Abstract
Seaweed cultivation has garnered significant interest, driven by its wide range of biomass benefits. However, comprehensive assessments from various perspectives are imperative to ensure the sustainable cultivation of seaweed. Biotic and Abiotic factors can significantly impact seaweed yield in complex commercial farming. Biotic factors include bacteria, fungi, viruses, and other algae, while abiotic factors include environmental conditions such as temperature, salinity, light intensity, and nutrient availability. Additionally, the susceptibility of seaweeds to pests and diseases further compounds the issue, leading to potential crop losses. This study endeavours to shed light on the immense potential of macroalgae cultivation and underscores the pressing need for scientific advancements in this field. The comprehensive review clearly explains the latest developments in seaweed cultivation and highlights significant advances from diverse seaweed research. Moreover, it provides insightful glimpses into possible future developments that could shape the trajectory of this promising industry.
Keywords: Seaweeds, Cultivation, Challenge, Biotic and abiotic, Productivity
Graphical abstract
1. Introduction
The world population is expected to surpass 9 billion people by 2050, presenting a significant challenge in providing food and sustaining everyone, especially with climate change and depletion of essential resources. Addressing the needs of the growing population, the Food and Agriculture Organization of the United Nations (FAO) estimates that world food production would need to increase by 60%. Nevertheless, it is imperative to undertake this challenge sustainably, avoiding any additional negative impact on the environment [1,2]. For decades, seaweeds have been a traditional food source. Still, its recent application in various sectors, including food, feed, biofuels, chemicals, nutraceuticals, medicines, cosmetics, and environmental bioremediation, has increased worldwide seaweed production [3,4]. The potential benefits of seaweed cultivation and products, as depicted in Fig. 1, highlight the sustainable benefits associated with this practice. The demand for seaweed has escalated to the point where natural seaweed stocks are no longer sufficient to fulfill demand. To generate more seaweeds, seaweed mariculture, or seaweed farming, is required. Although wild seaweed has a wide variety of nutritional value, seaweed farming may give new approaches to manage or standardize seaweed's nutritional content [5]. The global market for seaweed farming and its products was estimated at USD 5.9 billion in 2019 and is expected to increase at a compound annual growth rate (CAGR) of 9.1% until 2027 [6].
Fig. 1.
Potential benefits of seaweed cultivation and products.
Accordingly, seaweed farming can solve the projected food shortages for the world's ever-increasing population [7]. Seaweed farming is a model of development, climate action, and the environment that can all work together to add value to communities. It can aid in creating a globe free of poverty on a liveable planet. Seaweed farms may provide environmental services such as carbon storage, nitrogen recycling, and socioeconomic advantages to vulnerable coastal communities [8]. To develop seaweed aquaculture for various applications, it is critical to understand the possible constraints and possibilities associated with establishing and operating a production unit and market chain. This includes considering social, economic, cultural, political, and environmental concerns to guarantee that the activity is long-term and can provide employment, money, and food [9]. Seaweed farming is one of the world's fastest-growing industries. It is practiced in 132 nations, with 48 million square kilometers of farmed areas. Seaweed is actively produced in 37–44 countries [10]. Since the early 2000s, the fast rise of the seaweed farming business has resulted in a surge in seaweed diseases and pests. The giant commercial seaweed-producing countries are also the most vulnerable to seaweed disease outbreaks [2]. Due to the significant decrease in hydrocolloid output caused by these outbreaks, the seaweed farming business may collapse if viable treatments or mitigation techniques are not developed. Biosecurity measures must be implemented at all levels of the production chain, from farms to the national government, to prevent the spread of diseases and pests [11,12]. Establishing appropriate biosecurity measures to safeguard consumer health, ensure industrial viability, and preserve the environment is critical.
The concept of biosecurity is not new, yet most seaweed-producing countries pay little attention to it. Only lately research has begun to focus on understanding seaweed illnesses and pathogens, their routes of infection, and potential treatment strategies [13].
The main identified threats affecting seaweed cultivations are reported below [13].
-
1.
Biotic factors – Ice Ice disease, Epiphytic attachments or Epiphytes and grazing
-
2.
Abiotic factors include temperature, pH, salinity, and hydrodynamics.
Pests and illnesses have plagued seaweed producers for many years. As a result, farmers have devised several cost-cutting ways to address the problem. Washing seaweed blades in an acid solution for several minutes is a popular treatment to fight illness [14]. Others have resorted to handpicking any attached epiphytes from seaweed stock as soon as possible or removing seaweed with EFA attachment to prevent them from spreading [[15], [16], [17]] [[15], [16], [17]] [[15], [16], [17]].
When domestic seaweed farming was being pushed for commercialization in the 1990s, the notion of using genetic modification for strain selection in seaweed cultivation was already being studied. The objective was to create seaweed strains with quicker growth rates and larger carrageenan yields, especially in eucheumatoids [16]. New pathways in seaweed production can be explored by harnessing these improvements. Over the previous two decades, several research publications have bestowed on widely used molecular methods and developments in breeding tools to boost production and raise seaweed resilience to disease, predation, and epiphytism [[18], [19], [20], [21]] [[18], [19], [20], [21]] [[18], [19], [20], [21]].
Long-term genetic improvement solutions must be established to solve biological problems in seaweed aquaculture. The use of genetic engineering has the potential to bridge the gap between basic and applied seaweed science. Long-term genetic improvement efforts are critical for addressing biological problems in seaweed agriculture. The use of genetic engineering has the potential to bridge the gap between basic and applied seaweed science [22]. Existing and developing seaweed enterprises might benefit significantly from a well-designed genetic improvement strategy that minimizes genetic abnormalities and focuses selection on a few ranges of commercially valuable phenotypes. Selective breeding for seaweed output and quality is expected in the Republic of Korea, Japan, and China [23]. Still, it is rarely utilized in tropical developing countries, which may help to explain the recent decline in seaweed production in those nations [24,25].
To constantly improve seaweed aquaculture, significant research has been undertaken, including.
-
•
Breeding and genetic improvement: develop seaweed aquaculture by selecting novel seaweed strains that are more suitable for production, disease- and stress-resistant (due to climate change), and more nutritious for human consumption [26,27].
-
•
Automation and optimization of seaweed aquaculture systems: development of automated techniques for cultivation and harvesting and optimization of culture conditions to increase efficiency and cost-effectiveness [28].
-
•
Bio refining of seaweed biomass: enhanced technologies for extracting necessary chemicals for commercial purposes such as biofuels, medicines, and cosmetics, which can boost the economic worth of collected seaweed biomass [29].
It is also critical for most seaweed production worldwide to include critical regulatory components. Effective framework creation also depends on the availability of evidence-based research, which is scarce for seaweed farming and biosecurity threats. As a result, the industry's biosecurity problems are not directly addressed by present international frameworks. It has been challenging to link the seaweed sector with well-established frameworks for aquatic animal health and terrestrial crop biosecurity due to the industry's unusual focal host taxonomy (neither plant nor animal) and its presence in the water. The recent addition of algal species in IPPC guidelines is a crucial first step in bringing seaweed up in debates about biosecurity and global aquaculture. These procedures must now be expanded to include all produced seaweed species and consider the seaweed business's unique biosecurity issues [30]. In light of the significant geographical disparity in seaweed productivity and utilization (e.g., promoting seaweed consumption), developing a global program on seaweeds for improving seaweed agriculture and the value chain, or a focused one on addressing critical challenges, is a pressing necessity.
1.1. Global status of seaweed production
Asian nations like China, the Philippines, Indonesia, Japan, and Korea are among the prominent regions where macroalgae are effectively mass-cultivated for commercial purposes worldwide. The productivity of seaweed increased significantly from 10.6 million tonnes in 2000 to 35 million tonnes in 2020, dominated by five major species Undaria, Pyropia, Gracilaria, Eucheumatoids, Saccharina [31]. The cultivation of both Phaeophyceae (3.1–16.4 million tonnes) and Rhodophyta expanded (1–18.3 million tonnes), while production of Chlorophyta seaweed declined (31000–17000 million tonnes) [32]. Over 95 % of global macroalgae production was accounted for by Laminaria saccharina (35.4 percent), Kappaphycus Eucheuma (33.5 percent), Gracilaria (10.5 percent), Porphyra pyropia (8.6%), and Undaria (8.6%) (7.4 percent). Europe's seaweed production amounted to 287,033 tonnes, contributing approximately 0.8% to the global total in 2019 [33]. According to a new World Bank analysis of 10 emergent seaweed markets, its development potential might reach $11.8 billion by 2030. Despite this forecast, most of the extra value in the seaweed business remains untapped - it has strong development potential beyond its present markets [8]. Increasing seaweed cultivation by 14% per year would result in 500 million tonnes of dry weight or a 10% increase in the food supply, income generation, and quality of life by 2050, as reported in another World Bank report in 2016 [34]. Seaweed farming mainly focuses on limited countries, with East and Southeast Asia dominating.
The collection and utilization of seaweeds as human food and medicines in eastern Asian countries dates back at least 1500 years. In East Asia, modern seaweed cultivation was established in Korea, Japan, and China roughly from the 1950s–1970s [23]. Since then, it has expanded quickly, and seaweed farms are gaining popularity in other regions worldwide. Countries outside Asia produced less than 2% of the total farmed seaweed volumes in 2020 [35]. Nevertheless, the outlook for scaling seaweed production in other parts of the world is promising. However, East and Southeast Asian nations continue to produce most of the world's farmed seaweeds, with China, Indonesia, the Philippines, North and South Korea, Japan, and Malaysia contributing about 98% of the total produced [35]. In 2019, 98 countries made 2.65 billion US dollars by exporting macroalgae (909 million US dollars) and hydrocolloids (1.74 billion US dollars). According to the United Nations Comtrade database, exports of macroalgae and products in 2019 resulted in profits, as shown in Table 1.
Table 1.
Export of seaweeds and seaweed-based hydrocolloids, 2019 [36].
| Countries | Million USD |
|---|---|
| China | 578 |
| Indonesia | 329 |
| Republic of Korea | 320 |
| Philippines | 252 |
| Chile | 209 |
| Spain | 145 |
| France | 124 |
| USA | 102 |
| Germany | 82 |
| UK | 78 |
Using seaweed nutrients from ocean is an integral approach to combat eutrophication and achieve long-term sustainability. Additionally, a recent study demonstrated that extensive cultivation of Saccharina japonica (Phaeophyceae) could raise seawater oxygen levels and mitigate seawater acidification [37]. This research implies that seaweed might be extremely important for restoring and safeguarding the marine ecosystem. According to a life cycle impact study of seaweed farming and nutrient removal in Europe, seaweed may have a beneficial impact at a large scale (208 km2); bioremediation of nitrogen and phosphorus from anthropogenic activities in the aquatic system can enhance management methods at the water bodies [38]. According to Refs. [39,40], seaweed farms in Long Island Sound and the Bronx River Estua in the United States retained up to 300-1800 kg of carbon per hectare during cultivation. Across China [41], seaweed farms of diverse species store 421.78 tons of carbon per square kilometer annually. According to Refs. [41,42], most nitrogen and phosphorus are currently removed from Chinese waters by farmed kelp due to a combination of total harvest yield and tissue nutrient content. Red seaweed had the best capacity to remove nitrogen in an upscaling scenario [42] addition [43], showing that growing Gracilaria lemaneiformis could remove 128.10 tonnes of nitrogen, 15.89 tonnes of phosphorus, and 1192.03 tonnes of carbon from Yantian Bay seawater. Besides this, increasing macroalgae in industrial wastewater ponds can help reduce pollution [44].
Water scarcity may be one of the biggest obstacles to expanding global food production. The production of seaweed may help conserve up to a thousand liters of freshwater per kg compared to land crops [45]. Seaweed farming offers potential for "blue growth, but it also faces pioneering challenges due to the growing competition for ocean and coastal resources. Consequently, this emerging activity may escalate coastal disputes, encompassing various aspects of organizational, social, financial, and environmental sustainability. Hence, the economy and the environment benefit from the rise of the seaweed aquaculture business.
1.2. Objective of the review
This goal is to identify gaps in the existing literature and propose a viable solution for sustainable seaweed cultivation for the benefit of humanity. As a result, this article presents a thorough analysis of seaweed cultivation, emphasizing the following aspects.
-
o
To comprehensively assess the potential of macroalgae farming as a strategic pathway in addressing climate change and contributing to Sustainable Development Goals,
-
o
To provide an overview of current and emerging trends in macroalgae cultivation, including hatchery and open-sea methods, and assess their strengths and limitations.
-
o
To identify and explore possible solutions to address the macroalgae farming industry's biotic, abiotic, and socio-economic challenges.
-
o
To recommend strategies for driving innovation and fostering sustainable practices in macroalgae cultivation for long-term success.
2. Seaweed farming for addressing climate change and sustainable development goals
This section discusses the intricate relationship between seaweed farming and two critical global imperatives: addressing climate change and advancing Sustainable Development Goals (SDGs).
2.1. Seaweed's role in the climate change
Seaweed offers a promising natural solution for mitigating the effects of climate change as shown in Fig. 2.
Fig. 2.
Potential contribution of seaweed cultivation towards climate change [46,47].
Climate change mitigation refers to strategies and policies that aim to decrease the concentration of greenhouse gases in the atmosphere, either by reducing their emissions or increasing their capture. As a result of increased carbon dioxide emissions, global temperatures are increasing. The situation is deteriorating, mainly due to the rapid economic growth of developing nations whose carbon dioxide emissions are anticipated to rise soon. Seaweeds have the potential to serve as a renewable energy source and carbon sink; furthermore, seaweeds may play a significant role in climate change mitigation strategies [46].
Seaweed offers different potential pathways to mitigate climate change, as reported in the literature, such as.
-
•
Preserving and rebuilding wild seaweed forests may help mitigate climate change.
-
•
Increasing the sustainability of nearshore seaweed aquaculture with possible climate change mitigation co-benefits
-
•
Reducing industrial CO2 emissions by the use of seaweed products
-
•
Sequestering CO2 by cultivating seaweed in the deep sea [[48], [49], [50], [51]].
The carbon sequestration potential of certain seaweeds is as follows: Eucheuma spp. can sequester 68.43 tonnes of carbon/hectare/year, Kappaphycus striatus (Rhodophyta) can sequester 125.51tonnes of carbon/hectare/year, Laminaria spp. (Phaeophyceae) can mitigate 1156 tonnes of carbon/hectare/year, Ecklonia spp. (Phaeophyceae) can sequester 562 tonnes of carbon/per hectare/year, Sargassum spp. can sequester 346 (tonnes of carbon/hectare/year, and Gelidium spp. (Rhodphyta) can sequester 17 tonnes of carbon/hectare/year, as reported in Ref. [52]. According to Ref. [53], the total carbon sequestration by seaweed farming in Indonesia was 621,377 tonnes per year from pond/marine culture and 2.66 million tonnes of carbon per year from land culture. Thus, seaweed can sequester carbon and reduce atmospheric carbon dioxide levels, thereby mitigating the effects of global warming.
Taking all feasible measures to reduce atmospheric carbon dioxide load to prevent ecological damage is essential [54,55]. Climate change has prompted a blue carbon paradigm in which food and fuel can be obtained from aquatic environments through carbon harvesting, carbon sequestration, and carbon sinking [56]. Global climate change impacts are becoming more visible and affecting terrestrial and marine environments [57,58], all of which have significant economic consequences [59]. Some coastal and marine ecosystems' ability to absorb and sequestrate carbon can be crucial to mitigation attempts [60]. Considering these criteria, seaweed farming can be a viable approach for developing coastal countries to mitigate climate change as it requires meager investment and has many additional benefits. Seaweed wastes or biomass can be utilized as feedstocks for anaerobic digestion to create biomethane, which can be used as a bioenergy source in place of fossil fuels as a way to mitigate the effects of climate change. An inventive way to replace synthetic, non-biodegradable plastics and save the environment is to use bioplastic made from seaweed. Another strategy for reducing climate change is the conversion of seaweed biomass to biochar [61].
2.2. Seaweed farming towards sustainable development goals
Meeting UN SDGs goals on time and scale while the human population grows requires novel, potentially disruptive strategies to deliver the transformational change necessary. Specifically, there is a need to identify novel bioresources that can be grown sustainably, with minimal requirements of arable land, water and energy, support a net production of healthy food for humans and animals raised on land and at sea, sustainable and cost-effective energy, and provide sustainable materials harmless to the environment, while delivering positive, rather than negative impacts on biodiversity and the environment.
Seaweed farming is a viable and sustainable aquaculture method that can contribute significantly to fulfilling the UN Sustainable Development Goals (SDGs). It has the potential to meet SDG goals related to poverty eradication, food security, climate action, and sustainable consumption and production [62]. Seaweed aquaculture generates multiple ecosystem services that lead to direct benefits in advancing several SDGs, such as 1,2,3,5,6,7,8,12,13,14,15,17, as shown in Fig. 3. Investment in environmentally friendly seaweed farming can ensure poor and vulnerable people's access to productive resources, women's participation in food production and consumption, self-entrepreneurship in the creation of decent jobs for men and women, local economic growth, and the resilience of coastal poor and vulnerable communities [7].
Fig. 3.
Contribution of seaweed farming to sustainable development goals.
SDG 1 – No Poverty: Seaweed farming can address poverty (SDG 1) in various ways beyond producing revenue. Economic growth and revenue production empower people individually and in communities. From cultivation and processing to marketing and distribution, seaweed farming generates various revenue streams throughout the value chain. This encourages entrepreneurial skills, strengthens local economies, and allows people and families to leave poverty [63].
SDG2 - Zero Hunger: Currently, 90% of produced seaweeds are utilized directly for human consumption or as additives, with the primary latter consisting of hydrocolloids like agar, alginates, and carrageenans that are used in the food and pharmaceutical sectors to change the viscosity of their products [64].
SDG3 - Good Health: Seaweeds are beneficial additions to human diets because they include fiber, antioxidants, macro- and micronutrients, and good fatty acids that reduce the risk of several ailments [65]. Because certain seaweeds include up to 45% protein by weight, seaweed is a flexible food that may be utilized as an alternate source of protein in addition to adding nutrients to meals and dietary supplements [62].
