Abstract
The use of hemp as a forage source in livestock diets has been less studied because bioactive residues in animal tissues may pose a risk to consumers. This study investigated the effects of partial substitution of alfalfa hay (AH) with hemp forage (HF) in growing goat diets on growth performance, carcass traits, ruminal fermentation characteristics, rumen microbial communities, blood biochemistry, and antioxidant indices. Forty Xiangdong black goats with body weight (BW) 7.82 ± 0.57 kg (mean ± SD) were grouped by BW and randomly assigned into one of the four treatment diets (n = 10/treatment) in a completely randomized design. The goats were fed ad libitum total mixed rations containing 60% forage and 40% concentrate (DM basis). The diets included control (CON; 60% AH and 40% concentrate), 55% AH and 5% HF (HF5), 50% AH and 10% HF (HF10), and 40% AH and 20% HF (HF20). Increasing the substitution of HF for AH linearly decreased (P < 0.01) DM intake and improved feed conversion efficiency. However, final BW, average daily gain, carcass traits, meat quality, and most blood biochemistry indices did not differ among treatments. The ruminal NH3-N concentration and blood urine nitrogen linearly increased (P < 0.01) with increasing substitution rate of HF, whereas the total volatile fatty acids concentration quadratically changed (P < 0.01). Substitution of AH with HF had no effect on the diversity and richness of ruminal microbes, though it linearly decreased (P = 0.040) Prevotella_1 and linearly increased (P = 0.017) Rikenellaceae_RC9_gut_group. The cannabinoids and/or their metabolites were detected in both ruminal filtrates (8) and plasma (4), however, no detectable cannabinoid-related residues were observed in meat. These results indicate that the HF could be used to partially substitute AH in goat diets, whereas the effects vary between substitution rates of HF for AH. Although no cannabinoid-related residues were detected in meat, the presence of cannabinoids residues in blood warrants further study of HF feeding to confirm the cannabinoids residues are not present in the animal products.
Keywords: Alfalfa hay, Hemp forage, Growth performance, Ruminal microbiota, Cannabinoid metabolism, Goat
1. Introduction
Hemp (Cannabis sativa L.) is one of the most widely cultivated plants throughout world history (Bailoni et al., 2021; Schluttenhofer and Yuan, 2017). However, hemp production was once restricted in North America and Europe due to the compound Δ9-tetrahydrocannabinol (THC) and its ability to cause intoxication in humans (EFSA, 2011; Klir et al., 2019). Currently, new varieties of hemp characterized by their low contents (<0.3%) of THC have been bred, known as industrial hemp to distinguish them from marijuana (EFSA, 2011; Schluttenhofer and Yuan, 2017). Hemp is traditionally grown for either seed or fiber, while it now is considered a multifaceted plant as it has been widely used in various areas including industry, medicine and nutrition (Iannaccone et al., 2019; Semwogerere et al., 2020). Hemp has potential applications in the textile industry due to its high fiber contents, while its natural antioxidants and other bioactive components (peptides, phenolic compounds, non-intoxicating phytocannabinoids, terpenes, and phytosterols; Jin et al., 2020) given it applications in the production of medicines and functional foods. Currently, the hemp industry is experiencing a rapid growth creating the need to expand the development of hemp products, and it is important to deepen our understanding of the role of the functional bioactive components in hemp.
Hempseed and hempseed cakes, which are rich in oil and protein and free of THC, have received substantial attention and are widely utilized in both meat and dairy ruminant diets (Bailoni et al., 2021; Ianni et al., 2020; Klir et al., 2019). For instance, partially replacing soybean meal or extruded soybean with hempseed cake in goat diets had no negative effects on milk production and blood parameters (Salavardic et al., 2021), so it reduced the feed costs. In addition, supplementation of hempseed positively affected the energy production pathway in lactating ewes and improved animal resistance to adverse climatic conditions such as low temperatures (Iannaccone et al., 2019). However, the use of hemp as a forage source in livestock animal diets are less studied (Semwogerere et al., 2020), mainly because the concerns on the presence of bioactive residues in animal tissues may pose risks to consumers, especially THC that can have psychoactive effects on humans (EFSA, 2011; Kleinhenz et al., 2020a). Due to their lipophilic character, it was reported that THC and its metabolites can be distributed in different tissues and organs and can be excreted into milk (Escriva et al., 2017). Wagner et al. (2022) reported that Δ9-THC has a feed-to-milk transfer rate of 0.20% ± 0.03%. Thus, a systematic assessment of plasma pharmacokinetics and body residues of cannabinoids and their metabolites in ruminants after short- or long-term exposure to hemp feed is prerequisite for its permission of being used in ruminant feeds (Kleinhenz et al., 2020a; Krebs et al., 2021). Unfortunately, limited data are available on the bioavailability of the dominant bioactive compounds in hemp and their disposition in edible tissues (i.e., THC and cannabidiol [CBD]) of ruminant animals (EFSA, 2011; Kleinhenz et al., 2020a), which is a significant impediment to the utilization of hemp products as feed ingredients.
China has a long hemp cultivation history and is one of the largest hemp producers in the world (Schluttenhofer and Yuan, 2017). The shortage of forage resources has become one of the bottlenecks restricting ruminant husbandry in southern China, where climatic conditions are not suitable for growing high-protein and high-quality forage like alfalfa. There have been efforts made in seeking alternative grasses for alfalfa such as ramie (Tang et al., 2021) or stevia hay (Jiang et al., 2022). As a new type of economic crop with both nutritional and medicinal properties, hemp is expected to be a potential breakthrough point for the sustainable development of ruminant production in China and other countries. Although there are limited reports on the bioavailability and bioefficacy of hemp phytochemicals for improving ruminant health, production, and meat quality (Semwogerere et al., 2020), existing research shows that hemp has a favorable crude protein (CP) content and nutrient digestibility profile (Kleinhenz et al., 2022). Feeding hemp has been shown to increase lying activity and decrease stress and inflammation biomarkers in cattle (Kleinhenz et al., 2022), as well as lower cumulative gas production, suggesting mitigation of ruminal methane production (Vastolo et al., 2021). As mentioned previously, bioactive residues in animal products may pose risks to consumers, so special attention should be paid when hemp is included in animal feeds by considering both the nutritional and cannabinoid concentrations in the animal ration (Kleinhenz et al., 2020b).
Therefore, the objectives of this study were to determine the effects of increasing substitution rate of alfalfa hay (AH) with hemp forage (HF) in the diets of goats on 1) growth performance, carcass traits, meat quality, and organ index; 2) ruminal fermentation characteristics and microbiota; and 3) blood metabolites and hemp-related residues in the meat. We hypothesized that partially increasing the substitution of HF for AH in the diet of goats would increase growth performance and feed efficiency due to the higher protein content. We also hypothesized that, due to the presence of bioactive components with antioxidant and antimicrobial activity, ruminal fermentation and microbiota would be altered, and that the antioxidant status of goats would be improved.
2. Materials and methods
2.1. Animal ethic statement
Experimental protocols were reviewed and approved (No. IBFC202003) by the Institutional Animal Care and Use Committee of the Institute of Bast Fiber Crops, Chinese Academy of Agricultural Sciences, Changsha, China.
2.2. Experimental animals and diets
Forty growing Xiangdong black goats (a local breed of Hunan province, China; mean BW = 7.82 ± 0.57 kg) were grouped by BW and randomly assigned into 1 of 4 experimental diets (10 goats/treatment). Diets were control (CON; 60% AH and 40% concentrate), 55% AH and 5% HF (HF5), 50% AH and 10% HF (HF10), and 40% AH and 20% HF (HF20). The diets were formulated according to the recommendation of NRC (2007), and contained 60% forage and 40% concentrate (dry matter [DM] basis; Table 1). The substitution rates of HF for AF were mainly based on the favorable nutrient contents of HF versus AF (shown as footnotes of Table 1) and consideration of the potential detrimental effect of cannabinoids in HF on meat quality. The hemp was grown in June and harvested manually in October, 2019 in Derong County, Sichuan Province, China and the HF was prepared by crushing hemp stems and leaves into powder. The HF contained 4.35% CBD, 0.11% THC, and 0.28% cannabidiolic acid (analyzed on a wt/wt basis).
Table 1.
Feed ingredients and chemical composition of experimental diets (DM basis).
| Item | Diets1 |
|||
|---|---|---|---|---|
| CON | HF5 | HF10 | HF20 | |
| Ingredients, % of DM | ||||
| HF2 | 0 | 5 | 10 | 20 |
| AH3 | 60 | 55 | 50 | 40 |
| Corn grain4 | 20.4 | 20.4 | 20.4 | 20.4 |
| Soybean meal | 4.8 | 4.8 | 4.8 | 4.8 |
| Wheat bran | 12.72 | 12.72 | 12.72 | 12.72 |
| NaCl | 0.48 | 0.48 | 0.48 | 0.48 |
| Premix5 | 1.6 | 1.6 | 1.6 | 1.6 |
| Chemical composition, % of DM | ||||
| DM, % | 91.18 | 91.20 | 91.17 | 91.09 |
| OM | 95.11 | 94.92 | 94.67 | 94.54 |
| CP | 14.89 | 14.93 | 15.12 | 15.34 |
| NDF | 37.52 | 37.65 | 37.77 | 37.92 |
| ADF | 26.66 | 26.74 | 26.76 | 26.85 |
| Ca | 2.85 | 2.87 | 2.87 | 2.88 |
| P | 0.31 | 0.32 | 0.32 | 0.32 |
| ME,6 MJ/kg | 9.73 | 9.74 | 9.74 | 9.74 |
AH = alfalfa hay; HF = hemp forage; ME = metabolizable energy; DM = dry matter; OM = organic matter; CP = rude protein; NDF = neutral detergent fiber; ADF = acidic detergent fiber.
CON = 60% AH without HF; HF5 = 55% AH + 5% HF; HF10 = 50% AH + 10% HF; HF20 = 40% AH + 20% HF.
Composition (DM basis): 90.1% DM, 92.4% OM, 22.2% CP, 4.2% EE, 55.5% NDF and 33.2% ADF.
