Abstract
Background:
Recent reports have indicated that symptom exacerbation after a period of improvement, referred to as relapse, in early-stage psychosis could result in brain changes and poor disease outcomes. We hypothesized that substantial neuroimaging alterations may exist among patients who experience relapse in early-stage psychosis.
Methods:
We studied patients with psychosis within 2 years after the first psychotic event and healthy controls. We divided patients into 2 groups, namely those who did not experience relapse between disease onset and the magnetic resonance imaging (MRI) scan (no-relapse group) and those who did experience relapse between these 2 timings (relapse group). We analyzed 3003 functional connectivity estimates between 78 regions of interest (ROIs) derived from resting-state functional MRI data by adjusting for demographic and clinical confounding factors.
Results:
We studied 85 patients, incuding 54 in the relapse group and 31 in the no-relapse group, along with 94 healthy controls. We observed significant differences in 47 functional connectivity estimates between the relapse and control groups after multiple comparison corrections, whereas no differences were found between the no-relapse and control groups. Most of these pathological signatures (64%) involved the thalamus. The Jonckheere–Terpstra test indicated that all 47 functional connectivity changes had a significant cross-group progression from controls to patients in the no-relapse group to patients in the relapse group.
Limitations:
Longitudinal studies are needed to further validate the involvement and pathological importance of the thalamus in relapse.
Conclusion:
We observed pathological differences in neuronal connectivity associated with relapse in early-stage psychosis, which are more specifically associated with the thalamus. Our study implies the importance of considering neurobiological mechanisms associated with relapse in the trajectory of psychotic disorders.
Introduction
The trajectory of psychotic disorders after onset is heterogeneous and many patients show deteriorating courses.1,2 One of the major determinants of poor prognosis is the occurrence of relapse, defined as symptom exacerbations after a period of improvement.3,4 Relapse may be caused by intrinsic vulnerability but also by nonadherence to antipsychotic medication and comorbid substance use disorder.5–7 Patients with chronic psychosis consistently exhibit widespread alterations in brain functional connectivity, including the default mode network, salience network, and sensorimotor network.8–12 However, findings regarding the comparison between patients with first-episode psychosis and healthy individuals are less uniform.13,14 Relapses occurring at different times for different patients could be a key factor contributing to the variability during the early stages of psychosis.
A pioneering study described that relapse duration was related to significant decreases in both total cerebral volume and frontal brain measures.15 This study implied that extended periods of relapse may have a negative effect on patients with schizophrenia, suggesting the importance of preventing relapse and intervening when relapse occurs. This notion is consistent with the observation of changes in structural connectivity in different stages of psychosis related to relapse,16 and of altered functional connectivity after relapse among patients with psychosis, regardless of proper antipsychotic maintenance.17 Furthermore, a more recent study reported adverse effects of relapse on brain structure, independent of the effects of antipsychotic medications.18 Together, emerging evidence has consistently indicated that the occurrence of relapse in early-stage psychosis, regardless of reasons, could result in brain changes,15–18 which likely underlies poor disease outcomes. Although these studies are intriguing, most of them employed a hypothesis-driven approach that focused on a specific brain network.
In the present study, we hypothesized that relapse may affect brain functional connectivity, which may be well captured by a data-driven analysis of functional connectivity patterns in whole grey matter. We sought to identify neuroimaging alterations that are more specifically seen among patients with early-stage psychosis who experience relapse.
Methods
Study design
We conducted a cross-sectional study using medical records in a retrospective manner. We used hospitalization for psychotic symptom exacerbation as an objective indicator of relapse, according to many publications that used psychiatric hospitalization as a proxy of relapse.18–21 Accordingly, we used a cohort of patients with early-stage psychosis,22–26 and evaluated neuroimaging differences among patients who did not experience relapse before study enrolment (no-relapse group), patients who experienced relapse before the study enrolment (relapse group), and healthy controls.
Study cohort
We recruited patients within 2 years after their first psychotic event and confirmed diagnoses of psychotic disorders using the Structural Clinical Interview for DSM-IV Patient Edition (SCID).27 Exclusion criteria included a history of head trauma, nasal trauma, nasal surgery, neurologic disorder, cancer, or chronic viral infection (e.g., HIV, hepatitis), and reported history of intellectual disability. In addition, we excluded participants with an estimated intelligence quotient below 70 on the Hopkins Adult Reading Test.28 We excluded patients who reported active substance abuse or who screened positive for illicit substance use (excluding cannabis) during the enrolment process. We also excluded people who were pregnant, those taking anti-inflammatory agents, or those with any other psychiatric conditions. We excluded healthy controls who had a psychiatric diagnosis or a family history of schizophrenia-spectrum disorder.
