Abstract
The role of the herpes simplex virus type 1 tegument protein VP22 during infection is as yet undefined. We have previously shown that VP22 has the unusual property of efficient intercellular transport, such that the protein spreads from single expressing cells into large numbers of surrounding cells. We also noted that in cells expressing VP22 by transient transfection, the protein localizes in a distinctive cytoplasmic filamentous pattern. Here we show that this pattern represents a colocalization between VP22 and cellular microtubules. Moreover, we show that VP22 reorganizes microtubules into thick bundles which are easily distinguishable from nonbundled microtubules. These bundles are highly resistant to microtubule-depolymerizing agents such as nocodazole and incubation at 4°C, suggesting that VP22 has the capacity to stabilize the microtubule network. In addition, we show that the microtubules contained in these bundles are modified by acetylation, a marker for microtubule stability. Analysis of infected cells by both immunofluorescence and measurement of microtubule acetylation further showed that colocalization between VP22 and microtubules, and induction of microtubule acetylation, also occurs during infection. Taken together, these results suggest that VP22 exhibits the properties of a classical microtubule-associated protein (MAP) during both transfection and infection. This is the first demonstration of a MAP encoded by an animal virus.
The eukaryotic cytoskeleton, which comprises actin microfilaments, intermediate filaments (IFs), and microtubules (MTs), performs a broad range of complex activities within the cell. These include various aspects of cell motility (2, 3), the determination of cell shape and internal architecture (17, 32), and vesicle trafficking and chromosome movement during mitosis (18, 25, 29). Furthermore, the individual components of the cytoskeleton are interlinked to form a dynamic network accessing every area of the cytoplasm (41) and the plasma membrane (10, 39), providing a framework which coordinates multiple cellular processes. The involvement in so many cellular activities is likely to make the cytoskeleton a primary target for exploitation during virus infection of host cells. Surprisingly, however, there is relatively little detailed information on virus interactions with the host cytoskeleton, and it is only recently that data suggesting that viruses may utilize the positioning and dynamics of the cytoskeletal network to their own advantage have begun to emerge.
The majority of virus-induced cytoskeletal alterations documented to date involve the overall disruption of one or more elements of the cytoskeleton. For example, retroviruses and poliovirus encode proteases which induce the cleavage of cytoskeleton-associated proteins, thereby broadly increasing the dynamics of the cytoskeleton, resulting in disruption of the cell structure as infection progresses, and the appearance of well-characterized cytopathic effects (20, 43). A more specific disruption of the cytoskeleton occurs during infection by the rhabdovirus vesicular stomatitis virus, where the direct interaction of the virus matrix protein with tubulin results in the inhibition of MT assembly (33). Human immunodeficiency virus and papillomaviruses, on the other hand, encode activities which induce the collapse of the IF network, a property which may promote virus release from the cell (13, 23).
By contrast, examples of virus activities which induce cytoskeletal polymerization and/or stabilization are much rarer. One example is the baculovirus Autographa californica nuclear polyhedrosis virus, which has been shown to induce the appearance of thick actin cables between the plasma membrane and the nucleus at early times after infection (8) and to induce actin filaments in the nucleus at late times (7). These features have been proposed to be involved in virus transport from the cell surface to the nucleus and nucleocapsid morphogenesis, respectively. However, the best-characterized viral exploitation of the host cell cytoskeleton is that of vaccinia virus, which has been shown to induce actin polymerization directly behind its virus particle as a means of propelling the virus through the cell (11, 12). The virus protein(s) responsible for this activity has not yet been identified, but it has been shown that disruption of the actin cytoskeleton in infected cells inhibits virus release, indicating that actin is essential to the virus replicative cycle (35).
The herpes simplex virus type 1 (HSV-1) structural protein VP22, a component of the viral tegument, has an as yet undefined role in virus replication. However, we have recently shown that VP22 has the unusual property of intercellular transport when it is expressed during both infection and transient transfection (14). Moreover, we demonstrated that such VP22 transport occurs via a mechanism potentially involving actin microfilaments, suggesting that VP22 exhibits a cytoskeletal interaction. In this report, we demonstrate that VP22 interacts with another component of the cellular cytoskeleton, the MT network. We show that VP22 colocalizes with MTs in both transfected and infected cells and induces the appearance of thick MT bundles. Furthermore, we show that these VP22-induced MT bundles are highly stabilized in comparison to normal MTs and are resistant to both drug and cold treatment. As a consequence of VP22-induced stabilization, MTs are extensively modified by acetylation, a property also demonstrated in infected cells. Taken together, these results suggest that VP22 exhibits the properties of a classical cellular MT-associated protein (MAP) with powerful MT-stabilizing properties and represents the first demonstration of a MAP encoded by an animal virus.
MATERIALS AND METHODS
Plasmids.
The eukaryotic expression vector pGE109, described previously (15), contains the VP22 open reading frame under the control of the human cytomegalovirus immediate-early promoter.
Antibodies and reagents.
The polyclonal anti-VP22 antibody AGV30, described previously (14), was used at dilutions of 1:500 for immunofluorescence and 1:50,000 for Western blotting. Monoclonal antibodies against α-tubulin and acetylated tubulin (Sigma) were used at dilutions of 1:500 and 1:100, respectively, for immunofluorescence and 1:20,000 and 1:2000, respectively, for Western blotting. The monoclonal antibody against vimentin (Sigma) was used at a dilution of 1:200 for immunofluorescence.
