Abstract
Smoking is the most important risk factor for chronic obstructive pulmonary disease (COPD), however evidence from large-scale studies on whether secondhand smoke (SHS) increases the risk of COPD is still lacking. We conducted this large longitudinal study to investigate the association between SHS and the development of COPD. This is a longitudinal study. Data on 6519 subjects who were never-smokers, had no history of COPD, and had complete lung function records were extracted from the Taiwan Biobank. They were divided into two groups according to SHS exposure: no exposure and exposure groups. Data were collected when participants enrolled in the study and during regular follow-up. Cox proportional hazards regression models were used to estimate the relative risk (RR) and 95% confidence interval (CI) for the association between SHS and the risk of developing COPD. At 48 months of follow-up, 260 (4%) participants in the no exposure group and 34 (7%) participants in the exposure group developed COPD. The RR of incident COPD development was significantly higher in the exposure group than that in the no exposure group after adjusting for confounders (RR = 1.49; 95% CI 1.04 to 2.14; P value = 0.031). There is a dose–response relationship between the duration of exposure to SHS and the risk of incident COPD, which demonstrates that an additional hour of exposure to SHS per week was associated with a 1.03-fold increased likelihood of developing COPD after adjusting for confounders (RR = 1.03; 95% CI 1.00 to 1.05; P value = 0.027). SHS exposure contributes to the development of COPD. This finding can help raise awareness of the harms of SHS and provide a reference for formulating anti-smoking policies.
Subject terms: Environmental impact, Respiratory tract diseases
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality worldwide and is expected to become the 4th leading cause of disability-adjusted life years (DALY) by 20301,2. Although the reported prevalence of COPD varies between countries due to differences in definitions and methodologies, the global prevalence in adults aged over 30 was estimated to be 10.3% in 2019, which translates to 391.9 million people3. The Asia–Pacific region bears the highest burden of COPD in terms of deaths, DALY, and years of life lost (YLL)1. In Taiwan, the reported prevalence of COPD in adults aged over 40 years ranges from 2.48% to 9.5%4. According to 2021 statistics published by the Ministry of Health and Welfare in Taiwan, chronic lower respiratory tract diseases are the 8th leading cause of death, associated with an estimated mortality rate of 26.6 per 100,000 people. The same source indicated that COPD morbidity and mortality resulted in a total of 9.5 YLL in individuals below the age of 70 years, making it the 10th leading cause of YLL in 2017. The prevalence of COPD is high and is expected to keep growing due to cumulative exposure to risk factors and population aging, leading to increased psychological, economic, and social burden in the coming decades1,5.
Tobacco smoke remains the most significant cause of COPD, however an estimated 25–45% of patients with COPD have never smoked6. Other risk factors associated with the disease include genetics, childhood respiratory infections, asthma, and various environmental exposures such as occupational dust, outdoor and indoor air pollutants, and biomass fuels7. Second-hand smoke (SHS), which is another form of biomass smoke, has been linked to coronary heart disease, stroke, and lung cancer in adult non-smokers8. Recent studies have also linked SHS to a higher incidence and prevalence of chronic kidney disease and an increased overall risk of cancer, particularly lung and breast cancer, in never-smokers9,10.
According to an analysis of data from the 2019 Global Burden of Diseases (GBD) report, approximately 1.3 million fatalities worldwide were attributed to SHS exposure in 201911. The morbidity impact associated with SHS exposure, assessed through DALYs, revealed regional disparities, with elevated rates observed in low-income countries across Southeast Asia and the eastern Mediterranean region compared to Europe11. Moreover, an estimated 40% of children (aged 0–14 years), 33% of non-smoking men, and 35% of non-smoking women worldwide were reported to be exposed to SHS across various environments on a global scale12. Among adults, asthma and ischemic heart disease were identified as the predominant health concerns, while lower respiratory infections were the primary concern among children13. In addition, a study published in Nature Medicine in 2024 demonstrated that exposure to SHS increased the likelihood of ischemic heart disease, stroke, type 2 diabetes, and lung cancer by approximately 8%, 5%, 1%, and 1%, respectively14. Furthermore, evidence suggests detrimental associations between SHS and otitis media, asthma, lower respiratory infections, breast cancer and COPD14. These studies underscore the global imperative for individuals, governments, and the World Health Organization to recognize the urgency of controlling SHS.
Although the prevalence of SHS exposure has decreased substantially in Taiwan in the past decades15, SHS still poses a significant socioeconomic burden. A study evaluating the cost of smoking and SHS in Taiwan found that direct costs for treating tobacco-related diseases amounted to US$828 million in 2010, accounting for 3.4% of total personal healthcare expenditure. Furthermore, adding indirect costs measured by the value of lost productivity resulted in a total of US$1.67 billion, representing 0.4% of the gross domestic product in Taiwan15.
Previous studies have explored the relationship between SHS and COPD, and shown that SHS exposure is related to higher mortality from COPD16,17. In addition, in COPD patients who are not currently smoking, SHS exposure has been shown to cause higher exacerbation rates, worse respiratory symptoms, and poorer health status18–20. Both the Singapore Chinese Health Study and a 17-year cohort study in China corroborate these findings, demonstrating the adverse impact of SHS exposure on COPD risk16,21. However, conflicting evidence exists, with certain studies reporting no significant associations between SHS and COPD22–26. Notably, a case–control study in the United States22 and research involving COPD patients in Hong Kong23 found no increased risk associated with SHS exposure. Despite these discrepancies, the potential for SHS to cause COPD in non-smokers remains a subject of debate. Given the limited scope of current evidence, particularly from small cohorts, cross-sectional studies, and Western countries, there is a critical need for large-scale longitudinal studies in the Asia–Pacific region to comprehensively investigate the relationship between SHS and COPD. Therefore, the aim of this large-scale longitudinal study was to investigate the association between SHS and the development of COPD in Taiwan.
