Abstract
Background
Different progressions or prognoses of chronic obstructive pulmonary disease (COPD) have been reported according to structural abnormalities based on chest computed tomography (CT). This study aimed to investigate whether different structural abnormalities independently affect annual lung function changes and clinical prognosis in patients with COPD.
Methods
This longitudinal multicenter observational study was conducted using the KOCOSS cohort (NCT02800499) database in Korea from January 2012 to December 2019. For COPD patients with chest CT findings at baseline enrolment and longitudinal spirometric data, annual forced expiratory volume in 1 s (FEV1) decline rate (mL/year) and clinical outcomes were compared according to structural abnormalities, including emphysema, bronchiectasis (BE), and tuberculosis-destroyed lung (TDL). We estimated the adjusted annual FEV1 changes using a mixed-effect linear regression model.
Results
Among the enrolled 237 patients, 152 showed structural abnormalities. Emphysema, BE, and TDL were observed in 119 (78.3%), 28 (18.4%), and 27 (17.8%) patients, respectively. The annual decline in FEV1 was faster in COPD patients with structural abnormalities than those without (β = −70.6 mL/year, P-value = 0.039). BE/TDL-dominant or emphysema-dominant structural abnormality contributed to an accelerated annual FEV1 decline compared to no structural abnormality (BE/TDL-dominant, β = −103.7 mL/year, P-value = 0.043; emphysema-dominant, β = −84.1 mL/year, P-value = 0.018). Structural abnormalities made no significant differences in acute exacerbation rate and mortality.
Conclusion
The lung function decline rate in COPD differed according to structural abnormalities on CT. These findings may suggest that more focus should be placed on earlier intervention or regular follow-up with spirometry in COPD patients with BE or TDL on chest CT.
Keywords: Forced expiratory volume, Respiratory function tests, Bronchiectasis, Pulmonary emphysema, Pulmonary disease, Chronic obstructive, Cohort studies
1. Introduction
1.1. Background
Chronic obstructive pulmonary disease (COPD) is characterized by irreversible airflow limitation, causing progressive loss of lung function, reduced quality of life, and increased risk of acute exacerbation and mortality [1]. The clinical features of COPD often vary because the diagnosis is mainly based on lung function impairment, regardless of the cause or mechanism of disease development. Indeed, different lung function decline rates, response to treatment, and clinical prognosis have been reported in patients with COPD [2,3]. Recently, several subgroups based on clinical phenotypes have been identified in COPD patients. A personalized approach based on different COPD subtypes is now suggested to achieve a better response to treatment [4].
Previous studies have reported different progression or prognosis of COPD according to structural abnormalities, based on high-resolution chest computed tomography (CT) [5,6]. The increase in bronchial wall area or thickness is associated with a higher risk of acute exacerbation [7] and mortality [8], and reduced lung function [9]. The increase in bronchial wall area or thickness is associated with bronchiectasis due to the chronic inflammatory processes that contribute to structural changes in the airways. A history of tuberculosis is considered one of the main causes of bronchiectasis [10]. Bronchiectasis is significantly associated with poor prognosis in COPD patients [11]. Another structural abnormality in COPD is emphysema, resulting from alveolar and parenchymal destruction due to an imbalance between proteases and antiproteases [12]. There were differences in lung function changes [13] and mortality [8] according to the pattern and extent of emphysema in patients with COPD. However, there remains insufficient evidence to classify COPD patients with structural abnormalities into distinct subtypes. There are limited longitudinal studies on the differences in lung function decline rate and clinical prognosis according to different structural abnormalities in patients with COPD.
Therefore, we aimed to investigate whether different structural abnormalities independently affect annual lung function changes and clinical prognosis in patients with COPD.
2. Material and methods
Our study followed the guidelines of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [14].
2.1. Study design and eligibility criteria
This retrospective longitudinal study was performed using the Korea COPD Subgroup Study (KOCOSS) cohort database (NCT02800499), which prospectively enrolled patients diagnosed with COPD from January 2012 to December 2019 at 54 hospitals in South Korea. Detailed information on the methodology of the KOCOSS was described in a previous study [15]. COPD was diagnosed based on the spirometric criteria in the Global Initiative for Chronic Obstructive Lung Disease reports, which is a post-bronchodilator annual forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratio <0.7 [1]. The eligibility criteria were as follows: 1) age ≥40 years, 2) available information on baseline chest CT scan, 3) spirometric evaluation at baseline examination, and 4) follow-up with spirometric examination for 3 years.
2.2. Baseline information and lung function
In the baseline assessment, we obtained the demographic information of the eligible subjects, including age, sex, body mass index (BMI), smoking status, Charlson comorbidity index, and previous history of asthma. We acquired information on baseline symptoms or quality of life, including the COPD assessment test (CAT) score, St George's Respiratory Questionnaire for COPD Patients (SGRQ-C) score, and 6-min walking distance. We identified a history of acute exacerbations, including total and severe exacerbations.
Baseline spirometric information was obtained, including post-bronchodilator FEV1 and FVC (mL and % of the predicted value), diffusing capacity for carbon monoxide (DLCO) (%), DLCO/alveolar volume (VA) (%), and total lung capacity (mL and % of the predicted value). Regarding radiologic evaluation, we investigated structural abnormalities, including emphysema, bronchiectasis, and tuberculosis-destroyed lung (TDL), on chest CT. The structural abnormalities were identified based on the radiologist's reports.