SDG 5 - Gender equality: Beyond providing food and income, seaweed farming offers revolutionary potential for women's empowerment and aligns with SDG 5. Embracing seaweed farming opens for women to thrive alongside men in a more inclusive society and to make significant contributions. It also acts as a catalyst for the progress of gender equality. Tanzania and Kenya researched how seaweed cultivation empowers women living along the shore. Seaweed is grown, processed, and sold to give them a living and financial freedom. This enhances their living standard while bolstering their ability to make decisions and assume leadership positions within their communities. Seaweed farming offers these coastal communities a viable path toward gender equality and sustainable development [66].
SDG 6 – Clean water and Sanitation: Seaweed functions as a natural "biofilter" by drawing nutrients like nitrogen, phosphorus, and heavy metals from the water. This not only keeps our seas cleaner but some species may be used to absorb nitrogen runoff, which has been negatively affecting the health of reefs in places like the Great Barrier Reef. Seaweed's capacity to filter water may also be used to treat effluent from cities and aquaculture [62].
SDG 7 - Affordable and Clean Energy: Through a variety of processes, including fermentation, hydrogen release, transesterification, pyrolysis, liquefaction, and gasification, seaweed biomass may be utilized to create ethanol, butanol, biogas, biodiesel, bio-oil, or hydrogen [67]. Demands for seaweed-based biofuels, currently using about 1% of seaweed production [64], are likely to rise, driven by transportation demand. The shipping and aviation sectors are dedicated to maintaining current emissions from fossil fuels or reducing them, and they both anticipate growth of around 6% per year. Seaweed has emerged as a potential source in the hunt for energy-dense, zero-carbon fuels that these industries need to meet their energy demands, as the widespread production of green hydrogen is still a long way off [68].
SDG 8 - Potential to provide sustainable livelihoods: Large communities might benefit from a developed economy centered on small-scale seaweed farming (particularly for women). Furthermore, it will open up economic options for coastal villages that have been hindered by a lack of market for their products [69].
SDG 12 – Responsible consumption and production: Certain seaweeds can be utilized as alternative materials for a variety of purposes, including the production of bioplastics, due to their unique physical characteristics. Seaweed presents a viable alternative to petroleum-based materials for producing edible and biodegradable bioplastics, as plastic pollution in our oceans is a severe problem. The seaweed-based bioplastic produced by Perth-based Uluu has garnered media attention due to its remarkable attributes. The substitute plastic is said to be biodegradable if it ends up in the ocean, is compostable at home, and is carbon negative (since the creation of it absorbs more carbon than is released) [62].
SDG 13 - Biological sequestration of carbon dioxide: Seaweed cultivation has the potential to develop into a significant source of carbon sequestration as part of ecosystem services. Compared to other maritime plants, they likely have the most potential for this [70]. Growing seaweed may eliminate significant levels of phosphorus and nitrogen from aquatic environments. This contributes to the coastal ecosystem's stabilization. This prevents associated issues such as inadequate nourishment for marine life and low atmospheric oxygen levels [71]. It is commonly known that methane emissions from cattle play a significant role in climate change. Methane generated by ruminants is a pivotal contributor to the 10% of greenhouse gas (GHG) emissions from agriculture in the United Kingdom. It's a relatively recent concept to augment animal feed with seaweed to lower methane gas emissions. Small amounts of a red seaweed type were shown to reduce methane emissions by 80% when fed to cattle, according to research conducted in the US and Australia [72].
SDG 14 - Enhancement of habitat for coastal aquatic species: Research on seaweed aquaculture contributes to the enhancement of habitat for coastal marine species, demonstrating a positive impact on select environmental indicators. It has been observed that their adverse effects on the environment are minimal [73]. Seaweed farms may offer a low-cost adaptation approach to ocean acidification and deoxygenation, as well as crucial refuges from ocean acidification, according to a study conducted across a latitudinal range in China [69]. More greenhouse gasses may be extracted from water by seaweed than by mangroves or eelgrass. In actuality, seaweed may absorb these gases more than the combined biomass of eelgrass and mangroves. As a result, they can lessen the effects of ocean acidification locally [74].
SDG 15 – Life on Land: Seaweeds may have significant implications for terrestrial life as they are not terrestrial plants. Seaweed has several applications, including an environmentally friendly, sustainable pesticide and a fuel. Beyond this, seaweed benefits terrestrial ecosystem health. Furthermore, seaweed farming does not occupy limited agricultural land [75]. In addition, seaweed farming offers a solid way to accomplish SDG 15 by fostering responsible resource use, safeguarding ecosystems, and producing sustainable bio-based goods. We can fully realize the promise of this ocean-based solution for a healthier world and a more sustainable future by addressing the obstacles and encouraging cooperation [61,76].
SDG 17 – Partnerships for the Goals: Growing seaweed is a potent metaphor for SDG 17: Partnerships for the Goals. It encourages cooperation across different fields and specialties, illuminating how teamwork may solve complex problems and advance sustainable growth. Science and inventions are the primary drivers behind macroalgae or seaweed developments. Establishing strong partnerships between the algal sector and the interdisciplinary scientific community is vital to realizing seaweeds' vast potential and converting them into edible, accessible, and reasonably priced food or non-food items. The public sector can aid the process by funding fundamental research on diet, genetic resources, and illnesses [77]. An international coalition of governments, non-governmental organizations, and business executives, the Global Seaweed Alliance works to advance sustainable seaweed cultivation methods worldwide [78].
3. Overview of macroalgae (seaweed) cultivation
Before 1949, scientists and farmers did not know how to reproduce seaweed in a controlled environment. In 1949, Kathleen Mary Drew-Baker, a British botanist, researched the reproduction cycles of Porphyra umbilicalis, helping scientists and farmers to understand how to cultivate seaweed. Marine Colloids, the predecessor of Acadian Seaplants Limited, built a $1 million tank cultivation site in Charlesville, Nova Scotia, to produce Chondrus crispus, a type of seaweed used to extract carrageenan [79]. North America conducted several experiments to grow seaweed in offshore/nearshore systems in the late 1970s and early 1980s, but none culminated in industrial algae production [80]. Seaweed cultivation is now a significant industry, with various methods depending on the seaweed farmed. The taxonomical characteristics of the seaweed species solely determine the cultivation method. Certain species (Eucheuma, Kappaphycus, Chondrus, and Gracilaria) require a single-step farming approach by vegetative propagation, while others require two-step or multistep farming [81]. A seaweed cultivation system is made up of two main components (Fig. 4): (i) a hatchery and (ii) an on-growing site (either land-based tanks or at sea).
Fig. 4.
Classification of seaweed cultivation [82].
Some seaweeds can be cultivated vegetatively, while others can only be produced through a separate reproductive cycle involving the alternation of generations. In vegetative cultivation, small pieces of seaweed are taken and placed in an environment that will sustain their growth. Once they reach an optimal size, these plants are harvested by extracting the entire plant or trimming most of it while retaining a small portion capable of regrowth. When the whole plant is removed, small pieces are cut from it and used as feedstock for further cultivation. The suitable environment varies among species but must meet requirements for a salinity of the water, nutrients, water movement, water temperature and light. The seaweed can be held in this environment in several ways: pieces of seaweed may be tied to long ropes suspended in the water between wooden stakes or tied to ropes on a floating wooden framework (a raft); sometimes netting is used instead of ropes; in some cases, the seaweed is placed on the bottom of a pond and not fixed in any way; in more open waters, one kind of seaweed is either forced into the soft sediment on the sea bottom with a fork-like tool or held in place on a sandy bottom by attaching it to sand-filled plastic tubes [83].
The cultivation of seaweed has been identified as a ‘clean’ industry due to its potential environmental benefits. Macroalgal biomass is increasingly in demand across Europe. Its wide range of applications is moving beyond food (the main production focus in Asian countries, the most significant producers of cultivated seaweed) and animal feed (the primary focus for current production in Europe). At the moment, macroalgal biomass production is dominated (>95%) by cultivated strains rather than wild harvests, which accounted for only 4.5% of the world's total seaweed production in 2010 [84]. There is a growing need for a sustainable source of macroalgal biomass. Recent years have seen growth in the seaweed industry in Europe through the application of seaweed extracts in nutraceuticals, pharmaceuticals and as soil enhancers, as well as becoming increasingly of value as a food source or “sea vegetable” due to increased awareness of the health benefits of eating seaweed and seaweed extracts: improved weight loss [85], combating mineral deficiency [86], antioxidant [87] and anti-tumor properties [88].
3.1. Hatchery cultivation
There are several basic requirements for a seaweed hatchery: • filtered seawater • filtered air supply • lighting • chiller unit • tanks • seeders or rope • microscope • flasks • storage as depicted in Fig. 5. Attention to energy consumption is critical to minimize operating costs during the hatchery phase [89]. For instance, choosing an air blower with the correct power/output is crucial because it has to be operated continuously for several weeks to months. It is one of the most energy-consuming equipment used [82].
Fig. 5.
Layout of hatchery cultivation Setup [90].
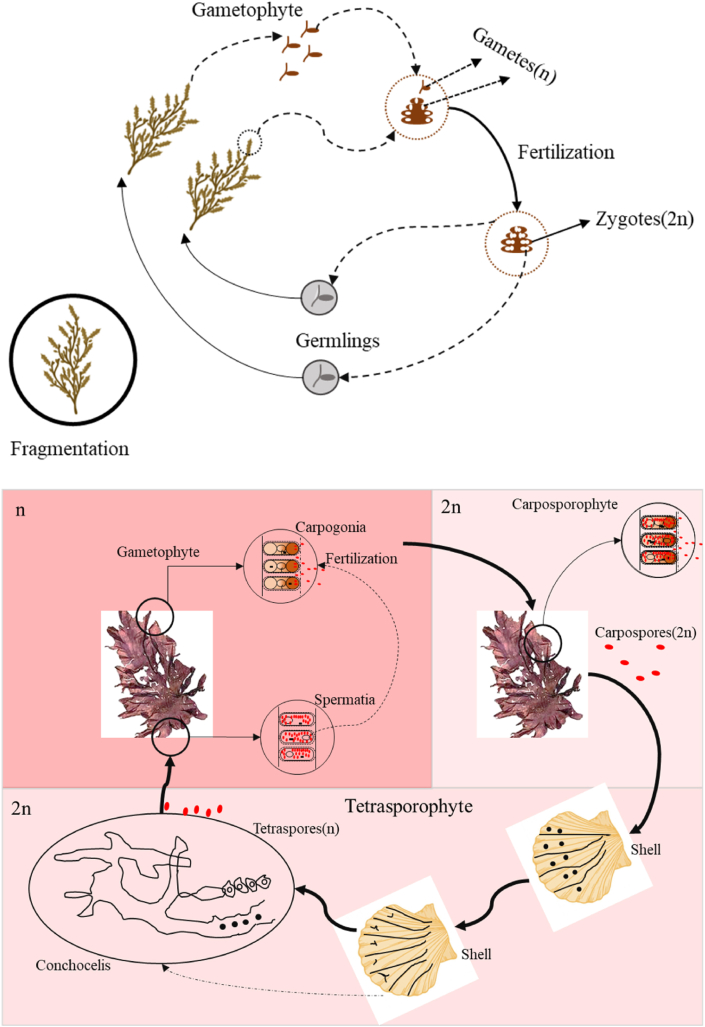
From an agronomic perspective, there are two types of seaweeds: clonal and nonclonal shown in Fig. 6. Non-clonal seaweeds are cultivated through vegetative propagation, a form of asexual reproduction. In contrast, unitary seaweeds (produces one frond from its hold fast) [91], such as kelps, require sexual reproduction in a hatchery or nursery for cultivation [3]. Non-clonal are typically farmed in a single phase, which involves attaching the fragments to the ropes and nets and setting them up in the farm site. Examples include Eucheuma, Kappaphycus, and Caulerpa while clonal seaweed before being installed on farm areas. Farming often entails in-vitro fertilization and nursery raising of young plants on nets. Several high-value seaweeds, like Porphyra, Gracillaria, Saccharina, Undaria, Ulva and Monostroma, are examples of clonal seaweed [92].
Fig. 6.
Summary of clonal and non-clonal life cycles. a Sargassum. b. Porphyra [92].
Hatcheries are the first step in clonal seaweed production before marine culture. This allows for continuous production with less seasonal impact, as well as reduced sensitivity to biomass degradation, diseases, and pests, such as the abrupt sporulation of mature thalli, which is common in disease-causing species [93], might be reduced by regulating the time of Ulva sp. germination or by retaining sporeling in a hatchery [94]. Attaching propagation of macroalgae along the ridges is the most widely used technique for producing seaweed close to the beach underwater.
A hatchery offers safe zones for seedlings and facilities for establishing grow-out arrays. Optimizing hatchery development methods is critical to the survival of seaweed species produced by sexual reproduction. Fertilization of seaweed juveniles during the hatchery is thought to sustain the initial phases of the macroalgae breeding and thus increase production yield during the develop-out phase. A hatchery must have adequate electrical capacity, transport links, a storage room, a laboratory, and other support services. The juvenile seaweeds are transplanted into the sea after a productive hatchery cycle, where they will live until harvest. In a seaweed hatchery, sporulation and the transition of generations from gametophyte to multicellular sporophytes are usually controlled during the first development process, depending on fertilizer supply, water quality, gas flow, and water exchange rates. The initial processing cycle typically (lasts 9–12 weeks) [95]. Research has found that the generation of juvenile sporophytes is a critical impediment to the establishment of seaweed cultivation for the generation of biofuel.
In the study by Ref. [96] in Bohol, central Philippines, the first experiment where Sargassum aquifolium (Paheophyceae) zygote was successfully created in the hatchery utilizing a variety of substrates. It raised its germlings before planting. Large-scale Saccharina japonica hatchery production was described by Ref. [97] as pre-treatment of parental seedlings, management of spore discharge, monitoring and modification of temperature and solar irradiance at various phases of the plant's growth, as well as the nutrient delivery regime over the length of the experiment. According to Ref. [98], to partially replace manual devices in various manufacturing phases, Hatchery must build mechanized equipment such as seedling curtain weaving machines, automatic curtain brushing machines, automated light adaption systems, and rope stretching devices.
3.2. At Sea Cultivation
Growing seaweed in real-life conditions with sunlight, heat, water motion energy, and nutrients is traditional. Seaweed production on natural and artificial substrata with native and exotic seaweeds can be grown on ropes or lines hanging along the water's edge or many meters beneath it. The off-bottom monoline technique is the most popular because of its accessibility, low price, installation, and maintenance efficiency. The conventional culture technique for Macrocystis pyrifera (Phaeophyceae) is long-line cultivation shown in Fig. 7, although lines are first coated with spores before being deployed offshore. This technique may produce very high biomass yields [99].
Fig. 7.
Schematics of long-line cultivation [90].
However, each seaweed species may necessitate developing and deploying new anchors, buoyancy systems, ropes, harvesting boats, and transportation. Brazilian viable production used the tie-tie technique at first. According to Ref. [16], the most systematic approach employed in commercial Kappaphycus alvarezii (Rhodophyta) farming is the vegetative multiplication of seedlings attached using a technique called the tie-tie method to long lines. Offshore production is likely to result in epiphytes, impairing biomass productivity. Nevertheless, this risk can be considerably diminished by regulating growing regions and maintaining clean environmental conditions [100].
This approach involved growing seaweeds in small natural water bodies with the help of natural or artificial light, nutrients, and phytohormones and controlling weeding, lighting, and water motion. Land-based tank systems, pioneered in the 1970s in Canada and the United States, revolutionized seaweed production. These species often possess morphologies or dimensions incompatible with standard open-ocean farming methods [101,102]. The input from several forms of energy and nutrients determines the efficiency of this system. Cultivation in tanks or ponds provides a more significant opportunity to regulate some factors driving seaweed vitality. Growth is artificially controlled by greenhouse covers and illumination (LED/HID lights with different spectrum emissions). More seawater can be pumped, or CO2 can be added to increase the carbon supply. Mixing fresh/seawater ratios can regulate salinity. Pumping pressurized water into the tanks causes the seaweeds to rotate, inducing water flow and, consequently, displacement of the seaweeds. Fig. 8 represents a pictorial description of various seaweed cultivation methods utilized at sea.
Fig. 8.
Schematic of at-sea cultivation.
Another promising advancement in seaweed cultivation is integrated multi-trophic aquaculture (IMTA) as shown in Fig. 9. This approach is an intensive type of cultivation in which one or more organisms are farmed together to maximize productivity, avoid wastage, and provide ecological functions like bio-remediation. Environmental protection and financial prosperity achieved by diversification, risk mitigation, acceptance via improved production methods, and social equity are all important factors to consider. Seaweeds are essential to IMTA systems because they support transforming nutrient-rich streams into useable resources, reduce eutrophication and maintain water quality [102,103]. Integrated seaweed mariculture is practiced in rivers, lakes, and tanks of effluents drained from reservoirs holding fish or invertebrates. However, there are concerns that co-cultured species like shellfish and seaweeds might serve as disease "reservoirs" for fish. Surprisingly, recent research by Ref. [104] and some findings suggest that adequately chosen secondary species in an IMTA context may have some disease control prospects. Mussels (Mytilus edulis) can decrease the water's infectious salmon anemia virus (ISAV). The growth of the alien species Codium fragile subsp. Fragile (formerly Codium fragile subsp. tomentosoides) (Chlorophyta) is one possible issue with the combined culture of seaweed-salmon farms. This intrusive alga competing with Gracilaria (Rhodophyta) for nutrients might affect biomass and agar quality [105].
Fig. 9.
Schematic of IMTA cultivation.
3.3. Prior studies on macroalgae cultivation
Although large scale seaweed cultivation is still in its initial stages, understanding the key limitations limiting the global seaweed production is crucial for its expansion. Table 2 presents an overview of various research work on seaweed cultivation techniques.
Table 2.
