Abstract
We describe the complete mitogenomes of the black corals Alternatipathesmirabilis Opresko & Molodtsova, 2021 and Parantipatheslarix (Esper, 1790) (Cnidaria, Anthozoa, Hexacorallia, Antipatharia, Schizopathidae). The analysed specimens include the holotype of Alternatipathesmirabilis, collected from Derickson Seamount (North Pacific Ocean; Gulf of Alaska) at 4,685 m depth and a potential topotype of Parantipatheslarix, collected from Secca dei Candelieri (Mediterranean Sea; Tyrrhenian Sea; Salerno Gulf; Italy) at 131 m depth. We also assemble, annotate and make available nine additional black coral mitogenomes that were included in a recent phylogeny (Quattrini et al. 2023b), but not made easily accessible on GenBank. This is the first study to present and compare two mitogenomes from the same species of black coral (Stauropathesarctica (Lütken, 1871)) and, thus, place minimum boundaries on the expected level of intraspecific variation at the mitogenome level. We also compare interspecific variation at the mitogenome-level across five different specimens of Parantipathes Brook, 1889 (representing at least two different species) from the NE Atlantic and Mediterranean Sea.
Key words: Antipatharian, genome skimming, holotype, intraspecific variation, Mitofinder, Parantipathes , Stauropathesarctica
Introduction
Black corals (Cnidaria, Anthozoa, Hexacorallia, Antipatharia) are found in all oceans and hold the record for the deepest (Schizopathesaffinis Brook, 1889 at 8,900 m; Molodtsova (2006)) and longest-lived (Leiopathesglaberrima (Esper, 1792) at 4,265 years; Roark et al. (2009)) coral and serve as underwater hosts for a diverse and staggering number of epibionts (Love et al. 2007). Black corals have historically been considered a deep-water group; however, only 31.57% of the 285 currently-described species occur at depths greater than 800 m (Molodtsova et al. 2022, 2023). While the black coral community continues to wait for the first antipatharian nuclear genome, black coral mitogenomics is gaining in popularity due to the ease of bioinformatically extracting whole mitogenomes from genome-skimming data (Quattrini et al. 2023a), its informativeness, cost-effectiveness and the availability of comparative data (Brugler and France 2007; Sinniger and Pawlowski 2009; Kayal et al. 2013; Figueroa et al. 2019; Barrett et al. 2020; Asorey et al. 2021; Bledsoe-Becerra et al. 2022; Feng et al. 2023; Quattrini et al. 2023a, b; Ramos et al. 2023). Herein, we describe two additional black coral mitogenomes (Alternatipathesmirabilis Opresko & Molodtsova, 2021 and Parantipatheslarix (Esper, 1790)), both from the family Schizopathidae and present them in a phylogenetic context. We analysed the holotype of Alternatipathesmirabilis, collected from Derickson Seamount (North Pacific Ocean; Gulf of Alaska) at 4,685 m depth and a potential topotype of Parantipatheslarix, collected from Secca dei Candelieri (Mediterranean Sea; Tyrrhenian Sea; Salerno Gulf; Italy) at 131 m depth. According to Esper (1792), the type locality of P.larix is in the “ocean near Naples,” which is adjacent to the Gulf of Salerno.
The genus Alternatipathes was established by Molodtsova and Opresko (2017) with the type species of the genus as Umbellapathesbipinnata Opresko, 2005 (Opresko and Molodtsova 2021). Species assigned to the genus include Umbellapathesbipinnata, Bathypathesalternata Brook, 1889, Alternatipathesvenusta Opresko & Wagner, 2020 and Alternatipathesmirabilis. Alternatipathesmirabilis is only known from a single specimen, which we analysed herein. The species name (mirabilis) is derived from Latin meaning “wonderful or strange.” The genus is broadly distributed in the Pacific, Indian, Atlantic and Southern Ocean basins at depths usually exceeding 2,500 m and often greater than 4,000 m (Opresko and Molodtsova 2021). DNA analysis of mitochondrial nad5-nad1 from the holotype suggested a close relationship to Schizopathes Brook, 1889 (Chery et al. 2018); however, the full mitogenome of Schizopathes is not currently available to test this hypothesis more robustly. The genus Parantipathes was established by Brook (1889) with the type species of the genus as Antipatheslarix Esper, 1790 (Opresko and Baron-Szabo 2001). In terms of its distribution, this species is only known from the Mediterranean Sea and eastern Atlantic Ocean.
In addition to describing the mitogenomes of Alternatipathesmirabilis and Parantipatheslarix, we also assembled, annotated and made available nine additional black coral mitogenomes that were included in a recent phylogeny (Quattrini et al. 2023b), but not made easily accessible on GenBank (i.e. mtDNA reads are embedded in non-annotated bulk Illumina whole genome shotgun fastq files). The taxa include Acanthopathesthyoides (Pourtalès, 1880) (USNM1288453), Aphanipathespedata (Gray, 1857) (USNM1288458), Bathypathesalaskensis Opresko & Molodtsova, 2021 (USNM1288462), Elatopathesabietina (Pourtalès, 1874) (USNM1288451), Parantipathes sp. (MSS29), Stauropathesarctica (Lütken, 1871) (DFONL ID #4089; Canadian Museum of Nature catalogue #CMNI 2023-0258), Stauropathes sp. Opresko, 2002 (USNM1404493), Telopathesmagna MacIsaac & Best, 2013 (MacIsaac et al. 2013) (USNM1204049) and Umbellapathes sp. Opresko, 2005 (USNM1404092).
Herein, we also compare two mitogenomes from the same species of black coral (Stauropathesarctica) and determine the expected level of intraspecific variation at the mitogenome level, which has not been done previously. We compare the results of this intraspecific comparison to the unexpectedly low mitogenome-level variation found within the trigeneric complex (Dendrobathypathes, Lillipathes and Parantipathes from the eastern North Pacific; Bledsoe-Becerra et al. (2022)). We also compare interspecific variation at the mitogenome-level across five different specimens of Parantipathes (representing at least two different species) from the northeast Atlantic and Mediterranean Sea.
