Abstract
Brefeldin A (BFA), an inhibitor of intracellular vesicle-dependent secretory transport, is a potent inhibitor of poliovirus RNA replication in infected cells. We have determined that the unknown mechanism of BFA inhibition of replication is reproduced in the cell-free poliovirus translation, replication, and encapsidation system. Furthermore, we provide evidence suggesting that the cellular mechanism targeted by BFA, the GTP-dependent synthesis of secretory transport vesicles, may be involved in viral RNA replication in the system via a soluble cellular GTP-binding and -hydrolyzing activity. This activity is related to the ARF (ADP-ribosylation factor) family of GTP-binding proteins. ARFs are required for the formation of several classes of secretory vesicles, and some family members are indirectly inactivated by BFA. Peptides that function as competitive inhibitors of ARF activity in cell-free transport systems also inhibit poliovirus RNA replication, and this inhibitory effect can be countered by the addition of exogenous ARF. We suggest that BFA inhibition of replication is diagnostic of a requirement for ARF activity in the cell-free system.
In many tissue culture cell types, infection by members of the genus Enterovirus of Picornaviridae results in the lysis of the cell and release of progeny virions. The progression of intracellular events during infection leads to characteristic morphological changes of the cell, generally described as cytopathic effect. In the late stages of infection, the host cells become rounded and enlarged and detach from the substrate. The cytoskeletal elements lose their normal organization (8, 11, 40), the nucleus appears to collapse, and cellular transcription and protein synthesis essentially cease (39). The mechanisms leading to these changes of the host cell are not well understood. The viral proteinase 2Apro is thought to be responsible for the shutoff of host translation partially through cleavage of the eIF-4G subunit of the eIF-4F cap-binding complex. eIF-4F is essential for translational initiation of capped cellular mRNAs (43). The other viral proteinase 3Cpro (or perhaps its precursor, 3CDpro) has been shown to cleave the TATA-binding protein (59) and the cyclic AMP-responsive element-binding protein (58), ostensibly resulting in a decrease in cellular transcription. 3Cpro also cleaves the microtubule-associated protein 4 (21), a phenomenon that may result in the destruction of the cytoskeletal system. In all, approximately 14 cellular proteins have been shown to be down-regulated or degraded in poliovirus-infected cells (54), although the identities of most of these targets remain obscure.
Perhaps one of the most dramatic changes in the organization of cellular ultrastructure is the rearrangement of the intracellular membranous organelles of the secretory system (Golgi complex and endoplasmic reticulum [ER]) and the formation of vesicular structures associated with viral RNA replication (5, 8). The perinuclear region of the cell becomes crowded with membranous vesicles of heterogeneous sizes. These virus-induced membranous structures are studded with the viral nonstructural proteins 2BC, 2B and 2C, which are found exclusively in this region of the infected cell (5, 6, 9). In addition, a membranous fraction of infected cells (known in its isolated form as the crude replication complex) has been shown to contain the genomic RNA and all of the nonstructural viral peptides. The crude replication complex is fully active in the initiation and synthesis of authentic viral RNA when isolated biochemically (8, 9, 45–48). In situ hybridization studies have also revealed the presence of viral RNA in this fraction (7, 52). Although there is no direct evidence that the vesicularization of intracellular membranes is a requirement for efficient genomic replication, it seems clear that this fraction of the infected cell constitutes a viral factory in which new RNA synthesis and the assembly of progeny virions take place.
The exact nature of the membranous vesicles is obscure, as is the mechanism underlying their generation. Characterization of the virally induced vesicles with immunological probes has demonstrated the presence of cellular markers of the ER, Golgi complex, and lysosomes (42). The structure of the vesicles has usually been described as generally spherical (6, 8), and in recent work they also appear to contain invaginated membranes reminiscent of autophagic vacuoles (42). Bienz et al. (9) have isolated assemblages of vesicles from infected cells in the form of rosette-like structures around electron-dense material that presumably constitutes the viral replication complex. At low temperature and in low-ionic-strength buffer, these rosettes disassemble into individual vesicles with viral products on the cytoplasmic face of tubular membranous protrusions; the dissociated rosettes are still functional in the in vitro synthesis of viral RNA (16), an observation suggesting that the rosette structure is not absolutely necessary to allow the replication complex to function in vitro.
Infection by poliovirus has been shown to cause a powerful block in the secretory transport of the cell, mediated by the 3A and 2B proteins (12). In the absence of the other poliovirus proteins, expression of 2C and 2BC from vaccinia virus vectors causes (i) the accumulation of membranous vesicles reminiscent of those seen in infected cells and (ii) the disappearance of the Golgi complex (10). 2BC has similar effects in yeast (2). The expression of 2B also results in disassembly of the Golgi complex (40). Collectively, these observations suggest that infection, through the activities of the viral proteins in question, causes the secretory pathway of the cell to shut down. It has been suggested that this effect may have evolved to overcome organismal antiviral responses (secretion of interferons and presentation of viral antigens by the major histocompatibility complex I) (12).
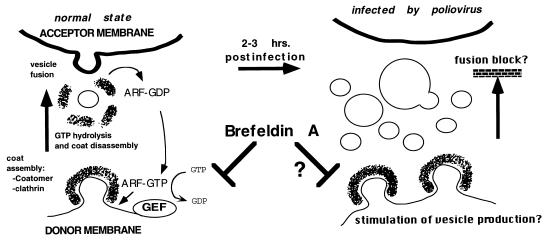
Paradoxically, there appears to be a requirement for some aspects of secretory function in the replication of poliovirus. This has been inferred by the inhibition of poliovirus replication by the fungal metabolite brefeldin A (BFA) (20, 30, 53), a drug usually characterized as an inhibitor of membrane traffic in the normal cells. BFA is a potent inhibitor of viral RNA synthesis in the infected cell, but not of entry, translation, or morphogenesis (20, 30, 53). This effect is dependent on a host-cell function, since replication proceeds normally in resistant cell lines, and attempts to isolate resistant poliovirus mutants have failed (11). The best-studied effect of BFA on the normal cell is the inhibition of vesicle-dependent transport at various stages in the secretory pathway, destroying the native functions of the Golgi complex, endosomes, and lysosomes (19, 25, 27, 57). BFA was ultimately shown to prevent the activation of some members of the ADP-ribosylation factor (ARF) family, a group of polypeptides that are key regulators in the synthesis of secretory transport vesicles (41). ARFs comprise a family of small (21-kDa) GTP-binding cytosolic proteins that localize to membranes when bound to GTP. This event prompts the recruitment and assembly of a protein coat (composed of either the coatomer or clathrin complexes), thereby deforming the underlying membrane into a budding vesicle (14, 44, 56) which eventually results in the release of a coated vesicle. Uncoating occurs upon GTP hydrolysis (50), which requires an additional factor (29). Binding of ARF to guanosine 5′-O-(3-thiotriphosphate) (GTPγS), a nonhydrolyzable GTP analog, results in the accumulation of coated buds and vesicles (50). When hydrolysis of GTP occurs, the coat components and ARF-GDP become cytosolic, but they can be recycled by a guanosine exchange factor (ARF-GEF), resulting in new ARF-GTP that renew the budding process. BFA inhibits the exchange step (although the effect on ARF-GEF may not be direct) (13, 18), effectively segregating ARF to the cytosol and preventing vesicle formation (Fig. 1). In a cell-free system, BFA prevents the synthesis of ARF-dependent coated transport vesicles from isolated Golgi membranes (35).