Composition (DM basis): 90.0% DM, 93.7% OM, 14.1% CP, 1.4% EE, 59.6% NDF, and 45.9% ADF.
Composition (DM basis): 90.3% DM, 92.6% OM, 70.1% starch, 8.4% CP, 4.4% EE.
Supplied per kilogram of dietary DM: 120 mg MgSO4·H2O, 100 mg CuSO4, 30 mg FeSO4·7H2O, 50 mg ZnSO4·H2O, 30 mg MnSO4·H2O, 0.4 mg KI, 0.9 mg CoCl2, 1.3 mg Na2SeO3, 9,000 IU vitamin A, 1,800 IU vitamin D3, 2,000 IU vitamin E.
ME was calculated according to NRC (2007).
2.3. Animal management, feed sampling, and chemical analysis
The experiment lasted 70 d, with 10 d of adaptation and 60 d of data and sample collection. Goats were housed in individual pens and fed a total mixed ration (TMR) ad libitum twice daily at 08:00 and 18:00, with free access to clean water. Daily feed offered and orts were recorded for each goat throughout the collection period to calculate DM intake (DMI). Goats were weighed at the beginning and end of the experiment before morning feeding and the average daily gain (ADG) was calculated by dividing the BW gain (final BW − initial BW) by the number of days on feed. The feed conversion efficiency (F:G) was estimated by dividing DMI by ADG. The AH, HF, and TMR samples were collected once weekly, whereas orts were collected daily, pooled, and subsampled weekly for each goat. All samples were air dried in an oven at 55 °C for 48 h and ground through a 1-mm screen (standard model 4, Arthur Thomas Co., Philadelphia, PA, USA) for chemical composition determination.
Analytical DM was determined by drying at 135 °C for 2 h (AOAC, 2005; method 930.15); the ash concentration was determined by combustion at 550 °C for 5 h and the OM concentration was calculated as 100 minus the ash concentration (AOAC, 2005; method 942.05). Neutral detergent fiber (NDF) was determined using heat-stable α-amylase (Termamyl 120 L, Novo Nordisk Biochem, Franklinton, NC, USA) and sodium sulfite (AOAC, 2005; method 2002.04). Ash-free acid detergent fiber (ADF) was determined according to AOAC (2005; method 973.18). Starch was analyzed via enzymatic hydrolysis of α-linked glucose polymers (Rode et al., 1999), and the total ether extract concentration was determined using method 920.39 (AOAC, 2005). Total N was analyzed using flash combustion and thermal conductivity detection (Model 1500, Carlo Erba Instruments, Milan, Italy), and protein concentration was calculated as N × 6.25. The ME was calculated from tabulated feed values according to NRC (2007).
2.4. Carcass characteristics and meat quality
At the end of the experiment, the goats were slaughtered in a commercial slaughterhouse and carcass traits and meat quality were measured. Dressing percentage was calculated individually as hot carcass weight divided by final live BW × 100. Organs including liver, lung, spleen, heart and kidney were weighed, and the organ index (OI) was calculated as percentage of live BW. Longissimus dorsi samples, kept at 4 °C, were collected to determine meat quality, including pH, eye muscle area, drip loss, cooking loss, shear force, and meat color. The pH of longissimus dorsi was determined 24 h after slaughter using a pH indicator (S210, Mettler Toledo, USA). The width and height of the cross section of longissimus 6 to 7 ribs were determined with a Vernier caliper, and the eye muscle area was calculated as width × height × 0.7. Analysis of the drip loss, cooking loss and shear force was conducted according to guidelines of 1995. Meat color was measured using a Minolta CR-410 color-meter (Minolta Camera Co., Ltd., Osaka, Japan), where L∗ represents lightness, a∗ redness, and b∗ yellowness.
2.5. Rumen fermentation and ruminal microbiota
Rumen fluid was collected from each goat immediately after slaughter, and was passed through four layers of cheesecloth. Ruminal pH was measured immediately using a portable pH meter (Model S210 SevenCompact pH; Mettler-Toledo Instruments Ltd., Shanghai, China). The filtrates were mixed and sub-sampled for measuring rumen fermentation parameters and microbial communities. Five milliliters of filtrate were preserved with 1 mL of 25% (wt/vol) HPO3 for analysis of total volatile fatty acids (VFA) and another 5 mL was preserved with 1 mL of 1% (wt/vol) H2SO4 for NH3-N analysis. The prepared samples were stored at −20 °C until analysis. Two additional ruminal filtrate samples (1.5 mL) were collected in 2-mL polypropylene centrifuge tubes, frozen in liquid nitrogen, and stored at −80 °C for analysis of rumen microbial community and metabolites, respectively.
The concentrations of VFA and NH3-N were determined using gas chromatography (GC7890A, Agilent, California, USA) and a spectrophotometer (UV-2600, Shimadzu Global Laboratory Consumables Co., LTD, Shanghai, China), respectively. For ruminal microbiota determination, the filtrate samples were thawed at 4 °C overnight and total DNA was extracted using a QIAamp Fast DNA stool mini kit (Qiagen, Hilden, Germany). After quality checking, the extracted DNA was used for 16S amplicon high-throughput sequencing analysis as previously reported (Ran et al., 2021a). In short, the V3-V4 hypervariable region of the 16S rRNA gene was amplified using the universal primers 341F and 806R (Zhu et al., 2018), followed by paired-end library preparation, cluster generation, and sequencing on Illumina MiSeq platform (Illumina, Inc., San Diego, CA, USA) according to the manufacturer's instructions. Raw sequencing data were processed using QIME2 (Bolyen et al., 2019) and the R-package DADA2 (version 1.4) denoising method. Richness (number of operational taxonomic units [OTUs]) and diversity (Shannon index) were calculated and principal coordinates analysis (PCoA) and non-metric multidimensional scaling (NMDS) were performed based on Bray-Curtis similarity distances using the R packages vegan (version 2.4.4) and phyloseq (version 1.20.0).
2.6. Blood regular indices, immune and antioxidant activity
Blood samples were collected from the jugular vein into vacuum tubes with and without clot activator (Shandong Aosaite Medical Devices Co., LTD, Heze, China) before the morning feeding on d 60. The samples were maintained at room temperature for 15 min. Then serum samples were obtained by centrifuging at 2,000 × g for 15 min at 4 °C and plasma samples were obtained by centrifuging at 3,000 × g for 20 min at 4 °C. Both serum and plasma were subsampled and frozen at −20 °C until analysis. Serum samples were used for analysis of blood regular indices, immune and antioxidant activity, while the plasma samples were used for analysis of cannabinoids and their metabolites.
The serum concentrations of regular blood metabolites including total protein (TP), albumin (ALB), glucose, triglyceride (TG), total cholesterol (TC), blood urea N (BUN), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) and antioxidant indices including malondialdehyde (MDA), superoxide dismutase (SOD), total antioxidant capacity (T-AOC), and glutathione peroxidase (GSH-PX) were measured using commercial kits (CUSABIO Biotech Co., Ltd, Wuhan, China). Blood immune indices including IgG, IL-1β, IL-2, IL-4, and interferon-γ (IFN-γ) were analyzed using ELISA kits (Nanjing Jiancheng Biology Co., LTD, Nanjing, China) according to the supplier's instructions.
2.7. Hemp-related cannabinoid metabolism and residues in meat
Cannabinoids and their metabolites in the rumen fluid, plasma, and meat (longissimus dorsi) were determined using ultra-high-performance liquid chromatography (UHPLC) tandem high-resolution mass spectrometry (MS; Thermo Fisher, USA), as reported previously (Jamwal et al., 2017; Kleinhenz et al., 2020a). Raw data were subjected to baseline filtering, peak identification, integration, retention time correction, peak alignment, and normalization using the metabolomics processing software Progenesis QI (V2.3 software; Nonlinear Dynamics, Newcastle, UK). Metabolite information was obtained from the Human Metabolome Database (http://www.hmdb.ca/), Metlin Database (https://metlin.scripps.edu/), and Bovine Metabolome Database (http://www.cowmetdb.ca/) (Artegoitia et al., 2017).
2.8. Statistical analysis
Data on growth performance, carcass characteristics, rumen fermentation and blood biochemistry were analyzed as a completely randomized design using the PROC MIXED procedure of SAS (version 9.2.0, SAS Institute Inc.), with the dietary treatment considered as a fixed effect and goats as a random effect. Orthogonal contrasts were used to test for linear and quadratic responses of the HF substitution rate with the orthogonal coefficients adjusted for unequal spaces using SAS. Least square means were compared using the Tukey's correction for multiple comparisons. Treatment effects were declared significant at P ≤ 0.05 and tendencies were discussed at 0.05 < P ≤ 0.10 unless otherwise stated.
3. Results
3.1. Growth performance and carcass characteristics
Effects of increasing the substitution rate of HF for AH on the growth performance, carcass traits, and meat quality of goats are shown in Table 2. The final BW and ADG did not differ (P > 0.1) among treatments; the DMI and F:G ratio linearly decreased (P < 0.01) with increasing substitution rates of HF. Carcass traits, including carcass weight, dressing percentage, and eye muscle area were also not affected (P > 0.1) by substitution rate of HF. Meat quality indices, such as pH, cooking loss, and meat color (L∗, a∗, and b∗), did not differ (P > 0.1) among treatments; however, the drip loss was greater (P ≤ 0.05) in the HF20 than in the CON diet. The shear force was quadratically (P < 0.01) changed by increasing inclusion rates of HF in the diet; the HF10 had the greatest shear force (P < 0.01). Organ indices including liver, lung, spleen, heart and kidney were not affected when AH was partially replaced by HF (Table S1).
Table 2.
