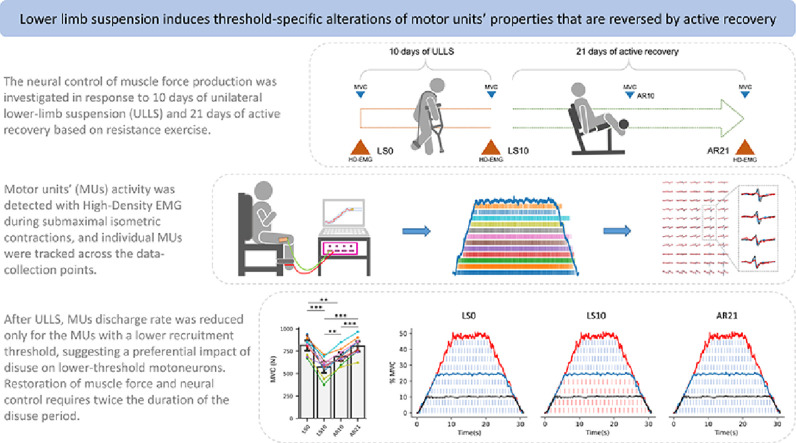
Highlights
-
•
Little is known about the consequences of disuse on the neural control of large muscles and how these can be reversed.
-
•
We studied changes in motor units properties in the vastus lateralis muscle after 10 days of unilateral lower limb suspension and after 21 days of active recovery.
-
•
A short period of muscle unloading reduces the discharge rate of lower- but not of higher-threshold motor units, suggesting a preferential impact of disuse on motoneurons with a lower depolarization threshold.
-
•
The restoration of neural control requires about twice the duration of the disuse period.
Keywords: Disuse, High-density EMG, Muscle disuse, Neural impairment, Neuromuscular degeneration
Abstract
Purpose
This study aimed to non-invasively test the hypothesis that (a) short-term lower limb unloading would induce changes in the neural control of force production (based on motor units (MUs) properties) in the vastus lateralis muscle and (b) possible changes are reversed by active recovery (AR).
Methods
Ten young males underwent 10 days of unilateral lower limb suspension (ULLS) followed by 21 days of AR. During ULLS, participants walked exclusively on crutches with the dominant leg suspended in a slightly flexed position (15°–20°) and with the contralateral foot raised by an elevated shoe. The AR was based on resistance exercise (leg press and leg extension) and executed at 70% of each participant's 1 repetition maximum, 3 times/week. Maximal voluntary isometric contraction (MVC) of knee extensors and MUs properties of the vastus lateralis muscle were measured at baseline, after ULLS, and after AR. MUs were identified using high-density electromyography during trapezoidal isometric contractions at 10%, 25%, and 50% of the current MVC, and individual MUs were tracked across the 3 data collection points.
Results
We identified 1428 unique MUs, and 270 of them (18.9%) were accurately tracked. After ULLS, MVC decreased by 29.77%, MUs absolute recruitment/derecruitment thresholds were reduced at all contraction intensities (with changes between the 2 variables strongly correlated), while discharge rate was reduced at 10% and 25% but not at 50% MVC. Impaired MVC and MUs properties fully recovered to baseline levels after AR. Similar changes were observed in the pool of total as well as tracked MUs.
Conclusion
Our novel results demonstrate, non-invasively, that 10 days of ULLS affected neural control predominantly by altering the discharge rate of lower-threshold but not of higher-threshold MUs, suggesting a preferential impact of disuse on motoneurons with a lower depolarization threshold. However, after 21 days of AR, the impaired MUs properties were fully restored to baseline levels, highlighting the plasticity of the components involved in neural control.
Graphical abstract
1. Introduction
Skeletal muscle disuse is a condition that can be experienced at any age and for a broad variety of reasons, including injury, hospitalization (e.g., during disease and after surgery), and even during space flight. In order to investigate the effects of disuse on neuromuscular health, and to identify potential countermeasures, different experimental models have been proposed1,2 (e.g., limb suspension, limb immobilization, bed-rest). Regardless of the implemented model or the investigated muscle, reduction in muscle force is considered a primary consequence of disuse.3 Currently, several mechanisms underlying disuse-induced muscle force reduction have been proposed, such as muscle atrophy3 and altered contractile properties of muscle fibers4 (i.e., excitation–contraction coupling) based on observations following limb suspension and bed-rest. However, these mechanisms are considered insufficient to fully explain the loss of muscle force, suggesting that other key processes might be involved.3,4
Disuse has been also defined as a reduction in the number of action potentials delivered by motoneurons;1 in light of this, it is surprising how little attention has been given to the role of neural signaling to the muscle in disuse conditions. Indeed, the available knowledge is limited to the seminal works of Duchateau and Hainaut5 and Seki et al,6,7 which are based on intramuscular electromyographic (EMG) recordings in hand muscles (i.e., adductor pollicis and first dorsal interosseous). These works consistently reported that hand cast immobilization results in a reduction of the discharge rate (DR) of motor units (MUs) across different contraction intensities, although discrepant conclusions were reached regarding whether the alterations in MUs DR were more pronounced for lower- or higher-threshold MUs. Duchateau and Hainaut5 directly observed that the gain in MUs DR was predominantly reduced in lower-threshold MUs while the results of Seki et al7 indirectly suggested that the higher-threshold MUs might be more affected, based on a reduced slope in their force–frequency relationship. These contrasting results may be attributed to different interpretations of the data or, perhaps, to the pioneering approaches adopted in these investigations, which only allowed for the manual identification of a small number of MUs so as to minimize variability in the results. Additionally, it is unknown whether the findings of these studies might be limited to small muscles since larger muscles use distinct neural strategies to control force production8 (hereafter referred to as neural control9).
Over the last years, the development of EMG technologies and the introduction of multi-channel recording systems, namely high-density EMG (HD-EMG),9 allowed for the non-invasive detection of a large and representative population of MUs active in different regions of the examined muscle.10 Moreover, these unique features allowed for the appropriate investigation of MUs activity in large muscles9 like the knee extensors, which are fundamental for locomotion and independence in daily tasks and for the identification and tracking of individual MUs over time.11 Besides, the concurrent recording of force may provide additional insights on the discharge characteristics of the simultaneously activated MUs and their common synaptic input, especially at lower intensities of contraction.12
Therefore, the aim of this study was to take advantage of HD-EMG to investigate how 10 days of unilateral lower limb suspension (ULLS), a well-established model of disuse induced with limb unloading,13 can alter the neural control in a large thigh muscle (i.e., vastus lateralis). Furthermore, we also investigated the condition of neural control after 21 days of active recovery (AR) based on moderate-to-high-intensity resistance exercise. Our hypotheses were that (a) 10 days of ULLS would be sufficient to induce detectable alterations of neural control, particularly via alterations of MUs DR, and (b) that 21 days of AR (about twice the duration of the ULLS) would be sufficient to restore neural control.
2. Methods
2.1. Participants and experimental protocol
This study was part of a larger investigation aimed at detecting early biomarkers of neuromuscular degeneration after short-term unloading.14
The investigation was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Department of Biomedical Sciences of the University of Padova (Italy), reference HEC-DSB/01-18. Volunteers were enrolled in the study after examination of medical history and signing the written consent form.
Twelve recreationally active young adults volunteered to participate in this study. To reduce the risk of deep venous thrombosis associated with ULLS, which is more common in females,15 only male individuals were accepted. Inclusion criteria were: 18–35 years of age, body mass index ranged 20–28 kg/m2, and involvement in recreational physical activities (1–3 times/week, self-reported). Exclusion criteria were: sedentary lifestyle, smokers, history of deep venous thrombosis, and any other condition preventing safe participation in the study.
