Abstract
Persistent inflammation is the key aggravator in many cardiovascular diseases, including atherosclerosis, aneurysm, injury/reperfusion, thrombosis, and neointimal hyperplasia following surgical or percutaneous interventions. Resolution is an active process orchestrated by specialized pro-resolving lipid mediators (SPMs) which tamp down acute inflammatory signals, promote healing and facilitate a return to homeostasis. SPMs are endogenously derived from poly-unsaturated fatty acids, and their biologic activity is mediated via specific G-protein coupled receptor binding. The potency of SPM in regulating the inflammatory response has encouraged investigation into their therapeutic and diagnostic use in cardiovascular pathologies. Herein we describe the translational groundwork which has established the synthesis and interactions of SPM in cardiovascular and hematologic cells, the therapeutic effects of SPM in animal models of cardiovascular disease, and some early technologies that harness and attempt to optimize SPM delivery and “resolution pharmacology”. Further studies are required to precisely determine the mechanisms of resolution in the cardiovascular system and to determine the clinical settings in which SPM can be utilized to optimize patient outcomes.
Keywords: cardiovascular disease, inflammation, resolution, lipid mediators, resolvins, atherosclerosis, intimal hyperplasia, drug delivery
Graphic Abstract
Vascular injury, inflammation, resolution, and repair. Injury to EC and underlying vSMC is the initiating event. The expression of cytokines and cell adhesion molecules is increased, resulting in leukocyte recruitment and the production of proinflammatory mediators. PUFA such as arachidonic acid (AA) are converted by lipoxygenases (LO) and cyclooxygenases (COX) into proinflammatory lipid mediators such as prostaglandin (PG) and leukotriene (LT). These in turn promote vascular permeability, platelet aggregation, further leukocyte recruitment, and augmentation of inflammatory cytokines. A lipid mediator class switch occurs in which LO and COX begin to convert PUFA (e.g. DPA, DHA, EPA) into SPM. SPM downregulate inflammatory processes, reducing cytokine production and promote clearance of debris and tissue repair via M1-M2 macrophage transition. If resolution is delayed or insufficient, inflammatory signals persist resulting in dysfunctional VSMC, fibrosis, and exaggerated neointimal hyperplasia.
1.1. Background
1.1.1. Traditional Paradigm of Cardiovascular Disease and Treatment
Acute and chronic inflammation play a major etiologic role in cardiovascular disease (atherosclerosis, aneurysmal disease, venous thrombosis, ischemia-reperfusion). Pathologic inflammation occurs in numerous clinical scenarios, both in native disease progression (e.g. atheromatous plaque growth, rupture, thrombosis, dissection, etc.) and iatrogenic settings (e.g. angioplasty, stenting, bypass surgery, etc.). Diet, exercise, and lipid lowering medications can slow progression or stabilize atherosclerotic disease, but invasive interventions are often necessary to treat symptoms or prevent catastrophic consequences. These procedures generally result in an acute-on-chronic inflammatory response. Following reperfusion of ischemic tissue, the resulting cascade often worsens the extent of local injury. If resolution does not occur promptly, protracted inflammation leads to further end-organ damage, recurrent disease and downstream clinical events. Current therapeutics used in the setting of vascular interventions have focused on the use of cytotoxic, anti-inflammatory, or anti-thrombotic drugs which are nonspecific and may cause undesired effects on the endothelium and immune system, possibly even increasing long-term mortality[1]. These agents can delay physiologic healing, placing areas of vascular injury at increased risk of thrombosis. While recent trials have demonstrated the potential benefits of altering the inflammatory response in cardiovascular disease, they have also shown the consequences of inhibiting upstream immunologic mechanisms, including fatal infectious complications[2]. Harnessing resolution mechanisms may represent an important new opportunity to modulate the vascular inflammatory response without impairing host defense and healing or increasing bleeding risk, first termed “resolution pharmacology” by Peretti et al[3]. This review will summarize the relevant biochemistry, cell biology, and translational studies that underpin the development of resolution pharmacology for cardiovascular disease.
1.1.2. Biology of Vascular Injury, Inflammation, and Remodeling
Numerous types of insults (metabolic, hemodynamic, traumatic, etc.) and risk factors integrate at the vasculature and lead to inflammation. The underlying key step in many cardiovascular pathologies is injury to the endothelial cell (EC) barrier, with variable damage to underlying matrix and vascular smooth muscle cells (vSMC), and exposure to circulating blood elements. This leads to a cascade of signals promoting thrombosis and inflammation. Apoptotic EC and vSMC, as well as recruitment of platelets, coagulation proteins, and leukocytes create a rapid influx of inflammatory cytokines (e.g. IL-1B, IL-6, TNF-a) and chemokines (e.g. MCP-1) as well as reactive oxygen species (ROS) to the site of injury. Proliferative growth factors are released from cellular elements and extracellular matrix (e.g. PDGF, FGF), causing vSMC activation to a state of increased migration, proliferation, resistance to apoptosis, expression of pro-inflammatory cytokines and synthesis of vascular matrix proteins. Failure to resolve this acute inflammatory process in a timely fashion leads to disease progression, clinical events, resistance to therapies, and/or recurrence of disease post-intervention, such as neointimal hyperplasia (NIH) leading to vessel narrowing [4].
1.1.3. Biochemistry of resolution
Resolution has been identified as an active process in the timeline of inflammation, involving a complex network of specific pro-resolving mediators, receptors, and downstream pathways. Researchers have investigated this framework to explore mechanisms of disease as well as potential therapeutic or diagnostic tools[5–8]. These studies identified distinct classes of specialized pro-resolving lipid mediators (SPM), endogenously derived from polyunsaturated fatty acids (PUFA) through molecular profiling in small animal models of self-limited inflammation. Precursor PUFAs for SPMs include n-6 arachidonic acid (AA) and the n-3 docosapentaenoic acid (DPA), docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). These SPM families include E- and D- series resolvins, protectins, maresins, and lipoxins, many of which have been produced via total organic synthesis[9–11]. The biologic activity of SPM are carried out by various GPCRs, including ALX/FPR2, DRV1/GPR32, DRV2/GPR18, ERV1/ChemR23, GPR37, GPR101 and LGR6[12–15]. Importantly, ongoing research has continued to uncover new SPM classes, mediators and receptors suggesting biochemical redundancy, time- and tissue-specificity in the resolution response to various stimuli[12]. There also exist non-lipid pro-resolving mediators, such as Annexin A1, a peptide which acts on ALX/FPR2[16]. This would be expected in a process so fundamental to organismal health. Whereas SPM share key defining biologic properties such as improving the “resolution-index” in small animal models of sterile peritonitis [17] we refer to them as a broad category in this review, while recognizing that specific SPMs may play critical roles in a tissue, time, and disease context.
In physiologic resolving inflammatory exudates, cell-cell interactions lead to the generation of active signals that limit leukocyte, namely PMN, accumulation and promote the clearance of apoptotic cells and cellular debris[18]. SPMs coordinate these interactions by enhancing M1-M2 phenotypic polarization in macrophages, promoting lipoxygenase (LO) activity, and upregulating SPM receptor expression[19–22]. The biochemical mechanisms underlying the observed “lipid mediator class switch” from pro-inflammatory mediators (e.g. prostaglandins, leukotrienes) to SPM within resolving exudates are of considerable interest and are actively being defined[19,21].
A deficiency or lack of resolution signals may thus contribute to many disease states and is of particular interest in the pathogenesis of cardiovascular disease. If the inflammatory cycle is left unchecked, a detrimental cycle of leukocyte recruitment, saturation of cytokines and growth factors, vSMC stimulation and proliferation leads to intimal hyperplasia and stenosis[4,23–26].
1.2. SPM Biosynthesis and Activity in the Cardiovascular System
1.2.1. Vascular Biosynthesis
The PUFA precursors AA, EPA, DPA and DHA are made available at local sites of injury through edema and the generation of tissue exudates. The conversion of these precursors into lipid mediators is carried out by lipoxygenase (LO) and cyclooxygenase (COX) enzymatic activity. These processes are likely carried out through the exchange of biochemical metabolites between cells, including leukocytes, platelets, and vascular cells, i.e. ECs and vSMCs. Early in the inflammatory response, AA-derived pro-inflammatory prostaglandins and leukotrienes account for the majority of synthesized lipid mediators. A lipid mediator class switch is then responsible for the subsequent synthesis of SPMs utilizing the same enzyme systems acting on a broader array of substrates including the n-3 PUFA. Subcellular localization of the key enzyme 5-LO appears to be one critical mechanism in this class switch that yields SPMs[22]. These mediators are synthesized locally and active transiently, as they are rapidly metabolized by available enzymes such as eicosanoid oxidoreductases and prostaglandin dehydrogenase[27]. The specific origins of SPMs in the vasculature have yet to be fully elucidated, but likely involve cell-cell interactions with exchange of intermediates. LXA4 was discovered intraluminally in a study of coronary angioplasty in humans[28]. Recent studies demonstrated that freshly harvested non-diseased human artery segments and cultured vascular cells are capable of producing D-series resolvins and maresins when exposed to precursors such as DHA, in the absence of intervening leukocytes or platelets [29]. Conditioned medium from vascular cells treated with DHA supplementation reduced leukocyte adhesion on cytokine activated target cells, and this action was carried out by the receptors ALX/FPR2 and GPR32. In the murine mesenteric microcirculation, LXA4 is produced by platelet-leukocyte and leukocyte-endothelial interactions as a protective response to vascular insult such as ischemia reperfusion[30]. A similar response is seen in the cerebral and renal microvasculature following ischemic insult[31,32]. These studies provide evidence that the vasculature is capable of endogenous production of SPM to counteract and balance acute inflammation.
