Background:
To assess the efficacy and safety of rituximab (RTX) in the treatment of neuromyelitis optica spectrum diseases (NMOSDs), and give a guideline on clinical medication
Methods:
The databases of Pubmed, Embase, Cochrane Library, CNKI, and Wan fang were systematically searched by computer, and the search period was from the establishment of the databases until January 2022. To collect the trials of RTX in the treatment of NMOSDs, two researchers completed literature screening, quality assessment, and data extraction independently. Statistical analysis was performed using Review Manager 5.3 and Stata 15.1 software.
Results:
There were 37 studies in the meta-analysis, including 5 randomized controlled trials (RCTs) and 32 observational studies. Meta-analysis results revealed that NMOSDs patients treated with RTX significantly reduced the annualized relapse rate (ARR) (weighted mean difference [WMD] = 1.45, 95% confidence interval [CI]: 1.24–1.66, P < .01) and the Expanded disability status scale (EDSS) scores (WMD = 1.34, 95%CI: 1.25–1.44, P < .01). RTX is more effective than azathioprine (AZA) in the treatment of NMOSDs (ARR: WMD = −0.54, 95% CI: −0.75 to −0.33; EDSS: WMD = −0.65, 95% CI: −0.83 to −0.48; P < .0001).There was no difference in ARR and EDSS scores between anti-aquapor in-4-antibody seropositive NMOSD and seronegative NMOSD patients treated with RTX (ARR: WMD = −0.01, 95% CI: −0.25 to 0.24, P = .96 > 0.05; EDSS: WMD = 0, 95% CI: −0.30 to 0.31, P = .99 > 0.05). In this study, 681 patients were recorded safety data of RTX therapy, 23% (156 patients) had adverse events, and 0.7% (5 patients) of NMOSDs discontinued due to severe adverse reactions.
Conclusions:
NMOSDs patients treated with RTX can significantly reduce the relapse frequency and EDSS scores, and also improve neurological dysfunction, besides the efficacy is better than azathioprine. RTX has a high incidence of adverse reactions, which are mild and with certain self limited, it should be cautious in clinical medication
Keywords: meta-analysis, neuromyelitis optica, neuromyelitis optica spectrum disorders, rituximab
1. Introduction
Neuromyelitis optica spectrum disorders are a type of primary inflammatory demyelinating disease of the central nervous system. The patients are mainly female, and the onset is usually between 5 and 50 years old, with an average onset age of 39 years.[1] At present, the pathogenesis of neuromyelitis optica spectrum diseases (NMOSD) is still unclear. Most scholars believe that aquaporin-4-antibody specifically binds to AQP-4 that causes necrosis of astrocytes, and releases of inflammatory mediators and infiltration of inflammatory response through complement dependent and antibody dependent cytotoxic pathways, which are eventually leading to injury of oligoendrocytes and demyelination.
Rituximab (RTX) is a human and mouse monoclonal chimeric antibody that binds to the CD20 antigen of B lymphocytes in the blood and initiates an immune response that mediates B cell lysis by depleting B lymphocytes. The study of Cabre et al[2] and Kim et al[3] showed that RTX could reduce the annual recurrence rate and the neurological dysfunction in patients with NMOSD. However, there are few randomized controlled trials (RCT) of RTX in the treatment of NMOSD, so it is necessary to conduct a meta-analysis on the efficacy and adverse drug reactions of RTX in the treatment of NMOSD, so as to provide evidence-based medicine basis for clinical medication.
2. Methods
2.1. Literature retrieval strategy
Embase, PubMed, The Cochrane Library, China National Knowledge Infrastructure (CNKI) database and Wan Fang Data were searched. The retrieval method is a combination of subject words (MesH table) and free words.
2.2. Inclusion and exclusion criteria
Inclusion criteria: The patients should meet the diagnostic criteria for neuromyelitis optic spectrum disease in 2015; RTX treatment; The language of the included literature was Chinese or English, and the study types included randomized controlled trial, cohort study, self-controlled before and after study, or case-control study; and Include at least one outcome indicator: Main indicators: Annual recurrence rate: the number of recurrences per year; Expanded Disability Status Scale score: EDSS is widely used in clinical evaluation of neurological dysfunction and disease severity in patients with NMOSD. The incidence of RTX adverse events (AEs) and discontinuation of treatment due to serious AEs. Secondary outcome indicators: Annual recurrence rate before and after treatment with RTX and AZA in patients with NMOSD; EDSS scores of NMOSD patients before and after treatment with RTX and AZA; The annual recurrence rate of patients with AQP-4 positive and AQP-4 negative NMO treated with RTX; EDSS scores of patients with AQP-4 positive and AQP-4 negative NMO treated with RTX.
Exclusion criteria: Case reports and review literature; ongoing studies, studies that did not provide outcome indicators, and duplicate studies; Exclude the literatures with the number of participants ≤ 5; unpublished literature; and Exclude the literatures whose annualized relapse rate (ARR) and EDSS data could not be extracted or whose full text could not be obtained.
2.3. Data extraction and quality evaluation
For continuous data (ARR and EDSS), crude data such as mean, standard deviation (SD), median, range, and sample size were directly extracted from the literature. The quality of the included RCT was assessed using the Cochrane Bias Risk Assessment Scale, and the literature quality of the non-RCT was assessed using the Newcastle-Ottawa Scale (NOS).
2.4. Statistical analysis
Review Manager 5.3 and Stata15.1 software were used for statistical analysis. Heterogeneity was detected by Q test and I2 test. Q test calculates chi-square value and P value, and the judgment standard is P value, P > .1, which indicates that the heterogeneity is small. If I2 < 50%, the fixed effects model was selected to combine the effect sizes of each study, and if I2 ≥ 50%, the random effects model was used. Sensitivity analysis, subgroup analysis or Meta regression were performed for outcome indicators with high heterogeneity to find out the reasons for the increase in heterogeneity. Since ARR and EDSS were continuous variable data, the Dersimonia–Laird (D–L) method[4] was used to calculate weighted mean difference (WMD) and 95% CI. Z (U) test was used for hypothesis test, and the test level was set at .05. P < .05 indicated statistical significance. Otherwise, it was not considered statistically significant. Funnel plot method and EGGER were used to test whether there was any publication bias.