Methods
Data source and study population
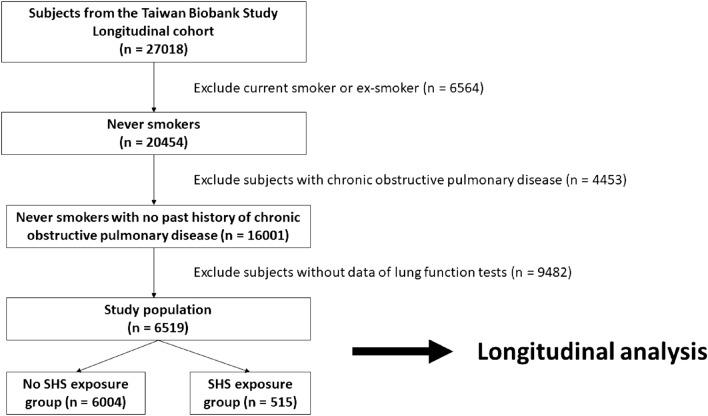
This study utilized the Taiwan Biobank (TWB), a substantial community research database, which has recruited cancer-free volunteers aged 20 to 70 years from more than 30 recruitment centers in Taiwan since 2008. Upon enrollment, individuals underwent questionnaire surveys, physical examinations, blood sampling, and lung function tests. Starting from 2016, recurrent assessments of these characteristics have been conducted during scheduled visits, typically occurring within 2–4 years following the initial appointment. By August 2021, the biobank had amassed a cumulative count of 151,406 participants, with 37,508 having undergone the initial follow-up assessment27. Detailed information about its methodology and development has been reported in previous studies28–32. As a result of the TWB releasing data in batches, with varying release dates and prices, we encountered difficulties in obtaining data for all subjects who completed the initial follow-up assessment. Consequently, a total of 27,018 follow-up participants were enrolled in the present study (Fig. 1). After excluding active and ex-smokers, individuals with a history of COPD, and those without complete lung function test results, the analysis included 6,519 participants (Fig. 1). Written informed consent was obtained from all participants, and the study adhered to the principles of the Declaration of Helsinki. The Institutional Review Board of Kaohsiung Medical University Hospital approved this study (Approval No. KMUHIRB-E(I)-20190398).
Figure 1.
Selection of study population.
The participants were first classified into three groups based on their smoking status, namely "never-smokers," "ex-smokers," and "active smokers," using self-reported questionnaires. The never-smokers were also asked about their exposure to SHS: "Have you been exposed to SHS?" Those who reported exposure to SHS were assigned to the exposure group, while the others were assigned to the no exposure group. Subsequently, participants in the exposure group were asked additional questions regarding their SHS exposure, including "How many hours per week have you been exposed to SHS?" and "Is your exposure to SHS at home or work or both?".
Variables and study outcomes
We gathered comprehensive demographic data on all participants, encompassing age, sex, education level, marital status, and living arrangements. We also meticulously assessed several factors associated with COPD, including obesity33, alcohol consumption34, blood pressure35, blood sugar36, blood lipids37, asthma38, chronic kidney disease39, coronary heart disease40, arrhythmia40, depression41, gout42, and peptic ulcer43. These variables served as confounding factors and were duly adjusted for in our analysis.
The primary endpoint was the development of COPD, defined as forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) < 0.7. Spirometry measurements of FEV1 and FVC were obtained using a MicroLab spirometer and Spida 5 software (Micro Medical Ltd., Rochester, Kent, UK), and administered by trained technicians following the 2005 technical standards of the American Thoracic Society and the European Respiratory Society44. Each participant underwent three lung function tests, all meeting the quality criteria standards (i.e., with differences within 5% or 100 mL), and the best result of the three tests was used for analysis. FVC-predicted (or FVC%-predicted) and FEV1-predicted (or FEV1%-predicted) values were calculated by dividing the measured values by the reference values based on formulas derived from the general Asian population regarding sex, age, and height. These population-specific formulas were integrated into the spirometry software, and individual participant details were entered to yield percent-predicted values. Post-bronchodilator measurements were not conducted.
Statistical analysis
We conducted a longitudinal study, administering a follow-up survey to all subjects every 2 years, starting from their enrollment. The subjects were categorized into two groups: the exposure and no exposure to SHS groups. The categorical variables are represented as numbers and percentages, while continuous variables are presented as means along with standard deviations. Group comparisons were carried out using chi-square tests (for categorical variables) and independent t tests (for continuous variables). Subsequently, we employed Cox proportional hazards regression models (both univariate and multivariate) to explore the association between SHS exposure and COPD. The multivariate Cox proportional hazards regression models included an age-adjusted model, adjusted model 1, and adjusted model 2. Age adjustment was crucial due to its significance as a risk factor for COPD development in several studies3,7. This adjustment helped us better account for the influence of age on COPD risk, ensure comparability across age groups, and obtain more interpretable and accurate estimates of the association between SHS exposure and COPD development within our study population. Adjusted model 1 included variables that were statistically significant in the univariate analysis, while adjusted model 2 accounted for variables with clinical relevance and the potential to confound the association of COPD33–43. Differences were presented as relative risk (RR) and 95% confidence interval (CI). Additionally, we conducted a dose analysis to assess the impact of varying durations of SHS exposure. P value < 0.05 was considered indicative of statistical significance. The statistical analysis was performed using R (version 3.6.2, R Foundation for Statistical Computing, Wien, Austria) and SPSS (version 20.0, IBM Corp, Armonk, NY, USA).