Importantly, several relevant drugs have been implicated in the biosynthesis of lipid mediators, including aspirin and statin. Oral administration of aspirin acetylates and alters the active site of COX-2. This inhibits the production of proinflammatory prostaglandins and promotes the production of aspirin-triggered (AT) forms of SPM such as 15-epi-lipoxin, a potent antiinflammatory and pro-resolving mediator[33] and AT-RvD1[27]. These stereoisomers have prolonged bioavailability and may account for some of the effects of aspirin. Atorvastatin increases the production of the 13-series resolvins (RvTs) via S-nitrosylation of COX-2, which improved survival in a mouse model of sepsis [34]. Conversely agents such as COX-2 inhibitors are “resolution-toxic”.
1.2.2. Endothelial Cells
In the cardiovascular system, ECs form the protective barrier between circulating blood elements and underlying vascular architecture, maintaining laminar blood flow, and regulating platelet, leukocyte, and other macromolecular activity. When this barrier is breached or activated, a thrombo-inflammatory response is initiated, which in an evolutionary sense may be protective against pathogens or traumatic exsanguination, but in chronic situations can lead to deleterious effects. Endothelial cells interact with a number of SPMs, such as RvD1, RvD2, MaR1, LXA4, and PD1 (Table I). The downstream effects include reduction of proinflammatory cytokines, adhesion molecule expression, and leukocyte-EC interaction, carried out by various SPM receptors, including ALX/FPR2, DRV1/GPR32, DRV2/GPR18, and ERV1/ChemR23 [9,29,35–41].
Table I.
Summary of effects of SPM on various cardiovascular and inflammatory cell types
| Cell Type | SPM Studied | Effects | References |
|---|---|---|---|
| Endothelial Cell | RvD1, RvD2, MaR1, LXA4, PD1 | Reduce proinflammatory cytokine production, adhesion molecule expression, leukocyte-EC adhesion | [9,35–38] |
| vSMC | RvD1, RvD2, RvE1, MaR1, LXA4 | Reduced proliferation, migration, vasoconstriction | [23,35,42,43] |
| Platelets | RvD1, RvE1, MaR1 | Enhanced hemostasis, reduced aggregation and inflammatory cytokine secretion | [36,56–58] |
| Macrophages | RvD1, RvD2, ATRvD1, AT-RvD3, ATPD1, AT-LXB4, MaR1 | Enhanced M2 polarization, efferocytosis | [19,20,47,52–55,64] |
| Neutrophils | RvD2, RvD4, RvE1 | Reduced NET release, recruitment | [42,47–51] |
1.2.3. Vascular Smooth Muscle Cells
vSMCs also play a critical role in cardiovascular disease. Physiologically, they constitute the majority of the vessel wall, providing mechanical integrity, regulating vascular tone, and contributing to both functional and dysfunctional vessel wall healing. In the resting state, vSMCs are quiescent and highly organized, surrounded by other architectural proteins in the extracellular matrix. In response to injury and the resultant availability of inflammatory cytokine and growth factors (e.g. TNF-α, IL-1, IL-6, PDGF, FGF), vSMCs assume an activated state prone to migration, proliferation, and secretion of proinflammatory factors. When this inflammatory state is left unchecked, neointimal hyperplasia is promoted and ultimately leads to stenosis of the affected blood vessel. In vitro, treatment with RvD1, RvD2, and MaR1 reduce leukocyte-VSMC interactions via downregulation of cell adhesion molecules and other proinflammatory genes[23,35]. Treatment with RvD1, RvD2, RvE1 and LXA4 attenuate vSMC migration, as well as proliferation to a modest degree, after exposure to promigratory and proliferative growth factors[23,42,43] (Table I). These effects are mediated at least in part by receptor-mediated activation of the cyclic AMP/protein kinase A pathway[44].
SPMs are also able to modulate the contractility of vSMC. RvE1 reduced vSMC hyperreactivity and calcium hypersensitivity induced by IL-1 and TNF-α[45], and pretreatment with RvE1, RvD1 or RvD2 resulted in significant inhibition of constriction in human pulmonary arteries and rat thoracic aortas in response to a thromboxane A2 mimetic[46].
1.2.4. Circulating Cells
Neutrophil recruitment is the hallmark of any acute inflammatory response, promoting the local production of inflammatory cytokines and radical oxygen species. Treatment with RvD1, RvD2, RvD4, and RvE1 reduces neutrophil recruitment and neutrophil extracellular trap (NET) activity [42,47–51] (Table I). Subsequently, monocytes are the second wave of leukocyte recruitment and transition to macrophages in tissue. Macrophages assist with tissue repair and clearance of apoptotic ECs, vSMCs, and neutrophils. Initially macrophages are activated into their M1 phenotype, which increase the production of proinflammatory cytokines and radical oxygen intermediates in an attempt to augment the inflammatory response and clear pathogens. SPM (e.g. RvD1, RvD2, AT-RvD1, AT-RvD3, AT-PD1, AT-LXB4, MaR1) encourage transition to the M2 phenotype, which is associated with clearance of apoptotic cells, wound healing and tissue repair [19,20,47,52–55] (Table I).
Platelet recruitment and aggregation is the initial response to cardiovascular events such as injury and plaque rupture when subendothelial vascular structures are exposed to the blood. They are also critical in the formation of pathologic thromboses. Treatment with various SPM produce different effects on platelet activity and function. MaR1 enhances the hemostatic function of platelets but reduces inflammatory activity[56]. RvD1 reduces platelet activation even in response to pro-aggregation stimuli such as ADP[57,58]. RvE1 similarly blocks ADP-mediated signals to reduce platelet aggregation [36] (Table I). Platelet interaction with leukocytes in vascular injury or thrombosis leads to enhanced production of MaR1, potentially promoting resolution pathways[59].
1.3. SPM in Cardiovascular Disease
1.3.1. Atherosclerosis
Impaired resolution of vascular inflammation plays a key role in atherosclerosis. Correlation between a lack of resolution mechanisms and atherosclerosis was first reported by Merched and colleagues, who demonstrated that mice deficient in 12/15-lipoxygenase activity had accelerated atherosclerosis[9]. Work by Ho and colleagues demonstrated that human patients with peripheral arterial disease had lower levels of the circulating SPM aspirin-triggered lipoxin A4 (ATL; 15-epi-LXA4)[60]. Since then, a deficiency in SPMs has been demonstrated in patients with coronary artery disease and cerebrovascular disease[52,61,62]. In a study of patients with known cardiovascular disease with significantly reduced levels of circulating RvDn-3 DPA, in vitro supplementation of RvDn-3 DPA with these patients’ blood resulted in significant dose-dependent reduction in platelet and leukocyte activation[63]. In a mouse model, deletion of ChemR23/ERV1 (receptor for SPM RvE1) caused proatherogenic signaling in macrophages, impaired efferocytosis, and increased plaque size and core necrosis[53].
SPMs have been identified in hyperlipidemic mouse atherosclerotic lesions, and a lack of SPM is associated with plaque instability. In these mice, treatment with RvD1, RvD2, and MaR1 improve plaque phenotype and increase fibrous cap thickness[64,65] (Table II). A study of ALX/FPR2 (receptor for RvD1) knockout mice revealed that these mice developed unstable atherosclerotic plaques with reduced collagen[40]. The same group demonstrated that treatment with ATL reduced atheroprogression in ApoE deficient mice through ALX/FPR2 signaling, and that this benefit was lost in ALX/FPR2 deficient mice[66]. In a rabbit model of periodontitis and high cholesterol diet, treatment with topical RvE1 reduced periodontitis and diminished atherogenesis with less overall arterial plaque, lower intima/media ratio, and decreased leukocyte infiltration[67].
Table II.
Summary of in vivo models demonstrating effects of SPM in cardiovascular disease
| Model Type | Species | SPMs studied | Effects | References |
|---|---|---|---|---|
| Atherosclerosis | Mouse, Rabbit | RvE1, RvD1, RvD2, MaR1 | Reduced atherosclerosis, thicker fibrous cap, enhanced plaque stability, reduced calcifications | [53,64–69] |
| Myocardial IR | Mouse, Rat | RvD1, RvE1 | Reduced size of infarct, improved myocardial recovery | [78–81] |
| Cerebral IR | Mouse | 15-Epi-LXA4, LXA4, RvD1, RvD2, MaR1 |
Reduced inflammation, mortality | [31,82–86] |
| Visceral IR | Mouse | RvD1–3, AT-RvD1, PD1, LXA4, 15-Epi-LXA4 | Reduced kidney, hepatic, lung, visceral injury and inflammation | [30,87–92] |
| Hind-limb ischemia | Mouse | LXA4, RvD2 | Improved skeletal muscle recovery | [51,93] |
| Aneurysm | Mouse | RvD1, RvD2 | Decreased aneurysm formation | [48,54,72] |
| Venous Thrombosis | Mouse | RvD2, RvD4 | Reduced thrombus burden | [49,73] |
A hallmark of advanced atherosclerosis is plaque calcification. Calcification is the deposition of calcium and phosphate into calcium-phosphate crystals due to the phenotypic transformation of vSMCs into osteoblast-like cells. Vascular calcification can occur in different patterns (e.g. intimal versus medial) but is generally associated with clinical disease progression and can present major therapeutic challenges. Currently there is no pharmacologic therapy that effectively targets this process. In a mouse model of vascular calcification, RvE1 and chemerin stimulation of ChemR23 resulted in reduced phosphate-induced calcification in vSMCs[68,69].