FIG. 1.
Possible mechanism for BFA inhibition of poliovirus RNA replication, first proposed by Maynell et al. (30) and supported by our observations in the cell-free system. The budding of nascent transport vesicles at several steps in secretory transport is dependent on the assembly of clathrin or coatomer complexes upon the budding donor membrane face (38, 41). This assembly is catalyzed by ARF-GTP, and disassembly of the coat on the fully formed vesicle occurs upon GTP hydrolysis. ARF can be recycled by a GEF, which is inhibited by BFA. Thus, the drug is an indirect inhibitor of transport vesicle budding. Because poliovirus RNA replication is strongly associated with (but not necessarily dependent on) the vesicularization of secretory organelle membranes, this process may be dependent on the BFA-sensitive mechanism for transport vesicle synthesis. Infection may cause a stimulation in the process, and/or a block in fusion of vesicles to target membranes, causing the disassembly of these organelles and the accumulation of virally induced vesicles. If this process is required for optimal levels of RNA replication to occur, then BFA may act as an indirect inhibitor of replication in the cell-free system and in the infected cell.
The apparently conflicting observations that poliovirus induces a secretory block but at the same time is inhibited by a drug that blocks transport suggest that viral genomic replication interacts with the secretory pathway in such a way as to disrupt its overall function. Simultaneously, it appears to recruit cellular components of the secretory pathway for its own use. In this study, we used a cell-free poliovirus replication system (31) to analyze this possible requirement. The system allows the faithful synthesis and processing of the polioviral polyprotein from input viral mRNA, genomic replication, and encapsidation of newly synthesized viral RNA to yield infectious particles (3, 4, 15, 31, 32, 49, 51). We have determined that poliovirus replication in the cell-free system is inhibited by 2 mM guanidine hydrochloride, a specific inhibitor of poliovirus replication in vivo (31). Barton et al. (3) have reported that a soluble host cell fraction is required for the recovery of cell-free replication from the block induced by guanidine hydrochloride.
Here, we demonstrate that cell-free replication is sensitive to BFA, an observation suggesting that the mechanism of BFA inhibition of replication is reproduced in the system. In addition, we report evidence of a cellular activity that is required for efficient genomic replication in the cell-free system. Available evidence suggests that the cellular activity is related to ARF proteins. We have determined that a class of peptides which are believed to act as competitive inhibitors of ARF activity in the formation of coated transport vesicles (1, 23) act as specific inhibitors of cell-free poliovirus RNA replication and that this effect can be antagonized by increasing the amount of ARF protein in the system. Our results suggest that the specific cellular mechanism that is inhibited by BFA, and that is dependent on the activity of ARF proteins, is required for genomic replication in the cell-free system. We propose that the same requirement may exist in vivo.
MATERIALS AND METHODS
Preparation of cytoplasmic HeLa extract.
Cytoplasmic extract was prepared as described previously (31, 32), with slight modification. Log-phase HeLa S3 spinner cultures were grown to a density of 7 × 107 cells/ml in Dulbecco modified Eagle medium (GIBCO BRL) supplemented with 5% fetal bovine serum, harvested by centrifugation at 284 × g for 10 min at 4°C, and washed in 15 ml of cold Hanks balanced salt solution (GIBCO BRL) three times. The last wash was performed in a 15-ml graduated Falcon centrifuge tube for accurate measurement of the packed cell volume. Packed cells were resuspended in 1.5 volumes (relative to the packed cell volume) of hypotonic buffer (10 mM K-HEPES [pH 7.4], 10 mM potassium acetate, 1.5 mM magnesium acetate, 2.5 mM dithiothreitol [DTT]), incubated on ice for 10 min, and lysed with 8 to 12 strokes of a 15-ml Dounce homogenizer with type B pestle (Bellco). The degree of lysis, determined visually by phase-contrast microscopy, was approximately 80 to 90% of the original number of cells. Cell debris and nuclei were removed by centrifugation at 12,000 × g for 20 min at 4°C, and the resulting postnuclear supernatant was dialyzed (12- to 14-kDa cutoff) against 500 volumes (relative to the supernatant volume) of dialysis buffer (10 mM K-HEPES [pH 7.4], 90 mM potassium acetate, 1.5 mM magnesium acetate, 2.5 mM DTT) for 2 h at 4°C. The dialyzed lysate was then centrifuged at 9,000 × g 10 min at 4°C, and 50% glycerol (1:1 in sterile dH2O) was added to a final concentration of 10%. Treatment with micrococcal nuclease (Pharmacia) at a concentration of 38 U/ml was carried out for 15 min at room temperature in the presence of 750 μM calcium chloride and stopped with the addition of EGTA to 3 mM. Protein concentration was measured by Bradford assay and normally found to range between 9 and 10 mg/ml.
Extracts treated with GTP, GTPγS, guanosine 5′-O-(3-thiodiphosphate) (GDPβS), and β:γ imidoguanosine 5′-triphosphate (Gpp[NH]p) (all purchased from Sigma) were processed the same way, except that the postnuclear supernatant was divided into equal aliquots, and the nucleotides (resuspended in sterile distilled water to a concentration of 100 mM) were each added to final concentrations of 1 mM. In each experiment, sterile distilled water was added to one aliquot to produce a mock-treated sample. The lysates were then mixed gently and incubated at 34° for 20 min. This was followed by dialysis and processing in the usual manner.
Soluble and insoluble fractions of the completed (dialyzed, nuclease-digested, and glycerol-supplemented) mock-treated extract were produced by centrifugation at 100,000 × g at 4°C for 60 min. The supernatant (S-100) was then removed, and the pellet (P-100) was resuspended in dialysis buffer (with 10% glycerol) to a final volume equal to that of the supernatant.
Immunoblotting for ARF.
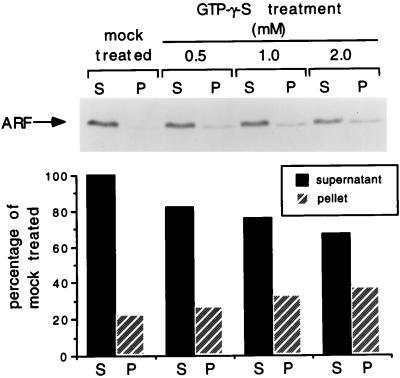
Changes in the localization of ARF due to GTPγS treatment were determined by preparing soluble and insoluble fractions of treated (using 0.5, 1.0, and 2.0 mM GTPγS) and mock-treated extract. Following dialysis, the extract was centrifuged in the usual manner, the supernatant was harvested, and an equal amount of 2× Laemmli buffer was added. The pellet was resuspended in a volume of 1× Laemmli buffer (minus bromophenol blue) equal to that of the supernatant, stirred by heavy vortexing, and boiled for 10 min, at which time all particulate matter had been completely solubilized.
The supernatant and pellet fractions (5 μl of each) were subjected to polyacrylamide gel electrophoresis (PAGE) on a sodium dodecyl sulfate (SDS)–15% polyacrylamide gel, and the protein bands were electrotransferred to a nitrocellulose membrane sheet (0.45-μm pore size) overnight. The membrane was then immunoblotted with anti-ARF monoclonal antibody 1D9 (generously provided by R. Kahn, Vanderbilt University). Detection of ARF-antibody complexes was carried out with horseradish peroxidase-conjugated anti-mouse antibody and ECL (enhanced chemiluminescence) Western blotting detection reagents (Amersham). ARF-specific bands on the resulting exposed film were analyzed by densitometry scanning (ScanAnalysis 68000; Biosoft).
