Highlights
-
•
Resistance training improves body adiposity, metabolic risk, and inflammation in postmenopausal and older females.
-
•
High-volume resistance training elicits greater improvements in metabolic risk and inflammation outcomes.
-
•
We identified an optimal dosage for resistance training prescription in this population.
Keywords: C-reactive protein, Fat mass, Lipid profile, Menopause, Strength training
Abstract
Purpose
This meta-analytical study aimed to explore the effects of resistance training (RT) volume on body adiposity, metabolic risk, and inflammation in postmenopausal and older females.
Methods
A systematic search was performed for randomized controlled trials in PubMed, Scopus, Web of Science, and SciELO. Randomized controlled trials with postmenopausal and older females that compared RT effects on body adiposity, metabolic risk, and inflammation with a control group (CG) were included. Independent reviewers selected the studies, extracted the data, and performed the risk of bias and certainty of the evidence (Grading of Recommendations, Assessment, Development, and Evaluation (GRADE)) evaluations. Total body and abdominal adiposity, blood lipids, glucose, and C-reactive protein were included for meta-analysis. A random-effects model, standardized mean difference (Hedges’ g), and 95% confidence interval (95%CI) were used for meta-analysis.
Results
Twenty randomized controlled trials (overall risk of bias: some concerns; GRADE: low to very low) with overweight/obese postmenopausal and older females were included. RT groups were divided into low-volume RT (LVRT, ∼44 sets/week) and high-volume RT (HVRT, ∼77 sets/week). Both RT groups presented improved body adiposity, metabolic risk, and inflammation when compared to CG. However, HVRT demonstrated higher effect sizes than LVRT for glucose (HVRT = –1.19; 95%CI: –1.63 to –0.74; LVRT = –0.78; 95%CI:–1.15 to –0.41) and C-reactive protein (HVRT = –1.00; 95%CI: –1.32 to –0.67; LVRT = –0.34; 95%CI, –0.63 to –0.04)) when compared to CG.
Conclusion
Compared to CG, HVRT protocols elicit greater improvements in metabolic risk and inflammation outcomes than LVRT in overweight/obese postmenopausal and older females.
Graphical abstract

1. Introduction
Post menopause is characterized by loss of ovarian function (i.e., low estrogen levels), which is associated with increased levels of body adiposity (particularly abdominal adiposity),1,2 and an impaired inflammatory and metabolic profile.3,4 Interestingly, during pathological expansion of the adipose tissue (i.e., obesity), abdominal adiposity has been associated with inflammatory and metabolic profile impairments5,6 and an increased risk of non-communicable diseases (e.g., type 2 diabetes mellitus, breast cancer, and cardiovascular disease).7, 8, 9, 10, 11, 12, 13, 14 Moreover, abdominal adiposity15,16 has been associated with an increased risk of premature mortality,17, 18, 19 regardless of body mass index.18 Thus, it is reasonable to assume that prevention, treatment, and control/maintenance of excess body adiposity levels, inflammation, and impairments in the metabolic profile are needed to reduce the burden of non-communicable diseases and premature mortality, particularly in postmenopausal and older females.
To promote successful aging in postmenopausal and older females,20 public health guidelines recommend resistance training (RT) as a non-pharmacological intervention to improve physical fitness (e.g., cardiorespiratory fitness, muscular fitness, and body composition) and limit the development and progression of chronic diseases and other disabling conditions.21, 22, 23, 24, 25 RT recommendations are usually based on moderate-to-high intensity (i.e., 50%–80% of 1 maximum repetition (1RM)), 5–10 exercises (major muscle groups), 1–3 sets of 8–15 repetitions per exercise, and 1–3 times per week (total volume: ∼15–90 sets/week).21, 22, 23,26 However, there is a lack of consensus regarding the efficacy of RT for improving body adiposity, metabolic risk, and inflammation outcomes, since meta-analytical studies demonstrated small-to-moderate effects27, 28, 29, 30, 31, 32, 33, 34 or null effects.35, 36, 37, 38, 39, 40 The lack of consensus may be related to the designs (e.g., non-randomized controlled trials (non-RCTs) and caloric restriction studies) and population characteristics (e.g., young, middle, and older adults; studies involving males and females) of the studies.
Specifically, postmenopausal and older females are characterized by a particular physiological condition due to low concentrations of estrogen, which is different from other populations (e.g., pre-menopausal females and males) regarding body adiposity, metabolic risk, and inflammation.1,4,41,42 Indeed, estrogen may protect against excess body fat storage since a reduction in estrogen signaling (e.g., estrogen receptor α signaling) has been associated with reductions in energy expenditure, which favors a positive energy balance and increased body adiposity.1 Moreover, estrogen levels have been associated with anti-inflammatory modulation due to mitigating reactive oxygen species and transcription factors that lead to an increase in pro-inflammatory cytokines.3 Thus, low estrogen levels may increase the pro-inflammatory profile, which can impair the metabolic profile.3,4 Indeed, some studies have demonstrated that postmenopausal and older females on estrogen replacement therapy present lower levels of body adiposity, metabolic risk, and inflammation when compared to postmenopausal and older females with no estrogen therapy.43, 44, 45 Collectively, this evidence suggests an important link between estrogen levels with body adiposity, metabolic risk, and inflammation outcomes. Therefore, it is reasonable to assume that postmenopausal and older females may have distinct RT adaptations compared to other populations regarding body adiposity, metabolic risk, and inflammation outcomes.
RCTs have demonstrated that RT volume with ∼50–90 sets/week promotes null-to-small effects on body adiposity, metabolic risk, and inflammation outcomes in postmenopausal and older females.46, 47, 48, 49, 50, 51 Interestingly, some evidence suggests that a higher RT volume, with ∼80–144 sets/week, is associated with greater improvements in these outcomes.30,52 In this regard, it is rational to expect that RT volume may modulate body adiposity, metabolic risk, and inflammation adaptations in postmenopausal and older females. However, there is no consensus on this topic. Recently, some meta-analytical studies demonstrated that although RT does not affect body adiposity (no RCT studies included), this type of exercise improves the inflammatory (i.e., reductions in interleukin-6 (IL-6), tumor necrosis factor-α, and C-reactive protein (CRP)) and metabolic profile (i.e., blood lipids) in postmenopausal and older females.53, 54, 55 However, these studies did not explore the RT volume (dosage), which makes it difficult to understand the efficacy of RT prescription.
Although qualitative synthesis has demonstrated benefits in favor of RT in postmenopausal and older females, no meta-analytical studies to date have evaluated the changes induced by low- and high-volume RT (HVRT) on body adiposity (particularly abdominal adiposity), metabolic risk, and inflammation outcomes. Furthermore, to the best of our knowledge, no meta-analytical studies have aimed to summarize the evidence on whether RT volume is a key factor for improving physiological adaptations in postmenopausal and older females. Therefore, the current systematic review with meta-analysis of RCTs aimed to compare the changes caused by low-volume RT (LVRT) or HVRT on body adiposity, metabolic risk, and inflammation outcomes compared to a control group (CG) in postmenopausal and older females. We hypothesized that both LVRT and HVRT would improve body adiposity, metabolic risk, and inflammation compared to CG, and that HVRT would result in superior changes compared to LVRT.
2. Methods
2.1. Data source and search strategy
This systematic review was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines56 (Supplementary Tables 1 and 2) and is registered on the International Prospective Register of Systematic Reviews (PROSPERO CRD42022322587). English language articles were retrieved by title and abstract from the earliest record up to August 2022 from PubMed, Scopus, Web of Science, and SciELO by 2 independent authors (MAdSC and PRPN). The search strategy (based on Medical Subject Headings) combined the following terms: “Post-Menopause”; “Resistance Training”; “Adiposity”; “Inflammatory profile”; “Metabolic Syndrome” (the full search strategy is reported in Supplementary Table 3). In addition, the grey literature (e.g., abstracts, conference papers, and editorials) was excluded. A third reviewer (PCS) evaluated the article in the case of disagreements.
2.2. Study selection
Two independent authors (MAdSC and PRPN) performed the systematic search and completed the study selection. The eligibility criteria were determined according to PICOS: Population (postmenopausal or older females), Intervention (RT), Comparators (CG participants who did not receive the RT intervention), Outcome (body adiposity, metabolic risk, and inflammation assessments), and Study design (RCTs).
Initially, duplicate records were excluded. Subsequently, the records were retrieved for screening by title and abstract. The articles were included in the review if they met the following criteria: (a) RCT study; (b) postmenopausal females, characterized by amenorrhea (at least 1 year) and aged ≥ 45 years, or older females with age ≥ 60 years old; (c) intervention group with supervised land based RT for the major muscle groups with specified intensity, volume, and exercises; (d) comparator CG with no exercise and/or placebo interventions (i.e., low energy expenditure that did not affect the outcomes, such as stretching exercises); and (e) measurements (literature established measurement methods) from baseline to the last available follow-up of body adiposity, serum/plasma inflammatory profile, and/or serum/plasma metabolic profile. Studies were excluded according to the following criteria: females that received any type of hormonal therapy or phytoestrogens; or were engaged any nutrition strategies (caloric restriction) for weight loss; or were sufficiently active females, according to the World Health Organization recommendations involving the practice of physical activity (e.g., aerobic or resistance exercises that could affect the outcomes) and were engaged experimental intervention using no structured physical exercise other than RT (e.g., aerobic exercises). The study selection agreement between MAdSC and PRPN presented a Cohen's κ result = 0.865, p < 0.001. Any study selection disagreements were discussed with a third author (PCS). The third author was responsible for the tiebreaking decision.
2.3. Data extraction and quality assessments of each study
One author (PRPN) extracted the following data from each study for analysis: author/year, number of participants within each group, baseline participant characteristics, intervention details, and pre- and post-data from all outcomes (Tables 1 and 2). For studies containing multiple intervention arms vs. CGs, only data from RT groups were extracted. After the data extraction, the authors (AAdO, BdFC, GCS, and LMVS) independently confirmed the precision of the extracted data. Contact with corresponding authors was performed to clarify data or obtain missing information. Each outcome for body adiposity (e.g., total body adiposity and abdominal adiposity) and serum/plasma inflammatory (e.g., CRP, IL-6, and tumor necrosis factor-α) and metabolic profile (e.g., total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), triglycerides (TG), and fasting glucose) were extracted separately for later analysis (Tables 3 and 4).
Table 1.
