Abstract
Purpose
To retrospectively assess the time course of complications after image-guided small renal mass biopsy using initial follow-up imaging.
Materials and methods
A total of 190 masses (mean, 2.1 ± 0.70 cm; range, 0.6–3.8 cm) were assessed using initial computed tomography (43 non-enhanced and 141 enhanced) or magnetic resonance imaging (five non-enhanced and one enhanced) after biopsy. Initial follow-up imaging was classified into two groups (i.e., with or without hematoma) and various factors were compared.
Results
The masses were histologically diagnosed in all patients except one. Post-procedural complications included 129 Grade I hematomas, 1 Grade I hemothorax, 9 Grade II hematomas, and 1 Grade IIIa pneumothorax. Residual 28 Grade I and 6 Grade II hematomas and 8 new complications (6 small hematomas, 1 pseudoaneurysm, and 1 arteriovenous fistula) were observed on the initial follow-up imaging obtained at a median of 21 days (3–90 days) after the biopsy. On the initial follow-up imaging, the groups with and without hematoma differed significantly in the following factors: age (P = 0.04), size (P = 0.02), guided images (P < 0.01), hematoma at the end of the procedure (P < 0.01), and days after biopsy (P < 0.01). Although three masses exhibited > 25% shrinkage, no significant change was observed in mass diameter on initial follow-up imaging (mean, 2.1 ± 0.71 cm; P = 0.90).
Conclusion
Initial follow-up imaging after a biopsy revealed improvements in most of the complications, a few new complications, and an unchanged mass diameter.
Keywords: Biopsy, Imaging, Complication, Renal neoplasms
Introduction
Approximately 20% of small renal masses (i.e., ≤ 4 cm) are benign [1], and it is crucial to avoid unnecessary surgical resection and local ablative therapy (e.g., cryoablation and radiofrequency ablation). When small renal masses are difficult to diagnose using imaging studies before surgery or when local ablative therapy is planned, an image-guided needle biopsy is performed for histological evaluation [2, 3].
Image-guided needle biopsy for renal masses was previously contraindicated due to its low diagnostic performance and the risk of some complications (e.g., bleeding and seeding) [4]. Conversely, recently this procedure has been frequently performed with high diagnostic yields and safety; > 90% of renal masses can be diagnosed histologically [5]. Because complications are often minor and asymptomatic in image-guided needle biopsy, no imaging follow-up is usually performed (e.g., follow-up computed tomography [CT] is performed 1 week or 1 month after biopsy). We speculated that follow-up imaging studies would show changes in the biopsy-related early complications as well as additional findings (e.g., occurrence of late complications and increase in mass diameter). However, few robust and large reports exist on post-biopsy imaging studies.
This study aimed to retrospectively assess the time course of complications on initial follow-up CT or magnetic resonance imaging (MRI) after renal mass biopsy.
Materials and methods
This study was approved by our ethics committee (approval number: KEN2202-039) and opt-out consent was obtained for the retrospective use of medical patient data. This study was conducted according to the principles of the Declaration of Helsinki. Informed consent was waived because of the retrospective use of patient data; however, written informed consent was obtained from all patients before they underwent percutaneous renal mass biopsy and imaging studies.
Patients
This study included patients who underwent percutaneous image-guided cutting needle biopsy of renal masses at our institution between April 2016 and August 2020. The inclusion criteria were as follows: (i) ≤ 4 cm renal mass (i.e., T1a renal cell carcinoma [RCC] equivalent); and (ii) after the biopsy, initial follow-up CT or MRI was conducted in the period between the next day to 90 days. The exclusion criteria were as follows: (i) patients in whom biopsy was performed for multiple renal masses during the study period; (ii) initial follow-up CT or MRI was performed after another abdominal interventional radiology procedure (e.g., transarterial embolization [TAE] and biopsy of another abdominal mass); (iii) masses for which re-biopsy after a non-diagnostic initial biopsy was performed; (iv) masses for which biopsy was performed after TAE in the same session; or (v) biopsy performed for masses with local progression after renal cryoablation.