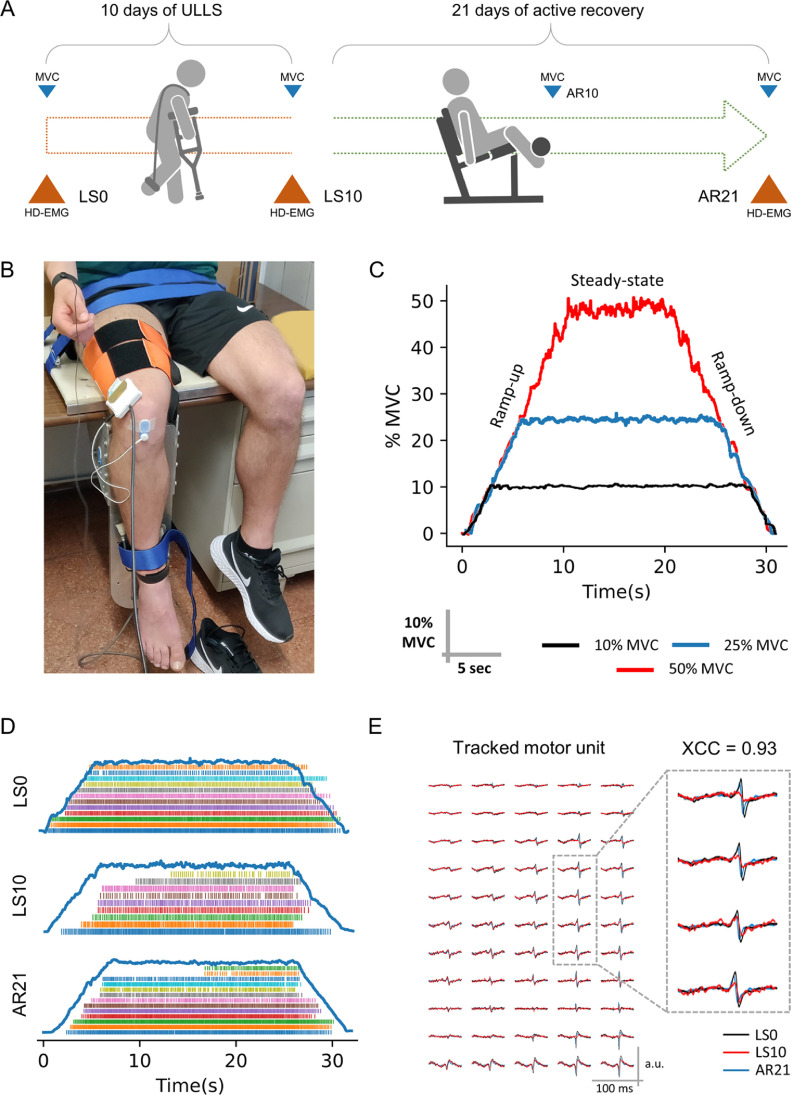
Prior to the beginning of the study, participants were familiarized with the study procedures, and they practiced carrying out daily tasks while performing ULLS.16 Measurements were conducted at baseline (Day 0 of limb suspension (LS0)), after 10 days of ULLS (LS10), and following 21 days of AR (AR21) (Fig. 1A). At LS10, participants were tested immediately after the interruption of the limb suspension. At AR21, the tests were executed 72 h after the last exercise session to avoid muscle fatigue.
Fig. 1.
Schematic representation of (A) the study design and (B–E) procedures of data collection and analysis. (A) data were collected at baseline (Day 0 of limb suspension), after 10 days of ULLS, and after 21 days of active recovery (data collection points were named LS0, LS10, and AR21, respectively). MVC was recorded also after 10 days of active recovery (AR10); (B) HD-EMG was recorded from the vastus lateralis muscle during ramp contractions at 10%, 25%, and 50% MVC; (C) ramp slope was standardized at 5% MVC/s. The recorded electrical activity of the muscle was decomposed to obtain (D) the pattern of discharge times of the MUs and (E) the MUs action potential shape was used to track the MUs longitudinally across the different data collection points. XCC is the measure of similarity between the MUs action potential shape. AR = active recovery; HD-EMG = high-density electromyography; LS = limb suspension; MUs = motor units; MVC = maximal voluntary contraction; ULLS = unilateral lower limb suspension; XCC = cross-correlation coefficient.
The duration of the AR phase was based on previous observations that full recovery of muscle function after a 2-week lower limb immobilization required an AR period lasting twice as long (4 weeks) as the disuse phase.17 In this study, an intermediate measurement of maximal voluntary isometric contraction (MVC) was also performed after 10 days of AR (AR10) to monitor how much force had been recovered since LS0 (Fig. 1A).
Participants were asked to refrain from intense exercise as well as coffee and alcohol intake during the 24 h preceding data collection.
2.2. Intervention
2.2.1. ULLS
The dominant lower limb (right leg for all participants) was suspended in a slightly flexed position (15°–20° of knee flexion) with straps connecting the shoulders and the suspended foot (Fig. 1A) while the opposite foot was fitted with an elevated shoe (50 mm sole) to prevent the suspended lower limb from touching the ground while moving, as originally described by Berg et al.13 Volunteers walked exclusively on crutches during the whole ULLS period and avoided any loading or active contraction of the suspended limb.13 The straps were always worn when participants had the necessity to walk but were removed while sitting or lying in bed. Participants were instructed on how to properly wear the straps during the familiarization session.
Precautionary measures to prevent deep venous thrombosis have been taken as previously described.14 Compliance was evaluated through daily calls and messages.
2.2.2. AR
The AR phase started 72 h after the end of the suspension period and was conducted for the following 21 days. The AR program was based on unilateral resistance exercise performed 3 times/week with at least 24 h of recovery between sessions. Every session was composed of 3 sets of 10 repetitions of leg press and leg extension at 70% 1 repetition maximum (RM) after a warm-up period at 30% 1RM. Both exercises were executed from full knee extension (0°) to ∼90° limb flexion. Sets were separated by a 2-min rest. The time under tension was set at ∼2 s both in the concentric and eccentric phases.
1RM was estimated from the 4RM–6RM during the first exercise session18 of each week, and the load employed was adjusted accordingly.
The choice of the intensity and the decision to indirectly estimate the 1RM were based on the participant characteristics (i.e., not previously involved in resistance exercise and coming from 10 days of complete unloading of the lower limb).
2.3. Measurements
2.3.1. MVC
MVC was assessed during MVC of the knee extensor muscles at a 90° knee angle using a custom-made knee dynamometer fitted with a load cell (RS 206–0290; Tedea Huntleigh, Selb, Germany) and attached above the ankle with straps (Fig. 1B), as previously described.4,14
In order to ensure correct assessment of MVC,19 participants practiced the task during the familiarization session supervised by an experienced operator. They were instructed to “push as hard as possible” by pushing the dominant leg against the load cell and maintaining the contraction for 3–4 s. After a standardized warm-up executed up to 70% of their perceived maximum, participants practiced the maximal task until they became able to consistently reach their maximum force values. During every contraction, participants were stabilized to the seat by straps at the waist in order to prevent any compensatory movement. Loud verbal encouragement was provided to encourage the maximum voluntary effort. At each data collection point, the test was repeated 3 times with 60 s of rest, and only the contraction with the maximum value was considered for the MVC calculation.
2.3.2. HD-EMG matrix placement
The HD-EMG signal was recorded from the vastus lateralis muscle (Fig. 1B) using a matrix of 64 equally spaced electrodes (GR08MM1305; OT Bioelettronica, Torino, Italy) filled with conductive cream (Ac cream; OT Bioelettronica) and arranged over 5 columns and 13 rows with 8 mm interelectrode distance, which corresponded to 30.72 cm2 of recording area.
The matrix was placed following the muscle fascicle orientation (detected with B-mode ultrasound; Mylab70; Esaote, Genoa, Italy)20 and with the central electrodes of the last 2 rows of the matrix over the innervation zone.21 The ultrasound recordings were used also to detect muscle borders and avoid the placement of the matrix across adjacent muscles.
The innervation zone was detected between 35% and 20% of femur length21 with low-intensity percutaneous electrical stimulation using a pen electrode with an electrical current of 8–16 mA (Digitimer Ltd, Welwyn Garden, Hertfordshire, UK).14
Before placing the matrix, the skin was shaved, cleaned with 70% ethanol, and then with abrasive-conductive paste (Spes medica, Salerno, Italy). Reference electrodes were placed on the malleolus and patella bones. After the recordings, the matrix border was marked with a permanent marker and emphasized by the operator at every meeting with the participants to allow the reproducible placement of the matrix in the following data collection points.22
2.3.3. HD-EMG recordings
The HD-EMG signal was recorded during trapezoidal contractions (Fig. 1C) at 3 different submaximal intensities (10%, 25%, and 50% MVC). All the contractions had a total duration of 30 s. The ramp-up and ramp-down phases were performed with a linear force increase/decrease set at 5% MVC/s,23 and the duration of the steady-state phase was adjusted accordingly (e.g., at 25% MVC, the ramps lasted 5 s each and the steady-state 20 s for a total 30 s contraction) (Fig. 1C). All the trapezoidal contractions were repeated twice with 60 s of rest in between, and the different intensities were proposed in random order. Participants received real-time visual feedback of the force produced and were instructed to match the trapezoidal template as precisely as possible.