Characteristics of the studies included in the systematic review and meta-analysis.
| Study (country) | Sample | Group | Outcome | Main result |
|---|---|---|---|---|
| Bouchard et al. (2009),61 Canada | Obese postmenopausal females (55–75 years), mean age = 63 years | CG (n = 12), RT (n = 11), machines and free weights |
Fat mass (DEXA) | No effect on any outcomes |
| Cavalcante et al. (2018),62 Brazil | Overweight/obese postmenopausal females, mean age = 67 years | CG (n = 19), RT-F2 (n = 18), RT-F3 (n = 20), machines and free weights |
Fat mass, android fat, trunk fat, and gynoid fat (DEXA) | Both RT groups decreased overall fat outcomes vs. CG |
| Coelho-Júnior et al. (2019),63 Brazil | Overweight/obese postmenopausal females (60–79 years), mean age = 67 years | CG (n = 14), RT-NP (n = 10), machines and free weights; RT-DUP (n = 12), machines and free weights and elastic bands |
F% (BIA) and waist | No effect on any outcomes |
| Conceição et al. (2013),64 Brazil | Overweight postmenopausal females (45–60 years), mean age = 53 years | CG (n = 10), RT (n = 10), machines and free weights |
F%, fat mass (skinfolds), waist, HDL-c, TG, and glucose | RT group decreased F%, fat mass, and glucose compared to CG No effects for the remaining variables |
| Cunha et al. (2019),65 Brazil | Overweight/obese older females (≥60 years), mean age = 70 years | CG (n = 23), RT (n = 25), machines and free weights |
HDL-c, LDL-c, VLDL-c, TC, TG, glucose, and CRP | RT group decreased LDL-c, TC, Glucose, and CRP compared to CG No effects for the remaining variables |
| Cunha et al. (2021),66 Brazil | Overweight/obese older females (≥60 years), mean age = 69 years | CG (n = 18), RT-1 set (n = 19) and RT-3 sets (n = 18), machines and free weights |
F%, trunk fat (DEXA), HDL-c, LDL-c, VLDL-c, TC, TG, glucose, and CRP | Both RT groups decreased LDL-c, TC, and F% compared to CG RT-3 decreased TG, VLDL-c, glucose, and F% compared to CG and RT-1 Only RT-3 decreased CRP and trunk fat compared to CG |
| Do Nascimento et al. (2018),67 Brazil | Overweight/obese older females (60–80 years), mean age = 66 years | CG (n = 22), RT (n = 22), machines and free weights |
F% and fat mass (DEXA) | No effect on any outcomes |
| Dos Santos et al. (2020),68 Brazil | Overweight/obese postmenopausal females, mean age = 67 years | CG (n = 19), RT-NPR (n = 20) and RT-WPR (n = 20), machines and free weights |
Fat mass, android fat, trunk fat, gynoid fat (DEXA), glucose, HDL-c, LDL-c, TC, TG, and CRP | Both RT groups decreased overall fat outcomes, LDL-c, TC, TG, CRP, and increased HLD-c, however only android fat, LDL-c, and CRP were different from CG Both RT groups showed blunted glucose increases compared to CG |
| Egana et al. (2010),69 Spain | Overweight postmenopausal females (59–79 years), mean age = 67 years | CG (n = 8), RT (n = 8), elastic bands |
F% (skinfolds) | RT group decreased F% compared to CG |
| Elliott et al. (2002),70 UK | Overweight/obese postmenopausal females (49–62 years), mean age = 55 years | CG (n = 7), RT (n = 8), machines and free weights |
F% (skinfolds), HDL-c, LDL-c, VLDL-c, TC, and TG |
No effect on any outcomes |
| Fritz et al. (2018),71 Spain | Overweight postmenopausal females (60–85 years), mean age = 68 years | CG (n = 20), RT-ET (n = 22) and RT-EB (n = 21), elastic bands |
F% and trunk fat % (BIA) | Both RT groups decreased overall fat outcomes compared to CG |
| Gambassi et al. (2016),72 Brazil | Overweight postmenopausal females, mean age = 65 years | CG (n = 13), RT (n = 13), machines and free weights |
F% and fat mass (BIA) | RT group decreased overall fat outcomes compared to CG |
| Nunes et al. (2016),52 Brazil | Overweight/obese postmenopausal females (49–79 years), mean age = 61 years | CG (n = 11), RT-3 sets (n = 10) and RT-6 sets (n = 11), machines and free weights |
F% (skinfolds), waist, HDL-c, LDL-c, VLDL-c, TC, TG, IL-1/6, TNF-α, and Hb1Ac | All groups decreased F% Only RT-3 reduced Hb1Ac Only RT-6 reduced LDL-c and TC Only RT-6 reduced waist compared to RT-3 and CG Only RT-06 showed a lower change in IL-6 compared to CG No effects for the remaining variables |
| Orsatti et al. (2008),73 Brazil | Overweight/obese postmenopausal females (45–70 years), mean age = 58 years | CG (n = 22), RT (n = 21), machines and free weights |
F% (BIA) and waist | No effect on any outcomes |
| Phillips et al. (2012),48 USA | Obese postmenopausal females, mean age = 65 years | CG (n = 12) RT (n = 11), machines and free weights |
F%, fat mass (skinfolds), IL-6, adiponectin, leptin, TNF-α, and CRP | RT group decreased Leptin, TNF-α and, CRP compared to CG CG group decreased IL-6 No effects for the remaining variables |
| Ribeiro et al. (2020),74 Brazil | Obese older females (≥60 years), mean age = 68 years | CG (n = 15), RT (n = 18), machines and free weights |
F% and fat mass (DEXA) | RT group decreased overall fat outcomes compared to CG |
| Tomeleri et al. (2016),75 Brazil | Obese older females (≥60 years), mean age = 68 years | CG (n = 19), RT (n = 19), machines and free weights |
F%, trunk fat (DEXA), HDL-c, LDL-c, TC, TG, glucose, IL-6, TNF-α, and CRP | RT group decreased overall fat outcomes, LDL-c, CRP, IL-6, TNF-α, and glucose and increased HDL-c compared to CG No effects for TC and TG |
| Tomeleri et al. (2018)a;76 Brazil | Overweight/obese older females (≥60 years), mean age = 70 years | CG (n = 22), RT (n = 24), machines and free weights |
F%, trunk fat % (DEXA), IL-6/IL-10, TNF-α, and CRP | RT group decreased overall fat outcomes, CRP, IL-6, and TNF-α, and increased IL-10 compared to CG |
| Tomeleri et al. (2018)b;77 Brazil | Overweight/obese older females (≥60 years), mean age = 70 years | CG (n = 23), RT (n = 22), machines and free weights |
F%, android fat (DEXA), waist, TG, HDL-c, glucose, HOMA-IR, IL-6, TNF-α, and CRP | RT group decreased overall fat outcomes, glucose, HOMA-IR, waist, and CRP compared to CG RT group decreased TNF-α No effects for IL-6 |
| Urzi et al. (2019),78 Slovenia | Overweight/obese postmenopausal females, mean age = 86 years, nursing home residents | CG (n = 9), RT (n = 11), elastic bands |
Fat mass (BIA), glucose, CRP, resistin, IL-8/15, and BDNF | RT group increased BDNF compared to CG No effects for the remaining variables |
Abbreviations: BDNF = brain-derived neurotrophic factor; BIA = bioelectrical impedance analysis; CG = control group; CRP = C-reactive protein; DEXA = dual energy X-ray absorptiometry; F% = body fat percentage; HDL-c = high-density lipoprotein; HOMA-IR = homeostatic model assessment for insulin resistance; IL = interleukin; LDL-c = low-density lipoprotein; RT = resistance training group; RT-DUP = resistance training performed with daily undulating periodization; RT-EB = resistance training performed with traditional elastic bands; RT-ET = resistance training performed with elastic tubes with handles; RT-F2 = resistance training performed 2 times per week; RT-F3 = resistance training performed 3 times per week; RT-NP = non-periodized resistance training; RT-NPR = resistance training performed with narrow-pyramid system; RT-WPR = resistance training performed with wide-pyramid system; TC = total cholesterol; TG = triglycerides; TNF-α = tumor necrosis factor-α; VLDL-c = very low-density lipoprotein.
Table 2.
Characteristics of the resistance training protocols of the studies included in the systematic review and meta-analysis.
