Abstract
Visual exploration paradigms involving object arrays have been used to examine salience of social stimuli such as faces in ASD. Recent work suggests performance on these paradigms may associate with clinical features of ASD. We evaluate metrics from a visual exploration paradigm in 4-to-11-year-old children with ASD (n=23; 18 males) and typical development (TD; n=23; 13 males). Presented with arrays containing faces and nonsocial stimuli, children with ASD looked less at (p=0.002) and showed fewer fixations to (p=0.022) faces than TD children, and spent less time looking at each object on average (p=0.004). Attention to the screen and faces correlated positively with social and cognitive skills in the ASD group (ps<.05). This work furthers our understanding of objective measures of visual exploration in ASD and its potential for quantifying features of ASD.
Keywords: Visual processing, eye-tracking, autism spectrum disorder, visual exploration, visual search
Introduction
Attenuated attention to social information, such as that presented by the faces, communication, and activities of other people, is a hallmark characteristic of autism spectrum disorder (ASD) (Chevallier et al. 2012; Dawson, Bernier, and Ring 2012). The extent to which social attention is affected in the visual domain in ASD has been evaluated using eye tracking methods (Frazier et al. 2017). Visual exploration and visual search paradigms, in particular, have been used to examine attentional biases, visual preferences, and orienting processes that lend insight into how individuals with ASD process socially-relevant stimuli, such as faces, relative to other competing stimuli (Elison et al. 2012; Frazier et al. 2016; Gliga et al. 2009; Sasson et al. 2008; Sasson and Touchstone 2014).
Visual search paradigms typically rely on explicit instructions to participants to identify the presence or absence of specific objects on the bases of their features or a conjunction of their features (Wolfe 1998). Individuals with ASD exhibit enhanced visual search performance represented by greater accuracy in identifying a unique target in a set of identical distractors (Brenner, Turner, and Müller 2007; Joseph et al. 2009; O’Riordan 2004). Enhanced visual discrimination and search performance has been leveraged as evidence in support of various cognitive models of ASD. One such model is weak central coherence, which in its original form posits the presence of a “detail-oriented” locally-biased information processing style and advantage in ASD arising from a relative weakness in context integration into a global/gestalt representation (Frith 1989, 2001; Happé 2005; Happé and Frith 2006). The reduced generalization hypothesis is an alternative model which posits that atypical or reduced perceptions of similarity lead simultaneously to both enhanced discrimination and reduced categorization ability (Plaisted 2001; Plaisted and Davis 2009). A third cognitive model which cites enhanced visual search performance is the enhanced perceptual functioning model, which posits that locally-oriented biases in ASD reflect in part the optional processing of higher-order information that is more mandatory in non-autistics (Mottron et al. 2006; Mottron, Dawson, and Soulières 2009; Mottron and Burack 2001). While there is mixed evidence regarding relationships between perceptual and socio-cognitive ability (Happé 1994, 1999) and the tradeoffs between local and global information processing (Plaisted and Davis 2009), overall evidence suggests that many individuals with ASD may use different strategies or show differential (sometimes superior) performance on elementary visual processing tasks (Samson et al. 2012).
In infants, and in comparative studies involving special populations where equivalent access to verbal instructions cannot be presumed, implicit visual search paradigms are often used instead of visual search paradigms with explicit (oral or written) instructions. Implicit visual search paradigms rely on prepotent differences in visual saliency (or “pop-out” effects) to examine low-level, elementary attentional biases (e.g. Cheung et al. 2016; Frank, Amso, and Johnson 2014; Gliga et al. 2009), or they claim to exemplify implicit training methods (Kaldy et al. 2011) including statistical learning to bias participants to find specific search targets.
Visual search, and specifically constructs indexed by implicit visual search paradigms, may reflect domain-general processes that facilitate visual navigation of the social world. For example, performance on elementary implicit visual search tasks correlates positively with attention to faces in typically-developing infants (Frank et al. 2014). Interestingly, a longitudinal study found that enhanced visual search abilities for identifying a unique target in a set of identical distractors at 9 months predicted greater severity of autism symptoms at 15 months and 24 months (Gliga et al. 2015). Kaldy and colleagues (2011) similarly describe improved ability to identify targets in toddlers with ASD compared to age-matched typically-developing controls as young as 2.5-years-of-age. These results suggest relationships between visual search and social ability may be different in children with or at-risk-for ASD, signifying atypical developmental processes or attentional strategies that may relate to known non-verbal advantages in performance observed in older children with ASD (Joseph, Tager-Flusberg, and Lord 2002).
Closely related to implicit search paradigms are visual object exploration and scene free-viewing paradigms. These paradigms are often constructed similarly to implicit visual search paradigms: both negate the need for specific verbal comprehension by excluding explicit instructions, but free-viewing paradigms do not focus on particular low-level pop-out effects nor evaluate pre-training biases. A common inclusion in visual object exploration paradigms involves the comparison of “social” versus “nonsocial” objects, with the class of social objects largely composed of people (e.g. bodies and faces), and the set of non-social objects comprising imagery without commonly-assumed anthropomorphic interpretation or context. A current question involves whether all visual object exploration paradigms involving faces could be considered implicit visual search paradigms due to the presence of faces, which some have argued engages specific fast-acting reflexive brain circuitry in human observers (Driver et al. 1999; Hershler and Hochstein 2005; though see VanRullen 2006; Hershler and Hochstein 2006).
Prior visual search studies primarily used nonsocial stimuli (e.g., letters) to evaluate visual discrimination and visual processing skills in ASD. However, using both social and nonsocial stimuli in a visual exploration paradigm can further inform understanding of attentional biases, as well as illuminate gaze behaviors that tap into visual information processing strategies and social motivation. There is an assumption that social information is particularly salient for most individuals, particularly stimuli of faces. Studies have demonstrated that infants as young as 6 months orient to faces in this type of social and nonsocial visual array more than would be expected by chance, and that they orient more often to faces than to other categories of objects. For example, 6 month-olds (and adults) maintained their attention to faces more than toward other objects, suggesting a developmental continuity in the perceived salience of faces (Di Giorgio et al. 2012; Gliga et al. 2009).
When presented with visual exploration paradigms that depict arrays of social stimuli (e.g., people) dispersed among nonsocial stimuli (e.g., computers, furniture, toys, food), children with ASD explored fewer stimuli within the visual array (i.e. evidenced “circumscribed” visual attentional behavior), were more likely to fixate longer on stimuli (social and nonsocial) they looked at (“perseverative” behavior), and were more likely to make several fixations within a stimuli (“detail-oriented” behavior) (Sasson et al. 2008). These results were consistent with greater featural-level tendencies observed in nonsocial visual search paradigms. Sasson and colleagues also found that greater circumscribed visual interest in objects was associated with ASD symptom severity, including repetitive and restricted behaviors.