Results
Baseline characteristics
A total of 1,453 men and 5,066 women were included in the study, and their baseline profiles are summarized in Table 1. There were 6,004 individuals in the no exposure group and 515 in the exposure group. Among the 515 participants exposed to SHS, the median weekly exposure was 1.2 h (interquartile range 0.5 to 5 h). SHS exposure was greater at home (n = 235, 46%) than at work (n = 94, 18%), while the remaining individuals were exposed in both settings (n = 174, 34%).
Table 1.
Clinical characteristics of the study participants (n = 6519).
| Characteristics | Secondhand Smoke Exposure | ||
|---|---|---|---|
| No Exposure, n = 6004 | Exposure, n = 515 | P value | |
| Secondhand smoke exposure | |||
| Location, % | |||
| At home only | N/A | 235 (46) | |
| At work only | N/A | 94 (18) | |
| At both home and work | N/A | 174 (34) | |
| Missing value | N/A | 12 (2) | |
| Exposure hours per week, median (IQR) | N/A | 1.2 (0.5 to 5) | |
| Demographic data | |||
| Age, yr | 51 ± 10 | 48 ± 10 | < 0.001*** |
| Women | 4682 (78) | 384 (75) | 0.077 |
| Body mass index, kg/m2 | 23.8 ± 3.5 | 24.2 ± 3.6 | 0.005** |
| Alcohol status, ever | 169 (3) | 34 (7) | < 0.001*** |
| Physical activity, yes | 2834 (47) | 192 (37) | < 0.001*** |
| Married, yes | 5543 (92) | 478 (93) | 0.795 |
| Educational status, ≧ college | 3108 (52) | 203 (39) | < 0.001*** |
| Systolic blood pressure, mm Hg | 117 ± 18 | 116 ± 18 | 0.242 |
| Diastolic blood pressure, mm Hg | 71 ± 11 | 71 ± 11 | 0.900 |
| Comorbidities | |||
| Hypertension | 677 (11) | 63 (12) | 0.515 |
| Diabetes mellitus | 288 (5) | 20 (4) | 0.388 |
| Dyslipidemia | 443 (7) | 41 (8) | 0.600 |
| Asthma | 234 (4) | 29 (6) | 0.061 |
| Chronic kidney disease | 66 (1) | 7 (1) | 0.515 |
| Coronary artery disease | 68 (1) | 4 (1) | 0.659 |
| Arrythmia | 290 (5) | 25 (5) | 1.000 |
| Peptic ulcer | 922 (15) | 79 (15) | 1.000 |
| Depression | 203 (3) | 14 (3) | 0.521 |
| Gout | 140 (2) | 11 (2) | 0.879 |
IQR = Interquartile range.
** means P value < 0.01; and *** means P value < 0.001.
The SHS exposure group were younger, had a higher body mass index (BMI), and demonstrated a tendency toward a higher prevalence of alcohol consumption, along with a lower prevalence of physical activity and educational attainment compared to the no exposure group. No significant differences were observed in terms of sex, marital status, or the prevalence of comorbidities listed in Table 1 between the two groups.
Associations between variables and the development of COPD
In the univariate analysis, BMI, systolic blood pressure (SBP), and diastolic blood pressure (DBP) demonstrated negative associations with the development of COPD (Table 2). Moreover, the risk of developing incident COPD was significantly higher the SHS exposure group than in the no exposure group (RR = 1.52; 95% CI 1.07 to 2.18; P value = 0.021). Conversely, no significant associations were identified between incident COPD and factors such as age, sex, or the comorbidities listed in Table 2.
Table 2.
Parameters associated with incident COPD (n = 6519).
| Parameters | Relative risk (95% CI) | P value |
|---|---|---|
| Age (per 1 year) | 1.00 (0.98 to 1.01) | 0.455 |
| Women (vs. men) | 0.95 (0.73 to 1.25) | 0.729 |
| Body mass index (per 1 kg/m2) | 0.96 (0.92 to 0.99) | 0.014* |
| Alcohol status, ever (vs. never) | 0.76 (0.36 to1.61) | 0.471 |
| Physical activity, yes (vs. no) | 0.95 (0.76 to1.20) | 0.685 |
| Married, yes (vs. no) | 1.02 (0.66 to 1.58) | 0.920 |
| Living alone, yes (vs. no) | 0.97 (0.60 to 1.56) | 0.906 |
| Education status (per higher level) | 0.93 (0.84 to 1.05) | 0.239 |
| Systolic blood pressure (per 1 mmHg) | 0.99 (0.98 to 0.99) | < 0.001*** |
| Diastolic blood pressure (per 1 mmHg) | 0.98 (0.97 to 0.99) | < 0.001*** |
| Hypertension, yes (vs. no) | 0.89 (0.61 to 1.29) | 0.535 |
| Diabetes mellitus, yes (vs. no) | 0.78 (0.43 to 1.43) | 0.428 |
| Dyslipidemia, yes (vs. no) | 0.68(0.38 to 1.21) | 0.193 |
| Asthma, yes (vs. no) | 1.46 (0.89 to 2.38) | 0.130 |
| Chronic kidney disease, yes (vs. no) | 0.91 (0.29 to 2.84) | 0.871 |
| Coronary artery disease, yes (vs. no) | 0.92 (0.30 to 2.88) | 0.890 |
| Arrythmia, yes (vs. no) | 0.62 (0.32 to 1.21) | 0.161 |
| Peptic ulcer, yes (vs. no) | 0.87 (0.62 to 1.21) | 0.406 |
| Depression, yes (vs. no) | 1.13 (0.62 to 2.06) | 0.693 |
| Gout, yes (vs. no) | 1.33 (0.69 to 2.59) | 0.397 |
| Secondhand Smoke Exposure, yes (vs. no) | 1.52 (1.07 to 2.18) | 0.021* |
COPD = Chronic obstructive pulmonary disease; CI = Confidence interval.