3. Results
3.1. Study identification and selection
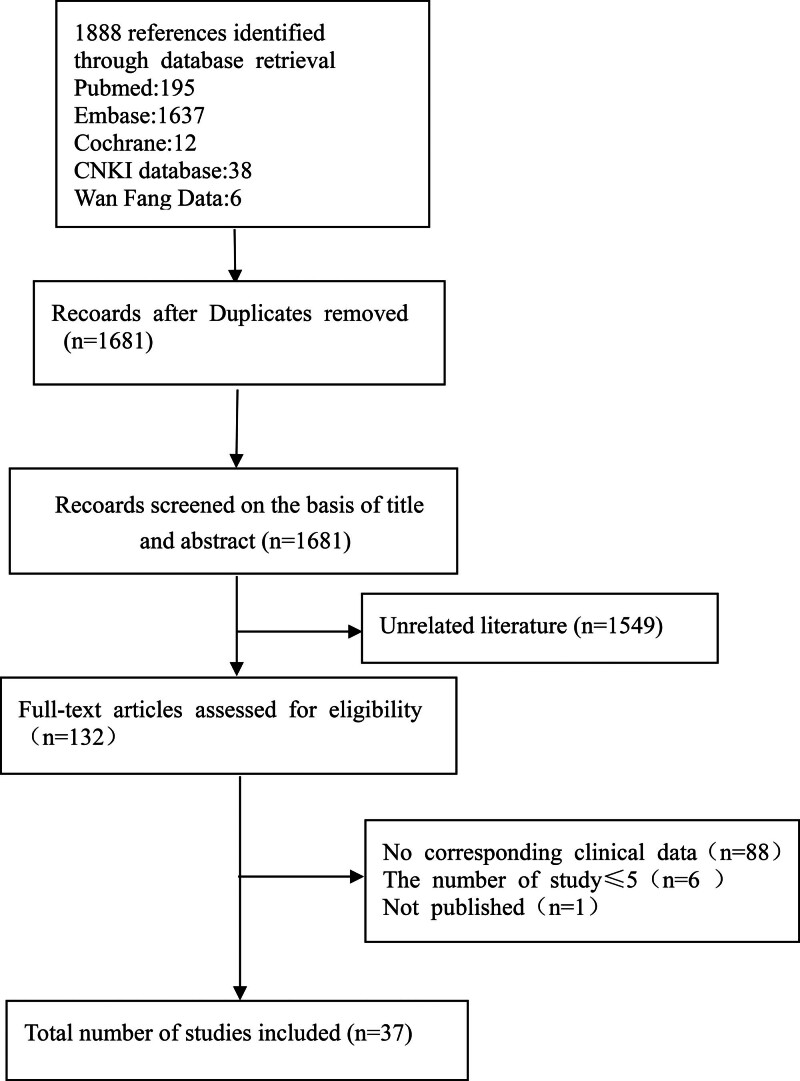
By searching Chinese and English databases, a total of 1888 relevant literatures were retrieved. Finally, 37 studies were included in this systematic review. Figure 1 for the process of literature inclusion.
Figure 1.
The selection process of documents.
3.2. Basic characteristics of the literature included in this study
The data extracted in this study included the name of the investigator, the publication time of the literature, the number of subjects, gender, age, the number of AQP-4 antibody positive patients, the course of disease, and the follow-up time. Table 1 for the information of the included literatures.
Table 1.
Main characteristics of the studies included in the meta-analysis.
| Included studies | Research type | Patient no. | Sex (F/M) | Age (yr) | AQP4-Ab (+) | Duration of disease (yr/mo) | Follow-up (yr/mo) |
|---|---|---|---|---|---|---|---|
| Cabre et al 2018[2] | Prospective | 32 | 30/2 | 39.9 (12.1) | 20 | NC | 2 yr |
| Dastjerdi et al 2018[5] | Prospective | 56 | 46/10 | 36.86 | 30 | 87.60 ± 59.65 mo | 6/12 mo |
| Fernández-Megía et al 2015[6] | Retrospective | 6 | 6/0 | 46 (38–58) | 3 | NC | NC |
| Jacob et al 2008[7] | Retrospective | 25 | 22/3 | 38 (7–65) | 14 | 4.5 (0.8–17) yr | 19 (6–40) mo |
| Kim et al 2015[3] | Retrospective | 100 | 92/8 | 43 (11) | 94 | 11 (5) yr | 67 (9–108) mo |
| Nikoo et al 2017[8] | RCT | 33 | 29/4 | 35.33 (8.98) | 13 | 6.23 (4.29) yr | 12 mo |
| Shaygannejad et al 2019[9] | Prospective | 44 | 35/9 | 37.2 ± 10.4 | 14 | 6.3 ± 4.1 yr | 31.6 ± 7.3 mo |
| Zephir et al 2015[10] | Retrospective | 32 | 27/5 | 45 ± 12.1 | 28 | 6.5 (1–410) mo | 28.7 ± 21 mo |
| Zhang et al 2017[11] | Retrospective | 31 | 23/8 | 42.2 ± 16.9 | 25 | 4.05 ± 2.11 yr | >2 yr |
| Annovazzi et al 2016[12] | Retrospective | 73 | 64/9 | 46.5 ± 12.5 | 53 | 6 ± 7.2 yr | 35.6 ± 27 |
| Pellkofer et al 2011[13] | Prospective | 9 | 8/1 | 36.1 (11.5) | 9 | 11 (7.7) yr | 29.6 (14.5) mo |
| Lindsey et al 2012[14] | Retrospective | 8 | 7/1 | 37.6 (14.4) | 4 | 65.1 (53.7) mo | 39.9 (40.7) mo |
| Ip et al 2013[15] | Retrospective | 7 | 6/1 | 52 (22–62) | 4 | 57 (40–272) mo | 24 (1–42) mo |
| Jeong et al 2016[16] | Retrospective | 55 | 50/5 | 42 (15–68) | 52 | 41.7 (2.1–231.5) mo | 64.7 (6.2–99.8) mo |
| Collongues et al 2016[17] | Retrospective | 21 | 19/2 | 37.8 (15.5) | 19 | 46.9 (51.2) mo | 31 (18) mo |
| Cohen et al 2017[18] | Prospective | 40 | 33/7 | 40.2 (22–62) | 20 | 40 (2–165) mo | 2 yr |
| Yang et al 2018[19] | Prospective | 20 | 19/1 | 40.7 (11.4) | 10 | 11 (0.2–240) mo | 29 (18–40) mo |
| Bedi et al 2011[20] | Retrospective | 23 | 21/2 | 37.1 ± 14.6 | 15 | 114 (13–266) mo | 32.5 (7–63) mo |
| Casallas-Vanegas et al 2020[21] | Retrospective | 66 | 54/12 | 36.2 ± 12.01 | 44 | 5.85 ± 4.03 yr | NC |
| Correa Diaz et al 2019[22] | Retrospective | 21 | 18/2 | 36.7 (13.3) | NC | NC | 24.8 (6–47) mo |
| Flores et al 2012[23] | NC | 13 | 12/1 | 33.3 ± 13.8 | NC | NC | 24 mo |
| Kim et al 2009[24] | NC | 27 | 24/3 | 33.5 ± 11.6 | NC | 5.6 ± 3.6 yr | NC |
| Kim et al 2013[25] | Retrospective | 30 | 27/3 | 38 (23–58) | NC | 61 (49–82) mo | NC |
| Lin et al 2018[26] | RCT | 14 | NC | 32.9 ± 13.6 | NC | 20.5 ± 6.8 mo | NC |
| Wu and Niu 2019[27] | Retrospective | 15 | 13/2 | 31.8 ± 13.2 | NC | 1–144 mo | NC |
| Xiao et al 2020[28] | Retrospective | 36 | NC | NC | NC | NC | 19.83 ± 7.74 mo |
| Bai 2016[29] | RCT | 9 | 9 | 38.28 | NC | NC | 7–40 mo |
| Li 2019[30] | Retrospective | 29 | 27/2 | 38.3 ± 13.9 | 27 | NC | 3.1/2.1 yr |
| Liu 2017[31] | RCT | 15 | 15 | 37.84 ± 4.12 | NC | 6.93 ± 1.52 yr | NC |
| Niu 2019[32] | Retrospective | 15 | 13/2 | 31.8 ± 13.2 | NC | 1–144 mo | NC |
| Wang et al 2018[33] | Prospective | 21 | 20/1 | 26.2 ± 12.3 | NC | 9.2 ± 5.9 yr | 28.4 ± 4.9 mo |
| Zhang 2016[34] | Prospective | 19 | NC | 21-59 | NC | NC | NC |
| Zhu 2018[35] | Retrospective | 8 | NC | 48.52 ± 3.25 | NC | NC | NC |
| Jia et al 2018[36] | RCT | 9 | 8/1 | 42.78 | NC | 4.7 yr | NC |
| Zhang 2020[37] | Retrospective | 29 | 25/4 | 29 (15–54) | 24 | 39 (12–260) mo | NC |
| Cao et al 2021[38] | Retrospective | 29 | 25/4 | 16 (55.2) | NC | NC | NC |
| Uzunköprü et al 2021[39] | Retrospective | 85 | 69/16 | 43.78 ± 13.75 | 58 | 10.15 ± 7.93 | 21 mo |
AQP4-Ab = aquaporin 4 antibody, NC = no clear, RCT = randomized clinical trial.