Nocodazole (used at concentrations ranging from 50 ng/ml to 10 μg/ml) and taxol (used at 50 μg/ml) were obtained from Sigma.
Transfections and infections.
COS-1 and Vero cells were maintained in Dulbecco’s modified minimal essential medium containing 10% newborn calf serum. Cells were plated into six-well trays (6 by 35 mm) at a density of either 2 × 105 cells per well for immunofluorescence or 4 × 105 cells per well for Western blotting. DNA mixes consisting of 200 ng of expression plasmid made up to 2 μg with pUC19 DNA were transfected by the calcium phosphate precipitation technique modified with BES [N,N-bis(2-hydroxyl)-2-aminoethanesulfonic acid]-buffered saline in place of HEPES-buffered saline. Transfected cells were analyzed 40 h posttransfection unless otherwise stated.
Virus infections were carried out with HSV-1 strain 17 at a multiplicity of 10.
Immunofluorescence and microscopy.
Cells to be processed for immunofluorescence were washed with phosphate-buffered saline (PBS) and fixed for 15 min at room temperature with 100% methanol. The samples were blocked with 10% calf serum in PBS for 10 min, and primary antibody was added in the same solution for a further 20 min. Following three 5-min washes with PBS, secondary antibodies (fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G used at a dilution of 1:100 [Vector] and/or tetramethyl rhodamine isothiocyanate-conjugated anti-rabbit immunoglobulin G used at a dilution of 1:200 [Sigma]) were added in blocking solution and incubated for 10 min. After three additional 5-min washes, the coverslips were mounted in 90% glycerol in PBS. Samples were examined in dual channels with a Bio-Rad MRC600 confocal microscope, and images were processed with Adobe Photoshop software.
Western blot analysis.
Proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose filters, and reacted with the appropriate primary antibody. A horseradish peroxidase-linked secondary conjugate was used, and reactive bands were visualized with enhanced chemiluminescence detection reagents (Amersham).
RESULTS
VP22 colocalizes with and reorganizes MTs.
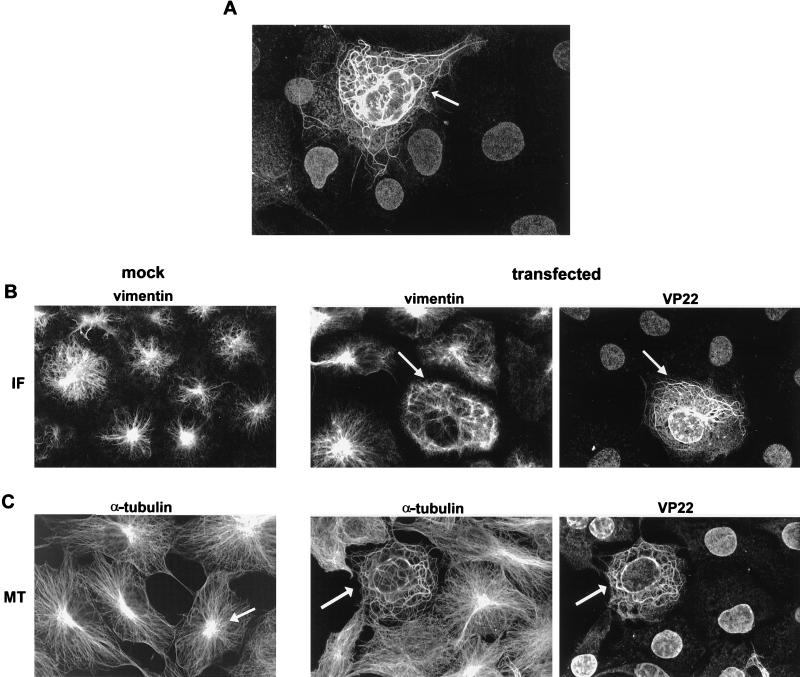
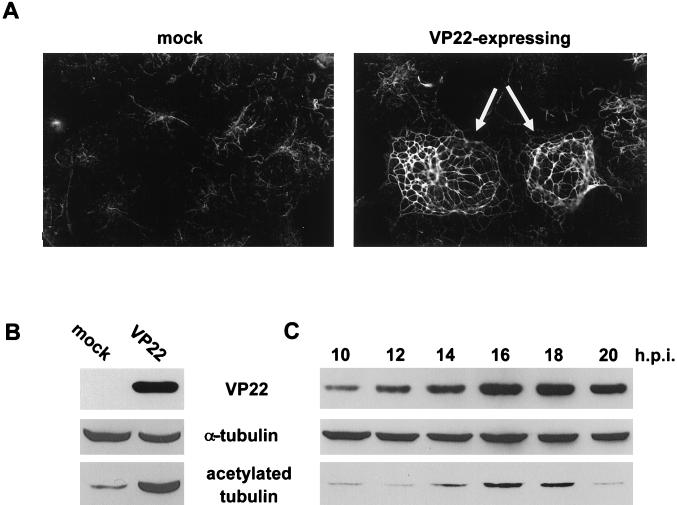
We have previously shown that VP22 exhibits heterogeneity in subcellular localization following delivery of the VP22 gene by either transfection, microinjection, or virus infection (14). This heterogeneity is the consequence of a mixed population of VP22-containing cells: those which synthesize VP22, in which VP22 is localized to the cytoplasm, and those surrounding cells which take up VP22, where it accumulates largely in the nucleus (Fig. 1A). Furthermore, we have demonstrated that within the cell that is synthesizing VP22, the protein forms distinctive cage-like filaments (arrowed in Fig. 1A). The nature of the VP22 filamentous pattern suggested to us that it may interact with some element of the cytoskeleton. However, the well-documented localizations of actin microfilaments, IFs, and MTs bear little resemblance to the pattern of localization observed for VP22 (Fig. 1B and C, mock, vimentin and α-tubulin). Nonetheless, to determine if VP22 was affecting the nature of any component of the cytoskeleton, we examined the location of both the IF protein vimentin and the MT protein α-tubulin in COS-1 cells expressing VP22. While IFs in VP22-expressing cells had a slightly altered pattern of localization in comparison to nonexpressing cells (Fig. 1B; compare vimentin in mock and transfected), there was no evidence of direct interaction between VP22 and vimentin, as there was a lack of any degree of colocalization of the two proteins in the VP22-expressing cell (Fig. 1B, transfected; compare vimentin and VP22 in arrowed cell). The localization of MTs, however, was dramatically altered in VP22-expressing cells (Fig. 1C; compare α-tubulin in mock and transfected cells; VP22-expressing cell arrowed in transfected cells) such that α-tubulin was reorganized into thick bundles which exactly colocalized with the VP22 filaments (Fig. 1B, transfected; compare α-tubulin and VP22 in arrowed cell). Moreover, the MT-organizing center (arrowed in Fig. 1C, mock), the site of nucleation for MTs within the cell, was absent from VP22-expressing cells (Fig. 1C; arrowed cell in transfected). In addition, it is noteworthy that there was no colocalization between VP22 and MTs in the cells which had taken up VP22 (Fig. 1C, transfected; compare α-tubulin and VP22), suggesting that VP22 behaves differently in synthesizing and recipient cells.