These associations suggest a pathway to the clinical phenotype of ASD that derives from early differences in an attentional bias for local or featural characteristics over global features. Attentional biases towards low-level visual features of objects over higher-level conceptual features (social, or otherwise) may account for visual preferences for physical contingencies or geometric shapes over social scenes in young children with ASD (Klin et al. 2009; Pierce et al. 2015). Associations between viewing behaviors and ASD symptomatology highlight the potential for eye-tracking methods to index clinically relevant facets of the ASD symptom profile. Prior work has found that visual exploration of social versus nonsocial visual stimuli can distinguish children with ASD from those with typical development with high sensitivity and specificity (up to 80% sensitivity and 98% specificity depending on samples), suggesting the potential for visual exploration behaviors to inform clinical judgment (Frazier et al. 2016; Loth et al. 2017; Pierce et al. 2016).
The current study had two key aims: to evaluate whether a visual exploration paradigm that included an array of social and nonsocial stimuli would reveal differences in attention between children with ASD and TD controls and to explore possible relations between visual search performance with social and repetitive behavioral features of the autism phenotype. We hypothesized that we would replicate prior work that reported diminished looking at faces in ASD as compared to controls (Frazier et al. 2017). As shown in prior studies of children with ASD (e.g. Sasson et al. 2008), we also hypothesized that children with ASD would show viewing behaviors reflective of circumscribed, perseverative, and detail-focused visual exploration strategies. Finally, we hypothesized that individual differences in visual exploration would associate with symptom severity.
Methods
Participants and clinical characterization
Participants were enrolled as part of a multi-site study that aimed to assess a battery of eye tracking and EEG paradigms to be used in clinical trials with children with ASD [Autism Biomarker Consortium—Clinical Trials (ABC-CT)] (McPartland et al. 2020). These paradigms tap into relevant domains of social functioning in ASD (e.g., preferential attention to social information) that are associated with social symptomatology in ASD. This methodology specifically employs technologies which automatically quantify responses (eye tracking and EEG) in order to objectively code specific reactions and behaviors. The data reported here are from an initial feasibility study of 51 children between the ages 4 and 11 years (26 ASD; 25 TD) from five sites (Yale University, University of California, Los Angeles, Boston Children’s Hospital, Duke University, and University of Washington). This feasibility phase, which occurred before the larger main study, ensured that equipment and experimental protocols were identical at each site and were applicable for the target population (Webb et al. 2020). All participants had either normal or corrected to normal vision. Parents provided written consent and participant assent was obtained.
Prior to inclusion in the study, participants were screened with a battery of autism and cognitive assessments. To be included in the ASD group, participants met diagnostic threshold on the Autism Diagnostic Observation Schedule (ADOS-2; Lord et al. 2012), Autism Diagnostic Interview-Revised (ADI-R; Rutter, LeCouteur, and Lord 2003), and on DSM-5 (American Psychiatric Association 2013), and had full scale IQ between 50–150 as assessed via the Differential Ability Scales, 2nd Edition (DAS-II; Elliott 2007). Individuals in the typically developing (TD) group had an IQ between 80–150 via the DAS-II and had no reported psychiatric conditions and no first degree relatives with ASD. To quantify autism symptoms, we used the calibrated severity scores from the ADOS (ADOS-2). To quantify social ability, we used the Socialization Standard Score from the Vineland Adaptive Behavior Scales-II (Vineland; Sparrow et al., 2005).
The age of children in the ASD group did not statistically differ from that of TD children (TDage=6.66 ± 1.96 years; ASDage=8.01 ± 2.23 years, t(44)=1.95, p = .056) but a medium effect size difference was evident (d=.643). In addition to having greater ASD symptom severity (ADOS CSS: TD=1.19 ± 0.42; ASD=7.73 ± 1.64, t(44)=19.76, p<.001), the ASD group also showed lower scores both on cognitive (FSIQ: TD=114.08 ± 9.34; ASD=91.27 ± 19.14, t(44)=5.46, p < .001) and adaptive social functioning (Vineland Socialization Standard Score: TD=103.58 ± 12.18; ASD= 75.21 ± 11.02, t(44)=8.61, p <.001), relative to TD controls (see Table 1).
Table 1.
Participant Demographics
| ASD (n = 23; 18 M) |
TD (n = 23; 13 M) |
t | p | |
|---|---|---|---|---|
| Age | 8.01 (2.23) |
6.66 (1.95) |
1.95 | 0.056 |
| ADOS CSS1 | 7.73 (1.64) |
1.19 (0.42) |
19.76 | <0.001 |
| Full Scale IQ3 | 91.27 (19.14) |
114.08 (9.34) |
5.46 | <0.001 |
| Verbal IQ3 | 89.54 (1.21) |
115.23 (13.79) |
5.22 | <0.001 |
| Nonverbal IQ3 | 93.08 (18.69) |
111.04 (8.03) |
4.50 | <0.001 |
| Communication Std Score2 | 77.50 (13.43) |
111.27 (14.27) |
8.59 | <0.001 |
| Socialization Std Score2 | 75.21 (11.02) |
103.58 (12.18) |
8.61 | <0.001 |
Notes.
ADOS Calibrated Severity Score;
Vineland Adaptive Behavior Scales;
Differential Abilities Scales-II (Nonverbal IQ from Special Nonverbal Composite)
Procedure
The visual exploration paradigm was among a battery of eye-tracking paradigms as part of the ABC-CT Feasibility Study. ET occurred on both days, but the visual exploration paradigm occurred on only one day. The day of administration (day 1 or day 2) and the occurrence of visual search trials among the other paradigms (within day order), was counterbalanced across subjects (Webb et al. 2020).
The eye-tracking equipment included the Neurobehavioral Systems Presentation 18.1 for experimental control. Eye movements were recorded using a SR Eyelink 1000 Plus eye tracker at 500 Hz. The eye-tracking session began after participants were seated approximately 65 cm from a 24” 1920 × 1200 pixel LED monitor for stimulus presentation in a quiet, dimly lit room. During set-up, child-friendly animated movies were shown onscreen to attract the child’s attention. One or more behavioral assistants was seated near the child during the session and provided verbal redirections toward the screen when necessary. An experimenter controlled the eye-tracking and the stimulus presentation computers. A 5-point calibration and validation scheme was completed prior to beginning the eye-tracking session, with periodic calibration validations presented throughout the session. Trials were excluded when uncertainty regarding point-of-gaze (POG) were greater than 2.5° (105 pixel) and for which less than 50% of eye-tracking data were obtained.