| Study (country) | Exercise | RT prescription | RT volume groupa |
|---|---|---|---|
| Bouchard et al. (2009),61 Canada | 9 exercises: leg press, chest press, leg extension, shoulder press, sit-up, seated row, triceps extension, arm curl, and calf extension | 80% 1RM, 8 reps/exercise, 3 sets/exercise, 1–1.5 min recovery between sets, 3 times/week for 12 weeks; No adherence reported | HVRT |
| Cavalcante et al. (2018),62 Brazil | 8 exercises: chest press, horizontal leg press, seated row, knee extension, preacher curl, leg curl, triceps pushdown, and seated calf raise | 10–15 RM, 10–15 reps/exercise, 1 set/exercise, 2–3 min recovery between exercises, 2 (RT-F2) or 3 (RT-F3) times/week for 12 weeks; Adherence ≥85% | LVRT |
| Coelho-Júnior et al. (2019),63 Brazil | NP and DUP – 9 exercises, Training A: seated row, leg press, chest press, seated leg curl, lateral arm raise, calf raise, arm curl, triceps pushdown, and abdominal crunch Training B: squat on the chair (until 90° knee flexion), chest press, seated leg curl, seated row, frontal arm raise, calf raise, arm curl, triceps pushdown, and abdominal crunch |
NP: 5–6 RPE (CR-10) trainings A and B DUP: 5–6 RPE (CR-10) training A and 3 RPE (CR-10) training B (power session with elastic bands) NP and DUP performed 8–10 reps/exercise, 3 sets/exercise, 1 min recovery between sets, 2 times/week for 22 weeks; Adherence ≥88% |
HVRT |
| Conceição et al. (2013),64 Brazil | 9 exercises: leg press, leg extension, leg curl, bench press, lat pulldown, lateral raise, triceps pushdown, arm curl, and basic abdominal crunch | 8–10 RM, 8–10 reps/exercise, 3 sets/exercise, 1–1.5 min recovery between sets, 3 times/week for 16 weeks; No adherence reported | HVRT |
| Cunha et al. (2019),65 Brazil | 8 exercises: chest press, horizontal leg press, seated row, knee extension, preacher curl (free weights), leg curl, triceps pushdown, and seated calf raise | 10–15 RM, 10–15 reps/exercise, 1 set/exercise, ∼2 min recovery between exercises, 3 times/week for 12 weeks; Adherence ≥85% | LVRT |
| Cunha et al. (2021),66 Brazil | 8 exercises: chest press, horizontal leg press, seated row, knee extension, preacher curl (free weights), leg curl, triceps pushdown, and seated calf raise | 10–15 RM, 10–15 reps/exercise, 1 (RT-1) or 3 (RT-3) sets/exercise, 1–2 min recovery between sets and 2–3 between exercises, 3 times/week for 12 weeks; Adherence ≥85% | RT-1: LVRT RT-3: HVRT |
| Do Nascimento et al. (2018),67 Brazil | 8 exercises: chest press machine, leg extension, wide-grip front lat pulldown, leg curl, preacher curl, seated calf raise, triceps pushdown, and abdominal crunches | 10–15 RM, 10–15 reps/exercise, 2 sets/exercise, 1–1.5 min recovery between sets and 2–3 min between exercises, 3 times/week for 12 weeks Abdominal crunches (only exception) were performed at 20–30 repetitions without additional overload; Adherence was ∼96% |
HVRT |
| Dos Santos et al. (2020),68 Brazil | 8 exercises: chest press, horizontal leg press, seated row, leg extension, preacher curl, leg curl, triceps pushdown, and seated calf raise | RT-NPR 12-10-8 RM, 12–8 reps/exercise and RT-WPR 15-10-5 RM, 15–5 reps/exercise; Both NPR and WPR performed 3 sets/exercise, 1–2 min recovery between sets and 2–3 min recovery between exercises, 3 times/week for 8 weeks; Adherence >85% |
LVRT |
| Egana et al. (2010),69 Spain | 12 exercises: double arm pull back, shoulder abduction, lateral pull down, bicep curl, triceps curl, upright row, one-legged press, calf dorsi flexion, ankle eversion, hip flexion, knee flexion, and knee extension | 10 RM, 10 reps/exercise, 2 sets/exercise, 2–3 s recovery between reps and 1 min between exercises, 2 times/week for 12 weeks; Adherence was ∼96% | LVRT |
| Elliott et al. (2002),70 UK | 5 exercises: bilateral leg extension and flexion, leg press, bench press, and lat pull down | 80% of 10RM, 8 reps/exercise, 3 sets/exercise, 2 min recovery between sets, 3 times/week for 8 weeks; Adherence was 100% | LVRT |
| Fritz et al. (2018),71 Spain | 6 exercises: upright rowing, incline rowing, elbow curl, narrow stance squat, lunge and wide stance squat | RT-ET and RT-EB: 7–9 RPE (OMNI-RES), 10 reps/exercise, 3–4 sets/exercise, 1.5 min recovery between sets, 2 times/week for 8 weeks; Adherence was ∼92% | LVRT |
| Gambassi et al. (2016),72 Brazil | 8 exercises: leg press at 180°, seated row, leg curl, bench press, abduction machine, push down, adduction machine, and biceps curl | 8 RM, 8 reps/exercise, 3 sets/exercise, 2 min recovery between sets, 2 times/week for 12 weeks; No adherence reported | LVRT |
| Nunes et al. (2016),52 Brazil | 8 exercises: squat, leg curl, leg extension, bench press, rowing machine, pull down, triceps pulley, and barbell curls | 70% 1RM, 8–12 reps/exercise, 3 (RT-3) or 6 (RT-6) sets/exercise, 1.5 min recovery between sets and exercises, 3 times/week for 16 weeks; Adherence was ∼89% | HVRT |
| Orsatti et al. (2008),73 Brazil | 10 exercises: leg press, leg extension, peck deck, bench press, seated row, lat pull down, triceps pulley and biceps curl, abdominal, and calf | 60%–80% 1RM, 8–12 reps/exercise, 3 sets/exercise, 1.5–2 min recovery between sets and exercises, 3 times/week for 16 weeks Abdominal and calf exercises (only exceptions) were performed with 30 and 20 repetitions, respectively; Adherence was 100% |
HVRT |
| Phillips et al. (2012),48 USA | 10 exercises: chest press, lat pull-down, seated rows, shoulder press, leg abduction, leg adduction, chest flys, leg press, leg curl, and leg extension | 8–12 RM, 8–12 reps/exercise, 3 sets/exercise, 1.5 min recovery between sets and 2 min recovery between exercises, 3 times/week for 12 weeks; Adherence was 100% | HVRT |
| Ribeiro et al. (2020),74 Brazil | 8 exercises: chest press, seated row, triceps push-down, preacher curl, horizontal leg press, knee extension, knee curl, and seated calf raise | 8–12 RM, 8–12 reps/exercise, 3 sets/exercise, 1–2 min recovery between sets and 2–3 min recovery between exercises, 3 times/week for 8 weeks; Adherence ≥85% | LVRT |
| Tomeleri et al. (2016),75 Brazil | 8 exercises: chest press, horizontal leg press, seated row, knee extension, preacher curl (free weights), leg curl, triceps pushdown, and seated calf raise | 10–15 RM, 10–15 reps/exercise, 3 sets/exercise, 1–2 min recovery between sets and 2–3 min recovery between exercises, 3 times/week for 8 weeks; Adherence ≥85% | HVRT |
| Tomeleri et al. (2018)a;76 Brazil | 8 exercises: chest press, seated row, triceps pushdown, preacher curl, horizontal leg press, knee extension, knee curl, and seated calf raise | 10–15 RM, 10–15 reps/exercise, 3 sets/exercise, 1–2 min recovery between sets and 2–3 min recovery between exercises, 3 times/week for 12 weeks; Adherence ≥85% | HVRT |
| Tomeleri et al. (2018)b;77 Brazil | 8 exercises: chest press, seated row, triceps pushdown, preacher curl, horizontal leg press, knee extension, knee curl, and seated calf raise | 10–15 RM, 10–15 reps/exercise, 3 sets/exercise, 1–2 min recovery between sets and 2–3 min recovery between exercises, 3 times/week for 12 weeks; Adherence ≥85% | HVRT |
| Urzi et al. (2019),78 Slovenia | 8 exercises: chair squats; band seated: biceps curl, seated row, knee extension, leg press, and hip abduction; and standing behind the chair: knee flexion and calf raise | 12–14 (BORG), 9 (5–12) reps/exercise, 2 (1–3) sets/exercise, 1–2 min recovery between sets, 3 times/week for 12 weeks; Adherence was ∼88% | LVRT |
Total volume = number of exercises × number of sets per exercise × number of reps per exercise × number of exercise sessions per week × weeks of intervention (LVRT and HVRT were separated by the median value, 6480 arbitrary units).
Abbreviations: BORG = Borg rate of perceived exertion scale; CR-10 = adapted Borg scale; DUP = daily undulating periodization; HVRT = high-volume resistance training; LVRT = low-volume resistance training; NP = non-periodized; OMNI-RES = Omni resistance scale; reps = repetitions; RM = repetition maximum; RPE = rating of perceived exertion; RT-DUP = resistance training performed with daily undulating periodization; RT-EB = resistance training performed with traditional elastic bands; RT-ET = resistance training performed with elastic tubes with handles; RT-F2 = resistance training performed 2 times per week; RT-F3 = resistance training performed 3 times per week; RT-NP = non-periodized resistance training; RT-NPR = resistance training performed with narrow-pyramid system; RT-WPR = resistance training performed with wide-pyramid system.
Table 3.
Meta-analysis performed on the effects of LVRT on body adiposity, metabolic risk, and inflammation in postmenopausal and older females.
| LVRT (n) | CG (n) | SMD (95%CI) | k | I2 | Overall effect (p) | GRADE | |
|---|---|---|---|---|---|---|---|
| Body adiposity | |||||||
| Total body adipositya | 198 | 186 | –0.29 (–0.50 to –0.09)* | 9 | 0%, p = 0.82 | <0.01 | Lowc,d |
| Abdominal adiposityb | 118 | 114 | –0.35 (–0.61 to –0.09)⁎,† | 4 | 0%, p = 0.66 | <0.01 | Lowc,d |
| Serum metabolic risk and inflammation | |||||||
| TC | 92 | 86 | –0.47 (–0.83 to –0.11)⁎ | 4 | 28%, p = 0.23 | 0.01 | Lowc,d |
| LDL-c | 92 | 86 | –0.76 (–1.15 to –0.36)⁎ | 4 | 37%, p = 0.17 | <0.01 | Lowc,d |
| HDL-c | 92 | 86 | 0.27 (–0.09 to 0.62) | 4 | 28%, p = 0.24 | 0.14 | Lowc,d |
| TG | 92 | 86 | –0.29 (–0.59 to 0.00) | 4 | 0%, p = 0.75 | 0.05 | Lowc,d |
| Glucose | 95 | 88 | –0.78 (–1.15 to –0.41)⁎ | 4 | 32%, p = 0.21 | <0.01 | Lowc,d |
| CRP | 95 | 88 | –0.34 (–0.63 to –0.04)⁎ | 4 | 0%, p = 0.99 | 0.02 | Lowc,d |
Note: Total volume = number of exercises × number of sets per exercise × number of reps per exercise × number of exercise sessions per week × weeks of intervention (LVRT median value was 5760 arbitrary units).
Outcome analyzed with anthropometric, dual energy X-ray absorptiometry and bioelectrical impedance analysis (kg and %). When the study had kg and % data, preference was given to data in kg.
Outcome analyzed with dual energy X-ray absorptiometry (kg), and bioelectrical impedance (%) analysis. To avoid redundancy outcomes, only trunk fat (kg) was extracted from the Cavalcante et al.62
Downgraded by 1 level due to indirectness of evidence (only 3 studies directly compared LVRT with HVRT and/or surrogate outcomes).
Downgraded by 1 level due to imprecision of results (small sample size and large 95%CI).
p < 0.05 favors RT compared to CG.
Without outlier studies.
Abbreviations: 95%CI = 95% confidence interval; CG = control group; CRP = C-reactive protein; GRADE = certainty of the evidence (Grading of Recommendations, Assessment, Development, and Evaluation); HDL-c = high-density lipoprotein cholesterol; I2 = heterogeneity; k = number of studies; LDL-c = low-density lipoprotein cholesterol; LVRT = low-volume resistance training group; SMD = standardized mean difference; TC = total cholesterol; TG = triglycerides.
Table 4.