1.3.2. Aortic Aneurysmal Disease
Aortic aneurysmal disease is dilation of the aorta at risk for rupture and life-threatening hemorrhage. The underlying pathophysiology is chronic inflammation within the aortic wall associated with increased protease activity and reduced stability of structural elements. Accumulation of leukocytes (lymphocytes and monocytes/macrophages) in the aortic wall leads to loss of structural integrity and depletion of vSMC. The only available treatment is surgery, as there are no pharmacologic treatments yet identified that affect the progression of aneurysmal disease. Several studies have demonstrated that PUFA supplementation in animal models of aortic aneurysm reduce inflammation and matrix degeneration[70]. In a mouse model of elastase induced aortic aneurysm, treatment with RvD1 and RvD2 promoted M2 polarization of macrophages and reduced the activity of matrix metalloproteinases (MMP), and attenuated aneurysm formation and progression[54] (Table II). In a mouse model of angiotensin II (AT-II) induced aortic aneurysm, treatment with RvD1 inhibited neutrophil extracellular trap phagocytosis (NETosis), reduced markers of inflammation, and decreased aneurysm progression[48]. Pillai et al demonstrated the clinical relevance of resolution biochemistry in aortic aneurysmal disease by revealing time dependent changes in circulating lipid mediators in patients undergoing aortic aneurysm repair[71]. Petri et al demonstrated that aneurysmal human aortas had significantly reduced levels of ALX/FPR2, and deletion of either ALX/FPR2 or the lipoxin-synthesizing 12/15 lipoxygenase resulted in enhanced aneurysm formation and increased aneurysmal leukocyte recruitment in a mouse model of abdominal aortic aneurysm[72]. The same study also demonstrated that pre-treatment with AT-LXA4 prior to UV light exposure resulted in pro-resolution signaling (inhibition of p38 phosphorylation) in murine neutrophils, but this was not seen in ALX/FPR2 deficient neutrophils.
1.3.3. Venous Thrombotic Disease
Deep vein thrombosis (DVT) is the formation of a blood clot in the deep veins of the legs or the pelvis as a result of some combination of impaired flow, injury to the blood vessel and hypercoagulability. Following the acute event, chronic inflammation and downstream venous remodeling often leads to post-thrombotic syndrome in the affected limb. This involves venous valvular insufficiency causing pain, swelling, dermatitis, and chronic wounds. SPM participate in the acute phase of DVT clearance and may modulate the severity of inflammation and reduce the likelihood or severity of post-thrombotic syndrome. In vitro, RvE1 reduces platelet activation in response to stimulants such as ADP[57,58] (Table II). In a mouse model of burn injury, RvD2 reduced dermal vessel thrombosis[73]. The coagulation process leads to a temporal production of SPM that is likely important for subsequent clearance[74]. More recently, treatment with RvD4 reduced thrombus burden and neutrophil recruitment and increased pro-resolving monocytes and the synthesis of other D-series resolvins in a mouse model of IVC thrombosis[49].
1.3.4. Ischemia/Reperfusion Injury
In ischemic disease, anoxia and deprivation of critical metabolic substrates leads to infarct. Without reperfusion or the development of significant collateral circulation, the injured cells die and are replaced with dysfunctional scar tissue. With reperfusion (spontaneous or percutaneous coronary intervention), ischemia is halted, but additional injury and tissue damage occurs as a result of reperfusion and exposure to radical oxygen species (ROS). This injury is mediated by microvascular obstruction, myocardial edema, and exposure to inflammatory factors[75–77]. With unchecked inflammation as a result of reperfusion, the extent of injury extends beyond the initial area affected by ischemia, resulting in increased morbidity and mortality. In murine models of cardiac[50,78–81], cerebral[31,82–86], liver[55,87–89], lung[90], kidney[91,92], visceral[30], and hind leg[51,93] ischemia, pre-treatment with SPM (RvD1–3, AT-RvD1, RvE1, PD1, LXA4, AT-LXA4) has reduced tissue injury by enhancing cell protection pathways and reducing the harmful effects of ROS (Table II).
Following myocardial ischemia, persistent inflammation leads to myocyte injury and worse heart failure. In a model of ischemic cardiomyopathy with ALX/FPR2 gene knockout mice, the loss of ALX/FPR2 (RvD1 receptor) resulted in magnified obesogenic cardiomyopathy and renal inflammation[94]. In a separate study, treatment with RvD1 promoted resolution and improved ventricular myocyte recovery and function[79]
1.3.5. Vascular Injury and Remodeling
Invasive interventions for cardiovascular disease entail mechanical injury or detouring focal areas of disease to improve or restore circulation. These include angioplasty, stenting, endarterectomy, thrombectomy, and bypass surgery. Unfortunately, these interventions necessitate acute injury on diseased tissue riddled with chronic inflammation. The severity of the resultant acute inflammatory response ultimately affects vessel healing and long-term clinical outcomes, resulting in excessive scarring and narrowing of the vessel lumen in 50% or more of patients in 2–3 years[4,24,95–100]. A current hypothesis is that locally generated SPMs are critical in resolving vascular inflammation after injury, and they represent an important mechanism that may be deficient in patients requiring cardiovascular interventions[6,11,36].
In a rabbit model of femoral artery angioplasty, intraarterial delivery of RvD1 or RvD2 led to reduced NIH at one month[23] (Table III). In murine models of carotid artery ligation, neointimal hyperplasia (NIH) was significantly reduced with systemic (intra-peritoneal) delivery of RvD2 or MaR1[47]. In a mouse model of femoral artery wire injury, treatment with intraperitoneal RvE1 or oral supplementation with eicosapentanoic acid (EPA), the precursor to E-series resolvins, attenuated NIH through multiple mechanisms, including reduced neutrophil recruitment, suppression of t-cell traffic through RANTES suppression, and M2 macrophage polarization[101].
Table III.
Summary of in vivo models demonstrating effects of SPM in neointimal hyperplasia
| Model Type | Species | SPMs studied | Effects | References |
|---|---|---|---|---|
| Artery ligation | Mouse | RvD2, MaR1, AT-LXA4 | Decreased neointima | [43,47] |
| Angioplasty | Rat, Rabbit | RvD1, RvD2, PD1iso | Decreased neointima | [23,42,101–103] |
| Vein Bypass | Rabbit, Rat (synthetic graft) | RvD1, AT-RvD1 | Reduced vein graft hyperplasia, reduces inflammation and promotes vascular regeneration | [104,105] |
Wu et al demonstrated reduced NIH (50–60%) in a rat model of carotid artery angioplasty with RvD1 using perivascular pluronic gel or a biodegradable scaffold delivery[42]. Similar reductions in NIH in this model have been demonstrated with intravenous delivery of RvD1 and PD1iso[102]. In contrast to local delivery, oral supplementation of RvD1 reduced leukocyte recruitment and markers of acute inflammation, but did not affect downstream NIH in this model [103].
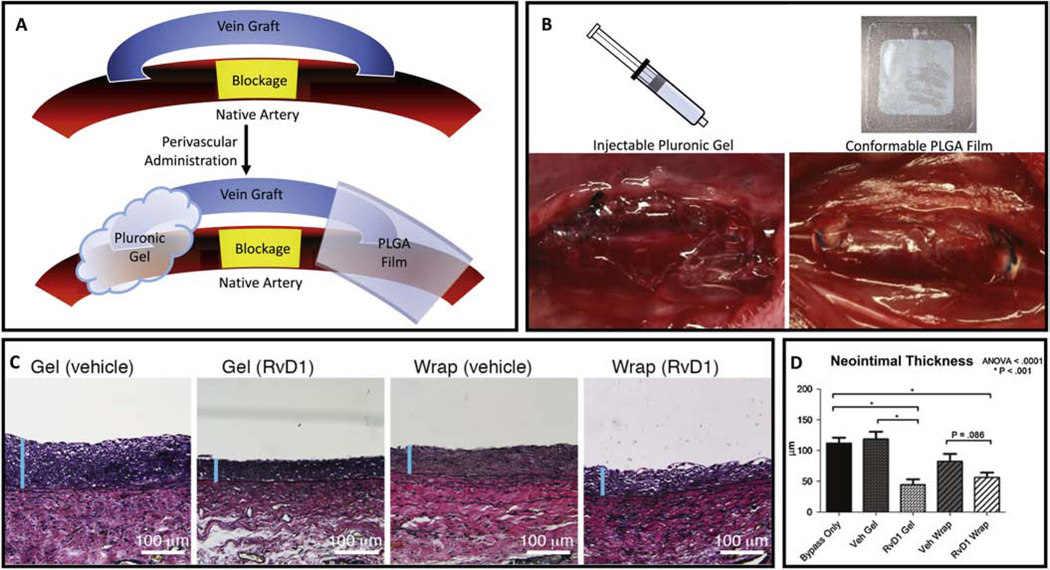
In a rabbit model of carotid artery bypass with autologous vein graft, Wu et al also demonstrated reduced NIH with perivascular RvD1 administration (Figure 1) [104]. In a rat model of aortic bypass with prosthetic polycaprolactone electrospun vascular graft, direct loading of AT-RvD1 into the graft fibers reduced inflammation and promoted vascular tissue regeneration[105]. Though much work remains to elucidate the optimal dosing, timing, and method of delivery, these studies provide encouraging evidence that SPMs may represent a new class of agents to improve vessel healing and reduce restenosis.
Figure 1:
Local delivery of SPM to improve vascular graft healing. Bypass grafts are subject to an acute inflammatory response that can lead to intimal hyperplasia, particularly at the anastomoses. The cartoon (A) depicts two methods of perivascular delivery of SPM using a gel or a thin film device, illustrated in panel B as applied to rabbit vein grafts in-vivo. PLGA films (“wraps”) were created with a thin bi-layered design for unidirectional release and loaded with 1 μg of RvD1. Delivery of RvD1 with gel or wrap decreased rabbit vein graft hyperplasia at 28 days post-implantation, while vehicle controls had no effect (C,D).
Adapted with permission from ref 104
1.4. Clinical Application
1.4.1. Human Research
Many studies have investigated the relationship between omega-3 PUFAs and cardiovascular health[106]. Randomized clinical trials testing omega-3 PUFA supplementation have demonstrated variable results[107,108]. A randomized trial conducted in Italy from 1993–1995 demonstrated that oral supplementation of n-3 PUFA in patients who recently suffered myocardial infarction reduced the relative risk of death, nonfatal myocardial infarction or stroke by 10%[109]. More recently, the REDUCE-IT trial demonstrated that twice daily oral supplementation of 2g icosapent ethyl, a highly purified EPA ethyl ester, significantly lowered the risk of ischemic events, including cardiovascular death, in patients with elevated triglyceride levels despite statin therapy[110]. On the other hand, the VITAL trial demonstrated that 1g of marine omega-3 fatty acid once daily did not reduce the risk of major cardiovascular events[111].