Visual Exploration Paradigm
The visual search paradigm was adapted from Gliga, Elsabbagh, Andravizou, and Johnson (2009). Participants were presented with full-color circular arrays consisting of 5 static image stimuli — a face with direct gaze, an outline of a face filled with a pattern, a bird, a mobile phone, and a motor vehicle (see Figure 1). Each array consisted of a different set of stimuli from each category, and the location for the stimuli from each category varied between arrays. All stimuli were equidistant from the center of the screen and of comparable size. Each circular array trial was presented for 20 seconds; there were 6 trials.
Figure 1.
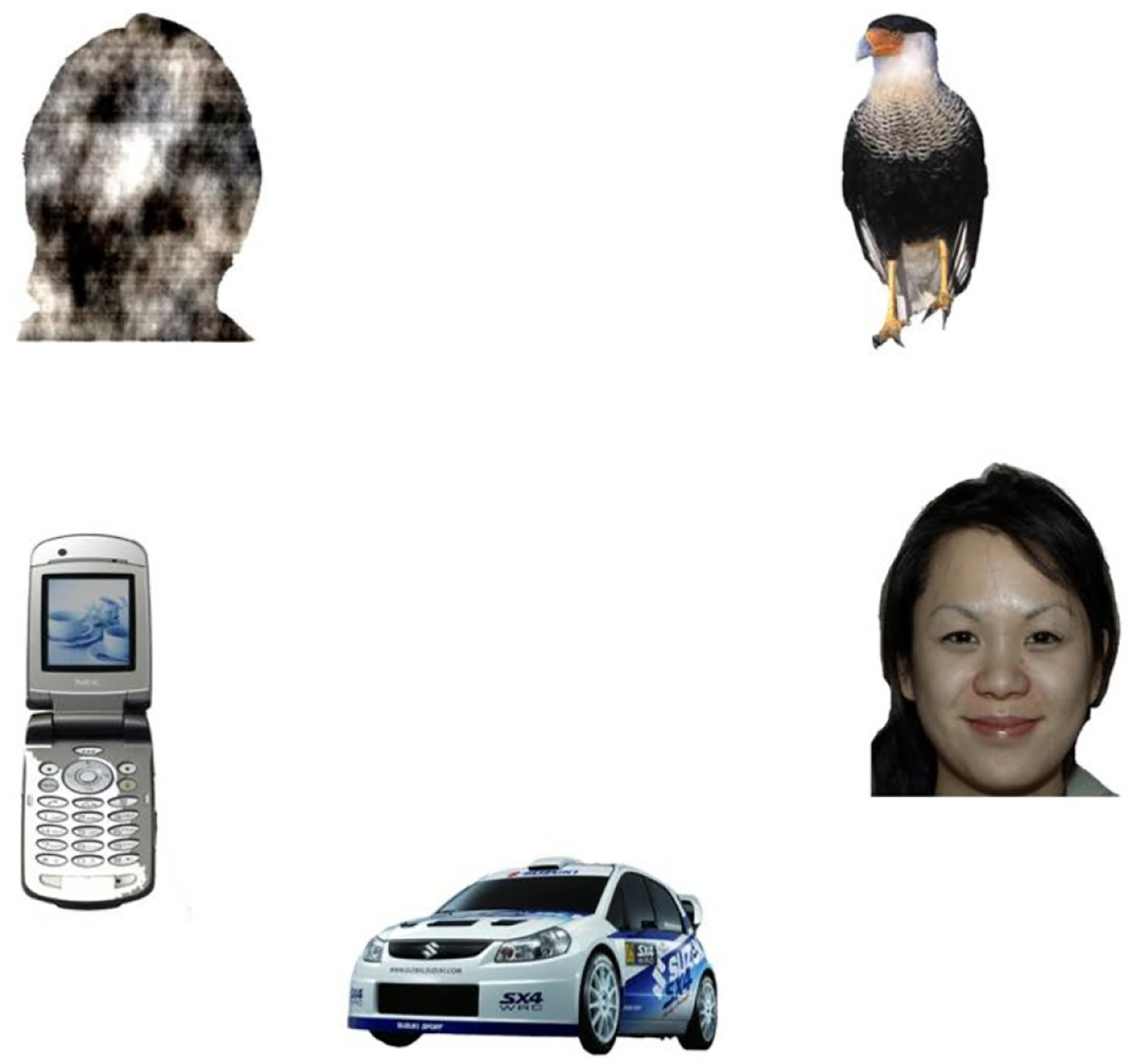
Sample stimulus from the visual exploration paradigm. Each circular array had 5 image stimuli featuring one colored photo from the following categories: faces, scrambled faces, mobile phones, birds, and cars. *Note, black bar over eyes of lower right face was not shown to participants.
A central fixation stimulus was displayed between trials to ensure that gaze was directed to the center of screen before array presentation onset. The array presentation was accompanied with music to assist in maintaining the child’s attention to the screen. Rectangular regions of interest (ROIs) were drawn around each image stimulus. Gaze data were extracted for each ROI, including number and duration of fixations, as well as total onscreen viewing time during the stimulus presentation.
Data Processing and Dependent Variables
Raw data from the eye tracker were periodically drift- and coordinate-corrected based on periodic 5-point recalibration targets and centering stimuli presented across the course of experiment delivery. Blinks were treated as periods of lost data and not interpolated. No additional filters were applied to interpolate, smooth, or otherwise adjust scanpath computations. Fixations were calculated by I-VT velocity threshold algorithm (Salvucci and Goldberg 2000), using a velocity threshold of 25 visual degrees per second and a minimum fixation duration requirement of 80 ms. Because fixations could span multiple ROIs, a partial weighted fixation count was used in all ROI-associated fixation count calculations, with the weights associated with a fixation allocated linearly relative to the number of gaze points collected within each ROI and summing to 1.
The primary variable of interest was percent looking time to the ROI corresponding to the face target relative to total trial-based onscreen looking time (“Face Looking Time Percent”). Analogous analyses were conducted for all other object classes and for the set of all objects together (“Non-face Object Looking Time Percent”) to provide additional context. The percent onscreen looking time (“Valid Looking Time Percent”), relative to total trial length, was also recorded as a measure of on-task visual engagement. These looking time variables were computed based on detected eye-tracking samples falling within associated ROIs (and not based on fixation duration aggregates). Additional variables of interest capturing circumscribed, perseverative, and detail-oriented exploration strategies were modeled after those used in similar visual exploration studies evaluating social attention in children with ASD (Gliga et al. 2009; Sasson et al. 2008, 2010). See Table 2 for a summary of eye-tracking variables.
Table 2.