Biopsy procedure
A biopsy was performed by 14 interventional radiologists with a median experience of 17 years (range, 5–32 years), using a coaxial system with an 18- or 20-gauge cutting needle (TEMNO Evolution, MERIT MEDICAL, UT, USA or STARCUT, TSK LABORATORY, Tochigi, Japan). Patients who were taking anticoagulants or antiplatelet medications underwent biopsy procedures either after discontinuation of these medications or after conversion to heparinization.
After confirming the target and setting the puncture route, all biopsies except one were performed either under CT fluoroscopy or ultrasound (US) guidance under local anesthesia. One biopsy was performed under the general anesthesia. Specimen acquisition was repeated until a sufficient sample was obtained for histological evaluation. After biopsy completion, complications were evaluated by US or plain conventional CT examination according to the Clavien–Dindo classification [6].
Follow-up imaging and angiography
The initial follow-up CT or MRI after the biopsy was performed in the period between the next day to 90 days. The sequence of events after a diagnosis of malignancy is shown in Fig. 1. In many patients with biopsy-proven RCC, angiography and TAE using lipiodol were performed before CT fluoroscopy-guided cryoablation to enhance tumor localization and reduce the risk of bleeding and seeding. First, a 4-F vascular sheath was placed via the common femoral artery. Next, digital subtraction angiography was performed after selecting the renal artery using a 4-F diagnostic catheter. In all TAE procedures, CT angiography was performed.
Fig. 1.
Sequence of events after a diagnosis of malignancy
Evaluation of the follow-up imaging results
Mass-related characteristics, such as size, laterality (right or left kidney), position (exophytic, parenchymal, mixed, or central), longitudinal location (upper or lower), and anteroposterior location (dorsal or ventral), were evaluated using CT or MRI performed before the biopsy. The positions and longitudinal and anteroposterior locations were categorized based on the definition by Gervais et al. [7] and Iguchi et al. [8], respectively.
The following findings were evaluated on CT or MRI: improvement in complications observed immediately after the biopsy, the occurrence of new complications not observed immediately after biopsy (i.e., delayed complications), and change in mass diameter. When angiography was performed, patients were evaluated for biopsy-related complications (e.g., occurrence of pseudoaneurysm and arteriovenous fistula [AVF]). If complications were detected, they were correlated with the initial follow-up CT or MRI findings. These images were reviewed by two board-certified diagnostic and interventional radiologists with 25 (T.I.) and 12 years (S.K.) of experience, respectively, by consensus.
Statistical analysis
Initial follow-up images were classified into two groups, with or without hematoma. Patient-, mass-, and biopsy procedure-related factors were then compared between the two groups using Fisher’s exact test for categorical variables and the Mann–Whitney U test for numerical variables. The diameters of the renal masses on pre-biopsy and initial follow-up imaging were compared using paired t tests. Statistical significance was set at P < 0.05. All statistical analyses were performed using SPSS software version 26 (IBM).
Results
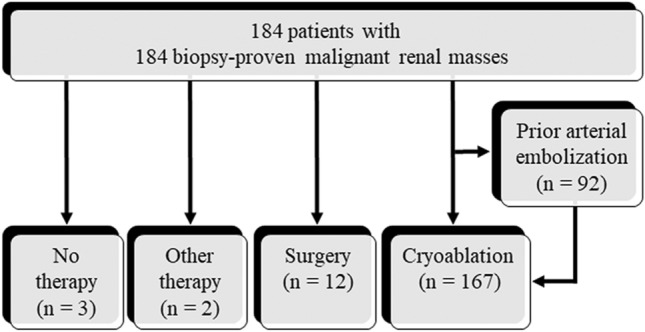
An image-guided cutting needle biopsy was performed for 342 renal masses in 327 patients at our institution between April 2016 and August 2020. Among them, 190 masses (mean diameter, 2.1 ± 0.70 [standard deviation] cm; range, 0.6–3.8 cm) in 190 patients (129 men, 61 women; mean age, 64.8 ± 13.4 years; age range, 25–91 years) were included in this study (Fig. 2). The characteristics of the 190 patients, 190 masses, and 190 biopsy procedures are summarized in Table 1.
Fig. 2.
Flow chart diagram with the number of masses
Table 1.