FIG. 1.
VP22 colocalizes with and reorganizes MTs in transfected cells. (A) Typical VP22 localization pattern in COS-1 cells transfected with plasmid pGE109 and stained with the anti-VP22 polyclonal antibody AGV30. The cell expressing VP22 (arrowed) is surrounded by cells which have taken up VP22 into their nuclei. (B and C) Localization of the cytoskeletal proteins vimentin (B) and α-tubulin (C) in untransfected (mock) and VP22-expressing (transfected) COS-1 cells. Immunofluorescence was carried out with the antivimentin (B) or anti-α-tubulin (C) monoclonal antibodies, which were used in conjunction with AGV30 for double labeling of transfected cells. VP22-expressing cells are arrowed. The cell MT-organizing center is arrowed in panel C, mock.
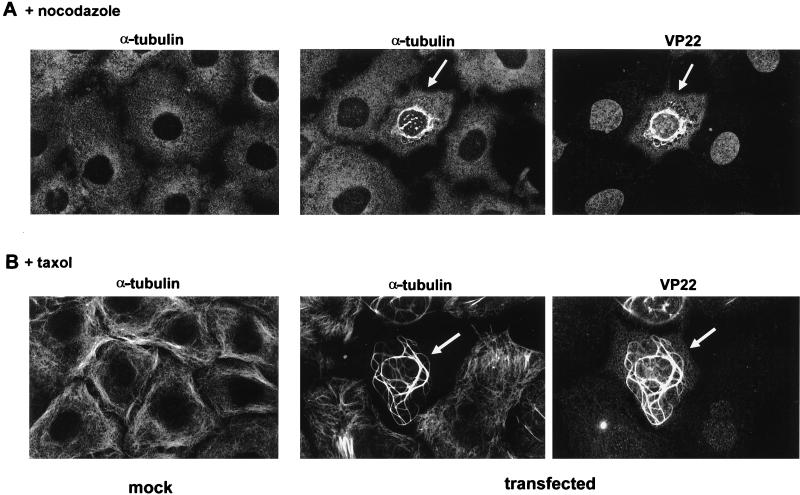
To confirm that the apparent VP22–α-tubulin colocalization represented an interaction between the two proteins, we used two drugs which are known to affect the stability of the MT network. First, both untransfected and transfected cells were treated with the MT-destabilizing drug nocodazole (Fig. 2A). In the presence of a high concentration of nocodazole (10 μg/ml), MTs in mock cells were entirely depolymerized, resulting in a diffuse pattern of α-tubulin staining within the cytoplasm (Fig. 2A, mock). Likewise in VP22-expressing cells, treatment with the same concentration of nocodazole resulted in the destabilization of both the MT and VP22 networks such that the two proteins behaved identically (Fig. 2A, transfected; compare α-tubulin and VP22). Second, both untransfected and transfected cells were treated with the MT-stabilizing drug taxol (Fig. 2B). Under these conditions, MTs in untransfected cells were stabilized in loose bundles which localized toward the edge of the cell (Fig. 2B, mock). By contrast, the taxol-treated MTs in VP22-expressing cells had an appearance very different from that of MTs observed in untransfected cells, localizing into substantial thick whorls of bundled MTs (Fig. 2B; compare α-tubulin in mock and transfected). However, as for the nocodazole treatment, both the MTs and VP22 filaments exhibited the same pattern of localization within the taxol-treated VP22-expressing cells (Fig. 2B, transfected; compare α-tubulin and VP22). Thus, we conclude that the MT network within VP22-expressing cells is reorganized into bundles which colocalize with VP22 filaments as a consequence of an interaction (either direct or indirect) of the two proteins.
FIG. 2.
Drugs which alter MT stability affect the localization of VP22. Untransfected (mock) and VP22-expressing (transfected) COS-1 cells were incubated in either nocodazole (10 μg/ml) (A) or taxol (50 μg/ml) (B) for 3 h prior to fixation. Cells were stained with AGV30 and/or anti-α-tubulin. VP22-expressing cells are arrowed.