3.3. Quality evaluation
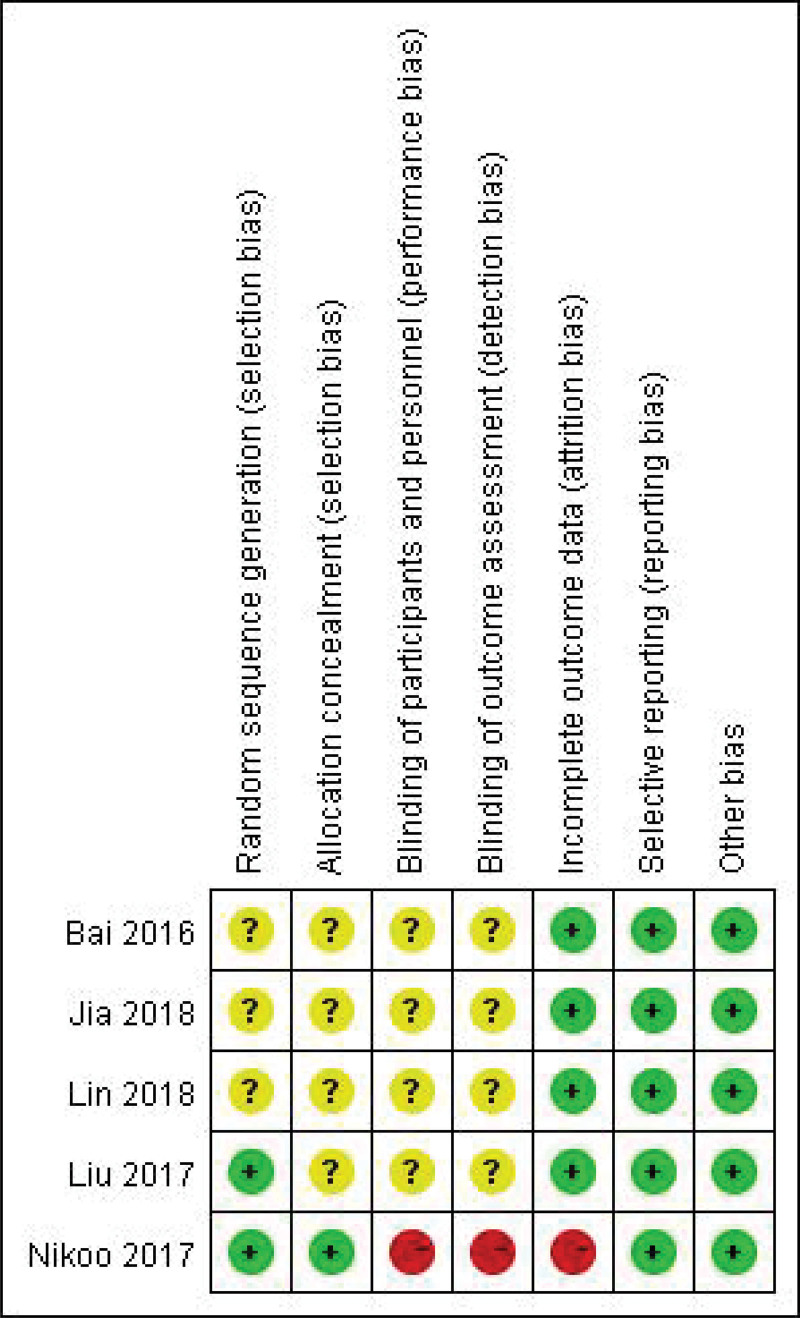
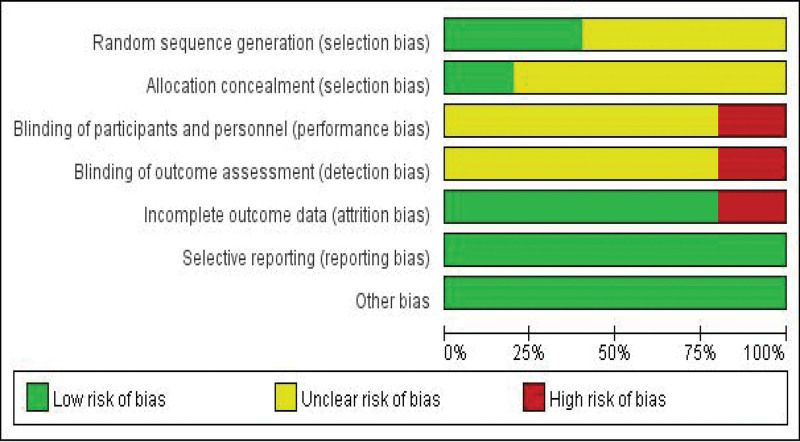
All RCTs included in this study met the evaluation criteria of medium literature quality, as shown in Figures 2 and 3. The Newcastle-Ottawa Quality Assessment Scale was used for the included case-control or cohort studies, as shown in Table 2.
Figure 2.
Risk of bias summary.
Figure 3.
Risk of bias graph.
Table 2.
Evaluation of the Newcastle-Ottawa Scale for the assessment of the quality of nonrandomized studies.