Variations in dosing, composition and formulation may affect the interpretation of these results. Most of these studies tested lower doses of marine oil supplements and focused on major clinical endpoints including myocardial infarction, stroke, and mortality.
With the discovery of SPM and their precursors, more contemporary studies have been able to study the effect of oral PUFA and SPM supplementation on the overall lipid and inflammatory profile of patients with vascular disease. Oral supplementation with omega-3 fatty acids increases circulating plasma levels of SPM and their intermediates [112,113]. The OMEGA-PAD-I trial demonstrated that 1-month daily supplementation with 4.4 g of fish oil in patients with peripheral arterial disease resulted in a decrease in triglycerides and an increase in the omega-3 index and pathway markers SPM production, such as 5-hydroxyeicosapentaenoate (5-HEPE), 15-HEPE, 18-HEPE, and 4-HDHA, but without a concomitant increase in circulating SPM levels or changes in systemic inflammatory markers[112]. Secondary analysis of this data demonstrated a strong correlation between the observed increase in omega-3 index and production of SPM production pathway markers[114]. The OMEGA-PAD-II trial continued the experiment for 3 months, and demonstrated significant increases in the SPMs lipoxin A4, and RvE3, although there were no changes in markers of systemic inflammation or clinical indicators of disease severity[113]. The OMEGA-SPM trial studied the effect of an oral supplement containing both marine oils and mono-hydroxylated SPM precursors, such as 17-HDHA and 18-HEPE, in PAD patients and found a shift in lipid profile favoring SPM over PG which was associated with an increase in phagocytic activity in circulating monocytes and macrophages, as well as a reduction in several pro-inflammatory markers on circulating monocytes[115].
1.4.2. Challenges to Clinical Application
The application of SPM as either therapeutics or diagnostic tools faces several challenges. SPM are unstable molecules which are rapidly metabolized by ubiquitously present dehydrogenases and reductases[27], limiting their durability. They are synthesized and active locally, hence the physiologic relevance of circulating plasma levels is not known. From a pharmacologic standpoint, the synthesis of many SPM is complex with unstable intermediates. Optimal pharmacologic dosing and duration of treatment for SPM therapeutics is unclear. With the multitude of enzymes and targets present in the biosynthesis and interactions of SPM, it is unclear what formulation might best promote resolution in the human physiology.
Clinical and in vivo studies demonstrate that the nutritional approach to supplement resolution mechanisms presents a variety of challenges. The balance between omega-3 and omega-6 metabolomes and their utilization of substrate and biosynthetic enzymes are complex and may involve direct competition and the production of varying downstream prostanoids and SPM [6,11,116–118]. The resultant lipid mediator isomers may have effects of varying potency or may not interact equally (or even competitively) with the analogous receptor[29,57,119,120]. Individual patient characteristics such as age and underlying disease (e.g. CAD, HTN, DMII, smoking) may adversely impact the pathways of lipid mediator synthesis and resolution biology[121]. Tissue specific context is also critically important for synthesis, degradation, and biologic activity of SPM. Thus, oral administration of precursors rather than active SPM may not achieve adequate local concentrations of lipid mediators[122]. In cases where localized disease is of interest, targeted delivery methods may lead to better outcomes.
Bioavailability and stability are important considerations for therapeutics and remain a key challenge for bioactive lipids. Several synthetic analogs of SPM and SPM receptor agonists have been described in an attempt to replicate the bioactivity of SPM with improved durability and simpler synthesis. To date four generations of LXA4 analogs have been produced which have reduced metabolic inactivation and improved stability and potency, including the BLXA4-ME analog which has been developed into an oral rinse to treat gingivitis[123]. An analog of RvE1 known as RX-10045, is being used in a stable aqueous micellar formulation for the topical treatment of corneal inflammation[124]. Analogs of other SPM have also been produced, including benzo-diacetylenic-17R-RvD1-methyl ester (BDA-RvD1) and DRV1/GPR32 agonists [125,126]. Further studies are required to determine their in-vivo potency.
Direct administration of SPM to areas of vascular disease may address the issue of achieving clinically significant local concentrations. As an example, investigators directly injected an SPM cocktail in a human blister model and demonstrated reduced PMN counts as a marker of enhanced resolution[21]. We developed a biodegradable poly(lactic-co-glycolic acid) thin film for controlled, directional release of RvD1. Use of this drug-eluting device was associated with reduced neointimal hyperplasia in a rabbit bypass grafting model (Figure 1) [104]. Shi et al directly loaded AT-RvD1 into the fibers of a prosthetic polycaprolactone electrospun vascular graft, which resulted in reduced inflammation and promoted vascular tissue regeneration in a rat model of aortic bypass[105]. These methods of local SPM delivery are relevant to surgical settings with direct exposure of the vessel of interest. Other carriers, such as nanoparticles, may provide protection of SPM from enzymatic degradation, and could be developed for intravascular administration or as coatings on catheter-based devices such as balloons or stents[127]. For example, in a mouse model of ischemic stroke, neutrophil membrane-derived nanovesicles loaded with RvD2 were administered intravenously to target and treat the acute inflammatory response following brain ischemia and reperfusion, which resulted in reduced inflammation and improved neurologic function[86]. Fredman et al developed a collagen-IV targeted nanoparticle containing Ac2–26, an Annexin A1 mimetic that activates the FPR2/ALX receptor, which was able to improve plaque stability, reduced oxidative stress, and decreased necrosis of the core in a mouse model of atherosclerosis[16].
Though there are some studies demonstrating relative deficiency of certain SPM in cardiovascular disease states, biomarkers correlating with improved resolution and clinical outcomes are yet to be identified[52,60–63]. This is an important need in the clinical arena, as it would provide a therapeutic resolution target analogous to the use of C-reactive protein for cardiovascular anti-inflammatory trials[2,128]. Circulating plasma levels of SPM have been measured in various studies [118,52,114,86] and are often indexed to either total prostaglandins or leukotriene B4. In a study of patients with known cardiovascular disease with significantly reduced levels of circulating RvDn-3 DPA, in vitro mixing of RvDn-3 DPA with these patients’ blood resulted in significant dose-dependent reduction in platelet and leukocyte activation[63]. Further work is required to validate such measurements in the context of clinical settings of inflammatory disease. As researchers elucidate the pharmacodynamics, pharmacokinetics, and expression of resolution receptors and enzymes in the cardiovascular system, more directed approaches to translating resolution biology to cardiovascular disease can take place.
1.5. Conclusion
Inflammation is central to cardiovascular diseases, and responses to therapeutic interventions. The identification of biochemical pathways that actively mediate resolution offers new opportunities for monitoring disease progression, treatment responses, and therapeutics. SPM and their receptors are critical drivers of resolution, supported by a strong foundation of basic and translational science. Much work is required to precisely identify the molecular and cellular pathways of resolution involved in specific cardiovascular pathologies. Acute clinical settings such as thrombosis, reperfusion, invasive procedures, or surgery may offer the optimal initial opportunities to leverage SPM or their analogues as therapeutics. The development of novel drug delivery platforms and improved SPM formulations is ongoing and heralds future clinical applications for the cardiovascular patient.