2.3. Study outcomes
The primary outcome was the annual change in post-bronchodilator FEV1 (mL/year) according to different structural abnormalities. The secondary outcome was the comparison between the 3-year total number of moderate-to-severe exacerbations, the annual rate of moderate-to-severe exacerbations, and mortality events according to different structural abnormalities.
2.4. Patient groups
Eligible patients were classified into two groups according to the radiological structural abnormalities on chest CT. Structural abnormalities on chest CT include bronchiectasis, TDL, and emphysema. TDL is commonly accompanied by bronchiectasis, and it is often difficult to clearly distinguish which is predominant between TDL and bronchiectasis in clinical practice. Therefore, the radiologic features of bronchiectasis or TDL were grouped together and defined as bronchiectasis or TDL (BE/TDL)-dominant structural abnormalities. The radiological features of emphysema were defined as emphysema-dominant structural abnormalities. Patients with both BE/TDL-dominant and emphysema-dominant structural abnormalities were excluded from the comparative analyses between TDL-dominant and emphysema-dominant structural abnormalities.
2.5. Statistical analyses
We used Student's t-test or Wilcoxon rank-sum test to compare continuous variables, and chi-square test or Fisher's exact test to compare categorical variables. We performed univariate and multivariate logistic regression analyses using the stepwise selection method to determine clinically important variables. We excluded variables with a variance inflation factor >4.0, which is considered significant multicollinearity. We calculated the annual changes in post-bronchodilator FEV1 (mL/year) at the individual level using the first and last follow-up spirometric results. We assessed the variables affecting repeated FEV1 measurements using a linear mixed-effect model. Statistical significance was set P-values <0.05. R statistical software, version 4.1.1 (R Core Team [2021], Vienna, Austria), was used for the statistical analyses.
2.6. Ethics
The Institutional Review Board at each hospital (Seoul Metropolitan Government-Seoul National University Boramae Medical Center IRB No. 06-2012-36) approved the study protocol. Written informed consent was obtained for all participants at enrolment. This study was conducted in accordance with the principles of the Declaration of Helsinki.
3. Results
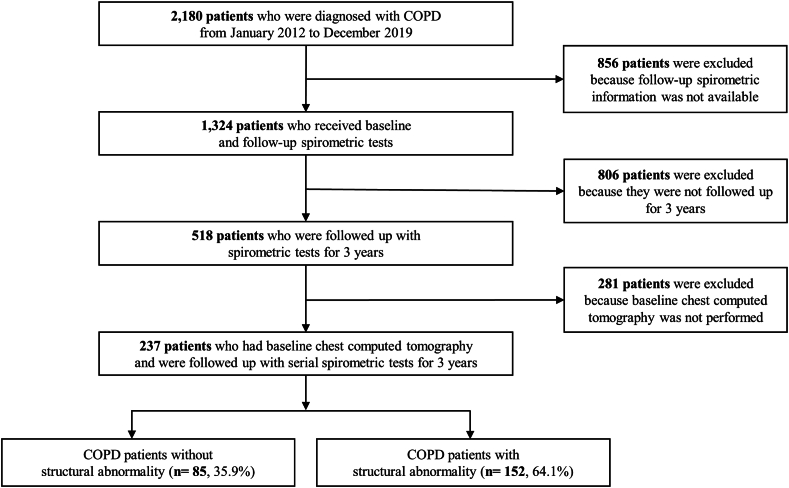
Among 2180 patients diagnosed with COPD, 1324 patients underwent one or more spirometry examinations. Of these, 518 patients were followed up for more than 3 years with spirometry, baseline chest CT was carried out for 237 patients. Eventually, the eligible 237 patients were classified into two groups: 85 without structural abnormalities and 152 with structural abnormalities (Fig. 1). Among patients with structural abnormalities, emphysema, bronchiectasis, and TDL were found in 119 (78.3 %), 28 (18.4%), and 27 (17.8%) patients, respectively.
Fig. 1.
Flow chart of inclusion for eligible patients BE/TDL, bronchiectasis or tuberculosis destroyed lung; COPD, chronic obstructive pulmonary disease.
3.1. Baseline characteristics and lung function
At baseline, more patients with structural abnormalities were men (Table 1). We found no significant difference in the symptomatic burden or exacerbation history between patients with and without structural abnormalities. In the baseline spirometric evaluation, FEV1 and DLCO did not differ according to structural abnormality. Whereas FEV1/FVC was lower, and FVC (L) and total lung capacity (TLC) (L) were higher in patients with structural abnormalities (Table 2).
Table 1.
Baseline demographic characteristics of COPD patients with and without structural abnormality.