Cell-free translation, RNA replication, and synthesis of poliovirus.
BFA was purchased from Epicentre Technologies, resuspended to 10 mg/ml in dimethyl sulfoxide (DMSO), and stored at 4°C. High-pressure liquid chromatography-purified ARF N-terminal peptides P-13, P-26, and P-28, a generous gift of R. Kahn, were resuspended to a final concentration of 10 mM in 10 mM K-HEPES (pH 7.4) and stored at −20°C. Purified recombinant human ARF1 (hARF1), expressed in SF9 insect cells from a baculovirus vector and therefore presumably myristoylated, was kindly provided by Andrew Morris (State University of New York at Stony Brook) and was dialyzed against a 100× volume of 10 mM K-HEPES (pH 7.4)–2.5 mM DTT for 2 h prior to use in the cell-free system.
The assembly of cell-free replication reactions was as described previously (31, 32), with slight modifications. As a preliminary step, we assembled a translation mix (TM) containing the following: 8 mM ATP (Pharmacia), 480 μM GTP (Pharmacia), 80 mM creatine phosphate (Sigma), creatine phosphokinase (200 μg/ml; Sigma), calf liver tRNA (200 μg/ml; Sigma), 10% amino acid mixture minus methionine (1 mM stock; Promega), 2 mM spermidine (pH 7.4) (Sigma), and 150 mM K-HEPES (pH 7.4). The TM was aliquoted and stored at −80°C.
Cell-free replication reactions were assembled by producing a 1.42× master mix (containing 55% cytoplasmic lysate, 18.5% TM, 545 μM magnesium acetate, 1.31 mM magnesium chloride, 159 mM potassium acetate, 0.01% complete amino acid mix, 436 U of RNase inhibitor per ml, 284 μM each CTP and UTP, and 204 μM GTP) and adding diethyl pyrocarbonate-treated distilled H2O and a concentrated stock of purified poliovirus RNA (16 μg/ml, final concentration) to 1× final master mix concentration. Final reaction volume was 12.5 μl (composed of 8.8 μl of master mix and 3.7 μl of distilled H2O, RNA stock, and other tested reagents combined). Incubation at 34°C for 12 h was followed by endpoint titration and standard plaque assay to determine resulting viral titer (31, 32).
Translation products were labeled with Tran 35S-label (ICN) by substituting the complete amino acid mixture in the final reaction with 8.8 μCi of Tran 35S-label (ICN). After incubation, the reaction mixture was diluted with 2× Laemmli buffer, boiled for 5 min, and analyzed by SDS-PAGE (12% gel) and autoradiography.
Pulse-labeling and analysis of newly synthesized RNA in the reaction were done as described by Barton et al. (3), with some modifications. Fifty microcuries of [α-32P]CTP (800 Ci/ml; ICN) was added at the 8-h time point to a reaction volume of 25 μl. After incubation for 1 h at 34°C, the reaction mixture was diluted to 200 μl with Tris-EDTA, and EDTA and SDS were added to 5 mM and 0.5%, respectively. The diluted reaction mixture was extracted with phenol-chloroform, precipitated with ethanol and ammonium acetate, and electrophoresed in 0.8% Tris-acetate-EDTA–agarose under native conditions. Under weight, the gel was flattened between sheets of blotting paper, dried, and analyzed by autoradiography.
RESULTS
Cell-free, de novo poliovirus synthesis is inhibited by BFA.
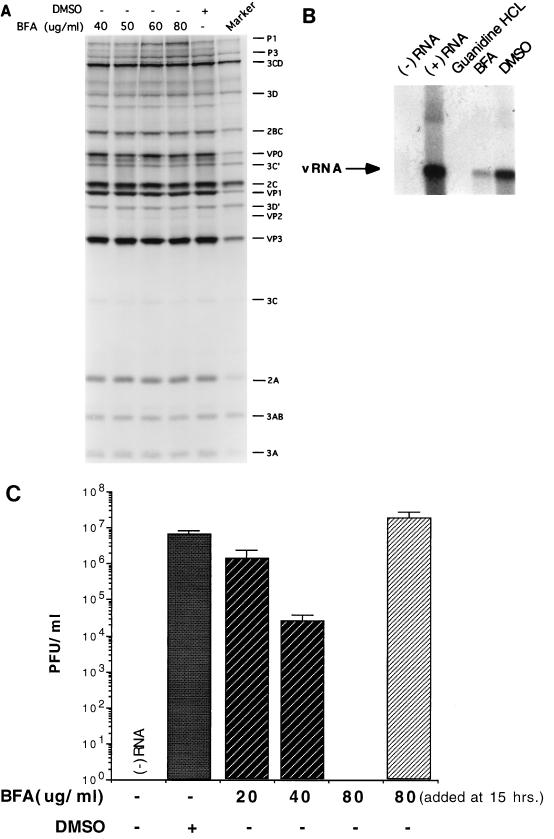
Previous work from other groups has demonstrated that the fungal metabolite BFA, an inhibitor of intracellular secretory transport, is a potent and specific inhibitor of enterovirus and rhinovirus replication in infected cells (20, 30, 53). We sought to study this effect biochemically by the use of an established cell-free replication system which recreates the entire infectious cycle of poliovirus in a cytoplasmic extract of HeLa cells (31). Addition of the drug to cell-free reactions had no effect on the translation of the input viral RNA and the processing of the resultant polyprotein up to a concentration of 80 μg/ml (Fig. 2A). However, the yield of infectious virus from such reactions was inhibited almost 10-fold starting at a BFA concentration of 20 μg/ml and was completely abrogated at 80 μg/ml. Addition of the drug at the end of incubation had no effect on viral titer (Fig. 2C).
FIG. 2.
BFA is an inhibitor of poliovirus RNA replication in the cell-free system. (A) 35S-labeled translation and processing products from the cell-free system, synthesized in the presence of increasing amounts of BFA, and resolved by SDS-PAGE (12.5% gel) and autoradiography. The DMSO-containing lane includes an amount equivalent to that in the lane with 80 μg of BFA per ml (0.8%). Size marker was produced by 35S-pulse-labeling of poliovirus-infected cells. (B) Newly synthesized viral RNA (vRNA) products from the cell-free system, pulse-labeled with [α-32P]CTP, and resolved by native agarose gel electrophoresis and autoradiography. Lanes: (−) RNA, no RNA; (+) RNA, no additions other than viral RNA; guanidine HCl, 2 mM guanidine-HCl; BFA, 80 μg of BFA per ml; brefeldin A; DMSO, 0.8% DMSO. (C) Viral yield from cell-free reactions in the presence of increasing amounts of BFA as determined by standard plaque assay; same conditions as for A. In the last reaction, BFA was added at the end of incubation.