Meta-analysis performed on the effects of HVRT on body adiposity, metabolic risk, and inflammation in postmenopausal and older females.
| HVRT (n) | CG (n) | SMD (95%CI) | k | I2 | Overall effect (p) | GRADE | |
|---|---|---|---|---|---|---|---|
| Body adiposity | |||||||
| Total body adipositya | 201 | 210 | –0.23 (–0.43 to –0.04)⁎ | 11 | 0%, p = 0.95 | 0.02 | Lowd,e |
| Abdominal adiposityb | 179 | 187 | –0.22 (–0.42 to –0.01)⁎ | 8 | 0%, p = 0.99 | 0.04 | Lowd,e |
| Serum metabolic risk and inflammation | |||||||
| TC | 40 | 41 | –0.38 (–0.82 to 0.06)† | 2 | 0%, p = 0.90 | 0.09 | Lowd,e |
| LDL-c | 39 | 40 | –0.73 (–1.84 to 0.38)† | 2 | 81%, p < 0.01 | 0.20 | Very lowcd,e, |
| HDL-c | 90 | 92 | 0.34 (0.04 to 0.63)⁎ | 5 | 2%, p = 0.41 | 0.03 | Lowd,e |
| TG | 90 | 92 | –0.28 (–0.63 to 0.08) | 5 | 29%, p = 0.22 | 0.12 | Lowd,e |
| Glucose | 47 | 47 | –1.19 (–1.63 to –0.74)⁎,† | 3 | 0%, p = 0.48 | <0.01 | Lowd,e |
| CRP | 83 | 82 | –1.00 (–1.32 to –0.67)⁎ | 4 | 0%, p = 0.79 | <0.01 | Lowd,e |
Note: Total volume = number of exercises × number of sets per exercise × number of reps per exercise × number of exercise sessions per week × weeks of intervention (LVRT median value was 5760 arbitrary units).
Outcome analyzed with anthropometric, dual energy X-ray absorptiometry and bioelectrical impedance analysis (kg and %). When the study had kg and % data, preference was given to data in kg.
Outcome analyzed with anthropometric (cm), dual energy X-ray absorptiometry (kg and %), and bioelectrical impedance (%) analysis.
Downgraded by 1 level due to unexplained heterogeneity (large heterogeneity).
Total volume = number of exercises × number of sets per exercise × number of reps per exercise × number of exercise sessions per week × weeks of intervention (HVRT median value was 7200 arbitrary units).
Downgraded by 1 level due to indirectness of evidence (only 3 studies directly compared LVRT with HVRT and/or surrogate outcomes).
Downgraded by 1 level due to imprecision of results (small sample size and large 95%CI).
p < 0.05 favors RT compared to CG.
Without outlier studies.
Abbreviations: 95%CI = 95% confidence interval; CG = control group; CRP = C-reactive protein; GRADE = certainty of the evidence (Grading of Recommendations, Assessment, Development, and Evaluation); HDL-c = high-density lipoprotein cholesterol; HVRT = high-volume resistance training group; I2 = heterogeneity; k = number of studies; LDL-c = low-density lipoprotein cholesterol; SMD = standardized mean difference; TC = total cholesterol; TG = triglycerides.
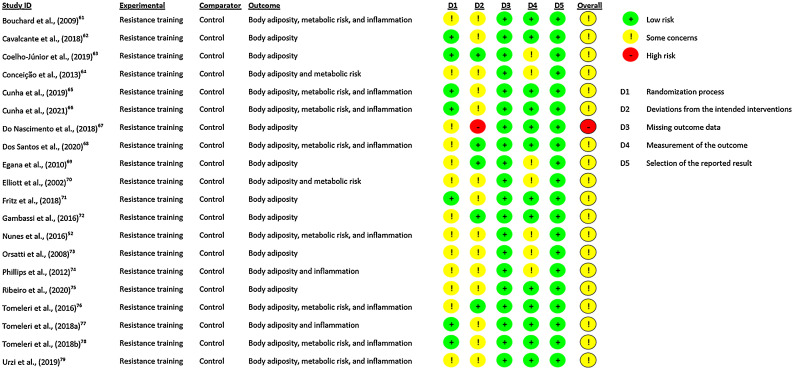
The quality of the included studies was assessed using the revised tool for assessing the risk of bias in randomized trials.57 The assessment was performed according to the evaluation of certain domains: (a) bias arising from the randomization process, (b) bias due to deviations from intended interventions, (c) bias due to missing outcome data, (d) bias in measurement of the outcome, and (e) bias in selection of the reported result. After evaluation of these domains, the overall bias was considered through the risk-of-bias judgment based on low/high/some concerns. The quality assessments of both reviewers (PRPN and PCS) were compared, and disagreements in the scores were resolved by discussion (unanimous agreement).
2.4. Certainty of the evidence: GRADE approach
Two authors (MAdSC and PRPN) independently performed the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach, which was followed to assess the certainty of the evidence supporting the effects of RT on each outcome.58 According to the GRADE approach, 5 factors reduce the certainty of the evidence: (a) risk of bias or limitations in the detailed design and implementation, (b) unexplained heterogeneity or inconsistency of results, (c) indirectness of evidence, (d) imprecision of results, and (e) high probability of publication bias. The certainty of evidence was rated as high, moderate, low, or very low. Disagreements in the GRADE approach were resolved by discussion.
2.5. Data syntheses and analyses
The meta-analysis was conducted using Review Manager Software (Version 5.4.; RevMan (Computer program), the Cochrane Collaboration, London, UK). RevMan was used to calculate the effect size of the RT intervention on each outcome (Tables 3 and 4) separately: body adiposity (total body adiposity and abdominal adiposity), and serum/plasma inflammatory (CRP) and metabolic profiles (TC, LDL-c, HDL-c, TG, and fasting glucose). The variation (pre- minus post-intervention) from all included studies was used to calculate the standardized mean difference and 95% confidence interval (95%CI), and these were conducted using the DerSimonian-Laird random-effects inverse variance model for all outcomes. Weighted percentages were based on the sample sizes of the respective studies. Statistical significance was assumed as p < 0.05 in a Z-test analysis to examine whether the effect size differed significantly from 0.
Study heterogeneity was evaluated using the I2 statistic and Cochrane's Q. Values of I2 higher than 50% and 75% were considered moderate and high heterogeneity, with a threshold p ≤ 0.1. For Cochrane's Q, significant heterogeneity exists when the Q value exceeds the degrees of freedom of the estimate. Moreover, publication bias was tested visually using a funnel plot when a sufficiently large sample of studies (i.e., ≥10 study groups) was available for the RT vs. CG comparison. Sensitivity analyses were performed by excluding 1 trial at a time, according to the risk of bias, to test the robustness of the pooled results. Effect sizes (Hedges’ g) were calculated, with values of 0.00–0.19 considered trivial, >0.19–0.49 small, >0.49–0.79 moderate, and >0.79 large.
The RT studies were coded according to the total training volume (dosage), calculated as: number of exercises × number of sets per exercise × number of reps per exercise × number of exercise sessions per week × weeks of intervention.59 Regarding RT prescription and data imputation, if a range of values was used in the studies, for example 2–3 days per week or 8–12 repetitions per set, we used the mean value (e.g., 2.5 and 10, respectively) to calculate the total volume. The LVRT and HVRT were divided according to median total volume (6480 arbitrary units).
The RT intensity was not included in the total training volume calculation for the following reasons:60 (a) although the %1RM–RM relationship may be used in field practice, research to date does not support the widespread use of these tables for establishing training loads for every exercise (e.g., equipment: machine and free weights; muscle groups: single joints and multiple joints); (b) the most accurate relationship between the %1RM–RM is for loads greater than 75% of the 1RM and fewer than 10 repetitions, which was not a common exercise intensity in the majority of the studies included in this review; and (c) some studies included in this review used exercises with elastic bands and rating of perceived exertion (RPE) intensity, which makes the use of the %1RM–RM relationship difficult. Moreover, the RT intensity zone was similar across the included studies, ranging from moderate to high intensity.
3. Results
3.1. Selection and quality assessment of the studies
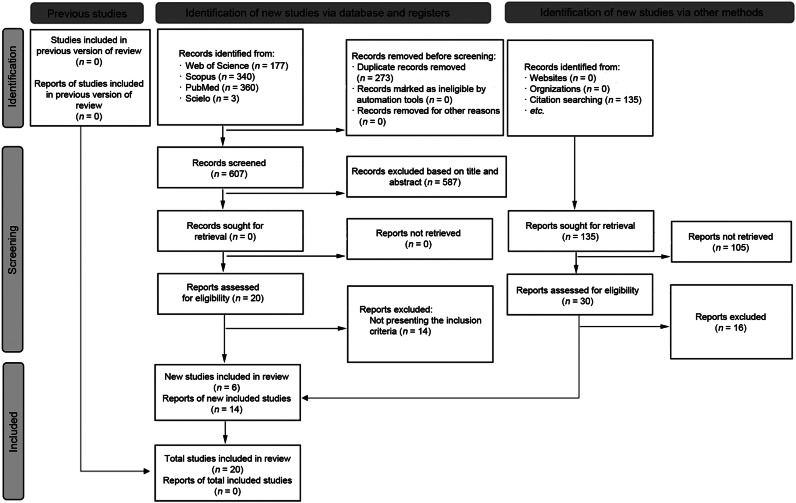
The initial selection processes retrieved 1015 full texts, as documented in the PRISMA flow diagram (Fig. 1). After excluding duplicate records and papers based on title and abstract, 50 studies were assessed according to the PICOS eligibility criteria. Subsequently, 30 studies were excluded for not meeting the inclusion criteria. Therefore, 20 RCTs (RT groups (n = 26) and CG (n = 20))52,61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78 were included in the qualitative and quantitative synthesis: 19 investigations with body adiposity data,52,61, 62, 63, 64,66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78 9 studies with serum inflammatory data,48,52,65,66,68,75, 76, 77, 78 and 9 with serum metabolic data52,64, 65, 66,68,70,75,77,78 (Table 1). Only 1 study did not provide inflammatory data.48 Moreover, all studies included in both the qualitative and quantitative synthesis presented an overall risk of bias for all outcomes characterized by some concerns, except 1 study which presented an overall high risk of bias (Fig. 2). A funnel plot was constructed using the standardized mean difference for the effects of LVRT and HVRT on total body adiposity against the standard error, and after the funnel plot inspection, no asymmetry was demonstrated, suggesting no publication bias (Supplementary Fig. 1).
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
Fig. 2.
Risk of bias in randomized trials.
3.2. Characteristics of the participants
The characteristics of the study participants included in the systematic review with meta-analysis are presented in Table 1. A total of 742 sedentary females (mean age = 66 years) who were overweight/obese and apparently healthy were included in the analysis. The mean values for blood markers were ∼220 mg/dL for TC, ∼135 mg/dL for LDL-c, ∼58 mg/dL for HDL-c, ∼130 mg/dL for TG, ∼100 mg/dL for fasting glucose, and ∼3 mg/L for CRP. One study was conducted with female nursing home residents78 (n = 20) (Table 1). According to the studies, a total of 309 females were characterized as older adults65, 66, 67,74, 75, 76, 77 (age ≥ 60 years) and the remainder as postmenopausal females48,52,61, 62, 63, 64,68, 69, 70, 71, 72, 73,78 (age ≥ 45 years and spontaneous amenorrhea).