Summary of ET Variables
| Variable Name | Computation/Definition |
|---|---|
| Valid Looking Time Percentage | Duration of gaze onscreen divided by stimulus presentation duration x 100 |
| ROI Looking Time Percentage | Duration of gaze within a Region-of-Interest (ROI) divided by total onscreen gaze duration (ROIs=Faces or Non-Face Objects) x 100 |
| Exploration | # of objects in the array for which ROI detected gaze was ≥ 500ms |
| Perseveration | Average of duration of detected gaze within ROIs across all ROIs for which detected gaze was ≥ 500ms |
| ROI Detail Orientation | Average number of fixations that fell within each ROI |
Sasson (2008) defined the terms exploration, perseveration, and detail-orientation in the context of quantifying eye-tracking gaze patterns towards a visual array. Exploration was defined as “how many different stimuli were explored” – quantified by the total number of stimuli per trial on which a participant recorded a fixation. Showing low exploration was interpreted as “circumscribed” attentional behavior. Perseveration was defined as “how long individual items were explored” – quantified by the total fixation time per stimulus explored. Lastly, detail-orientation was described as “how many different times individual stimuli were explored” – quantified by the number of discrete fixations per stimulus explored. While these definitions were chosen for historical reasons, we note some tension between the interpretation of these variables as specified by their variable name and their specific mathematical definitions. We revisit this in the discussion.
These definitions, originally developed on complex arrays with many more targets than ours (Sasson et al. 2008, 2010), were adapted to the current study as follows. First, exploration was defined as the number of targets looked at for at least 500ms during each trial. Unlike the original Sasson definition, a temporal total gaze duration requirement was used rather than a single fixation requirement. This was due to uncertainty caused by partial fixations as well as the much smaller number of stimuli presented: combined with the relatively long duration of stimulus presentation, most trials involved the exploration of all stimuli despite the more restrictive 500 ms looking requirement. Second, perseveration was defined as the total looking time to each individual target, averaged across all objects which were explored. This differed from percentage looking time directed towards each object in that it reflected an overall aggregate characteristic of the exploration of all targets. Finally, detail orientation was defined as the number of fixations directed to each individual target. See Table 2 for a summary of ET variables.
Participants were excluded from the analysis if they provided fewer than 2 valid trials. Following this criterion, 46 (23 ASD [18 male], 23 TD [17 male]) out of 51 participants were included in the analyses below.
Statistical Analysis
The data were analyzed using linear mixed models in SPSS (IBM). Each of the aforementioned dependent variables (i.e., percent face target looking, percent non-face object looking, percent onscreen looking, exploration, detail orientation, and perseveration) were separately modeled with stimulus as the repeated measure nested within the subject, diagnosis (ASD vs TD) as the predictor, and full scale IQ (FSIQ) and age at enrollment as covariates. Pearson correlations examined the associations among our primary variables of interest (e.g., percent looking to face targets) and phenotypic characteristics with and without controlling for of FSIQ and age (Ben-Itzchak and Zachor 2007).
Results
Face, Non-Face, and Valid Onscreen Looking Time Percentages
Children with ASD had lower face looking time percentages than TD controls, over and above the effects of FSIQ and age (F1,37.0=11.4, p=0.002). There were no group differences in looking time percentage at non-face, object targets (F1, 38.6=1.4, p=0.239) or on-screen valid looking time percentage (F1, 39.1=2.0, p=0.163) (see Figure 2A).
Figure 2.
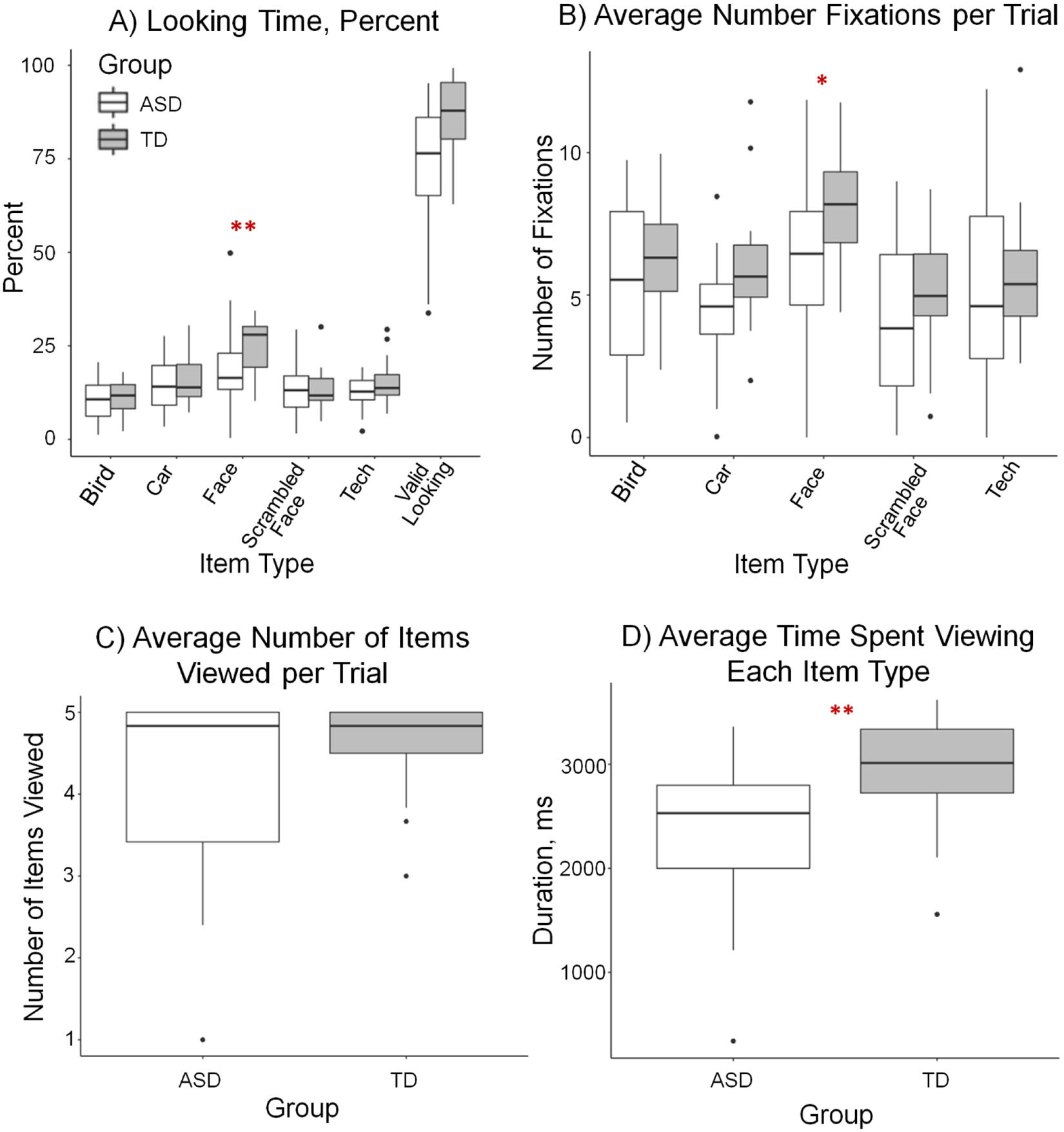
A) Percentage of on-screen looking time ASD and TD groups spent looking at face and non-face objects, with on-screen looking time (Valid Looking) percentage of stimulus display time also shown for reference. B) Number of fixations on objects by item type and group (i.e. detail orientation). C) Number of objects viewed per trial out of a maximum of 5 (i.e. exploration). D) Amount of time spent looking at a viewed object on average (i.e., perseveration). *p<.05, **p<.01
Exploration
ASD and TD children did not differ in exploration (i.e., number of objects viewed per trial) over and above the effects of FSIQ and age (F1, 40.4=1.9, p=0.178, see Figure 2C). Both groups looked at most objects within the circular array.