EMG and force signals were sampled at 2048 Hz with the EMG-Quattrocento (OT Bioelettronica). The EMG signal was recorded in monopolar configuration, amplified (× 150), and band-pass filtered (10–500 Hz) at source.23 Force was recorded synchronously with the EMG signal, and the offset was removed before starting the recording.
2.3.4. Force and HD-EMG signal analyses
The force signal was converted to Newton (N) and low-pass filtered (fourth-order, zero-lag, Butterworth, and 15 Hz cut-off).23 Force steadiness was computed as the coefficient of variation of force recorded during the steady-state phase (COVsteady) of the trapezoidal contractions and expressed as percentage (i.e., the ratio of the SD to the mean).24
The HD-EMG signal was band-pass filtered between 20 Hz and 500 Hz (second-order; Butterworth) and decomposed into discharge times of the MUs with the validated convolutive blind source separation technique (Fig. 1D) (OTBioLab+; OT Bioelettronica).25 After the decomposition, the pattern of discharge times for each MU was visually inspected and manually edited.26 Only the identified MUs with a pulse to noise ratio (PNR) of ≥28 decibel (dB) (sensitivity of >85%) were maintained for further analyses.27
All MUs decomposed from the 2 trapezoidal contractions recorded at the same intensity during the same data collection point (for each participant) were pooled and analyzed together after the removal of duplicated MUs (as explained in Section 2.3.5.). This approach allowed us to increase the number of unique MUs, to reduce variability induced by the inability of a participant to reproduce the trapezoidal path, and to avoid results biased by the duplication of values, which would occur if duplicated MUs were not removed.
For each identified MU, we computed the absolute and relative (as percentage of MVC) recruitment threshold (RT) and derecruitment threshold (DERT) as well as the average DR at recruitment, derecruitment, and during the steady-state phase.
MUs DR was calculated over the first and last 4 discharges at recruitment and derecruitment and during the entire steady-state phase.23
DR modulation was defined as the difference between the DR at recruitment and the DR at the start (first 10 discharges) of the steady-state phase (delta DR from recruitment to target, ΔDRR–T). The difference between the target force (i.e., 10%, 25%, and 50%) and the force at recruitment (ΔForceR–T) was also computed.
MUs properties were first analyzed based on contraction intensity and then by RT. In particular, MUs with an RT of ≤25% MVC were compared to those with an RT of >25% MVC; they were then defined as lower- and higher-threshold MUs, respectively. The analyses comparing lower- and higher-threshold MUs have been performed both in the pool of total MUs and that of tracked MUs.
Signal processing and analyses were performed with custom Python scripts (Release 3.9.7; Python Software Foundation, Fredericksburg, VA, USA).
2.3.5. MUs tracking and duplicates removal
MUs action potential waveforms and their spatial distribution (Fig. 1E) were used to recognize the same MUs across different recording sessions, as previously described.10,11,28 Briefly, the tracking method is based on the normalized 2-dimensional cross-correlation value (XCC) between the waveforms of individual MUs generated by spike-triggered average on a 25 ms time-window; this accounts for both the shape and the location of the waveforms.11,29 Different thresholds of similarity were set based on whether MUs tracking was used to remove duplicated MUs within the same recording session or to track them across the different data collection points. Pairs of MUs from the same recording session with XCC of ≥0.9 were considered duplicates28 and, therefore, the MU with the lowest PNR was removed from the following analyses.27 To track the MUs longitudinally, the XCC threshold was set at ≥0.8 to account for minor differences in the matrix placement.11,28
2.4. Statistical analysis
Concerning MVC and force steadiness, normality of the distribution of variables was assessed through the Shapiro–Wilk test. Since the normality assumption was satisfied, a one-way repeated measure analysis of variance was used. Sphericity was tested with Mauchly's test, and when the assumption of sphericity was violated, the correction of Greenhouse–Geisser was applied. Post hoc pairwise t tests were conducted with Holm's correction when the overall model had a p < 0.05.
All the MUs properties were analyzed using linear models (time as fixed effect, random intercept, and clustered by participant) as multiple MUs were recorded from each participant.14,30 Normality for the residuals of each variable was assessed through visual inspection of the Q–Q plot and histogram. If normality of the residuals was not confirmed, a generalized linear mixed effect model was used instead. Post hoc comparisons were conducted with Holm's correction when the overall model had a p < 0.05.
Repeated measures correlation was used to determine the common within-individual association for paired measures assessed at the 3 data collection points.31 For this analysis, participants’ average values were used as a representation of the clustered values (i.e., MUs properties), as is commonly done in studies with HD-EMG.32,33 Fixed slopes were employed to estimate a single correlation coefficient for all subjects, which simplifies the model and improves the stability and accuracy of the estimates.31
Mixed models were computed with Jamovi 2.2.2 (The Jamovi project, Sydney, Australia) while the other analyses were performed using Python (Release 3.9.7; pingouin package; Python Software Foundation34). Statistical significance was accepted at p < 0.05. The results are reported and plotted as mean ± SEM for linear models and as mean ± SD for the analyses of variance. Partial eta squared (ηp²) was also reported for analyses of variance.
3. Results
3.1. Participants
Out of 12 participants, 1 dropped out after baseline measures for personal reasons. All the others successfully completed the study without any adverse event. Participants characteristics were as follows: age = 22.1 ± 2.9 years; height =178.0 ± 3.1 cm; body mass = 72.1 ± 7.1 kg; BMI = 22.9 ± 2.1 kg/m2 (mean ± SD). Due to the inability to decompose MUs with a PNR above 28 dB at LS10 and AR21, 1 participant was excluded from the analyses. Hence, a total of 10 participants were included in the final analyses.
3.2. MVC and force control
Complete statistical summary of MVC and COVsteady is available as Supplementary Table 1.
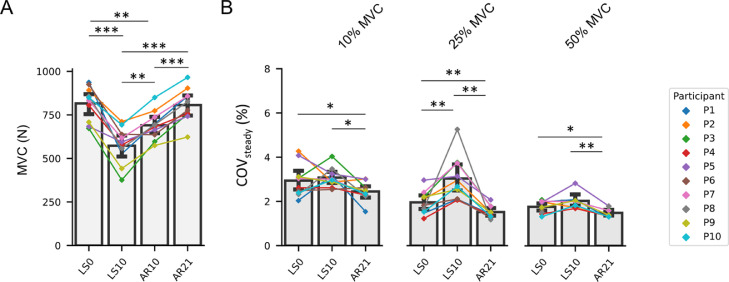
For MVC, a main effect of time was observed (ηp² = 0.842, p < 0.001). Compared to LS0, MVC decreased at LS10 (–29.77%, p < 0.001), remained lower at AR10 (–15.28%, p = 0.002), and returned to LS0 values at AR21 (Fig. 2A).
Fig. 2.
Bar plots representing (A) the MVC and (B) the COV of the steady state phase at the different data collection points. COV of the steady state phase is also represented at the 3 different submaximal contraction intensities (i.e., 10%, 25%, and 50% MVC). Data are displayed as mean ± SD, and the changes for every participant are highlighted by a connected point plot. Significance levels are: * p < 0.05, ** p < 0.01, *** p < 0.001. AR = active recovery; COV = coefficient of variation of force; LS = limb suspension; MVC = maximal voluntary isometric contraction; N = Newton.
COVsteady showed a main effect of time at all contraction intensities (p = 0.017 and ηp² = 0.364 at 10% MVC, p = 0.001 and ηp² = 0.656 at 25% MVC, p = 0.001 and ηp² = 0.699 at 50% MVC). Compared to LS0, COVsteady increased at LS10 at 25% MVC (+54.95%, p = 0.008) and decreased at AR21 for all contraction intensities (–16.77%, p = 0.020 at 10% MVC; –22.43%, p = 0.002 at 25% MVC; and –15.36%, p = 0.033 at 50% MVC) (Fig. 2B).
3.3. MUs decomposition and tracking
A total of 1428 unique MUs (616 at 10% MVC, 541 at 25% MVC, and 271 at 50% MVC) were identified, with an average PNR of 35.04 ± 2.23 dB at 10% MVC, 33.00 ± 1.87 dB at 25% MVC, and 31.80 ± 1.36 dB at 50% MVC (mean ± SD). Of these, 270 MUs (18.9% of the total pool) were tracked (Fig. 1E) across the 3 data collection points with an average XCC of 0.88 ± 0.04 at 10% MVC, 0.92 ± 0.03 at 25% MVC, and 0.93 ± 0.03 at 50% MVC (mean ± SD). The obtained values are in line with the methodological validation of the technique.11
3.4. MUs properties
A complete statistical summary of MUs properties is available in Supplementary Tables 2–4.