MTs are bundled in HSV-1-infected cells.
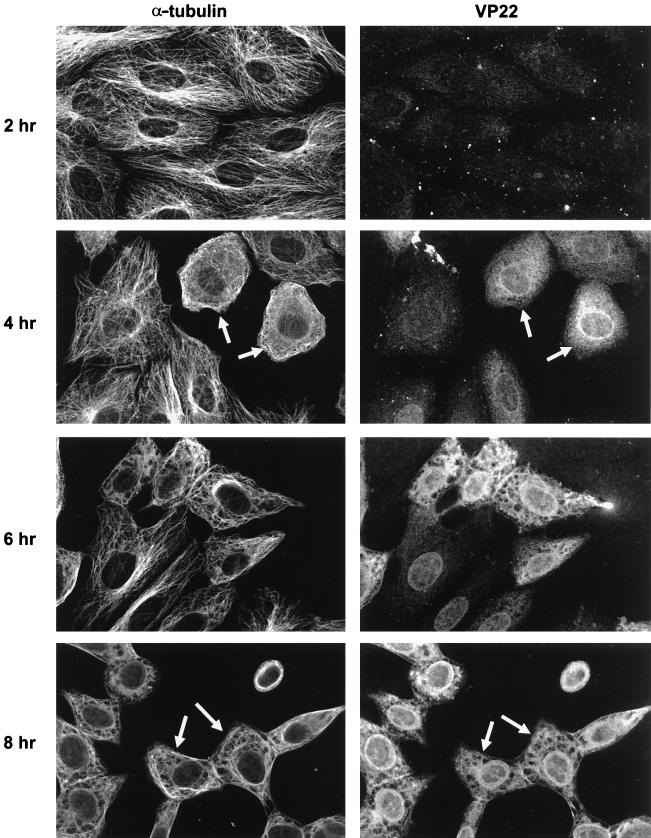
It has previously been demonstrated that MTs become reorganized during infection by HSV-1 (1). To determine if such reorganization is related to the effects of VP22 on MTs observed during transient expression, a time course of infection was carried out in Vero cells. Cells were infected at a multiplicity of 10, fixed at various times after infection, and stained for both α-tubulin and VP22. Under these conditions VP22 was initially detected in the cell at 4 h after infection (Fig. 3, VP22, 4 h), increasing in concentration over the next 4 h (Fig. 3, VP22, 6 h and 8 h). Notably, MT reorganization began only at the time when VP22 was first detected (Fig. 3, α-tubulin; compare 2 h and 4 h) and strikingly was apparent only in the cells which were expressing VP22 to any detectable level (Fig. 3, 4 h, arrowed cells). Over the next 4 h, MT reorganization became more evident (Fig. 3, α-tubulin, 6 h and 8 h) and always correlated with those cells in which VP22 was highly expressed. Moreover, a degree of colocalization was apparent between VP22 and α-tubulin at these times, although not so dramatically as that seen during transient transfection (Fig. 3, 8 h, arrowed cells). At later times in infection, the level of cytopathic effect in the cells prevented any meaningful colocalization studies (data not shown). While these results do not directly demonstrate that VP22 is responsible for MT reorganization during infection, they clearly demonstrate that MTs are altered similarly in infected cells and in transfected cells expressing the individual VP22 protein.
FIG. 3.
VP22 colocalizes with MTs in HSV-1-infected cells. Vero cells were infected with HSV-1 (strain 17) at a multiplicity of 10 and fixed at various times after infection. Double immunofluorescence was carried out with AGV30 and anti-α-tubulin antibodies. Cells showing colocalization of VP22 with MTs are arrowed at 4 and 8 h postinfection.
VP22-induced MT bundles are highly stabilized.
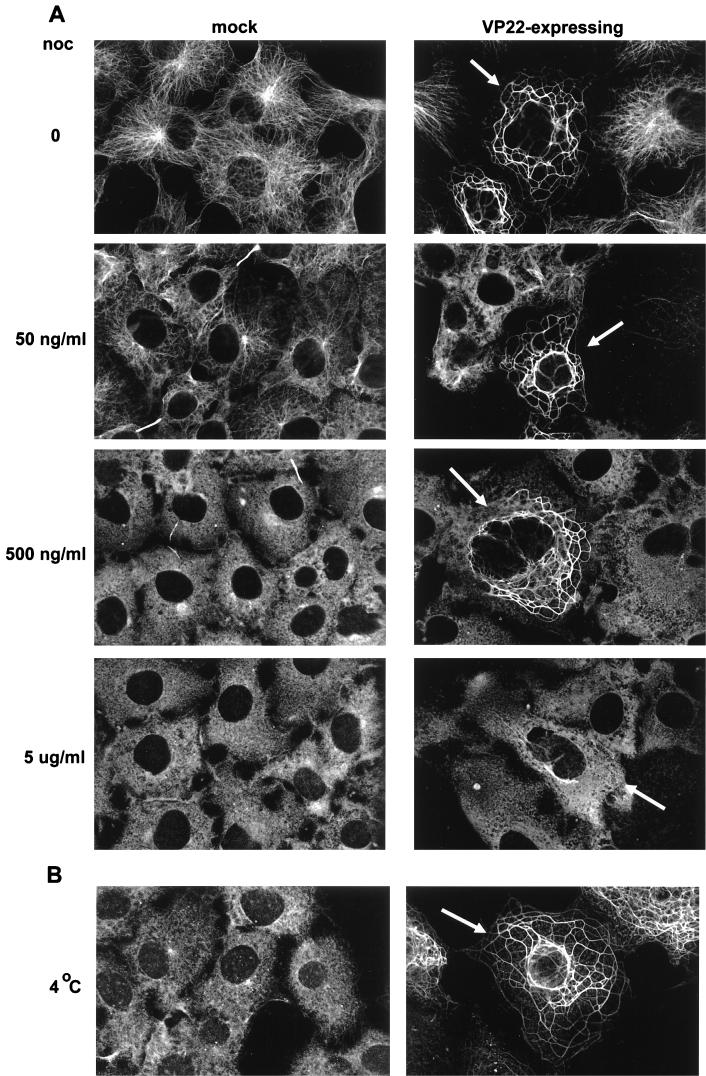
While conducting the experiment represented in Fig. 2, in which VP22-expressing cells were treated with nocodazole to destabilize the MT network, we noted that even at the relatively high concentration of the drug used, VP22-induced MT bundles were not completely depolymerized at times when those in non-VP22-expressing cells were clearly disrupted (Fig. 2A). To examine the possibility that VP22 was altering the dynamics of these MTs and increasing their stability, both mock- and VP22-transfected cells were treated with increasing concentrations of nocodazole (Fig. 4A). In the case of untransfected cells, concentrations as low as 50 ng/ml had a demonstrable effect on the nature of the MTs (Fig. 4A, mock; compare 0 and 50 ng/ml). At 500 ng/ml, the only region of the MT network left intact appeared to be the MT bridges between the cells (Fig. 4A, mock, 500 ng/ml), while at 5 μg/ml even these had been disrupted (Fig. 4A, mock, 5 μg/ml). By contrast, the MT bundles in VP22-expressing cells (arrowed in Fig. 4A, VP22-expressing) remained relatively unaffected by the drug at concentrations as high as 500 ng/ml (Fig. 4A, VP22-expressing; compare 0 and 500 ng/ml). Only at the concentration of 5 μg/ml did nocodazole begin to have an effect on these VP22-induced bundles (Fig. 4A, VP22-expressing, 5 μg/ml).