| Without structural abnormality (n = 85) | With structural abnormality (n = 152) | P-value | |
|---|---|---|---|
| Age, year, mean (SD) | 68.0 (7.2) | 67.8 (7.1) | 0.819 |
| Age category, year, n (%) | |||
| 40-64 | 26 (30.6) | 54 (35.5) | 0.530 |
| 65-69 | 24 (28.2) | 28 (18.4) | 0.112 |
| 70-74 | 21 (24.7) | 40 (26.3) | 0.907 |
| ≥75 | 14 (16.5) | 30 (19.7) | 0.656 |
| Male, n (%) | 71 (83.5) | 142 (93.4) | 0.028 |
| Body mass index, kg/m2, mean (SD) | 23.4 (3.6) | 22.72 (3.5) | 0.167 |
| Smoking status, n (%) | |||
| Never smoker | 13 (15.3) | 10 (6.6) | 0.052 |
| Ex-smoker | 54 (63.5) | 109 (71.7) | 0.247 |
| Current smoker | 18 (21.2) | 33 (21.7) | 1.000 |
| CCI, category, n (%) | |||
| 0 | 34 (40.0) | 57 (37.5) | 1.000 |
| 1–2 (mild) | 28 (32.9) | 47 (30.9) | 0.197 |
| ≥3 (moderate to severe) | 23 (27.1) | 48 (31.6) | 0.566 |
| Previous history of asthma, n (%) | 38 (45.2) | 53 (35.6) | 0.189 |
| Symptoms and quality of life | |||
| CAT score, mean (SD) | 13.9 (7.8) | 15.1 (7.7) | 0.283 |
| ≥10, n (%) | 58 (68.2) | 110 (72.4) | 0.601 |
| SGRQ-C, mean (SD) | 32.6 (21.1) | 37.1 (20.6) | 0.112 |
| ≥25, n (%) | 48 (56.5) | 105 (69.1) | 0.071 |
| 6MWD, mean (SD) | 386 (105) | 388 (112) | 0.892 |
| Previous exacerbation history, n (%) | |||
| Moderate-to-severe | 10 (11.8) | 35 (23.0) | 0.051 |
| Severe | 5 (5.9) | 15 (9.9) | 0.415 |
Data are expressed as mean (±standard deviation) or number (percentage).
CAT, COPD assessment test; CCI, Charlson comorbidity index; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; SD, standard deviation; SGRQ-C, St. George's respiratory questionnaire for COPD patients; 6MWD, 6 min walking distance.
Table 2.
Baseline lung function of COPD patients with and without structural abnormality.
| Without structural abnormality (n = 85) | With structural abnormality (n = 152) | P-value | |
|---|---|---|---|
| Baseline lung function | |||
| Post-BDR FEV1, L, mean (SD) | 1.7 (0.6) | 1.7 (0.5) | 0.886 |
| Post-BDR FEV1, % of predicted value, mean (SD) | 59.5 (17.3) | 56.4 (16.6) | 0.174 |
| GOLD grade 1, n (%) | 12 (14.1) | 14 (9.2) | 0.346 |
| GOLD grade 2, n (%) | 46 (54.1) | 86 (56.6) | 0.819 |
| GOLD grade 3, n (%) | 25 (29.4) | 48 (31.6) | 0.835 |
| GOLD grade 4, n (%) | 2 (2.4) | 4 (2.6) | 1.000 |
| Post-BDR FVC, L, mean (SD) | 3.0 (0.8) | 3.2 (0.8) | 0.048 |
| Post-BDR FVC, % of predicted value, mean (SD) | 76.6 (13.8) | 78.3 (15.4) | 0.382 |
| <80%, n (%) | 52 (61.2) | 84 (55.3) | 0.456 |
| Post-BDR FEV1/FVC, %, mean (SD) | 54.5 (11.5) | 50.3 (11.5) | 0.007 |
| DLCO, % of predicted value, mean (SD) | 69.7 (19.4) | 64.3 (18.9) | 0.058 |
| DLCO/VA, % of predicted value, mean (SD) | 80.2 (20.6) | 76.0 (22.8) | 0.194 |
| TLC, L, mean (SD) | 5.3 (1.0) | 5.7 (0.8) | 0.005 |
| TLC, % of predicted value, mean (SD) | 96.2 (13.9) | 97.0 (13.7) | 0.744 |
Data are expressed as mean (±standard deviation) or number (percentage).
COPD, chronic obstructive pulmonary disease; DLCO, diffusing capacity for carbon monoxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; LAMA, long-acting muscarinic antagonist; Post-BDR, Post-bronchodilator; SD, standard deviation; TLC, total lung capacity; VA, alveolar volume.
After excluding patients with both BE/TDL-dominant and emphysema-dominant structural abnormalities, baseline characteristics and lung function were compared between COPD patients without structural abnormalities and those with BE/TDL-dominant or emphysema-dominant structural abnormalities (Supplementary information 1 and 2). Male sex, lower BMI, ever-smoker, and higher FVC (L) or TLC (L) were more likely to be found in those with emphysema-dominant structural abnormalities than those without structural abnormalities. We found that the SGRQ-C was higher in COPD patients with BE/TDL-dominant structural abnormalities than those without structural abnormalities.
In comparing BE/TDL-dominant and emphysema-dominant structural abnormalities, male and ever-smoker patients were more likely to have emphysema-dominant structural abnormalities (Supplementary Information 3). Meanwhile, a higher BMI and CAT score were found in COPD patients with BE/TDL-dominant structural abnormalities. In the comparison of clinical features, those with emphysema-dominant structural abnormalities showed a higher FVC (L) or TLC (L) (Supplementary Information 4).
3.2. Lung function change in the overall population
In all eligible COPD patients, the estimated median annual change in post-bronchodilator FEV1 was −38.3 (interquartile range (IQR) = −107.5–33.3] ml/year during 3 years of follow-up (Supplementary Information 5). We found that the annual FEV1 change was −30.0 (IQR = −90.0–50.0) mL/year in COPD patients without structural abnormality and −41.7 (IQR = −113.8–23.3) mL/year in those with structural abnormalities. In COPD patients with structural abnormality, we found that annual FEV1 change was −41.7 (IQR = −110.0–10.0) mL/year in BE/TDL-dominant structural abnormality and −40.0 (IQR = −109.2–28.3) mL/year in emphysema-dominant structural abnormality.
