The classic features of giant-cell myocarditis (GCM) are subacute, refractory congestive heart failure (CHF), and arrhythmia, although atypical presentations are common. Here, we report the case of a 62-year-old woman with CHF symptoms after a recent coronavirus disease 19 (COVID-19) vaccination, initially misdiagnosed as post-vaccine myocarditis. Rapid deterioration into cardiogenic shock resulted in endomyocardial biopsy, revealing GCM. Immunosuppressive therapy was initiated, and her cardiac function improved. This case illustrates the importance of considering GCM in the differential diagnosis in cases of acute heart failure refractory to standard therapy, even in patients with recent history of COVID-19 vaccination.
Case
A 62-year-old woman presented to hospital with a 5-day history of exertional dyspnea and intermittent, sharp chest discomfort, 2 weeks after her third dose of the SPIKEVAX (Moderna, Cambridge, MA) COVID-19 mRNA vaccine. She endorsed paroxysmal nocturnal dyspnea and orthopnea but no other systemic symptoms. She had no known medical or familial history, took no prescription medications, and denied use of alcohol, tobacco, and illicit substances.
Her vitals were remarkable for hypoxia requiring 6 L per minute of supplemental oxygen, tachycardia at 114 beats per minute, and blood pressure of 104/82 mm Hg. Her physical examination was suggestive of volume overload, with bilateral rales, peripheral edema, and an elevated jugular venous pressure. Her laboratory investigations were significant for elevated high-sensitivity troponin (13,317 ng/L, reference range [RR] 0-16 ng/L), B-type natriuretic peptide (BNP) (2170 ng/L, RR ≤ 160 ng/L), and serum lactate (2.9 mmol/L, RR 0.5-2.2 mmol/L). Her electrocardiogram demonstrated sinus tachycardia with a right bundle branch block (Supplemental Fig. S1). Her computed tomography pulmonary angiogram results were negative for pulmonary embolism but did show bilateral ground-glass opacities, large pleural effusions, and pulmonary edema. Her COVID-19 polymerase chain reaction test results were negative.
She was admitted and treated as CHF exacerbation secondary to presumed acute coronary syndrome. She received aspirin, ticagrelor, and heparin and intravenous furosemide for volume overload. Her oxygen requirements continued to escalate.
A transthoracic echocardiogram early in admission demonstrated normal sized left ventricle (LV) with severely decreased global systolic function (left ventricular ejection fraction [LVEF] 26%), moderate right ventricular systolic dysfunction, and moderate-severe functional mitral regurgitation.
Her clinical course continued to worsen over the subsequent few days requiring transfer to the cardiac intensive care unit. Her respiratory status worsened on maximal high flow nasal oxygen. She was intolerant to bilevel positive airway pressure ventilation, ultimately requiring intubation and mechanical ventilation on day 6 of admission. She underwent a coronary angiogram, which demonstrated no significant coronary artery disease. The left ventricular end-diastolic pressure was markedly elevated at 36 mm Hg. Because of the overall clinical picture, an intra-aortic balloon pump (IABP) was inserted during the procedure.
After ruling out significant obstructive coronary artery disease, her CHF was thought to be secondary to post-vaccine myocarditis. She underwent aggressive diuresis with furosemide, with norepinephrine and dobutamine support, which resulted in significant ectopy, necessitating suppression with amiodarone. A repeat echocardiogram performed on day 10 postadmission demonstrated ongoing biventricular failure with an LVEF of 25% and moderate mitral regurgitation. A pulmonary artery catheter was inserted, and measurements (Table 1) were consistent with cardiogenic shock, on the basis of a reduced cardiac index (1.7 L/min/m2) and an elevated wedge pressure (27 mm Hg).
Table 1.
Pulmonary artery catheter measurements
| Time (hh:mm) | CO (4-8 L/min) | CI (2.5-4 L/min/m2) | SV (60-100 mL/beat) | SVI (33-47 mL/beat/m2) | SVR (800-1200 dynes-sec/cm5) | PVR (< 250 dynes-sec/cm5) | LVSWI (50-62 g × min/m2) | RVSWI (5-10 g/m/beat/m2) | CVP (2-6 mm Hg) | PAWP (6-18 mm Hg) |
|---|---|---|---|---|---|---|---|---|---|---|
| 11:27 | 3.2 | 1.7 | 29 | 15 | 1448 | 250 | 8.7 | 5.4 | 11 | 27 |
| 11:34 | 3.6 | 1.9 | 35 | 19 | 1354 | 422 | 11.3 | 8.8 | 11 | 27 |
| 21:28, on milrinone | 4.9 | 2.6 | 49 | 26 | 799 | 261 | 14.6 | 8.6 | 10 | 18 |
CI, cardiac index; CO, cardiac output; CVP, central venous pressure; LVSWI, left ventricular stroke work index; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; RVSWI, right ventricular stroke work index; SV, stroke volume; SVI, stroke volume index; SVR, systemic vascular resistance.