3.3. Characteristics of the interventions and comparators
The characteristics of the interventions and comparators included in the systematic review with meta-analysis are presented in Table 2. The LVRT (n = 223 females) presented the following variables in mean ± SD: 7.7 ± 1.6 exercises performed with 10.3 ± 1.6 repetitions/exercise, 2.3 ± 1.0 sets/exercise, 1.7 ± 0.4 min recovery, 2.6 ± 0.5 times/week, for 10.1 ± 2.0 weeks. The number of total sets/week was 44.5 ± 19.1. The intensity was reported as RPE (moderate and moderate-to-high), %10RM (moderate), and RMs (moderate-to-high and high) in 3, 1, and 9 LVRT groups, respectively. LVRT was performed with machines and free weights (9 LVRT groups) and elastic bands (4 LVRT groups). Adherence to LVRT was ≥85% and was reported by most of the studies. No potential harm from LVRT was reported.
The HVRT (n = 201 females) presented the following variables in mean ± SD: 8.6 ± 0.7 exercises performed with 10.5 ± 1.6 repetitions/exercise, 3.1 ± 0.8 sets/exercise, 1.3 ± 0.2 min recovery, 2.8 ± 0.3 times/week, for 14.4 ± 4.0 weeks. The number of total sets/week was 77.0 ± 23.9. The intensity was reported as %1RM (moderate-to-high and high), and RMs (moderate-to-high and high) in 3 and 8 HVRT groups, respectively. All HVRT groups performed RT with machines and free weights, except 1 HVRT study group that used a combination of machines, and free weights with elastic bands. Adherence to HVRT was ≥85% and was reported by most of the studies. No potential harm from HVRT was reported.
In general, all CG were instructed to maintain their usual lifestyle (i.e., maintenance of daily physical activity) and not to perform any type of structured exercise program that could affect the outcomes (e.g., RT), although 2 studies52,67 reported stretching activities (placebo intervention) in CG.
3.4. LVRT effects on body adiposity, metabolic risk, and inflammation
The effects of LVRT on body adiposity, metabolic risk, and inflammation included in the systematic review with meta-analysis are presented in Table 3. LVRT reduced total body adiposity and abdominal adiposity when compared to CG, with small effect sizes. Regarding serum metabolic risk, LVRT reduced TC, LDL-c, and glucose when compared to CG, with small, moderate and moderate effect sizes, respectively. For inflammatory outcomes, LVRT reduced CRP, with a small effect size when compared to CG. In the sensitivity analysis (heterogeneity removed), Fritz et al.71 study group was removed from the abdominal adiposity outcome (Supplementary Fig. 2).
No effect was observed for TG and HDL-c. No meta-analysis was performed for the remaining metabolic and inflammatory outcomes (Table 1) due to insufficient data.
3.5. HVRT effects on body adiposity, metabolic risk, and inflammation
The HVRT effects on body adiposity, metabolic risk, and inflammation included in the systematic review with meta-analysis are presented in Table 4. We observed a reduction in total body fat adiposity and abdominal adiposity when compared to CG, with small effect sizes. Regarding serum metabolic risk, HVRT reduced glucose when compared to CG, with large effect size. Moreover, HVRT increased HDL-c when compared to CG, with a small effect size. For inflammatory outcomes, HVRT reduced CRP, with a large effect size when compared to CG. In the sensitivity analysis (heterogeneity removed), the study by Tomeleri et al.77 was removed for the glucose outcome (Supplementary Fig. 3).
No effect was observed for TC, LDL-c, or TG. In the sensitivity analysis (heterogeneity removed), the study by Cunha et al.66 was removed for the TC outcome, and the study by Tomeleri et al.75 was removed for the LDL-c outcome (Supplementary Fig. 3), and no effect was observed after the sensitivity analysis. No meta-analysis was performed for the remaining metabolic or inflammatory outcomes (Table 1) due to insufficient data.
4. Discussion
4.1. Main findings
To the best of our knowledge, this paper is the first to summarize the evidence comparing low and high RT volume in overweight/obese postmenopausal and older females. More specifically, to date, this is the first systematic review with meta-analysis that sought to explore the efficacy of RT volume on body adiposity, metabolic risk, and inflammation outcomes. The main finding of our study was that both RT volume groups (LVRT and HVRT) presented reduced total body adiposity and abdominal adiposity, glucose, and CRP when compared to CG. Furthermore, only the LVRT presented reduced TC and LDL-c when compared to CG, whereas only the HVRT presented increased HDL-c when compared to CG. However, HVRT demonstrated higher effect sizes than LVRT for glucose and CRP when compared to CG. Thus, collectively, despite the positive adaptations induced by RT in the outcomes analyzed in this meta-analysis of RCTs, the higher-volume protocols elicited greater improvements in metabolic and inflammatory outcomes than the lower-volume protocols.
4.2. Body adiposity
Obesity (i.e., body mass index ≥ 30 kg/m2 and body fat ≥ 40%) and abdominal obesity (i.e., waist circumference > 88 cm for females)79,80 are common in postmenopausal and older females.1,2 Moreover, this condition has been associated with premature mortality.17, 18, 19 In this sense, the public health guidelines recommending exercise interventions against obesity often focus on moderate-to-high intensity aerobic training as the first option instead of moderate-to-high intensity RT, which is more commonly recommended as a complementary intervention.20, 21, 22, 23 These recommendations are based on previous studies on the management of overweight and obese patients, which mainly focus on body weight loss rather than overall body composition (i.e., lean-soft tissue and fat mass) and, therefore, limit the effectiveness of RT.81 Indeed the position of the American College of Sports Medicine on physical activity for weight loss and prevention of weight regain is that RT will not reduce body weight.81 However, this body weight loss approach may be misleading since RT is typically associated with body recomposition aspects,82 such as an increase in lean-soft tissue mass (∼0.8 kg) and a decrease in fat body mass (∼1 kg), which may not reflect meaningful body weight loss.34 Indeed, in the present study, both RT groups (LVRT and HVRT) presented slightly reduced total body adiposity (∼1 kg and 1.3%, data not shown), with small effect sizes (Tables 3 and 4) in overweight/obese postmenopausal and older females. Our data are in accordance with recent studies on the effects of RT on total body adiposity reduction (0.55–1.00 kg and 1.4%–1.6%).34,83 In addition, our results are similar to those of recent studies on the effects of aerobic and combined training (aerobic plus RT) on total body adiposity reduction (1.7%–2.8%).34,40 Interestingly, the average body adiposity gain during menopause is estimated to be 0.8 kg and 0.8% in the short-term (1.3 years) and 2.6 kg and 2.5% in the long-term (3.9 years).2 Moreover, long-term RT (6 years) prevented total body adiposity gain (1.5 kg) when compared to non-exercisers (controls) in overweight/obese postmenopausal females,25 indicating that RT may be sufficient to offset some menopause-related alterations in body adiposity. Therefore, RT alone may be important (like aerobic training or combined training24,34,40), particularly in postmenopausal and older females, to prevent gains in body adiposity and combat obesity associated risks.25
Interestingly, epidemiological evidence has shown that a reduction of ∼0.06 kg in visceral adipose tissue or 5 cm in waist circumference is clinically important, as it is associated with a reduction in metabolic risk factors for all-cause mortality.84, 85, 86 Moreover, the risk of death increased by 13% in females for every 5-cm increase in waist circumference.87 In the present study, both RT volume groups presented slightly reduced abdominal adiposity (waist circumference, android, and trunk fat), with small effect sizes (Tables 3 and 4). Our data are in accordance with recent studies on RT effects on abdominal adiposity reduction (small effect sizes: 0.24–0.49).28,34,83 Additionally, waist circumference reduced by ∼2 cm (small effect size) when both RT volume groups were compared to CG (data not shown). Our results corroborate those of Loaiza-Betancur and colleagues,55 who demonstrated similar reductions in waist circumference (∼2.9 cm) in postmenopausal and older females after RT. In addition, similar reductions in waist circumference (∼2–3 cm) were observed after moderate-to-vigorous aerobic training and combined training (aerobic training plus RT).40,86 Collectively, these studies suggest that LVRT and HVRT may have a similar clinical relevance to aerobic training in terms of its ability to combat obesity in postmenopausal and older females, although with small effect sizes and low-quality evidence. Therefore, RT should be more strongly encouraged by public health guidelines as a go-to non-pharmacological intervention.
The effects of physical activity (e.g., exercise and non-exercise physical activity) on body adiposity reduction may be related to a caloric deficit, at least in part, through the imbalance between energy intake and energy expenditure.28,34,81 Thus, RT volume (exercise physical activity) is an important training variable, responsible for increased energy expenditure.88,89 Indeed, some RCT studies suggest that a higher RT volume is associated with greater improvements in body adiposity reduction in postmenopausal and older females.52,66 However, in the present study, both RT groups (LVRT and HVRT) presented similarly reduced body adiposity when compared to CG. Interestingly, recent studies reported that RT training volume did not predict body adiposity improvements following RT.28,83 Thus, it seems that energy expenditure increased by RT volume may not always directly affect body adiposity reduction. Although this study was not designed to explore this complex question, a possible explanation for this phenomena may be related to physical activity (reduced levels) and/or dietary (increased levels) compensation90,91 since the higher RT volume may contribute to energy balance by exceeding the caloric deficit. Interestingly, most of the studies included in our systematic review did not control physical activity and dietary intake, although they reported asking volunteers to maintain their usual physical activity and dietary intake. Thus, future studies should explore these issues.
4.3. Metabolic and inflammatory profile
Metabolic and inflammatory impairments are hallmarks of obesity92 and the postmenopausal period in females.3,4 Previous studies have associated metabolic and inflammatory impairments with increased risk for non-communicable diseases (e.g., type 2 diabetes mellitus, breast cancer, and cardiovascular disease),7, 8, 9, 10, 11, 12, 13, 14 which are associated with ∼74% of all deaths globally.93 Thus, combating the deleterious effects provoked by metabolic and inflammatory impairments is a crucial concern in terms of public health. In our study, we demonstrated that both RT volume groups improved metabolic and inflammatory profiles when compared to CG. Interestingly, it has been shown that improvements of blood cholesterols (TC reduction of ∼38 mg/dL and HDL-c increments of ∼13 mg/dL) reduces the risk of mortality from cardiovascular disease by 33%.94 Furthermore, previous data showed that glucose increments of 18 mg/dL (for people with fasting glucose levels above 100 mg/dL) increased cancer and vascular death by 5% and 13%, respectively.95 Additionally, median values of CRP greater than 1.2 mg/L were shown to increase the risk of breast cancer in postmenopausal and older females by 42%, and every 1 mg/L reduction in CRP reduced breast cancer mortality risk by 22%.96 Our data showed that both RT volume groups improved blood cholesterols with small to moderate effect sizes (LVRT reduced TC and LDL-c by ∼18 mg/dL and 26 mg/dL, respectively; HVRT increased HDL-c by ∼5 mg/dL); reduced glucose by 13 mg/dL, with moderate to large effect sizes; and CRP by 1.1 mg/L, with small-to-large effect sizes (∼0.6 mg/L in LVRT and ∼1.6 mg/dL in HVRT) when compared to CG (data not shown). Again, our results corroborated those of Loaiza-Betancur and colleagues,55 who demonstrated similar improvements in metabolic risk and inflammation in postmenopausal and older females after RT. Although the collective evidence is low quality, it suggests that RT (LVRT and HVRT) may have clinical relevance for metabolic risk and inflammation among postmenopausal and older females.