| Study | Selection | Comparability | Outcome |
|---|---|---|---|
| Prospective cohort study | |||
| Cabre 2018 | ★★★ | ★★ | ★★★ |
| Dastjerdi 2018 | ★★★ | ★★ | ★★ |
| Shaygannejad 2019 | ★★★ | ★★ | ★★★ |
| Pellkofer 2011 | ★★★ | ★★ | ★★★ |
| Cohen 2017 | ★★★ | ★★ | ★★★ |
| Yang 2018 | ★★★ | ★★ | ★★★ |
| Wang 2018 | ★★★ | ★★ | ★★ |
| Zhang 2016 | ★★★ | ★★ | ★★ |
| Retrospective cohort study | |||
| Fernández-Megía 2015 | ★★★ | ★★ | ★★ |
| Jacob 2008 | ★★★ | ★★ | ★★ |
| Kim 2015 | ★★★ | ★★ | ★★★ |
| Zephir 2015 | ★★★ | ★★ | ★★★ |
| Zhang 2017 | ★★★ | ★★ | ★★★ |
| Annovazzi 2016 | ★★★ | ★★ | ★★★ |
| Lindsey 2012 | ★★★ | ★★ | ★★★ |
| Ip 2013 | ★★★ | ★★ | ★★★ |
| Jeong 2016 | ★★★ | ★★ | ★★★ |
| Collongues 2016 | ★★★ | ★★ | ★★★ |
| Bedi 2011 | ★★★ | ★★ | ★★★ |
| Casallas-Vanegas 2020 | ★★★ | ★★ | ★★ |
| Correa Diaz 2019 | ★★★ | ★★ | ★★★ |
| Kim 2013 | ★★★ | ★★ | ★★ |
| Wu 2019 | ★★★ | ★★ | ★★ |
| Xiao 2020 | ★★★ | ★★ | ★★ |
| Li 2019 | ★★★ | ★★ | ★★★ |
| Niu 2019 | ★★★ | ★★ | ★★ |
| Zhu 2018 | ★★★ | ★★ | ★★ |
| Zhang 2020 | ★★★ | ★★ | ★★ |
| Cao 2021 | ★★★ | ★★ | ★★ |
| Uzunköprü 2021 | ★★★ | ★★ | ★★ |
3.4. Results of meta-analysis
3.4.1. Effects on ARR.
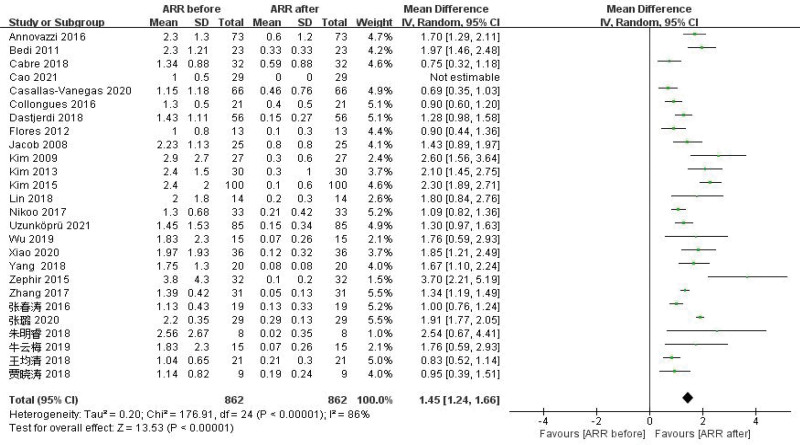
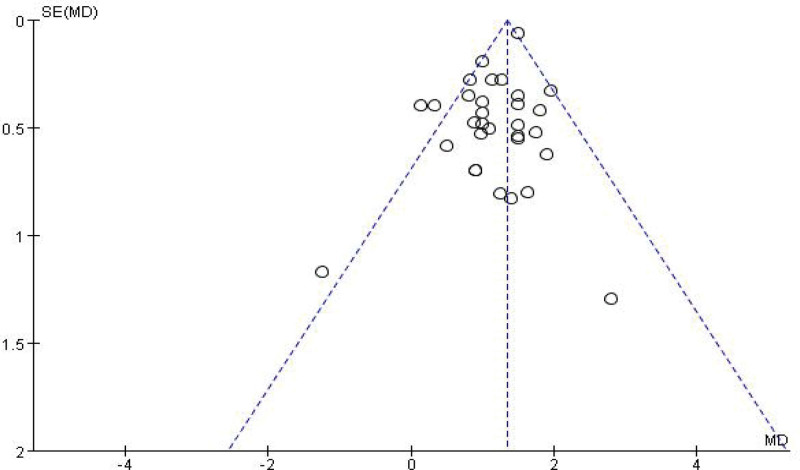
A total of 26 studies[2,3,5,7,8,10–12,17,19–21,23–28,32–39] were reported ARR before and after RTX therapy in patients with NMOSD. The results of Meta-analysis showed that the ARR of NMOSD patients after RTX treatment was significantly decreased by 1.45, and the difference was statistically significant (WMD = 1.45, 95% CI: 1.24–1.66, P < .01). Figure 4 was for the forest plot. Since I2 = 86% > 50% and the Q test P < .1; it was suggested that there was a high degree of heterogeneity in the included literatures, and the random-effect model was selected for analysis. In order to ensure the stability of the study, sensitivity analysis was carried out, and the results showed that there was no significant interference from any literature on the results of this meta-analysis, indicating that this study had good stability. Figure 5 was for sensitivity analysis. Next, Meta regression was continued to investigate the factors influencing effect size, in that effect size was the dependent variable and age and EDSS baseline were independent variables. No significant correlation was found between the ARR difference and the following variables: age (P = .32; 95% CI: −0.02 to 0.07), baseline EDSS (P = .31; 95% CI: −0.098 to 0.31). Funnel plots were drawn by RevMan 5.3 software to investigate the existence of publication bias, as shown in Figure 6. It can be clearly seen from the funnel plot, which is asymmetric. Egger test was conducted with Stata 15.1 software, and the result showed that P = .004 < 0.1, so it was judged that there was publication bias in the literature of this study.
Figure 4.
Forest plots of ARR. ARR = annualized relapse rate.
Figure 5.
Sensitivity analysis of ARR. ARR = annualized relapse rate.
Figure 6.
Bias figure of ARR. ARR = annualized relapse rate.