We evaluated the presence and severity of positive and negative symptoms among patients with the Scale for the Assessment of Negative Symptoms (SANS) and the Assessment of Positive Symptoms (SAPS). More details about clinical recruitment can be found in previous publications.22–26
Clinical characterization
Board-certified psychiatrists assessed self-reports and the Johns Hopkins electronic medical database for patients. Hospitalization for psychotic symptom exacerbation has been used as an objective outcome indicator of relapse by many groups with a track of successful applications.18–21,29 Accordingly, we categorized patients into the no-relapse and relapse groups using psychiatric hospitalization as a proxy for relapse, namely those who experienced no psychiatric hospitalizations after the onset of psychosis and before magnetic resonance imaging, and patients who experienced psychiatric hospitalizations for psychotic exacerbation in this period. We counted only hospitalizations directly associated with psychotic symptom exacerbation for relapse. In addition, we carefully examined medical records for treatment adherence, duration between hospitalizations, and cannabis use to determine recovery or symptom stability before psychotic symptom exacerbation. We obtained medication records, as well as the duration of illness (the interval between onset and resting-state functional magnetic resonance imaging [rs-fMRI] scan) through self-reports and confirmed through the Johns Hopkins electronic medical database. We converted the antipsychotic dosages to chlorpromazine equivalents using the defined daily dose method.30
While the main research goal of this study was to identify common signatures of relapse in psychosis from a dimensional viewpoint, we also evaluated potential confounding effects associated with affective status. Accordingly, we divided the patients into nonaffective and affective groups; the former included those with schizophrenia or schizoaffective disorder, whereas the latter included those with bipolar disorder with psychotic features or major depressive disorder with psychotic features. We did not include schizophreniform disorder and not otherwise specified psychotic disorder in this dichotomic categorization.
Brain imaging
We acquired the synchrony of fMRI time courses and the T1-weighted images from the brain cortex and deep grey matter (average volume 1254.2 mL) using a Philips dStream Achieva 3 T MRI. Participants were instructed to close their eyes during the scan, monitored by camera. The image parameters for rs-fMRI were an axial orientation, original matrix 80 × 80, 36 slices, voxel size of 3 × 3 × 4 mm, repetition time 2000 ms, echo time 30 ms, and 210 time points; for high-resolution, T1-weighted images, image parameters were sagittal orientation, original matrix 170 × 170, 256 slices, voxel size 1 × 1 × 1.2 mm, repetition time 6700 ms, and echo time 3.1 ms.
We used MRICloud (www.MRICloud.org), an automated cloud platform, to process the brain images.31 We conducted global brain signal correction with CompCor, packaged with MRICloud. We aligned rs-fMRI time courses to the mean image using rigid body registration by following standard procedures to minimize the effects of head motion. We identified and excluded motion and intensity outlier frames using the Artifact Detection Tools toolbox. To further limit the potential confounding effects of head motion on this study, we calculated framewise displacement using 6 motion parameters.32 Following the criteria in published studies,24,33,34 we excluded 1 participant with a framewise displacement larger than 0.3 from the analysis. Framewise displacement was further adjusted in downstream analyses.
We also used MRICloud to segment and process structural MRI images.31 The detailed protocols for obtaining and processing MRI scans are described in our previous publication.35 High-resolution, T1-weighted images (magnetization-prepared rapid acquisition with gradient echo [MP-RAGE]) from each participant were parceled using MRICloud, resulting in the definition of 78 grey matter regions of interest (ROIs) (Appendix 1, Table S1, available at www.jpn.ca/lookup/doi/10.1503/jpn.230115/tab-related-content). For the segmentation, we used a multi-atlas set that matched the demography of study participants in our cohort. The MP-RAGE images and their respective segmentations were then warped to the rs-fMRI. Through this process, we obtained 3003 Fisher z-transformed resting-state functional connectivity estimates between the 78 ROIs.