Effects of increasing substitute alfalfa hay (AH) with hemp forage (HF) on growth performance, carcass traits and meat quality of Xiangdong black goats.
| Item | Treatments1 |
SEM |
P-value2 |
|||||
|---|---|---|---|---|---|---|---|---|
| CON | HF5 | HF10 | HF20 | T | L | Q | ||
| Growth performance | ||||||||
| Initial BW, kg | 7.72 | 7.98 | 7.85 | 7.75 | 0.355 | 0.876 | 0.903 | 0.535 |
| Final BW, kg | 12.88 | 13.62 | 13.27 | 13.08 | 0.309 | 0.406 | 0.996 | 0.207 |
| ADG, g/d | 86.11 | 93.89 | 90.28 | 88.89 | 4.022 | 0.594 | 0.904 | 0.323 |
| DMI, g/d | 432.9a | 404.4ab | 384.7bc | 365.8c | 14.48 | 0.023 | <0.01 | 0.371 |
| F:G, kg/kg | 5.09a | 4.30b | 4.27b | 4.15b | 0.163 | <0.01 | <0.01 | 0.210 |
| Carcass traits | ||||||||
| Carcass weight, kg | 4.86 | 4.96 | 5.19 | 5.01 | 0.280 | 0.871 | 0.705 | 0.522 |
| Dressing, % | 37.71 | 36.51 | 39.14 | 38.33 | 2.123 | 0.854 | 0.723 | 0.914 |
| Eye muscle area, cm2 | 5.28 | 4.44 | 5.95 | 4.65 | 0.522 | 0.196 | 0.695 | 0.433 |
| Meat quality | ||||||||
| pH | 6.35 | 6.58 | 6.37 | 6.54 | 0.752 | 0.989 | 0.994 | 0.999 |
| Drip loss, % | 8.17b | 9.67ab | 8.67ab | 12.50a | 1.568 | 0.045 | 0.074 | 0.556 |
| Cooking loss, % | 34.33 | 38.33 | 31.83 | 34.00 | 2.016 | 0.178 | 0.472 | 0.926 |
| Shear force, N | 65.66b | 73.15b | 92.85a | 58.40b | 6.570 | <0.01 | 0.445 | <0.01 |
| Meat color | ||||||||
| L∗ | 51.42 | 48.07 | 50.14 | 50.32 | 1.733 | 0.592 | 0.957 | 0.408 |
| a∗ | 17.34 | 19.28 | 18.06 | 17.66 | 1.075 | 0.608 | 0.860 | 0.382 |
| b∗ | 6.92 | 5.91 | 6.51 | 6.09 | 0.515 | 0.524 | 0.435 | 0.632 |
a, b Means least square of means within a row with different superscripts differ (P ≤ 0.05).
CON = 60% AH without HF; HF5 = 55% AH + 5% HF; HF10 = 50% AH + 10% HF; HF20 = 40% AH + 20% HF; AH, alfalfa hay; HF, hemp forage.
T = treatment; L = linear; Q = quadratic; CON was included in L and Q analysis.
3.2. Ruminal fermentation and microbial community
The ruminal pH did not differ (P > 0.1) among diets, whereas the NH3-N concentration linearly (P < 0.01) increased with increasing substitution rate of HF for AF, with greater (P = 0.022) NH3-N concentration in HF20 than in the other diets (Table 3). Total VFA concentration quadratically (P < 0.01) changed with increasing HF substitution rates, with the greatest concentration observed in the HF10 diet. The molar proportion of acetate quadratically (P < 0.01) changed; whereas the molar proportion of propionate linearly increased (P = 0.024) with increasing HF substitution rates. As a result, the acetate-to-propionate ratio linearly (P = 0.032) decreased with increasing substitution rates of HF. The molar proportion of butyrate tended (P = 0.081) to increase linearly with increasing HF substitution rates. The molar proportion of branched-chain VFA (BCVFA) tended to quadratically (P = 0.061) change with increasing substitution rate of HF, with greater values in the CON than in the HF5 and HF10 diets.
Table 3.
Effects of increasing substitute alfalfa hay (AH) with hemp forage (HF) in the diet on rumen fermentation characteristics of Xiangdong black goats.
| Item | Treatments1 |
SEM |
P-value2 |
|||||
|---|---|---|---|---|---|---|---|---|
| CON | HF5 | HF10 | HF20 | T | L | Q | ||
| pH | 6.49 | 6.39 | 6.21 | 6.30 | 0.072 | 0.685 | 0.896 | 0.275 |
| NH3-N, mg/dL | 11.46b | 12.38b | 12.38b | 14.34a | 0.606 | 0.022 | <0.01 | 0.657 |
| VFA | ||||||||
| Total, mmol/L | 39.52b | 43.78ab | 53.10a | 46.02ab | 5.232 | 0.036 | 0.279 | <0.01 |
| Acetate (A), mol/100 mol | 61.33 | 65.20 | 65.03 | 62.00 | 1.961 | 0.502 | 0.756 | <0.01 |
| Propionate (P), mol/100 mol | 14.00b | 15.60a | 15.67a | 16.17a | 0.525 | 0.042 | 0.024 | 0.893 |
| Butyrate, mol/100 mol | 8.67 | 10.40 | 9.33 | 10.83 | 0.680 | 0.142 | 0.081 | 0.873 |
| BCVFA3, mol/100 mol | 16.03a | 7.94b | 9.78b | 11.25ab | 2.024 | 0.051 | 0.312 | 0.061 |
| A:P4 | 4.37 | 4.17 | 4.15 | 3.89 | 0.150 | 0.082 | 0.032 | 0.501 |
a, b Means least square of means within a row with different superscripts differ (P ≤ 0.05).
CON = 60% AH without HF; HF5 = 55% AH + 5% HF; HF10 = 50% AH + 10% HF; HF20 = 40% AH + 20% HF.
T = treatment; L = linear; Q = quadratic; CON was included in L and Q analysis.
BCVFA = branched-chain volatile fatty acids.
A:P = acetate to propionate ratio.
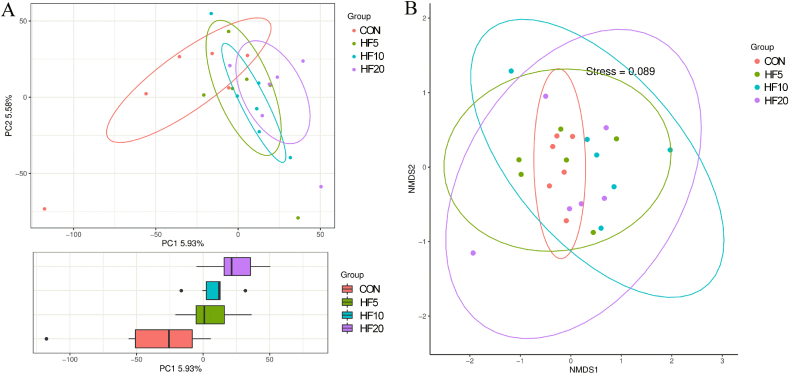
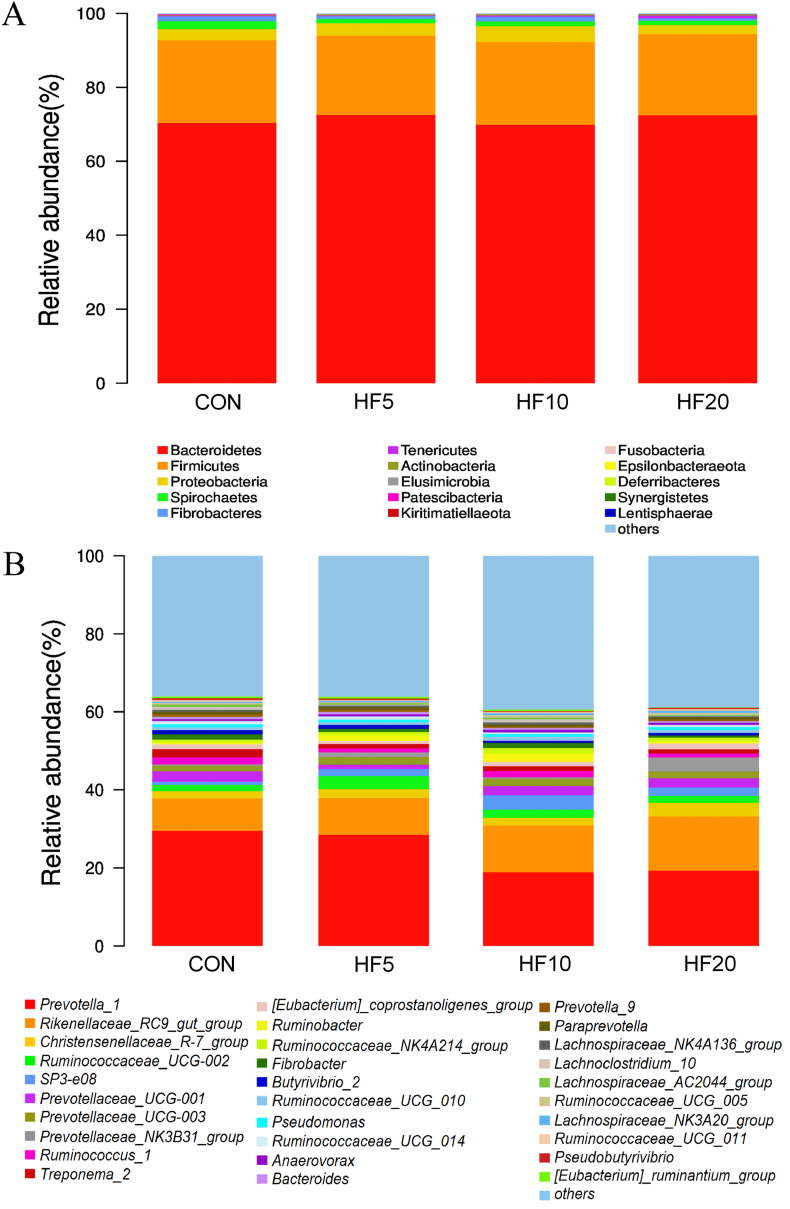
The ruminal microbiota was determined using 16S rDNA sequencing. An average of 62,362 valid tags per sample were obtained, with sufficient coverage of each sample to accurately describe the microbial composition, as indicated by the rarefaction curves (Fig. S1). No difference (P > 0.1) was observed in the numbers of OTUs among treatments (Fig. S2) or among the α-diversity indices including observed species, Shannon, and Simpson indexes (Fig. S3). Furthermore, goats receiving different HF levels could not be separated from each other using PCoA and NMDS analysis, meaning HF5, HF10 and HF20 had similar community structure. Goats in the HF groups showed a much more diffuse distribution pattern as compared to CON (Fig. 1) and no clear clustering of the ruminal microbes, suggesting a greater distance within the groups. The taxonomic composition of the ruminal microbiota by diets was analyzed at the phylum (Fig. 2A) and genus (Fig. 2B) levels by means of relative abundance. Except those lower than 0.1% and unclassified, more than 99.5% of rumen bacteria belonged to the following seven phyla: Bacteroidetes, Firmicutes, Proteobacteria, Spirochaetes, Fibrobacteres, Tenericutes, and Actinobacteria. Bacteroidetes (average of four treatments, 71.26%) and Firmicutes (average of four treatments, 22.07%) were the most abundant, resulting in an Firmicutes-to-Bacteroidetes ratio around 0.30 (Table 4). The relative abundance of Tenericutes increased linearly (P = 0.028) with increasing substitution rate of HF, with a greater relative abundance in HF20 than in the other treatments, whereas the relative abundances of the other phyla were not affected by treatment.