3.3. Lung function decline and clinical outcomes
In a multivariable analysis with a linear mixed model, FEV1 decline was significantly accelerated in COPD patients with structural abnormalities (beta-coefficient = −70.6 mL/year, P-value = 0.039, Table 3). Significant clinical factors related to an accelerated annual decline in FEV1 in COPD patients were older age, female sex, a higher CAT score, moderate-to-severe exacerbation history within the previous 1 year, and a lower baseline FVC. During the 3-year follow-up period, there was no significant difference in the 3-year total number of moderate-to-severe exacerbations, the annual rate of moderate-to-severe exacerbations, and mortality events between COPD patients with and without structural abnormalities (Supplementary Information 6). Our multivariable regression analyses found no difference in the risk of moderate-to-severe exacerbation or mortality between COPD patients with and without structural abnormalities (Supplementary Information 7 and 8).
Table 3.
Adjusted effect of clinical factors contributing to the annual FEV1 change in COPD patients.
| Annual FEV1 change, mL/year |
P-value | ||
|---|---|---|---|
| Beta-coefficient | Standard error | ||
| Age | −16.73 | 2.29 | <0.001 |
| Sex, female | −458.15 | 70.29 | <0.001 |
| Current smoker | −23.637 | 72.47 | 0.744 |
| CCI ≥3 | 124.1 | 39.08 | 0.001 |
| CAT score | −6.45 | 2.10 | 0.002 |
| Moderate-to-severe exacerbation history within previous 1 year | −238.95 | 41.50 | <0.001 |
| Baseline FVC, % of predicted value | 17.15 | 1.12 | <0.001 |
| Structural abnormality on chest CT | −70.59 | 34.08 | 0.039 |
The results of multivariable linear mixed effect model were summarized as slope estimate %/year (standard error).
BMI and baseline FEV1 were not included for multivariable analysis because of multicollinearity with structural abnormality.
CAT, COPD assessment test; CCI, Charlson comorbidity index; COPD, chronic obstructive pulmonary disease; CT, computed tomography; FVC, forced vital capacity.
3.4. Lung function change according to structural abnormality
COPD patients with BE/TDL-dominant structural abnormality showed an accelerated annual FEV1 decline compared to those without structural abnormality (beta-coefficient = −103.7 mL/year, P-value = 0.043, Table 4). In addition, the annual FEV1 decline rate was worse in those with emphysema-dominant structural abnormalities than in COPD patients without structural abnormalities (beta-coefficient = −84.1 mL/year, P-value = 0.018, Table 5). There was no significant difference in the 3-year total number of moderate-to-severe exacerbations, the annual rate of moderate-to-severe exacerbations, and mortality events between COPD patients without structural abnormalities and those with BE/TDL-dominant or emphysema-dominant structural abnormalities (Supplementary Information 9). Our multivariable regression analyses found that the risk of moderate-to-severe exacerbation or mortality was not significantly higher in COPD patients with BE/TDL-dominant or emphysema-dominant structural abnormalities than those without structural abnormalities (Supplementary Information 10 and 11).
Table 4.
Effect of BE/TDL-dominant structural abnormality contributing to the annual FEV1 change compared to no structural abnormality.
| Annual FEV1 change, mL/year |
P-value | ||
|---|---|---|---|
| Beta-coefficient | Standard error | ||
| Age | −21.6 | 3.26 | <0.001 |
| Sex, female | −496.74 | 78.54 | <0.001 |
| BMI | 13.53 | 7.00 | 0.054 |
| Current smoker | −91.00 | 84.97 | 0.285 |
| CAT score | 5.21 | 2.97 | 0.080 |
| Moderate-to-severe exacerbation history within previous 1 year | −179.06 | 67.20 | 0.008 |
| Baseline FVC, % of predicted value | 15.85 | 1.71 | <0.001 |
| DLCO, % of predicted value | 10.17 | 1.40 | <0.001 |
| BE/TDL-structural abnormality (reference: no structural abnormality) | −103.65 | 50.91 | 0.043 |
The results of multivariable linear mixed effect model were summarized as slope estimate %/year (standard error).
Baseline FEV1 were not included for multivariable analysis because of multicollinearity with structural abnormality.
BE/TDL, bronchiectasis or tuberculosis destroyed lung; BMI, body mass index; CAT, COPD assessment test; CCI, Charlson comorbidity index; COPD, chronic obstructive pulmonary disease; DLCO, diffusing capacity for carbon monoxide; FVC, forced vital capacity.
Table 5.
Effect of emphysema-dominant structural abnormality contributing to the annual FEV1 change compared to no structural abnormality.
| Annual FEV1 change, mL/year |
P-value | ||
|---|---|---|---|
| Beta-coefficient | Standard error | ||
| Age | −15.53 | 2.45 | <0.001 |
| Sex, female | −445.92 | 60.75 | <0.001 |
| Current smoker | 0.334 | 41.54 | 0.994 |
| CAT score | −11.44 | 2.24 | <0.001 |
| Moderate-to-severe exacerbation history within previous 1 year | −258.74 | 43.30 | <0.001 |
| Baseline FVC, % of predicted value | 18.34 | 1.18 | <0.001 |
| Emphysema-dominant structural abnormality (reference: no structural abnormality) | −84.13 | 35.53 | 0.018 |
The results of multivariable linear mixed effect model were summarized as slope estimate %/year (standard error).
BMI and baseline FEV1 were not included for multivariable analysis because of multicollinearity with structural abnormality.
CAT, COPD assessment test; COPD, chronic obstructive pulmonary disease; FVC, forced vital capacity.