MRICloud is designed to reduce data dimensions by defining the ROIs based on established biological knowledge. Thus, we transitioned from voxels to ROIs through the application of anatomic atlases that represented the specific structures with clinical importance, and analyzed the rs-fMRI signal between ROIs. Consequently, the interpretation of the results and possible clinical translation becomes more straightforward.35 Furthermore, it facilitates the combination of various features, including fMRI correlations and other variables.36 This method demonstrated reliability and robustness against artifactual noise.37
Statistical analysis
We used R version 3.5.1 and SPSS version 24.0 to perform statistical analysis. We calculated group comparisons of demographic and clinical data using analysis of variance (ANOVA) for continuous variables and χ2 tests for categorical variables.
We conducted ANOVA and Tukey post hoc tests to compare differences in functional connectivity among the control, no-relapse, and relapse groups. We adjusted for age, sex, race, and framewise displacement in the analysis. Cross-group progression in significant differences in functional connectivity, identified by ANOVA and Tukey post hoc tests, was further tested using the Jonckheere–Terpstra test.16,38 The Jonckheere–Terpstra test is a nonparametric method for comparing 3 or more groups under the null hypothesis of equal outcomes among the groups against the alternative that outcomes follow a definite ordering. Lastly, we conducted partial Pearson correlation — adjusted for age, sex, race, and framewise displacement — to evaluate potential confounding effects of clinical variables (i.e., chlorpromazine equivalent dose, duration of illness, and SAPS and SANS total scores) on significant results.
We performed the Benjamini–Hochberg procedure, a method for controlling the false discovery rate (FDR), for multiple comparison correction of all the analyses where multiple statistical tests were involved.39 Results with adjusted p values (also called q values) smaller than 0.05 were considered significant.
Ethics approval
This study was approved by the Johns Hopkins Medicine Institutional Review Boards and in accordance with the Code of Ethics of the World Medical Association (1964 Declaration of Helsinki). All study participants provided written informed consent. Parental consent and assent were obtained for all participants younger than 18 years.
Results
Demographic and clinical data
After excluding 4 patients in the relapse group based on their medical records and 1 patient in the relapse group with outlying brain imaging data, the final sample included 94 controls and 85 patients (54 in the no-relapse group and 31 in the relapse group) (Table 1). Most patients were medicated; 5 were treated with first-generation antipsychotics, 67 with second-generation antipsychotics, and 6 with a combination of both first- and second-generation antipsychotics. Four patients (2 in each of relapse and no-relapse groups) were treated with clozapine. In addition, 22 (40.7%) patients in the no-relapse group and 13 (41.9%) in the relapse group took mood stabilizers or antidepressants. Seven patients were unmedicated at the first visit. Among patients in the relapse group, the average interval between the onset and first relapse was 359 days.
Table 1.
Characteristics of the study participants
| Characteristic | No. (%) of participants* | p value | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Healthy controls n = 94 |
No-relapse n = 54 |
Relapse n = 31 |
No-relapse v. healthy controls | Relapse v. healthy controls | No-relapse v. relapse | |
| Age, mean ± SD, yr | 23.4 ± 3.9 | 22.2 ± 4.8 | 22.4 ± 4.1 | 0.1 | 0.2 | 0.9 |
| Sex, male | 42 (44.7) | 39 (72.2) | 22 (71.0) | 0.002 | 0.02 | 1 |
| Race, Black | 59 (62.8) | 23 (42.6) | 20 (64.5) | 0.03 | 1 | 0.08 |
| CPZ, mg, mean ± SD | NA | 238.0 ± 219.7 | 373.1 ± 247.6 | NA | NA | 0.02 |
| Duration of illness, mo, mean ± SD | NA | 12.4 ± 9.1 | 18.6 ± 7.6 | NA | NA | 0.001 |
| SAPS, mean ± SD | NA | 3.1 ± 3.5 | 4.7 ± 4.2 | NA | NA | 0.07 |
| SANS, mean ± SD | NA | 7.6 ± 4.6 | 7.5 ± 5.5 | NA | NA | 1.0 |
| Diagnosis, affective group | NA | 13 (24.1) | 10 (32.2) | NA | NA | 0.7 |
| Diagnosis details | NA | NA | 0.2 | |||
| Schizophrenia | NA | 30 (55.6) | 17 (54.8) | |||
| Schizoaffective disorder | NA | 5 (9.3) | 3 (9.7) | |||
| Schizophreniform disorder | NA | 3 (5.6) | 0 (0.0) | |||
| Bipolar disorder with psychotic features | NA | 8 (14.8) | 10 (32.2) | |||
| Major depressive disorder with psychotic features | NA | 5 (9.3) | 0 (0.0) | |||
| Other psychotic disorder, not otherwise specified | NA | 3 (5.6) | 1 (3.2) | |||
CPZ = chlorpromazine equivalent dose; NA = not applicable; SANS = Scale for the Assessment of Negative Symptoms; SAPS = Scale for the Assessment of Positive Symptoms; SD = standard deviation.
