [PubMed] [Google Scholar]
- [18].Sahin A, Bayer A, Ozge G, et al. Corneal biomechanical changes in diabetes mellitus and their influence on intraocular pressure measurements. Invest Ophthalmol Vis Sci. 2009;50:4597–604. [DOI] [PubMed] [Google Scholar]
- [19].Scheler A, Spoerl E, Boehm AG. Effect of diabetes mellitus on corneal biomechanics and measurement of intraocular pressure. Acta Ophthalmol. 2012;90:e447–51. [DOI] [PubMed] [Google Scholar]
- [20].Castro DP, Prata TS, Lima VC, et al. Corneal viscoelasticity differences between diabetic and nondiabetic glaucomatous patients. J Glaucoma. 2010;19:341–3. [DOI] [PubMed] [Google Scholar]
- [21].Perez-Rico C, Gutierrez-Ortiz C, Gonzalez-Mesa A, et al. Effect of diabetes mellitus on Corvis ST measurement process. Acta Ophthalmol. 2015;93:e193–8. [DOI] [PubMed] [Google Scholar]
- [22].Ramm L, Herber R, Spoerl E, et al. Measurement of corneal biomechanical properties in diabetes mellitus using the ocular response analyzer and the corvis ST. Cornea. 2019;38:595–9. [DOI] [PubMed] [Google Scholar]
- [23].Ramm L, Herber R, Spoerl E, et al. Factors influencing corneal biomechanics in diabetes mellitus. Cornea. 2020;39:552–7. [DOI] [PubMed] [Google Scholar]
- [24].Bak-Nielsen S, Pedersen IB, Ivarsen A, et al. Repeatability, reproducibility, and age dependency of dynamic scheimpflug-based pneumotonometer and its correlation with a dynamic bidirectional pneumotonometry device. Cornea. 2015;34:71–7. [DOI] [PubMed] [Google Scholar]
- [25].Lopes BT, Roberts CJ, Elsheikh A, et al. Repeatability and reproducibility of intraocular pressure and dynamic corneal response parameters assessed by the corvis ST. J Ophthalmol. 2017;2017:8515742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vellara HR, Ali NQ, Gokul A, et al. Quantitative analysis of corneal energy dissipation and corneal and orbital deformation in response to an air-pulse in healthy eyes. Invest Ophthalmol Vis Sci. 2015;56:6941–7. [DOI] [PubMed] [Google Scholar]
- [27].Matalia J, Francis M, Tejwani S, et al. Role of age and myopia in simultaneous assessment of corneal and extraocular tissue stiffness by air-puff applanation. J Refract Surg. 2016;32:486–93. [DOI] [PubMed] [Google Scholar]
- [28].Roberts CJ, Mahmoud AM, Bons JP, et al. Introduction of two novel stiffness parameters and interpretation of air puff-induced biomechanical deformation parameters with a dynamic scheimpflug analyzer. J Refract Surg. 2017;33:266–73. [DOI] [PubMed] [Google Scholar]
- [29].Huseynova T, Waring GO, Roberts C, et al. Corneal biomechanics as a function of intraocular pressure and pachymetry by dynamic infrared signal and Scheimpflug imaging analysis in normal eyes. Am J Ophthalmol. 2014;157:885–93. [DOI] [PubMed] [Google Scholar]
- [30].Vinciguerra R, Elsheikh A, Roberts CJ, et al. Influence of pachymetry and intraocular pressure on dynamic corneal response parameters in healthy patients. J Refract Surg. 2016;32:550–61. [DOI] [PubMed] [Google Scholar]
- [31].Krueger RR, Ramos-Esteban JC. How might corneal elasticity help us understand diabetes and intraocular pressure? J Refract Surg. 2007;23:85–8. [DOI] [PubMed] [Google Scholar]
- [32].Bao F, Deng M, Zheng X, et al. Effects of diabetes mellitus on biomechanical properties of the rabbit cornea. Exp Eye Res. 2017;161:82–8. [DOI] [PubMed] [Google Scholar]
- [33].Last JA, Pan T, Ding Y, et al. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest Ophthalmol Vis Sci. 2011;52:2147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sinha Roy A, Kurian M, Matalia H, et al. Air-puff associated quantification of non-linear biomechanical properties of the human cornea in vivo. J Mech Behav Biomed Mater. 2015;48:173–82. [DOI] [PubMed] [Google Scholar]