Article Highlights:
SPM are generated locally, resolve inflammation, and promote tissue repair
Biologic activity of SPM is mediated via G-protein coupled receptor interactions
SPM are beneficial in animal models of cardiovascular disease
SPM-based therapeutics will require novel formulation and delivery platforms
More research is needed into mechanisms of resolution in cardiovascular disease
Funding:
This work was supported by the National Institutes of Health [HL119508, HHSN268201700005C to MSC; 1R25EB023856-04, A129688 to ASK]
Table of Abbreviations
- SPM
Specialized pro-resolving lipid mediator
- EC
Endothelial cell
- vSMC
Vascular smooth muscle cell
- PUFA
Polyunsaturated fatty acid
- EPA
Eicosapentaenoic acid
- DPA
Docosapentaenoic acid
- DHA
Docosahexaenoic acid
- RvD#
D-series resolvin #
- RvE#
E-series resolvin #
- Mar1
Maresin1
- GPCR
G-protein coupled receptor
- LO
Lipoxygenase
- COX
Cyclooxygenase
- AA
Arachidonic acid
- PDGF
Platelet derived growth factor
- FGF
Fibroblast growth factor
- PMN
Polymorphonucelar cells/neutrophils
- MCP-1
Monocyte chemoattractant protein-1
- IL-#
Interleukin-#
- LXA4
Lipoxin A4
- PD1
Protectin D1
- NIH
Neointimal hyperplasia
- AT
Aspirin triggered
- CV
Cardiovascular
- AMP/ADP
Adenosine mono/di phosphate
- HEPE
Hydroxyeicosapentaenoate
- HDHA
Hydroxydocosahexaenoic acid
- IR
Ischemia/reperfusion
Footnotes
Conflict of interest: MSC is co-inventor on patents (USPTO 9,463,177; 10,111,847) assigned to Regents of the University of California and Brigham and Women’s Hospital. MSC is a co-founder of VasaRx.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D, Risk of Death Following Application of Paclitaxel‐Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta‐Analysis of Randomized Controlled Trials, J. Am. Heart Assoc 7 (2018). 10.1161/JAHA.118.011245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease, N. Engl. J. Med 377 (2017) 1119–1131. 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- [3].Perretti M, Leroy X, Bland EJ, Montero-Melendez T, Resolution Pharmacology: Opportunities for Therapeutic Innovation in Inflammation, Trends Pharmacol. Sci 36 (2015) 737–755. 10.1016/j.tips.2015.07.007. [DOI] [PubMed] [Google Scholar]
- [4].Shah PK, Inflammation, Neointimal Hyperplasia, and Restenosis: As the Leukocytes Roll, the Arteries Thicken, Circulation. 107 (2003) 2175–2177. 10.1161/01.CIR.0000069943.41206.BD. [DOI] [PubMed] [Google Scholar]
- [5].Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN, Molecular Circuits of Resolution: Formation and Actions of Resolvins and Protectins, J. Immunol 174 (2005) 4345–4355. 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- [6].Serhan CN, Pro-resolving lipid mediators are leads for resolution physiology, Nature. 510 (2014) 92–101. 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac R-L, Resolvins J. Exp. Med 196 (2002) 1025–1037. 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Serhan CN, Levy BD, Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators, J. Clin. Invest 128 (2018) 2657–2669. 10.1172/JCI97943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L, Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators, FASEB J. 22 (2008) 3595–3606. 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Spite M, Clària J, Serhan CN, Resolvins, Specialized Proresolving Lipid Mediators, and Their Potential Roles in Metabolic Diseases, Cell Metab. 19 (2014) 21–36. 10.1016/j.cmet.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Serhan CN, Resolution Phase of Inflammation: Novel Endogenous Anti-Inflammatory and Proresolving Lipid Mediators and Pathways, Annu. Rev. Immunol 25 (2007) 101–137. 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- [12].Chiang N, Serhan CN, Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors, Mol. Aspects Med 58 (2017) 114–129. 10.1016/j.mam.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Park J, Langmead CJ, Riddy DM, New Advances in Targeting the Resolution of Inflammation: Implications for Specialized Pro-Resolving Mediator GPCR Drug Discovery, ACS Pharmacol. Transl. Sci 3 (2020) 88–106. 10.1021/acsptsci.9b00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chiang N, Libreros S, Norris PC, de la Rosa X, Serhan CN, Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions, J. Clin. Invest 129 (2019) 5294–5311. 10.1172/JCI129448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Flak MB, Koenis DS, Sobrino A, Smith J, Pistorius K, Palmas F, Dalli J, GPR101 mediates the pro-resolving actions of RvD5n-3 DPA in arthritis and infections, J. Clin. Invest 130 (2019) 359–373. 10.1172/JCI131609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fredman G, Kamaly N, Spolitu S, Milton J, Ghorpade D, Chiasson R, Kuriakose G, Perretti M, Farokhzad O, Tabas I, Targeted nanoparticles containing the proresolving peptide Ac2–26 protect against advanced atherosclerosis in hypercholesterolemic mice, Sci. Transl. Med 7 (2015) 275ra20–275ra20. 10.1126/scitranslmed.aaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Serhan CN, Novel Lipid Mediators and Resolution Mechanisms in Acute Inflammation, Am. J. Pathol 177 (2010) 1576–1591. 10.2353/ajpath.2010.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K, Novel Functional Sets of Lipid-Derived Mediators with Antiinflammatory Actions Generated from Omega-3 Fatty Acids via Cyclooxygenase 2–Nonsteroidal Antiinflammatory Drugs and Transcellular Processing, J. Exp. Med 192 (2000) 1197–1204. 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Werz O, Gerstmeier J, Libreros S, De la Rosa X, Werner M, Norris PC, Chiang N, Serhan CN, Human macrophages differentially produce specific resolvin or leukotriene signals that depend on bacterial pathogenicity, Nat. Commun 9 (2018) 59. 10.1038/s41467-017-02538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dalli J, Serhan CN, Pro-Resolving Mediators in Regulating and Conferring Macrophage Function, Front. Immunol 8 (2017) 1400. 10.3389/fimmu.2017.01400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Motwani MP, Colas RA, George MJ, Flint JD, Dalli J, Richard-Loendt A, De Maeyer RPH, Serhan CN, Gilroy DW, Pro-resolving mediators promote resolution in a human skin model of UV-killed Escherichia coli–driven acute inflammation, JCI Insight. 3 (2018) e94463. 10.1172/jci.insight.94463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fredman G, Ozcan L, Spolitu S, Hellmann J, Spite M, Backs J, Tabas I, Resolvin D1 limits 5-lipoxygenase nuclear localization and leukotriene B 4 synthesis by inhibiting a calcium-activated kinase pathway, Proc. Natl. Acad. Sci 111 (2014) 14530–14535. 10.1073/pnas.1410851111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Miyahara T, Runge S, Chatterjee A, Chen M, Mottola G, Fitzgerald JM, Serhan CN, Conte MS, D-series resolvin attenuates vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury, FASEB J. 27 (2013) 2220–2232. 10.1096/fj.12-225615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tanaka H, Sukhova GK, Swanson SJ, Clinton SK, Ganz P, Cybulsky MI, Libby P, Sustained activation of vascular cells and leukocytes in the rabbit aorta after balloon injury., Circulation. 88 (1993) 1788–1803. 10.1161/01.CIR.88.4.1788. [DOI] [PubMed] [Google Scholar]
- [25].Lacolley P, Regnault V, Nicoletti A, Li Z, Michel J-B, The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles, Cardiovasc. Res 95 (2012) 194–204. 10.1093/cvr/cvs135. [DOI] [PubMed] [Google Scholar]
- [26].Inoue T, Croce K, Morooka T, Sakuma M, Node K, Simon DI, Vascular Inflammation and Repair, JACC Cardiovasc. Interv 4 (2011) 1057–1066. 10.1016/j.jcin.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sun Y-P, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN, Resolvin D1 and Its Aspirin-triggered 17 R Epimer: STEREOCHEMICAL ASSIGNMENTS, ANTI-INFLAMMATORY PROPERTIES, AND ENZYMATIC INACTIVATION, J. Biol. Chem 282 (2007) 9323–9334. 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- [28].Brezinski DA, Nesto RW, Serhan CN, Angioplasty triggers intracoronary leukotrienes and lipoxin A4. Impact of aspirin therapy., Circulation. 86 (1992) 56–63. 10.1161/01.CIR.86.1.56. [DOI] [PubMed] [Google Scholar]