Characteristics of 190 patients, 190 renal masses, and 190 biopsy procedures
| Variable | Value | |
|---|---|---|
| Patient | ||
| Age (y) | Mean ± SD (range) | 64.8 ± 13.4 (25–91) |
| Sex | Man/woman | 129/61 |
| Anticoagulants or antiplatelet medications | Yes/no | 47/143 |
| Initial follow-up image | Plain CT/Contrast-enhanced CT/Dynamic CT/Plain MRI/Dynamic MRI/CT angiography scans during TAE | 43/7/98/5/1/36 |
| Renal mass | ||
| Size (cm) | Mean ± SD (range) | 2.1 ± 0.70 (0.6–3.8) |
| Laterality | Right kidney/Left kidney/Renal allograft | 102/86/2 |
| Position | Exophytic/Parenchymal/Mixed/Central | 140/10/22/18 |
| Longitudinal location | Upper/Lower | 99/91 |
| Antero-posterior location | Dorsal/Ventral | 105/85 |
| Biopsy procedure | ||
| Patient position | Prone/Supine/Lateral | 183/2/5 |
| Guiding image | Ultrasound/CT fluoroscopy (with contrast medium)/Both | 42/140 (53)/8 |
| Needle gauge | 18-gauge/20-gauge/Both | 186/3/1 |
| Number of fires | Mean ± SD (range) | 3.4 ± 1.1 (1–9) |
SD: standard deviation, CT: computed tomography, MRI: magnetic resonance imaging, TAE: transarterial embolization
CT or US immediately after the biopsy showed 130 Grade I complications (129 hematomas and 1 hemothorax), 9 Grade II hematomas, and 1 Grade IIIa pneumothorax in 138 patients. In 189 masses (99.5%), a histological diagnosis was obtained revealing 184 malignancies and 5 benign lesions (Table 2). In one non-diagnostic biopsy, the histological finding was normal tissue (i.e., renal tissue with inflammation).
Table 2.
Biopsy diagnosis of 190 renal masses
| Histological diagnosis | Number |
|---|---|
| Malignancy | |
| Renal cell carcinoma | 180 |
| Metastasis | 3 |
| Unclassified carcinoma | 1 |
| Benign lesion | |
| Angiomyolipoma | 1 |
| Cyst | 1 |
| Oncocytoma | 1 |
| Papillary neoplasia | 1 |
| Renomedullary interstitial cell tumor | 1 |
| Non-diagnosis | |
| Renal tissue with inflammation | 1 |
Initial follow-up imaging was performed at a median of 21 days (3–90 days) after biopsy (Table 1). Fifty-six of 92 patients performed TAE already had initial CT or MRI before the TAE procedure. In the remaining 36 patients, initial follow-up CT was performed at the time of the TAE procedure. On the initial follow-up imaging, one hemothorax and one pneumothorax had disappeared after 21 and 33 days, respectively, and eight new complications (i.e., six small hematomas, one pseudoaneurysm, and one AVF) occurred. Of the 138 hematomas, 104 disappeared (Fig. 3), whereas 28 Grade I and 6 Grade II hematomas were reduced in size but remained. On the initial follow-up imaging, the groups with and without hematoma differed significantly in the following factors: age (P = 0.04), size (P = 0.02), guided images (P < 0.01), hematoma at the end of the procedure (P < 0.01), and days after biopsy (P < 0.01) (Table 3).
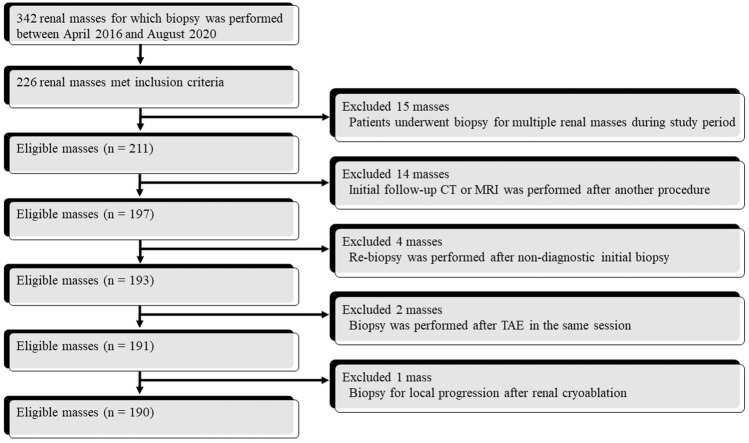
Fig. 3.