However, HVRT demonstrated higher effect sizes than LVRT for glucose and CRP when compared to CG (Tables 3 and 4). The higher effect sizes observed in HVRT for metabolic risk and inflammation may be explained by the RT dosage prescription. The RT volume protocols (LVRT and HVRT) were similar regarding intensity (moderate to high), repetitions per exercise (8–12), and weekly frequency (2–3 times) (Table 2 and Results section). In contrast, HVRT demonstrated a higher number of weekly sets (∼32.5 sets, a 73% increase) and intervention lengths (∼4.3 weeks, a 42% increase) when compared to LVRT. Thus, it is reasonable to accept that HVRT produced more energy expenditure (i.e., a higher number of sets per week, increased length of intervention) when compared to LVRT in postmenopausal and older females. Indeed, the energy cost of a single set of 8–15 repetitions of 8 RT exercises is ∼70–80 kcal in young and older females.88,89 This suggests, from a practical perspective and based on the number of weekly sets, that the LVRT and HVRT energy expenditure values were about 420 kcal/week and 720 kcal/week, respectively.
In addition, epidemiological studies have demonstrated associations between a higher level of energy expenditure (kcal/week) from physical activity and reduced levels of metabolic risk and inflammation.97, 98, 99, 100 Interestingly, RT volume has been shown to be positively associated with the activation of signaling pathways that regulate aerobic capacity (e.g., mitochondrial biogenesis, glucose, and fatty acid oxidation), myokine secretion (e.g., IL-6, follistatin), and anti-inflammatory and metabolic balance (e.g., insulin sensitivity, adipose tissue browning, and optimizing of the status of metabolic diseases).92,101, 102, 103 Our results suggest that HVRT may be associated with better responses to metabolic risk and inflammation, particularly in postmenopausal and older females.
4.4. Limitations and strengths
Our data demonstrated that both LVRT and HVRT (supervised RT with high adherence) improved body adiposity (total and abdominal), metabolic risk, and inflammation in overweight/obese postmenopausal and older females. However, HVRT seems to be necessary for greater improvements in metabolic risk and inflammation. On the other hand, some issues must be considered when choosing HVRT as this modality requires more physical effort and time commitment, which may decrease real-world adherence. The monotony (e.g., higher number of sets, exercises, and weekly frequency) of HVRT may decrease motivation in some individuals. Thus, HVRT may not be appropriate for all postmenopausal and older females.
While the literature appears to demonstrate that RT (HVRT, in particular) is a promising strategy to improve body adiposity, metabolic risk, and inflammation in postmenopausal and older females, some limitations of this meta-analytic study must be recognized. First, the overall risk of bias was characterized as showing some concerns in most of these studies (a single study presented an overall high risk of bias), and this was likely due to a robust methodological approach. Second, caution should be taken with the general interpretation of the results because the included studies presented high heterogeneity for some outcomes (metabolic and inflammatory profile), which may limit the generalization of the results. However, when the heterogeneity was removed from the analysis by removing outlier studies, the effects of RT volume on analyzed outcomes were maintained regardless of the studies with high heterogeneity. Third, all results demonstrated low certainty of evidence for most outcomes due to unexplained heterogeneity, indirectness of evidence (only 3 studies directly compared LVRT with HVRT), and imprecision of results (small sample size and large CI). Thus, the results of this meta-analytic study should be interpreted with caution.
On the other hand, a strong point of this systematic review with meta-analysis is its approach to exploring the efficacy of RT volume on body adiposity, metabolic risk, and inflammation markers in postmenopausal and older females through RCT studies. In addition, the sample characteristics and RT protocols across LVRT and HVRT were homogeneous, and the methodological processes used in the current review demonstrated high agreement between all authors.
4.5. Future perspectives
Postmenopausal and older females submitted to RT using LVRT and HVRT may gain significant protection against the harmful effects associated with excess body adiposity, metabolic risk, and inflammation. More specifically, postmenopausal and older females may demonstrate superior effects regarding metabolic risk and inflammation after HVRT as compared to LVRT. However, some perspectives should be suggested for future studies. First, more care should be taken with the randomization process (e.g., the allocation sequence should be concealed) and deviations from the intended intervention (e.g., appropriate analysis to estimate the effect of assignment), as these were some concerns in the risk of bias in the RCTs. Second, larger RCTs involving the effects of RT on body adiposity, metabolic risk, and inflammation in postmenopausal and older females should compare volume directly against CG and prioritize the control of dietary intake and levels of physical activity during the intervention to ensure that adaptive responses are not affected by confounding variables. Third, more metabolic risk and inflammation markers should be analyzed since there were insufficient data for meta-analysis of many outcomes.
5. Conclusion
This systematic review with meta-analysis of RCTs suggests that both LVRT and HVRT are effective strategies for improving body adiposity (total and abdominal), metabolic risk, and inflammation in overweight/obese postmenopausal and older females. Thus, RT may be included in the public health guidelines as one of the first options for non-pharmacological intervention against obesity, metabolic risk, and inflammation impairments. However, HVRT appears to facilitate greater improvements in metabolic risk and inflammation than LVRT. Therefore, our findings endorse the importance of HVRT for overweight/obese postmenopausal and older females to optimize improvements in metabolic risk and inflammation in order to combat non-communicable diseases.
Acknowledgments
This study was supported by the Minas Gerais State University (UEMG/Brazil). PRPN received a Research Productivity Scholarship Program (UEMG-PQ08/2021). MAdSC received a doctorate scholarship from the National Council of Technological and Scientific Development (CNPq/Brazil - Process 140473/2020–3). PCS received a doctorate scholarship from the Coordination of Improvement of Higher Education Personnel (CAPES/Brazil – Code 001).
Authors’ contributions
PRPN and MAdSC were responsible for the conceptualization and design of the study, screening process, data collection and extraction, risk of bias assessment, and data analysis, as well as the drafting of the original version of the manuscript; PCS participated in the study design, screening process, data collection and extraction, and risk of bias assessment, and helped to review the manuscript draft; AAdO, BdFC, GCS, and LMVS participated in the study design, checked the information in the selected articles, and helped to review the manuscript draft. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials associated with this article can be found in the online version at doi:10.1016/j.jshs.2023.09.012.
Supplementary materials
References
- 1.Van Pelt RE, Gavin KM, Kohrt WM. Regulation of body composition and bioenergetics by estrogens. Endocrinol Metab Clin North Am. 2015;44:663–676. doi: 10.1016/j.ecl.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juppi HK, Sipila S, Fachada V, et al. Total and regional body adiposity increases during menopause-evidence from a follow-up study. Aging Cell. 2022;21:e13621. doi: 10.1111/acel.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfeilschifter J, Koditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23:90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- 4.Mumusoglu S, Yildiz BO. Metabolic syndrome during menopause. Current Vasc Pharmacol. 2019;17:595–603. doi: 10.2174/1570161116666180904094149. [DOI] [PubMed] [Google Scholar]
- 5.Maury E, Ehala-Aleksejev K, Guiot Y, Detry R, Vandenhooft A, Brichard SM. Adipokines oversecreted by omental adipose tissue in human obesity. Am J Physiol Endocrinol Metab. 2007;293:E656–E665. doi: 10.1152/ajpendo.00127.2007. [DOI] [PubMed] [Google Scholar]
- 6.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: Depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 7.Grossmann V, Schmitt VH, Zeller T, et al. Profile of the immune and inflammatory response in individuals with prediabetes and type 2 diabetes. Diabetes Care. 2015;38:1356–1364. doi: 10.2337/dc14-3008. [DOI] [PubMed] [Google Scholar]
- 8.Donath MY. Targeting inflammation in the treatment of type 2 diabetes: Time to start. Nat Rev Drug Discov. 2014;13:465–476. doi: 10.1038/nrd4275. [DOI] [PubMed] [Google Scholar]
- 9.Wu M, Chou Y, Chou W, et al. Circulating levels of leptin, adiposity and breast cancer risk. Br J Cancer. 2009;100:578–582. doi: 10.1038/sj.bjc.6604913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ollberding NJ, Kim Y, Shvetsov YB, et al. Prediagnostic leptin, adiponectin, C-reactive protein, and the risk of postmenopausal breast cancer. Cancer Prev Res (Phila) 2013;6:188–195. doi: 10.1158/1940-6207.CAPR-12-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niu J, Jiang L, Guo W, Shao L, Liu Y, Wang L. The association between leptin level and breast cancer: A meta-analysis. PloS One. 2013;8:e67349. doi: 10.1371/journal.pone.0067349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedenreich CM, Langley AR, Speidel TP, et al. Case-control study of markers of insulin resistance and endometrial cancer risk. Endocr Relat Cancer. 2012;19:785–792. doi: 10.1530/ERC-12-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shapiro Y, Mashavi M, Luckish E, Shargorodsky M. Diabetes and menopause aggravate age-dependent deterioration in arterial stiffness. Menopause. 2014;21:1234–1238. doi: 10.1097/GME.0000000000000231. [DOI] [PubMed] [Google Scholar]
- 14.Camilleri G, Borg M, Brincat S, Schembri-Wismayer P, Brincat M, Calleja-Agius J. The role of cytokines in cardiovascular disease in menopause. Climacteric. 2012;15:524–530. doi: 10.3109/13697137.2012.700743. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez-Ono A, Monter-Carreola G, Zamora-Gonzalez J, et al. Association of visceral fat with coronary risk factors in a population-based sample of postmenopausal women. Int J Obes Relat Metab Disord. 2002;26:33–39. doi: 10.1038/sj.ijo.0801842. [DOI] [PubMed] [Google Scholar]