Fig. 1.
Beta diversity analysis through (A) principal coordinates analysis (PCoA) and (B) non-metric multidimensional scaling (NMDS) for ruminal microbiota of Xiangdong black goats fed alfalfa hay (AH)-based diets (60% of diet, DM basis) substituted with different rates of hemp forage (HF). CON = 60% AH without HF; HF5 = 55% AH + 5% HF; HF10 = 50% AH + 10% HF; HF20 = 40% AH + 20% HF; AH = alfalfa hay; HF = hemp forage.
Fig. 2.
Bar plots of ruminal microbiota at (A) phylum and (B) genus levels of Xiangdong black goats fed alfalfa hay (AH)-based diets (60% of diet, DM basis) substituted with different rates of hemp forage (HF). CON = 60% AH without HF; HF5 = 55% AH + 5% HF; HF10 = 50% AH + 10% HF; HF20 = 40% AH + 20% HF; AH = alfalfa hay; HF = hemp forage.
Table 4.
Effects of increasing substitute alfalfa hay (AH) with hemp forage (HF) in the diet on the ruminal microbiota of Xiangdong black goats at phylum level (relative abundance, %).
| Item | Treatments1 |
SEM |
P-value2 |
|||||
|---|---|---|---|---|---|---|---|---|
| CON | HF5 | HF10 | HF20 | T | L | Q | ||
| Bacteroidetes | 70.32 | 72.53 | 69.83 | 72.36 | 1.914 | 0.671 | 0.624 | 0.825 |
| Firmicutes | 22.43 | 21.44 | 22.41 | 22.01 | 1.804 | 0.978 | 0.969 | 0.931 |
| F:B ratio3 | 0.32 | 0.30 | 0.33 | 0.31 | 0.032 | 0.917 | 0.951 | 0.931 |
| Proteobacteria | 2.97 | 3.37 | 4.28 | 2.49 | 0.886 | 0.543 | 0.691 | 0.202 |
| Spirochaetes | 2.16 | 1.13 | 1.31 | 1.20 | 0.432 | 0.330 | 0.228 | 0.269 |
| Fibrobacteres | 1.34 | 0.79 | 1.32 | 0.58 | 0.297 | 0.208 | 0.150 | 0.702 |
| Tenericutes | 0.21b | 0.23b | 0.32b | 0.83a | 0.201 | 0.045 | 0.028 | 0.388 |
| Actinobacteria | 0.15 | 0.16 | 0.22 | 0.17 | 0.022 | 0.153 | 0.327 | 0.121 |
| Others | 0.43 | 0.33 | 0.33 | 0.37 | 0.061 | 0.635 | 0.589 | 0.267 |
a, b Means least square of means within a row with different superscripts differ (P ≤ 0.05).
CON = 60% AH without HF; HF5 = 55% AH + 5% HF; HF10 = 50% AH + 10% HF; HF20 = 40% AH + 20% HF; AH, alfalfa hay; HF, hemp forage.
T = treatment; L = linear; Q = quadratic; CON was included in L and Q analysis.
F:B = Firmicutes to Bacteroidetes ratio.
At the genus level, the averaged relative abundances of ruminal bacteria among treatments >0.5% are shown in Table 5. Prevotella_1 (average of four treatments, 23.99%) and Rikenellaceae_RC9_gut_group (average of four treatments, 10.92%) from phylum Bacteroidetes had the first and second highest relative abundances, respectively. The relative abundance of Prevotella_1 linearly (P = 0.040) decreased but that of Rikenellaceae_RC9_gut_group (P = 0.017) increased with increasing substitution rates of HF. Similarly, the relative abundance of Bacteroides decreased linearly as the HF substitution rate increased. However, there were no significant effects (P > 0.1) of HF substitution rates on the relative abundances of the other genera. The treatment effect was observed for the relative abundances of Ruminococcaceae_NK4A214_group and Fibrobacter, which were the highest under HF10 treatment.
Table 5.
Effects of increasing substitute alfalfa hay (AH) with hemp forage (HF) in the diet on the ruminal microbiota of Xiangdong black goats at genus level (relative abundance, %).
| Phylum | Genus | Treatments1 |
SEM |
P-value2 |
|||||
|---|---|---|---|---|---|---|---|---|---|
| CON | HF5 | HF10 | HF20 | T | L | Q | |||
| Bacteroidetes | Prevotella_1 | 29.52a | 28.36a | 18.81b | 19.26b | 3.826 | 0.045 | 0.040 | 0.422 |
| Rikenellaceae_RC9_gut_group | 8.28b | 9.46b | 12.04ab | 13.90a | 1.649 | 0.050 | 0.017 | 0.746 | |
| Prevotellaceae_UCG-001 | 2.67 | 1.13 | 2.33 | 2.32 | 0.853 | 0.609 | 0.913 | 0.508 | |
| Prevotellaceae_UCG-003 | 1.41 | 1.89 | 1.89 | 1.72 | 0.442 | 0.852 | 0.731 | 0.451 | |
| Prevotellaceae_NK3B31_group | 0.39 | 1.26 | 0.39 | 3.67 | 1.869 | 0.568 | 0.237 | 0.583 | |
| SP3-e08 | 0.85 | 1.81 | 3.69 | 2.16 | 1.274 | 0.477 | 0.445 | 0.220 | |
| Bacteroides | 0.69a | 0.61ab | 0.48bc | 0.38c | 0.048 | <0.01 | <0.01 | 0.628 | |
| Prevotella_9 | 0.69 | 0.56 | 0.36 | 0.50 | 0.104 | 0.195 | 0.203 | 0.103 | |
| Paraprevotella | 0.49 | 0.60 | 0.43 | 0.52 | 0.075 | 0.467 | 0.895 | 0.765 | |
| Firmicutes | Christensenellaceae_R-7_group | 1.85 | 2.27 | 1.97 | 3.49 | 0.878 | 0.547 | 0.205 | 0.620 |
| Ruminococcaceae_UCG-002 | 1.61 | 3.32 | 2.06 | 1.79 | 0.656 | 0.274 | 0.694 | 0.255 | |
| Ruminococcus_1 | 1.82 | 1.07 | 1.65 | 1.02 | 0.360 | 0.312 | 0.245 | 0.931 | |
| Ruminococcaceae_NK4A214_group | 0.81b | 0.90ab | 1.48a | 1.04ab | 0.154 | 0.050 | 0.420 | 0.167 | |
| Butyrivibrio_2 | 1.11 | 1.07 | 0.55 | 0.68 | 0.221 | 0.225 | 0.118 | 0.385 | |
| Ruminococcaceae_UCG-010 | 0.70 | 0.65 | 0.99 | 0.77 | 0.122 | 0.228 | 0.479 | 0.248 | |
| Ruminococcaceae_UCG-014 | 0.82 | 0.83 | 0.49 | 0.55 | 0.183 | 0.424 | 0.206 | 0.580 | |
| Anaerovorax | 0.46 | 0.54 | 0.67 | 0.56 | 0.057 | 0.111 | 0.219 | 0.104 | |
| Fibrobacteres | Fibrobacter | 1.34a | 0.79ab | 1.31a | 0.57b | 0.297 | 0.045 | 0.148 | 0.703 |
| Spirochaetaceae | Treponema_2 | 2.09 | 1.09 | 1.23 | 1.07 | 0.422 | 0.294 | 0.176 | 0.278 |
| Proteobacteria | Ruminobacter | 0.40 | 1.39 | 2.05 | 0.40 | 0.745 | 0.344 | 0.889 | 0.078 |
| Pseudomonas | 0.79 | 0.68 | 0.70 | 0.72 | 0.042 | 0.314 | 0.482 | 0.151 | |
| Unclassified | 23.12 | 24.66 | 25.68 | 24.48 | 4.019 | 0.847 | 0.871 | 0.392 | |
| Others | 18.14 | 15.08 | 18.77 | 18.44 | 1.361 | 0.228 | 0.447 | 0.613 | |
a, b, c Means least square of means within a row with different superscripts differ (P ≤ 0.05).
CON = 60% AH without HF; HF5 = 55% AH + 5% HF; HF10 = 50% AH + 10% HF; HF20 = 40% AH + 20% HF; AH, alfalfa hay; HF, hemp forage.
T = treatment; L = linear; Q = quadratic; CON was included in L and Q analysis.