Perseveration
Perseveration was operationalized as the average gaze duration spent looking at an object, averaged across all objects explored in a trial. This average amount of time spent looking at an object was lower in children with ASD than TD controls, over and above the effects of FSIQ and age (F1,39.6=9.1, p=0.004, see Figure 2D).
Detail Orientation
Children with ASD showed less detail orientation towards faces (i.e. made significantly fewer fixations to faces) than TD controls, over and above the effects of age and FSIQ (F1, 34.7=5.8, p=0.022, see Figure 2B). However, children with ASD and TD controls were comparable in detail orientation directed to other non-social object targets (ps>.266).
Association between Visual Exploration and Social Profiles
We examined the associations between visual engagement by faces and phenotypic characteristics (ADOS CSS, IQ, and Vineland Adaptive Behavior Scales) in children with ASD. Without partialling for age and IQ, greater general attention to the scene (i.e. higher valid looking time percentage) was associated with lower ADOS CSS (r=−.456, p=.029) and higher IQ (r=.467, p=.025); greater percentage looking times at faces were associated with decreased IQ (r=−.571, p=.004). No relationships with age were noted. After controlling for age and IQ, no relationships with any other phenotypic characteristics or eye-tracking variables were noted.
Discussion
The aim of the current study was to evaluate the performance of children with ASD and TD on a visual exploration paradigm that featured social (i.e. face) and nonsocial objects. Consistent with other literature (Frazier et al. 2017), children with ASD in our study looked less and made fewer fixations to faces than their typical peers, suggesting reduced salience of faces relative to other visual stimuli.
As opposed to other eye-tracking measures of face processing, visual exploration paradigms allow us to directly measure the detection of and preference for faces in competition with other objects (Gliga et al. 2009). A reduced preference for social stimuli has been observed early in development in ASD (Chawarska, Macari, and Shic 2013) as well as later in life (Frazier et al. 2017). The current study did not observe significant correlations between metrics of visual exploration (other than general attention) and direct behavioral measurement of ASD symptom severity. Therefore, it may be that performance on visual exploration tasks indexes a categorical difference in ASD and is less sensitive to between-subject variability within ASD (vis ADOS CSS scores). Attenuated attention to faces may reflect specific impairments in social attention in the ASD group, which is largely consistent with work studying naturalistic scanning patterns (Frazier et al. 2017; Pierce et al. 2016). By extending these findings prioritization of faces within visual arrays, our results suggest that attenuated attention to faces may be a feature of ASD that is context independent. Along this line, the relationships between higher IQ and lower percentage of time looking at faces may suggest diminished social-attentional prioritization unique to average-to-high IQ children with ASD.
The current study elicited some contrary results to the findings of prior visual exploration paradigm studies (Sasson et al. 2008). In the present study, children with ASD, as compared to TD children, showed similar (rather than less) exploration, less (rather than more) perseveration, and less (rather than more) detail-orientation. Furthermore, this atypical behavior extended only to faces, as opposed to both faces and objects. Several methodological differences could account for the observed differences. First, Sasson et al. (2008) employed arrays with 24 objects per trial, whereas our study used an array of only 5 objects. For this reason, measures of exploration in the present study reached ceiling at 5, and, as evident from our results, may not have provided enough variability in participants for differences to emerge. Second, in Sasson et al. (2008), objects were full-color image stimuli spanning across broad categories of social and non-social imagery, with non-social imagery additionally including items the authors considered to be of high interest to individuals with ASD. It is thus possible that children with ASD in the current study found focusing on the stimuli less engaging due to the simplicity and relatively low number of stimuli presented, impacting fixation and time-per-stimulus measurements. Third, the objects in this study were larger than those typically shown in the Sasson study, which may have allowed for more fine-grained distinction of fixation behavior on individual objects.
In our study, decreased detail orientation (i.e. fewer fixations per trial) when looking at faces, but not other objects, in ASD, can be seen as an extension of overall decreased saliency for faces. It suggests that children with ASD may examine faces in these scenes with a potentially local as compared to a holistic or global examination strategy (López et al. 2004). These results may also be associated with decreased perseveration visual strategies (i.e. lower time spent focusing overt attention towards each object), which could indicate more superficial processing of or decreased engagement with characteristics of presented objects. Alternatively, they could be related to greater efficiency in visual information processing, consistent with enhanced visual search capabilities in individuals with ASD as evidenced across a range of studies (Kaldy et al. 2011; Mottron et al. 2006; Plaisted, O’Riordan, and Baron‐Cohen 1998). Future work will seek to examine the strength of these relations in a larger sample with an extended battery of potentially convergent clinical measures.
Metrics of visual behaviors may provide a proximal measure (relative to parent report and clinical ratings) of cognitive processes that underlie social behaviors, offering potential utility as biomarkers. Some intervention studies have found relatively small effect sizes of treatment on ADOS symptom severity (Fletcher‐Watson and McConachie 2010); however, eye-tracking measures in free-viewing paradigms highlight the potential to tap into subtle differences in social functioning, which may reflect cognitive processes involved in social learning and reward processing (Clements et al. 2018; Sepeta et al. 2012). Correlations between attention to face targets and social communicative skills did not hold in our sample within ASD and TD groups separately, perhaps due to a restricted range of skills. In addition, the sample may be underpowered for examining more nuanced clinical relations. Finally, we note that the interpretation of exploration, perseveration, and detail-orientation follow original guidance presented historically (Sasson et al. 2008, 2010). However, in general, there is a divorce between specific eye-tracking variables of interest and their ultimate, high-level interpretation. For example, being “detailed oriented” could be defined as “the desire to inspect multiple local features of an object or scene”. This definition would be consistent with an increased number of fixations used in the examination. However, it could also be described by longer average fixations spent in presumably greater concentration or deeper processing of specific, spatially-localized, visual characteristics. The product of the number of fixations and the mean fixation duration, in turn, is an exact definition of total fixation duration. Interpretive complexities introduced through high-level perspectives intersecting low-level scanpath features are a continuing challenge and future research direction.