Various research work on macroalgae farming.
| Cultivation Type | Species | Method | Result | Reference |
|---|---|---|---|---|
| Onshore | Ulva lactuca (formerly Ulva fasciata) (Chlorophyta) | Pulse-fertilization, nitrogen enrichment strategy. | Reduce epithelization, Productivity 18.8 and 6.8g dry wt m∼2 day-1 |
[106] |
| Offshore | Saccharina latissima (formerly Laminaria saccharina) (Phaeophyceae) | Ring carrier | Resistance to inclement weather. | [107] |
| Onshore | Gracilaria cornea (Rhodophyta). | 1000 L tanks, pH 8.0 , four treatments designated (1) N + P, (2) N + P + CO2, (3) flue gas, and (4) N + P + flue gas. |
Average annual growth rates were 91.3% and 94.1% per week for N + P + CO2 and N + P + flue gas, respectively, and 82.9% and 77.5% per week for N + P and flue gas. Reduced manufacturing costs |
[108] |
| Onshore | Ulva prolifera (Chlorophyta). | Germling clusters were transplanted to tank cultivation, where deep seawater (DSW) was supplied. (12–25 °C). | Average daily growth rate 37%. Culture remains free of significant epiphytic development for more than a year. |
[109] |
| Onshore | Ulva clathrate (Chlorophyta). | Cultivation with shrimp effluent, regular aeration | Effective at removing inorganic nutrients from effluent water. | [110] |
| Offshore |
Eucheuma denticulatum (Rhodophyta), Kappahycus alvarezii (Rhodophyta). |
Cultivation in Floating cages. |
Eucheuma denticulatum, K. alvarezii specific growth rate (SGR) 3.32%, and 3.1%. Herbivore predation decrease. |
[111] |
| Offshore | Gracilaria dura (Rhodophyta). | Cultivation in tube-net | Mean daily growth rate from 1.88 ± 0.23% day−1 to 3.30 ± 0.25% day−1. | [112] |
| Onshore | Ulva fasciata, Ulva compressa (Chlorophyta),Hypnea musciformis (Rhodophyta). | Spray and pond culture | 84 g FWm−2 day−1, 44–84% of yield and quality of spray-grown U. fasciata was obtained on 6°-inclined trays. H. musciformis and U. compressa, yielded up to 286 g FW m−2 day−1 and 172 g FWm−2 day−1. Spray culture method could provide markets with seaweed of acceptable quality, quantity, and price. |
[113] |
| Onshore | Ulva lactuca (formerly Ulva fasciata) (Chlorophyta) | Cultivation in a multi-tubular airlift photobioreactor | Averaged productivity of 0.87 kg m−2 d−1. | [114] |
| Offshore | Ulva fenestrate (Chlorophyta) | Hatchery temperature (10 and 15 °C), nutrient addition (PES and 3xPES), swarmer density (500 and 10,000 swarmers ML−1), off-shore rope cultivation. | Total fatty acid content (3.2–3.55% dw), mean total protein content (16.6–20.7% dw), average total chlorophyll a (1.29–1.69 mg g−1), chlorophyll b (0.73–1.32 mg g −1) and carotenoids (0.44–0.85 mg g−1). Prove as successful and cost-effective. |
[115] |
4. Recent trends in seaweed cultivation
Table 3 summarises innovative projects and research that are looking to create new methods to boost the profitability of seaweed production. To lessen the demand for seaweed, the governments of different countries launched several seaweed culture programs centered on research and pilot-scale production. Further projects are designed to create a culture system for the mass production of seaweed.
Table 3.
Status of commercial seaweed projects.
| Project name | Project Location | Developer | Innovation | Reference |
|---|---|---|---|---|
| GENIALG | Europe | GENIALG/EU Horizon 2020 | Developed innovative solutions to help production of seaweed biomass in Europe to become more economically and environmentally sustainable | [116] |
| REBECA | Macaronesia Region | Banco Español de Algas (BEA –Spanish Bank of Algae | Collection of seaweed from Macaronesia to conserve the biological diversity of the local seaweed and ocean life. | [117] |
| SeaStrains | Europe | The Alfred Wegener Institute | to lead the transition from decentralized, poorly documented seaweed stock cultures to a centralized, easily accessible biobank network of genetically and phenotypically characterized seaweed strains resources under the threat of global change through a centralized biobank. | [118] |
| SUBMARINER | Baltic sea region | SUBMARINER Network for Blue Growth EEIG | envision algae as a transformative tool to mitigate eutrophication, bolster aquatic ecosystems, and provide an eco-friendly source of nourishment for both human and animal consumption. | [47] |
| SIMBA project | Spain | CTAQUA | Cultivation of sea lettuce in open exchange with natural seawater in indoor cylinders, outdoor tanks, and salt evaporation ponds. | [119] |
| SIMBA project | Europe | WP3 leader NIOZ/NWO-I | The development of the microbiome growing on the seaweed Thalli (the plant body of algae that has a simple structure that does not have specialized tissues typical of higher plants, such as a stem, leaves and conducting tissues) will be determined, to compare this among cultivation systems and to study its potential relation with seaweed growth rate and biochemical composition. The final goal is to come to a formulation of the optimal microbiome composition for sustainable Ulva production in outdoor tanks. | [119] |
The Global Challenge Research Fund of the UK Research and Innovation financed a four-year, challenge-driven Global Seaweed STAR initiative [120] from October 1, 2017 to December 31, 2021. The study conducted by Global Seaweed STAR focused on the identification of pathogens and pests, biosecurity procedures and guidelines, genetic resources for algae, and socioeconomic stability in the seaweed business. By offering stakeholders in developing nations practical solutions and training, Global Seaweed STAR has solved the issues plaguing the seaweed industry, ensuring the long-term viability of this significant sector [120].
In its most recent endeavor, the Blue Natural Capital Financing Facility teamed up with the entrepreneurial business Seatech, which makes underwater habitats for seaweed. Sentech Energy offers underdeveloped areas the chance to boost their seaweed sector's competitiveness or launch thriving economic growth [121].
Grow-Trees.com helps to boost the income and self-sufficiency of the coastal community in Munaikkadu, Mandapam Camp, Ramnad District, Tamil Nadu. The organization offers equipment and experience to fishing communities through a seaweed farming initiative. The recently announced Algae Demo project, which the EU sponsors, intends to show that it is possible to cultivate specific seaweed varieties on a big scale and sustainably in open waters off the coast of Europe. With the help of the European Maritime Fisheries and Aquaculture Fund (EMFAF), the Algae Demo project aims to industrialize and commercialize seaweed farming while ensuring long-term survival capacity [122].
The project proposed off Duqm Omani coastal waters is tipped to host a large-scale seaweed cultivation project. The proposed offshore seaweed project is a collaborative venture between the Port of Duqm and DEME Concessions (a Belgian-based global provider of dredging, marine infrastructure and offshore energy solutions) with the support of Sultan Qaboos University (SQU) [123]. A large-scale, specialized seaweed cultivation vessel was developed as part of the Norwegian project Seaweed Vessel 2020, completed with the help of many business partners. Currently in the design phase, the ships are meant to be used throughout the cultivation process, from installing farm infrastructure to harvesting and transporting biomass [123].
A Company established in Belgium called AtSeaNova provides complete solutions for seaweed farms. They unveiled the SeaHarvester I product before the end of January 2020. It automates the 2D substrata and long-line cultivation procedures of seeding, harvesting, and cleaning. Additionally, At SeaNova provides methods for direct seeding of both brown and green seaweeds, gluing the young to the growth medium with an algae binder [124].
A fishing boat was transformed into a motorized harvester by North Sea Farmers. A cutting arm that was 8 m tall and propelled by electricity moved into the water when the boat positioned itself next to the plastic tube. The 2-m-wide net's long strands of seaweed were cut from it when the tubing was brought up. Following that, the seaweed was automatically packed and placed on the deck. Dutch seaweed farmers boast their first offshore mechanical harvest [125]. Automation innovation is essential for seeding, analyzing biomass, harvesting, and processing to improve economic resilience. Recent developments in this area include intelligent management systems for IMTA and automated biomass density estimation in land-based systems utilizing spectral reflectance imaging [126].
The procedure for utilizing an underwater robot (AUV) in a macroalgae plantation, encompassing initial placement through dead reckoning navigation with a preliminary estimate and scanning the entire farm for comprehensive data collection, is demonstrated [127]. Underwater robots can assist with infrastructure, water quality assessments, and crop monitoring [128]. The side-scan sonar is also a perfect sensor for observing seaweed farms since it can offer high-resolution images of ropes and buoys and sound signals for seaweed growth rate [129].
The University of East Anglia (UEA), the Centre for Environment, Fisheries and Aquaculture Science (Cefas), and Hethel Innovation are collaborators on the Seaweed in East Anglia (SEA) project. Businesses, investors, and local governments will use the project to understand the potential for Norfolk to develop a seaweed economy, concentrating on three areas: a. Examining seaweed aquaculture production techniques, species, and potential co-location sites b. Recognizing Norfolk's capacity to produce seaweed-based goods, including food and bioplastics, c. Creating a roadmap for developing the sector and the algae cluster [129].
A group of scientists and partners from the seaweed business, led by a non-profit organization, run the North Sea Farm 1 initiative. The project North Sea Farm 1 aims to test and enhance seaweed farming techniques while investigating the capacity of seaweed to store carbon. It will be situated on a wind farm off the coast of the Netherlands [130].
5. Macroalgae cultivation: challenges and remedial solutions
As the world's population increases exponentially, the natural environment continues to be exploited, disrupting the marine ecosystem. The need to sustainably accommodate a growing population is a significant issue. Seaweed aquaculture beds (SABs) can provide CO2 sequestration, fuel, and added product value, among other benefits. However, despite the numerous advantages, various biotic and abiotic problems threaten seaweed farming. Environmental threats due to climate change, viruses, epibionts, and grazers potentially influence seaweed's physiological efficiency, productivity, and sustainability. Below are some biotic and abiotic factors changing seaweed population dynamics.
5.1. Biotic factors
5.1.1. Ice-ice disease (IID)
A seaweed is called diseased when biological stressors as an etiological agent cause irregular physiological processes to interrupt the typical form structure, development, and production, including reproductive success [131]. confirmed the effect of "ice-ice" (the name ice-ice comes from the thalli's unprecedented bleaching to white sickness) in the Philippines in 1974. I-ice disease is the most pervasive issue encountered in Eucheumatoids seaweed production worldwide [132]. Ice-ice disease is not contagious and could be brought on by adverse environmental factors like severe temperature, pH, salinity, and opportunistic bacterial infections like Vibrio species [133]. Epiphyte outbreaks with concurrent IID can result in 25–75% harvest losses or worse. In Zanzibar, approximately 1000 tonnes of seaweed were lost in just seven years, from over 1000 tonnes in 2001 to nearly 2008 [134]. Due to ice-ice and epiphyte illnesses, Carrageenan features such as viscosity and gel strength can be reduced [135]. From 2012 to 2018, Malaysia's production of Eucheumatoid seaweed decreased dramatically from 331 to 173 thousand tonnes of fresh weight (FW) [33]. This drop has been chiefly brought on by ice-ice sickness outbreaks that continue to occur. Typically, environmental pressures make the thallus susceptible to disease-causing opportunistic microorganisms, which causes the thallus to bleach and the damaged tissues to disintegrate [136]. Ice-Ice illness, compared to normal seaweed thalli from the biosecure farm, showed a tenth to fourteen percent lower carrageenan output and a 16–45% lower gel strength [137]. Recently, there has been an ongoing discussion on the exact origin of the ice-ice illness in seaweed. It was found that ice-ice sickness inflicted by bacteria Alteromonas and Pseudoalteromonas, as well as the Cytophaga-Flavobacterium complex, has been connected to contaminated seaweeds [15]. IID-promoting microbes and environmental stresses were investigated by Refs. [138,139]. Both findings demonstrated that whereas Vibrio strains P11 and ABI-TU15 are capable of producing IID in vitro in the apparent lack of other known causal influences, these same strains may quickly cause more severe IID indications when stressful situations are present. Aside from marine bacteria, it was found by Ref. [140] that marine-derived fungi (Aspergillus terreus, A. ochraceus, Phoma sp.) also play a crucial role as ice-ice inducers in healthy, non-axenic K. alvarezii cultures, conditions, such as summer temperatures coupled with high light intensity and poor flow of water, as well as anthropogenic impacts, are major elements that cause ice-ice growth in the cultivated site. These findings contribute to a better understanding of how stress caused by cultural conditions may contribute to disease pathogenesis. Numerous techniques have been tried worldwide to control seaweed illnesses due to frequent outbreaks and economic losses caused by the bacterial community.
5.1.2. Grazers
Grazers that consume seaweed naturally are viewed as pests in the seaweed aquaculture industry. Depending on their body size, they are either mesograzers or micrograzers. Birds, urchins, gastropods, crabs, and various smaller herbivores such as amphipods, isopods, and polychaetes consume seaweeds as shown in Fig. 10. Herbivore damage to cultivated seaweeds can result in drastic reductions in biomass, lower yields per initiative, and economic damages. Furthermore, mounting evidence shows that tiny herbivores, even at low abundance, can significantly affect individual macrophytes. For instance, isopods consuming photosynthetically active tissue inhibit macrophytes' kelp growth [141]. Gastropods are likely to graze on kelps due to their high biomass and abundance [142]. On commercially grown varieties of seaweed in Japan, researchers discovered marine amphipod population increase due to ocean warming and nutrient enrichment [143] that resulted in the consumption of Undaria pinnatifida by isopod Cymodocea japonica. In temperate reef habitats, destructive grazing by macro grazers frequently causes population phase changes from high-productivity macroalgal forests to sparsely productive barren lands [144,145]. Through structural damage to stipes, which enhances the susceptibility of algae to being displaced by decisive wave action, the activity of these mesograzers can also result in a drop in macroalgal biomass [146].
Fig. 10.
Effect of grazing on seaweed cultivation.
Many freshwater fish, including catfish, bristlenose plecos, and amano shrimp, are primarily herbivores and rely on a diet almost entirely composed of plant-based items. Tropical fish have developed to digest aquatic-based vegetables better than we can as individuals. Fish from tropical and marine areas consume seaweed and other aquatic-based algae, which have anti-inflammatory and immune-boosting qualities. Historically, uncontrolled grazing by fishlings and juveniles like Acanthurus dussumieri (surgeonfish) have resulted in a loss of 50–80% of Eucheuma at a depth of 0.5–2 m in seaweed farms [147,148], tropical Lessepsian rabbitfishes (Siganus spp.) overgraze macroalgae and seagrasses [148] in contrast, chubs and rabbit fishes (Kyphosus spp. and Siganus spp., Siganidae) overgraze kelp forests in Australia and Japan [145]. Turtles are also indeed a worry as, in addition to eating large meals, they frequently crawl throughout a farm, inflicting significant physical damage. According to Ref. [149], the kelp Saccharina lattisima has experienced 40–80% dieback owing to sea urchin exploitation in many areas of Norway.
Several ideas have been suggested and evaluated to understand better how the feeding preferences of indigenous and alien species influence biological incursions. According to the enemy release hypothesis (ERH), non-native species encounter low predation/grazing pressure, considering they left their co-evolved enemies behind and native opponents fail to discover them as a potential food source [150]. However, the depiction is not clear, and it is still debatable [151]. The research found that native Fucus vesiculosus was preferred by North Sea mesograzers over non-native Sargassum muticum (introduced to the North Sea), S. fusiforme, and S. horneri (native to Japan but not present in the North Sea), signifying that Enemy Release plays a significant role in invasion achievement in this location. In addition, the recent expansion of exotic herbivores has considerably impacted macrophyte establishment and survival in numerous regions worldwide.
5.1.3. Epiphytes
Epiphytic disease is a parasitic disease that causes crop loss in seaweed [152], confirmed in farmed Kappaphycus in 1975. Sessile life forms (epibionts) such as bacteria, protozoa, and invertebrates colonize most marine species, resulting in epibiosis. It has been stated that epibionts can restrict the absorption of O2, CO2, and nutrients through the thallus membrane, (ii) reduce the quantity of light that can be used for photosynthesis, (iii) impair sporulation, (iv) reduce thallus mobility (v) enhance the palatability of the thallus [153].
Epiphytes conquer the layer of cultivated seaweed fronds, reducing production levels and [154]quality. Unguja, "Zanzibar Island," has had the bottommost seaweed output since epiphyte infection emerged in the 1990s, resulting in a decline in seaweed productivity [155]. According to Ref. [156], the emergence of the epiphytic parasite caused a sharp drop in Kappaphycus alvarezii output. Pathogen outbreaks in Tanzania have severely affected the seaweed business, endangering the lives of 30,000–36,000 seaweed farmers [154,157,158]. Epiphytic filamentous algae (EFA) and the "ice-ice" condition are the most prevalent diseases associated with these outbreaks, which are typically related to stress brought on by climate change [154,159,160]. [161] clarified that a rise in water temperature, radiation exposure, and the movement of water and low currents causes the development of epiphytes in seaweed cultivation. Epiphytic diversity peaks in the spring; for example, epiphytic algae growth bursts during the warmer months, as witnessed in Malaysia in March and June [162]. As per the hypothesis [163], native species should be more resistant to local enemies than non-native species since they have natural biochemical defenses that local antagonists have not yet developed. For example, brown alga F. evanescens was identified to be less populated by sessile invertebrates and filamentous algae in its non-native habitats in northern Europe than its native congener F. vesiculosus [164].
5.1.4. Red tide and Brown tide
Harmful algal blooms (HABs) stand out as a scientifically challenging and globally significant coastal challenge facing the globe today. Specific algae species can attain high densities, or "blooms," that cause brown (and red) tides. Brown (red) tides can occur when certain algae species reach high concentrations, or "blooms," that discolor water also. The blooms are so dense (as shown in Fig. 11) that they prevent adequate light from reaching the surface water. In addition to making temporarily hazardous oceans, brown (red) tides can also reduce the amount of dissolved oxygen in the water, a condition known as a dead zone. According to studies, toxic algal blooms are becoming more common, most likely due to global warming and eutrophication from agriculture and landscaping. Besides these factors, inefficient methods of growing seaweed may also be one factor in macroalgae blooms. For instance, 95% of Pyropia yezoensis (formerly Porphyra yezoensis) farming in China's coastal region was responsible for the frequent blooms of Enteromorpha (Ulva) prolifera in the Yellow Sea and the East China Sea [165,166]. Sargassum (brown macroalgae) has been inundating the Caribbean, Gulf of Mexico, and Florida coastlines nearly every year since 2011, peaking in June and July [167]. The worst sargassum invasions happened in 2018 and 2022, and in 2023, the problem will intensify [168], with the Caribbean being impacted earlier in the season than expected. Miami-Dade County paid US$6 million 2022 to remove sargassum from only four well-known beaches [167].