FIG. 4.
VP22 stabilizes MTs in transfected cells. (A) Untransfected (mock) or transfected (VP22-expressing) COS-1 cells were exposed to different concentrations (ranging from 0 to 5 μg/ml) of nocodazole (noc) for 1 h prior to fixation. The cells were then stained with the anti-α-tubulin monoclonal antibody, and VP22-expressing cells (arrowed) were identified by the presence of MT bundles. (B) Untransfected (mock) or transfected (VP22-expressing) COS-1 cells were incubated at 4°C for 15 min prior to fixation. Staining was carried out as for panel A.
As an alternative and more stringent mechanism of destabilizing MTs (30), we incubated both mock-transfected and VP22-expressing cells for a short period of time at 4°C (Fig. 4B). Under these conditions, MTs in untransfected cells were almost entirely depolymerized (Fig. 4B, mock). However, once again VP22-induced MT bundles were readily observed (Fig. 4B, arrowed in VP22-expressing). Taken together, these results indicate that the bundles induced by the interaction of MTs with VP22 are highly stabilized in comparison to normal MT filaments.
VP22-stabilized MTs are hyperacetylated.
To confirm MT stabilization by VP22, we assessed the level of MT modification by acetylation in untransfected and VP22-expressing cells. Acetylation is known to occur on MTs stabilized by normal cellular mechanisms (37), although the role played by acetylation is not yet clear. Acetylated MTs were identified initially by immunofluorescence using an antibody specific for the modified form of tubulin (Fig. 5A). This demonstrated that while all mock-transfected cells contained a low level of acetylated MTs (Fig. 5A, mock), the MT bundles present in VP22-expressing cells (Fig. 5A, VP22-expressing, arrowed) were heavily acetylated (Fig. 5A; compare mock and VP22-expressing). Moreover, the acetylated MTs colocalized with the VP22 filaments (data not shown), confirming that the increased modification was occurring on the VP22-interacting MTs. This effect was also demonstrated by determining the relative amounts of acetylated tubulin in total cell extracts from mock- and VP22-transfected cells (Fig. 5B). While the overall level of α-tubulin was the same in each sample (Fig. 5B, α-tubulin), the level of acetylated tubulin increased around 10-fold in VP22-transfected cells in comparison to mock-transfected cells (Fig. 5B, acetylated tubulin; compare mock and VP22).
FIG. 5.
VP22 induces the hyperacetylation of MTs in transfected and infected cells. (A) Untransfected (mock) or transfected (VP22-expressing) COS-1 cells were stained with an antibody specific for the acetylated form of tubulin. VP22-expressing cells are arrowed. (B) Western blot of total extracts of untransfected (mock) or transfected (VP22) COS-1 cells reacted with AGV30, anti-α-tubulin, and anti-acetylated tubulin. (C) Western blot of HSV-1 (strain 17 at a multiplicity of 10)-infected Vero cell total extracts, harvested at 10 to 20 h postinfection (h.p.i.) and reacted with AGV30, anti-α-tubulin, and anti-acetylated tubulin.