3.4.1. Total pool of MUs
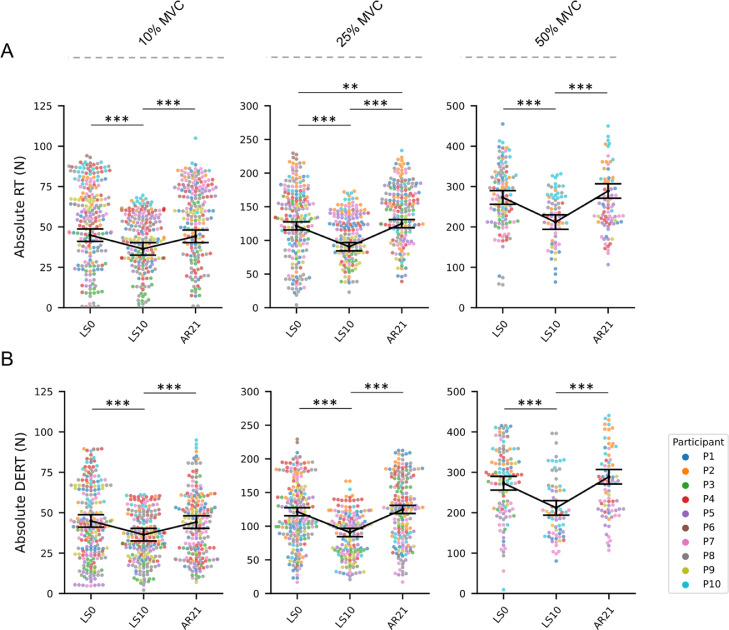
MUs absolute RT (Fig. 3A) and DERT (Fig. 3B) were reduced at LS10 (compared to LS0) for all contraction intensities (–20.69% at 10% MVC, –15.15% at 25% MVC, and –19.56% at 50% MVC; all p < 0.001 for RT; –18.93% at 10% MVC, –25.43% at 25% MVC, and –22.34% at 50% MVC; all p < 0.001 for DERT) and completely recovered to LS0 values at AR21 at 10% and 50% MVC. On the other hand, at 25% MVC, the RT at AR21 exceeded the LS0 level (+10.61%, p = 0.002).
Fig. 3.
Swarm plots representing the (A) absolute MUs RT and (B) DERT at the 3 data collection points. From left to right, MUs properties are presented for the 3 different submaximal contraction intensities (i.e., 10% MVC, 25% MVC, and 50% MVC). Individual MUs are represented by dots and clustered by subject. Summary data are presented as mean ± SEM, and the direction of the changes is highlighted by a connection line. Significance levels are: ** p < 0.01, *** p < 0.001. AR = active recovery; DERT = derecruitment threshold; LS = limb suspension; MUs = motor units; MVC = maximal voluntary isometric contraction; N = Newton; RT = recruitment threshold; SEM = standard error of the mean.
Compared to LS0, MUs relative RT (Supplementary Fig. 1A) was increased at LS10 at 10% and 25% MVC (+11.10%, p = 0.008 at 10% MVC; +19.10%, p < 0.001 at 25% MVC). At AR21, relative RT returned to baseline values at 10% but not at 25% MVC (+11.10%, p = 0.003). MUs relative DERT (Supplementary Fig. 1B) was increased at LS10 at 10% MVC (+16.10%, p < 0.01) and returned to baseline values at AR21.
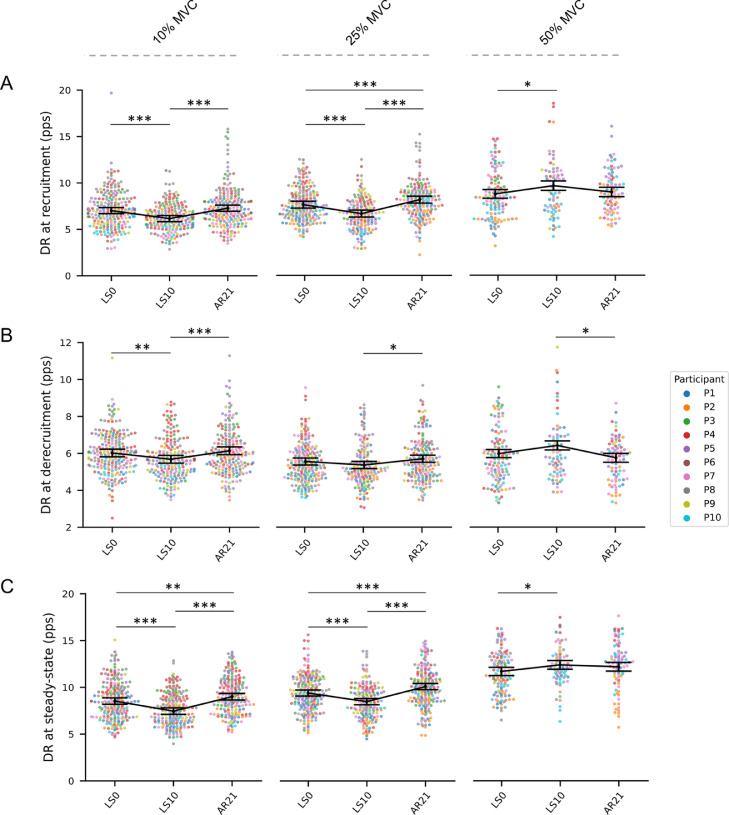
Compared to LS0, MUs DR at recruitment (Fig. 4A) was reduced at LS10 at 10% and 25% (–12.25% at 10% and –12.53% at 25% MVC; both p < 0.001) but increased at 50% MVC (+10.09%, p = 0.039). At AR21, DR at recruitment returned to baseline values at 10% and 50% while exceeding the LS0 values at 25% MVC (+7.05%, p < 0.001). At 10% MVC, DR at derecruitment was reduced at LS10 (–5.65%, p = 0.002) and returned to baseline values at AR21. At 25% and 50% MVC, DR at derecruitment did not change between LS0 and LS10 (Fig. 4B). The DR of the steady-state phase (Fig. 4C) decreased at LS10 at 10% MVC (–12.54%, p < 0.001) and 25% MVC (–9.80%, p < 0.001) and exceeded the LS0 values at AR21 (+5.51%, p = 0.006 at 10% MVC and +7.35%, p < 0.001 at 25% MVC). At 50% MVC, the DR of the steady-state phase increased at LS10 (+5.98%, p = 0.044) and returned to LS0 values at AR21.
Fig. 4.
Swarm plots representing the MUs DR at (A) recruitment, (B) derecruitment, and (C) during the steady-state phase at the 3 data collection points. From left to right, MUs properties are presented for the 3 different submaximal contraction intensities (i.e., 10%, 25%, and 50% MVC). Individual MUs are represented by dots and clustered by subject. Summary data are presented as mean ± SEM, and the direction of the changes is highlighted by a connection line. Significance levels are: * p < 0.05, ** p < 0.01, *** p < 0.001. AR = active recovery; DR = discharge rate; LS = limb suspension; MUs = motor units; MVC = maximal voluntary isometric contraction; pps = pulses per second; SEM = standard error of the mean.
Changes in the total pool of MUs classified as lower- and higher-threshold reflected closely what we observed for MUs at 10% and 25% MVC and those at 50% MVC, and they are presented in detail in the supplementary materials (Supplementary Fig. 3 and Supplementary Table 3). Briefly, ULLS affected the RT and DERT of both lower- and higher-threshold MUs while DR was affected only for lower-threshold MUs.
3.4.2. Correlations
Repeated-measures correlations were used to describe the strength of the association between 2 variables of interest and whether these associations were statistically significant.