Fig. 11.
Bloom of Ulva intestinalis (Picture taken at Somatheeram Beach, Kerala).
The Sargassum Operational Detection Algorithms (SODA) project is a collaboration between CLS, Hygeos, and Mercator Ocean through the Copernicus Marine Service. SODA aims to create state-of-the-art algorithms for sargassum detection and share this data with the wider Copernicus Marine community [169]. Due to these techniques, geostationary satellites can deliver crisp photos of the Caribbean every 10 min, providing precise information on the presence and migration of sargassum [170]. Eco-friendly techniques can also reduce the possible significance of growing seaweed to algal blooms by prohibiting the incorrect emergence of invasive alien species and preventing the removal of significant amounts of epiphytes into the water. For example, adjusting harvest timing and growing techniques for P. yezoensis has assisted in reducing the size of U. prolifera blooms in the Yellow Sea [171]. Some rhodophyte (red algae) species can prevent the development of HABs by chemical secretion or healthy competition that is toxic to HABs. For instance, it has been demonstrated that some Gracilaria species indigenous to Asian waters limit the formation of numerous HABs via allelopathy [172].
Table 4 explores the relationship between different biotic factors and proposes solutions to address the associated challenges.
Table 4.
Biotic factors and potential solution.
| Biotic Factors | Suggested Solution |
|---|---|
| Ice-Ice Disease |
|
| Grazing |
|
| Epiphytes |
|
5.2. Abiotic factors
5.2.1. Temperature
According to Ref. [57], the human-caused temperature increase has achieved a 1-degree Celsius overhead pre-industrial level, with a realistic range of 0.8–1.2 °C. It will likely meet 1.5 °C by 2030 and 2052 if trends persist. The water temperature for seaweed production is between 27 and 30 °C. Many cultivated seaweed species hit their fertile peak from winter to spring, with increased nutrient levels, low temperatures, and abundant sunlight. Extreme temperatures can cause cellular and subcellular damage, resulting in destructive stress, although mild temperature variations can limit development and production. When Eucheuma denticulatum was stressed by high temperature or pH, the research found that the species generated volatile, halogenated chemical compounds [181]. High temperatures can also cause chloroplast swelling, dilation, and the rupture of the chloroplast membrane [182]. A study by [183] found that the antioxidant activity and phytochemical composition of macroalgae were affected by high temperatures. A study by [184] found that uncertain temperature fluctuations can disrupt the life cycles, growth, and productivity of Gracilaria seaweed. In both China and Indonesia. Farmers claim that seaweed development is inhibited for extended periods when the water temperature rises above 33 °C. This could be a crucial stage in the growth of Gracilaria, affecting harvest yields and potentially impacting the farmers' ability to make a profit [98]. According to Refs. [185,186], within brown seaweed, the reproduction and sorus induction time and the enhancement of the reproductive traits depend on temperature. Due to the production of crystals in cells at low temperatures, membrane proteins and fats can be harmed, impacting seaweed life in terms of metabolism, photosynthesis, growth, and development [187]. Furthermore, numerous algal physiologic mechanisms, including diffusive concentrations and carrier-mediated nutrient absorption, are affected by temperature. For example, the rate of NO3- absorption by Gracilaria tikvahiae was observed to increase with rising temperature [188]. Climate change also impacts interactions between marine creatures: for example, increased warmth decreases herbivore defenses in Fucus vesiculosus [189], making microbial illnesses easier [190]. Loss of chlorophyll and other photosynthetic pigments (shown in Fig. 12), unexpectedly in high seawater temperatures, can lead to partial or even complete bleaching of algae thalli, followed by growth inhibition [191,192].
Fig. 12.
Bleaching of sea lettuce by the sun (Picture taken at Hawa Beach, Kerala).
5.2.2. Biofouling
Marine biofouling is the unintended deposition of aquatic microorganisms, animals, and plants on seawater surfaces. Fluctuations in environmental variables (temperature, light, and salinity), geography, hydrodynamics, and depth impact have all been found to influence frond fouling. A lack of economic value [193], decreased development as a result of hindered nutrient uptake [194], and limited light existence are all effects of biofouling [195]. In 2015, researchers on the Faroe Islands reported S. latissima farms heavily fouled at wave-exposed and protected sites instead of a location site exposed to strong velocities [195]. The cortical cells of Laurencia translucida (Rhodophyta) can synthesize FA derivatives (e.g., HCs and FAs) and release them to the thallus surface, offering an innovative defensive technique for preventing microfouling [196].
5.2.3. Hydro dynamism
It refers to the tidal cycle's water motions caused by airstreams and changes in the water bodies' densities [197]. Compared to subtidal locations, the beach has immediate and harsh changing surroundings, with water movement among several surrounding stressors affecting algae development. Fig. 13 depicts the strong tide crashing against the seaweed, ultimately breaking it apart and washing it away.The intricate interplay between auxiliary ecological variables, including light, nutrients, and fluid movement, makes water movement investigations involve the rate of macroalgal proliferation. Correlate an increase in the kelp species Macrocystis pyrifera growth rate during autumn with elevated wave action [198]. According to Ref. [199], no direct impact of flow rate on the rate of algal development was found for Saccharina latissima (as Laminaria longicruris) (Phaeophyceae) and Adamsiella chauvinii (Rhodophyta) respectively. In tanks, Gracilaria parvispora (Rhodophyta) formed at a much slower pace (2.6% per day−1) as compared to the lagoon (8–10 percent per day− 1) [200]. A rise in water flow is favorable for resource acquisition, resulting in increased photosynthesis and development. In contrast, a decrease in water motion (i.e., slow flow) will surge gases and restrict nutrient mass transfer, potentially causing adverse effects on metabolic rates [201]. Seawater's increased viscosity and density provide stresses on seaweed development from drag and acceleration forces far more than those that land plants encounter, limiting macroalgae's maximum growth [202].
Fig. 13.
Tide hitting the seaweed (Picture taken at Hawa Beach, Kerala).
Previous methods, such as the directional wave-fetch approach [203], depended on coarse-scale predictors that lacked spatial and temporal precision for precise assessments of the impacts of wave velocity on seaweed of water motion on macroalgae. Nowadays, oceanographic tools like current meters have been effectively used. According to Ref. [204], storm activity will rise owing to enhanced temperature variations between the water and the land. Seaweed aquaculture beds (SABs) can be vulnerable to storm-related destruction in the forthcoming atmosphere, possibly requiring a greater focus on designing structures for the cultivation of seaweed that can withstand more substantial waves and storm forces.
5.2.4. Salinity
Another significant component that affects seaweed growth is salinity. It is expressed in practical salinity units (PSU), an electrical conductivity measurement used to determine the water's ionic content. The salinity in the open sea is somewhat constant between 33 and 37 PSU. Salinity levels in marine waters fluctuate with geography and time. Evaporation and sea ice freezing cause a rise in salinity and precipitation, while freshwater intake from rivers causes a drop in salinity [205]. Water salinity differences frequently cause water circulation, evaporation, rainfall, and river movement. A rise or fall in salinity may impact the turgor pressure that affects the growth of seaweed [16]. Low salinity can produce oxidative stress, leading to peroxide formation in explants and thallus stiffness reduction; as demonstrated in Gracilaria corticata subjected to 15 ppt in perspective, the limit of acceptable and ideal salinity for Eucheuma cottoni seaweed development is 28–34 ppt [206,207]. The impact of season fluctuations on carrageenan's yield and gel mechanical strength in K. alvarezii was investigated [208]. The best yield of K. alvarezii's carrageenan was harvested at 30 salinities; results were either decreased or increased below or above this point. According to Ref. [209], Kappaphycus alvarezii showed an unsaturated fatty acid up-regulation in high-saline situations compared to hypo-saline settings. These findings revealed distinct salinity acclimation pathways in Kappaphycus alvarezii and varied metabolomic responses in control, hypo-, and hyper-saline environments. Producing reactive oxygen species, generated in reaction with numerous perturbations and may cause cell injury by inducing oxidative stress, is one of the most significant impacts of salt stress on seaweed physiology [210]. In hypersaline water, the emergence of seaweed is typically inhibited by both combined enzyme effects and decreased turgor strain, which impedes the division of cells [211]. Many tidewater algae use diverse physiological and metabolic processes and pathways to acclimate to oscillating salinities in the surroundings when exposed to increases in salinity [197]. Seaweed may develop and sustain in tropical areas with 32 and 34 psμ water salinity levelsas per [212].
5.2.5. Light
Light availability and water transparency strongly influence seaweed photosynthetic activity and biosynthetic ability, resulting in financial and environmental suffering from seaweed farms [213]. The photon fluence rate correlates to the photosynthetic light compensation point. The maximum light capacity can be attained if the light is raised far beyond the compensation point, enhanced by photosynthesis. Enzymatic reactions limit photosynthesis at this time, and if light levels rise again, photosynthesis is lowered (photoinhibition). The optimum growth ranges and light resource tolerances of different strains or kinds of seaweed can vary. For Kappaphycus alvarezii strains from Sabah, Malaysia, a photon flux density of 75 mol photons m−2 s−1, and a DGR of 4.3–0.5 percent per day−1 was found to be appropriate [214]. Reduction in protein concentration and a rise in lipid percentage in green algae Dunaliela tertiolecta, when light intensities were elevated to saturation, were found by Ref. [215]. The distribution of fatty acids in each species differed due to the different photon flux density and temperature growing circumstances. In Gracilaria species, unsaturated fatty acid levels rise as photon flux density rises [216]. According to Ref. [217], growing Ulva australis (formerly Ulva pertusa) (Chlorophyta) and Sargassum fusiforme, under white LED light was the best auxiliary light because it encouraged the proliferation of macroalgae while retaining the synthesis of proteins. In comparison, red LED was not recommended for Sargassum fusiforme, culture because it inhibited seaweed development and had a reduced proportion of residual energy beneath the waterline. To tackle the challenges posed by different abiotic factors, Table 5 explores their relationships and proposes solutions.
Table 5.
Abiotic factors and suggested solution.
| Abiotic Factors | Suggested Solution |
|---|---|
| Temperature |
|
| Biofouling |
|
| Hydro dynamism |
|
| Salinity |
|
| Light |
|
5.3. Socio-economic factor
Despite the enormous potential market opportunities for seaweed on a global scale, there are significant issues with seaweed cultivation, precisely price swings. Considering the scarcity of global market pricing, most seaweed is sold through direct contractual agreements, and significant price variations emerge, i.e., rate of Kappaphycus alvarezii has almost doubled in Indonesia, jumping from rough costs from $0.60 to $1.80 per kilo. As a result, several producers hurried to collect juvenile or low-quality seaweed, resulting in a surplus on the market. Thus, triggering a market collapse in response to the unexpected price rise [223]. Consequently, the value of seaweed drops during the year; according to a report conducted by Ref. [224], during lean development cycles, seaweed's yearly average price increased by 20%, whereas prices held steady during middle production times. The cost of seaweed decreased by 10% during peak production. Rainy seasons impair farmers' capability to dry seaweed, leading to higher moisture levels and a lower-quality crop with reduced costs. In the Konawe Selatan District, seaweed production decreased from 105,072 tons to 100,710 tons. Four thousand three hundred sixty-two tons less seaweed was produced in Konawe Selatan on account of the declining cost of seaweed in the region, which fluctuated from IDR 12,500 to IDR 15,000 per kilogram in 2010 to IDR 10,000 per kilogram in 2013, and finally down to IDR 6000 per kilogram in 2015 and 2016 [225]. Farmers in South Korea have a lot of difficulties since they sell fresh biomass, and there may be an excess of supplies during particular harvest periods. The farmers are now financially insecure, while Japanese farmers do not experience the same market difficulties [98]. The growing scheme has lowered financial risk for growers by reducing dried seaweed price fluctuations and avoiding longer market chains.
Due to a lack of marketing networks, growers cannot communicate with retailers and must depend on intermediaries, who typically organize transportation for the farmers to the mainland. There is a lack of confidence between processors, mediators, and growers in macroalgae superiority, culminating in farmers being penalized. Government departments and third-party observers view mediators as exploiting poor seaweed farmers (alternatively working as artisanal fishermen). When capacity costs are sufficiently high, these prices are the high and low bounds for middlemen's asking and bidding prices. The price of the productive output is one of the elements that can motivate seaweed growers and fishermen to produce more. Anglers will be more driven to boost their produce if seaweed has a higher selling price. Proper management is required to raise seaweed selling prices from the input sector to the output.
Social opposition has also been a major stumbling block to aquaculture development. Nearshore waterways are famous for entertaining (boating, fishing, and swimming) and aesthetic purposes. As a result, offshore cultivation has been recommended as a potential option for resolving stakeholder conflicts. To establish a Macroalgae farm in the United Kingdom, a license from the regulatory authority may be required [226]. The macroalgae sector frequently mentions legal restrictions as a barrier to its development [227] primarily referring to the formal and substantive requirements for acquiring an operating license [228]. Establishing a relationship between macroalgae growers and other stakeholders and consultation with locals on the most effective ways to set up an operation may be necessary to obtain the social license to operate [229]. Seaweed aquaculture is expected to expand rapidly over the next few years. However, there aren't many licenses, particularly for the farming of species with rising demand and significant potential value for which the excavation from wild stocks is presently forbidden because of environmental security measures (such as for Saccharina latissima and Palmaria palmata) or the scarcity of wild biomass (e.g., Porphyra sensu lato) [228].
5.3.1. Aging, work fatigue, and health problems of seaweed workers
Workers for seaweed production are needed for various tasks, including sowing, planting, upkeep, harvesting, processing, and drying. Due to seaweed farmers' advanced ages, seaweed farming faces many quality human resource challenges. According to Ref. [230] younger persons were less likely to cultivate seaweed in South Konawe than older farmers, who had an average age of 35 or above, demonstrating that adolescents were less drawn to the industry and that the aging of the farmers in the South Konawe Regency limited the growth of the seaweed agribusiness.
Farmers who grow seaweed face worse health conditions. They complained of fatigue, low energy, physical aches, and issues with their eyes, skin, and respiratory systems [231]. According to Ref. [231], more than half of the seaweed workers, 67.1%.in Takalar Regency, South Sulawesi, Indonesia, feel fatigued. Lifting seaweed from the harvest and spreading it on land covered in plastic sheeting is the task. Seaweed sorting requires hours of repeated hand labor in an uncomfortable sitting position. These primary working postures may result in MSDs and occupational. According to Ref. [232] seaweed workers in Takalar Regency carried out their duties in non-ergonomic positions that could lead to MSDs. The study recommended redesigning instruments and the work process to implement ergonomic rules and principles.
Respiratory issues also occur in seaweed removal workers working in the hot sun. Media reports claim that owing to nature's wrath, some of Mexico's beaches are witnessing smelly sargassum seaweed. The amount has surpassed the worst performance level since 2018. Sargassum has an irreversible influence on workers' health, even though it poses no hazard to tourists from a health perspective [233]. It is suggested to encourage all entities, notably policymakers, to take action to lessen the health issues associated with algal blooms.
5.3.2. Old technique
Due to several restrictions, the seaweed industry struggles to continue as a significant economic activity. One of the most important things that contribute to developing high-quality and adequate seaweed is using the proper techniques for cultivation. Seaweed growers, primarily located in coastal regions, severely lack access to contemporary technology and essential resources. Farmers have inadequate agricultural equipment and inadequate drying facilities. For instance, deeper water farming of seaweed was recommended as the best option, but sadly, most of Zanzibar's seaweed growers are women, and women historically have trouble swimming [234]. Since 2006, ZaSCI and the Institute of Marine Sciences have been educating farmers about alternate agricultural methods that will enable them to relocate from the warm shallow seas. However, acceptance is low due to women's lack of swimming ability and the absence of readily available boats [157,235]. One of the programs to support seaweed farmers in improved farming techniques was the Seaweed Training Programme by the African Women Educationalists (FAWE). FAWE is an NGO that provides education and technical vocational skills and competence to girls and young women to achieve their full potential [236]. Training programs intended to transfer technical knowledge to seaweed farmers should provide flexible training schedules that accommodate women's domestic duties given their prominent role in the industry [237].
Only a few nations, including China, have achieved industrialized seedling growing, extensive offshore cultivation, and mechanical harvesting, creating an industrial chain from product processing to sales. However, most nations primarily concentrate on basic agriculture, and there is little automation in large-scale harvesting and processing. It leads to unreliable quality, ineffectiveness, high labor costs, and processing waste. Large-scale interventions include laws to control biosecurity concerns and government-sponsored programs to educate farmers about innovative farming methods and supply them with new seed sources. Such measures could lessen the exposure and susceptibility of seaweed growers to environmental stress while maintaining long-term seaweed output. In Madagascar, farmers receive biosecurity training from the private sector [238]. Zanzibar recently established about 136 projects worth TZS 3.2 trillion to improve production and productivity [239]. The main components of these investments targeted technological innovation, farmers' and processors' capacity building, policies, regulation, and gender issues along the value chain [238]. Only a few nations, including China, have achieved industrialized seedling growing, extensive offshore cultivation, and mechanical harvesting, creating an industrial chain from product processing to sales.
However, most nations primarily concentrate on primary agriculture, and there is little automation in large-scale harvesting and processing. It leads to unreliable quality, ineffectiveness, high labor costs, and processing waste. Numerous bioactive substances from seaweed have been researched recently. For instance, Bigelow Laboratory for Ocean Sciences, the University of Connecticut, and Woods Hole Oceanographic Institution (WHOI) have signed a license agreement for a kelp germplasm collection, or collection of microscopic cells known as gametophytes, encompassing more than 1200 samples [240]. However, the value-added products are typically low due to lacking cutting-edge extraction and purification methods [241]. For instance, GreenWave's Kelp Climate Fund is a subsidy for ocean farmers to support a bundle of climate impact. GreenWave's 10-year goal is to provide training, tools, and support to a baseline of 10,000 farmers to catalyze the planting of regenerative ocean crops and yield meaningful economic and climate impacts [242].
5.3.3. Suggested solution
-
•
Accurate, trustworthy market intelligence and manufacturing must be used to mitigate price volatility.
-
•
Established marketplaces to give international reference pricing for seaweed.
-
•
Economic incentives and regulations, such as nutrient trading schemes and streamlined marine licensing processes, can potentially support the seaweed farming industry [243,244].