To confirm that MTs in infected cells were behaving similarly to those present in VP22-transfected cells, we assessed the level of MT acetylation during infection. Western blotting of cell lysates harvested at different times after infection indicated that while the overall level of α-tubulin remained the same throughout the infectious cycle (Fig. 5C, α-tubulin), the level of acetylated tubulin increased approximately fourfold at late times in infection (Fig. 5C, acetylated tubulin; compare 10 and 18 h.p.i.). This increase in tubulin acetylation correlated with the synthesis of VP22 during infection (Fig. 5C; compare VP22 and acetylated tubulin), suggesting that the presence of VP22 within the infected cell may be involved in MT acetylation. Thus, it would appear that MT localization and dynamics within infected cells are similar to those in cells transfected with VP22, implying that VP22-induced alteration of the MT network is a feature of the HSV-1 replicative cycle.
DISCUSSION
The fundamental role played by the cytoskeleton in orchestrating many aspects of cell growth and communication, including physical connections, intracellular signalling pathways, and the coordination of cell division, implies that the cytoskeleton may be a major target of virus-cell interactions. In this report we show that the herpesvirus structural protein VP22, which we have previously shown to have the property of intercellular spread (14), has the additional property of MT interaction and rearrangement. While several animal viruses have now been shown to interact in some way with either the actin cytoskeleton (11, 12) or IFs (13, 23), this is the first demonstration of an animal virus-encoded protein capable of stabilizing the MT network.
There are several examples of viruses which cause a generalized disruption of the MT network as infection progresses, resulting in well-characterized cytopathic effects. Such disruption usually occurs either via a direct interaction between a viral component and the cytoskeleton (33) or by virus-induced cleavage of MAPs, thereby broadly increasing cytoskeletal dynamics (20, 43). VP22 is unusual in that rather than disrupting the MT network, it actively stabilizes it. It has previously been shown that MTs are reorganized during HSV-1 infection (1), and our results from both transfections and infections strongly suggest that VP22 is responsible for this activity. It is noteworthy that in contrast to our results which demonstrate that VP22 stabilizes MTs against exposure to nocodazole, the above-mentioned study concluded that the effects of nocodazole on MTs were dominant over the effects of viral infection (1). However, the single concentration of nocodazole used in these studies was around 5 μg/ml, which in our hands was high enough to overcome the stabilizing effect of VP22 (Fig. 4A). Moreover, it is clear from both our colocalization and MT acetylation studies that VP22-induced MT stabilization in transfected cells is more efficient than that observed in HSV-1-infected cells. This may be explained by the fact that VP22 has multiple roles to play during the replicative cycle, and in particular it must be incorporated into newly assembling virus particles. Therefore, while the majority of the VP22 population in transfected cells is available for assembly into MT bundles, it is likely that a fine balance exists between the different activities of VP22 in an infected cell.
While the precise role of MT stabilization during HSV-1 infection remains to be defined, we propose several possibilities which are not mutually exclusive. For instance, MTs have been implicated in the transport of HSV-1 capsids at the initial stage of infection in both neurons (42) and tissue culture (40). As VP22 is a structural component of the HSV-1 virion tegument, and by definition could still be associated with the viral capsid at such early times, it is conceivable that the VP22-MT interaction is in some way involved in capsid trafficking. Localized MT stabilization and/or assembly could be particularly important in the neuron, in which the viral capsid has a considerably greater distance to travel from the periphery of the cell to the nucleus. MT assembly and stability may also be involved in virus egress from the cell, facilitating the transport of naked capsids to their point of envelopment within the cell. In relation to this, it is noteworthy that MTs have previously been shown to be involved in virus egress in the neuron model (36).
MTs are structures which exhibit dynamic instability within the cell, in that they undergo alternating growth and shrinkage (34). Our results demonstrate that VP22 interacts with and rearranges MTs into bundles which are highly stabilized to cold and drug treatment and therefore are likely to be much less dynamic than normal MTs. The ability of VP22 to stabilize MTs suggests that it behaves similarly to a group of cellular proteins known as MAPs, which include the neuronal proteins tau (9) and MAP2 (28) and the nonneuronal protein MAP4 (4). These proteins, which are considered to be regulators of MT dynamics within the cell, have been shown to promote MT assembly in vitro, and are involved in nucleation and stabilization of MTs in vivo (16, 19, 22). Moreover, the overexpression of several MAPs by transient transfection results in the production of MT bundles similar to those observed during the expression of VP22 (21, 24, 27). Members of the tau/MAP2/MAP4 family share an element of structural homology, including a proline-rich sequence and a repeated motif which are both thought to be essential for MT binding and bundling (6, 16, 21, 28, 44). While VP22 is a generally proline-rich protein (14), there is no obvious homology with the conserved regions of the cellular MAPs.
The exact mechanism of MT bundling by MAPs remains unclear. It was initially suggested that MAPs physically cross-linked individual MTs (21, 26, 27) in the same manner as certain microfilament-cross-linking proteins (31). However, more recently it has been proposed that MT bundles are the result purely of increased but localized MT nucleation and assembly within a restricted space, resulting in a mass of parallel MTs (5, 38). VP22 clearly possesses an MT bundling property, and the loss of the centrosome in VP22-expressing cells, from which MTs are normally nucleated, together with the appearance of MT junctions throughout the cytoplasm (Fig. 1C, transfected), suggests that MT nucleation in these cells may no longer be dependent on the organizing center. Taken together, these results suggest that VP22 behaves similarly to classical cellular MAPs, although we do not yet know if VP22-induced MT bundles are the result of a direct interaction with MTs or are generated by an alternative indirect mechanism involving another partner.