Among COPD patients with structural abnormalities, there was no significant difference in the annual FEV1 decline between BE/TDL-dominant structural abnormalities and emphysema-dominant structural abnormalities (Supplementary Information 12). Significant clinical factors related to an accelerated annual decline in FEV1 in COPD patients with structural abnormalities were older age, female sex, lower BMI, higher CAT score, and higher baseline FVC. During the 3-year follow-up period, there was no significant difference in the 3-year total number of moderate-to-severe exacerbations, the annual rate of moderate-to-severe exacerbations, and mortality events between BE/TDL-dominant and emphysema-dominant structural abnormalities (Supplementary Information 13).
4. Discussion
Our study found that structural abnormalities were significantly associated with lung function changes, but did not affect clinical prognosis in patients with COPD. Patients with structural abnormalities had a faster annual FEV1 decline rate during the 3-year observation period, even after adjusting for clinically important variables. Patients with BE/TDL-dominant or emphysema-dominant structural abnormalities showed an accelerated FEV1 decline compared to those without structural abnormalities. We found no significant difference in annual FEV1 changes between the BE/TDL-dominant and emphysema-dominant structural abnormalities. The morphological features of chest CT need to be considered as a clinical subtype related to longitudinal lung function changes in COPD patients.
Radiologic subtyping of structural abnormalities is related to the pathophysiology of structural changes in COPD. Visually defined radiological findings in COPD patients can be divided into two subtypes: airway-predominant disease and emphysema-predominant disease [6,16]. Pathological mechanisms of chronic bronchitis include infiltration of mononuclear cells and influx of neutrophils in the airway, which produce airway inflammation and cause mucus hypersecretion [17]. In emphysema, the lung parenchyma is destroyed by apoptosis of endothelial or epithelial cells and a dysregulated protease-antiprotease balance [17,18]. Subtyping using chest CT can provide information on clinical features and may help predict treatment responses or clinical outcomes [6,19]. In our study, COPD patients with BE/TDL or emphysema showed a more rapid FEV1 decline than those without structural abnormalities. Considering these structural abnormalities, including emphysema, bronchiectasis, and TDL, are increasingly detected in clinical practice with the increasing use of chest CT for lung cancer screening, doctors may find and treat more COPD patients at an early stage.
Bronchiectasis can lead to chronic and indolent airway inflammatory progression via a vicious cycle [20]. Although it has not yet been elucidated whether bronchiectasis causes COPD, it aggravates airflow limitation and the clinical course in COPD patients [21]. Neutrophil elastase activity may be related to an accelerated decrease in FEV1 in patients with bronchiectasis [22]. A recent report showed that brensocatib that inhibits neutrophil serine protease activity significantly reduces exacerbation risk and is potentially beneficial in preserving lung function [23]. In addition, clinical factors, such as Pseudomonas aeruginosa colonization, exacerbation events, and systemic inflammation, can further accelerate lung function decline in patients with bronchiectasis [24]. Considering the faster rate of lung function decline, further studies on appropriate therapeutic strategies to preserve lung function in COPD patients with bronchiectasis are required.
TDL is radiologically diagnosed as a destructive lung parenchymal sequela due to pulmonary tuberculosis. Many studies have reported lung function impairment after treatment for pulmonary tuberculosis [25]. TDL causes chronic respiratory symptoms, accelerated lung function decline, and more exacerbations [26,27]. Although the mechanism of airflow limitation by TDL is poorly understood, multiple factors, including host immune response, genetic predisposition, comorbidities, and environmental exposure, may affect lung function decline in patients with TDL [25]. Many studies have reported a higher risk of COPD development in patients with TDL [[28], [29], [30]]. Our study showed that TDL was found in 18% of patients and was related to accelerated FEV1 decline in COPD patients. Therefore, COPD patients with TDL need to be considered a high-risk group for rapid lung function decline.
The relationship between emphysema and rapid decline in lung function has been reported in well-designed studies [5,13,31,32]. Dysregulated host immune responses, including protease and antiprotease imbalance, inhibition of histone deacetylase, excessive airway inflammation, and oxidative stress, can contribute to the development and progression of emphysema [12]. Inflammatory and mechanical factors involved in the pathogenesis of emphysema can worsen lung function in patients [33]. Lung function decline is related to emphysema severity in patients with COPD [31]. Recently, the concept of pre-COPD has been proposed, which includes patients with emphysema and normal lung function because airflow limitation progresses in this population [34]. In our study, even after adjusting for smoking status and exacerbation history, emphysema was significantly correlated with accelerated FEV1 decline. This finding suggests that host factors are related to an accelerated lung function decline in emphysema. Therefore, it is necessary to investigate genetic factors related to various host responses to airway inflammation or parenchymal destruction caused by noxious exposure or infection.
In our study, structural abnormalities did not impact on prognosis. There seems several reasons for the negative results. First, while structural abnormalities contribute to the deterioration of chronic airway inflammation or the progression of COPD, their direct association with exacerbation or mortality may not be clinically evident. Indeed, our findings align with recent studies that demonstrated no correlation between BE or TDL and the severity of acute exacerbations in COPD [35]. Furthermore, emphysema also showed no association with moderate to severe or severe exacerbation of COPD in multivariable analysis [36]. Second, in our study, there was a small number of exacerbation and mortality events, which might have resulted in insufficient statistical power. Third, our study had a relatively short observation period of 3 years, which would be insufficient duration for evaluating mortality. Fourth, exacerbations may be influenced more by factors beyond structural abnormalities, such as previous exacerbation history, symptom severity, and treatment.