Unless indicated otherwise.
In comparisons of demographic characteristics across groups, we observed no differences in age. Higher proportions of males were present in the relapse and no-relapse groups, compared with the control group. The no-relapse group included more White people than the control group (Table 1). No differences in demographic data were observed between the no-relapse and relapse groups. All these demographic variables were adjusted for in the analyses of functional connectivity. We observed shorter duration of illness and lower chlorpromazine equivalent doses among patients in the no-relapse group, compared with the relapse group (Table 1). The relapse group had a higher mean value of the SAPS total score than the no-relapse group, although this difference did not reach statistical significance. When dividing patients into affective (n = 23) and nonaffective (n = 55) groups, we did not observe any significant difference in the proportion of the no-relapse and relapse patients (13 affective and 35 non-affective patients in the no-relapse group and 10 affective and 20 non-affective patients in the relapse group) (Table 1).
Functional connectivity
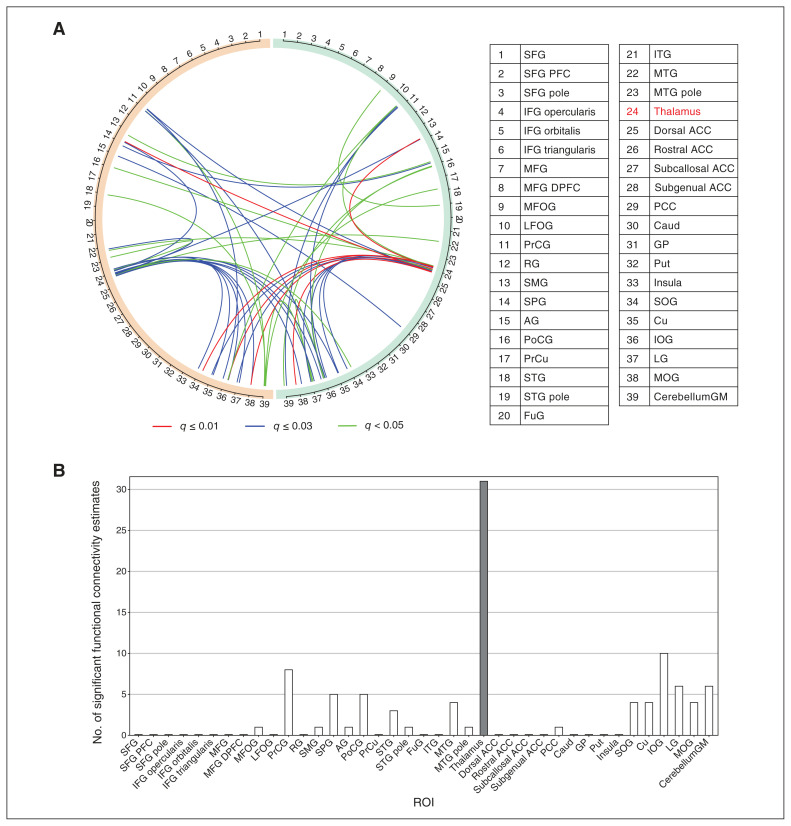
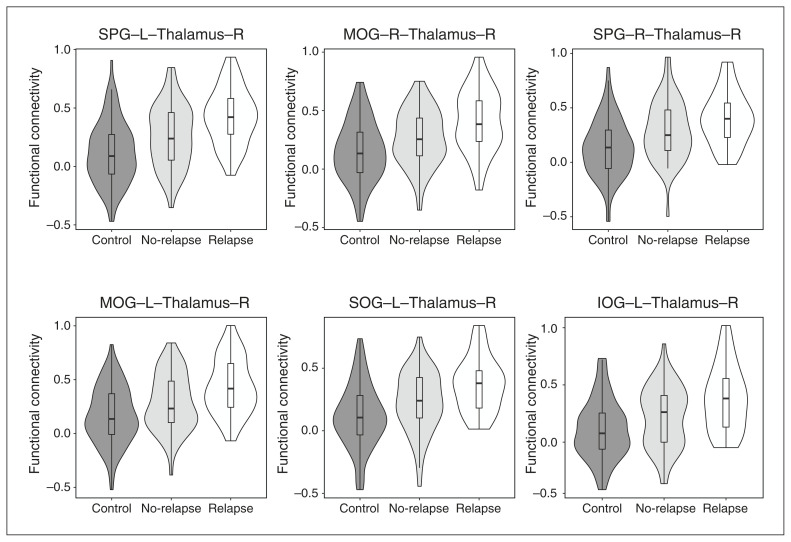
We hypothesized that more robust pathological signatures of functional connectivity would exist in the comparison between the relapse and control groups, in contrast to that between the no-relapse and control groups, as a reflection of relapse-related pathology. To test this hypothesis, we compared functional connectivity data derived from rs-fMRI of the control, no-relapse, and relapse groups. Before correction for multiple comparisons, 383 functional connectivity estimates were different between the relapse and control groups (the relapse/control comparison), whereas 143 and 23 estimates were different in the no-relapse/control and relapse/no-relapse comparisons, respectively. Consistent with our hypothesis, we observed 47 significant differences in functional connectivity in the relapse/control comparison after corrections for multiple comparisons (Figure 1A and Appendix 1, Figure S1), whereas no differences were found in other group comparisons. Interestingly, most pathological differences in functional connectivity (30 of 47) involved the thalamus (Figure 1B). We further performed the Jonckheere–Terpstra test and found that all these differences had a significant cross-group progression from controls to no-relapse patients to relapse patients (Figure 2 and Appendix 1, Figure S2, Table S2).
Figure 1.