The patient remained in cardiogenic shock despite management. She was transferred to a specialized cardiac institution for consideration of mechanical circulatory support and endomyocardial biopsy. At the quaternary care centre, the cardiac surgery team inserted an emergency Impella CP heart pump device (Abiomed, Danvers, MA). Impella was selected over extracorporeal membrane oxygenation (ECMO) because the patient had moderate-to-severe mitral regurgitation, which could be made worse by ECMO-associated increase in afterload. Her clinical condition began to improve with the Impella device, and her IABP was removed the following day.
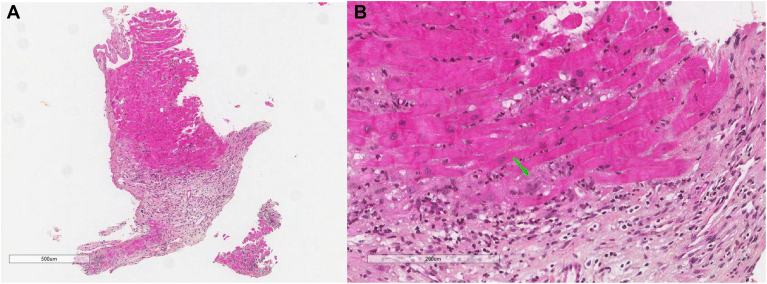
The decision was made to proceed directly to endomyocardial biopsy, foregoing cardiac magnetic resonance imaging and other advanced imaging, in consideration of the patient’s rapid clinical deterioration, tachycardia, and inability to remain in position for the scan without intubation. She underwent an endomyocardial biopsy 12 days postadmission, which demonstrated cardiomyocyte necrosis, giant cells, lymphocytes, histiocytes, and eosinophils consistent with active GCM (Fig. 1). She was therefore started on methylprednisolone, mycophenolate mofetil, tacrolimus, antithymocyte globulin, and appropriate prophylactic antimicrobials. The patient’s clinical status significantly improved with the treatments. The Impella device was removed 19 days after initiation of therapy, and she remained in stable condition. A repeat echocardiogram was performed 39 days after initiation of therapy and showed an improvement in her LVEF to 35%. She was discharged to a rehabilitation facility, pending consideration for cardiac transplant.
Figure 1.
(A) Microscopic image of the biopsy showing loss of cardiac myocytes and replacement by an inflammatory infiltrate and granulation tissue (hematoxylin & eosin [H&E] stain, magnification ×500 μm). (B) Higher magnification shows a mixed inflammatory infiltrate composed of lymphocytes, eosinophils, histiocytes, and giant cells (green arrow) (H&E stain, magnification ×200 μm).
Discussion
GCM is a rare but lethal autoimmune myocarditis that generally affects relatively young, healthy people. It is characterized by refractory CHF of subacute onset and frequently by refractory ventricular arrhythmias or high-degree atrioventricular block. It requires a high degree of clinical suspicion, particularly in the absence of arrhythmia. The diagnosis is confirmed by an endomyocardial biopsy showing extensive necrosis and a polymorphous inflammatory response of multinucleated giant cells.1 Immunosuppressive therapy should be initiated after biopsy results. However, given the fatality associated with the condition, the most effective therapy is cardiac transplantation.
Symptoms of GCM may be initially misdiagnosed as an inflammatory myocarditis from recent vaccination, particularly during the COVID-19 pandemic. Post-vaccine myocarditis is a recognized phenomenon that appears, in younger patients, to be more common with the mRNA COVID-19 vaccines compared with any other vaccination.2 The risk is highest after the second vaccination dose3; however, a case after the third dose has been reported.4 Conversely, there are few reported cases of GCM following COVID-19 vaccination.5,6 Distinguishing between these 2 entities is crucial because of the drastic difference in their clinical courses and treatments. This case presentation had several features not in keeping with post-vaccine myocarditis, including that the patient was an older woman and the onset of GCM occurred after her third dose and more than 2 weeks after administration of the vaccine.