The inhibition of viral yield by BFA is probably indicative of a block in genome replication, since the synthesis of infectious virus is completely dependent on replication, and efficient encapsidation of the input template RNA does not occur (31, 32). Moreover, the block in BFA-treated cells is related to poliovirus RNA synthesis (20, 30). Therefore, we directly monitored RNA replication in the presence of the drug. Barton et al. (3) have examined the kinetics of viral genomic replication in the cell-free system and shown that addition of [α-32P]CTP at various time points results in the accumulation of radiolabeled material with the properties of poliovirus RNA. Pulse-labeling from the 8- to 10-h time point in the presence of 80 μg of BFA per ml resulted in a severe drop in the synthesis of viral RNA, as assayed by native agarose gel electrophoresis (Fig. 2B). Thus, the sensitivity of virus yield to BFA in the cell-free system appears to be at the level of RNA synthesis. This finding suggests that the mechanism underlying the in vivo BFA effect can be reproduced in vitro. However, cell-free replication was much less sensitive to the drug than the in vivo case, where concentrations as low as 2 μg/ml are sufficient to nearly abolish viral reproduction (20, 30, 53).
Cell-free replication is dependent on a cellular GTP-binding and hydrolyzing activity.
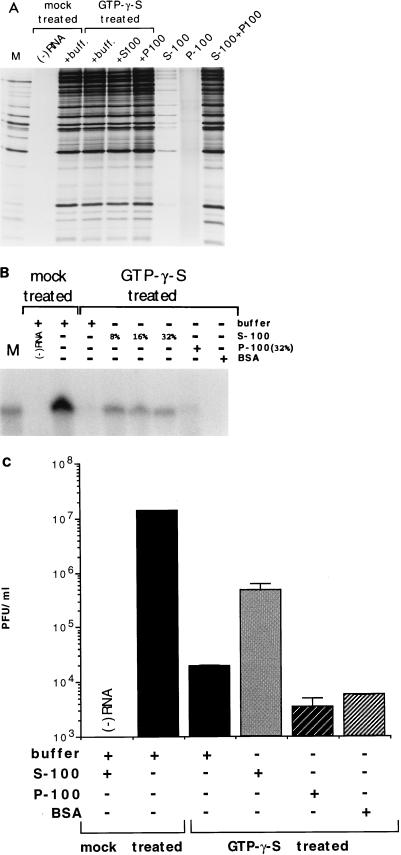
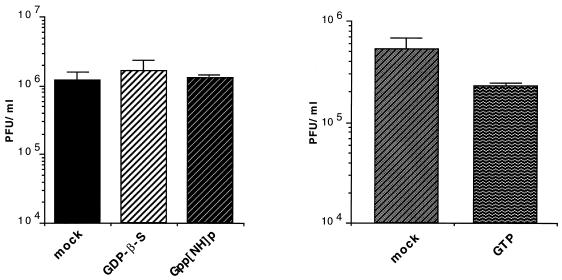
The mode of BFA inhibition of polioviral RNA replication is unknown. We considered it possible that BFA inhibition could indicate a requirement for ARF activity in RNA replication. Therefore, we designed an experiment aimed at detecting a requirement for cellular GTP-binding and -hydrolyzing activities in the cytoplasmic HeLa extract. Accordingly, the extract was incubated in the presence of 1 mM GTPγS (a nonhydrolyzable GTP analog) at 34°C for 20 min, followed by the normal dialysis step (see Materials and Methods). Insoluble aggregates that normally form during dialysis were removed by centrifugation at 8,000 × g for 10 min. The supernatant was then used in cell-free viral synthesis reactions, assaying for translation and processing, RNA replication, and yield of infectious virus. Under the conditions of the experiment, the incubation of the extract with GTPγS had no effect on translation and proteolytic processing compared to a mock-treated extract (Fig. 3A). RNA replication, however, was severely impaired, with little detectable labeled product seen from reactions containing treated extract (Fig. 3B). The viral titer was likewise reduced in such reactions, by approximately 1,000-fold (Fig. 3C). Treatment of the extract with GDPβS and Gpp[NH]p had no effect. The latter is a stable GTP analog which induces ARF membrane binding with a 10-fold-lower efficiency than GTPγS (37). Treatment with GTP caused only a twofold drop in the synthesis of virus (Fig. 4). The data indicate that the cytoplasmic extract contains a GTP-binding and -hydrolyzing activity that is partially lost or inactivated upon treatment with GTPγS, and which is required for genomic replication in the cell-free system. The lack of inhibition by Gpp[NH]p treatment suggests that a member(s) of the ARF family may be the activity in question, and the loss of function induced by treatment with GTPγS may be caused by an irreversible association of ARF with membrane.
FIG. 3.
Treatment of the HeLa cytoplasmic extract with GTPγS results in the loss or inactivation of a cellular activity involved in cell-free RNA replication. Treatment is described in Materials and Methods. Dialysis buffer was added to reactions not containing supplementary fractions. (A) 35S-labeled translation and processing products from reactions assembled with mock-treated extract, GTPγS-treated extract alone or supplemented with 0.8% S-100 or P-100, S-100 alone, P-100 alone, and a mixture of equal amounts of S-100 and P-100. Products were resolved as for Fig. 2A. M, size marker. (B) Pulse-labeled viral RNA synthesized in cell-free reactions assembled with mock-treated extract, or with GTPγS-treated extract alone or supplemented with indicated percentages of S-100, 32% P-100, and 1.6 mg of BSA per ml, equivalent to 32% S-100. Products were resolved as for Fig. 2B. (C) Viral yield from cell-free reactions assembled with mock-treated extract, GTPγS-treated extract alone or supplemented with 0.8% S-100, P-100, and BSA. Bars represent means of duplicate samples.
FIG. 4.
Treatment of extract with other forms of guanosine nucleotides has little or no effect on cell-free RNA synthesis. HeLa cytoplasmic extracts were divided evenly and treated with GDPβS, Gpp[NH]p, or GTP, or were mock treated, as detailed in Materials and Methods. Cell-free replication reactions were assembled with the resulting extracts, and viral titer was determined postincubation by standard plaque assay. Bars represent means of duplicate samples.
To determine whether the GTPγS-sensitive activity resided in a soluble or insoluble cellular fraction, the mock-treated extract was separated into S-100 and P-100 fractions. Aliquots of each fraction were then used to supplement reactions containing the GTPγS-treated extract. Addition of the S-100 at a final reaction concentration of 8% resulted in the partial recovery of viral titer from the treated extract (Fig. 3C) and, similarly, a partial recovery of RNA replication (Fig. 3B). The addition of higher levels (16 to 32%) of S-100 to the reaction did not further stimulate vRNA synthesis (Fig. 3B), an observation that we cannot explain at present. The addition of the resuspended P-100 or of equivalent (by protein content) amounts of bovine serum albumin (BSA) did not stimulate viral yield or replication. Furthermore, the S-100 had no stimulatory effect on translation and processing in such reactions, as expected. The S-100, in the absence of the normally used S-10 extract, exhibited only slight translational activity (Fig. 3A). These results suggest that the recovery of RNA synthesis was not due to stimulation of translation. Interestingly, the P-100 fraction stimulated translation slightly, whereas it slightly inhibited replication and viral yield. When equal amounts were mixed in the reaction, translation was reconstituted, as expected (Fig. 3A). The data indicate that a cellular activity (or activities) specifically required for genomic replication is present in the cell-free system. This activity is lost or inactivated by GTPγS treatment, and it is normally found among the cytosolic (soluble) components of the mock-treated S-10 extract.
ARF proteins are partially lost from the GTPγS-treated extract.