Materials and methods
Specimen collection and species identification
The holotype of Alternatipathesmirabilis Opresko & Molodtsova, 2021 (USNM1070972) was collected by Dr. Amy Baco-Taylor on 20 July 2004, from Derickson Seamount (North Pacific Ocean; Gulf of Alaska; Station # JD-093) at 4,685 m depth using the Jason II ROV (Latitude, Longitude: 53.0419, -161.183). The holotype of A.mirabilis was deposited into the black coral collection at the Smithsonian Institution’s National Museum of Natural History (NMNH). Specimens accessioned into the SI NMNH’s Invertebrate Zoology collection are freely available to researchers to access and study. A.mirabilis was identified by Drs. Dennis Opresko and Tina Molodtsova, the leading authorities on black coral taxonomy and systematics. Parantipatheslarix (Esper, 1788) (USNM1280881) was collected in July 2012 from Secca dei Candelieri (Mediterranean Sea; Tyrrhenian Sea; Salerno Gulf; Italy) at 131 m depth (Latitude, Longitude: 40.0744, 15.8765). P.larix was also deposited into the SI NMNH’s Invertebrate Zoology collection. P.larix was identified by Dr. Marzia Bo of the Universita di Genova in Italy, also an authority on black coral taxonomy and systematics.
Specimen preparation and sequencing
Tissues from Alternatipathesmirabilis (OGL-E27108; USNM1070972) and Parantipatheslarix (OGL-E27184; USNM1280881) were initially stored in 95% ethanol. DNA was isolated from these samples using a modified CTAB extraction protocol (France et al. 1996). Specifically, tissue samples were incubated in 750 μl of 2X-CTAB with 50 μl Proteinase K (Qiagen, Hilden, Germany) overnight before digestion at 56 °C for 3 hours. Ceramic beads (200 μl, 0.1 mm) were added to each sample and tubes were placed in a BeadBug microtube homogeniser (Benchmark Scientific, South Plainfield, NJ, USA) for two 30 second intervals at 2,800 rpm. Next, particulate material was precipitated by centrifugation at 17K RCF (Relative Centrifugal Force or g-force) for 5 minutes and the supernatants were transferred to new tubes with 750 μl of -20 °C chloroform, vortexed until cloudy and phases were separated by centrifugation at 17K RCF for 10 minutes. Supernatants were then transferred to tubes with 750 μl of -20 °C absolute ethanol, inverted and phases were separated by centrifugation at 17K RCF for 5 minutes. Supernatants were discarded and precipitated DNA was washed with 750 μl of 70% ethanol and then pelleted by centrifugation at 17K RCF for 5 min. Supernatants were again discarded and pellets were dried using a Savant DNA 120 Speedvac Concentrator (Thermo Scientific, Waltham, MA, USA) before suspension in 50 μl of Buffer AE (Qiagen, Hilden, Germany). DNA extracts were subsequently treated with RNase A and purified using a Zymo Research DNA Clean & Concentrator (Irvine, CA, USA). To visualise DNA, 2 μl of each extract was loaded on to a horizontal slab gel (1% agarose, 1X TAE buffer containing 1% Biotium GelRed nucleic acid gel stain; Freemont, CA, USA) and separated at approximately 175 V for 5 min then 130 V for 30 min and visualised using a Bio-Rad Gel Doc XR + Molecular Imager and Image Lab software (Hercules, CA, USA). To quantify DNA present in each extract, 5 μl of each sample was analysed using a Promega QuantiFluor ONE dsDNA System with a Quantus Fluorometer (Madison, WI, USA). DNA extractions were sent to the New York Genome Center for whole genome shotgun (WGS) sequencing on an Illumina HiSeqX (2x150 bp). Library preparation utilised a TruSeq PCR-free kit (450 bp).
Bioinformatics
Mitochondrial genomes were bioinformatically extracted from the WGS runs using MitoFinder v.1.4 (Allio et al. 2020). MitoFinder employed MEGAHIT v.3.0 (Li et al. 2015) for mitogenome assembly and tRNAscan-SE (Chan and Lowe 2019) for tRNA annotation. The following command was used to run MitoFinder on an iMac: ./mitofinder --megahit --override --new-genes -j [file name] -1 [left_reads.fastq.gz] -2 [right_reads.fastq.gz] -r [genbank_reference.gb] -o [genetic_code] -p [threads] -m [memory] -t trnascan. Stichopathesluetkeni (GenBank Accession # NC_018377) was used as the reference and translation table 4 (Mold, Protozoan and Coelenterate Mitochondrial Code and the Mycoplasma/Spiroplasma Code) was used as the genetic code. Newly-assembled mitogenomes were annotated using the MITOS Web Server (Bernt et al. 2013).
Phylogenetic analysis
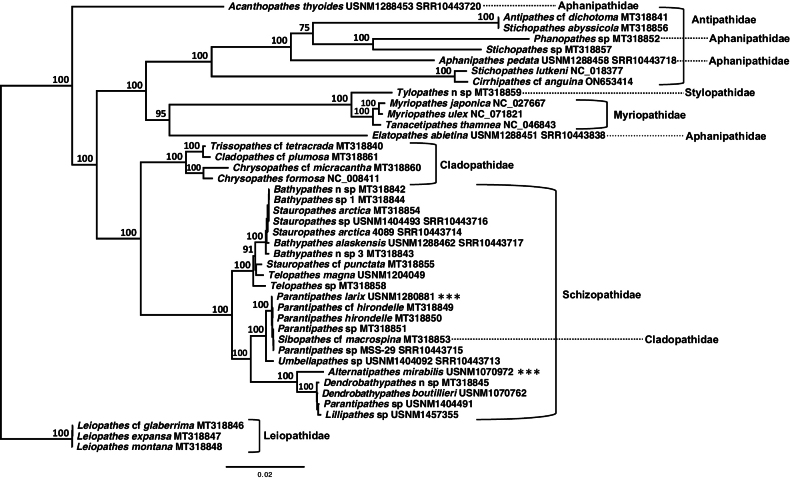
The newly-obtained mitogenomes of Alternatipathesmirabilis (USNM1070972; GenBank Accession Number OR398473) and Parantipatheslarix (USNM1280881; GenBank Accession Number OR398474) were added to the phylogeny presented in Bledsoe-Becerra et al. (2022) that contained 29 mitogenomes. We then assembled, annotated and added nine black coral mitogenomes that were included in a recent phylogeny (Quattrini et al. 2023b), but not made easily accessible on GenBank. We also included two newly-released black coral mitogenomes: Myriopathesulex (Ellis & Solander, 1786) (NC_071821) and Cirrhipathescf.anguina (Dana, 1846) (ON653414; Shizuru et al. (2024)) for a total of 42 taxa. Each of the 13 protein-coding genes (cox1-3, nad1-6, nad4L, atp6, atp8 and cytb) and two ribosomal RNAs (12S and 16S) from all 42 mitogenomes were placed in individual AliView v.1.23 (Larsson 2014) files, individually aligned using MAFFT LINS-i v.7 (Katoh et al. 2019) and subsequently concatenated into a single file using Seqotron v.1.0.1 (Fourment and Holmes 2016), treating the mitogenome as a single locus. Significant length variation was encountered within each of the 18 intergenic regions (IGRs) across the seven families, resulting in ambiguous alignments within these regions; thus, IGRs were not considered. The final dataset consisted of 42 taxa and 16,416 sites (alignment available upon request to co-author Brugler). The Akaike Information Criterion within jModelTest