* means P value < 0.05; and *** means P value < 0.001.
Association between SHS and the development of COPD
After a follow-up period of 48 months, COPD developed in 260 (4%) participants in the no exposure group and 34 (7%) participants in the exposure group (Table 3). After adjusting for confounding variables, the RR of incident COPD development was significantly higher in the exposure group than in the no exposure group (RR = 1.49; 95% CI 1.04 to 2.14; P value = 0.031) (Table 3).
Table 3.
Relative risk for incident COPD by exposure of secondhand smoke after adjusting for confounders (n = 6519).
| No Exposure | Secondhand smoke exposure | P value | |
|---|---|---|---|
| Presence of COPD, n (%) | 260 (4) | 34 (7) | |
| Age-adjusted relative risk (95% CI) | 1.00 (reference) | 1.51 (1.05 to 2.16) | 0.025* |
| Adjusted model 1, relative risk (95% CI) | 1.00 (reference) | 1.55 (1.08 to 2.21) | 0.017* |
| Adjusted model 2, relative risk (95% CI) | 1.00 (reference) | 1.49 (1.04 to 2.14) | 0.031* |
COPD = Chronic obstructive pulmonary disease; CI = Confidence interval.
Model 1 adjusts for age, body mass index, systolic blood pressure and diastolic blood pressure.
Model 2 adjusts for age, sex, body mass index, alcohol status, physical activity, marital status, living alone, educational status, systolic blood pressure, diastolic blood pressure, history of hypertension, dyslipidemia, diabetes mellitus, asthma, chronic kidney disease, coronary artery disease, arrythmia, peptic ulcer, depression, and gout.
* means P value < 0.05.
Association between SHS Frequency and the Development of COPD
To further investigate the potential dose–response relationship between the duration of exposure to SHS and the risk of incident COPD, we analyzed the association using data from Table 4. The results revealed that each additional hour of weekly exposure to SHS was associated with a 1.03-fold increased likelihood of developing COPD (unadjusted RR = 1.03; 95% CI 1.01 to 1.05; P value = 0.013). This association remained significant after adjusting for various covariates, including age, sex, BMI, alcohol consumption, physical activity, marital status, living arrangements, educational level, SBP, DBP, and a range of medical conditions (adjusted model 2: RR = 1.03; 95% CI 1.00 to 1.05; P value = 0.027). Thus, these findings suggest that even relatively low levels of SHS exposure may contribute to an elevated risk of COPD development (Table 4).
Table 4.
Relative risk for incident COPD according to weakly hours of secondhand smoke exposure.
| Weekly hours of secondhand smoke exposure | Unadjusted relative risk (95% CI) | Age-adjusted relative risk (95% CI) |
Adjusted model 1, relative risk (95% CI) | Adjusted model 2, relative risk (95% CI) |
|---|---|---|---|---|
| Per 1 h /week |
1.03* (1.01 to 1.05) |
1.03* (1.01 to 1.05) |
1.03* (1.00 to 1.05) |
1.03* (1.00 to 1.05) |
| P value | 0.013 | 0.016 | 0.021 | 0.027 |
COPD = Chronic obstructive pulmonary disease; CI = Confidence interval.
Model 1 adjusts for age, body mass index, systolic blood pressure and diastolic blood pressure.
Model 2 adjusts for age, sex, body mass index, alcohol status, physical activity, marital status, living alone, educational status, systolic blood pressure, diastolic blood pressure, history of hypertension, dyslipidemia, diabetes mellitus, asthma, chronic kidney disease, coronary artery disease, arrythmia, peptic ulcer, depression, and gout.
* means P value < 0.05.
Discussion
In this longitudinal study of Taiwanese never-smokers, a notable association was established between SHS exposure and an increased risk of COPD after accounting for confounding variables. In addition, there is a dose–response relationship between the duration of exposure to SHS and the risk of incident COPD, which demonstrates that an additional hour of exposure to SHS per week was associated with a 1.03-fold increased likelihood of developing COPD after adjusting for confounders. These findings demonstrate the interplay between SHS exposure and the incidence of COPD in the Asia–Pacific region.
SHS poses a significant public health threat due to its widely recognized detrimental effects on health, and it is important to note that no level of SHS exposure is risk-free8–10,45,46. Even brief encounters with SHS can have considerable adverse impacts on the respiratory, cardiovascular, immune, and endocrine systems45,46. Numerous studies have explored the connection between COPD and SHS, of which many have primarily focused on the effects of SHS exposure in patients already diagnosed with COPD18,47,48. These investigations have reported associations between exposure to SHS with unfavorable patient-centered outcomes, such as dyspnea and chronic cough. In addition, SHS exposure has been linked to a reduced disease-specific quality of life in COPD patients who are not current smokers18,47. Furthermore, SHS has been shown to have detrimental effects on active smokers who have already been diagnosed with COPD48.