Altered functional connectivity in the relapse group, compared with the control group. (A) Visualization of 47 significant functional connectivity estimates. Orange and green semicircles represent the left and right brain hemispheres, respectively. Numbers on the semicircles denote regions of interest (ROIs). The thalamus, number 24, is highlighted in red. Lines between numbers represent functional connectivity. Estimates with q-values smaller than 0.01, 0.01–0.03, or 0.03–0.05 are in red, blue, or green, respectively. (B) Number of significant functional connectivity estimates of each ROI. Most estimates (30 of 47) involved the thalamus (highlighted with a dark bar). Note: ACC = anterior cingulate cortex; AG = angular gyrus; Caud = caudate nucleus; CerebellumGM = cerebellum grey matter; Cu = cuneus; FuG = fusiform gyrus; GP = globus pallidus; IFG = inferior frontal gyrus; IOG = inferior occipital gyrus; ITG = inferior temporal gyrus; LFOG = lateral fronto-orbital gyrus; LG = lingual gyrus; MFG = middle frontal gyrus; DPFC = dorsolateral prefrontal cortex; MFOG = middle fronto-orbital gyrus; MOG = middle occipital gyrus; MTG = middle temporal gyrus; PCC = posterior cingulate cortex; PFC = prefrontal cortex; PoCG = postcentral gyrus; PrCG = precentral gyrus; PrCu = precuneus; Put = putamen; RG = gyrus rectus; SMG = supramarginal gyrus; SOG = superior occipital gyrus; SPG = superior frontal gyrus; STG = superior temporal gyrus.
Figure 2.
Top 6 significant functional connectivity estimates (ranked based on p values) altered between the relapse and healthy control groups. Note: IOG = inferior occipital gyrus; L = left; MOG = middle occipital gyrus; R = right; SOG = superior occipital gyrus; SPG = superior frontal gyrus.
Confounding effects of clinical variables
Clinical variables (i.e., chlorpromazine equivalent dose, duration of illness, and SAPS and SANS total scores) may potentially confound the group comparison results of functional connectivity. Therefore, we tested the correlation between clinical variables and those 47 functional connectivity estimates that were significantly different between the relapse and control groups. No significant correlation was identified in the entire patient group (Appendix 1, Table S3), suggesting that altered functional connectivity signatures may more directly reflect the disease-associated changes among patients who experienced relapse.
Discussion
We evaluated neuroimaging alterations associated with relapse among patients with early-stage psychosis. We observed significant differences in functional neuronal connectivity, particularly involving the thalamus, between patients who experienced relapse in early-stage psychosis and healthy controls. Several clinical factors, such as duration of illness, antipsychotic medication, and symptomatic features, do not seem to affect this conclusion.