3.3. Blood biochemistry
Results of blood biochemistry, including regular blood metabolites, antioxidant activity, and immune activity, are shown in Table 6. The serum concentrations of TP, TG, ALT and AST did not differ (P > 0.10) among treatments. However, the concentrations of ALB and TC linearly (P < 0.01) decreased, and that of BUN linearly (P = 0.050) increased with increasing HF substitution rate. Greater serum glucose concentration was detected in HF10 than in other treatments. The serum T-AOC linearly (P = 0.014) decreased with increasing HF substitution rate, whereas neither a linear/quadratic effect nor treatment effect (P > 0.1) was observed for the antioxidant indices measured as MDA, SOD, and GSH-PX among treatments. Regarding the immune indices, serum concentrations of antibody (IgG), pro-inflammatory (IL-1β, IL-2, and IFN-γ), and anti-inflammatory factors (IL-4) were greatly affected (P ≤ 0.05) by dietary HF contents, with the greatest concentration of immune indices consistently observed in goats fed HF5 diet and no differences (P > 0.1) between CON and HF20. The serum concentration of IgG tended to linearly (P = 0.065) decrease, but the concentrations of IL-2, IL-4, and IFN-γ quadratically (P ≤ 0.05) changed with increasing HF substitution rates, with a sharp increase from CON to HF5 and then decreased to the original level as the dietary HF contents increased.
Table 6.
Effects of increasing substitute alfalfa hay (AH) with hemp forage (HF) on blood biochemistry, antioxidant and immune indices of Xiangdong black goats.
| Item | Treatments1 |
SEM |
P-value2 |
|||||
|---|---|---|---|---|---|---|---|---|
| CON | HF5 | HF10 | HF20 | T | L | Q | ||
| Biochemistry | ||||||||
| TP, g/L | 66.76 | 62.64 | 63.28 | 61.52 | 2.509 | 0.502 | 0.211 | 0.567 |
| ALB, g/L | 24.52a | 22.66a | 22.13a | 18.52b | 1.087 | <0.01 | <0.01 | 0.799 |
| Glucose, mmol/L | 2.52b | 2.46b | 4.03a | 2.69b | 0.227 | <0.01 | 0.267 | 0.107 |
| TG, mmol/L | 0.55 | 0.59 | 0.61 | 0.47 | 0.051 | 0.278 | 0.197 | 0.144 |
| TC, mmol/L | 0.89a | 0.70b | 0.51c | 0.39c | 0.053 | <0.01 | <0.01 | 0.112 |
| BUN, mmol/L | 4.94b | 5.74ab | 6.42a | 5.90a | 0.313 | 0.025 | 0.050 | 0.091 |
| ALT, U/L | 4.45 | 4.29 | 4.86 | 4.60 | 0.409 | 0.958 | 0.697 | 0.824 |
| AST, U/L | 12.41 | 11.98 | 11.30 | 12.61 | 1.398 | 0.914 | 0.905 | 0.512 |
| Antioxidant indices | ||||||||
| MDA, nmol/mL | 2.15 | 1.68 | 1.99 | 1.97 | 0.192 | 0.400 | 0.860 | 0.358 |
| SOD, U/mL | 142.83 | 133.72 | 154.83 | 147.26 | 7.019 | 0.226 | 0.352 | 0.672 |
| T-AOC, μmol/mL | 0.20 | 0.18 | 0.16 | 0.15 | 0.017 | 0.071 | 0.014 | 0.362 |
| GSH-PX, U/mL | 97.72 | 101.24 | 92.79 | 90.11 | 6.048 | 0.577 | 0.255 | 0.868 |
| Immune indices | ||||||||
| IgG, g/L | 9.09ab | 10.26a | 8.52b | 8.35b | 0.465 | 0.036 | 0.065 | 0.521 |
| IL-1β, ng/L | 19.20b | 21.86a | 19.36b | 18.54b | 0.751 | 0.027 | 0.136 | 0.118 |
| IL-2, ng/L | 32.24b | 39.59a | 36.90a | 33.04b | 1.473 | <0.01 | 0.564 | <0.01 |
| IL-4, ng/L | 73.70b | 91.33a | 76.52b | 73.21b | 3.478 | <0.01 | 0.201 | 0.039 |
| IFN-γ, ng/L | 50.80b | 61.60a | 55.74ab | 52.35b | 2.634 | 0.041 | 0.668 | 0.033 |
TP = total protein; ALB = albumin; BUN = blood urea nitrogen; TC = total cholesterol; TG = triglyceride; ALT = alanine aminotransferase; AST = aspartate aminotransferase; MDA = malondialdehyde; SOD = superoxide dismutase; T-AOC = total antioxidant capacity; GSH-PX = glutathione peroxidase; IFN-γ = interferon-γ.
a, b, c Means least square of means within a row with different superscripts differ (P ≤ 0.05).
CON = 60% AH without HF; HF5 = 55% AH + 5% HF; HF10 = 50% AH + 10% HF; HF20 = 40% AH + 20% HF; AH, alfalfa hay; HF, hemp forage.
T = treatment; L = linear; Q = quadratic; CON was included in L and Q analysis.
3.4. Metabolism of hemp-related cannabinoids
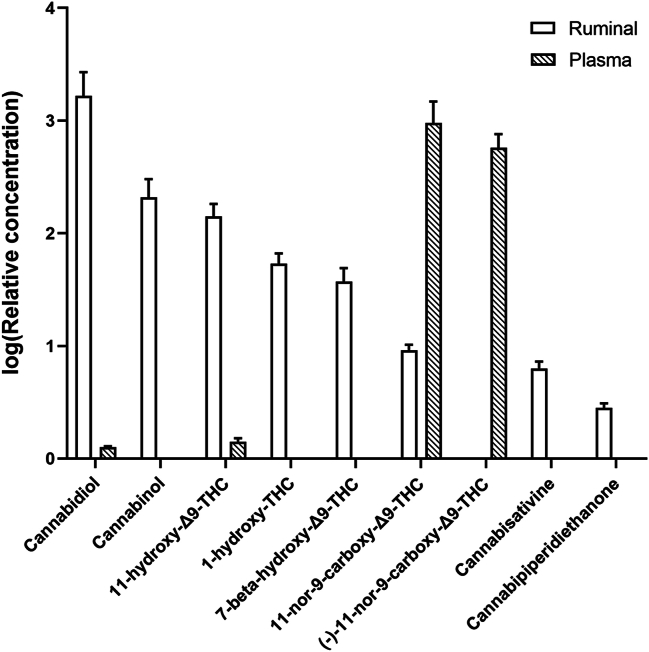
The content of hemp-related bioactive compounds was measured in ruminal, plasma, and meat samples. Eight cannabinoids and their metabolites were detected in ruminal filtrates and four cannabinoids and their metabolites in plasma (Fig. 3; Table S2), whereas no residues were detected in the longissimus dorsi samples. In the ruminal filtrates, the detected cannabinoids and their metabolites content tended to decrease in the order of CBD, cannabinol, 11-hydroxy-Δ9-THC (THC-OH), 1-hydroxy-THC, 7-beta-hydroxy-Δ9-THC, 11-nor-9-carboxy-Δ9-THC (THC-COOH), cannbisativine, and cannabipiperidiethanone. In the plasma, low concentrations of CBD and THC-OH, but high concentrations of THC-COOH and (−)-THC-COOH were detected. Among these compounds, CBD, THC-OH, and THC-COOH were detected in both ruminal filtrates and plasma, with CBD and THC-OH being extremely low and the THC-COOH concentrations being higher in plasma than in ruminal filtrates. (−)-THC-COOH was the only hemp-related metabolite detected in plasma but not in the rumen, with a relative concentration comparable to that of THC-COOH.
Fig. 3.
The relative concentrations of hemp-related residues or derivatives in ruminal filtrates and plasma of Xiangdong black goats. Results were expressed as logarithmic of relative concentration.
4. Discussion
Hemp and its by-products generated during cannabinoid production are ideally suited as novel sources of forage for ruminants (Kleinhenz et al., 2020a); however, few studies evaluated their feeding value and residues in animal products. There is an urgent need to bridge the gap in understanding the impact of hemp and its by-products on growth performance, nutrient digestibility, ruminal fermentation, blood metabolites, carcass, and meat quality (Semwogerere et al., 2020). In addition, although not detected in the current study, special attention should be paid to the blood half-lives of cannabinoids, which will allow ruminant producers to establish withdrawal intervals to ensure that animals consuming hemp and its by-products can enter the food chain (Kleinhenz et al., 2020a).
4.1. Effects of substitution of AH with HF on growth performance and carcass traits
In the present study, increasing levels of HF in the diet resulted in a linear decrease in DMI and improvement in the feed conversion rate without decreasing ADG. These results suggest that the feeding value of HF may be higher than that of AH when partially substituted for AH under current experimental conditions. The linear reduction in DMI with increasing HF inclusion rate may be related to the specific odor of hemp-related bioactive compounds. Wagner et al. (2022) also reported a decreased feed intake when corn silage was partially replaced with industrial hemp silage. In contrast, Krebs et al. (2021) reported no effects on DMI when hemp stubble was included in the pelleted diets of sheep. The discrepancy among these studies could be explained by the differences in the composition and form of the hemp used. The HF used in the current study contained mainly stems and leaves and might have greater amounts of hemp-related bioactive compounds than hemp stubble used in the study by Krebs et al. (2021). Furthermore, in Krebs et al. (2021), the hemp stubble was included in the pelleted ration. The pelleting process at high temperature and pressure may have reduced the activity of the bioactive components. In the present study, the improved feed conversion ratio with increasing dietary HF inclusion may have been due to improved nutrient digestibility with HF addition. Krebs et al. (2021) reported greater DM and OM digestibility in hemp stubble than in oat straw. In the present study, although feed digestibility was not measured, the greater ruminal total VFA concentration and higher molar proportion of acetate in the HF10 group suggested an improvement in feed digestibility with HF feeding. The presence of secondary metabolites may favor ruminal microbial activity. This speculation is supported by the enhanced relative abundances of Rikenellaceae_RC9_gut_group and Ruminococcaceae_NK4A214. Similarly, substituting AH with stevia hay, which is also rich in secondary metabolites, increased the digestibility of NDF and ADF (Jiang et al., 2022).