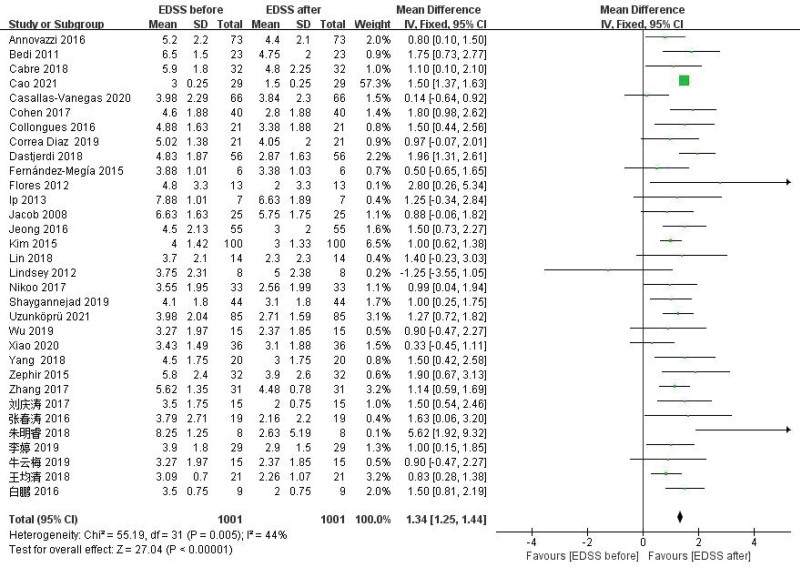
3.4.2. Efficacy on the EDSS score.
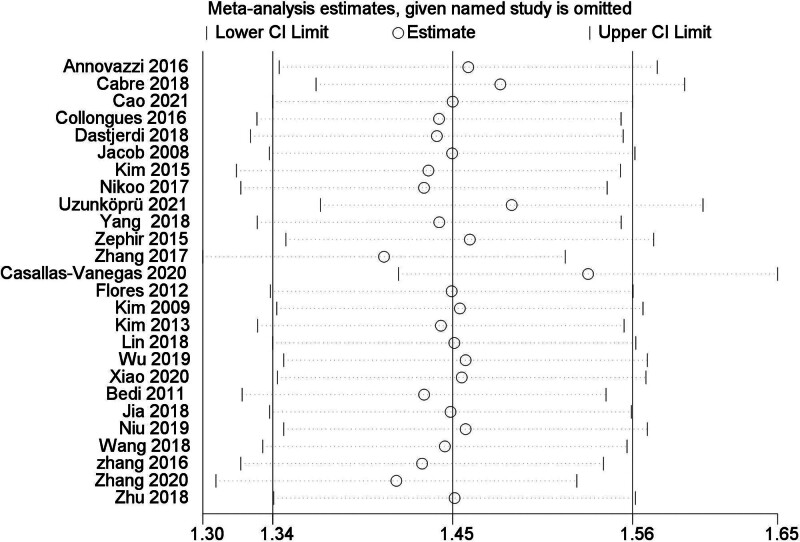
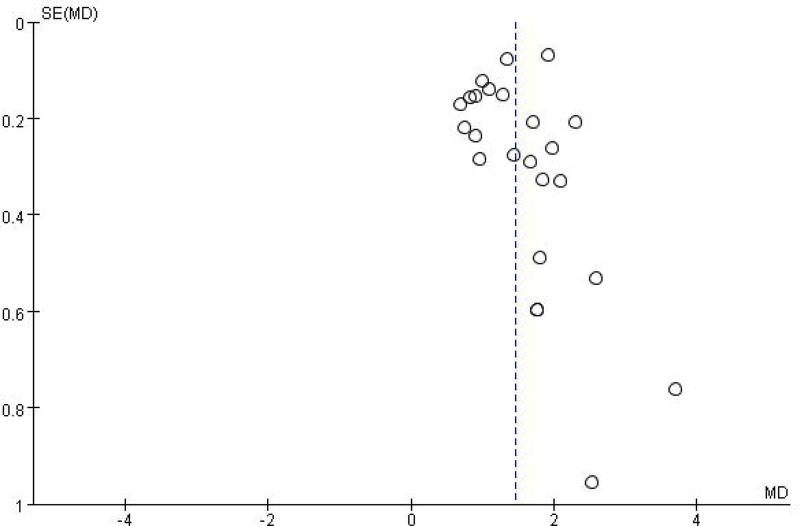
Meta-analysis results showed that the average EDSS score of NMOSD patients after RTX treatment decreased by 1.34 points, and the difference was statistically significant (WMD = 1.34, 95% CI: 1.25–1.44, P < .01). Figure 7 for the forest plot. Since I2 = 44% < 50%, it was suggested that there was a low degree of heterogeneity in the included literatures, and the fixed-effect model was selected for analysis. The results of sensitivity analysis showed that there was no significant interference from any literature on the results of this meta-analysis, indicating that the results of this meta-study were stable. Figure 8 for Sensitivity analysis of EDSS. The funnel plot of this study is shown in Figure 9. It could be clearly seen from the funnel plot that it is asymmetric. At the same time, Egger test was conducted with Stata 15.1 software, and the result showed that P = .039 < .1, so it was judged that there was publication bias in the literature of this study.
Figure 7.
Forest plot of EDSS. EDSS = expanded disability status scale.
Figure 8.
Sensitivity analysis of EDSS. EDSS = expanded disability status scale.
Figure 9.
Bias figure of EDSS. EDSS = expanded disability status scale.
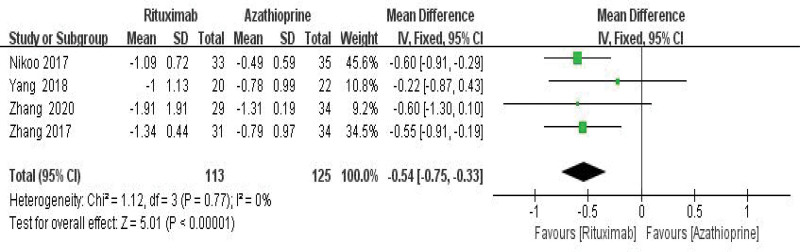
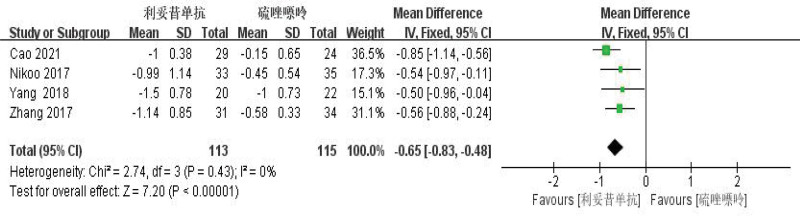
3.5. Comparison of efficacy between RTX and AZA in the treatment of NMOSD
RTX was better than azathiopine in the treatment of NMOSD and significantly reduced ARR (WMD = −0.54, 95% CI: −0.75 to −0.33, Z = 5.01, P < .01) and EDSS score (WMD = −0.65, 95% CI: −0.83 to −0.48), with Z = 7.20, P = .0001 < .01). Its forest plot could be seen in Figures 10 and 11. Since I2 = 0% < 50%, it was suggested that there was no heterogeneity in the included literatures. Egger test was conducted with Stata 15.1 software, and the results showed that (ARR P = .526 > 0.1, EDSS P = .423 > .1), so it was judged that there was no publication bias.
Figure 10.
Forest plot of ARR comparison between rituximab and azathioprine. ARR = annualized relapse rate.
Figure 11.
Forest plot of EDSS comparison between rituximab and azathioprine. EDSS = expanded disability status scale.
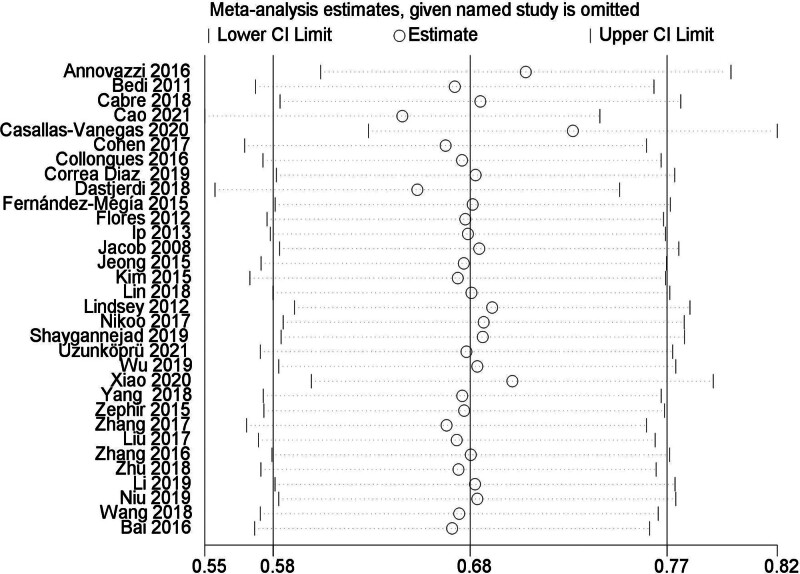
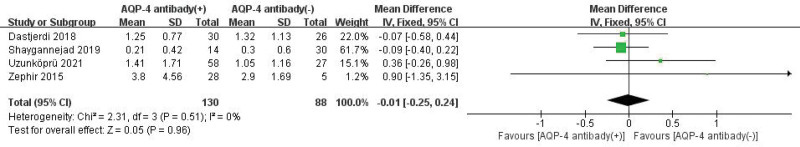
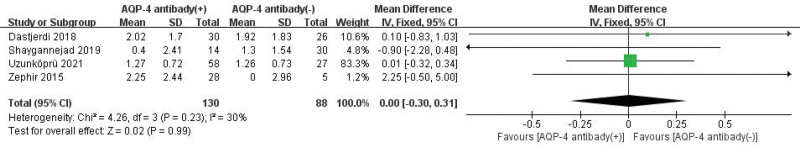
3.6. Efficacy of RTX in patients with anti-aquapor in-4-antibody-seropositive NMO and seronegative NMO
ARR comparison results showed that there was no statistical significance between patients with anti-aquapor in-4-antibody-seropositive NMO and seronegative NMO (WMD = −0.01, 95% CI: −0.25 to 0.24, Z = 0.05, P = .96 > .05). EDSS comparison results showed that there was no statistical significance between patients with anti-aquapor in-4-antibody-seropositive NMO and seronegative NMO (WMD = 0, 95% CI: −0.30 to 0.31, and Z = 0.02, P = .99 > .05). Figures 12 and 13 were for the forest plot. Since I2 = 0% < 50% and I2 = 30% < 50%, it was suggested that there was a low degree of heterogeneity in the included literatures. Egger test was conducted with Stata 15.1 software, and the results showed that ARR: P = .850 > .1, EDSS P = .535 > .1, suggesting that there was no publication bias among the studies.