v.2.1.10 (Guindon and Gascuel 2003; Darriba et al. 2012) selected the GTR + I + G model of sequence evolution (p-inv: 0.4670; gamma: 1.0920). XSEDE on the CIPRES Science Gateway v.3.3 (Miller et al. 2011) was used to construct a Maximum Likelihood phylogeny using IQ-Tree v.2.2.2.5 with the GTR+I+G model of sequence evolution, a BioNJ starting tree and 1,000 ultrafast bootstrap replicates (Hoang et al. 2018; Minh et al. 2020). The resulting phylogenetic tree was visualised using FigTree v.1.4.4 (by Andrew Rambaut; https://github.com/rambaut/figtree/releases). The ML tree (Fig. 1) was rooted internally to the Leiopathidae. This decision was based on: 1) the mitogenome-based phylogeny presented in Barrett et al. (2020) that included nine hexacoral outgroups (4 actiniarians, 3 zoantharians, 1 scleractinian and 1 corallimorpharian) and 2) a time-calibrated phylogeny by Horowitz et al. (2023), based on target-capture enrichment of 2,380 ultraconserved elements and exonic loci from 83 species of black coral and nine outgroups, both of which recovered the Leiopathidae as an early branching, monophyletic group sister to all other antipatharian families (but see DeSalle et al. (2023)). The number of variable sites (or single nucleotide polymorphisms; SNPs) and pairwise distance estimates were calculated using MEGA X (Kumar et al. 2018; Stecher et al. 2020) and included the Kimura 2-Parameter model (K2P), uniform rates amongst sites and pairwise deletion of gaps/missing data.
Figure 1.
Maximum Likelihood phylogenetic tree, based on 13 protein-coding genes and two ribosomal RNAs (42 taxa and 16,416 sites). The mitogenomes of Alternatipathesmirabilis (USNM1070972; OR398473) and Parantipatheslarix (USNM1280881; OR398474) are indicated with three asterisks. The families Aphanipathidae and Cladopathidae are polyphyletic with representatives indicated with a horizontal dotted line. The tree is rooted internally to the Leiopathidae. Node support values are based on 1,000 ultrafast bootstrap replicates. Species IDs are followed by museum voucher codes (e.g. USNM) and/or GenBank accession numbers (e.g. MT, NC, ON or SRR).
Results
The Alternatipathesmirabilis mitogenome (OR398473) is 17,632 bp in length and contains the typical 13 protein-coding genes (cox1-3, nad1-6, nad4L, atp6, atp8 and cytb), two ribosomal RNAs (12S and 16S) and two transfer RNAs (Met and Trp). Intergenic regions account for 8.72% (1,538 bp) of the mitogenome, with the longest IGR between nad5 (5’) and nad1 (365 bp; Table 1). The Parantipatheslarix mitogenome (OR398474) is 17,734 bp in length and contains the typical 13 protein-coding genes (cox1-3, nad1-6, nad4L, atp6, atp8 and cytb), two ribosomal RNAs (12S and 16S) and two transfer RNAs (Met and Trp). Intergenic regions account for 9.41% (1,669 bp) of the mitogenome, with the longest IGR between nad5 (5’) and nad1 (367 bp; Table 1). Both A.mirabilis and P.larix have the typical black coral mitochondrial gene order; thus, to date, one unique gene order has been observed within the Order Antipatharia. Base composition was similar between A.mirabilis (A: 5841, T: 4701, G: 3180, C: 3910) and P.larix (A: 5895, T: 4708, G: 3212, C: 3919) and both mitogenomes are AT-rich (59.79% each).
Table 1.
Lengths of protein-coding genes, ribosomal RNAs, transfer RNAs and intergenic regions (IGRs) within the Alternatipathesmirabilis (17,632 bp; OR398473) and Parantipatheslarix (17,734 bp; OR398474) mitogenomes.
| Gene | Parantipatheslarix | Alternatipathesmirabilis |
|---|---|---|
| 12S | 1141 | 1141 |
| IGR | 197 | 197 |
| nad2 | 1518 | 1518 |
| IGR | 19 | 19 |
| tRNA Trp | 70 | 70 |
| IGR | 27 | 27 |
| nad5-3’ | 1131 | 1131 |
| IGR | 115 | 115 |
| nad3 | 357 | 357 |
| IGR | 48 | 32 |
| nad1 | 984 | 984 |
| IGR | 367 | 365 |
| nad5-5’ | 708 | 708 |
| IGR | 108 | 46 |
| atp6 | 699 | 699 |
| IGR | 82 | 82 |
| atp8 | 213 | 213 |
| IGR | 24 | 24 |
| nad6 | 564 | 564 |
| IGR | 104 | 87 |
| nad4 | 1476 | 1476 |
| IGR | 61 | 61 |
| cox2 | 750 | 750 |
| IGR | 82 | 68 |
| nad4L | 300 | 300 |
| IGR | 92 | 92 |
| cox1 | 1590 | 1590 |
| IGR | 34 | 34 |
| cox3 | 789 | 789 |
| IGR | 96 | 74 |
| 16S | 2561 | 2590 |
| IGR | 64 | 74 |
| tRNA Met | 71 | 71 |
| IGR | 49 | 40 |
| cytb | 1143 | 1143 |
| IGR | 100 | 101 |
After assembling nine mitogenomes that were included in a phylogeny in Quattrini et al. (2023b), mitogenome sizes ranged from 17,699 (Bathypathesalaskensis USNM1288462) to 20,066 bp (Acanthopathesthyoides USNM1288453). Quattrini et al. (2023b) simply uploaded Illumina fastq files and each of the 13 protein-coding genes individually, but did not upload the two ribosomal RNAs (12S and 16S), two transfer RNAs (Met and Trp) or any of the intergenic regions. To make the data more easily accessible, we pulled data from the Illumina fastq files to assemble and annotate full mitogenomes. Complete nucleotide sequence data are now available in the Third Party Annotation (TPA) section of the DDBJ/ENA/GenBank databases under the following accession numbers: BK063761 (Acanthopathesthyoides; USNM1288453), BK063759 (Aphanipathespedata; USNM1288458), BK063764 (Bathypathesalaskensis; USNM1288462), BK063760 (Elatopathesabietina; USNM1288451), BK063757 (Parantipathes sp.; MSS29), BK063763 (Stauropathesarctica; DFONL ID #4089; Canadian Museum of Nature catalogue #CMNI 2023-0258), BK063762 (Stauropathes sp.; USNM1404493), OR398475 (Telopathesmagna; USNM1204049) and BK063758 (Umbellapathes sp.; USNM1404092). The two newly-released black coral mitogenomes ranged in size from 17,711 bp (MyriopathesulexNC_071821 [OP104910]; released 3 April 2023) to 20,452 bp (Cirrhipathescf.anguinaON653414; released 24 December 2022; Shizuru et al. (2024)). Elatopathesabietina (BK063760) and Myriopathesulex (NC_071821) were the only taxa that contained a LAGLI-DADG homing endonuclease in the cox1 gene (Beagley et al. 1996).