A 77-year-old woman with a renal mass in the left kidney. A Supine dynamic CT image (arterial phase) before biopsy shows a 2.1 cm renal mass (arrow). B Prone plain CT image immediately after US-guided needle biopsy shows a small hematoma (arrow). This mass is histologically diagnosed as clear cell RCC. C Prone plain CT image 25 days after biopsy shows disappearance of hematoma
Table 3.
Variables in presence and absence of hematoma on the initial follow-up image
| Hematoma | |||||
|---|---|---|---|---|---|
| Variable | Presence (n = 37) | Absence (n = 153) | P value | ||
| Patient | |||||
| Age (y) | Mean ± SD (range) | 69.3 ± 12.8 (44–91) | 63.8 ± 13.2 (25–86) | 0.04 | |
| Sex | Man/Woman | 28/9 | 101/52 | 0.33 | |
| Post-biopsy anticoagulant or platelet medication | Yes/No | 11/26 | 36/117 | 0.52 | |
| Initial follow-up image days after biopsy | Mean ± SD (range) | 13.1 ± 5.3 (3–23) | 30.9 ± 18.6 (5–90) | < 0.01 | |
| Renal mass | |||||
| Size (cm) | Mean ± SD (range) | 2.3 ± 0.69 (1.1–3.6) | 2.0 ± 0.69 (0.6–3.8) | 0.02 | |
| Laterality* | Right kidney/Left kidney | 25/12 | 77/74 | 0.10 | |
| Position | Exophytic and mixed/Others | 34/3 | 128/25 | 0.30 | |
| Longitudinal location | Upper/Lower | 14/23 | 85/68 | 0.07 | |
| Antero-posterior location | Dorsal/Ventral | 25/12 | 80/73 | 0.10 | |
| Biopsy procedure | |||||
| Guiding image** | Ultrasound/CT fluoroscopy | 14/19 | 28/121 | < 0.01 | |
| Number of fires | Mean ± SD (range) | 3.4 ± 1.2 (2–9) | 3.4 ± 1.0 (1–8) | 0.62 | |
| Hematoma at the end of procedure | Presence/Absence | 34/3 | 104/49 | < 0.01 | |
| SD: standard deviation, CT: computed tomography, MRI: magnetic resonance imaging | |||||
*Two renal masses in the renal allograft were excluded from the analysis
**Eight procedures performed under both image guidance were excluded from the analysis
After the biopsy, 167 RCCs were treated with cryoablation, and 92 underwent TAE before cryoablation. Of the 92 patients who underwent angiography, 1 had a pseudoaneurysm and AVF 13 days after the biopsy, and 2 had AVFs 31 and 78 days after the biopsy (Fig. 4). Of these complications, two AVFs could not be detected on the initial follow-up CT. All pseudoaneurysms and AVFs were successfully treated with TAE using the microcoils.
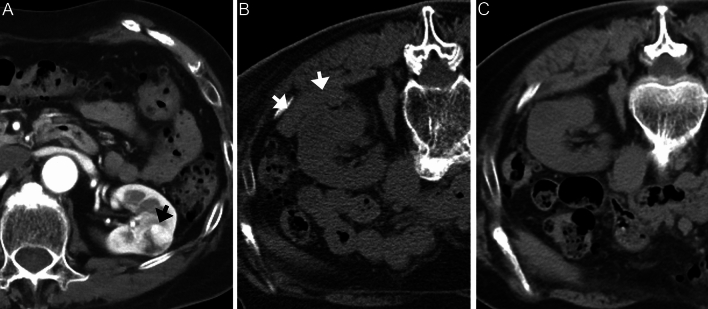
Fig. 4.