6. Conclusion
This review has explored the immense potential of macroalgae farming in addressing climate change and achieving the UN's sustainable development goals. A comprehensive understanding of this growing seaweed industry is provided by introducing the global status of seaweed production, various cultivation methods, recent trends, and challenges. Specific fundamental challenges must be addressed to unlock the full potential benefits that macroalgae farming can offer worldwide. The following are the key findings from the study.
-
1.
Innovations in seaweed farming: To meet the increasing demand for macroalgae production, advancing state-of-the-art, economical, and ecologically sustainable practices is essential. Responsible farming techniques that balance production with environmental preservation must be developed. Furthermore, the industry must explore adaptive farming practices to thrive in a changing climate. By implementing suitable strategies, countries can harness the immense potential of macroalgae cultivation and contribute to sustainable seaweed production on a global scale.
-
2.
Overcoming the challenges: While immense opportunities exist, seaweed farming faces several challenges. It is crucial to carefully select suitable cultivation sites to mitigate challenges arising from climate change, epiphytes, and the emergence of infectious species. Biotic and abiotic factors can significantly affect production, demanding continuous research and innovation. Developing disease-resistant strains, improved cultivation technologies, and effective mitigation strategies are essential for overcoming these hurdles. Establishing disease-resistant macroalgae and germplasm banks can safeguard genetic diversity, ensuring sustainable growth.
-
3.
Empowering Seaweed communities: Macroalgae farmers can be encouraged and supported through well-coordinated public funding to facilitate potential commercialization and value production. Farmers need access to educational programs on managing threats and difficulties while assisting in obtaining planting supplies and farming equipment at reduced or subsidized prices.
-
4.
Setting Country-Specific Standards and Targets: Governments should enforce globally acceptable norms and protocols for monitoring insect breeding and disease outbreaks. Implementing consistent practices across nations will minimize risks of biosecurity threats and promote responsible resource management. The country-specific target is reaching a certain production level to meet market demands while driving carbon neutrality through seaweed cultivation. By establishing clear targets and monitoring progress, each nation can contribute to a sustainable and vibrant global seaweed Industry.
-
5.
Collaboration and Knowledge Sharing: Collaboration between scientists, researchers, policymakers, and industry stakeholders is vital for driving the full potential of seaweed cultivation for a sustainable and resilient future. Encourage scientists to prioritize research on specific challenges, policymakers to implement supportive regulations, and investors to embrace seaweed innovation. By sharing knowledge, resources, and best practices, we can navigate the challenges and realize the full potential of this sustainable and versatile resource.
In essence, macroalgae farming stands at the crossroads of opportunity and challenge. Overcoming the challenges requires concentrated efforts, innovative solutions, and a commitment to sustainability. This study illuminates the path forward, offering insights and perspectives that can guide the future of a collaborative ocean where seaweed thrives, empowering communities, mitigating climate change, and paving the way for a brighter future.
Data availability statement
N/A for this article.
CRediT authorship contribution statement
Nida Khan: Writing – original draft. K. Sudhakar: Writing – review & editing, Supervision, Resources. R. Mamat: Writing – review & editing, Supervision, Project administration.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Sudhakar Kumarasamy reports financial support was provided by University of Malaysia Pahang Al-Sultan Abdullah. "The corresponding author, Sudhakar Kumarasamy, discloses membership on the Editorial Board of Heliyon Journal If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements and Funding
The authors would like to thank the Universiti Malaysia Pahang Al-Sultan Abdullah (UMPSA), Malaysia (www.umpsa.edu.my) for laboratory facilities as well as additional financial support under Internal Research grant RDU223310 and PGRS210348.
References
- 1.United Nations Feeding the world sustainably | united nations. 2012. https://www.un.org/en/chronicle/article/feeding-world-sustainably
- 2.Ferdouse F., Holdt S.L., Smith R., Murua P., Yang L. Globefish Research Programme; Rome, Italy: 2018. The Global Status of Seaweed Production, Trade and Utilization. [Google Scholar]
- 3.Buschmann A.H., Camus C., Infante J., Neori A., Israel Á., Hernández-González M.C., et al. vol. 52. 2017. pp. 391–406. (Seaweed Production: Overview of the Global State of Exploitation, Farming and Emerging Research Activity). Https://DoiOrg/101080/0967026220171365175. [DOI] [Google Scholar]
- 4.Ścieszka S., Klewicka E. Algae in food: a general review. Crit. Rev. Food Sci. Nutr. 2019;59:3538–3547. doi: 10.1080/10408398.2018.1496319. [DOI] [PubMed] [Google Scholar]
- 5.Leandro A., Pacheco D., Cotas J., Marques J.C., Pereira L., Gonçalves A.M.M. Seaweed's bioactive Candidate compounds to food industry and global food security. Life. 2020;10:1–37. doi: 10.3390/LIFE10080140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Commercial Seaweed Market Size, Share, Trends Report. 2023. https://www.grandviewresearch.com/industry-analysis/commercial-seaweed-market n.d. [Google Scholar]
- 7.Sultana F., Wahab M.A., Nahiduzzaman M., Mohiuddin M., Iqbal M.Z., Shakil A., et al. Seaweed farming for food and nutritional security, climate change mitigation and adaptation, and women empowerment: a review. Aquac Fish. 2023;8:463–480. doi: 10.1016/J.AAF.2022.09.001. [DOI] [Google Scholar]
- 8.Global Seaweed New and Emerging Markets Report 2023. World Bank; 2023. https://www.worldbank.org/en/topic/environment/publication/global-seaweed-new-and-emerging-markets-report-2023 [Google Scholar]
- 9.Rebours C., Marinho-Soriano E., Zertuche-González J.A., Hayashi L., Vásquez J.A., Kradolfer P., et al. Seaweeds: an opportunity for wealth and sustainable livelihood for coastal communities. J. Appl. Phycol. 2014;265:1939–1951. doi: 10.1007/S10811-014-0304-8. 2014;26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Froehlich H.E., Afflerbach J.C., Frazier M., Halpern B.S. Blue growth potential to mitigate climate change through seaweed Offsetting. Curr. Biol. 2019;29:3087–3093.e3. doi: 10.1016/J.CUB.2019.07.041. [DOI] [PubMed] [Google Scholar]
- 11.FAO . 2007. FAO Biosecurity Toolkit.https://www.fao.org/3/a1140e/a1140e.pdf [Google Scholar]
- 12.Mackinnon B., Hao B., Reantaso M.B. Innovative Biosecurity Approaches for a Healthier Aquaculture Industry. 2020. The progressive management pathway for improving aquaculture biosecurity (PMP/AB) FAO COFI Virtual Dialogue Webinar. [Google Scholar]
- 13.Sugumaran R., Padam B.S., Thau W., Yong L., Saallah S., Ahmed K., et al. A Retrospective review of global commercial seaweed production-current challenges, biosecurity and mitigation measures and Prospects. Publ. Health. 2022;19:7087. doi: 10.3390/ijerph19127087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim G.H., Moon K.H., Kim J.Y., Shim J., Klochkova T.A. A revaluation of algal diseases in Korean Pyropia (Porphyra) sea farms and their economic impact. ALGAE. 2014;29:249–265. doi: 10.4490/ALGAE.2014.29.4.249. [DOI] [Google Scholar]
- 15.Ward G.M., Faisan J.P., Cottier-Cook E.J., Gachon C., Hurtado A.Q., Lim P.E., et al. A review of reported seaweed diseases and pests in aquaculture in Asia. J World Aquac Soc. 2020;51:815–828. doi: 10.1111/JWAS.12649. [DOI] [Google Scholar]
- 16.Ask E.I., Azanza R.V. Advances in cultivation technology of commercial eucheumatoid species: a review with suggestions for future research. Aquaculture. 2002;206:257–277. doi: 10.1016/S0044-8486(01)00724-4. [DOI] [Google Scholar]
- 17.Pang T., Liu J., Liu Q., Li H., Li J. Observations on pests and diseases affecting a eucheumatoid farm in China. J. Appl. Phycol. 2015;27:1975–1984. doi: 10.1007/s10811-014-0507-z. [DOI] [Google Scholar]
- 18.Cheney D., Rudolph B., Wang L.Z., Metz B., Watson K., Roberts K., et al. Genetic Manipulation and strain improvement in commercially valuable red seaweeds. New Dev Mar Biotechnol. 1998:101–104. doi: 10.1007/978-1-4757-5983-9_22. [DOI] [Google Scholar]
- 19.Zuccarello G.C., Critchley A.T., Smith J., Sieber V., Lhonneur G.B., West J.A. Systematics and genetic variation in commercial <Emphasis Type="Italic">Kappaphycus</Emphasis> and <Emphasis Type="Italic">Eucheuma</Emphasis> (Solieriaceae, Rhodophyta) Eighteenth Int Seaweed Symp. 2006:417–425. doi: 10.1007/978-1-4020-5670-3_50. [DOI] [Google Scholar]
- 20.Hayashi L., Yokoya N.S., Kikuchi D.M., Oliveira E.C. Callus induction and micropropagation improved by colchicine and phytoregulators in Kappaphycus alvarezii (Rhodophyta, Solieriaceae) J. Appl. Phycol. 2008;20:653–659. doi: 10.1007/S10811-007-9234-Z/FIGURES/4. [DOI] [Google Scholar]
- 21.Bindu M.S., Levine I.A. The commercial red seaweed Kappaphycus alvarezii-an overview on farming and environment. J. Appl. Phycol. 2011;23:789–796. doi: 10.1007/s10811-010-9570-2. [DOI] [Google Scholar]
- 22.Khan N., Sudhakar K., Mamat R. Seaweed farming: a perspectives of genetic engineering and nano-technology application. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e15168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kyoung Hwang E., Yotsukura N., Jun Pang S., Su L., Feng Shan T. Seaweed breeding programs and progress in eastern Asian countries. Phycologia. 2019;58:484–495. doi: 10.1080/00318884.2019.1639436. [DOI] [Google Scholar]
- 24.Hurtado A.Q., Neish I.C., Critchley A.T. vol. 58. 2019. pp. 472–483. (Phyconomy: the Extensive Cultivation of Seaweeds, Their Sustainability and Economic Value, with Particular Reference to Important Lessons to Be Learned and Transferred from the Practice of Eucheumatoid Farming). Https://DoiOrg/101080/0031888420191625632. [DOI] [Google Scholar]
- 25.The State of World Fisheries and Aquaculture 2020 . Brief. State World Fish Aquac 2020 Br. 2020. [DOI] [Google Scholar]
- 26.Wichard T., Charrier B., Mineur F., Bothwell J.H., Clerck O De, Coates J.C. The green seaweed Ulva: a model system to study morphogenesis. Front. Plant Sci. 2015;0:72. doi: 10.3389/FPLS.2015.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fort A., Lebrault M., Allaire M., Esteves-Ferreira A.A., McHale M., Lopez F., et al. Extensive variations in Diurnal growth patterns and metabolism among Ulva spp. strains. Plant Physiol. 2019;180:109–123. doi: 10.1104/PP.18.01513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solvang T., Bale E.S., Broch O.J., Handå A., Alver M.O. Automation concepts for industrial-scale production of seaweed. Front. Mar. Sci. 2021;8 doi: 10.3389/FMARS.2021.613093/BIBTEX. [DOI] [Google Scholar]
- 29.Sadhukhan J., Gadkari S., Martinez-Hernandez E., Ng K.S., Shemfe M., Torres-Garcia E., et al. Novel macroalgae (seaweed) biorefinery systems for integrated chemical, protein, salt, nutrient and mineral extractions and environmental protection by green synthesis and life cycle sustainability assessments. Green Chem. 2019;21:2635–2655. doi: 10.1039/C9GC00607A. [DOI] [Google Scholar]
- 30.Campbell I., Kambey C.S.B., Mateo J.P., Rusekwa S.B., Hurtado A.Q., Msuya F.E., et al. Biosecurity policy and legislation for the global seaweed aquaculture industry. J. Appl. Phycol. 2020;32:2133–2146. doi: 10.1007/S10811-019-02010-5/FIGURES/5. [DOI] [Google Scholar]
- 31.Hatch Innovation services HATCH seaweed insights - seaweed insights n.d. https://seaweedinsights.com/
- 32.Cai J., Lovatelli A., Manjarrez Aguilar, Cornish L., Dabbadie L. Seaweeds and microalgae: an overview for unlocking their potential in global aquaculture development. Seaweeds Microalgae an Overv Unlocking Their Potential Glob. Aquac Dev. 2021 doi: 10.4060/CB5670EN. [DOI] [Google Scholar]
- 33.FAO . FAO; Rome, Italy: 2020. The State of World Fisheries and Aquaculture 2020. [DOI] [Google Scholar]
- 34.Bjerregaard R., Valderrama D., Radulovich R., Diana J., Capron M., Mckinnie C.A., et al. World Bank; 2016. Seaweed Aquaculture for Food Security, Income Generation and Environmental Health in Tropical Developing Countries.https://documents.worldbank.org/en/publication/documents-reports/documentdetail/947831469090666344/seaweed-aquaculture-for-food-security-income-generation-and-environmental-health-in-tropical-developing-countries [Google Scholar]
- 35.SeaweedInsights . 2022. Global Production Overview.https://seaweedinsights.com/global-production/ [Google Scholar]
- 36.Cai J. 2021. Global Status of Seaweed Production, Trade and Utilization. [Google Scholar]
- 37.Xiao X., Agustí S., Yu Y., Huang Y., Chen W., Hu J., et al. Seaweed farms provide refugia from ocean acidification. Sci. Total Environ. 2021;776 doi: 10.1016/J.SCITOTENV.2021.145192. [DOI] [PubMed] [Google Scholar]
- 38.Seghetta M., Tørring D., Bruhn A., Thomsen M. Bioextraction potential of seaweed in Denmark - an instrument for circular nutrient management. Sci. Total Environ. 2016;563–564:513–529. doi: 10.1016/j.scitotenv.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Kim J.K., Kraemer G.P., Yarish C. Use of sugar kelp aquaculture in long Island sound and the Bronx River Estuary for nutrient extraction. Mar. Ecol. Prog. Ser. 2015;531:155–166. doi: 10.3354/MEPS11331. [DOI] [Google Scholar]
- 40.Kim J.K., Yarish C., Hwang E.K., Park M., Kim Y. Seaweed aquaculture: cultivation technologies, challenges and its ecosystem services. ALGAE. 2017;32:1–13. doi: 10.4490/algae.2017.32.3.3. [DOI] [Google Scholar]
- 41.Zheng Y., Jin R., Zhang X., Wang Q., Wu J. The considerable environmental benefits of seaweed aquaculture in China. Stoch. Environ. Res. Risk Assess. 2019;33:1203–1221. doi: 10.1007/S00477-019-01685-Z/TABLES/9. [DOI] [Google Scholar]
- 42.Gao G., Gao L., Jiang M., Jian A., He L. The potential of seaweed cultivation to achieve carbon neutrality and mitigate deoxygenation and eutrophication. Environ. Res. Lett. 2021;17 doi: 10.1088/1748-9326/AC3FD9. [DOI] [Google Scholar]
- 43.Duan Y., Yang N., Hu M., Wei Z., Bi H., Huo Y., et al. Growth and nutrient uptake of Gracilaria lemaneiformis under different nutrient conditions with implications for ecosystem services: a case study in the laboratory and in an enclosed mariculture area in the East China Sea. Aquat. Bot. 2019;153:73–80. doi: 10.1016/J.AQUABOT.2018.11.012. [DOI] [Google Scholar]
- 44.Iy S., Ch R., Gt J., Sk K. Evaluation of ethanol production and bioadsorption of heavy metals by various red seaweeds. Bioprocess Biosyst Eng. 2016;39:915–923. doi: 10.1007/S00449-016-1571-3. [DOI] [PubMed] [Google Scholar]
- 45.Radulovich R. Massive freshwater gains from producing food at sea. Water Pol. 2011;13:547–554. doi: 10.2166/WP.2011.137. [DOI] [Google Scholar]
- 46.Farghali M., Mohamed I.M.A., Osman A.I., Rooney D.W. Seaweed for climate mitigation, wastewater treatment, bioenergy, bioplastic, biochar, food, pharmaceuticals, and cosmetics: a review. Environ. Chem. Lett. 2022;211:97–152. doi: 10.1007/S10311-022-01520-Y. 2022;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Submariner. Algae Cultivation, Processing & Products. Submar Netw Blue Growth n.d..https://submariner-network.eu/our-solutions/algae-cultivation-processing-products/(accessed January 5, 2024)..