- 16.Roriz AKC, de Oliveira CC, Moreira PA, Eickemberg M, Medeiros JMB, Sampaio LR. Methods of predicting visceral fat in Brazilian adults and older adults: A comparison between anthropometry and computerized tomography. Arch Latinoam Nutr. 2011;61:5–12. [PubMed] [Google Scholar]
- 17.Kaichi Y, Sakane H, Higashibori H, et al. Relationship between sudden natural death and abdominal fat evaluated on postmortem CT scans. Obesity Sci Pract. 2017;3:219–223. doi: 10.1002/osp4.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahakyan KR, Somers VK, Rodriguez-Escudero JP, et al. Normal-weight central obesity: Implications for total and cardiovascular mortality risk in persons with normal-weight central obesity. Ann Intern Med. 2015;163:827–835. doi: 10.7326/M14-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dagenais GR, Yi Q, Mann JF, et al. Prognostic impact of body weight and abdominal obesity in women and men with cardiovascular disease. Am Heart J. 2005;149:54–60. doi: 10.1016/j.ahj.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Steele J, Fisher J, Skivington M, et al. A higher effort-based paradigm in physical activity and exercise for public health: Making the case for a greater emphasis on resistance training. BMC Public Health. 2017;17:300. doi: 10.1186/s12889-017-4209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 22.Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54:1451–1462. doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 24.Taylor J, Walsh S, Kwok W, et al. A scoping review of physical activity interventions for older adults. Int J Behav Nutr Phys Act. 2021;18:82. doi: 10.1186/s12966-021-01140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bea JW, Cussler EC, Going SB, Blew RM, Metcalfe LL, Lohman TG. Resistance training predicts 6-yr body composition change in postmenopausal women. Med Sci Sport Exerc. 2010;42:1286–1295. doi: 10.1249/MSS.0b013e3181ca8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratamess NA, Alvar BA, Evetoch TE, et al. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41:687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 27.Vissers D, Hens W, Taeymans J, Baeyens JP, Poortmans J, Van Gaal L. The effect of exercise on visceral adipose tissue in overweight adults: A systematic review and meta-analysis. PloS One. 2013;8:e56415. doi: 10.1371/journal.pone.0056415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khalafi M, Malandish A, Rosenkranz SK, Ravasi AA. Effect of resistance training with and without caloric restriction on visceral fat: A systemic review and meta-analysis. Obes Rev. 2021;22:e13275. doi: 10.1111/obr.13275. [DOI] [PubMed] [Google Scholar]
- 29.Yarizadeh H, Eftekhar R, Anjom-Shoae J, Speakman JR, Djafarian K. The effect of aerobic and resistance training and combined exercise modalities on subcutaneous abdominal fat: A systematic review and meta-analysis of randomized clinical trials. Adv Nutr. 2021;12:179–196. doi: 10.1093/advances/nmaa090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sardeli AV, Tomeleri CM, Cyrino ES, Fernhall B, Cavaglieri CR, Chacon-Mikahil MPT. Effect of resistance training on inflammatory markers of older adults: A meta-analysis. Exp Gerontol. 2018;111:188–196. doi: 10.1016/j.exger.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 31.Ashton RE, Tew GA, Aning JJ, Gilbert SE, Lewis L, Saxton JM. Effects of short-term, medium-term and long-term resistance exercise training on cardiometabolic health outcomes in adults: Systematic review with meta-analysis. Br J Sports Med. 2020;54:341–348. doi: 10.1136/bjsports-2017-098970. [DOI] [PubMed] [Google Scholar]
- 32.Strasser B, Siebert U, Schobersberger W. Resistance training in the treatment of the metabolic syndrome: A systematic review and meta-analysis of the effect of resistance training on metabolic clustering in patients with abnormal glucose metabolism. Sports Med. 2010;40:397–415. doi: 10.2165/11531380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 33.Qadir R, Sculthorpe NF, Todd T, Brown EC. Effectiveness of resistance training and associated program characteristics in patients at risk for type 2 diabetes: A systematic review and meta-analysis. Sports Med Open. 2021;7:38. doi: 10.1186/s40798-021-00321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez P, Taaffe DR, Galvao DA, et al. Resistance training effectiveness on body composition and body weight outcomes in individuals with overweight and obesity across the lifespan: A systematic review and meta-analysis. Obes Rev. 2022;23:e13428. doi: 10.1111/obr.13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ismail I, Keating SE, Baker MK, Johnson NA. A systematic review and meta-analysis of the effect of aerobic vs. resistance exercise training on visceral fat. Obes Rev. 2012;13:68–91. doi: 10.1111/j.1467-789X.2011.00931.x. [DOI] [PubMed] [Google Scholar]
- 36.Chang YH, Yang HY, Shun SC. Effect of exercise intervention dosage on reducing visceral adipose tissue: A systematic review and network meta-analysis of randomized controlled trials. Int J Obes (Lond) 2021;45:982–997. doi: 10.1038/s41366-021-00767-9. [DOI] [PubMed] [Google Scholar]
- 37.Alizaei Yousefabadi H, Niyazi A, Alaee S, Fathi M, Mohammad Rahimi GR. Anti-inflammatory effects of exercise on metabolic syndrome patients: A systematic review and meta-analysis. Biol Res Nurs. 2021;23:280–292. doi: 10.1177/1099800420958068. [DOI] [PubMed] [Google Scholar]
- 38.Wewege MA, Thom JM, Rye KA, Parmenter BJ. Aerobic, resistance or combined training: A systematic review and meta-analysis of exercise to reduce cardiovascular risk in adults with metabolic syndrome. Atherosclerosis. 2018;274:162–171. doi: 10.1016/j.atherosclerosis.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Pattyn N, Cornelissen VA, Eshghi SR, Vanhees L. The effect of exercise on the cardiovascular risk factors constituting the metabolic syndrome: A meta-analysis of controlled trials. Sports Med. 2013;43:121–133. doi: 10.1007/s40279-012-0003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Donoghue G, Blake C, Cunningham C, Lennon O, Perrotta C. What exercise prescription is optimal to improve body composition and cardiorespiratory fitness in adults living with obesity? A network meta-analysis. Obes Rev. 2021;22:e13137. doi: 10.1111/obr.13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sirola J, Rikkonen T. Muscle performance after the menopause. J Br Menopause Soc. 2005;11:45–50. doi: 10.1258/136218005775544561. [DOI] [PubMed] [Google Scholar]
- 42.Toth M, Tchernof A, Sites C, Poehlman E. Effect of menopausal status on body composition and abdominal fat distribution. Int J Obes Relat Metab Disord. 2000;24:226–231. doi: 10.1038/sj.ijo.0801118. [DOI] [PubMed] [Google Scholar]
- 43.Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. Meta-analysis: Effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab. 2006;8:538–554. doi: 10.1111/j.1463-1326.2005.00545.x. [DOI] [PubMed] [Google Scholar]
- 44.Costa GBC, Carneiro G, Umeda L, Pardini D, Zanella MT. Influence of menopausal hormone therapy on body composition and metabolic parameters. Biores Open Access. 2020;9:80–85. doi: 10.1089/biores.2019.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahtiainen M, Alen M, Pöllänen E, et al. Hormone therapy is associated with better body composition and adipokine/glucose profiles: A study with monozygotic co-twin control design. Menopause. 2012;19:1329–1335. doi: 10.1097/gme.0b013e31825a3344. [DOI] [PubMed] [Google Scholar]
- 46.Lera Orsatti F, Nahas EA, Maesta N, et al. Effects of resistance training frequency on body composition and metabolics and inflammatory markers in overweight postmenopausal women. J Sports Med Phys Fitness. 2014;54:317–325. [PubMed] [Google Scholar]
- 47.Maesta N, Nahas EA, Nahas-Neto J, et al. Effects of soy protein and resistance exercise on body composition and blood lipids in postmenopausal women. Maturitas. 2007;56:350–358. doi: 10.1016/j.maturitas.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 48.Phillips MD, Patrizi RM, Cheek DJ, Wooten JS, Barbee JJ, Mitchell JB. Resistance training reduces subclinical inflammation in obese, postmenopausal women. Med Sci Sports Exerc. 2012;44:2099–2110. doi: 10.1249/MSS.0b013e3182644984. [DOI] [PubMed] [Google Scholar]
- 49.Senechal M, Bouchard DR, Dionne IJ, Brochu M. The effects of lifestyle interventions in dynapenic-obese postmenopausal women. Menopause. 2012;19:1015–1021. doi: 10.1097/gme.0b013e318248f50f. [DOI] [PubMed] [Google Scholar]
- 50.Orsatti FL, Nahas EA, Nahas-Neto J, Maesta N, Orsatti CL, Fernandes CE. Effects of resistance training and soy isoflavone on body composition in postmenopausal women. Obstet Gynecol Int. 2010;2010 doi: 10.1155/2010/156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ward LJ, Nilsson S, Hammar M, et al. Resistance training decreases plasma levels of adipokines in postmenopausal women. Sci Rep. 2020;10:19837. doi: 10.1038/s41598-020-76901-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nunes PR, Barcelos LC, Oliveira AA, et al. Effect of resistance training on muscular strength and indicators of abdominal adiposity, metabolic risk, and inflammation in postmenopausal women: Controlled and randomized clinical trial of efficacy of training volume. Age (Dordr) 2016;38:40. doi: 10.1007/s11357-016-9901-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khalafi M, Malandish A, Rosenkranz SK. The impact of exercise training on inflammatory markers in postmenopausal women: A systemic review and meta-analysis. Exp Gerontol. 2021;150 doi: 10.1016/j.exger.2021.111398. [DOI] [PubMed] [Google Scholar]
- 54.Thomas E, Gentile A, Lakicevic N, et al. The effect of resistance training programs on lean body mass in postmenopausal and elderly women: A meta-analysis of observational studies. Aging Clin Exp Res. 2021;33:2941–2952. doi: 10.1007/s40520-021-01853-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loaiza-Betancur AF, Gómez-Tomás C, Blasco JM, Chulvi-Medrano I, Iglesias-González LE. Effects of resistance training on C-reactive protein in menopausal and postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Menopause. 2022;29:1430–1440. doi: 10.1097/GME.0000000000002076. [DOI] [PubMed] [Google Scholar]
- 56.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sterne JAC, Savovic J, Page MJ, et al. Rob 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 58.Schünemann HJ, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH. Cochrane GRADEing Methods Group; Hoboken, NJ: 2022. Completing “summary of findings” tables and grading the certainty of the evidence. [Google Scholar]