Figure 12.
Forest plot of ARR comparison between patients with AQP-4 positive and negative NMO treated with rituximab. ARR = annualized relapse rate, AQP-4 = anti-aquapor in-4-antibody, NMO = neuromyelitis optica.
Figure 13.
Forest plot of EDSS comparison between patients with AQP-4 positive and negative NMO treated with rituximab. AQP-4 = anti-aquapor in-4-antibody, EDSS = expanded disability status scale, NMO = neuromyelitis optica.
3.7. Safety
A total of 1105 people were enrolled in 37 studies, of which 681 patients documented safety data for RTX therapy. AEs occurred in 156 patients (23%), including 5 patients (0.7%) who were discontinued due to severe adverse reactions. The most common adverse reactions were infusion reactions and infection. Table 3 was for specific information.
Table 3.
Adverse events on RTX in neuromyelitis optica spectrum disorders.
| Study | Total number of patients | Number of patients with adverse events | Adverse events | Number of events | Total number of discontinuation due to adverse effects |
|---|---|---|---|---|---|
| Cabre 2018 | 32 | 7 | Lower urinary tract infection | 1 | 1 |
| Pharyngitis | 1 | ||||
| Xerostomia | 1 | ||||
| Moderate headache | 1 | ||||
| Moderate fever | 1 | ||||
| Laryngeal dysesthesia | 1 | ||||
| Fernández-Megía 2015 | 6 | 2 | Infusion reactions | 2 | – |
| Jacob 2008 | 25 | 12 | Infusion reactions | 7 | – |
| Herpes simplex and positive tuberculin skin test herpes zoster | 1 | ||||
| Recurrent Clostridium difficile colitis | 1 | ||||
| A cutaneous fungal infection | 1 | ||||
| Fatal urinary tract–related septicemia | 1 | ||||
| 1 | |||||
| Kim 2015 | 100 | 34 | Infusion-related reactions | 26 | – |
| Herpes zoster infection | 5 | ||||
| Pneumonia | 2 | ||||
| Thyroid cancer | 1 | ||||
| Nikoo 2017 | 33 | 4 | Allergic reactions | 4 | 1 |
| Shaygannejad 2019 | 44 | 15 | Infusion reactions | 14 | 1 |
| Severe allergic reaction | 1 | ||||
| Zephir 2015 | 32 | 0 | No relevant side effect | 0 | – |
| Zhang 2017 | 31 | 4 | Infusion reactions | 4 | – |
| Annovazzi 2016 | 73 | 19 | Urinary tract infections | 6 | 2 |
| Respiratory infections | 4 | ||||
| Infusion reactions | 7 | ||||
| Died | 2 | ||||
| Pellkofer 2011 | 9 | 4 | Urogenital infection, thrombosis, death | 1 | – |
| Adnexitis | 1 | ||||
| Pneumonia | 1 | ||||
| Urosepsis | 1 | ||||
| Ip 2013 | 7 | 2 | Infusion reactions | 2 | – |
| Yang 2018 | 20 | 1 | Transit hyperpyrexia | 1 | – |
| Bedi 2011 | 23 | 7 | Herpes zoster infection | 1 | – |
| Urinary tract infection | 1 | ||||
| Respiratory infections | 2 | ||||
| Fatigue | 1 | ||||
| Transient leukopenia | 1 | ||||
| Transient elevations of hepatic transaminases | 1 | ||||
| Casallas-Vanegas 2020 | 66 | 3 | Respiratory infections | 2 | – |
| Urinary tract infection | 1 | ||||
| Flores 2012 | 13 | 3 | Tetter | 3 | – |
| Kim 2013 | 30 | 12 | Infusion reactions | 12 | – |
| Lin 2018 | 14 | 2 | Phthisis | 1 | 1 |
| Respiratory infections | 1 | ||||
| Wu 2019 | 15 | 3 | No clear | – | |
| Xiao 2020 | 36 | 8 | Infusion reactions | 5 | – |
| Urinary tract infection | 3 | ||||
| Jia 2018 | 9 | 2 | Infusion reactions | 1 | – |
| Herpes zoster infection | 1 | ||||
| Niu 2019 | 15 | 1 | Respiratory infections | 1 | – |
| Wang 2018 | 21 | 8 | No clear | – | |
| Zhang 2016 | 19 | 1 | Herpes zoster infection | 1 | – |
| Zhu 2018 | 8 | 2 | Infusion reactions | 2 | – |
4. Discussion
NMOSD is an inflammatory autoimmune demyelinating disease of the central nervous system, and tends to occur in young people, which with other autoimmune diseases, such as systemic lupus erythematosus, sjogren syndrome, myasthenia gravis, thyroid function hyperfunction, hashimoto thyroiditis, mixed connective tissue disease, tuberous polyarteritis, and so on. Antinuclear antibodies, anti-SSA/SSB antibodies, and anti-cardiolipin antibodies could be detected in serum. Although relatively unknown due to the small number of patients with NMOSD, significant progress has been made in recent years in the study of NMOSD, such as the discovery of MOG-Ab, the NMOSDs diagnostic criteria developed by the International NMO Diagnostic Group (IPND) in 2015, and the development of new drugs, which deepened people’s understanding of NMOSDs. It is worth noting that NMOSD has a risk of recurrence and accumulation of disability. If NMOSD is not treated, nearly half of the patients will develop limb weakness and blindness, and one-third of the patients may die within 5 years.[40] Therefore, prevention of recurrence has become the focus of treatment. Moreover, many studies have shown that certain immunosuppressants can reduce the recurrence of NMOSD and the degree of disability.
At present, the clinical treatment of NMOSD includes acute treatment, sequential treatment (immunosuppressive therapy), symptomatic treatment, and rehabilitation treatment, which can effectively improve the condition of patients. If NMOSD patients have symptoms of neurological impairment or relapse, not only acute treatment is needed, but also the main treatment methods should include glucocorticoid, plasma exchange, intravenous immunoglobulin, etc, and are needed to reduce clinical symptoms and prevent and treat complications. In contrast to Disease modification therapy for multiple sclerosis, sequential treatment of NMOSD is required to prevent recurrence, so patients with AQP-4 antibody positive NMOSD or those with AQP-4 antibody negative NMOSD should be treated with immunosuppressant therapy, which actively reduces the annual recurrence rate, the degree of disability accumulation, and improves the prognosis. Due to the lack of effective means to distinguish between single-phase and multi-phase NMOSD at present, excessive immune intervention is not necessary for AQP4-IgG negative single-phase NMOSD. At present, Mycophenolate mofetil (MMF), AZA, RTX, and Methotrexate (MTX) are mainly used clinically, which can effectively prevent NMOSD recurrence, but these have not been verified by high quality RCT. Espiritu and Pasco[41] have published an article, which showed that AZA improves relapses and disability in patients with NMOSD. Damato et al[42] and Mirmosayyeb et al[43] also have published an article titled A systematic review and meta-analysis of Efficacy and Safety of Rituximab Therapy in Neuromyelitis Optica Spectrum Disorders, which showed that RTX therapy reduces the frequency of NMOSD relapses and neurological disability in patients with NMOSDs.