Discussion
The Maximum Likelihood phylogeny (Fig. 1), consisting of 42 taxa and 16,416 sites, largely mirrors the phylogeny presented in Brugler et al. (2013) that was based on three mitochondrial gene regions (igrW, igrN and cox3-cox1); however, the mitogenome-based phylogeny, presented here, yields greater bootstrap support. In our new mitogenome-based phylogeny, the holotype of Alternatipathesmirabilis is sister to a clade containing Dendrobathypathes Opresko, 2002, Parantipathes (from the North Pacific Ocean) and Lillipathes Opresko, 2002 (bootstrap support: 100), while a putative topotype of Parantipatheslarix is placed within a clade containing additional Parantipathes (all from the Northeast Atlantic), Sibopathes van Pesch, 1914 and Umbellapathes (bootstrap support: 100). Sibopathes is currently classified in the family Cladopathidae yet falls within the Schizopathidae in our analyses. However, any potential reclassification of this genus should include data from the type specimen of Sibopathes. These data were not available at the time of this analysis.
According to our analyses, the family Aphanipathidae is polyphyletic with representatives forming a group sister to the majority of antipatharians (Acanthopathesthyoides USNM1288453; bootstrap support: 100), sister to the Myriopathidae (Elatopathesabietina USNM1288451; bootstrap support: 95) or sister to different representatives of the Antipathidae (Aphanipathespedata USNM1288458 and Phanopathes sp. Opresko, 2004 MT318852; bootstrap support: 100; taxon sampling within the Antipathidae is very limited as our phylogeny only includes five of 122+ species within the family).
In Brugler et al. (2013), Acanthopathesthyoides (USNM1288453) and Elatopathesabietina (USNM1288451) were considered “wandering taxa” as their phylogenetic relationship shifted depending on the dataset or tree-building algorithm. It appears that our new mitogenome-based phylogeny has stabilised their position and revealed more strongly-supported phylogenetic affiliations for both taxa.
Only one representative from the family Stylopathidae was included in the phylogeny (Tylopathes sp. nov. Brook, 1889 MT318859) and is sister to the Myriopathidae (bootstrap support: 100). Any potential reclassification of these genera within the Myriopathidae will require sequence data from the remaining genera within the Stylopathidae (Stylopathes Opresko, 2006 and Triadopathes Opresko, 2006). These data were not available at the time of this analysis.
To our knowledge, this study is the first to compare two mitogenomes from the same species of black coral (StauropathesarcticaMT318854 and CMNI 2023-0258) and thus we can, for the first time, place lower limits on the expected level of intraspecific variation at the mitogenome level. Both mitogenomes are 17,700 bp in length and a comparison revealed 12 SNPs (K2P distance: 0.0678%). Stauropathesarctica (MT318854) was collected at 1,446 m depth from North Porcupine Bank (NE Atlantic; Irish Margin). Stauropathesarctica (CMNI 2023-0258) was collected at 600 m depth from Treworgie Canyon (NW Atlantic; Grand Banks of Newfoundland). Bledsoe-Becerra et al. (2022) compared the mitogenomes of the trigeneric complex (Dendrobathypathes, Lillipathes and Parantipathes from the eastern North Pacific) and only found 32 SNPs across 17,687 bp. Pairwise comparisons revealed 18 (Dendrobathypathes and Parantipathes) and 23 (Lillipathes and Parantipathes; Lillipathes and Dendrobathypathes) SNPs. If future mitogenomic studies show that approximately 12 SNPs are typical of intraspecific comparisons within the Antipatharia, then 18 and 23 SNPs may be indicative of interspecific variation and, thus, Dendrobathypathes, Lillipathes and Parantipathes (from the eastern North Pacific) could be consolidated into a single genus. However, a black coral nuclear genome is not available at this time, which could fundamentally change our understanding of species relationships within this group. Therefore, a major consolidation of multiple genera is not advised until nuclear genomes are also sequenced and analysed. It is also important to note that the mitogenome-level comparisons noted above (for Stauropathes, Dendrobathypathes, Lillipathes and Parantipathes) are all for taxa within the family Schizopathidae and, thus, variation within, or thresholds between, other families may differ given their different evolutionary histories. As per Horowitz et al. (2023), 95% of extant black corals were recovered in two distinct clades that diverged ~ 295 million years ago (during the Carboniferous-Permian) on the continental slope. The first clade contained members of the Antipathidae, Aphanipathidae, Myriopathidae and Stylopathidae with crown node at 242 My; these taxa largely stayed on the slope or moved up on to the shelf. The second clade contained members of the Schizopathidae and Cladopathidae with crown node at 202 My; these taxa are largely found at slope and abyssal depths.