The lack of adverse effects of substituting AH with HF on carcass traits in the present study is consistent with the similar treatment effects on growth rate. Our findings also agree with those of a previous study that fed sheep pelleted rations containing hemp stubble (Krebs et al., 2021). In the current trial, substitution of AH with HF had no impact on meat pH, color, and cooking loss. However, there was greater drip loss when one-third of the AH was replaced by HF, indicating lowered tenderness. A previous study reported that hempseed cake had effects comparable to soybean meal on ruminant carcass and meat quality attributes (Semwogerere et al., 2020); moreover, feeding hempseed cake increased the total antioxidant capacity of sheep milk, perhaps due to the bioactive compounds in the hemp, such as terpenes, CBD, α-tocopherol, and polyphenol (Mierlita, 2018). Although there were no adverse effects of partially substituting AH with HF on meat quality in the current study, further studies are needed to determine whether feeding hemp-based products positively affects meat oxidative stability (Semwogerere et al., 2020).
4.2. Effects of substitution of AH with HF on ruminal pH and fermentation
Ruminal pH reflects the fermentation pattern and internal environment of the rumen and is considered an important determinant of rumen health. In the current study, the substitution of AH with HF in roughage-based diets did not affect ruminal pH. On the current trial, ruminal pH ranged from 6.29 to 6.37 among treatments, which is consistent with the high-forage feeding. Similarly, Krebs et al. (2021) reported that the inclusion hemp stubble did not affect ruminal pH.
The ruminal NH3-N concentration reflects the dynamics between protein degradation and microbial protein synthesis in the rumen (Reynolds and Kristensen, 2008). In the present study, the ruminal NH3-N concentration was within the optimal range suggested by Owens and Bergen (1983), at 0.39 to 29 mg/dL. The higher concentration of NH3-N with the HF20 diet is likely due to higher dietary CP contents, but the lower DMI than that of CON may have reduced microbial protein synthesis due to low energy availability. Because nutrient digestibility was not measured in the current study, it is unknown whether diets containing HF had greater CP degradability than the CON diet, in which AH was the sole forage source. However, when oat chaff was substituted with hemp stubble in sheep diets, Krebs et al. (2021) reported a greater NH3-N concentration and CP digestibility.
The ruminal VFA are the main energy source for ruminants, providing up to 70% of their energy requirement (Srinivas and Gupta, 1997). Greater total VFA concentrations, especially of acetate, in the HF diets may indicate an improvement in DM and fiber digestibility. This may explain the improved feed conversion rate with increasing HF substitution rates. Krebs et al. (2021) reported greater total VFA concentrations in diets containing hemp stubble, possibly because of the higher OM digestibility when substituting oat chaff with hemp stubble. We found that the higher molar proportion of acetate with HF10 agreed with the results of Krebs et al. (2021), who also reported an increased concentration of acetate when hemp stubble was included in sheep diets. The results indicated that hemp inclusion may promote acetic acid fermentation in the rumen. The lower BCVFA proportion with HF inclusion could not be explained by the lower proteolytic activity with HF because the lower BCVFA did not influence the total VFA concentration.
4.3. Effects of substitution of AH with HF on ruminal microbial community
The sequencing results showed that the substitution of AH with HF had little effect on the diversity and richness of ruminal bacteria, suggesting no detrimental effects from hemp-related bioactive compounds including cannabinoids (Δ9-THC and CBD) and their metabolites; however, substitution with HF altered the microbial community profiles, with greater changes observed under higher HF substitution ratios.
This alteration was mainly reflected in the fluctuations in the relative abundances of several major bacterial genera. More specifically, the relative abundances of Prevotella_1 and Rikenellaceae_RC9_gut_group, both belonging to the phylum Bacteroidetes, were greatly decreased and increased, respectively, with increasing HF substitution rates. Many studies have consistently identified these genera as the core genera in the rumen (Henderson et al., 2015; Xue et al., 2018), suggesting that they play critical roles in rumen fermentation. Bacteria of Prevotella genus are known to be the predominant proteolytic bacteria with a great diversity of extracellular proteolytic activities in the rumen (Griswold et al., 1999); thus, the decreased relative abundance of Prevotella_1 could reduce the degradation of dietary protein in the rumen and increase the amount of protein that enters the small intestine. This suggests that feeding HF may increase by-pass rumen protein. However, this effect was not observed in the present study, likely because AH contains highly degradable non-protein nitrogen, while corn protein is less degradable. Prevotella_1 also plays an essential role in polysaccharide degradation and sugar fermentation (Accetto and Avgustin, 2019) and its relative abundance increases with greater concentrations of dietary hemicellulose and water-soluble carbohydrates when corn silage replaced with sweet sorghum silage (Ran et al., 2021b). Therefore, the reduced relative abundance of Prevotella_1 was likely related to the different concentrations of dietary hemicellulose and water-soluble carbohydrates between AH and HF.
The Rikenellaceae_RC9_gut_group are fiber digesters that increase propionate production and enables more efficient energy recovery by lowering methane emissions through hydrogen scavenging (Conte et al., 2022; Daghio et al., 2021). It was reported that Rikenellaceae_RC9_gut_group was positively correlated with the production of propionate in heat-tolerant dairy cows, providing a more effective energy recovery system than heat-susceptible cows (Wang et al., 2022). Therefore, the enhanced molar proportion of propionate and decreased acetate-to-propionate ratio were largely related to the increased relative abundance of Rikenellaceae_RC9_gut_group when AH was substituted with HF. This suggests that the inclusion of HF in the diet of goats promoted propionic acid fermentation by upregulating the relative abundance of Rikenellaceae_RC9_gut_group. The relative abundance of Rikenellaceae_RC9_gut_group was consistently higher in animals with better growth performances (Conte et al., 2022; Daghio et al., 2021; Liu et al., 2022) and health status (Liu et al., 2022; Wang et al., 2022). We speculate that the inclusion of HF in the diet of goats may improve their health and growth performance. Further research is needed to verify the exact role of Rikenellaceae_RC9_gut_group played in ruminant performance.
Ruminococcaceae have a dominant role in cellulose and hemicellulose degradation (Pang et al., 2022) and rumen biohydrogenation pathways (Huws et al., 2011). The enhanced abundance of Ruminococcaceae_NK4A214 may have contributed to the greater production of propionate and improved feed efficiency in the current study, ultimately improving animal performance (Wang et al., 2022). As important cellulose and hemicellulose digesters, the abundance of some unclassified Ruminococcaceae could be used as potential biomarkers of subacute ruminal acidosis risk (Zhang et al., 2022). Rikenellaceae_RC9_gut_group and Ruminococcaceae_NK4A214 were negatively associated with residual feed intake (Liu et al., 2022), suggesting that they are beneficial for improving feed efficiency. In brief, the alteration of microbial community profiles by substituting AH with HF was consistent with the effects of promoting propionate fermentation and improving feed efficiency.
4.4. Effects of substitution of AH with HF on blood metabolites and antioxidant capacities
Hemp and its by-products are rich in bioactive compounds (e.g., terpenes, flavonoids, and cannabinoids) and are commonly studied as medicine due to their therapeutic potential in many diseases. Specifically, these bioactive compounds have the potential to alter the blood biochemistry, antioxidant capacity, and immune status. However, few studies have evaluated hemp as a forage source and investigated the effects of hemp bioactive compounds on blood biochemistry in ruminants. Kleinhenz et al. (2020a, 2022) reported no effect on serum biochemistry when feeding industrial hemp to beef cattle and Holstein steers for a short or long term. Similarly, although the differences in some values were observed among treatments in the current study, all values remained within their suitable reference ranges for goats, suggesting the overall safety of HF inclusion in the diet of goats. The linear increase in BUN with increasing dietary HF content was consistent with the linear elevated ruminal NH3-N concentrations. This is likely due to the numerically greater dietary CP content in the HF.
Hemp-related bioactive compounds have been reported to have antioxidant capacity (Semwogerere et al., 2020); however, no difference in antioxidant indices, including MDA, SOD, and GSH-PX, were observed among treatments in the present study, suggesting that goats did not suffer from oxidative stress during the experimental period. In livestock production, stressful conditions may result from nutrition (e.g., high-grain diets), environmental conditions, and management (weaning, transportation, and feedlot entry; Olagaray and Bradford, 2019). Stress events have been implicated in promoting oxidative stress through excessive production of reactive oxygen species or decreased antioxidant defenses (Olagaray and Bradford, 2019). In the present study, the goats were fed high-forage diets under well-adapted environmental condition. Therefore, stress was minimal. Interestingly, Holstein steers consuming cannabinoids from industrial hemp have lower stress biomarkers and improved lying times, suggesting increased animal welfare (Kleinhenz et al., 2022). Moreover, administration of industrial hemp had little impact on serum amyloid A and haptoglobin (Kleinhenz et al., 2022), which are acute-phase proteins recognized as indicators of inflammation. In the current study, pro-inflammatory (IL-1β, IL-2, and IFN-γ) and anti-inflammatory factors (IL-4) were elevated when AH diets were substituted with low levels of HF, but no such elevations were observed when replacing AH with increasing amounts of HF.
4.5. Effects of substitution of AH with HF on cannabinoid residues
The potential of cannabinoid residues, especially psychoactive cannabinoid Δ9-THC, in edible tissue, milk, or milk products has greatly hindered the use of industrial hemp by-products, such as flowers, leaves and stubbles, in ruminant diets. Approximately 144 cannabinoids have been identified in cannabis plants using HPLC-MS/MS. The THC and CBD are the most prominent, with particularly high contents in hemp flowers and leaves (Andre et al., 2016; Pourseyed Lazarjani et al., 2020). Several pioneer studies have been conducted to elucidate the pharmacokinetics of cannabinoids in beef cattle (Kleinhenz et al., 2020a), Holstein steers (Kleinhenz et al., 2022), and sheep (Krebs et al., 2021) fed diets containing hemp by-products for a short- or long-term period. These studies suggested that hemp-related cannabinoids can be absorbed from the rumen wall and distributed throughout the body via the blood stream. However, little information is available on cannabinoid metabolism in the rumen. It has been speculated that rumen microbes can potentially degrade or metabolize cannabinoids (Kleinhenz et al., 2020a). The present study is the first to determine the metabolites of hemp-related cannabinoids in the rumen and plasma using UHPLC-MS. High concentrations of CBD, cannabinol and THC-OH were detected in ruminal filtrates, and extremely high levels of (±)-THC-COOH were detected in plasma of goats. Human studies have revealed that THC-OH is the principal active metabolite with higher psychotropic activity than other cannabinoids, and is further oxidized to the inactive form of THC-COOH by liver enzymes (Escriva et al., 2017; Schwilke et al., 2009). Our results suggest that ruminal microbes might have played an important role in metabolizing psychoactive cannabinoids into inactive (±)-THC-COOH, which were then absorbed via ruminal epithelia and transferred into the blood. THC-COOH has a long half-life in the body of up to several days, making it the main metabolite tested in the blood or urine for cannabis use (Jamwal et al., 2017; Schwilke et al., 2009). Similarly, (±)-THC-COOH can also be used to monitor if animals were illegally provided hemp-related by-products. However, the inclusion of hemp in ruminant diets may have beneficial effects on animal welfare and health status, such as the increased lying behavior and decreased biomarkers of stress and inflammation observed in cattle (Kleinhenz et al., 2022) and reduced symptoms of severe gastric ulcers observed in pigs (Madsen et al., 2022). Due to this, future studies are warranted on hemp by-products utilization in stressful situations.