In line with our findings, numerous investigations have consistently emphasized a notable increase in the risk of COPD linked to SHS exposure, with odds ratios (ORs) ranging from 1.31 to 2.2420,49,50. An analysis conducted by Yin and colleagues of 6,497 Chinese individuals who had never smoked revealed that exposure to 40 h of passive smoking per week for more than 5 years was correlated with a 48% higher odds of spirometrically defined COPD20. Similarly, a cross-sectional study conducted in the United States involving 2,113 adults reported a 36% greater odds of developing COPD associated with exposure to SHS49. Three systematic reviews and meta-analyses, incorporating data from 21, 15, and 8 studies respectively, provided evidence supporting a correlation between SHS exposure and the risk of COPD, with relative risks of 1.44 (95% CI 0.67 to 3.12), 2.02 (95% CI 1.52 to 2.67), and 1.66 (95% CI 1.38 to 2.00), respectively14,51,52.
In contrast, other studies have reported a different perspective, with no direct link between exposure to SHS and the risk of developing COPD19,53–55. For example, a 20-year longitudinal study of 3,011 European adults—both never-smokers and ex-smokers—suggested a potential association between SHS exposure and respiratory symptoms; however, it did not establish a definitive link to changes in lung function or the occurrence of COPD55. Similarly, a study conducted by Kim et al. in Korea found no noticeable difference in the prevalence of COPD between never-smokers exposed and not exposed to SHS53. Moreover, a systematic review and meta-analysis of observations from seven prospective cohorts found no statistically significant evidence of an association between SHS exposure and COPD (RR = 1.21; 95% CI 0.93 to 1.57)14.
The disparities in outcomes noted among studies may originate from variances in study populations and inclusion criteria, notably accounting for the incorporation of ex-smokers in certain studies, potentially altering susceptibility to the effects of SHS. In addition, covariates integrated into the analyses may exacerbate potential bias and exert influence on the observed results. Furthermore, the prevalent utilization of cross-sectional designs in preceding studies necessitates careful consideration when drawing conclusive inferences. Therefore, the findings of this community-based, longitudinal investigation elucidating the association between SHS and COPD harbor significant potential for informing and shaping future research endeavors in this domain.
We also found a dose–response relationship between SHS and COPD, with a 1.03-fold increased risk of developing COPD with each additional hour of SHS exposure per week. Similar to our findings, several previous studies have also suggested a correlation between a longer duration of exposure to SHS with a higher prevalence of COPD50,56. In study conducted in Estonia, individuals exposed to more than 5 h of SHS per day (equivalent to over 35 h per week) exhibited a 1.54-fold higher risk (95% CI 1.13 to 3.00) of physician-diagnosed chronic bronchitis or emphysema56. Conversely, for those exposed to 1 to 5 h per day (equivalent to 7 to 35 h per week), the risk was lower (OR = 1.16; 95% CI 0.88 to 1.53)56. Similarly, a study from England reported that greater exposure to passive smoke was independently associated with an elevated risk of COPD, with an adjusted OR, 1.05 (95% CI 0.93 to 1.18) for 1 to 19 h of exposure per week, and 1.18 (95% CI 1.01 to 1.39) for 20 or more hours of exposure per week50.
SHS consists of 15% mainstream smoke, inhaled and exhaled by smokers, and 85% sidestream smoke emitted from the burning tip of a cigarette. Sidestream smoke is notably more toxic than mainstream smoke, and both contain a complex array of over 4,000 chemical compounds, including carcinogens and respiratory toxins57. Woodruff et al.58, and more recently, Goldklang et al.59, investigated into SHS-induced emphysema mechanisms using mouse models. Their research demonstrated that SHS exposure triggers an increase in alveolar macrophage recruitment and activation markers, mirroring responses observed in human smokers and ultimately leading to emphysema58. SHS exposure has also been demonstrated to contribute to the degradation of elastin and lung structure, further bolstering its association with the development of emphysema57. Recent research has also linked SHS to thicker airway walls in COPD patients, correlating with respiratory symptoms47,48. Taken together, these findings may explain the mechanisms underlying the association between chronic SHS exposure and COPD.
Some studies have investigated the short-term impact of SHS exposure on lung function and COPD, and others have examined the long-term effects. Notably, the TackSHS study found that acute SHS exposure (within 60 min) had no significant impact on COPD symptoms or spirometric indices after 24 hours60. In addition, Flouris et al. observed that decreases in lung function caused by 1 h of exposure to SHS receded within 60 min. However, they also found that elevated inflammatory cytokine levels persisted for at least 3 h post-SHS exposure, suggesting chronic low-grade inflammation in individuals consistently exposed to SHS45. This underscores that even brief SHS exposure can initiate mechanisms leading to COPD development.
Anti-smoking policies implemented worldwide have successfully reduced SHS exposure and improved respiratory symptoms, especially among bar and other hospitality workers19,61,62. A systematic review conducted by Callinan et al. found that the introduction of a smoking ban led to improved health outcomes through a reduction in SHS exposure, particularly in relation to cardiovascular disease61. However, consistent evidence on the reduction in active smoking is lacking, and exposure to SHS at home and in private cars has not changed significantly since implementing smoking bans63. These findings emphasize the numerous harms of SHS, and underscore the health-promoting effects of smoking bans. Consequently, our results and those of the previous studies reiterate the importance of smoking cessation and provide a foundation for governments to develop pertinent anti-smoking policies.