The possibility that a BFA-sensitive ARF protein is responsible for the cellular activity observed in the treated extract predicts that ARF is either inactivated during treatment with GTPγS or, as a result of the treatment, sedimented during the low-speed centrifugation which follows dialysis. To determine how treatment with GTPγS affects the localization of ARF proteins, we carried out immunoblotting of both sedimented and soluble fractions of either treated or mock-treated extracts, using a monoclonal antibody (1D9) which recognizes several members of the mammalian ARF family (ARF1, -3, -4, -5, and -6). Immunoreactive ARF proteins are mostly soluble in the mock-treated extract (Fig. 5). This observation was not surprising, given that ARF was originally purified from a soluble tissue fraction (22) and is cytosolic unless bound to GTP (38, 50). Treatment with increasing concentrations of GTPγS resulted in the progressive loss of ARF proteins from the supernatant (Fig. 5), as would be expected if a subpopulation of the proteins were complexed with GTPγS and became irreversibly membrane associated. The loss of ARFs from the supernatant is only partial, but it covaries with the loss in replicational activity in the corresponding supernatants. These results support the possibility that cell-free viral replication directly or indirectly requires the activity of a BFA-sensitive member(s) of the ARF family.
FIG. 5.
ARF proteins are partially lost from the cytoplasmic extract through treatment with GTPγS. Extract was divided evenly and either treated with indicated concentrations of GTPγS or mock treated as described in Materials and Methods. Following dialysis and centrifugation, equal aliquots of the soluble and insoluble fractions of all four extracts were analyzed by SDS-PAGE (15% gel) and ECL immunoblotting with anti-ARF monoclonal antibody. Quantitation of resulting signal was carried out by densitometry scanning of short-exposure films of the immunoblot and is presented as a percentage of ARF found in the supernatant fraction of the mock-treated extract.
Competitive inhibitors of ARF activity specifically inhibit cell-free poliovirus RNA replication.
The N terminus of ARF has been characterized as an effector domain in the generation of coated transport vesicles. Deletion of the first 17 amino acid residues results in a protein that lacks ARF activity, and synthetic peptides encoding the same residues were found to act as potent competitive inhibitors of the generation of coated vesicles from isolated Golgi membranes (23). They are believed to compete for membrane-associated binding partners of ARF, which binds to the budding membrane in a saturable, site-specific manner (17).
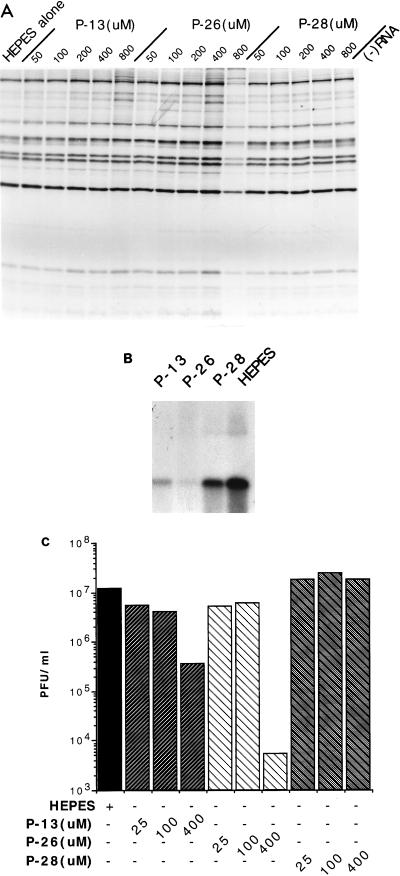
We have determined the effects of two such peptides in the cell-free replication system. These consist of 16 N-terminal residues (2 to 17) of either murine ARF1 (mARF1) (P-13) or hARF4 (P-26). We also used a truncated form consisting of 12 residues (6 to 17) of mARF1 (P-28). The peptide sequences and their effects on transport and cell-free poliovirus replication are summarized in Table 1. P-13 and P-26 were found to severely inhibit viral yield from the system starting at a concentration of 25 μM, showing severe inhibition at 400 μM (Fig. 6C). The inhibition occurs at the level of replication, since incorporation of [α-32P]CTP was severely abrogated in the presence of 400 μM P-13 and P-26 (Fig. 6B), but translation and processing at several different concentrations were unaffected (Fig. 6A). In contrast, P-28 had no effect on virus yield (Fig. 3C) and showed only slight inhibition of replication (Fig. 6B), a result correlating with its inability to inhibit ARF activity. It should be mentioned that the levels of inhibition by P-13 and P-26 were variable, often fluctuating with different extract preparations. We also noted a drop in the potency of inhibition over time when the peptide solutions were stored at −20°C. However, the overall effect was consistent, and the results shown are representative of three different experiments.
TABLE 1.
Peptide inhibitors of ARF function
FIG. 6.
ARF N-terminal peptides are specific inhibitors of cell-free poliovirus RNA replication. (A) 35S-labeled translation and processing products from the cell-free system in the presence of the indicated concentrations of the P-13, P-26, and P-28 peptides. Products were resolved as for Fig. 1A. (B) RNA products from cell-free reactions containing 400 μM P-13, P-26, and P-28, plus an equivalent amount of HEPES buffer (800 μM) added with the peptides. Products were resolved as for Fig. 2B. (C) Viral yield from cell-free reactions containing indicated concentrations of P-13, P-26, and P-28 and 800 μM HEPES. Results are generally representative of several independent experiments.
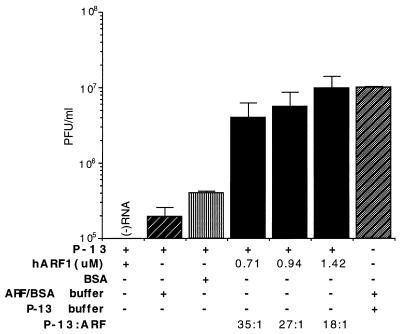
Weidman and Winter (55) have previously demonstrated detergent-like properties of P-13, which can be interpreted to mean that its inhibition of in vitro transport may be a nonspecific effect resulting from the denaturation of the involved membranes, and not through competition for binding partners of ARF. Arguing against this conclusion is the observation that the ARF-inhibiting activity of P-13 can be partially antagonized by the addition of purified ARF (1, 23) or cellular cytosolic fractions (1, 23), although a combination of excess ARF with P-13 was found to inhibit transport in permeable cell systems (1, 23). To address this possible complication, we titrated purified recombinant hARF1 (in which residues 2 to 17 are identical to those in mARF-1) into cell-free reaction mixtures containing 25 μM P-13 to determine whether the inhibition of viral synthesis effected by the peptide could be reversed by the addition of ARF protein. We observed a surprisingly high level of peptide inhibition (which, as mentioned previously, was highly variable) and rescue of viral synthesis when hARF1 was present, even though the peptide was in significant excess in all experimental conditions (Fig. 7). BSA, added at an amount equal by weight to the lowest ARF concentration, raised the yield only slightly in the presence of P-13. The data can be interpreted to suggest that the two inhibitory peptides P-13 and (by extension) P-26 inhibit replication through specific competition with ARF for a cellular effector that binds to the N-terminal domain and that this interaction is required for replication of the input viral RNA in the cell-free system. The observation that ARF effects rescue even in the presence of an excess of P-13 may indicate that the native protein has a higher affinity than the peptide for the common binding partner(s).
FIG. 7.
Inhibition of cell-free replication by P-13 can be antagonized by increasing amounts of purified recombinant hARF1. Reaction mixtures containing 25 μM P-13 were supplemented with indicated concentrations of hARF1, with ARF buffer (see Materials and Methods), or with BSA. Bars represent the means of duplicate samples and two independent experiments.