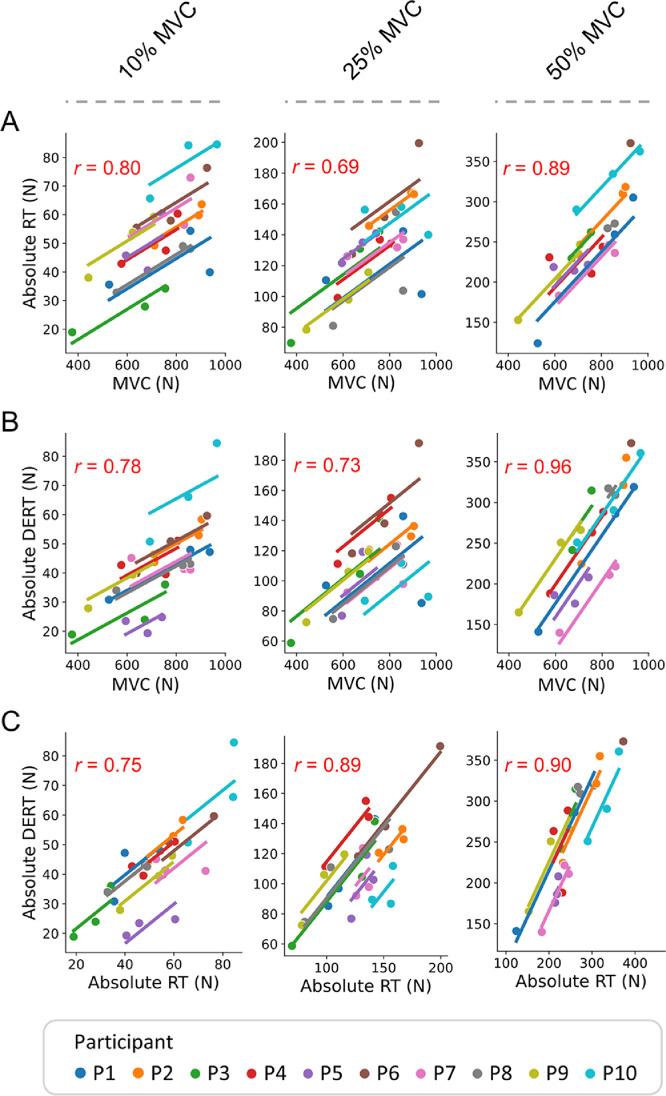
A moderate-to-strong positive correlation was observed between MVC and absolute RT at 10%, 25%, and 50% MVC (r = 0.80, 0.69, and 0.89, respectively) (Fig. 5A) as well as between MVC and absolute DERT (r = 0.78, 0.73, and 0.96, respectively) (Fig. 5B) and between absolute RT and DERT (r = 0.75, 0.89, and 0.90, respectively) (Fig. 5C). For all the correlations, significance levels were p < 0.001.
Fig. 5.
Plots of the repeated-measures correlation describing the common within-individual association between (A) MVC and absolute RT, (B) MVC and absolute DERT, as well as (C) absolute RT and absolte DERT across the different data collection points. From left to right, correlations are presented at the 3 different submaximal contraction intensities (i.e., 10%, 25%, and 50% MVC). The r value is reported in the upper left of each figure. Significance level is p < 0.001 for all correlations. DERT = derecruitment threshold; MVC = maximal voluntary isometric contraction; N = Newton; RT = recruitment threshold.
3.4.3. DR modulation
A reduced ΔDRR–T between LS0 and LS10 was observed at 10% MVC (–21.19%, p = 0.035) and 25% MVC (–41.6%, p < 0.001), but not at 50% MVC. The ΔDRR–T returned to LS0 at AR21 at 10% MVC and 25% MVC but exceeded the LS0 values at 50% (+23.73%, p = 0.039) (Supplementary Fig. 2A). The relative ΔForceR–T (Supplementary Fig. 2B) was reduced at LS10 compared to LS0 at 10% MVC (–23.50%, p = 0.005) and at 25% MVC (–35.00%, p < 0.001), but not at 50% MVC. The ΔForceR–T returned to LS0 at AR21 at 10% MVC but not at 25% MVC (–20.10%, p = 0.003).
3.4.4. Pool of tracked MUs
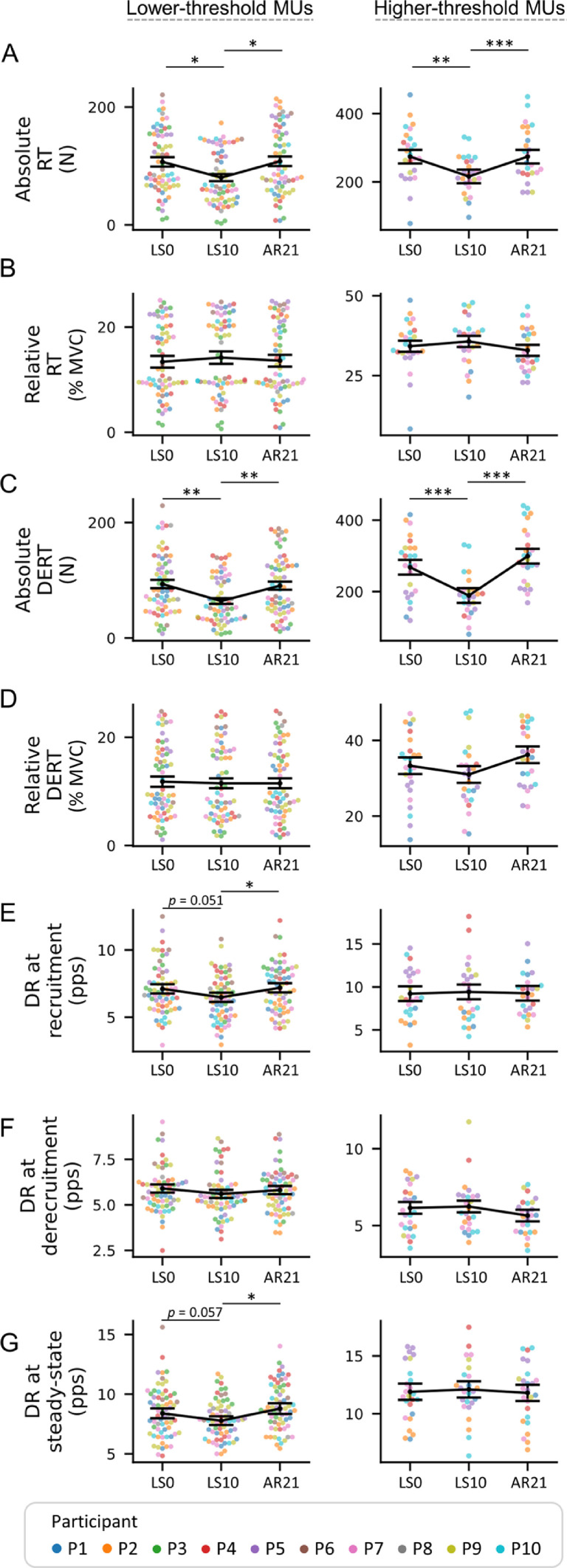
Absolute RT (Fig. 6A) was reduced at LS10 compared to LS0 (–24.95%, p = 0.017 for lower-threshold and –21.17%, p = 0.003 for higher-threshold MUs) and returned to the LS0 values at AR21. The same trend was observed for DERT (–31.55%, p = 0.003 for lower-threshold and –29.48%, p < 0.001 for higher-threshold MUs) (Fig. 6C). Neither the relative RT and DERT of the tracked MUs (Fig. 6B and 6D) differed at any measurement.
Fig. 6.
Swarm plots representing the MUs properties obtained from the pool of tracked MUs. (A and C) MUs RT and DERT are presented in both absolute and (B and D) relative terms (as percent of the MVC). MUs DR is shown at (E) recruitment, (F) derecruitment, and (G) during the steady-state phase, at the 3 data collection points. From left to right, MUs properties are presented based on the classification of lower- and higher-threshold (i.e., recruited below or above 25% MVC). Individual MUs are represented by dots and clustered by subject. Summary data are presented as mean ± SEM, and the direction of the changes is highlighted by a connection line. Significance levels are: * p < 0.05, ** p < 0.01, *** p < 0.001. AR = active recovery; DERT = derecruitment threshold; DR = discharge rate; LS = limb suspension; MUs = motor units; MVC = maximal voluntary isometric contraction; N = Newton; pps = pulses per second; RT = recruitment threshold; SEM = standard error of the mean.
Comparing LS10 with LS0, DR at recruitment (–8.86%, p = 0.051; Fig. 6E) and during the steady-state phase (–7.27%, p = 0.057; Fig. 6G) approached the borderline of a significant reduction for lower-threshold but not for higher-threshold MUs that did not change. DR at derecruitment was not affected by the interventions (Fig. 6F). For both lower- and higher-threshold MUs, ∆DRR–T was not affected by the intervention (Supplementary Fig. 4A). A visual representation of the between- and within-participants variability of tracked MUs is available in Supplementary Fig. 5.