- [29].Chatterjee A, Komshian S, Sansbury BE, Wu B, Mottola G, Chen M, Spite M, Conte MS, Biosynthesis of proresolving lipid mediators by vascular cells and tissues, FASEB J. 31 (2017) 3393–3402. 10.1096/fj.201700082R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Brancaleone V, Gobbetti T, Cenac N, le Faouder P, Colom B, Flower RJ, Vergnolle N, Nourshargh S, Perretti M, A vasculo-protective circuit centered on lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 operative in murine microcirculation, Blood. 122 (2013) 608–617. 10.1182/blood-2013-04-496661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Vital SA, Becker F, Holloway PM, Russell J, Perretti M, Granger DN, Gavins FNE, Formyl-Peptide Receptor 2/3/Lipoxin A 4 Receptor Regulates Neutrophil-Platelet Aggregation and Attenuates Cerebral Inflammation: Impact for Therapy in Cardiovascular Disease, Circulation. 133 (2016) 2169–2179. 10.1161/CIRCULATIONAHA.115.020633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wu S-H, Wang M-J, Lü J, Chen X-Q, Signal transduction involved in lipoxin A4-induced protection of tubular epithelial cells against hypoxia/reoxygenation injury, Mol. Med. Rep 15 (2017) 1682–1692. 10.3892/mmr.2017.6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Spite M, Serhan CN, Novel Lipid Mediators Promote Resolution of Acute Inflammation: Impact of Aspirin and Statins, Circ. Res 107 (2010) 1170–1184. 10.1161/CIRCRESAHA.110.223883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dalli J, Chiang N, Serhan CN, Elucidation of novel 13-series resolvins that increase with atorvastatin and clear infections, Nat. Med 21 (2015) 1071–1075. 10.1038/nm.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chatterjee A, Sharma A, Chen M, Toy R, Mottola G, Conte MS, The Pro-Resolving Lipid Mediator Maresin 1 (MaR1) Attenuates Inflammatory Signaling Pathways in Vascular Smooth Muscle and Endothelial Cells, PLoS ONE. 9 (2014) e113480. 10.1371/journal.pone.0113480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sansbury BE, Spite M, Resolution of Acute Inflammation and the Role of Resolvins in Immunity, Thrombosis, and Vascular Biology, Circ. Res 119 (2016) 113–130. 10.1161/CIRCRESAHA.116.307308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Filep JG, Zouki C, Petasis NA, Hachicha M, Serhan CN, Anti-inflammatory actions of lipoxin A(4) stable analogs are demonstrable in human whole blood: modulation of leukocyte adhesion molecules and inhibition of neutrophil-endothelial interactions, Blood. 94 (1999) 4132–4142. [PubMed] [Google Scholar]
- [38].Chattopadhyay R, Mani AM, Singh NK, Rao GN, Resolvin D1 blocks H 2 O 2 -mediated inhibitory crosstalk between SHP2 and PP2A and suppresses endothelial-monocyte interactions, Free Radic. Biol. Med 117 (2018) 119–131. 10.1016/j.freeradbiomed.2018.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chiang N, Dalli J, Colas RA, Serhan CN, Identification of resolvin D2 receptor mediating resolution of infections and organ protection, J. Exp. Med 212 (2015) 1203–1217. 10.1084/jem.20150225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Petri MH, Laguna-Fernández A, Gonzalez-Diez M, Paulsson-Berne G, Hansson GK, Bäck M, The role of the FPR2/ALX receptor in atherosclerosis development and plaque stability, Cardiovasc. Res 105 (2015) 65–74. 10.1093/cvr/cvu224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Norling LV, Dalli J, Flower RJ, Serhan CN, Perretti M, Resolvin D1 Limits Polymorphonuclear Leukocyte Recruitment to Inflammatory Loci: Receptor-Dependent Actions, Arterioscler. Thromb. Vasc. Biol 32 (2012) 1970–1978. 10.1161/ATVBAHA.112.249508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wu B, Mottola G, Chatterjee A, Lance KD, Chen M, Siguenza IO, Desai TA, Conte MS, Perivascular delivery of resolvin D1 inhibits neointimal hyperplasia in a rat model of arterial injury, J. Vasc. Surg 65 (2017) 207–217.e3. 10.1016/j.jvs.2016.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Petri MH, Laguna-Fernandez A, Tseng C-N, Hedin U, Perretti M, Bäck M, Aspirin-triggered 15-epi-lipoxin A4 signals through FPR2/ALX in vascular smooth muscle cells and protects against intimal hyperplasia after carotid ligation, Int. J. Cardiol 179 (2015) 370–372. 10.1016/j.ijcard.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mottola G, Chatterjee A, Wu B, Chen M, Conte MS, Aspirin-triggered resolvin D1 attenuates PDGF-induced vascular smooth muscle cell migration via the cyclic adenosine monophosphate/protein kinase A (cAMP/PKA) pathway, PLOS ONE. 12 (2017) e0174936. 10.1371/journal.pone.0174936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hiram R, Rizcallah E, Marouan S, Sirois C, Sirois M, Morin C, Fortin S, Rousseau E, Resolvin E 1 normalizes contractility, Ca 2+ sensitivity and smooth muscle cell migration rate in TNF-α- and IL-6-pretreated human pulmonary arteries, Am. J. Physiol.-Lung Cell. Mol. Physiol 309 (2015) L776–L788. 10.1152/ajplung.00177.2015. [DOI] [PubMed] [Google Scholar]
- [46].Jannaway M, Torrens C, Warner JA, Sampson AP, Resolvin E1, resolvin D1 and resolvin D2 inhibit constriction of rat thoracic aorta and human pulmonary artery induced by the thromboxane mimetic U46619, Br. J. Pharmacol 175 (2018) 1100–1108. 10.1111/bph.14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Akagi D, Chen M, Toy R, Chatterjee A, Conte MS, Systemic delivery of proresolving lipid mediators resolvin D2 and maresin 1 attenuates intimal hyperplasia in mice, FASEB J. 29 (2015) 2504–2513. 10.1096/fj.14-265363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Spinosa M, Su G, Salmon MD, Lu G, Cullen JM, Fashandi AZ, Hawkins RB, Montgomery W, Meher AK, Conte MS, Sharma AK, Ailawadi G, Upchurch GR, Resolvin D1 decreases abdominal aortic aneurysm formation by inhibiting NETosis in a mouse model, J. Vasc. Surg 68 (2018) 93S–103S. 10.1016/j.jvs.2018.05.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Cherpokova D, Jouvene CC, Libreros S, DeRoo EP, Chu L, de la Rosa X, Norris PC, Wagner DD, Serhan CN, Resolvin D4 attenuates the severity of pathological thrombosis in mice, Blood. 134 (2019) 1458–1468. 10.1182/blood.2018886317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Liu G, Liu Q, Shen Y, Kong D, Gong Y, Tao B, Chen G, Guo S, Li J, Zuo S, Yu Y, Yin H, Zhang L, Zhou B, Funk CD, Zhang J, Yu Y, Early treatment with Resolvin E1 facilitates myocardial recovery from ischaemia in mice: Early therapy with RvE1 improves cardiac recovery after MI, Br. J. Pharmacol 175 (2018) 1205–1216. 10.1111/bph.14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhang MJ, Sansbury BE, Hellmann J, Baker JF, Guo L, Parmer CM, Prenner JC, Conklin DJ, Bhatnagar A, Creager MA, Spite M, Resolvin D2 Enhances Postischemic Revascularization While Resolving Inflammation, Circulation. 134 (2016) 666–680. 10.1161/CIRCULATIONAHA.116.021894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Elajami TK, Colas RA, Dalli J, Chiang N, Serhan CN, Welty FK, Specialized proresolving lipid mediators in patients with coronary artery disease and their potential for clot remodeling, FASEB J. 30 (2016) 2792–2801. 10.1096/fj.201500155R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Laguna-Fernandez A, Checa A, Carracedo M, Artiach G, Petri MH, Baumgartner R, Forteza MJ, Jiang X, Andonova T, Walker ME, Dalli J, Arnardottir H, Gisterå A, Thul S, Wheelock CE, Paulsson-Berne G, Ketelhuth DFJ, Hansson GK, Bäck M, ERV1/ChemR23 Signaling Protects Against Atherosclerosis by Modifying Oxidized Low-Density Lipoprotein Uptake and Phagocytosis in Macrophages, Circulation. 138 (2018) 1693–1705. 10.1161/CIRCULATIONAHA.117.032801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Pope NH, Salmon M, Davis JP, Chatterjee A, Su G, Conte MS, Ailawadi G, Upchurch GR, D-series resolvins inhibit murine abdominal aortic aneurysm formation and increase M2 macrophage polarization, FASEB J. 30 (2016) 4192–4201. 10.1096/fj.201600144RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kang J-W, Lee S-M, Resolvin D1 protects the liver from ischemia/reperfusion injury by enhancing M2 macrophage polarization and efferocytosis, Biochim. Biophys. Acta BBA - Mol. Cell Biol. Lipids 1861 (2016) 1025–1035. 10.1016/j.bbalip.2016.06.002. [DOI] [PubMed] [Google Scholar]
- [56].Lannan KL, Spinelli SL, Blumberg N, Phipps RP, Maresin 1 induces a novel pro-resolving phenotype in human platelets, J. Thromb. Haemost 15 (2017) 802–813. 10.1111/jth.13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Dona M, Fredman G, Schwab JM, Chiang N, Arita M, Goodarzi A, Cheng G, von Andrian UH, Serhan CN, Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets, Blood. 112 (2008) 848–855. 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Fredman G, Van Dyke TE, Serhan CN, Resolvin E1 Regulates Adenosine Diphosphate Activation of Human Platelets, Arterioscler. Thromb. Vasc. Biol 30 (2010) 2005–2013. 10.1161/ATVBAHA.110.209908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Abdulnour R-EE, Dalli J, Colby JK, Krishnamoorthy N, Timmons JY, Tan SH, Colas RA, Petasis NA, Serhan CN, Levy BD, Maresin 1 biosynthesis during platelet–neutrophil interactions is organ-protective, Proc. Natl. Acad. Sci 111 (2014) 16526–16531. 10.1073/pnas.1407123111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ho KJ, Spite M, Owens CD, Lancero H, Kroemer AHK, Pande R, Creager MA, Serhan CN, Conte MS, Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis, Am. J. Pathol 177 (2010) 2116–2123. 10.2353/ajpath.2010.091082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Thul S, Labat C, Temmar M, Benetos A, Bäck M, Low salivary resolvin D1 to leukotriene B 4 ratio predicts carotid intima media thickness: A novel biomarker of non-resolving vascular inflammation, Eur. J. Prev. Cardiol 24 (2017) 903–906. 10.1177/2047487317694464. [DOI] [PubMed] [Google Scholar]
- [62].Bazan HA, Lu Y, Jun B, Fang Z, Woods TC, Hong S, Circulating inflammation-resolving lipid mediators RvD1 and DHA are decreased in patients with acutely symptomatic carotid disease, Prostaglandins Leukot. Essent. Fatty Acids 125 (2017) 43–47. 10.1016/j.plefa.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Colas RA, Souza PR, Walker ME, Burton M, Zasłona Z, Curtis AM, Marques RM, Dalli J, Impaired Production and Diurnal Regulation of Vascular RvD n-3 DPA Increase Systemic Inflammation and Cardiovascular Disease, Circ. Res 122 (2018) 855–863. 10.1161/CIRCRESAHA.117.312472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Fredman G, Hellmann J, Proto JD, Kuriakose G, Colas RA, Dorweiler B, Connolly ES, Solomon R, Jones DM, Heyer EJ, Spite M, Tabas I, An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques, Nat. Commun 7 (2016) 12859. 10.1038/ncomms12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Viola JR, Lemnitzer P, Jansen Y, Csaba G, Winter C, Neideck C, Silvestre-Roig C, Dittmar G, Döring Y, Drechsler M, Weber C, Zimmer R, Cenac N, Soehnlein O, Resolving Lipid Mediators Maresin 1 and Resolvin D2 Prevent Atheroprogression in Mice, Circ. Res 119 (2016) 1030–1038. 10.1161/CIRCRESAHA.116.309492. [DOI] [PubMed] [Google Scholar]