- 48.Krause-Jensen D., Lavery P., Serrano O., Marba N., Masque P., Duarte C.M. Sequestration of macroalgal carbon: the elephant in the Blue Carbon room. Biol. Lett. 2018;14:23955–26900. doi: 10.1098/RSBL.2018.0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duarte C.M., Bruhn A., Krause-Jensen D. A seaweed aquaculture imperative to meet global sustainability targets. Nat. Sustain. 2022;5:185–193. doi: 10.1038/S41893-021-00773-9. [DOI] [Google Scholar]
- 50.Troell M., Henriksson P.J.G., Buschmann A.H., Chopin T., Quahe S. Farming the ocean–seaweeds as a Quick Fix for the climate? Rev Fish Sci Aquac. 2023;31:285–295. doi: 10.1080/23308249.2022.2048792/SUPPL_FILE/BRFS_A_2048792_SM2563.JPG. [DOI] [Google Scholar]
- 51.Ross F.W.R., Boyd P.W., Filbee-Dexter K., Watanabe K., Ortega A., Krause-Jensen D., et al. Potential role of seaweeds in climate change mitigation. Sci. Total Environ. 2023;885 doi: 10.1016/J.SCITOTENV.2023.163699. [DOI] [PubMed] [Google Scholar]
- 52.Jagtap A.S., Meena S.N. Seaweed farming: a perspective of sustainable agriculture and socio-economic development. Nat Resour Conserv Adv Sustain. 2022:493–501. doi: 10.1016/B978-0-12-822976-7.00022-3. [DOI] [Google Scholar]
- 53.Liu Y., Cao L., L Cheung W.W., al, Wu J., Yang H., et al. Cultivated seaweed carbon sequestration capacity. IOP Conf. Ser. Earth Environ. Sci. 2019;370 doi: 10.1088/1755-1315/370/1/012017. [DOI] [Google Scholar]
- 54.Jhariya M.K., Meena R.S., Banerjee A. Ecological Intensification of natural resources towards sustainable productive system. Ecol Intensif Nat Resour Sustain Agric. 2021;1–28 doi: 10.1007/978-981-33-4203-3_1/COVER. [DOI] [Google Scholar]
- 55.Banerjee A., Jhariya M.K., Raj A., Yadav D.K., Khan N., Meena R.S. Agroecol Footprints Manag Sustain Food Syst; 2020. Energy and Climate Footprint towards the Environmental Sustainability; pp. 415–443. [DOI] [Google Scholar]
- 56.Yong W.T.L., Thien V.Y., Rupert R., Rodrigues K.F. Seaweed: a potential climate change solution. Renew. Sustain. Energy Rev. 2022;159 doi: 10.1016/J.RSER.2022.112222. [DOI] [Google Scholar]
- 57.Hoegh-Guldberg O., Jacob D., Taylor M., Bindi M., Brown S., Camilloni I., et al. Impacts of 1.5oC Global Warming on Natural and Human Systems. In: global Warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of. IPCC. 2018:175–312. doi: 10.1017/9781009157940.005. [DOI] [Google Scholar]
- 58.Poloczanska E.S., Brown C.J., Sydeman W.J., Kiessling W., Schoeman D.S., Moore P.J., et al. Global imprint of climate change on marine life. Nat Clim Chang. 2013;310:919–925. doi: 10.1038/nclimate1958. 2013;3. [DOI] [Google Scholar]
- 59.DeFries R., Edenhofer O., Halliday A., Heal G., Lenton T., Puma M., et al. The missing economic risks in assessments of climate change impacts. Grantham Res Inst Clim Chang Environ. 2019 https://www.lse.ac.uk/granthaminstitute/publication/the-missing-economic-risks-in-assessments-of-climate-change-impacts/ [Google Scholar]
- 60.Duarte C.M., Losada I.J., Hendriks I.E., Mazarrasa I., Marbà N. The role of coastal plant communities for climate change mitigation and adaptation. Nat Clim Chang. 2013;311:961–968. doi: 10.1038/nclimate1970. 2013;3. [DOI] [Google Scholar]
- 61.Wageningen Food & Biobased Research MACROFUELS: macro-algae as a sustainable source for biofuels - WUR 2020. https://www.wur.nl/en/project/macrofuels-macro-algae-as-a-sustainable-source-for-biofuels.htm
- 62.NICK O’SULLIVAN. BLOG Top 5 ways the seaweed industry is supporting the SDGs - UN Global Compact Network Australia. UN Glob Compact Netw Aust. 2023 https://unglobalcompact.org.au/blog-top-5-ways-the-seaweed-industry-is-supporting-the-sdgs/ [Google Scholar]
- 63.Rimmer M.A., Larson S., Lapong I., Purnomo A.H., Pong‐masak P.R., Swanepoel L., et al. Seaweed aquaculture in Indonesia contributes to social and economic aspects of livelihoods and community Wellbeing. Sustain. Times. 2021;13 doi: 10.3390/SU131910946. 2021;13:10946. [DOI] [Google Scholar]
- 64.Mazarrasa I., Olsen Y.S., Mayol E., Marbà N., Duarte C.M. Rapid growth of seaweed biotechnology provides opportunities for developing nations. Nat. Biotechnol. 2013;317:591–592. doi: 10.1038/nbt.2636. 2013;31. [DOI] [PubMed] [Google Scholar]
- 65.Holdt S.L., Kraan S. Bioactive compounds in seaweed: functional food applications and legislation. J. Appl. Phycol. 2011;233:543–597. doi: 10.1007/S10811-010-9632-5. 2011;23. [DOI] [Google Scholar]
- 66.Keah R., Kitomari S. Seaweed farming Brings economic empowerment to coastal women in Tanzania and Kenya. Talk Africa. 2023 https://www.talkafrica.co.ke/seaweed-farming-brings-economic-empowerment-to-coastal-women-in-tanzania-and-kenya/ [Google Scholar]
- 67.Guedes A.C., Amaro H.M., Sousa-Pinto I., Malcata F.X. Applications of spent biomass. Biofuels from Algae. 2014:205–233. doi: 10.1016/B978-0-444-59558-4.00010-3. [DOI] [Google Scholar]
- 68.Duarte C.M., Bruhn A., Krause-Jensen D. A seaweed aquaculture imperative to meet global sustainability targets. Nat. Sustain. 2021 53 2021;(5):185–193. doi: 10.1038/s41893-021-00773-9. [DOI] [Google Scholar]
- 69.Sharma N., Bapuly N. Seaweed farming: a powerful way to implement the G20 sustainable development. Obs Res Found. 2023 https://www.orfonline.org/hindi/research/seaweed-cultivation-as-a-means-to-realise-the-g20-agenda-sustainability#_edn19 [Google Scholar]
- 70.Duarte C.M., Wu J., Xiao X., Bruhn A., Krause-Jensen D. Can seaweed farming play a role in climate change mitigation and adaptation? Front. Mar. Sci. 2017;4 doi: 10.3389/FMARS.2017.00100/FULL. [DOI] [Google Scholar]
- 71.Racine P., Marley A.C., Froehlich H.E., Gaines S.D., Ladner I., MacAdam-Somer I., et al. A case for seaweed aquaculture inclusion in U.S. nutrient pollution management. Mar Policy. 2021;129 doi: 10.1016/J.MARPOL.2021.104506. [DOI] [Google Scholar]
- 72.Huws S. Using Seaweed Supplements To Significantly Reduce Livestock Methane Emissions. Sch Biol Sci Queens Univ Belfast n.d. https://www.qub.ac.uk/Research/case-studies/seaweed-supplements-reducing-methane-emissions.html (accessed January 1, 2024).
- 73.Visch W., Kononets M., Hall P.O.J., Nylund G.M., Pavia H. Environmental impact of kelp (Saccharina latissima) aquaculture. Mar. Pollut. Bull. 2020;155 doi: 10.1016/J.MARPOLBUL.2020.110962. [DOI] [PubMed] [Google Scholar]
- 74.NOAA Fisheries. Seaweed Aquaculture Natl Ocean Atmos Adm US Dep Commer Gov United States Am. 2020 https://www.fisheries.noaa.gov/national/aquaculture/seaweed-aquaculture [Google Scholar]
- 75.Dutch Seaweed Group Sustainable development goals 2022. https://www.dutchseaweedgroup.com/en/about-seaweed/sustainable-development-goals/
- 76.Innovation News Network Sustainable innovation – the rise of seaweed-based bioplastics in Europe 2020. https://www.innovationnewsnetwork.com/sustainable-innovation-the-rise-of-seaweed-based-bioplastics-in-europe/6513/
- 77.FAO . Food Agric Organ United Nations; 2023. Recognizing and Enhancing the Contribution of Algae to Global Aquaculture Development.https://knowledge4policy.ec.europa.eu/publication/recognizing-enhancing-contribution-algae-global-aquaculture-development_en [Google Scholar]
- 78.Global Seaweed Coalition Annual report. 2023. https://drive.google.com/file/d/1WJx_z-axjRd6H84BRC0QzgUVaxuZ39Vf/view
- 79.Acadian Seaplants Limited Land-based seaweed cultivation was pioneered by Acadian Seaplants. 2022. https://www.acadianseaplants.com/land-based-seaweed-cultivation/
- 80.Bird K.T. J. Benson. Seaweed cultivation for renewable resources 1987. https://agris.fao.org/agris-search/search.do?recordID=XF2015041947
- 81.Prasad Behera D., Vadodariya V., Veeragurunathan V., Sigamani S., Moovendhan M., Srinivasan R., et al. Seaweeds cultivation methods and their role in climate mitigation and environmental cleanup. Total Environ Res Themes. 2022;3–4 doi: 10.1016/J.TOTERT.2022.100016. [DOI] [Google Scholar]
- 82.Mooney-McAuley K.M., Edwards M.D., Champenois J., Gorman E. 2016. Best Practice Guidelines for Seaweed Cultivation and Analysis: Public Output Report Report [WP1A5.01] of the EnAlgae Project. [DOI] [Google Scholar]
- 83.McHugh D.J. A guide to the seaweed industry. FAO Fish Pap Rome. 2003 doi: 10.1007/s00343-015-4332-2. [DOI] [Google Scholar]
- 84.FAO The state of food and agriculture, 2012. Food agric organ united nations. 2012. https://www.fao.org/3/i3028e/i3028e00.htm Rome.
- 85.Hall A.C., Fairclough A.C., Mahadevan K., Paxman J.R. Ascophyllum nodosum enriched bread reduces subsequent energy intake with no effect on post-prandial glucose and cholesterol in healthy, overweight males. A pilot study. Appetite. 2012;58:379–386. doi: 10.1016/J.APPET.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 86.Flores S.R.L., Dobbs J., Dunn M.A. Mineral nutrient content and iron bioavailability in common and Hawaiian seaweeds assessed by an in vitro digestion/Caco-2 cell model. J. Food Compos. Anal. 2015;43:185–193. doi: 10.1016/J.JFCA.2015.06.008. [DOI] [Google Scholar]
- 87.García-Casal M.N., Ramírez J., Leets I., Pereira A.C., Quiroga M.F. Antioxidant capacity, polyphenol content and iron bioavailability from algae (Ulva sp., Sargassum sp. and Porphyra sp.) in human subjects. Br. J. Nutr. 2008;101:79–85. doi: 10.1017/S0007114508994757. [DOI] [PubMed] [Google Scholar]
- 88.Kuda T., Yano T., Matsuda N., Nishizawa M. Inhibitory effects of laminaran and low molecular alginate against the putrefactive compounds produced by intestinal microflora in vitro and in rats. Food Chem. 2005;91:745–749. doi: 10.1016/J.FOODCHEM.2004.06.047. [DOI] [Google Scholar]
- 89.Taelman S.E., Champenois J., Edwards M.D., De Meester S., Dewulf J. Comparative environmental life cycle assessment of two seaweed cultivation systems in North West Europe with a focus on quantifying sea surface occupation. Algal Res. 2015;11:173–183. doi: 10.1016/J.ALGAL.2015.06.018. [DOI] [Google Scholar]
- 90.Mcelligott D, Mackey M, Maguire J. Technical report on seaweed hatchery and sea grow-out site design. Seaf Tech Serv Bord Iascaigh Mhara n.d. https://bim.ie/wp-content/uploads/2023/05/BIM-Technical-Report.pdf (accessed December 31, 2023).
- 91.Scrosati R. Review of studies on biomass-density relationships (including self-thinning lines) in seaweeds: main contributions and persisting misconceptions. Phycol. Res. 2005;53:224–233. doi: 10.1111/J.1440-183.2005.00390.X. [DOI] [Google Scholar]
- 92.Felix B. Agronomy and cultivation methods for edible seaweeds 2013. https://www.ripublication.com/ijafst_spl/ijafstv4n7spl_04.pdf
- 93.Niesenbaum R.A. The ecology of sporulation by the macroalga Ulva lactuca L. (chlorophyceae) Aquat. Bot. 1988;32:155–166. doi: 10.1016/0304-3770(88)90095-2. [DOI] [Google Scholar]
- 94.Gao G., Clare A.S., Rose C., Caldwell G.S. Non-cryogenic preservation of thalli, germlings, and gametes of the green seaweed Ulva rigida. Aquaculture. 2017;473:246–250. doi: 10.1016/J.AQUACULTURE.2017.02.012. [DOI] [Google Scholar]
- 95.Kraan S. Mass-cultivation of carbohydrate rich macroalgae, a possible solution for sustainable biofuel production. Mitig Adapt Strateg Glob Chang. 2010;181:27–46. doi: 10.1007/S11027-010-9275-5. 2010;18. [DOI] [Google Scholar]
- 96.Aaron-Amper J., Largo D.B., Handugan E.R.B., Nini J.L., Alingasa K.M.A., Gulayan S.J. Culture of the tropical brown seaweed Sargassum aquifolium: from hatchery to field out-planting. Aquac Reports. 2020;16 doi: 10.1016/J.AQREP.2019.100265. [DOI] [Google Scholar]
- 97.Su L., Pang S.J., Shan T.F., Li X. Large-scale hatchery of the kelp Saccharina japonica: a case study experience at Lvshun in northern China. J. Appl. Phycol. 2017 296 2017;29:3003–3013. doi: 10.1007/S10811-017-1154-Y. [DOI] [Google Scholar]
- 98.SeaweedInsights . 2022. Future of Farm.https://seaweedinsights.com/future-of-farm/ [Google Scholar]
- 99.Gutierrez A., Correa T., Muñoz V., Santibañez A., Marcos R., Cáceres C., et al. Farming of the giant kelp Macrocystis pyrifera in Southern Chile for development of novel food products. J. Appl. Phycol. 2006;183:259–267. doi: 10.1007/S10811-006-9025-Y. 2006;18. [DOI] [Google Scholar]
- 100.Roesijadi G., Copping A.E., Huesemann M.H., Forster J. 2008. Techno-Economic Feasibility Analysis of Offshore Seaweed Farming for Bioenergy and Biobased Products. [Google Scholar]
- 101.Kumar S.A., Magnusson M., Ward L.C., Paul N.A., Brown L. Seaweed supplements normalise metabolic, cardiovascular and liver responses in high-carbohydrate, high-fat fed rats. Mar. Drugs. 2015;13:788–805. doi: 10.3390/MD13020788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schuenhoff A., Shpigel M., Lupatsch I., Ashkenazi A., Msuya F.E., Neori A. A semi-recirculating, integrated system for the culture of fish and seaweed. Aquaculture. 2003;221:167–181. doi: 10.1016/S0044-8486(02)00527-6. [DOI] [Google Scholar]
- 103.Neori A., Chopin T., Troell M., Buschmann A.H., Kraemer G.P., Halling C., et al. Integrated aquaculture: rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture. 2004;231:361–391. doi: 10.1016/J.AQUACULTURE.2003.11.015. [DOI] [Google Scholar]
- 104.Bak U.G., Mols-Mortensen A., Gregersen O. Production method and cost of commercial-scale offshore cultivation of kelp in the Faroe Islands using multiple partial harvesting. Algal Res. 2018;33:36–47. doi: 10.1016/J.ALGAL.2018.05.001. [DOI] [Google Scholar]
- 105.Neill P.E., Alcalde O., Faugeron S., Navarrete S.A., Correa J.A. Invasion of Codium fragile ssp. tomentosoides in northern Chile: a new threat for Gracilaria farming. Aquaculture. 2006;259:202–210. doi: 10.1016/j.aquaculture.2006.05.009. [DOI] [Google Scholar]
- 106.DeBusk T.A., Blakeslee M., Ryther J.H. Studies on the outdoor cultivation of Ulva lactuca L. Bot. Mar 1986;29:381–386. doi: 10.1515/BOTM.1986.29.5.381/MACHINEREADABLECITATION/RIS. [DOI] [Google Scholar]
- 107.Buck B.H., Buchholz C.M. The offshore-ring: a new system design for the open ocean aquaculture of macroalgae. J. Appl. Phycol. 2004 165 2004;16:355–368. doi: 10.1023/B:JAPH.0000047947.96231.EA. [DOI] [Google Scholar]
- 108.Israel A., Gavrieli J., Glazer A., Friedlander M. Utilization of flue gas from a power plant for tank cultivation of the red seaweed Gracilaria cornea. Aquaculture. 2005;249:311–316. doi: 10.1016/J.AQUACULTURE.2005.04.058. [DOI] [Google Scholar]
- 109.Hiraoka M., Oka N. Tank cultivation of Ulva prolifera in deep seawater using a new “germling cluster” method. J. Appl. Phycol. 2007;201:97–102. doi: 10.1007/S10811-007-9186-3. 2007;20. [DOI] [Google Scholar]
- 110.Copertino M.D.S., Tormena T., Seeliger U. Biofiltering efficiency, uptake and assimilation rates of Ulva clathrata (Roth) J. Agardh (Clorophyceae) cultivated in shrimp aquaculture waste water. J. Appl. Phycol. 2008;21:31–45. doi: 10.1007/S10811-008-9357-X. 2008 211. [DOI] [Google Scholar]
- 111.Kasim M., Mustafa A., Male I., M, Jalil W. New methods on cultivation of eucheuma denticulatum and Kappahycus alvarezii in Indonesia. J. Fish. Aquat. Sci. 2017;12:207–217. doi: 10.3923/JFAS.2017.207.217. [DOI] [Google Scholar]
- 112.Mantri V.A., Shah Y., Thiruppathi S. vol. 1. 2020. pp. 12–19. (Feasibility of Farming the Agarose-Yielding Red Alga Gracilaria Dura Using Tube-Net Cultivation in the Open Sea along the Gujarat Coast of NW India). Https://DoiOrg/101080/2638808120191648181. [DOI] [Google Scholar]
- 113.Neori A., Bronfman Y., van Rijn J., Guttman L., Krupnik N., Shpigel M., et al. The suitability of Ulva fasciata, Ulva compressa, and Hypnea musciformis for production in an outdoor spray cultivation system, with respect to biomass yield and protein content. J. Appl. Phycol. 2020;325:3183–3197. doi: 10.1007/S10811-020-02130-3. 2020;32. [DOI] [Google Scholar]
- 114.Savvashe P., Mhatre-Naik A., Pillai G., Palkar J., Sathe M., Pandit R., et al. High yield cultivation of marine macroalga Ulva lactuca in a multi-tubular airlift photobioreactor: a scalable model for quality feedstock. J. Clean. Prod. 2021;329 doi: 10.1016/J.JCLEPRO.2021.129746. [DOI] [Google Scholar]
- 115.Steinhagen S., Enge S., Larsson K., Olsson J., Nylund G.M., Albers E., et al. Sustainable large-scale aquaculture of the northern Hemisphere sea lettuce, Ulva fenestrata, in an off-shore Seafarm. J. Mar. Sci. Eng. 2021;9:615. doi: 10.3390/JMSE9060615. 2021;9:615. [DOI] [Google Scholar]
- 116.GENIALG GENIALG project results help to boost the European seaweed sector. 2021. https://genialgproject.eu/2021/08/19/genialg-project-results-help-to-boost-the-european-seaweed-sector/
- 117.BEA REBECA-CCT marca la ‘hoja de ruta’ a los emprendedores en biotecnología azul. Banco Español de Algas. 2023 https://marinebiotechnology.org/en/actuality/news/350-rebeca-cct-marca-la-hoja-de-ruta-a-los-emprendedores-en-biotecnologia-azul.html [Google Scholar]
- 118.Alfred-Wegener-Institut . 2023. The SeaStrains Project and Workshop.https://www.awi.de/forschung/besondere-gruppen/aquakultur/marine-aquakultur/forschung/seastrains.html [Google Scholar]
- 119.SIMBA Project progress: seaweed cultivation in SIMBA - SIMBA 2019. https://simbaproject.eu/project-progress-seaweed-cultivation-in-simba/
- 120.GlobalSeaweedSTAR. Aquaculture n.d..https://www.globalseaweed.org/(accessed January 4, 2024)..