RTX is the first anti-CD20 monoclonal antibody approved for the treatment of B-cell lymphoma in 1997. B cells are usually almost completely depleted two weeks after the infusion of RTX, which lasts 6 to 12 months. Therefore, patients with NMOSD should receive regular maintenance therapy every 6 months. However, there were significant differences in the initial dose of RTX required for B cell depletion and the time to B cell reproliferation in patients. In a study of patients with NMOSD, it took 17% of patients 6 months to regain B cells.[44]However, B-cell consumption lasting more than 3 years after the administration of RTX has also been reported,[45] so the administration time should be set according to the situation of B-cell reproliferation to prevent overtreatment of patients with persistent B-cell failure, so as to prevent complications and reduce costs. The results of this meta-analysis proved that RTX could significantly reduce the ARR and EDSS scores of NMOSD patients, and its therapeutic effect was better than AZA. There was no statistical difference between RTX in the treatment of NMOSD patients with anti-aquapor in-4-antibody (AQP-4)-seropositive and seronegative. In addition, the results show that 156 cases (23%) experienced AEs, and 5 cases (0.7%) with severe adverse reactions caused by drug withdrawal, which indicated higher incidence of adverse reactions in RTX, but the vast majority of AEs for mild-to-moderate, have certain self-limited. It should be careful to use in the clinical application of rituxan for NMOSD patients.
This meta-analysis evaluated the efficacy and safety of RTX in the treatment of NMOSD from 37 included studies. In addition, the study assessed the efficacy of RTX and AZA in the treatment of NMOSD, indicating that there were no clinical differences between patients with AQP-4 positive and AQP-4 negative neuromyelitis optic spectrum disease treated with RTX. We could get some updated conclusion and help to develop a more reasonable treatment in clinical work.
4.1. Limitations
Although the quality of all the included studies was moderate, there were differences in terms of intervention and protocol design, and the results may have some limitations. There is publication bias in the influence of RTX on the recurrence rate and EDSS of NMOSD patients, which may be related to the high heterogeneity among included studies and the small sample size of some studies. Methods such as inclusion of high-quality homogeneous studies and expansion of sample size can reduce publication bias and make the results more accurate. Besides, there are few RCT on the efficacy and safety of RTX in the treatment of NMOSD, so more large multicenter RCTs are needed to further verify the accuracy of the conclusions.
Acknowledgments
The authors would like to thank Professor Wang for his help in designing the search strategies.
Author contributions
G-YD and X-JX designed the analysis. G-YD and X-JX collected and abstracted the data and carried out the statistical analysis. G-YD drafted the manuscript. All authors analyzed and interpreted the data and critically revised the manuscript for important intellectual content. The contents of this study are solely the responsibility of the authors and do not necessarily represent the official view of their institutions or any other party. G-YD and X-JX have full access to all the data and take full responsibility for the data, the analysis and the interpretation. All authors reviewed and approved the final report.
Conceptualization: Xiang-Jian Xiao.
Data curation: Gu-Yi Dong.
Formal analysis: Gu-Yi Dong.
Investigation: Gu-Yi Dong.
Methodology: Gu-Yi Dong.
Software: Xiang-Jian Xiao.
Writing – original draft: Gu-Yi Dong, Yan-Hong Meng, Xiang-Jian Xiao.
Writing – review & editing: Gu-Yi Dong, Yan-Hong Meng.
Abbreviations:
- 95% CI =
- 95% confidence interval
- AEs =
- adverse events
- AQP-4 =
- anti-aquapor in-4-antibody
- ARR =
- annualized relapse rate
- AZA =
- azathioprine
- CNKI =
- China National Knowledge Infrastructure
- EDSS =
- expanded disability status scale
- IPND =
- International NMO Diagnostic Group
- MMF =
- mycophenolate mofetil
- MTX =
- methotrexate
- NMOSDs =
- neuromyelitis optica spectrum diseases
- NOS =
- Newcastle-Ottawa Scale
- RCTs =
- randomized controlled trials
- RTX =
- rituximab
- SD =
- standard deviation
- WMD =
- weighted mean difference
This article does not contain any studies with human or animal subjects performed by any of the authors.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available. The data and materials are searched in the Pubmed, Embase, Cochrane Library, CNKI, Wan fang in public and checked by Professor Xiao.
How to cite this article: Dong G-Y, Meng Y-H, Xiao X-J. A meta-analysis on efficacy and safety of rituximab for neuromyelitis optica spectrum disorders. Medicine 2022;101:36(e30347).
Contributor Information
Gu-Yi Dong, Email: 18330103864@163.com.
Yan-Hong Meng, Email: 13833169540@163.com.
References
- [1].Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of neuromyelitis Optica. Lancet Neurol. 2007;6:805–15. [DOI] [PubMed] [Google Scholar]
- [2].Cabre P, Mejdoubi M, Jeannin S, et al. Treatment of neuromyelitis Optica with rituximab: a 2-year prospective multicenter study. J Neurol. 2018;265:917–25. [DOI] [PubMed] [Google Scholar]
- [3].Kim SH, Jeong IH, Hyun JW, et al. Treatment outcomes with rituximab in 100 patients with neuromyelitis Optica: influence of FCGR3A polymorphisms on the therapeutic response to rituximab. JAMA Neurol. 2015;72:989–95. [DOI] [PubMed] [Google Scholar]
- [4].Dersimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [5].Dastjerdi RM, Ashtari F, Shaygannejad V. Disease course, progression and activity among neuromyelitis Optica (NMOSD) patients who treated with rituximab, 6 and 12 months after the first dose, in Isfahan. Mult Scler Relat Disord. 2018;26:262–3. [DOI] [PubMed] [Google Scholar]
- [6].Fernández-Megía MJ, Casanova-Estruch B, Pérez-Miralles F, et al. Clinical evaluation of rituximab treatment for neuromyelitis Optica. Neurologia. 2015;30:461–4. [DOI] [PubMed] [Google Scholar]
- [7].Jacob A, Weinshenker BG, Violich I, et al. Treatment of neuromyelitis Optica with rituximab: retrospective analysis of 25 patients. Arch Neurol. 2008;65:1443–8. [DOI] [PubMed] [Google Scholar]
- [8].Nikoo Z, Badihian S, Shaygannejad V, et al. Comparison of the efficacy of azathioprine and rituximab in neuromyelitis Optica spectrum disorder: a randomized clinical trial. J Neurol. 2017;264:2003–9. [DOI] [PubMed] [Google Scholar]
- [9].Shaygannejad V, Fayyazi E, Badihian S, et al. Long-term tolerability, safety and efficacy of rituximab in neuromyelitis Optica spectrum disorder: a prospective study. J Neurol. 2019;266:642–50. [DOI] [PubMed] [Google Scholar]
- [10].Zéphir H, Bernard-Valnet R, Lebrun C, et al. Rituximab as first-line therapy in neuromyelitis Optica: efficiency and tolerability. J Neurol. 2015, 262: 2329–35 [DOI] [PubMed] [Google Scholar]
- [11].Zhang M, Zhang C, Bai P, et al. Effectiveness of low dose of rituximab compared with azathioprine in Chinese patients with neuromyelitis Optica: an over 2-year follow-up study. Acta Neurol Belg. 2017;117:695–702. [DOI] [PubMed] [Google Scholar]