3.5. Summary of MUs properties
In the total pool of MUs, absolute RT and DERT were reduced at LS10 at all contraction intensities and recovered to baseline levels at AR21. In contrast, MUs DR was reduced at LS10 but only at 10% and 25% MVC while, at 50% MVC, DR increased compared to LS0. At AR21, DR returned to baseline levels at 50% MVC while it exceeded the baseline levels at 10% and 25% MVC. The changes in MUs absolute RT, DERT, and DR were confirmed in the pool of tracked MUs.
At LS10, relative RT was increased at 10% and 25% but not at 50% MVC. At AR21, it recovered to baseline levels at 10% but not at 25% MVC. Relative RT did not change in the pool of tracked MUs.
The ranges of DR (ΔDRR–T) and force modulation (ΔForceR–T) were reduced at LS10 compared to LS0 at 10% and 25% but not at 50% MVC. At both 10% and 25% MVC, ΔDRR–T returned to baseline levels at AR21. At AR21at 10% but not at 25% MVC, ΔForceR–T returned to baseline levels. No changes were identified in the pool of tracked MUs.
4. Discussion
The main finding of this study was that a short period of unloading of the dominant lower limb (i.e., 10 days of ULLS) in young healthy men is sufficient to induce a detectable alteration of the neural strategies used to control muscle force production (referred to here as neural control9,10) in the vastus lateralis muscle predominantly by reducing the DR of lower- but not higher-threshold MUs. Moreover, 21 days of AR (about twice the duration of ULLS) based on resistance exercise is sufficient to restore neural control and even to suggest the beginning of an exercise-induced overcompensation, highlighting the remarkable functional plasticity of the neural components.
The threshold-specific alterations of MUs DR demonstrate a different impact of disuse on the motoneurons innervating lower- and higher-threshold MUs in the large vastus lateralis muscle. This finding shows a similarity with the seminal work of Duchateau and Hainaut5 on small hand muscles and suggests that the preferential impact of disuse on the motoneurons innervating lower-threshold MUs is a general phenomenon. In other words, this response appears as a fundamental mechanism independent from the size or the anatomical location and characteristics of the considered muscle.8,35
Unveiling the effects of unloading on the neural control of vastus lateralis, a large component in the group of knee extensor muscles that are fundamental for daily life motor tasks and with key metabolic roles, may have relevance for developing practices aimed at preventing or recovering disuse-induced neuromuscular impairments. For example, knowing the preferential deterioration of the motoneurons innervating lower-threshold MUs, and the rapid onset of this alteration, might drive the development of rehabilitation protocols specifically designed to target these motoneurons (low intensities of exercise) and suitable to be carried out shortly after the cause of disuse (e.g., injury or surgery).
4.1. MUs DR is affected by ULLS
Our data indicate a difference between the ULLS-induced changes in absolute RT and DERT, observed both in lower- and higher-threshold MUs, and the changes in DR that differed for lower- and higher-threshold MUs. Notably, all these changes were confirmed in the pool of tracked MUs, where there are no confounding effects due to different populations of decomposed MUs,11,22 thereby strengthening the reliability of the results.
The strong correlation between absolute RT and DERT suggests that the order of recruitment and derecruitment was not altered by disuse; in other words, short-term disuse seems to not cause deviations from the Henneman's size principle.36 Additionally, the correlation between MVC and absolute RT and DERT highlights a synergic change of the 2 parameters and indicates that the reduced RT observed at LS10 at all contraction intensities should be a consequence of internal muscular impairments equally affecting all MUs. Indeed, recent evidence suggests that short periods of bed rest and ULLS are sufficient to impair muscle fibers contractility by altering intracellular calcium handling4 and muscle fibers specific force.37 Interestingly, these changes were identified evenly in slow and fast muscle fibers. In this case, the recruitment of MUs with altered contractile properties will generate lower contractile force and, therefore, all MUs will be recruited at lower absolute intensities.
Regarding the different effect of ULLS on DR of lower- and higher-threshold MUs, we hypothesized the possibility of alterations specific to the motoneurons with a lower depolarization threshold (which are expected to innervate smaller MUs possibly composed by a prevalence of slow-type muscle fibers22,36,38,39). Indeed, it was demonstrated that 3 weeks of knee immobilization induced a phenotype shift from slow to fast fibers.40,41 This phenomenon might be related to, and anticipated by, a different functional preservation of lower- and higher-threshold motoneurons. In this case, although higher-threshold motoneurons might retain largely unaltered DR activity (at least for 10 days), muscle fibers are unable to effectively translate the neural signaling in force production due to impaired contractile properties.4
In addition, mechanisms such as the recurrent inhibition42 (i.e., motoneuron inhibition mediated by the Renshaw cells) or synaptic noise43, 44, 45 (i.e., random disturbing synaptic activity in neurons that can alter the common input to the motoneurons) could contribute to the modulation of the lower-threshold motoneurons’ activity. Indeed, the reduced force steadiness that we observed at LS10 at 25% MVC suggests the presence of synaptic noise induced by ULLS, in light of recent evidence suggesting that altered synaptic noise might be responsible for altered steadiness at lower intensities of contraction.43, 44, 45
We hypothesized that as a consequence of peripheral impairment of the muscle fibers mechanical contractile properties after disuse,4 MUs are recruited at lower absolute intensities. At the same time, the reduced DR for the lower-threshold MUs introduces an additional neural limitation to force production. By increasing contraction intensity and recruiting higher-threshold MUs, the higher-threshold motoneurons with preserved functionality may in principle try to compensate for the loss of force caused by impaired lower-threshold motoneurons (already recruited and sustaining force production); in practice, however, they would have little or no effect due to mechanical constraints of muscle fibers.4,37 This last hypothesis suggests that DR reduction of lower-threshold MUs, alongside impaired muscle fibers mechanical contractile properties4 and other factors, could be responsible for the reduction in muscle force commonly observed after disuse.
4.2. Reduced DR, but not DR modulation, might be responsible for reduced muscle force
To further investigate the changes in MUs DR, we analyzed the DR modulation (∆DRR–T) of single MUs. In the total pool of MUs, the DR modulation was reduced for lower- but not higher-threshold MUs. However, in the same pool, lower-threshold MUs were recruited at a higher intensity in relative terms (i.e., the relative RT of the MUs decomposed at 10% and 25% MVC at LS10 was higher than at LS0). Therefore, we concluded that DR modulation was only partially affected by ULLS and that the observed difference was mainly a consequence of a differently balanced population of MUs decomposed at LS10 compared to LS0. This was also confirmed in the pool of tracked MUs, where no significant difference in DR modulation was observed. It should be noted, however, that tracked MUs showed a trend similar to that observed in the total pool, suggesting that significant alteration of DR modulation might become apparent with longer-term unloading or more severe models of disuse, as previously suggested.5 Indeed, the work of Duchateau and Hainaut5 on hand muscles reported a larger reduction in DR modulation for lower- compared to higher-threshold MUs after 6–8 weeks of hand cast immobilization. This finding suggests that the preferential impact of disuse on the motoneurons innervating lower-threshold MUs should be expected for both smaller and larger muscles, despite different utilization of MUs recruitment strategies and DR modulation to sustain force production (i.e., compared to large muscles, smaller muscles are expected to achieve the full MUs recruitment at lower intensity of contraction and to rely more on DR modulation to achieve the MVC8,35). Unfortunately, this information is not available in the study of Seki et al,7 which also reported a reduction in DR modulation after 6 weeks of cast immobilization but did not investigate whether this was more pronounced in lower- or higher-threshold MUs.
Altogether, our findings suggest reduced DR, but not DR modulation, as an early determinant of force loss after short periods of unloading. It also suggests that impaired DR modulation might become a limiting factor at later stages.