This study has several limitations. First, we assessed SHS exposure through self-reported surveys, introducing the potential for recall bias. To more comprehensively evaluate the dose-related association, obtaining information on the number of cigarettes smoked by others or assessing biomarkers such as urine cotinine or NNAL level could be valuable. It is important to note that these biomarkers are suitable for assessing short-term and medium-term exposure, respectively47. Second, we were unable to gather information on changes in exposure during the follow-up period and the total years of SHS exposure due to the simplicity of the survey questions. Given that COPD is associated with continuous and long-term exposure to risk factors, tracking changes in exposure and total exposure duration is crucial. A study with an extended follow-up period could provide further insights. Third, the participants may have had additional risk factors contributing to COPD that were not adjusted for as confounders in our study, such as occupational exposure or residing in industrial areas. Considering the potential impact of urban air pollution on incident COPD, adjustment for urbanization level may also be necessary. Lastly, no post-bronchodilator measurements were conducted; however, we did account for self-reported asthma in the adjustments.
In conclusion, the findings of this longitudinal study with 4 years of follow-up suggest a potential causal effect between SHS and COPD among non-smokers. We also identified a dose–response relationship between SHS exposure and the development of COPD. While randomized controlled trials remain the gold standard for establishing causality, they may present ethical challenges. Therefore, future prospective multi-racial, large-scale studies should be undertaken to further validate the impact of SHS on the development of COPD in individuals who have never smoked.
Author contributions
Data curation, J.-I.L. and J.-H.G.; formal analysis, H.-L.J. and J.-H.G.; investigation, S.-C.C. and J.-H.G.; methodology, H.-L.J. and J.-H.G.; project administration, J.-H.G.; resources, J.-H.G.; software, J.-H.G.; supervision, S.-C.C., S.-P H., and J.-H.G.; validation, J.-H.G.; writing—original draft, W.-C.S. and J.-H.G.; writing—review and editing, Wen-Chi Su and J.-H.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the Research Center for Environmental Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan, from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan and by Kaohsiung Medical University Research Center Grant (KMU-TC109A01-1 and KMUTC111IFSP01).
Data availability
The data underlying this study are from the Taiwan Biobank. Due to restrictions placed on the data by the Personal Information Protection Act of Taiwan, the minimal data set cannot be made publicly available. Data may be available upon request to interested researchers. Please send data requests to Szu-Chia Chen, Division of Nephrology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Wen-Chi Su and Huai-Lei Juan.
References
- 1.Cheng S-L, et al. COPD in Taiwan: a national epidemiology survey. Int. J. Chron. Obstruct. Pulmonary Disease. 2015;1:2459–2467. doi: 10.2147/COPD.S89672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez AD, Mathers CD. Measuring the global burden of disease and epidemiological transitions: 2002–2030. Ann. Trop. Med. Parasitol. 2006;100:481–499. doi: 10.1179/136485906X97417. [DOI] [PubMed] [Google Scholar]
- 3.Adeloye D, et al. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir. Med. 2022;10:447–458. doi: 10.1016/S2213-2600(21)00511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng S-L, et al. Update on guidelines for the treatment of COPD in Taiwan using evidence and GRADE system-based recommendations. J. Formos. Med. Assoc. 2021;120:1821–1844. doi: 10.1016/j.jfma.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Adeloye D, et al. Global and regional estimates of COPD prevalence: Systematic review and meta–analysis. Journal of global health. 2015;5:1. doi: 10.7189/jogh.05.020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. The Lancet. 2009;374:733–743. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- 7.Dm M. Global burden of COPD: Risk factors, prevalence, and future trends. Lancet. 2007;370:765–773. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 8.Tsai J, et al. Exposure to secondhand smoke among nonsmokers—United States, 1988–2014. Morbid. Mortal. Week. Rep. 2018;67:1342. doi: 10.15585/mmwr.mm6748a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jhee JH, et al. Secondhand smoke and CKD. Clin. J. Am. Soc. Nephrol.: CJASN. 2019;14:515. doi: 10.2215/CJN.09540818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim A-S, Ko H-J, Kwon J-H, Lee J-M. Exposure to secondhand smoke and risk of cancer in never smokers: A meta-analysis of epidemiologic studies. Int. J. Environ. research Public Health. 2018;15:1981. doi: 10.3390/ijerph15091981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhai C, et al. Global, regional, and national deaths, disability-adjusted life years, years lived with disability, and years of life lost for the global disease burden attributable to second-hand smoke, 1990–2019: A systematic analysis for the Global Burden of Disease Study. Sci. Total Environ. 2023;862:160677. doi: 10.1016/j.scitotenv.2022.160677. [DOI] [PubMed] [Google Scholar]
- 12.Lubick N. Global estimate of SHS burden. Environ. Health Perspect. 2011;119:A66–67. doi: 10.1289/ehp.119-a66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yousuf H, et al. Estimated worldwide mortality attributed to secondhand tobacco smoke exposure, 1990–2016. JAMA Network Open. 2020;3:e201177. doi: 10.1001/jamanetworkopen.2020.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flor LS, et al. Health effects associated with exposure to secondhand smoke: A Burden of Proof study. Nat. Med. 2024;30:149–167. doi: 10.1038/s41591-023-02743-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sung H-Y, Chang L-C, Wen Y-W, Tsai Y-W. The costs of smoking and secondhand smoke exposure in Taiwan: A prevalence-based annual cost approach. BMJ Open. 2014;4:e005199. doi: 10.1136/bmjopen-2014-005199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He Y, et al. Secondhand smoke exposure predicted COPD and other tobacco-related mortality in a 17-year cohort study in China. Chest. 2012;142:909–918. doi: 10.1378/chest.11-2884. [DOI] [PubMed] [Google Scholar]