Thus, as we have previously shown that VP22 spreads between cells during HSV-1 infection, by a mechanism potentially involving the actin microfilaments (14), we have now shown that VP22 may interact with two elements of the cytoskeleton. Although we do not yet know if VP22 trafficking also involves its MT interaction, a connection between the two activities would provide an elegant mechanism for coordinated delivery of VP22 into surrounding cells during virus infection and would further emphasize the intracellular connections within the cytoskeleton.
ACKNOWLEDGMENT
This work was funded by Marie Curie Cancer Care.
REFERENCES
- 1.Avitabile E, Di Gaeta S, Torrisi M R, Ward P L, Roizman B, Campadelli-Fiume G. Redistribution of microtubules and Golgi apparatus in herpes simplex virus-infected cells and their role in viral exocytosis. J Virol. 1995;69:7472–7482. doi: 10.1128/jvi.69.12.7472-7482.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayscough K R, Drubin D G. ACTIN: general principles from studies in yeast. Annu Rev Cell Dev Biol. 1996;12:129–160. doi: 10.1146/annurev.cellbio.12.1.129. [DOI] [PubMed] [Google Scholar]
- 3.Barkalow K, Hartwig J H. Actin cytoskeleton. Setting the pace of cell movement. Curr Biol. 1995;5:1000–1002. doi: 10.1016/s0960-9822(95)00200-4. [DOI] [PubMed] [Google Scholar]
- 4.Bulinsky J C, Borisy G G. Widespread distribution of a 210,000 mol wt microtubule-associated protein in cells and tissues of primates. J Cell Biol. 1980;87:802–808. doi: 10.1083/jcb.87.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgin K E, Ludin B, Feralli J, Matus A. Bundling of microtubules in transfected cells does not involve an autonomous dimerization site on the MAP2 molecule. Mol Cell Biol. 1994;5:511–517. doi: 10.1091/mbc.5.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapin S, Bulinski J C. Non-neuronal 210 kDa microtubule associated protein (MAP4) contains a domain homologous to the microtubule-binding domain of MAP2 and tau. J Cell Sci. 1991;98:27–36. doi: 10.1242/jcs.98.1.27. [DOI] [PubMed] [Google Scholar]
- 7.Charlton C A, Volkman L E. Sequential rearrangement and nuclear polymerization of actin in baculovirus-infected Spodoptera frugiperida cells. J Virol. 1991;65:1219–1227. doi: 10.1128/jvi.65.3.1219-1227.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charlton C A, Volkman L E. Penetration of Autographa californica nuclear polyhedrosis virus nucleocapsids into IPLB Sf 21 cells induces actin cable formation. Virology. 1993;197:245–254. doi: 10.1006/viro.1993.1585. [DOI] [PubMed] [Google Scholar]
- 9.Cleveland D W, Hwo S-Y, Kirschner M W. Physical and chemical properties of purified tau factor and the role of tau in microtubule assembly. J Mol Biol. 1977;116:227–247. doi: 10.1016/0022-2836(77)90214-5. [DOI] [PubMed] [Google Scholar]
- 10.Cowin P, Burke B. Cytoskeleton-membrane interactions. Curr Opin Cell Biol. 1996;8:56–65. doi: 10.1016/s0955-0674(96)80049-4. [DOI] [PubMed] [Google Scholar]
- 11.Cudmore S, Cossart P, Griffiths G, Way M. Actin-based motility of vaccinia virus. Nature. 1995;378:636–638. doi: 10.1038/378636a0. [DOI] [PubMed] [Google Scholar]
- 12.Cudmore S, Reckmann I, Griffiths G, Way M. Vaccinia virus: a model system for actin-membrane interactions. J Cell Sci. 1996;109:1739–1747. doi: 10.1242/jcs.109.7.1739. [DOI] [PubMed] [Google Scholar]
- 13.Doorbar J, Ely S, Sterling J, McClean C, Crawford L. Specific interaction between HPV 16 E1-E4 and cytokeratins results in collapse of the epithelial cell intermediate filament network. Nature. 1991;352:824–827. doi: 10.1038/352824a0. [DOI] [PubMed] [Google Scholar]
- 14.Elliott G, O’Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- 15.Elliott G D, Meredith D M. The herpes simplex virus type 1 tegument protein VP22 is encoded by gene UL49. J Gen Virol. 1992;73:723–726. doi: 10.1099/0022-1317-73-3-723. [DOI] [PubMed] [Google Scholar]
- 16.Feralli J, Doll T, Matus A. Sequence analysis of MAP2 function in living cells. J Cell Sci. 1994;107:3115–3125. doi: 10.1242/jcs.107.11.3115. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs E. Intermediate filaments and disease mutations that cripple cell strength. J Cell Biol. 1994;125:511–516. doi: 10.1083/jcb.125.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodson H V, Valetti C, Kreis T E. Motors and membrane traffic. Curr Opin Cell Biol. 1997;9:18–28. doi: 10.1016/s0955-0674(97)80147-0. [DOI] [PubMed] [Google Scholar]
- 19.Hirokawa N. Microtubule organisation and dynamics dependent on microtubule-associated proteins. Curr Opin Cell Biol. 1994;6:74–81. doi: 10.1016/0955-0674(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 20.Joachims M, Harris K S, Etchison D. Poliovirus protease 3C mediates cleavage of microtubule-associated protein 4. Virology. 1995;211:451–461. doi: 10.1006/viro.1995.1427. [DOI] [PubMed] [Google Scholar]