A 59-year-old man with a renal mass in the right kidney. A Supine dynamic CT image (arterial phase) before biopsy shows a 1.1 cm mass (arrow). B Prone CT fluoroscopy image with contrast medium during biopsy shows needle (arrowhead) penetration of the target mass (arrow). This mass is histologically diagnosed as clear cell RCC. C Selective renal angiography 78 days after biopsy shows AVF (arrow). D Selective renal angiography after coil embolization (arrow) shows the disappearance of AVF
No significant difference was observed in diameters of renal mass on images before biopsy and on initial follow-up imaging (mean, 2.1 ± 0.71 cm; range, 0.8–3.8 cm; P = 0.90). Initial follow-up imaging showed three masses (1.6%) with > 25% shrinkage, including two clear cell RCCs and one papillary neoplasm (Fig. 5 and Table 4). One of the three masses had a reduced contrast effect on CT images.
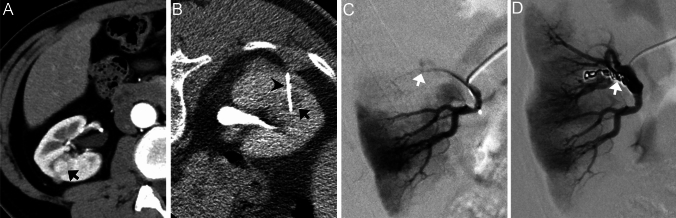
Fig. 5.
A 74-year-old woman with a cystic renal mass in the left kidney. A Coronal dynamic CT image (arterial phase) before biopsy shows a 2.3 cm mass (arrow). B Prone CT fluoroscopy image during biopsy shows needle (arrow) penetration of the mass. This mass is histologically diagnosed as clear cell RCC. C Coronal CT during arteriography image 63 days after biopsy shows that the biopsied mass shrinks (arrow). Its diameter is 1.7 cm, and the shrinkage rate is 26.1%
Table 4.
Information on three shrinking masses
| Age (y)/ Sex |
Diameter (cm) before biopsy/ initial follow-up |
Shrinkage rate (%) | Histological diagnosis |
Procedural complication | Initial follow-up imaging | ||
|---|---|---|---|---|---|---|---|
| Case | Modality | Timing | |||||
| 1 | 57/M | 1.1/0.8 | 72.7 | Clear cell RCC | Small hematoma | Dynamic CT | 29 days later |
| 2 | 74/W | 2.3/1.7 | 73.9 | Clear cell RCC | Small hematoma | CT during arteriography | 63 days later |
| 3 | 85/M | 2.0/1.2 | 60.0 | Papillary neoplasia | Small hematoma | Dynamic CT | 64 days later |
| W: woman, M: man, RCC: renal cell carcinoma, CT: computed tomography | |||||||
Discussion
This study showed that all 140 complications detected after the biopsy resolved or relieved, and 8 new complications (i.e., 6 small hematomas, 1 pseudoaneurysm, and 1 AVF) occurred on the initial follow-up CT or MRI. The initial follow-up image with the hematoma was performed at an earlier time, with a biopsy performed on a larger renal mass. The mean diameter of the renal masses did not significantly differ between the pre-biopsy and initial follow-up imaging; however, three masses (1.6%) shrank by more than 25% after the biopsy.
A high level of safety must be ensured for aggressive renal mass biopsies. In a systematic review of 5,228 patients, dissemination was observed in only two cases, and other complications were mainly back pain and hematoma/hematuria, most of which did not require any treatment [5]. The most common complication of a biopsy is hematoma, and its reported frequency varies (i.e., 8.5–67.9%) [8–10]. The CT was more sensitive than the US for detecting small and post-biopsy hematomas [11]. Our initial follow-up imaging showed six small new hematomas. These might not be visible on US or were negligible immediately after the biopsy and may have subsequently enlarged. Most biopsy-related hematomas are minor and asymptomatic; therefore, follow-up imaging may not usually be possible. In our study, such small hematomas spontaneously improved without treatment (hematomas were less likely to be observed over time), suggesting that strict imaging follow-up may not be necessary.