- 121.Seatech Energy. Industrial scale Seaweed farming & Anaerobic Digestion n.d..https://seatechinnovation.com/(accessed January 4, 2024)..
- 122.The Fish Site EU backs seaweed farming success 2022. https://thefishsite.com/articles/eu-backs-seaweed-farming-success
- 123.Prabhu Conrad. Oman Obs; 2022. Major Seaweed Cultivation Project Proposed off Duqm - Oman Observer.https://www.omanobserver.om/article/1117930/business/economy/major-seaweed-cultivation-project-proposed-off-duqm [Google Scholar]
- 124.Kerrison P.D., Innes M., Macleod A., McCormick E., Elbourne P.D., Stanley M.S., et al. Comparing the effectiveness of twine- and binder-seeding in the Laminariales species Alaria esculenta and Saccharina latissima. J. Appl. Phycol. 2020;32:2173–2181. doi: 10.1007/S10811-020-02069-5/FIGURES/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.The fish site Dutch seaweed farmers boast first offshore mechanical harvest | the Fish Site. Fish Site. 2022 https://thefishsite.com/articles/dutch-seaweed-farmers-boast-first-offshore-mechanical-harvest#tags [Google Scholar]
- 126.Praeger C., Vucko M.J., McKinna L., de Nys R., Cole A. Estimating the biomass density of macroalgae in land-based cultivation systems using spectral reflectance imagery. Algal Res. 2020;50 doi: 10.1016/J.ALGAL.2020.102009. [DOI] [Google Scholar]
- 127.Stenius I., Folkesson J., Bhat S., Sprague C.I., Ling L., Özkahraman Ö., et al. A system for autonomous seaweed farm Inspection with an underwater robot. Sensors. 2022;22 doi: 10.3390/S22135064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fischell E., Stanton T.K., Kukulya A., Lavery A.C. Monitoring of macroalgae (kelp) farms with autonomous underwater vehicle-based split-beam sonar. J. Acoust. Soc. Am. 2018;144:1806. doi: 10.1121/1.5067972. [DOI] [Google Scholar]
- 129.Thefishsite Project aims to kickstart East Anglia's seaweed sector. Fish Site Ltd. 2023 https://thefishsite.com/articles/project-aims-to-kickstart-east-anglias-seaweed-sector [Google Scholar]
- 130.Amazon Introducing the world's first commercial-scale seaweed farm located between offshore wind turbines. AboutamazonEu. 2023 https://www.aboutamazon.eu/news/sustainability/introducing-the-worlds-first-commercial-scale-seaweed-farm-located-between-offshore-wind-turbines [Google Scholar]
- 131.Trono Gavino C., Jr. Euchema seaweed farming in the Philippines. Int Union Conserv Nat Nat Resour. 1973 [Google Scholar]
- 132.Tahiluddin A.B., Ertugrul Terzi. Ice-ice disease in commercially cultivated seaweeds Kappaphycus spp. and eucheuma spp.: a review on the causes, Occurrence, and control measures. Mar Sci Technol Bull. 2021;10:234–243. doi: 10.33714/MASTEB.917788. [DOI] [Google Scholar]
- 133.Arasamuthu A., Patterson Edward &j K. Occurrence of ice-ice disease in seaweed Kappaphycus alvarezii at Gulf of Mannar and Palk Bay, Southeastern India. Indian J Geo Mar Sci. 2018;47:1208–1216. [Google Scholar]
- 134.Msuya Flower E. In: Soc. Econ. Dimens. Carrageenan Seaweed Farming. Valderrama D., Cai J., Hishamunda N., Ridler N., editors. 2013. Social and economic dimensions of carrageenan seaweed farming in the United Republic of Tanzania. Soc Econ Dimens Carrageenan Farming; p. 204. [Google Scholar]
- 135.Mendoza W.G., Montaño N.E., Ganzon-Fortes E.T., Villanueva R.D. Chemical and gelling profile of ice-ice infected carrageenan from Kappaphycus striatum (Schmitz) Doty “sacol” strain (Solieriaceae, Gigartinales, Rhodophyta) J. Appl. Phycol. 2002;14:409–418. doi: 10.1023/A:1022178119120. [DOI] [Google Scholar]
- 136.Ward G.M., Kambey C.S.B., Faisan J.P., Tan P.L., Daumich C.C., Matoju I., et al. Ice-Ice disease: an environmentally and microbiologically driven syndrome in tropical seaweed aquaculture. Rev Aquac. 2022;14:414–439. doi: 10.1111/RAQ.12606. [DOI] [Google Scholar]
- 137.Kambey C.S.B., Campbell I., Cottier-Cook E.J., Nor A.R.M., Kassim A., Sade A., et al. Seaweed aquaculture: a preliminary assessment of biosecurity measures for controlling the ice-ice syndrome and pest outbreaks of a Kappaphycus farm. J. Appl. Phycol. 2021;33:3179–3197. doi: 10.1007/S10811-021-02530-Z/TABLES/8. [DOI] [Google Scholar]
- 138.Largo D.B., Fukami K., Nishijima T. Occasional pathogenic bacteria promoting ice-ice disease in the carrageenan-producing red algae Kappaphycus alvarezii and Eucheuma denticulatum (Solieriaceae, Gigartinales, Rhodophyta) J. Appl. Phycol. 1995 76 1995;7:545–554. doi: 10.1007/BF00003941. [DOI] [Google Scholar]
- 139.Azizi A., Mohd Hanafi N., Basiran M.N., Teo C.H. Evaluation of disease resistance and tolerance to elevated temperature stress of the selected tissue-cultured Kappaphycus alvarezii Doty 1985 under optimized laboratory conditions. 3 Biotech. 2018;8 doi: 10.1007/S13205-018-1354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Solis M.J.L., Draeger S., Dela Cruz T.E.E. Marine-derived fungi from Kappaphycus alvarezii and K. striatum as potential causative agents of ice-ice disease in farmed seaweeds. Bot. Mar 2010;53:587–594. doi: 10.1515/BOT.2010.071. [DOI] [Google Scholar]
- 141.Poore A.G.B., Gutow L., Pantoja J.F., Tala F., Jofré Madariaga D., Thiel M. Major consequences of minor damage: impacts of small grazers on fast-growing kelps. Oecologia. 2013 1743 2013;174:789–801. doi: 10.1007/S00442-013-2795-4. [DOI] [PubMed] [Google Scholar]
- 142.Barnes R.S.K. Spatial structure of a multi-species guild: the dominant biofilm-grazing microgastropods of seagrass. Hydrobiol. (Sofia) 2018 8271 2018;827:293–307. doi: 10.1007/S10750-018-3781-Y. [DOI] [Google Scholar]
- 143.Endo H., Sato Y., Kaneko K., Takahashi D., Nagasawa K., Okumura Y., et al. Ocean warming combined with nutrient enrichment increases the risk of herbivory during cultivation of the marine macroalga Undaria Pinnatifida. ICES J. Mar. Sci. 2021;78:402–409. doi: 10.1093/ICESJMS/FSAA069. [DOI] [Google Scholar]
- 144.Steneck R.S., Graham M.H., Bourque B.J., Corbett D., Erlandson J.M., Estes J.A., et al. Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ. Conserv. 2002;29:436–459. doi: 10.1017/S0376892902000322. [DOI] [Google Scholar]
- 145.Vergés A., Steinberg P.D., Hay M.E., Poore A.G.B., Campbell A.H., Ballesteros E., et al. The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proc R Soc B Biol Sci. 2014;281 doi: 10.1098/RSPB.2014.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Krumhansl K.A., Lee J.M., Scheibling R.E. Grazing damage and encrustation by an invasive bryozoan reduce the ability of kelps to withstand breakage by waves. J Exp Mar Bio Ecol. 2011;407:12–18. doi: 10.1016/J.JEMBE.2011.06.033. [DOI] [Google Scholar]
- 147.Ennis D J., Ussell R. Oahu; Hawaii: 2008. Ecology of the Imported Red Seaweed Eucheuma Striatum Schmitz on Coconut Island. [Google Scholar]
- 148.Vergés A., Tomas F., Cebrian E., Ballesteros E., Kizilkaya Z., Dendrinos P., et al. Tropical rabbitfish and the deforestation of a warming temperate sea. J. Ecol. 2014;102:1518–1527. doi: 10.1111/1365-2745.12324. [DOI] [Google Scholar]
- 149.Moy F.E., Christie H. Large-scale shift from sugar kelp (Saccharina latissima) to ephemeral algae along the south and west coast of Norway. Mar. Biol. Res. 2012;8:309–321. doi: 10.1080/17451000.2011.637561. [DOI] [Google Scholar]
- 150.Keane R.M., Crawley M.J. Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol. 2002;17 [Google Scholar]
- 151.Schwartz N., Rohde S., Hiromori S., Schupp P. Understanding the invasion success of Sargassum muticum: herbivore preferences for native and invasive Sargassum spp. Mar Biol. 2016;163:1–13. doi: 10.1007/s00227-016-2953-4. [DOI] [Google Scholar]
- 152.Doty M., Vb A. Status, problems, advances and economics of eucheuma farms. Mar Technol Soc. 1975;9:30–35. [Google Scholar]
- 153.Wahl M., Hay M.E., Enderlein P. Effects of epibiosis on consumer-prey interactions. Hydrobiologia. 1997;355:49–59. doi: 10.1023/A:1003054802699. [DOI] [Google Scholar]
- 154.Msuya F.E., Porter M. Impact of environmental changes on farmed seaweed and farmers: the case of Songo Songo Island, Tanzania. J. Appl. Phycol. 2014;265:2135–2141. doi: 10.1007/S10811-014-0243-4. 2014;26. [DOI] [Google Scholar]
- 155.Davis E. Seaweed farmer education: is it Enough to sustain the industry? Analyzing the status of stakeholder investment in Muungoni and Jambiani. Unguja. Indep Study Proj Collect. 2011 [Google Scholar]
- 156.Tsiresy G., Preux J., Lavitra T., Dubois P., Lepoint G., Eeckhaut I. Phenology of farmed seaweed Kappaphycus alvarezii infestation by the parasitic epiphyte Polysiphonia sp. Madagascar. J Appl Phycol. 2016 285 2016;28:2903–2914. doi: 10.1007/S10811-016-0813-8. [DOI] [Google Scholar]
- 157.Msuya F.E. Seaweed resources of Tanzania: status, potential species, challenges and development potentials. Bot. Mar 2020;63:371–380. doi: 10.1515/BOT-2019-0056/MACHINEREADABLECITATION/RIS. [DOI] [Google Scholar]
- 158.Rusekwa S.B., Campbell I., Msuya F.E., Buriyo A.S., Cottier-Cook E.J. Biosecurity policy and legislation of the seaweed aquaculture industry in Tanzania. J. Appl. Phycol. 2020;326:4411–4422. doi: 10.1007/S10811-020-02194-1. 2020;32. [DOI] [Google Scholar]
- 159.Largo Danilo B., Msuya Flower E., Menezes Ana. FAO; 2020. Understanding Diseases and Control in Seaweed Farming in Zanzibar. FAO Fish Aquac Tech Pap No 662 Rome. 10.4060/ca9004en. [Google Scholar]
- 160.Cottier-Cook EJ, Nagabhatla N, Badis Y, Campbell ML, Chopin T, Dai W, et al. Safeguarding the Future of the Global Seaweed Aquaculture Industry.n.d.).
- 161.Muñoz J., Fotedar R. Epiphytism of Gracilaria cliftonii (Withell, millar & Kraft) from Western Australia. J. Appl. Phycol. 2010;22:371–379. doi: 10.1007/s10811-009-9469-y. [DOI] [Google Scholar]
- 162.Vairappan C.S., Chung C.S., Hurtado A.Q., Soya F.E., Lhonneur G.B., Critchley A. Distribution and symptoms of epiphyte infection in major carrageenophyte-producing farms. J. Appl. Phycol. 2008;20:477–483. doi: 10.1007/s10811-007-9299-8. [DOI] [Google Scholar]
- 163.Hierro J.L., Callaway R.M. Allelopathy and exotic plant invasion. Plant Soil. 2003 2561 2003;256:29–39. doi: 10.1023/A:1026208327014. [DOI] [Google Scholar]
- 164.Wikström S.A., Pavia H. Chemical settlement inhibition versus post-settlement mortality as an explanation for differential fouling of two congeneric seaweeds. Oecologia. 2004 1382 2003;138:223–230. doi: 10.1007/S00442-003-1427-9. [DOI] [PubMed] [Google Scholar]
- 165.Zhang Y., He P., Li H., Li G., Liu J., Jiao F., et al. Ulva prolifera green-tide outbreaks and their environmental impact in the Yellow Sea, China. Natl. Sci. Rev. 2019;6:825–838. doi: 10.1093/NSR/NWZ026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hu C., Li D., Chen C., Ge J., Muller-Karger F.E., Liu J., et al. On the recurrent Ulva prolifera blooms in the Yellow Sea and East China sea. J Geophys Res Ocean. 2010;115 doi: 10.1029/2009JC005561. [DOI] [Google Scholar]
- 167.Leatherman S.P. FIU News - Florida International University; 2023. The Great Atlantic Sargassum Belt Is Carrying a Massive Bloom of Brown Seaweed toward Florida and the Caribbean.https://news.fiu.edu/2023/the-great-atlantic-sargassum-belt-is-carrying-a-massive-bloom-of-brown-seaweed-toward-florida-and-the-caribbean [Google Scholar]
- 168.NASA Earth Observatory A massive seaweed bloom in the Atlantic. NASA Earth Obs. 2023 https://earthobservatory.nasa.gov/images/151188/a-massive-seaweed-bloom [Google Scholar]
- 169.Marion Sutton. SODA | CMEMS.n.d. https://marine.copernicus.eu/about/research-development-projects/2022-2024/soda (accessed July 17, 2023)..
- 170.Copernicus services. Copernicus marine service evolution projects | CMEMS. 2022. https://marine.copernicus.eu/about/research-development-projects#Copernicus21stServiceEvolutionCallforTenders20222024
- 171.Zhang J., Shi J., Gao S., Huo Y., Cui J., Shen H., et al. Annual patterns of macroalgal blooms in the Yellow Sea during 2007–2017. PLoS One. 2019;14 doi: 10.1371/JOURNAL.PONE.0210460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yang Y., Liu Q., Chai Z., Tang Y. Inhibition of marine coastal bloom-forming phytoplankton by commercially cultivated Gracilaria lemaneiformis (Rhodophyta) J. Appl. Phycol. 2014;276:2341–2352. doi: 10.1007/S10811-014-0486-0. 2014;27. [DOI] [Google Scholar]
- 173.Msuya F.E., Buriyo A., Omar I., Pascal B., Narrain K., Ravina J.J.M., et al. Cultivation and utilisation of red seaweeds in the Western Indian ocean (WIO) region. J. Appl. Phycol. 2014;26:699–705. doi: 10.1007/s10811-013-0086-4. [DOI] [Google Scholar]
- 174.Biag D.C., Cuadro J.C., Nolial J.C.C., Lemios RO De, Edoria C.L.T., Hombre R.S., et al. How to prevent early onset of Epiphytes and ‘Ice-Ice’ disease in cultivated seaweeds (Kappaphycus) Camarines Norte, Philippines. 2022;21 doi: 10.30574/GSCBPS.2022.21.1.0382. 074–9. [DOI] [Google Scholar]
- 175.Pfister C.A., Hay M.E. Associational plant refuges: convergent patterns in marine and terrestrial communities result from differing mechanisms. Oecologia. 1988;77:118–129. doi: 10.1007/BF00380934. [DOI] [PubMed] [Google Scholar]
- 176.Wijayanto D., Bambang A.N., Nugroho R.A., Kurohman F., Wijayanto D. vol. 13. 2020. (The Impact of Planting Distance on Productivity and Profit of Eucheuma Cottonii Seaweed Cultivation in Karimunjawa Islands, Indonesia). [Google Scholar]
- 177.Wijayanto D., Bambang A.N., Nugroho R.A., Kurohman F. vol. 13. 2020. (The Growth Model of Eucheuma Cottonii Cultivated in Karimunjawa Islands). Indonesia. [Google Scholar]
- 178.Peteiro C., Freire O. Epiphytism on blades of the edible kelps Undaria pinnatifida and Saccharina latissima farmed under different abiotic conditions. J World Aquac Soc. 2013;44:706–715. doi: 10.1111/JWAS.12065. [DOI] [Google Scholar]
- 179.Loureiro R.R., Reis R.P., Critchley A.T. In vitro cultivation of three Kappaphycus alvarezii (Rhodophyta, Areschougiaceae) variants (green, red and brown) exposed to a commercial extract of the brown alga Ascophyllum nodosum (Fucaceae, Ochrophyta) J. Appl. Phycol. 2009;221:101–104. doi: 10.1007/S10811-009-9412-2. 2009;22. [DOI] [Google Scholar]
- 180.Shang Y.C. Economic aspects of Gracilaria culture in Taiwan. Aquaculture. 1976;8:1–7. doi: 10.1016/0044-8486(76)90014-4. [DOI] [Google Scholar]
- 181.Mtolera M.S.P., Collén J., Pedersén M., Ekdahl A., Abrahamsson K., Semesi A.K. vol. 31. 2010. pp. 89–95. (Stress-induced Production of Volatile Halogenated Organic Compounds in Eucheuma Denticulatum (Rhodophyta) Caused by Elevated pH and High Light Intensities). 101080/09670269600651241. [DOI] [Google Scholar]
- 182.Chen W.R., Zheng J.S., Li Y.Q., Guo W.D. Effects of high temperature on photosynthesis, chlorophyll fluorescence, chloroplast ultrastructure, and antioxidant activities in fingered citron. Russ. J. Plant Physiol. 2012;59:732–740. doi: 10.1134/S1021443712060040. [DOI] [Google Scholar]
- 183.Ling A.L.M., Yasir S., Matanjun P., Abu Bakar M.F. Effect of different drying techniques on the phytochemical content and antioxidant activity of Kappaphycus alvarezii. J. Appl. Phycol. 2015;27:1717–1723. doi: 10.1007/s10811-014-0467-3. [DOI] [Google Scholar]
- 184.Raikar S., Iima M., Fujita Y. Effect of temperature, salinity and light intensity on the growth of Gracilaria spp. (Gracilariales, Rhodophyta) from Japan, Malaysia and India. Undefined. 2001;30:98–104. [Google Scholar]
- 185.Marinho-Soriano E., Fonseca P.C., Carneiro M.A.A., Moreira W.S.C. Seasonal variation in the chemical composition of two tropical seaweeds. Bioresour. Technol. 2006;97:2402–2406. doi: 10.1016/J.BIORTECH.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 186.Abdullah N., Wibowo E.S., Irfan M., Muchdar F., Malan S. Seaweed Kappaphycus alvarezii cultivation using longline method in Kastela waters, Ternate Island, Indonesia. AACL Bioflux. 2020;13:2336–2342. [Google Scholar]
- 187.Kumar Y.N., Poong S.W., Gachon C., Brodie J., Sade A., Lim P.E. Impact of elevated temperature on the physiological and biochemical responses of Kappaphycus alvarezii (Rhodophyta) PLoS One. 2020;15 doi: 10.1371/JOURNAL.PONE.0239097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lapointe B.E., Tenore K.R., Dawes C.J. Interactions between light and temperature on the physiological ecology of Gracilaria tikvahiae (Gigartinales: Rhodophyta) - I. Growth, photosynthesis and respiration. Mar Biol. 1984;80:161–170. doi: 10.1007/BF02180183. [DOI] [Google Scholar]
- 189.Weinberger F, Rohde S, Oschmann Y, Shahnaz L, Dobretsov S, Wahl M. Effects of limitation stress and disruptive stress on induced antigrazing defense in the bladder wrack Fucus vesiculosus n.d..https://agris.fao.org/agris-search/search.do?recordID=AV2012064605 (accessed July 24, 2021)..