- 59.Nunes JP, Kassiano W, Costa BDV, Mayhew JL, Ribeiro AS, Cyrino ES. Equating resistance-training volume between programs focused on muscle hypertrophy. Sports Med. 2021;51:1171–1178. doi: 10.1007/s40279-021-01449-2. [DOI] [PubMed] [Google Scholar]
- 60.Haff GG, Triplett NT. 4th ed. Human Kinetics; Champaign, IL: 2015. Essentials of strength training and conditioning. [Google Scholar]
- 61.Bouchard DR, Soucy L, Sénéchal M, Dionne IJ, Brochu M. Impact of resistance training with or without caloric restriction on physical capacity in obese older women. Menopause. 2009;16:66–72. doi: 10.1097/gme.0b013e31817dacf7. [DOI] [PubMed] [Google Scholar]
- 62.Cavalcante EF, Ribeiro AS, do Nascimento MA, et al. Effects of different resistance training frequencies on fat in overweight/obese older women. Int J Sports Med. 2018;39:527–534. doi: 10.1055/a-0599-6555. [DOI] [PubMed] [Google Scholar]
- 63.Coelho-Júnior HJ, de Oliveira Goncalvez I, Sampaio RAC, et al. Periodized and non-periodized resistance training programs on body composition and physical function of older women. Exp Gerontol. 2019;121:10–18. doi: 10.1016/j.exger.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 64.Conceicao MS, Bonganha V, Vechin FC, et al. Sixteen weeks of resistance training can decrease the risk of metabolic syndrome in healthy postmenopausal women. Clin Interv Aging. 2013;8:1221–1228. doi: 10.2147/CIA.S44245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cunha PM, Ribeiro AS, Nunes JP, et al. Resistance training performed with single-set is sufficient to reduce cardiovascular risk factors in untrained older women: The randomized clinical trial. Active aging longitudinal study. Arch Gerontol Geriatr. 2019;81:171–175. doi: 10.1016/j.archger.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 66.Cunha PM, Tomeleri CM, Nascimento MA, et al. Comparison of low and high volume of resistance training on body fat and blood biomarkers in untrained older women: A randomized clinical trial. J Strength Cond Res. 2021;35:1–8. doi: 10.1519/JSC.0000000000003245. [DOI] [PubMed] [Google Scholar]
- 67.do Nascimento MA, Gerage AM, Januario RS, et al. Resistance training with dietary intake maintenance increases strength without altering body composition in older women. J Sports Med Phys Fitness. 2018;58:457–464. doi: 10.23736/S0022-4707.16.06730-X. [DOI] [PubMed] [Google Scholar]
- 68.Dos Santos L, Ribeiro AS, Nunes JP, et al. Effects of pyramid resistance-training system with different repetition zones on cardiovascular risk factors in older women: A randomized controlled trial. Int J Environ Res Public Health. 2020;17:6115. doi: 10.3390/ijerph17176115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Egana M, Reilly H, Green S. Effect of elastic-band-based resistance training on leg blood flow in elderly women. Appl Physiol Nutr Metab. 2010;35:763–772. doi: 10.1139/H10-071. [DOI] [PubMed] [Google Scholar]
- 70.Elliott KJ, Sale C, Cable NT. Effects of resistance training and detraining on muscle strength and blood lipid profiles in postmenopausal women. Br J Sports Med. 2002;36:340–344. doi: 10.1136/bjsm.36.5.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fritz NB, Juesas A, Gargallo P, et al. Positive effects of a short-term intense elastic resistance training program on body composition and physical functioning in overweight older women. Biol Res Nurs. 2018;20:321–334. doi: 10.1177/1099800418757676. [DOI] [PubMed] [Google Scholar]
- 72.Gambassi BB, Rodrigues B, Feriani DJ, et al. Effects of resistance training of moderate intensity on heart rate variability, body composition, and muscle strength in healthy elderly women. Sport Sci Health. 2016;12:389–395. [Google Scholar]
- 73.Orsatti FL, Nahas EA, Maesta N, Nahas-Neto J, Burini RC. Plasma hormones, muscle mass and strength in resistance-trained postmenopausal women. Maturitas. 2008;59:394–404. doi: 10.1016/j.maturitas.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 74.Ribeiro AS, Schoenfeld BJ, Dos Santos L, et al. Resistance training improves a cellular health parameter in obese older women: A randomized controlled trial. J Strength Cond Res. 2020;34:2996–3002. doi: 10.1519/JSC.0000000000002773. [DOI] [PubMed] [Google Scholar]
- 75.Tomeleri CM, Ribeiro AS, Souza MF, et al. Resistance training improves inflammatory level, lipid and glycemic profiles in obese older women: A randomized controlled trial. Exp Gerontol. 2016;84:80–87. doi: 10.1016/j.exger.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 76.Tomeleri CM, Ribeiro AS, Cavaglieri CR, et al. Correlations between resistance training-induced changes on phase angle and biochemical markers in older women. Scand J Med Sci Sports. 2018;28:2173–2182. doi: 10.1111/sms.13232. [DOI] [PubMed] [Google Scholar]
- 77.Tomeleri CM, Souza MF, Burini RC, et al. Resistance training reduces metabolic syndrome and inflammatory markers in older women: A randomized controlled trial. J Diabetes. 2018;10:328–337. doi: 10.1111/1753-0407.12614. [DOI] [PubMed] [Google Scholar]
- 78.Urzi F, Marusic U, Licen S, Buzan E. Effects of elastic resistance training on functional performance and myokines in older women-A randomized controlled trial. J Am Med Dir Assoc. 2019;20:830–834. doi: 10.1016/j.jamda.2019.01.151. [DOI] [PubMed] [Google Scholar]
- 79.Bray GA, Ryan DH. Clinical evaluation of the overweight patient. Endocrine. 2000;13:167–186. doi: 10.1385/ENDO:13:2:167. [DOI] [PubMed] [Google Scholar]
- 80.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72:694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- 81.Donnelly J, Blair S, Jakicic JM, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 82.Barakat C, Pearson J, Escalante G, Campbell B, De Souza EO. Body recomposition: Can trained individuals build muscle and lose fat at the same time? Strength Cond J. 2020;42:7–21. [Google Scholar]
- 83.Wewege MA, Desai I, Honey C, et al. The effect of resistance training in healthy adults on body fat percentage, fat mass and visceral fat: A systematic review and meta-analysis. Sports Med. 2022;52:287–300. doi: 10.1007/s40279-021-01562-2. [DOI] [PubMed] [Google Scholar]
- 84.Okauchi Y, Nishizawa H, Funahashi T, et al. Reduction of visceral fat is associated with decrease in the number of metabolic risk factors in Japanese men. Diabetes Care. 2007;30:2392–2394. doi: 10.2337/dc07-0218. [DOI] [PubMed] [Google Scholar]
- 85.Kashiwagi R, Iwahashi H, Yamada Y, et al. Effective waist circumference reduction rate necessary to avoid the development of type 2 diabetes in Japanese men with abdominal obesity. Endocr J. 2017;64:881–894. doi: 10.1507/endocrj.EJ17-0113. [DOI] [PubMed] [Google Scholar]
- 86.Tan A, Thomas RL, Campbell MD, Prior SL, Bracken RM, Churm R. Effects of exercise training on metabolic syndrome risk factors in post-menopausal women—A systematic review and meta-analysis of randomised controlled trials. Clin Nutr. 2023;42:337–351. doi: 10.1016/j.clnu.2023.01.008. [DOI] [PubMed] [Google Scholar]
- 87.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 88.Phillips WT, Ziuraitis JR. Energy cost of single-set resistance training in older adults. J Strength Cond Res. 2004;18:606–609. doi: 10.1519/1533-4287(2004)18<606:ECOSRT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 89.Haddock BL, Wilkin LD. Resistance training volume and post exercise energy expenditure. Int J Sports Med. 2006;27:143–148. doi: 10.1055/s-2005-865601. [DOI] [PubMed] [Google Scholar]
- 90.Swelam BA, Verswijveren S, Salmon J, Arundell L, Ridgers ND. Exploring activity compensation amongst youth and adults: A systematic review. Int J Behav Nutr Phys Act. 2022;19:25. doi: 10.1186/s12966-022-01264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Drenowatz C. Reciprocal compensation to changes in dietary intake and energy expenditure within the concept of energy balance. Adv Nutr. 2015;6:592–599. doi: 10.3945/an.115.008615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gonzalez-Gil AM, Elizondo-Montemayor L. The role of exercise in the interplay between myokines, hepatokines, osteokines, adipokines, and modulation of inflammation for energy substrate redistribution and fat mass loss: A review. Nutrients. 2020;12:1899. doi: 10.3390/nu12061899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martinez R, Lloyd-Sherlock P, Soliz P, et al. Trends in premature avertable mortality from non-communicable diseases for 195 countries and territories, 1990–2017: A population-based study. Lancet Glob Health. 2020;8:e511–e523. doi: 10.1016/S2214-109X(20)30035-8. [DOI] [PubMed] [Google Scholar]
- 94.Lewington S, Whitlock G, Clarke R, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: A meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. The Lancet. 2007;370:1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 95.Rao Kondapally Seshasai S, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Frydenberg H, Thune I, Lofterod T, et al. Pre-diagnostic high-sensitive C-reactive protein and breast cancer risk, recurrence, and survival. Breast Cancer Res Treat. 2016;155:345–354. doi: 10.1007/s10549-015-3671-1. [DOI] [PubMed] [Google Scholar]
- 97.Geffken DF, Cushman M, Burke GL, Polak JF, Sakkinen PA, Tracy RP. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol. 2001;153:242–250. doi: 10.1093/aje/153.3.242. [DOI] [PubMed] [Google Scholar]
- 98.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Rimm EB. Leisure-time physical activity and reduced plasma levels of obesity-related inflammatory markers. Obes Res. 2003;11:1055–1064. doi: 10.1038/oby.2003.145. [DOI] [PubMed] [Google Scholar]
- 99.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: A systematic review. J Am Coll Cardiol. 2005;45:1563–1569. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 100.Major GC, Piche ME, Bergeron J, Weisnagel SJ, Nadeau A, Lemieux S. Energy expenditure from physical activity and the metabolic risk profile at menopause. Med Sci Sports Exerc. 2005;37:204–212. doi: 10.1249/01.mss.0000152702.57291.fa. [DOI] [PubMed] [Google Scholar]
- 101.Zunner BEM, Wachsmuth NB, Eckstein ML, et al. Myokines and resistance training: A narrative review. Int J Mol Sci. 2022;23:3501. doi: 10.3390/ijms23073501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ahtiainen JP, Walker S, Silvennoinen M, et al. Exercise type and volume alter signaling pathways regulating skeletal muscle glucose uptake and protein synthesis. Eur J Appl Physiol. 2015;115:1835–1845. doi: 10.1007/s00421-015-3155-3. [DOI] [PubMed] [Google Scholar]
- 103.Garcia-Hermoso A, Ramirez-Velez R, Diez J, Gonzalez A, Izquierdo M. Exercise training-induced changes in exerkine concentrations may be relevant to the metabolic control of type 2 diabetes mellitus patients: A systematic review and meta-analysis of randomized controlled trials. J Sport Health Sci. 2023;12:147–157. doi: 10.1016/j.jshs.2022.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.