- [66].Petri MH, Laguna-Fernandez A, Arnardottir H, Wheelock CE, Perretti M, Hansson GK, Bäck M, Aspirin-triggered lipoxin A4 inhibits atherosclerosis progression in apolipoprotein E−/− mice, Br. J. Pharmacol 174 (2017) 4043–4054. 10.1111/bph.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hasturk H, Abdallah R, Kantarci A, Nguyen D, Giordano N, Hamilton J, Van Dyke TE, Resolvin E1 (RvE1) Attenuates Atherosclerotic Plaque Formation in Diet and Inflammation-Induced Atherogenesis, Arterioscler. Thromb. Vasc. Biol 35 (2015) 1123–1133. 10.1161/ATVBAHA.115.305324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Carracedo M, Artiach G, Witasp A, Clària J, Carlström M, Laguna-Fernandez A, Stenvinkel P, Bäck M, The G-protein coupled receptor ChemR23 determines smooth muscle cell phenotypic switching to enhance high phosphate-induced vascular calcification, Cardiovasc. Res 115 (2019) 1557–1566. 10.1093/cvr/cvy316. [DOI] [PubMed] [Google Scholar]
- [69].Carracedo M, Witasp A, Qureshi AR, Laguna‐Fernandez A, Brismar T, Stenvinkel P, Bäck M, Chemerin inhibits vascular calcification through ChemR23 and is associated with lower coronary calcium in chronic kidney disease, J. Intern. Med 286 (2019) 449–457. 10.1111/joim.12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Meital LT, Sandow SL, Calder PC, Russell FD, Abdominal aortic aneurysm and omega-3 polyunsaturated fatty acids: Mechanisms, animal models, and potential treatment, Prostaglandins Leukot. Essent. Fatty Acids 118 (2017) 1–9. 10.1016/j.plefa.2017.02.001. [DOI] [PubMed] [Google Scholar]
- [71].Pillai PS, Leeson S, Porter TF, Owens CD, Kim JM, Conte MS, Serhan CN, Gelman S, Chemical Mediators of Inflammation and Resolution in Post-Operative Abdominal Aortic Aneurysm Patients, Inflammation. 35 (2012) 98–113. 10.1007/s10753-0119294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Petri MH, Thul S, Andonova T, Lindquist-Liljeqvist M, Jin H, Skenteris N-T, Arnardottir H, Maegdefessel L, Caidahl K, Perretti M, Roy J, Bäck M, Resolution of Inflammation Through the Lipoxin and ALX/FPR2 Receptor Pathway Protects Against Abdominal Aortic Aneurysms, JACC Basic Transl. Sci 3 (2018) 719–727. 10.1016/j.jacbts.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Bohr S, Patel SJ, Sarin D, Irimia D, Yarmush ML, Berthiaume F, Resolvin D2 prevents secondary thrombosis and necrosis in a mouse burn wound model: RvD2 prevents secondary necrosis in burns, Wound Repair Regen. 21 (2013) 35–43. 10.1111/j.1524-475X.2012.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Norris PC, Libreros S, Chiang N, Serhan CN, A cluster of immunoresolvents links coagulation to innate host defense in human blood, Sci. Signal 10 (2017) eaan1471. 10.1126/scisignal.aan1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Iliceto S, Galiuto L, Marchese A, Cavallari D, Colonna P, Biasco G, Rizzon P, Analysis of microvascular integrity, contractile reserve, and myocardial viability after acute myocardial infarction by dobutamine echocardiography and myocardial contrast echocardiography, Am. J. Cardiol 77 (1996) 441–445. 10.1016/S0002-9149(97)89334-4. [DOI] [PubMed] [Google Scholar]
- [76].Bell RM, Yellon DM, There is More to Life than Revascularization: Therapeutic Targeting of Myocardial Ischemia/Reperfusion Injury: Strategies to Attenuate Lethal Reperfusion Injury, Cardiovasc. Ther 29 (2011) e67–e79. 10.1111/j.1755-5922.2010.00190.x. [DOI] [PubMed] [Google Scholar]
- [77].Hausenloy DJ, Bøtker HE, Condorelli G, Ferdinandy P, Garcia-Dorado D, Heusch G, Lecour S, van Laake LW, Madonna R, Ruiz-Meana M, Schulz R, Sluijter JPG, Yellon DM, Ovize M, Translating cardioprotection for patient benefit: position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology, Cardiovasc. Res 98 (2013) 7–27. 10.1093/cvr/cvt004. [DOI] [PubMed] [Google Scholar]
- [78].Liu R, Li Z, Wang Q, Resolvin D1 Attenuates Myocardial Infarction in a Rodent Model with the Participation of the HMGB1 Pathway, Cardiovasc. Drugs Ther 33 (2019) 399–406. 10.1007/s10557-019-06884-y. [DOI] [PubMed] [Google Scholar]
- [79].Kain V, Ingle KA, Colas RA, Dalli J, Prabhu SD, Serhan CN, Joshi M, Halade GV, Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function, J. Mol. Cell. Cardiol 84 (2015) 24–35. 10.1016/j.yjmcc.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Gilbert K, Bernier J, Godbout R, Rousseau G, Resolvin D1, a Metabolite of Omega-3 Polyunsaturated Fatty Acid, Decreases Post-Myocardial Infarct Depression, Mar. Drugs 12 (2014) 5396–5407. 10.3390/md12115396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Keyes KT, Ye Y, Lin Y, Zhang C, Perez-Polo JR, Gjorstrup P, Birnbaum Y, Resolvin E1 protects the rat heart against reperfusion injury, Am. J. Physiol.-Heart Circ. Physiol 299 (2010) H153–H164. 10.1152/ajpheart.01057.2009. [DOI] [PubMed] [Google Scholar]
- [82].Zuo G, Zhang D, Mu R, Shen H, Li X, Wang Z, Li H, Chen G, Resolvin D2 protects against cerebral ischemia/reperfusion injury in rats, Mol. Brain 11 (2018) 9. 10.1186/s13041-018-0351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Han J, Liu C, Wang Z, Liu L, Cheng L, Fan Y, Anti-inflammatory properties of lipoxin A4 protect against diabetes mellitus complicated by focal cerebral ischemia/reperfusion injury, Neural Regen. Res 11 (2016) 636. 10.4103/1673-5374.180750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Xian W, Wu Y, Xiong W, Li L, Li T, Pan S, Song L, Hu L, Pei L, Yao S, Shang Y, The pro-resolving lipid mediator Maresin 1 protects against cerebral ischemia/reperfusion injury by attenuating the pro-inflammatory response, Biochem. Biophys. Res. Commun 472 (2016) 175–181. 10.1016/j.bbrc.2016.02.090. [DOI] [PubMed] [Google Scholar]
- [85].Bisicchia E, Sasso V, Catanzaro G, Leuti A, Besharat ZM, Chiacchiarini M, Molinari M, Ferretti E, Viscomi MT, Chiurchiù V, Resolvin D1 Halts Remote Neuroinflammation and Improves Functional Recovery after Focal Brain Damage Via ALX/FPR2 Receptor-Regulated MicroRNAs, Mol. Neurobiol 55 (2018) 6894–6905. 10.1007/s12035-018-0889-z. [DOI] [PubMed] [Google Scholar]
- [86].Kooij G, Derada Troletti C, Leuti A, Norris PC, Riley I, Albanese M, Ruggieri S, Libreros S, van der Pol SMA, van het Hof B, Schell Y, Guerrera G, Buttari F, Mercuri NB, Centonze D, Gasperini C, Battistini L, de Vries HE, Serhan CN, Chiurchiù V, Specialized pro-resolving lipid mediators are differentially altered in peripheral blood of patients with multiple sclerosis and attenuate monocyte and blood-brain barrier dysfunction, Haematologica. (2019) haematol.2019.219519. 10.3324/haematol.2019.219519. [DOI] [PMC free article] [PubMed]
- [87].Zhang T, Shu H-H, Chang L, Ye F, Xu K-Q, Huang W-Q, Resolvin D1 protects against hepatic ischemia/reperfusion injury in rats, Int. Immunopharmacol 28 (2015) 322–327. 10.1016/j.intimp.2015.06.017. [DOI] [PubMed] [Google Scholar]
- [88].Zhang T, Xiu H-H, Liu J-X, Ma Y, Xu K-Q, Huang W-Q, Protective effect of aspirin-triggered resolvin D1 on hepatic ischemia/reperfusion injury in rats: The role of miR-146b, Int. Immunopharmacol 51 (2017) 140–147. 10.1016/j.intimp.2017.08.008. [DOI] [PubMed] [Google Scholar]
- [89].Kang J-W, Choi H-S, Shin J-K, Lee S-M, Resolvin D1 activates the sphingosine-1-phosphate signaling pathway in murine livers with ischemia/reperfusion injury, Biochem. Biophys. Res. Commun 514 (2019) 1058–1065. 10.1016/j.bbrc.2019.05.041. [DOI] [PubMed] [Google Scholar]
- [90].Xia J, Xue JY, Du J, Wu GW, Hu XT, Zhao QF, [Role and related mechanism of resolvin D1 in lung ischemia reperfusion injury in rats], Zhonghua Yi Xue Za Zhi. 99 (2019) 1111–1115. 10.3760/cma.j.issn.0376-2491.2019.14.015. [DOI] [PubMed] [Google Scholar]
- [91].Jaworska K, Ratajczak J, Huang L, Whalen K, Yang M, Stevens BK, Kinsey GR, Both PD-1 Ligands Protect the Kidney from Ischemia Reperfusion Injury, J. Immunol 194 (2015) 325–333. 10.4049/jimmunol.1400497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Duffield JS, Hong S, Vaidya VS, Lu Y, Fredman G, Serhan CN, Bonventre JV, Resolvin D Series and Protectin D1 Mitigate Acute Kidney Injury, J. Immunol 177 (2006) 5902–5911. 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- [93].Zong H, Li X, Lin H, Hou C, Ma F, Lipoxin A4 pretreatment mitigates skeletal muscle ischemia-reperfusion injury in rats, Am. J. Transl. Res 9 (2017) 1139–1150. [PMC free article] [PubMed] [Google Scholar]
- [94].Tourki B, Kain V, Pullen AB, Norris PC, Patel N, Arora P, Leroy X, Serhan CN, Halade GV, Lack of resolution sensor drives age-related cardiometabolic and cardiorenal defects and impedes inflammation-resolution in heart failure, Mol. Metab 31 (2020) 138–149. 10.1016/j.molmet.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Muto A, Model L, Ziegler K, Eghbalieh SDD, Dardik A, Mechanisms of Vein Graft Adaptation to the Arterial Circulation: – Insights Into the Neointimal Algorithm and Management Strategies –, Circ. J 74 (2010) 1501–1512. 10.1253/circj.CJ-10-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kornowski R, Hong MK, Tio FO, Bramwell O, Wu H, Leon MB, In-Stent Restenosis: Contributions of Inflammatory Responses and Arterial Injury to Neointimal Hyperplasia, J. Am. Coll. Cardiol 31 (1998) 224–230. 10.1016/S0735-1097(97)00450-6. [DOI] [PubMed] [Google Scholar]