- 17.Diver WR, Jacobs EJ, Gapstur SM. Secondhand smoke exposure in childhood and adulthood in relation to adult mortality among never smokers. Am. J. Prev. Med. 2018;55:345–352. doi: 10.1016/j.amepre.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Eisner MD, et al. Directly measured secondhand smoke exposure and COPD health outcomes. BMC Pulmonary Med. 2006;6:1–11. doi: 10.1186/1471-2466-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisner MD, et al. The impact of SHS exposure on health status and exacerbations among patients with COPD. Int. J. Chron. Obstruct. Pulmonary Disease. 2009;1:169–176. doi: 10.2147/COPD.S4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin P, et al. Passive smoking exposure and risk of COPD among adults in China: The Guangzhou Biobank Cohort Study. The Lancet. 2007;370:751–757. doi: 10.1016/S0140-6736(07)61378-6. [DOI] [PubMed] [Google Scholar]
- 21.David GL, Koh WP, Lee HP, Yu MC, London SJ. Childhood exposure to environmental tobacco smoke and chronic respiratory symptoms in non-smoking adults: The Singapore Chinese Health Study. Thorax. 2005;60:1052–1058. doi: 10.1136/thx.2005.042960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Behrendt CE. Mild and moderate-to-severe COPD in nonsmokers: Distinct demographic profiles. Chest. 2005;128:1239–1244. doi: 10.1378/chest.128.3.1239. [DOI] [PubMed] [Google Scholar]
- 23.Chan-Yeung M, et al. Determinants of chronic obstructive pulmonary disease in Chinese patients in Hong Kong. Int. J. Tubercul. Lung Dis. 2007;11:502–507. [PubMed] [Google Scholar]
- 24.Diver WR, Jacobs EJ, Gapstur SM. Secondhand smoke exposure in childhood and adulthood in relation to adult mortality among never smokers. Am J Prev Med. 2018;55:345–352. doi: 10.1016/j.amepre.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Gerbase MW, et al. Respiratory effects of environmental tobacco exposure are enhanced by bronchial hyperreactivity. Am. J. Respir. Crit. Care Med. 2006;174:1125–1131. doi: 10.1164/rccm.200512-1890OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pahwa P, et al. Incidence and longitudinal changes in prevalence of chronic bronchitis in farm and non-farm rural residents of Saskatchewan. J. Occup. Environ. Med. 2019;61:347–356. doi: 10.1097/jom.0000000000001560. [DOI] [PubMed] [Google Scholar]
- 27.Feng YA, et al. Taiwan Biobank: A rich biomedical research database of the Taiwanese population. Cell Genom. 2022;2:100197. doi: 10.1016/j.xgen.2022.100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei CY, et al. Genetic profiles of 103,106 individuals in the Taiwan Biobank provide insights into the health and history of Han Chinese. NPJ Genom. Med. 2021;6:10. doi: 10.1038/s41525-021-00178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li JY, et al. Hyperuricemia and its association with osteoporosis in a large Asian cohort. Nutrients. 2022;14:1. doi: 10.3390/nu14112206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang TY, et al. The association between menopause, postmenopausal hormone therapy, and kidney stone disease in Taiwanese women. Ann. Epidemiol. 2023;78:13–18. doi: 10.1016/j.annepidem.2022.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Ho CY, Lee JI, Huang SP, Chen SC, Geng JH. A genome-wide association study of metabolic syndrome in the Taiwanese population. Nutrients. 2023;16:1. doi: 10.3390/nu16010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen YH, et al. The impact of secondhand smoke on the development of kidney stone disease is not inferior to that of smoking: A longitudinal cohort study. BMC Publ Health. 2023;23:1189. doi: 10.1186/s12889-023-16116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verberne LD, et al. Overweight in patients with chronic obstructive pulmonary disease needs more attention: a cross-sectional study in general practice. NPJ Primary Care Respirat. Med. 2017;27:63. doi: 10.1038/s41533-017-0065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaluza J, Harris HR, Linden A, Wolk A. Alcohol consumption and risk of chronic obstructive pulmonary disease: A prospective cohort study of men. Am. J. Epidemiol. 2019;188:907–916. doi: 10.1093/aje/kwz020. [DOI] [PubMed] [Google Scholar]
- 35.Kim S-H, et al. Chronic obstructive pulmonary disease is independently associated with hypertension in men: A survey design analysis using nationwide survey data. Medicine. 2017;96:1. doi: 10.1097/MD.0000000000006826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur. Respirat. J. 2008;32:962–969. doi: 10.1183/09031936.00012408. [DOI] [PubMed] [Google Scholar]
- 37.Kahnert K, et al. Relationship of hyperlipidemia to comorbidities and lung function in COPD: Results of the COSYCONET cohort. PLoS One. 2017;12:e0177501. doi: 10.1371/journal.pone.0177501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cukic V, Lovre V, Dragisic D, Ustamujic A. Asthma and chronic obstructive pulmonary disease (COPD)–differences and similarities. Materia Socio-medica. 2012;24:100. doi: 10.5455/msm.2012.24.100-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen C-Y, Liao K-M. Chronic obstructive pulmonary disease is associated with risk of chronic kidney disease: a nationwide case-cohort study. Sci. Rep. 2016;6:25855. doi: 10.1038/srep25855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabe KF, Hurst JR, Suissa S. Cardiovascular disease and COPD: dangerous liaisons? Eur. Respirat. Rev. 2018;27:1. doi: 10.1183/16000617.0057-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Negi H, Sarkar M, Raval AD, Pandey K, Das P. Presence of depression & its risk factors in patients with chronic obstructive pulmonary disease. Indian J. Med. Res. 2014;139:402. [PMC free article] [PubMed] [Google Scholar]