Sung et al.6 describe a case of GCM in a patient presenting with fever, shortness of breath, and cough 1 week after receiving his second dose of the BNT162b2 (Pfizer-BioNTech, New York, New York, USA) vaccine. Notably, the patient had ST-segment elevation on electrocardiogram in the setting of clinical CHF. Similar to our case, their patient required an IABP and improved with immunosuppression therapy alone. In contrast, Kang et al.5 reported a patient with dull chest pain and dyspnea 4 days after the second dose of the BNT162b2 vaccine. Their patient underwent percutaneous coronary intervention and developed sudden ventricular tachycardia requiring cardiopulmonary resuscitation; that patient eventually received heart transplantation. In both cases, as with ours, the patients had elevated troponin and BNP and severe LV dysfunction on echocardiogram that was new onset and rapidly progressive. The latter is a hallmark of GCM. Interestingly, neither our patient nor the patient of Sung et al.6 presented with ventricular arrhythmia, highlighting that this is not an essential feature of GCM presentation.
The American Heart Association and the American College of Cardiology Foundation suggest endomyocardial biopsy to exclude GCM in new-onset rapidly progressive CHF associated with ventricular tachycardia, high-grade atrioventricular block, or hemodynamic instability after excluding common etiologies such as ischemia.1 However, studies have shown that high-degree atrioventricular block and ventricular arrhythmias may be absent up to 50% of the time.1,7 Therefore, a rapid decline in LVEF over several days, with subacute CHF of 2 weeks' to 3 months’ duration that is refractory to 1 to 2 weeks of standard treatment, should raise suspicion for GCM. An endomyocardial biopsy should be considered—including early transfer to specialized centres when necessary—in these circumstances, irrespective of vaccination status, and even in the absence of arrhythmia and classic echocardiographic features of GCM.
Novel Teaching Points.
-
•
Although the initial presentation of GCM may resemble other types of myocarditis, the ensuing rapid clinical deterioration and lack of response to standard treatment should prompt consideration of the diagnosis.
-
•
The classic features of GCM are rapidly progressive CHF, associated with ventricular tachycardia or high-grade atrioventricular block; however, cases can present without arrhythmia.
-
•
Clinicians must maintain a high index of suspicion for GCM and consider biopsy even in the absence of some clinical features, given the high mortality and the necessity for early intervention.
Acknowledgments
Ethics Statement
This research has adhered to the relevant ethical guidelines.
Patient Consent
The authors confirm that a patient consent form(s) has been obtained for this article.
Funding Sources
No funding was provided for this article.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 546 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2023.11.025.
Supplementary Material
References
- 1.Bang V., Ganatra S., Shah S.P., et al. Management of patients with giant cell myocarditis: JACC Review Topic of the Week. J Am Coll Cardiol. 2021;77:1122–1134. doi: 10.1016/j.jacc.2020.11.074. [DOI] [PubMed] [Google Scholar]
- 2.Centres for Disease Control and Prevention Clinical considerations: myocarditis after mRNA COVID-19 vaccines. 2021. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html Available at: Accessed October 1, 2023.
- 3.Oster M.E., Shay D.K., Su J.R., et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327:331–340. doi: 10.1001/jama.2021.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mengesha B., Asenov A.G., Hirsh-Raccah B., Amir O., Pappo O., Asleh R. Severe acute myocarditis after the third (booster) dose of mRNA COVID-19 vaccination. Vaccines (Basel) 2022;10:575. doi: 10.3390/vaccines10040575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang D.-H., Na J.-Y., Yang J.-H., et al. Fulminant giant cell myocarditis following heterologous vaccination of ChAdOx1 nCoV-19 and Pfizer-BioNTech COVID-19. Medicina (Kaunas) 2022;58:449. doi: 10.3390/medicina58030449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sung K., McCain J., King K.R., et al. Biopsy-proven giant cell myocarditis following the COVID-19 vaccine. Circ Heart Fail. 2022;15 doi: 10.1161/CIRCHEARTFAILURE.121.009321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berthelot-Richer M., O'Connor K., Bernier M., et al. When should we consider the diagnosis of giant cell myocarditis? Revisiting “classic” echocardiographic and clinical features of this rare pathology. Exp Clin Transplant. 2014;12:565–568. doi: 10.6002/ect.2013.0218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.