Our study had several limitations. First, it was a retrospective study with a small number of patients. Our results need to be validated in a larger cohort of patients with COPD. Second, bronchiectasis and emphysema have heterogeneous features; however, our study did not quantify or qualify their extent, severity, and pattern. We classified the included patients according to the presence or absence of bronchiectasis and emphysema, as described in the radiologist's report. Currently, image data are being collected for further research. Third, lung function decline rate is affected by various factors other than structural abnormalities, such as health behavior, air pollution, and second-hand smoking. Even if the FEV1 change is adjusted based on available clinical information, our results should be carefully interpreted.
In conclusion, the lung function decline rate was faster in COPD patients with BE/TDL-dominant or emphysema-dominant structural abnormalities than in those without. An individualized approach for preserving lung function in COPD patients with structural abnormalities should be investigated further.
Ethics approval and consent to participate
The Institutional Review Board at each hospital (Seoul Metropolitan Government-Seoul National University Boramae Medical Center IRB No. 06-2012-36) approved the study protocol. Written informed consent was obtained for all participants at enrolment. This study was conducted in accordance with the principles of the Declaration of Helsinki.
Consent for publication
Not applicable.
Availability of data and materials
The data that support the findings of this study are available from the KOrea COpd Subgroup Study (KOCOSS) team but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the KOCOSS team.
Funding
This work was supported by the Research Program funded Korea National Institute of Health (Fund CODE 2016ER670100, 2016ER670101,2016ER670102, 2018ER67100,2018ER67101,2018ER67102, 2021ER120500, and 2021ER120501).
CRediT authorship contribution statement
Hyun Woo Lee: Writing – original draft, Visualization, Software, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Jung-Kyu Lee: Writing – review & editing, Resources, Data curation. Youlim Kim: Writing – review & editing, Resources, Data curation. An-Soo Jang: Writing – review & editing, Resources, Data curation. Yong il Hwang: Writing – review & editing, Resources, Data curation. Jae Ha Lee: Writing – review & editing, Resources, Data curation. Ki-Suck Jung: Writing – review & editing, Resources, Funding acquisition, Data curation. Kwang Ha Yoo: Writing – review & editing, Resources, Funding acquisition, Data curation. Hyoung Kyu Yoon: Writing – review & editing, Resources, Data curation. Deog Kyeom Kim: Writing – review & editing, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Kwang Ha Yoo reports financial support was provided by Korea National Institute of Health. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e27683.
List of Abbreviations
- BE/TDL
bronchiectasis or tdl
- BMI
body mass index
- CAT
COPD assessment test
- COPD
chronic obstructive pulmonary disease
- CT
computed tomography
- DLCO
diffusing capacity for carbon monoxide
- FEV1
forced expiratory volume in 1 s
- FVC
forced vital capacity
- IQR
interquartile range
- SGRQ-C
st george's respiratory questionnaire for COPD patients
- STROBE
strengthening the reporting of observational studies in epidemiology
- TDL
tuberculosis-destroyed lung; VA, alveolar volume
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Global Initiative For Chronic Obstructive Lung Disease . 2021. Global Strategy for Prevention, Diagnosis and Management of Chronic Obstructive Pulmonary Disease (2021 Report. [Google Scholar]
- 2.Castaldi P.J., Benet M., Petersen H., Rafaels N., Finigan J., Paoletti M., Marike Boezen H., Vonk J.M., Bowler R., Pistolesi M., Puhan M.A., Anto J., Wauters E., Lambrechts D., Janssens W., Bigazzi F., Camiciottoli G., Cho M.H., Hersh C.P., Barnes K., Rennard S., Boorgula M.P., Dy J., Hansel N.N., Crapo J.D., Tesfaigzi Y., Agusti A., Silverman E.K., Garcia-Aymerich J. Do COPD subtypes really exist? COPD heterogeneity and clustering in 10 independent cohorts. Thorax. 2017;72(11):998–1006. doi: 10.1136/thoraxjnl-2016-209846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee H.W., Lee J.K., Lee M.G., Shin K.C., Ra S.W., Kim T.H., Hwang Y.I., Jung K.S., Yoo K.H., Kim D.K. Risk factors of rapid FEV1 decline in a real-world chronic obstructive pulmonary disease cohort. Respiration. 2022;101(12):1078–1087. doi: 10.1159/000525871. [DOI] [PubMed] [Google Scholar]
- 4.Singh D., Miravitlles M., Vogelmeier C. Chronic obstructive pulmonary disease individualized therapy: tailored approach to symptom management. Adv. Ther. 2017;34(2):281–299. doi: 10.1007/s12325-016-0459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park J., Hobbs B.D., Crapo J.D., Make B.J., Regan E.A., Humphries S., Carey V.J., Lynch D.A., Silverman E.K. Subtyping COPD by using visual and quantitative CT imaging features. Chest. 2020;157(1):47–60. doi: 10.1016/j.chest.2019.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benlala I., Laurent F., Dournes G. Structural and functional changes in COPD: what we have learned from imaging. Respirology. 2021;26(8):731–741. doi: 10.1111/resp.14047. [DOI] [PubMed] [Google Scholar]