- 42.Bartziokas K, et al. Serum uric acid as a predictor of mortality and future exacerbations of COPD. Eur. Respirat. J. 2014;43:43–53. doi: 10.1183/09031936.00209212. [DOI] [PubMed] [Google Scholar]
- 43.Christensen S, et al. Impact of COPD on outcome among patients with complicated peptic ulcer. Chest. 2008;133:1360–1366. doi: 10.1378/chest.07-2543. [DOI] [PubMed] [Google Scholar]
- 44.Huang CH, et al. Betel nut chewing was associated with obstructive lung disease in a large Taiwanese population study. J. Personaliz. Med. 2021;11:1. doi: 10.3390/jpm11100973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flouris AD, et al. Acute and short-term effects of secondhand smoke on lung function and cytokine production. Am. J. Respirat. Crit. Care Med. 2009;179:1029–1033. doi: 10.1164/rccm.200812-1920OC. [DOI] [PubMed] [Google Scholar]
- 46.Flouris AD, Vardavas CI, Metsios GS, Tsatsakis AM, Koutedakis Y. Biological evidence for the acute health effects of secondhand smoke exposure. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2010;298:L3–L12. doi: 10.1152/ajplung.00215.2009. [DOI] [PubMed] [Google Scholar]
- 47.Fu Z, et al. Objective secondhand smoke exposure in chronic obstructive pulmonary disease patients without active smoking: The US National Health and Nutrition Examination Survey (NHANES) 2007–2012. Ann. Transl. Med. 2020;8:1. doi: 10.21037/atm.2020.03.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Putcha N, et al. Understanding the impact of second-hand smoke exposure on clinical outcomes in participants with COPD in the SPIROMICS cohort. Thorax. 2016;71:411–420. doi: 10.1136/thoraxjnl-2015-207487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eisner MD, et al. Lifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary disease. Environ. Health. 2005;4:1–8. doi: 10.1186/1476-069X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jordan RE, Cheng KK, Miller MR, Adab P. Passive smoking and chronic obstructive pulmonary disease: Cross-sectional analysis of data from the Health Survey for England. BMJ Open. 2011;1:e000153. doi: 10.1136/bmjopen-2011-000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen P, Li Y, Wu D, Liu F, Cao C. Secondhand smoke exposure and the risk of chronic obstructive pulmonary disease: A systematic review and meta-analysis. Int. J. Chron. Obstruct. Pulmon. Dis. 2023;18:1067–1076. doi: 10.2147/copd.s403158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fischer F, Kraemer A. Meta-analysis of the association between second-hand smoke exposure and ischaemic heart diseases, COPD and stroke. BMC Public Health. 2015;15:1202. doi: 10.1186/s12889-015-2489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim WJ, et al. The effects of secondhand smoke on chronic obstructive pulmonary disease in nonsmoking Korean adults. Kor. J. Internal Med. 2014;29:613. doi: 10.3904/kjim.2014.29.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jaakkola MS, Jaakkola JJ. Effects of environmental tobacco smoke on the respiratory health of adults. Scandinav. J. Work Environ. Health. 2002;1:52–70. [PubMed] [Google Scholar]
- 55.Flexeder C, et al. Second-hand smoke exposure in adulthood and lower respiratory health during 20 year follow up in the European Community Respiratory Health Survey. Respirat. Res. 2019;20:1–15. doi: 10.1186/s12931-019-0996-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larsson ML, et al. Passive smoking and respiratory symptoms in the FinEsS Study. Eur. Respirat. J. 2003;21:672–676. doi: 10.1183/09031936.03.00033702. [DOI] [PubMed] [Google Scholar]
- 57.Salvi S. Tobacco smoking and environmental risk factors for chronic obstructive pulmonary disease. Clin. Chest Med. 2014;35:17–27. doi: 10.1016/j.ccm.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 58.Woodruff PG, et al. Alveolar macrophage recruitment and activation by chronic second hand smoke exposure in mice. COPD J. Chron. Obstruct. Pulmonary Dis. 2009;6:86–94. doi: 10.1080/15412550902751738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldklang MP, Marks SM, D'Armiento JM. Second hand smoke and COPD: Lessons from animal studies. Front. Physiol. 2013;4:30. doi: 10.3389/fphys.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keogan S, et al. Lung function changes in patients with chronic obstructive pulmonary disease (COPD) and asthma exposed to secondhand smoke in outdoor areas. J. Asthma. 2021;58:1169–1175. doi: 10.1080/02770903.2020.1766062. [DOI] [PubMed] [Google Scholar]
- 61.Frazer K, et al. Legislative smoking bans for reducing harms from secondhand smoke exposure, smoking prevalence and tobacco consumption. Cochrane Database Syst. Rev. 2016;1:1. doi: 10.1002/14651858.CD005992.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Menzies D, et al. Respiratory symptoms, pulmonary function, and markers of inflammation among bar workers before and after a legislative ban on smoking in public places. JAMA. 2006;296:1742–1748. doi: 10.1001/jama.296.14.1742. [DOI] [PubMed] [Google Scholar]
- 63.Callinan, J. E., Clarke, A., Doherty, K. & Kelleher, C. Legislative smoking bans for reducing secondhand smoke exposure, smoking prevalence and tobacco consumption. Cochrane Database Syst. Rev. (2010). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this study are from the Taiwan Biobank. Due to restrictions placed on the data by the Personal Information Protection Act of Taiwan, the minimal data set cannot be made publicly available. Data may be available upon request to interested researchers. Please send data requests to Szu-Chia Chen, Division of Nephrology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University.