We also had the unique opportunity to compare interspecific variation at the mitogenome-level across five different specimens of Parantipathes from the NE Atlantic and Mediterranean Sea (Parantipathescf.hirondelleMT318849; Parantipatheshirondelle Molodtsova, 2006 MT318850; Parantipatheslarix USNM1280881; Parantipathes sp. MSS-29; Parantipathes sp. MT318851). We also included Sibopathescf.macrospina (MT318853) in this analysis as it groups phylogenetically amongst these five Parantipathes. All six mitogenomes are 17,734 bp in length and a comparison revealed only 18 SNPs (K2P distances ranged from 0.00564% [Sibopathescf.macrospinaMT318853 vs. Parantipathes sp. MT318851 and Parantipathes sp. MSS-29 and Parantipathescf.hirondelleMT318849 vs. ParantipatheshirondelleMT318850] to 0.0843% [Parantipatheslarix USNM1280881 vs. Parantipathes sp. MSS-29]). These results also support consolidating Dendrobathypathes, Lillipathes and Parantipathes (from the eastern North Pacific) into a single genus. Again, obtaining sequence data from the type specimen of Sibopathes will be necessary prior to the potential reclassification of this genus.
We encourage future black coral mitogenomic studies to focus on obtaining mitogenomes from type species (where possible) and continue to fill in missing taxonomic gaps, particularly in the Antipathidae, Aphanipathidae, Myriopathidae and Stylopathidae.
While morphological characteristics are the gold standard for delineating relationships amongst organisms, the combined use of morphology and genetics is a powerful combination to better understand evolutionary relationships (e.g. Wagner et al. (2010); Horowitz et al. (2020)). In fact, many fields must entirely rely on genetics to characterise diversity at the family level and below because morphology is lacking (e.g. Blank and Trench (1985); LaJeunesse et al. (2014)) and/or morphological characteristics are problematic (e.g. Pinzón and Lajeunesse (2011); Pinzón et al. (2013); Rodríguez et al. (2014); Bledsoe-Becerra et al. (2022); Opresko et al. (2022); Molodtsova et al. (2023)). Based on the data presented here and clear ambiguities created when using morphological characteristics of black coral, we strongly advocate that the black coral community preferentially use diversity at the molecular level to delineate evolutionary relatedness between groups and morphology only be used to support relationships revealed by molecular analyses. We also urge the International Commission on Zoological Nomenclature (ICZN), which is responsible for producing the International Code of Zoological Nomenclature, to incorporate robust molecular comparisons into species descriptions to account for instances where morphology fails.
Acknowledgements
We thank our reviewers, Anthony Montgomery and Jeremy Horowitz, for greatly improving an earlier version of the manuscript. Our ZooKeys subject editor, James Reimer, also deserves special recognition for providing a very positive experience for our team. MRB is a Research Associate at the American Museum of Natural History and the Smithsonian Institution’s National Museum of Natural History and gratefully acknowledges these affiliations.
Citation
Cruz BA, Cappelmann A, Chutjian H, Roman JC, Reid MA, Wright J, Gonzalez AD, Keyman T, Griffith KM, Appiah-Madson HJ, Distel DL, Hayes VE, Drewery J, Pettay DT, Staton JL, Brugler MR (2024) Complete mitochondrial genomes of the black corals Alternatipathes mirabilis Opresko & Molodtsova, 2021 and Parantipathes larix (Esper, 1788) (Cnidaria, Anthozoa, Hexacorallia, Antipatharia, Schizopathidae). ZooKeys 1196: 79–93. https://doi.org/10.3897/zookeys.1196.116837
Funding Statement
Port Royal Sound Foundation; USCB’s Summer Research Experience Scholarship Program; University of South Carolina SMART Program
Additional information
Conflict of interest
The authors have declared that no competing interests exist.
Ethical statement
An institutional animal care and use committee (IACUC) permit was not necessary as black corals are not vertebrates or cephalopods (Phylum Mollusca). Black corals are protected under appendix II of the Convention on International Trade of Endangered Species (CITES; www.cites.org). Organisms listed in appendix II require an export permit as well as a Certificate of Scientific Exchange (COSE) on the receiving end. The Smithsonian Institution’s National Museum of Natural History (NMNH) maintains an active COSE permit and, thus, can receive black corals that are shipped to them with the appropriate export permit. All black corals in the SI NMNH collection have been vetted for proper export permits. The SI NMNH is the institution from which the specimens studied herein were obtained.
Funding
Sequencing was conducted at the New York Genome Center using funds provided to MRB through a Cycle 47 PSC-CUNY Research Award (#69191-00-47). Financial support was provided to MRB by the Port Royal Sound Foundation and to the Ocean Genome Legacy Center of Northeastern University by a grant from the National Fish and Wildlife Foundation. Resources purchased with funds from the NSF FSML programme (DBI 1722553, to Northeastern University) were used to generate data for this manuscript. Financial support was provided to BAC, HC, MR, JW and ADG through USCB’s Summer Research Experience Scholarship Program and to JW and ADG through the University of South Carolina SMART Program.
Author contributions
Conceptualisation: MRB. Provided samples and accessioned them into a museum: JD, VEWH. DNA extraction, DNA quantification, and shipping samples: HJAM, DLD. Data analysis: BAC, AC, HC, JCR, MAR, JW, ADG, JLS, MRB. Data interpretation: BAC, AC, HC, JCR, MAR, JW, ADG, TK, KG, DTP, JLS, MRB. Submitted data to GenBank: KG, JLS, MRB. Significant intellectual contributions: DTP. Wrote original draft of manuscript: BAC, AC, JCR, MAR, MRB. Revised manuscript: all authors.
Author ORCIDs
Brendan A. Cruz https://orcid.org/0009-0008-4422-6489
Anneau Cappelmann https://orcid.org/0009-0007-8700-5726
Hope Chutjian https://orcid.org/0009-0008-5821-9335
Jude C. Roman https://orcid.org/0009-0002-9297-8008
Mason A. Reid https://orcid.org/0009-0009-6794-3947
Jacob Wright https://orcid.org/0009-0007-3743-6181
Aydanni D. Gonzalez https://orcid.org/0009-0007-7049-1019
Taylor Keyman https://orcid.org/0009-0006-0844-8485
Kierstin M. Griffith https://orcid.org/0009-0003-6800-4091
Hannah J. Appiah-Madson https://orcid.org/0000-0001-8408-7729
Daniel L. Distel https://orcid.org/0000-0002-3860-194X
Vonda E. Hayes https://orcid.org/0000-0001-8153-5629
Jim Drewery https://orcid.org/0000-0003-4308-1798
D. Tye Pettay https://orcid.org/0000-0002-2060-3226
Joseph L. Staton https://orcid.org/0009-0002-8695-5563
Mercer R. Brugler https://orcid.org/0000-0003-3676-1226
Data availability
Mitogenomic data are available in GenBank under accession numbers OR398473 (Alternatipathesmirabilis USNM1070972), OR398474 (Parantipatheslarix USNM1280881), BK063761 (Acanthopathesthyoides USNM1288453), BK063759 (Aphanipathespedata USNM1288458), BK063764 (Bathypathesalaskensis USNM1288462), BK063760 (Elatopathesabietina USNM1288451), BK063757 (Parantipathes sp. MSS29), BK063763 (Stauropathesarctica CMNI 2023-0258), BK063762 (Stauropathes sp. USNM1404493), OR398475 (Telopathesmagna USNM1204049) and BK063758 (Umbellapathes sp. USNM1404092). The phylogenetic tree can be found on figshare: https://doi.org/10.6084/m9.figshare.25130414.