4.3. Neural control is restored by an AR period
Twenty-one days of AR (about twice the duration of the ULLS intervention) with a resistance-exercise protocol executed at 70% 1RM completely restored the LS0 levels of muscle force and neural control. Our hypothesis is that increased neural drive to the muscle during the AR period, induced by increased demand of motor tasks,46 stimulated both the recovery of intrinsic properties of the motoneurons and of the contractile muscle tissue. Additionally, some parameters (e.g., force steadiness and MUs DR) also exceeded the LS0 levels, suggesting the early onset of some exercise-induced overcompensatory adaptations.46 In particular, the improved force steadiness might suggest an enhanced adaptive response of the superior centers of neuromuscular control.45
The complete recovery observed after 21 days of AR, at least in young and healthy men, highlights the plasticity of the components involved in the regulation of neural control and the complete reversibility of the changes induced by short periods of unloading. It should be noted, however, that although the full recovery was achieved, a longer time (compared to the duration of unloading) was necessary, likely suggesting a sort of unloading-induced hysteresis in the recovery process. Notably, the necessity of a prolonged recovery period for the restoration of the neural control in young and healthy men could have profound implications for fragile populations (e.g., older people), for which the recovery after a period of disuse could be difficult and further delayed.17
4.4. Methodological considerations
In line with previous studies,23,32,47 potential alterations in MUs behavior could not be assessed during very high contraction intensities or at the MVC due to a progressive increase in the number of superimposed and overlapping MUs action potentials in the recorded EMG signal. Nevertheless, the range of intensity analyzed in this study is crucial for most of the daily tasks and has, therefore, clinical and physiological relevance.
The differences in relative RT and DERT between LS0 and LS10 in the total pool of MUs indicate that the populations of MUs decomposed at the 2 data collection points might differ slightly, suggesting that alterations in the MUs action potential properties14,48,49 (e.g., shape, complexity2) might have affected the results of the decomposition favoring the detection of MUs with a higher RT. This observation carries at least 2 implications: (a) the decomposition algorithm successfully identified active MUs even after an unloading period, and (b) while comparing MUs properties before and after severe interventions, close attention has to be paid to the comparability of the decomposed pool of MUs.50 Therefore, longitudinal tracking of the same MUs provides stronger evidence of changes affecting the single MU, although it significantly reduces the number of investigated MUs (about 20% of the total pool) and the statistical power. In addition, MUs tracking might preferentially identify MUs with a better-preserved action potential shape and, therefore, those less affected by the intervention.50 For this reason, the discussion of the paper focused mainly on the interpretation of the results obtained from the total pool, while analysis of the tracked pool of MUs was used to validate and support the results.
We acknowledge as a limitation in the study design the absence of a control group, which could have been useful for experimentally demonstrating the maintenance of the MUs properties across the study period and for verifying the performance of decomposition and MUs tracking in absence of intervention.
Finally, even though 10 days of ULLS were sufficient to induce noticeable impairments in neural control, longer duration,51 more severe models of disuse,52 or different populations (e.g., older adults)53 might highlight different adaptations.
5. Conclusion
Our novel results demonstrate, for the first time in large muscles, that ULLS induces a preferential deterioration of motoneurons innervating lower-threshold MUs; they also suggest this deterioration is one of the early determinants of force loss after periods of disuse. Furthermore, integrating our findings with the available literature, we propose that the preferential impact of disuse on the motoneurons innervating lower-threshold MUs is a fundamental physiological mechanism independent from the size, location, and function of the considered muscle.
Additionally, the ability to non-invasively investigate the changes in neural control after ULLS and AR with HD-EMG could have relevance for the development of prospective countermeasures aimed at delaying or recovering the disuse-induced impairments.
Acknowledgments
The present study was funded by the Italian Space Agency, MARcatori biologici e funzionali per la biomeccanica aStronautica di PREcisione (Project number DC-VUM-2017-006). The study sponsor/funder was not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report. We thank the volunteers for their participation in the study.
Authors’ contributions
GV conceptualized and designed the study, was in charge of the HD-EMG recordings and analysis, and drafted the manuscript; FS conceptualized and designed the study and was involved in data collection and analysis; AC and ADV were involved in data collection and analysis; MVF, MVN, and GDV conceptualized and designed the study. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials associated with this article can be found in the online version at doi:10.1016/j.jshs.2023.06.004.
Supplementary materials
References
- 1.Reggiani C. Not all disuse protocols are equal: New insight into the signalling pathways to muscle atrophy. J Physiol. 2015;593:5227–5228. doi: 10.1113/JP271613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarto F, Valli G, Monti E. Motor unit alterations with muscle disuse: What's new? J Physiol. 2022;600:4811–4813. doi: 10.1113/JP283868. [DOI] [PubMed] [Google Scholar]
- 3.Clark BC. In vivo alterations in skeletal muscle form and function after disuse atrophy. Med Sci Sports Exerc. 2009;41:1869–1875. doi: 10.1249/MSS.0b013e3181a645a6. [DOI] [PubMed] [Google Scholar]
- 4.Monti E, Reggiani C, Franchi MV, et al. Neuromuscular junction instability and altered intracellular calcium handling as early determinants of force loss during unloading in humans. J Physiol. 2021;599:3037–3061. doi: 10.1113/JP281365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duchateau J, Hainaut K. Effects of immobilization on contractile properties, recruitment and firing rates of human motor units. J Physiol. 1990;422:55–65. doi: 10.1113/jphysiol.1990.sp017972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seki K, Kizuka T, Yamada H. Reduction in maximal firing rate of motoneurons after 1-week immobilization of finger muscle in human subjects. J Electromyogr Kinesiol. 2007;17:113–120. doi: 10.1016/j.jelekin.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Seki K, Taniguchi Y, Narusawa M. Effects of joint immobilization on firing rate modulation of human motor units. J Physiol. 2001;530:507–519. doi: 10.1111/j.1469-7793.2001.0507k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Luca CJ. Control properties of motor units. J Exp Biol. 1985;115:125–136. doi: 10.1242/jeb.115.1.125. [DOI] [PubMed] [Google Scholar]
- 9.Farina D, Negro F, Muceli S, Enoka RM. Principles of motor unit physiology evolve with advances in technology. Physiology (Bethesda) 2016;31:83–94. doi: 10.1152/physiol.00040.2015. [DOI] [PubMed] [Google Scholar]
- 10.Del Vecchio A, Holobar A, Falla D, Felici F, Enoka RM, Farina D. Tutorial: Analysis of motor unit discharge characteristics from high-density surface EMG signals. J Electromyogr Kinesiol. 2020;53 doi: 10.1016/j.jelekin.2020.102426. [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Valdes E, Negro F, Laine CM, Falla D, Mayer F, Farina D. Tracking motor units longitudinally across experimental sessions with high-density surface electromyography. J Physiol. 2017;595:1479–1496. doi: 10.1113/JP273662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enoka RM, Duchateau J. Rate coding and the control of muscle force. Cold Spring Harb Perspect Med. 2017;7 doi: 10.1101/cshperspect.a029702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berg HE, Dudley GA, Häggmark T, Ohlsén H, Tesch PA. Effects of lower limb unloading on skeletal muscle mass and function in humans. J Appl Physiol (1985) 1991;70:1882–1885. doi: 10.1152/jappl.1991.70.4.1882. [DOI] [PubMed] [Google Scholar]
- 14.Sarto F, Stashuk DW, Franchi MV, et al. Effects of short-term unloading and active recovery on human motor unit properties, neuromuscular junction transmission and transcriptomic profile. J Physiol. 2022;600:4731–4751. doi: 10.1113/JP283381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bleeker MWP, Hopman MTE, Rongen GA, Smits P. Unilateral lower limb suspension can cause deep venous thrombosis. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1176–R1177. doi: 10.1152/ajpregu.00718.2003. [DOI] [PubMed] [Google Scholar]