Pseudoaneurysms and AVF are rare vascular complications of renal mass biopsies. Pseudoaneurysm is generally related to renal parenchymal biopsy, nephrectomy, renal transplantation, or percutaneous procedures [12]. Renal AVFs can also result from renal parenchymal biopsy, trauma, inflammation, surgery, masses, and atherosclerosis [13]. Sosa-Barrios et al. reported that observation using color Doppler US after renal parenchymal biopsy of native kidneys showed AVF in 5.2% of patients, and 95% of them were asymptomatic [14]. The gold standard for diagnosis is angiography [15], and only one of our three AVFs could be detected on dynamic CT. In patients with renal AVF, possible symptoms include hematuria, hypertension, flank pain, vascular murmur in the renal arteries, heart failure, and decreased renal function due to hemodynamic changes [13, 14]. If patients are asymptomatic after renal mass biopsy, no follow-up imaging (e.g., color Doppler US and angiography) will be performed to diagnose the pseudoaneurysm and AVF, and it may be difficult to detect these completely on CT and MRI. All three patients with AVF were asymptomatic, and it is possible that there were other unnoticed AVFs. In our study, the frequencies of pseudoaneurysms and AVF were 1.1% and 3.3%, respectively, in patients who underwent angiography. More AVFs could have been detected if all patients had undergone angiography, because only 48.4% (92/190 patients) underwent angiography after the biopsy. In addition, it is important to perform color Doppler US for the diagnosis of renal AVF before angiography.
In our study, none of the 190 masses were enlarged (> 25%) on the initial follow-up imaging within 90 days. One active surveillance from multicenter research showed that the mean annual RCC growth rate for the entire cohort was 0.25 cm/year: masses that are ≥ 2.45 cm in the largest diameter at diagnosis grow faster than smaller masses [16]. The incidence of spontaneous regression of primary RCC is unknown; however, some English case reports have been published [17–19]. Hypothetical mechanisms for its spontaneous regression were suggested as following: humoral, immunological, and vascular factors (e.g., autoinfarction) [17]. The incidence of mass shrinkage after renal biopsy is unknown and was 1.6% (3/190 masses) in this study. One of the three masses showed a reduced contrast effect. Possible reasons for renal mass shrinkage after the biopsy include the follows: i) the mass is an inflammation-related change, not a neoplasm; ii) biopsy causes injury to feeding arteries resulting in ischemia/infarction; or iii) spontaneous regression of the mass.
This retrospective study conducted at a single institution has several limitations. First, the timing of the initial imaging follow-up was not uniform. Second, the protocol for the initial imaging follow-up was not standardized (e.g., CT or MRI or plain, contrast-enhanced, or dynamic study). Prospective follow-up with a more unified protocol may resolve these limitations; however, excessive imaging follow-up would be unnecessary because most complications are minor, asymptomatic, and improve spontaneously. Third, unenhanced CT or MRI may have overlooked pseudoaneurysms and AVFs. Finally, although 92 angiographies showed 1 pseudoaneurysm and 3 AVFs, angiography was not performed before the biopsy. Therefore, biopsy-related changes were not accurately assessed.
In conclusion, the initial follow-up imaging after the biopsy of small renal masses showed improvements in most of the complications, a few new complications, and an unchanged mass diameter.
Funding
This study was not supported by any funding.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study design was approved by the appropriate ethics review board.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Remzi M, Ozsoy M, Klingler HC, Susani M, Waldert M, Seitz C, et al. Are small renal tumors harmless? Analysis of histopathological features according to tumors 4 cm or less in diameter. J Urol. 2006;176:896–899. doi: 10.1016/j.juro.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 2.Amaral BS, Macek P, Arora A, Pazeto CL, Zugail AS, Mombet A, et al. Renal tumor biopsy: rationale to avoid surgery in small renal masses. Curr Urol Rep. 2021;22:46. doi: 10.1007/s11934-021-01064-8. [DOI] [PubMed] [Google Scholar]