- 7.Han M.K., Kazerooni E.A., Lynch D.A., Liu L.X., Murray S., Curtis J.L., Criner G.J., Kim V., Bowler R.P., Hanania N.A., Anzueto A.R., Make B.J., Hokanson J.E., Crapo J.D., Silverman E.K., Martinez F.J., Washko G.R. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011;261(1):274–282. doi: 10.1148/radiol.11110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johannessen A., Skorge T.D., Bottai M., Grydeland T.B., Nilsen R.M., Coxson H., Dirksen A., Omenaas E., Gulsvik A., Bakke P. Mortality by level of emphysema and airway wall thickness. Am. J. Respir. Crit. Care Med. 2013;187(6):602–608. doi: 10.1164/rccm.201209-1722OC. [DOI] [PubMed] [Google Scholar]
- 9.Nakano Y., Muro S., Sakai H., Hirai T., Chin K., Tsukino M., Nishimura K., Itoh H., Paré P.D., Hogg J.C., Mishima M. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am. J. Respir. Crit. Care Med. 2000;162(3 Pt 1):1102–1108. doi: 10.1164/ajrccm.162.3.9907120. [DOI] [PubMed] [Google Scholar]
- 10.Palwatwichai A., Chaoprasong C., Vattanathum A., Wongsa A., Jatakanon A. Clinical, laboratory findings and microbiologic characterization of bronchiectasis in Thai patients. Respirology. 2002;7(1):63–66. doi: 10.1046/j.1440-1843.2002.00367.x. [DOI] [PubMed] [Google Scholar]
- 11.Martínez-García M.A., de la Rosa Carrillo D., Soler-Cataluña J.J., Donat-Sanz Y., Serra P.C., Lerma M.A., Ballestín J., Sánchez I.V., Selma Ferrer M.J., Dalfo A.R., Valdecillos M.B. Prognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013;187(8):823–831. doi: 10.1164/rccm.201208-1518OC. [DOI] [PubMed] [Google Scholar]
- 12.Sharafkhaneh A., Hanania N.A., Kim V. Pathogenesis of emphysema: from the bench to the bedside. Proc. Am. Thorac. Soc. 2008;5(4):475–477. doi: 10.1513/pats.200708-126ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vestbo J., Edwards L.D., Scanlon P.D., Yates J.C., Agusti A., Bakke P., Calverley P.M., Celli B., Coxson H.O., Crim C., Lomas D.A., MacNee W., Miller B.E., Silverman E.K., Tal-Singer R., Wouters E., Rennard S.I. Changes in forced expiratory volume in 1 second over time in COPD. N. Engl. J. Med. 2011;365(13):1184–1192. doi: 10.1056/NEJMoa1105482. [DOI] [PubMed] [Google Scholar]
- 14.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet (London, England) 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 15.Lee J.Y., Chon G.R., Rhee C.K., Kim D.K., Yoon H.K., Lee J.H., Yoo K.H., Lee S.H., Lee S.Y., Kim T.E., Kim T.H., Park Y.B., Hwang Y.I., Kim Y.S., Jung K.S. Characteristics of patients with chronic obstructive pulmonary disease at the first visit to a pulmonary medical center in Korea: the KOrea COpd subgroup study team cohort. J. Kor. Med. Sci. 2016;31(4):553–560. doi: 10.3346/jkms.2016.31.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subramanian D.R., Gupta S., Burggraf D., Vom Silberberg S.J., Heimbeck I., Heiss-Neumann M.S., Haeussinger K., Newby C., Hargadon B., Raj V., Singh D., Kolsum U., Hofer T.P., Al-Shair K., Luetzen N., Prasse A., Müller-Quernheim J., Benea G., Leprotti S., Boschetto P., Gorecka D., Nowinski A., Oniszh K., Castell W.Z., Hagen M., Barta I., Döme B., Strausz J., Greulich T., Vogelmeier C., Koczulla A.R., Gut I., Hohlfeld J., Welte T., Lavae-Mokhtari M., Ziegler-Heitbrock L., Brightling C., Parr D.G. Emphysema- and airway-dominant COPD phenotypes defined by standardised quantitative computed tomography. Eur. Respir. J. 2016;48(1):92–103. doi: 10.1183/13993003.01878-2015. [DOI] [PubMed] [Google Scholar]
- 17.Bourdin A., Burgel P.R., Chanez P., Garcia G., Perez T., Roche N. Recent advances in COPD: pathophysiology, respiratory physiology and clinical aspects, including comorbidities, European respiratory review. an official journal of the European Respiratory Society. 2009;18(114):198–212. doi: 10.1183/09059180.00005509. [DOI] [PubMed] [Google Scholar]
- 18.Kim W.D., Chi H.S., Choe K.H., Kim W.S., Hogg J.C., Sin D.D. The role of Granzyme B Containing cells in the progression of chronic obstructive pulmonary disease. Tuberc. Respir. Dis. 2020;83(Supple 1):S25–s33. doi: 10.4046/trd.2020.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitaguchi Y., Fujimoto K., Kubo K., Honda T. Characteristics of COPD phenotypes classified according to the findings of HRCT. Respir. Med. 2006;100(10):1742–1752. doi: 10.1016/j.rmed.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 20.King P.T. The pathophysiology of bronchiectasis. Int. J. Chronic Obstr. Pulm. Dis. 2009;4:411–419. doi: 10.2147/copd.s6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martínez-García M., Soler-Cataluña J.J., Donat Sanz Y., Catalán Serra P., Agramunt Lerma M., Ballestín Vicente J., Perpiñá-Tordera M. Factors associated with bronchiectasis in patients with COPD. Chest. 2011;140(5):1130–1137. doi: 10.1378/chest.10-1758. [DOI] [PubMed] [Google Scholar]