DISCUSSION
Possible significance of BFA inhibition of enterovirus RNA replication.
The mechanism of inhibition of poliovirus genomic replication by BFA (20, 30) is unknown. However, the effect does suggest that genomic replication requires a BFA-sensitive aspect of secretory transport for efficient function. On the other hand, infection by poliovirus causes a block in protein secretion (12). This implies that replication does not require secretory transport in general, as would be expected of a naked virus that does not encode glycosylated envelope proteins. In fact, poliovirus-based expression vectors encoding a gene specifying a glycoprotein (containing a signal sequence) were found to be nonviable in HeLa cells (28). It could be argued that BFA inhibition of poliovirus replication results from generally toxic effects of the drug on the host cell. This interpretation can be excluded by the observation that in the cell-free system, BFA inhibits replication in the absence of a metabolically active host cell. In addition, the drug does not inhibit all picornavirus replication (encephalomyocarditis virus is not affected) (20), an observation which strongly argues against general toxicity by BFA.
A simple interpretation is that BFA directly inhibits a cellular process that is required for replication to occur, a phenomenon that is also reproduced in vitro. However, the concentrations of BFA that effect inhibition in vitro (starting at 20 μg/ml) are significantly higher than those that do so in vivo (2 μg/ml). This effect may be intrinsic to the rather inefficient nature of cell-free replication compared to the in vivo case (31, 32). Similarly high concentrations were required in experiments utilizing cell-free secretory transport systems (35). These observations may be explained by a general dilution of the molecular target of BFA when cell extracts are prepared. The sensitivity to BFA of the replication system developed by us may also be a feature of our preparation procedure of the cell-free system, since Tang et al. (49) reported that the drug had no effect on virus yield in a similar cell-free system. Differences in the preparation of the cytoplasmic cell extracts or in the reactions conditions may inactivate BFA-degrading activities in our system. Such activities exist in intact cells. For example, forskolin, an activator of adenylate cyclase, prompts the detoxification of BFA in vivo by an unknown cellular enzyme (33) and reverses its toxic effects (26).
The data obtained with the use of GTP analogs indicate that cell-free RNA replication has an apparent requirement for a cellular GTP-binding and -hydrolyzing activity, the loss of which can be partially complemented by a soluble fraction of the normal extract. This activity is inactivated by incubation of the cytoplasmic extract with GTPγS, but not Gpp[NH]p, an observation suggesting that it may be related to ARF. Indeed, the GTPγS treatment results in the partial loss of ARF proteins from the soluble fraction of the cytoplasmic extract. This could be interpreted to mean that ARF is the activity whose function is impaired through treatment with GTPγS. If so, our results suggest that inhibition of RNA replication by BFA in the cell-free system is diagnostic of a requirement for ARF activity in the general process.
We have observed that peptides which encode the N-terminal sequence of ARF proteins, and which are thought to act as competitive inhibitors for ARF interaction with binding partners during coated vesicle formation, are inhibitors of cell-free RNA replication. One possible explanation for this effect is that the peptides, which form amphipathic helices in aqueous solution, have a general detergent-like effect on membranes in the extract (55), disrupting replication indirectly. Arguing against this possibility is the observation that P-13 and P-26 have unequal effects at equal concentrations, implying a specific mechanism of inhibition. Moreover, a truncated peptide (P-28) had little or no effect at equivalent concentrations. More importantly, inhibition by the P-13 peptide (consisting of the mARF1 N-terminal residues 2 to 17, identical to the corresponding human sequence) can be antagonized by the addition of increasing amounts of purified recombinant hARF1. This result is consistent with the inhibition occurring through competition and specifically suggests that the activity of ARF proteins is somehow required for viral RNA replication to occur efficiently in the cell-free system.
How may ARF activity be required during replication?
It is unclear whether ARF acts as a direct player in RNA replication. However, the role of ARF may be indirect since inhibition of ARF by BFA is itself indirect. It is more likely that the specific process in which ARF is normally involved, perhaps the activation of a step in the transport pathway, is relevant for replication in vivo. We hypothesize that infection somehow deregulates the transport pathway such that overall secretion is blocked, but the step inhibited by BFA, the ARF-dependent synthesis of secretory vesicles, remains active and is perhaps even stimulated by virus-encoded proteins such as 2BC, 2C, and/or 2B. As has been proposed by Maynell et al. (30) and Doedens et al. (11), a block in the fusion of secretory vesicles with target membranes would result in their accumulation (11, 30), leading to the progressive disassembly of the donor organelle. These coated vesicles would eventually be uncoated through GTP hydrolysis, and the naked membranes may fuse nonspecifically, giving rise to the larger vacuolar structures seen during poliovirus infection of target cells (Fig. 1). Viral replication complexes assemble at these membrane structures, forming the rosette structures described by Bienz et al. (9). The recent observation that the replication-associated vesicles may originate primarily from the ER, Golgi, and lysosomes is consistent with a requirement for ARF activity in their generation, since evidence exists for possible ARF involvement in anterograde and retrograde transport from the ER to the Golgi (1), intra-Golgi transport (34), anterograde transport from the trans-Golgi network (44), and endosomal function (24, 56). These transport steps are also affected by BFA (19, 27).
As yet, there is no direct evidence that virus-induced vesicular structures form in the cell-free replication system described here. However, the sensitivity of replication to BFA, coupled with an apparent requirement for a soluble cellular GTP-binding and -hydrolyzing activity and the inhibition of RNA replication by competitive inhibitors of ARF, suggests that the cellular pathway that produces transport vesicles in the normal cell, or a specific step thereof, is involved in RNA replication in vitro. We suggest that this may also be the case in vivo. It is unlikely that there is a functional secretory apparatus in the cell-free system, because we do not observe glycosylation and processing of a reporter protein (28). However, our model requires only that the BFA-sensitive mechanism for generating transport vesicles be active, as it is in cell-free systems that reproduce the process (35, 36). These systems are similar in many respects to the cell-free replication system, making our interpretation of the results presented here, and their possible significance with regard to poliovirus RNA replication, a reasonable explanation of why BFA inhibits poliovirus RNA replication.
ACKNOWLEDGMENTS
We thank Thomas Pfister for critical reading of the manuscript, Richard Kahn for the generous gift of ARF N-terminal peptides and ARF antibody and for helpful discussions, and Andrew Morris for purified recombinant hARF1 and helpful discussion.
A.C. was a member of the graduate training program of the Department of Molecular Genetics and Microbiology. This work was supported in part by NIH grant 5R37AI15122.