The numbers and types of secondary metabolites of hemp-related cannabinoids detected in the plasma differed greatly among the studies. For instance, Kleinhenz et al. (2020a) reported no detection of (−)-THC-COOH in any plasma sample at any sampling time, whereas a high concentration of (−)-THC-COOH was detected in a comparable concentration to THC-COOH in plasma in our study. The tetrahydrocannabinolic acid was the only cannabinoid detected in sheep plasma by Krebs et al. (2021), whereas four and five cannabinoids were detected in goats in the present study, and in cattle (Kleinhenz et al., 2020a), respectively. This inconsistency may have been caused by differences in the dietary contents of hemp, types of cannabinoids offered, and microbial communities in cattle, goats, and sheep. Our results also indicated that CBD and THC-OH could be absorbed directly from ruminal fluids into the blood via the ruminal epithelia.
Due to its lipophilic character, THC is rapidly absorbed into the fat and muscle tissues shortly after assimilation via the blood, resulting in a rapid decrease in plasma concentrations (Sharma et al., 2012). The Δ9-THC was detected in both the kidney and subcutaneous fat in all sheep fed hemp stubble, while Δ9-THC was only detected in a few of meat samples from sheep fed hemp stubble (Krebs et al., 2021). This suggests that Δ9-THC is more likely to be deposited in fat tissue other than muscle. In the current study, no detectable Δ9-THC was observed in all meat samples of goats receiving diets containing HF; however, detection was not carried out in fat samples. Our results indicate that inclusion of 20% HF in a high-forage diet did not lead to Δ9-THC residue in goat meat. The difference between the finding of the current and those of Krebs et al. (2021) could be because of the lower proportion of HF included in the present study, which resulted in lower contents of Δ9-THC. Jakubovic et al. (1974) reported that radioactivity appeared in the feces and urine of a lamb suckling milk from an ewe injected with 14C labeled THC, indicating the transfer of THC and its metabolites via milk. Even though the feed-to-milk transfer rate of THC was low (0.20% ± 0.03%) when using industrial hemp to feed dairy cows, the acute reference doses in milk and dairy products for specific consumer groups might be excessive (Wagner et al., 2022).
The presence of any cannabinoid residue in commercial products, such as meat and milk, is prohibited by many countries; thus, the inclusion of hemp-related by-products in livestock diets should be performed with caution. It has been suggested that the determination of a withholding period is required to enable the safe feeding of hemp stubbles to sheep (Krebs et al., 2021). Further research is required to gain a greater understanding of how the digestion of hemp by-products in ruminants affects the bioavailability of terpenes, cannabinoids, lignans, and polyphenols (Semwogerere et al., 2020), as well as their deposition in edible tissues, excretion pathways, and the time required for full excretion.
5. Conclusion
Increasing the substitution of AH with HF at rates of 5%, 10% and 20% in high-forage goat diets linearly reduced feed intake and improved feed efficiency without detrimental effects on growth performance and carcass traits. The substitution of AH with HF also improved ruminal fermentation, through a greater VFA concentration, higher propionate production, and nitrogen utilization. Enhanced ruminal fermentation, especially propionate production, was associated with an increased relative abundance of ruminal bacteria from genus Rikenellaceae_RC9_gut_group. The results indicated that ruminal microbes play an important role in hemp-related cannabinoid metabolism and most are absorbed into the blood in the form of (±)-THC-COOH. Substitution of AH with HF had small detrimental effects on the serum biochemistry of goats and no cannabinoid residues were found in meat samples, suggesting the overall safety of the inclusion of HF in the diet of goats. Based on the results, the HF10 (50% AH + 10% HF) diet is recommended due to the growth performance and ruminal fermentation characteristics under the current experimental conditions.
Author contributions
Duanqin Wu and Dalin Liu organized the experiment and gave some advice on experiment idea. Zhipeng Xu conducted the animal experiment and Tao Ran wrote the manuscript. Tao Ran and Wenzhu Yang conducted the experimental analysis. Duanqin Wu and Wenzhu Yang reviewed the manuscript and gave some advice. All authors read and approved the final manuscript.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This research was funded by the National Key Research and Development Program of China (2021YFD1300504), “Double First Class” Special Guidance Project, Team Building Funds, Research Startup Funds (561120213), Lanzhou University, Lanzhou, China, High-end Foreign Experts Recruitment Plan (G2023175005L), and The Agricultural Science and Technology Innovation Program, Chinese Academy of Agricultural Sciences (ASTIP-IBFC).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2024.01.003.
Contributor Information
Dalin Liu, Email: liudl@yzu.edu.cn.
Duanqin Wu, Email: wuduanqin@caas.cn.
Appendix supplementary data
The following is the Supplementary data to this article:
References
- Accetto T., Avgustin G. The diverse and extensive plant polysaccharide degradative apparatuses of the rumen and hindgut prevotella species: a factor in their ubiquity? Syst Appl Microbiol. 2019;42:107–116. doi: 10.1016/j.syapm.2018.10.001. [DOI] [PubMed] [Google Scholar]
- AMSA (American Meat Science Association) Research guidelines for cookery, sensory evaluation and instrumental tenderness measurement of fresh meat. 1995. Chicago, IL. [Google Scholar]
- Andre C.M., Hausman J.F., Guerriero G. Cannabis sativa: the plant of the thousand and one molecules. Front Plant Sci. 2016;7:19. doi: 10.3389/fpls.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC . 18th ed. AOAC International; Gaithersburg, MD: 2005. Official Methods of Analysis. [Google Scholar]
- Artegoitia V.M., Foote A.P., Lewis R.M., Freetly H.C. Rumen fluid metabolomics analysis associated with feed efficiency on crossbred steers. Sci Rep. 2017;7:2864. doi: 10.1038/s41598-017-02856-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailoni L., Bacchin E., Trocino A., Arango S. Hemp (cannabis sativa l.) seed and co-products inclusion in diets for dairy ruminants: a review. Animals. 2021;11:856. doi: 10.3390/ani11030856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., et al. Reproducible, interactive, scalable and extensible microbiome data science using qiime 2. Nat Biotechnol. 2019;37:1091. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte G., Dimauro C., Daghio M., Serra A., Mannelli F., Mcammond B.M., Van Hamme J.D., Buccioni A., Viti C., Mantino A., Mele M. Exploring the relationship between bacterial genera and lipid metabolism in bovine rumen. Animal. 2022;16 doi: 10.1016/j.animal.2022.100520. [DOI] [PubMed] [Google Scholar]
- Daghio M., Ciucci F., Buccioni A., Cappucci A., Casarosa L., Serra A., Conte G., Viti C., Mcammond B.M., Van Hamme J.D., Mele M. Correlation of breed, growth performance, and rumen microbiota in two rustic cattle breeds reared under different conditions. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.652031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escriva U., Andres-Costa M.J., Andreu V., Pico Y. Analysis of cannabinoids by liquid chromatography-mass spectrometry in milk, liver and hemp seed to ensure food safety. Food Chem. 2017;228:177–185. doi: 10.1016/j.foodchem.2017.01.128. [DOI] [PubMed] [Google Scholar]
- Griswold K.E., White B.A., Mackie R.I. Diversity of extracellular proteolytic activities among prevotella species from the rumen. Curr Microbiol. 1999;39:187–194. doi: 10.1007/s002849900443. [DOI] [PubMed] [Google Scholar]
- Henderson G., Cox F., Ganesh S., Jonker A., Young W., Global Rumen Census C., Janssen P.H. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci Rep. 2015;5 doi: 10.1038/srep14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huws S.A., Kim E.J., Lee M.R., Scott M.B., Tweed J.K., Pinloche E., Wallace R.J., Scollan N.D. As yet uncultured bacteria phylogenetically classified as Prevotella, Lachnospiraceae incertae sedis and unclassified Bacteroidales, Clostridiales and Ruminococcaceae may play a predominant role in ruminal biohydrogenation. Environ Microbiol. 2011;13:1500–1512. doi: 10.1111/j.1462-2920.2011.02452.x. [DOI] [PubMed] [Google Scholar]
- Iannaccone M., Ianni A., Contaldi F., Esposito S., Martino C., Bennato F., et al. Whole blood transcriptome analysis in ewes fed with hemp seed supplemented diet. Sci Rep. 2019;9:16192. doi: 10.1038/s41598-019-52712-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianni A., Di Domenico M., Bennato F., Peserico A., Martino C., Rinaldi A., Candeloro L., Grotta L., Camma C., Pomilio F., Martino G. Metagenomic and volatile profiles of ripened cheese obtained from dairy ewes fed a dietary hemp seed supplementation. J Dairy Sci. 2020;103:5882–5892. doi: 10.3168/jds.2019-17954. [DOI] [PubMed] [Google Scholar]
- Jakubovic A., Tait R.M., Mcgeer P.L. Excretion of thc and its metabolites in ewes' milk. Toxicol Appl Pharmacol. 1974;28:38–43. doi: 10.1016/0041-008x(74)90128-8. [DOI] [PubMed] [Google Scholar]