References
- Allio R, Schomaker‐Bastos A, Romiguier J, Prosdocimi F, Nabholz B, Delsuc F. (2020) MitoFinder: Efficient automated large‐scale extraction of mitogenomic data in target enrichment phylogenomics. Molecular Ecology Resources 20(4): 892–905. 10.1111/1755-0998.13160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asorey CM, Sellanes J, Wagner D, Easton EE. (2021) Complete mitochondrial genomes of two species of Stichopathes Brook, 1889 (Hexacorallia: Antipatharia: Antipathidae) from Rapa Nui (Easter Island). Mitochondrial DNA, Part B, Resources 6(11): 3226–3228. 10.1080/23802359.2021.1990150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett NJ, Hogan RI, Allcock AL, Molodtsova T, Hopkins K, Wheeler AJ, Yesson C. (2020) Phylogenetics and mitogenome organisation in black corals (Anthozoa: Hexacorallia: Antipatharia): an order-wide survey inferred from complete mitochondrial genomes. Frontiers in Marine Science 7: 440. 10.3389/fmars.2020.00440 [DOI]
- Beagley CT, Okada NA, Wolstenholme DR. (1996) Two mitochondrial group I introns in a metazoan, the sea anemone Metridium senile: One intron contains genes for subunits 1 and 3 of NADH dehydrogenase. Proceedings of the National Academy of Sciences of the United States of America 93(11): 5619–5623. 10.1073/pnas.93.11.5619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. (2013) MITOS: Improved de novo metazoan mitochondrial genome annotation. Molecular Phylogenetics and Evolution 69(2): 313–319. 10.1016/j.ympev.2012.08.023 [DOI] [PubMed] [Google Scholar]
- Blank RJ, Trench RK. (1985) Speciation and symbiotic dinoflagellates. Science 229(4714): 656–658. 10.1126/science.229.4714.656 [DOI] [PubMed] [Google Scholar]
- Bledsoe-Becerra YM, Whittaker IS, Horowitz J, Naranjo KM, Johnson-Rosemond J, Mullins KH, Cunningham KM, Shetty S, Messinides SN, Behney MS, Fehsal JA, Watson AN, McKnight KE, Nasiadka TW, Popa H, Pettay DT, Appiah-Madson HJ, Distel DL, Brugler MR. (2022) Mitogenomics reveals low variation within a trigeneric complex of black corals from the North Pacific Ocean. Organisms, Diversity & Evolution 22(2): 1–11. 10.1007/s13127-021-00537-5 [DOI] [Google Scholar]
- Brook G. (1889) Report on the Antipatharia. Report on the scientific results of the Voyage Challenger. Zoology : Analysis of Complex Systems, ZACS 32: 5–222. [Google Scholar]
- Brugler MR, France SC. (2007) The complete mitochondrial genome of the black coral Chrysopathesformosa (Cnidaria: Anthozoa: Antipatharia) supports classification of antipatharians within the subclass Hexacorallia. Molecular Phylogenetics and Evolution 42(3): 776–788. 10.1016/j.ympev.2006.08.016 [DOI] [PubMed] [Google Scholar]
- Brugler MR, Opresko DM, France SC. (2013) The evolutionary history of the order Antipatharia (Cnidaria: Anthozoa: Hexacorallia) as inferred from mitochondrial and nuclear DNA: implications for black coral taxonomy and systematics. Zoological Journal of the Linnean Society 169(2): 312–361. 10.1111/zoj.12060 [DOI] [Google Scholar]
- Chan PP, Lowe TM. (2019) tRNAscan-SE: searching for tRNA genes in genomic sequences. In: Kollmar M (Еd.) Gene Prediction. Methods in Molecular Biology, vol 1962. Humana, New York, 1–14. 10.1007/978-1-4939-9173-0_1 [DOI] [PMC free article] [PubMed]
- Chery N, Parra K, Evankow A, Stein D, Distel D, Appiah-Madson H, Ross R, Sanon E, Alomari N, Johnson R, Vasovic A, Horowitz A, Popa H, Short B, Kourehjan D, Vasquez DM, Rodriguez E, Opresko DM, Brugler MR. (2018) Partnering with the Ocean Genome Legacy to advance our understanding of black corals (Order Antipatharia). 15th Deep-Sea Biology Symposium, Monterey, California, 9–14 September 2018. Poster presentation. [preprint available at] https://tidalmarshtaskforce.wixsite.com/uscb/publications
- Darriba D, Taboada GL, Doallo R, Posada D. (2012) jModelTest 2: More models, new heuristics and parallel computing. Nature Methods 9(8): 772–772. 10.1038/nmeth.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSalle R, Narechania A, Tessler M. (2023) Multiple outgroups can cause random rooting in phylogenomics. Molecular Phylogenetics and Evolution 184: 107806. 10.1016/j.ympev.2023.107806 [DOI] [PubMed]
- Esper EJC. (1788–1830) Die Pflanzenthiere in Abbildungen nach der Natur mit Farben erleuchtet nebst Beschreibungen. Raspischen Buchhandlung, Nuremberg. 3 vols text, 2 vols pls.