REFERENCES
- 1.Balch W E, Kahn R A, Schwaninger R. ADP-ribosylation factor is required for vesicular trafficking between the endoplasmic reticulum and the cis-Golgi compartment. J Biol Chem. 1992;267:13053–13061. [PubMed] [Google Scholar]
- 2.Barco A, Carrasco L. A human virus protein, poliovirus protein 2BC, induces membrane proliferation and blocks the exocytic pathway in the yeast Saccharomyces cerevisiae. EMBO J. 1995;14:3349–3364. doi: 10.1002/j.1460-2075.1995.tb07341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barton D J, Black E P, Flanegan J B. Complete replication of poliovirus in vitro: preinitiation RNA replication complexes require soluble cellular factors for the synthesis of VPg-linked RNA. J Virol. 1995;69:5516–5527. doi: 10.1128/jvi.69.9.5516-5527.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barton D J, Flanegan J B. Coupled translation and replication of poliovirus RNA in vitro: synthesis of functional 3D polymerase and infectious virus. J Virol. 1993;67:822–831. doi: 10.1128/jvi.67.2.822-831.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bienz K, Egger D, Pasamontes L. Association of polioviral proteins of the P2 genomic region with the viral replication complex and virus-induced membrane synthesis as visualized by electron microscopic immunocytochemistry and autoradiography. Virology. 1987;160:220–226. doi: 10.1016/0042-6822(87)90063-8. [DOI] [PubMed] [Google Scholar]
- 6.Bienz K, Egger D, Pfister T. Characteristics of the poliovirus replication complex. Arch Virol Suppl. 1994;9:147–157. doi: 10.1007/978-3-7091-9326-6_15. [DOI] [PubMed] [Google Scholar]
- 7.Bienz K, Egger D, Pfister T, Troxler M. Structural and functional characterization of the poliovirus replication complex. J Virol. 1992;66:2740–2747. doi: 10.1128/jvi.66.5.2740-2747.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bienz K, Egger D, Rasser Y, Bossart W. Intracellular distribution of poliovirus proteins and the induction of virus-specific cytoplasmic structures. Virology. 1983;131:39–48. doi: 10.1016/0042-6822(83)90531-7. [DOI] [PubMed] [Google Scholar]
- 9.Bienz K, Egger D, Troxler M, Pasamontes L. Structural organization of poliovirus RNA replication is mediated by viral proteins of the P2 genomic region. J Virol. 1990;64:1156–1163. doi: 10.1128/jvi.64.3.1156-1163.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho M W, Teterina N, Egger D, Bienz K, Ehrenfeld E. Membrane rearrangement and vesicle induction by recombinant poliovirus 2C and 2BC in human cells. Virology. 1994;202:129–145. doi: 10.1006/viro.1994.1329. [DOI] [PubMed] [Google Scholar]
- 11.Doedens J, Maynell L A, Klymkowsky M W, Kirkegaard K. Secretory pathway function, but not cytoskeletal integrity, is required in poliovirus infection. Arch Virol Suppl. 1994;9:159–172. doi: 10.1007/978-3-7091-9326-6_16. [DOI] [PubMed] [Google Scholar]
- 12.Doedens J R, Kirkegaard K. Inhibition of cellular protein secretion by poliovirus proteins 2B and 3A. EMBO J. 1995;14:894–907. doi: 10.1002/j.1460-2075.1995.tb07071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donaldson J, Finazzi D, Klausner R D. Brefeldin A inhibits Golgi membrane-catalyzed exchange of guanine nucleotide onto ARF protein. Nature. 1992;360:350–352. doi: 10.1038/360350a0. [DOI] [PubMed] [Google Scholar]
- 14.Donaldson J G, Cassel D, Kahn R A, Klausner R D. ADP-ribosylation factor, a small GTP binding protein, is required for binding of the coatomer protein β-COP to Golgi membranes. Proc Natl Acad Sci USA. 1992;89:6408–6412. doi: 10.1073/pnas.89.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duggal R, Cuconati A, Gromeier M, Wimmer E. Genetic recombination of poliovirus in a cell-free system. Proc Natl Acad Sci USA. 1997;94:13786–13791. doi: 10.1073/pnas.94.25.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egger D, Pasamontes L, Bolten R, Boyko V, Bienz K. Reversible dissociation of the poliovirus replication complex: functions and interactions of its components in viral RNA synthesis. J Virol. 1996;70:8675–8683. doi: 10.1128/jvi.70.12.8675-8683.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helms J B, Palmer D J, Rothman J E. Two distinct populations of ARF bound to Golgi membranes. J Cell Biol. 1993;121:751–760. doi: 10.1083/jcb.121.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helms J B, Rothman J E. Inhibition by brefeldin A of a Golgi membrane enzyme that catalyzes exchange of guanine nucleotide bound to ARF. Nature. 1992;360:352–352. doi: 10.1038/360352a0. [DOI] [PubMed] [Google Scholar]
- 19.Hunziker W, Whitney J A, Mellman I. Selective inhibition of transcytosis by brefeldin A in MDCK cells. Cell. 1991;67:617–627. doi: 10.1016/0092-8674(91)90535-7. [DOI] [PubMed] [Google Scholar]
- 20.Irurzun A, Perez L, Carrasco L. Involvement of membrane traffic in the replication of poliovirus genomes: effects of brefeldin A. Virology. 1992;191:166–175. doi: 10.1016/0042-6822(92)90178-r. [DOI] [PubMed] [Google Scholar]
- 21.Joachims M, Harris K S, Etchison D. Poliovirus protease 3C mediates cleavage of microtubule-associated protein 4. Virology. 1995;211:451–461. doi: 10.1006/viro.1995.1427. [DOI] [PubMed] [Google Scholar]
- 22.Kahn R A, Gilman A G. The protein cofactor necessary for ADP-ribosylation of Gs by cholera toxin is itself a GTP binding protein. J Biol Chem. 1986;261:7906–7911. [PubMed] [Google Scholar]
- 23.Kahn R A, Randazzo P, Serafini T, Weiss O, Rulka C, Clark J, Amherdt M, Roller P, Orci L, Rothman J E. The amino terminus of ADP-ribosylation factor (ARF) is a critical determinant of ARF activities and is a potent and specific inhibitor of protein transport. J Biol Chem. 1992;267:13039–13046. [PubMed] [Google Scholar]
- 24.Lenhard J M, Kahn R A, Stahl P D. Evidence for ADP-ribosylation factor (ARF) as a regulator of in vitro endosome-endosome fusion. J Biol Chem. 1992;267:13047–13052. [PubMed] [Google Scholar]
- 25.Lippincott-Schwartz J, Donaldson J, Schweizer A, Berger E G, Hauri H-P, Yuan L C, Klausner R D. Microtubule dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990;60:821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- 26.Lippincott-Schwartz J, Glickman J, Donaldson J G, Robbins J, Kreis T E, Seamon K B, Sheetz M P, Klausner R D. Forskolin inhibits and reverses the effects of brefeldin A on Golgi morphology by a cAMP-independent mechanism. J Cell Biol. 1991;112:567–577. doi: 10.1083/jcb.112.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lippincott-Schwartz J, Yuan L, Tipper C, Amherdt M, Orci L, Klausner R D. Brefeldin A’s effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991;67:601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- 28.Lu H H, Alexander L, Wimmer E. Construction and genetic analysis of dicistronic polioviruses containing open reading frames for epitopes of human immunodeficiency virus type 1 gp120. J Virol. 1995;69:4797–4806. doi: 10.1128/jvi.69.8.4797-4806.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makler V, Cukierman E, Rotman M, Admon A, Cassel D. ADP-ribosylation factor-directed GTPase-activating protein. Purification and partial characterization. J Biol Chem. 1995;270:5232–5237. doi: 10.1074/jbc.270.10.5232. [DOI] [PubMed] [Google Scholar]