- Jamwal R., Topletz A.R., Ramratnam B., Akhlaghi F. Ultra-high performance liquid chromatography tandem mass-spectrometry for simple and simultaneous quantification of cannabinoids. J Chromatogr B. 2017;1048:10–18. doi: 10.1016/j.jchromb.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M.C., Datsomor O., Cheng Z.Q., Meng Z.T., Zhan K., Yang T.Y., et al. Partial substitution of alfalfa hay by stevia (stevia rebaudiana) hay can improve lactation performance, rumen fermentation, and nitrogen utilization of dairy cows. Front Vet Sci. 2022;9:899148. doi: 10.3389/fvets.2022.899148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D., Dai K.P., Xie Z., Chen J. Secondary metabolites profiled in cannabis inflorescences, leaves, stem barks, and roots for medicinal purposes. Sci Rep. 2020;10:3309. doi: 10.1038/s41598-020-60172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhenz M.D., Magnin G., Lin Z.M., Griffin J., Kleinhenz K.E., Montgomery S., Curtis A., Martin M., Coetzee J.F. Plasma concentrations of eleven cannabinoids in cattle following oral administration of industrial hemp (Cannabis sativa) Sci Rep. 2020;10:12753. doi: 10.1038/s41598-020-69768-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhenz M.D., Magnin G., Ensley S.M., Griffin J.J., Goeser J., Lynch E., Coetzee J.F. Nutrient concentrations, digestibility, and cannabinoid concentrations of industrial hemp plant components. Appl Anim Sci. 2020;36:489–494. [Google Scholar]
- Kleinhenz M.D., Weeder M., Montgomery S., Martin M., Curtis A., Magnin G., et al. Short term feeding of industrial hemp with a high cannabidiolic acid (cbda) content increases lying behavior and reduces biomarkers of stress and inflammation in holstein steers. Sci Rep. 2022;12:3683. doi: 10.1038/s41598-022-07795-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klir Z., Novoselec J., Antunovic Z. An overview on the use of hemp (cannabis sativa l.) in animal nutrition. Poljoprivreda. 2019;25:52–61. [Google Scholar]
- Krebs G.L., De Rosa D.W., White D.M., Blake B.L., Dods K.C., May C.D., et al. Intake, nutrient digestibility, rumen parameters, growth rate, carcase characteristics and cannabinoid residues of sheep fed pelleted rations containing hemp (Cannabis sativa l.) stubble. Trans Anim Sci. 2021;5:txab213. doi: 10.1093/tas/txab213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wu H., Chen W., Liu C., Meng Q., Zhou Z. Rumen microbiome and metabolome of high and low residual feed intake angus heifers. Front Vet Sci. 2022;9 doi: 10.3389/fvets.2022.812861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen P.A., Curtasu M.V., Canibe N., Hedemann M.S., Pedersen M.L.M., Lauridsen C. Non-targeted metabolomics of saliva to explore potential biomarkers for gastric ulceration in pigs fed hemp. Animal. 2022;16 doi: 10.1016/j.animal.2022.100477. [DOI] [PubMed] [Google Scholar]
- Mierlita D. Effects of diets containing hemp seeds or hemp cake on fatty acid composition and oxidative stability of sheep milk. S Afr J Anim Sci. 2018;48:504–515. [Google Scholar]
- EFSA Scientific opinion on the safety of hemp (cannabis genus) for use as animal feed.Panel on additives and products or substances used in animal feed (feedap) EFSA J. 2011;9:2011. [Google Scholar]
- NRC (National Research Council) 3rd ed. National Academy Press; Washington (DC): 2007. Nutrient requirements of small ruminants, sheep, goats, cervids, and new world camelids. [Google Scholar]
- Olagaray K.E., Bradford B.J. Plant flavonoids to improve productivity of ruminants - a review. Anim Feed Sci Technol. 2019;251:21–36. [Google Scholar]
- Owens F.N., Bergen W.G. Nitrogen metabolism of ruminant animals: historical perspective, current understanding and future implications. J Anim Sci. 1983;57(Suppl 2):498–518. [PubMed] [Google Scholar]
- Pang K., Chai S., Yang Y., Wang X., Liu S., Wang S. Dietary forage to concentrate ratios impact on yak ruminal microbiota and metabolites. Front Microbiol. 2022;13 doi: 10.3389/fmicb.2022.964564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourseyed Lazarjani M., Torres S., Hooker T., Fowlie C., Young O., Seyfoddin A. Methods for quantification of cannabinoids: a narrative review. J Cannabis Res. 2020;2:35. doi: 10.1186/s42238-020-00040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran T., Jin L., Abeynayake R., Saleem A.M., Zhang X.M., Niu D.Y., Chen L.Y., Yang W.Z. Effects of brewers' spent grain protein hydrolysates on gas production, ruminal fermentation characteristics, microbial protein synthesis and microbial community in an artificial rumen fed a high grain diet. J Anim Sci Biotechnol. 2021;12:1. doi: 10.1186/s40104-020-00531-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran T., Tang S.X., Yu X., Hou Z.P., Hou F.J., Beauchemin K.A., Yang W.Z., Wu D.Q. Diets varying in ratio of sweet sorghum silage to corn silage for lactating dairy cows: feed intake, milk production, blood biochemistry, ruminal fermentation, and ruminal microbial community. J Dairy Sci. 2021;104:12600–12615. doi: 10.3168/jds.2021-20408. [DOI] [PubMed] [Google Scholar]
- Reynolds C.K., Kristensen N.B. Nitrogen recycling through the gut and the nitrogen economy of ruminants: an asynchronous symbiosis. J Anim Sci. 2008;86:E293–E305. doi: 10.2527/jas.2007-0475. [DOI] [PubMed] [Google Scholar]
- Rode L.M., Yang W.Z., Beauchemin K.A. Fibrolytic enzyme supplements for dairy cows in early lactation. J Dairy Sci. 1999;82:2121–2126. doi: 10.3168/jds.S0022-0302(99)75455-X. [DOI] [PubMed] [Google Scholar]
- Salavardic Z.K., Novoselec J., Didara M., Steiner Z., Cavar S., Sabic A.M., et al. Effect of dietary hempseed cake on milk performance and haemato-chemicals in lactating alpine dairy goats. Animal. 2021;15:100255. doi: 10.1016/j.animal.2021.100255. [DOI] [PubMed] [Google Scholar]
- Schluttenhofer C., Yuan L. Challenges towards revitalizing hemp: a multifaceted crop. Trends Plant Sci. 2017;22:917–929. doi: 10.1016/j.tplants.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Schwilke E.W., Schwope D.M., Karschner E.L., Lowe R.H., Darwin W.D., Kelly D.L., Goodwin R.S., Gorelick D.A., Huestis M.A. Delta9-tetrahydrocannabinol (thc), 11-hydroxy-thc, and 11-nor-9-carboxy-thc plasma pharmacokinetics during and after continuous high-dose oral thc. Clin Chem. 2009;55:2180–2189. doi: 10.1373/clinchem.2008.122119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semwogerere F., Katiyatiya C.L.F., Chikwanha O.C., Marufu M.C., Mapiye C. Bioavailability and bioefficacy of hemp by-products in ruminant meat production and preservation: a review. Front Vet Sci. 2020;7:572906. doi: 10.3389/fvets.2020.572906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P., Murthy P., Bharath M.M. Chemistry, metabolism, and toxicology of cannabis: clinical implications. Iran J Psychiatry. 2012;7:149–156. [PMC free article] [PubMed] [Google Scholar]
- Srinivas B., Gupta B.N. Rumen fermentation, bacterial and total volatile fatty acid (TVFA) production rates in cattle fed on urea-molasses-mineral block licks supplement. Anim Feed Sci Technol. 1997;65:275–286. [Google Scholar]
- Tang S., He Y., Zhang P., Kang J., Yan Q., Han X., Tan Z., Wang H., Wu D., Yu L., Wang M., Zhou C., Jiao J. Substitution of ramie (boehmeria nivea) for alfalfa in improving the carcass and meat quality of Liuyang Black goats. Anim Nutr. 2021;7:688–694. doi: 10.1016/j.aninu.2020.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastolo A., Calabro S., Pacifico S., Koura B.I., Cutrignelli M.I. Chemical and nutritional characteristics of Cannabis sativa L. co-products. J Anim Physiol Anim Nutr. 2021;105:1–9. doi: 10.1111/jpn.13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner B., Gerletti P., Fürst P., Keuth O., Bernsmann T., Martin A., Schäfer B., Numata J., Lorenzen M.C., Pieper R. Transfer of cannabinoids into the milk of dairy cows fed with industrial hemp could lead to Δ9-THC exposure that exceeds acute reference dose. Nat Food. 2022;3:921–932. doi: 10.1038/s43016-022-00623-7. [DOI] [PubMed] [Google Scholar]
- Wang Z., Liu L., Pang F., Zheng Z., Teng Z., Miao T., Fu T., Rushdi H.E., Yang L., Gao T., Lin F., Liu S. Novel insights into heat tolerance using metabolomic and high-throughput sequencing analysis in dairy cows rumen fluid. Animal. 2022;16 doi: 10.1016/j.animal.2022.100478. [DOI] [PubMed] [Google Scholar]
- Xue M., Sun H., Wu X., Guan L.L., Liu J. Assessment of rumen microbiota from a large dairy cattle cohort reveals the pan and core bacteriomes contributing to varied phenotypes. Appl Environ Microbiol. 2018;84 doi: 10.1128/AEM.00970-18. e00970-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Mu Y., Zhang R., Xue Y., Guo C., Qi W., Zhang J., Mao S. Responsive changes of rumen microbiome and metabolome in dairy cows with different susceptibility to subacute ruminal acidosis. Anim Nutr. 2022;8:331–340. doi: 10.1016/j.aninu.2021.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z.G., Kristensen L., Difford G.F., Poulsen M., Noel S.J., Abu Al-Soud W., Sorensen S.J., Lassen J., Lovendahl P., Hojberg O. Changes in rumen bacterial and archaeal communities over the transition period in primiparous holstein dairy cows. J Dairy Sci. 2018;101:9847–9862. doi: 10.3168/jds.2017-14366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.