Conclusion
Data from this study lends support for the existence of systematic differences in how children with ASD and TD view visual arrays, which may tap into attenuated preference for socially relevant stimuli in individuals with ASD. Specifically, we showed that when presented with arrays of objects, including faces, children with ASD, as compared to TD peers, look less and show fewer fixations to faces, and spend less time looking at objects in general (including when covarying for age and cognitive ability). These results have implications for understanding the strategies by which children with ASD visually process their social world. The visual exploration paradigm also requires minimal instruction, only takes approximately 2 minutes, and is well-tolerated by children with a broad range of cognitive and social abilities. Further, biomarkers developed around this task may be able to help provide greater insight into alterations in social attention shared by individuals with ASD, with subsequent implications for clinical trials.
Acknowledgments
Support was provided by the U19 Consortium on Biomarker and Outcome Measures of Social Impairment for use in Clinical Trials in Autism Spectrum Disorder (ABC-CT) NIMH U19 MH108206 (PI: McPartland) and by NIMH K01 MH104739 (PI: Shic). Additional important contributions were provided by members of the ABC-CT consortium including: Heather Borland, Cynthia Brandt, Shou An Chang, Scott Compton, Susan Faja, Alyssa Gateman, Simone Hasselmo, Bailey Heit, Gerhard Helleman, Julie Holub, Toni Howell, Ann Harris, Alexander Hoslet, Kathryn Hutchins, Kelsey Jackson Dommer, Lily Katsovitch, Minah Kim, April Levin, Beibin Li, Samantha Major, Samuel Marsan, Andriana S. Méndez Leal, Lisa Nanamaker, Leon Rozenblit, Maura Sabatos-DeVito, Megha Santhosh, Damla Senturk, Laura Simone, Dylan Stahl, Cindy Voghell, Quan Wang, and Andrew Yuan.
The authors Tawny Tsang, Adam Naples, Erin Barney, Minhang Xie, Raphael Bernier, James Dziura, Susan Faja, Shafli Jeste, Charles A. Nelson, Michael Murias, Helen Seow, Catherine Sugar, Sara J. Webb, and Scott P. Johnson declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
James McPartland has received funding from Janssen Research and Development, receives book Royalties from Guilford, Springer, and Lambert Press, and is a consultant with BlackThorn Therapeutics.
Geraldine Dawson is on the Scientific Advisory Boards of Janssen Research and Development, Akili, Inc., LabCorp, Inc., and Roche Pharmaceutical Company, a consultant for Apple, Inc, Gerson Lehrman Group, and Axial Ventures, has received grant funding from Janssen Research and Development, is CEO of DASIO, LLC, which focuses on digital phenotyping tools, and receives book royalties from Guilford Press, Springer, and Oxford University Press.
Frederick Shic consults for Roche Pharmaceutical Company, Janssen Research and Development, and BlackThorn Therapeutics.
No company contributed to funding of this study. A representative from Janssen served on the FNIH Biomarkers Consortium Project Team and provided in kind support in terms of sharing experiences and preliminary results of the JAKE study.
References
- American Psychiatric Association. 2013. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Arlington, Va.: American Psychiatric Association. [Google Scholar]
- Ben-Itzchak Esther, and Zachor Ditza A.. 2007. “The Effects of Intellectual Functioning and Autism Severity on Outcome of Early Behavioral Intervention for Children with Autism.” Research in Developmental Disabilities 28(3):287–303. doi: 10.1016/j.ridd.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Brenner Laurie a, Turner Katherine C., and Müller Ralph-Axel. 2007. “Eye Movement and Visual Search: Are There Elementary Abnormalities in Autism?” Journal of Autism and Developmental Disorders 37(7):1289–1309. [DOI] [PubMed] [Google Scholar]
- Chawarska Katarzyna, Macari Suzanne L., and Shic Frederick. 2013. “Decreased Spontaneous Attention to Social Scenes in 6-Month-Old Infants Later Diagnosed with Autism Spectrum Disorders.” Biological Psychiatry 74(3):195–203. doi: 10.1016/j.biopsych.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CHM, Bedford R, Johnson MH, Charman T, and Gliga T. 2016. “Visual Search Performance in Infants Associates with Later ASD Diagnosis.” Developmental Cognitive Neuroscience. doi: 10.1016/j.dcn.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier Coralie, Kohls Gregor, Troiani Vanessa, Brodkin Edward S., and Schultz Robert T.. 2012. “The Social Motivation Theory of Autism.” Trends in Cognitive Sciences 16(4):231–39. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements Caitlin C., Zoltowski Alisa R., Yankowitz Lisa D., Yerys Benjamin E., Schultz Robert T., and Herrington John D.. 2018. “Evaluation of the Social Motivation Hypothesis of Autism: A Systematic Review and Meta-Analysis.” JAMA Psychiatry 75(8):797–808. doi: 10.1001/jamapsychiatry.2018.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson Geraldine, Bernier Raphael, and Ring Robert H.. 2012. “Social Attention: A Possible Early Indicator of Efficacy in Autism Clinical Trials.” Journal of Neurodevelopmental Disorders 4(1):11. doi: 10.1186/1866-1955-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio Di, Elisa Chiara Turati, Altoè Gianmarco, and Simion Francesca. 2012. “Face Detection in Complex Visual Displays: An Eye-Tracking Study with 3- and 6-Month-Old Infants and Adults.” Journal of Experimental Child Psychology 113(1):66–77. doi: 10.1016/j.jecp.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Driver Jon, Davis Greg, Ricciardelli Paola, Kidd Polly, Maxwell Emma, and Baron-Cohen Simon. 1999. “Gaze Perception Triggers Reflexive Visuospatial Orienting.” Visual Cognition 6(5):509. doi: 10.1080/135062899394920. [DOI] [Google Scholar]
- Elison Jed T., Sasson Noah J., Turner-Brown Lauren M., Dichter Gabriel S., and Bodfish James W.. 2012. “Age Trends in Visual Exploration of Social and Nonsocial Information in Children with Autism.” Research in Autism Spectrum Disorders 6(2):842–51. doi: 10.1016/j.rasd.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott CD 2007. “Differential Ability Scales—Second Edition (DAS-II).” San Antonio, TX: Psychological Corporation. [Google Scholar]