- Feng H, Lv S, Li R, Shi J, Wang J, Cao P. (2023) Mitochondrial genome comparison reveals the evolution of cnidarians. Ecology and Evolution 13(6): e10157. 10.1002/ece3.10157 [DOI] [PMC free article] [PubMed]
- Figueroa DF, Hicks D, Figueroa NJ. (2019) The complete mitochondrial genome of Tanacetipathesthamnea Warner, 1981 (Antipatharia: Myriopathidae). Mitochondrial DNA, Part B, Resources 4(2): 4109–4110. 10.1080/23802359.2019.1692701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourment M, Holmes EC. (2016) Seqotron: A user-friendly sequence editor for Mac OS X. BMC Research Notes 9(1): 1–4. 10.1186/s13104-016-1927-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- France SC, Rosel PE, Ewann J. (1996) DNA sequence variation of mitochondrial large-subunit rRNA. Molecular Marine Biology and Biotechnology 5(1): 15–28. https://pubmed.ncbi.nlm.nih.gov/8869515/ [PubMed] [Google Scholar]
- Guindon S, Gascuel O. (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52(5): 696–704. 10.1080/10635150390235520 [DOI] [PubMed] [Google Scholar]
- Hoang DT, Chernomor O, Von Haeseler A, Minh BQ, Vinh LS. (2018) UFBoot2: Improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35(2): 518–522. 10.1093/molbev/msx281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J, Brugler MR, Bridge TC, Cowman PF. (2020) Morphological and molecular description of a new genus and species of black coral (Cnidaria: Anthozoa: Hexacorallia: Antipatharia: Antipathidae: Blastopathes) from Papua New Guinea. Zootaxa 4821(3): 553–569. 10.11646/zootaxa.4821.3.7 [DOI] [PubMed] [Google Scholar]
- Horowitz J, Quattrini AM, Brugler MR, Miller DJ, Pahang K, Bridge TC, Cowman PF. (2023) Bathymetric evolution of black corals through deep time. Proceedings of the Royal Society B, Biological Sciences 290(2008): 20231107. 10.1098/rspb.2023.1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Rozewicki J, Yamada KD. (2019) MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics 20(4): 1160–1166. 10.1093/bib/bbx108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayal E, Roure B, Philippe H, Collins AG, Lavrov DV. (2013) Cnidarian phylogenetic relationships as revealed by mitogenomics. BMC Evolutionary Biology 13(1): 1–18. 10.1186/1471-2148-13-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. (2018) MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution 35(6): 1547–1549. 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaJeunesse TC, Wham DC, Pettay DT, Parkinson JE, Keshavmurthy S, Chen CA. (2014) Ecologically differentiated stress-tolerant endosymbionts in the dinoflagellate genus Symbiodinium (Dinophyceae) Clade D are different species. Phycologia 53(4): 305–319. 10.2216/13-186.1 [DOI] [Google Scholar]
- Larsson A. (2014) AliView: A fast and lightweight alignment viewer and editor for large datasets. Bioinformatics (Oxford, England) 30(22): 3276–3278. 10.1093/bioinformatics/btu531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Liu CM, Luo R, Sadakane K, Lam TW. (2015) MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics (Oxford, England) 31(10): 1674–1676. 10.1093/bioinformatics/btv033 [DOI] [PubMed] [Google Scholar]
- Love MS, Yoklavich MM, Black BA, Andrews AH. (2007) Age of black coral (Antipathesdendrochristos) colonies, with notes on associated invertebrate species. Bulletin of Marine Science 80(2): 391–399. https://www.ingentaconnect.com/content/umrsmas/bullmar/2007/00000080/00000002/art00008# [Google Scholar]
- Lütken C. (1871) Antipathes antarctica, en ny Sortkoral fra Polarhavet. Oversigt over det Kongelige Danske videnskabernes selskabs forhandlinger 2: 18–26. https://www.biodiversitylibrary.org/page/29561248 [Google Scholar]
- MacIsaac KG, Best M, Brugler MR, Kenchington ELR, Anstey LJ, Gordan T. (2013) Telopathesmagna gen. nov., spec. nov. (Cnidaria: Anthozoa: Antipatharia: Schizopathidae) from deep waters off Atlantic Canada and the first molecular phylogeny of the deep-sea family Schizopathidae. Zootaxa 3700(2): 237–258. 10.11646/zootaxa.3700.2.3 [DOI] [PubMed] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. (2011) The CIPRES science gateway: a community resource for phylogenetic analyses. In: Proceedings of the 2011 TeraGrid Conference: extreme digital discovery, 1–8. 10.1145/2016741.2016785 [DOI]
- Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, Von Haeseler A, Lanfear R. (2020) IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution 37(5): 1530–1534. 10.1093/molbev/msaa015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molodtsova TN. (2006) Black corals (Antipatharia: Anthozoa: Cnidaria) of North-East Atlantic. Biogeography of the North Atlantic Seamounts. KMK Press, Moscow, 141–151. https://scholar.google.com/scholar?hl=en&as_sdt=0%2C41&q=Black+corals+%28Antipatharia%3A+Anthozoa%3A+Cnidaria%29+of+North-East+Atlantic.+&btnG=
- Molodtsova TN, Opresko DM. (2017) Black corals (Anthozoa: Antipatharia) of the Clarion-Clipperton fracture zone. Marine Biodiversity 47(2): 349–365. 10.1007/s12526-017-0659-6 [DOI] [Google Scholar]
- Molodtsova TN, Opresko DM, Wagner D. (2022) Description of a new and widely distributed species of Bathypathes (Cnidaria: Anthozoa: Antipatharia: Schizopathidae) previously misidentified as Bathypathesalternata Brook, 1889. PeerJ 10: e12638. 10.7717/peerj.12638 [DOI] [PMC free article] [PubMed]
- Molodtsova TN, Opresko DM, O’Mahoney M, Simakova UV, Kolyuchkina GA, Bledsoe YM, Nasiadka TW, Ross RF, Brugler MR. (2023) One of the Deepest Genera of Antipatharia: Taxonomic Position Revealed and Revised. Diversity 15(3): 436. 10.3390/d15030436 [DOI] [Google Scholar]
- Opresko DM. (2002) Revision of the Antipatharia (Cnidaria: Anthozoa). Part II. Schizopathidae. Zoölogische Mededeelingen 76: 411–442. https://repository.naturalis.nl/pub/217481 [Google Scholar]