- [12].Annovazzi P, Capobianco M, Moiola L, et al. Rituximab in the treatment of neuromyelitis Optica: a multicentre Italian observational study. J Neurol. 2016;263:1727–35. [DOI] [PubMed] [Google Scholar]
- [13].Pellkofer HL, Krumbholz M, Berthele A, et al. Long-term follow-up of patients with neuromyelitis Optica after repeated therapy with rituximab. Neurology. 2011;76:1310–5. [DOI] [PubMed] [Google Scholar]
- [14].Lindsey JW, Meulmester KM, Brod SA, et al. Variable results after rituximab in neuromyelitis Optica. J Neurol Sci. 2012;317:103–5. [DOI] [PubMed] [Google Scholar]
- [15].Ip VH, Lau AY, Au LW, et al. Rituximab reduces attacks in Chinese patients with neuromyelitis Optica spectrum disorders. J Neurol Sci. 2013;324:38–9. [DOI] [PubMed] [Google Scholar]
- [16].Jeong IH, Park B, Kim SH, et al. Comparative analysis of treatment outcomes in patients with neuromyelitis Optica spectrum disorder using multifaceted endpoints. Mult Scler J. 2016;22:329–39. [DOI] [PubMed] [Google Scholar]
- [17].Collongues N, Brassat D, Maillart E, et al. Efficacy of rituximab in refractory neuromyelitis Optica. Mult Scler. 2016;22:955–9. [DOI] [PubMed] [Google Scholar]
- [18].Cohen M, Romero G, Bas J, et al. Monitoring CD27+ memory B-cells in neuromyelitis Optica spectrum disorders patients treated with rituximab: results from a bicentric study. J Neurol Sci. 2017;373:335–8. [DOI] [PubMed] [Google Scholar]
- [19].Yang Y, Wang CJ, Wang BJ, et al. Comparison of efficacy and tolerability of azathioprine, mycophenolate mofetil, and lower dosages of rituximab among patients with neuromyelitis Optica spectrum disorder. J Neurol Sci. 2018;385:192–7. [DOI] [PubMed] [Google Scholar]
- [20].Bedi GS, Brown AD, Delgado SR, et al. Impact of rituximab on relapse rate and disability in neuromyelitis Optica. Mult Scler. 2011;17:1225–30. [DOI] [PubMed] [Google Scholar]
- [21].Casallas-Vanegas A, Gomez-Figueroa E, De Saráchaga AJ, et al. Rituximab efficacy at different initial and maintenance doses in neuromyelitis Optica spectrum disorder: experience from a national health institute in México. J Neurol Sci. 2020;419:117204. [DOI] [PubMed] [Google Scholar]
- [22].Correa Diaz EP, Torres Herran GE, Zambrano FC. Impact of rituximab on relapse rate and disability in a cohort of patients with neuromyelitis Optica from Quito. Mult Scler J. 2019;25:118. [DOI] [PubMed] [Google Scholar]
- [23].Flores J, Aguirre L, Salinas L, et al. Use of rituximab in Devic’s disease, two-year follow-up. Mexican experience. Mult Scler. 2012;18:296. [Google Scholar]
- [24].Kim HJ, Kim W, Park MS, et al. B cell depletion therapy using rituximab in neuromyelitis Optica spectrum disorders. Mult Scler. 2009;15:S253–4. [Google Scholar]
- [25].Kim SH, Huh SY, Lee SJ, et al. A 5-year follow-up of rituximab treatment in patients with neuromyelitis Optica spectrum disorder. JAMA Neurol. 2013;70:1110–7. [DOI] [PubMed] [Google Scholar]
- [26].Lin J, Li X, Xue B, et al. Low-dosage of rituximab in Chinese patients with neuromyelitis Optica spectrum disorder. J Neuroimmunol. 2018;317:1–4. [DOI] [PubMed] [Google Scholar]
- [27].Wu Z, Niu Y. Preliminary observation of efficacy and safety of 15 patients with neuromyelitis optic spectrum disorder treated with rituximab. Eur J Immunol. 2019;49:1774–5. [Google Scholar]
- [28].Xiao H, Zeng W, Li L, et al. Retrospective observation of low-dose rituximab treatment in Chinese patients with neuromyelitis Optica spectrum disorders in a real-world setting. Front Neurol. 2020;11:642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bai P. Effectiveness of rituximab in the treatment of neuromyelitis Optica spectrum diseases. Shanxi Medical University. 2016. [Google Scholar]
- [30].Li T. Study on efficacy, immunogenicity and application time of low dose rituximab in NMOSD. Tianjin Medical University. 2019. [Google Scholar]
- [31].Liu QT. Role of rituximab in the treatment of refractory neuromyelitis optic spectrum disease. Med Front. 2017;19:125–6. [Google Scholar]
- [32].Niu YM. Preliminary observation on the efficacy and safety of rituximab in the treatment of 15 patients with neuromyelitis optic spectrum disease. China Med Med Univ. 2019. [Google Scholar]
- [33].Wang JJ, Xu QG, Zhou HF, et al. Effectiveness and safety of low dose rituximab in prevention of recurrent neuromyelitis optic spectrum disease. Chin J Fundus Dis. 2018;34:155–8. [Google Scholar]
- [34].Zhang CT. Clinical observation of different doses of rituximab in the treatment of neuromyelitis optic spectrum diseases. Shanxi Medical University. 2016. [Google Scholar]
- [35].Zhu MJ. To analyze the clinical efficacy and safety of rituximab in the treatment of refractory neuromyelitis optic spectrum diseases. Chin Med Guide. 2018;16:77–8. [Google Scholar]
- [36].Jia XT, Gu NB, Liu ZQ, et al. Clinical observation of low-dose rituximab sequential treatment of neuromyelitis optic spectrum diseases. J Cardiovascular Cerebrovascular Dis Integrated Traditional Chin Western Med. 2018;16:3256–8. [Google Scholar]
- [37].Zhang L. Efficacy and prognostic factors of azathioprine, mortimecofenate and rituximab in the treatment of neuromyelitis optic spectrum diseases. Hebei Med Univ. 2020. [Google Scholar]
- [38].Cao S, Yu H, Tian J, et al. Efficacy and safety of modified reduced-dose rituximab in Chinese patients with neuromyelitis Optica spectrum disorder: a retrospective cohort study. J Neurol Sci. 2021;429:117616. [DOI] [PubMed] [Google Scholar]
- [39].Uzunköprü C, Tütüncü M, Gündüz T, et al. The efficacy of rituximab in patients with neuromyelitis Optica spectrum disorder: a real-world study from Turkey. Int J Clin Pract. 2021;75:e14158. [DOI] [PubMed] [Google Scholar]
- [40].Wingerchuk DM, Hogancamp WF, O’brien PC, et al. The clinical course of neuromyelitis Optica (Devic’s syndrome). Neurology. 1999;53:1107–14. [DOI] [PubMed] [Google Scholar]
- [41].Espiritu AI, Pasco PMD. Efficacy and tolerability of azathioprine for neuromyelitis Optica spectrum disorder: a systematic review and meta-analysis. Mult Scler Relat Disord. 2019;33:22–32. [DOI] [PubMed] [Google Scholar]
- [42].Damato V, Evoli A, Iorio R. Efficacy and safety of rituximab therapy in neuromyelitis Optica spectrum disorders: a systematic review and meta-analysis. JAMA Neurol. 2016;73:1342–8. [DOI] [PubMed] [Google Scholar]
- [43].Mirmosayyeb O, Shaygannejad V, Barzegar M, et al. Efficacy and safety of rituximab in treating patients with neuromyelitis Optica spectrum disorder (NMOSD): a systematic review and meta-analysis. Autoimmun Rev. 2021;20:102727. [DOI] [PubMed] [Google Scholar]
- [44].Greenberg BM, Graves D, Remington G, et al. Rituximab dosing and monitoring strategies in neuromyelitis Optica patients: creating strategies for therapeutic success. Mult Scler. 2012;18:1022–6. [DOI] [PubMed] [Google Scholar]
- [45].Yi JS, Decroos EC, Sanders DB, et al. Prolonged B-cell depletion in MuSK myasthenia gravis following rituximab treatment. Muscle Nerve. 2013;48:992–3. [DOI] [PMC free article] [PubMed] [Google Scholar]