- Fletcher‐Watson S, and McConachie H. 2010. “Interventions Based on the Theory of Mind Cognitive Model for Autism Spectrum Disorder (ASD).” The Cochrane Library (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank Michael C., Amso Dima, and Johnson Scott P.. 2014. “Visual Search and Attention to Faces during Early Infancy.” Journal of Experimental Child Psychology 118:13–26. doi: 10.1016/j.jecp.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier Thomas W., Klingemier Eric W., Beukemann Mary, Speer Leslie, Markowitz Leslie, Parikh Sumit, Wexberg Steven, Giuliano Kimberly, Schulte Elaine, Delahunty Carol, Ahuja Veena, Eng Charis, Manos Michael J., Hardan Antonio Y., Youngstrom Eric A., and Strauss Mark S.. 2016. “Development of an Objective Autism Risk Index Using Remote Eye Tracking.” Journal of the American Academy of Child & Adolescent Psychiatry. doi: 10/f8f2w7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier Thomas W., Strauss Mark, Klingemier Eric W., Zetzer Emily E., Hardan Antonio Y., Eng Charis, and Youngstrom Eric A.. 2017. “A Meta-Analysis of Gaze Differences to Social and Nonsocial Information Between Individuals With and Without Autism.” Journal of the American Academy of Child and Adolescent Psychiatry 56(7):546–55. doi: 10.1016/j.jaac.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith Uta. 1989. Autism: Explaining the Enigma. Basil Blackwell. [Google Scholar]
- Frith Uta. 2001. “Mind Blindness and the Brain in Autism.” Neuron 32(6):969–79. doi: 10.1016/S0896-6273(01)00552-9. [DOI] [PubMed] [Google Scholar]
- Gliga Teodora, Bedford Rachael, Charman Tony, and Johnson Mark H.. 2015. “Enhanced Visual Search in Infancy Predicts Emerging Autism Symptoms.” Current Biology 25(13):1727–30. doi: 10.1016/j.cub.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliga Teodora, Elsabbagh Mayada, Andravizou Athina, and Johnson Mark. 2009. “Faces Attract Infants’ Attention in Complex Displays.” Infancy 14(5):550–62. doi: 10.1080/15250000903144199. [DOI] [PubMed] [Google Scholar]
- Happé Francesca. 1994. “Wechsler IQ Profile and Theory of Mind in Autism: A Research Note.” Journal of Child Psychology and Psychiatry 35(8):1461–71. doi: 10/fcqt4k. [DOI] [PubMed] [Google Scholar]
- Happé Francesca. 1999. “Autism: Cognitive Deficit or Cognitive Style?” Trends in Cognitive Sciences 3(6):216–22. [DOI] [PubMed] [Google Scholar]
- Happé Francesca. 2005. “The Weak Central Coherence Account of Autism.” Pp. 640–49 in Handbook of Autism and Pervasive Developmental Disorders. John Wiley & Sons, Ltd. [Google Scholar]
- Happé Francesca, and Frith Uta. 2006. “The Weak Coherence Account: Detail-Focused Cognitive Style in Autism Spectrum Disorders.” Journal of Autism and Developmental Disorders 36(1):5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- Hershler Orit, and Hochstein Shaul. 2005. “At First Sight: A High-Level Pop out Effect for Faces.” Vision Research 45(13):1707–24. doi: 10.1016/j.visres.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Hershler Orit, and Hochstein Shaul. 2006. “With a Careful Look: Still No Low-Level Confound to Face Pop-Out.” Vision Research 46(18):3028–35. doi: 10.1016/j.visres.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Joseph Robert M., Keehn Brandon, Connolly Christine, Wolfe Jeremy M., and Horowitz Todd S.. 2009. “Why Is Visual Search Superior in Autism Spectrum Disorder?” Developmental Science 12(6):1083–96. doi: 10.1111/j.1467-7687.2009.00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph Robert M., Tager-Flusberg Helen, and Lord Catherine. 2002. “Cognitive Profiles and Social-Communicative Functioning in Children with Autism Spectrum Disorder.” Journal of Child Psychology and Psychiatry, and Allied Disciplines 43(6):807–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldy Zsuzsa, Kraper Catherine, Carter Alice S., and Blaser Erik. 2011. “Toddlers with Autism Spectrum Disorder Are More Successful at Visual Search than Typically Developing Toddlers.” Developmental Science 14(5):980–88. doi: 10.1111/j.1467-7687.2011.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin Ami, Lin David, Gorrindo Phillip, Ramsay Gordon, and Jones Warren. 2009. “Two-Year-Olds with Autism Orient to Non-Social Contingencies Rather than Biological Motion.” Nature 459(7244):257–61. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López Beatriz, Donnelly Nick, Hadwin Julie, and Leekam Susan. 2004. “Face Processing in High‐functioning Adolescents with Autism: Evidence for Weak Central Coherence.” Visual Cognition 11(6):673–88. doi: 10.1080/13506280344000437. [DOI] [Google Scholar]
- Lord Catherine, Rutter Michael, DiLavore Pamela C., Risi Susan, Gotham Katherine, and Bishop S. 2012. Autism Diagnostic Observation Schedule: ADOS-2. Western Psychological Services; Los Angeles, CA. [Google Scholar]
- Loth Eva, Charman Tony, Mason Luke, Tillmann Julian, Jones Emily J. H., Wooldridge Caroline, Ahmad Jumana, Auyeung Bonnie, Brogna Claudia, Ambrosino Sara, Banaschewski Tobias, Baron-Cohen Simon, Baumeister Sarah, Beckmann Christian, Brammer Michael, Brandeis Daniel, Bölte Sven, Bourgeron Thomas, Bours Carsten, De Bruijn Yvette, Chakrabarti Bhismadev, Crawley Daisy, Cornelissen Ineke, Dell Acqua Flavio, Dumas Guillaume, Durston Sarah, Ecker Christine, Faulkner Jessica, Frouin Vincent, Garces Pilar, Goyard David, Hayward Hannah, Ham Lindsay M., Hipp Joerg, Holt Rosemary J., Johnson Mark H., Isaksson Johan, Kundu Prantik, Lai Meng Chuan, D’Ardhuy Xavier Liogier, Lombardo Michael V., Lythgoe David J., Mandl René, Meyer-Lindenberg Andreas, Moessnang Carolin, Mueller Nico, O’Dwyer Laurence, Oldehinkel Marianne, Oranje Bob, Pandina Gahan, Persico Antonio M., Ruigrok Amber N. V., Ruggeri Barbara, Sabet Jessica, Sacco Roberto, San José Cáceres Antonia, Simonoff Emily, Toro Roberto, Tost Heike, Waldman Jack, Williams Steve C. R., Zwiers Marcel P., Spooren Will, Murphy Declan G. M., and Buitelaar Jan K.. 2017. “The EU-AIMS Longitudinal European Autism Project (LEAP): Design and Methodologies to Identify and Validate Stratification Biomarkers for Autism Spectrum Disorders.” Molecular Autism 8(1):1–19. doi: 10.1186/s13229-017-0146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland James C., Bernier Raphael A., Jeste Shafali S., Dawson Geraldine, Nelson Charles A., Chawarska Katarzyna, Earl Rachel, Faja Susan, Johnson Scott P., Sikich Linmarie, Brandt Cynthia A., Dziura James D., Rozenblit Leon, Hellemann Gerhard, Levin April R., Murias Michael, Naples Adam J., Platt Michael L., Sabatos-DeVito Maura, Shic Frederick, Senturk Damla, Sugar Catherine A., Webb Sara J., and the Autism Biomarkers Consortium for Clinical Trials. 