- [35].Vinciguerra R, Ambrosio R, Jr., Elsheikh A, et al. Detection of keratoconus with a new biomechanical index. J Refract Surg. 2016;32:803–810. [DOI] [PubMed] [Google Scholar]
- [36].Francis M, Pahuja N, Shroff R, et al. Waveform analysis of deformation amplitude and deflection amplitude in normal, suspect, and keratoconic eyes. J Cataract Refract Surg. 2017;43:1271–80. [DOI] [PubMed] [Google Scholar]
- [37].Jung Y, Park HL, Yang HJ, et al. Characteristics of corneal biomechanical responses detected by a non-contact scheimpflug-based tonometer in eyes with glaucoma. Acta Ophthalmol. 2017;95:e556–63. [DOI] [PubMed] [Google Scholar]
- [38].Aoki S, Murata H, Matsuura M, et al. The effect of air pulse-driven whole eye motion on the association between corneal hysteresis and glaucomatous visual field progression. Sci Rep. 2018;8:2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Metzler KM, Mahmoud AM, Liu J, et al. Deformation response of paired donor corneas to an air puff: intact whole globe versus mounted corneoscleral rim. J Cataract Refract Surg. 2014;40:888–96. [DOI] [PubMed] [Google Scholar]
- [40].Terai N, Spoerl E, Haustein M, et al. Diabetes mellitus affects biomechanical properties of the optic nerve head in the rat. Ophthalmic Res. 2012;47:189–94. [DOI] [PubMed] [Google Scholar]
- [41].Bailey AJ, Paul RG, Knott L. Mechanisms of maturation and ageing of collagen. Mech Ageing Dev. 1998;106:1–56. [DOI] [PubMed] [Google Scholar]
- [42].Albon J, Karwatowski WS, Avery N, et al. Changes in the collagenous matrix of the aging human lamina cribrosa. Br J Ophthalmol. 1995;79:368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Albon J, Purslow PP, Karwatowski WS, et al. Age related compliance of the lamina cribrosa in human eyes. Br J Ophthalmol. 2000;84:318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Coudrillier B, Pijanka J, Jefferys J, et al. Effects of age and diabetes on scleral stiffness. J Biomech Eng. 2015;137:0710071–07100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sigal IA, Flanagan JG, Ethier CR. Factors influencing optic nerve head biomechanics. Invest Ophthalmol Vis Sci. 2005;46:4189–99. [DOI] [PubMed] [Google Scholar]
- [46].Norman RE, Flanagan JG, Sigal IA, et al. Finite element modeling of the human sclera: influence on optic nerve head biomechanics and connections with glaucoma. Exp Eye Res. 2011;93:4–12. [DOI] [PubMed] [Google Scholar]
- [47].Eilaghi A, Flanagan JG, Simmons CA, et al. Effects of scleral stiffness properties on optic nerve head biomechanics. Ann Biomed Eng. 2010;38:1586–92. [DOI] [PubMed] [Google Scholar]
- [48].Kimball EC, Nguyen C, Steinhart MR, et al. Experimental scleral cross-linking increases glaucoma damage in a mouse model. Exp Eye Res. 2014;128:129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Nathan DM, Singer DE, Hurxthal K, et al. The clinical information value of the glycosylated hemoglobin assay. N Engl J Med. 1984;310:341–6. [DOI] [PubMed] [Google Scholar]
- [50].Gomel N, Barequet IS, Lipsky L, et al. The effect of the glycemic control on the aqueous humor glucose levels in diabetic patients undergoing elective cataract surgery. Eur J Ophthalmol. 2021;31:415–21. [DOI] [PubMed] [Google Scholar]
- [51].Serbecic N, Beutelspacher S, Markovic L, et al. Repeatability and reproducibility of corneal biomechanical parameters derived from corvis ST. Eur J Ophthalmol. 2020;30:1287–94. [DOI] [PubMed] [Google Scholar]