- 30.Maynell L A, Kirkegaard K, Klymkowsky M W. Inhibition of poliovirus RNA synthesis by brefeldin A. J Virol. 1992;66:1985–1994. doi: 10.1128/jvi.66.4.1985-1994.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molla A, Paul A V, Wimmer E. Cell-free, de novo synthesis of poliovirus. Science. 1991;254:1647–1651. doi: 10.1126/science.1661029. [DOI] [PubMed] [Google Scholar]
- 32.Molla A, Paul A V, Wimmer E. Effects of temperature and lipophilic agents on poliovirus formation and RNA synthesis in a cell-free system. J Virol. 1993;67:5932–5938. doi: 10.1128/jvi.67.10.5932-5938.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nickel W, Helms J B, Kneusel R E, Wieland F T. Forskolin stimulates detoxification of brefeldin A. J Biol Chem. 1996;271:15870–15873. doi: 10.1074/jbc.271.27.15870. [DOI] [PubMed] [Google Scholar]
- 34.Orci L, Stamnes M, Ravazzola M, Amherdt M, Perrelet A, Sollner T H, Rothman J E. Bidirectional transport by distinct populations of COPI-coated vesicles. Cell. 1997;90:335–349. doi: 10.1016/s0092-8674(00)80341-4. [DOI] [PubMed] [Google Scholar]
- 35.Orci L, Tagaya M, Amherdt M, Perrelet A, Donaldson J G, Lippincott-Schwartz J, Klausner R D, Rothman J E. Brefeldin A, a drug that blocks secretion, prevents the assembly of non-clathrin-coated buds on Golgi cisternae. Cell. 1991;64:1183–1195. doi: 10.1016/0092-8674(91)90273-2. [DOI] [PubMed] [Google Scholar]
- 36.Ostermann J, Orci L, Tani K, Amherdt M, Ravazzola M, Elazar Z, Rothman J E. Stepwise assembly of functionally active transport vesicles. Cell. 1993;75:1015–1025. doi: 10.1016/0092-8674(93)90545-2. [DOI] [PubMed] [Google Scholar]
- 37.Regazzi R, Ulrich S, Kahn R A, Wollheim C B. Redistribution of ADP-ribosylation factor during stimulation of permeabilized cells with GTP analogues. Biochem J. 1991;275:639–644. doi: 10.1042/bj2750639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothman J E, Wieland F T. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- 39.Rueckert R R. Picornaviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. I. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 609–654. [Google Scholar]
- 40.Sandoval I V, Carrasco L. Poliovirus infection and expression of the poliovirus protein 2B provoke the dissassembly of the Golgi complex, the organelle target for the antipoliovirus drug Ro-090179. J Virol. 1997;71:4679–4693. doi: 10.1128/jvi.71.6.4679-4693.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schekman R, Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- 42.Schlegel A, Giddings T H, Jr, Ladinsky M S, Kirkegaard K. Cellular origin and ultrastructure of membranes induced during poliovirus infection. J Virol. 1996;70:6576–6588. doi: 10.1128/jvi.70.10.6576-6588.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sonenberg N. Regulation of translation by poliovirus. Adv Virol Res. 1987;33:175–204. doi: 10.1016/s0065-3527(08)60318-8. [DOI] [PubMed] [Google Scholar]
- 44.Stamnes M A, Rothman J E. The binding of AP-1 clathrin adaptor particles to Golgi membranes requires ADP-ribosylation factor, a small GTP-binding protein. Cell. 1993;73:999–1005. doi: 10.1016/0092-8674(93)90277-w. [DOI] [PubMed] [Google Scholar]
- 45.Takeda N, Kuhn R J, Yang C F, Takegami T, Wimmer E. Initiation of poliovirus plus-strand RNA synthesis in a membrane complex of infected HeLa cells. J Virol. 1986;60:43–53. doi: 10.1128/jvi.60.1.43-53.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takeda N, Yang C F, Kuhn R J, Wimmer E. Uridylylation of the genome-linked protein of poliovirus in vitro is dependent upon an endogenous RNA template. Virus Res. 1987;8:193–204. doi: 10.1016/0168-1702(87)90015-3. [DOI] [PubMed] [Google Scholar]
- 47.Takegami T, Kuhn R J, Anderson C W, Wimmer E. Membrane-dependent uridylylation of the genome-linked protein VPg of poliovirus. Proc Natl Acad Sci USA. 1983;80:7447–7451. doi: 10.1073/pnas.80.24.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takegami T, Semler B L, Anderson C W, Wimmer E. Membrane fractions active in poliovirus RNA replication contain VPg precursor polypeptides. Virology. 1983;128:33–47. doi: 10.1016/0042-6822(83)90316-1. [DOI] [PubMed] [Google Scholar]
- 49.Tang R S, Barton D J, Flanegan J B, Kirkegaard K. Poliovirus RNA recombination in cell-free extracts. RNA. 1997;3:624–633. [PMC free article] [PubMed] [Google Scholar]
- 50.Tanigawa G, Orci L, Amherdt M, Ravazzola M, Helms J B, Rothman J E. Hydrolysis of bound GTP by ARF protein triggers uncoating of Golgi-derived COP-coated vesicles. J Cell Biol. 1993;123:1365–1371. doi: 10.1083/jcb.123.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Todd S, Towner J S, Semler B L. Translation and replication properties of the human rhinovirus genome in vivo and in vitro. Virology. 1997;229:90–97. doi: 10.1006/viro.1996.8416. [DOI] [PubMed] [Google Scholar]
- 52.Troxler M, Egger D, Pfister T, Bienz K. Intracellular localization of poliovirus RNA by in situ hybridization at the ultrastructural level using single-stranded riboprobes. Virology. 1992;191:687–697. doi: 10.1016/0042-6822(92)90244-j. [DOI] [PubMed] [Google Scholar]
- 53.Tucker S P, Thornton C L, Wimmer E, Compans R W. Vectorial release of poliovirus from polarized human intestinal epithelial cells. J Virol. 1993;67:4274–4282. doi: 10.1128/jvi.67.7.4274-4282.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Urzainqui A, Carrasco L. Degradation of cellular proteins during poliovirus infection: studies by two-dimensional gel electrophoresis. J Virol. 1989;63:4729–4735. doi: 10.1128/jvi.63.11.4729-4735.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weidman P J, Winter W M. The G protein-activating peptide, mastoparan, and the synthetic NH2-terminal ARF peptide, ARFp13, inhibit in vitro Golgi transport by irreversibly damaging membranes. J Cell Biol. 1994;127:1815–1827. doi: 10.1083/jcb.127.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whitney J A, Gomez M, Sheff D, Kreis T E, Mellman I. Cytoplasmic coat proteins involved in endosome function. Cell. 1995;83:703–713. doi: 10.1016/0092-8674(95)90183-3. [DOI] [PubMed] [Google Scholar]
- 57.Wood S A, Park J E, Brown W J. Brefeldin A causes a microtubule-mediated fusion of the trans-Golgi network and early endosomes. Cell. 1991;67:591–600. doi: 10.1016/0092-8674(91)90533-5. [DOI] [PubMed] [Google Scholar]
- 58.Yalamanchili P, Datta U, Dasgupta A. Inhibition of host cell transcription by poliovirus: cleavage of transcription factor CREB by poliovirus-encoded protease 3Cpro. J Virol. 1997;71:1220–1226. doi: 10.1128/jvi.71.2.1220-1226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yalamanchili P, Harris K, Wimmer E, Dasgupta A. Inhibition of basal transcription by poliovirus: a virus-encoded protease (3Cpro) inhibits formation of TBP-TATA box complex in vitro. J Virol. 1996;70:2922–2929. doi: 10.1128/jvi.70.5.2922-2929.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]