- 16.Tesch PA, Lundberg TR, Fernandez-Gonzalo R. Unilateral lower limb suspension: From subject selection to “omic” responses. J Appl Physiol (1985) 2016;120:1207–1214. doi: 10.1152/japplphysiol.01052.2015. [DOI] [PubMed] [Google Scholar]
- 17.Suetta C, Hvid LG, Justesen L, et al. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol (1985) 2009;107:1172–1180. doi: 10.1152/japplphysiol.00290.2009. [DOI] [PubMed] [Google Scholar]
- 18.Brzycki M. Strength testing—Predicting a one-rep max from reps-to-fatigue. J Phys Educ Recreat Danc. 1993;64:88–90. [Google Scholar]
- 19.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 20.Hug F, Del Vecchio A, Avrillon S, Farina D, Tucker K. Muscles from the same muscle group do not necessarily share common drive: Evidence from the human triceps surae. J Appl Physiol (1985) 2021;130:342–354. doi: 10.1152/japplphysiol.00635.2020. [DOI] [PubMed] [Google Scholar]
- 21.Botter A, Oprandi G, Lanfranco F, Allasia S, Maffiuletti NA, Minetto MA. Atlas of the muscle motor points for the lower limb: Implications for electrical stimulation procedures and electrode positioning. Eur J Appl Physiol. 2011;111:2461–2471. doi: 10.1007/s00421-011-2093-y. [DOI] [PubMed] [Google Scholar]
- 22.Casolo A, Farina D, Falla D, Bazzucchi I, Felici F, Del Vecchio A. Strength training increases conduction velocity of high-threshold motor units. Med Sci Sports Exerc. 2020;52:955–967. doi: 10.1249/MSS.0000000000002196. [DOI] [PubMed] [Google Scholar]
- 23.Del Vecchio A, Casolo A, Negro F, et al. The increase in muscle force after 4 weeks of strength training is mediated by adaptations in motor unit recruitment and rate coding. J Physiol. 2019;597:1873–1887. doi: 10.1113/JP277250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enoka RM, Farina D. Force steadiness: From motor units to voluntary actions. Physiology (Bethesda) 2021;36:114–130. doi: 10.1152/physiol.00027.2020. [DOI] [PubMed] [Google Scholar]
- 25.Negro F, Muceli S, Castronovo AM, Holobar A, Farina D. Multi-channel intramuscular and surface EMG decomposition by convolutive blind source separation. J Neural Eng. 2016;13 doi: 10.1088/1741-2560/13/2/026027. [DOI] [PubMed] [Google Scholar]
- 26.Hug F, Avrillon S, Del Vecchio A, et al. Analysis of motor unit spike trains estimated from high-density surface electromyography is highly reliable across operators. J Electromyogr Kinesiol. 2021;58 doi: 10.1016/j.jelekin.2021.102548. [DOI] [PubMed] [Google Scholar]
- 27.Holobar A, Minetto MA, Farina D. Accurate identification of motor unit discharge patterns from high-density surface EMG and validation with a novel signal-based performance metric. J Neural Eng. 2014;11 doi: 10.1088/1741-2560/11/1/016008. [DOI] [PubMed] [Google Scholar]
- 28.Maathuis EM, Drenthen J, van Dijk JP, Visser GH, Blok JH. Motor unit tracking with high-density surface EMG. J Electromyogr Kinesiol. 2008;18:920–930. doi: 10.1016/j.jelekin.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 29.McManus L, Lowery M, Merletti R, et al. Consensus for experimental design in electromyography (CEDE) project: Terminology matrix. J Electromyogr Kinesiol. 2021;59 doi: 10.1016/j.jelekin.2021.102565. [DOI] [PubMed] [Google Scholar]
- 30.Yu Z, Guindani M, Grieco SF, Chen L, Holmes TC, Xu X. Beyond t test and ANOVA: Applications of mixed-effects models for more rigorous statistical analysis in neuroscience research. Neuron. 2022;110:21–35. doi: 10.1016/j.neuron.2021.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bakdash JZ, Marusich LR. Repeated measures correlation. Front Psychol. 2017;8:456. doi: 10.3389/fpsyg.2017.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nuccio S, Del Vecchio A, Casolo A, et al. Deficit in knee extension strength following anterior cruciate ligament reconstruction is explained by a reduced neural drive to the vasti muscles. J Physiol. 2021;599:5103–5120. doi: 10.1113/JP282014. [DOI] [PubMed] [Google Scholar]
- 33.Del Vecchio A, Negro F, Holobar A, et al. You are as fast as your motor neurons: Speed of recruitment and maximal discharge of motor neurons determine the maximal rate of force development in humans. J Physiol. 2019;597:2445–2456. doi: 10.1113/JP277396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vallat R. Pingouin: Statistics in Python. J Open Source Softw. 2018;3:1026. doi: 10.21105/joss.01026. [DOI] [Google Scholar]
- 35.Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- 36.Mendell LM. The size principle: A rule describing the recruitment of motoneurons. J Neurophysiol. 2005;93:3024–3026. doi: 10.1152/classicessays.00025.2005. [DOI] [PubMed] [Google Scholar]
- 37.Brocca L, Longa E, Cannavino J, et al. Human skeletal muscle fibre contractile properties and proteomic profile: Adaptations to 3 weeks of unilateral lower limb suspension and active recovery. J Physiol. 2015;593:5361–5385. doi: 10.1113/JP271188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heckman CJ, Mottram C, Quinlan K, Theiss R, Schuster J. Motoneuron excitability: The importance of neuromodulatory inputs. Clin Neurophysiol. 2009;120:2040–2054. doi: 10.1016/j.clinph.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burke RE. Handbook of physiology. American Physiological Society; Bethesda, MD: 1981. Motor units: Anatomy, physiology, and functional organization. In: Brooks VB, editor; pp. 345–422. [Google Scholar]
- 40.Hortobágyi T, Dempsey L, Fraser D, et al. Changes in muscle strength, muscle fibre size and myofibrillar gene expression after immobilization and retraining in humans. J Physiol. 2000;524:293–304. doi: 10.1111/j.1469-7793.2000.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ciciliot S, Rossi AC, Dyar KA, Blaauw B, Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol. 2013;45:2191–2199. doi: 10.1016/j.biocel.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 42.Piotrkiewicz M, Kudina L, Mierzejewska J. Recurrent inhibition of human firing motoneurons (experimental and modeling study) Biol Cybern. 2004;91:243–257. doi: 10.1007/s00422-004-0507-1. [DOI] [PubMed] [Google Scholar]
- 43.Dideriksen JL, Negro F, Enoka RM, Farina D. Motor unit recruitment strategies and muscle properties determine the influence of synaptic noise on force steadiness. J Neurophysiol. 2012;107:3357–3369. doi: 10.1152/jn.00938.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faisal AA, Selen LPJ, Wolpert DM. Noise in the nervous system. Nat Rev Neurosci. 2008;9:292–303. doi: 10.1038/nrn2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dideriksen JL, Negro F, Farina D. The optimal neural strategy for a stable motor task requires a compromise between level of muscle cocontraction and synaptic gain of afferent feedback. J Neurophysiol. 2015;114:1895–1911. doi: 10.1152/jn.00247.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Škarabot J, Brownstein CG, Casolo A, Del Vecchio A, Ansdell P. The knowns and unknowns of neural adaptations to resistance training. Eur J Appl Physiol. 2021;121:675–685. doi: 10.1007/s00421-020-04567-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Casolo A, Del Vecchio A, Balshaw TG, et al. Behavior of motor units during submaximal isometric contractions in chronically strength-trained individuals. J Appl Physiol (1985) 2021;131:1584–1598. doi: 10.1152/japplphysiol.00192.2021. [DOI] [PubMed] [Google Scholar]
- 48.Piasecki M, Garnés-Camarena O, Stashuk DW. Near-fiber electromyography. Clin Neurophysiol. 2021;132:1089–1104. doi: 10.1016/j.clinph.2021.02.008. [DOI] [PubMed] [Google Scholar]
- 49.Inns TB, Bass JJ, Hardy EJO, et al. Motor unit dysregulation following 15 days of unilateral lower limb immobilisation. J Physiol. 2022;600:4753–4769. doi: 10.1113/JP283425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Power KE, Lockyer EJ, Botter A, Vieira T, Button DC. Endurance-exercise training adaptations in spinal motoneurones: Potential functional relevance to locomotor output and assessment in humans. Eur J Appl Physiol. 2022;122:1367–1381. doi: 10.1007/s00421-022-04918-2. [DOI] [PubMed] [Google Scholar]
- 51.de Boer MD, Maganaris CN, Seynnes OR, Rennie MJ, Narici MV. Time course of muscular, neural and tendinous adaptations to 23 day unilateral lower-limb suspension in young men. J Physiol. 2007;583:1079–1091. doi: 10.1113/jphysiol.2007.135392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Widrick JJ, Trappe SW, Romatowski JG, Riley DA, Costill DL, Fitts RH. Unilateral lower limb suspension does not mimic bed rest or spaceflight effects on human muscle fiber function. J Appl Physiol (1985) 2002;93:354–360. doi: 10.1152/japplphysiol.01245.2001. [DOI] [PubMed] [Google Scholar]
- 53.Mahmassani ZS, Reidy PT, McKenzie AI, Stubben C, Howard MT, Drummond MJ. Age-dependent skeletal muscle transcriptome response to bed rest-induced atrophy. J Appl Physiol (1985) 2019;126:894–902. doi: 10.1152/japplphysiol.00811.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.