2020. “The Autism Biomarkers Consortium for Clinical Trials (ABC-CT): Scientific Context, Study Design, and Progress Toward Biomarker Qualification.” Frontiers in Integrative Neuroscience 14. doi: 10/gg2gmp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottron L, Dawson M, and Soulières I. 2009. “Enhanced Perception in Savant Syndrome: Patterns, Structure and Creativity.” Philosophical Transactions B 364(1522):1385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottron L, Dawson M, Soulieres I, Hubert B, and Burack J. 2006. “Enhanced Perceptual Functioning in Autism: An Update, and Eight Principles of Autistic Perception.” Journal of Autism and Developmental Disorders 36(1):27–43. [DOI] [PubMed] [Google Scholar]
- Mottron Laurent, and Burack Jacob A.. 2001. “Enhanced Perceptual Functioning in the Development of Autism.” Pp. 131–48 in The development of autism: Perspectives from theory and research. Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers. [Google Scholar]
- O’Riordan Michelle A. 2004. “Superior Visual Search in Adults with Autism.” Autism 8(3):229–48. doi: 10.1177/1362361304045219. [DOI] [PubMed] [Google Scholar]
- Pierce Karen, Marinero Steven, Hazin Roxana, McKenna Benjamin, Barnes Cynthia Carter, and Malige Ajith. 2015. “Eye Tracking Reveals Abnormal Visual Preference for Geometric Images as an Early Biomarker of an Autism Spectrum Disorder Subtype Associated with Increased Symptom Severity.” Biological Psychiatry. doi: 10.1016/j.biopsych.2015.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce Karen, Marinero Steven, Hazin Roxana, McKenna Benjamin, Barnes Cynthia Carter, and Malige Ajith. 2016. “Eye Tracking Reveals Abnormal Visual Preference for Geometric Images as an Early Biomarker of an Autism Spectrum Disorder Subtype Associated with Increased Symptom Severity.” Biological Psychiatry 79(8):657–66. doi: 10.1016/j.biopsych.2015.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaisted Kate. 2001. “Reduced Generalization in Autism: An Alternative to Weak Central Coherence.” The Development of Autism: Perspectives from Theory and Research 149–169. [Google Scholar]
- Plaisted Kate, and Davis Greg. 2009. “Perception and Apperception in Autism: Rejecting the Inverse Assumption.” Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 364(1522):1393–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaisted Kate, O’Riordan Michelle, and Baron‐Cohen Simon. 1998. “Enhanced Visual Search for a Conjunctive Target in Autism: A Research Note.” Journal of Child Psychology and Psychiatry 39(5):777–83. doi: 10.1111/1469-7610.00376. [DOI] [PubMed] [Google Scholar]
- Rutter M, LeCouteur A, and Lord C. 2003. “Autism Diagnostic Interview-Revised (ADI-R).” Western Psychological Services, Los Angeles, CA. [Google Scholar]
- Salvucci DD, and Goldberg JH. 2000. “Identifying Fixations and Saccades in Eye-Tracking Protocols.” Proceedings of the 2000 Symposium on Eye Tracking Research & Applications 71–78. [Google Scholar]
- Samson Fabienne, Mottron Laurent, Soulières Isabelle, and Zeffiro Thomas A.. 2012. “Enhanced Visual Functioning in Autism: An ALE Meta-Analysis.” Human Brain Mapping 33(7):1553–81. doi: 10.1002/hbm.21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson NJ, Elison JT, Turner-Brown LM, Dichter GS, and Bodfish JW. 2010. “Brief Report: Circumscribed Attention in Young Children with Autism.” Journal of Autism and Developmental Disorders 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson Noah J., and Touchstone Emily W.. 2014. “Visual Attention to Competing Social and Object Images by Preschool Children with Autism Spectrum Disorder.” Journal of Autism and Developmental Disorders 44(3):584–92. doi: 10.1007/s10803-013-1910-z. [DOI] [PubMed] [Google Scholar]
- Sasson Noah J., Turner-Brown Lauren M., Holtzclaw Tia N., Lam Kristen S. L., and Bodfish James W.. 2008. “Children with Autism Demonstrate Circumscribed Attention during Passive Viewing of Complex Social and Nonsocial Picture Arrays.” Autism Research 1(1):31–42. doi: 10.1002/aur.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepeta Leigh, Tsuchiya Naotsugu, Davies Mari S., Sigman Marian, Bookheimer Susan Y., and Dapretto Mirella. 2012. “Abnormal Social Reward Processing in Autism as Indexed by Pupillary Responses to Happy Faces.” Journal of Neurodevelopmental Disorders 4(1):17. doi: 10.1186/1866-1955-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, and Balla DA. 2005. “Vineland Adaptive Behavior Scales, (Vineland-II).” AGS, Circle Pines, MN. [Google Scholar]
- VanRullen Rufin. 2006. “On Second Glance: Still No High-Level Pop-out Effect for Faces.” Vision Research 46(18):3017–27. doi: 10.1016/j.visres.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Webb Sara Jane, Shic Frederick, Murias Michael, Sugar Catherine A., Naples Adam J., Barney Erin, Borland Heather, Hellemann Gerhard, Johnson Scott, Kim Minah, Levin April R., Sabatos-DeVito Maura, Santhosh Megha, Senturk Damla, Dziura James, Bernier Raphael A., Chawarska Katarzyna, Dawson Geraldine, Faja Susan, Jeste Shafali, McPartland James, and the Autism Biomarkers Consortium for Clinical Trials. 2020. “Biomarker Acquisition and Quality Control for Multi-Site Studies: The Autism Biomarkers Consortium for Clinical Trials.” Frontiers in Integrative Neuroscience 13. doi: 10.3389/fnint.2019.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe Jeremy M. 1998. “What Can 1 Million Trials Tell Us About Visual Search?” Psychological Science 9(1):33–39. doi: 10.1111/1467-9280.00006. [DOI] [Google Scholar]