- 22.Chalmers J.D., Moffitt K.L., Suarez-Cuartin G., Sibila O., Finch S., Furrie E., Dicker A., Wrobel K., Elborn J.S., Walker B., Martin S.L., Marshall S.E., Huang J.T., Fardon T.C. Neutrophil elastase activity is associated with exacerbations and lung function decline in bronchiectasis. Am. J. Respir. Crit. Care Med. 2017;195(10):1384–1393. doi: 10.1164/rccm.201605-1027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chalmers J.D., Haworth C.S., Metersky M.L., Loebinger M.R., Blasi F., Sibila O., O'Donnell A.E., Sullivan E.J., Mange K.C., Fernandez C., Zou J., Daley C.L. Phase 2 Trial of the DPP-1 inhibitor brensocatib in bronchiectasis. N. Engl. J. Med. 2020;383(22):2127–2137. doi: 10.1056/NEJMoa2021713. [DOI] [PubMed] [Google Scholar]
- 24.Martínez-García M.A., Soler-Cataluña J.J., Perpiñá-Tordera M., Román-Sánchez P., Soriano J. Factors associated with lung function decline in adult patients with stable non-cystic fibrosis bronchiectasis. Chest. 2007;132(5):1565–1572. doi: 10.1378/chest.07-0490. [DOI] [PubMed] [Google Scholar]
- 25.Ravimohan S., Kornfeld H., Weissman D., Bisson G.P. Tuberculosis and lung damage: from epidemiology to pathophysiology. Eur. Respir. Rev. : an official journal of the European Respiratory Society. 2018;27(147) doi: 10.1183/16000617.0077-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meghji J., Lesosky M., Joekes E., Banda P., Rylance J., Gordon S., Jacob J., Zonderland H., MacPherson P., Corbett E.L., Mortimer K., Squire S.B. Patient outcomes associated with post-tuberculosis lung damage in Malawi: a prospective cohort study. Thorax. 2020;75(3):269–278. doi: 10.1136/thoraxjnl-2019-213808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhee C.K., Yoo K.H., Lee J.H., Park M.J., Kim W.J., Park Y.B., Hwang Y.I., Kim Y.S., Jung J.Y., Moon J.Y., Rhee Y.K., Park H.K., Lim J.H., Park H.Y., Lee S.W., Kim Y.H., Lee S.H., Yoon H.K., Kim J.W., Kim J.S., Kim Y.K., Oh Y.M., Lee S.D., Kim H.J. Clinical characteristics of patients with tuberculosis-destroyed lung. Int. J. Tubercul. Lung Dis. : the official journal of the International Union against Tuberculosis and Lung Disease. 2013;17(1):67–75. doi: 10.5588/ijtld.12.0351. [DOI] [PubMed] [Google Scholar]
- 28.Byrne A.L., Marais B.J., Mitnick C.D., Lecca L., Marks G.B. Tuberculosis and chronic respiratory disease: a systematic review. Int. J. Infect. Dis. : IJID : official publication of the International Society for Infectious Diseases. 2015;32:138–146. doi: 10.1016/j.ijid.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 29.Menezes A.M., Hallal P.C., Perez-Padilla R., Jardim J.R., Muiño A., Lopez M.V., Valdivia G., Montes de Oca M., Talamo C., Pertuze J., Victora C.G. Tuberculosis and airflow obstruction: evidence from the PLATINO study in Latin America. Eur. Respir. J. 2007;30(6):1180–1185. doi: 10.1183/09031936.00083507. [DOI] [PubMed] [Google Scholar]
- 30.Lee C.H., Lee M.C., Lin H.H., Shu C.C., Wang J.Y., Lee L.N., Chao K.M. Pulmonary tuberculosis and delay in anti-tuberculous treatment are important risk factors for chronic obstructive pulmonary disease. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishimura M., Makita H., Nagai K., Konno S., Nasuhara Y., Hasegawa M., Shimizu K., Betsuyaku T., Ito Y.M., Fuke S., Igarashi T., Akiyama Y., Ogura S. Annual change in pulmonary function and clinical phenotype in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2012;185(1):44–52. doi: 10.1164/rccm.201106-0992OC. [DOI] [PubMed] [Google Scholar]
- 32.Mohamed Hoesein F.A., de Hoop B., Zanen P., Gietema H., Kruitwagen C.L., van Ginneken B., Isgum I., Mol C., van Klaveren R.J., Dijkstra A.E., Groen H.J., Boezen H.M., Postma D.S., Prokop M., Lammers J.W. CT-quantified emphysema in male heavy smokers: association with lung function decline. Thorax. 2011;66(9):782–787. doi: 10.1136/thx.2010.145995. [DOI] [PubMed] [Google Scholar]
- 33.Suki B., Lutchen K.R., Ingenito E.P. On the progressive nature of emphysema: roles of proteases, inflammation, and mechanical forces. Am. J. Respir. Crit. Care Med. 2003;168(5):516–521. doi: 10.1164/rccm.200208-908PP. [DOI] [PubMed] [Google Scholar]
- 34.Han M.K., Agusti A., Celli B.R., Criner G.J., Halpin D.M.G., Roche N., Papi A., Stockley R.A., Wedzicha J., Vogelmeier C.F. From GOLD 0 to pre-COPD. Am. J. Respir. Crit. Care Med. 2021;203(4):414–423. doi: 10.1164/rccm.202008-3328PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee H.J., Lee J.K., Park T.Y., Heo E.Y., Kim D.K., Lee H.W. Validation of the Rome proposal for severity of acute exacerbation of chronic obstructive pulmonary disease. Ther. Adv. Respir. Dis. 2023;17 doi: 10.1177/17534666231172917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park H., Lee H.J., Lee J.K., Park T.Y., Jin K.N., Heo E.Y., Kim D.K., Lee H.W. Diffusing capacity as an independent predictor of acute exacerbations in chronic obstructive pulmonary disease. Sci. Rep. 2024 doi: 10.1038/s41598-024-51593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the KOrea COpd Subgroup Study (KOCOSS) team but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the KOCOSS team.