The staging model is a potential model of disease progression of psychotic disorders.16 When a diffusion spectrum imaging data set was analyzed using this model, structural connectivity among patients with first-episode psychosis (stage II) was similar to that of controls, whereas significant differences were observed between patients who relapsed and those with incomplete remission (stage III).16 This study reported a decreasing trend of global and nodal brain efficiency from healthy controls, to patients in stage II, to patients in stage III. Consistently, using a Jonckheere–Terpstra test, we also observed a significant cross-group progression from healthy controls, from healthy controls to patients in the no-relapse group to patients in the relapse group. Meanwhile, we did not observe any difference between the control and no-relapse groups, as well as between the no-relapse and relapse groups. The difference between the farthest groups ( relapse and control) was significant, while the changes from the control to the no-relapse group and from the no-relapse group to the relapse group were in the same direction with smaller effect sizes that did not reach statitsical significance. Taken together, these results may highlight progressive brain changes along the psychosis disease trajectory, particularly in association with relapse.
We observed that the thalamus was an important brain region that may be associated with relapse in early-stage psychosis. Aberrant functional connectivity involving the thalamus has been reproducibly reported among patients with psychosis,25,40–43 reflecting the role of the thalamus in connecting sensory and cognitive processes. We observed differences in functional connectivity between the thalamus and cerebral cortex, such as the parietal, occipital, and temporal lobes. Among these, we pay particular attention to the superior parietal gyrus in conjunction with the thalamus because of its implication in several cognitive domains in association with the role of the prefrontal cortex among patients with schizophrenia.44–46
Limitations
Relapse is a complex condition to which many factors — such as antipsychotic medication cessation, substance abuse, and stressful life events — may contribute. Thus, we emphasize the importance of longitudinal studies with a larger sample size to validate the neuronal connectivity differences reported here. Although the global brain signal was corrected during processing of the rs-fMRI data using MRICloud, some studies have shown that the global signal may reflect neural functions beyond its conventional recognition as a noise.47 Thus, future analyses without the global correction may also be considered. We interpreted hospitalization as an end point for symptom exacerbation, but other factors such as suicidality (v. psychotic symptoms) and family pressure may play a role in symptom exacerbation and relapse. However, a systematic review of relapse in schizophrenia reported that hospitalization was the most widely used factor as a proxy for relapse by representing symptom exacerbation (62% of publications).21 The merit of using hospitalization is that it is objective, scalable, quantitative, and useful as a proxy for large multi-institutional studies, including those using a cross-sectional design.20 Taken together, in this cross-sectional study, which we expect to be a prototype for scalable and multi-institutional projects (including data from multiple countries), we used the proxy of relapse to study brain alternations specifically found in a subgroup of patients in early-stage psychosis who experienced relapse.
Conclusion
By employing rs-fMRI, we identified changes in pathological neuronal connectivity linked to relapse in early-stage psychosis. In particular, we found that functional connectivity estimates involving the thalamus were significantly enriched in these pathological signatures, characterized by increased correlation in activity patterns between the thalamus and other brain regions in the relapse group compared with the control group. These findings imply neuronal hyperactivity following relapse. Our study underscores the importance of further research to investigate the potential disruptions that relapse may induce in the brain.
Acknowledgements
The authors acknowledge the contributions of Ms. Yukiko Lema, Dr. Koko Ishizuka, and Dr. Mellisa A. Landek-Salgado.
Footnotes
Competing interests: Frederik Nucifora reports participation on an advisory board with Newron Pharmaceuticals. No other competing interests were declared.
Contributors: Akira Sawa conceived and design this study. Kun Yang designed the analytic strategy. Marina Mihaljevic reviewed patients’ records and defined the relapse and no-relapse groups. Anna Ross, Rebecca Schaub, Nicola Cascella, Jennifer Coughlin, Gerald Nestadt, Frederik Nucifora, Thomas Sedlak, and Andreia Faria contributed to data acquisition. Marina Mihaljevic, Anisha Nagpal, Semra Etyemez, Zui Narita, and Kun Yang contributed to data analysis. Vince Calhoun and Andreia Faria contributed to the interpretation of imaging data. Marina Mihaljevic, Anisha Nagpal, Kun Yang, and Akira Sawa drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Funding: This study is supported by grants from the National Institutes of Health (MH-092443, MH-094268, MH-105660, and MH-107730, to Akira Sawa); foundation grants from Stanley and RUSK/S-R (to Akira Sawa), an award from Brain and Behavior Research Foundation (to Kun Yang), and a Fulbright fellowship (to Marina Mihaljevic). Study recruitment was in part funded by Mitsubishi Tanabe Pharma Corporation.