- Opresko DM. (2004) Revision of the Antipatharia (Cnidaria: Anthozoa). Part IV. Establishment of a new family, Aphanipathidae. Zoologische Mededelingen, Leiden 78(11): 209–240. http://www.repository.naturalis.nl/document/51441 [Google Scholar]
- Opresko DM. (2005) New genera and species of antipatharian corals (Cnidaria: Anthozoa) from the North Pacific. Zoologische Mededelingen, Leiden 79: 129–165. https://repository.naturalis.nl/pub/210737/ZM79-02_129-166.pdf [Google Scholar]
- Opresko DM. (2006) Revision of the Antipatharia (Cnidaria: Anthozoa). Part V. Establishment of a new family, Stylopathidae. Zoologische mededeelingen, Leiden 80-4(11): 109–138. https://repository.si.edu/bitstream/handle/10088/6225/Opresko_2006_Part_5_Stylopathidae.pdf
- Opresko DM, Baron-Szabo RC. (2001) Re-descriptions of the antipatharian coral described by EJC Esper with select English translations of the original German text. Senckenbergiana Biologica 81: 1–21. http://miseryukyu.com/MISE@University_of_the_Ryukyus/Zoantharia_literature_files/Opresko_Baron-Szabo-2001.pdf [Google Scholar]
- Opresko DM, Molodtsova TN. (2021) New species of deep-sea antipatharians from the North Pacific (Cnidaria: Anthozoa: Antipatharia), Part 2. Zootaxa 4999(5): 401–422. 10.11646/zootaxa.4999.5.1 [DOI] [PubMed] [Google Scholar]
- Opresko DM, Wagner D. (2020) New species of black corals (Cnidaria: Anthozoa: Antipatharia) from deep-sea seamounts and ridges in the North Pacific. Zootaxa 4868(4): 543–559. 10.11646/zootaxa.4868.4.5 [DOI] [Google Scholar]
- Opresko DM, Stewart R, Voza T, Tracey DI, Brugler MR. (2022) New genus and species of black coral from the SW Pacific and Antarctica (Cnidaria: Anthozoa: Antipatharia: Schizopathidae). Zootaxa 5169(1): 31–48. 10.11646/zootaxa.5169.1.3 [DOI] [PubMed] [Google Scholar]
- Pinzón JH, Lajeunesse TC. (2011) Species delimitation of common reef corals in the genus Pocillopora using nucleotide sequence phylogenies, population genetics and symbiosis ecology. Molecular Ecology 20(2): 311–325. 10.1111/j.1365-294X.2010.04939.x [DOI] [PubMed] [Google Scholar]
- Pinzón JH, Sampayo E, Cox E, Chauka LJ, Chen CA, Voolstra CR, LaJeunesse TC. (2013) Blind to morphology: Genetics identifies several widespread ecologically common species and few endemics among Indo‐Pacific cauliflower corals (Pocillopora, Scleractinia). Journal of Biogeography 40(8): 1595–1608. 10.1111/jbi.12110 [DOI] [Google Scholar]
- Quattrini AM, McCartin LJ, Easton EE, Horowitz J, Wirshing HH, Bowers H, Mitchell K, Sei M, McFadden CS, Herrera S. (2023a) Skimming genomes for systematics and DNA barcodes of corals. bioRxiv. 10.1101/2023.10.17.562770 [DOI] [PMC free article] [PubMed]
- Quattrini AM, Snyder KE, Purow-Ruderman R, Seiblitz IG, Hoang J, Floerke N, Ramos NI, Wirshing HH, Rodriguez E, McFadden CS. (2023b) Mito-nuclear discordance within Anthozoa, with notes on unique properties of their mitochondrial genomes. Scientific Reports 13(1): 7443. 10.1038/s41598-023-34059-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos NI, DeLeo DM, Horowitz J, McFadden CS, Quattrini AM. (2023) Selection in coral mitogenomes, with insights into adaptations in the deep sea. Scientific Reports 13(1): 6016. 10.1038/s41598-023-31243-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roark EB, Guilderson TP, Dunbar RB, Fallon SJ, Mucciarone DA. (2009) Extreme longevity in proteinaceous deep-sea corals. Proceedings of the National Academy of Sciences of the United States of America 106(13): 5204–5208. 10.1073/pnas.0810875106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez E, Barbeitos MS, Brugler MR, Crowley LM, Grajales A, Gusmão L, Häussermann V, Reft A, Daly M. (2014) Hidden among sea anemones: The first comprehensive phylogenetic reconstruction of the order Actiniaria (Cnidaria, Anthozoa, Hexacorallia) reveals a novel group of hexacorals. PLOS ONE 9(5): e96998. 10.1371/journal.pone.0096998 [DOI] [PMC free article] [PubMed]
- Shizuru LEK, Montgomery AD, Wagner D, Freel EB, Toonen RJ. (2024) The complete mitochondrial genome of a species of Cirrhipathes de Blainville, 1830 from Kaua‘i, Hawai‘i (Hexacorallia: Antipatharia). Mitochondrial DNA, Part B, Resources 9(2): 223–226. 10.1080/23802359.2024.2310130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinniger F, Pawlowski J. (2009) The partial mitochondrial genome of Leiopathesglaberrima (Hexacorallia: Antipatharia) and the first report of the presence of an intron in COI in black corals. Galaxea 11(1): 21–26. 10.3755/galaxea.11.21 [DOI] [Google Scholar]
- Stecher G, Tamura K, Kumar S. (2020) Molecular evolutionary genetics analysis (MEGA) for macOS. Molecular Biology and Evolution 37(4): 1237–1239. 10.1093/molbev/msz312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D, Brugler MR, Opresko DM, France SC, Montgomery AD, Toonen RJ. (2010) Using morphometrics, in situ observations and genetic characters to distinguish among commercially valuable Hawaiian black coral species; a redescription of Antipathesgrandis Verrill, 1928 (Antipatharia: Antipathidae). Invertebrate Systematics 24(3): 271–290. 10.1071/IS10004 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Mitogenomic data are available in GenBank under accession numbers OR398473 (Alternatipathesmirabilis USNM1070972), OR398474 (Parantipatheslarix USNM1280881), BK063761 (Acanthopathesthyoides USNM1288453), BK063759 (Aphanipathespedata USNM1288458), BK063764 (Bathypathesalaskensis USNM1288462), BK063760 (Elatopathesabietina USNM1288451), BK063757 (Parantipathes sp. MSS29), BK063763 (Stauropathesarctica CMNI 2023-0258), BK063762 (Stauropathes sp. USNM1404493), OR398475 (Telopathesmagna USNM1204049) and BK063758 (Umbellapathes sp. USNM1404092). The phylogenetic tree can be found on figshare: https://doi.org/10.6084/m9.figshare.25130414.