- 190.Harley C.D.G., Anderson K.M., Demes K.W., Jorve J.P., Kordas R.L., Coyle T.A., et al. Effects of climate change on global seaweed communities. J. Phycol. 2012;48:1064–1078. doi: 10.1111/J.1529-8817.2012.01224.X. [DOI] [PubMed] [Google Scholar]
- 191.Tchernov D., Gorbunov M.Y., De Vargas C., Yadav S.N., Milligant A.J., Häggblom M., et al. Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc Natl Acad Sci U S A. 2004;101:13531–13535. doi: 10.1073/PNAS.0402907101/SUPPL_FILE/02907TABLE3.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chalanika De Silva H.C., Asaeda T. Effects of heat stress on growth, photosynthetic pigments, oxidative damage and competitive capacity of three submerged macrophytes. Http://McManuscriptcentralCom/Tjpi. 2017;12:228–236. doi: 10.1080/17429145.2017.1322153. [DOI] [Google Scholar]
- 193.Park C.S., Hwang E.K. Seasonality of epiphytic development of the hydroid Obelia geniculata on cultivated Saccharina japonica (Laminariaceae, Phaeophyta) in Korea. J. Appl. Phycol. 2011;243:433–439. doi: 10.1007/S10811-011-9755-3. 2011;24. [DOI] [Google Scholar]
- 194.Hurd C.L., Durante K.M., Harrison P.J. vol. 39. 2019. pp. 435–440. (Influence of Bryozoan Colonization on the Physiology of the Kelp Macrocystis Integrifolia (Laminariales, Phaeophyta) from Nitrogen-Rich and -poor Sites in Barkley Sound, British Columbia, Canada). 102216/I0031-8884-39-5-4351. [DOI] [Google Scholar]
- 195.Mols-Mortensen A., Ortind E.Á.G., Jacobsen C., Holdt S.L. Variation in growth, yield and protein concentration in Saccharina latissima (Laminariales, Phaeophyceae) cultivated with different wave and current exposures in the Faroe Islands. J. Appl. Phycol. 2017;29:2277–2286. doi: 10.1007/S10811-017-1169-4/FIGURES/7. [DOI] [Google Scholar]
- 196.Paradas W.C., Salgado L.T., Pereira R.C., Hellio C., Atella G.C., De Lima Moreira D., et al. A novel antifouling defense strategy from red seaweed: exocytosis and deposition of fatty acid derivatives at the cell wall surface. Plant Cell Physiol. 2016;57:1008–1019. doi: 10.1093/PCP/PCW039. [DOI] [PubMed] [Google Scholar]
- 197.Hurd C.L., Harrison P.J., Bischof K., Lobban C.S. Seaweed ecology and physiology. Seaweed Ecol Physiol Second Ed. 2014:1–551. doi: 10.1017/CBO9781139192637. [DOI] [Google Scholar]
- 198.Hepburn C.D., Holborow J.D., Wing S.R., Frew R.D., Hurd C.L. Exposure to waves enhances the growth rate and nitrogen status of the giant kelp Macrocystis pyrifera. Mar. Ecol. Prog. Ser. 2007;339:99–108. doi: 10.3354/MEPS339099. [DOI] [Google Scholar]
- 199.Kregting L.T., Hepburn C.D., Hurd C.L., Pilditch C.A. Seasonal patterns of growth and nutrient status of the macroalga Adamsiella chauvinii (Rhodophyta) in soft sediment environments. J Exp Mar Bio Ecol. 2008;360:94–102. doi: 10.1016/J.JEMBE.2008.04.001. [DOI] [Google Scholar]
- 200.Nagler P.L., Glenn E.P., Nelson S.G., Napolean S. Effects of fertilization treatment and stocking density on the growth and production of the economic seaweed Gracilaria parvispora (Rhodophyta) in cage culture at Molokai, Hawaii. Aquaculture. 2003;219:379–391. doi: 10.1016/S0044-8486(02)00529-X. [DOI] [Google Scholar]
- 201.Atkinson M.J., Bilger R.W. Effects of water velocity on phosphate uptake in coral reef-hat communities. Limnol. Oceanogr. 1992;37:273–279. doi: 10.4319/LO.1992.37.2.0273. [DOI] [Google Scholar]
- 202.Gaylord B., Blanchette C.A., Denny M.W. Mechanical consequences of size in wave-swept algae. Ecol. Monogr. 1994;64:287–313. doi: 10.2307/2937164. [DOI] [Google Scholar]
- 203.Burrows M.T., Harvey R., Robb L. Wave exposure indices from digital coastlines and the prediction of rocky shore community structure. Mar. Ecol. Prog. Ser. 2008;353:1–12. doi: 10.3354/MEPS07284. [DOI] [Google Scholar]
- 204.Stocker T.F., Qin D., Plattner G.-K., Tignor M.M.B., Allen S.K., Boschung J., et al. 2013. Climate Change 2013 the Physical Science Basis Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. [Google Scholar]
- 205.Tomascik T. vol. vol. III. Oxford Univ Press; 1997. pp. 13–23. (The Ecology of the Indonesian Seas, Vol. VIII, Part. 2, Chapters). 643–1388. [Google Scholar]
- 206.Talley L.D., Maccracken M.C., Perry J.S., Munn T. In: Encycl. Glob. Environ. Chang. MacCracken M.C., Perry J.S., editors. John Wiley & Sons, Ltd; Chichester, UK: 2002. Salinity patterns in the ocean editor-in-chief; pp. 629–640. [Google Scholar]
- 207.Kumar M., Kumari P., Gupta V., Reddy C.R.K., Jha B. Biochemical responses of red alga Gracilaria corticata (Gracilariales, Rhodophyta) to salinity induced oxidative stress. J Exp Mar Bio Ecol. 2010;391:27–34. doi: 10.1016/J.JEMBE.2010.06.001. [DOI] [Google Scholar]
- 208.Periyasamy C., Rao P.V.S., Anantharaman P. Spatial and temporal variation in carrageenan yield and gel strength of cultivated Kappaphycus alvarezii (Doty) Doty in relation to environmental parameters in Palk Bay waters, Tamil Nadu, Southeast coast of India. J. Appl. Phycol. 2015;281:525–532. doi: 10.1007/S10811-015-0536-2. 2015;28. [DOI] [Google Scholar]
- 209.Siddiqui S.A., Agrawal S., Brahmbhatt H., Rathore M.S. Metabolite expression changes in Kappaphycus alvarezii (a red alga) under hypo- and hyper-saline conditions. Algal Res. 2022;63 doi: 10.1016/J.ALGAL.2022.102650. [DOI] [Google Scholar]
- 210.R M., S V., M G., F V.B. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–498. doi: 10.1016/J.TPLANTS.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 211.Lee T.-M., Liu C.-H. Correlation of decreased calcium contents with proline accumulation in the marine green macroalga Ulva fasciata exposed to elevated NaCl contents in seawater. J. Exp. Bot. 1999;50:1855–1862. doi: 10.1093/JXB/50.341.1855. [DOI] [Google Scholar]
- 212.Ding L., Ma Y., Huang B., Chen S. Effects of seawater salinity and temperature on growth and pigment contents in Hypnea cervicornis J. Agardh (Gigartinales, Rhodophyta) BioMed Res. Int. 2013;2013 doi: 10.1155/2013/594308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Orfanidis S. Light requirements for growth of six shade-acclimated Mediterranean macroalgae. Mar Biol. 1992 1123 1992;112:511–515. doi: 10.1007/BF00356298. [DOI] [Google Scholar]
- 214.Yong W.T.L., Ting S.H., Yong Y.S., Thien V.Y., Wong S.H., Chin W.L., et al. Optimization of culture conditions for the direct regeneration of Kappaphycus alvarezii (Rhodophyta, Solieriaceae) J. Appl. Phycol. 2014;26:1597–1606. doi: 10.1007/s10811-013-0191-4. [DOI] [Google Scholar]
- 215.Cuhel R.L., Ortner P.B., Lean D.R.S. Night synthesis of protein by algae1. Limnol. Oceanogr. 1984;29:731–744. doi: 10.4319/LO.1984.29.4.0731. [DOI] [Google Scholar]
- 216.Levy I., Maxim C., Friedlander M. Fatty acid distribution among some red algal macrophytes. J. Phycol. 1992;28:299–304. doi: 10.1111/J.0022-3646.1992.00299.X. [DOI] [Google Scholar]
- 217.Huang S., Li K., Pan Y., Yu Y., Wernberg T., de Bettignies T., et al. Artificial light source selection in seaweed production: growth of seaweed and biosynthesis of photosynthetic pigments and soluble protein. PeerJ. 2021;9 doi: 10.7717/PEERJ.11351/SUPP-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Msuya F E., Brugere C., Jiddawi N., Nyonje B., Maly R. Livelihoods and Environmental Protection Photo Credit: Cecile Brugere. Inst Mar Sci Zanzibar; 2018. The introduction of an improved seaweed farming technology for women's empowerment. [DOI] [Google Scholar]
- 219.Clare A.S. vol. 9. 2009. pp. 211–229. (Marine Natural Product Antifoulants: Status and Potential). 101080/08927019609378304. [DOI] [Google Scholar]
- 220.D F., C K., S L., C L., F G. Pyrethroids as promising marine antifoulants: laboratory and field studies. Mar. Biotechnol. 2009;11:153–160. doi: 10.1007/S10126-008-9130-9. [DOI] [PubMed] [Google Scholar]
- 221.Reith J.H., Deurwaarder E.P., Hemmes K., Lettings G., Curvers A.P.W.M., Brandenburg W., et al. 2005. BIO-OFFSHORE: Grootschalige teelt van zeewieren in combinatie met offshore windparken in de Noordzee. [Google Scholar]
- 222.Fernández P.A., Leal P.P., Henríquez L.A. vol. 58. 2019. pp. 542–551. (Co-culture in Marine Farms: Macroalgae Can Act as Chemical Refuge for Shell-Forming Molluscs under an Ocean Acidification Scenario). 101080/0031888420191628576. [DOI] [Google Scholar]
- 223.Barta P. WALL Str J; 2008. Indonesia Got Soaked when the Seaweed Bubble Burst.https://www.wsj.com/articles/SB122454073909251775 [Google Scholar]
- 224.Zamroni A., Yamao M. Coastal resource management: fishermen-s perceptions of seaweed farming in Indonesia. Int J Nutr Food Eng. 2011;12 doi: 10.5281/ZENODO.1330433. [DOI] [Google Scholar]
- 225.Fausayana I. 2017. Habitus, Modal Dan Kelembagaan Pembudidayaan Rumput Laut : (Dalam Meningkatkan Ekonomi Masyarakat Pesisir) [Google Scholar]
- 226.Wood D., Capuzzo E., Kirby D., Mooney-McAuley K., Kerrison P. UK macroalgae aquaculture: what are the key environmental and licensing considerations? Mar Policy. 2017;83:29–39. doi: 10.1016/J.MARPOL.2017.05.021. [DOI] [Google Scholar]
- 227.Barbier Michèle, Charrier Bénédicte. Pegasus - phycomorph European guidelines for a SUstainable aquaculture of seaweeds - phycomorph n.d. https://www.phycomorph.org/pegasus-phycomorph-european-guidelines-for-a-sustainable-aquaculture-of-seaweeds
- 228.Araújo R., Vázquez Calderón F., Sánchez López J., Azevedo I.C., Bruhn A., Fluch S., et al. Current status of the algae production industry in Europe: an emerging sector of the blue bioeconomy. Front. Mar. Sci. 2021;7:1247. doi: 10.3389/FMARS.2020.626389/BIBTEX. [DOI] [Google Scholar]
- 229.Billing S.L., Rostan J., Tett P., Macleod A. Is social license to operate relevant for seaweed cultivation in Europe? Aquaculture. 2021;534 doi: 10.1016/j.aquaculture.2020.736203. [DOI] [Google Scholar]
- 230.Nuryadi A.M., Sara L., Rianda L., Bafadal A. vol. 12. Southeast Sulawesi; Indonesia: 2019. (A Model for Developing Seaweed Agribusiness in South Konawe). [Google Scholar]
- 231.Thamrin Y., Muis M., Wahyu A., Hardianti A. Seaweed farmers and work fatigue: a mixed-method approach. Open Access Maced J Med Sci. 2020;8:192–195. doi: 10.3889/oamjms.2020.5226. [DOI] [Google Scholar]
- 232.Thamrin Y., Wahyu A., Russeng S.S., Wahyuni A., Hardianti A. Ergonomics and musculoskeletal disorders among seaweed workers in Takalar Regency: a mixed method approach. Med Clínica Práctica. 2020;3 doi: 10.1016/J.MCPSP.2020.100110. [DOI] [Google Scholar]
- 233.Outlook India Seaweed mountains in Mexican beach towns affect tourism and pose health risks. Outlook India. 2022 https://www.outlookindia.com/travel/seaweed-mountains-in-mexican-beach-towns-affect-tourism-and-pose-health-risks-news-220086 [Google Scholar]
- 234.Makame M.O., Hamad A.R., Said M.S., Mushi A., Sharif K. Moving seaweed farms from shallow to deep seawater to cope with warming and diseases in zanzibar. Current socio-economic and cultural barriers. J. Sustain. Dev. 2021;14:p29. doi: 10.5539/JSD.V14N5P29. [DOI] [Google Scholar]
- 235.Msuya F.E., Hurtado A.Q. vol. 52. 2017. pp. 482–494. (The Role of Women in Seaweed Aquaculture in the Western Indian Ocean and South-East Asia). 101080/0967026220171357084. [DOI] [Google Scholar]
- 236.Lumenyela R.A., Magasha O., Dimosso P. Adoption of seaweed improved farming techniques among farmers in Zanzibar: an application of Adoption and diffusion Outcome prediction tool. Soc Sci Humanit Open. 2023;7 doi: 10.1016/J.SSAHO.2023.100472. [DOI] [Google Scholar]
- 237.UNEP . United Nations Off Nairob; 2022. Gender Mainstreaming in Coastal and Marine Ecosystems Management: Principles, Case Studies and Lessons Learned.https://www.unep.org/resources/report/gender-mainstreaming-coastal-and-marine-ecosystems-management-principles-case [Google Scholar]
- 238.Msuya F.E., Bolton J., Pascal F., Narrain K., Nyonje B., Cottier-Cook E.J. Seaweed farming in Africa: current status and future potential. J. Appl. Phycol. 2022;34:985–1005. doi: 10.1007/S10811-021-02676-W/FIGURES/3. [DOI] [Google Scholar]
- 239.Citizen The. Sh3.2 trillion invested in Zanzibar's Blue economy so far. 2022. https://www.thecitizen.co.tz/tanzania/zanzibar/sh3-2-trillion-invested-in-zanzibar-s-blue-economy-so-far-3966560
- 240.Woods Hole Oceanographic Institution . Woods Hole Oceanogr Inst; 2022. Innovative, New “Road Map” for Kelp Crop Improvement.https://www.newswise.com/articles/innovative-new-road-map-for-kelp-crop-improvement [Google Scholar]
- 241.Pérez M.J., Falqué E., Domínguez H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs. 2016;14 doi: 10.3390/MD14030052. Page 52 2016;14:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Smith B. Our story — GreenWave n.d. https://www.greenwave.org/story
- 243.Chopin T., Tacon A.G.J. Importance of seaweeds and extractive species in global. Aquaculture Production. 2020;29:139–148. doi: 10.1080/23308249.2020.1810626. Https://DoiOrg/101080/2330824920201810626. [DOI] [Google Scholar]
- 244.Duarte C.M., Bruhn A., Krause-Jensen D. A seaweed aquaculture imperative to meet global sustainability targets. Nat. Sustain. 2021 53 2021;(5):185–193. doi: 10.1038/s41893-021-00773-9. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
N/A for this article.