References
- 1.Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet 2016;388:86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn RS, Sommer IE, Murray RM, et al. Schizophrenia. Nat Rev Dis Primers 2015;1:15067. [DOI] [PubMed] [Google Scholar]
- 3.Suvisaari J, Mantere O, Keinänen J, et al. Is it possible to predict the future in first-episode psychosis? Front Psychiatry 2018;9:580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wunderink L, van Bebber J, Sytema S, et al. Negative symptoms predict high relapse rates and both predict less favorable functional outcome in first episode psychosis, independent of treatment strategy. Schizophr Res 2020;216:192–9. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez-Jimenez M, Priede A, Hetrick SE, et al. Risk factors for relapse following treatment for first episode psychosis: a systematic review and meta-analysis of longitudinal studies. Schizophr Res 2012;139:116–28. [DOI] [PubMed] [Google Scholar]
- 6.Bowtell M, Ratheesh A, McGorry P, et al. Clinical and demographic predictors of continuing remission or relapse following discontinuation of antipsychotic medication after a first episode of psychosis. A systematic review. Schizophr Res 2018;197:9–18. [DOI] [PubMed] [Google Scholar]
- 7.Caseiro O, Pérez-Iglesias R, Mata I, et al. Predicting relapse after a first episode of non-affective psychosis: a three-year follow-up study. J Psychiatr Res 2012;46:1099–105. [DOI] [PubMed] [Google Scholar]
- 8.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A 2009;106:1279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry 2012;169:1092–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandl F, Avram M, Weise B, et al. Specific substantial dysconnectivity in schizophrenia: a transdiagnostic multimodal meta-analysis of resting-state functional and structural magnetic resonance imaging studies. Biol Psychiatry 2019;85:573–83. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Zeng L-L, Chen Y, et al. Evidence of a dissociation pattern in default mode subnetwork functional connectivity in schizophrenia. Sci Rep 2015;5:14655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palaniyappan L, White TP, Liddle PF. The concept of salience network dysfunction in schizophrenia: from neuroimaging observations to therapeutic opportunities. Curr Top Med Chem 2012;12: 2324–38. [DOI] [PubMed] [Google Scholar]
- 13.Sarpal DK, Robinson DG, Lencz T, et al. Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry 2015;72:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganella EP, Seguin C, Pantelis C, et al. Resting-state functional brain networks in first-episode psychosis: A 12-month follow-up study. Aust N Z J Psychiatry 2018;52:864–75. [DOI] [PubMed] [Google Scholar]
- 15.Andreasen NC, Liu D, Ziebell S, et al. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. Am J Psychiatry 2013;170:609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffa A, Baumann PS, Klauser P, et al. Brain connectivity alterations in early psychosis: from clinical to neuroimaging staging. Transl Psychiatry 2019;9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubio JM, Lencz T, Barber A, et al. Striatal functional connectivity in psychosis relapse: a hypothesis generating study. Schizophr Res 2022;243:342–8. [DOI] [PubMed] [Google Scholar]
- 18.Voineskos AN, Mulsant BH, Dickie EW, et al. Effects of antipsychotic medication on brain structure in patients with major depressive disorder and psychotic features: neuroimaging findings in the context of a randomized placebo-controlled clinical trial. JAMA Psychiatry 2020;77:674–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Csernansky JG, Mahmoud R, Brenner R, et al. A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med 2002;346:16–22. [DOI] [PubMed] [Google Scholar]
- 20.Addington DE, Patten SB, McKenzie E, et al. Relationship between relapse and hospitalization in first-episode psychosis. Psychiatr Serv 2013;64:796–9. [DOI] [PubMed] [Google Scholar]
- 21.Olivares JM, Sermon J, Hemels M, et al. Definitions and drivers of relapse in patients with schizophrenia: a systematic literature review. Ann Gen Psychiatry 2013;12:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamath V, Lasutschinkow P, Ishizuka K, et al. Olfactory functioning in first-episode psychosis. Schizophr Bull 2018;44:672–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang AM, Pradhan S, Coughlin JM, et al. Assessing brain metabolism with 7-T proton magnetic resonance spectroscopy in patients with first-episode psychosis. JAMA Psychiatry 2019;76:314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narita Z, Yang K, Kuga H, et al. Face processing of social cognition in patients with first episode psychosis: its deficits and association with the right subcallosal anterior cingulate cortex. Schizophr Res 2021;238:99–107. [DOI] [PubMed] [Google Scholar]