- 3.Iguchi T, Hiraki T, Gobara H, Fujiwara H, Sakurai J, Matsui Y, et al. Value of percutaneous needle biopsy of small renal tumors in patients referred for cryoablation. Minim Invasive Ther Allied Technol. 2017;26:86–91. doi: 10.1080/13645706.2016.1249889. [DOI] [PubMed] [Google Scholar]
- 4.Ortiz-Alvarado O, Anderson JK. The role of radiologic imaging and biopsy in renal tumor ablation. World J Urol. 2010;28:551–557. doi: 10.1007/s00345-010-0549-z. [DOI] [PubMed] [Google Scholar]
- 5.Marconi L, Dabestani S, Lam TB, Hofmann F, Stewart F, Norrie J, et al. Systematic review and meta-analysis of diagnostic accuracy of percutaneous renal tumour biopsy. Eur Urol. 2016;69:660–673. doi: 10.1016/j.eururo.2015.07.072. [DOI] [PubMed] [Google Scholar]
- 6.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Renal cell carcinoma: clinical experience and technical success with radio-frequency ablation of 42 tumors. Radiology. 2003;226:417–424. doi: 10.1148/radiol.2262012062. [DOI] [PubMed] [Google Scholar]
- 8.Iguchi T, Hiraki T, Matsui Y, Tomita K, Uka M, Tanaka T, et al. Image-guided core biopsy of 2-cm or smaller renal tumors. Diagn Interv Imaging. 2020;101:715–720. doi: 10.1016/j.diii.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Richard PO, Jewett MA, Bhatt JR, Kachura JR, Evans AJ, Zlotta AR, et al. Renal tumor biopsy for small renal masses: a single-center 13-year experience. Eur Urol. 2015;68:1007–1013. doi: 10.1016/j.eururo.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Iguchi T, Hiraki T, Matsui Y, Fujiwara H, Sakurai J, Masaoka Y, et al. CT fluoroscopy-guided renal tumor cutting needle biopsy: retrospective evaluation of diagnostic yield, safety, and risk factors for diagnostic failure. Eur Radiol. 2018;28:283–290. doi: 10.1007/s00330-017-4969-7. [DOI] [PubMed] [Google Scholar]
- 11.Seager MJ, Patel U, Anderson CJ, Gonsalves M. Image-guided biopsy of small (≤ 4 cm) renal masses: the effect of size and anatomical location on biopsy success rate and complications. Br J Radiol. 2018;91:20170666. doi: 10.1259/bjr.20170666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roman LI, Feel CF, França VT, Merten CM, Dummer CD. Renal artery pseudoaneurysm J Bras Nefrol. 2017;39:458–461. doi: 10.5935/0101-2800.20170080. [DOI] [PubMed] [Google Scholar]
- 13.Belczak SQ, Pedroso GD, Atihe LF, Vilela ABF, Melice RS, Benedito C, Marques GG. Renal arteriovenous fistula after renal biopsy: a case report and literature review. J Vasc Bras. 2019;18:e20180112. doi: 10.1590/1677-5449.011218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sosa-Barrios RH, Burguera V, Rodriguez-Mendiola N, Galeano C, Elias S, Ruiz-Roso G, et al. Arteriovenous fistulae after renal biopsy: diagnosis and outcomes using Doppler ultrasound assessment. BMC Nephrol. 2017;18:365. doi: 10.1186/s12882-017-0786-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lubomirova M, Krasteva R, Bogov B, Paskalev E. Incidence of A-V fistulas after renal biopsy of native and transplanted kidney-two centers experience. Maced J Med Sci. 2015;3:241–4. doi: 10.3889/oamjms.2015.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manson RJ, Abdolell M, Trottier G, Pringle C, Lawen JG, Bell DG, et al. Growth kinetics of renal masses: analysis of prospective cohort of patients undergoing active surveillance. Eur Urol. 2011;59:863–867. doi: 10.1016/j.eururo.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi K, Sato T, Sunaoshi K, Takahashi A, Tamakawa M. Spontaneous regression of primary renal cell carcinoma with inferior vena caval tumor thrombus. J Urol. 2002;167:242–243. doi: 10.1016/S0022-5347(05)65424-9. [DOI] [PubMed] [Google Scholar]
- 18.Edwards MJ, Anderson JA, Angel JR, Harty JI. Spontaneous regression of primary and metastatic renal cell carcinoma. J Urol. 1996;155:1385. doi: 10.1016/S0022-5347(01)66275-X. [DOI] [PubMed] [Google Scholar]
- 19.Lacquaniti S, Pierconti F, Servello C, Pisanti F, Destito A. Spontaneous partial fibrotic regression of a primary renal carcinoma: a case report. Arch Ital Urol Androl. 1999;71:35–36. [PubMed] [Google Scholar]