- 21.Kanai Y, Chen J, Hirokawa N. Microtubule bundling by tau proteins in vivo: analysis of functional domains. EMBO J. 1992;11:3953–3961. doi: 10.1002/j.1460-2075.1992.tb05489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanai Y, Takemura R, Oshima T, Mori H, Ihara Y, Yanangisawa M, Masiki T, Hirokawa N. Expression of multiple isoforms and microtubule bundle formation in fibroblasts transfected with a single tau cDNA. J Cell Biol. 1989;109:1173–1184. doi: 10.1083/jcb.109.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karczewski M K, Strebel K. Cytoskeleton association and virion incorporation of the human immunodeficiency virus type 1 Vif protein. J Virol. 1996;70:494–507. doi: 10.1128/jvi.70.1.494-507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knops J, Kosik K, Lee G, Pardee J, Cohengould L, McConlogue L. Overexpression of tau in a nonneuronal cell induces long cellular processes. J Cell Biol. 1991;114:725–733. doi: 10.1083/jcb.114.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuriyama R, Nislow C. Molecular components of the mitotic spindle. Bioessays. 1992;14:81–88. doi: 10.1002/bies.950140203. [DOI] [PubMed] [Google Scholar]
- 26.Lee G, Brandt R. Microtubule-bundling studies revisited: is there a role for MAPs. Trends Cell Biol. 1992;2:286–289. doi: 10.1016/0962-8924(92)90106-w. [DOI] [PubMed] [Google Scholar]
- 27.Lewis S A, Ivanov I E, Lee G H, Cowan N J. Organisation of microtubules in dendrites and axons is determined by a short hydrophobic zipper in microtubule-associated proteins MAP2 and tau. Nature. 1989;342:498–505. doi: 10.1038/342498a0. [DOI] [PubMed] [Google Scholar]
- 28.Lewis S A, Wang D, Cowan N J. Microtubule-associated protein MAP2 shares a microtubule binding motif with tau protein. Science. 1988;242:936–939. doi: 10.1126/science.3142041. [DOI] [PubMed] [Google Scholar]
- 29.MacRae T H. Towards an understanding of microtubule function and cell organisation: an overview. Biochem Cell Biol. 1992;70:835–841. doi: 10.1139/o92-131. [DOI] [PubMed] [Google Scholar]
- 30.Margolis R L, Rauch C T, Pirollet F, Job D. Specific association of STOP protein with microtubules in vitro and with stable microtubules in mitotic spindles of cultured cells. EMBO J. 1990;9:4095–4102. doi: 10.1002/j.1460-2075.1990.tb07631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsudaira P. Modular organisation of actin cross-linking proteins. Trends Biochem Sci. 1991;16:87–92. doi: 10.1016/0968-0004(91)90039-x. [DOI] [PubMed] [Google Scholar]
- 32.McLean W H, Lane E B. Intermediate filaments in disease. Curr Opin Cell Biol. 1995;7:118–125. doi: 10.1016/0955-0674(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 33.Melki R, Gaudin Y, Blondel D. Interaction between tubulin and the viral matrix protein of vesicular stomatitis virus: possible implications in the viral cytopathic effect. Virology. 1994;202:339–347. doi: 10.1006/viro.1994.1350. [DOI] [PubMed] [Google Scholar]
- 34.Mitchison T J, Kirschner M W. Dynamic instability in microtubule growth. Nature. 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- 35.Payne L G, Kristensson K. The effect of cytochalasin D and monensin on enveloped vaccinia virus release. Arch Virol. 1982;74:11–20. doi: 10.1007/BF01320778. [DOI] [PubMed] [Google Scholar]
- 36.Penfold M E, Armati P, Cunningham A L. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc Natl Acad Sci USA. 1994;91:6529–6533. doi: 10.1073/pnas.91.14.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piperno G, LeDizet M, Chang X. Microtubules containing acetylated α-tubulin in mammalian cells in culture. J Cell Biol. 1987;104:289–302. doi: 10.1083/jcb.104.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Preuss U, Biernat J, Mandelkow E-M, Mandelkow E. The ‘jaws’ model of tau-microtubule interaction examined in CHO cells. J Cell Sci. 1997;110:789–800. doi: 10.1242/jcs.110.6.789. [DOI] [PubMed] [Google Scholar]
- 39.Pumplin D W, Bloch R J. The membrane skeleton. Trends Cell Biol. 1993;3:113–117. doi: 10.1016/0962-8924(93)90173-x. [DOI] [PubMed] [Google Scholar]
- 40.Sodeik B, Ebersold M W, Helenius A. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J Cell Biol. 1997;136:1007–1021. doi: 10.1083/jcb.136.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Svitkina T M, Verkhovsky A B, Borisy G G. Plectin sidearms mediate interaction of intermediate filaments with microtubules and other components of the cytoskeleton. J Cell Biol. 1996;135:991–1007. doi: 10.1083/jcb.135.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Topp K S, Meade L B, LaVail J H. Microtubule polarity in the peripheral processes of trigeminal ganglion cells: relevance to the retrograde transport of herpes simplex virus. J Neurosci. 1994;14:318–325. doi: 10.1523/JNEUROSCI.14-01-00318.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallin M, Deinum J, Goobar L, Danielson U H. Proteolytic cleavage of microtubule-associated proteins by retroviral proteinases. J Gen Virol. 1990;71:1985–1991. doi: 10.1099/0022-1317-71-9-1985. [DOI] [PubMed] [Google Scholar]
- 44.West R R, Tenbarge K M, Olmsted J B. A model for microtubule-associated protein 4 structure. J Biol Chem. 1991;266:21886–21896. [PubMed] [Google Scholar]