- 25.Faria AV, Zhao Y, Ye C, et al. Multimodal MRI assessment for first episode psychosis: a major change in the thalamus and an efficient stratification of a subgroup. Hum Brain Mapp 2021;42:1034–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang K, Longo L, Narita Z, et al. A multimodal study of a first episode psychosis cohort: potential markers of antipsychotic treatment resistance. Mol Psychiatry 2022;27:1184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kübler U. Structured clinical interview for DSM-IV (SCID). In: Gellman MD, Turner JR, editors. Encyclopedia of behavioral medicine. New York: Springer; 2013:1919–20. Available: 10.1007/978-1-4419-1005-9_66 (accessed 2021 Feb. 10). [DOI] [Google Scholar]
- 28.Schretlen DJ, Winicki JM, Meyer SM, et al. Development, psychometric properties, and validity of the hopkins adult reading test (HART). Clin Neuropsychol 2009;23:926–43. [DOI] [PubMed] [Google Scholar]
- 29.Bhattacharyya S, Schoeler T, Di Forti M, et al. Stressful life events and relapse of psychosis: analysis of causal association in a 2-year prospective observational cohort of individuals with first-episode psychosis in the UK. Lancet Psychiatry 2023;10:414–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leucht S, Samara M, Heres S, et al. Dose equivalents for antipsychotic drugs: the DDD method. Schizophr Bull 2016;42(Suppl 1):S90–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mori S, Wu D, Ceritoglu C, et al. MRICloud: delivering high-throughput MRI neuroinformatics as Cloud-based software as a service. Comput Sci Eng 2016;18:21–35. [Google Scholar]
- 32.Power JD, Barnes KA, Snyder AZ, et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012;59:2142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Achterberg M, van der Meulen M. Genetic and environmental influences on MRI scan quantity and quality. Dev Cogn Neurosci 2019;38:100667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeSerisy M, Musial A, Comer JS, et al. Functional connectivity of the anterior insula associated with intolerance of uncertainty in youth. Cogn Affect Behav Neurosci 2020;20:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faria AV, Liang Z, Miller MI, et al. Brain MRI pattern recognition translated to clinical scenarios. Front Neurosci 2017;11:578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faria AV, Joel SE, Zhang Y, et al. Atlas-based analysis of resting-state functional connectivity: evaluation for reproducibility and multi-modal anatomy-function correlation studies. Neuroimage 2012;61:613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang Z, He X, Ceritoglu C, et al. Evaluation of cross-protocol stability of a fully automated brain multi-atlas parcellation tool. PLoS One 2015;10:e0133533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bewick V, Cheek L, Ball J. Statistics review 10: further nonparametric methods. Crit Care 2004;8:196–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol 2014;67:850–7. [DOI] [PubMed] [Google Scholar]
- 40.Pergola G, Selvaggi P, Trizio S, et al. The role of the thalamus in schizophrenia from a neuroimaging perspective. Neurosci Biobehav Rev 2015;54:57–75. [DOI] [PubMed] [Google Scholar]
- 41.Woodward ND, Heckers S. Mapping thalamocortical functional connectivity in chronic and early stages of psychotic disorders. Biol Psychiatry 2016;79:1016–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao H, Chén OY, Chung Y, et al. Cerebello-thalamo-cortical hyperconnectivity as a state-independent functional neural signature for psychosis prediction and characterization. Nat Commun 2018;9:3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andreasen NC. The role of the thalamus in schizophrenia. Can J Psychiatry 1997;42:27–33. [DOI] [PubMed] [Google Scholar]
- 44.Guo S, Kendrick KM, Yu R, et al. Key functional circuitry altered in schizophrenia involves parietal regions associated with sense of self. Hum Brain Mapp 2014;35:123–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen X, Jiang F, Fang X, et al. Cognitive dysfunction and cortical structural abnormalities in first-episode drug-naïve schizophrenia patients with auditory verbal hallucination. Front Psychiatry 2022;13:998807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen J, Kim W-S, Tsogt U, et al. Neuronal signatures of anger and fear in patients with psychosis. Psychiatry Res Neuroimaging 2023; 333:111658. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J, Northoff G. Beyond noise to function: reframing the global brain activity and its dynamic topography. Commun Biol 2022;5:1350. [DOI] [PMC free article] [PubMed] [Google Scholar]