- [97].de Vries MR, Quax PHA, Inflammation in Vein Graft Disease, Front. Cardiovasc. Med 5 (2018) 3. 10.3389/fcvm.2018.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, Namini H, Hamdan AD, Roddy SP, Belkin M, Berceli SA, DeMasi RJ, Samson RH, Berman SS, Results of PREVENT III: A multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery, J. Vasc. Surg 43 (2006) 742–751.e1. 10.1016/j.jvs.2005.12.058. [DOI] [PubMed] [Google Scholar]
- [99].Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FGR, Gillespie I, Ruckley CV, Raab GM, Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: A survival prediction model to facilitate clinical decision making, J. Vasc. Surg 51 (2010) 52S–68S. 10.1016/j.jvs.2010.01.077. [DOI] [PubMed] [Google Scholar]
- [100].Baumann F, Ozdoba C, Gröchenig EN, The Importance of Patency in Patients with Critical Limb Ischemia Undergoing Endovascular Revascularization for Infrapopliteal Arterial Disease, Front. Cardiovasc. Med 1 (2015). 10.3389/fcvm.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Liu G, Gong Y, Zhang R, Piao L, Li X, Liu Q, Yan S, Shen Y, Guo S, Zhu M, Yin H, Funk CD, Zhang J, Yu Y, Resolvin E1 attenuates injury-induced vascular neointimal formation by inhibition of inflammatory responses and vascular smooth muscle cell migration, FASEB J. 32 (2018) 5413–5425. 10.1096/fj.201800173R. [DOI] [PubMed] [Google Scholar]
- [102].Makino Y, Miyahara T, Nitta J, Miyahara K, Seo A, Kimura M, Suhara M, Akai A, Akagi D, Yamamoto K, Hoshina K, Proresolving Lipid Mediators Resolvin D1 and Protectin D1 Isomer Attenuate Neointimal Hyperplasia in the Rat Carotid Artery Balloon Injury Model, J. Surg. Res 233 (2019) 104–110. 10.1016/j.jss.2018.07.049. [DOI] [PubMed] [Google Scholar]
- [103].Mottola G, Werlin EC, Wu B, Chen M, Chatterjee A, Schaller MS, Conte MS, Oral Resolvin D1 attenuates early inflammation but not intimal hyperplasia in a rat carotid angioplasty model, Prostaglandins Other Lipid Mediat. 146 (2020) 106401. 10.1016/j.prostaglandins.2019.106401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Wu B, Werlin EC, Chen M, Mottola G, Chatterjee A, Lance KD, Bernards DA, Sansbury BE, Spite M, Desai TA, Conte MS, Perivascular delivery of resolvin D1 inhibits neointimal hyperplasia in a rabbit vein graft model, J. Vasc. Surg 68 (2018) 188S–200S.e4. 10.1016/j.jvs.2018.05.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Shi J, Zhang X, Jiang L, Zhang L, Dong Y, Midgley AC, Kong D, Wang S, Regulation of the inflammatory response by vascular grafts modified with Aspirin-Triggered Resolvin D1 promotes blood vessel regeneration, Acta Biomater. 97 (2019) 360–373. 10.1016/j.actbio.2019.07.037. [DOI] [PubMed] [Google Scholar]
- [106].Siscovick DS, Barringer TA, Fretts AM, Wu JHY, Lichtenstein AH, Costello RB, Kris-Etherton PM, Jacobson TA, Engler MB, Alger HM, Appel LJ, Mozaffarian D, Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease: A Science Advisory From the American Heart Association, Circulation. 135 (2017). 10.1161/CIR.0000000000000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].The ORIGIN Trial Investigators, n–3 Fatty Acids and Cardiovascular Outcomes in Patients with Dysglycemia, N. Engl. J. Med 367 (2012) 309–318. 10.1056/NEJMoa1203859. [DOI] [PubMed] [Google Scholar]
- [108].Rauch B, Schiele R, Schneider S, Diller F, Victor N, Gohlke H, Gottwik M, Steinbeck G, Del Castillo U, Sack R, Worth H, Katus H, Spitzer W, Sabin G, Senges J, for the OMEGA Study Group, OMEGA, a Randomized, Placebo-Controlled Trial to Test the Effect of Highly Purified Omega-3 Fatty Acids on Top of Modern Guideline-Adjusted Therapy After Myocardial Infarction, Circulation. 122 (2010) 2152–2159. 10.1161/CIRCULATIONAHA.110.948562. [DOI] [PubMed] [Google Scholar]
- [109].Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial, The Lancet. 354 (1999) 447–455. 10.1016/S0140-6736(99)07072-5. [DOI] [PubMed] [Google Scholar]
- [110].Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Juliano RA, Jiao L, Granowitz C, Tardif J-C, Ballantyne CM, Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia, N. Engl. J. Med 380 (2019) 11–22. 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- [111].Manson JE, Cook NR, Lee I-M, Christen W, Bassuk SS, Mora S, Gibson H, Albert CM, Gordon D, Copeland T, D’Agostino D, Friedenberg G, Ridge C, Bubes V, Giovannucci EL, Willett WC, Buring JE, Marine n−3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer, N. Engl. J. Med 380 (2019) 23–32. 10.1056/NEJMoa1811403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Grenon SM, Owens CD, Nosova EV, Hughes‐Fulford M, Alley HF, Chong K, Perez S, Yen PK, Boscardin J, Hellmann J, Spite M, Conte MS, Short‐Term, High‐Dose Fish Oil Supplementation Increases the Production of Omega‐3 Fatty Acid–Derived Mediators in Patients With Peripheral Artery Disease (the OMEGA‐PAD I Trial), J. Am. Heart Assoc 4 (2015). 10.1161/JAHA.115.002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Ramirez JL, Gasper WJ, Khetani SA, Zahner GJ, Hills NK, Mitchell PT, Sansbury BE, Conte MS, Spite M, Grenon SM, Fish Oil Increases Specialized Pro-resolving Lipid Mediators in PAD (The OMEGA-PAD II Trial), J. Surg. Res 238 (2019) 164–174. 10.1016/j.jss.2019.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Schaller MS, Zahner GJ, Gasper WJ, Harris WS, Conte MS, Hills NK, Grenon SM, Relationship between the omega-3 index and specialized pro-resolving lipid mediators in patients with peripheral arterial disease taking fish oil supplements, J. Clin. Lipidol 11 (2017) 1289–1295. 10.1016/j.jacl.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Schaller MS, Chen M, Colas RA, Sorrentino TA, Lazar AA, Grenon SM, Dalli J, Conte MS, Treatment with a Marine Oil Supplement Alters Lipid Mediators and Leukocyte Phenotype in Healthy Subjects and those with Peripheral Arterial Disease, J Am Heart Assoc. (in press) (2020). [DOI] [PMC free article] [PubMed]
- [116].Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN, Lipid mediator class switching during acute inflammation: signals in resolution, Nat. Immunol 2 (2001) 612–619. 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- [117].Poorani R, Bhatt AN, Dwarakanath BS, Das UN, COX-2, aspirin and metabolism of arachidonic, eicosapentaenoic and docosahexaenoic acids and their physiological and clinical significance, Eur. J. Pharmacol 785 (2016) 116–132. 10.1016/j.ejphar.2015.08.049. [DOI] [PubMed] [Google Scholar]
- [118].Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN, Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue, Am. J. Physiol.-Cell Physiol 307 (2014) C39–C54. 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN, Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis, Nature. 461 (2009) 1287–1291. 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Wittwer J, Hersberger M, The two faces of the 15-lipoxygenase in atherosclerosis, Prostaglandins Leukot. Essent. Fatty Acids 77 (2007) 67–77. 10.1016/j.plefa.2007.08.001. [DOI] [PubMed] [Google Scholar]
- [121].Halade GV, Kain V, Black LM, Prabhu SD, Ingle KA, Aging dysregulates D- and E-series resolvins to modulate cardiosplenic and cardiorenal network following myocardial infarction, Aging. 8 (2016) 2611–2634. 10.18632/aging.101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Endo J, Sano M, Isobe Y, Fukuda K, Kang JX, Arai H, Arita M, 18-HEPE, an n-3 fatty acid metabolite released by macrophages, prevents pressure overload-induced maladaptive cardiac remodeling, J. Exp. Med 211 (2014) 1673–1687. 10.1084/jem.20132011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Fu T, Mohan M, Brennan EP, Woodman OL, Godson C, Kantharidis P, Ritchie RH, Qin CX, Therapeutic Potential of Lipoxin A 4 in Chronic Inflammation: Focus on Cardiometabolic Disease, ACS Pharmacol. Transl. Sci 3 (2020) 43–55. 10.1021/acsptsci.9b00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Cholkar K, Gilger BC, Mitra AK, Topical delivery of aqueous micellar resolvin E1 analog (RX-10045), Int. J. Pharm 498 (2016) 326–334. 10.1016/j.ijpharm.2015.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Chiang N, Barnaeva E, Hu X, Marugan J, Southall N, Ferrer M, Serhan CN, Identification of Chemotype Agonists for Human Resolvin D1 Receptor DRV1 with Pro-Resolving Functions, Cell Chem. Biol 26 (2019) 244–254.e4. 10.1016/j.chembiol.2018.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Orr SK, Colas RA, Dalli J, Chiang N, Serhan CN, Proresolving actions of a new resolvin D1 analog mimetic qualifies as an immunoresolvent, Am. J. Physiol.-Lung Cell. Mol. Physiol 308 (2015) L904–L911. 10.1152/ajplung.00370.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Conte MS, Desai TA, Wu B, Schaller M, Werlin E, Pro-resolving lipid mediators in vascular disease, J. Clin. Invest 128 (2018) 3727–3735. 10.1172/JCI97947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, Gupta M, Tsigoulis M, Verma S, Clearfield M, Libby P, Goldhaber SZ, Seagle R, Ofori C, Saklayen M, Butman S, Singh N, Le May M, Bertrand O, Johnston J, Paynter NP, Glynn RJ, Low-Dose Methotrexate for the Prevention of Atherosclerotic Events, N. Engl. J. Med 380 (2019) 752–762. 10.1056/NEJMoa1809798. [DOI] [PMC free article] [PubMed] [Google Scholar]